Abstract
Background & Aims
Several steps in the HBV life cycle remain obscure because of a lack of robust in vitro infection models. These steps include particle entry, formation and maintenance of covalently closed circular (ccc) DNA, kinetics of gene expression and viral transmission routes. This study aimed to investigate infection kinetics and cccDNA dynamics during long-term culture.
Methods
We selected a highly permissive HepG2-NTCP-K7 cell clone engineered to express sodium taurocholate cotransporting polypeptide (NTCP) that supports the full HBV life cycle. We characterized the replication kinetics and dynamics of HBV over six weeks of infection.
Results
HBV infection kinetics showed a slow infection process. Nuclear cccDNA was only detected 24 h post-infection and increased until 3 days post-infection (dpi). Viral RNAs increased from 3 dpi reaching a plateau at 6 dpi. HBV protein levels followed similar kinetics with HBx levels reaching a plateau first. cccDNA levels modestly increased throughout the 45-day study period with 5–12 copies per infected cell. Newly produced relaxed circular DNA within capsids was reimported into the nucleus and replenished the cccDNA pool. In addition to intracellular recycling of HBV genomes, secondary de novo infection events resulted in cccDNA formation. Inhibition of relaxed circular DNA formation by nucleoside analogue treatment of infected cells enabled us to measure cccDNA dynamics. HBV cccDNA decayed slowly with a half-life of about 40 days.
Conclusions
After a slow infection process, HBV maintains a stable cccDNA pool by intracellular recycling of HBV genomes and via secondary infection. Our results provide important insights into the dynamics of HBV infection and support the future design and evaluation of new antiviral agents.
Lay summary
Using a unique hepatocellular model system designed to support viral growth, we demonstrate that hepatitis B virus (HBV) has remarkably slow infection kinetics. Establishment of the episomal transcription template and the persistent form of the virus, so called covalently closed circular DNA, as well as viral transcription and protein expression all take a long time. Once established, HBV maintains a stable pool of covalently closed circular DNA via intracellular recycling of HBV genomes and through infection of naïve cells by newly formed virions.
Keywords: HBV, Hepatitis B virus, cccDNA, NTCP, Replenishment, Viral spread, Transmission, Intracellular recycling
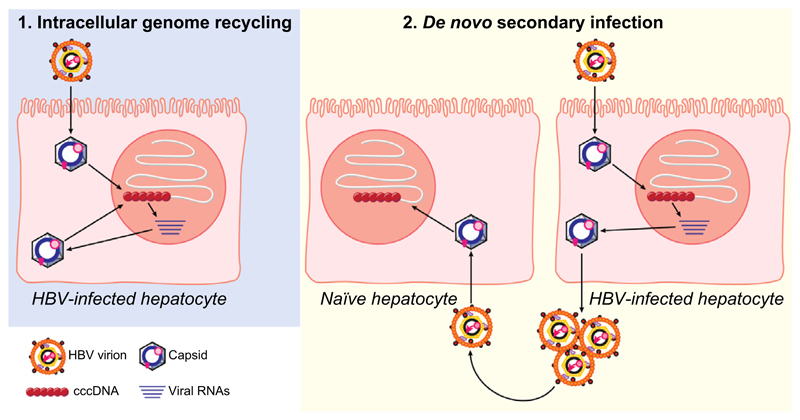
Graphic abstract.
Introduction
Hepatitis B virus (HBV) chronically infects 257 million individuals worldwide and is a major driver of end-stage liver diseases such as cirrhosis and hepatocellular carcinoma [WHO 2017]. The immense death toll of 887,000 individuals/year has driven an intensive search for curative treatment approaches. However, a more detailed understanding of infection kinetics and the genesis and maintenance of episomal nuclear DNA pools, so called covalently closed circular (ccc) DNA, is needed to guide the development of an HBV cure.
HBV is a hepatotropic enveloped DNA virus consisting of a 3.2 kb partially double-stranded genome, termed relaxed circular (rc) DNA.1 Initial interaction with heparan sulfate proteoglycans enables the virus to attach to the plasma membrane2,3 and is critical for subsequent interactions with sodium taurocholate co-transporting polypeptide (NTCP) as a functional receptor required for HBV infection.4,5 Upon interaction with NTCP, the viral particle is internalized and following fusion of the viral and cellular membranes the capsid is released into the cytoplasm and transported to the nucleus. At the nuclear pore, rcDNA is released into the nucleus where it is converted into cccDNA. cccDNA serves as the transcriptional template for pregenomic RNA (pgRNA) and subgenomic RNAs and permits the persistence of HBV infection (summarized in6). The pgRNA is packaged together with HBV polymerase into newly formed capsids and reverse transcribed, giving rise to progeny rcDNA. Mature rcDNA-containing capsids are enveloped at the endoplasmic reticulum and secreted via multivesicular bodies.7 Alternatively, these capsids can traffic back to the nucleus, where they release newly formed rcDNA that can replenish the cccDNA pool (termed intracellular recycling or intracellular amplification).8,9 This cycle can be prevented by inhibitors of reverse transcription or by core protein allosteric modulators that have not only been reported to inhibit formation of rcDNA-containing capsids but also to perturb cccDNA biosynthesis (unpublished data and10).
An alternative pathway to maintain the cccDNA pool is via secondary infection of naïve cells or pre-infected cells by extracellular viral particles. Enveloped viruses can initiate new infection events via the transfer of extracellular particles to naïve target cells or via direct cell-to-cell contacts.11 Cell-free spread of extracellular progeny virus allows transition throughout the body to infect distant cells or organs or even a new host. It can be prevented by neutralizing antibodies or entry inhibitors targeting virus-receptor interaction. In contrast, direct cell-to-cell transmission routes enable a virus to evade neutralizing antibodies or complement and are resistant to entry inhibitors. For hepatitis C virus (HCV) it has been proposed that cell-to-cell spread facilitates immune escape and circumvents rate-limiting steps of the viral life cycle, like attachment and entry.12
HBV transmission routes that establish and maintain a cccDNA pool are poorly understood, reflecting the paucity of in vitro culture systems that support long-term and secondary infection events. Neither the longevity nor the size of the cccDNA established by HBV has been accurately measured.6 In this study, we developed a highly permissive HepG2 clone stably expressing NTCP, designated HepG2-NTCP-K7, that enabled us to determine the kinetics of the viral life cycle and to study HBV transmission pathways that underly cccDNA persistence.
In this study, we highlighted the slow infection kinetics and distinct order of expression of viral RNAs and proteins, defined the size of the cccDNA pool and cccDNA half-life and importantly showed modest increases in cccDNA levels over several weeks of cell culture. Furthermore, we demonstrated the role of both rcDNA recycling pathway and extracellular particle-mediated infection in maintaining cccDNA levels.
Materials and methods
Cell lines
HepG2-NTCP cells were generated using a lentiviral vector encoding human NTCP under the control of a cytomegalovirus promoter. NTCP cDNA was synthesized by reverse transcription of mRNA from differentiated HepaRG cells. Transduced cells were selected with blasticidin (30 lg/ml) and the best growing single cell clones were screened for their ability to support HBV infection. The clone HepG2-NTCP-K7 was selected for our studies (Fig. S1). Other cell lines and clones used are detailed in the Supplementary methods. All cells were maintained in Dul-becco’s Modified Eagles Medium supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, penicillin/streptomycin, 1 mM sodium pyruvate and non-essential amino acids (Thermo Fisher Scientific, Waltham, MA, USA).
