Abstract
Malaria pathogenesis results from the asexual replication of Plasmodium falciparum within human red blood cells, which relies on a precisely timed cascade of gene expression over a 48-h life cycle. Although substantial post-transcriptional regulation of this hardwired program has been observed, it remains unclear how these processes are mediated on a transcriptome-wide level. To this end, we identified mRNA modifications in the P. falciparum transcriptome and performed a comprehensive characterization of N6-methyladenosine (m6A) over the course of blood-stage development. Using mass spectrometry and m6A RNA sequencing, we demonstrate that m6A is highly developmentally regulated, exceeding m6A levels known in any other eukaryote. We characterize a distinct m6A writer complex and show that knockdown of the putative m6A methyltransferase, PfMT-A70, by CRISPR interference leads to increased levels of transcripts that normally contain m6A. In accordance, we find an inverse correlation between m6A methylation and mRNA stability or translational efficiency. We further identify two putative m6A-binding YTH proteins that are likely to be involved in the regulation of these processes across the parasite’s life cycle. Our data demonstrate unique features of an extensive m6A mRNA methylation programme in malaria parasites and reveal its crucial role in dynamically fine-tuning the transcriptional cascade of a unicellular eukaryote.
Malaria, a mosquito-borne human disease caused by the unicellular apicomplexan parasite Plasmodium falciparum, remains a major global health threat1. Human pathogenesis results from the 48-h intra-erythrocytic developmental cycle (IDC), during which each parasite undergoes schizogony within red blood cells (RBCs) to create up to 32 new daughter cells (Fig. 1a). Each step of the IDC—from RBC invasion (0 h post-infection (h.p.i.)) to egress—is effected by a precisely timed cascade of gene expression. Throughout this process, mRNAs from the majority of genes expressed reach peak abundance only once, which is thought to correspond to the time point when its encoded product is most required2. While gene silencing is partially achieved via heterochromatinization of genes encoding variant surface antigens3 and the inducer of sexual stage development4, the large majority of the P. falciparum genome is in a euchromatic and transcriptionally permissive state throughout the IDC5. Here, dynamic chromatin accessibility in promoter regions strongly correlates with mRNA abundance, and these sequences can be recognized by transcription factors in vitro6,7. While the parasite’s periodic profile of mRNA abundance appears to be hardwired and unresponsive to perturbation throughout the IDC8,9, changes in mRNA turnover have been found to substantially contribute to overall mRNA abundances on top of transcriptional activity during the IDC10. Therefore, it has been suggested that while transcription may dictate the initial level of mRNA abundance, modulation at the RNA level could provide a more sensitive and/or responsive layer of regulation10–12. Such fine-tuned post-transcriptional regulation in P. falciparum is most evident in the dynamics of mRNA degradation rates. mRNA stability was shown to be highly dynamic during the IDC10 and to increase gradually over a single developmental cycle13. Moreover, extensive post-transcriptional repression of protein synthesis occurs throughout the IDC14, and translational efficiency was found to differ substantially among mRNA transcripts at the same time point during the IDC15. However, the underlying mechanisms orchestrating these processes on a transcriptome-wide scale are as yet unknown.
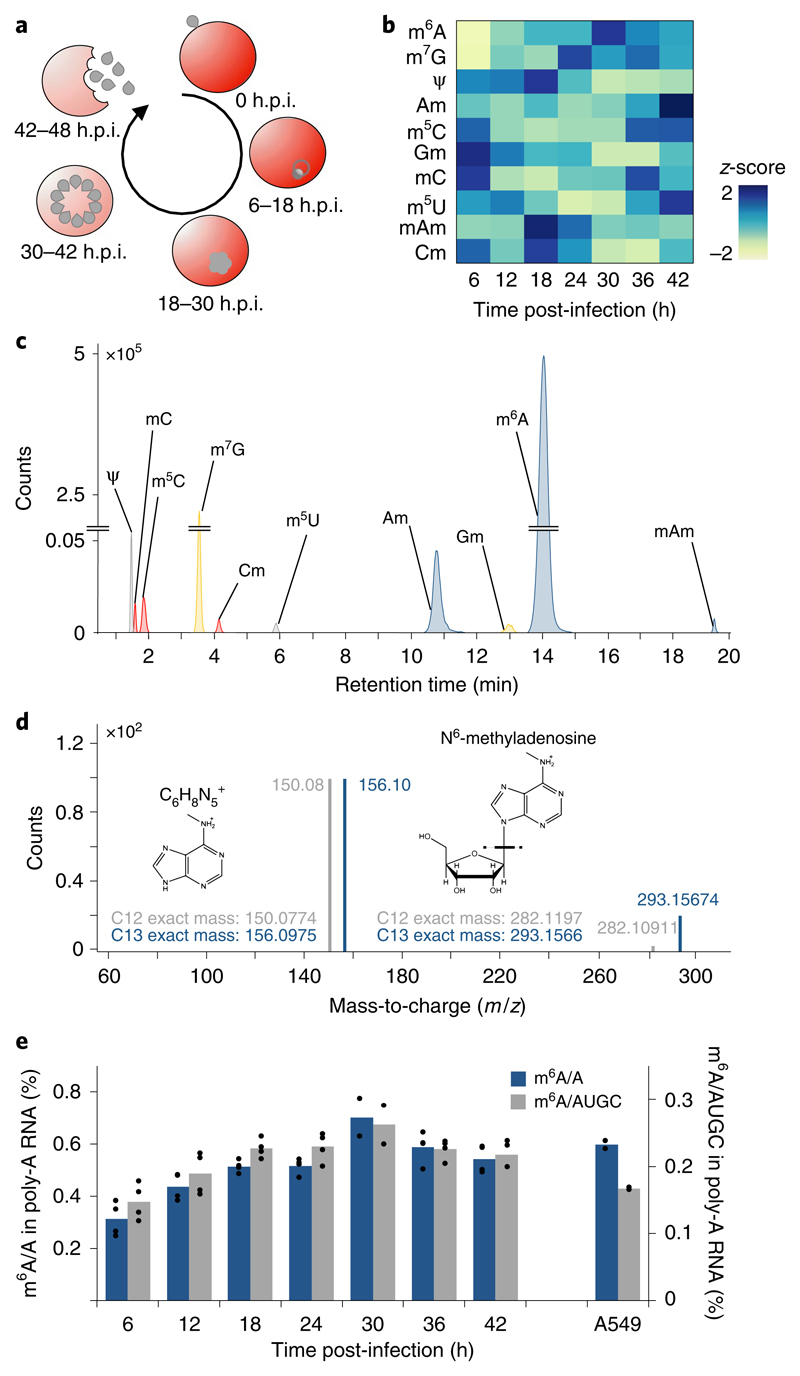
Fig. 1. global dynamics of mRNA modifications during the P. falciparum iDC.
a, Diagram illustrating the asexual replicative cycle of P. falciparum inside human RBCs including RBC invasion, host cell remodelling, replication via schizogony and RBC egress. b, Heatmap of normalized abundances (modification/canonical nucleotide) of ten mRNA modifications measured by LC-MS/MS at 6-h intervals over the course of the IDC (see also Supplementary Fig. 1d). Average of four biological replicates for each modification and timepoint, except 30 h.p.i. (n = 2). Blue indicates a high z-score while yellow indicates a low z-score (see Methods). c, LC-MS/MS extracted ion chromatograms of modified nucleotides analysed in parasite mRNA collected at 36 h.p.i. rC, cytosine; rU, uracil; rG, guanosine; rA, adenosine; m6A, N6-methyladenosine; m7G, N7-methyl-2’-guanosine; Ψ, pseudouridine; Am, 2’-O-methyladenosine; m5C, 5-methylcytosine; Gm, 2’-O-methylguanosine; mC, (3 or 4)-methylcytosine; m5U, 5-methyluridine; mAm, *-methyl-2’-O-methyladenosine; Cm, 2’-O-methylcytosine. d, Analysis of m6A by high-resolution mass spectrometry. Product ion spectrum of m6A in P. falciparum mRNA (grey) using heavy (C13) labelled E. coli tRNA (blue) as a standard. Fragmentation of m/z 282.12 (C12-red) and m/z 293.16 (C13-blue) leads to the breakage of the glycosidic bond resulting in the loss of the ribose sugar (132 Da) to form a daughter ion having a m/z 150.08 (C12-red) and m/z 156.10 (C13-blue). e, Global dynamics of calibrated m6A/A (blue) and m6A/AUGC (grey) levels across the asexual replicative cycle in 6-h intervals. As a reference, human A549 mRNA was analysed in parallel39. n = 4 for all time points except 30 h.p.i. (n = 2); n = 2 for human A549 samples. Points represent individual biological replicates with bars showing the mean.
Several recent studies in model organisms have shown that chemical nucleotide modifications located at internal positions of mRNA can act as mediators of global post-transcriptional regulation of gene expression16. Most notably, the relatively abundant N6-methyladenosine (m6A) has been shown to affect a multitude of cellular processes. In mammalian cells, m6A is catalysed by a methyltransferase complex consisting of a catalytically active m6A methyltransferase (METTL3, methyltransferase-like 3), a second methyltransferase-like protein (METTL14) and the METTL3 adaptor protein WTAP (Wilms-tumour-1 associated protein)17. METTL14 does not have enzymatic activity by itself18, but provides the binding site for substrate mRNA and allosterically induces the activity of METTL319. A key function of WTAP is the localization of the m6A ‘writer’ complex to nuclear speckles20. While other proteins can assemble with the m6A methyltransferase complex in mammalian cells, orthologues of METTL3, METTL14 and WTAP form an evolutionarily conserved m6A core complex among eukaryotes17.
m6A can change the secondary structure of RNAs21, making adjacent binding sites accessible for RNA-binding proteins (RBPs), or directly recruit specific m6A-binding proteins17. In mammalian cells, m6A-binding proteins include proteins of the YTH (YT521-B homology) family and eIF3 (eukaryotic initiation factor 3). m6A-mediated recruitment of eIF3 initiates translation independent of the canonical cap-binding eIF4E22, whereas YTHDF (YTH domain family) proteins can decrease mRNA stability23 and enhance trans-lation24. The cellular and organismal processes affected by m6A are widespread and include cell differentiation and cancer cell progression in mammalian cells25, sex determination in Drosophila 26, and meiosis in yeast27.
The P. falciparum genome has the highest AT content (~80%) of any organism sequenced so far28. More intriguing yet is the even stronger adenosine bias in the mRNA transcriptome, with adenosines constituting ~45% of all mRNA nucleotides, compared to ~32% in yeast or ~27% in humans. The reason for this high AT bias remains puzzling, but given this unique nucleotide composition and high level of post-transcriptional regulation during the IDC, we hypothesized that mRNA nucleotide modifications, especially on adenosines, could provide an additional layer of gene regulation in P. falciparum.
Here, we use mass spectrometry to identify P. falciparum mRNA modifications and show that m6A mRNA methylation is a crucial layer of post-transcriptional regulation during the IDC. We identify unique features of the parasite’s methylation program, including a distinct m6A writer complex, preferential m6A site localization, m6A-mediated translational repression and a diverse set of putative m6A-binding proteins. Using CRISPR interference, we characterize the methyltransferase PfMT-A70 as an integral component in m6A methylation and show that m6A dynamically fine-tunes a hardwired transcriptional program across P. falciparum blood-stage development.
Results
Identification of a dynamic m6A methylation program during P. falciparum blood-stage development
We first identified mRNA modifications in an unbiased manner with chromatography-coupled triple quadrupole mass spectrometry (LC-MS/MS) from synchronized parasites harvested over the course of the IDC (Supplementary Fig. 1a,b). Ten modifications previously identified in eukaryotic mRNAs were detected in our analysis over the course of the IDC (Fig. 1b,c and Supplementary Fig. 1c,d). These modifications include N7-methylguanosine (m7G), pseudouridine (Ψ), 5-methylcytidine (m5C), and N6-methyladenosine (m6A) (Supplementary Table 1 and Fig. 1d). m6A was previously reported to be the most abundant internal mRNA modification in mammalian cells16. While global m6A/A levels in P. falciparum are comparable to those in human mRNA (Fig. 1e and Supplementary Table 2), overall m6A levels in the parasite’s mRNA (45% adenosine) are higher throughout almost the entire IDC (Fig. 1e). Moreover, m6A mRNA methylation in P. falciparum is highly developmentally regulated, with a continuous increase from 0.3% at early stages of the IDC up to 0.7% m6A/A at 30 h.p.i. (Fig. 1e and Supplementary Fig. 1e).
