Abstract
We report the detection of the oxygen-bearing complex organic molecules propenal (C2H3CHO), vinyl alcohol (C2H3OH), methyl formate (HCOOCH3), and dimethyl ether (CH3OCH3) toward the cyanopolyyne peak of the starless core TMC-1. These molecules are detected through several emission lines in a deep Q-band line survey of TMC-1 carried out with the Yebes 40m telescope. These observations reveal that the cyanopolyyne peak of TMC-1, which is the prototype of cold dark cloud rich in carbon chains, contains also O-bearing complex organic molecules like HCOOCH3 and CH3OCH3, which have been previously seen in a handful of cold interstellar clouds. In addition, this is the first secure detection of C2H3OH in space and the first time that C2H3CHO and C2H3OH are detected in a cold environment, adding new pieces in the puzzle of complex organic molecules in cold sources. We derive column densities of (2.2 ± 0.3) × 1011 cm™2, (2.5 ± 0.5) × 1012 cm−2, (1.1 ± 0.2) × 1012 cm−2, and (2.5 ± 0.7) × 1012 cm−2 for C2H3CHO, C2H3OH, HCOOCH3, and CH3OCH3, respectively. Interestingly, C2H3OH has an abundance similar to that of its well known isomer acetaldehyde (CH3CHO), with C2H3OH/CH3CHO ~ 1 at the cyanopolyyne peak. We discuss potential formation routes to these molecules and recognize that further experimental, theoretical, and astronomical studies are needed to elucidate the true mechanism of formation of these O-bearing complex organic molecules in cold interstellar sources.
Keywords: astrochemistry, line: identification, ISM: individual objects (TMC-1), ISM: molecules, radio lines: ISM
1. Introduction
Complex organic molecules (COMs) like methyl formate (HCOOCH3) and dimethyl ether (CH3OCH3) have been traditionally observed in the warm gas around protostars, the so-called hot cores and corinos, where they are thought to form upon thermal desorption of ice mantles on grains (Herbst & van Dishoeck 2009). In the last decade, these molecules have been observed as well in a few cold sources, like the dense cores B1-b (Öberg et al. 2010; Cernicharo et al. 2012) and L483 (Agúndez et al. 2019), the dark cloud Barnard 5 (Taquet et al. 2017), the pre-stellar cores L1689B (Bacmann et al. 2012) and L1544 (Jiménez-Serra et al. 2016), and the starless core TMC-1 (Soma et al. 2018). The low temperatures in these environments inhibit thermal desorption, and it is still an active subject of debate how are these molecules formed, whether in the gas phase or on grain surfaces followed by some non-thermal desorption process (Vasyunin & Herbst 2013; Ruaud et al. 2015; Balucani et al. 2015; Chang & Herbst 2016; Vasyunin et al. 2017; Shin-gledecker et al. 2018; Jin & Garrod 2020).
The cyanopolyyne peak of TMC-1, TMC-1(CP), is characterized by a carbon-rich chemistry, with high abundances of carbon chains and a poor content of O-bearing COMs (e.g., Agúndez & Wakelam 2013). Here we report the detection of four O-bearing COMs toward TMC-1(CP). Propenal (C2H3CHO) has been reported previously toward massive star-forming regions in the Galactic center (Hollis et al. 2004; Requena-Torres et al. 2008) and in the hot corino IRAS 16293-2422B (Manigand et al. 2021). Vinyl alcohol (C2H3OH) has been only seen toward Sagittarius B2(N), where the high spectral density complicates the identification (Turner & Apponi 2001). Therefore, this is the first clear detection of C2H3OH in space and the first time that C2H3CHO and C2H3OH are detected in a cold environment. We also report the detection of HCOOCH3 and CH3OCH3, recently detected (the latter tentatively) toward the methanol peak of TMC-1 (Soma et al. 2018) but not toward TMC-1(CP).
2. Astronomical observations
The data presented here belong to a Q-band line survey of TMC-1(CP), αJ2000 = 4h41m41.9s and δJ2000 = +25°41′27.0″, performed with the Yebes 40m telescope. The cryogenic receiver for the Q band, built within the Nanocosmos project1 and which covers the 31.0-50.4 GHz frequency range with horizontal and vertical polarizations, was used connected to FFTS spectrometers, which cover a bandwidth of 8×2.5 GHz in each polarization with a spectral resolution of 38.15 kHz. The system is described in Tercero et al. (2021). The half power beam width (HPBW) of the Yebes 40m telescope ranges from 36.4″ to 54.4″ in the Q band. The intensity scale is antenna temperature, for which we estimate an uncertainty of 10 %, which can be converted to main beam brightness temperature, T mb, by dividing by B eff/F eff (see Table 1). The line survey was carried out during several observing runs and various results have been already published. Data taken during November 2019 and February 2020 allowed to detect the negative ions C3N™ and C5N™ (Cernicharo et al. 2020a), and to discover HC4NC (Cernicharo et al. 2020b), HC3O+ (Cernicharo et al. 2020c), and HC5NH+ (Marcelino et al. 2020). A further observing run, carried out in October 2020, resulted in the detection of HDCCN (Cabezas et al. 2021), HC3S+ (Cernicharo et al. 2021a), CH3CO+ (Cernicharo et al. 2021b), and various C4H3N isomers (Marcelino et al. 2021). Additional observations were taken in December 2020 and January 2021, which led to the discovery of vinyl acetylene (CH2CHCCH; Cernicharo et al. 2021c), allenyl acetylene (CH2CCHCCH; Cernicharo et al. 2021d), and propargyl (CH2CCH; Agúndez et al. 2021), and a final run was carried out in March 2021. All observations were carried out using the frequency switching technique, with a frequency throw of 10MHz during the two first observing runs and 8MHz in the later ones. All data were reduced with the program CLASS of GILDAS (Pety 2005)2.
