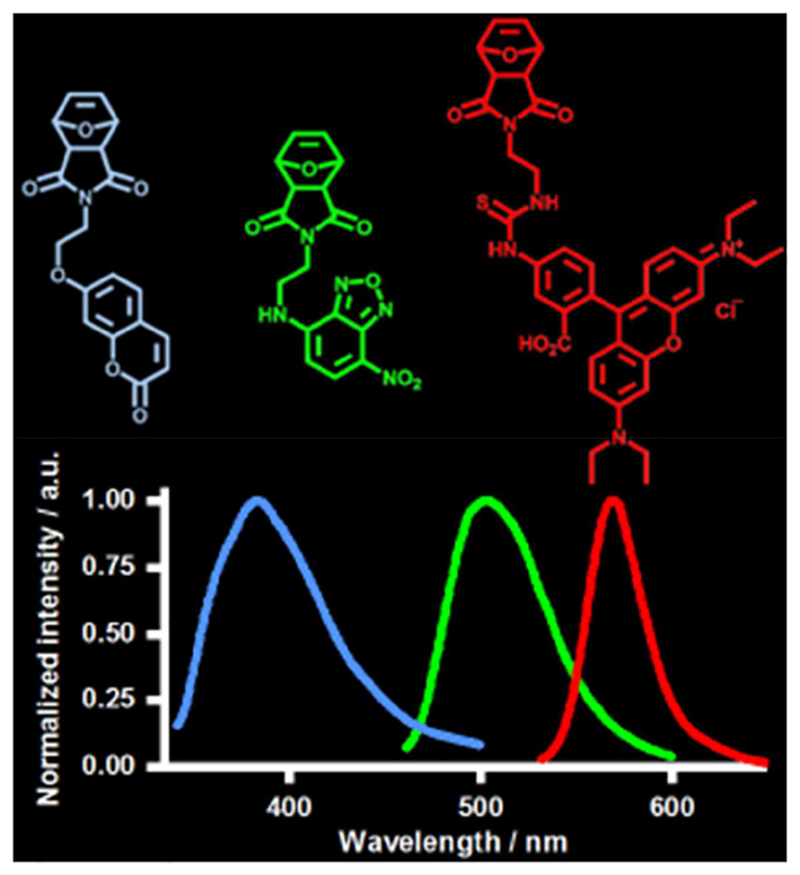
Abstract
The synthesis and characterization of a series of green, blue and red-fluorescent exo-oxanorbornene acid and imide monomers carrying nitrobenzofurazan, coumarin, and Rhodamin B, respectively, as fluorophores is presented. These monomers carry oxanorbornene as polymerizable unit, and were readily copolymerized with bioactive functional oxanorbornene monomers by ring-opening metathesis polymerization (ROMP), as demonstrated by gel permeation chromatography and NMR spectroscopy. Due to the ease of synthesis of these monomers, and their cost-effectiveness compared many to other fluorescent probes, they are useful for biomaterials applications.
Keywords: fluorescent polymers, functionalization of polymers, monomer synthesis, ring-opening metathesis polymerization (ROMP)
Figure For Toc.
1. Introduction
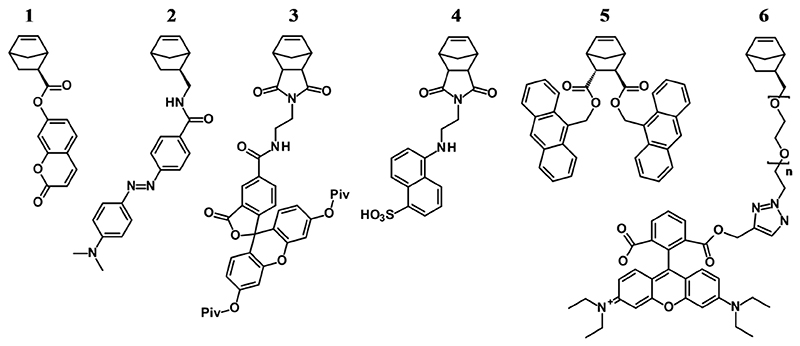
Fluorescent molecules have long been used to visualize (nano)particles, to stain cells or distinct cell parts, or parts of living organisms.[1–3] In the context of polymer science, fluorescent labels have been used to visualize the interaction of bioactive polymers with cells,[4–6] or to image micro- and nanostructures using fluorescent or confocal microscopy.[7–10] As with other functional groups, there are three basic strategies to include fluorescent probes into a polymer during synthesis: the fluorophore can either be part of the initiator,[11–13] of a (co)monomer,[14–18] or of the terminating agents[19–23] of the target polymer. Additionally, the fluorophore can be introduced by post-polymerization functionalization.[20, 24–26] For ring-opening metathesis polymerization (ROMP), this has recently been summarized and illustrated.[27, 28] In the context of visualization of polymers and polymeric materials in biological environments, each of these approaches has different advantages and disadvantages. Fluorescent initiators can be quite convenient if the initiator reacts quantitatively, and if there are no chain transfer reactions (which is true for living polymerizations like anionic polymerization and ROMP). Under these conditions, every chain is fluorescently labeled and elaborate purification is unnecessary, as there are no low molecular weight fluorescent species. Additionally, there is excellent control over the precise placement of the fluorophore. The latter is also true for polymers that are terminated by fluorescent quenching agents. In the termination approach, however, there is often uncertainty whether all polymer chains are functionalized because of potential side reactions (chain end quenching by impurities, branching, back-biting etc.). Additionally, any excess of fluorescent termination agent has to be carefully removed from the polymer because fluorescent low molecular weight impurities would cause artefacts in the fluorescence microscopy image. Another limitation of the initiation and termination approach is that there is only one fluorophore per polymer chain. This might not always be enough - especially for high molecular weight polymers, the fluorophore density might be too low. For such polymers, it is worthwhile to consider copolymerization of the desired functional monomer with a fluorescent monomer. Assuming that the functional and fluorescent monomers copolymerized statistically, this yields a polymer with the desired density of fluorescent repeat units and an even distribution of the fluorescence intensity over the entire polymer. For polymerizations that can go to quantitative conversion (like ROMP), no unbound fluorescent species remain in the polymerization mixture when using the fluorescent comonomer approach. This is the advantage over post-polymerization functionalization, where careful removal of low-molecular-weight fluorescent impurities is required. Another advantage of the fluorescent comonomer approach, in particular in the context of biomedical applications, is that it allows the precise placement of different chromophores and therapeutic agents in different parts of the polymer by serial addition of different fluorescent species, for example in block copolymer synthesis.[29, 30] Several fluorescent monomers that can be used for ROMP are available, including dye-labeled norbornenes,[15, 16, 27, 31–33] norbornadienes,[34] and dithio- and aminomaleimides.[35, 36] Figure 1 shows examples of a coumarin-labeled norbornene monomer (1),[27] a DABCYL-labeled norbornene monomer (2),[27] a fluorescein-labeled norbornene imide monomer (3),[27] an EDANS-labeled norbornene imide monomer (4),[27] an anthracene-labeled norbornene monomer (5),[17] and a Rhodamine B-functionalized monomer (6).[33]
Figure 1.
