Abstract
Objectives
Increased central artery stiffness associates with cardiovascular disease. Among other factors, hypertension and aging are strong contributors to central artery stiffening, yet it has been difficult to separate their effects. Herein, we study isolated and combined effects of hypertension and aging on central artery remodeling in multiple mouse models as a function of sex.
Methods
We biomechanically phenotyped the aorta as a function of two different methods of inducing hypertension [infusion of angiotensin II (AngII) or combining a high salt diet with inhibition of endothelial-derived nitric oxide synthase using l-NAME] in male and female wild-type and fibulin-5 null mice, the latter of which models aspects of aortic aging.
Results
Despite increasing blood pressure similarly, salt + l-NAME led to adaptive and maladaptive remodeling in the abdominal and thoracic aorta, respectively, whereas AngII caused luminal dilatation but little remodeling of the wall. Importantly, effects of aging were more dramatic than those because of induced hypertension and, consequently, superimposing hypertension on aging led to modest additional changes in luminal radius and wall thickness, though wall stress and stiffness increased mainly because of the elevated pressure.
Conclusion
Our results suggest that effects of hypertension on aortic remodeling are modest when superimposed on aging in mice, largely independent of sex. These findings are consistent with general observations in humans and in spontaneously hypertensive rats, though separated here for the first time in a rodent model characterized by a severe loss of elastic fiber integrity similar to that found in the aged human aorta.
Keywords: angiotensin, aorta, nitric oxide synthase, salt, stiffness
Introduction
Hypertension and aging are strong risk factors for cardiovascular disease, and both associate with marked changes in central arterial structure, properties, and function. Although these two factors have long been studied [1–3], their separate effects on central arteries remain unclear [4,5]. It is thought, however, that two hallmarks of central arterial aging are compromised elastic fibers, because of fatigue-induced damage from the relentless pulsatile loading [6–9], and dysfunctional endothelial cells, which leads to reduced nitric oxide bioavailability and inflammation-induced intimal thickening [5,10]. One reason that it is difficult to delineate the biomechanical mechanisms of hypertension and aging is that aging also typically associates with elevated blood pressure, which separately associates with inflammation [11]. Diverse mouse models nevertheless offer hope in trying to disentangle effects of hypertension and aging on central arteries.
Effects of aging are many and diverse [12] and different mouse models exhibit one or more of these hallmarks [13]. Herein, we focus on phenotypic, rather than biological or functional, consequences of aging [14]. Specifically, our study was designed to quantify separate and combined effects of hypertension and aging on the biomechanical phenotype of the aorta by examining effects of two different hypertensive stimuli on two different regions (thoracic vs. abdominal) in both sexes of two mouse models, one wild-type and one genetically modified. Because of the long half-life of vascular elastin (decades) under normal conditions, natural/biological aging in mice does not phenocopy the loss of elastic fiber integrity seen in human aging (Supplemental Fig. S1, http://links.lww.com/HJH/B281 [15]). Fortunately, genetically modified mouse models enable graded losses in elastic fiber integrity, with fibulin-5 null (Fbln5 –/–) mice exhibiting changes in central artery wall mechanics and hemodynamics that mimic human aging phenotypically (Fig. S1, http://links.lww.com/HJH/B281 [16,17]). Fibulin-5 is a glycoprotein that associates with elastin to form elastic fibers; lack of fibulin-5 compromises elastogenesis [18] and yields a relatively stable model of an aged aorta over long periods in maturity [19]. Herein we use male and female Fbln5 –/– mice as a model of central artery aging. We also consider the aforementioned role of endothelial dysfunction as well as two other contributors to aortic aging that manifest in hypertension: increased salt sensitivity and altered angiotensin II (AngII) signaling [4,5]. Specifically, we combine pharmacologic inhibition of endothelial nitric oxide with a high-salt diet as well as chronic AngII infusion to superimpose these clinically relevant aspects of hypertension on arterial aging to assess separate or possibly additive effects of these risk factors. Finally, we use biaxial biomechanical phenotyping to delineate changes in intrinsic material stiffness from those of structural stiffness, noting that altered central artery stiffness is a clinical indicator and initiator of cardiovascular disease associated with hypertension and aging [20,21].
Methods
Detailed materials and methods are provided in Supplemental Materials, http://links.lww.com/HJH/B281. Briefly, male and female mice were rendered hypertensive and the descending thoracic aorta (DTA) and infrarenal abdominal aorta (IAA) were excised, subjected to custom biomechanical testing ex vivo, phenotyped in terms of eight key biomechanical metrics, and examined histologically.
Results
Data were analyzed and compared for two genotypes (Fbln5 +/+ and Fbln5 –/–) × three experimental conditions (control as well as salt + l-NAME-induced or AngII-induced hypertension) × two sexes (male and female) at two aortic locations (abdominal – IAA, and thoracic – DTA), thus yielding 24 groups for comparison, with n = 5 or more for all groups except male Fbln5 –/– mice treated with salt + l-NAME for which mortality was 55% (Fig. S2, http://links.lww.com/HJH/B281). Data for the control condition alone are from our prior report on male and female wild-type and fibulin-5 null mice [16]. For ease of discussion, and because we sought to evaluate effects of hypertension superimposed on a model of marked aortic aging, we present results separately for the IAA and DTA, focusing first on males and then females as there are more data in the literature for male mice with which to compare. We previously reported [16] values of SBPs/DBPs for 20-week-old male and female wild-type mice (120 ± 6/84 ± 6 and 121 ± 4/86 ± 3 mmHg, respectively, as mean ± standard error) and similarly for age-matched male and female fibulin-5 null mice (121 ± 7/85 ± 7 and 123 ± 5/85 ± 5 mmHg). The high salt diet + l-NAME raised SBP/DBP, on average, in both sexes of wild-type (to 160 ± 4/118 ± 3 mmHg in males and 171 ± 7/131 ± 8 in females) and fibulin-5 null (to 152 ± 13/110 ± 11 mmHg in male and 174 ± 8/133 ± 7 in female) mice. Chronic infusion with AngII also raised blood pressure in wild-type (153 ± 7/ 110±6mmHg in males and 176±9/125± 11 in females) and fibulin-5 null (168 ± 10/120 ± 8 mmHg in males and 153 ± 8/112 ± 8 in females) mice. Hence, with the exception of Angll infusion in fibulin-5 null mice, salt + l-NAME and AngII elevated blood pressure more in female than in male mice.
Cardiac output was measured in the salt + l-NAME-treated mice using ultrasound (Vevo 2100; Fujifilm VisualSonics, Inc., Toronto, Canada) and found not to differ significantly from that in untreated controls, independent of sex, consistent with prior reports for AngII infusion in wild-type mice [22]. Hence, with the fold-change in blood flow ε~1, inner radius a should be preserved to maintain mean wall shear stress (a→ε 1/3 ao, see Supplemental Methods, http://links.lww.com/HJH/B281). Conversely, because the fold-change in blood pressure γ > 1 in hypertension, wall thickness h should increase proportionally (h → ε 1/3 γho = γho, see Supplemental Methods, http://links.lww.com/HJH/B281) if locally mechanoadapted.
