Abstract
Diagnosing relapse after radiotherapy for lung cancer is challenging. The specificity of both CT and 18F-FDG PET/CT is low because of radiation-induced changes. 3′-deoxy-3′-18F-fluorothymidine (18F-FLT) PET has previously demonstrated higher specificity for malignancy than 18F-FDG PET. We investigated the value of 18F-FLT PET/CT for diagnosing relapse in irradiated lung cancer. Methods: Patients suspected of relapse of lung cancer after definitive radiotherapy (conventional fractionated radiotherapy [cRT] or stereotactic body radiotherapy [SBRT]) were included. Sensitivity and specificity were analyzed both within the irradiated high-dose volume (HDV) and on a patient basis. Marginal differences and interobserver agreement were assessed. Results: Sixty-three patients who had received radiotherapy in 70 HDVs (34 cRT; 36 SBRT) were included. The specificity of 18F-FLT PET/CT was higher than that of 18F-FDG PET/CT (HDV, 96% [95% CI, 87–100] vs. 71% [95% CI, 57–83] [P = 0.0039]; patient-based, 90% [95% CI, 73–98] vs. 55% [95% CI, 36–74] [P = 0.0020]). The difference in specificity between 18F-FLT PET/CT and 18F-FDG PET/CT was higher after cRT than after SBRT. The sensitivity of 18F-FLT PET/CT was lower than that of 18F-FDG PET/CT (HDV, 69% [95% CI, 41–89] vs. 94% [95% CI, 70–100] [P = 0.1250]; patient-based, 70% [95% CI, 51–84] vs. 94% [95% CI, 80–99] [P = 0.0078]). Adding 18F-FLT PET/CT when 18F-FDG PET/CT was positive or inconclusive improved the diagnostic value compared with 18F-FDG PET/CT alone. In cRT HDVs, the probability of malignancy increased from 67% for 18F-FDG PET/CT alone to 100% when both tracers were positive. Conclusion: 18F-FLT PET/CT adds diagnostic value to 18F-FDG PET/CT in patients with suspected relapse. The diagnostic impact of 18F-FLT PET/CT was highest after cRT. We suggest adding 18F-FLT PET/CT when 18F-FDG PET/CT is inconclusive or positive within the previously irradiated volume to improve diagnostic value in patients for whom histologic confirmation is not easily obtained.
Keywords: lung cancer, radiotherapy, relapse, 18F-FDG PET/CT, 18F-FLT PET/CT
Disease control after definitive radiotherapy is initially high, but 15%–40% of the patients will eventually experience locoregional failure (1–5). Many patients experience radiation-induced pneumonitis (3%–35%) or fibrosis (30%–50%) after radiotherapy (3,6), and distinguishing local recurrence from radiation-induced lung injuries is challenging. Active surveillance with CT is recommended (7), but changes on CT after radiotherapy may mimic recurrence (8). 18F-FDG PET/CT is recommended if relapse is suspected (9). Posttreatment inflammation may, however, cause high 18F-FDG uptake, thus reducing the specificity of 18F-FDG PET (10).
3′-deoxy-3′-18F-fluorothymidine (18F-FLT) is a marker of proliferation (11). 18F-FLT PET has a higher specificity than 18F-FDG PET and performed better in differential diagnosis of inflammatory lesions in the lung (12). The potential of 18F-FLT PET to differentiate malignancy from radiation-induced changes is less well described (13–15).
One small study showed correct diagnosis of disease progression with 18F-FLT PET/CT in 7 of 8 patients after stereotactic body radiotherapy (SBRT) for lung cancer (14). To our knowledge, no publications have addressed the diagnostic value of 18F-FLT PET after conventional fractionated radiotherapy (cRT) in patients with lung cancer.
In the current study, we hypothesized that 18F-FLT PET/CT could better diagnose relapse after radiotherapy for lung cancer.
MATERIALS AND METHODS
Patients
Patients were prospectively included if meeting the following criteria: histologically confirmed non–small cell or small cell lung cancer, treatment with definitive radiotherapy within the last 24 mo, and current suspicion of relapse warranting an 18F-FDG PET/CT examination. The causes of relapse suspicion are specified in Table 1. Patients were analyzed according to treatment regime: cRT (i.e., normo- and hyperfractionated radiotherapy) or SBRT.
TABLE 1.
