Abstract
Chondrites are meteorites from undifferentiated parent bodies that provide fundamental information about early Solar System evolution and planet formation. The element Cr is highly suitable for deciphering both the timing of formation and the origin of planetary building blocks because it records both radiogenic contributions from 53Mn-53Cr decay and variable nucleosynthetic contributions from the stable 54Cr nuclide. Here, we report high-precision measurements of the massindependent Cr isotope compositions (ε53Cr and ε54Cr) of chondrites (including all carbonaceous chondrites groups) and terrestrial samples using for the first time a multi-collection inductively-coupled-plasma mass-spectrometer to better understand the formation histories and genetic relationships between chondrite parent bodies. With our comprehensive dataset, the order of decreasing ε54Cr (per ten thousand deviation of the 54Cr/52Cr ratio relative to a terrestrial standard) values amongst the carbonaceous chondrites is updated to CI = CH ≥ CB ≥ CR ≥ CM ≈ CV ≈ CO ≥ CK > EC > OC. Chondrites from CO, CV, CR, CM and CB groups show intra-group ε54Cr heterogeneities that may result from sample heterogeneity and/or heterogeneous accretion of their parent bodies. Resolvable ε54Cr (with 2SE uncertainty) differences between CV and CK chondrites rule out an origin from a common parent body or reservoir as has previously been suggested. The CM and CO chondrites share common ε54Cr characteristics, which suggests their parent bodies may have accreted their components in similar proportions. The CB and CH chondrites have low-Mn/Cr ratios and similar ε53Cr values to the CI chondrites, invalidating them as anchors for a bulk 53Mn-53Cr isochron for carbonaceous chondrites. Bulk Earth has a ε53Cr value that is lower than the average of chondrites, including enstatite chondrites. This depletion may constrain the timing of volatile loss from the Earth or its precursors to be within the first million years of Solar System formation and is incompatible with Earth’s accretion via any of the known chondrite groups as main contributors, including enstatite chondrites.
Keywords: Chondrites; Genetic relationship; 54Cr systematics; CV-CK, CH-CB and CO-CM clans; CV subgroups; 53Mn-53Cr chronometry; Condensation history; Volatile depletion; Early Earth; Solar System
1. Introduction
Chondrites are the oldest cosmic sedimentary rocks, the most pristine of which preserve information about the origin ofthe Solar System (e.g.,Krot et al., 2014). Most of the parent bodies of chondrites did not undergo significant melting (though some underwent varying degrees of thermal metamorphism) and thus did not differentiate. Therefore, bulk chondrite samples have chemical compositions that are thought to be representative of the bulk parent body and have been taken as proxies for the composition of bulk differentiated planets such as the Earth (Allègre et al., 1995). Thus, investigating the chemical and isotopic compositions of chondrites is central to better understand the evolution of the Solar System and planet formation. Chondrites also record large chemical and isotopic variations amongst them (Alexander, 2019a,b; Braukmüller et al., 2018; Hellmann et al., 2020; Palme and O’Neill, 2014). In particular, O and Cr isotopes have been key in evaluating genetic links between meteorites and planets (e.g., Clayton and Mayeda, 1999;Qin et al., 2010; Trinquier et al., 2007; Warren, 2011). Although there are several studies utilizing Cr isotopes in this manner, the kinship between different groups of carbonaceous chondrites is still debated. The possible genetic links between the Vigarano-type (CV) and Karoonda-type (CK) chondrites (Dunn and Gross, 2017; Dunn et al., 2016; Greenwood et al., 2010), as well as those of chondrites in Ornans-type (CO) and Mighei-type (CM) groups (e.g., Schrader and Davidson, 2017) and Bencubbin-type (CB) and high-metal (CH) groups are still unresolved (e.g., Krot et al., 2014).
The Cr isotope system may be one of the best suited isotopic systems for studying the timing of chondrite formation and the genetic relationships between meteorite groups (Birck and Allègre, 1988; Trinquier et al., 2007). The short-lived radionuclide 53Mn, with a half-life of 3.7 ± 0.2 Myrs (Holden, 1990), decays to 53Cr and was present in the early Solar System (Lugmair and Shukolyukov,1998). Therefore, the 53Mn-53Cr decay system is a useful chronometer to date early Solar System events such as chondrule (precursor) formation (Nyquist et al., 2001; Yamashita et al., 2010; Zhu et al., 2019a; Zhu et al.,2020a), differentiation of planets/asteroids (Lugmair and Shukolyukov, 1998; Trinquier et al., 2008b; Wadhwa et al., 2003; Yamakawa et al., 2010; Zhu et al., 2019b; Zhu et al., 2020b), as well as the aqueous alteration (Fujiya et al., 2012; Fujiya et al., 2013) and possibly the metamorphic processes in chondrite parent bodies (Trinquier et al., 2008b; Göpel et al., 2015). Furthermore, previous Cr isotope measurements have suggested that the various carbonaceous chondrite (CC) groups define a bulk isochron with a slope defining an initial 53Mn/55Mn ratio of (8.5 ± 1.5) × 10-6, which was used to suggest that a volatile fractionation in the solar nebular occurred as early as 4568.6 ± 1.1 Ma (Shukolyukov and Lugmair 2006, Moynier et al. 2007) when the date is anchored to the U isotope corrected age of the D’Orbigny angrite (Amelin, 2008; Brennecka and Wadhwa, 2012; Glavin et al., 2004). This whole-rock 53Mn-53Cr isochron, reported in Shukolyukov and Lugmair (2006), is primarily controlled by two extreme endmembers representing the Ivuna-type carbonaceous (CI; with the highest 55Mn/52Cr ratio) and the CB chondrites (with the lowest 55Mn/52Cr ratio). Based on more recent measurements of the Cr isotopes in CI chondrites (Qin et al., 2010; Trinquier et al., 2008b), Qin et al. (2010) suggested a lower slope of (5.4 ± 2.4) × 10-6 and a correspondingly younger age of 4566.1 ± 2.4 Ma age for the Mn/Cr fractionation (also anchored to U-corrected D’Orbigny), although this younger age is still consistent within uncertainty with that of Shukolyukov and Lugmair (2006). Finally, the slope of the isochron was later updated to [(6.2 ± 1.9) × 10-6] (Göpel et al., 2015). However, all of these isochrons are mostly controlled by the data point for one CB chondrite, Hammadah al Hamra (HaH) 237 (Shukolyukov and Lugmair, 2006) and to date no Cr isotope data for the CH chondrites, which have comparably low Mn/Cr ratios (Lodders et al., 1998), have been reported. Therefore, providing high-precision Cr isotope data for CB and CH chondrites is critical to further evaluate the timing of Mn/Cr fractionation in the early Solar System.
Variable 54Cr nucleosynthetic anomalies, expressed as ε54Cr (the parts per 10,000 deviation of the mass fractionation corrected 54Cr/52Cr ratio from a terrestrial standard) have been used as a tracer of potential genetic relationships between Solar System materials (Trinquier et al., 2007) including between Earth and Moon (Mougel et al. 2018). The published ε54Cr values for several chondrite groups with the mean values following the sequence: CI > CB ≥ CR ≥ CH ≥ CM ≥ CV ≥ CO ≥ CK > EC (Enstatite Chondrites) ≈ RC (Rumuruti Chondrites) > OC (Ordinary Chondrites) (Göpel et al., 2015; Mougel et al.,2018; Pedersen et al., 2019; Qin et al., 2010; Shukolyukov and Lugmair, 2006; Trinquier et al., 2007; Van Kooten et al., 2016; Zhu et al., 2021). These isotopic differences between chondrites have been compared to the composition of the Earth’s mantle (Mougel et al. 2018) to detect and identify the likely sources of the impact-related extraterrestrial materials included in terrestrial rocks (e.g., Koeberl et al., 2007; Magna et al., 2017; Mougel et al.,2017; Mougel et al., 2019; Schmitz et al., 2016; Trinquier et al., 2006) and to test magma ocean models for asteroids (e.g., Zhu et al., 2019b, 2020b). To date, the most 54Cr-rich phases analyzed in chondrites are 10 s to 100 s of nanometer-size presolar spinel grains that can be concentrated in acid residues (e.g., Podosek et al., 1997; Rotaru et al., 1992) and have been identified by NanoSIMS (Dauphas et al., 2010; Nittler et al., 2018; Qin et al.,2011). These grains probably formed in the ejecta of one or more supernova.
However, although Cr-isotopic homogeneity within each chondrite group is generally assumed, this assertion is only based on a limited number of measurements, often of the same meteorites. In several instances, when two or more meteorites from the same group have been analyzed their ε54Cr values differ by more than the reported uncertainties of the measurements, including: Renazzo-type carbonaceous (CR) (1.06 ± 0.08 to 1.32 ± 0.11 [2SE] for Northwest Africa [NWA] 7837 and Graves Nunataks [GRA] 06100); CO (0.57 ± 0.11 to 0.87 ± 0.18, Lance’ and Kainsaz); CK (0.33 ± 0.12 to 0.63 ± 0.09 for Elephant Moraine [EET] 92002 and Karoonda) and CV (0.71 ± 0.15 to 1.10 ± 0.08, for Leoville and Allende) (Qin etal., 2010; Trinquier et al., 2007; Zhu etal., 2020b). Hence, a more comprehensive Cr isotope dataset is required to better understand the extent of the ε54Cr variability within and between chondrite groups and to better determine the ε54Cr sequence for chondrites, especially for the CH and CB chondrites, which lack systematic ε54Cr studies. Additionally, the CK and CV chondrites share many similar features, including: chondrule sizes and abundances (Weisberg et al., 2006), petrological and chemical compositions (Isa et al., 2014), cosmic-ray exposure ages (Scherer andSchultz, 2000), O-isotope compositions (Greenwood et al.,2010) and Ti isotopic anomalies (Trinquier et al., 2009; Zhang et al., 2012) and are considered a clan (the CV-CK clan). Different models have been proposed for their origins, including the single parent body hypothesis where CV and CK chondrites originate from different depths within the same parent body, with the CK3 to CK6 petrologic types at progressively greater depths (Greenwood et al., 2010). This common origin for CV and CK chondrite groups can be tested using the ε54Cr systematics. ε54Cr can also be used to track the relationships between chondrites in CM-CO (e.g., Schrader and Davidson, 2017) and CB-CH clans (e.g., Krot et al., 2014).
The mass-independent Cr isotope compositions of meteorites are traditionally measured by thermal ionization mass spectrometry (TIMS) (Birck and Allègre, 1988; Lugmair and Shukolyukov, 1998; Qin et al., 2010; Shukolyukov and Lugmair, 2006; Trinquier et al., 2007; Trinquier et al., 2008a). However, it appears that there are small residual mass-dependent fractionations that cannot be corrected for, which are evident in the correlation of ε53Cr and ε54Cr for multiple measurements of standards with a slope of ~2 (Bourdon and Fitoussi, 2020; Qin et al.,2010; Trinquier et al., 2006). It has been suggested that such residual mass-dependent isotopic fractionations could potentially arise from isotopic fractionation between different oxidized Cr gas species during evaporation from the filaments during TIMS analysis (Bourdon and Fitoussi,2020). This would mimic equilibrium isotope fractionation that occurred as Cr evaporated during the formation of bodies such as the Moon and the asteroid 4 Vesta (Sossi et al., 2018; Zhu et al., 2019c). Moreover, the column chemistry in some previous studies can only reach a Cr yield of ~80% (e.g., Qin et al., 2010; Trinquier et al., 2008a; Trinquier et al., 2008b; Zhu et al., 2019a), and sometimes the yield can be as low as ~60% (Kruijer et al., 2020). However, the equilibrium Cr stable isotope fractionation on the column cannot be fully corrected if the yield is low [e.g., <70%; (Larsen et al., 2016; Qin et al., 2010; Trinquier et al., 2008a)], since the different Cr cuts from the columns show mass-independent fractionation with ε53Cr ranging from —0.2 to +0.2 and ε54Cr ranging from —0.5 to +0.4 (Trinquier et al., 2008a). In order to avoid this problem, we have utilized a high-yield (~95%) four-step column chemistry and employ multiple-collector inductively-coupled-plasma mass-spectrometry (MC-ICP-MS) to measure the mass-independent fractionation of Cr isotopes for a self-consistent comprehensive set of chondrite group compositions, including CI, CB (both CBa and CBb subgroups), CH, CR, CM, CV (including the oxidized, oxA after Allende and oxB after Bali, and reduced, Red after Vigarano, subgroups), CO, CK and high-Fe enstatite (EH) chondrite groups (Pedersen et al., 2019; Qin et al.,2010; Trinquier et al., 2007; Trinquier et al., 2008b; Zhu et al., 2021). This study aims to better constrain the genetic relationship between chondrite parent bodies, chondrite parent body processes (redox, thermal metamorphism and aqueous alteration), the 53Mn-53Cr “isochron” for CCs and other chondrites, and the radiogenic Cr isotopic deficits between chondrites and Earth.
2. Samples and Methods
2.1. Samples and digestion
The sample suite analyzed in this study includes: one CI1 chondrite (Orgueil), three CB3 chondrites (Miller Range [MIL] 05082, Quebrada Chimborazo [QC] 001 and Hammadah al Hamra [HaH] 237), two CH3 chondrites (Pecora Escarpment [PCA] 91467 and Asuka [A] 881020), two CR chondrites (Grosvenor Mountains [GRO] 95577 [CR1] and Al Rais an anomalous [CR2]), five CM chondrites (Scott Glacier [SCO] 06043 [CM1], Nogoya [CM2], Banten [CM2], Jbilet Winselwan [CM2] and Aguas Zarcas, a new CM2 fall from 2019), three CO3 chondrites (Ornans, MIL 07193 and Dominion Range [DOM] 10104), one CV3oxA chondrite (Allende), three CV3oxB chondrites (Bali, Mokoia, and Kaba), two CV3red chondrites (Leoville and Vigarano), four CK chondrites (Allan Hills [ALH] 85002 [CK4], Karoonda [CK4], Elephant Moraine [EET] 92002 [CK5] and Lewis Cliff [LEW] 87009 [CK6]), and one enstatite chondrite, Sahara 97096 [EH3]. We note that, CB and CH chondrites are highly heterogeneous due to their metal-rich nature (Krot et al., 2014) and that the samples analyzed here (<100 mg) may not represent the bulk parent meteorites. We also selected the United States Geological Survey (USGS) terrestrial rock standard DTS-1 (along with Allende) as a reference material to test the precision and accuracy of the data. Furthermore, the Cr isotope compositions of two widely used artificial standards, NIST 3112a and SCP-Cr (ICP-MS elemental standard for Cr), were measured to test for potential non-mass dependent isotopic fractionation of Cr induced during production of the standards and to calibrate possible offsets between studies using different standards [e.g., NIST 3112a was used by Qin et al. (2010) and Zhu et al. (2019a)]. Among these samples, MIL 05082, Aguas Zarcas, Ornans and Bali were chunks, while the other samples are powders. Based on our recording information, the powder of Jbilet Winselwan, Mokoia, Leoville, Vigarano and Sahara 97096 were from original sample masses of 1.01 g, 1.08 g, 1 g, 0.16 g, and ~0.5 g respectively.
The samples were dissolved following the protocol described in Inglis et al. (2018) using Teflon bombs and an Analab EvapoClean, which has been successfully applied in previous studies (Zhu et al., 2019b, 2020b). The procedure involved heating the samples in concentrated HF and HNO3 (2:1) at 140 °C for two days, drying down the samples and subsequent dissolution of the solid residues in 6 N HCl (also at 140 °C) for another two days to ensure complete digestion of fluorides, and refractory phases such as chromite and spinel. The combination of Teflon bombs and Analab EvapoClean for chondrite dissolution is simple and convenient, resulting in lower blanks compared to traditional dissolution methods, such as PARR™ bomb dissolution (e.g., Zhu et al., 2019c) and alkaline fusion (e.g., Qin et al., 2010). Before the chemical separation of Cr (see below), ~10% aliquots were preserved for subsequent determination of the 55Mn/52Cr ratio and major element contents.
2.2. Determination of the 55Mn/52Cr ratios
High-precision 53Mn-53Cr chronology requires the accurate determination of the 55Mn/52Cr ratios, which were measured here on a MC-ICP-MS Neptune Plus, using a method that was similar to those employed in previous studies (Göpel et al., 2015; Trinquier et al., 2008a; Zhuet al., 2019b; Zhu et al., 2020b). We initially prepared three Mn-Cr doped artificial standard solutions gravitationally, with Mn-Cr contents of 10-100 ppb, 50-100 ppb and 100-100 ppb and Mn/Cr ratios of ~0.1, ~0.5 and ~1.0. The unpurified sample solutions were diluted to a Cr content of ~100 ppb. The intensities for 55Mn and 52Cr on Faraday detectors obtained when analyzing the standard and sample solutions ranged from 0.5 V to 5 V, and 10 cycles of 4 seconds each were measured in each analysis to obtain a target precision for the 55Mn/52Cr ratios better than 0.1%. After establishing a calibration curve (R2> 0.999) based on the true and measured 55Mn/52Cr ratios of the three artificial standards, the 55Mn/52Cr ratios of the chondrite samples could be calculated. The external precisions for the 55Mn/52Cr ratios are better than 0.5% (2SD, N = 6) as determined from multiple measurements of the USGS standards PCC-1 and DTS-1. The final estimated precision of <5% (2σ) was determined from a comparison of the PCC-1 and Allende meteorite results with those in the literature (Moynier et al., 2007; Qin et al.,2010; Shukolyukov and Lugmair, 2006; Trinquier et al.,2008a). The determination ofthe Mn/Cr ratios on Neptune Plus is faster than the standard-addition method (that requires preparation and analysis of at least four solutions per sample; (e.g., Qin et al., 2010; Zhu et al., 2019a) and more accurate than the Mn and Cr content determination (e.g., Pedersen et al., 2019) by quadrupole ICP-MS (5–10%, 2σ). Additionally, introduction of the low-concentration unpurified samples into the MC-ICP-MS does not result in measurable memory effects.
2.3. Column chemistry
Low Cr yields from column chemistry, where Cr isotopes typically fractionate via equilibrium processes, can result in apparent mass-independent Cr isotope variations resulting from inappropriate mass fractionation corrections (Larsen et al., 2016; Qin et al., 2010; Trinquier et al.,2008a). To avoid this issue, a four-step column chemistry for Cr purification with high yield broadly following previous approaches (Bizzarro et al., 2011; Larsen et al., 2018; Larsen et al., 2016; Pedersen et al., 2019; Schiller et al.,2014; Trinquier et al., 2008a) was employed (Table 1). Only ~5 mg of samples were dissolved in 10 M HCl and dried down three times before the following column chemistry step to purify the Cr. First, we used an anion chromatographic purification column to efficiently remove Fe in 6 M HCl. Prior to sample loading on cation exchange columns, we used a Cr pre-treatment procedure involving dissolution in 10 M HCl at >120 °C to efficiently promote the formation of Cr3+-Cl species, which have a low affinity for the cation exchanger and thus elute early (Larsen et al.,2016; Trinquier et al., 2008a). This was followed by elution of Cr on a 1 ml cation exchange column in 20 ml of 0.5 M HNO3 to remove the major elements including Mg, Ca, Al, Ni (Bizzarro et al., 2011) and collect all the Cr species (major Cr0 and minor Cr2+ and Cr3+) to reach a >99% recovery. The samples were then exposed to 0.5 MHNO3+ 0.6% H2O2 at room temperature for >1 day to promote the formation of Cr3+ (Larsen et al. 2016). However, it is difficult to transform all Cr to Cr3+, so the Cr0-bearing material is collected in 0.5 ml of the loading solution and 0.5 ml of 0.5 N HNO3 elution to increase the recovery to >95% in the next column. The third clean-up column involved Cr purification from Al, Fe, V, Ti (and other high-field-strength elements) and Na, K on a small (0.33 ml) cation exchange column using 0.5 M HNO3, 1 M HF and 6 M HCl (Larsen et al., 2018). Finally, for the fourth column, 0.7 ml of TODGA resin were used in 8 N HCl to remove the residual Fe, V and Ti (stuck on the column) which have isobaric isotopes with 54Cr (54Fe) and 50Cr (50V and 50Ti) (Pedersen et al., 2019; Schiller et al., 2014). The full procedure typically reaches a total yield between 95% and 99%, and effectively removes any matrix, especially Fe, V and Ti. Low abundances of matrix elements are important for analyses by MC-ICP-MS as all elements present are ionized (unlike the selective thermal ionization ofTIMS) generating potential isobaric interferences and altering the mass fractionation behavior. Artificial standards including NIST 979, NIST 3112a and SCP-Cr (The standard for ICP-MS measurements) were passed through the first column chemistry ste<5 ng, which is negligible com-paredtothe 10–20 μg of Cr processed through the columns. The final Cr solution was dried in ~100 μl of concentrated HNO3 three times to transform the acid media and remove residual organics (i.e., those from the cation exchange resin).