The P. falciparum genome encodes a highly conserved putative orthologue (PF3D7_0729500) of the m6A mRNA methyltransferase18 characterized in other eukaryotes (METTL3 in mammalian cells, Ime4 in Saccharomyces cerevisiae, and MTA in Arabidopsis thaliana) (Fig. 2a). The encoded protein contains the functional domain of its putative orthologues (MT-A70) and has been designated PfMT-A70. To investigate a potentially conserved role in P. falciparum, we generated a cell line expressing an HA-tagged PfMT-A70 protein, which localized to the nucleus and the cytoplasm (Fig. 2b).
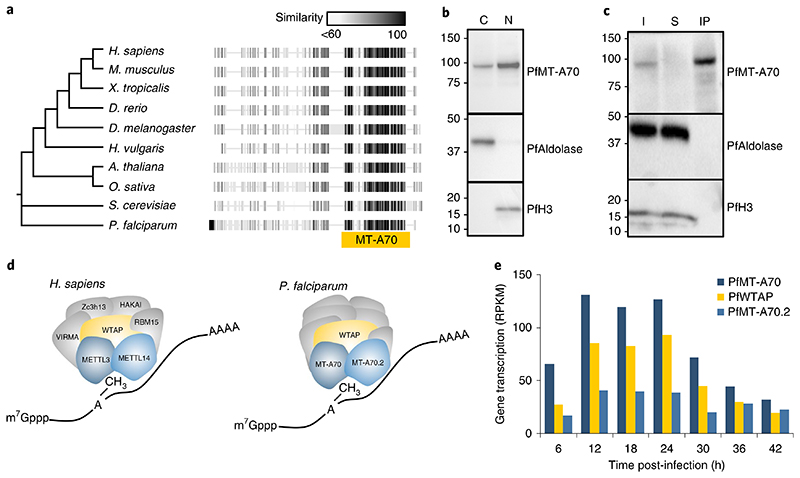
Fig. 2. Characterization of the P. falciparum m6A writer complex.
a, Phylogenetic tree and protein alignments of PfMT-A70 with orthologues in other eukaryotes. The location of the MT-A70 domain is shown below the alignment (yellow bar). The grey-scale gradient indicates the percentage similarity across all proteins at an aligned position. b, Western blot analysis showing the enrichment of HA-tagged PfMT-A70 in the cytoplasmic (C) and nuclear (N) cell fractions at 12 h.p.i. PfAldolase and histone H3 serve as controls for the cytoplasmic and nuclear fraction, respectively. Numbers on the left indicate molecular weight in kilodaltons. Data are representative of three independent biological experiments. c, Western blot analysis of PfMT-A70-HA co-IP with anti-HA antibodies. PfAldolase and histone H3 demonstrate efficacy of the anti-HA co-IP. Numbers on the left indicate molecular weight in kilodaltons. I, input; S, supernatant; IP, co-IP fraction. Data are representative of three independent biological experiments. d, Schematic of the human m6A writer complex (left) and the P. falciparum proteins that were co-immunoprecipitated with PfMT-A70. Orthologous proteins are highlighted in the same colour; that is, METTL3/PfMT-A70, METTL14/PfMT-A70.2 and WTAP/PfWTAP. Co-factors of the human core m6A writer complex (left) and putative, non-conserved co-factors of P. falciparum (right) are shown in grey. e, Gene transcription (in reads per kilobase of exon per one million mapped reads (RPKM))82 of putative orthologues of the P. falciparum m6A writer complex at 6-h intervals over the course of the IDC.
We next performed immunoprecipitation of PfMT-A70-HA followed by LC-MS and identified 16 specific proteins (Fig. 2c,d and Supplementary Table 3), one of which (PF3D7_1230800) is highly conserved with eukaryotic orthologues of WTAP (Fig. 2d and Supplementary Fig. 2a). The P. falciparum orthologue contains a WTAP/MUM2 domain and was designated PfWTAP. We identified a second putative mRNA methyltransferase containing an MT-A70 domain (PF3D7_1235500, designated PfMT-A70.2) that shows high sequence conservation with orthologues of the bilaterian METTL14 (Fig. 2d and Supplementary Fig. 2a). All three P. falciparum orthologues reach peak transcription levels between 12 and 24 h.p.i., shortly before maximal m6A/A ratios are reached (Figs. 1e and 2e). Among the other co-immunoprecipitated proteins are known RNA/DNA binding proteins such as Alba3, an ATP-dependent RNA helicase, and a FoP-domain-containing protein. Three proteins identified with high confidence (PF3D7_0707200, PF3D7_1036900 and PF3D7_1366300) have unknown functions and show no homology to other eukaryotic proteins (Supplementary Table 3).
CRISPR interference of PfMT-A70 decreases global m6A mRNA methylation
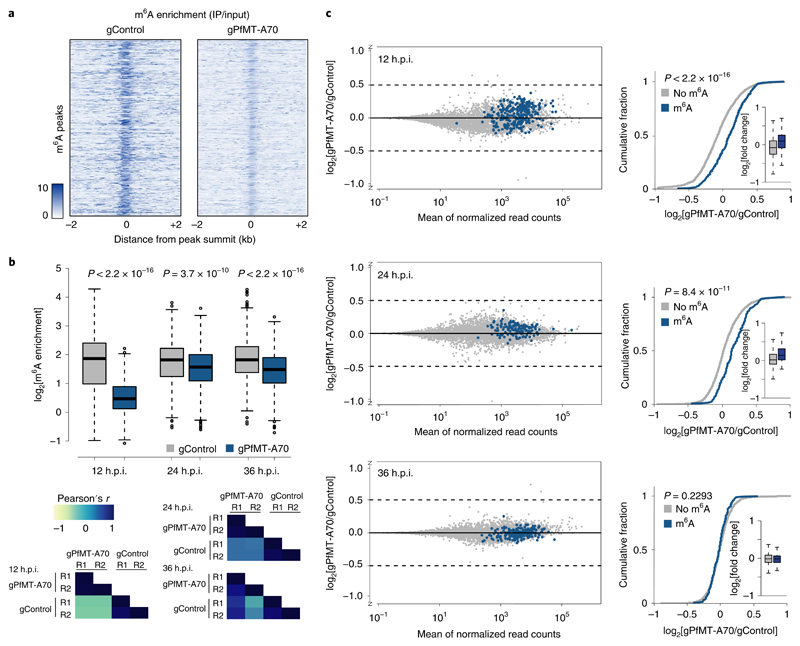
We targeted PfMT-A70 to gain insight into the functional role of m6A mRNA methylation in P. falciparum. As attempted CRISPR/Cas9-targeted deletion was unsuccessful, we utilized CRISPR interference29 (CRISPRi; Supplementary Fig. 2b), which has recently been adapted for P. falciparum 30. For CRISPRi of PfMT-A70, we targeted its promoter downstream of the transcription start site and ~100 bp upstream of the translation start site with a gRNA complementary to the non-template strand (‘gPfMT-A70’, Fig. 3a). A cell line expressing a non-specific gRNA served as a negative control (‘gControl’, Fig. 3a). gPfMT-A70-targeted dCas9 chromatin immunoprecipitation followed by sequencing (ChIP-seq)31 showed a robust and highly specific enrichment at the target site, which overlaps with a nucleosome-depleted region (FAIRE)32 enriched for H3K9ac5 and H2A.Z33 (that is, hallmarks of promoter regions in P. falciparum 34; Fig. 3b,c).
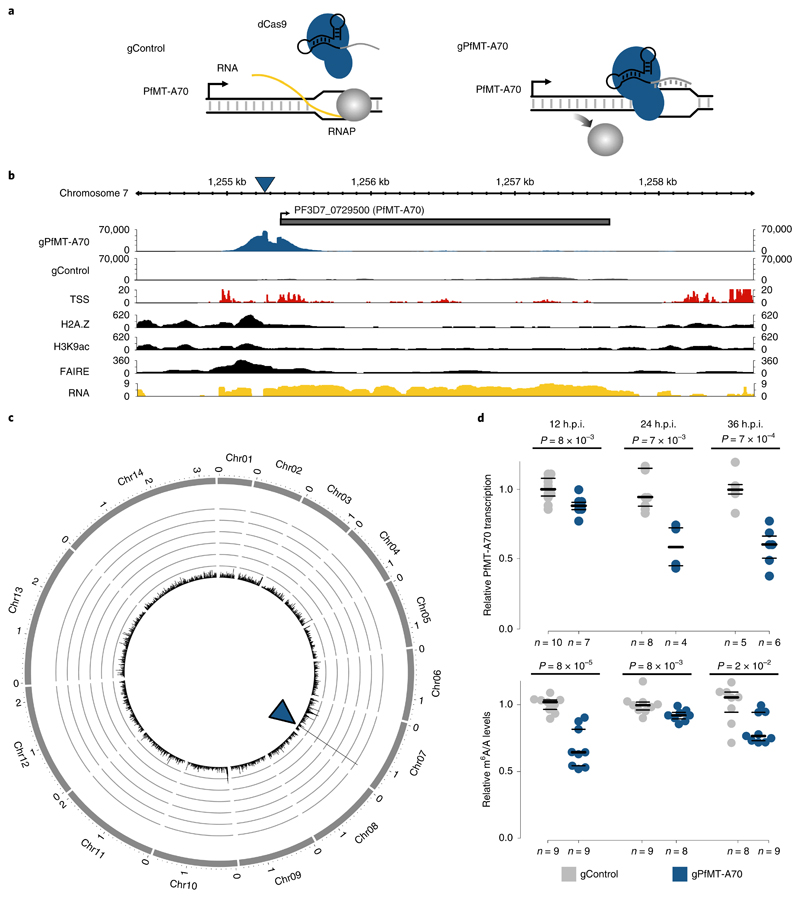
Fig. 3. Knockdown of the PfMT-A70 m6A methyltransferase by CRiSPRi.
a, Diagram of the CRISPR interference system for targeted transcriptional knockdown of PfMT-A70. A non-specific gRNA (gControl) without a binding site in the P. falciparum genome is used as a negative control in all experiments (left). The specific gRNA (gPfMT-A70) targets the PfMT-A70 promoter on the non-template strand downstream of the putative transcription start site, blocking the elongating RNA polymerase II (RNAP) and silencing the target gene (right). b, dCas9-ChIP sequencing (12 h.p.i.) shows enrichment for gPfMT-A70-targeted dCas9 (blue), but not non-targeted dCas9 (gControl in grey) at the targeted upstream region of the PfMT-A70 gene (indicated at the top with a grey bar). Arrow indicates direction of transcription. The blue triangle indicates the location of the gRNA target site. Genomic features investigated in previous studies that define the putative promoter region are shown below: transcription start site (TSS)34, histone 2A variant (H2A.Z)33, acetylation of histone H3 at lysine 9 (H3K9ac)5, nucleosome depletion as determined by FAIRE-seq32 (FAIRE), and RNA-seq coverage (RNA) at the genomic locus6. c, Circos plot of gPfMT-A70 dCas9 ChIP-seq (normalized to gControl) across all 14 nuclear chromosomes (exterior grey bars) shows no substantial enrichment of dCas9-binding other than at the PfMT-A70 promoter (blue arrowhead), demonstrating the high specificity of the method. Chromosome name and length (in megabases) are indicated at the exterior of the plot. The y axis indicates ChIP enrichment on a scale from 0 to 150 RPKM in 1,000 nt windows. dCas ChIP-seq data (b,c) are representative of two independent biological experiments. d, Comparison of PfMT-A70 transcription measured by RT-qPCR (top) and m6A/A measured by LC-MS/MS (bottom) between gControl (grey) and gPfMT-A70 (blue) parasites at three different time points over the IDC (indicated at the top). PfMT-A70 transcript levels were normalized to those of the housekeeping gene serine tRNA ligase (PF3D7_0717700). The number of biological replicates is indicated on the bottom of the graph. P values were calculated using two-sided independent-samples t-test (RT-qPCR) and a two-sided Mann-Whitney U-test (LC-MS/MS). The average of PfMT-A70 transcription and m6A/A levels in gControl parasites were set to 1. Horizontal lines represent median and interquartile ranges.
To determine the effects of PfMT-A70 CRISPRi, we harvested mRNA from synchronized gPfMT-A70 and gControl parasites at 12, 24 and 36 h.p.i. RT-qPCR revealed a significant downregulation of PfMT-A70 transcripts throughout the IDC, with knockdown levels ranging from ~15% at 12 h.p.i. to 40% at 24 and 36 h.p.i. (Fig. 3d, top). While PfMT-A70 CRISPRi did not have a discernible effect on parasite growth (Supplementary Fig. 2c), LC-MS/MS revealed a significant 10–30% decrease in global m6A/A levels at all three time points (Fig. 3d, bottom), further identifying PfMT-A70 as an integral component of the m6A writer complex in P. falciparum.
mRNA transcripts are differentially m6A-methylated during the IDC
We measured the dynamics of m6A mRNA methylation on a transcript-specific level across the IDC using m6A-methylated mRNA immunoprecipitation (IP) followed by sequencing (m6A-seq) (Supplementary Fig. 2d,e). m6A-IP followed by LC-MS/MS demonstrated high antibody specificity (Fig. 4a and Supplementary Fig. 2f). We next prepared strand-specific RNA sequencing libraries from m6A eluates (‘m6A-IP’) and mRNA input (‘m6A-input’, used for peak normalization) from two replicates of gPfMT-A70 and gControl parasites at 12, 24 and 36 h.p.i. More than 99% of reads map to protein-coding regions, confirming that the m6A LC-MS/MS measurements result from mRNA and not ribosomal or transfer RNAs.