Patient Characteristics
| Characteristic | All patients (n = 63) | cRT patients (n = 34)* | SBRT patients (n = 30)* |
|---|---|---|---|
| Age at 18F-FLT PET/CT (y) | 70 (55–86) | 68 (58–86) | 75 (55–86) |
| Sex | |||
| Male | 36 (57%) | 18 (53%) | 19 (63%) |
| Female | 27 (43%) | 16 (47%) | 11 (37%) |
| Histology | |||
| Adenocarcinoma | 30 (47.6%) | 15 (44.1%) | 15 (50%) |
| Squamous cell carcinoma | 25 (39.7%) | 13 (38.2%) | 13 (43.3%) |
| NSCLC not otherwise specified | 4 (6.3%) | 2 (5.9%) | 2 (6.7%) |
| SCLC | 2 (3.2%) | 2 (5.9%) | 0 |
| Mixed NSCLC/SCLC | 2 (3.2%) | 2 (5.9%) | 0 |
| Stage at diagnosis | |||
| Ia | 13 (20.6%) | 0 | 13 (43.3%) |
| Ib | 6 (9.5%) | 1 (2.9%) | 5 (16.7%) |
| IIa | 2 (3.2%) | 0 | 2 (6.7%) |
| IIb | 5 (7.9%) | 1 (2.9%) | 4 (13.3%) |
| IIIa | 15 (23.8%) | 14 (41.2%) | 2 (6.7%) |
| IIIb | 16 (25.4%) | 15 (44.1%)* | 1 (3.3%)* |
| IV | 6 (9.5%) | 3 (8.8%) | 3 (10.0%) |
| Radiotherapy | |||
| Normofractionated, 60 Gy (24–33 F) | 30 (47.6%) | 31 (91.2%) | |
| Hyperfractionated, 45–60 Gy (30–40 F) | 3 (4.8%) | 3 (8.8%) | |
| SBRT | |||
| 50 Gy (5 F) | 2 (3.2%) | 2 (6.7%) | |
| 45–72 Gy (3 F) | 28 (44.4%) | 28 (93.3%) | |
| Chemotherapy | 35 (55.6%) | 32 (94.1%) | 3 (10%) |
| Cause of relapse suspicion | |||
| Symptoms | 1 (1.6%) | 1 (2.9%) | 0 |
| CT (surveillance) | 53 (84.1%) | 30 (88.2%) | 24 (80%) |
| CT and symptoms | 2 (3.2%) | 2 (5.9%) | 0 |
| 18F-FDG PET/CT (surveillance) | 6 (9.5%) | 0 | 6 (20%) |
| 18F-FDG PET/CT and symptoms | 1 (1.6%) | 1 (2.9%) | 0 |
| Days between radiotherapy end and 18F-FLT PET/CT | 237 (34–729) | 277 (34–626) | 236 (108–729) |
| Days between 18F-FDG and 18F-FLT PET/CT | 6 (1–30) | 6 (1–22) | 6 (1–30) |
One patient was included in both subgroups.
NSCLC = non–small cell lung cancer; SCLC = small-cell lung cancer; F = fractions.
Qualitative data are numbers and percentages; continuous data are medians and ranges.
Patients were recruited from Copenhagen University Hospital, Rigshospitalet, Bispebjerg University Hospital, and Herlev University Hospital in Denmark from January 2015 to January 2019. The study protocol was approved by the local Ethics Committee (approval H-4-2014-060) and by institutional review boards. All patients gave written informed consent. The study was registered at clinicaltrials.gov (identifier NCT029995889).
Imaging
18F-FDG PET/CT was conducted as a routine clinical investigation at the referring hospital according to local procedures. Details are available in Supplemental Table 1 (supplemental materials are available at http://jnm.snmjournals.org). Patients fasted at least 4 h before receiving an injection of 18F-FDG (200 MBq or 4 MBq/kg, according to institutional protocol) and rested 60 min between injection and scan. Images were reconstructed following vendor recommendations or international clinical guidelines for 18F-FDG PET imaging.
18F-FLT PET/low-dose CT was performed at Rigshospitalet on a Siemens Biograph TruePoint TrueV 40 or 64 PET/CT scanner. 18F-FLT (5 MBq/kg; maximum, 350 MBq) was injected 60 ± 10 min before PET/CT without restrictions regarding fasting or resting. Static regional imaging was obtained from the skull base to the iliac bone. 18F-FLT PET images were reconstructed using ordered-subset expectation maximization with point-spread-function modeling, 3 iterations, 21 subsets, and a gaussian postreconstruction filter of 2 mm in full width at half maximum.
Image Analysis
All images were analyzed on a Mirada Medical Ltd. XD 3.6 workstation.
The PET/CT images were interpreted retrospectively as project readings, independently of subsequent management of the patients. The interpreters were unaware of the clinical data and previous PET results but not of previous CT results. Project readings were performed qualitatively and jointly by an experienced nuclear medicine physician and a radiologist. The 18F-FLT PET/CT images were double-read by 2 observer-teams. Interpretation of 18F-FDG PET/CT and 18F-FLT PET/CT images from the same patient by the same observer-team was separated by a minimum of 3 mo.
Up to 3 lesions in each PET/CT scan were evaluated for malignancy using a 5-point scale: definitely benign, probably benign, inconclusive, probably malignant, and definitely malignant (patient-based analysis). From the previous radiotherapy plan, the high-dose volume (HDV) was defined within the 50% isodose curve, and PET-evaluated lesions within the HDV were identified (HDV-based analysis). If an HDV lesion was not matched with a PET-evaluated lesion, the HDV lesion was classified as definitely benign.
SUVmax from 18F-FDG PET and 18F-FLT PET was measured in the evaluated lesions and in the HDV.
Endpoint and Reference Standard
The endpoint was relapse status (relapse or no relapse) within 6 mo after 18F-FLT PET/CT. Confirmation by histology was encouraged in the protocol. However, if histology was not clinically feasible, a compound reference standard was applied. Use of this standard was assigned by an experienced clinical oncologist and was based on a review of patient records, including histology, imaging, invasive procedures, and conference decisions. The clinical oncologist did not know the name or age of the patient, the dates of the exams, or the names of involved physicians.
Statistics
The study size was determined from a power calculation based on previous studies suggesting different results from 18F-FDG PET and 18F-FLT PET in at least 20% of lung cancer patients (16–18). With a power of 80% and a 2-sided α-level of 0.05 for significance, there needed to be at least 29 patients in each group. Taking the possibility of dropouts into account, each group was appointed up to 35 patients.