Table 1. Four-step column chemistry used in this study to purify chromium.
| Eluent | Volume (ml) | Procedural step | Elements eluted |
|---|---|---|---|
| Step 1: 0.5 ml Biorad AG1-X8 200-400 mesh resin | |||
| 6M HCl | 2 | Condition | |
| 6M HCl | 0.5 | Sample Load | |
| 6M HCl | 3 | Cr collection | Cr + matrix |
| H2O | 5 | Wash | Fe + Ni |
| Step 2: 1 ml Biorad AG50W-X8 200-400 mesh resin | |||
| 0.5 M HCl | 3 | Condition | |
| 0.5 M HCl | 3 | *1Sample Load | |
| 0.5 M HNO3 | 20 | Cr collection | Cr + matrix |
| 6M HCl | 5 | Wash | Mg +; Ca + Al +; Mn + Ni |
| Step 3: 0.33 ml Biorad AG50W-X8 200-400 mesh resin | |||
| H2O | 1 | Condition | |
| 0.5 M HNO3 | 0.5 | *2Sample Load and Cr collection | Cr |
| 0.5 M HNO3 | 0.5 | Cr collection | Cr |
| 1M HF | 2.5 | Matrix elution | Ti + V + K + Na + Fe + Al |
| 1M HCl | 6 | Matrix elution | K+ Na |
| 6M HCl | 3 | Cr collection + Wash | Cr |
| Step 4: 0.75 ml Eichrom TODGA resin | |||
| 8M HCl | 2 | Condition | |
| 8M HCl | 0.5 | Sample Load | Cr |
| 8M HCl | 2 | Cr collection | Cr |
| H2O | 4 | Wash | V + Fe + Ti |
Note: Sample pretreatment: dissolving sample in 0.25 ml 6 M HCl (in 7 ml beaker) with heating at >120 °C for more than 2 h, then added 2.75 ml H2O before loading. This is to transform Cr as Cr0
Sample pretreatment: dissolving sample in 0.125 ml 2 M HNO3 (in 3 ml beaker) with heating at 100 ° C for 2 h, then added 0.01 ml H2O2 and 0.365 ml H2O, and put the mixture at room temperature for more than 24 h. This is to maximize the oxidation of Cr as Cr3+.
2.4. Isotope analysis
The Cr isotopic compositions of all the samples were determined using an MC-ICP-MS Neptune Plus located at the Centre for Star and Planet Formation, Globe Institute, University of Copenhagen. Detailed analytical and data reduction methods are described in Zhu et al. (2021),Schiller et al. (2014) and Pedersen et al. (2019). Each sample was measured by sample-standard bracketing using the NIST SRM 979 Cr standard. Sample solutions with ~0.5 ppm of Cr were introduced to the plasma via an ESI Apex IR resulting in 52Cr signals of 20-40 V at an uptake rate of ~0.06 mL/min. Each sample was measured five times. The 53Cr/52Cr and 54Cr/52Cr ratios were normalized to a constant 50Cr/52Cr ratios of 0.051859 using an exponential law (Lugmair and Shukolyukov, 1998). All the measured isotopic ratios are expressed relative to NIST SRM 979 in the epsilon notations:
| (1) |
with x = 53 or 54.
In order to control the influence of the potential isobaric interferences from Fe, V and Ti (54Fe to 54Cr, 50V and 50Ti to 50Cr) and major alkali elements (Na and K), we also performed doping tests for these elements (the doped samples are the corresponding SCP elemental standards of ICP-MS). The external precision was tested on five Allende, five DTS-1 and two Orgueil from aliquots that were each individually purified from the same digestion. We also provide the ε53Cr data for another Ivuna sample of which ε54Cr has been reported in Van Kooten et al. (2016) that described the related analytical methods.
3. Results
The Cr isotope data for doping and external precision tests are reported in Table 2 and combined with literature data from Schiller et al. (2014). In Table 3, we compare the 55Mn/52Cr and Cr isotope data for the same chondrites measured in this and previous studies (Göpel et al., 2015; Jenniskens et al., 2012; Kadlag et al., 2019; Langbroeket al., 2019; Mougel et al., 2018; Moynier et al., 2007; Petitat et al., 2011; Qin et al., 2010; Sanborn et al., 2019; Schiller et al., 2014; Trinquier et al., 2007; 2008b; van Kooten et al., 2020, 2016; Williams et al., 2020; Zhu et al., 2020a, 2020b). The averaged group Cr isotope data and 55Mn/52Cr ratios of all the chondrites are reported in Table 4. We also summarize the Cr isotope data for Rumu-ruti (R) chondrites, Earth, Moon, Mars, Vesta and other achondrite parent bodies in Table 5. Relevant literature O isotope data are shown alongside the new Cr isotope data in Tables 4 and 5 (the samples measured for O and Cr isotope compositions are never from the same aliquots). The Cr isotope data (including literature data) for the terrestrial samples are listed in Table 6. We also re-measured the 55Mn/52Cr ratios on unprocessed dissolution aliquots for the H chondrites reported in Pedersen et al. (2019) using our MC-ICP-MS approach.
Table 2. Doping test for V, Ti, Fe, Na and K and external precision tests by Allende, DTS-1 and Orgueil.
| Samples | Interference Contribution (ppm)* | ε53Cr | 2se | ε54Cr | 2se | N/[ref.] |
|---|---|---|---|---|---|---|
| Cr-V-0% | 0 | 0.00 | 0.00 | –0.01 | 0.02 | 5 |
| Cr-V-S14 | 24 | 0.00 | 0.02 | 0.00 | 0.02 | [1] |
| Cr-V-0.5% | 269 | 0.00 | 0.03 | 0.04 | 0.02 | 5 |
| Cr-V-2.5% | 1438 | –0.06 | 0.02 | –0.07 | 0.03 | 5 |
| Cr-V-5% | 2764 | –0.09 | 0.01 | –0.12 | 0.04 | 5 |
| Cr-Ti-0% | 0 | –0.01 | 0.02 | 0.02 | 0.03 | 5 |
| Cr-Ti-S14 | 1609 | 0.01 | 0.02 | 0.00 | 0.02 | [1] |
| Cr-Ti-0.5% | 7590 | 0.01 | 0.05 | 0.03 | 0.05 | 5 |
| Cr-Ti-1% | 14,269 | 0.02 | 0.03 | 0.06 | 0.08 | 5 |
| Cr-Ti-2% | 27,904 | 0.04 | 0.05 | 0.11 | 0.06 | 5 |
| Cr-Fe-0% | 0 | 0.00 | 0.01 | 0.01 | 0.02 | 5 |
| Cr-Fe-0.01% | 205 | 0.00 | 0.01 | –0.08 | 0.04 | 5 |
| Cr-Fe-S14 | 558 | 0.02 | 0.02 | 0.01 | 0.02 | [1] |
| Cr-Fe-0.1% | 2505 | 0.01 | 0.01 | –0.04 | 0.05 | 5 |
| Cr-Fe-0.5% | 11,846 | –0.01 | 0.02 | –0.19 | 0.04 | 5 |
| Cr-Na-50% | 0.04 | 0.05 | 0.11 | 0.05 | 4 | |
| Cr-Na-100% | 0.01 | 0.02 | 0.02 | 0.09 | 4 | |
| Cr-K-50% | 0.03 | 0.05 | 0.04 | 0.02 | 4 | |
| Cr-K-100% | 0.01 | 0.02 | 0.01 | 0.10 | 4 | |
| Allende-1 | 0.08 | 0.01 | 0.87 | 0.08 | 5 | |
| Allende-2 | 0.10 | 0.02 | 0.90 | 0.02 | 5 | |
| Allende-3 | 0.11 | 0.02 | 0.94 | 0.02 | 5 | |
| Allende-4 | 0.14 | 0.03 | 0.95 | 0.08 | 5 | |
| Allende-5 | 0.10 | 0.02 | 0.96 | 0.05 | 5 | |
| Average | 0.10 | 0.04 | 0.92 | 0.07 | 2SD | |
| DTS-1-1 | 0.05 | 0.01 | 0.11 | 0.07 | 5 | |
| DTS-1-2 | 0.05 | 0.01 | 0.16 | 0.05 | 5 | |
| DTS-1-3 | 0.06 | 0.03 | 0.18 | 0.07 | 5 | |
| DTS-1-4 | 0.04 | 0.03 | 0.15 | 0.03 | 5 | |
| DTS-1-5 | 0.07 | 0.02 | 0.19 | 0.07 | 5 | |
| Average | 0.05 | 0.03 | 0.16 | 0.06 | 2SD | |
| Orgueil-1 | 0.19 | 0.05 | 1.51 | 0.05 | 5 | |
| Orgueil-2 | 0.19 | 0.01 | 1.50 | 0.08 | 5 | |
| Average | 0.19 | 0.00 | 1.50 | 0.01 | 2SD |
Note: The references [1] Schiller et al. (2014). The doped samples are the corresponding SCP elemental standards of ICP-MS.
The corresponding V, Ti and Fe interference is for 50Cr, 50Cr and 54Cr respectively.
Table 3. Comparison of 55Mn/52Cr and Cr isotope data for meteorites that have been measured multiple times here and/or in the literature.
| Mass (mg) | 55Mn/52Cr | ε53Cr | Error | ε54Cr | Error | N | Instruments | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Orgueil | 0.87 | 0.43 | 0.10 | 11 | Nu Plasma HR MC-ICP-MS | (Moynier et al. 2007) | |||||||
| Orgueil | 992 | 0.81 | 0.39 | 0.10 | 1.51 | 0.20 | 110 | VG-54E and Micromass Sector 54 TIMS | (Shukolyukov and Lugmair, 2006) | ||||
| Orgueil | 39.0 | 0.93 | 0.46 | 0.06 | 1.94 | 0.12 | n.d. | Triton TIMS | (Kadlag et al., 2019) | ||||
| Orgueil | 22.6 | 0.86 | 0.19 | 0.05 | 1.51 | 0.05 | 5 | Neptune Plus MC-ICP-MS | This study | ||||
| Orgueil | 22.6 | 0.19 | 0.01 | 1.50 | 0.08 | 5 | Neptune Plus MC-ICP-MS | This study | |||||
| Orgueil | 46 | 0.80 | 0.25 | 0.06 | 1.56 | 0.06 | 3-5 | Triton TIMS | (Trinquier et al., 2007, 2008a, 2008b) | ||||
| Orgueil | ~100 | 0.85 | 0.20 | 0.05 | 1.55 | 0.13 | 6-10 | Triton TIMS | (Qin et al., 2010) | ||||
| Orgueil | 0.24 | 0.05 | 1.69 | 0.09 | 6-10 | Triton TIMS | (Qin et al., 2010) | ||||||
| Orgueil | 320 | 0.81 | 0.25 | 0.03 | 1.56 | 0.06 | n.d. | Triton TIMS | (Petitat et al., 2011) | ||||
| Averaged value | 0.85 | 0.29 | 0.07 | 1.60 | 0.10 | ||||||||
| Ivuna | 225 | 0.82 | 0.41 | 0.11 | 1.59 | 0.24 | 71 | VG-54E and Micromass Sector 54 TIMS | (Shukolyukov and Lugmair, 2006) | ||||
| Ivuna | 30.5 | 0.94 | 0.30 | 0.17 | 1.79 | 0.20 | n.d. | Triton TIMS | (Kadlag et al., 2019) | ||||
| Ivuna | 0.75 | 0.16 | 0.02 | 1.55 | 0.05 | 10 | Neptune Plus MC-ICP-MS | (Schiller et al., 2014) | |||||
| Ivuna | ~200 | 0.85 | 0.25 | 0.06 | 1.59 | 0.14 | 12 | Triton TIMS (Total Evaporation) | This study; (van Kooten et al., 2016) | ||||
| 1.30 | 0.09 | Triton Plus TIMS | (Williams et al., 2020) | ||||||||||
| Averaged value | 0.84 | 0.28 | 0.10 | 1.56 | 0.15 | ||||||||
| Allende | ~100 | 0.42 | 0.08 | 0.01 | 0.87 | 0.08 | 5 | Neptune Plus MC-ICP-MS | This study | ||||
| Allende | ~100 | 0.10 | 0.02 | 0.90 | 0.02 | 5 | Neptune Plus MC-ICP-MS | This study | |||||
| Allende | ~100 | 0.11 | 0.02 | 0.94 | 0.02 | 5 | Neptune Plus MC-ICP-MS | This study | |||||
| Allende | ~100 | 0.14 | 0.03 | 0.95 | 0.08 | 5 | Neptune Plus MC-ICP-MS | This study | |||||
| Allende | ~100 | 0.10 | 0.02 | 0.96 | 0.05 | 5 | Neptune Plus MC-ICP-MS | This study | |||||
| Allende | ~50 | 0.42 | 0.10 | 0.06 | 1.10 | 0.08 | 8 | Neptune Plus MC-ICP-MS | (Zhu et al., 2020b) | ||||
| Allende | 0.42 | 0.16 | 0.06 | 0.88 | 0.17 | 15 | Triton TIMS (Total Evaporation) | (Zhu et al., 2020a) | |||||
| Allende | 81 | 0.45 | 0.04 | 0.06 | 0.86 | 0.09 | 3-5 | Triton TIMS | (Trinquier et al., 2007, 2008a, 2008b) | ||||
| Allende | ~100 | 0.14 | 0.04 | 0.98 | 0.14 | 6-10 | Triton TIMS | (Qin et al., 2010) | |||||
| Allende | ~100 | 0.13 | 0.05 | 0.92 | 0.13 | 6-10 | Triton TIMS | (Qin et al., 2010) | |||||
| Allende | 0.43 | 0.14 | 0.11 | 5 | Nu Plasma HR MC-ICP-MS | (Moynier et al., 2007) | |||||||
| Allende | 2380 | 0.43 | 0.10 | 0.09 | 0.85 | 0.17 | 70 | VG-54E and Micromass Sector 54 TIMS | (Shukolyukov and Lugmair, 2006) | ||||
| Allende | 44.3 | 0.51 | 0.07 | 0.08 | 1.24 | 0.24 | n.d. | Triton TIMS | (Kadlag et al., 2019) | ||||
| Allende | 0.86 | 0.09 | 4 | Triton Plus TIMS | (Williams et al., 2020) | ||||||||
| Averaged value | 0.44 | 0.11 | 0.02 | 0.95 | 0.06 | ||||||||
| Vigarano | 21.0 | 0.42 | 0.08 | 0.02 | 0.84 | 0.04 | 5 | Neptune Plus MC-ICP-MS | This study | ||||
| Vigarano | ~100 | 0.57 | 0.22 | 0.07 | 0.91 | 0.12 | 6-10 | Triton TIMS | (Qin et al., 2010) | ||||
| Vigarano | ~100 | 0.14 | 0.05 | 0.82 | 0.13 | 6-10 | Triton TIMS | (Qin et al., 2010) | |||||
| Vigarano | 0.23 | 0.08 | 12 | Nu Plasma HR MC-ICP-MS | (Moynier et al. 2007) | ||||||||
| Averaged value | 0.49 | 0.17 | 0.07 | 0.86 | 0.05 | ||||||||
| Leoville | 24.8 | 0.41 | 0.08 | 0.04 | 0.81 | 0.10 | 5 | Neptune Plus MC-ICP-MS | This study | ||||
| Leoville | ~100 | 0.46 | 0.12 | 0.04 | 0.71 | 0.15 | 6-10 | Triton TIMS | (Qin et al., 2010) | ||||
| Averaged value | 0.43 | 0.10 | 0.04 | 0.76 | 0.10 | ||||||||
| Renazzo | 12 | 0.54 | 0.20 | 0.10 | 1.30 | 0.21 | 3-5 | Triton TIMS | (Trinquier et al., 2007, 2008a, 2008b) | ||||
| Renazzo | 1.22 | 0.10 | 4 | Triton Plus TIMS | (Sanborn et al., 2019) | ||||||||
| Averaged value | 0.54 | 0.20 | 0.10 | 1.26 | 0.08 | ||||||||
| Jbilet Winselwan | 19.5 | 0.56 | 0.12 | 0.02 | 0.82 | 0.04 | 5 | Neptune Plus MC-ICP-MS | This study | ||||
| Jbilet Winselwan | ~150 | 0.63 | 0.19 | 0.06 | 1.01 | 0.12 | 16 | Triton TIMS (Total Evaporation) | (van Kooten et al. 2020) | ||||
| Averaged value | 0.59 | 0.16 | 0.07 | 0.92 | 0.18 | ||||||||
| Murchison | 0.64 | 0.27 | 0.06 | 1.01 | 0.05 | 3-5 | Triton TIMS | (Trinquier et al., 2007, 2008a, 2008b) | |||||
| Murchison | ~150 | 0.67 | 0.19 | 0.04 | 0.93 | 0.07 | 16 | Triton TIMS (Total Evaporation) | (van Kooten et al. 2020) | ||||
| Murchison | ~100 | 0.60 | 0.17 | 0.08 | 0.97 | 0.20 | 6-10 | Triton TIMS | (Qin et al., 2010) | ||||
| Murchison | 0.16 | 0.04 | 0.89 | 0.08 | 4 | Triton Plus TIMS | (Jenniskens et al., 2012) | ||||||
| Averaged value | 0.64 | 0.20 | 0.05 | 0.95 | 0.05 | ||||||||
| Murray | 101 | 0.64 | 0.27 | 0.09 | 1.13 | 0.21 | 93 | VG-54E and Micromass Sector 54 TIMS | (Shukolyukov and Lugmair, 2006) | ||||
| Murray | ~150 | 0.63 | 0.18 | 0.03 | 0.85 | 0.10 | 16 | Triton TIMS (Total Evaporation) | (van Kooten et al. 2020) | ||||
| Averaged value | 0.63 | 0.23 | 0.09 | 0.99 | 0.27 | ||||||||
| Kainsaz | 1030 | 0.54 | 0.20 | 0.10 | 1.02 | 0.24 | 91 | VG-54E and Micromass Sector 54 TIMS | (Shukolyukov and Lugmair, 2006) | ||||
| Kainsaz | ~100 | 0.52 | 0.13 | 0.06 | 0.87 | 0.18 | 6-10 | Triton TIMS | (Qin et al., 2010) | ||||
| Averaged value | 0.53 | 0.17 | 0.07 | 0.95 | 0.15 | ||||||||
| Lance’ | 8 | 0.47 | -0.04 | 0.07 | 0.57 | 0.11 | 3-5 | Triton TIMS | (Trinquier et al., 2007, 2008a, 2008b) | ||||
| Lance’ | 0.50 | 0.20 | 0.09 | 15 | Nu Plasma HR MC-ICP-MS | (Moynier et al. 2007) | |||||||
| Karoonda | 33.9 | 0.41 | 0.04 | 0.02 | 0.50 | 0.09 | 5 | Neptune Plus MC-ICP-MS | This study | ||||
| Karoonda | 25 | 0.48 | 0.14 | 0.06 | 0.63 | 0.09 | 3-5 | Triton TIMS | (Trinquier et al., 2007, 2008a, 2008b) | ||||
| Averaged value | 0.44 | 0.09 | 0.10 | 0.57 | 0.13 | ||||||||
| EET 92002 | 34.6 | 0.41 | 0.10 | 0.04 | 0.52 | 0.09 | 5 | Neptune Plus MC-ICP-MS | This study | ||||
| EET 92002 | ~100 | 0.46 | 0.02 | 0.05 | 0.33 | 0.12 | 6-10 | Triton TIMS | (Qin et al., 2010) | ||||
| Averaged value | 0.43 | 0.06 | 0.08 | 0.43 | 0.19 | ||||||||
| Qingzhen | 0.12 | 0.04 | 0.00 | 0.05 | 5 | Triton TIMS | (Mougel et al., 2018) | ||||||
| Qingzhen | 260 | 0.72 | 0.20 | 0.06 | 3-5 | Triton TIMS | (Trinquier et al., 2007, 2008a, 2008b) | ||||||
| Qingzhen | 260 | 0.72 | 0.17 | 0.06 | -0.02 | 0.08 | 3-5 | Triton TIMS | (Trinquier et al., 2007, 2008a, 2008b) | ||||
| Average value | 0.72 | 0.16 | 0.05 | -0.01 | 0.02 | Triton TIMS | (Trinquier et al., 2007, 2008a, 2008b) | ||||||
| Kota-Kota | n.d. | 0.11 | 0.04 | 0.00 | 0.08 | 6 | Triton TIMS | (Mougel et al., 2018) | |||||
| Kota-Kota | 0.69 | 0.18 | 0.06 | -0.02 | 0.21 | 3-5 | Triton TIMS | (Trinquier et al., 2007, 2008a, 2008b) | |||||
| Kota-Kota | 0.69 | 0.17 | 0.06 | 0.04 | 0.07 | 3-5 | Triton TIMS | (Trinquier et al., 2007, 2008a, 2008b) | |||||
| Averaged value | 0.69 | 0.15 | 0.04 | 0.01 | 0.03 | ||||||||
| Abee | 0.05 | 0.04 | -0.02 | 0.08 | 4 | Triton TIMS | (Mougel et al., 2018) | ||||||
| Abee | 18 | 0.93 | 0.26 | 0.08 | -0.06 | 0.12 | 3-5 | Triton TIMS | (Trinquier et al., 2007, 2008a, 2008b) | ||||
| Averaged value | 0.93 | 0.16 | 0.21 | -0.04 | 0.04 | ||||||||
| SAH 97096 | 33.2 | 0.65 | 0.25 | 0.03 | 0.17 | 0.08 | 5 | Neptune Plus MC-ICP-MS | This study | ||||
| SAH 97096 | 0.65 | 0.19 | 0.04 | -0.01 | 0.14 | 14 | Triton TIMS (Total Evaporation) | (Zhu et al., 2020a) | |||||
| Averaged value | 0.65 | 0.22 | 0.06 | 0.08 | 0.18 | ||||||||
| MIL 05082 | 96.4* | 0.37 | 0.20 | 0.01 | 1.50 | 0.09 | 5 | Neptune Plus MC-ICP-MS | This study | ||||
| QC 001 | 39.8 | 0.47 | 0.19 | 0.04 | 1.45 | 0.06 | 5 | Neptune Plus MC-ICP-MS | This study | ||||
| HaH 237 | 32.9 | 0.09 | 0.05 | 0.03 | 1.42 | 0.04 | 5 | Neptune Plus MC-ICP-MS | This study | ||||
| HaH 237 | 679 | 0.08 | -0.15 | 0.09 | 0.87 | 0.19 | 148 | VG-54E and Micromass Sector 54 TIMS | (Shukolyukov and Lugmair,2006) | ||||
| Bencubbin (silicate<metal) | 0.04 | -0.05 | 0.06 | 1.11 | 0.09 | 3-5 | Triton TIMS | (Trinquier et al., 2007, 2008a, 2008b) | |||||
| Bencubbin(metal<silicate) | 0.48 | 0.12 | 0.09 | 1.13 | 0.09 | 3-5 | Triton TIMS | (Trinquier et al., 2007, 2008a, 2008b) | |||||
| Gujba metal chondrule | 0.20 | -0.03 | 0.13 | 1.07 | 0.27 | 3-5 | Triton TIMS | (Trinquier et al., 2007, 2008a, 2008b) | |||||
Note: The error for Mn/Cr ratios is 5%.The mass marked*meansachip.The uncertainty of average values are 95% confidence intervals.