Abdominal aorta
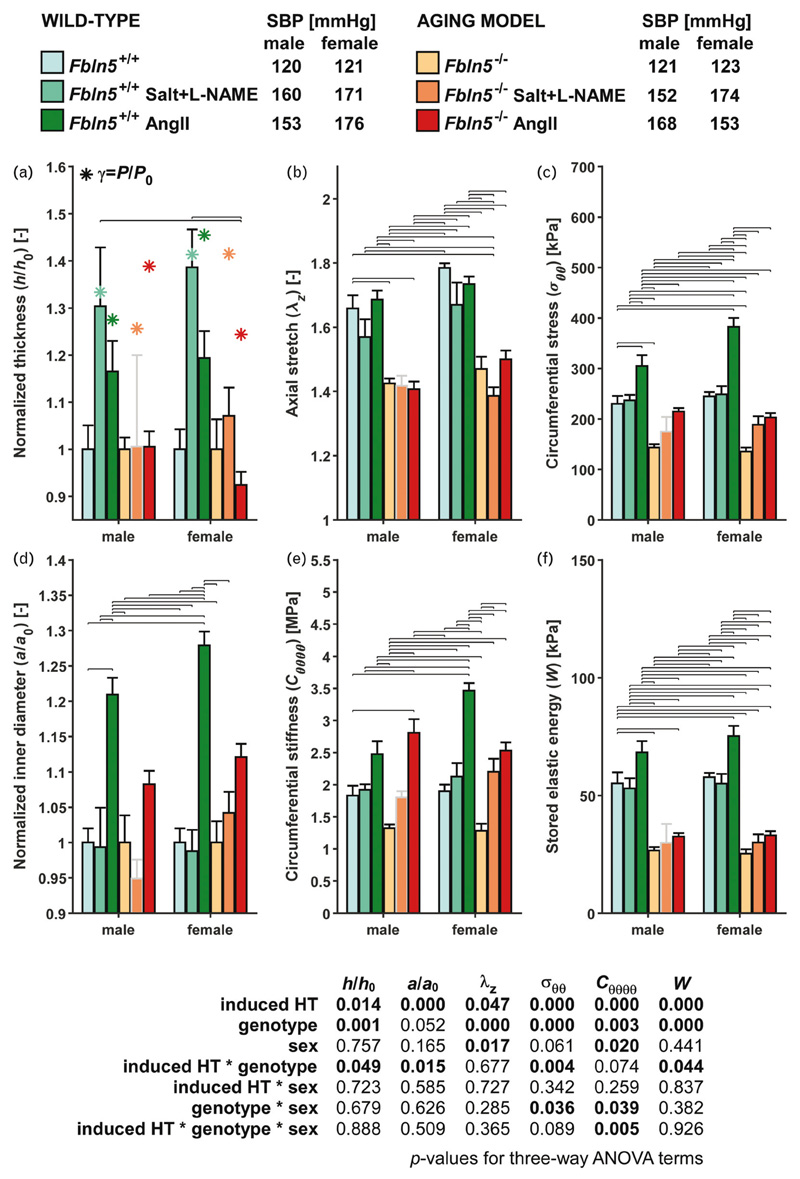
Individual (symbols) and mean (vertical solid lines) values of select geometrical and mechanical metrics are contrasted in Fig. S3 (http://links.lww.com/HJH/B281) for each IAA from all six groups of male mice at group-specific systolic pressures and individual values of axial stretch; associated values are in Supplemental Table S1, http://links.lww.com/HJH/B281 for these and additional metrics.
On average, wild-type IAAs nearly mechanoadapted in response to salt + l-NAME-induced hypertension (Fig. 1). Luminal radius was preserved to within 1% (though highly variable) and the wall thickened, on average, by 30% in response to a γ= 1.33-fold increase in systolic pressure, with only a ~5% reduction in axial stretch and no change in cardiac output. Consequently, circumferential wall stress and material stiffness were restored to within 3–5% of original values and elastic energy storage was nearly maintained (only 4% lower). Other metrics similarly changed little (Fig. S3, http://links.lww.com/HJH/B281 and Table S1, http://links.lww.com/HJH/B281). To isolate the influence of an (acute) increase in pressure alone, independent of possible remodeling, we re-evaluated all metrics using constitutive properties from normotensive control mice at blood pressures equal to the hypertensive values (gray rows in Fig. S3, http://links.lww.com/HJH/B281). In these cases, circumferential stress and stiffness increased markedly as expected. Comparison with data for the wild-type salt + l-NAME group confirmed that the IAA had remodeled and restored circumferential mechanics.
Figure 1.
Select biomechanical metrics for the infrarenal abdominal aorta, computed at group-specific SBPs and individual axial stretch, for all six primary groups and both sexes (12 groups). A subscript ‘o’ denotes original/homeostatic. Bars and whiskers indicate arithmetic means and standard errors; asterisks in panel a represent values for a perfect mechanoadaptation given a constant cardiac output (ε = 1, h/h 0 = γ ≡ P/P o). Horizontal lines indicate significant differences [P < 0.05, Bonferroni post hoc test following three-way analysis of variance (ANOVA); see Table S6]. The male Fbln5 –/– + salt + l-NAME group (bar and whisker outlined in gray) was not included in post hoc testing because of the low sample size (n = 2; see Fig. S2, http://links.lww.com/HJH/B281). Group sizes: males: n = 5, 9, 5, 5, 2, 5; females: n = 5, 5, 5, 5, 6, 5. Note the near mechanoadaptation only for the salt + l-NAME-treated wild-type IAA, independent of sex. Angll, angiotensin II; HT, hypertension; IAA, infrarenal abdominal aorta. l-NAME, Nω-nitro-l-arginine methyl ester.
The situation was different in the wild-type male mice when hypertension was induced with AngII infusion for 2 weeks (Fig. 1). In response to a lower (γ = 1.28) fold increase in systolic pressure, the IAA dilated dramatically (21% increase in luminal radius) but thickened modestly (17% on average), not sufficiently to preserve circumferential wall stress or material stiffness near original values (33 and 35% increases, respectively). Given that the in-vivo axial stretch was largely preserved, the thin wall distended and stored more (24%) elastic energy at systolic pressure than normal. Other differences are revealed in Fig. S3 (http://links.lww.com/HJH/B281) and Table S1 (http://links.lww.com/HJH/B281).
