Abstract
Introduction
ZNF384 gene fusions resulting from translocations with several partner genes have been described in B cell acute lymphoblastic leukemia (B-ALL) with a characteristic immunophenotype (aberrant CD13 and or CD33 with dim CD10). The prognosis of patients with this rearrangement appears to depend on the fusion partner. ZNF384 rearrangements have been identified by high through put technologies such as RNA sequencing in most of the studies published. We tested the feasibility of using the characteristic immunophenotype as a tool to screen for patients with ZNF384 translocations which can be subsequently confirmed by cytogenetic / molecular methodologies.
Methods
ZNF384 rearrangements in B-ALL patients at diagnosis with CD10 <80% and were negative for the BCR-ABL1 fusion (n = 109) were identified by fluorescence in situ hybridization followed by confirmation by reverse transcriptase-polymerase chain reaction and Sanger sequencing. The end of induction measurable residual disease evaluated by flow cytometry for these patients was obtained from patient records.
Results
ZNF384 translocations were identified in 14 patients and were cytogenetically cryptic in 13. EP300-ZNF384 was the most common fusion partner (n = 12), while TAF15-ZNF384 and TCF3-ZNF384 were identified in 1 patient each. End of induction MRD by flow cytometry was positive in 5 of 8 patients with the EP300-ZNF384 fusion treated at our center.
Conclusion
Our findings show a practical approach for the identification of ZNF384 gene rearrangements by widely available technologies and indicate that the response to therapy may be heterogeneous even in this subset, which has been reported as having a favorable prognosis.
Keywords: B cell acute lymphoblastic leukemia, end of induction MRD, EP300-ZNF384, ZNF384 fusions
1. Introduction
B cell acute lymphoblastic leukemia (B-ALL) is a heterogeneous disease characterized by the presence of distinct genetic subtypes which impact response to therapy.1,2 Gene fusions involving the zinc-finger protein 384 (ZNF384) gene on chromosome 12p13.31 have been reported in up to 6% of children, 7.3% adults, and 15% of adolescents and young adults(AYA) with B-ALL.3–5 The ZNF384 gene encodes a transcription factor that regulates promoters of the extracellular matrix genes6 and is known to be involved in B-ALL and in mixed phenotype acute leukemia (MPAL) through fusions with several partner genes.5,7–10 Fusions involving the ZNF384 gene have been reported to be mutually exclusive of other genetic subtypes and thus constitute a distinct subgroup in B-ALL.6 ZNF384 rearranged (ZNF384r) leukemia shows a unique gene expression profile and displays a characteristic immunophenotype with weak CD10 and aberrant expression of the myeloid markers, CD13, and/ or CD33.11–13
The genes most frequently involved in fusions with ZNF384 include EP300 (22q13.2), TCF3 (19p13.3), and TAF15 (17q12), and the prognosis appears to vary by fusion partner. The EP300-ZNF384 fusion appears to be favorable while the TCF3-ZNF384 fusion is frequently associated with late relapses and a poor prognosis.11,14 Since the ZNF384 gene and several of the fusion partner gene loci are present adjacent to the telomeric ends of their respective chromosome arms, ZNF384 gene fusions are cytogenetically cryptic and hence cannot be identified by analysis of G-banded chromosomes.10
ZNF384r leukemia’s are characterized by overexpression of the FLT3 gene15 and sunitinib, a FLT3 inhibitor, has been shown to induce molecular response in a patient with EP300-ZNF384 rearrangement.16 Although the biological basis of FLT3 overexpression in such patients is not well understood, identification of such fusions may thus help in providing the option for targeted therapy in patients who remain refractory/relapse on conventional chemotherapy. There is a paucity of data in this unique subtype of patients from India. We describe a series of B-ALL patients with ZNF384 gene rearrangements diagnosed and treated at our center.
2. Methods
2.1. Patients
Consecutive B-ALL patients diagnosed in the Department of Haematology, Christian Medical College, Vellore between January 1, 2015, and September 30, 2020, with CD10 expression <80% and who were negative for the BCR-ABL1 fusion were selected for analysis.11 Diagnosis and risk stratification of B cell ALL were based on morphology, immunophenotyping by multiparameter flow cytometry, cytogenetic analysis, and the RT-PCR detection of common fusion transcripts (BCR-ABL1, ETV6-RUNX1, TCF3-PBX1, and KMT2A-AFF1), as described previously.17 The clinical details including age, WBC count, bone marrow (BM) blast percentage, MRD status (positive >0.01%),18,19 and treatment details were collected from the patients’ records.