Table 4. The Mn/Cr ratio, and Cr and O isotope data for all chondrites.
| Carbonaceous Chondrites | Fall/Find | Mass (mg) | Type | 55Mn/52Cr | ε53Cr | Error | ε54Cr | Error | N/[ref.] | Δ17O | Error | [ref.] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Orgueil | fall | 22.6 | CI1 | 0.85 | 0.29 | 0.07 | 1.60 | 0.10 | [This study, 2, 4, 5, 21, 30, 31, 32] | 0.39 | 0.08 | [9] |
| Ivuna | fall | CI1 | 0.84 | 0.28 | 0.10 | 1.56 | 0.15 | [1, 22, 29, 30, 32] | 0.47 | 0.08 | [9] | |
| CI average | 0.84 | 0.28 | 0.01 | 1.56 | 0.05 | 2SD | ||||||
| PCA 91467* | find | 28.5 | CH3 | 0.36 | 0.13 | 0.00* | 1.50 | 0.08 | 5 | -1.47 | 0.08 | [9] |
| A-881020* | find | 40.5 | CH3 | 0.33 | 0.11 | 0.03 | 1.49 | 0.06 | 5 | |||
| CH average | 0.34 | 0.12 | 0.03 | 1.50 | 0.01 | 2SD | ||||||
| MIL 05082* | find | 96.4 | CB3 | 0.37 | 0.20 | 0.01 | 1.50 | 0.09 | 5 | |||
| QC 001* | find | 39.8 | CBa3 | 0.47 | 0.19 | 0.04 | 1.45 | 0.06 | 5 | |||
| Bencubbin* | find | CBa3 | 1.12 | 0.03 | [2] | -1.75 | 0.08 | [9] | ||||
| Gujba* | fall | CBa3 | 1.29 | 0.07 | [3] | -2.38 | 0.08 | [10] | ||||
| HaH 237* | find | 32.9 | CBb3 | 0.09 | 0.05 | 0.03 | 1.42 | 0.04 | 5 | -0.77 | 0.08 | [11] |
| CB average | 0.42 | 0.20 | 0.01 | 1.36 | 0.30 | 2SD | ||||||
| GRO 95577 | find | 20.5 | CR1 | 0.51 | 0.15 | 0.02 | 1.25 | 0.06 | 5 | -0.45 | 0.08 | [9] |
| Al Rais | fall | 23.2 | CR2-an | 0.60 | 0.19 | 0.01 | 1.24 | 0.11 | 5 | -1.01 | 0.08 | [9] |
| Renazzo | fall | CR2 | 0.54 | 0.20 | 0.10 | 1.26 | 0.08 | [2, 4, 24] | -0.96 | 0.08 | [9] | |
| GRA 06100 | find | CR2 | 0.58 | 0.25 | 0.05 | 1.32 | 0.11 | [5] | -1.80 | 0.03 | [20] | |
| NWA 6043 | find | CR2 | 0.58 | 0.07 | 0.07 | 1.24 | 0.10 | [22, 23] | ||||
| EET 92161 | find | CR2 | 0.50 | 0.21 | 0.04 | 1.19 | 0.12 | [22, 23] | ||||
| NWA 7837 | find | CR2 | 0.39 | 0.02 | 0.04 | 1.06 | 0.08 | [22, 23] | ||||
| GRA 95229 | find | CR2 | 1.18 | 0.07 | [29] | -2.19 | 0.01 | [29] | ||||
| QUE 99177 | find | CR2 | 1.43 | 0.12 | [29] | -2.89 | 0.12 | [29] | ||||
| LAP 02342 | find | CR2 | 1.49 | 0.11 | [29] | -2.45 | 0.06 | [29] | ||||
| NWA 6921 | find | CR6 | 1.32 | 0.09 | [24,29] | -1.74 | 0.01 | [29] | ||||
| NWA 7317 | find | CR6 | 1.32 | 0.09 | [24] | |||||||
| CR average | 0.53 | 0.16 | 0.16 | 1.28 | 0.23 | 2SD | ||||||
| SCO 06043 | find | 21.7 | CM1 | 0.60 | 0.22 | 0.02 | 1.13 | 0.12 | 5 | |||
| Nogoya | fall | 24.3 | CM2 | 0.59 | 0.18 | 0.05 | 0.76 | 0.04 | 5 | -2.00 | 0.08 | [9] |
| Banten | fall | 21.7 | CM2 | 0.58 | 0.12 | 0.03 | 0.86 | 0.05 | 5 | -2.97 | 0.08 | [9] |
| Aguas Zarcas | fall | 87.7 | CM2 | 0.60 | 0.15 | 0.03 | 0.86 | 0.03 | 5 | -2.78 | 0.20 | [18] |
| Jbilet Winselwan | find | 19.5 | CM2 | 0.59 | 0.16 | 0.10 | 0.92 | 0.18 | [This study, 23] | -4.03 | 0.55 | [18] |
| Paris | find | CM2 | 0.62 | 0.16 | 0.05 | 0.93 | 0.09 | [6] | -3.39 | 0.39 | [19] | |
| NWA 8157 | find | CM2 | 0.62 | 0.20 | 0.11 | 1.01 | 0.18 | [6] | -4.05 | 0.55 | [18] | |
| Murchison | fall | CM2 | 0.64 | 0.20 | 0.11 | 0.95 | 0.04 | [2, 4, 5, 23, 28] | -2.60 | 0.08 | [12] | |
| Mighei | fall | CM2 | 0.63 | 0.18 | 0.03 | 0.74 | 0.10 | [23] | -2.50 | 0.08 | [9] | |
| Cold Bokkeveld | fall | CM2 | 0.65 | 0.07 | 0.03 | 0.81 | 0.12 | [23] | -2.45 | 0.08 | [9] | |
| Murray | fall | CM2 | 0.63 | 0.23 | 0.09 | 0.99 | 0.27 | [23, 30] | -3.07 | 0.08 | [9] | |
| Maribo | fall | CM2 | 0.65 | 0.29 | 0.04 | 1.13 | 0.15 | [23] | ||||
| Diepenveen | fall | CM2-an | 0.13 | 0.05 | 0.85 | 0.10 | [27] | |||||
| CM average | 0.62 | 0.17 | 0.11 | 0.92 | 0.26 | 2SD | ||||||
| Allende | fall | ~100 | CV3-oxA | 0.44 | 0.11 | 0.02 | 0.95 | 0.06 | [This study, 2, 4, 5, 25, 26, 28, 30, 31, 32] | -3.62 | 0.06 | [13] |
| Bali | fall | 42.3 | CV3-oxB | 0.45 | 0.13 | 0.04 | 1.10 | 0.06 | 5 | -3.30 | 0.17 | [13] |
| Mokoia | fall | 20.5 | CV3-oxB | 0.45 | 0.11 | 0.04 | 1.00 | 0.01 | 5 | -3.18 | 0.07 | [13] |
| Leoville | find | 24.8 | CV3-red | 0.43 | 0.10 | 0.04 | 0.76 | 0.10 | [This study, 5] | -4.69 | 0.17 | [13] |
| Vigarano | fall | 21.0 | CV3-red | 0.49 | 0.13 | 0.10 | 0.85 | 0.02 | [This study, 5, 31] | -4.25 | 0.03 | [13] |
| Kaba | fall | 19.7 | CV3-oxB | 0.43 | 0.08 | 0.05 | 0.70 | 0.07 | 5 | -3.43 | 0.31 | [13] |
| CV average | 0.45 | 0.11 | 0.04 | 0.89 | 0.30 | 2SD | ||||||
| MIL 07193 | find | 20.9 | CO3 | 0.48 | 0.16 | 0.02 | 1.22 | 0.04 | 5 | |||
| DOM 10104 | find | 24.7 | CO3 | 0.48 | 0.09 | 0.03 | 0.80 | 0.06 | 5 | |||
| Ornans | fall | 66.2 | CO3 | 0.50 | 0.12 | 0.01 | 0.90 | 0.03 | 5 | -4.45 | 0.08 | [9] |
| Felix | fall | CO3 | 0.47 | 0.07 | 0.06 | 0.63 | 0.09 | [2, 4] | -4.59 | 0.08 | [9] | |
| Kainsaz | fall | CO3 | 0.53 | 0.17 | 0.07 | 0.95 | 0.15 | [5, 31] | -4.72 | 0.08 | [9] | |
| CO average | 0.49 | 0.11 | 0.07 | 0.90 | 0.43 | 2SD | ||||||
| ALH 85002 | find | 45.8 | CK4 | 0.42 | 0.06 | 0.02 | 0.46 | 0.05 | 5 | |||
| Karoonda | fall | 33.9 | CK4 | 0.44 | 0.09 | 0.10 | 0.57 | 0.13 | [This study, 2, 4] | -4.55 | 0.00 | [13] |
| EET 92002 | find | 34.6 | CK5 | 0.43 | 0.06 | 0.08 | 0.43 | 0.19 | [This study, 5] | |||
| LEW 87009 | find | 43.8 | CK6 | 0.44 | 0.08 | 0.04 | 0.58 | 0.05 | 5 | -4.32 | 0.08 | [9] |
| CK average | 0.43 | 0.07 | 0.03 | 0.51 | 0.15 | 2SD | ||||||
| Ordinary Chondrites | ||||||||||||
| Roosevelt | find | H3.4 | 0.65 | 0.17 | 0.02 | -0.44 | 0.03 | [7] | ||||
| Brownsflied | find | H3.7 | 0.67 | 0.18 | 0.02 | -0.44 | 0.03 | [7] | ||||
| Ochansk | fall | H4 | 0.64 | 0.15 | 0.02 | -0.40 | 0.03 | [7] | 0.82 | 0.08 | [14] | |
| LAP 03601 | find | H4 | 0.78 | 0.21 | 0.06 | -0.28 | 0.11 | [5] | ||||
| Ste. Marguerite | fall | H4 | 0.63 | 0.13 | 0.06 | -0.39 | 0.07 | [2, 4] | ||||
| Beaver Creek | fall | H4 | 0.70 | 0.17 | 0.02 | -0.40 | 0.04 | [7] | 0.76 | 0.08 | [14] | |
| Bath | fall | H4 | 0.68 | 0.17 | 0.02 | -0.36 | 0.04 | [7] | 0.71 | 0.08 | [14] | |
| Menow | fall | H4 | 0.66 | 0.12 | 0.02 | -0.43 | 0.03 | [7] | ||||
| Forest city | fall | H5 | 0.71 | 0.18 | 0.02 | -0.36 | 0.03 | [7] | 0.75 | 0.08 | [14] | |
| Estacado | find | H6 | 0.67 | 0.15 | 0.02 | -0.35 | 0.06 | [7] | ||||
| Aarhus | fall | H6 | 0.68 | 0.17 | 0.01 | -0.41 | 0.03 | [7] | ||||
| Kernouve | fall | H6 | 0.72 | 0.19 | 0.06 | -0.37 | 0.07 | [2, 4] | ||||
| Portales Valley | fall | H6/7 | 0.74 | 0.19 | 0.02 | -0.37 | 0.04 | [7] | ||||
| H average | 0.69 | 0.17 | 0.05 | -0.38 | 0.09 | 2SD | ||||||
| QUE 97008 | find | L3 | 0.72 | 0.17 | 0.06 | -0.42 | 0.14 | [5] | ||||
| Bjurböle | fall | L4 | 0.81 | 0.20 | 0.06 | [4] | 1.00 | 0.08 | [14] | |||
| Knyahinya | fall | L5 | 0.70 | 0.15 | 0.06 | -0.38 | 0.08 | [2, 4] | 1.05 | 0.08 | [14] | |
| Holbrook | fall | L6 | 0.80 | 0.23 | 0.06 | [4] | ||||||
| L average | 0.76 | 0.19 | 0.07 | -0.40 | 0.06 | 2SD | ||||||
| Chainpur | fall | LL3-4 | 0.85 | 0.24 | 0.06 | -0.47 | 0.07 | [2, 4] | ||||
| Soko-Banja | fall | LL4 | 0.82 | 0.34 | 0.06 | [4] | 1.32 | 0.08 | [14] | |||
| GRO 95552 | find | LL4 | 0.78 | 0.19 | 0.04 | -0.33 | 0.10 | [5] | ||||
| Olivenza | fall | LL5 | 0.76 | 0.23 | 0.06 | [4] | 1.11 | 0.08 | [14] | |||
| Guidder | fall | LL5 | 0.99 | 0.21 | 0.06 | [4] | 1.19 | 0.08 | [14] | |||
| Saint-Se’verin | fall | LL6 | 0.77 | 0.28 | 0.06 | -0.41 | 0.10 | [2, 4] | 1.16 | 0.08 | [14] | |
| LL average | 0.83 | 0.25 | 0.04 | -0.40 | 0.14 | 2SD | ||||||
| OC average | 0.74 | 0.19 | 0.10 | -0.39 | 0.09 | 2SD | ||||||
| Enstatite Chondrites | ||||||||||||
| SAH 97096 | find | 33.2 | EH3 | 0.65 | 0.22 | 0.06 | 0.08 | 0.18 | [This study, 26] | -0.07 | 0.02 | [17] |
| Qingzhen | fall | EH3 | 0.72 | 0.15 | 0.07 | -0.01 | 0.02 | [2,4,8] | -0.03 | 0.08 | [15] | |
| Kota-Kota | find | EH3 | 0.69 | 0.14 | 0.07 | 0.01 | 0.01 | [2,4,8] | -0.15 | 0.08 | [15] | |
| ALHA 77295 | find | EH3 | 0.71 | 0.14 | 0.06 | 0.05 | 0.14 | [5] | ||||
| Abee | fall | EH4 | 0.93 | 0.16 | 0.21 | -0.04 | 0.04 | [2,4,8] | 0.19 | 0.19 | [17] | |
| Indarch | fall | EH4 | 0.91 | 0.21 | 0.07 | 0.05 | 0.14 | [5] | 0.12 | 0.08 | [15] | |
| EH average | 0.77 | 0.17 | 0.07 | 0.02 | 0.09 | 2SD | ||||||
| MAC 88136 | find | EL3 | 0.72 | 0.15 | 0.03 | 0.02 | 0.09 | [5] | -0.11 | 0.04 | [17] | |
| MAC 88184 | find | EL3 | 0.20 | 0.03 | 0.11 | 0.07 | [8] | |||||
| Hvittis | fall | EL6 | 0.67 | 0.14 | 0.07 | -0.01 | 0.17 | [2,4] | 0.07 | 0.08 | [15] | |
| Pillistfer | fall | EL6 | 0.59 | 0.15 | 0.05 | 0.09 | 0.08 | [2,4] | 0.02 | 0.08 | [15] | |
| LON 94100 | find | EL6 | 0.56 | 0.17 | 0.04 | -0.02 | 0.14 | [5] | ||||
| Eagle | fall | EL6 | 0.14 | 0.05 | -0.07 | 0.07 | [8] | |||||
| EL average | 0.63 | 0.16 | 0.05 | 0.02 | 0.14 | 2SD | ||||||
| EC average | 0.71 | 0.16 | 0.06 | 0.02 | 0.11 | 2SD | ||||||
Note: The references: [1] Schiller et al. (2014), [2] Trinquier et al. (2007), [3] Yamashita etal. (2010), [4] Trinquier et al. (2008a), [5]Qin etal. (2010), [6] Göpel et al. (2015), [7] Pedersen et al. (2019), [8] Mougel etal. (2018), [9] Clayton and Mayeda (1999), [10] Rubin et al. (2001), [11] Weisberg et al. (2001), [12] Clayton and Mayeda (1984), [13] Greenwood et al. (2010), [14] Clayton et al. (1991), [1 5] Clayton et al. (1984),[16] Greenwood etal. (2017),[17] Newton et al. (2000), [1 8] Meteoritical Bulletin, [19] Hewins et al. (2014), [20] Schrader et al. (2011),[21] Petitat et al. (2011),[22] Van Kooten et al. (2016),[23] van Kooten et al. (2020), [24] Sanborn et al. (2019), [25] Zhu etal. (2020b), [26] Zhu et al. (2020a), [27] Langbroek etal. (2019), [28] Jenniskens et al. (2012), [29] Williams et al. (2020), [30] Shukolyukov and Lugmair (2006), [31] Moynier et al. (2007) and [32] Kadlag et al. (2019).
The errors for 55Mn/52Cr ratios are 5%. The 55Mn/52Cr for H chondrite samples in [7] Pedersen et al. (2019) are re-measured (same solution) by MC-ICP-MS in this study.
The Fe/Cr (atom) ratios for CB and CH chondrites: PCA 91467, 97; A-881020, 115; QC 001, 25; HaH 237, 235
The errors for O isotope data in [18] are 1SD of multiple O isotope data, and in [10] and [11] are quoted as 0.08 from [9].
The ε53Cr 2SE uncertainty for PCA 91467 is less than 0.004. The CB and CH samples in this study, including PCA 91467, MIL 05082, A-881020, QC 001 and HaH 237 may not represent the bulk composition of the parent chondrites given the clear evidence for sample heterogeneity in CB and CH chondrites and the fact that the mass of our samples is relatively small. The ε54Cr data for Bencubbin is the average (with 2SD uncertainty) of two data (silicate > metal and silicate < metal) in Trinquier et al. (2007). The ε54Cr data for Gujba is the average (with 2SD uncertainty) of the data for chondrules and metals in Yamashita et al. (2010).
Table 5. The Cr and O isotope compositions of various Solar System materials not analyzed here (including: Rumuruti chondrites, terrestrial planets and achondrite parent bodies).
| Meteorites/Planets | ε54Cr | Error (2SD) | N | Ref. | Δ17O | error | N | Ref. |
|---|---|---|---|---|---|---|---|---|
| Rumuruti (R) chondrites | –0.06 | 0.08 | 12 | [35] | 2.72 | 0.31 | 24 | [36] |
| Earth | 0.09 | 0.12 | 15 | [2, 8, 25, this study] | -0.01 | 0.01 | 14 | [26] |
| Moon | 0.09 | 0.08 | / | [8] | –0.01 | 0.02 | 22 | [26] |
| Aubrites | –0.16 | 0.19 | 1 | [2] | –0.01 | 0.11 | 13 | [26] |
| Mars (SNC meteorites) | –0.17 | 0.15 | 18 | [2, 33] | 0.28 | 0.08 | [34] | |
| Angrite parent body (APB) | –0.42 | 0.13 | 8 | [2, 22] | –0.07 | 0.01 | 5 | [26] |
| Brachinites | –0.44 | 0.23 | 2 | [29] | –0.17 | 0.09 | 2 | [29] |
| Winonaites | –0.53 | 0.02 | 3 | [23] | –0.51 | 0.08 | 16 | [26] |
| Acapulcoite-lodranite Clan | –0.61 | 0.19 | 6 | [23] | –1.12 | 0.36 | 23 | [26] |
| Vesta (HED meteorites) | –0.73 | 0.08 | 9 | [2] | –0.24 | 0.02 | 105 | [26] |
| Ureilite parent body (UPB) | –0.95 | 0.15 | 18 | [24, 25] | –0.96 | 1.00 | 42 | [26] |
Note: The reference sources are: [2] Trinquier et al. (2007), [8] Mougel et al. (2018), [22] Zhu et al. (2019b), [23]Lietal. (2018), [24] Zhu etal. (2020b), [25] Yamakawa et al. (2010), [26] Greenwood et al. (2017), [25] Zhu et al. (2020b), [29] Williams et al. (2020), [33] Kruijer et al. (2020), [34] Ireland etal. (2020), [35] Zhu et al. (2021) and [36] Bischoff et al. (2011). The ε54Cr values are calculated by mean average. The O isotope data did not consider Cumberland falls for aubrites and some isotopically anomalous eucrites for Vesta. The N (/) of ε54Cr value of Moon means it was modeled by the correlation ofthe ε53Cr and ε54Cr oflunar samples that had been influenced by cosmogenic effects. The ε54Cr for Nakhla (Mars) is chosen from [2], due to the smaller uncertainty.