Findings for the male fibulin-5 null model of marked aortic aging were very different (Fig. 1; Fig. S3, http://links.lww.com/HJH/B281 and Table S1, http://links.lww.com/HJH/B281). In response to either a γ = 1.26 (salt + l-NAME) or a γ = 1.39 (AngII) fold increase in systolic pressure, the IAA exhibited modest changes in luminal radius (5% decrease and 8% increase, respectively) and essentially no further thickening (0%) or change in axial stretch (0 and –1%). Because of the elevated pressure and lack of favorable remodeling, circumferential wall stress and material stiffness were higher than normal (especially in AngII-induced hypertension), with a slightly increased elastic energy storage as expected of an aortic wall that did not thicken sufficiently. Indeed, metrics for both the salt + l-NAME and AngII groups were strikingly similar to those calculated for an acute increase in blood pressure without remodeling (Fig. S3, gray rows, http://links.lww.com/HJH/B281).
As wall stress, material stiffness, and energy storage are pressure-dependent metrics, it is useful to compare values at a common normotensive value closer to mean arterial pressure. Table S1 (http://links.lww.com/HJH/B281) lists values for the IAA from all six groups at 100mmHg. Observe that circumferential wall stress, material stiffness, and energy storage were within ±11% of normotensive values in the fibulin-5 null mice, again suggesting modest hypertension-induced remodeling in this model of marked aortic aging. In contrast, stress, stiffness, and energy at 100 mmHg were typically lower for the hypertensive wild-type mice, particularly for the salt + l-NAME group because of adaptive remodeling.
Findings for the IAA of female mice were similar to those for male mice, including mechanoadaptive remodeling to salt + l-NAME induced hypertension and under-adaptive remodeling to AngII in wild-type mice (Fig. 1; Fig. S4, http://links.lww.com/HJH/B281 and Table S2, http://links.lww.com/HJH/B281). On average, salt+ l-NAME led to a 41% increase in pressure and similar wall thickening (39%), with a nearly preserved luminal radius (–1%), thus maintaining circumferential stress (+2%). As in males, stored energy was reduced slightly (–5%), consistent with a modest decrease in axial stretch (–6%). AngII treatment caused a γ = 1.45-fold increase in pressure, with a marked dilatation (28%) but modest thickening of the wall (19%), which did not offset the pressure increase, leading to a significant increase in circumferential stress (+56%). Female fibulin-5 null mice remodeled little given salt + l-NAME or AngII-induced pressure increases of 41 and 24%: wall thickness changed +7 and –8%, respectively, whereas inner radius changed 4 and 12%, respectively.
Thoracic aorta
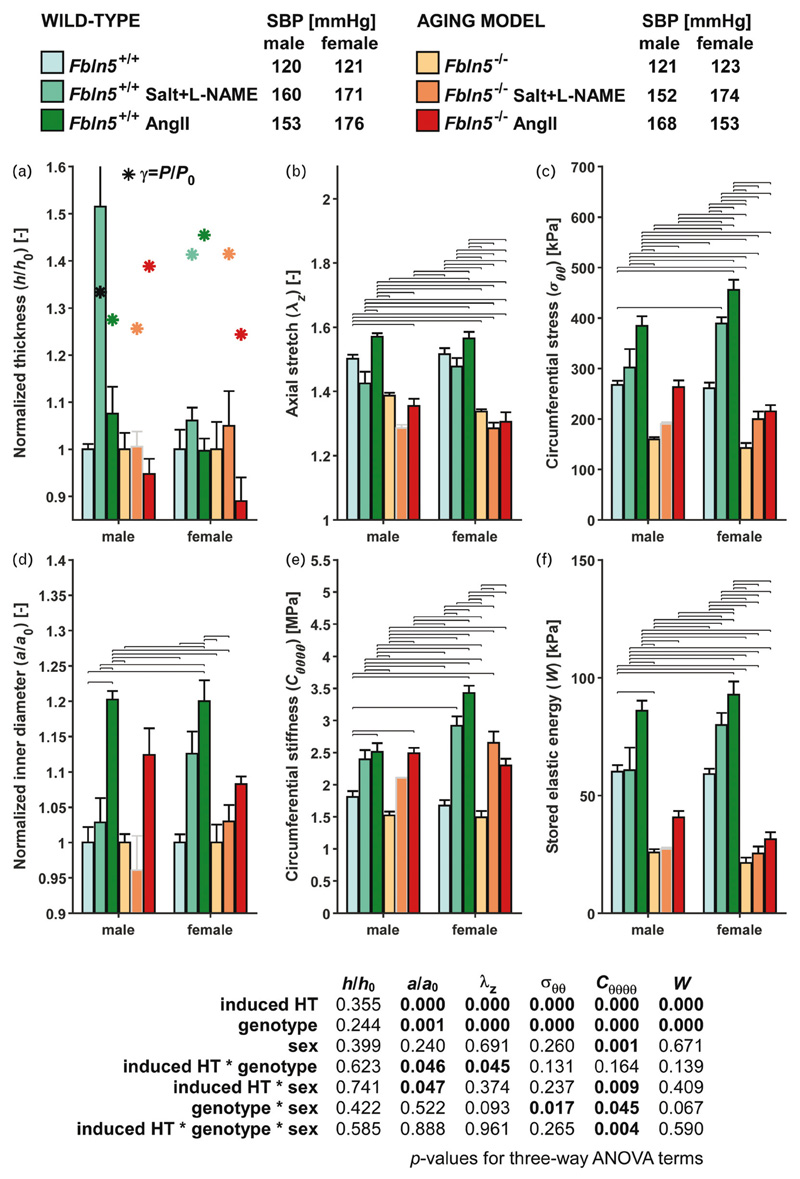
Values of geometrical and mechanical metrics are shown for the DTA of male mice at group-specific values of systolic pressure and individual values of in-vivo axial stretch (Fig. 2); additional data are in Fig. S5 (http://links.lww.com/HJH/B281) and Table S3 (http://links.lww.com/HJH/B281). In contrast with the response of the wild-type IAA, which nearly mechanoadapted to salt + l-NAME-induced hypertension, the DTA exhibited a stronger bimodal response to salt + l-NAME. Seven of nine DTA samples exhibited little wall thickening (increase of ~6%), with associated increases in circumferential stress and material stiffness of 32 and 41% (under-adaptation); axial stretch was nearly maintained (2% decrease), but stored energy increased (+24%) because of the pressure elevation. In contrast, two of the nine DTA samples showed dramatic maladaptation to salt + l-NAME, with increases in thickness of 140 and 280% (Fig. S6, http://links.lww.com/HJH/B281 and Table S4, http://links.lww.com/HJH/B281), which markedly decreased circumferential stress (–38 and –72%), axial stretch (–15 and –19%), and energy storage (–69 and –89%). Changes in material stiffness were less pronounced (+22 and –17%). Note that it was the IAAs of these two mice that also showed greater thickening than did the other seven (+86 and +141%; Fig. S3, http://link-s.lww.com/HJH/B281). Their larger dilatation resulted in changes in circumferential stretch that were within the spread of that for the other seven mice, however. Similar to the IAA, the DTA in the AngII-infused wild-type mice exhibited a marked dilatation (~20% increase in luminal radius) but not sufficient thickening (~8%) in response to hypertension (γ = 1.28). Axial stretch remained close to normal (within 5%), thus these changes collectively left circumferential stress and stiffness higher than normal (+44 and +39%, respectively) and so too energy storage (+43% higher).