2.2. Detection of ZNF384 rearrangement by FISH
FISH analysis was performed using a dual-color break apart probe, SPEC ZNF384 (ZytoLight dual-color break apart probe, Zytovision) on destained G-banded slides of bone marrow/ archived cell pellets. Slides were analyzed using the Axioimager M1 (Carl Zeiss) microscope and ISIS software (Metasystems).20
2.3. Molecular analysis of ZNF384 fusion partners
Total RNA was extracted from bone marrow or peripheral blood samples at diagnosis. RNA (2 μg) was reverse transcribed to cDNA using random hexamers and Superscript RT Kit (Thermo Scientific). RT-PCR was performed to identify the ZNF384 fusion partner as described previously.12,17 The PCR product was sequenced using the Big Dye Terminator v3.1 Cycle Sequencing kit (Applied Biosystems) in an ABI Prism 3130 Genetic Analyzer (Applied Biosystems).
2.4. Treatment
Modified BFM 95 and GMALL protocols with risk stratification were used for treating children and adults, respectively.21 Postinduction, flow cytometry-based measurable residual disease (MRD) testing was performed.22 Patients with high-risk disease were offered allogeneic transplant.21
3. Results
3.1. Detection of ZNF384 rearrangements in BCP-ALL patients by FISH analysis
Of the 891 patients with B-ALL diagnosed during the study period (January 2015-June 2020), 158(68 children and 90 adults) had CD10 expression <80% after exclusion of patients with the BCR/ABL1 fusion (n = 16). Samples were not available for analysis in 49 patients. The algorithm of sample selection and evaluation is illustrated in Figure S1. ZNF384 gene rearrangements were identified in 14 of the 109 patients tested (12.8%). All 14 patients had weak to moderate expression of CD10 with aberrant CD13 and CD33 in 9 and either CD13/CD33 in 2 and 3 patients, respectively(Table 1).
Table 1. Clinical characteristics and laboratory findings in patients with ZNF384 gene rearrangements.
| UPN.NO | Age/ Sex | Total WBC (/cu.mm) | Blast (%) | CD10 (%) | CD13 (%) | CD33 (%) | Karyotype | Fusion partner (RT-PCR) | End Induction MRD |
|---|---|---|---|---|---|---|---|---|---|
| FS 39/19 | 59/M | 16 900 | 94 | 37.7 | 66.4 | 93.1 | 46,XY,t(12;17)(p13.3;q11.2) [16]/ 47,idem,+8[4] | TAF15 | Positive (0.202%) |
| FS 42/19 | 33/M | 6200 | 29 | 34.1 | 61.6 | 85 | 46,XY,add(7)(p11.1),del(7) (p11.1)[12]/46,XY[8] | TCF3 FISH only | aNot applicable |
| FS 52/19 | 23/M | 34 000 | 88 | 47.6 | 60.4 | 15 | 46,XY[20] | EP300 | Positive (0.411%) |
| FS 84/19 | 13/F | 11 100 | 56 | 58 | 1.7 | 91.1 | 46,XX,del(7)(q22)[9]/ 46,XX[11] | EP300 | Positive (1.735%) |
| FS 62/19 | 2/M | 10 100 | 79 | 44.1 | 13.5 | 84.4 | 46,XY[20] | EP300-FISH only | aNot applicable |
| F1002/19 | 9/F | 18 600 | 83 | 11.8 | 94.8 | 97.1 | 46,XX[20] | EP300 | Positive (0.500%) |
| FS124/19 | 2/M | 45 700 | 73 | 55.2 | 73.9 | 17.5 | 46,XY[22] | EP300 | Negative |
| FS04/20 | 25/M | 12 100 | 39 | 1.7 | 21.9 | 86.7 | 46 ~ 47,XY,del(1)(q32),?der (16), add(17)(q25), -20,+mar, +mar [cp7]/ 46,XY[19] | EP300 | Positive (2.247%) |
| FS113/20 | 48/M | 3200 | 96 | 17.9 | 90.4 | 80.4 | 46,XY,t(2;5)(p12;q35), del(13) (q13q32)[15]/ 46,XY[5] | EP300 | Negative |
| FS124/20 | 22/M | 5400 | 61 | 43.1 | 44.5 | 84.6 | 46,XY[20] | EP300 | Negative |
| FS125/20 | 52/M | 2000 | 90 | 7.5 | 42.3 | 83.9 | 46,XY,add(20)(q13.3) [2]/ 46,XY[18] | EP300-FISH only | Not available |
| FS127/20 | 20/F | 5500 | 92 | 56.9 | 6.9 | 70.9 | 46,XX,ins(5;13) (q31;q13q33) [11]/ 46,XX[9] | EP300 | aNot applicable |
| FS162/20 | 41/F | 9100 | 82 | 0.3 | 46.8 | 86.4 | 46,XX[21] | EP300 | Positive (0.463%) |
| FL 54/20 | 33/M | 16 900 | 97 | 14 | 52.2 | 51 | 47,XY,+X[11]/48,idem, +12[4]/46,XY[6] | EP300-FISH only | aNot applicable |
Note: Abbreviations: CD, Cluster of differentiation; MRD, Measurable residual disease; RT-PCR, Reverse transcriptase-polymerase chain reaction; WBC, White blood cell.