Table 6. Cr isotope data for artificial standards and terrestrial samples.
| Sample Name | Petrology | ε53Cr | 2se | ε54Cr | 2se | N/[ref.] |
|---|---|---|---|---|---|---|
| NIST 3112a | Artificial standard | 0.04 | 0.03 | 0.03 | 0.05 | 5 |
| SCP-Cr | Artificial standard | 0.02 | 0.02 | 0.05 | 0.08 | 5 |
| DTS-1 | Dunite | 0.05 | 0.03 | 0.16 | 0.06 | 5, 2SD |
| DTS-2b | Dunite | 0.04 | 0.01 | 0.08 | 0.06 | [1] |
| PCC-1 | Peridotite | 0.05 | 0.03 | 0.10 | 0.07 | [25] |
| KOLA15-UB | Peridotite | 0.00 | 0.03 | 0.05 | 0.06 | [8] |
| BM31 | Peridotite | 0.07 | 0.04 | 0.13 | 0.08 | [8] |
| BM23 | Peridotite | -0.01 | 0.05 | 0.03 | 0.08 | [8] |
| Tibet chromite | Chromite | 0.03 | 0.07 | 0.08 | 0.08 | [2, 4] |
| Deccan basalt | Basalt | 0.03 | 0.08 | 0.08 | 0.15 | [2, 4] |
| Erta Ale tholeite | Basalt | -0.02 | 0.06 | -0.02 | 0.08 | [2, 4] |
| 10PUB22-07 | Basalt | 0.08 | 0.05 | 0.19 | 0.07 | [8] |
| KBD408729 | Basalt | 0.07 | 0.03 | 0.14 | 0.07 | [8] |
| NIST 688 | Basalt | 0.06 | 0.04 | 0.11 | 0.07 | [8] |
| CV-SN-98-19 | Basalt | 0.01 | 0.05 | 0.12 | 0.11 | [8] |
| BE-N | Basalt | 0.12 | 0.03 | 0.17 | 0.07 | [8] |
| BHVO-2 | Basalt | 0.02 | 0.03 | 0.07 | 0.07 | [8] |
| Average | 0.04 | 0.02 | 0.09 | 0.03 | 2SE, N =15 | |
| 0.08 | 0.12 | 2SD, N =15 | ||||
Note: The average data for terrestrial samples (N = 15) do not include the two artificial standards. The reference sources are [1] Schiller et al.(2014), [2] Trinquier et al. (2007), [4] Trinquier et al. (2008a), [8]Mougel et al. (2018) and [25] Zhu et al. (2020b).
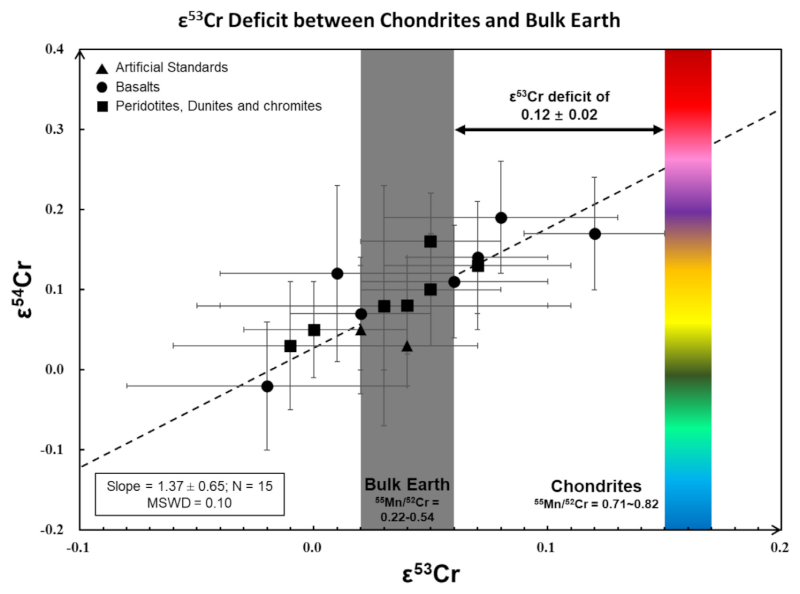
The higher precision Cr isotope data (than those typically obtained by TIMS) for DTS-1 (USGS standards), Allende and as well as the Orgueil meteorites reported here are consistent with most previously reported values (Mougel et al., 2018; Qin et al., 2010; Schiller et al., 2014; Trinquier et al., 2007; Trinquier et al., 2008b; Zhu et al.,2019a; Zhu et al., 2019b; Zhu et al., 2020b), providing confidence in the accuracy of our protocol. Based on multiple individually processed aliquots of Allende (5), DTS-1 (5) and Orgueil digestions (2), we estimate the external reproducibility of our data to be better than 0.04 and 0.07 for ε53Cr and ε54Cr, respectively (Table 2), which is consistent with the estimates from Schiller et al. (2014). The doping tests show that isobaric interferences do not result in resolvable effects when Fe, V and Ti interferences represent less than ~0.1% (2505 ppm to 54Cr), ~2.5% (1438 ppm to 50Cr) and ~1% (14269 ppm to 50Cr), respectively (Table 2). Finally, Na and K have very limited effects even when their concentrations are sub similar to Cr in the analyzed solutions, and they cause no drift in the Cr mass fractionation.
There are no resolvable mass-independent Cr isotope shifts between chondrite falls and finds from the same group, implying that the mass-independent Cr isotope compositions preserved in the meteorites is robust against limited terrestrial weathering. As such, we conclude that the Cr isotope data reported here is accurate within the reported uncertainties.
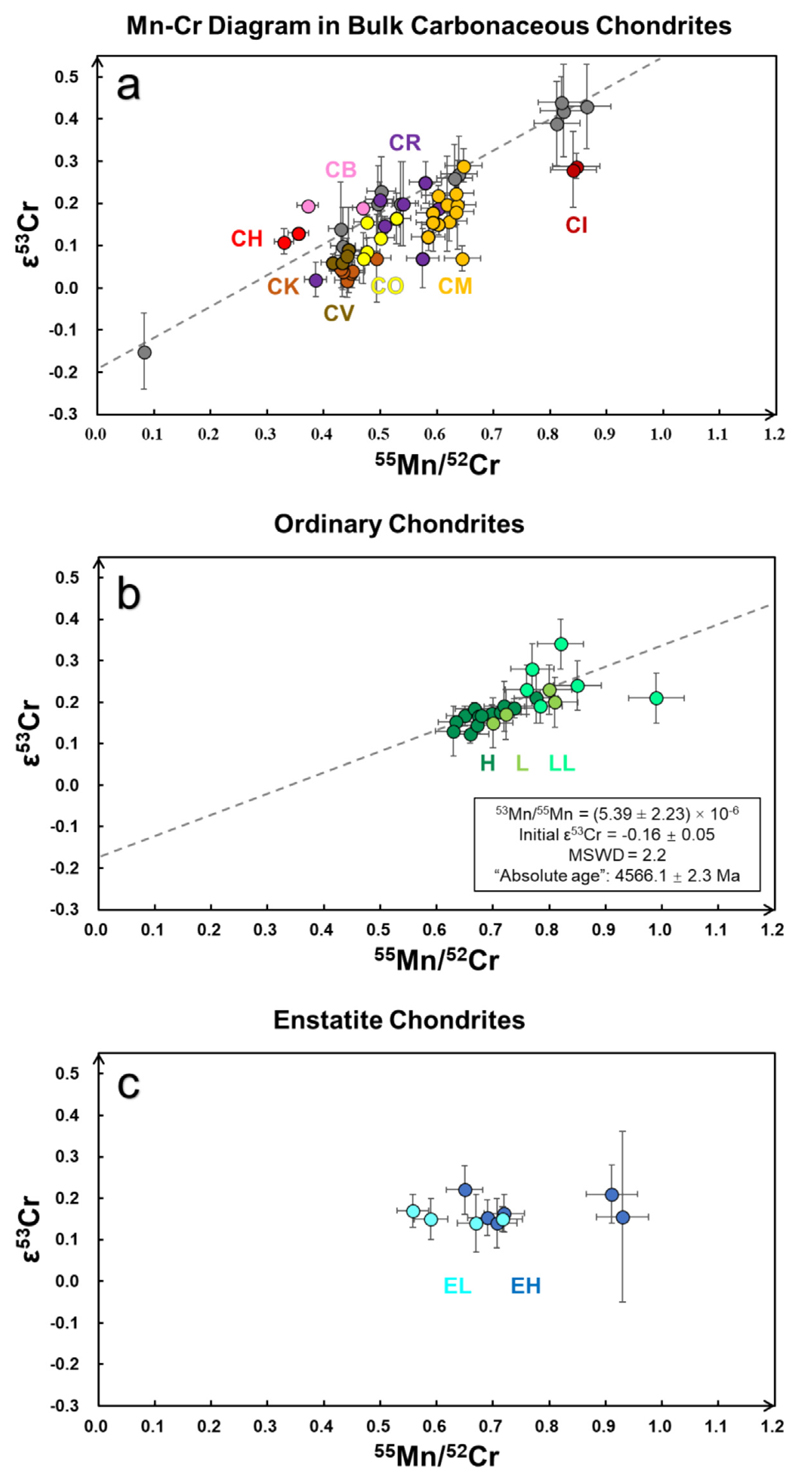
The ε54Cr values of the studied CC groups decrease in the following sequence (mean ± 2SD): CI (1.56 ± 0.07) >CH (1.50 ± 0.07) ≥ CB (1.36 ± 0.30) ≥ CR (1.28 ± 0.23) >CM (0.92 ± 0.26) ≈ CV (0.89 ± 0.30) ≈ CO (0.90 ±0.43) ≥ CK (0.51 ±0.15) (Figs. 1 and 2). Here, the calculated 2SD uncertainty for grouping CI and CH chondrites are 0.05 and 0.01 respectively (Table 4), which are less than the external uncertainty of 0.07 in this study. Thus, we quote the external reproducibility of 0.07 for the ε54Cr uncertainty of CI and CH chondrites rather than the calculated 2SD. CC reservoirs have a ε54Cr + 0.3 higher than Non-CC reservoirs as terrestrial samples and ECs have ε54Cr values in the range 0-0.2. Resolved intragroup ε54Cr variability exists within CB, CM, CV and CO chondrite data, whereas no significant intra-group ε54Cr variability was found amongst CI, CH, CR and CK chondrite data at the level of our precision. The ε54Cr values are not correlated with the degree of aqueous alteration (CR2 to CR1, CM2 to CM1) and, at least amongst the CK chondrites, they are also not correlated with the extent of thermal metamorphism (CK4 to CK6) (Table 3). Furthermore, no systematic correlation between ε53Cr and ε54Cr values is observed among the CCs, contradicting the claim of a correlation suggested by Shukolyukov and Lugmair (2006).
Fig. 1.
The ε54Cr variations in different groups of chondrites (the data and reference sources can be found in Tables 4–6), which occur in the order CI = CH ≥ CB ≥ CR > CM ≈ CV ≈ CO ≥CK > EC ≈ RC > OC. The color shades indicate the 2SD variation of different chondrite groups (the blue and green shades show the 2SD uncertainty of all the ECs and OCs respectively; we use the external reproducibility 0.07 to represent the grouping uncertainties ofCIs and CHs since the 2SD uncertainty are less than 0.07. The middle bold dashed line at ε54Cr = +0.3 is the ε54Cr boundary for non-carbonaceous chondrite (NC) and carbonaceous chondrite (CC) reservoirs. All CK, RC, OC and EC groups have homogeneous ε54Cr within their respective groups. The CV and CK chondrites have distinct ε54Cr values from one another, whereas the ε54Cr for CV, CM and CO chondrites overlap. EC and RC have similar ε54Cr features with the Earth-Moon system.
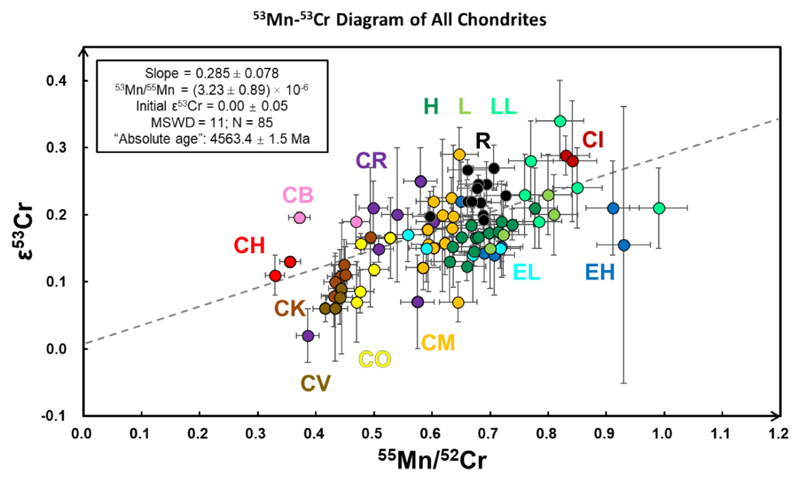
The carbonaceous chondrites have decreasing 55Mn/52Cr ratios in the order: CI > CM ≥ CR ≥ CO > CV = CK ≥ CB = CH (Fig. 3a). Despite these differences, the CCs with the highest and lowest 55Mn/52Cr ratios, the CI and CB-CH chondrites, respectively, have the highest ε54Cr values (Fig. 1). More importantly, when considering all CC groups there is no systematic increase in ε53Cr values with increasing 55Mn/52Cr ratios, based on the data in this study (Fig. 3a). Most CCs have ε53Cr values ranging from 0 to 0.2, with an average value of 0.15 (±0.13, 2SD; ± 0.02, 2SE; N = 4l; excluding the silicate separate of CB chondrites). The ε53Cr values of CI, CR, CM and CB chondrites tend to be slightly higher compared to CH, CO, CV and CK chondrites, although some CO and CM chondrites exhibit indistinguishable ε53Cr values. Limited intra-group differences in ε53Cr values that are not correlated with their respective 55Mn/52Cr ratios also exist in, for example, the CR, CM and CO groups. The 55Mn/52Cr and ε53Cr values for most OCs are correlated (Fig. 3b) and a model 1 regression of Isoplot R (Vermeesch, 2018) of these data results in a slope of 0.48 ± 0.20 that corresponds to an initial 53Mn/55- Mn of 5.39 ± 2.23 (MSWD = 2.2, 2SE, N = 23) and an initial ε53Cr (intercept of y axis) of -0.16 ±0.05. All the regressions reported in this paper are calculated in the same way. The Cr data for all the ECs (with literature data) reveals no (positive) correlation between Mn/Cr ratio and ε53Cr (Fig. 3c).
Fig. 3. 55Mn/52Cr vs. ε53Cr in the CCs (a), OCs (b) and ECs (c).
In (a), the gray circles and dashed line are from Moynier et al. (2007) and Shukolyukov and Lugmair (2006), who reported well-defined 53Mn-53Cr isochrons. However, the additional data presented here (colored data points; the colors for chondrite groups are the same as in Fig. 1) do not reproduce this correlation line. (a) Does not include the 53Mn-53Cr data for the silicate parts of CB chondrites. The best fit 53Mn-53Cr correlation line in (b) may be a mixing line rather than an isochron.
When combined with literature data (Mougel et al., 2018; Trinquier et al., 2007; Trinquier et al., 2008b), the terrestrial samples show minor Cr isotopic heterogeneity (Fig. 4), with average ε53Cr = 0.04 (±0.08, 2SD; ±0.02, 2SE; N= 15) and ε54Cr = 0.07 (±0.12, 2SD; ±0.03, 2SE; N = 15). There is no obvious difference between samples with different petrology or chemistry (e.g., the basalt and peridotite). The Cr metal standards NIST SRM 3112a and SCP-Cr have indistinguishable Cr isotope compositions from the NIST SRM 979 standard.
Fig. 4.
All of the 55Mn/52Cr and ε53Cr data for the different chondrite groups. The warm-color circles are the CCs, while the black, green and blue ones are the RCs, OCs and ECs, respectively. The data are from Table 4, and regressed by Model 3 (maximum likelihood regression with overdispersion), Isoplot R (Vermeesch, 2018). This figure does not include the 53Mn-53Cr data for the silicate parts ofCB chondrites. Thereisa positive trend (gray dashed line) between 55Mn/52Cr and ε53Cr with a slope of 0.285 ± 0.078 (MSWD = 11, N = 85; some literature data do not have 55Mn/52Cr ratio information) regressed by model 3, Isoplot R. This 53Mn-53Cr correlation line is interpreted as a mixing trend and, as such, does not have chronological significance.
4. Discussion
4.1. Comparison of Cr isotope data for chondrites with literature
CI chondrites are dominated by matrix materials and contain very few chondrules and refractory inclusions (Barrat et al., 2012; Krot et al., 2014), making them more homogenous than other types of chondrites. Hence, CI chondrites are good candidates to compare the Cr isotope data from this study with those in the literature. We have listed all the reported Mn/Cr ratios and Cr isotope data for Orgueil in Table 3. For Orgueil, ε54Cr values generally fall between 1.50 - 1.60, except for the data reported in Kadlag et al. (2019), which are higher (ε54Cr= 1.94 ±0.12). For ε53Cr, some studies (Trinquier et al., 2008a, 2008b; Qin et al., 2010; Schiller et al., 2014) report values of around 0.20, except for those in Kadlag et al. (2019), Moynier et al. (2007) and Shukolyukov and Lugmair (2006), all of which report slightly higher values around 0.40. These elemental and isotope inconsistencies cannot be attributed to the influence from carbonates that have high Mn/Cr ratios and high ε53Cr values, because the 55- Mn/52Cr ratios of CI chondrites reported in all studies are similar (0.80-0.85). As for 55Mn/52Cr ratios, the data for Allende (0.51), Orgueil (0.94) and Ivuna (0.93) in Kadlag et al. (2019) are systematically higher than those reported in this study and other literatures (see Table 3). The cause of the inconsistency between Kadlag et al.(2019) and other recent studies is unknown. Stracke et al.(2012) showed that all the 38 Allende samples have an similar 55Mn/52Cr values, averaging at 0.41 ± 0.02 (that is consistent with the value from our study, 0.42 ± 0.02), while Kadlag et al. (2019) reported a value of 0.51 ± 0.03. Since Kadlag et al. (2019) used Parr bombs in their dissolution procedures, it is unlikely that incomplete sample dissolution can account for the data inconsistency.
A similar data inconsistency is also be observed in another CI chondrite, Ivuna. Kadlag et al. (2019) reported a ε54Cr value (1.79 ± 0.20) for Ivuna, which is higher than that reported in other literature (Schiller et al., 2014; Shukolyukov and Lugmair, 2006; Van Kooten et al.,2016), while Williams et al. (2020) reported a lower ε54Cr value of 1.30 ± 0.09. As for ε53Cr values, Shukolyukov and Lugmair (2006) report a higher ε53Cr value than that of Schiller et al. (2014) and this study, and the data in Kadlag et al. (2019) has a large error (0.17). Both higher ε53Cr and ε54Cr values in Kadlag et al. (2019) might be caused by a residual mass-dependent Cr isotope fractionation using TIMS. However, since the authors do not provide data for terrestrial samples, it is difficult to evaluate this hypothesis. Similarly, Shukolyukov and Lugmair (2006) and Williams et al. (2020) did not report data for terrestrial samples and, as such, the accuracy of their data is difficult to evaluate. The reason for the slightly lower ε54Cr data in Williams et al. (2020) is also not clear since the authors did not report the ε53Cr data. Note that a residual massdependent fractionation effect would shift both ε53Cr and ε54Cr with a factor of ~1:2 [see Discussion inSection 4.5, and literature (Bourdon and Fitoussi, 2020; Qin et al.,2010; Shukolyukov and Lugmair, 2006; Trinquier et al.,2006, 2008a)].