HBV infection
HBV was purified and concentrated from the culture medium of stable HepAD38 producer cells by heparin affinity chromatography followed by sucrose gradient ultracentrifugation. HBV inocula were diluted to 50% FBS/20% sucrose to a final concentration of 2–3 × 1010 DNA-containing virus particle (vp)/ml. Unless otherwise indicated, HepG2-NTCP-K7 cells were seeded on collagen-coated plates and pre-differentiated with 2.5% DMSO for two days prior to infecting with HBV in the presence of 4% polyethylene glycol (PEG) 6000 for 20–24 h. The inoculum was removed by extensive washing with PBS and cells were cultured in the presence of 2.5% DMSO.
Real-time PCR (qPCR) quantification of HBV genomes
Total cellular DNA was extracted using NucleoSpin Tissue kit (Macherey Nagel). For selective cccDNA PCR, isolated DNA was treated with 5 units of T5 exonuclease (NEB, Frankfurt, Germany) for 30 min in 10 μl reaction volume followed by heat-inactivation at 95 °C for 5 min and fourfold dilution with distilled water.13 Two different primer sets were used to detect total intracellular HBV-DNA (HBV1844F: 5′-GTTGCCCGTTTGTC CTCTAATTC-3′ and HBV1745R: 5′-GGAGGGATACATAG-AGGTTC CTTGA-3′) and cccDNA (cccDNA92F:5′-GCCTATTGATTGGAAAG TATGT-35′ and cccDNA2251R: 5′-AGCTGAGGCGGTATCTA-3′).13 For HBV-DNA quantification, an external plasmid standard was used. For relative quantification, a dilution series of infected cells was used with the human prion protein (PRNP) gene serving as a reference (PRNPF: 5’-TGCTGGGAAGTGCCATGAG-3’ and PRNPR: 5′-CGGTGCATGTTTTCACGATAGTA-3′). To determine cccDNA half-life, cccDNA and intracellular total HBV-DNA levels were normalized by two reference genes encoding PRNP and mitochondrial cytochrome c oxidase subunit 3 (MT-CO3).14 qPCR was performed using LightCycler480 instrument (Roche, Basel, Switzerland)
Imaging HBV core protein expressing cells
HBV-infected cells seeded on 12 mm coverslips were fixed with 4% paraformaldehyde and permeabilized with 0.5% saponin. Cells were then incubated with rabbit anti-core serum (DAKO) followed by Alexa Flour 594-coupled secondary antibody incubation (Invitrogen, Carlsbad, CA, USA). After immunostaining, coverslips were mounted with Flouromount-G containing DAPI (SouthernBiotech, Birmingham, AL, USA) and images collected by Fluoview FV10i microscope (Olympus, Tokyo, Japan). The number of core protein expressing cells was manually counted in 3–5 randomly selected fields of view.
Southern blot analysis of HBV-DNA
To detect protein-free forms of HBV-DNA including cccDNA, a modified Hirt extraction procedure was used.4 Intracellular capsid-associated DNA was prepared as described.15 Viral DNA forms were separated on an agarose gel, transferred onto a nylon membrane, and hybridized with a digoxigenin-labeled HBV-specific probe.13,15 DNA signal was detected by DIG Luminescent Detection Kit (Roche).
Western blot analysis
Western blot analysis was performed essentially as described.15 For HBx detection, HBV-infected cells in a 35 mm dish were lysed with Pierce RIPA buffer (ThermoFisher Scientific) in the presence of protease inhibitor cocktail (Roche) on ice for 10 min. Primary antibodies used in this study include anti-HBx (5F9 from ViroStat, Portland, Maine, USA), anti-HBVcore (in-house hybridoma supernatant 8C9), polyclonal rabbit anti-HBs serum H863 (kindly provided by S. Urban), anti-actin (SIGMA, St. Louis, MO, USA), and rabbit anti-NTCP serum K9 (kindly provided by B. Stieger).16
Results
Optimizing the HBV infection system
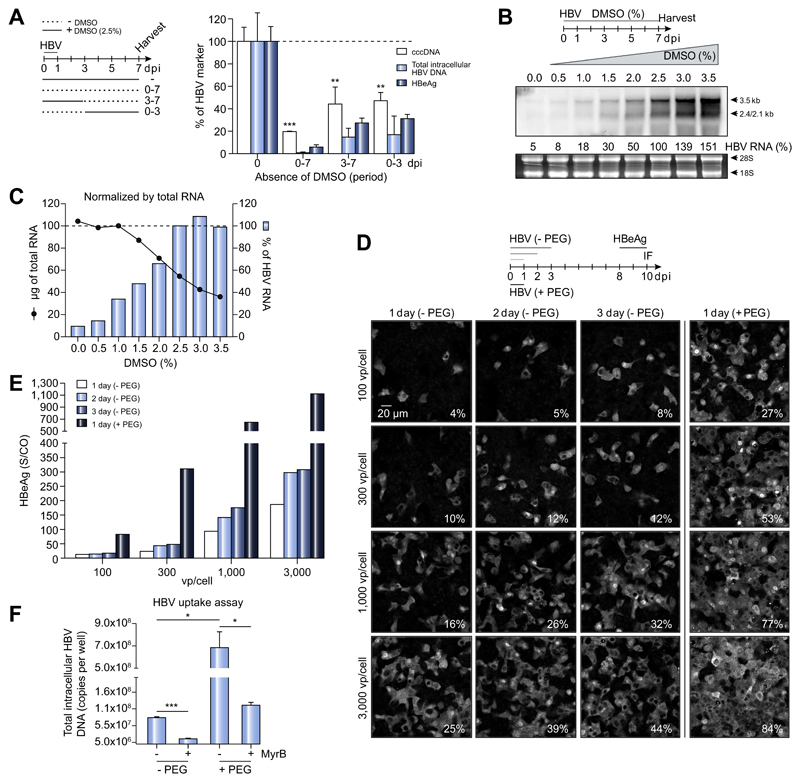
Having selected the HepG2-NTCP-K7 clone (Fig. S1), we sought to optimize the infection protocol and to investigate the role of DMSO and PEG on different steps of the viral life cycle.5,17 We analyzed various HBV markers, including cccDNA, through an experiment in which cells were cultured with or without 2.5% DMSO during and after infection (Fig. 1A). Interestingly, we observed a significant reduction in cccDNA, HBV-DNA and HBV e antigen (HBeAg) expression following DMSO withdrawal, during (0–3 dpi) or after establishment of infection (3–7 dpi), suggesting a role for DMSO in regulating different steps of the viral life cycle. In the absence of DMSO, cccDNA was reduced fivefold but the most pronounced effect was on intracellular HBV-DNA. Since pgRNA is reverse transcribed into HBV-DNA, we analyzed HBV transcripts by northern blot (Fig. 1B). DMSO treatment resulted in a dose-dependent increase of all HBV-RNAs. With 2.5% DMSO, HBV 3.5 kb RNA (including pgRNA) and 2.4-/2.1 kb subgenomic RNA levels were 10-fold greater than in DMSO-free cultures (Fig. 1C). These data suggest that DMSO has a dual effect on viral cccDNA and RNA levels and 2.5% DMSO was selected for future experimentation.
Fig. 1. Role of DMSO and PEG in HepG2-NTCP infection model.