Table 1. Observed line parameters of the target O-bearing COMs of this study in TMC-1.
| Molecule | Transition | Eup (K) | v calc (MHz) | T*A peak (mK) | △va (km s−) | VLSR (km s−1) | ∫ T*Adv (mK km s−1) | S/N b (σ) | B eff/F eff c |
|---|---|---|---|---|---|---|---|---|---|
| trans-C2H3CHO | 41,4-31,3 | 6.2 | 34768.987 | 3.06 ± 0.31 | 0.62 ± 0.08 | 5.77 ± 0.03 | 2.03 ± 0.21 | 14.5 | 0.603 |
| 40,4-30,3 | 4.3 | 35578.136 | 3.77 ± 0.33 | 0.81 ± 0.08 | 5.79 ± 0.03 | 3.27 ± 0.27 | 19.4 | 0.597 | |
| 41,3 −31,2 | 6.4 | 36435.990 | 3.17 ± 0.29 | 0.85 ± 0.09 | 5.73 ± 0.04 | 2.87 ± 0.25 | 19.2 | 0.590 | |
| 51,5-41,4 | 8.3 | 43455.469 | 1.81 ± 0.49 | 0.72 ± 0.27 | 5.78 ± 0.08 | 1.38 ± 0.37 | 6.5 | 0.530 | |
| 50,5-40,4 | 6.4 | 44449.749 | 3.67 ± 0.54 | 0.54 ± 0.08 | 5.80 ± 0.04 | 2.11 ± 0.31 | 10.5 | 0.521 | |
| 51,4-41,3 | 8.6 | 45538.994 | 4.72 ± 0.66 | 0.69 ± 0.09 | 5.80 ± 0.04 | 3.45 ± 0.43 | 12.6 | 0.511 | |
| syn-C2H3OH | 40,4-31,3 | 9.3 | 32449.221 | 1.17 ± 0.32 | 1.07 ± 0.21 | 5.61 ± 0.11 | 1.34 ± 0.27 | 6.8 | 0.622 |
| 21,2-11,1 | 5.1 | 37459.184 d | – | – | – | – | – | 0.581 | |
| 20,2-10,1 | 2.8 | 39016.387 | 1.90 ± 0.39 | 0.89 ± 0.16 | 5.81 ± 0.07 | 1.79 ± 0.29 | 9.0 | 0.568 | |
| 21,1-11,0 | 5.3 | 40650.606 | 1.71 ± 0.41 | 0.65 ± 0.19 | 5.88 ± 0.08 | 1.18 ± 0.28 | 6.7 | 0.554 | |
| HCOOCH3 | 31,3-21,2 E | 4.0 | 34156.889 | 1.92 ± 0.29 | 0.62 ± 0.12 | 5.93 ± 0.06 | 1.27 ± 0.23 | 9.6 | 0.608 |
| 31,3-21,2 A | 3.9 | 34158.061 | 1.86 ± 0.29 | 0.80 ± 0.16 | 5.84 ± 0.07 | 1.59 ± 0.28 | 10.6 | 0.608 | |
| 30,3-20,2 E | 3.5 | 36102.227e | – | – | – | – | – | 0.593 | |
| 30,3-20,2 A | 3.5 | 36104.775 | 2.61 ± 0.29 | 0.74 ± 0.09 | 5.80 ± 0.04 | 2.05 ± 0.22 | 14.6 | 0.592 | |
| 31,2-21,1 E | 4.4 | 38976.085 | 1.81 ± 0.35 | 0.66 ± 0.14 | 5.93 ± 0.07 | 1.28 ± 0.25 | 8.3 | 0.568 | |
| 31,2-21,1 A | 4.4 | 38980.803 | 2.45 ± 0.35 | 0.57 ± 0.09 | 5.92 ± 0.04 | 1.49 ± 0.21 | 10.4 | 0.568 | |
| 41,4-31,3 E | 6.1 | 45395.802 | 2.82 ± 0.57 | 0.63 ± 0.18 | 6.00 ± 0.07 | 1.89 ± 0.40 | 8.3 | 0.512 | |
| 41,4-31,3 A | 6.1 | 45397.360 | 2.39 ± 0.57 | 0.42 ± 0.19 | 5.91 ± 0.10 | 1.07 ± 0.32 | 5.8 | 0.512 | |
| 40,4-30,3 E | 5.8 | 47534.116 | 3.19 ± 0.75 | 0.59 ± 0.12 | 5.92 ± 0.07 | 2.01 ± 0.44 | 7.1 | 0.493 | |
| 40,4-30,3 A | 5.8 | 47536.905 | 2.76 ± 0.75 | 0.98 ± 0.23 | 5.99 ± 0.10 | 2.88 ± 0.60 | 7.9 | 0.493 | |
| CH3OCH3 | 21,1 −20,2 AE + EA | 4.2 | 31105.223 | 1.35 ± 0.39 | 0.92 ± 0.25 | 5.72 ± 0.13 | 1.31 ± 0.36 | 5.8 | 0.632 |
| 21,1-20,2 EE | 4.2 | 31106.150 | 1.52 ± 0.39 | 0.54 ± 0.34 | 5.94 ± 0.08 | 0.87 ± 0.29 | 5.0 | 0.632 | |
| 31,2-30,3 AE + EA | 7.0 | 32977.276 | 1.37 ± 0.25 | 0.63 ± 0.13 | 5.84 ± 0.07 | 0.91 ± 0.19 | 7.8 | 0.618 | |
| 31,2-30,3 EE | 7.0 | 32978.232 | 1.37 ± 0.25 | 0.95 ± 0.23 | 5.84 ± 0.09 | 1.38 ± 0.27 | 9.6 | 0.618 | |
| 31.2-30,3 AA | 7.0 | 32979.187 | 1.06 ± 0.25 | 0.85 ± 0.32 | 5.99 ± 0.12 | 0.96 ± 0.29 | 7.1 | 0.618 | |
| 41.3-40,4 EE | 10.8 | 35593.408 | 1.22 ± 0.27 | 0.56 ± 0.13 | 5.82 ± 0.07 | 0.73 ± 0.16 | 6.4 | 0.597 | |
| 11,1-00,0 EE | 2.3 | 47674.967 | 3.31 ± 0.62 | 0.59 ± 0.12 | 6.02 ± 0.06 | 2.08 ± 0.38 | 8.9 | 0.492 |
The line parameters T*A peak, Δv, VLSR, and ∫ T*Adv and the associated errors are derived from a Gaussian fit to each line profile.