ROMP monomers labeled with fluorescent coumarin (1), DABCYL (2), fluorescein (3), EDANS (4), anthracene (5) and Rhodamin B (6), respectively.[17, 27, 28, 33]
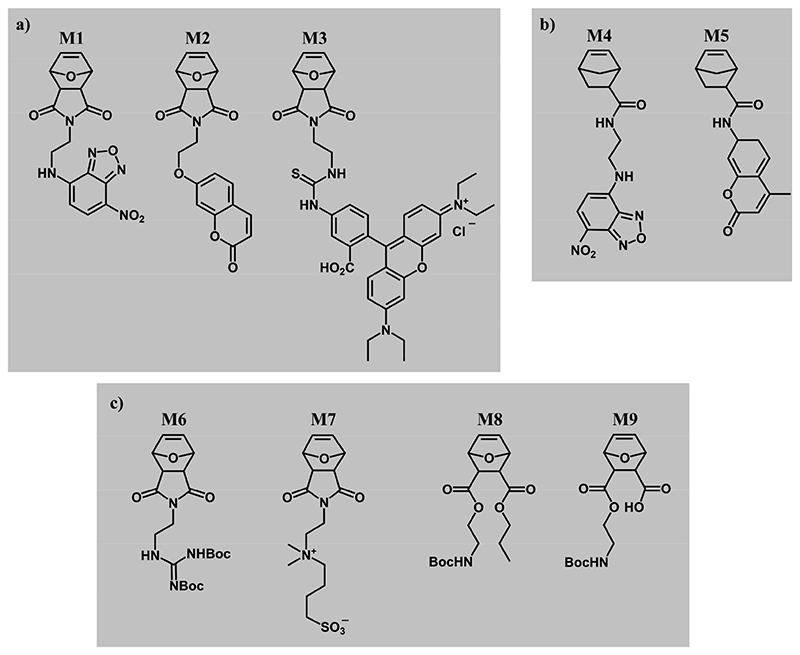
In this paper, we present the synthesis and ring-opening metathesis copolymerization of five fluorescent monomers with functional bioactive monomers, resulting in bioactive fluorescent copolymers for biological studies and materials applications, e.g. surface coatings.[37–42] These polymers were based on exo-oxanorbornene imide (M1, M2 and M3, Figure 2) and exo-5-norbornene 2-carboxylic acid (M4 and M5, Figure 2), i.e. they had polymerizable units similar to those of functional bioactive monomers frequently used in our research (M6, M7, M8 and M9, respectively, all also in the exo configuration Figure 2). Further design criteria for the fluorescent monomers were that the fluorophores should be as small as possible and that the monomers could not contain hydrophobic spacers, as this would have changed the overall bioactivity of the target copolymers. Also, distinctly different fluorophores, i.e. emitting green (M1 and M4), blue (M2 and M5), and red fluorescence (M3), were needed to ensure ease of the intended optical imaging.
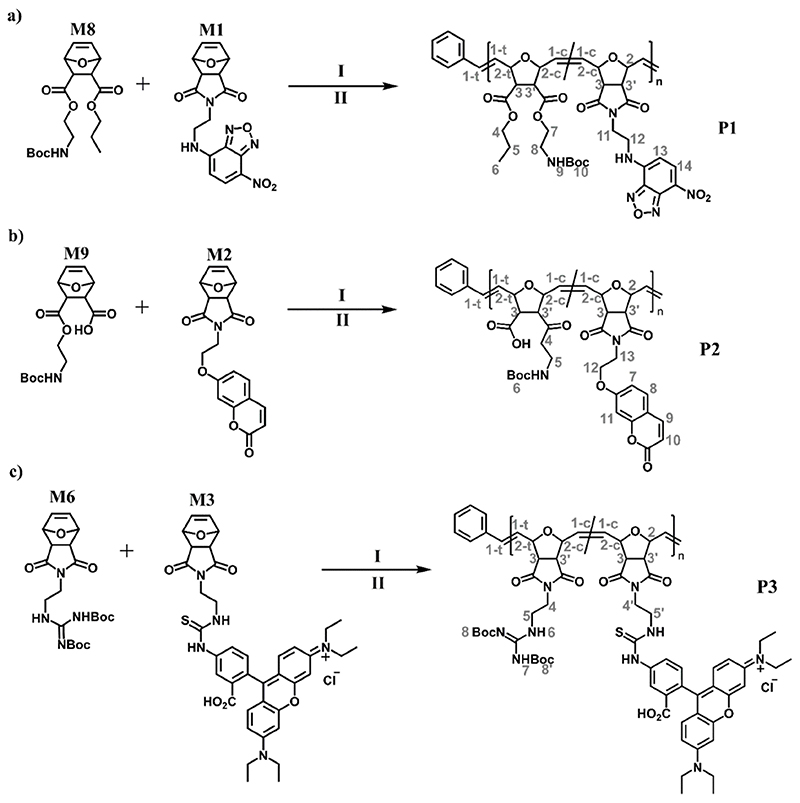
Figure 2. ROMP monomers for bioactivity studies.
a) Fluorescently labeled oxanorbornene-imide monomers M1, M2 and M3 with nitrobenzofurazan (NBD), coumarin (COU), Rhodamine B (RHB) fluorophores, respectively. b) Fluorescently labeled norbornene-amidate monomers M4 and M5 with NBD and COU fluorophores, respectively. The fluorescent monomers in a) and b) were copolymerized with structurally similar bioactive ROMP monomers c): the guanidinium oxanorbornene M6, the sulfobetaine monomer M7, the propyl-oxanorbornene M8, and the protected zwitterionic monomer M9. All monomers have exo-configuration.