The diagnostic values of 18F-FDG PET/CT and 18F-FLT PET/CT were analyzed within the HDV and on a patient basis as a whole-body analysis. For the HDV-based analysis, all HDVs from each patient were included. For the patient-based analysis, the worst grading on the 5-point scale in each patient was selected. Sensitivity, specificity, negative predictive value, positive predictive value, and accuracy were calculated. Inconclusive PET results were included in the analysis one time as a positive result and one time as a negative result, and the 2 scenarios were analyzed separately. Patients or HDVs with an inconclusive reference standard were excluded from the diagnostic analysis. Marginal differences in sensitivity and specificity between 18F-FDG PET/CT and 18F-FLT PET/CT were calculated by McNemar tests for all 4 combinations of handling inconclusive 18F-FDG PET and 18F-FDG PET results. Interobserver agreement was calculated with κ-statistics for positive versus negative or inconclusive 18F-FLT PET/CT results.
A model combining 18F-FDG PET/CT and 18F-FLT PET/CT was suggested, and the diagnostic value of combined 18F-FDG PET/CT and 18F-FLT PET/CT was calculated.
Statistical analyses were performed in SPSS, version 25. With MedCalc (version 19.2; MedCalc Software Ltd.), 95% CIs for diagnostic value and marginal differences were determined.
RESULTS
Patients
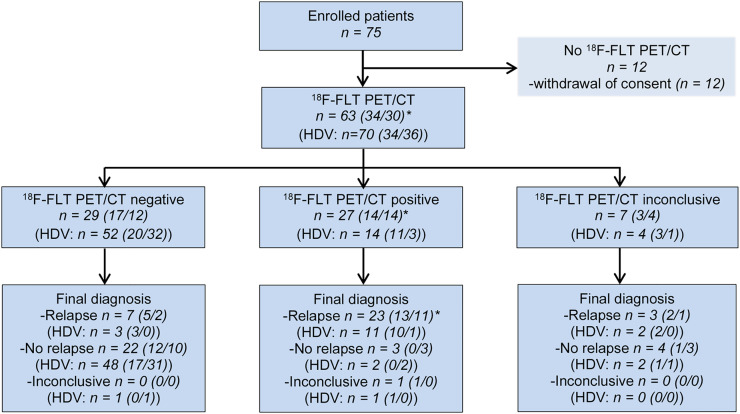
In total, 75 patients were enrolled. However, 12 patients withdrew consent; thus, 63 patients were evaluable (Fig. 1). Two patients participated twice in the study; the second time was due to a newly suspected relapse. One patient received both cRT and SBRT and was included in both subgroups. This patient was treated initially with cRT and later with SBRT because of a new malignant lesion. Accordingly, 34 patients had been treated with cRT and 30 patients with SBRT. In accordance with the indications for the radiotherapy regimes, the stage at the time of diagnosis was higher for the cRT group than for the SBRT group. Patient characteristics are presented in Table 1.
FIGURE 1.
Patient flow in study. Numbers in parentheses refer to subgroups (cRT/SBRT). *One patient was included in both subgroups.
In total, 70 HDVs (34 cRT HDVs; 36 SBRT HDVs) from the 63 patients were included in the analysis. Two patients were treated with radiotherapy twice; the second time was due to local relapse. Four patients received SBRT in 2 (n = 3) or 3 (n = 1) SBRT HDVs at initial diagnosis because of several lung lesions. In each patient, cRT HDV was coherent; thus, 1 cRT HDV was included per cRT patient.
Diagnostic Value of 18F-FDG PET/CT and 18F-FLT PET/CT in Irradiated HDV
During the 6 mo of follow-up, relapse was diagnosed in 16 HDVs, as confirmed by biopsy or, in 10 of the 16 cases, by subsequent progression. Nonrelapse was confirmed by 6 mo of follow-up without progression or by negative biopsy in 45 of 52 HDVs; in the remaining HDVs, the confirmation level was low (Table 2).
TABLE 2.
Clinical Outcome and Basis for Confirmation
| Outcome | Confirmation basis | All | cRT | SBRT |
|---|---|---|---|---|
| HDVs | 70 | 34 | 36 | |
| Relapse | 16 | 15 | 1 | |
| Histology | 4 | 4 | 0 | |
| Subsequent progression | 6 | 5 | 1 | |
| 18F-FDG PET/CT only | 6 | 6 | 0 | |
| No relapse | 52 | 18 | 34 | |
| No subsequent progression | 44 | 15 | 29 | |
| Negative biopsy (follow-up not applicable because of systematic treatment) | 1 | 0 | 1 | |
| 18F-FDG PET/CT only (follow-up not applicable because of systematic treatment) | 7 | 3 | 4 | |
| Inconclusive | 2* † | 1* | 1 † | |
| Patients | 63 | 34 ‡ | 30 ‡ | |
| Relapse | 33 | 20 | 14 | |
| Histology | 8 | 4 | 4 | |
| Subsequent progression | 11 | 5 | 7 | |
| Disseminated disease | 6 | 4 | 2 | |
| 18F-FDG PET/CT only | 8 | 7 | 1 | |
| No relapse | 29 | 13 | 16 | |
| No subsequent progression | 29 | 13 | 16 | |
| Inconclusive | 1† | 1† | 0 |
Biopsies were performed twice; both were suggestive but not conclusive of malignancy. Two months after end of follow-up, relapse was diagnosed on basis of metastatic adenocarcinoma cells in exudate from pericardium.
Clinical PET report described “progression of radiation-induced changes,” and biopsy was suggested although not performed. Follow-up was not applicable, as patient received systemic treatment due to distant relapse.