Δv is the full width at half maximum (FWHM).
Signal-to-noise ratio is computed as S/N = ∫T*Adv / [rms × -, where c is the speed of light, δv is the spectral resolution (0.03815 MHz), the rms is given in the uncertainty of T*A peak, and the rest of parameters are given in the table.
Beff is given by the Ruze formula B eff = 0.738 exp [™(v/72.2)2], where v is the frequency in GHz, and F eff = 0.97.
Line overlaps with a hyperfine component of CH2CCH (see Agúndez et al. 2021).
Line detected but not fitted because it overlaps with a negative frequency-switching artifact.
3. Molecular spectroscopy
C2H3CHO has two conformers. The most stable, and the only one reported in space (Hollis et al. 2004; Requena-Torres et al. 2008; Manigand et al. 2021), is the trans form, which is the one reported here as well. Level energies and transition frequencies were obtained from the rotational constants derived by Daly et al. (2015). The dipole moment along the a axis (all transitions observed here are of a-type) is 3.052D (Blom et al. 1984).
C2H3OH has also two conformers, named syn and anti. Turner & Apponi (2001) assigned various emission features to the two conformers in the crowded spectra of Sgr B2(N). Here we report an unambiguous detection of the syn form, which is the most stable one, in a colder source that is much less affected by line confusion. Level energies and transition frequencies were computed from the rotational constants given by Melosso et al. (2019). The components of the dipole moment along the a and b axes are 0.616 D and 0.807 D, respectively (Saito 1976). Both a- and b-type transitions are observed here.
HCOOCH3 is a well known interstellar molecule in which the internal rotation, or torsion, of the methyl group makes each rotational level to split into A and E substates, with statistical weights A:E = 1:1. We used the spectroscopy from a fit to the lines measured by Ogata et al. (2004) implemented in MADEX (Cernicharo et al. 2012). Here all observed transitions are of a-type and thus we adopted μa = 1.63 D (Curl 1959).
CH3OCH3 is an asymmetric rotor in which the large amplitude internal motion of the two equivalent methyl groups leads to level splitting into AA, EE, EA, and AE substates, with nontrivial statistical weights (Endres et al. 2009). We adopted the spectroscopy from the CDMS catalog (Müller et al. 2005) 3. The geometry of the molecule makes it to have non-zero dipole moment only along the b axis, with a measured value of 1.302 D (Blukis et al. 1963).
4. Results
We detected various lines for each of the O-bearing COMs target of this study (see Table 1), with signal-to-noise ratios (S/N) well above 3 σ. The position of the lines is consistent with the calculated frequencies based on laboratory data (see Sec. 3) and the systemic velocity of the source, VLSR = 5.83 km s−1 (Cernicharo et al. 2020b). Moreover, the relative intensities of the lines are those expected for rotational temperatures in the range 3-10 K, which are typical of TMC-1 (Gratier et al. 2016), and there are no missing lines. We thus consider that the detection of the four O-bearing COMs in TMC-1 is secure. Hereafter we discuss the particularities of each molecule.