Figure 2.
Select biomechanical metrics for the descending thoracic aorta (DTA), computed at group-specific systolic blood pressures (SBPs) and individual axial stretch, for all six primary groups and both sexes (12 groups). A subscript ‘o’ denotes original/homeostatic. Bars and whiskers indicate arithmetic means and standard errors; asterisks in panel (a) represent values for a perfect mechano-adaptation given a constant cardiac output (). Horizontal lines indicate significant differences (P < 0.05). Bonferroni post-hoc test following three-way analysis of variance (ANOVA); see Table S6]. The whisker for the male Fbln5 +/+ + Salt+l-NAME group in panel A (representing a standard error of 0.32) was truncated to avoid compression of the other bars. The male Fbln5 –/– + Salt+l-NAME group (bar and whisker outlined in gray) was not included in post-hoc testing due to low sample size (n = 2). Group sizes: males: females. Note the lack of mechano-adaptation of all hypertensive groups, independent of sex-NAME, Nv-nitro-L-arginine methyl ester.
The DTA in the male fibulin-5 null mice exhibited changes similar to those of the IAA. There was modest remodeling in response to salt + l-NAME (γ = 1.26) and AngII (γ = 1.39) induced hypertension (Fig. 2), with luminal radius changing –4 and +12%, respectively, and wall thickness (0 and –5%) and axial stretch (–7 and –2%) changing little; see also Fig. S5 (http://links.lww.com/HJH/B281) and Table S3 (http://links.lww.com/HJH/B281). Collectively, therefore, these modest geometric changes elevated circumferential wall stress (19-65%, respectively), circumferential material stiffness (39-64%), and energy storage (4-58%). Table S3 (http://links.lww.com/HJH/B281) also lists results for a constant pressure of 100 mmHg to remove any pressure dependency. As expected, given the less than optimal remodeling, circumferential wall stress, material stiffness, and elastic energy storage were generally lower than normal for the hypertensive wild-type and fibulin-5 null mice, especially for the salt + l-NAME wild-type mice.
Finally, consider results for the DTA of female mice (Fig. 2; Fig. S7, http://links.lww.com/HJH/B281 and Table S5, http://links.lww.com/HJH/B281). As in seven of the nine wild-type male mice on salt + l-NAME, the wild-type female mive showed little thickening (6%), leading to a significant increase in circumferential stress (49%), thus under-adaptation. Also similar was the small change in axial stretch (–3%) and increase in stored energy (35%) consistent with the pressure elevation. Wild-type female mice given AngII showed no thickening (0%) but significant dilatation (20%), which increased circumferential stress (75%). In line with the lack of remodeling, axial stretch remained nearly unchanged (+3%) and stored energy increased with blood pressure (57%). Similar to fibulin-5 null male mice, the female fibulin-5 null DTA did not thicken in response to either salt + l-NAME or AngII (+5 and –11% changes, respectively). Changes in inner radius were also small (+3 and +8%), which together with the elevated blood pressure increased circumferential stress (40 and 51%) and stiffness (78 and 54%). Hypertension induced little change in axial stretch (–4 and –2%). Stored energy increased (19%) in the salt + l-NAME group, but less than that for an acute increase in blood pressure alone (32%). Conversely, stored energy increased (47%) in the AngII group more than that for an acute increase in blood pressure (+19%). These differences appear to stem from the different luminal responses, namely, dilatation in AngII-induced hypertension.
Comparisons with prior results
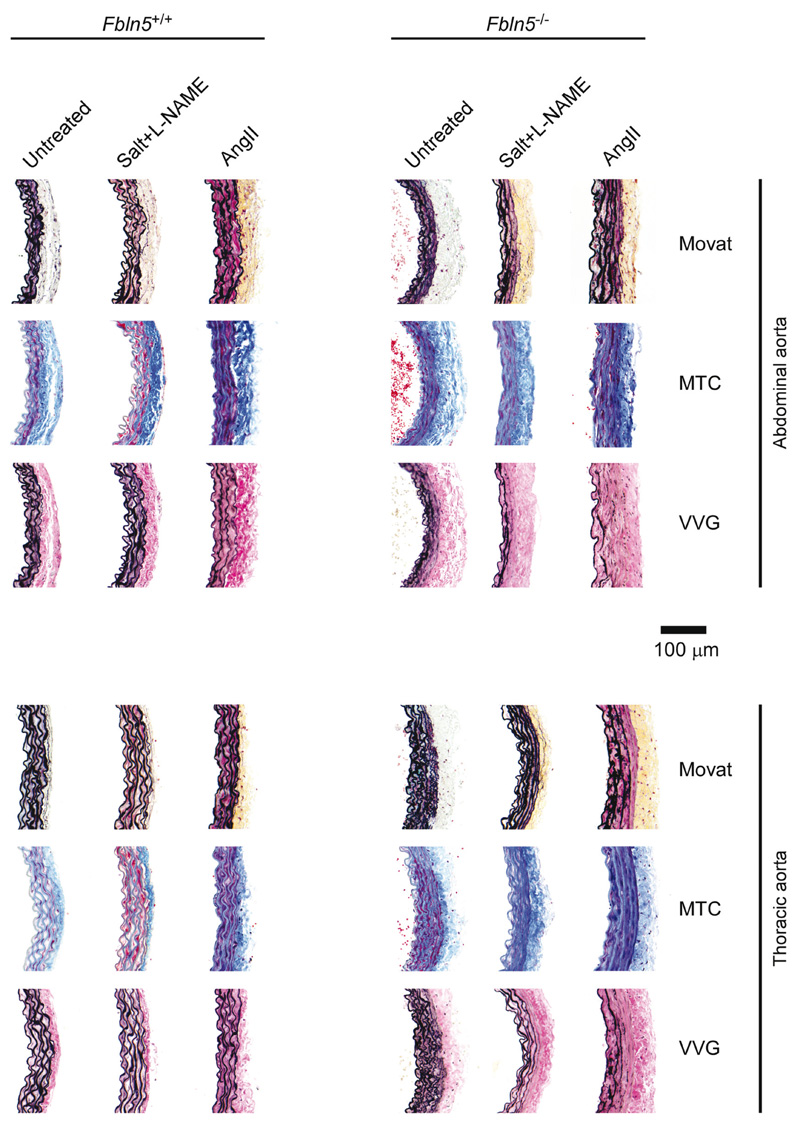
Figure 3 shows representative histological sections for the 12 groups of male mice; results were similar for female mice. As we used consistent experimental methods and data analyses, we compared our new data directly with prior results for IAAs and DTAs from normotensive male and female Fbln5 +/+ and Fbln5 –/– mice [16] (controls herein). We also contrasted our findings with data for IAAs and DTAs from naturally aged male Fbln5 +/+ mice [15] as well as DTAs from normotensive and hypertensive (same salt + l-NAME combination) male Myh11 +/+ and Myh11R247C/R247C mice [23] [Tables S1 (http://link-s.lww.com/HJH/B281) and S3 (http://links.lww.com/HJH/B281)]. Although tail-cuff-measured SBPs decreased in wild-type mice that aged naturally to 100 weeks (~107mmHg), the biaxial data revealed that absence of fibulin-5 results in a biomechanical phenotype similar to, but more dramatic than, that of natural aging, as desired. Relative to young wild-type controls, when evaluated at group-specific systolic pressures, the IAAs and DTAs from both naturally aged and fibulin-5 null mice exhibit luminal encroachment (luminal radius decreased by 9-10% in the IAA and 17-3% in the DTA for Fbln5 –/– and aged mice, respectively), marked thickening of the wall (45-44% in the IAA, 40-28% in the DTA), a reduced in-vivo axial stretch (decreases of 14-7% in the IAA, 8-9% in the DTA), decreases in circumferential wall stress (38-45% in the IAA, 40-32% in the DTA) and material stiffness (28-55% in the IAA, 16-37% in the DTA), and a dramatic reduction in elastic energy storage capability (52-42% in the IAA, 5735% in the DTA). That is, loss of elastic fiber integrity and natural aging dramatically reduce circumferential wall stress and material stiffness due primarily to luminal encroachment, thickening of the wall, and a reduced axial stretch. In contrast, using the same breeders herein, salt + l-NAME-induced hypertension (γ = 1.33) led to marked thickening of the aortic wall in only two of nine wild-type mice (with 30-52% increases on average in the IAA and DTA, respectively), but without luminal encroachment or marked reductions in axial stretch. Interestingly, AngII-induced hypertension (γ = 1.27) did not cause the thickening of the wall that was expected (only 17 and 8% in the IAA and DTA), but rather resulted in marked luminal enlargement (increases of 21 and 20%) while changing axial stretch little (2 and 5%, respectively). Consequently, effects of hypertension on wild-type Fbln5 +/+ mice were generally more modest and quantitatively very different biaxially from those for either a loss of elastic fiber integrity or natural aging, particularly the tendency of the lumen to dilate rather than encroach.