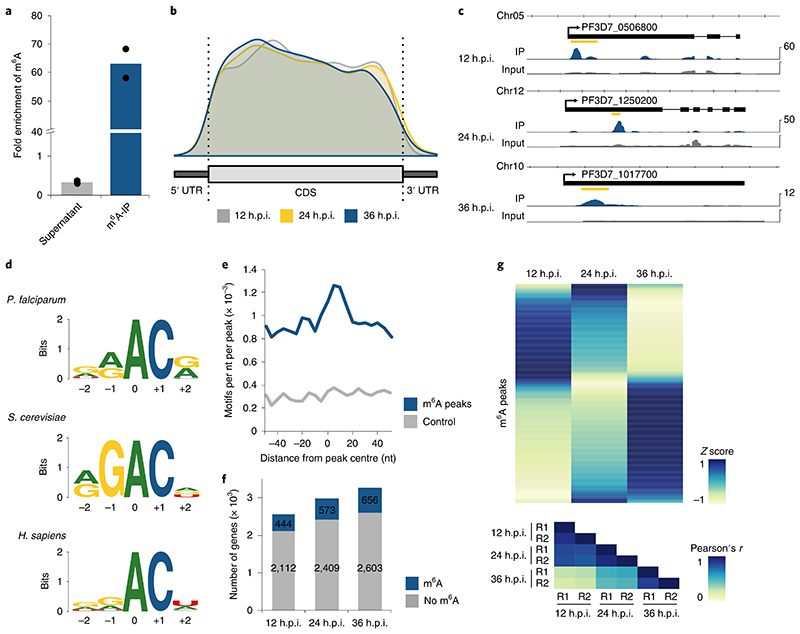
Fig. 4. Differential m6A methylation during the P. falciparum iDC.
a, Fold enrichment of m6A levels as measured by LC-MS/MS in different fractions of the m6A-IP experiment, showing a three-fold depletion in the supernatant and 63-fold enrichment in the eluate. m6A levels were normalized to the total number of adenosines (that is, m6A/A) for each fraction, and fold enrichments were calculated as ratios over the m6A/A levels in the input mRNA sample (collected at 36 h.p.i.). Points represent individual biological replicates, with bars showing the mean. b, Distribution of m6A peak location along a normalized transcript coding sequence (CDS), 500 nt upstream of the start (5’ UTR) and 500 nt downstream of the stop codon (3’ UTR) for peaks identified at 12 (grey), 24 (yellow) and 36 (blue) h.p.i. The total number of identified m6A sites by m6A-seq is likely to be an underestimate. Besides those that were not called due to the conservative annotation approach, m6A sites in transcripts reaching peak expression at different time points or sites in low complexity regions are likely to have been missed. c, Exemplary RNA sequence coverage of m6A-IP (blue) and input (grey) gControl samples showing m6A peaks identified at 12 h.p.i. in PF3D7_0506800 (transcription factor 25; RPKM, 107), 24 h.p.i. in PF3D7_1250200 (CSC1-like protein; RPKM, 53) and at 36 h.p.i. in PF3D7_1017700 (conserved protein of unknown function; RPKM, 10). The location of the m6A peak is indicated by the yellow bar. Data are representative of two independent biological experiments. d, Comparison of the deduced sequence motif of m6A methylation sites in P. falciparum (top), S. cerevisiae (middle)27 and human (bottom)35. The relative height of each nucleotide indicates frequency and the total height at each position represents sequence conservation (‘bits’). The N6-methylated adenosine is located at position 0. e, Density plot showing the occurrence of the consensus motif within ±50 nt of all significant m6A-seq peak summits (blue) and size-matched random control sequences (grey) in 5 nt windows. f, Bar charts indicating the number of transcripts (RPKM ≥5) with no (grey) or at least one significant (blue) m6A peak at three different time points over the IDC. g, Heatmap of average row Z score normalized m6A enrichments in gControl parasites (average derived from n = 2 biologically independent experiments) displaying changes in transcript-specific methylation at three time points throughout the IDC (top). Each row represents an m6A peak (n = 840) in a transcript expressed with RPKM ≥ 5 at all three time points. Bottom: heatmap showing Pearson’s r correlation coefficients of m6A enrichments, demonstrating a high degree of reproducibility between the two replicates (R1 and R2) at each time point. Blue indicates a high Z score while yellow indicates a low Z score (see Methods).
We stringently mapped m6A methylation sites in gPfMT-A70 and gControl samples (two replicates each) collected at 12, 24 and 36 h.p.i. We identified 603, 873 and 996 significant m6A peaks at 12, 24 and 36 h.p.i., which parallels the global increase in m6A/A levels as measured by LC-MS/MS. At all time points, the majority of transcripts contain only one m6A peak (Supplementary Fig. 3a) with an average of ~0.7 m6A peaks per kilobase of exon (Supplementary Fig. 3b). The main determinant of m6A peak number per transcript is transcript length: transcripts with two or more peaks are ~70% longer than transcripts with a single m6A peak. In total, we identified 1,520 non-overlapping m6A peaks across all time points, present in 1,043 distinct transcripts (Supplementary Tables 4–6). 256 peaks located in 205 distinct transcripts were identified at all three time points independently. These transcripts are enriched in functions related to RNA processing (Q = 8.5 × 10−3) and include multiple pre-mRNA splicing factors (Supplementary Table 7).
m6A peak summits are almost exclusively (n = 1,514) found within protein-coding sequences or 200 nucleotides (nt) upstream of the translation start or downstream of the translation stop site. While five m6A peaks were identified in intergenic regions and one in an intron, no enrichment of m6A peaks was observed near intron–exon boundaries (Supplementary Fig. 3c). In contrast to the topology of m6A sites in metazoans and plants, m6A peaks in P. falciparum are not enriched near the stop codon and 3’ UTR, but show a tendency for localization towards the 5’ end (Fig. 4b,c).
We searched for sequence motifs associated with m6A peaks and found a GGACA motif to be most significantly enriched (P = 1 × 10−12; Supplementary Table 8). This motif is similar to the RGAC and DRACH (D = G/A/U; R = G/A; H = C/A/U) motifs identified at yeast27 and human m6A sites35 (Fig. 4d). Compared to a control sequence set of similar length and size, motifs in an RAC context are generally enriched (Supplementary Fig. 3d), with the deduced consensus motif occurring in 87% of all m6A peaks. Similar to m6A peaks, the motif occurs twice as often within the CDS compared to the highly AT-rich 5’ and 3’ UTRs (Supplementary Fig. 3e). This motif is also centrally enriched in a region ±20 nt around the m6A peak summit (Fig. 4e), suggesting a certain context dependency for specific m6A methylation.
For an inherently dynamic transcriptional program such as that underlying the IDC of P. falciparum, changes in transcript-specific methylation might provide a means of modulating the transcriptome independently of initial gene transcription. Throughout the IDC, there is a modest increase in the total number of transcribed genes that have at least one m6A peak (Fig. 4f). However, the m6A enrichment (that is, m6A-IP/m6A-input), or fraction of transcripts from one gene containing a specific m6A peak, changes extensively throughout the IDC (Fig. 4g). Most m6A peaks reach maximum m6A enrichment at only one time point, suggesting that transcriptspecific methylation is indeed an actively regulated mechanism.
m6A inversely correlates with mRNA stability and translational efficiency
To determine the effect of m6A on individual transcripts, we first compared m6A enrichment of individual m6A peaks between gControl and gPfMT-A70 parasites and found a significant reduction in the gPfMT-A70 cell line (Fig. 5a,b, top) at all three time points, mirroring the decrease in global m6A/A levels in gPfMT-A70 parasites as measured by LC-MS/MS (Fig. 3d, bottom). These decreases are consistent, with two replicates each of gControl and gPfMT-A70 showing a high degree of correlation at each time point (Fig. 5b, bottom, and Supplementary Tables 4–6).
Fig. 5. PfMT-A70 knockdown leads to upregulation of m6A-methylated transcripts.
a, Heatmap of m6A enrichment at 12 h.p.i. depicting the decrease in m6A enrichment for individual m6A peaks (n = 603, y axis) between gControl (left) and gPfMT-A70 (right) parasites calculated in 10 nt windows in a region ± 2 kb around m6A peak summits. Darker colour indicates higher enrichment. b, Boxplot of m6A enrichment for all m6A peaks in gControl (grey) and gPfMT-A70 (blue) parasites showing a significant decrease in m6A enrichment following PfMT-A70 depletion at all three time points. m6A enrichment for each peak was averaged over two replicates in each condition and time point (see correlation between replicates below). Number of m6A peaks: 603, 873 and 996 at 12, 24 and 36 h.p.i., respectively. Centre line, median; box limits, first and third quartile; whiskers, 1.5× interquartile range. P values were calculated using a two-sided Mann-Whitney U-test. Bottom: heatmap depicting Pearson’s r correlation coefficients of m6A enrichment among replicates of gControl and gPfMT-A70 parasites at all three time points (see Supplementary Tables 4–6). Blue indicates a high score while yellow indicates a low score. c, MA-plots (left) of log2[gPfMT-A70/gControl, M] plotted over the mean abundance of each gene (A) at 12 (top), 24 (middle) and 36 (bottom) h.p.i. n = 3 for gPfMT-A70 and n = 2 for gControl at all time points. Transcripts containing m6A peaks with a more than two-fold decrease in m6A enrichment following PfMT-A70 depletion are highlighted in blue. Cumulative fraction plots (right) comparing the distribution of log2[gPfMT-A70/gControl] between non-methylated transcripts (grey; n = 2,131, 3,208 and 3,334 at 12, 24 and 36 h.p.i., respectively) and transcripts with a more than two-fold decrease in m6A enrichment following PfMT-A70 depletion (blue; n = 284, 132 and 199 at 12, 24 and 36 h.p.i., respectively) at 12 (top), 24 (middle) and 36 (bottom) h.p.i. P values were calculated using a two-sided Mann-Whitney U-test. Insets: boxplots comparing the log2 fold-changes between the two classes of transcripts. Centre line, median; box limits, first and third quartile; whiskers, 1.5× interquartile range.
We performed strand-specific mRNA sequencing in gControl and gPfMT-A70 parasites at 12, 24 and 36 h.p.i. and observed minimal changes in global transcript abundance following PfMT-A70 knockdown, with only a small percentage of genes showing significant differential expression (Supplementary Tables 9 and 10). However, at 12 and 24 h.p.i., we found a significant increase in overall abundance of transcripts that show the most pronounced decrease in m6A enrichment (≥two-fold) following PfMT-A70 depletion (Fig. 5c).
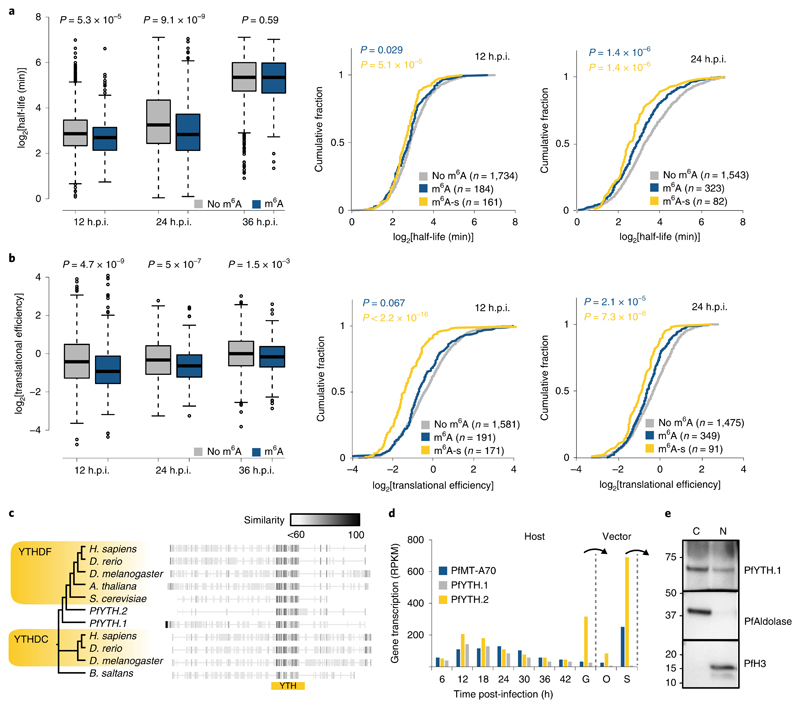
Theoretically, this observed increase in transcript abundance following PfMT-A70 knockdown could be due either to higher transcription rates or lower transcript degradation rates. Because m6A methylation was previously shown to affect mRNA degradation23, we compared mRNA stability data (measured as mRNA half-life) previously obtained from wild-type parasites13 between m6A-methylated and non-methylated transcripts found in our study. Strikingly, m6A-methylated transcripts have significantly lower mRNA stabilities than non-methylated transcripts (Fig. 6a, left). These differences are significant at 12 (P = 5.3 × 10−5) and 24 h.p.i. (P = 9.1 × 10−9), but not at the 36 h.p.i. time point (P = 0.59). We found even lower mRNA stabilities for the ‘m6A-sensitive’ transcripts (≥two-fold decrease in m6A enrichment and increased transcript abundance (log2 fold-change >0)) at 12 and 24 h.p.i. (Fig. 6a, right). These data suggest that the increase of transcript abundances following PfMT-A70 knockdown might indeed reflect decreased rates of m6A-mediated mRNA degradation at the 12 and 24 h.p.i. time points. A further comparison of m6A methylation with additional mRNA stability data10 revealed a similar pattern, showing lower mRNA stabilities for m6A-methylated than non-methylated transcripts (Supplementary Fig. 4a).