Patients who discontinued treatment at the study center.
3.2. EP300 was the most frequent fusion partner
Archived cDNA/RNA samples were available for 10 of the 14 patients with ZNF384 gene rearrangements. The EP300-ZNF384 fusion was identified in 9 and the TAF15-ZNF384 in one patient, respectively.
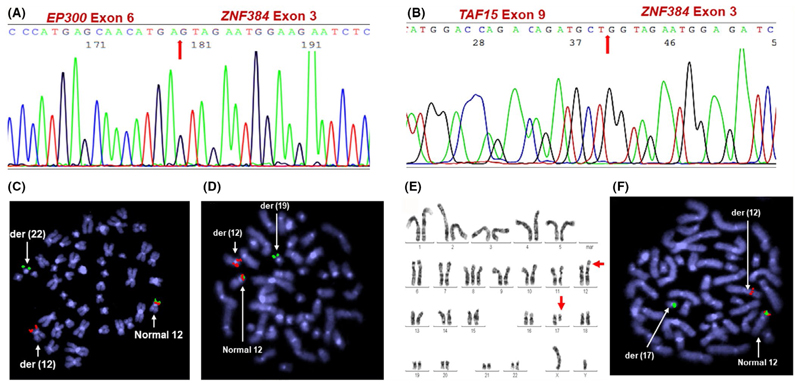
The breakpoints were identified by sequencing to characterize the fusion genes. In all the 9 patients with the EP300-ZNF384 fusion, the resulting sequence revealed that the exon 6 of EP300 was fused to exon 3 of ZNF384 (Figure 1A). In the patient with the t(12;17), exon 9 of the TAF15 gene was fused to exon 3 of ZNF384 (Figure 1B).
Figure 1. Identification of ZNF384 gene fusions.
Sequence of the (A) EP300-ZNF384 fusion involving exon 6 of EP300 and exon 3 of ZNF384 (UPN. No FS 84/19 ) (B) TAF15-ZNF384 fusion involving exon 9 of TAF15 and exon 3 of ZNF384 (UPN. No FS 39/19) (C-D) Inverted DAPI view of metaphase FISH image (1Fusion 1Red 1Green) showing the (C) t(12;22) with the 3’ZNF384 signal(spectrum green) on the derivative(der) chromosome 22 (UPN. No FS 62/19) (D) t(12;19) with the 3’ZNF384 signal (spectrum green) on the derivative chromosome 19 (UPN. No FS 42/19) (E) Representative image of G-banded karyotype showing the reciprocal t(12;17) (F) Inverted DAPI view of metaphase FISH image showing the t(12;17) with the 3ZNF384 signal(spectrum green) on the derivative chromosome 17 (UPN. No FS 39/19)
Matched metaphase FISH analysis in 4 patients for whom samples were not available for RT-PCR analysis showed the t(12;22)- EP300-ZNF384 in 3(Figure 1C), and the t(12;19)- TCF3-ZNF384 in 1(Figure 1D).