Figure 3.
Representative histology for all 12 groups of aortas from male mice; results were similar for females. Movat’s pentachrome, MTC (Masson’s trichrome), and VVG (Verhoeff-Van Gieson) stains; AngII, angiotensin II. l-NAME, Nω-nitro-L-arginine methyl ester. See Fig. S6 (http://links.lww.com/HJH/B281 for counter-examples.
Interestingly, DTAs from male Myh11 +/+ mice showed a slight dilatation (11%) in response to salt + l-NAME (Fig. S5, http://links.lww.com/HJH/B281), though thickness mechanoadapted well (41% increase for σ = 1.41), which was not the case for Fbln5 +/+ mice. It is noteworthy that DTAs from untreated Fbln5 +/+ and Myh11 +/+ mice were biomechanically similar, with all metrics within 10% except circumferential material stiffness, which was 25% higher in the Myh11 +/+ mice. Finally, DTAs from the Myh11R247C/R247C mice thickened in response to salt + l-NAME (37% for γ = 1.58), though with luminal dilatation (12%). Their circumferential material stiffness was elevated 2.24-fold; however, consistent with the common elevation of this metric in mice with a propensity toward thoracic aortic aneurysm or dissection [24]. In summary, neither salt + l-NAME-induced nor AngII-induced hypertension elicited much aortic remodeling in male or female Fbln5 –/– mice, our model of advanced aortic aging that exhibited characteristics similar too but more dramatic than those of natural aging to 100 weeks.
Discussion
Rodent models – particularly spontaneously hypertensive rats (SHRs) – have long been used to study effects of hypertension and aging on central arterial structure, properties, and function. Pulse wave velocity (PWV), an integrative measure of in-vivo structural stiffness, is higher in young (13-week) and old (65-week) SHRs relative to age-matched controls, but this elevation is because of a pressure effect [2]. That is, assessing properties at equal pressures (achieved in vivo using vasodilators, which do not change stiffness [25]) reveals that hypertension does not increase intrinsic aortic stiffness; rather, aging seems to associate best with this increase in the SHRs (33% increase based on Moens–Korteweg) relative to controls. Whereas PWV integrates information over finite lengths of the aorta, local measures provide more insight. For example, our local measures also suggested that aging associated best with the altered properties, but furthermore, that remodeling in hypertension tends to be more effective (closer to adaptive) in the IAA than in the DTA, a result also observed in AngII-infused apolipoprotein-E null (Apoe –/–) mice [26].
In-vitro ring tests and atomic force microscopy have been used to assess local stiffness of the aortic wall and isolated smooth muscle cells in SHRs relative to young (16-week) and old (64-weeks) controls [3]. Although ring tests cannot account for changes in in-vivo axial stretch [27,28], an important determinant of in-vivo properties [29], these data also suggest that stiffening results more from aging in SHRs than from hypertension. Notwithstanding the importance of these and related studies in aged SHRs, the elastic laminae remain largely intact, though straighter and more separated, in aged controls and SHRs. Hence, the associated aging does not phenocopy the breakdown of elastic fibers that characterizes aortic aging in humans [6,30]. It was for these reasons that we focused on fibulin-5 null mice as a model of murine aortic aging [15,17] (also see Fig. S1, http://links.lww.com/HJH/B281).
Indeed, with the availability of genetically modified mice, most recent basic science studies use mouse models of hypertension and aging. Mice lacking the α1 subunit of the α1ß1 integrin (which binds collagen) tend not to exhibit marked remodeling of the carotid artery in response to Angll-induced (200 ng/kg/min) hypertension [31], thus suggesting the importance of cellular mechanosensing and mechanoregulation of matrix in such remodeling [32]. Interestingly, despite elevating blood pressure similarly, norepinephrine-induced hypertension did not induce significant remodeling in either wild-type or α1-null mice. Thus, the method of inducing hypertension matters. Elevating blood pressure using acute transverse aortic constriction causes dramatic remodeling of the ascending aorta, including luminal dilatation and thickening of the wall because of an accumulation of collagen that depends on AngII since a type 1 AngII receptor antagonist (Losartan) reduced much of the remodeling [33]. Collagen accumulation in, and thus thickening of, the DTA in AngII-induced (490 ng/kg/min) hypertension results largely from T-cell activity and IL-17a [10], though such remodeling can be less in female mice [34]. Notwithstanding all that has been learned using mouse models, the elastic lamellae in all of these prior mouse models remained largely intact, unlike that characteristic of large artery aging in humans [6,30].