(A) HepG2-NTCP-K7 cells were infected with HBV and maintained with or without 2.5% DMSO from the time of infection as indicated. Viral DNA and HBeAg levels were measured by qPCR and ELISA, respectively. (B) Cells were cultured with increasing concentrations of DMSO (up to 3.5%) one day after HBV infection. Total RNA was extracted at 7 dpi and subjected to Northern blot analysis using an HBV-DNA probe. Viral RNA was quantified with 2.5% DMSO condition set to 100%. Ribosomal RNA (28S and 18S) stained by ethidium bromide is shown as a loading control. (C) The amount of total RNA from a 35 mm dish extracted at respective conditions (black line with solid circle) and the relative amount of HBV-RNA normalized to total RNA (blue bar) are given. (D, E) HepG2-NTCP-K7 cells were infected with either increasing moi (100, 300, 1,000, and 3,000 vp/cell) of HBV in the absence of PEG or at an moi of 100 vp/cell in the presence of PEG for 1, 2 and 3 days as indicated. (D) Cells were fixed and stained for HBV core protein and the percentage of infected cells is denoted at the bottom-right corner of each image. (E) HBeAg was determined in extracellular media. (F) HBV uptake was determined with or without PEG in the presence or absence of the entry inhibitor MyrB. HBV genomes inside the cells were quantified by qPCR. Statistical significance was determined using Student’s t test (*p <0.05, **p <0.01, ***p <0.001). dpi, day post-infection; HBeAg, HBV e antigen; HBV, hepatitis B virus; moi, multiplicity of infection; MyrB, Myrcludex-B; PEG, polyethylene glycol; qPCR, real-time PCR; vp, virus particle.
PEG has been reported to enhance HBV infection by facilitating viral attachment to cell surface heparan sulfate proteoglycans.2,3,17 To evaluate whether PEG is required for HBV infection of HepG2-NTCP-K7 cells, cells were inoculated with HBV at increasing multiplicity of infection (moi) in the presence of PEG for one day or without PEG for one to three days before the inoculum was removed (Fig. 1D). At 10 dpi, HBeAg secretion over 48 h and the frequency of HBV core expressing cells were determined (Fig. 1D-E). Infection rates were significantly higher if PEG was added and reached 77% and 84% at moi 1,000 and 3,000 vp/cell, respectively, while the time of exposure had a minimal effect (Fig. 1D). The number of HBV-infected cells was determined by intracellular core protein staining and immunofluorescence microscopy or flow cytometry, which gave similar results (Fig. S2). HBeAg expression increased with the frequency of HBV core expressing cells (Fig. 1E). HBV internalization in a synchronized uptake assay increased by 1log10 when PEG was added but was still inhibited by the synthetic pre-S1 peptide, Myrcludex-B (MyrB), demonstrating NTCP dependence (Fig. 1F). In summary, these data show that PEG enhances HBV infection, however, a high moi is still required. Therefore, we used PEG at the time of HBV inoculation and maintained cells in media containing 2.5% DMSO to achieve optimal infection.
HBV infection kinetics
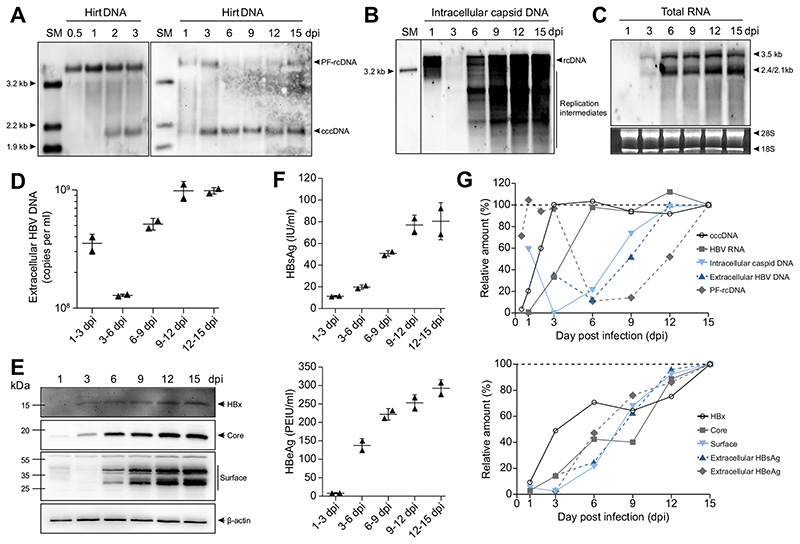
To determine the kinetics of HBV infection, we analyzed the expression of viral DNA and RNA species and proteins over time. Southern blot analysis detected cccDNA after 24 h and a marked increase was found between 1 and 2 dpi (Fig. 2A), suggesting that cccDNA formation is an inefficient and slow process. cccDNA increased further until 3 dpi and thereafter remained at a constant level. The identity of cccDNA was confirmed by both T5 exonuclease treatment where non-cccDNA species are digested13 and restriction enzyme digestion that linearizes cccDNA (Fig. S3).18 High levels of protein-free rcDNA (PF-rcDNA) were detected 12 h after infection and further increased by 1 dpi before declining to almost undetectable levels, suggesting that PF-rcDNA is the precursor of cccDNA (Fig. 2A).18,19 PF-rcDNA was detected again from 12 dpi after substantial amounts of intracellular rcDNA had been produced (Fig. 2B), suggesting that it is derived from both incoming and newly synthesized rcDNA.
Fig. 2. Kinetics of the HBV-DNA forms, viral RNA, and protein expression in HepG2-NTCP-K7 cells.
(A-F) HepG2-NTCP-K7 cells were infected with HBV at an moi of 1,000 vp/cell. At indicated time points, (A) DNA extracted after Hirt lysis and (B) intracellular capsid-associated DNA, and (C) total RNA were detected by Southern or northern blot analysis, respectively, using an HBV-DNA probe. (D) Extracellular HBV-DNA was quantified by qPCR. (E) Intracellular HBx, core, and surface proteins were detected by western blot analysis. (F) Secreted HBsAg and HBeAg were quantified by immunoassay. (G) Each band or value determined at different time points in panel A to F was quantified and plotted. Values from 15 dpi were set to 100%. dpi, day post-infection; HBeAg, HBV e antigen; HBsAg, HBV surface antigen; HBV, hepatitis B virus; moi, multiplicity of infection; qPCR, real-time PCR; SM, size marker; vp, virus particle.
To quantify cccDNA levels in our infection model, we used two independent methods, Southern blot and qPCR (Table 1). HepG2-NTCP-K7 cells were infected with increasing moi of HBV and harvested at 3 dpi when cccDNA was fully established from incoming virus, whereas rcDNA was barely detectable (Fig. 2A-B). cccDNA copies increased with increasing moi, and 8.2–9.6 copies per cell (i.e., 10.7–12.5 copies per infected cell) were formed following inoculation with 1,000 vp/cell (Table 1). These data show that HepG2-NTCP-K7 cells support infection of multiple viral particles.
Table 1. cccDNA copy number in HBV-infected HepG2-NTCP-K7 cells.
| Multiplicity of infection | Southern blota | qPCRb | ||
|---|---|---|---|---|
| per cell | per infected cell | per cell | per infected cell | |
| 100 vp/cell | 2.5 ± 1.0 | 9.2 ±3.8 | 1.4±0.6 | 5.0±2.3 |
| 300 vp/cell | 5.3 ± 0.9 | 9.9 ± 1.8 | 3.0 ± 1.2 | 5.7±2.2 |
| 1000 vp/cell | 9.6 ± 0.9 | 12.5 ±1.2 | 8.2 ± 2.1 | 10.7±2.7 |
HBV-infected HepG2-NTCP-K7 cells were harvested at 3 dpi for DNA extraction and subjected to cccDNA copy number determination. Prior to DNA extraction, cells were trypsinized and counted. The number of infected cells was determined by counting core protein-positive cells (Fig. 1D). Mean ± SD is given.