Fig. 6. Correlation of m6A with mRNA stability and translational efficiency.
a, Boxplots (left) depicting mRNA half-lives for transcripts without (grey; n = 1,734, 1,534 and 1,495 at 12, 24 and 36 h.p.i., respectively) or with (blue; n = 301, 377 and 383 at 12, 24 and 36 h.p.i., respectively) m6A peaks at 12, 24 and 36 h.p.i. Centre line, median; box limits, first and third quartile; whiskers, 1.5× interquartile range. P values were calculated with a two-sided Mann-Whitney U-test. Right: cumulative fraction plots of mRNA half-lives at 12 and 24 h.p.i. for transcripts without m6A peak (grey), with m6A peak (blue), and ‘m6A-sensitive’ (yellow) transcripts. P values were calculated with a two-sided Mann-Whitney U-test against mRNA half-lives of non-methylated transcripts. b, Boxplots (left) depicting translation efficiencies for transcripts without (grey; n = 1,584, 1,475 and 1,486 at 12, 24 and 36 h.p.i., respectively) or with (blue; n = 317, 412 and 435 at 12, 24 and 36 h.p.i., respectively) m6A peaks at 12, 24, and 36 h.p.i. Centre line, median; box limits, first and third quartile; whiskers, 1.5× interquartile range. P values were calculated with a two-sided Mann-Whitney U-test. Right: cumulative fraction plots of translation efficiencies at 12 and 24 h.p.i. for transcripts without m6A peak (grey), with m6A peak (blue), and ‘m6A-sensitive’ (yellow) transcripts. P values were calculated with a two-sided Mann-Whitney U-test against translation efficiencies of non-methylated transcripts. c, Phylogenetic tree and protein alignment of the putative m6A reader protein PfYTH and orthologues in other eukaryotes. The location of the YTH domain is indicated below (yellow bar). The grey scale gradient indicates the percentage similarity across all proteins at an aligned position. YTHDC, YTH domain-containing protein; YTHDF, YTH domain-containing family protein. d, Transcription (RPKM) of the P. falciparum PfYTH proteins and PfMT-A70 over the course of the IDC6, stage V gametocytes (G), oocysts (O) and salivary gland sporozoites (S). Arrows indicate transmission from host to vector and vice versa. e, Western blot analysis showing the enrichment of HA-tagged PfYTH in the cytoplasmic (C) and nuclear (N) fractions at 12 h.p.i. PfAldolase and histone H3 serve as controls for the cytoplasmic and nuclear fractions, respectively. Numbers on the left indicate molecular weight in kilodaltons. Data are representative of two independent biological experiments.
We next compared translational efficiencies (TE) between m6A-methylated and non-methylated transcripts throughout the IDC. TEs were calculated previously for P. falciparum as the ratio of ribosome-bound RNA versus steady state mRNA15. At each developmental stage, m6A methylation correlates with significantly lower translational efficiencies for a transcript (Fig. 6b, left). This correlation is most pronounced at the 12 (P = 4.7 × 10−9) and 24 h.p.i. (P = 5 × 10−7) time points, but is still significant at 36 h.p.i. (P = 1.5 × 10−3). Similarly to the pattern seen for mRNA stability, we observed an additional significant decrease in translational efficiencies between m6A-methylated and the subset of ‘m6A-sensitive’ transcripts at 12 and 24 h.p.i. (Fig. 6b, right). Notably, the ‘m6A-sensitive’ transcripts show no preference for m6A peak location across the coding sequence (Supplementary Fig. 4b)
A GO enrichment analysis of the ‘m6A-sensitive’ transcripts shows that these are enriched for biological processes that are highly relevant for their respective time points of development. At 12 h.p.i., these processes include regulation of gene expression (Q = 3 × 10−3), DNA-templated transcription and regulation of RNA biosynthesis (Q = 3 × 10−3). Among these gene transcripts are four members of the AP2 transcription factor family, two histone deacetylases, one histone-lysine methyltransferase, and one bromodomain-containing protein (Supplementary Table 11). At 24 h.p.i., binding to chromatin (Q = 6.1 × 10−3) is among the most significantly enriched molecular functions, including SNF2 helicases and two SET- and one bromodomain-containing protein (Supplementary Table 11).
P. falciparum encodes an evolutionarily conserved, diverse set of putative m6A-binding proteins
In several eukaryotes, proteins containing a YTH domain have been identified as specific m6A ‘reader’ proteins, mediating both mRNA degradation and translational activation in an m6A-dependent manner24. The P. falciparum genome encodes two distinct proteins containing a YTH domain: PfYTH.1 (PF3D7_1419900, previously annotated as ‘cleavage and polyadenylation specificity factor subunit 4, CPSF4)36 and PfYTH.2 (PF3D7_0309800, Fig. 6c). The YTH domain of both proteins shows high conservation of key residues known to bind mRNA and the methylated adenosine in other eukaryotes24 (Supplementary Fig. 4c). PfYTH.1 also features the C-terminal arrangement of the YTH domain and an N-terminal low-complexity disordered region that is characteristic of metazoan YTHDF proteins (Supplementary Fig. 4d)24. In contrast, PfYTH.2 does not show overall similarity to other known m6A reader proteins. Both proteins are highly expressed early during the IDC (Fig. 6d), and ectopic expression of PfYTH.1 revealed localization of the protein to both the nucleus and cytoplasm (Fig. 6e). In comparison to the dynamic expression during the IDC, PfYTH.2 shows even higher expression in the transmission stages of the parasite, namely gametocytes and salivary gland sporozoites, the latter of which also highly expresses PfMT-A70 (Fig. 6d).
Discussion
In this study, we characterize a dynamic and extensive m6A mRNA methylation program that fine-tunes the transcriptional cascade during blood-stage development of P. falciparum. We generated a high-resolution map of m6A methylation sites throughout the IDC and identify PfMT-A70 as an integral member of the putative m6A-methyltransferase complex.
Although gene knockout was unsuccessful, CRISPRi allowed us to reduce PfMT-A70 transcript levels to observe consistently decreased m6A levels via LC-MS/MS and m6A-seq, as well as increased abundance of transcripts with decreased m6A enrichment. Higher knockdown levels might be possible with multiple gRNAs, and reveal an effect on parasite growth or more drastic changes in global transcript abundances. However, even using a dCas9-repressor fusion protein did not achieve higher knockdown efficiencies of an essential gene in P. falciparum 30. Thus, it is likely that long transfection and cloning periods favour transgenic clones with lower knockdown levels and possibly lower phenotypic defects. Notably, knockdown or even knockout of the m6A-methyltransferase in D. melanogaster 26 or mouse cells37, respectively, resulted in only moderate changes in gene expression.
The P. falciparum m6A methylation program is set apart from those in other eukaryotes by several key aspects. First, while MT-A70 methyltransferases can be found throughout eukaryotes38 and the core members of the P. falciparum m6A writer complex are conserved, we did not identify other co-factors that are similarly conserved in metazoans and plants (for example, HAKAI and VIRMA39–41) in our PfMT-A70 co-immunoprecipitation. Thus, the putative P. falciparum co-factors are likely to be lineage-specific, and several of them do not share any sequence homology to other eukaryotic proteins. In line with this divergence of the extended m6A writer complex, we find that the m6A motif differs from those found in other eukaryotes27,35, showing a higher prevalence of adenosine at positions −1 and +2 that mirrors the higher adenosine content of the parasite’s mRNA transcriptome. Most identified co-factors show nuclear localization or are found exclusively in the nuclear proteome42 (for example, PfMT-A70.2), suggesting that m6A methylation in P. falciparum occurs prior to nuclear mRNA export. Unlike in other eukaryotes, there is no preference for m6A methylation sites close to the stop codon and the 3’ UTR in P. falciparum. It was proposed that this 3’ enrichment largely depends on the protein VIRMA, which in mammalian cells specifically recruits the m6A writer complex to the 3’ UTR43; however, we did not identify an orthologue in the P. falciparum genome.
With the high adenosine content in the parasite’s protein-coding transcriptome, overall m6A levels markedly exceed those measured in human mRNA, suggesting a key role for this modification during asexual replication. It is tempting to speculate that m6A methylation might represent one evolutionary driving force of high adenosine content observed at gene loci. In addition, the functional diversity of transcripts containing m6A suggests that this modification is involved in various important cellular processes during the IDC. While m6A may directly affect the fate of an mRNA transcript and its encoded protein, the modulation of transcripts encoding major regulatory proteins such as AP2 transcription factors or chromatin remodellers might further amplify its modulatory potential.
In mammalian cells, m6A levels are mostly static even during key developmental stages such as stem cell differentiation39,44, with deregulation of METTL3 and increases in global m6A of only 25% being sufficient to inhibit differentiation, promote cell growth, and maintain tumorigenicity25. Although the m6A-demethylases Alkbh5 and FTO may modulate methylation levels in some cases17, m6A is generally deposited early and maintained throughout the life of an mRNA transcript45. In contrast, the most unique feature of the P. falciparum m6A methylation program is its dynamic nature. Parallel LC-MS/MS measurements and transcript-specific m6A-seq demonstrated similar m6A dynamics, with global m6A/A levels doubling and the number of identified m6A peaks gradually increasing towards the end of the IDC. Since we did not find a potential m6A demethylase in the P. falciparum genome, m6A methylation dynamics during the IDC could result from different rates of active m6A methylation by the writer complex and the subsequent rate of m6A-methylated transcript degradation. Indeed, the observed gradual increase of global m6A/A levels measured by LC-MS/MS might be partially due to the m6A-mediated mRNA degradation at earlier but not later stages of the IDC, as is evidenced by the inverse correlation of m6A status and mRNA stability at 12 and 24 h.p.i., but not 36 h.p.i It follows that transcripts with decreased m6A enrichment are overabundant at only the earlier (and not the late) time point following PfMT-A70 depletion.
These differences in m6A-dependent mRNA degradation rates might be due to m6A reader proteins mediating mRNA turnover. Both PfYTH.1 and PfYTH.2 transcription decreases over the course of the IDC (Fig. 6d), providing a potential link between the gradual increase in global m6A/A levels and the decrease in m6A-mediated transcript degradation over the course of parasite development. Similarly, we find the strongest association between m6A and translational efficiency at 12 h.p.i., with a continuous decrease during the IDC (Fig. 6b, left). Thus, the timely action of m6A writers and the combinatorial or exclusive expression of putative reader proteins tightly regulate m6A dynamics, probably ensuring the maximal modulatory potential of m6A at each step of the lifecycle.
Mammalian YTHDF proteins 1 to 3 have been linked to mRNA degradation and translational activation24. However, based on their sequence similarity and the overlap of binding sites46, it was suggested that these proteins might be partially redundant17. In contrast, P. falciparum encodes only two YTH proteins, and while their roles are unknown, the divergence of these two proteins beyond their YTH domain and the distinct expression patterns during the parasite lifecycle suggest that they mediate two distinct functions. m6A has not been associated with translational repression in any eukaryote yet, but this post-transcriptional regulation is of particular importance for P. falciparum during the IDC15 and especially during transmission between the human host and mosquito vector47,48. During the latent gametocyte and salivary gland sporozoite stages, mRNAs are kept in a translationally repressed state and protein synthesis is only initiated following transmission, readily ensuring rapid continuation of development12,49. The co-expression of PfMT-A70 and PfYTH.2 in sporozoites suggests that m6A could also contribute to translational repression during this transmission stage (Fig. 6d).