Despite some inconsistency between the various studies, we list and consider all published data in Table 3. Averaging the all the data in literature and this study gives: Orgueil: ε53Cr = 0.29 ± 0.07 and ε54Cr = 1.60 ± 0.10; Ivuna: ε53Cr = 0.28 ± 0.10 and ε54Cr = 1.56 ± 0.15 (the uncertainty reflects the 95% confidence interval, which is also used for other chondrite samples with multiple literature data; Tables 3 and 4).
Allende, which is a large fall (mass of ~2 tons), has been the subject of extensive Cr isotopic studies. All the Allende data show relatively homogeneous ε53Cr values (ranging from 0.04 ± 0.06 to 0.16 ± 0.06) but heterogeneous ε54Cr values, ranging from 0.86 ± 0.09 to 1.10 ± 0.08 (Trinquier et al., 2007; Zhu et al., 2020b). Since the CVs are the chon-drites with the highest abundances of refractory inclusions (CAIs – Ca, Al-rich inclusions and AOAs – amoeboid olivine aggregates), which have extreme ε54Cr values (Trinquier et al., 2009), and they also have chondrules with variable ε54Cr values (Olsen et al., 2016), the ε54Cr variability between Allende measurements likely reflect sample heterogeneity at the scales sampled. The average data for Allende: ε53Cr 0.11 ± 0.02 and ε54Cr = 0.95 ± 0.07 is reported in Table 4.
We also compared the Cr isotope data for other chondrites analyzed by various workers (samples are listed in Table 3). In Table 4, we list the averages and the 95% confidence interval uncertainties for the averaged values. Within the uncertainties, most analyses of the same meteorites are consistent. Two exceptions are the ε54Cr values of Jbilet Winselwan (CM2) (van Kooten et al., 2020) and the ε53Cr values of Lance’ (CO3) (Moynier et al., 2007; Trinquier et al., 2008b). The small-degree of ε54Cr heterogeneity (0.82 ± 0.04–1.01 ± 0.12) in Jbilet Winselwan could also result from sample heterogeneity, given that CM chondrites also contain abundant refractory inclusions (Krot et al., 2014), and their chondrules possess variable ε54Cr values (van Kooten et al., 2020). Note that the ε53Cr of Lance, -0.04 ± 0.07 in Trinquier et al. (2008b), is lower than that of most other CO chondrites and even those of all other measured chondrites, and its ε54Cr value is also lower than all the other CO chondrites. Because the mass of the Lance’ aliquot analyzed by Trinquier et al. (2008b) is only 9 mg, it is possible that it is not representative of the bulk parent meteorite. As such, we have not included the ε53Cr and ε54Cr values for Lance’ in Table 4and do not consider it in further discussion.
It is also noteworthy to discuss the Cr isotope variation in CB chondrites. The three CB chondrites measured in this study (with different subgroups, i.e., CB, CBa and CBb), MIL 05082, QC 001 and HaH 237 (mostly silicate), have homogeneous ε54Cr values (1.46 ± 0.08; 2SD, N = 3), which is inconsistent with those of other previously reported CB chondrites HaH 237 (0.87 ± 0.19) (Shukolyukov and Lugmair, 2006) and Bencubbin (bulk, average of silicate and metal; 1.12 ± 0.03) (Trinquier et al., 2007). Here, since the metal and silicate parts for Ben-cubbin (Trinquier et al., 2007) and Gujba (Yamashita et al.,2010) have consistent ε54Cr values, we averaged the ε54Cr values of their different components to represent their bulk ε54Cr compositions. The bulk ε54Cr values of HaH 237 and Bencubbin are different from the average ε54Cr value of the Gujba components, 1.29 ± 0.07, (Yamashita et al., 2010), but overlap within uncertainty with the ε54Cr value (1.07 ± 0.27) of the metal spherules from Gujba (Trinquier et al., 2008b). We do not interpret this ε54Cr inconsistency as inter-laboratory biases because the Cr isotope data for terrestrial samples from the different studies (including different instruments within the same laboratory) are all consistent within error (Qin et al., 2010; Trinquier et al., 2007, 2008b; Zhu et al., 2019a, 2020a, 2020b). Instead, it is likely that there is some ε54Cr variability between CB chondrite samples.
The ε54Cr data for the CO chondrites analyzed in this study (Table 4), ranging from 0.80 ± 0.06 to 1.22 ± 0.04, are higher than for Fe’lix (0.63 ± 0.09) and Lance’ (0.57 ± 0.11) reported in Trinquier et al. (2007), but similar to that of Kainsaz (0.87 ± 0.18) reported in Qin et al. (2010) (Table 4).
4.2. Updated ε54Cr sequence and intra-group ε54Cr heterogeneity of carbonaceous chondrites
Chondrites typically have low cosmic ray exposure ages (CREA, less than 100 Ma) and relatively low Fe/Cr ratios (Eugster, 2003; Weber et al., 2001), which limits the potential cosmogenic effects on their Cr isotope compositions. Even amongst angrites with Fe/Cr ratios of up to 600 and CREA up to 60 Ma (Eugster, 2003; Zhu et al.,2019b) and mesosiderites with CREA up to 300 Ma (Eugster, 2003; Trinquier et al., 2007), their ε54Cr values remain relatively homogeneous and show no correlation with CREA. As for the Fe-rich CB chondrites, the metal and silicate parts of Bencubbin have the same ε54Cr values, again showing that cosmogenic effects are not detectable in them with current measurement precisions. Therefore, ε54Cr signatures are a robust tool for tracing general genetic relationships of chondrite parent bodies.
In Table 4, combining these new measurements with literature data (Göpel et al., 2015; Qin et al., 2010; Trinquier et al., 2007; van Kooten et al., 2020; Zhu et al., 2021) results in an updated ε54Cr sequence: CI ≥ CH ≥ CB ≥ CR > CM ≈ CV ≈ CO ≥ CK > EC ≈ RC > OC (Fig. 1). We note that the CB and CH chondrite samples in this study may not represent a bulk sample due to the sample heterogeneity. However, the CB chondrite components (e.g., chondrules, silicates and metal) have homogeneous ε54Cr values (Trinquier et al., 2007; Yamashita et al., 2010), perhaps owing to their formation mechanism by impact (Krot et al., 2005). Thus, the ε54Cr data reported here for CB chondrites can be used to estimate the bulk value ofthe parent meteorite. We note that the ε54Cr systematics in CH chondrite components needs to be studied in the future. This updated sequence provides new insights into confirming the classification of meteorites linked to recognized meteorite groups and determining potential genetic affinities between ungrouped chondrites and recognized meteorite groups. However, since our new data show an overlap in the ε54Cr values of CM, CV and CO chondrites, the applicability of the ε54Cr systematics as a tracer in carbonaceous meteorites is weaker than originally suggested (e.g., Trinquier et al., 2007). Aguas Zarcas fell on April 23, 2019 in Costa Rica and was classified as a CM2 chondrite (the Meteorite Bulletin #108). This chondrite is of significant interest as it contains abundant prebiotic compounds similar to other CM chondrites (Glavin et al.,2020). The Cr isotopic data for Aguas Zarcas from this study, with ε53Cr = 0.15 ± 0.03; ε54Cr = 0.86 ± 0.03, is consistent with the ε54Cr variation range of CM chondrites (e54Cr = 0.92 ± 0.24, 2SD).
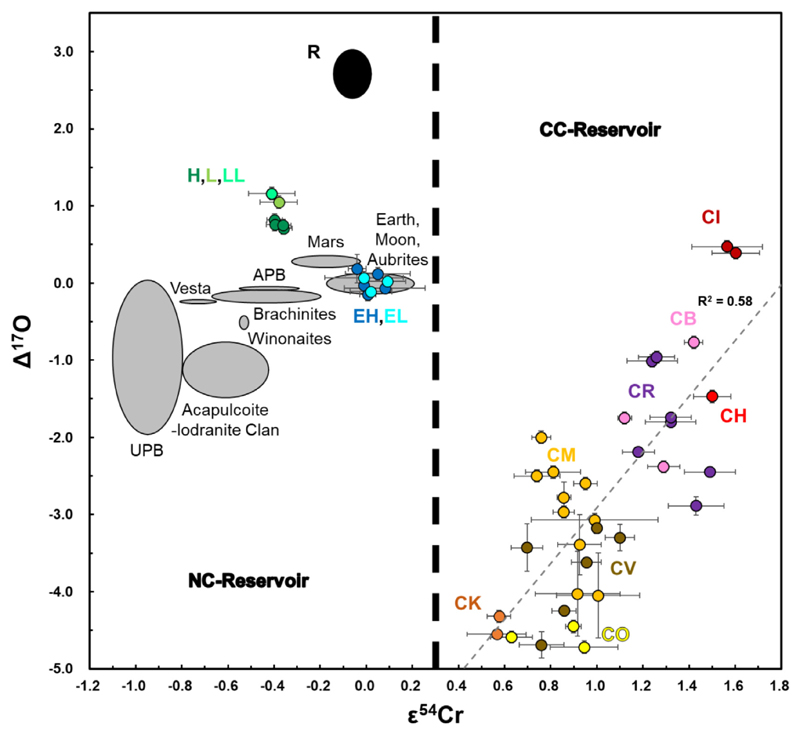
The ε54Cr and D17O values of CCs were previously reported to be correlated (Trinquier et al., 2007). However, this correlation was based on a limited data set and the Cr and O isotope data were often not from the same chondrites (see Fig. 2; Trinquier et al., 2007). Our new data (same chondrites, but not the same sample aliquots for Cr and O isotope measurements) show that this correlation (R2 = 0.58) is not as robust as previously observed. Nonetheless, our data confirm the Cr isotopic difference between the CCs and most other (e.g., OCs, RCs and ECs) meteorites (Trinquier et al., 2007; Warren, 2011), which is also observed for Ti (Trinquier et al., 2009; Zhang et al., 2012), Ca (Dauphas et al., 2014; Schiller et al., 2018), Ni (Steele et al., 2012); Mo (Budde et al.,2016; Spitzer et al., 2020; Yokoyama et al., 2019), and Ru (Fischer-Gö dde et al., 2015; Fischer-Gö dde and Kleine,2017).
Fig. 2.
The ε54Cr and Δ17O compositions of the CCs, OCs, ECs (filled circles), as well as achondrites, terrestrial and lunar samples (gray ellipses). The data sources for planets and achondrites are shown in Table 5. NC: non-CCs; APB: angrite parent body; UPB: ureilite parent body. The colored circles represent the same samples that are shown in Fig. 1. It should be noted that the correlation between ε54Cr and Δ17O values inCCsis notasstrong(R2=0.58) as that described in Trinquier et al. (2007) and Warren (2011). The ε54Cr gap betweentheCCand NC reservoirs is + 0.3, because some terrestrial samples and the ECs have ε54Cr values in the range 0 - 0.2, and no CCs possess ε54Cr values <0.4.
An important new observation shown by our extended database is the intragroup ε54Cr variability among the CB, CM, CO, and CV chondrites (Figs. 1 and 2). This is consistent with reported intra-group heterogeneities for bulk Ti isotope anomalies (expressed as ε50Ti) amongst the CM, CV and CO chondrites (Trinquier et al., 2009; Zhang et al., 2012). One of the best examples of this is the Allende CV chondrite whose published ε54Cr values range from 0.86 ± 0.09 to 1.10 ± 0.08 (Qin et al., 2010; Trinquier et al., 2007; Zhu et al., 2020b). Similarly, the three CB chondrites studied here: MIL 05082 (CB), QC 001 (CBa), and HaH 237 (CBb) have indistinguishable ε54Cr values with a mean of 1.46 ± 0.08 (2SD, N = 3). This is in contrast to the previously reported data for CBs that are significantly different in their absolute values and more variable, e.g., 1.29 ± 0.07 (2SD; average value of the chondrules and metal in Gujba) for Gujba, and 1.12 ± 0.03 (2SD) for Bencubbin (Trinquier et al., 2007; Yamashita et al., 2010), but marginally overlaps with the ε54Cr data (1.07 ± 0.27) for one metal chondrule in Gujba (Trinquier et al., 2008a, 2008b). We also find that some CH and CB chondrites have comparable ε54Cr compositions to CIs, which until now were considered the most 54Cr-enriched chondrites in bulk. The intragroup ε54Cr heterogeneity of the CB, CM, CO, and CV chondrites likely result from sample heterogeneity at the scale of most measured samples that is also reflected by intragroup (e.g., CM, CB, CV, or CR) or even intra-chondrite (e.g., Jbilet Winselwan, Paris, and NWA 8157) O isotope variability (see Fig. 2 and Table 4). However, the O isotope compositions (D17O) can also change as a result of aqueous alteration (Schrader et al., 2014; Schrader et al., 2011) and terrestrial weathering (Alexander et al., 2018). Mineral and acid leachates exhibit very large Cr isotope variability (Göpelet al., 2015; Podosek et al., 1997; Qin et al., 2010; Rotaru et al., 1992;Schiller et al., 2014; Trinquier et al., 2007; Wang et al., 2011). The CM, CO, and CV chondrites also contain large fractions of CAIs and AOAs (5 vol.%, 13 vol.%, and 10 vol.%, respectively; Krot et al., 2014; Weisberg et al., 2006) that have high ε54Cr values of up to ~6 (Larsen et al., 2011; Trinquier et al., 2009) but relatively low Cr contents. Although chondrules recorded heterogeneous ε54Cr values (e.g.,Bollard et al., 2019; Olsen et al., 2016; Qin et al., 2011; Schneider et al., 2020; van Kooten et al., 2020; Zhu et al., 2019a, 2020a), it is unlikely that they are the cause the ε54Cr heterogeneity between bulk chondrites from same groups because OCs, ECs and CK chondrites, which have chondrules showing clear ε54Cr heterogeneity (Bollard et al., 2019; Williams et al.,2020; Zhu et al., 2020a), have homogeneous ε54Cr values. Schneider et al. (2020) report that various matrix material in Allende have a fairly narrow range of ε54Cr values (1.06 ± 0.22; 2SD, N = 3) and, as such, a variable chon-drule/matrix ratio could also contribute to the variability of the ε54Cr values between CCs groups. It should be noted that the OCs and ECs have high abundances (60-80%) of chondrules (Krot et al., 2014), which is consistent with the ε54Cr homogeneity in OCs and ECs, since the chondrule/matrix-ratio effect should be less in OCs and ECs than that in CCs. Alternatively, the limited ε54Cr heterogeneity within the OCs, ECs, RCs and CKs (maybe also CR; Bunch et al., 2008) could also be the result of metamorphic homogenization. Since they are not as metamorphosed, the apparent intragroup ε54Cr heterogeneity of CM, CO and CV chondrites (CV chondrules possess the most variable ε54Cr values, ranging from -0.79 to 2.01; Olsen et al., 2016) may be mainly due to the relatively small sample size and non-representative sampling. This is consistent with the observation that 0.6-1 g of Allende may not be enough to be representative for the average bulk composition (Stracke et al., 2012).
It is unlikely that metamorphism can explain the ε54Cr homogeneity in type-3 OCs, ECs and RCs. Also, the effect of sample size and CAI-AOA abundance cannot explain the ε54Cr heterogeneity amongst the CR and CB chondrites, because they have low combined CAI and AOA contents (less than 0.5 vol%.; Scott and Krot, 2005) and chondrules with almost homogeneous ε54Cr values (Olsen et al., 2016; Yamashita et al., 2010). Alternatively, it is possible that the intra-group ε54Cr heterogeneity in CCs (mainly CV, CO, CM, CB and CR groups) reflects ε54Cr heterogeneity in their parent bodies at scales that are larger than represented by the meteorites. This is also consistent with the variable major element compositions, abundances of their components (e.g., CAIs) and O isotope compositions (Fig. 2) in different chondrite samples from the same group (Ebel et al., 2016; Hezel et al., 2008). Note that ε54Cr heterogeneities have also been found within achondrite groups, e.g., ureilites (Zhu et al., 2020b), so it is also possible that some parent bodies (e.g., CV, CO, CM and CB) are isotopically heterogeneous.
4.3. Constraints on parent body processes and possible genetic relationships (CV-CK, CM-CO and CB-CH clans) inferred from ε54Cr systematics
The lack of correlated intragroup ε54Cr values with the degree of aqueous alteration (i.e., CR2 to CR1, CM2 to CM1) or thermal metamorphism (i.e., CK4 to CK6) also suggests that parent body processes did not redistributethe Cr at the scales we have sampled (e.g., Qin et al., 2010; Trinquier et al., 2007). This is also consistent with previous studies, indicating that petrologic types 3 to 6 in both the OCs and ECs have the same ε54Cr values within their uncertainties (Mougel et al., 2018; Qin et al., 2010; Trinquier et al., 2007).
Our new ε54Cr data also allow us to evaluate if the CV sub-groups originate from an isotopically homogeneous, common parent body. The three CV subgroups primarily differed from one another in their redox states: oxidized CVoxA, CVoxB and the reduced CVred. These CV subgroups may either originate from two or three different parent bodies (Gattacceca et al., 2020; Greenwood et al., 2010) or, alternatively, from different regions of a single parent body (Greenwood et al., 2010). Based on the presence of CVoxB-type clasts in the CVred chondrite Vigarano (Krot et al.,2000) and clasts of CVoxB and CVoxA lithologies in the oxidized CV Mokoia (Krot et al., 1998), the origins of these subgroups from separate parent bodies already appears unlikely. Our data show that meteorites from these three subgroups have indistinguishable ε54Cr signatures (e.g., CVoxA: Allende with ε54Cr of 0.95 ± 0.06; CVoxB: Bali, Mokoia and Kaba with ε54Cr of 0.70 ± 0.07 to 1.10 ± 0.06; CVred: Leoville and Vigarano with ε54Cr of 0.76 ± 0.10 and 0.85 ± 0.02, respectively, Table 3), consistent with their origin from a single parent body and that internal redox variations did not alter their bulk ε54Cr compositions. This is inconsistent with the systematically different O isotope compositions between oxidized and reduced CV chondrites (Clayton and Mayeda, 1999; Gattacceca et al.,2020). Combined with their distinct chondrule sizes and matrix abundances, Gattacceca et al. (2020) argued there are multiple CV chondrite parent bodies. Since CVox and CVred chondrites have similar ε54Cr values, and O isotopes are sensitive to aqueous alteration (e.g.,Farquhar et al.,1998), an alternative explanation for the relationship between CVox and CVred sub-groups is the regional heterogeneity in water/rock ratio within a single parent body.
Based on chemical and petrological similarities and similar O-isotope compositions, the CV and CK chondrites have been grouped into the CV-CK clan and are considered by some to have originated from the same parent body (Greenwood et al., 2010). Nonetheless, the CV and CK chondrites are still considered different chondrite groups based on relative differences in the abundances of their bulk refractory lithophile elements and CAIs, and the presence of coarse-grained igneous rims around chondrules in CV chondrites that are almost absent in the CK chondrites (Kallemeyn et al., 1991). To reconcile the similar O-isotope compositions but the small chemical and textural differences between CV and CK chondrites, it has been proposed that the CK chondrites (which exist as petrologic types 3 to 6) formed deeper in the same parent body as the CV chondrites (which are exclusively low petrologic type 3 s) (Greenwood et al., 2010; Wasson et al., 2013). This scenario is consistent with thermal modeling (Elkins-Tanton et al., 2011). Our high-precision Cr isotope data reveal that the CK chondrites have similar ε54Cr values to one another, 0.51 ± 0.15 (2SD), ±0.08 (2SE, N = 4), but are significantly lower than those of the CV chondrites (ε54Cr=0.89±0.30[2SD],±0.12[2SE,N=6]).Thiscon-firms the first resolvable difference between the ε54Cr values of one CV and one CK chondrite reported in Trinquieret al. (2007). Given the lack of evidence that secondary parent body processes, including aqueous alteration, thermal metamorphism and redox processes can significantly affect ε54Cr at the scale we sampled, the different ε54Cr values for CV and CK chondrites imply that they did not originate from the same parent reservoir. This is also supported by the different chemical composition of magnetites in CV and CK chondrites, suggesting that they experienced different metamorphic histories (Dunn et al., 2016).
Similarly, CM and CO chondrites, which are in the CM-CO clan (Weisberg et al., 2006), share mineralogical and geochemical similarities, including anhydrous mineral compositions and bulk elemental and O-isotope compositions (Clayton and Mayeda, 1999; Greenwood and Franchi,2004; Kallemeyn and Wasson, 1981; Weisberg et al.,2006), which is consistent with their similar ε54Cr compositions. As such, our Cr isotope data further supports the idea that the CM and CO chondrite precursors formed from similar materials. However, they cannot derive from the same parent body, because (1) their mean chondrule sizes are resolvably different and their chondrules (mainly type II chondrules) have different petrologic characteristics (Schrader and Davidson, 2017), (2) the CM chondrite parent body likely formed approximately 1 million years after the CO chondrite parent body (Sugiura and Fujiya, 2014).