Protein-free DNAs extracted by modified Hirt method were subjected to Southern blot analysis. Standard curves were generated by preparing serial dilution of a 3.2 kb double-stranded linear HBV genome. The experiment was performed twice.
Total cellular DNA was subjected to cccDNA-selective qPCR without prior T5 exonuclease treatment. Standard curves were generated by preparing serial dilution of a plasmid DNA-containing HBV monomer for qPCR. The experiment was repeated three times. cccDNA, covalently closed circular DNA; dpi, days post infection; HBV, hepatitis B virus; qPCR, real-time PCR; vp, virus particle.
Northern blot analysis showed that viral transcripts corresponding to HBV pgRNA and subgenomic RNAs were detectable from 3 dpi, increased by 6 dpi and remained constant thereafter (Fig. 2C). In parallel, the level of capsid-associated intracellular DNA (i.e., rcDNA and replication intermediates that stem from reverse transcription) and extracellular HBV-DNA increased from 3 to 12 dpi (Fig. 2B, D). The high levels of rcDNA observed at 1 dpi are mostly likely derived from input virus associating with cell membranes or vesicles. We observed increasing expression of intracellular HBx, core and surface proteins together with secreted HBV surface antigen (HBsAg) and HBeAg reaching a plateau between 12 and 15 dpi (Fig. 2E-F). These data highlight the accumulation of viral products over time starting with cccDNA (plateau at 3 dpi), viral RNAs (plateau at 6 dpi) to newly produced rcDNA (plateau at 12 dpi) (Fig. 2G). Interestingly, HBx expression reached maximum levels earlier than the expression of other viral proteins. This may imply that HBx could serve as an early viral protein required immediately after infection. The accumulation of viral proteins, on the other hand, could either be a consequence of accumulating transcripts or indicate secondary infection events.
cccDNA persistence during long-term HBV infection
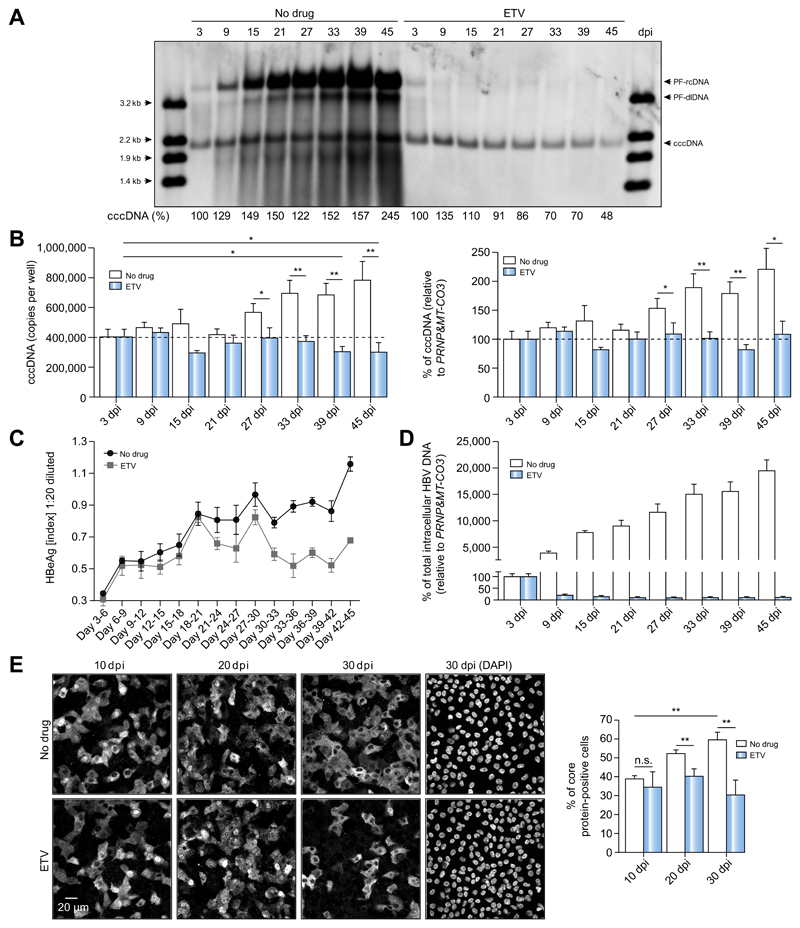
Having shown the HepG2-NTCP infection model supports robust HBV infection, we investigated how cccDNA is maintained and whether it can be amplified during long-term culture. HBV-infected HepG2-NTCP-K7 cells were cultured for up to 45 days in the presence of 2.5% DMSO, which induces cell cycle arrest20 and prevents cccDNA loss to cell division,21 and harvested at indicated time points (Fig. 3A). In parallel, cells were treated with entecavir (ETV) at a 2,000-fold IC50 from 3 dpi to inhibit reverse transcription and formation of new rcDNA. Southern blot analysis revealed that cccDNA levels modestly increased over time. Treating cells with ETV diminished PF-rcDNA and reduced cccDNA levels by 70% and 48% at 39 and 45 dpi, respectively (Fig. 3A). Notably, PF-rcDNA accumulated to high levels over time, which was inhibited with ETV, suggesting that rcDNA-containing capsids refill nuclear HBV-DNA to maintain cccDNA levels.9,18,19 However, the conversion of PF-rcDNA to cccDNA was rate-limiting (Fig. 3A). To validate these findings, intracellular cccDNA and total HBV-DNA were determined by qPCR and HBeAg secretion by ELISA (Fig. 3B-D). cccDNA levels slowly doubled over time in untreated cells, whereas ETV treatment prevented a cccDNA increase (Fig. 3B). HBeAg secretion was gradually increasing until 45 dpi, whereas it peaked at 30 dpi and decreased thereafter under ETV treatment (Fig. 3C). As shown in Fig. 3D, continuous ETV treatment drastically lowered total intracellular HBV-DNA levels, but without effecting cell viability or morphology (Fig. S4). Quantifying the number of HBV core expressing cells during the infection also showed a twofold difference between control and ETV treatment at 30 dpi (60 ± 4% vs. 30 ± 8%) (Fig. 3E), confirming our cccDNA data. These results demonstrate that HepG2-NTCP-K7 cells support persistent HBV replication, highlight the long-term stability of cccDNA and indicate that low-level cccDNA amplification occurs.
Fig. 3. Dynamics of cccDNA during long-term culture of HBV-infected HepG2-NTCP-K7 cells.
(A-D) HepG2-NTCP-K7 cells were infected with HBV at an moi of 300 vp/cell (A) or 100 vp/cell (B-D) and kept with or without ETV (1 lM) treatment from 3 dpi onwards. (A) DNA was isolated after Hirt extraction at different time points and subjected to Southern blot analysis using an HBV-DNA probe. Restriction fragments of HBV-DNA (3.2 kb to 1.4 kb) in the first and last lanes serve as size markers and as controls for Southern blot transfer efficiency. Percentage values below each lane indicate the relative amount of cccDNA. (B-D) cccDNA and total intracellular HBV-DNA, and HBeAg levels were measured by qPCR and ELISA, respectively. For cccDNA measurement, both absolute copy numbers and relative cccDNA values to PRNP and MT-CO3 are given. (E) Cells were infected with HBV at an moi of 300 vp/cell and treated with ETV from 3 dpi. Intracellular HBV core protein was visualized by immunofluorescence staining at 10, 20, and 30 dpi. The number of core protein-positive cells at each time point was counted and is plotted in a bar graph. Statistical significance was determined using Student’s t test (**p <0.01). cccDNA, covalently closed circular DNA; dpi, day post-infection; ETV, entecavir; HBeAg, HBV e antigen; HBV, hepatitis B virus; moi, multiplicity of infection; qPCR, real-time PCR; vp, virus particle.