One patient was included in both subgroups.
18F-FLT PET/CT and 18F-FDG PET/CT were positive in 14 and 29 HDVs, respectively. Sensitivity and negative predictive value were lower for 18F-FLT PET/CT than for 18F-FDG PET/CT, and the specificity and positive predictive value were higher for 18F-FLT PET/CT than for 18F-FDG PET/CT, both when considering inconclusive PET results positive and when considering inconclusive PET results negative. The results from all diagnostic analysis are presented in Table 3. A cross-tabulation of PET results relative to clinical outcome is available in Supplemental Table 2.
TABLE 3.
Diagnostic Value of 18F-FDG PET/CT and 18F-FLT PET/CT Within Irradiated HDV
| HDV group | Tracer | Handling of inconclusive PET results | Sensitivity | Specificity | Positive predictive value | Negative predictive value | Accuracy |
|---|---|---|---|---|---|---|---|
| All (n = 68) | 18F-FDG | As positive | 94 (70–100) | 71 (57–83) | 50 (39–61) | 97 (85–100) | 76 (65–86) |
| As negative | 94 (70–100) | 75 (61–86) | 54 (41–65) | 98 (85–100) | 79 (68–88) | ||
| 18F-FLT | As positive | 81 (54–96) | 92 (81–98) | 76 (55–90) | 94 (85–98) | 90 (80–96) | |
| As negative | 69 (41–89) | 96 (87–100) | 85 (58–96) | 91 (80–96) | 90 (80–96) | ||
| cRT (n = 33) | 18F-FDG | As positive | 93 (68–100) | 61 (36–83) | 67 (52–78) | 92 (62–99) | 76 (58–89) |
| As negative | 93 (68–100) | 67 (41–87) | 70 (54–82) | 92 (64–99) | 79 (61–91) | ||
| 18F-FLT | As positive | 80 (52–96) | 94 (73–100) | 92 (64–99) | 85 (67–94) | 88 (72–97) | |
| As negative | 67 (38–88) | 100 (81–100) | 100 | 78 (64–88) | 85 (68–95) | ||
| SBRT (n = 35) | 18F-FDG | As positive | 100 (3–100) | 76 (59–89) | 11 (6–19) | 100 | 77 (60–90) |
| As negative | 100 (3–100) | 79 (62–91) | 13 (7–22) | 100 | 80 (63–92) | ||
| 18F-FLT | As positive | 100 (3–100) | 91 (76–98) | 25 (10–50) | 100 | 91 (77–98) | |
| As negative | 100 (3–100) | 94 (80–99) | 33 (12–68) | 100 | 94 (81–99) |
Inconclusive PET results were handled as positive or negative. Results are from masked PET evaluations. Data are percentages, with 95% CIs in parentheses.
For simplification, this and the following subsection describe results from analyses considering inconclusive 18F-FDG PET/CT results as positive and inconclusive 18F-FLT PET/CT results as negative.
The specificity of 18F-FLT PET/CT within the HDV was 25% (95% CI, 13%–37%) higher than the specificity of 18F-FDG PET/CT (P = 0.0039); that is, 18F-FDG PET/CT was false-positive in 25% more cases than 18F-FLT/PET/CT. The difference in specificity was largest in the cRT HDVs (cRT HDV, 39% [95% CI, 16%–61%] [P = 0.0156]; SBRT HDV, 18% [95% CI, 5%–30%] [P = 0.0313]).
Though the sensitivity of 18F-FDG PET/CT was higher than the sensitivity of 18F-FLT PET/CT, the difference was not significant (all, 25% [95% CI, 4%–46%] [P = 0.1250]; cRT HDV, 27% [95% CI, 4%–49%] [P = 0.1250]; SBRT HDV, inconclusive because there was only one relapse). Cross-tabulations of 18F-FLT PET results versus 18F-FDG PET results and results from all McNemar analyses with variant handlings of inconclusive results are available in Supplemental Tables 3 and 4.
18F-FLT SUVmax in relapsed HDVs was 1.8–9.7 (median, 2.4), compared with 0.4–4.5 (median, 2.2) in benign HDVs. 18F-FDG SUVmax in relapsed HDVs was 4.0–20.5 (median, 12.8), compared with 0.7–17.5 (median, 4.1) in benign HDVs.
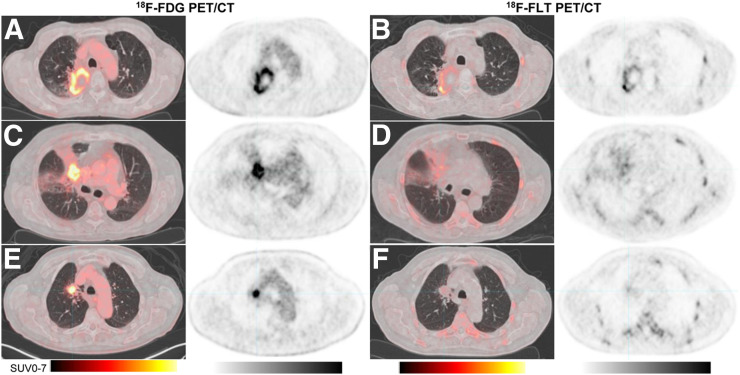
PET images illustrating the diagnostic strengths and weaknesses are shown in Figure 2.
FIGURE 2.