Among the 14 patients who showed ZNF384 gene rearrangement by FISH, 9 had karyotypic abnormalities of which only 1 showed an abnormality involving the ZNF384 locus on 12p13.31, namely the t(12;17) (Figure 1E,F). Normal karyotypes were reported in 7 patients while the rest had structural abnormalities that did not involve the ZNF384 gene locus (Table 1).
3.3. Treatment outcome
Of the 10 patients for whom molecular characterization was done, 3 children were treated with a modified BFM protocol while 7 adult patients received the modified GMALL protocol. MRD status at the end of induction was available for 9 patients of whom 6 (66.7%) were positive (Table 1). This included one patient with the TAF15-ZNF384 fusion and 5 with the ZNF384-EP300 fusion. The median follow-up since treatment initiation for the 10 patients who were treated at our center was 13.4 months (interquartile range: 9.0-39.1 months). Hence, although, we noted high rates of detectable MRD at the end of induction therapy, we did not see any morphologic relapses. This was despite only 2 patients being able to undergo stem cell transplantation (Supplementary results).
4. Discussion
Advanced genomic approaches have led to the classification of BALL, specifically B-other ALL into novel genetic subtypes, some with a clear potential for targeted therapeutic approaches.2,15 In our cohort, we used a strategy wherein Ph-negative patients with low CD10 expression were tested for ZNF384 rearrangements. In one of the largest studies on ZNF384 rearranged B-ALL, the CD10 expression was reported to range from 0.39% to 67.34%.14 Since the phenotypic association has been well described previously, we tested for this abnormality in a selected group of 109 patients. Using this strategy, among the tested samples, we identified ZNF384 rearrangements in 6% of children and 11% of adults. ZNF384 fusions constituted 2% (4/193) of children and 5% (10/192) of adults with B-other ALL in the study group. There is a wide range of frequencies of ZNF384 gene fusion in literature depending on the specific subtype of B-ALL that is analyzed. Studies on B-ALL show the ZNF384 in 1%-5% of children and 7.3% in adults, whereas it is seen in 5% and 6% of children with B-other ALL and adults with Philadelphia-negative ALL, respectively. Although the frequencies reported by us are comparable to published reports, a limitation in this study is that the efficiency of this selection strategy based on immunophenotype has not been compared with a control group with CD10 >80%.
Similar to previous reports, EP300 was the most frequent fusion partner in our cohort of patients with ZNF384 gene rearrangements.11 ZNF384r ALLs do not exhibit other unique presenting features except for the distinct immunophenotype.
The EP300-ZNF384 and the TCF3-ZNF384 fusions identified in this study were the result of cytogenetically cryptic translocations as has been reported previously,10 while only the TAF15-ZNF384 fusion could be identified by karyotyping. A striking difference is the presence of chromosomal abnormalities in 9 patients in contrast to the majority of previous reports which have shown cryptic ZNF384 rearrangements in patients with normal karyotypes.10,14 The clinical significance of this observation has to be explored further.
The prognosis of patients with ZNF384r may be dependent on the fusion partner. Previous studies have shown that the EP300-ZNF384 group to be associated with good prognosis and the TCF3-ZNF384 to be associated with late relapse.12 However, 6 of the 9 patients treated in our center were MRD positive at the end of induction including 5 with EP300-ZNF384 suggesting that this subgroup may require a longer follow-up to assess their prognostic impact. Due to a short follow-up period, this does not appear to translate into an adverse clinical outcome in our cohort.
The limitations of this study include the fact that the initial screening was done by FISH analysis followed by RT-PCR for only the patients who were positive by FISH. Cryptic insertions might not have been identified by FISH. In addition, we identified only one patient each with the TCF3 and TAF15- ZNF384 gene fusions, and hence, the clinical outcome of this subgroup needs to be validated in a larger cohort with long-term follow-up data.
Screening for ZNF384 rearrangements in patients with B-other ALL who show an aberrant expression of CD13 and /or CD33 with dim to moderate CD10 expression may help in identifying this subgroup of patients. Aberrant expression of CD13 and CD33 has also been reported in Philadelphia chromosome-positive B-ALL.23 Hence, in B-ALL patients with low CD10 and aberrant CD13/CD33, a possible reflex test could be either FISH using a break part probe for ZNF384 or RT-PCR for the commonly reported ZNF384 gene fusions after excluding BCR-ABL1 fusion.