HBV transmission via extracellular particles
Since the frequency of infected cells increased between 10 and 30 dpi from 39 ± 2 to 60 ± 4% but slightly decreased during ETV treatment (Fig. 3E), we hypothesized that secondary infection contributed to maintaining persistent infection. High titer HBV released into the cell culture medium (1 × 109 copies/ml i.e. per 1–2 × 106 cells) would allow this (Fig. 2D).
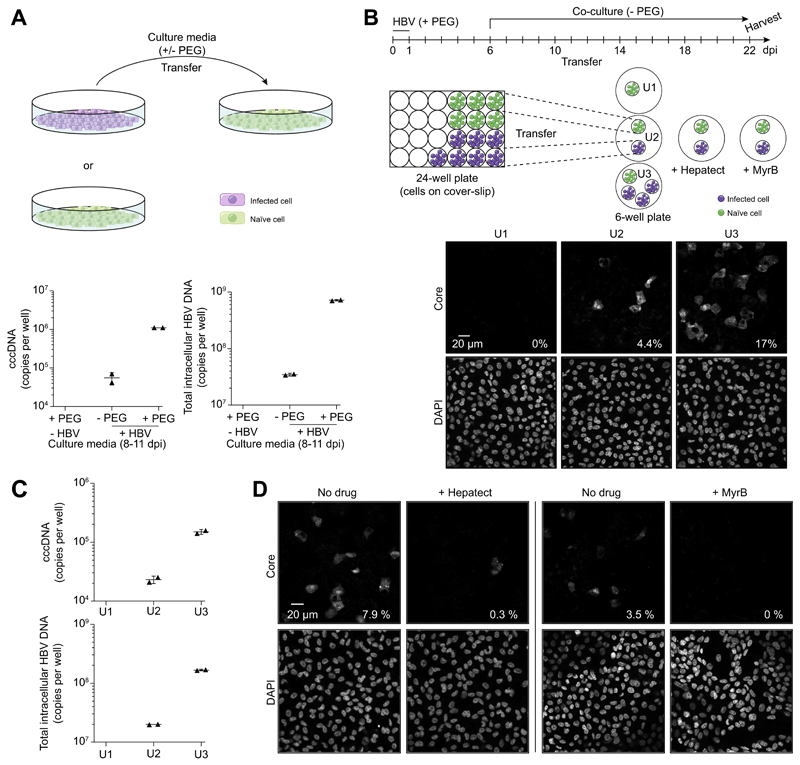
Transfer of media from HBV-infected HepG2-NTCP-K7 cells resulted in cccDNA formation and infection of naïve cells, and – as expected – the efficiency of second round infection was enhanced by addition of PEG (Fig. 4A).22 To confirm that HBV transmission is mediated by extracellular virions, HepG2-NTCP-K7 cells were seeded on coverslips and infected with HBV (Fig. 4B). After six days, coverslips were transferred into new wells together with naïve cells. When co-cultured with one (U2) or three (U3) coverslips of infected cells for 16 days, 4.4% and 17% of naïve target cells expressed viral core protein, respectively, (Fig. 4B) and had cccDNA and intracellular HBV-DNA established (Fig. 4C). Neutralizing anti-HBs serum (Hepatect) or MyrB blocked HBV transmission (Fig. 4D), demonstrating extracellular particle transmission. Transmission of extracellular particles was further supported by a transwell experiment that physically separated HBV-infected producer cells from naïve target cells and prevented cell-cell contact dependent transmission but facilitated the diffusion of extracellular particles (Fig. S5). These data demonstrate that progeny virus released from infected HepG2-NTCP-K7 cells can infect naïve cells and support de novo cccDNA formation in the absence of PEG.
Fig. 4. Analysis of de novo infection mediated by extracellular virions.
(A) Cell culture media from HBV-infected HepG2-NTCP-K7 cells were transferred onto naïve cells with or without PEG. At 11 days post-transfer, cccDNA and total intracellular HBV-DNA were analyzed by qPCR. (B) For co-culture experiments in a physically separated setting, HepG2-NTCP-K7 cells were seeded on coverslips and either treated with PEG only or infected with HBV at an moi of 1,000 vp/cell with PEG. At 6 dpi, coverslips with infected cells were transferred into new wells together with coverslips with non-infected cells as depicted and subsequently co-cultured for 16 days in the absence of PEG. Naïve target cells from each condition (denoted U1, U2 and U3) were subjected to immunofluorescence staining with an anti-core antibody. (C) cccDNA and total intracellular HBV-DNA contents were analyzed by qPCR. Target cells were treated with trypsin for 5 min to remove cell surface bound HBV prior to DNA isolation. (D) The same experiment performed (condition U2) in the presence or absence of entry inhibitors (0.5 IU/ml Hepatect CP or 200 nM MyrB). The percentage of infected cells is denoted at the bottom-right corner of each image. cccDNA, covalently closed circular DNA; dpi, day post-infection; HBV, hepatitis B virus; moi, multiplicity of infection; MyrB, Myrcludex-B; PEG, polyethylene glycol; qPCR, real-time PCR; vp, virus particle. (This figure appears in colour on the web.)
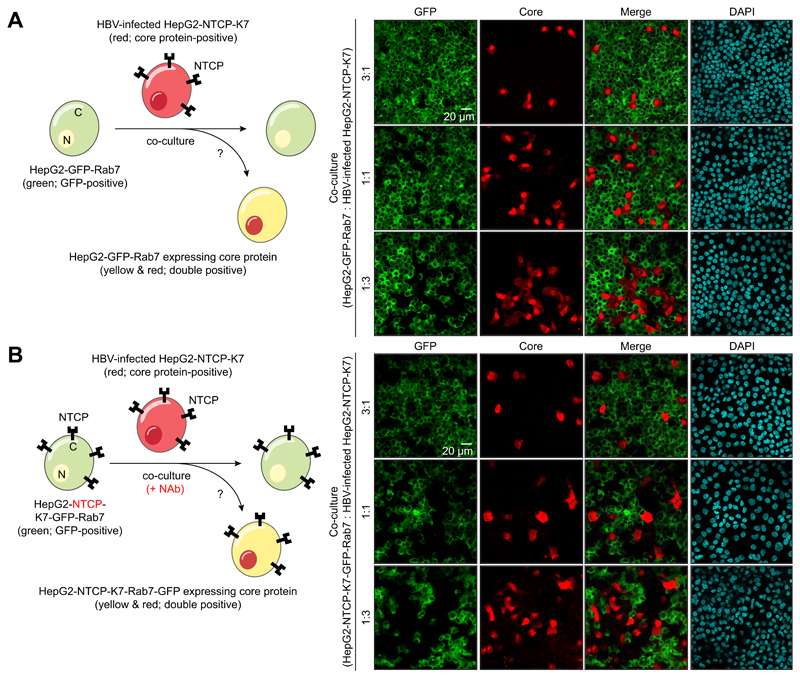
Since we observed infected cell pairs and groups of three to five adjacent HBV core expressing cells (Fig. 4B), we wondered whether intracellular particles, presumably mature capsids or enveloped virions, were transmitted to neighboring cells via direct cell-to-cell contact. To investigate this, we co-cultured HBV-infected HepG2-NTCP-K7 cells with HepG2-GFP-Rab7 target cells and monitored the frequency of cells co-expressing GFP-Rab7 and core protein (Fig. 5A). However, we could not detect any double positive cells. To evaluate potential NTCP-dependent cell-to-cell transmission, an analogous co-culture experiment was performed with naïve HepG2-NTCP-K7 cells expressing GFP-Rab7 in the continuous presence of a neutralizing antibody (Fig. 5B). In this setting, very few double positive cells were detected. Importantly, most of them were not in direct contact with HBV-infected cells (Fig. S6A), indicating insufficient blockage of de novo infection by the anti-HBs antiserum rather than direct cell-to-cell transmission (Fig. S6B). Collectively, these data suggest that extracellular virus rather than direct cell-to-cell transmission contributes to viral persistence in our in vitro infection model.