2. Experimental Section
2.1. Materials
All chemicals were purchased from Fluka, Sigma-Aldrich or TCI Europe, and were used as received. Solvents for gel-permeation chromatography (GPC) were HPLC quality and obtained from Carl Roth. Grubbs’ third generation catalyst was synthesized as reported previously.[43] The exo-oxanorbornene-imide precursors 4-(2-aminoethyl)-3,6-epoxy-1,2,3,6-tetrahydrophthalimide S1 [44] and exo-4-(2-bromoethyl)-3,6-epoxy-1,2,3,6-tetrahydrophthalimide S2 [45–47] were synthesized analogously to literature procedures, as described in the Supporting Information (SI). The syntheses of monomers M4 and M5 and polymers P4 and P5 are given in the Supporting Information.
The synthesis of the guanidinium oxanorbornene M6, [48] the sulfobetaine monomer M7, [49] the propyl-oxanorbornene M8,[50] and the protected zwitterionic monomer M9 [42] were performed as reported previously.
2.2. Methods
NMR spectra were recorded on a Bruker 250 MHz spectrometer (Bruker, Madison, WI, USA). Gel permeation chromatography was measured on a SECcurity system with PSS SDV columns (PSS Polymer Standards Service, Mainz, Germany). Polymers were measured at 30 °C in CHCl3 or in THF at room temperature. at a flow rate of 1 mL min-1. The molecular mass distribution was calibrated against poly(methyl methacrylate) (PMMA) standards. Electrospray ionization mass spectrometry (ESI/MS) was measured on a Thermo LCQ Advantage (Thermo Fisher Scientific, Waltham, MA, USA). Fluorescence spectra were recorded on a LS 55 fluorescence spectrometer (Perkin Elmer, Waltham/MA, USA). Fluorescence microscopy images were taken on a Nikon Eclipse Ti-S inverted microscope (Nikon GmbH, Düsseldorf, Germany) using a green-fluorescent-protein, DAPI and Texas red filter, respectively, at 60x magnification. The imaging time was varied between 80 ms and 1 s and the images were processed with the software ImageJ. Brightness and image contrast were manually adjusted for better visualization.
2.3. Fluorescent Monomers
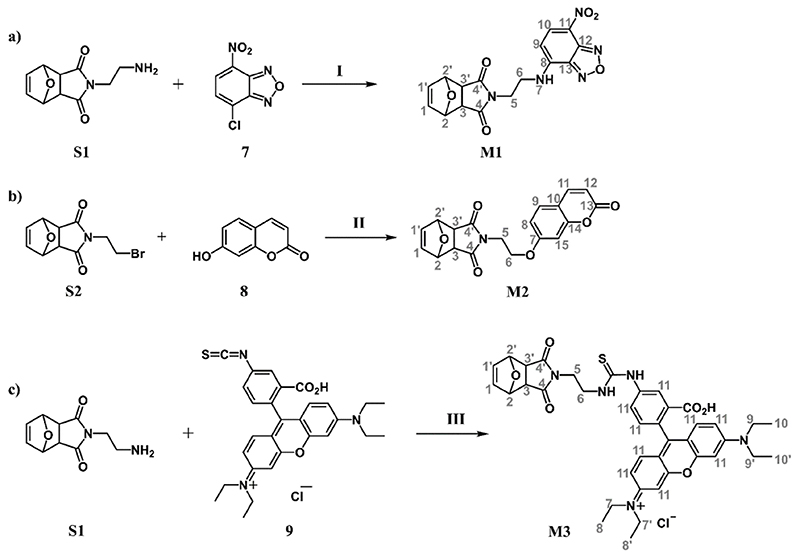
The peak assignments of the NMR spectra of monomers M1 to M3 refer to the atom numbering shown in Figure 3.
Figure 3. Synthesis and NMR peak assignment of fluorescent oxanorbornene imide monomers M1, M2 and M3.
a) Green-fluorescing NBD-monomer M1 (I: CH2Cl2, diisopropylethylamine); b) blue-fluorescing COU-monomer M2 (II: acetone, K2CO3); c) red-fluorescing RHB-monomer M3 (III: MeOH). The numbering of atoms of the products refers to the peak assignments in the Experimental.
Green-fluorescing Nitrobenzofurazan (NBD)-labeled Monomer M1
Exo-4-(2-aminoethyl)-3,6-epoxy-1,2,3,6-tetrahydrophthalimide (S1, 489 mg, 2.25 mmol, 1 eq) was dissolved in dichloromethane (15 mL). Diisopropylethylamine (1.57 mL, 9.0 mmol, 4 eq) and 4-chloro-7-nitrobenzofurazan (7, 449 mg, 2.25 mmol, 1 eq) were added under nitrogen atmosphere. The mixture turned dark orange and was stirred for 1.5 hours. The reaction was monitored via thin layer chromatography. The solvents were removed by vacuum and a dark solid was obtained. A filter-column (CH2Cl2:ethyl acetate 1:1) gave the NBD-labeled monomer M1 (yield: 342.5 mg, 0.92 mmol, 41%).
1H-NMR (250 MHz, DMSO-d6) δ / ppm: 9.33 (s, 1 H, H7), 8.57 (d, J = 8.4 Hz, 1 H, H10), 6.53 (s, 2 H, H1 & H1’), 6.45 (d, J = 8.8 Hz, 1 H, H9), 5.06 (s, 2 H, H2 & H2’), 3.68 (m, 4 H, H5 & H6), 2.93 (s, 2 H, H3 & H3’);
13C-NMR (63 MHz, DMSO-d6) δ / ppm: 176.44 (C4 & C4’), 136.40 (C1 & C1’), 80.26 (C2 & C2’), 47.24 (C3 & C3’);
MS (ESI) calculated for C16H13N5O6 [M + Na]: 394.08, found: 394.08.
Blue-fluorescing Coumarin (COU)-labeled Monomer M2
7-Hydroxycoumarin (8, 8.10 g, 50 mmol, 5 eq) was dissolved in acetone (50 mL). K2CO3 (6.91 g, 50 mmol, 5 eq) and exo-N-(2-bromoethyl)-3,6-epoxy-1,2,3,6-tetrahydrophthalimide (S2, 2.72 g, 10 mmol, 1 eq) were slowly added and the reaction mixture was refluxed for 24 h. After cooling down, the reaction mixture was poured into 300 mL of water under stirring. The COU-labeled product M2 was precipitated from the solution and was used without additional purification steps (yield: 1.41 g, 4 mmol, 40%).