The major factor controlling mRNA transcript abundance during the IDC is certainly the transcriptional activity at the genic locus7,10. However, here we show that the fate of individual transcripts from the same gene can be changed, possibly by decreasing mRNA stability and/or repressing translation through specific m6A methylation. Our findings that (1) transcripts with decreased m6A enrichment following PfMT-A70 knockdown were consistently more abundant and (2) there is an increased association between ‘m6A-sensitivity’ and mRNA stability and translational repression suggest that m6A serves as a balance to modulate the outcome of protein synthesis independent of initial transcriptional rates. Thus, temporally dynamic changes of transcript-specific m6A methylation rates during the IDC have, to a certain extent, the potential to finetune a possibly imprecise, hardwired transcriptional program.
Collectively, our study reveals an additional layer of dynamic and widespread post-transcriptional modulation of gene expression in P. falciparum. The conservation of core features of m6A mRNA methylation makes P. falciparum an excellent system to study the interplay between m6A methylation and gene transcription. Moreover, our results add m6A as a major player in the malaria parasite ‘epitranscriptome’ code and open new avenues for drug development in malaria parasites.
Methods
Parasite culture
Asexual blood-stage P. falciparum parasites (strain 3D7) were cultured as described previously50. Briefly, parasites were cultured in human RBCs in RPMI-1640 culture medium (Thermo Fisher no. 53400-025) supplemented with 10% v/v Albumax I (Thermo Fisher no. 11020039), hypoxanthine (0.1 mM final concentration, CC-Pro no. Z-41-M) and 10 mg gentamicin (Sigma no. G1397-10ML) at 4% haematocrit and under 5% O2, 3% CO2 at 37 °C. Parasite development was monitored by Giemsa staining. RBCs were obtained from the Etablissement Français du Sang with approval number HS 2016-24803.
For synchronization, late stage parasites were enriched with plasmagel flotation followed by ring stage enrichment via sorbitol (5%) lysis 6 h later. For sampling of highly synchronous parasites during the IDC, the synchronous schizonts were enriched by plasmagel flotation shortly before reinvasion, followed by a sorbitol lysis 6 h later. The 0 h time point was considered to be 3 h after plasmagel flotation. Parasites were collected at 4% haematocrit and ~2–3% parasitaemia.
Parasite growth assay
Parasite growth kinetics were measured as described previously51. Briefly, two clones of gControl and gPfMT-A70 P. falciparum parasites were tightly synchronized and diluted to 0.2% parasitaemia at ring stage (0 h). The growth curve was replicated in three distinct batches of RBCs on a 96-well plate (200 μl complete culture media per well). Parasitaemia was measured every 24 h by staining parasite nuclei using SYBR Green I (Sigma no. S9430) and counting (un-) infected RBCs using a Guava easyCyte flow cytometer (Merck Millipore).
Total RNA extraction
For RNA extraction, infected RBCs (iRBCs) were collected by centrifugation and washed once with Dulbecco’s phosphate-buffered saline (DPBS) (Thermo Fisher no. 14190-144) at 37 °C. iRBCs were lysed in 0.075% saponin in DPBS at 37 °C, and the parasite cell pellet was washed once with DPBS and then resuspended in 700 μl QIAzol reagent (Qiagen no. 79306). Total RNA was extracted using the miRNeasy Mini Kit (Qiagen no. 217004), including an on-column DNase I digestion and exclusion of small RNA (<200 nt) according to the manufacturer’s protocol.
Analysis of mRNA modifications by LC-MS/MS
Total RNA from highly synchronous parasites was extracted from samples collected during the IDC at seven time points at 6-h intervals (that is, 6 h.p.i. to 42 h.p.i.). To minimize human RNA and DNA contamination, parasites were cultured in leukocyte-depleted RBCs. Total RNA from human A549 cells was extracted as described above. Poly(A) RNA from P. falciparum or human A549 total RNA was enriched by two successive rounds of purification with the Dynabeads mRNA purification kit (Thermo Fisher no. 61006) (Supplementary Fig. 1b).
Purified P. falciparum RNA was hydrolysed enzymatically as described with a slightly modified protocol using the following components in the buffer mix (10 mM Tris-HCl (pH 7.9), 1 mM MgCl2, 5 U benzonase (Merck no. 71206), 50 μM desferroxamine (Sigma no. D9533), 0.1 μg μl−1 pentostatin (Sigma no. SML0508), 100 μM butylated hydroxytoluene (Sigma no. W218405), 0.5 μg μl−1 tetrahydrouridine (Calbiochem no. 584222), 5 U bacterial alkaline phosphatase (Thermo Fisher no. 18011015), 0.05 U phosphodiesterase I (Sigma no. P3243)) and [15N]5-2’-deoxyadenosine ([15N]-dA) (Cambridge Isotope Laboratories no. NLM-3895-25) as the internal standard to account for variations in sample handling and mass spectrometer response52. Hypersil GOLD aQ column (100 × 2.1 mm, 1.9 μm (Thermo Scientific no. 25305)) was used to resolve the digested ribonucleosides in a two-buffer eluent system (buffer A = 0.1% formic acid in water; buffer B = 0.1% formic acid in acetonitrile). HPLC was performed at a flow rate of 300 μl m−1 at 25 °C. The gradient of 0.1% formic acid in acetonitrile was as follows: 0–12 min, held at 0%; 12–15.3 min, 0–1%; 15.3–18.7 min, 1–6%; 18.7–20 min, held at 6%; 20–24 min, 6–100%; 24–27.3 min, held at 100%; 27.3–28 min, 100–0%; 28–41 min, 0%. The HPLC column was directly connected to an Agilent 6490 triple quadrupole mass spectrometer or Agilent 6530 q-ToF with ESI Jetstream ionization operated in positive ion mode. The voltages and source gas parameters were as follows: gas temperature, 50 °C; gas flow, 11 l min−1; nebulizer, 20 psi; sheath gas temperature, 300 °C; sheath gas flow, 12 l min−1; capillary voltage, 1,800 V; and nozzle voltage, 2,000 V. The molecular transition ions were quantified in multiple-reaction monitoring (MRM) mode (Supplementary Table 1). For the collision-induced dissociation using the q-ToF, data was acquired from 200–500 m/z with an acquisition rate of 5 spectra s−1 in MS mode and from 50 to 500 m/z with an acquisition rate of 2 spectra s−1 in MS/MS mode.
Generation of tagged PfMT-A70 and PfYTH P. falciparum strains
PfMT-A70 and PfYTH open reading frames (ORF) without stop codon were PCR-amplified from P. falciparum genomic DNA (strain 3D7) using primers MT-A70_F/MT-A70_R and YTH1_F/YTH1-R (Supplementary Table 12), respectively. A 3×FLAG-2×HA tag was synthesized by GenScript (Piscataway, USA) and PCR-amplified using the primer pair MT-A70_HA-F/HA_R for PfMT-A70 and YTH1_HA-F/HA_R for PfYTH (Supplementary Table 12). The resulting tag fragments were fused to the C terminus of the corresponding ORF fragment with PCR using primer pairs MT-A70_F/HA_R for PfMT-A70 and YTH1_F/HA_R for PfYTH (Supplementary Table 12). The final PCR fragments were cloned into the XhoI/KpnI restriction sites of the pARL-STEVORfull vector backbone53 using the In-Fusion HD cloning kit (Clontech no. 639649). The resulting plasmids were verified by Sanger sequencing. All PCR reactions were performed using the KAPA HiFi DNA Polymerase (Roche no. 07958846001) following the manufacturer’s protocol, but with lower elongation temperature (62 °C or 68 °C). Cloning and plasmid amplification were performed using XL10-Gold ultracompetent E. coli (Agilent Technologies no. 200315) following the manufacturer’s protocol. The final plasmids (pARL-PfMT-A70-3×FLAG-2×HA and pARL-PfYTH-3×FLAG-2×HA) contain the drug-selectable marker human dihydrofolate reductase (hDHFR), conferring resistance to the antifolate drug WR99210. The final plasmids encode a full length PfMT-A70 or PfYTH protein with a C-terminal 3×FLAG-2×HA tag under the chloroquine resistance transporter promoter (Supplementary Fig. 2b).
Ring-stage parasites were transfected with 50 μg plasmid by electroporation as described previously54 and drug-resistant parasites emerged after four weeks of continuous culture in the presence of 2.67 nM WR99210 (Jacobus Pharmaceuticals).
Protein fractionation and western blot analysis
One ml iRBCs containing synchronous parasites expressing HA-tagged PfMT-A70 or PfYTH at ring stage (2–3% parasitaemia) were washed once with DPBS at 37 °C, and RBCs were lysed with 0.075% saponin in DPBS. Parasites were then washed once again with DPBS. For separation of the cytoplasmic and nuclear protein fractions, the cell pellet was first resuspended in 1 ml cytoplasmic lysis buffer (CLB: 25 mM Tris-HCl pH 7.5, 10 mM NaCl, 1% IGEPAL CA-630, 1 mM DTT, 1.5 mM MgCl2, 1× protease inhibitor (PI, Roche no. 11836170001)) and incubated on ice for 30 min. Cells were further homogenized in a glass douncer, and the cytoplasmic lysate was cleared with centrifugation (13,500g, 10 min, 4 °C). The pellet (containing the nuclei) was resuspended in 100 μl nuclear extraction buffer (25 mM Tris-HCl pH 7.5, 1 mM DTT, 1.5 mM MgCl2, 600 mM NaCl, 1% IGEPAL CA-630, PI) supplemented with 1 μl benzonase (Sigma Aldrich no. E1014-5KU) and sonicated for 10 cycles with 30 s (on/off) intervals (5 min total sonication time) in a Diagenode Bioruptor Pico (Diagenode no. B01060010). This nuclear lysate was cleared with centrifugation (13,500g, 10 min, 4 °C), and the nuclear fraction was diluted with 300 μl CLB.
Protein samples were supplemented with NuPage Sample Buffer (Thermo Fisher no. NP0008) and NuPage Reducing Agent (Thermo Fisher no. NP0004) and denatured for 10 min at 70 °C. The samples were separated on a NuPage 4–12% Bis-Tris gel using MOPS running buffer at 150 V for 1.5 h and transferred to a PVDF membrane overnight at 15 V at 4 °C. The membrane was blocked for 1 h in 1% milk in 0.1% Tween20 in PBS (PBST). The HA-tagged proteins and histone H3 were detected with anti-HA (Abcam no. ab9110; 1:1,000 in 1% milk-PBST) and anti-H3 (Abcam no. ab1791: 1:1,000 in 1% milk-PBST) primary antibodies, respectively, followed by donkey anti-rabbit (GE no. NA934-1ML) secondary antibodies conjugated to HRP (1:5,000). PfAldolase was detected using an HRP conjugated anti-PfAldolase (Abcam no. ab38905, 1:5,000 in 1% milk PBST) antibody. The HRP signal was developed using the SuperSignal West Pico chemiluminescent substrate (Thermo Fisher no. 34580) and imaged with a ChemiDoc XRS+ (Bio-Rad).
PfMT-A70 co-immunoprecipitation
P. falciparum PfMT-A70-HA (n = 3 replicates) expressing parasites (cultured with 2.67 nM WR99210 (Jacobus Pharmaceuticals)) and wild-type P. falciparum 3D7 (as a negative control, cultured in standard growth medium) were sorbitol-synchronized. After 36 h, the parasites were harvested after Percoll (Sigma no. P4937) enrichment, washed twice with RPMI and crosslinked with 0.5 mM dithiobissuccinimidyl propionate (DSP) (Thermo Fisher no. 22585) in PBS for 60 min at 37 °C55. The reaction was quenched with PBS containing 25 mM Tris-HCl. These trophozoites were lysed with RIPA buffer (10 mM Tris HCl pH 7.5, 150 mM NaCl, 0.1% SDS, 1% Triton) containing protease and phosphatase inhibitor cocktail (Thermo Fisher no. 78440). The lysates were cleared by centrifugation at 16,000 g for 10 min. Supernatants were incubated with 25 μl of anti-HA Dynabeads (Thermo Fisher no. 88836) overnight at 4 °C. 20 μl of sample were taken prior to separation of the beads for Western blot analysis (‘Input’, Fig. 2c and Supplementary Fig. 5). Beads were magnetically isolated and 20 μl of sample were taken from the supernatant for Western blot analysis (‘Supernatant’, Fig. 2c and Supplementary Fig. 5). The beads were washed five times with 1 ml RIPA buffer following five washes with 1 ml PBS and one wash with 1 ml 100 mM ammonium bicarbonate (Sigma no. 09830). The beads were reduced with 10 mM dithiothreitol (Sigma no. D9779), alkylated with 55 mM iodoacetamide (Sigma no. I1149) and subjected to on-bead digestion using 50 μg of trypsin (Thermo Fisher no. 90059). The resulting peptides were desalted using C18 cartridges (Thermo Fisher no. 89852) and sent for MS analysis.