Fig. 5. Evaluation of cell-to-cell transmission of HBV.
(A) HepG2-GFP-Rab7 cells and HBV-infected HepG2-NTCP-K7 cells were co-seeded into a 24-well plate at three different ratios and co-cultured. (B) HepG2-NTCP-K7-GFP-Rab7 cells and HBV-infected HepG2-NTCP-K7 cells were co-cultured at three different ratios in the continuous presence of neutralizing antibodies (NAb; 0.5 IU/ml Hepatect CP). After 21 days, cells were fixed, stained with an anti-core antibody, and analyzed by immunofluorescence staining. GFP signal was visualized in parallel. HBV, hepatitis B virus.
cccDNA is formed via intracellular recycling of HBV genomes
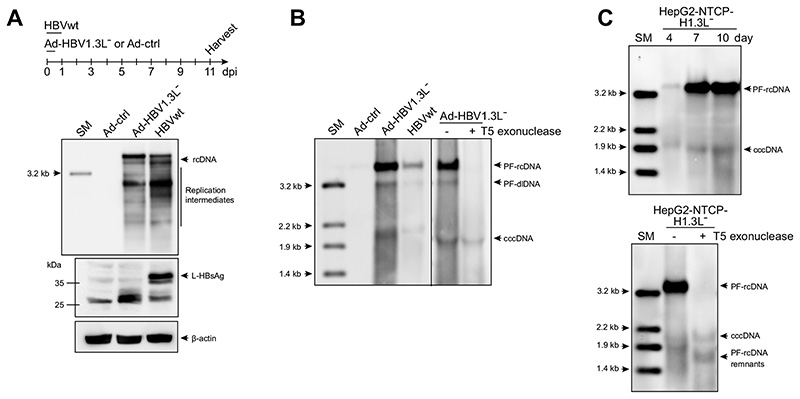
We next investigated the contribution of intracellular recycling pathway by using a L-HBsAg-deficient, but replication-competent overlength HBV genome (HBV1.3L−). Since L-HBsAg is required for infectious progeny virus production and secretion, the usage of HBV1.3L− genome excludes de novo infection via extracellular particles. HBV1.3L− genome was delivered via an adenoviral vector (Ad-HBV1.3L−) (Fig. 6A-B)23 or integrated into HepG2-NTCP-K7 cells (Fig. 6C) requiring nuclear recycling of HBV rcDNA for establishment of cccDNA. As expected, L-HBsAg was only detected in the sample infected with wild-type HBV (HBVwt), whereas rcDNA and replication intermediates were detected in both Ad-HBV1.3L− -transduced and HBVwt-infected cells (Fig. 6A). Importantly, Ad-mediated delivery as well as integration of an HBV1.3L− genome resulted in PF-rcDNA sensitive and cccDNA resistant to T5 exonuclease treatment (Fig. 6B-C). Since cccDNA formation can only result from nuclear import of capsids, this demonstrates that the intracellular recycling pathway of rcDNA-containing capsids is active and is a driver of cccDNA replenishment.
Fig. 6. Nuclear recycling of HBV1.3L− genomes.
(A, B) HepG2-NTCP-K7 cells were either transduced with Ad-HBV1.3L− or Ad-ctrl at an moi of 20 infectious units/cell or infected with HBV at an moi of 500 vp/cell. (A) Intracellular capsid-associated HBV-DNA was analyzed by Southern blotting at day 11. An aliquot of cell lysate prepared during the HBV-DNA isolation was subjected to Western blot analysis using an anti-HBsAg serum. β-actin served as a loading control. (B) DNA extracted after Hirt lysis was assayed by Southern blot following T5 exonuclease digestion, if indicated. (C) HepG2-NTCP-K7-H1.3L− cells were cultured and harvested at the indicated time points and Hirt-extracted DNA was analyzed by Southern blotting. The identity of cccDNA was confirmed by T5 exonuclease digestion. cccDNA, covalently closed circular DNA; HBsAg, HBV surface antigen; HBV, hepatitis B virus; moi, multiplicity of infection; SM, size marker; vp, virus particle.
Discussion
Viruses employ multiple strategies to establish and maintain infection, however, our understanding of the HBV transmission pathways that establish chronic infection is limited. In this study, we used the HepG2-NTCP-K7 cell clone that supports efficient HBV replication and enabled us to define HBV infection kinetics, to study for the first time cccDNA dynamics and define pathways maintaining the pool of episomal viral DNA.
Infection kinetics were remarkably slow, requiring three days to establish the cccDNA pool and initiate transcription (Fig. 2). RNA levels reached a plateau after six days, but protein expression and intracellular HBV-DNA replication took more than twelve days to reach a steady-state. We found that the cccDNA pool once established was very stable for more than a month and that cccDNA was replenished during the course of infection.
In contrast to previous reports,5,24 our HepG2-NTCP-K7 cells support secondary HBV infection and an intracellular recycling pathway that results in a net cccDNA increase. The K7 clone selected showed more stable NTCP expression and higher HBV susceptibility than the parental cell population and other clones derived thereof (Fig. S1, 7).
Since the longevity and size of an HBV cccDNA pool has not been accurately measured,6 we attempted to determine cccDNA half-life by Southern blot analysis, as the gold standard of cccDNA measurement, and cccDNA-selective qPCR following inhibition of viral DNA synthesis in our cell culture model (Fig. 3). These analyses revealed a cccDNA half-life of approximately 40 days determined by Southern blot analysis and even longer than 40 days when measured by qPCR. This difference could be due to variation between assays, DNA isolation methods (Hirt DNA for Southern blot vs. total cellular DNA for qPCR), and/or alteration of reference gene amounts and stability during long-term culture. Of note, controlling cell growth during longterm culture (i.e., 2.5% DMSO, optimal cell seeding density, and regular media exchange) is the critical factor allowing stable maintenance and even low-level amplification of cccDNA. Consistently, the same strategy was applied to determine cccDNA longevity in vivo in woodchucks and ducks infected with their respective HBV relatives and resulted in similar cccDNA halflives of 33–50 and 35–57 days, respectively.25,26
Next, we determined that on average 1.4–9.6 copies of cccDNA per cell, which corresponds to 5.0–12.5 copies per infected cell (as determined by positive core staining), are formed and that the copy number is mainly defined by the amount of virus in the inoculum (Table 1). cccDNA quantification from two independent methods (Southern blot analysis and selective qPCR) provided very similar data and adds confidence in the use of a well-controlled qPCR to quantify cccDNA levels in clinical material.