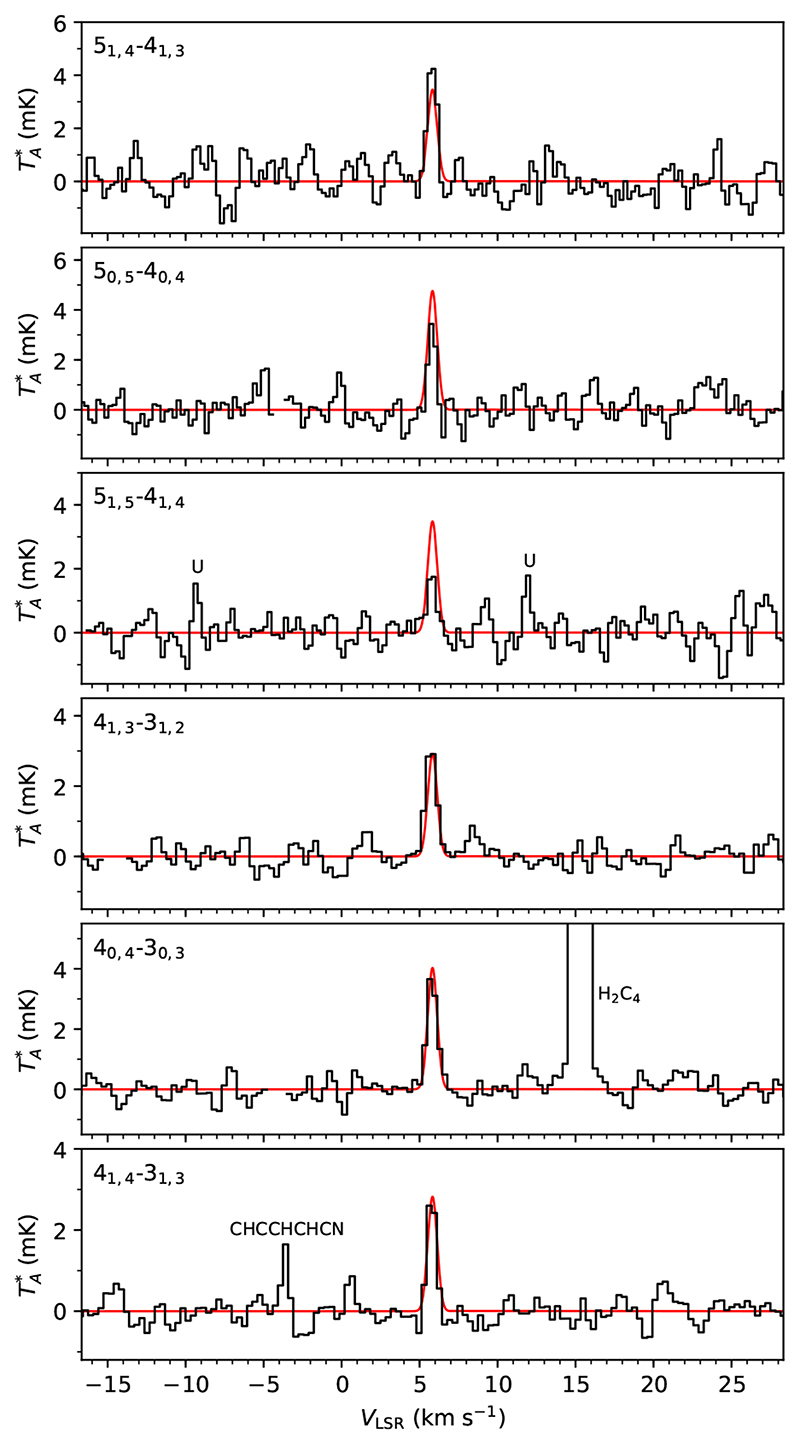
The detection of the trans form of C2H3CHO is very solid since the six observed lines are detected with S/N between 6.5 σ and 19.4 σ and the VLSR of the lines are fully consistent with the systemic velocity of TMC-1 (see Table 1 and Fig. 1). The rotational temperature (T rot) of C2H3CHO is not very precisely determined, 7.5 ± 3.5K from a rotation diagram, but synthetic spectra computed under thermodynamic equilibrium indicate that values in the range 5-10K are consistent with the relative intensities observed. We thus adopted T rot = 7.5 K to compute the synthetic spectra and derive the column density. The arithmetic mean of the observed C2H3CHO linewidths, 0.71 km s−1, is adopted when computing the synthetic spectra (see Table 1). We also assumed a circular emission distribution with a diameter θs = 80”, as observed for various hydrocarbons in TMC-1 (Fossé et al. 2001). For the other three molecules we followed the same convention, adopting as linewidth the average of the observed values and assuming the same emission distribution. The column density derived for C2H3CHO is (2.2 ± 0.3) × 1011 cm-2.
Fig. 1.
Lines of C2H3CHO observed in TMC-1 (see parameters in Table 1). In red computed synthetic spectra for N = 2.2 × 1011 cm−2, Trot = 7.5K, FWHM = 0.71 km s−1, and θs = 80”.
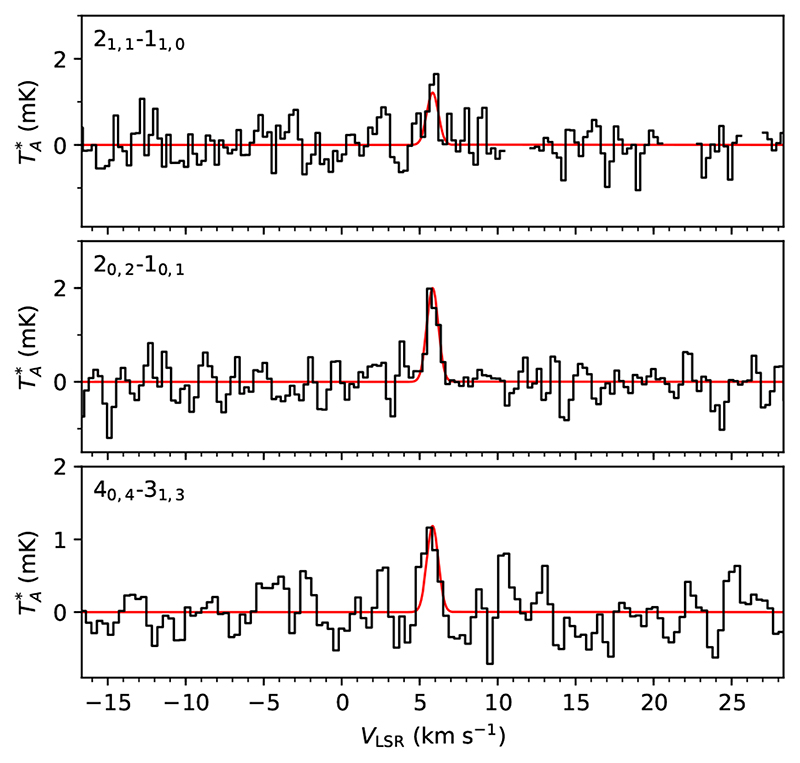
In the case of C2H3OH (see Table 1 and Fig. 2), three lines are clearly detected, with S/N of 6.7 σ and 9.0 σ. The frequency of the 21,2−11,1 transition, 37459.184 MHz, coincides with a hyperfine component of CH2CCH, recently reported in TMC-1 (Agúndez et al. 2021). We predict = 1.1 mK for the 21,2−11,1 transition, which indicates that the observed line (see Agúndez et al. 2021) has contributions from both CH2CCH and C2H3OH. The high detection significance of three lines, the overlap of a fourth line with CH2CCH, and the fact that there are no missing lines, makes us to consider the detection of C2H3OH secure. The rotational temperature is not well constrained for C2H3OH, although the observed relative intensities indicate that it must be in the high range of the values typically observed in TMC-1. We thus adopted Trot = 10K and a linewidth of 0.87 km s−1 to compute the synthetic spectra. We derive a column density of (2.5 ± 0.5) × 1012 cm−2 for C2H3OH.
Fig. 2.
Lines of C2H3OH observed in TMC-1 (see parameters and note on line 21,1–11,0 in Table 1). In red computed synthetic spectra for N = 2.5 × 1012 cm−2, T rot = 10 K, FWHM = 0.87 km s−1, and θs = 80”.