Protein mass spectrometry
Peptides were separated by reverse phase HPLC (Thermo Easy nLC1000) using a precolumn (made in house, 6 cm of 10 μm C18) and a self-pack 5 μm tip analytical column (12 cm of 5 μm C18, New Objective) over a 150 minute gradient before nanoelectrospray using a Q-Exactive HF-X mass spectrometer (Thermo Scientific). The mass spectrometer was operated in a data-dependent mode. The parameters for the full scan MS were: resolution of 70,000 across 350–2000 m/z, AGC 3e6, and maximum IT 50 ms. The full MS scan was followed by MS/MS for the top 15 precursor ions in each cycle with a NCE of 28 and dynamic exclusion of 30 s. Raw mass spectral data files (.raw) were searched using Proteome Discoverer (Thermo Fisher) and Mascot (version 2.4.1)56. Mascot search parameters were: 10 ppm mass tolerance for precursor ions; 15 milli mass units for fragment ion mass tolerance; two missed cleavages of trypsin; fixed modification was carbamidomethylation of cysteine; variable modifications were methionine oxidation and serine, threonine and tyrosine phosphorylation. Only peptides with a Mascot score greater than or equal to 25 and an isolation interference less than or equal to 30 were included in the data analysis.
CRISPRi knockdown of PfMT-A70
An enzymatically inactive or ‘dead’ Cas9 protein (dCas9) was expressed from the pUF-dCas9 plasmid, which is derived from the pUF-Cas9 plasmid backbone described in ref. 54 with the following modifications: the Cas9 was mutated at the RuvC and HNH positions, as described in ref. 57 and the 3×FLAG tag present at the N terminus of the Cas9 protein in pUF-Cas9 was removed and a 3xHA tag followed by a glmS ribozyme was added to the C terminus of the dCas9 protein in pUF-dCas9 (Supplementary Fig. 2b). Candidate target sites for dCas9 had to be (1) specific, (2) close to the transcription start site, and (3) on the non-template strand and were identified using ‘Protospacer’58.
The guide RNA targeting the PfMT-A70 promoter (gPfMT-A70) (Supplementary Table 12) was synthesized by Eurofins Genomics (Ebersberg, Germany) and cloned into the BtgZ1 restriction site of the pL6-egfp plasmid as described elsewhere54 using the In-Fusion HD cloning kit (Clontech no. 639649). For the gControl gRNA, the pL6-egfp plasmid was unaltered. Cloning and plasmid amplification were performed using XL10-Gold ultracompetent E. coli (Agilent Technologies no. 200315) following the manufacturer’s protocol and plasmids were purified using the NucleoBond Xtra Maxi Plus kit (Macherey Nagel no. 740416.10) according to the manufacturer’s instructions. Ring stage parasites were transfected with 25 μg of pL6 and pUF plasmids by electroporation as described previously54 and drug-resistant parasites emerged after four weeks of continuous culture in the presence of 2.67 nM WR99210 (Jacobus Pharmaceuticals) and 1.5 μM DSM1. Parasite cloning was performed by limiting dilution as described previously54.
Quantitative PCR with reverse transcription
Total RNA was extracted from gPfMT-A70 and gControl parasites collected at 12, 24, and 36 h.p.i., as described above. cDNA was generated using the Superscipt VILO cDNA synthesis kit (Thermo Fisher no. 11754050) following the manufacturer’s protocol. PfMT-A70 was amplified in technical triplicates using Power Sybr Green PCR Master Mix (Thermo Fisher no. 4367659) and primers PfMT-A70_qPCR-F/PfMT-A70_ qPCR-R (Supplementary Table 12) on a BioRad CFX qPCR machine. A no-RT control (substitution of RT during cDNA synthesis with H2O) and a no template control (no cDNA added during qPCR amplification) were included in all experiments. Mean starting quantity (SQ-mean) values of technical triplicates were extrapolated from a standard curve made with serial dilutions of genomic DNA. PfMT-A70 levels were normalized to those of an internal housekeeping control gene serine-tRNA ligase (PF3D7_0717700) using primer pair Seryl_qPCR-F/Seryl_qPCR-R (Supplementary Table 7).
dCas9 chromatin immunoprecipitation (dCas9 ChIP)
ChIP experiments were performed for gPfMT-A70 and gControl parasites at 12 h.p.i. at ~2% parasitaemia. RBC cultures (~4% haematocrit) were crosslinked for 10 min by direct addition of methanol-free formaldehyde (1% final concentration) (Thermo Fisher no. 28908). The cross-linking reaction was quenched using a final concentration of 0.125 M glycine for an additional 5 min at room temperature. The iRBC pellet was washed once with DPBS at 4 °C and lysed using 0.15% saponin in DPBS at 4 °C. Parasite cells were collected by centrifugation (3,250g, 4 °C, 5 min), washed twice in DPBS at 4 °C, snap frozen in liquid nitrogen, and stored until further use at −80 °C.
For each ChIP experiment, ~2 × 108 parasites were resuspended in 2 ml cytoplasmic lysis buffer (10 mM HEPES pH 8, 10 mM KCl, 0.1 mM EDTA pH 8, plus complete protease inhibitor (PI, Roche no. 11836170001)), transferred to a prechilled 2 ml douncer homogenizer and set on ice for 30 min. 50 μl 10% IGEPAL CA-630 were added (0.25% final concentration) and parasites were subjected to dounce homogenization. The nuclei were pelleted by centrifugation (10 min, 13,500g, 4 °C), and the supernatant (cytoplasmic fraction) was removed. The nuclei pellet was resuspend in 300 μl SDS Lysis buffer (50 mM Tris-HCl pH 8, 10 mM EDTA pH 8, 1% SDS, PI) and transferred to a Diagenode 1.5 ml sonication tube. Chromatin was sonicated to ~ 1,000 bp DNA fragments in a Bioruptor Pico Plus for 24 cycles with 30 s (on/off) intervals at high intensity (12 min total sonication time), and remaining debris were removed by centrifugation (10 min at 13,500g at 4 °C). 30 μl of sample were stored as input control at −80 °C. 120 μl of sample were diluted 1:10 in ChIP dilution buffer (16.7 mM Tris-HCl pH 8, 150 mM NaCl, 1.2 mM EDTA pH 8, 1% Triton X-100, 0.01% SDS, PI).
For each sample, 25 μl of protein G magnetic beads (Thermo Fisher no. 10004D) were used and pre-washed three times in 500 μl ChIP dilution buffer. Beads were resuspended in 100 μl ChIP dilution buffer and incubated with 1 μg anti-HA (Abcam no. ab9110) antibody for 2 h with constant rotation at 4 °C. Following antibody binding, beads were washed twice in 1 ml ChIP dilution buffer and resuspended in 25 μl ChIP dilution buffer. The ChIP sample was added to the antibody-conjugated beads and incubated overnight at 4 °C with constant rotation. Following immunoprecipitation, the beads were washed sequentially in 1 ml of low salt wash buffer (20 mM Tris-HCl pH 8, 150 mM NaCl, 2 mM EDTA pH 8, 1% Triton X-100, 0.1% SDS, PI), 1 ml high salt wash buffer (20 mM Tris-HCl pH 8, 500 mM NaCl, 2 mM EDTA pH 8, 1% Triton X-100, 0.1% SDS, PI), one mL LiCl wash buffer (10 mM Tris-HCl pH 8, 250 mM NaCl, 1 mM EDTA pH 8, 0.5% IGEPAL CA-630, 0.5% sodium deoxycholate, PI) and 1 ml TE buffer (10 mM Tris-HCl pH 8, 1 mM EDTA pH 8). For each wash, the beads were rotated for 5 min at 4 °C except for the last TE wash, which was performed at room temperature. The beads were subsequently resuspended in 210 μl elution buffer (50 mM Tris-HCl pH 8, 10 mM EDTA pH 8, 1% SDS) and protein-DNA complexes were eluted by 30 min incubation at 65 °C in an agitator (900 rpm 1 min, still 30 s). At this step, the input sample was recovered from storage, diluted with 170 μl elution buffer, and processed in parallel with the IP sample.
Protein-DNA complexes were reverse crosslinked by incubation at 65 °C for 6 h. Samples were diluted with 200 μl of TE buffer and incubated with RNaseA (0.2 mg ml−1 final concentration) (Thermo Fisher no. EN0531) at 37 °C for 2 h. Proteinase K (0.2 μg ml−1 final concentration) (NEB no. P8107S) was then added and samples were incubated at 55 °C for 2 h. DNA was purified by adding 400 μl phenol:chloroform:isoamyl alcohol, and phases were separated by centrifugation (10 min, 13,500g, 4 °C) after vigorous vortexing. 16 μl of 5 M NaCl (200 mM final concentration) and 30 μg glycogen were added to the aqueous phase, and DNA was precipitated by adding 800 μl 100% EtOH 4 °C and incubating for 30 min at −20 °C. DNA was pelleted by centrifugation (20,000g, 10 min, 4 °C) and washed with 500 μl 80% EtOH 4 °C. After centrifugation, the DNA pellet was air-dried and resuspended in 30 μl of 10 mM Tris-HCl pH 8.0.
m6A immunoprecipitation and sequencing
Two replicates were sampled for both cell lines expressing dCas9 and either a non-targeting control gRNA (gControl) or the PfMT-A70 promoter-targeting gRNA (gPfMT-A70). Total RNA from highly synchronous parasites was collected at 12, 24 and 36 h.p.i. as described above, followed by poly(A) RNA enrichment using the Dynabeads mRNA purification kit (Thermo Fisher no. 61006).
300–500 ng purified mRNA was fragmented for 15 min at 70 °C to ~ 100 nt using the NEBNext fragmentation module (NEB no. E6150S) and purified using the Qiagen MinElute kit (Qiagen no. 74204) with slight modifications: to also purify small RNA <200 nt, 100 μl of RNA sample was supplemented with 350 μl of lysis buffer (provided in the kit) followed by the addition of 700 μl of 100% ethanol. 20 ng fragmented mRNA were stored as input sample at −80 °C.
For each m6A-IP, 25 μl of protein A/G magnetic beads (Thermo Fisher no. 88802) were diluted in 250 μl reaction buffer (150 mM NaCl, 10 mM Tris-HCl pH 7.5, 0.1% IGEPAL CA-630 in nuclease-free H2O) and washed twice in 250 μl reaction buffer. The beads were resuspended in 100 μl reaction buffer and incubated with 2.5 μg anti-m6A antibody (Abcam no. ab151230) for 30 min at room temperature with constant rotation. The beads were subsequently washed three times with 0.5 ml reaction buffer. For each m6A-IP, 300–500 ng fragmented mRNA were diluted with nuclease-free H2O to a final volume of 395 μl, supplemented with 5 μl RNase inhibitor (Promega no. N2615), 100 μl 5× reaction buffer (750 mM NaCl, 50 mM Tris-HCl pH 7.5, 0.5% IGEPAL CA-630 in nuclease-free H2O) and added to the antibody-bead mixture. The sample was incubated at 4 °C with constant rotation for 2 h. Following immunoprecipitation, the supernatant was removed and the beads were washed twice in 250 μl reaction buffer, twice in 250 μl low salt wash buffer (50 mM NaCl, 10 mM Tris-HCl pH 7.5, 0.1% IGEPAL CA-630 in nuclease-free H2O), and twice in 250 μl high salt wash buffer (500 mM NaCl, 10 mM Tris-HCl pH 7.5, 0.1% IGEPAL CA-630 in nuclease-free H2O), all at 4 °C. m6A mRNA fragments were competitively eluted by two successive incubations (1 h, 1,000 rpm continuous shaking at 4 °C) with 100 μl elution buffer (6.7 mM m6A 5-monophosphate sodium salt (Sigma no. M2780-10MG), 150 mM NaCl, 10 mM Tris-HCl pH 7.5, 0.1%, 400 U RNase inhibitor (Promega no. N2615) in nuclease-free H2O). RNA was purified using the Qiagen MinElute kit (Qiagen no. 74204) as described above.
RNA sequencing libraries were prepared using the Illumina TruSeq stranded RNA Library Prep Kit (Illumina no. RS-122-2101) according to the manufacturer’s instructions with slight modifications. To account for the AT-richness of cDNA fragments, we used the KAPA Hifi polymerase (Roche no. 07958846001) at the library amplification step. The libraries were sequenced on an Illumina NextSeq 500 platform with a 1 × 150 bp single-end read layout.