18F-FDG PET/CT and 18F-FLT PET/CT in 3 representative patients with suspected relapse after cRT of lung cancer. (A and B) Relapse 19 mo after end of cRT detected by 18F-FDG PET/CT (A) and 18F-FLT PET/CT (B). (C and D) No relapse 4 mo after end of cRT; 18F-FDG PET/CT was false-positive (C) and 18F-FLT PET/CT true-negative (D). (E and F) Relapse 15 mo after end of cRT; 18F-FDG PET/CT was true-positive (E) and 18F-FLT PET/CT false-negative (F). Relapse was located in lung tissue as confirmed by biopsy, not in lymph node as it may appear on these images.
Patient-Based Diagnostic Value of 18F-FDG PET/CT and 18F-FLT PET/CT
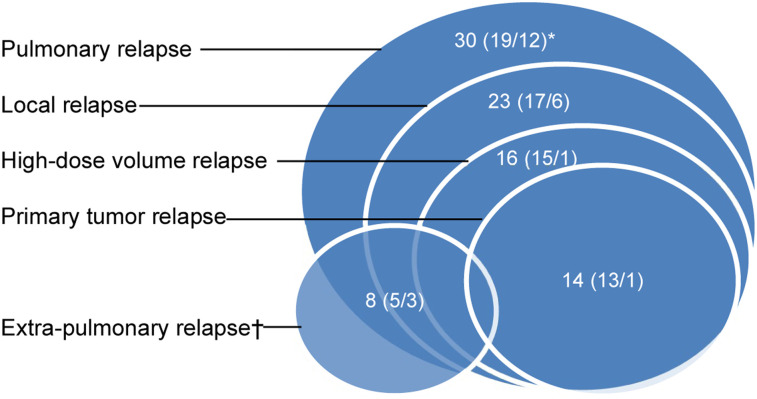
During follow-up, 33 patients (52%) were diagnosed with relapse. Figure 3 illustrates the location of the relapse. In 19 of the 33 patients, relapse was confirmed by biopsy or subsequent progression according to RECIST 1.1. Nonrelapse was confirmed by 6 mo of follow-up without progression according to RECIST 1.1 in all patients (Table 2).
FIGURE 3.
Location of relapse. In total, 33 patients had relapse; of these, 30 had pulmonary relapse. Numbers in parentheses refer to subgroups (cRT/SBRT). *One patient was included in both subgroups. †Three patients (1/2) had only extrapulmonary relapse.
18F-FLT PET/CT and 18F-FDG PET/CT were positive in 27 and 43 patients, respectively. Cross-tabulations and diagnostic value are available in Supplemental Tables 2 and 5.
The specificity of 18F-FLT PET/CT was 34% (95% CI, 17%–52%) higher than the specificity of 18F-FDG PET/CT in all patients (90% [95% CI, 73%–98%] vs. 55% [95% CI, 36%–74%]; P = 0.0020). In cRT patients, 18F-FLT PET/CT outperformed 18F-FDG PET/CT, with a 54% (95% CI, 27%–81%) higher specificity (100% [95% CI, 75%–100%] vs. 46% [95% CI, 19%–75%]; P = 0.0156). The specificity was not significantly different in SBRT patients (19% [95% CI, −0.4%–38%]; P = 0.2500).
The sensitivity of 18F-FDG PET/CT was 24% (95% CI, 10%–39%) higher than the sensitivity of 18F-FLT PET/CT in all patients (94% [95% CI, 80–99] vs. 70% [95% CI, 51–84]; P = 0.0078). In the subgroups, the difference in sensitivity did not reach statistical significance (cRT patients, 25% [95% CI, −6%–44%] [P = 0.0625]; SBRT patients, 21% [95% CI, −0.1%–43%] [P = 0.2500]). Cross-tabulations and McNemar analyses with variant handlings of inconclusive PET results are available in Supplemental Tables 3 and 6.
18F-FLT SUVmax in patients with pulmonary relapse was 0.9–9.7 (median, 3.7), compared with 0.8–4.5 (median, 2.5) in patients without pulmonary relapse. measured in the lowest-grade lesions from the 5-point grading scale 18F-FDG SUVmax in patients with pulmonary relapse was 1.2–20.5 (median, 8.6), compared with 1.9–17.5 (median, 4.6) in patients without pulmonary relapse.
Interobserver Agreement of 18F-FLT PET/CT
Patient-based and HDV-based interobserver agreement was moderate (0.47 and 0.57, respectively). Interobserver agreement was highest in cRT patients (0.68) and cRT HDVs (0.70) and only moderate or poor in SBRT patients (0.45) and SBRT HDVs (−0.04).