However, it should be noted that cccDNA detection by qPCR is selective and not specific for cccDNA, and that decent selectivity can be only achieved when the amount of non-cccDNA species (i.e., rcDNA) is relatively low (ideally cccDNA:rcDNA ≤1:1,000);13 otherwise, false-positive signals arise in late PCR cycles and interfere with conclusions. At 3 dpi from which cccDNA copy number was determined (Table 1), the ratio of non-cccDNA species to cccDNA was <50, whereas it reaches >1,000 at 15 dpi (data not shown). Selective cccDNA qPCR in samples containing >1,000 copies rcDNA per cccDNA requires pretreatment for instance with T5 exonuclease to remove non-cccDNA species.13 T5 exonuclease treatment, however, results in a >20% loss of cccDNA when the level of non-cccDNA species is low (data not shown).
Knowing that a stable cccDNA pool must be refilled, the question arose as to how cccDNA is replenished during the course of infection. The mechanism of cccDNA replenishment could be explained by: (i) secondary infection via extracellular particles, (ii) direct cell-to-cell transmission, or (iii) intracellular trafficking of mature, HBV rcDNA-containing capsids back to the nucleus resulting in nuclear reimport of newly formed rcDNA.
Several lines of evidence demonstrate transmission via extracellular virus particles in our HepG2-NTCP-K7 cells. Firstly, extracellular media from HBV-infected cells were capable of infecting naïve target cells in an NTCP-dependent manner (Fig. 4A). Secondly, the co-culture experiment resulted in cccDNA and core protein expression in naïve target cells that was blocked by entry inhibitors (Fig. 4B-D). Finally, physically separating HBV-infected cells from naïve target cells using a transwell assay resulted in HBV-infected target cells (Fig. S5).
In a chimpanzee, HBV spreads through the liver during acute infection concomitant with a rapid rise of serum HBV-DNA.27 Similarly, in humanized liver chimeric mice, the frequency of HBV-infected human hepatocytes increases rapidly between weeks three and nine post-infection.28 Recently, Xia et al. reported evidence of HBV spread in hepatocyte-like cells derived from pluripotent stem cells.24 A recent study showed that NTCP-expressing HepG2 cells support the complete HBV life cycle including viral spread, if PEG is continuously added.22 Although this is consistent with our finding, PEG may mediate cell fusion and thereby deliver HBV proteins.29 Thus, our observations are in line with previous reports and highlight the importance of a second round de novo infection for maintaining persistent HBV infection.
In our cell culture model, there was no evidence of direct cell-to-cell transmission contributing to the cccDNA increase, when assessed by co-culture experiments with NTCP-positive and -negative HBV-naïve recipient cells (Fig. 5. and Fig. S6A). Nonetheless, our findings cannot completely exclude this possibility in vivo. Endogenous factors required for this process may be lost during transformation into cancer cells (i.e., HepG2), as in the case of NTCP.4 Alternatively, two-dimensional cell culture does not adequately reflect the polarized structure of hepatocytes and its surrounding extracellular milieu, which may be critical to establish contact between the infected and adjacent uninfected cells. However, our findings are consistent with the efficacy of anti-HBs antibodies in the post-liver transplant setting and vaccine-induced neutralizing antibodies that efficiently prevent HBV infection in contrast to HCV which can spread via cell-to-cell transmission12 and for which antibody responses efficiently neutralizing the virus in the clinical setting are lacking.
HBV replication begins from cccDNA which as an episomal, nuclear DNA serves as the transcriptional template and permits persistent infection.1 In duck HBV infection, cccDNA can be amplified via recycling of mature capsid to the nucleus and import of the rcDNA contained within the capsid.8,9 Reverse transcriptase inhibitors such as ETV block maturation of rcDNA inside the viral capsid preventing the formation of infectious virions and thus secondary infection, as well as inhibiting the nuclear import of rcDNA from mature capsid and the regeneration of cccDNA.
To investigate the contribution of an intracellular recycling pathway in our infection model, we delivered a replication-competent HBV1.3L− genome. Lack of the L-HBsAg (also called envelope protein L) excludes formation of infectious virus and thus secondary infection, but still allows formation of mature HBV capsids that cannot be enveloped and thus are prone to recycle to the nucleus.23 We demonstrated that both transient and stable delivery of HBV1.3L− genomes resulted in cccDNA formation in HepG2-NTCP-K7 cells (Fig. 6), which can only be explained by nuclear reimport of HBV-DNA from newly formed capsids. Notably, PF-rcDNA drastically accumulated during long-term culture following HBV infection and after delivery of the HBV1.3L− genome, whereas cccDNA levels did not increase accordingly (Fig. 3A and Fig. 6). In other studies, the extent of cccDNA accumulation observed in HBV was also markedly lower than that observed in duck HBV infection.9,18,30 This indicates that the conversion of PF-rcDNA to cccDNA is a ratelimiting step for cccDNA formation, and our HepG2-NTCP-K7 cells would be an ideal model to identify host factor(s) involved in this process. In addition, the data indicate that preventing rcDNA to cccDNA conversion is an interesting target in attempts to eradicate cccDNA.
In summary, we have generated and characterized a HepG2-NTCP cell clone supporting robust HBV infection. Using this model, we showed that our cells support the complete HBV life cycle from particle entry to infectious particle secretion and determined longevity of an HBV cccDNA pool. Our data sheds light on two major routes of cccDNA replenishment utilized by HBV (i.e., de novo infection and intracellular recycling of HBV genomes). The infection model will allow us to extend our understanding of poorly characterized steps in the HBV life cycle, for instance cccDNA formation and replenishment and viral dissemination, which could eventually be targeted in an attempt to eradicate HBV persistence.
Highlights.
Studying HBV has been limited by the availability of in vitro and in vivo models.
A selected HepG2 cell clone expressing NTCP supports longterm HBV infection.
HBV has slow infection kinetics requiring 3 days for full establishment of infection.
HBV establishes 1–9 copies of cccDNA per cell which have an estimated half-life of 40 days.
cccDNA levels remain stable by intracellular genome recycling and secondary infection.
Acknowledgements
We are grateful to Stephan Urban for Myrcludex-B and antiserum H863, Bruno Stieger for rabbit anti-NTCP serum, and Christoph Seeger for HepAD38 cell line. We thank Samuel Jeske, Konstantin Wolf, Antje Malo, and Thomas Michler for their support and constructive discussion.
Financial support
The work was supported by the German Research foundation via project 14 in collaborative research center TRR 179. UP and JAM receive funding by the Institute for Advanced Study with the support of the Technical University of Munich via the German Excellence Initiative and EU 7th Framework Program under grant agreement number 291,763 and via the EU network Hepcar within Horizon 2020.
Footnotes
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Authors’ contributions
CK, WR, UP designed the study; CK, AC, WC, JH, RB performed the experiments; JMW, DS, TA, KZ, KW provided key materials; CK, AC, JAM, UP wrote and finalized the manuscript.
Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jhep.2018.08.012.