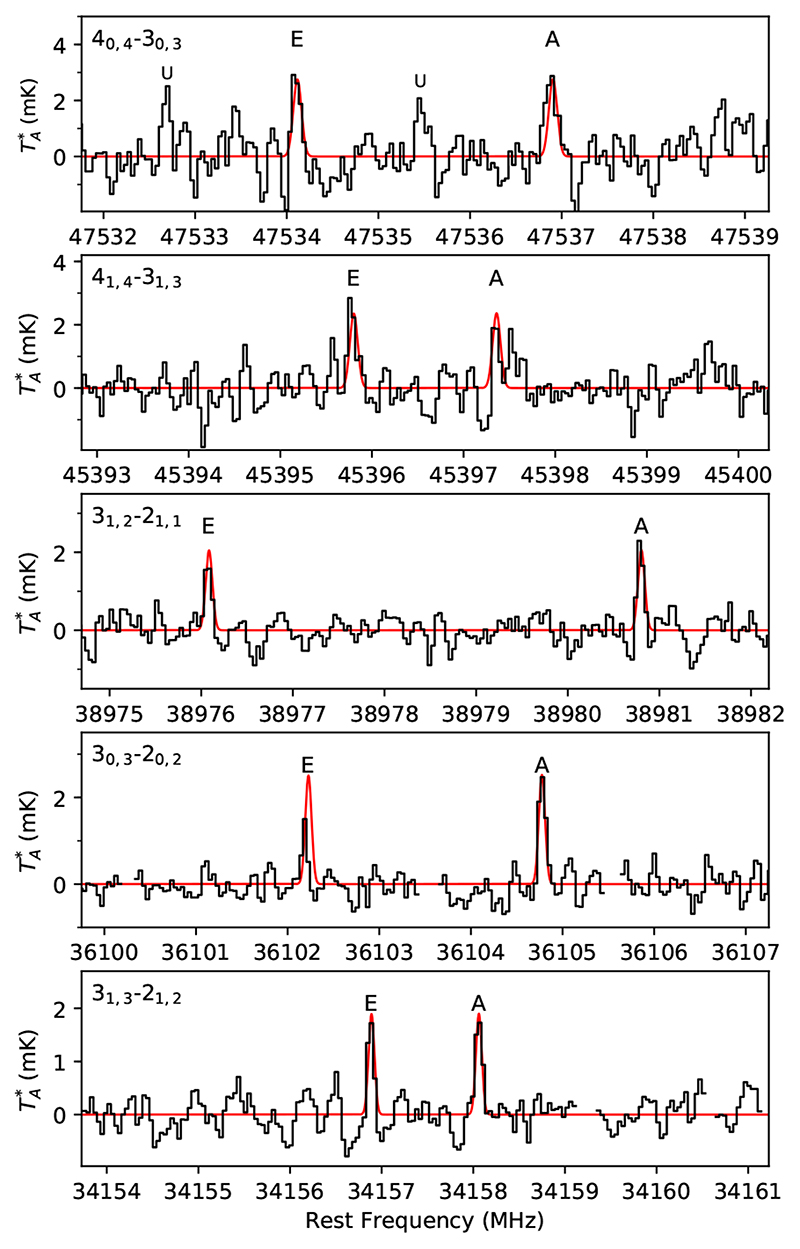
We detected five A/E doublets of HCOOCH3 with S/N in the range 5.8-14.6 σ (see Table 1 and Fig. 3). The only line affected by a problem is the 30,3−20,2 transition of the E substate, which accidentally lies close to a negative frequency-switching artifact, making it to appear less intense and slightly shifted from the correct position. We thus did not fit this line. The rotational temperature derived is 5.1 ± 2.5 K, and we thus adopted T rot = 5 K, and a linewidth of 0.67 km s−1, to compute the synthetic spectra. The total column density obtained for HCOOCH3, including both A and E substates, is (1.1 ± 0.2) × 1012 cm−2.
Fig. 3.
Lines of HCOOCH3 observed in TMC-1 (see parameters and note on line 30,3-20,2 E in Table 1). In red computed synthetic spectra for a N = 1.1 × 1012cm-2, T rot = 5 K, FWHM = 0.67 km s−1, and θs = 80”.
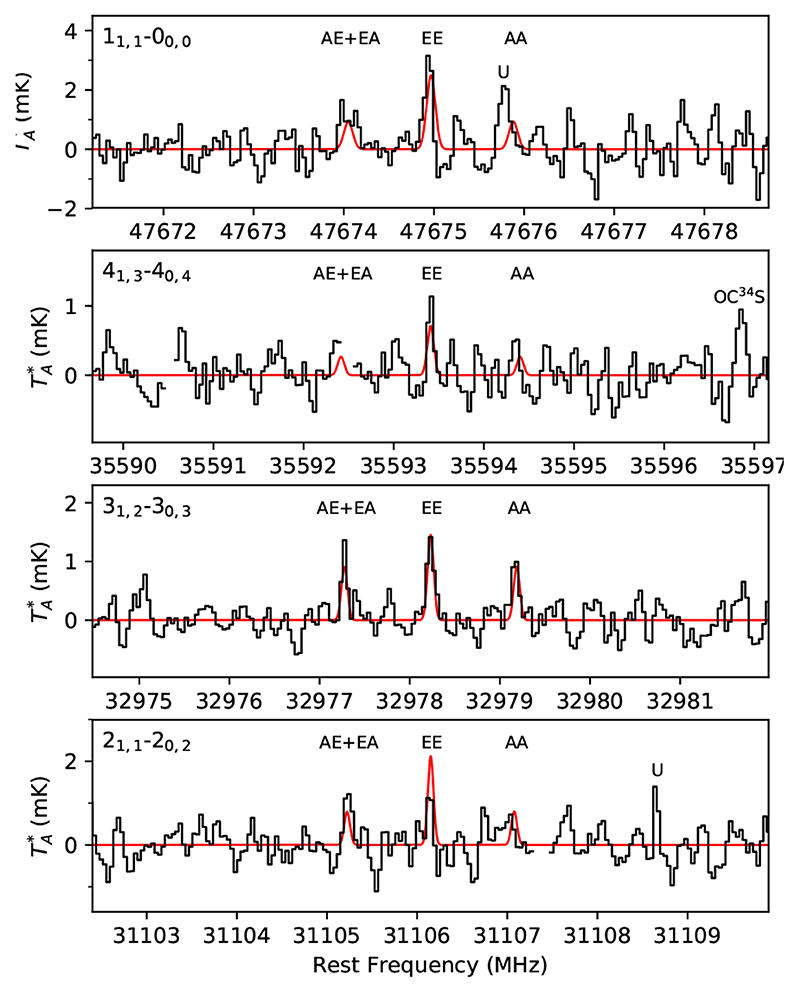
For CH3OCH3, we observed four triplets with the characteristic structure of an intense component corresponding to the EE substate lying between two equally intense components corresponding to the AE+EA and AA substates. The EE component of the four triplets is detected with good confidence levels, between 5.0 σ and 9.6 σ (see Table 1 and Fig. 4). The weaker components corresponding to the AE+EA and AA substates are sometimes found to lie within the noise, although the computed synthetic spectra is consistent with this fact. We consider that the detection of CH3OCH3 is secure. From a rotation diagram we derive a low rotational temperature of 3.6 ± 0.6 K, which is well constrained by the availability of transition covering upper level energies from 2.3K to 10.8 K. We thus adopted Trot = 3.6K and a linewidth of 0.72 km s−1 to compute the synthetic spectra, which implies a total column density, including the four substates, of (2.5 ± 0.7) × 1012 cm−2 for CH3OCH3.