Combined Diagnostic Value of 18F-FDG PET/CT and 18F-FLT PET/CT
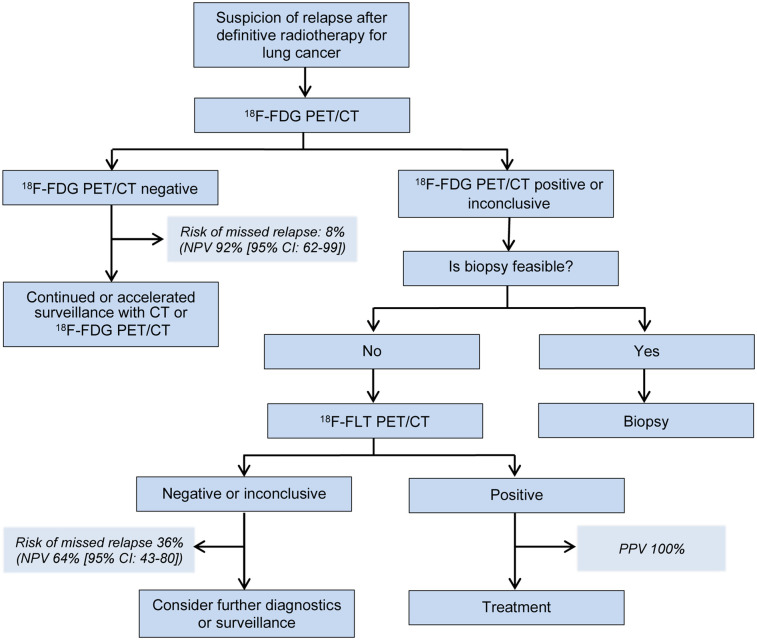
To exploit the high negative predictive value of 18F-FDG PET/CT and the high positive predictive value of 18F-FLT PET/CT, we suggest adding 18F-FLT PET/CT when the 18F-FDG PET/CT results are positive or inconclusive. When 18F-FDG PET/CT was negative, 18F-FLT PET/CT provided no additional value, as all negative 18F-FDG PET/CT results were accompanied by negative 18F-FLT PET/CT results. The suggested diagnostic flow is illustrated in Figure 4.
FIGURE 4.
Suggested diagnostic flow for patients suspected for having relapse within irradiated HDV. Positive predictive value (PPV) and negative predictive value (NPV) are given for HDVs treated with cRT.
Diagnostic accuracy was improved in the combined model when compared with a single positive or inconclusive 18F-FDG PET/CT result (Table 4). The impact of adding 18F-FLT PET/CT to positive or inconclusive 18F-FDG PET/CT results was highest in cRT patients, raising the probability of malignancy from 72% after positive or inconclusive 18F-FDG PET/CT results to 100% when 18F-FLT PET/CT results were positive.
TABLE 4.
Diagnostic Value of 18F-FLT PET/CT After Positive 18F-FDG PET/CT
| Group | Pretest probability of malignancy* | Sensitivity | Specificity | Positive likelihood ratio | Negative likelihood ratio | Positive predictive value | Negative predictive value | Accuracy |
|---|---|---|---|---|---|---|---|---|
| Patients | ||||||||
| All (n = 44) | 70% | 74% (55%–88%) | 77% (46%–95%) | 3.2 (1.2–8.9) | 0.3 (0.2–0.7) | 88% (74%–95%) | 56% (39%–71%) | 75% (60%–87%) |
| cRT (n = 25) | 72% | 72% (47%–90%) | 100% (59%–100%) | NA | 0.3 (0.1–0.6) | 100% | 58% (40%–75%) | 80% (59%–93%) |
| SBRT (n = 20) | 70% | 79% (49%–95%) | 50% (12%–88%) | 1.6 (0.7–3.7) | 0.4 (0.1–1.5) | 79% (61%–90%) | 50% (22%–78%) | 70% (46%–88%) |
| HDVs | ||||||||
| All (n = 30) | 50% | 73% (45%–92%) | 87% (60%–98%) | 5.5 (1.5–20.7) | 0.3 (0.1–0.7) | 85% (59%–95%) | 76% (58%–89%) | 80% (61%–92%) |
| cRT (n = 21) | 67% | 71% (42%–92%) | 100% (59%–100%) | NA | 0.3 (0.1–0.7) | 100% | 64% (43%–80%) | 81% (58%–95%) |
| SBRT (n = 9) | 11% | 100% (3%–100%) | 75% (35%–97%) | 4.0 (1.2–13.3) | 0 | 33% (13%–62%) | 100% | 78% (40%–97%) |
Positive predictive value for positive or inconclusive 18F-FDG PET/CT results.
NA = not applicable.
Data in parentheses are 95% CIs.
DISCUSSION
The main finding of this study was that 18F-FLT PET/CT with a high specificity and positive predictive value adds value to 18F-FDG PET/CT for the detection of relapse of lung cancer after radiotherapy. The sensitivity of 18F-FLT PET/CT was, in most settings, not significantly different from that of 18F-FDG PET/CT.
The superior specificity of 18F-FLT PET/CT is consistent with results from pretreatment studies (12) and results after SBRT (14). In the small study of Hiniker et al., sensitivity (80% [4/5]) and specificity (100% [3/3]) were high, with only one false-negative 18F-FLT PET/CT result after SBRT for lung cancer (14). Hiniker et al. included patients with suggestive 18F-FDG PET/CT results, and the rate of local relapse was higher than in our study (5/8 vs. 1/35). With only one SBRT HDV relapse, our study did not have the statistical power to allow conclusions on sensitivity in this group. Similar results have also been demonstrated after concomitant chemoradiotherapy for esophageal cancer; 18F-FLT PET/CT was superior to 18F-FDG PET/CT in distinguishing malignant tissue from esophagitis (13).
We demonstrated a higher difference in specificity between 18F-FLT PET/CT and 18F-FDG PET/CT after cRT than after SBRT, because of a combination of lower specificity for 18F-FDG PET/CT and higher specificity for 18F-FLT PET/CT after cRT than after SBRT. Different patterns of injuries in the surrounding lung tissue from different radiotherapy regimes (3,6) may explain this difference. Toxicity is related to dose deposited in surrounding lung tissues; larger HDVs in cRT regimes cause larger volumes of lung tissue to be exposed. Smaller HDVs from SBRT regimes spare the surrounding lung tissue to a higher extent. A higher prevalence of radiation-induced changes may explain the lower specificity of 18F-FDG PET/CT after cRT. The lower specificity and positive predictive value of 18F-FLT PET/CT in the SBRT HDVs than in the cRT HDVs might be caused by the very low prevalence of relapse in SBRT HDVs.