1H-NMR (250 MHz, DMSO-d6) δ / ppm: 7.98 (d, J = 9.64 Hz, 1 H, H11), 7.61 (d, J = 8.69 Hz, 1 H, H9), 6.96 (d, J = 2.40 Hz, 1 H, H15), 6.87 (d, J = 8.53, 2.37 Hz, 1 H, H12), 6.55 (s, 2 H, H1 & H1’), 6.29 (d, J = 9.48 Hz, 1 H, H8), 5.14 (s, 2 H, H2 & H2’), 4.19 (t, J = 5.61 Hz, 2 H, H6), 3.76 (t, J = 5.69 Hz, 2 H, H5), 2.97 (s, 2 H, H3 & H3’);
13C-NMR (63 MHz, DMSO-d6) δ / ppm: 176.33 (C4 & C4’), 161.08 (C7), 160.21 (C13), 155.25 (C14), 144.25 (C9), 136.47 (C1 & C1’), 129.49 (C11), 112.76 (C8), 112.68 (C12), 112.61 (C10), 101.37 (C15), 80.34 (C2 & C2’), 64.58 (C6), 47.15 (C3 & C3’), 37.15 (C5);
MS (ESI) calculated for C19H15NO6 [M+Na]: 376.08, found: 376.08.
Red-fluorescing Rhodamin B (RHB)-labeled Monomer M3
Exo-4-(2-aminoethyl)-3,6-epoxy-1,2,3,6-tetrahydrophthalimide (S1, 7.68 mg, 0.037 mmol, 1 eq) and Rhodamine B (RHB) isothiocyanate (9, 20.0 mg, 0.037 mmol, 1 eq) were placed in dry Schlenk flask. Dry methanol (5 mL) was added under nitrogen atmosphere. The solution was stirred overnight. Afterwards, methanol was removed by vacuum. The resulting solid was dissolved in dichloromethane and precipitated in hexane to give a violet solid (M3, yield: 19 mg, 0.025 mmol, 70%).
1H-NMR (250 MHz, MeOD) δ / ppm: 7.3-6.9 (m, 9 H, H11), 6.60 (s, 2 H, H1 & H1’), 5.22 (s, 2 H, H2 & H2’), 3.80 (t, J = 6 Hz, 2 H, H5), 3.68 (m, 8 H, H7 & H7’, H9 & H9’), 3.14 (t, J = 6.1 Hz, 2 H, H6), 3.02 (s, 2 H, H3 & H3’), 1.32 (m, 12 H, H8 & H8’, H10 & H10’);
13C-NMR (63 MHz, CD2Cl2) δ / ppm: 177.75 (C4 & C4’), 136.86 (C1 & C1’), 81.20 (C2 & C2’), 48.79 (C3 & C3’), 46.09 (C7 & C7’), 45.76 (C9 & C9’), 38.56 (C6), 36.66 (C5), 12.94 (C8 & C8’, C10 & C10’);
MS (ESI) calculated for C39H42N5O6 [M]+: 708.29, found: 708.29.
2.4. Fluorescent Copolymers
The peak assignments of the NMR spectra of polymers P1 to P3 refer to the atom numbering shown in Figure 4.
Figure 4. Synthesis and NMR peak assignments for fluorescent polymers P1, P2 and P3.
a) Green-fluorescing NBD-polymer P1 (I: CH2Cl2, Grubbs’ 3rd Generation catalyst; II: ethylvinylether); b) blue-fluorescing COU-polymer P2; c) red-fluorescing RHB-polymer P3. All monomers are in the exo configuration. The numbering refers to the peak assignment in the Experimental; t and c refer to the NMR signals of the trans and cis protons, respectively.
Green-fluorescent Propyl-SMAMP P1 (Boc-protected form)
The reaction was conducted under nitrogen atmosphere. NBD-monomer M1 (55.9 mg, 0.15 mmol, 1 eq) and boc-protected propyl-SMAMP monomer M8 (500 mg, 1.4 mmol, 9 eq) were dissolved in 8 mL dry CH2Cl2. The monomer solution was added to a solution of Grubbs 3rd generation catalyst (4.02 mg in 2 mL dry CH2Cl2, 0.0037 eq; resulting in a target molecular mass of 100 kg·mol-1) and stirred for 1.5 h at room temperature. Ethyl vinyl ether (1 mL) was added to terminate the polymerization reaction and the polymer solution was stirred for additional 1 h. The CH2Cl2 solution containing the polymer was precipitated into cold hexane. The precipitated polymer P1 was filtered and dried under high vacuum.
GPC (CHCl3, PMMA): Mn 75 kg mol-1; Mw 95 kg mol-1; PDI 1.27;
1H-NMR (250 MHz, CDCl3) δ / ppm: 8.53 (s, 1 H, H14), 6.26 (s, 1 H, H13), 5.92 (br s, H1-t), 5.62 (br s, H1-c), 5.42 (br s, 1 H, Boc-NH), 5.14 (br s, H2-c), 4.72 (br s, H2-t), 4.17 (br s, 2 H, H7), 4.09 (br s, 2 H, H4), 3.38 (br s, 2 H, H8), 3.15 (br s, 2 H, H3 & H3’), 1.66 (m, 2 H, H5), 1.46 (s, 9 H, H10), 0.95 (s, 3 H, H6).
Blue-fluorescing Polyzwitterion P2 (Boc-protected form)
Polymer P2 was synthesized and purified in the same manner as P1, except that the reaction components were: COU-labeled monomer M2 (27.3 mg, 0.077 mmol, 1 eq) and monomer M9 (250.0 mg, 0.677 mmol, 8.8 eq) in dry CH2Cl2 (5 mL); Grubbs 3rd generation catalyst (1.80 mg, 0.002 mmol, 0.026 eq; target molecular mass 125 kg mol-1) in 2 mL dry CH2Cl2; ethyl vinyl ether (1 mL). After 1 h stirring with ethyl vinyl ether, the solvent was evaporated. The residue was taken up in a small amount of CH2Cl2, and was precipitated into cold hexane. The precipitated polymer P2 was filtered off and dried under high vacuum.
GPC (THF, PMMA): Mn 116 kg mol-1; Mw 171 kg mol-1; PDI 1.5. For GPC measurement, the polyzwitterion was derivatized with trimethylsilyldiazomethane according to a literature procedure.[51]
1H-NMR (250 MHz, tetrahydrofurane-d8) δ / ppm: 7.74 (br s, 1 H, H9), 7.46 (br s, 1 H, H8), 6.88 (s, 1 H, H10 & H11), 6.17 (br s, 1 H, H7), 5.92 (br s, H1-t), 5.60 (br s, H1-c), 5.12 (br s, H2-c), 4.69 (br s, H2-t), 4.26 (br s, 2 H, H13), 4.08 (m, 2 H, H4), 3.88 (br s, 2 H, H12), 3.28 (br s, 2 H, H5), 3.12 (br s, 2 H, H3 & H3’), 1.41 (s, 9 H, H6).