Our study appears to be the first in rodents to address the potential combination of lost elastic fiber integrity and multiple methods of superimposing hypertension, including effects of salt sensitivity, altered AngII, and decreased nitric oxide bioavailability, each relevant to human hypertension. When considering prior data on normotensive Fbln5 –/–, naturally aged Fbln5 +/+ (to 100 weeks), and hypertensive Myh11 +/+ mice, plus associated mutants [15,16,23], our biaxial comparisons across 30 groups of mice suggest three primary findings (Tables S1–S3, http://links.lww.com/HJH/B281 and S5, http://links.lww.com/HJH/B281). First, a high salt diet combined with nitric oxide inhibition elevated systolic pressures similar to AngII infusion in male Fbln5 +/+ mice (160 vs. 153mmHg) and yet elicited very different responses. Salt + l-NAME resulted in a near mechanoadaptation of the IAA (preserved circumferential stress) but maladaptation of the DTA, characterized by under-adaptation (n = 7) or severe maladaptation (n = 2). In contrast, AngII infusion caused modest (insufficient) thickening of the wall but marked luminal dilatation of the IAA and DTA. Although the duration of hypertension may have contributed to these differences (13 weeks for salt + l-NAME, 2 weeks for AngII), our finding that the IAA tends to mechanoadapt on average whereas the DTA maladapts in Fbln5 +/+ mice rendered hypertensive with salt + l-NAME is similar to our prior finding for 2-4 weeks of AngII infusion in Apoe –/– mice [26]. Together, these findings suggest that duration of pressure elevation alone is not as important as the differential remodeling responses between the abdominal and thoracic aorta [35]. Indeed, we previously observed slightly greater remodeling of the IAA than the DTA in natural aging [15]. It is not clear, however, why salt + l-NAME resulted in an adaptive response in the DTA of the Myh11 +/+ mice or why AngII failed to elicit marked remodeling in the thoracic aorta in wild-type mice herein, particularly given that maladaptive remodeling has been observed in the DTA [36]. Diverse factors clearly affect the ability of a particular vessel to adapt or not.
Second, effects of natural aging and absence of fibulin-5 on aortic properties were more dramatic than those because of induced hypertension in wild-type mice, with aging leading to luminal encroachment and reduced circumferential wall stress and stiffness as well as energy storage. In contrast, hypertension generally resulted in mild dilatation and increased circumferential stress, stiffness, and energy storage. Again, these results are qualitatively consistent with those for aged SHRs, with effects of aging generally more severe than those because of hypertension alone [2,3].
Third, hypertension superimposed on a model of marked aortic aging (compromised elastic fiber integrity) induced modest additional changes in luminal radius and wall thickness but increased wall stress and material stiffness because of the pressure effect. Whereas modest changes in luminal radius may reflect potentially off-setting effects – encroachment in aging, dilatation in hypertension – it is not clear why wall thickening was modest in response to the superimposed pressure elevation. That is, the marked thickening of the wall because of natural aging and com-promised elastic fiber integrity (28–65% for the IAA and DTA) changed modestly (a further ±11%) for both methods of inducing hypertension (γ = 1.26–1.45), though switching wall stress and stiffness from lower to higher than normal. Such a transition to higher stresses could render the wall vulnerable to other conditions, perhaps not unlike the increased vulnerability to dissection in naturally aged Fbln5 +/+ and hypertensive Myh11R247C/R247C mice [15,23].
That hypertension superimposed on our model of aortic aging did not elicit dramatic changes in the wall also appears qualitatively similar to findings for humans. Clinical data suggest that ‘blood pressure seemed to be less important than age’ in modifying central arteries [1] and that hypertension-induced stiffening of the carotid artery is modest in older individuals but marked in young individuals [37]. Although a large population-based study reported a substantial cross-sectional association between PWV and the presence/grade of hypertension in young and old individuals [38], regression analyses in such studies cannot delineate direct (through nonlinear mechanics) and indirect (via arterial remodeling) effects of blood pressure on PWV [39]. Indeed, when accounting for the direct pressure dependency of PWV (e.g. as in [2]), the stiffening effect of hypertension superimposed on aging is modest [40], again similar to findings in SHRs and our present study.
As Fbln5 –/– mice exhibit a more severe biomechanical phenotype than do naturally aged Fbln5 +/+ mice (Fig. S1, http://links.lww.com/HJH/B281, Tables S1–S3, and S5, http://links.lww.com/HJH/B281), we expect similar but less marked effects of hypertension in natural murine aging. Although such a study would be interesting, we used a mouse model that better mimicked the human condition biomechanically (Fig. S1, http://links.lww.com/HJH/B281), namely, compromised elastic fiber integrity (Fig. S8–S9, http://links.lww.com/HJH/B281) [41]. Note, therefore, that the ascending aorta tends to be structurally stiffer in patients with Marfan Syndrome (which compromises elastic fiber integrity) than in age-matched controls up to 40 years of age, but thereafter stiffness is comparable between Marfan and non-Marfan individuals [42]. As blood pressure tends to increase with age, these data may suggest that loss of elastic fiber integrity results in more dramatic aortic changes than those because of superimposed increases in blood pressure. Finally, computational simulations of the enlargement of abdominal aortic aneurysms suggest that rates of enlargement should be greater in younger patients as their aortic wall contains more elastin that can be damaged [43]. It thus appears that our results are also congruent with observations for other elastopathies, though with greater detail via multiple directly compared biaxial metrics.
Notwithstanding insights gained during this study, there are limitations. Blood pressure was measured using a tail-cuff, yet central pressures tend to be more relevant in cases of hypertension and aging. In the male Fbln5 –/– salt + l-NAME group, only two mice survived and were included in the study results. The observed SBP in those two mice may not be representative for the entire group, noting that the most severely hypertensive animals may have been the ones that died prior to testing. Biomechanical testing was performed under passive conditions; hence we did not account for possible differences in basal aortic tone across groups. There is currently no way to assess basal tone in vivo under conscious conditions, hence we used a protocol that allowed all vessels to be compared equally. Consistent with most prior studies, we used a single-layered continuum model to compute transmurally averaged material properties, again allowing a consistent comparison across groups. A bilayered model would distinguish medial and adventitial properties, noting that cases of exuberant collagen deposition tend to occur mainly in the adventitia. Finally, vessels were tested under quasi-static conditions without supporting perivascular tissue (tethering, [44]). The higher strain rates in vivo could elicit a more viscoelastic response, potentially suggesting different changes in stiffness as aging tends also to be characterized by increased glycosaminoglycans [15].