The difference in specificity between 18F-FDG PET/CT and 18F-FLT/CT was higher on a patient basis than within HDVs, as a result of the low specificity of 18F-FDG PET/CT on a patient basis. 18F-FDG PET and 18F-FLT PET were evaluated in a masked manner to make comparable and unbiased evaluations. However, in the clinical setting, knowledge of previous treatment and possible inflammatory sites is essential for evaluation of 18F-FDG PET/CT (10), and a masked reading might have a higher impact on 18F-FDG PET/CT than on 18F-FLT PET/CT. With several lesions evaluated in each patient in the patient-based analysis, the consequence of masking was more pronounced on a patient basis than in the HDV-based analysis. To quantify the consequence of masking, we compared the masked 18F-FDG PET/CT results with results from the clinical 18F-FDG PET/CT report. The specificity of 18F-FDG PET/CT was 10% (95% CI, −12%–32%) higher in the clinical report than in the masked results, but the difference was not significant (P = 0.549). Masking did not affect sensitivity (94%; P = 1). Applying 18F-FLT PET in a patient-based analysis is controversial, as 18F-FLT PET has limited use for diagnosing distant metastases due to high background-uptake in the liver and bone (19), and false positive results in lymph nodes may be caused by proliferative B lymphocytes (20). Our project was not designed to investigate the diagnostic value of 18F-FLT PET/CT on metastases; however, 8 patients were diagnosed with metastases in bones or liver. In 5 patients, 18F-FLT PET/CT missed bone or liver metastases, but because of other malignant lesions detected by 18F-FLT PET/CT, only 3 patients had false-negative 18F-FLT PET/CT results due to distant metastases. Accordingly, extrapulmonary metastases had no impact on specificity in this study but some impact on patient-based sensitivity.
There were some limitations to our study. Patients in whom recurrence was strongly suspected could be referred directly for biopsy or oncologic treatment and thus not included in this project. Masked reading of PET scans is a deviation from clinical guidelines (10) but was applied to make 18F-FDG PET/CT and 18F-FLT PET/CT evaluations comparable. Combining 18F-FDG PET with diagnostic CT and combining 18F-FLT PET with low-dose CT potentially gave 18F-FDG PET/CT an advantage over 18F-FLT PET/CT. Project readings were, however, not masked to CT, and previous diagnostic CT scans could therefore be accessed. Combining an added 18F-FLT PET scan with low-dose CT seems sufficient and reduces excessive ionizing irradiation and cost. Neither 18F-FDG nor 18F-FLT PET/CT was done with respiratory gating. Lack of gating could potentially lead to misregistration between PET and CT and a potential underestimation of tracer uptake, especially in small nodules. Relapse status was in most cases confirmed by either histology or follow-up with subsequent progression or nonprogression. In some patients, the relapse diagnosis was based solely on 18F-FDG PET/CT, as decided by a multidisciplinary conference, because of an obvious outcome on 18F-FDG PET/CT or the patient’s being unfit for invasive procedures. 18F-FDG PET/CT is recommended as a second-step test for patients with suspected relapse after radiotherapy (7), and therefore we did not exclude patients without further confirmation than 18F-FDG PET/CT. Thus, in these cases, the test result of 18F-FDG PET/CT and the reference were not independent, potentially overestimating the sensitivity and specificity of 18F-FDG PET/CT. When 18F-FLT PET/CT and 18F-FDG PET/CT results agreed, a potential overestimate would concern the absolute values of sensitivity and specificity of 18F-FLT PET/CT and 18F-FDG PET/CT but not their differences. However, when 18F-FLT PET/CT and 18F-FDG PET/CT results did not agree, and the reference was based solely on 18F-FDG PET/CT, 18F-FLT PET/CT results would always be false. Only in 2 patients in whom relapse was based solely on the 18F-FDG PET/CT did the 18F-FLT PET/CT results not agree with the 18F-FDG PET/CT results. Although 18F-FDG PET/CT was favored when further confirmation of relapse status was not obtained, the specificity of 18F-FLT PET/CT was significantly higher than that of 18F-FDG PET/CT.
Early and precise diagnosis of lung cancer relapse are essential, as surgery or reirradiation with curative intent might be feasible (1). To improve diagnosis of relapse, we suggest adding 18F-FLT PET/CT when 18F-FDG PET/CT is positive or inconclusive within the HDV. We acknowledge that in many cases renewed biopsy is required because of the possibility of a pathologic transition, which potentially changes the treatment of choice. When biopsy is feasible and favored, 18F-FLT PET/CT does not outperform invasive procedures; 18F-FLT PET might have a place for guiding biopsies, but further investigations are needed. In the many patients in whom biopsy is not feasible because of poor lung condition or difficult location, 18F-FLT PET/CT adds valuable diagnostic information.
CONCLUSION
18F-FLT PET/CT has a higher specificity than 18F-FDG PET/CT in patients who have been treated with radiotherapy, both within the HDV and on a patient basis. The diagnostic impact of 18F-FLT PET/CT was highest after cRT. We suggest adding 18F-FLT PET/CT when the results of 18F-FDG PET/CT are inconclusive or positive within the HDV in patients who are unfit for invasive procedures and when renewed histology is not essential for the further course.
DISCLOSURE
This project received funding from the Danish Cancer Society (grant R134-A8543-15-S42) and the Department of Clinical Physiology, Nuclear Medicine, and PET, Rigshospitalet, University of Copenhagen, Denmark. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is 18F-FLT PET valuable in diagnosing relapse of irradiated lung cancer?