The CB and CH chondrites form the same clan (Krot et al., 2014) and both of them have high ε54Cr values (>1.1). However, the CBs have heterogeneous ε54Cr values, while those of the CHs are homogeneous. Furthermore, the CB (MIL05082) and CH (PCA 91467 and A-881020) chondrites have similar 55Mn/52Cr ratios but different ε53Cr values, which is likely caused by their different initial ε53Cr values and/or formation times. This suggests different origins for the CB and CH chondrites, which is consistent with their different ε54Cr features, i.e. the ε54Cr heterogeneity and homogeneity in the CB and CH chondrite parent bodies, respectively. However, the numbers of CB and CH samples that have been measured are still small and measurements of more CBs and CHs are needed to confirm our conclusions.
4.4. 53Mn-53Cr systematics of bulk chondrites
The previously postulated CC 53Mn-53Cr isochron was mostly controlled by a single CB chondrite, HaH 237 with low 55Mn/52Cr of 0.08 and ε53Cr of -0.15 ± 0.09, and two CI chondrites, Orgueil and Ivuna, with high 55Mn/52Cr of ~0.8 and ε53Cr of ~0.4 (see the grey points and line in Fig. 3a) (Moynier et al., 2007; Shukolyukov and Lugmair, 2006). Subsequently, Trinquier et al. (2008b) and Qin et al. (2010) determined that using an updated ε53Cr values for CI chondrites of ~0.2, the age of the “isochron”, decreases from 4568.6 ± 1.1 Ma to 4566.1 ± 2.4 Ma (the two ages are still consistent within uncertainty). At that time, no CH chondrites had been studied. Here we show that our sample of HaH 237 (mostly silicates) has a similar 55Mn/52Cr (0.09) to that reported by Shukolyukov and Lugmair (2006) but a higher ε53Cr value (0.05 ± 0.03). The two CB chondrites MIL 05082 and QC 001 have 55Mn/52Cr ratios of 0.37-0.47 and ε53Cr values of 0.20 ± 0.01 (2SD, N = 2) that are indistinguishable from CI chondrites (0.21 ± 0.04; 2SD, N = 2). Moreover, theCH chondrites in this study with the lowest Mn/Cr ratios have ε53Cr values of 0.12 ± 0.03 that are too high to be consistent with the postulated isochron. Although our new values suggest that the CH and CB chondrites are not consistent with a common CC isochron, a potential caveat with this observation is the difficulty in obtaining samples that are representative of the bulk meteorites. We note that while there is a broad correlation between bulk CC 55Mn/52Cr ratios and ε53Cr values, these data do not form a single well-defined 53Mn-53Cr isochron even when the CH and CB chondrites are excluded (Fig. 3a).
There are several likely reasons for the lack of a single, well-defined 53Mn-53Cr isochron among the CCs. Firstly, unlike other CCs, the CH and CB chondrites probably formed via impacts that postdated the formation of other CCs (Krot et al., 2005; Yamashita et al., 2010), and the impact, e.g., ~5 Ma after CAIs (Krot et al., 2005), may potentially and secondarily modify the Mn/Cr ratios (Krot and Nagashima, 2017; Weisberg et al., 2001). Thus, there is no a priori reason why they should form a common 53Mn-53Cr isochron with the other CCs. Secondly, all eight CC groups have distinct photochemical and/or nucleosyn-thetic isotope anomalies (Clayton and Mayeda, 1999; Schiller et al., 2018; Trinquier et al., 2009; Zhang et al.,2012) reflecting variability in the makeup of their precursors and/or their formation environments within the proto-planetary disk. Finally, chondrites are complex assemblages of CAIs, AOAs, chondrules and matrix (Alexander, 2019a) that formed at different times and under varying conditions (Connelly et al., 2012). The chondritic components (i.e., chondrules, matrix, metals, CAIs and AOAs) do not all have the same ages and/or source regions (initial ε53Cr values). For example, although CAIs and some chondrules did form at the same time (see the internal isochron ages; Connelly et al., 2012; Bollard et al., 2017), these objects have distinct nucleosynthetic anomalies (Cr and Ti: Trinquier, et al. 2009; Olsen et al., 2016; Gerber et al.,2017; Schneider et al., 2020; Williams et al., 2020; Zhu et al., 2019a; Zhu et al., 2020a), and, as such, must have formed in distinct reservoirs. Some CO and CM chondrites have similar 55Mn/52Cr ratios but distinct ε53Cr values, which indicates that on average their components experienced Mn/Cr fractionation at different times and/or conditions. Their different radiogenic Cr isotopic compositions also supports CM and CO chondrites originating from different parent bodies (e.g.,Schrader and Davidson, 2017). The matrix also has a distinct origin based on its variable nucleosynthetic isotope signatures (Cr, Ti, Mo and W; Budde et al., 2016; Schneider et al., 2020). This has also been discussed in Zhu et al. (2020a) and Alexander (2019a). Overall, the 53Mn-53Cr correlation for most chondrites is almost certainly a multiple-component mixing trend. However, this mixing for CC 53Mn-53Cr correlation line is not limited to only chondrule-matrix (e.g., Anders, 1964), because CK chondrites with high abundance of matrix (~75%; Krot et al., 2014) also have low Mn/Cr ratios compared to mostofother CC groups (Fig. 3a). Considering that the half-life of 53Mn is comparable to the accretion ages of chondrites, it is perhaps unsurprising that a general trend resembling a 53Mn-53Cr isochron between chondrite groups exists.
Such a correlation is stronger for the OCs than the CCs (Fig. 3b). The calculated initial 53Mn/55Mn ratio for this OC trend is (5.39 ± 2.23) × 10-6. When anchored to D’Orbigny with the absolute age (U isotopecorrected) of 4563.37 ± 0.25 Ma and an initial 53Mn/55Mn of (3.24 ± 0.04) × 10-6 (Amelin, 2008; Brennecka and Wadhwa, 2012; Glavin et al., 2004), this is equivalent to an age of 4566.1 ± 2.3 Ma. However, this apparent isochron for the OCs may not have chronological significance, even considering that the ε54Cr and △17O values suggest a common origin for this group, because the correlation line can also result from mixing of different proportions of chondritic components. Compared to the CCs, the OCs have fewer refractory inclusions, more chondrules, less matrix and variable amount of metal (H > L > LL). The mixing end-members are thus chondrules, matrix and metal, and the metal should play a more important role in the mixing. This is because the metal-rich H chondrites possess lower 55Mn/52Cr ratios and lower ε53Cr values, compared to metal-poor LL chondrites, with intermediate L chondrites (Fig. 3b) (Krot et al., 2014). Fig. 3c shows there is no resolved positive slope in a 53Mn-53Cr correlation diagram. Note that ECs are composed of chondrules, metals (Krot et al., 2014) and many Mn- or Cr-rich sulfide minerals (e.g., Piani et al., 2016; Zhu et al., 2020a). Metals are poor in both Mn and Cr, and silicate usually possess more Mn than Cr (Piani et al., 2016) such that, their sulfide minerals have large variability for Mn and Cr contents (e.g., Cr-rich troilite and Mn-rich niningerite; Piani et al., 2016), so their complicated 53Mn-53Cr mixing budgets may obscure any 53Mn-53Cr correlation.
When comparing the 53Mn-53Cr data for all chondrite groups (Fig. 4), there is no well-defined 53Mn-53Cr isochron. Actually, nearly half of the data points do not fit on the regression line. However, all the different groups of chondrites here define a positive trend between ε53Cr values and 55Mn/52Cr ratios. For example, the CV, CK, CO, and CH chondrites mostly possess low 55Mn/52Cr ratios (<~0.5) and ε53Cr values, <~0.12, while the CI, CM, CR (except one in van Kooten et al., 2020), and OCs mostly have high 55Mn/52Cr ratios (>~0.5) and ε53Cr values, >~0.12. These variations may be caused by a multiple-endmember mixing of the different chondritic components (chondrules, matrix, CAIs and AOAs) that on average experienced Mn/Cr fractionations at different times, reflecting a general relationship between 55Mn/52Cr ratios and ε53Cr values. Regressing all the 53Mn-53Cr data for all the chondrites [by model 3 (maximum likelihood regression with overdispersion) due to the MSWD ≫ 1, Isoplot R (Vermeesch, 2018)], the slope is 0.285 ± 0.078 with initial ε53Cr=0.00 ± 0.05 (MSWD =11, N=85).This slope corresponds to a 53Mn/55Mn = (3.23 ± 0.89) × 10-6 and an absolute age of 4563.4 ± 1.5 Ma, when anchored to the U isotope-corrected D’Orbigny angrite. However, this 53Mn-53Cr correlation line likely represent a mixing line that does not have any chronological significance.
Trinquier et al. (2008a, 2008b) included a number of chondrites (OC, EC, CI, CV, and CO groups) and planets/asteroids (including Earth, Mars and Vesta) on the same 53Mn-53Cr diagram with a slope of (6.53 ± 1.93) × 10-6 and an absolute age of 4567.3 ± 1.9 Ma. They interpreted this age as the last isotopic equilibration of Mn and Cr in the protoplanetary disk. The chondrites and planets had distinct origins and formation times, which violates the primary assumption of an isochron that all components formed at the same time from the same reservoir. Thus, any 53Mn-53Cr correlation is a mixing line that does not have any chronological significance either. Here, it should also be mentioned that the age from a 53Mn-53Cr “isochron” established by acid leachates in chondrites (Göpelet al., 2015; Trinquier et al., 2008b) are also questionable and mostly reflect mixing lines. This is because (1) The chondritic components, e.g., CAIs, chondrules, matrix, metal and carbonates, have different origins and formed at different periods, which has been discussed in the previous section. For example, only five leachates, out of 15, of Orgueil CI chondrite fall on a single 53Mn-53Cr correlation line (Trinquier et al., 2008b). This possibly reflects the fact that CI1 chondrites experienced secondary aqueous alteration and thereby forming resulting in the formation of younger carbonates (that would be dissolved by the weak acid, e.g., acetic acid, in the first step of leaching) with high Mn/Cr ratios (e.g., Fujiya et al., 2012; Fujiya et al., 2013), although CI chondrites are mostly composed of matrix material, and contain few CAIs and chondrules (Krot et al., 2014). The chondrites that have abundant CAIs, AOAs and chondrules, e.g., CV and CO chondrites, have a more complex 53Mn-53Cr mixing process; (2) Mn is rich in the easily dissolved minerals with high Mn/Cr ratios, e.g., carbonates, while Cr is rich in the refractory phases with low Mn/Cr ratios, e.g., chromites, so the acid leachates potentially represent the components of high-Mn/Cr and low-Mn/Cr reservoirs, which mostly reflects mixing lines that do not have chronological meaning. This is consistent with the anomalous 53Mn/55Mn [(13.64 ± 0.01) × 10-6; corresponding to 4571.1 ± 0.9 Ma that is older than CAIs] and initial ε53Cr values (-0.61 ± 0.05) obtained from a 53Mn-53Cr correlation line of acid leachates of the Paris chondrite (Göpel et al., 2015).
4.5. The ε53Cr deficit between chondrites and Earth suggests early volatile depletion of Earth precursors
The 15 terrestrial samples (including basalts, peridotites and chromites; including both MC-ICP-MS data in this study and TIMS literature data; see Table 6) measured relative to NIST SRM 979 and corrected for mass bias using the kinetic fractionation law exhibit ε53Cr and ε54Cr values that are slightly positive with 0.04 ± 0.08 (2SD); 0.02 (2SE) and 0.09 ± 0.12 (2SD); 0.03 (2SE), respectively (Fig. 5). The slightly positive ε53Cr and ε54Cr for terrestrial samples has been linked to an isotope fractionation behavior that differs from the kinetic fractionation of atomic Cr in the mass spectrometers that was induced during the preparation of the NIST 979 standard (Schiller et al., 2014). Similar mass-independent isotopic difference between terrestrial rocks and purified metal standards (i.e., NIST 3112a and SCP-Cr) have also been observed for other elements like Ni (Steele et al., 2011), Sr (Moynier et al., 2012), Ti (Zhang et al., 2012), and Mo (Budde et al., 2019). As such, the reported average ε54Cr values of terrestrial samples are currently the best estimate of the bulk terrestrial massindependent Cr isotope composition. The residual variability in terrestrial rock analyses highlights Cr isotope fractionation in nature and/or during Cr purification and TIMS analysis that do not follow the kinetic law for Cr, perhaps because they involved molecular species (e.g., CrO2) (see Fig. 6).
Fig. 5.
The ε53Cr and ε54Cr values for terrestrial rocks (Table 5) and chondrites. The black circles and squares are terrestrial crustal and mantle rocks respectively, while the black triangles are artificial standards (i.e., NIST SRM 3112a and SCP-Cr). The ε53Cr and ε54Cr values for terrestrial rocks are correlated with a slope of 1.37 ± 0.65 (N = 15, MSWD = 0.1), regressed by Model 1, Isoplot R (Vermeesch, 2018). This correlation indicates the residual mass-dependent Cr isotope fractionation. The gray and colorful bars indicate the average ε53Cr values (with 2SE uncertainty) for bulk silicate Earth (BSE; N = 15) and chondrites (N = 88), respectively. The two artificial standards are not considered to represent the Cr isotope composition of Earth. There is a ε53Cr deficit of 0.12 ± 0.02 between BE and chondrites, which potentially indicate an early volatile fractionation of proto-Earth. The 55Mn/52Cr for BE of 0.22-0.54, was estimated by Sun (1982), Wang et al. (2018), Wänke and Dreibus (1988) and (Palme and O’Neill, 2014), while the 55Mn/52Cr for chondrites are from those ofthe ECs (0.71; having the same isotope compositions with Earth for multiple elements) and CIs (0.82; are regarded to represent the chemical composition of the Solar System). In this figure, 55Mn/52Cr ratios of0.71-0.82 are only from ECs and CI chondrites, but note that some other groups of chondrites (e.g., CV, CO and CK in Fig. 4) may have an Earth-like 55Mn/52Cr ratios. No systematic differences were observed in data measured by MC-ICP-MS in this study and by TIMS in the literature.isotope-corrected D’Orbigny angrite. However, this 53Mn-53Cr correlation line likely represent a mixing line that does not have any chronological significance.
Fig. 6.
Calculated Δ53Cr and Δ54Cr (for evaporation of only Cr+ by kinetic isotope fractionation) using different Cr oxides (CrO, CrO2 and CrO3) and different fractionation laws (kinetic and equilibrium). The data sources are from Tables 7a and 7b. It should be noted that the variation factor (slope) between Δ53Cr and Δ54Cr is 2.61 that is highly consistent with the slope (2.6) between ε53Cr and ε54Cr values of multiple measurements for NIST 3112a (Qin et al., 2010).
This is supported by the fact that theoretical calculations predict that inappropriate mass fractionation correction should result in apparent mass-independent effects on ε53Cr and ε54Cr. Chromium evaporates not only as Cr+, but multiple oxidized species such as CrO, CrO2 and CrO3 may also be present during heating and evaporation during TIMS ionization. Based on masses and abundances stated in Table 7a, we firstly calculated the fractionation factors, β53Cr and β54Cr for different Cr species following Young and Galy (2004). In detail, the factors for equilibrium and kinetic fractionation laws are calculated from Eqs. (9) and (12), respectively, in Young and Galy (2004). Then, we determined the 53Cr/52Cr and 54Cr/52Cr ratios for a given 50Cr/52Cr fractionation and fractionating species. We subsequently derive the relative deviations resulting from non-kinetic and/or non-atomic Cr isotope fractionation from the predicted Cr isotope ratios resulting from the typically applied mass bias correction based on kinetic laws and atomic Cr for these isotope ratios (Δ53Cr and Δ54Cr) (Table 7b). Irrespective of the fractionating Cr species, the apparent anomalies in the 53Cr/52Cr and 54Cr/52Cr ratios (Δ53Cr and Δ54Cr) co-vary with a factor of ~2.6, which also mimics the slope (2.6) of the reported ε53Cr and ε54Cr values of multiple measurements for NIST 3112abyTriton TIMS (Qin etal.,2010).Thisratioisalso broadly consistent with the ε53Cr vs. ε54Cr values amongst the terrestrial samples (Fig. 5) that exhibit a slope of ~2 considering that the magnitude of isotopic variability is typically on the order of the analytical uncertainty. However, small variations in ε53Cr and ε54Cr in natural terrestrial samples may not solely be due to instrumental effects but maybethe product ofnatural equilibrium Cr stable isotope fractionation, i.e., the Cr stable isotope fractionation during magmatic process where fractionation occurs based on exchange of Cr2+ and Cr3+.
Table 7a. Parameters for theoretical calculation for Cr isotope fractionation.
| Mass of isotopes | Isotope abundance | CrO | CrO2 | CrO3 | |
|---|---|---|---|---|---|
| 50Cr | 49.946 | 4.345% | 65.941 | 81.936 | 97.931 |
| 52Cr | 51.941 | 83.789% | 67.935 | 83.930 | 99.925 |
| 53Cr | 52.941 | 9.501% | 68.936 | 84.930 | 100.925 |
| 54Cr | 53.939 | 2.365% | 69.934 | 85.929 | 101.924 |
| 16O | 15.995 | ||||
Table 7b. The calculated Cr isotope data based on different Cr oxides and different laws (kinetic and equilibrium), assuming the initial 50Cr/52Cr fractionation is 1000ppm.
| 50/52 | 53/52 | 54/52 | β53Cr | β54Cr | Δ53Cr | Δ54Cr | 54/53 factors | |
|---|---|---|---|---|---|---|---|---|
| Kin. Cr | 1000 | –487.09 | –964.17 | –2.05 | –1.04 | 0.00 | 0.00 | |
| Kin. CrO | 1000 | –490.46 | –972.94 | –2.04 | –1.03 | –3.37 | –8.77 | 2.606 |
| Kin. CrO2 | 1000 | –492.55 | –978.41 | –2.03 | –1.02 | –5.45 | –14.24 | 2.611 |
| Kin. CrO3 | 1000 | –493.97 | –982.14 | –2.02 | –1.02 | –6.88 | –17.97 | 2.614 |
| Equ. Cr | 1000 | –473.09 | –927.79 | –2.11 | –1.08 | 14.00 | 36.38 | 2.598 |
| Equ. CrO | 1000 | –479.68 | –944.75 | –2.08 | –1.06 | 7.42 | 19.41 | 2.617 |
| Equ. CrO2 | 1000 | –483.78 | –955.40 | –2.07 | –1.05 | 3.32 | 8.76 | 2.643 |
| Equ. CrO3 | 1000 | –486.58 | –962.71 | –2.06 | –1.04 | 0.51 | 1.46 | 2.838 |
Note: The fractionation factors, β53Cr and β54Cr, are calculated by Eqs. (9) and (12) in Young and Galy (2004).
The average ε53Cr value, 0.04± 0.02 (2SE, N= 15), of all terrestrial samples provides the current best estimate for the Cr isotopic composition of the BSE. It should be noted that Cr is more siderophile than Mn during core formation (Mann et al., 2009), resulting in elevated 55Mn/52Cr ratios in the BSE compared to the core. If the Earth core formation process occurred early (e.g.,chiller et al.,2020), i.e. before 53Mn extinction, the higher Mn/Cr ratio of BSE compared to the core would theoretically produce higher ε53Cr in the BSE relative to the core. Based on a mass balance calculation:
| (2) |
the ε53Cr values of the bulk earth (BE) should be similar to (if Earth core formed after 53Mn extinction) or slightly lower than (if Earth core formed before 53Mn extinction) that of BSE.
Since the ε53Cr value of the BSE is lower than that of chondrites, the ε53Cr of BE must also be lower. The ε53Cr value of BSE of 0.04 ± 0.02 (2SE, N = 15) is systematically lower than the average ε53Cr value of all the chondrites, ε53Cr= 0.16 ± 0.01 [2SE, N= 88; see Fig. 5; (Trinquier et al., 2008b)]. We tested the significance of the ε53Cr isotopic difference between chondrites (N = 88) and Earth (N = 15) by running an unpaired student t-test using Prism 8, which returned a P-value <0.0001 confirming the statistical difference between the two groups (statistical difference is considered for P-value <0.05). The BE also has a lower ε53Cr value compared to ECs (e53Cr = 0.15 ± 0.03; 2SE, N= 12; average 55Mn/52Cr = 0.71) that have been used as analogues for the isotopic composition ofEarth’s precursors via protracted stochastic collisional accretion given their otherwise close nucleosynthetic match to the BSE (Javoy et al., 2010), and CI chondrites (e53Cr = 0.22 ± 0.02; 2SE, N = 2; average 55Mn/52Cr = 0.82) that represent the bulk chemical composition of Solar System (Alle`gre et al., 1995; Krot et al., 2014). This ε53Cr deficit between chondrites and Earth has been already observed in previous studies (Qin et al., 2010; Trinquier et al.,2008b), and interpreted as representing early Mn/Cr volatile fractionation (Palme and O’Neill, 2014). Here, we combined all the chondrite data in this study and literature to better constrain the early volatile depletion of the Earth.