Fig. 4.
Lines of CH3OCH3 observed in TMC-1 (see parameters in Table 1). In red computed synthetic spectra for N = 2.5 × 1012cm−2, T rot = 3.6 K, FWHM = 0.72 km s−1, and θs = 80”.
The variation in the column densities due to the uncertainty in T rot is small, ~ 15 %, for C2H3CHO and C2H3OH, and higher, a factor of two, for HCOOCH3 and CH3OCH3.
5. Discussion
The abundances derived for C2H3CHO, C2H3OH, HCOOCH3, and CH3OCH3 are 2.2 × 10−11, 2.5 × 10−10, 1.1 × 10−10, and 2.5 × 10−10, respectively, relative to H2, if we adopt a column density of H2 of 1022 cm−2 (Cernicharo & Guélin 1987). Now, how are these four O-bearing COMs formed in TMC-1?.
C2H3CHO and C2H3OH could be formed by gas-phase neutral-neutral reactions between reactive radicals like OH, CH, or C2H and abundant closed-shell molecules. However, among the potential sources of C2H3OH, the reactions OH + C2H4 and OH + CH2CHCH3 seem to have activation barriers (Zhu et al. 2005; Zádor et al. 2009) and the reaction CH + CH3OH seems to yield H2CO and CH3 as products (Zhang et al. 2002). In the case of C2H3CHO, a potential formation reaction is OH + allene, but the main products are H2CCO + CH3 (Daranlot et al. 2012). Two more promising routes to C2H3CHO are the reactions CH + CH3CHO, which has been found to produce C2H3CHO (Goulay et al. 2012), and C2H + CH3OH, which to our knowledge has not been studied experimentally or theoretically.
C2H3CHO and C2H3OH are not specifically considered in the chemical networks UMIST RATE12 (McElroy et al. 2013) or KIDA uva.kida.2014 (Wakelam et al. 2015), but acetaldehyde (CH3CHO), which is an isomer of C2H3OH, is included. Since it often happens that astrochemical databases do not distinguish between different isomers because information is not available, it is conceivable that some of the reactions that are considered to produce CH3CHO could also form C2H3OH. According to a standard pseudo-time-dependent gas-phase chemical model of a cold dark cloud (e.g., Agúndez & Wakelam 2013), there are two main reactions of formation of CH3CHO. The first is O + C2H5, for which formation of C2H3OH does not seem to be an important channel, according to experiments (Slagle et al. 1988) and theory (Jung et al. 2011; Vazart et al. 2020). The second is the dissociative recombination of CH3CHOH+ with electrons, in which case experiments show that the CCO backbone is preserved with a branching ratio of 23% (Hamberg et al. 2010), so that it is possible that both CH3CHO and C2H3OH are formed. A different route to CH3CHO starting from abundant ethanol (C2H5OH) has been proposed (Skouteris et al. 2018; Vazart et al. 2020), but in L483, the only cold environment where C2H5OH has been detected, its abundance is twice smaller than that of CH3CHO (Agúndez et al. 2019). The column density of CH3CHO at TMC-1(CP) is (2.7-3.5) × 1012 cm−2 (Gratier et al. 2016; Cernicharo et al. 2020c), which implies a C2H3OH/CH3CHO ratio of ~ 1. Therefore, if the two isomers are formed by the same reaction, then the branching ratios should be similar. The reaction CH3 + + H2CO produces CH4 + HCO+ (Smith & Adams 1978), and thus it is unlikely to form C2H3OH, as suggested by Turner & Apponi (2001).
Grain-surface processes could also form C2H3CHO and C2H3OH in TMC-1. Experiments show that C2H3OH is formed upon proton irradiation of H2O/C2H2 ices (Hudson & Moore 2003), and electron irradiation of CO/CH4 and H2O/CH4 ices (Abplanalp et al. 2016; Bergantini et al. 2017), while C2H3CHO is produced after electron irradiation of CO/C2H4 ices (Abplanalp et al. 2015). Non-energetic processing of C2H2 ices, in which reactions with H atoms and OH radicals occur on the surface, also produces C2H3OH (Chuang et al. 2020). It remains however uncertain whether these experimental setups (e.g., in terms of irradiation fluxes and ice composition) resemble those of cold dark clouds. Abplanalp et al. (2016) made an effort in this sense by incorporating the results of electron irradiation experiments in a chemical model of a cold dark cloud, and found that cosmic rays could drive the formation of C2H3OH on grain surfaces. Recently, Shingledecker et al. (2019) proposed that C2H3CHO can be efficiently formed on grain surfaces by successive reactions of addition of an H atom to HC3O. This process would also produce propynal (HCCCHO), which make these authors to propose a chemical connection, and thus a potential correlation, between HCCCHO and C2H3CHO. If this mechanism is correct, then C2H3CHO would be more likely detected in those sources with intense HCCCHO emission (Loison et al. 2016).