In conclusion, consistent biomechanical phenotyping across multiple mouse models suggests that compromised elastic fiber integrity characteristic of extreme aging of the human aorta tends to affect wall properties more dramatically than does hypertension and, moreover, effects on wall properties because of the superimposition of hypertension onto this model of severe aging tend to be modest, independent of sex. This is not to say that hypertension superimposed on aging is not problematic; it likely increases wall stress, exacerbates adverse hemodynamics, and heightens effects on end organs. There is, therefore, a need to understand better how local changes in mechanics/mechanobiology affect global changes in hemodynamics/physiology [4,20,21]. Toward this end, there is also a need to quantify hemodynamic consequences of regional changes in aortic properties across the many different murine models of hypertension and aging.
Supplementary Material
Acknowledgements
We thank Chang-Shun He, MD, and Kirk Donegan for expert technical assistance and Maile Thayer-Phillips at the Yale StatLab for consultative support.
Funding
This work was supported by the US NIH (R01 HL105297 to C.A.F. and J.D.H., P01 HL134605 to D.B.R.); the Netherlands Organisation for Scientific Research (Rubicon 452172006 to B.S.); and the European Union’s Horizon 2020 Research and Innovation program (grant 793805 to B.S.).
Abbreviations
- a
deformed inner radius
- AngII
angiotensin II
- Cθθ
circumferential material stiffness
- DTA
descending thoracic aorta
- Fbln5
fibulin-5
- h
deformed wall thickness
- HT
hypertension
- IAA
infrarenal abdominal aorta
- l-NAME
Nω-nitro-l-arginine methyl ester
- Mov
Movat’s pentachrome
- MTC
Masson’s trichrome
- Myh11
smooth muscle myosin heavy chain
- P
pressure
- PWV
pulse wave velocity
- SHR
spontaneously hypertensive rat
- VVG
Verhoeff-Van Gieson
- W
stored energy density
- γ
fold increase in pressure
- ε
fold increase in blood flow
- λz
in-vivo axial stretch
- σZ
axial Cauchy stress
- σθ
circumferential Cauchy stress
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Benetos A, Laurent S, Hoeks AP, Boutouyrie PH, Safar ME. Arterial alterations with aging and high blood pressure. A noninvasive study of carotid and femoral arteries. Arterioscler Thromb. 1993;13:90–97. doi: 10.1161/01.atv.13.1.90. [DOI] [PubMed] [Google Scholar]
- 2.Marque V, Kieffer P, Atkinson J, Lartaud-Idjouadiene I. Elastic properties and composition of the aortic wall in old spontaneously hypertensive rats. Hypertension. 1999;34:415–422. doi: 10.1161/01.hyp.34.3.415. [DOI] [PubMed] [Google Scholar]
- 3.Sehgel NL, Sun Z, Hong Z, Hunter WC, Hill MA, Vatner DE, Meininger GA. Augmented vascular smooth muscle cell stiffness and adhesion when hypertension is superimposed on aging. Hypertension. 2015;65:370–377. doi: 10.1161/HYPERTENSIONAHA.114.04456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.AlGhatrif M, Wang M, Fedorova OV, Bagrov AY, Lakatta EG. The pressure of aging. Med Clin North Am. 2017;101:81–101. doi: 10.1016/j.mcna.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Safar ME. Arterial aging: hemodynamic changes and therapeutic options. Nat Rev Cardiol. 2010;7:442–449. doi: 10.1038/nrcardio.2010.96. [DOI] [PubMed] [Google Scholar]
- 6.O’Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50:1–13. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 7.Sherratt MJ. Tissue elasticity and the ageing elastic fibre. Age (Dordr) 2009;31:305–325. doi: 10.1007/s11357-009-9103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsamis A, Krawiec JT, Vorp DA. Elastin and collagen fibre microstructure of the human aorta in ageing and disease: a review. J R Soc Interface. 2013;10:20121004. doi: 10.1098/rsif.2012.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duca L, Blaise S, Romier B, Laffargue M, Gayral S, El Btaouri H, et al. Matrix ageing and vascular impacts: focus on elastin fragmentation. Cardiovasc Res. 2016;110:298–308. doi: 10.1093/cvr/cvw061. [DOI] [PubMed] [Google Scholar]
- 10.Wu J, Thabet SR, Kirabo A, Trott DW, Saleh MA, Xiao L, et al. Inflammation and mechanical stretch promote aortic stiffening in hypertension through activation of p38 mitogen-activated protein kinase. Circ Res. 2014;114:616–625. doi: 10.1161/CIRCRESAHA.114.302157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lakatta EG, Wang M, Najjar SS. Arterial aging and subclinical arterial disease are fundamentally intertwined at macroscopic and molecular levels. Med Clin North Am. 2009;93:583–604. doi: 10.1016/j.mcna.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Folgueras AR, Freitas-Rodriguez S, Velasco G, Lopez-Otin C. Mouse models to disentangle the hallmarks of human aging. Circ Res. 2018;123:905–924. doi: 10.1161/CIRCRESAHA.118.312204. [DOI] [PubMed] [Google Scholar]
- 14.Ferrucci L, Levine ME, Kuo PL, Simonsick EM. Time and the metrics of aging. Circ Res. 2018;123:740–744. doi: 10.1161/CIRCRESAHA.118.312816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferruzzi J, Madziva D, Caulk AW, Tellides G, Humphrey JD. Compromised mechanical homeostasis in arterial aging and associated cardiovascular consequences. Biomech Model Mechanobiol. 2018;17:1281–1295. doi: 10.1007/s10237-018-1026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferruzzi J, Bersi MR, Uman S, Yanagisawa H, Humphrey JD. Decreased elastic energy storage, not increased material stiffness, characterizes central artery dysfunction in fibulin-5 deficiency independent of sex. J Biomech Eng. 2015;137:031007. doi: 10.1115/1.4029431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuomo F, Ferruzzi J, Agarwal P, Li C, Zhuang ZW, Humphrey JD, Figueroa CA. Sex-dependent differences in central artery haemodynamics in normal and fibulin-5 deficient mice: implications for ageing. Proc R Soc A. 2019;475:20180076. doi: 10.1098/rspa.2018.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papke CL, Yanagisawa H. Fibulin-4 and fibulin-5 in elastogenesis and beyond: insights from mouse and human studies. Matrix Biol. 2014;37:142–149. doi: 10.1016/j.matbio.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wan W, Gleason RL., Jr Dysfunction in elastic fiber formation in fibulin-5 null mice abrogates the evolution in mechanical response of carotid arteries during maturation. Am J Physiol Heart Circ Physiol. 2013;304:H674–H686. doi: 10.1152/ajpheart.00459.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laurent S, Boutouyrie P. The structural factor of hypertension: large and small artery alterations. Circ Res. 2015;116:1007–1021. doi: 10.1161/CIRCRESAHA.116.303596. [DOI] [PubMed] [Google Scholar]