Red-fluorescent Poly(guanidinium oxanorbornene imide) P3 (Boc-protected form)
Polymer P3 was synthesized and purified in the same manner as P1, except that the reaction components were: RHB-labeled monomer M3 (10 mg, 0.0134 mmol, 0.05 eq) and guanidinium oxanorbornene monomer M6 (115 mg, 0.255 mmol, 0.95 eq) in dry CH2Cl2 (5 mL); Grubbs 3rd generation catalyst (0.92 mg, 0.00125 mmol, 0.0047 eq; target molecular mass 100 kg mol-1) in 2 mL dry CH2Cl2; ethyl vinyl ether (1 mL). The work-up to obtain P3 was the same as described for P2.
GPC (CHCl3, PMMA): Mn 80 kg mol-1; Mw 208 kg mol-1; PDI 2.6;
1H-NMR (250 MHz, CDCl3) δ / ppm: 11.45 (s, 1 H, H7), 8.68 (s, 1 H, H6), 6.08 (br s, H1-t), 5.81 (br s, H1-c), 5.05 (br s, H2-c), 4.49 (br s, H2-t), 3.74 (br s, 4 H, H4 & H4’, H5 & H5’), 3.36 (br s, H3 & H3’), 1.51 (s, 18 H, H8 & H8’).
3. Results and Discussion
The synthesis schemes for the oxanorbornene-imide monomers M1, M2 and M3 with green (Nitrobenzofurazan, NBD), blue (Coumarin, COU), and red (Rhodamine B, RHB) fluorophores are shown in Figure 3. As the high costs of functional fluorophores is a problem in materials applications, where much larger amounts are needed than in biology or biochemistry, we chose fluorescent starting materials that are easily available and not too expensive. For the synthesis of the green-fluorescent NBD monomer M1, NBD-chloride 7 was used. This compound is abundant and thus cost-effective because it is used as a staining reagent in thin layer chromatography, e.g. to visualize amino acids. It was coupled to oxanorbornene imide S1 in a base-catalyzed aromatic nucleophilic substitution reaction (Figure 3a). The reaction was monitored by thin-layer chromatography (TLC). When the TLC indicated that the fluorescent starting material had vanished, the reaction was worked up and the product was purified by filtration column chromatography. The product eluted first, although some part (and unreacted amine) remained on the column.
To obtain the blue-fluorescent, coumarin-labeled monomer M2, the bromoethyl imide S2 was synthesized as described in the literature and the Supporting Information. It was coupled with 7-hydroxycoumarin 8, also known as umbelliferone, in a Williamson ether synthesis. Even though the yield of M2 was only ~ 40%, this was not critical because umbelliferone is abundant and relatively inexpensive, being used at large scale for sun screen products. Also, the work-up by precipitation was straight-forward.
Finding a cost-effective red fluorophore for biomaterials applications was a challenge. Additionally, most suitable substance classes are relatively large molecules, in light of the larger conjugated systems that are needed to obtain red fluorescence. Yet hydrophobic systems had to be avoided to prevent toxicity to mammalian cells.[50, 52] Rhodamine B, the fluorophore used, was a compromise with regard to both aspects: although it is quite big, it has enough polar groups to ensure the lowest possible toxicity, yet the available Rhodamine B (RHB) isothiocyanate 9 was more than a factor of 20 times more expensive than the other selected chromophores. It was reacted with oxanorbornene imide S1 in a nucleophilic addition reaction, which gave monomer M3 in 70% yield. The ease of purification (by precipitation) and the better yields compared to the syntheses of M1 and M2 somewhat compensated the higher costs of the starting material.
The structure of all monomers was confirmed by 1H-NMR and 13C-NMR spectroscopy, as well as mass spectrometry (described in the Experimental). The syntheses of monomers M4 and M5, and the corresponding polymers P4 and P5 (by copolymerization of M4 with the propyloxanorbornene M8, and of M5 with the sulfobetaine monomer M7) is described in the Supporting Information.
The monomers M1, M2 and M3 were readily polymerizable. The target structures are shown in Figure 4. For each polymer, the amount of dye labeled monomer was between 5-10 mol% to obtain sufficient fluorescence intensity of the dye component without disturbing the bioactivity of the resulting copolymer. Monomer M1 was copolymerized with the propyl-oxanorbornene M8, giving a green-fluorescent copolymer P1 (Figure 4a). This polymer was antimicrobial upon removal of the tert-butoxycarbamate (Boc) protective group. Monomer M2 was copolymerized with the protected zwitterionic monomer M9, yielding the blue-fluorescent polymer P2 (Figure 4b). This polymer was antimicrobial and protein-repellent upon removal of the Boc-group.[42] Monomer M3 was copolymerized with the guanidinium oxanorbornene M6, yielding the red-fluorescent polymer P3, which gave a red-fluorescent antimicrobial polymer upon removal of the Boc-group (Figure 4c).