PERTINENT FINDINGS: 18F-FLT PET/CT had a higher specificity and positive predictive value than 18F-FDG PET/CT on a patient basis and within the irradiated HDV.
IMPLICATIONS FOR PATIENT CARE: Adding 18F-FLT PET to 18F-FDG PET/CT when relapse is suspected in previously irradiated lung cancers improves diagnostic accuracy significantly.
REFERENCES
- 1. Matsuo Y. A systematic literature review on salvage radiotherapy for local or regional recurrence after previous stereotactic body radiotherapy for lung cancer. Technol Cancer Res Treat. 2018;17:1533033818798633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Prezzano KM, Ma SJ, Hermann GM, Rivers CI, Gomez-Suescun JA, Singh AK. Stereotactic body radiation therapy for non-small cell lung cancer: a review. World J Clin Oncol. 2019;10:14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ball D, Mai GT, Vinod S, et al. Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non-small-cell lung cancer (TROG 09.02 CHISEL): a phase 3, open-label, randomised controlled trial. Lancet Oncol. 2019;20:494–503. [DOI] [PubMed] [Google Scholar]
- 4. Nakajima N, Sugawara Y, Kataoka M, et al. Differentiation of tumor recurrence from radiation-induced pulmonary fibrosis after stereotactic ablative radiotherapy for lung cancer: characterization of 18F-FDG PET/CT findings. Ann Nucl Med. 2013;27:261–270. [DOI] [PubMed] [Google Scholar]
- 5. Aupérin A, Le Pechoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2181–2190. [DOI] [PubMed] [Google Scholar]
- 6. Nyman J, Hallqvist A, Lund JA, et al. SPACE: a randomized study of SBRT vs conventional fractionated radiotherapy in medically inoperable stage I NSCLC. Radiother Oncol. 2016;121:1–8. [DOI] [PubMed] [Google Scholar]
- 7. Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv1–iv21. [DOI] [PubMed] [Google Scholar]
- 8. Huang K, Dahele M, Senan S, et al. Radiographic changes after lung stereotactic ablative radiotherapy (SABR): can we distinguish recurrence from fibrosis? A systematic review of the literature. Radiother Oncol. 2012;102:335–342. [DOI] [PubMed] [Google Scholar]
- 9. Sheikhbahaei S, Mena E, Yanamadala A, et al. The value of FDG PET/CT in treatment response assessment, follow-up, and surveillance of lung cancer. AJR. 2017;208:420–433. [DOI] [PubMed] [Google Scholar]
- 10. Boellaard R, O’Doherty MJ, Weber WA, et al. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging—version 1.0. Eur J Nucl Med Mol Imaging. 2010;37:181–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shen G, Ma H, Pang F, Ren P, Kuang A. Correlations of 18F-FDG and 18F-FLT uptake on PET with Ki-67 expression in patients with lung cancer: a meta-analysis. Acta Radiol. 2018;59:188–195. [DOI] [PubMed] [Google Scholar]
- 12. Wang Z, Wang Y, Sui X, et al. Performance of FLT-PET for pulmonary lesion diagnosis compared with traditional FDG-PET: a meta-analysis. Eur J Radiol. 2015;84:1371–1377. [DOI] [PubMed] [Google Scholar]
- 13. Yue J, Chen L, Cabrera AR, et al. Measuring tumor cell proliferation with 18F-FLT PET during radiotherapy of esophageal squamous cell carcinoma: a pilot clinical study. J Nucl Med. 2010;51:528–534. [DOI] [PubMed] [Google Scholar]
- 14. Hiniker SM, Sodji Q, Quon A, et al. FLT-PET-CT for the detection of disease recurrence after stereotactic ablative radiotherapy or hyperfractionation for thoracic malignancy: a prospective pilot study. Front Oncol. 2019;9:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Everitt SJ, Ball DL, Hicks RJ, et al. Differential 18F-FDG and 18F-FLT uptake on serial PET/CT imaging before and during definitive chemoradiation for non-small cell lung cancer. J Nucl Med. 2014;55:1069–1074. [DOI] [PubMed] [Google Scholar]
- 16. Yap CS, Czernin J, Fishbein MC, et al. Evaluation of thoracic tumors with 18F-fluorothymidine and 18F-fluorodeoxyglucose-positron emission tomography. Chest. 2006;129:393–401. [DOI] [PubMed] [Google Scholar]
- 17. Yang W, Zhang Y, Fu Z, et al. Imaging of proliferation with 18F-FLT PET/CT versus 18F-FDG PET/CT in non-small-cell lung cancer. Eur J Nucl Med Mol Imaging. 2010;37:1291–1299. [DOI] [PubMed] [Google Scholar]
- 18. Buck AK, Halter G, Schirrmeister H, et al. Imaging proliferation in lung tumors with PET: 18F-FLT versus 18F-FDG. J Nucl Med. 2003;44:1426–1431. [PubMed] [Google Scholar]
- 19. Tehrani OS, Shields AF. PET imaging of proliferation with pyrimidines. J Nucl Med. 2013;54:903–912. [DOI] [PubMed] [Google Scholar]
- 20. Troost EG, Vogel WV, Merkx MA, et al. 18F-FLT PET does not discriminate between reactive and metastatic lymph nodes in primary head and neck cancer patients. J Nucl Med. 2007;48:726–735. [DOI] [PubMed] [Google Scholar]