- 21.Humphrey JD, Harrison DG, Figueroa CA, Lacolley P, Laurent S. Central artery stiffness in hypertension and aging: a problem with cause and consequence. Circ Res. 2016;118:379–381. doi: 10.1161/CIRCRESAHA.115.307722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akishita M, Yamada H, Dzau VJ, Horiuchi M. Increased vasoconstrictor response of the mouse lacking angiotensin II type 2 receptor. Biochem Biophys Res Commun. 1999;261:345–349. doi: 10.1006/bbrc.1999.1027. [DOI] [PubMed] [Google Scholar]
- 23.Bellini C, Wang S, Milewicz DM, Humphrey JD. Myh11(R247C/ R247C) mutations increase thoracic aorta vulnerability to intramural damage despite a general biomechanical adaptivity. J Biomech. 2015;48:113–121. doi: 10.1016/j.jbiomech.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellini C, Bersi MR, Caulk AW, Ferruzzi J, Milewicz DM, Ramirez F, et al. Comparison of 10 murine models reveals a distinct biomechanical phenotype in thoracic aortic aneurysms. JR Soc Interface. 2017;14:20161036. doi: 10.1098/rsif.2016.1036. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butlin M, Lindesay G, Viegas KD, Avolio AP. Pressure dependency of aortic pulse wave velocity in vivo is not affected by vasoactive sub-stances that alter aortic wall tension ex vivo. Am J Physiol Heart Circ Physiol. 2015;308:H1221–1228. doi: 10.1152/ajpheart.00536.2014. [DOI] [PubMed] [Google Scholar]
- 26.Bersi MR, Khosravi R, Wujciak AJ, Harrison DG, Humphrey JD. Differential cell-matrix mechanoadaptations and inflammation drive regional propensities to aortic fibrosis, aneurysm or dissection in hypertension. J R Soc Interface. 2017;14:20170327. doi: 10.1098/rsif.2017.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Humphrey JD, Eberth JF, Dye WW, Gleason RL. Fundamental role of axial stress in compensatory adaptations by arteries. J Biomech. 2009;42:1–8. doi: 10.1016/j.jbiomech.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caulk AW, Humphrey JD, Murtada SI. Fundamental roles of axial stretch in isometric and isobaric evaluations of vascular contractility. J Biomech Eng. 2019;141 doi: 10.1115/1.4042171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spronck B, Humphrey JD. Arterial stiffness: different metrics, different meanings. J Biomech Eng. 2019;141:0910041–09100412. doi: 10.1115/1.4043486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenwald SE. Ageing of the conduit arteries. J Pathol. 2007;211:157–172. doi: 10.1002/path.2268. [DOI] [PubMed] [Google Scholar]
- 31.Louis H, Kakou A, Regnault V, Labat C, Bressenot A, Gao-Li J, et al. Role of alpha1beta1-integrin in arterial stiffness and angiotensin-induced arterial wall hypertrophy in mice. Am J Physiol Heart Circ Physiol. 2007;293:H2597–2604. doi: 10.1152/ajpheart.00299.2007. [DOI] [PubMed] [Google Scholar]
- 32.Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol. 2014;15:802–812. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuang SQ, Geng L, Prakash SK, Cao JM, Guo S, Villamizar C, et al. Aortic remodeling after transverse aortic constriction in mice is attenuated with AT1 receptor blockade. Arterioscler Thromb Vasc Biol. 2013;33:2172–2179. doi: 10.1161/ATVBAHA.113.301624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ji H, Zheng W, Li X, Liu J, Wu X, Zhang MA, et al. Sex-specific T-cell regulation of angiotensin II -dependent hypertension. Hypertension. 2014;64:573–582. doi: 10.1161/HYPERTENSIONAHA.114.03663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Korneva A, Humphrey JD. Maladaptive aortic remodeling in hypertension associates with dysfunctional smooth muscle contractility. Am J Physiol Heart Circ Physiol. 2019;316:H265–H278. doi: 10.1152/ajpheart.00503.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bersi MR, Bellini C, Wu J, Montaniel KRC, Harrison DG, Humphrey JD. Excessive adventitial remodeling leads to early aortic maladaptation in angiotensin-induced hypertension. Hypertension. 2016;67:890–896. doi: 10.1161/HYPERTENSIONAHA.115.06262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bussy C, Boutouyrie P, Lacolley P, Challande P, Laurent S. Intrinsic stiffness of the carotid arterial wall material in essential hypertensives. Hypertension. 2000;35:1049–1054. doi: 10.1161/01.hyp.35.5.1049. [DOI] [PubMed] [Google Scholar]
- 38.Reference Values for Arterial Stiffness Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardio-vascular risk factors: ’establishing normal and reference values’. Eur Heart J. 2010;31:2338–2350. doi: 10.1093/eurheartj/ehq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spronck B, Delhaas T, Butlin M, Reesink KD, Avolio AP. Options for dealing with pressure dependence of pulse wave velocity as a measure of arterial stiffness: an update of cardio-ankle vascular index (CAVI) and CAVI0. Pulse (Basel) 2018;5:106–114. doi: 10.1159/000479322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spronck B, Heusinkveld MH, Vanmolkot FH, Roodt JO, Hermeling E, Delhaas T, et al. Pressure-dependence of arterial stiffness: potential clinical implications. J Hypertens. 2015;33:330–338. doi: 10.1097/HJH.0000000000000407. [DOI] [PubMed] [Google Scholar]
- 41.Yanagisawa H, Davis EC, Starcher BC, Ouchi T, Yanagisawa M, Richardson JA, Olson EN. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature. 2002;415:168–171. doi: 10.1038/415168a. [DOI] [PubMed] [Google Scholar]
- 42.de Wit A, Vis K, Jeremy RW. Aortic stiffness in heritable aortopathies: relationship to aneurysm growth rate. Heart Lung Circ. 2013;22:3–11. doi: 10.1016/j.hlc.2012.08.049. [DOI] [PubMed] [Google Scholar]
- 43.Wilson JS, Humphrey JD. Evolving anisotropy and degree of elastolytic insult in abdominal aortic aneurysms: potential clinical relevance? J Biomech. 2014;47:2995–3002. doi: 10.1016/j.jbiomech.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferruzzi J, Di Achille P, Tellides G, Humphrey JD. Combining in vivo and in vitro biomechanical data reveals key roles of perivascular tethering in central artery function. PLoS One. 2018;13:e0201379. doi: 10.1371/journal.pone.0201379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.