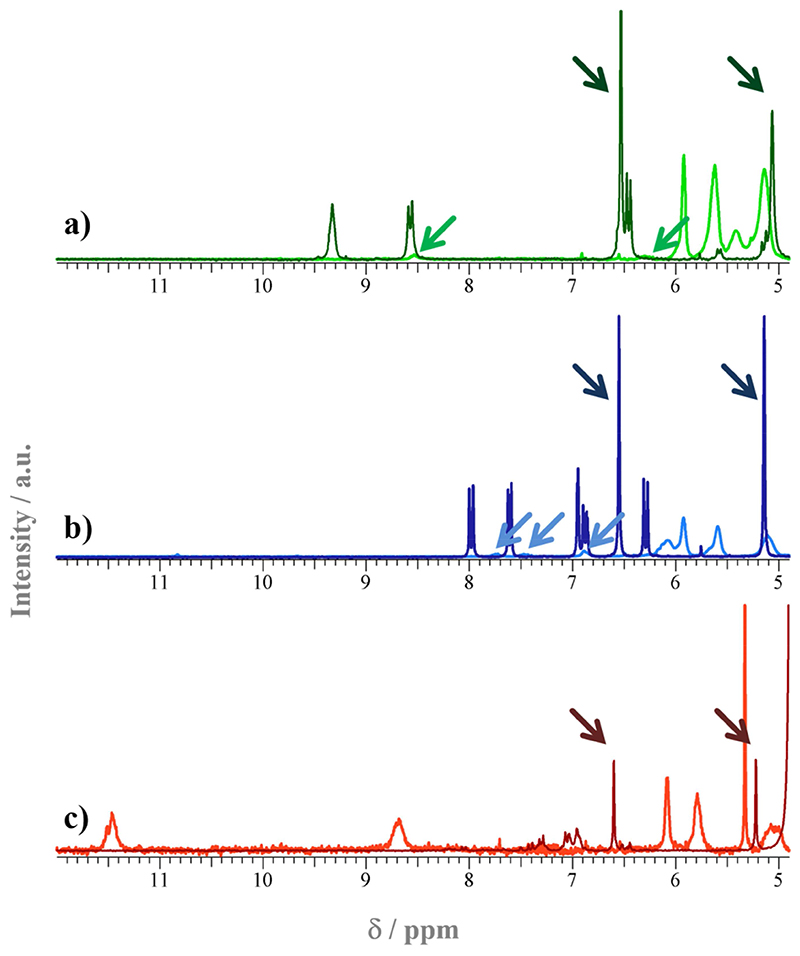
The polymers were characterized by gel permeation chromatography (GPC) in chloroform and tetrahydrofuran (THF), respectively, using poly(methyl methacrylate) (PMMA) standards. The results obtained for P1 to P3 (Mn = 75 kg mol-1, 116 kg mol-1, and 80 kg mol-1, respectively) were in reasonable agreement with the targeted molecular masses (Mn = 100 kg mol-1, 125 kg mol-1, and 100 kg mol-1, respectively), in light of the different structures of the target polymers and the PMMA standards. An overlay of the monomer and polymer NMR spectra of M1/P1, M2/P2, and M3/P3, respectively, is found in Figure 5. The darker arrows (pointing left to right) in each overlay set show the signals of the double bonds of the norbornene group in the fluorescent monomers. Expectedly, these vanished after polymerization and thus confirm that low-molecular weight fluorophores were no longer present in the product. The lighter arrows pointing right to left highlight characteristic peaks of the fluorescent chromophores in the polymers, and thus confirm that the fluorescent comonomers copolymerized with the functional comonomers.
Figure 5.
Detail of the 1H-NMR spectra of a) M1/P1, b) M2/P2, and c) M3/P3. The dark curves in each overlay set correspond to the monomer spectra, the lighter curves to the polymer spectra. The peak assignment is given in the Experimental. The arrows pointing left to right in each overlay set show the signals of the double bonds in the fluorescent monomers. These vanished after polymerization. The arrows pointing right to left highlight characteristic peaks of the fluorescent chromophores in the polymers.
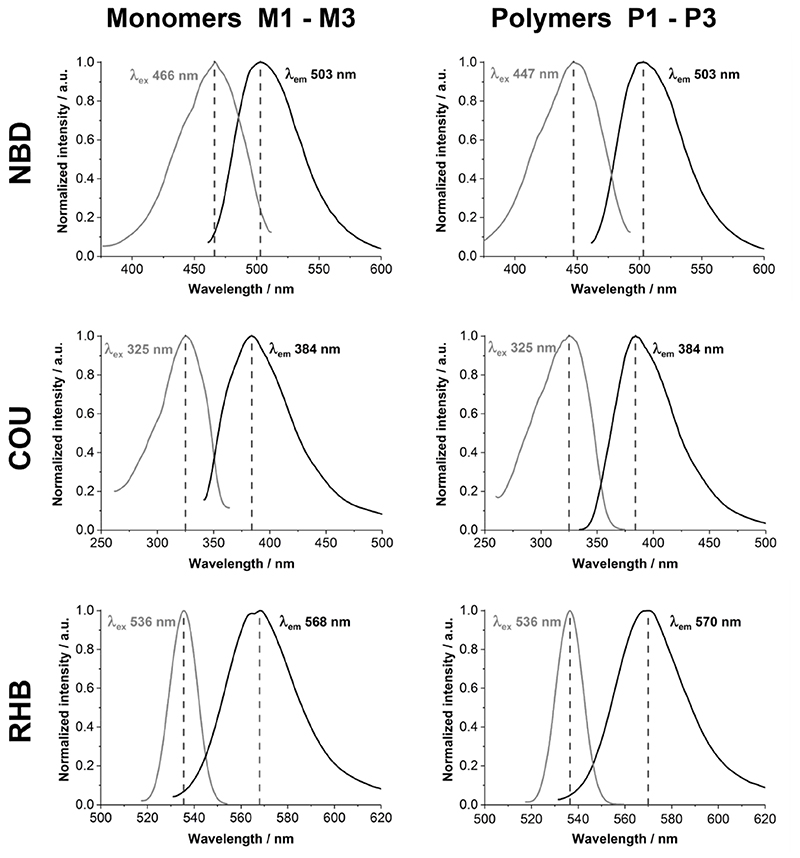
The fluorescence spectra of polymers P1 to P3, in comparison to the respective fluorescent monomers M1 to M3, are shown in Figure 6. The data also confirm that the fluorescent monomers were successfully incorporated into the corresponding polymers, and show that there is a close match between their respective excitation and emission peaks.
Figure 6.
Fluorescence spectra of monomers M1, M2, and M3 and the corresponding polymers P1, P2, and P3 (grey: extinction; black: emission). The left column shows the monomer spectra and the right column the respective polymer spectra. The dashed line indicates the wavelength of maximal extinction and emission, respectively.