Variability in the initial abundance of the 53Mn/55Mn within the early Solar System is unlikely to cause this discrepancy given that the CCs and non-CCs have similar ε53Cr values (Trinquier et al., 2008b), and the relative 53Mn-53Cr ages of some achondrites (e.g.,Sanborn et al.,2019; Zhu et al., 2019b) and chondrules (Bollard et al.,2015; Krot et al., 2005; Yamashita et al., 2010; Zhu et al.,2019a, 2020a) are consistent with absolute Pb-Pb chronometry. As such, the cause for this ε53Cr difference between the BSE and chondrites must be due to Mn/Cr fractionation prior to the extinction of 53Mn (half-life of 3.7 Myrs). The 55Mn/52Cr ratio of the BE is difficult to estimate precisely. However, it has been estimated that the 55Mn/52Cr ratio of the bulk Earth is between 0.22 and 0.54 (Palme and O’Neill, 2014; Sun, 1982; Wang et al., 2018; Wänke Dreibus, 1988), which is lower than those of the CIs (~0.82) and ECs (~0.71). The ECs have similar isotopic compositions as Earth for multiple elements (Claytonet al., 1984; Javoy et al., 2010; Mougel et al., 2018; Piani et al., 2020; Trinquier et al., 2007; Zhu et al., 2020a), and CIs are proxies for the chemical composition of the bulk Solar System (Alle`gre et al., 1995). The lower 55Mn/52Cr ratio for BE is likely caused by preferential evaporative loss of Mn relative to Cr either prior to or during Earth’s accretion. We can estimate the timing of the volatile depletion by calculating a minimum age of volatile loss. To that end, we calculate the evolving ε53Cr signature ofBE using the initial ε53Cr and 53Mn/55Mn abundance of the Solar System (Trinquier et al., 2008a, 2008b) assuming a single stage Mn-loss that reduced the initial Mn/Cr ratio of the accreting Earth to its current value between 0.22 and 0.54. From this estimate, it becomes apparent that lowering of the Mn/Cr ratio of the Earth must have occurred within 0-3 Myr after CAI formation. This age is also consistent with another volatile-sensitive chronometer, the 87Rb-87Sr system that indicates that the planetary volatile depletion events happened very early, i.e., in the first few Myrs after CAIs (Gray et al., 1973; Hans et al., 2013; Moynier et al.,2012).
It should be noted that decreasing the Mn/Cr ratios from chondrites to Earth would be consistent with stronger volatilization of Mn than Cr (Sossi et al., 2019). In fact, this age of proto-Earth volatilization is consistent with the 53Mn-53Cr age of chondrule precursors (Zhu et al., 2019a,2020a) that have been considered as potential candidates for the building blocks of terrestrial planets by the mechanism of pebble accretion (Johansen et al., 2015; Schiller et al., 2018; Schiller et al., 2020). In particular, pebble accretion inevitably results in hot planetary atmospheres and proto-planets (Popovas et al., 2018) providing a natural mechanism of early volatile loss of the accreting Earth at early times (Mahan et al., 2018; Rubin et al., 1988). Therefore, our data suggest that the terrestrial volatile depletion more likely occurred during the formation of Earth precursors as opposed to later volatile loss during planetary evolution. In particular, evaporation under planetary conditions is predicted to occur under more oxidizing conditions (Visscher and Fegley Jr, 2013), which could make Cr more volatile than Mn (Sossi et al., 2019). This would result in an increase of the Mn/Cr ratio and subsequently the ε53Cr of the devolatilized planet compared to chondrites, which is the opposite of what is observed.
5. Conclusion
This study reports a new comprehensive mass-independent Cr isotope dataset of numerous chondrite types as well as Earth, using improved methods for high-yield purification method and high-precision Cr isotope measurements by MC-ICP-MS. The main conclusion of our work can be summarized as follows:
Our ε54Cr chondrite data complement earlier work, and provide an updated decrease of ε54Cr as follows: CI ≥ CH ≥ CB ≥ CR > CM ≈ CV ≈ CO > CK > E-C > OC. Our data also show that CB, CM, CV, CR and CO chondrites have intra-group ε54Cr heterogeneities that are likely caused by unrepresentative sampling or heterogeneous accretion of chondrite parent bodies.
The distinct ε54Cr for CV and CK chondrites suggest that they do not come from the same parent body, whereas the indistinguishable ε54Cr values of CM and CO chondrites suggest their parent bodies share common precursor materials and, thus, may have formed under similar conditions. Moreover, heterogeneous ε54Cr values for CB chondrites compared to the homogeneous ε54Cr values for CH chondrites suggests they may have different origins.
The low-Mn/Cr CH and CB chondrites have indistinguishable ε53Cr values from those of high-Mn/Cr CI chondrites and, as such, these data are inconsistent with the proposed bulk CC 53Mn-53Cr isochron. The broad correlation between Mn/Cr ratios and ε53Cr values in chondrites may simply reflect a mixing line between high-and low-Mn/Cr ratio reservoirs.
When compared to the NIST SRM 979 Cr standard, bulk (silicate) Earth is characterized by ε53Cr and ε54Cr values of 0.04 ± 0.02 and 0.09 ± 0.03 (2SE), respectively. The correlated and slightly positive values reflect non-kinetic isotopic fractionation of the NIST SRM 979 Cr standard and some terrestrial samples. The lower ε53Cr of bulk silicate Earth compared to chondrites requires early volatile loss of Earth’s precursor materials. Based on the half-life of 53Mn (3.7 Ma), this volatile loss likely occurred within 3 Myr of Solar System formation.
Acknowledgements
We deeply appreciate Thorsten Kleine for efficient editorial handling and detailed and constructive comments from Herbert Palme, Anne Trinquier and one anonymous reviewer, which greatly improved this manuscript. F. M. acknowledges funding from the European Research Council under the H2020 framework pro-gram/ERC grant agreement (#637503-PRISTINE) and financial support of the UnivEarthS Labex program at Sorbonne Paris Cite’ (#ANR-10-LABX-0023 and #ANR-11-IDEX-0005-02), and the ANR through a chaire d’excellence Sorbonne Paris Cite’. Parts of this work were supported by IPGP multidisciplinary program PARI, and by Paris-IdF region SESAME (#12015908). M. B. acknowledges funding from the Carlsberg Foundation (CF18-1105), the Danish National Research Foundation (DNRF97) and the European Research Council (ERC Advanced Grant Agreement, #833275-DEEPTIME). M. S. acknowledges funding from the Villum Fonden (#00025333). E.v.K. acknowledges funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie Grant Agreement No786081. We also appreciate the Arizona State University Center for Meteorite Studies for providing some ofthe samples used in this study. US Antarctic meteorite samples are recovered by the Antarctic Search for Meteorites (ANSMET) program which has been funded by NSF and NASA, and characterized and curated by the Department of Mineral Sciences of the Smithsonian Institution and Astromaterials Acquisition and Curation Office at NASA
Johnson Space Center. Timothy Mock is appreciated for providing the Cr isotope standard of NIST SRM 3112a. K. Z. thanks the China Scholarship Council (CSC) and IPGP for a PhD fellowship (#201706340161) and the Aide a` la MOBILITE INTERNATIONALE des doctorants de l’IPGP (2019), respectively.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Alexander CMOD. Quantitative models for theelemental and isotopic fractionations in chondrites: The car-bonaceouschondrites. Geochim Cosmochim Acta. 2019a;254:277–309. [Google Scholar]
- Alexander CMOD. Quantitative models for theelemental and isotopic fractionations in the non-carbonaceouschondrites. Geochim Cosmochim Acta. 2019b;254:246–276. [Google Scholar]
- Alexander CMOD, Greenwood RC, Bowden R, Gibson JM, Howard KT, Franchi IA. A mutli-technique search for the most primitive CO chondrites. Geochim Cos-mochim Acta. 2018;221:406–420. [Google Scholar]
- Allègre CJ, Poirier J-P, Humler E, Hofmann AW. The chemical composition of the Earth. Earth Planet Sci Lett. 1995;134:515–526. [Google Scholar]
- Amelin Y. U-Pb ages of angrites. Geochim Cosmochim Acta. 2008;72:221–232. [Google Scholar]
- Anders E. Origin, age, and composition ofmeteorites. Space Sci Rev. 1964;3:583–714. [Google Scholar]
- Barrat JA, Zanda B, Moynier F, Bollinger C, Liorzou C, Bayon G. Geochemistry of CI chondrites: Major and trace elements, and Cu and Zn Isotopes. Geochim Cosmochim Acta. 2012;83:79–92. [Google Scholar]
- Birck J-L, Allègre CJ. Manganese—chromium isotopesystematics and the development of the early Solar System. Nature. 1988;331:579–584. [Google Scholar]
- Bischoff A, Vogel N, Roszjar J. The Rumurutichondrite group. Geochemistry. 2011;71:101–133. [Google Scholar]
- Bizzarro M, Paton C, Larsen K, Schiller M, Trinquier A, Ulfbeck D. High-precision Mg-isotope measurements ofterrestrial and extraterrestrial material by HR-MC-ICPMS—implications for the relative and absolute Mg-isotope composition of the bulk silicate Earth. J Anal At Spectrom. 2011;26:565–577. [Google Scholar]
- Bollard J, Connelly JN, Bizzarro M. Pb-Pb dating ofindividual chondrules from the CBa chondrite Gujba: Assess ment of the impact plume formation model. Meteorit Planet Sci. 2015;50:1197–1216. doi: 10.1111/maps.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollard J, Connelly JN, Whitehouse MJ, Pringle EA, Bonal L, Jørgensen JK, Nordlund A˚, Moynier F, Bizzarro M. Early formation of planetary building blocks inferredfrom Pb isotopic ages of chondrules. Sci Adv. 2017;3:e1700407. doi: 10.1126/sciadv.1700407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollard J, Kawasaki N, Sakamoto N, Olsen M, Itoh S, Larsen K, Wielandt D, Schiller M, Connelly JN, Yurimoto H, Bizzarro M. Combined U-corrected Pb-Pb dating and26Al-26Mg systematics of individual chondrules – Evidence fora reduced initial abundance of 26Al amongst inner SolarSystem chondrules. Geochim Cosmochim Acta. 2019;260:62–83. [Google Scholar]
- Bourdon B, Fitoussi C. Isotope fractionation duringcondensation and evaporation during planet formation processes. ACS Earth Space Chem. 2020;4:1408–1423. [Google Scholar]
- Braukmüller N, Wombacher F, Hezel DC, Escoube R, Münker C. The chemical composition of carbonaceouschondrites: Implications for volatile element depletion, complementarity and alteration. Geochim Cosmochim Acta. 2018;239:17–48. [Google Scholar]
- Brennecka GA, Wadhwa M. Uranium isotopecompositions of the basaltic angrite meteorites and the chronological implications for the early Solar System. Proc Natl Acad Sci. 2012;109:9299–9303. doi: 10.1073/pnas.1114043109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde G, Burkhardt C, Brennecka GA, Fischer-Gödde M, Kruijer TS, Kleine T. Molybdenum isotopicevidence for the origin of chondrules and a distinct geneticheritage of carbonaceous and non-carbonaceous meteorites. Earth Planet Sci Lett. 2016;454:293–303. [Google Scholar]
- Budde G, Burkhardt C, Kleine T. Molybdenumisotopic evidence for the late accretion of outer Solar Systemmaterial to Earth. Nat Astron. 2019;1 [Google Scholar]
- Bunch TE, Irving AJ, Wittke JH, Rumble D, Gellissen M, Palme H. Evidence for pervasive metamorphism onthe CR chondrite parent body from highly equilibrated CR6chondrites Northwest Africa 2994 and Northwest Africa 3100. LPI. 2008:1991. [Google Scholar]
- Clayton RN, Mayeda TK. The oxygen isotope record in Murchison and other carbonaceous chondrites. Earth Planet Sci Lett. 1984;67:151–161. [Google Scholar]
- Clayton RN, Mayeda TK. Oxygen isotope studies ofcarbonaceous chondrites. Geochim Cosmochim Acta. 1999;63:2089–2104. [Google Scholar]
- Clayton RN, Mayeda TK, Goswami J, Olsen EJ. Oxygen isotope studies of ordinary chondrites. Geochim Cosmochim Acta. 1991;55:2317–2337. [Google Scholar]
- Clayton RN, Mayeda TK, Rubin AE. Oxygen isotopic compositions of enstatite chondrites and aubrites. J Geophys Res Solid Earth. 1984;89:C245–C249. [Google Scholar]
- Connelly JN, Bizzarro M, Krot AN, Nordlund A˚, Wielandt D, Ivanova MA. The absolute chronology andthermal processing of solids in the solar protoplanetary disk. Science. 2012;338:651–655. doi: 10.1126/science.1226919. [DOI] [PubMed] [Google Scholar]
- Dauphas N, Chen JH, Zhang J, Papanastassiou DA, Davis AM, Travaglio C. Calcium-48 isotopic anomalies inbulk chondrites and achondrites: evidence for a uniform isotopic reservoir in the inner protoplanetary disk. Earth Planet Sci Lett. 2014;407:96–108. [Google Scholar]
- Dauphas N, Remusat L, Chen J, Roskosz M, Papanastassiou D, Stodolna J, Guan Y, Ma C, Eiler J. Neutronrich chromium isotope anomalies in supernova nanoparticles. Astrophys J. 2010;720:1577. [Google Scholar]
- Dunn TL, Gross J. Reclassification ofHart and Northwest Africa 6047: Criteria for distinguishingbetween CV and CK3 chondrites. Meteorit Planet Sci. 2017;52:2412–2423. [Google Scholar]
- Dunn TL, Gross J, Ivanova MA, Runyon SE, Bruck AM. Magnetite in the unequilibrated CK chondrites:Implications for metamorphism and new insights into the relationship between the CV and CK chondrites. Meteorit Planet Sci. 2016;51:1701–1720. [Google Scholar]
- Ebel DS, Brunner C, Konrad K, Leftwich K, Erb I, Lu M, Rodriguez H, Crapster-Pregont EJ, Friedrich JM, Weisberg MK. Abundance, major element compositionand size of components and matrix in CV, CO and Acfer 094chondrites. Geochim Cosmochim Acta. 2016;172:322–356. [Google Scholar]
- Elkins-Tanton LT, Weiss BP, Zuber MT. Chondrites as samples of differentiated planetesimals. Earth Planet Sci Lett. 2011;305:1–10. [Google Scholar]
- Eugster O. Cosmic-ray exposure ages of meteorites andlunar rocks and their significance. Geochemistry. 2003;63:3–30. [Google Scholar]
- Farquhar J, Thiemens MH, Jackson T. Atmospheresurface interactions on Mars: D17O measurements of carbonatefrom ALH 84001. Science. 1998;280:1580–1582. doi: 10.1126/science.280.5369.1580. [DOI] [PubMed] [Google Scholar]
- Fischer-Gödde M, Burkhardt C, Kruijer TS, Kleine T. Ru isotope heterogeneity in the solar protoplanetarydisk. Geochim Cosmochim Acta. 2015;168:151–171. [Google Scholar]
- Fischer-Gödde M, Kleine T. Ruthenium isotopicevidence for an inner Solar System origin of the late veneer. Nature. 2017;541:525. doi: 10.1038/nature21045. [DOI] [PubMed] [Google Scholar]
- Fujiya W, Sugiura N, Hotta H, Ichimura K, Sano Y. Evidence for the late formation of hydrous asteroids fromyoung meteoritic carbonates. Nat Commun. 2012;3:627. doi: 10.1038/ncomms1635. [DOI] [PubMed] [Google Scholar]
- Fujiya W, Sugiura N, Sano Y, Hiyagon H. Mn-Crages of dolomites in CI chondrites and the Tagish Lakeungrouped carbonaceous chondrite. Earth Planet Sci Lett. 2013;362:130–142. [Google Scholar]
- Gattacceca J, Bonal L, Sonzogni C, Longerey J. CVchondrites: More than one parent body. Earth Planet Sci Lett. 2020;547:116467 [Google Scholar]
- Gerber S, Burkhardt C, Budde G, Metzler K, Kleine T. Mixing and transport of dust in the early solar nebula asinferred from titanium isotope variations among chondrules. Astrophys J Lett. 2017;841:L17. [Google Scholar]
- Glavin D, Kubny A, Jagoutz E, Lugmair G. Mn-Cr isotope systematics of the D’Orbigny angrite. Meteorit Planet Sci. 2004;39:693–700. [Google Scholar]
- Glavin DP, Elsila JE, McLain HL, Aponte JC, Parker ET. Evidence for Extraterrestrial L-Amino AcidExcesses in the CM2 Aguas Zarcas and Murchison Carbona ceous Chondrites: Predictions for Ryugu and Bennu; 51st Lunar and Planetary Science Conference; 2020. p. 1025. [Google Scholar]
- Göpel C, Birck J-L, Galy A, Barrat J-A, Zanda B. Mn-Cr systematics in primitive meteorites: Insights from mineral separation and partial dissolution. Geochim Cos-mochim Acta. 2015;156:1–24. [Google Scholar]
- Gray C, Papanastassiou D, Wasserburg G. Theidentification of early condensates from the solar nebula. Icarus. 1973;20:213–239. [Google Scholar]
- Greenwood RC, Burbine TH, Miller MF, Franchi IA. Melting and differentiation of early-formed asteroids:The perspective from high precision oxygen isotope studies. Chem Erde. 2017;77:1–43. [Google Scholar]
- Greenwood RC, Franchi IA. Alteration andmetamorphism of CO3 chondrites: Evidence from oxygen andcarbon isotopes. Meteorit Planet Sci. 2004;39:1823–1838. [Google Scholar]
- Greenwood RC, Franchi IA, Kearsley AT, Alard O. The relationship between CK and CV chondrites. Geochim Cosmochim Acta. 2010;74:1684–1705. [Google Scholar]
- Hans U, Kleine T, Bourdon B. Rb-Sr chronology ofvolatile depletion in differentiated protoplanets: BABI, ADORand ALL revisited. Earth Planet Sci Lett. 2013;374:204–214. [Google Scholar]
- Hellmann JL, Hopp T, Burkhardt C, Kleine T. Origin of volatile element depletion among carbonaceouschondrites. Earth Planet Sci Lett. 2020;549:116508 [Google Scholar]
- Hewins RH, Bourot-Denise M, Zanda B, Leroux H, Barrat J-A, Humayun M, Göpel C, Greenwood RC, Franchi IA, Pont S. The Paris meteorite, the least altered CMchondrite so far. Geochim Cosmochim Acta. 2014;124:190–222. [Google Scholar]
- Hezel DC, Russell SS, Ross AJ, Kearsley AT. Modal abundances of CAIs: Implications for bulk chondriteelement abundances and fractionations. Meteorit Planet Sci. 2008;43:1879–1894. [Google Scholar]
- Holden NE. Total half-lives for selected nuclides. PureAppl Chem. 1990;62:941–958. [Google Scholar]
- Inglis EC, Creech JB, Deng Z, Moynier F. High-precision zirconium stable isotope measurements of geologicalreference materials as measured by double-spike MC-ICPMS. Chem Geol. 2018;493:544–552. [Google Scholar]
- Ireland TR, Avila J, Greenwood RC, Hicks LJ, Bridges JC. oxygen isotopes and sampling of the solar system. Space Sci Rev. 2020;216:25. [Google Scholar]
- Isa J, Rubin AE, Wasson JT. R-chondrite bulkchemical compositions and diverse oxides: Implications forparent-body processes. Geochim Cosmochim Acta. 2014;124:131–151. [Google Scholar]
- Javoy M, Kaminski E, Guyot F, Andrault D, Sanloup C, Moreira M, Labrosse S, Jambon A, Agrinier P, Davaille A. The chemical composition of the Earth: Enstatitechondrite models. Earth Planet Sci Lett. 2010;293:259–268. [Google Scholar]
- Jenniskens P, Fries MD, Yin Q-Z, Zolensky M, Krot AN, Sandford SA, Sears D, Beauford R, Ebel DS, Friedrich JM, Nagashima K, et al. Radar-enabled recovery of the Sutter’s millmeteorite, a carbonaceous chondrite regolith breccia. Science. 2012;338:1583–1587. doi: 10.1126/science.1227163. [DOI] [PubMed] [Google Scholar]
- Johansen A, Low M-M-M, Lacerda P, Bizzarro M. Growth of asteroids, planetary embryos, and Kuiper beltobjects by chondrule accretion. Sci Adv. 2015;1:e1500109. doi: 10.1126/sciadv.1500109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadlag Y, Becker H, Harbott A. Cr isotopes inphysically separated components of the Allende CV3 andMurchison CM2 chondrites: Implications for isotopic heterogeneity in the solar nebula and parent body processes. Meteorit Planet Sci. 2019;54:2116–2131. [Google Scholar]