Supplementary Material
Additional supporting information may be found online in the Supporting Information section.
Acknowledgements
The authors would like to thank the staff of the cytogenetics, flow cytometry, and leukemia labs of the department of Haematology. This study is funded in part by the CMC Fluid Research grant (No 11236: dated 28.03.2018) to NBJ and by the Centre of Excellence grant from Department of Biotechnology India: BT/COE/34/ SP13432/2015 to PB. VM, UK, and PB are supported by Wellcome DBT India Alliance (IA/CPHS/18/1/503930, IA/CPHE/17/1/503351, and IA/S/15/1/501842, respectively).
Funding Information
CMC Fluid Research grant, Grant/Award Number: 11236; Centre of Excellence grant from Department of Biotechnology India, Grant/Award Number: BT/COE/34/ SP13432/2015; Wellcome DBT India Alliance, Grant/Award Number: IA/CPHE/17/1/503351, IA/CPHS/18/1/503930 and IA/S/15/1/501842
Footnotes
Conflict Of Interest
Nil.
Author Contributions
NBJ: Conceptual development of the study, performed research, collected, and analyzed the data, and wrote the paper. UK: performed research, analyzed clinical data, and reviewed paper. AKA: performed research, analyzed flow cytometry data, and reviewed paper. BB: performed research and analyzed cytogenetic data. AK: performed research, clinical data accrual, and analysis. AJD: performed research, clinical data accrual, and analysis. AA: performed research, clinical data accrual, and analysis. BG: performed research, clinical data accrual, and analysis. VM: provided the clinical inputs, analyzed the data, and reviewed the paper. PB: Conceptual development of the study, performed research, analyzed the data, wrote, and reviewed the paper.
Data Availability Statement
Data available on request from the authors.
References
- 1.Mrózek K, Harper DP, Aplan PD. Cytogenetics and molecular genetics of acute lymphoblastic leukemia. Hematol Oncol Clin North Am. 2009;23(5):991–1010. doi: 10.1016/j.hoc.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moorman AV. New and emerging prognostic and predictive genetic biomarkers in B-cell precursor acute lymphoblastic leukemia. Haematologica. 2016;101(4):407–416. doi: 10.3324/haematol.2015.141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwab C, Harrison CJ. Advances in B-cell precursor acute lymphoblastic leukemia genomics. Hemasphere. 2018;2(4):e53. doi: 10.1097/HS9.0000000000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirabayashi S, Butler ER, Ohki K, et al. Clinical characteristics and outcomes of B-ALL with ZNF384 rearrangements: a retrospective analysis by the Ponte di Legno childhood ALL working group. Leukemia. 2021:l–6. doi: 10.1038/s41375-021-01199-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y-F, Wang B-Y, Zhang W-N, et al. Genomic profiling of adult and pediatric B-cell acute lymphoblastic leukemia. EBioMedicine. 2016;8:173–183. doi: 10.1016/j.ebiom.2016.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bidwell JP, Torrungruang K, Alvarez M, et al. Involvement of the nuclear matrix in the control of skeletal genes: the NMP1 (YY1), NMP2 (Cbfa1), and NMP4 (Nmp4/CIZ) transcription factors. Crit Rev Eukaryot Gene Expr. 2001;11(4):279–297. [PubMed] [Google Scholar]
- 7.Chen X, Wang F, Cao P, et al. Novel three-way fusions among ZNF384, EWSR1 and EHMT1 genes in paediatric B cell precursor acute lymphoblastic leukaemia with translocations resembling Philadelphia chromosomes. Br J Haematol. 2019;187(3):e75–e79. doi: 10.1111/bjh.16199. [DOI] [PubMed] [Google Scholar]