RNA-sequencing for differential gene expression analysis
For RNA sequencing, three replicates of highly synchronous gPfMT-A70 and two replicates of gControl parasites were collected at 12, 24 and 36 h.p.i. Total RNA was extracted as described above and poly(A) RNA was enriched using the Dynabeads mRNA purification kit (Thermo Fisher no. 61006).
RNA sequencing libraries were prepared using the Illumina TruSeq stranded RNA Library Prep Kit (Illumina no. RS-122-2101) according to the manufacturer’s instructions with slight modifications. To account for the AT-richness of cDNA fragments, we used the KAPA Hifi polymerase (Roche no. 07958846001) at the library amplification step. The libraries were sequenced on an Illumina NextSeq 500 platform with a 1 × 150 bp single-end read layout.
Phylogenetic analysis of the m6A writer and reader complex
Candidate proteins of the P. falciparum m6A writer complex were identified by searching known members of the m6A writer complex from other eukaryotes against the P. falciparum proteome using blastp59. Protein alignments were generated using mafft60 and visualized in Geneious61. For the phylogenetic analysis, gaps in the protein alignments were removed with trimal62 and the best phylogenetic model was calculated using protTest363. Phylogenetic trees were constructed using MEGA (v7)64 with 1,000 bootstrap replicates, and the bootstrap consensus trees were visualized in FigTree (v1.4.3, http://tree.bio.ed.ac.uk/software/figtree/). Low-complexity disorder regions were annotated using D2P265
Identification and quantification of mRNA modifications by LC-MS/MS
LC/MS data was extracted using the MassHunter Qualitative and Quantitative Analysis Software (version B06.00) and further analysed as follows. Briefly, to account for any background signal that could be a contribution from the salts and enzymes in the digestion buffer, the signal intensity (that is area under the curve) for each ribonucleoside is first subtracted from a matrix sample (without any RNA). To calculate relative levels of each modified ribonucleoside and to adjust for different injection amounts of RNA in each sample, the matrix-corrected intensity of the modified ribonucleosides (for example m6A) was then divided by the intensity of the respective canonical ribonucleoside (for example rA). To adjust day-to-day fluctuation in retention time of the mass spectrometer, an internal standard ([15N]-2’-deoxyadenosine) was spiked into each sample during RNA digestion. The absolute values for N6-methyladenosine (Fig. 1e) were calculated by using an external calibration curve prepared using known standards to account for the difference in ionization efficiency in the mass spectrometer (Supplementary Table 2).
For visualization of lifecycle-dependent changes in the quantities of modified ribonucleosides (Fig. 1b), the modified nucleotide ratios (for example mλ/A) were scaled to row Z-sores following the formula z = (x-u)/s, with x = actual measured value; u = average over all samples and s = standard deviation over all samples.
dCas9 ChIP sequencing and analysis
DNA sequencing libraries were prepared using the Diagenode MicroPlex Library Preparation kit (Diagenode no. C05010014) according to the manufacturer’s instructions and sequenced on an Illumina NextSeq 500 platform with a 1 × 150 bp single-end read layout. Raw image files were converted to fastq sequence files using Illumina’s bcl2fastq (v2.19). Reads were aligned to the P. falciparum genome (plasmoDB.org, v3, release v28)28,66 using bwa ‘mem’67 allowing only unique read mappings (option ‘-c 1’). Optical duplicates and alignments with a quality score <20 were removed using samtools’ ‘rmdup’ and ‘view’68, respectively.
For coverage plots of gPfMT-A70 and gControl (Fig. 3b) ChIP-seq experiments, deeptool’s bamCompare69 was used to normalize the read coverage per genome position (option ‘–bs 1’) in the respective input and ChIP samples to the total number of mapped reads in each library (option ‘– normalizeUsingRPKM’). Normalized input coverage per bin was subtracted from the ChIP values (option ‘–ratio subtract’) and coverage plots were visualized in plasmoDB’s build-in genome browser together with other chromatin features (plasmoDB.org).
For genome-wide coverage plots (Fig. 3c), the same approach was used as above, but genome coverage was calculated not per genome position but for 1000 nt windows. To further remove background levels of unspecific dCas9 binding, the gControl ChIP-input coverage was subtracted from the gPfMT-A70 ChIP-input coverage and visualized using circos70.
RNA sequencing read mapping
Raw image files were converted to fastq sequence files using Illumina’s bcl2fastq (v2.19). The reads were aligned to the P. falciparum genome (plasmoDB.org, v3, release 28)28 using bwa ‘mem’67 allowing only unique read mappings (option ‘-c 1’). Optical duplicates and alignments with a quality score <20 were removed using samtools’ ‘rmdup’ and ‘view’68, respectively.
m6A peak calling
At each time point, we separately identified significant (FDR ≤0.05) m6A peaks for each replicate (that is, 2× gControl and 2× gPfMT-A70) using macs271 with default settings but without prior peak modelling (option ‘– nomodel’), the fragment size set to 150 bp (option ‘–extsize 150’) and the genome size (option -g) set to 11 × 106. To generate a single robust set of m6A peaks for each time point, we identified overlapping m6A peaks using bedtools ‘multiinter’72 and only retained those that were present in ≥two of the four samples at each time point. Overlaps of m6A peaks and genic regions were identified using bedtools ‘intersect’72.
m6A motif identification
For the m6A motif analysis, sequences ±100 bp of the m6A peak summit were extracted from the P. falciparum genome using bedtools ‘getfasta’72, taking the strandedness of the underlying transcript into account (option ‘-s’). A random set of control sequences was constructed as following. For each m6A peak in a transcript, a random position in the same transcript was calculated using bash ‘shuffle’ implemented in a custom script. Sequences ±100 bp surrounding the random site were extracted using bedtools ‘getfasta’ as described above. Significantly enriched motifs in the m6A peak region were subsequently identified with homer2 ‘findMotif.pl’73.
5-mers in the m6A peak region and controls sequence set were counted using jellyfish74. The deduced sequence motif was generated using all 5-mers with an AC context that were enriched ≥two-fold in the m6A peak region compared to the random control using weblogo75, taking their frequency in the m6A peak region into account. The central enrichment of the deduced sequence motif was calculated using homer2 ‘annotatePeaks.pl’.
m6A enrichment calculation
m6A enrichments were calculated following the approach originally reported39,76. Briefly, for each time point, the number of reads mapping to an m6A peak in the m6A-IP and m6A-input sample was calculated using bedtools ‘coverage-count’ and normalized to the total number of mapped reads in each sample. m6A enrichments were then calculated as m6A-IP/input for each peak and in each individual sample. To calculate decreases in global m6A enrichment in gPfMT-A70 (Fig. 5b, top) at each time point, m6A enrichments of every peak were averaged over the two replicates of each condition. Of note, m6A enrichments were highly reproducible (Fig. 5b, bottom) and the individual m6A enrichment values of each m6A peak and replicate are shown in Supplementary Tables 4–6).
For visualization of changes in m6A enrichments (Fig. 5a), we used cgat’s ‘bam2geneprofile’77. Briefly, the read coverage from m6A-IP and m6A-input libraries of each sample was calculated and normalized to the total size of mapped reads in each library in 10 nt windows and in a region ±2 kb surrounding the m6A peak summit. m6A enrichments in each 10 nt window were calculated as the ration of m6A-IP over m6A-input.
Differential gene expression analysis
RNA sequencing reads for three replicates of the gPfMT-A70 and two replicates of the gControl cell lines were mapped to the P. falciparum genome and quality filtered as described above. Strand specific gene counts were calculated using htseq-count78.
Differential gene expression analysis was performed using DESeq279 using all replicates of gPfMT-A70 and gControl at each time point. Due to their monoallelic nature, genes encoding variant surface antigens (var, rifin and stevor) were excluded from the analysis. MA plots were generated using the ‘baseMean’ (mean normalized read count over all replicates and conditions) and ‘log2FoldChange’ values (gPfMT-A70 over gControl) as determined by DESeq2 for each time point.
For the comparison of transcript abundances (Fig. 5c, left), we first averaged the m6A enrichment of every m6A peak over the two replicates of each condition as described above. We then retained only those m6A-methylated transcripts that harbour m6A peaks with the most pronounced decreases in m6A enrichment (that is, ≥two-fold decrease). For the direct comparison of transcript log2 fold changes (Fig. 5c, right), the set of transcripts with reduced m6A enrichments was compared to non-methylated transcripts being expressed in the same range of mean normalized read counts (that is, baseMean value).
RPKM (reads per kilobase per exon per one million mapped reads) values were calculated in R using the command rpkm() from the package edgeR80.
mRNA stability and translational efficiency analysis
For the comparative analysis of m6A methylation and mRNA stability or translation efficiency, we used m6A-methylated transcripts that were expressed in the top 50% of all transcripts at a given time point as calculated by RNA-seq of the m6A-input samples and with a peak density of ≥ 0.2 m6A peaks per kilobase of exon. Corresponding mRNA stability (measured as mRNA half-life) and translational efficiency values of the same parasite developmental stages were extracted from refs. 13,15, respectively. Independent measurements of mRNA stability data were retrieved from ref. 10.
Statistical analysis
All statistical analysis were performed in R81. To test for a normal distribution of the data, the Shapiro–Wilk normality test was used. To test for significance between two groups, a two-sided independent-samples t-test or two-sided Mann-Whitney U-test were performed as indicated. Gene Ontology enrichments were calculated using the build-in tool at plasmoDB.org. Correlations between replicates were calculated in R using function cor() with default settings (calculation of Pearson correlation coefficient r).
All heatmaps were visualized using the function heatmap2() in the R gplots package with row z-score normalization. Z-scores were calculated over all samples (‘rows’) following the formula z = (x−u)/s, with x = given value of a specific sample; u = average over all samples and s = standard deviation over all samples.
Supplementary Material
Supplementary information is available for this paper at https://doi.org/10.1038/s41564-019-0521-7.
Acknowledgements
We thank A. Claёs, C. Scheidig-Benatar and P. Chen for help with parasite culture. Protein mass-spectrometry was performed at the Biopolymers and Proteomics core of The Koch Institute Swanson Biotechnology Center. This work was supported by a European Research Council Advanced Grant (PlasmoSilencing 670301) and the French Parasitology consortium ParaFrap (ANR-11-LABX0024) to A.Scherf. Work in the labs of P.R.P. and P.C.D. was funded by the National Research Foundation Singapore under its Singapore-MIT Alliance for Research and Technology (SMART) Centre, Infectious Disease and Antimicrobial Resistance IRGs. S.B. and J.M.B. were supported by an EMBO fellowship (S.B.: ALTF 1444-2016; J.M.B.: ALTF 180-2015). A.Si. acknowledges financial support from the Singapore-MIT Alliance (SMA) Graduate Fellowships.
Footnotes
Reporting Summary. Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Author contributions
P.R.P, P.C.D. and A.Sc. conceptualized the project. S.B., J.M.B. and A.Sc. conceived experiments. J.M.B. developed and performed CRISPR interference and dCas9 ChIP-seq experiments. S.B. performed m6A-seq and RT-qPCR experiments. A.Si. performed and analysed LC-MS/MS and protein co-IP experiments. S.B., J.M.B. and T.R. generated constructs, transfectants and parasite material. S.B. performed bioinformatic analyses. P.R.P., P.C.D. and A.Sc. supervised and helped interpret analyses. All authors discussed and approved the manuscript.
Competing interests
The authors declare no competing interests.
Reprints and permissions information is available at www.nature.com/reprints.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Data availability
All sequencing data are accessible on the Gene Expression Omnibus database (https://www.ncbi.nlm.nih.gov/geo) under study accession number GSE123839. Raw sequence data are accessible on the NCBI Sequence Read Archive (SRA) under BioProject accession number PRJNA473770. Proteomics and mRNA modification LC-MS/MS data are deposited at the Chorus database with accession number 1579.