There is no yet consensus on how are HCOOCH3 and CH3OCH3 formed in cold sources. Models where the synthesis relies on chemical desorption and gas-phase radiative associations usually require a chemical desorption efficiency as high as 10 % (Vasyunin & Herbst 2013; Balucani et al. 2015; Chang & Herbst 2016), which can be relaxed if Eley-Rideal processes (Ruaud et al. 2015), radiation chemistry (Shingledecker et al. 2018), or nondiffussive grain-surface processes (Jin & Garrod 2020) are considered. These models can account for abundances relative to H2 around 10−10 for HCOOCH3 and/or CH3OCH3 under certain assumptions, although they rely on yet poorly constrained chemical and physical processes. Astronomical observations pointed out that HCOOCH3 and CH3OCH3 could have a chemical connection with CH3OH, based on the slight abundance enhancement inferred for these O-bearing COMs at the CH3OH peak with respect to the dust peak in the pre-stellar core L1544 (Jiménez-Serra et al. 2016). The column densities derived here for HCOOCH3 and CH3OCH3 at TMC-1(CP) are similar, within a factor of two, to those reported by Soma et al. (2018) at the CH3OH peak of TMC-1. A coherent study using the same telescope and a detailed radiative transfer model is needed to see if there is a significant abundance enhancement of HCOOCH3 and CH3OCH3 at the CH3OH peak of TMC-1.
6. Conclusions
We reported the detection of C2H3CHO, C2H3OH, HCOOCH3, and CH3OCH3 toward the cyanopolyyne peak of TMC-1. This region, which is a prototypical cold dark cloud with abundant carbon chains, has been revealed as a new cold source where the O-bearing COMs HCOOCH3 and CH3OCH3 are present. In addition, we provide the first evidence of two other O-bearing COMs, C2H3CHO and C2H3OH, in a cold source, the latter being identified unambiguously for the first time in space here. The abundances relative to H2 derived are a few 10−11 for C2H3CHO and a few 10−10 for the three other molecules. Interestingly, C2H3OH has a similar abundance to its isomer CH3CHO, with C2H3OH/CH3CHO ~ 1. We discuss potential formation routes to these molecules and conclude that further experimental, theoretical, and astronomical studies are needed to shed light on the origin of these COMs in cold interstellar sources.
Acknowledgements
We acknowledge funding support from Spanish MICIU through grants AYA2016-75066-C2-1-P, PID2019-106110GB-I00, PID2019-106235GB-I00, and PID2019-107115GB-C21 and from the European Research Council (ERC Grant 610256: NANOCOSMOS). M.A. also acknowledges funding support from the Ramón y Cajal programme of Spanish MICIU (grant RyC-2014-16277). We thank the anonymous referee for a constructive report that helped to improve this manuscript.
Footnotes
Contributor Information
M. Agúndez, Email: marcelino.agundez@csic.es.
J. Cernicharo, Email: jose.cernicharo@csic.es.
References
- Abplanalp MJ, Borsuk A, Jones BM, Kaiser RI. ApJ. 2015;814:45. [Google Scholar]
- Abplanalp MJ, Gozem S, Krylov AI, et al. PNAS. 2016;113:7727. doi: 10.1073/pnas.1604426113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agúndez M, Wakelam V. Chem Rev. 2013;113:8710. doi: 10.1021/cr4001176. [DOI] [PubMed] [Google Scholar]
- Agúndez M, Marcelino N, Cernicharo J, et al. A&A. 2019;625:A147. doi: 10.1051/0004-6361/201935164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agúndez M, Cabezas C, Tercero B, et al. A&A. 2021;647:L10. doi: 10.1051/0004-6361/202140553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacmann A, Taquet V, Faure A, et al. A&A. 2012;541:L12. [Google Scholar]
- Balucani N, Ceccarelli C, Taquet V. MNRAS. 2015;449:L16. [Google Scholar]
- Bergantini A, Maksyutenko P, Kaiser RI. ApJ. 2017;841:96. [Google Scholar]
- Blom CE, Grassi G, Bauder A. J Am Chem Soc. 1984;106:7427 [Google Scholar]
- Blukis U, Kasai PH, Myers RJ. J Chem Phys. 1963;38:2753 [Google Scholar]
- Cabezas C, Endo Y, Roueff E, et al. A&A. 2021;646:L1. [Google Scholar]
- Cernicharo J, Guélin M. A&A. 1987;176:299. [Google Scholar]
- Cernicharo J. In: Stehlé C, Joblin C, d’Hendecourt L, editors. European Conference on Laboratory Astrophysics; 2012. p. 251. [Google Scholar]
- Cernicharo J, Marcelino N, Roueff E, et al. ApJ. 2012;759:L43. [Google Scholar]