References
Author names in bold designate shared co-first authorship
- [1].Seeger C, Mason WS. Molecular biology of hepatitis B virus infection. Virology. 2015;479–480C:672–686. doi: 10.1016/j.virol.2015.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Schulze A, Gripon P, Urban S. Hepatitis B virus infection initiates with a large surface protein-dependent binding to heparan sulfate proteoglycans. Hepatology. 2007;46:1759–1768. doi: 10.1002/hep.21896. [DOI] [PubMed] [Google Scholar]
- [3].Verrier ER, Colpitts CC, Bach C, Heydmann L, Weiss A, Renaud M, et al. A targeted functional RNA interference screen uncovers glypican 5 as an entry factor for hepatitis B and D viruses. Hepatology. 2016;63:35–48. doi: 10.1002/hep.28013. [DOI] [PubMed] [Google Scholar]
- [4].Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife. 2012;1:e00049. doi: 10.7554/eLife.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ni Y, Lempp FA, Mehrle S, Nkongolo S, Kaufman C, Falth M, et al. Hepatitis B and D viruses exploit sodium taurocholate co-transporting polypeptide for species-specific entry into hepatocytes. Gastroenterology. 2014;146:1070–1083. doi: 10.1053/j.gastro.2013.12.024. [DOI] [PubMed] [Google Scholar]
- [6].Nassal M. HBV cccDNA: viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut. 2015;64:1972–1984. doi: 10.1136/gutjnl-2015-309809. [DOI] [PubMed] [Google Scholar]
- [7].Watanabe T, Sorensen EM, Naito A, Schott M, Kim S, Ahlquist P. Involvement of host cellular multivesicular body functions in hepatitis B virus budding. Proc Natl Acad Sci USA. 2007;104:10205–10210. doi: 10.1073/pnas.0704000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tuttleman JS, Pourcel C, Summers J. Formation of the pool of covalently closed circular viral DNA in hepadnavirus-infected cells. Cell. 1986;47:451–460. doi: 10.1016/0092-8674(86)90602-1. [DOI] [PubMed] [Google Scholar]
- [9].Kock J, Rosler C, Zhang JJ, Blum HE, Nassal M, Thoma C. Generation of covalently closed circular DNA of hepatitis B viruses via intracellular recycling is regulated in a virus specific manner. PLoS Pathog. 2010;6:e1001082. doi: 10.1371/journal.ppat.1001082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Guo F, Zhao Q, Sheraz M, Cheng J, Qi Y, Su Q, et al. HBV core protein allosteric modulators differentially alter cccDNA biosynthesis from de novo infection and intracellular amplification pathways. PLoS Pathog. 2017;13:e1006658. doi: 10.1371/journal.ppat.1006658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sattentau Q. Avoiding the void: cell-to-cell spread of human viruses. Nat Rev Microbiol. 2008;6:815–826. doi: 10.1038/nrmicro1972. [DOI] [PubMed] [Google Scholar]
- [12].Timpe JM, Stamataki Z, Jennings A, Hu K, Farquhar MJ, Harris HJ, et al. Hepatitis C virus cell-cell transmission in hepatoma cells in the presence of neutralizing antibodies. Hepatology. 2008;47:17–24. doi: 10.1002/hep.21959. [DOI] [PubMed] [Google Scholar]
- [13].Xia Y, Stadler D, Ko C, Protzer U. Analyses of HBV cccDNA quantification and modification. Methods Mol Biol. 2017;1540:59–72. doi: 10.1007/978-1-4939-6700-1_6. [DOI] [PubMed] [Google Scholar]
- [14].Liu C, Cai D, Zhang L, Tang W, Yan R, Guo H, et al. Identification of hydrolyzable tannins (punicalagin, punicalin and geraniin) as novel inhibitors of hepatitis B virus covalently closed circular DNA. Antiviral Res. 2016;134:97–107. doi: 10.1016/j.antiviral.2016.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ko C, Shin YC, Park WJ, Kim S, Kim J, Ryu WS. Residues Arg703, Asp777, and Arg781 of the RNase H domain of hepatitis B virus polymerase are critical for viral DNA synthesis. J Virol. 2014;88:154–163. doi: 10.1128/JVI.01916-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Appelman MD, Chakraborty A, Protzer U, McKeating JA, van de Graaf SF. N-Glycosylation of the Na+-taurocholate cotransporting polypeptide (NTCP) determines its trafficking and stability and is required for hepatitis b virus infection. PLoS ONE. 2017;12:e0170419. doi: 10.1371/journal.pone.0170419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gripon P, Diot C, Guguen-Guillouzo C. Reproducible high level infection of cultured adult human hepatocytes by hepatitis B virus: effect of polyethylene glycol on adsorption and penetration. Virology. 1993;192:534–540. doi: 10.1006/viro.1993.1069. [DOI] [PubMed] [Google Scholar]
- [18].Guo H, Jiang D, Zhou T, Cuconati A, Block TM, Guo JT. Characterization of the intracellular deproteinized relaxed circular DNA of hepatitis B virus: an intermediate of covalently closed circular DNA formation. J Virol. 2007;81:12472–12484. doi: 10.1128/JVI.01123-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gao W, Hu J. Formation of hepatitis B virus covalently closed circular DNA: removal of genome-linked protein. J Virol. 2007;81:6164–6174. doi: 10.1128/JVI.02721-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nikolaou N, Green CJ, Gunn PJ, Hodson L, Tomlinson JW. Optimizing human hepatocyte models for metabolic phenotype and function: effects of treatment with dimethyl sulfoxide (DMSO) Physiol Rep. 2016;4 doi: 10.14814/phy2.12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Allweiss L, Volz T, Giersch K, Kah J, Raffa G, Petersen J, et al. Proliferation of primary human hepatocytes and prevention of hepatitis B virus reinfection efficiently deplete nuclear cccDNA in vivo. Gut. 2018;67:542–552. doi: 10.1136/gutjnl-2016-312162. [DOI] [PubMed] [Google Scholar]
- [22].Michailidis E, Pabon J, Xiang K, Park P, Ramanan V, Hoffmann HH, et al. A robust cell culture system supporting the complete life cycle of hepatitis B virus. Sci Rep. 2017;7:16616. doi: 10.1038/s41598-017-16882-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sprinzl MF, Oberwinkler H, Schaller H, Protzer U. Transfer of hepatitis B virus genome by adenovirus vectors into cultured cells and mice: crossing the species barrier. J Virol. 2001;75:5108–5118. doi: 10.1128/JVI.75.11.5108-5118.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Xia Y, Carpentier A, Cheng X, Block PD, Zhao Y, Zhang Z, et al. Human stem cell-derived hepatocytes as a model for hepatitis B virus infection, spreading and virus-host interactions. J Hepatol. 2017;66:494–503. doi: 10.1016/j.jhep.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhu Y, Yamamoto T, Cullen J, Saputelli J, Aldrich CE, Miller DS, et al. Kinetics of hepadnavirus loss from the liver during inhibition of viral DNA synthesis. J Virol. 2001;75:311–322. doi: 10.1128/JVI.75.1.311-322.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Addison WR, Walters KA, Wong WW, Wilson JS, Madej D, Jewell LD, et al. Half-life of the duck hepatitis B virus covalently closed circular DNA pool in vivo following inhibition of viral replication. J Virol. 2002;76:6356–6363. doi: 10.1128/JVI.76.12.6356-6363.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH, et al. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol. 2003;77:68–76. doi: 10.1128/JVI.77.1.68-76.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Volz T, Allweiss L, Ben MM, Warlich M, Lohse AW, Pollok JM, et al. The entry inhibitor Myrcludex-B efficiently blocks intrahepatic virus spreading in humanized mice previously infected with hepatitis B virus. J Hepatol. 2013;58:861–867. doi: 10.1016/j.jhep.2012.12.008. [DOI] [PubMed] [Google Scholar]
- [29].Lempp FA, Mutz P, Lipps C, Wirth D, Bartenschlager R, Urban S. Evidence that hepatitis B virus replication in mouse cells is limited by the lack of a host cell dependency factor. J Hepatol. 2016;64:556–564. doi: 10.1016/j.jhep.2015.10.030. [DOI] [PubMed] [Google Scholar]
- [30].Summers J, Smith PM, Huang MJ, Yu MS. Morphogenetic and regulatory effects of mutations in the envelope proteins of an avian hepadnavirus. J Virol. 1991;65:1310–1317. doi: 10.1128/jvi.65.3.1310-1317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]