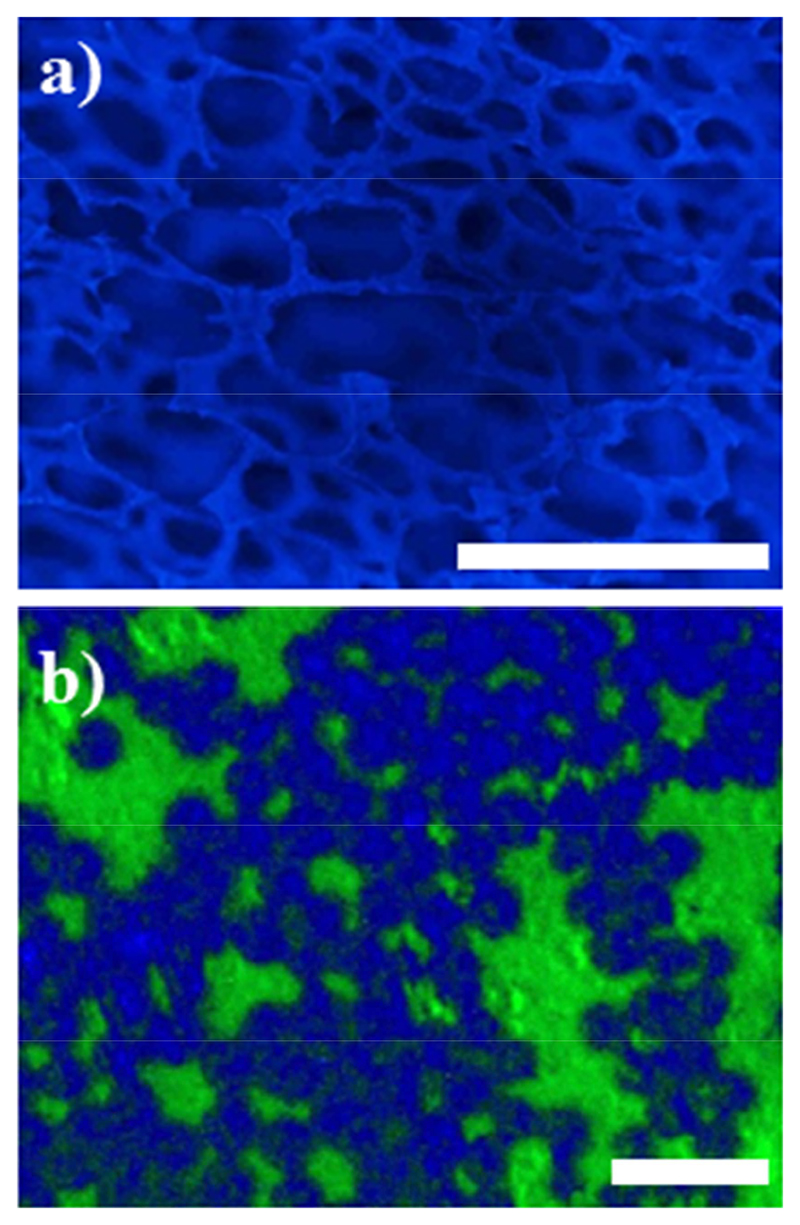
Potential applications for these polymers are shown in Figure 7. A polyurethane wound foam was coated with a variant of the blue-fluorescent polymer P2 (containing an additional cross-linker repeat unit)[42] to demonstrate that it could be immobilized on the entire porous structure of the foam (Figure 7a). Polymers P4 and P5 were selectively immobilized on a micro-patterned surface with a chemical contrast to establish structure-property relationships for micro-patterned antimicrobial and protein-repellent polymer surfaces (Figure 7b). [37, 42]
Figure 7. Fluorescence microscopy images.
a) Polyurethane wound foam was coated with a variant of the blue-fluorescent polymer P2 (containing an additional cross-linker repeat unit, scale bar = 500 μm). b) Polymers P4 (green-fluorescent) and P5 (blue-fluorescent), selectively immobilized on a micro-patterned surface with a chemical contrast (scale bar = 5 μm).
4. Conclusion
In this paper, the synthesis of a series of fluorescent monomers with different fluorophores, and their suitability for ring-opening metathesis polymerization, is presented. The resulting bioactive copolymers can be used for biomaterials applications.
As mentioned, the yields of the isolated compounds M1, M2, and M3 were lower than one would wish for (only 40-70%). The problem here was not the conversions of the reactions, which was much higher, but the work-up. Fluorescent compounds are notoriously difficult to handle. In column chromatography, the fluorescent compounds M1 to M3, as well as the fluorescent starting materials, caused excessive tailing. We therefore purified them by short filtration columns or precipitation, which is much more time-efficient but reduced the yield quite significantly. Since we could not accept any fluorescent impurities from the starting materials for our applications for fear of image artefacts, we would rather “waste” some product than carry over starting material impurities to the next step in an attempt to maximize the monomer yield. The fluorescence micrographs obtained from a surface functionalized with two of these fluorescent polymers (Figure 7b) demonstrates that no spill-over of the two different fluorophores used outside their allocated domains was observed.
Thus the here presented monomers provide a “construction kit” for researchers looking for structurally similar fluorescent monomers to label different parts of one polymer, or different polymers in hierarchical materials systems.
Supplementary Material
Acknowledgements
Alicia Malek-Luz is gratefully acknowledged for helping to compile data for the manuscript. This work was funded by the ministry for science, research and art of the state of Baden-Württemberg, Germany (GenMik I + II graduate school); the Emmy-Noether program of the Deutsche Forschungsgemeinschaft (German research foundation, DFG; LI1714/5-1), and the European Research Council (ERC-StG 637920 REGENERATE).