- Kallemeyn GW, Rubin AE, Wasson JT. The compositional classification of chondrites: V. The Karoonda(CK) group of carbonaceous chondrites. Geochim Cosmochim Acta. 1991;55:881–892. [Google Scholar]
- Kallemeyn GW, Wasson JT. The compositionalclassification of chondrites—I. The carbonaceous chondritegroups. Geochim Cosmochim Acta. 1981;45:1217–1230. [Google Scholar]
- Koeberl C, Shukolyukov A, Lugmair GW. Chromiumisotopic studies of terrestrial impact craters: Identification ofmeteoritic components at Bosumtwi, Clearwater East, Lap-pajärvi, and Rochechouart. Earth Planet Sci Lett. 2007;256:534–546. [Google Scholar]
- Krot AN, Amelin Y, Cassen P, Meibom A. Youngchondrules in CB chondrites from a giant impact in the earlysolar system. Nature. 2005;436:989–992. doi: 10.1038/nature03830. [DOI] [PubMed] [Google Scholar]
- Krot AN, Keil K, Scott ERD, Goodrich CA, Weisberg MK. 1.1 – Classification ofmeteorites and their genetic relationships A2 – Holland, Heinrich D. In: Turekian KK, editor. Treatise on Geochemistry. second. Elsevier; Oxford: 2014. pp. 1–63. [Google Scholar]
- Krot AN, Meibom A, Keil K. A clast of Bali-likeoxidized CV material in the reduced CV chondrite brecciaVigarano. Meteorit Planet Sci. 2000;35:817–825. [Google Scholar]
- Krot AN, Nagashima K. Constraints on mechanismsof chondrule formation from chondrule precursors andchronology of transient heating events in the protoplanetarydisk. Geochem J. 2017;51:45–68. [Google Scholar]
- Krot AN, Petaev MI, Scott ER, Choi BG, Zolensky ME, Keil K. Progressive alteration in CV3 chondrites:More evidence for asteroidal alteration. Meteorit Planet Sci. 1998;33:1065–1085. [Google Scholar]
- Kruijer TS, Borg LE, Wimpenny J, Sio CK. Onsetof magma ocean solidification on Mars inferred from Mn-Crchronometry. Earth Planet Sci Lett. 2020;542:116315 [Google Scholar]
- Langbroek M, Jenniskens P, Kriegsman LM, Nieuwenhuis H, De Kort N, Kuiper J, Van Westrenen W, Zolensky ME, Ziegler, Yin QZ. The CM carbonaceous chondriteregolith Diepenveen. Meteorit Planet Sci. 2019;54:1431–1461. [Google Scholar]
- Larsen KK, Trinquier A, Paton C, Schiller M, Wielandt D, Ivanova MA, Connelly JN, Nordlund A˚, Krot AN, Bizzarro M. Evidence for magnesium isotope heterogeneity in the solar protoplanetary disk. Astrophys J Lett. 2011;735:L37. [Google Scholar]
- LarsenK K, Wielandt D, Bizzarro M. Multi-elemention-exchange chromatography and high-precision MC-ICP-MSisotope analysis of Mg and Ti from sub-mm-sized meteoriteinclusions. J Anal At Spectrom. 2018;33:613–628. [Google Scholar]
- Larsen KK, Wielandt D, Schiller M, Bizzarro M. Chromatographic speciation of Cr(III)-species, inter-speciesequilibrium isotope fractionation and improved chemical purification strategies for high-precision isotope analysis. J Chromatogr A. 2016;1443:162–174. doi: 10.1016/j.chroma.2016.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Yin Q-Z, Bao H, Sanborn ME, Irving A, Ziegler K, Agee C, Marti K, Miao B, Li X, Li Y, et al. Evidence for a multilayered internal structure of the chondritic acapulcoite-lodranite parent asteroid. Geochim Cosmochim Acta. 2018;242:82–101. [Google Scholar]
- Lodders K, Fegley B, Lodders F. The planetaryScientist’s Companion. Oxford University Press on Demand; 1998. [Google Scholar]
- Lugmair G, Shukolyukov A. Early solar system timescales according to 53 Mn-53 Cr systematics. Geochim Cosmochim Acta. 1998;62:2863–2886. [Google Scholar]
- Magna T, Zˇaák K, Pack A, Moynier F, Mougel B, Peters S, Skaála R, Jonaásˇovaá Sˇ, Mizera J, Rˇanda Z. Zhamanshin astrobleme provides evidence for carbonaceouschondrite and post-impact exchange between ejecta and Earth’satmosphere. Nat Commun. 2017;8:227. doi: 10.1038/s41467-017-00192-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahan B, Moynier F, Siebert J, Gueguen B, Agranier A, Pringle EA, Bollard J, Connelly JN, Bizzarro M. Volatile element evolution of chondrules through time. Proc Natl Acad Sci. 2018;115:201807263. doi: 10.1073/pnas.1807263115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann U, Frost DJ, Rubie DC. Evidence for high-pressure core-mantle differentiation from the metal-silicatepartitioning of lithophile and weakly-siderophile elements. Geochim Cosmochim Acta. 2009;73:7360–7386. [Google Scholar]
- Mougel B, Moynier F, Göpel C. Chromium isotopichomogeneity between the Moon, the Earth, and enstatitechondrites. Earth Planet Sci Lett. 2018;481:1–8. [Google Scholar]
- Mougel B, Moynier F, Göpel C, Koeberl C. Chromiumisotope evidence in ejecta deposits for the nature of Paleoproterozoic impactors. Earth Planet Sci Lett. 2017;460:105–111. [Google Scholar]
- Mougel B, Moynier F, Koeberl C, Wielandt D, Bizzarro M. Identification of a meteoritic component usingchromium isotopic composition of impact rocks from theLonar impact structure, India. Meteorit Planet Sci. 2019;54:2592–2599. [Google Scholar]
- Moynier F, Day JM, Okui W, Yokoyama T, Bouvier A, Walker RJ, Podosek FA. Planetary-scalestrontium isotopic heterogeneity and the age of volatiledepletion of early Solar System materials. Astrophys J. 2012;758:45. [Google Scholar]
- Moynier F, Yin Q-Z, Jacobsen B. Dating the first stageof planet formation. Astrophys J Lett. 2007;671:L181. [Google Scholar]
- Newton J, Franchi IA, Pillinger CT. The oxygenisotopic record in enstatite meteorites. Meteorit Planet Sci. 2000;35:689–698. [Google Scholar]
- Nittler LR, Alexander CMD, Liu N, Wang J. Extremely 54Cr-and 50Ti-rich presolar oxide grains in aprimitive meteorite: Formation in rare types of supernovaeand implications for the astrophysical context of solar systembirth. Astrophys J Lett. 2018;856:L24. doi: 10.3847/2041-8213/aab61f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyquist L, Lindstrom D, Mittlefehldt D, Shih CY, Wiesmann H, Wentworth S, Martinez R. Manganese-chro-mium formation intervals for chondrules from the Bishunpurand Chainpur meteorites. Meteorit Planet Sci. 2001;36:911–938. [Google Scholar]
- Olsen MB, Wielandt D, Schiller M, Van Kooten EMME, Bizzarro M. Magnesium and 54Cr isotope compositions of carbonaceous chondrite chondrules – Insights intoearly disk processes. Geochim Cosmochim Acta. 2016;191:118–138. doi: 10.1016/j.gca.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palme H, O’Neill HSC. 3.1 – Cosmochemicalestimates of mantle composition. In: Holland HD, Turekian KK, editors. Treatise on Geochemistry. second. Elsevier; Oxford: 2014. pp. 1–39. [Google Scholar]
- Pedersen SG, Schiller M, Connelly JN, Bizzarro M. Testing accretion mechanisms of the H chondrite parent bodyutilizing nucleosynthetic anomalies. Meteorit Planet Sci. 2019;54:1215–1227. [Google Scholar]
- Petitat M, Birck J-L, Luu T, Gounelle M. Thechromium isotopic composition of the ungrouped carbonaceous chondrite Tagish Lake. Astrophys J. 2011;736:23. [Google Scholar]
- Piani L, Marrocchi Y, Libourel G, Tissandier L. Magmatic sulfides in the porphyritic chondrules of EH enstatitechondrites. Geochim Cosmochim Acta. 2016;195:84–99. [Google Scholar]
- Piani L, Marrocchi Y, Rigaudier T, Vacher LG, Thomassin D, Marty B. Earth’s water may have been inheritedfrom material similar to enstatite chondrite meteorites. Science. 2020;369:1110–1113. doi: 10.1126/science.aba1948. [DOI] [PubMed] [Google Scholar]
- Podosek F, Ott U, Brannon J, Neal C, Bernatowicz T, Swan P, Mahan S. Thoroughly anomalous chromium inOrgueil. Meteorit Planet Sci. 1997;32:617–627. [Google Scholar]
- Popovas A, Nordlund A˚, Ramsey JP, Ormel CW. Pebble dynamics and accretion on to rocky planets-I. Adiabaticand convective models. MNRAS. 2018;479:5136–5156. [Google Scholar]
- Qin L, Alexander CMOD, Carlson RW, Horan MF, Yokoyama T. Contributors to chromium isotope variation of meteorites. Geochim Cosmochim Acta. 2010;74:1122–1145. [Google Scholar]
- Qin L, Nittler LR, Alexander CMOD, Wang J, Stadermann FJ, Carlson RW. Extreme 54Cr-rich nano-oxidesin the CI chondrite Orgueil – Implication for a late supernovainjection into the solar system. Geochim Cosmochim Acta. 2011;75:629–644. [Google Scholar]
- Rotaru M, Birck JL, Allègre CJ. Clues to early solarsystem history from chromium isotopes in carbonaceouschondrites. Nature. 1992;358:465. [Google Scholar]
- Rubin AE, Fegley B, Brett R. Oxidation state in chondrites. Meteorit Early Solar Syst. 1988:488–511. [Google Scholar]
- Rubin AE, Kallemeyn GW, Wasson JT, Clayton RN, Mayeda TK, Grady M, Verchovsky A. Gujba: A new Bencubbin-like meteorite fall from Nigeria. LPI Contributions; 2001. p. 1779. [Google Scholar]
- Sanborn ME, Wimpenny J, Williams CD, Yamakawa A, Amelin Y, Irving AJ, Yin Q-Z. Carbonaceousachondrites Northwest Africa 6704/6693: Milestones for early solar system chronology and genealogy. Geochim CosmochimActa. 2019;245:577–596. [Google Scholar]
- Scherer P, Schultz L. Noble gas record, collisionalhistory, and pairing of CV, CO, CK, and other carbonaceouschondrites. Meteorit Planet Sci. 2000;35:145–153. [Google Scholar]
- Schiller M, Bizzarro M, Fernandes VA. Isotopicevolution of the protoplanetary disk and the building blocks ofEarth and the Moon. Nature. 2018;555:507. doi: 10.1038/nature25990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller M, Bizzarro M, Siebert J. Iron isotope evidencefor very rapid accretion and differentiation of the proto-Earth. Sci Adv. 2020;6:eaay7604. doi: 10.1126/sciadv.aay7604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller M, Van Kooten E, Holst JC, Olsen MB, Bizzarro M. Precise measurement of chromium isotopes by MC-ICPMS. J Anal At Spectrom. 2014;29:1406–1416. doi: 10.1039/C4JA00018H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz B, Yin Q-Z, Sanborn M, Tassinari M, Caplan C, Huss G. A new type of solar-system material recoveredfrom Ordovician marine limestone. Nat Commun. 2016;7:ncomms11851. doi: 10.1038/ncomms11851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JM, Burkhardt C, Marrocchi Y, Brennecka GA, Kleine T. Early evolution of the solar accretion diskinferred from Cr-Ti-O isotopes in individual chondrules. EarthPlanet Sci Lett. 2020;551:116585 [Google Scholar]
- Schrader DL, Davidson J. CM and CO chondrites: Acommon parent body or asteroidal neighbors? Insights fromchondrule silicates. Geochim Cosmochim Acta. 2017;214:157–171. [Google Scholar]
- Schrader DL, Davidson J, Greenwood RC, Franchi IA, Gibson JM. A water-ice rich minor body from the earlySolar System: The CR chondrite parent asteroid. Earth Planet Sci Lett. 2014;407:48–60. [Google Scholar]
- Schrader DL, Franchi IA, Connolly HC, Greenwood RC, Lauretta DS, Gibson JM. The formation andalteration of the Renazzo-like carbonaceous chondrites I:Implications of bulk-oxygen isotopic composition. Geochim Cosmochim Acta. 2011;75:308–325. [Google Scholar]
- Scott ER, Krot AN. Chondrites and the Protoplanetary Disk. 2005. Chondritic meteorites and the high-temperature nebular origins of their components; p. 15. [Google Scholar]
- Shukolyukov A, Lugmair G. Manganese-chromiumisotope systematics of carbonaceous chondrites. Earth Planet Sci Lett. 2006;250:200–213. [Google Scholar]
- Sossi P, Moynier F, van Zuilen K. Volatile lossfollowing cooling and accretion of the Moon revealed bychromium isotopes. Proc Natl Acad Sci. 2018;115:10920–10925. doi: 10.1073/pnas.1809060115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossi A, Klemme S, O’NeillH SC, Berndt J, Moynier F. Evaporation of moderately volatile elements fromsilicate melts: experiments and theory. Geochim Cosmochim Acta. 2019;260:204–231. [Google Scholar]
- Spitzer F, Burkhardt C, Budde G, Kruijer TS, Morbidelli A, Kleine T. Isotopic evolution of the inner solarsystem inferred from molybdenum isotopes in meteorites. Astrophys J Lett. 2020;898:L2. [Google Scholar]
- Steele RCJ, Elliott T, Coath CD, Regelous M. Confirmation of mass-independent Ni isotopic variability iniron meteorites. Geochim Cosmochim Acta. 2011;75:7906–7925. [Google Scholar]
- Steele RC, Coath CD, Regelous M, Russell S, Elliott T. Neutron-poor nickel isotope anomalies in meteorites. Astrophys J. 2012;758:59. [Google Scholar]
- Stracke A, Palme H, Gellissen M, Münker C, Kleine T, Birbaum K, Günther D, Bourdon B, Zipfel J. Refractory element fractionation in the Allende meteorite:Implications for solar nebula condensation and the chondriticcomposition of planetary bodies. Geochim Cosmochim Acta. 2012;85:114–141. [Google Scholar]
- Sugiura N, Fujiya W. Correlated accretion ages ande54Cr of meteorite parent bodies the evolution of the solarnebula. Meteorit Planet Sci. 2014;49:772–787. [Google Scholar]
- Sun S-S. Chemical composition and origin of the Earth’sprimitive mantle. Geochim Cosmochim Acta. 1982;46:179–192. [Google Scholar]
- Trinquier A, Birck J-L, Allègre CJ. The nature of theKT impactor. A 54 Cr reappraisal. Earth Planet Sci Lett. 2006;241:780–788. [Google Scholar]
- Trinquier A, Birck J-L, Allègre CJ. Widespread 54Crheterogeneity in the inner solar system. Astrophys J. 2007;655:1179–1185. [Google Scholar]
- Trinquier A, Birck J-L, Allègre CJ. High-precisionanalysis of chromium isotopes in terrestrial and meteoritesamples by thermal ionization mass spectrometry. J Anal At Spectrom. 2008a;23:1565–1574. [Google Scholar]
- Trinquier A, Birck JL, Allègre CJ, Göpel C, Ulfbeck D. 53Mn-53Cr systematics of the early Solar Systemrevisited. Geochim Cosmochim Acta. 2008b;72:5146–5163. [Google Scholar]
- Trinquier A, Elliott T, Ulfbeck D, Coath C, Krot AN, Bizzarro M. Origin of nucleosynthetic isotope heterogeneity in the solar protoplanetary disk. Science. 2009;324:374–376. doi: 10.1126/science.1168221. [DOI] [PubMed] [Google Scholar]
- van Kooten E, Cavalcante L, Wielandt D, Bizzarro M. The role of Bells in the continuous accretion between the CMand CR chondrite reservoirs. Meteorit Planet Sci n/a. 2020 doi: 10.1111/maps.13459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanKooten EMME, Wielandt D, Schiller M, Nagashima K, Thomen A, Larsen KK, Olsen MB, Nordlund A˚, KrotA N, Bizzarro M. Isotopic evidence for primordialmolecular cloud material in metal-rich carbonaceous chondrites. Proc Natl Acad Sci. 2016;113:2011–2016. doi: 10.1073/pnas.1518183113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeesch P. IsoplotR: A free and open toolbox forgeochronology. Geosci Front. 2018;9:1479–1493. [Google Scholar]
- Visscher C, Fegley B., Jr Chemistry of impactgenerated silicate melt-vapor debris disks. Astrophys J Lett. 2013;767:L12. [Google Scholar]
- Wadhwa M, Shukolyukov A, Davis AM, Lugmair GW, Mittlefehldt DW. Differentiation history of themesosiderite parent body: constraints from trace elements andmanganese-chromium isotope systematics in Vaca Muertasilicate clasts. Geochim Cosmochim Acta. 2003;67:5047–5069. [Google Scholar]
- Wang HS, Lineweaver CH, Ireland TR. Theelemental abundances (with uncertainties) of the most Earthlike planet. Icarus. 2018;299:460–474. [Google Scholar]
- Wang K, Moynier F, Podosek F, Foriel J. 58Fe and54Cr in early solar system materials. Astrophys J Lett. 2011;739:L58. [Google Scholar]
- Wänke H, Dreibus G. Chemical composition andaccretion history of terrestrial planets. Phil Trans R Soc Lond A. 1988;325:545–557. [Google Scholar]
- Warren PH. Stable-isotopic anomalies and the accre-tionary assemblage of the Earth and Mars: A subordinate rolefor carbonaceous chondrites. Earth Planet Sci Lett. 2011;311:93–100. [Google Scholar]
- Wasson JT, Isa J, Rubin AE. Compositional andpetrographic similarities of CV and CK chondrites: A singlegroup with variations in textures and volatile concentrationsattributable to impact heating, crushing and oxidation. Geochim Cosmochim Acta. 2013;108:45–62. [Google Scholar]
- Weber HW, Franke L, Schultz L. Subsolar noblegases in metal-rich carbonaceous (CH) chondrites. Meteorit Planet Sci. 2001;36:A220 [Google Scholar]
- Weisberg MK, McCoy TJ, Krot AN. Systematicsand evaluation of meteorite classification. Meteorites and theEarly Solar System II. 2006 [Google Scholar]
- Weisberg MK, Prinz M, Clayton RN, Mayeda TK, Sugiura N, Zashu S, Ebihara M. A new metal-rich chondritegrouplet. Meteorit Planet Sci. 2001;36:401–418. [Google Scholar]
- Williams CD, Sanborn ME, Defouilloy C, Yin Q-Z, Kita NT, Ebel DS, Yamakawa A, Yamashita K. Chondrules reveal large-scale outward transport of inner SolarSystem materials in the protoplanetary disk. Proc Natl AcadSci. 2020;117:23426–23435. doi: 10.1073/pnas.2005235117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakawa A, Yamashita K, Makishima A, Nakamura E. Chromium isotope systematics of achondrites: Chronology and isotopic heterogeneity of the inner solar system bodies. Astrophys J. 2010;720:150. [Google Scholar]
- Yamashita K, Maruyama S, Yamakawa A, Nakamura E. 53Mn-53Cr chronometry of CB chondrite: Evidence foruniform distribution of 53Mn in the early solar system. Astrophys J. 2010;723:20. [Google Scholar]
- Yokoyama T, Nagai Y, Fukai R, Hirata T. Origin andevolution of distinct molybdenum isotopic variabilities withincarbonaceous and noncarbonaceous reservoirs. Astrophys J. 2019;883:62. [Google Scholar]
- Young ED, Galy A. The isotope geochemistry andcosmochemistry of magnesium. Rev Mineral Geochem. 2004;55:197–230. [Google Scholar]
- Zhang J, Dauphas N, Davis AM, Leya I, Fedkin A. The proto-Earth as a significant source of lunar material. Nat Geosci. 2012;5:251–255. [Google Scholar]
- Zhu K, Liu J, Moynier F, Qin L, Alexander CMOD, He Y. Chromium isotopic evidence for an early formationof chondrules from the Ornans CO chondrite. Astrophys J. 2019a;873:82. [Google Scholar]
- Zhu K, Moynier F, Barrat J-A, Wielandt D, Larsen K, Bizzarro M. Timing and origin of the angrite parentbody inferred from Cr isotopes. Astrophys J Lett. 2019b;877:L13. [Google Scholar]
- Zhu K, Moynier F, Schiller M, Alexander CMOD, Barrat J-A, Bischoff A, Bizzarro M. Mass-independent andmass-dependent Cr isotopic composition of the Rumuruti (R)chondrites: Implications for their origin and planet formation. Geochim Cosmochim Acta. 2021;293:598–609. [Google Scholar]
- Zhu K, Moynier F, Schiller M, Bizzarro M. Datingand tracing the origin of enstatite chondrite chondrules with Crisotopes. Astrophys J Lett. 2020a;894:L26. [Google Scholar]
- Zhu K, Moynier F, Schiller M, Wielandt D, Larsen K, vanKooten E, Bizzarro M. Chromium isotopic constraints on the origin the ureilite parent body. Astrophys J. 2020b;888:126. [Google Scholar]
- Zhu K, Sossi PA, Siebert J, Moynier F. Tracking thevolatile and magmatic history of Vesta from chromium stable isotope variations in eucrite and diogenite meteorites. Geochim Cosmochim Acta. 2019c;266:598–610. [Google Scholar]