- 8.Georgakopoulos N, Diamantopoulos P, Micci F, et al. An adult patient with early pre-B acute lymphoblastic leukemia with t(12;17) (p13;q21)/ZNF384-TAF15. Vivo. 2018;32(5):1241–1245. doi: 10.21873/invivo.11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qian M, Zhang H, Kham SK-Y, et al. Whole-transcriptome sequencing identifies a distinct subtype of acute lymphoblastic leukemia with predominant genomic abnormalities of EP300 and CREBBP. Genome Res. 2017;27(2):185–195. doi: 10.1101/gr.209163.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shago M, Abla O, Hitzler J, Weitzman S, Abdelhaleem M. Frequency and outcome of pediatric acute lymphoblastic leukemia with ZNF384 gene rearrangements including a novel translocation resulting in an ARID1B/ZNF384 gene fusion. Pediatr Blood Cancer. 2016;63(11):1915–1921. doi: 10.1002/pbc.26116. [DOI] [PubMed] [Google Scholar]
- 11.Hirabayashi S, Ohki K, Nakabayashi K, et al. ZNF384-related fusion genes define a subgroup of childhood B-cell precursor acute lymphoblastic leukemia with a characteristic immunotype. Haematologica. 2017;102(1):118–129. doi: 10.3324/haematol.2016.151035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gocho Y, Kiyokawa N, Ichikawa H, et al. A novel recurrent EP300-ZNF384 gene fusion in B-cell precursor acute lymphoblastic leukemia. Leukemia. 2015;29(12):2445–2448. doi: 10.1038/leu.2015.111. [DOI] [PubMed] [Google Scholar]
- 13.Alexander TB, Gu Z, lacobucci I, et al. The genetic basis and cell of origin of mixed phenotype acute leukaemia. Nature. 2018;562(7727):373–379. doi: 10.1038/s41586-018-0436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirabayashi S, Butler E, Ohki K, et al. Acute lymphoblastic leukemia with Zinc-Finger Protein 384 (ZNF384)-related rearrangements: a retrospective analysis from the Ponte di Legno childhood ALL working group. Blood. 2019;134(Supplement_1):652. doi: 10.1182/blood-2019-123236. [DOI] [Google Scholar]
- 15.Mullighan CG. How advanced are we in targeting novel subtypes of ALL? Best Pract Res Clin Haematol. 2019;32(4):101095. doi: 10.1016/j.beha.2019.101095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffith M, Griffith OL, Krysiak K, et al. Comprehensive genomic analysis reveals FLT3 activation and a therapeutic strategy for a patient with relapsed adult B-lymphoblastic leukemia. Exp Hematol. 2016;44(7):603–613. doi: 10.1016/j.exphem.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Dongen J, Macintyre EA, Gabert JA, et al. Standardized RT-PCR analysis of fusion gene transcripts from chromosome aberrations in acute leukemia for detection of minimal residual disease. Leukemia. 1999;13(12):1901–1928. doi: 10.1038/sj.leu.2401592. [DOI] [PubMed] [Google Scholar]
- 18.Campana D. Minimal residual disease in acute lymphoblastic leukemia. Hematology. 2010;2010(1):7–12. doi: 10.1182/asheducation-2010.1.7. [DOI] [PubMed] [Google Scholar]
- 19.Short NJ, Jabbour E. Minimal residual disease in acute lymphoblastic leukemia: how to recognize and treat it. Curr Oncol Rep. 2017;19(1):6. doi: 10.1007/sll912-017-0565-x. [DOI] [PubMed] [Google Scholar]
- 20.Jain PP, Parihar M, Ahmed R, et al. Fluorescence in situ hybridization patterns of BCR/ABL1 fusion in chronic myelogenous leukemia at diagnosis. Indian J Pathol Microbiol. 2012;55(3):347–351. doi: 10.4103/0377-4929.101742. [DOI] [PubMed] [Google Scholar]
- 21.Jain P, Korula A, Deshpande P, et al. Adult acute lymphoblastic leukemia: limitations of intensification of therapy in a developing Country. J Glob Oncol. 2018;4:1–12. doi: 10.1200/JGO.17.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patkar N, Alex AA, B B, et al. Standardizing minimal residual disease by flow cytometry for precursor B lineage acutelymphoblastic leukemia in a developing country. Cytometry B Clin Cytom. 2012;82B(4):252–258. doi: 10.1002/cyto.b.21017. [DOI] [PubMed] [Google Scholar]
- 23.Corrente F, Bellesi S, Metafuni E, et al. Role of flow-cytometric immunophenotyping in prediction of BCR/ABL1 gene rearrangement in adult B-cell acute lymphoblastic leukemia. Cytometry B Clin Cytom. 2018;94(3):468–476. doi: 10.1002/cyto.b.21605. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available on request from the authors.