- Cernicharo J, Marcelino N, Pardo JR, et al. A&A. 2020a;641:L9. doi: 10.1051/0004-6361/202039231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernicharo J, Marcelino N, Agúndez M, et al. A&A. 2020b;642:L8. doi: 10.1051/0004-6361/202039274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernicharo J, Marcelino N, Agúndez M, et al. A&A. 2020c;642:L17. doi: 10.1051/0004-6361/202039351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernicharo J, Cabezas C, Endo Y, et al. A&A. 2021a;646:L3. doi: 10.1051/0004-6361/202040013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernicharo J, Cabezas C, Bailleux S, et al. A&A. 2021b;646:L7. doi: 10.1051/0004-6361/202040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernicharo J, Agúndez M, Cabezas C, et al. A&A. 2021c;647:L2. doi: 10.1051/0004-6361/202140434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernicharo J, Cabezas C, Agúndez M, et al. A&A. 2021d;647:L3. doi: 10.1051/0004-6361/202140482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q, Herbst E. ApJ. 2016;819:145. [Google Scholar]
- Chuang K-J, Fedoseev G, Qasim D, et al. A&A. 2020;635:A199. [Google Scholar]
- Curl RF. J Chem Phys. 1959;30:1529 [Google Scholar]
- Daly AM, Bermúdez C, Kolesniková L, Alonso JL. ApJS. 2015;218:30. [Google Scholar]
- Daranlot J, Hickson KM, Loison J-C, et al. J Phys Chem A. 2012;116:10871. doi: 10.1021/jp304831x. [DOI] [PubMed] [Google Scholar]
- Endres CP, Drouin BJ, Pearson JC, et al. A&A. 2009;504:635. [Google Scholar]
- Fossé D, Cernicharo J, Gerin M, Cox P. ApJ. 2001;552:168. [Google Scholar]
- Goulay F, Trevitt AJ, Savee JD, et al. J Phys Chem A. 2012;116:6091. doi: 10.1021/jp2113126. [DOI] [PubMed] [Google Scholar]
- Gratier P, Majumdar L, Ohishi M, et al. ApJS. 2016;225:25. [Google Scholar]
- Hamberg M, Zhaunerchyk V, Vigren E, et al. A&A. 2010;522:A90. [Google Scholar]
- Herbst E, van Dishoeck EF. ARA&A. 2009;47:427. [Google Scholar]
- Hollis JM, Jewell PR, Lovas FJ, et al. ApJ. 2004;610:L21. [Google Scholar]
- Hudson RL, Moore MH. ApJ. 2003;586:L107 [Google Scholar]
- Jiménez-Serra I, Vasyunin AI, Caselli P, et al. ApJ. 2016;830:L6. doi: 10.3847/2041-8205/830/1/L6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Garrod RT. ApJS. 2020;249:26. [Google Scholar]
- Jung S-H, Park Y-P, Kang K-W, et al. Theor Chem Acc. 2011;129:105. [Google Scholar]
- Loison J-C, Agúndez M, Marcelino N, et al. MNRAS. 2016;456:4101. doi: 10.1093/mnras/stv2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manigand S, Coutens A, Loison J-C, et al. A&A. 2021;645:A53. [Google Scholar]
- Marcelino N, Agúndez M, Tercero B, et al. A&A. 2020;643:L6. doi: 10.1051/0004-6361/202039251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelino N, Tercero B, Agúndez M, Cernicharo J. A&A. 2021;646:L9. doi: 10.1051/0004-6361/202040177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy D, Walsh C, Markwick AJ, et al. A&A. 2013;550:A36. [Google Scholar]
- Melosso M, McGuire BA, Tamassia F, et al. ACS Earth Space Chem. 2019;3:1189 [Google Scholar]
- Müller HSP, Schlöder F, Stutzki J, Winnewisser G. J Mol Struct. 2005;742:215. [Google Scholar]
- Öberg KI, Bottinelli S, Jørgensen JK, van Dishoeck EF. ApJ. 2010;716:825. [Google Scholar]
- Ogata K, Odashima H, Takagi K, Tsunekawa S. J Mol Spectr. 2004;225:14. [Google Scholar]
- Pety J. In: SF2A-2005: Semaine de l’Astrophysique Francaise. Ca-soli F, et al., editors. EDP; Les Ulis: 2005. p. 721. [Google Scholar]
- Requena-Torres MA, Martín-Pintado J, Martín S, Morris MR. ApJ. 2008;672:352. [Google Scholar]
- Ruaud M, Loison J-C, Hickson KM, et al. MNRAS. 2015;447:4004 [Google Scholar]
- Saito S. Chem Phys Lett. 1976;42:399. [Google Scholar]
- Shingledecker CN, Tennis J, Le Gal R, Herbst E. ApJ. 2018;861:20. [Google Scholar]
- Shingledecker CN, Álvarez-Barcia S, Korn VH, Kästner J. ApJ. 2019;878:80. [Google Scholar]
- Skouteris D, Balucani N, Ceccarelli C, et al. ApJ. 2018;854:135. [Google Scholar]
- Slagle IR, Sarzynński D, Gutman D, et al. J Chem Soc Faraday Trans. 1988;2:84–491. [Google Scholar]
- Smith D, Adams NG. Chem Phys Lett. 1978;54:535. [Google Scholar]
- Soma T, Sakai N, Watanabe Y, Yamamoto S. ApJ. 2018;854:116. [Google Scholar]
- Taquet V, Wirström ES, Charnley SB, et al. A&A. 2017;607:A20. [Google Scholar]
- Tercero F, López-Pérez JA, Gallego JD, et al. A&A. 2021;645:A37. doi: 10.1051/0004-6361/202038701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BE, Apponi AJ. ApJ. 2001;561:L207 [Google Scholar]
- Vasyunin AI, Herbst E. ApJ. 2013;769:34. [Google Scholar]
- Vasyunin AI, Caselli P, Dulieu F, Jiménez-Serra I. ApJ. 2017;842:33. [Google Scholar]
- Vazart F, Ceccarelli C, Balucani N, et al. MNRAS. 2020;499:5547 [Google Scholar]
- Wakelam V, Loison J-C, Herbst E, et al. ApJS. 2015;217:20. [Google Scholar]
- Zádor J, Jasper AW, Miller JA. Phys Chem Chem Phys. 2009;11:11040. doi: 10.1039/b915707g. [DOI] [PubMed] [Google Scholar]
- Zhang X-b, Liu J-J, Li Z-s, et al. J Phys Chem A. 2002;106:3814 [Google Scholar]
- Zhu RS, Park J, Lin MC. Chem Phys Lett. 2005;408:25. [Google Scholar]