References
- 1.World Malaria Report 2018. World Health Organization; 2018. [Google Scholar]
- 2.Bozdech Z, et al. The transcriptome of the intraerythrocytic developmental cycle of Plasmodium falciparum . PLoS Biol. 2003;1:85–100. doi: 10.1371/journal.pbio.0000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scherf A, Lopez-Rubio JJ, Riviere L. Antigenic variation in Plasmodium falciparum . Annu Rev Microbiol. 2008;62:445–470. doi: 10.1146/annurev.micro.61.080706.093134. [DOI] [PubMed] [Google Scholar]
- 4.Kafsack BFC, et al. A transcriptional switch underlies commitment to sexual development in malaria parasites. Nature. 2014;507:248–252. doi: 10.1038/nature12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salcedo-Amaya AM, et al. Dynamic histone H3 epigenome marking during the intraerythrocytic cycle of Plasmodium falciparum . Proc Natl Acad Sci USA. 2009;106:9655–9660. doi: 10.1073/pnas.0902515106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kensche PR, et al. The nucleosome landscape of Plasmodium falciparum>reveals chromatin architecture and dynamics of regulatory sequences. Nucleic Acids Res. 2016;44:2110–2124. doi: 10.1093/nar/gkv1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toenhake CG, et al. Chromatin accessibility-based characterization of the gene regulatory network underlying lasmodium falciparum blood-stage development. Cell Host Microbe. 2018;23:557–569. doi: 10.1016/j.chom.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganesan K, et al. A genetically hard-wired metabolic transcriptome in Plasmodium falciparum fails to mount protective responses to lethal antifolates. PLoS Pathog. 2008;4:e1000214. doi: 10.1371/journal.ppat.1000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Llinás M, Bozdech Z, Wong ED, Adai AT, DeRisi JL. Comparative whole genome transcriptome analysis of three Plasmodium falciparum strains. Nucleic Acids Res. 2006;34:1166–1173. doi: 10.1093/nar/gkj517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Painter HJ, et al. Genome-wide real-time in vivo transcriptional dynamics during Plasmodium falciparum blood-stage development. Nat Commun. 2018;9:2656. doi: 10.1038/s41467-018-04966-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes KR, Philip N, Starnes GL, Taylor S, Waters AP. From cradle to grave: RNA biology in malaria parasites. WIRES RNA. 2010;1:287–303. doi: 10.1002/wrna.30. [DOI] [PubMed] [Google Scholar]
- 12.Vembar SS, Droll D, Scherf A. Translational regulation in blood stages of the malaria parasite Plasmodium spp.: systems-wide studies pave the way. WIRES RNA. 2016;7:772–792. doi: 10.1002/wrna.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shock JL, Fischer KF, DeRisi JL. Whole-genome analysis of mRNA decay in Plasmodium falciparum reveals a global lengthening of mRNA half-life during the intra-erythrocytic development cycle. Genome Biol. 2007;8:R134. doi: 10.1186/gb-2007-8-7-r134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foth BJ, Zhang N, Mok S, Preiser PR, Bozdech Z. Quantitative protein expression profiling reveals extensive post-transcriptional regulation and post-translational modifications in schizont-stage malaria parasites. Genome Biol. 2008;9:R177. doi: 10.1186/gb-2008-9-12-r177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caro F, Ahyong V, Betegon M, DeRisi JL. Genome-wide regulatory dynamics of translation in the Plasmodium falciparum asexual blood stages. eLife. 2014;3:e04106. doi: 10.7554/eLife.04106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roundtree IA, Evans ME, Pan T, He C. Dynamic RNA modifications in gene expression regulation. Cell. 2017;169:1187–1200. doi: 10.1016/j.cell.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer KD, Jaffrey SR. Rethinking m6A readers, writers, and erasers. Annu Rev Cell Dev Biol. 2017;33:319–342. doi: 10.1146/annurev-cellbio-100616-060758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Śledź P, Jinek M. Structural insights into the molecular mechanism of the m(6)A writer complex. eLife. 2016;5:e18434. doi: 10.7554/eLife.18434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, et al. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat Cell Biol. 2014;16:191–198. doi: 10.1038/ncb2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ping X-L, et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24:177–189. doi: 10.1038/cr.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu N, et al. N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature. 2015;518:560–564. doi: 10.1038/nature14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer KD, et al. 5’ UTR m(6)A promotes cap-independent translation. Cell. 2015;163:999–1010. doi: 10.1016/j.cell.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505:117–120. doi: 10.1038/nature12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patil DP, Pickering BF, Jaffrey SR. Reading m6A in the transcriptome: m6A-binding proteins. Trends Cell Biol. 2018;28:113–127. doi: 10.1016/j.tcb.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vu LP, et al. The N6-methyladenosine (m6A)-forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat Med. 2017;23:1369–1376. doi: 10.1038/nm.4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lence T, et al. m6A modulates neuronal functions and sex determination in Drosophila . Nature. 2016;540:242–247. doi: 10.1038/nature20568. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz S, et al. High-resolution mapping reveals a conserved, widespread, dynamic mRNA methylation program in yeast meiosis. Cell. 2013;155:1409–1421. doi: 10.1016/j.cell.2013.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gardner MJ, et al. Genome sequence of the human malaria parasite Plasmodium falciparum . Nature. 2002;419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larson MH, et al. CRISPR interference (CRISPRi) for sequence-specific control of gene expression. Nat Protoc. 2013;8:2180–2196. doi: 10.1038/nprot.2013.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao B, et al. Epigenetic editing by CRISPR/dCas9 in Plasmodium falciparum . Proc Natl Acad Sci USA. 2019;116:255–260. doi: 10.1073/pnas.1813542116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujita T, Fujii H. Efficient isolation of specific genomic regions and identification of associated proteins by engineered DNA-binding molecule-mediated chromatin immunoprecipitation (enChIP) using CRISPR. Biochem Biophys Res Commun. 2013;439:132–136. doi: 10.1016/j.bbrc.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 32.Ponts N, et al. Nucleosome landscape and control of transcription in the human malaria parasite. Genome Res. 2010;20:228–238. doi: 10.1101/gr.101063.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bártfai R, et al. H2A.Z demarcates intergenic regions of the Plasmodium falciparum epigenome that are dynamically marked by H3K9ac and H3K4me3. PLoS Pathog. 2010;6:e1001223. doi: 10.1371/journal.ppat.1001223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adjalley SH, Chabbert CD, Klaus B, Pelechano V, Steinmetz LM. Landscape and dynamics of transcription Initiation in the malaria parasite Plasmodium falciparum . Cell Rep. 2016;14:2463–2475. doi: 10.1016/j.celrep.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linder B, et al. Single-nucleotide-resolution mapping of m6A and m6Am throughout the transcriptome. Nat Methods. 2015;12:767–772. doi: 10.1038/nmeth.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stevens AT, Howe DK, Hunt AG. Characterization of mRNA polyadenylation in the apicomplexa. PLoS One. 2018;13:e0203317. doi: 10.1371/journal.pone.0203317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Engel M, et al. The role of m6A/m-RNA methylation in stress response regulation. Neuron. 2018;99:389–403. doi: 10.1016/j.neuron.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iyer LM, Zhang D, Aravind L. Adenine methylation in eukaryotes: apprehending the complex evolutionary history and functional potential of an epigenetic modification. Bioessays. 2016;38:27–40. doi: 10.1002/bies.201500104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwartz S, et al. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5’ sites. Cell Rep. 2014;8:284–296. doi: 10.1016/j.celrep.2014.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wen J, et al. Zc3h13 regulates nuclear RNA m6A methylation and mouse embryonic stem cell self-renewal. Mol Cell. 2018;69:1028–1038. doi: 10.1016/j.molcel.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Růžička K, et al. Identification of factors required for m6A mRNA methylation in Arabidopsis reveals a role for the conserved E3 ubiquitin ligase HAKAI. New Phytol. 2017;215:157–172. doi: 10.1111/nph.14586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oehring SC, et al. Organellar proteomics reveals hundreds of novel nuclear proteins in the malaria parasite Plasmodium falciparum . Genome Biol. 2012;13:R108. doi: 10.1186/gb-2012-13-11-r108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yue Y, et al. VIRMA mediates preferential m6A mRNA methylation in 3’UTR and near stop codon and associates with alternative polyadenylation. Cell Disco. 2018;4:10. doi: 10.1038/s41421-018-0019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia-Campos MA, et al. Deciphering the ‘m6A code’ via quantitative profiling of m6A at single-nucleotide resolution. 2019 doi: 10.1101/571679. Preprint. [DOI] [PubMed] [Google Scholar]
- 45.Ke S, et al. m6A mRNA modifications are deposited in nascent pre-mRNA and are not required for splicing but do specify cytoplasmic turnover. Genes Dev. 2017;31:990–1006. doi: 10.1101/gad.301036.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patil DP, et al. M6A RNA methylation promotes XIST-mediated transcriptional repression. Nature. 2016;537:369–373. doi: 10.1038/nature19342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lasonder E, et al. Integrated transcriptomic and proteomic analyses of P. falciparum gametocytes: molecular insight into sex-specific processes and translational repression. Nucleic Acids Res. 2016;44:6087–6101. doi: 10.1093/nar/gkw536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lindner SE, et al. Extensive transcriptional and translational regulation occur during the maturation of malaria parasite sporozoites. 2019 doi: 10.1101/642298. Preprint. [DOI] [Google Scholar]
- 49.Cui L, Lindner S, Miao J. Translational regulation during stage transitions in malaria parasites. Ann New York Acad Sci. 2015;1342:1–9. doi: 10.1111/nyas.12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lopez-Rubio JJ, Mancio-Silva L, Scherf A. Genome-wide analysis of heterochromatin associates clonally variant gene regulation with perinuclear repressive centers in malaria parasites. Cell Host Microbe. 2009;5:179–190. doi: 10.1016/j.chom.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 51.Vembar SS, Macpherson CR, Sismeiro O, Coppée J-Y, Scherf A. The PfAlba1 RNA-binding protein is an important regulator of translational timing in Plasmodium falciparum blood stages. Genome Biol. 2015;16:212. doi: 10.1186/s13059-015-0771-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ng CS, et al. tRNA epitranscriptomics and biased codon are linked to proteome expression in Plasmodium falciparum . Mol Syst Biol. 2018;14:e8009. doi: 10.15252/msb.20178009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crabb BS, et al. Transfection of the human malaria parasite Plasmodium falciparum . Methods Mol Biol. 2004;270:263–276. doi: 10.1385/1-59259-793-9:263. [DOI] [PubMed] [Google Scholar]
- 54.Ghorbal M, et al. Genome editing in the human malaria parasite Plasmodium falciparum using the CRISPR-Cas9 system. Nat Biotechnol. 2014;32:819–821. doi: 10.1038/nbt.2925. [DOI] [PubMed] [Google Scholar]
- 55.Mesén-Ramírez P, et al. Stable translocation intermediates jam global protein export in Plasmodium falciparum parasites and link the PTEX component EXP2 with translocation activity. PLoS Pathog. 2016;12:e1005618. doi: 10.1371/journal.ppat.1005618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perkins DN, Pappin DJ, Creasy DM, Cottrell JS. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999;20:3551–3567. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 57.Qi LS, et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell. 2013;152:1173–1183. doi: 10.1016/j.cell.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.MacPherson CR, Scherf A. Flexible guide-RNA design for CRISPR applications using Protospacer Workbench. Nat Biotechnol. 2015;33:805–806. doi: 10.1038/nbt.3291. [DOI] [PubMed] [Google Scholar]
- 59.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 60.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kearse M, et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Capella-Gutierrez S, Silla-Martinez JM, Gabaldon T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 2009;25:1972–1973. doi: 10.1093/bioinformatics/btp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Darriba D, Taboada GL, Doallo R, Posada D. ProtTest 3: fast selection of best-fit models of protein evolution. Bioinformatics. 2011;27:1164–1165. doi: 10.1093/bioinformatics/btr088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oates ME, et al. D2P2: database of disordered protein predictions. Nucleic Acids Res. 2013;41:D508–D516. doi: 10.1093/nar/gks1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aurrecoechea C, et al. EuPathDB: the eukaryotic pathogen genomics database resource. Nucleic Acids Res. 2017;45:D581–D591. doi: 10.1093/nar/gkw1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li H, Durbin R. Fast and accurate short read alignment with BurrowsWheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li H, et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ramírez F, et al. deepTools2: a next generation web server for deepsequencing data analysis. Nucleic Acids Res. 2016;44:W160–W165. doi: 10.1093/nar/gkw257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krzywinski M, et al. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19:1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Y, et al. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heinz S, et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38:576–589. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marçais G, Kingsford C. A fast, lock-free approach for efficient parallel counting of occurrences of k-mers. Bioinformatics. 2011;27:764–770. doi: 10.1093/bioinformatics/btr011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Crooks GE, Hon G, Chandonia J-M, Brenner SE. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meyer KD, et al. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell. 2012;149:1635–1646. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sims D, et al. CGAT: computational genomics analysis toolkit. Bioinformatics. 2014;30:1290–1291. doi: 10.1093/bioinformatics/btt756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Anders S, Pyl PT, Huber W. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.R: A language and environment for statistical computing. R Development Core Team; 2012. [Google Scholar]
- 82.Broadbent KM, et al. Strand-specific RNA sequencing in Plasmodium falciparum malaria identifies developmentally regulated long non-coding RNA and circular RNA. BMC Genom. 2015;16:454. doi: 10.1186/s12864-015-1603-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All sequencing data are accessible on the Gene Expression Omnibus database (https://www.ncbi.nlm.nih.gov/geo) under study accession number GSE123839. Raw sequence data are accessible on the NCBI Sequence Read Archive (SRA) under BioProject accession number PRJNA473770. Proteomics and mRNA modification LC-MS/MS data are deposited at the Chorus database with accession number 1579.