References
- [1].Robin MP, O’Reilly RK. Polymer International. 2015;64:174. [Google Scholar]
- [2].Landau MJ, Gould DJ, Patel KM. Annals of Translational Medicine. 2016;4 doi: 10.21037/atm.2016.10.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhu H, Fan JL, Du JJ, Peng XJ. Accounts of Chemical Research. 2016;49:2115. doi: 10.1021/acs.accounts.6b00292. [DOI] [PubMed] [Google Scholar]
- [4].Jonca J, Tukaj C, Werel W, Mizerska U, Fortuniak W, Chojnowski J. J Mater Sci Mater Med. 2016;27:1. doi: 10.1007/s10856-016-5669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Weilbaecher M, Allmeroth M, Hemmelmann M, Ritz S, Mailaender V, Bopp T, Barz M, Zentel R, Becker C. J Biomed Nanotechnol. 2014;10:81. doi: 10.1166/jbn.2014.1693. [DOI] [PubMed] [Google Scholar]
- [6].Freichels H, Danhier F, Preat V, Lecomte P, Jerome C. Int J Artif Organs. 2011;34:152. doi: 10.5301/ijao.2011.6420. [DOI] [PubMed] [Google Scholar]
- [7].Breul AM, Hager MD, Schubert US. Chem Soc Rev. 2013;42:5366. doi: 10.1039/c3cs35478d. [DOI] [PubMed] [Google Scholar]
- [8].Jeffet J, Kobo A, Su T, Grunwald A, Green O, Nilsson AN, Eisenberg E, Ambjornsson T, Westerlund F, Weinhold E, Shabat D, et al. ACS Nano. 2016;10:9823. doi: 10.1021/acsnano.6b05398. [DOI] [PubMed] [Google Scholar]
- [9].Lopez-Barron CR, Macosko CW. J Microsc (Oxford UK) 2011;242:242. doi: 10.1111/j.1365-2818.2010.03462.x. [DOI] [PubMed] [Google Scholar]
- [10].Yoon B, Oh EH, Lee CW, Kim J-M. Bull Korean Chem Soc. 2013;34:1282 [Google Scholar]
- [11].Burtscher D, Saf R, Slugovc C. Journal of Polymer Science Part A: Polymer Chemistry. 2006;44:6136 [Google Scholar]
- [12].Burtscher D, Lexer C, Mereiter K, Winde R, Karch R, Slugovc C. Journal of Polymer Science Part A: Polymer Chemistry. 2008;46:4630 [Google Scholar]
- [13].Scheinhardt B, Trzaskowski J, Baier MC, Stempfle B, Oppermann A, Wöll D, Mecking S. Macromolecules. 2013;46:7902 [Google Scholar]
- [14].Leitgeb A, Wappel J, Slugovc C. Polymer. 2010;51:2927 [Google Scholar]
- [15].Sandholzer M, Lex A, Trimmel G, Saf R, Stelzer F, Slugovc C. Journal of Polymer Science Part A: Polymer Chemistry. 2007;45:1336 [Google Scholar]
- [16].Gallas K, Knall A-C, Scheicher SR, Fast DE, Saf R, Slugovc C. Macromolecular Chemistry and Physics. 2014;215:76. [Google Scholar]
- [17].Radl SV, Roth M, Gassner M, Wolfberger A, Lang A, Hirschmann B, Trimmel G, Kern W, Griesser T. Eur Polym J. 2014;52:98. [Google Scholar]
- [18].Boyd TJ, Schrock RR. Macromolecules. 1999;32:6608 [Google Scholar]
- [19].Gordon EJ, Gestwicki JE, Strong LE, Kiessling LL. Chemistry & Biology. 2000;7:9. doi: 10.1016/s1074-5521(00)00060-0. [DOI] [PubMed] [Google Scholar]
- [20].Mangold SL, Carpenter RT, Kiessling LL. Organic letters. 2008;10:2997. doi: 10.1021/ol800932w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chien M-P, Thompson MP, Barback CV, Ku T-H, Hall DJ, Gianneschi NC. Advanced Materials. 2013;25:3599. doi: 10.1002/adma.201300823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hilf S, Kilbinger AFM. Nat Chem. 2009;1:537. doi: 10.1038/nchem.347. [DOI] [PubMed] [Google Scholar]
- [23].Lexer C, Saf R, Slugovc C. J Polym Sci Part A Polym Chem. 2009;47:299. [Google Scholar]
- [24].Kolonko EM, Pontrello JK, Mangold SL, Kiessling LL. Journal of the American Chemical Society. 2009;131:7327. doi: 10.1021/ja809284s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kolonko EM, Kiessling LL. Journal of the American Chemical Society. 2008;130:5626. doi: 10.1021/ja8001716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kilbinger AFM. Functional Polymers by Post-Polymerization Modification. Wiley-VCH Verlag GmbH & Co. KGaA; 2012. The Synthesis of End-Functional Ring-Opening Metathesis Polymers; p. 153. [Google Scholar]
- [27].Thompson MP, Randolph LM, James CR, Davalos AN, Hahn ME, Gianneschi NC. Polymer Chemistry. 2014;5:1954. doi: 10.1039/C3PY01338C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hollauf M, Trimmel G, Knall A-C. Monatshefte für Chemie - Chemical Monthly. 2015;146:1063 [Google Scholar]
- [29].Feng K, Xie N, Chen B, Tung C-H, Wu L-Z. Biomacromolecules. 2016;17:538. doi: 10.1021/acs.biomac.5b01450. [DOI] [PubMed] [Google Scholar]
- [30].Ahmed E, Morton SW, Hammond PT, Swager TM. Advanced Materials. 2013;25:4504. doi: 10.1002/adma.201301656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sandholzer M, Fritz-Popovski G, Slugovc C. Journal of Polymer Science Part A Polymer Chemistry. 2008;46:401. [Google Scholar]
- [32].Roberts KS, Sampson NS. Organic letters. 2004;6:3253. doi: 10.1021/ol048935y. [DOI] [PubMed] [Google Scholar]
- [33].Gueugnon F, Denis I, Pouliquen D, Collette F, Delatouche R, Héroguez V, Grégoire M, Bertrand P, Blanquart C. Biomacromolecules. 2013;14:2396. doi: 10.1021/bm400516b. [DOI] [PubMed] [Google Scholar]
- [34].Miki K, Oride K, Kimura A, Kuramochi Y, Matsuoka H, Harada H, Hiraoka M, Ohe K. Small. 2011;7:3536. doi: 10.1002/smll.201101637. [DOI] [PubMed] [Google Scholar]
- [35].Robin MP, Osborne SAM, Pikramenou Z, Raymond JE, O’Reilly RK. Macromolecules. 2016;49:653. doi: 10.1021/acs.macromol.5b02152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mabire AB, Robin MP, Quan W-D, Willcock H, Stavros VG, O’Reilly RK. Chemical Communication. 2015;51:9733. doi: 10.1039/c5cc02908b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Vöhringer M, Hartleb W, Lienkamp K. ACS Biomaterials Science & Engineering. 2017 doi: 10.1021/acsbiomaterials.7b00140. [DOI] [PubMed] [Google Scholar]
- [38].Dorner F, Boschert D, Schneider A, Hartleb W, Al-Ahmad A, Lienkamp K. ACS Macro Lett. 2015;4:1337. doi: 10.1021/acsmacrolett.5b00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zou P, Laird D, Riga EK, Deng Z, Perez-Hernandez H-R, Guevara-Solarte DL, Steinberg T, Al-Ahmad A, Lienkamp K. Journal of Materials Chemistry B: Materials for Biology and Medicine. 2015;3:6224. doi: 10.1039/c5tb00906e. [DOI] [PubMed] [Google Scholar]
- [40].Dorner F, Malek-Luz A, Saar JS, Bonaus S, Al-Ahmad A, Lienkamp K. Macromol Chem Phys. 2016 Ahead of Print. [Google Scholar]
- [41].Hartleb W, Saar JS, Zou P, Lienkamp K. Macromol Chem Phys. 2016;217:225. [Google Scholar]
- [42].Kurowska M, Eickenscheidt A, Guevara-Solarte DL, Widyaya VT, Marx F, Al-Ahmad A, Lienkamp K. Biomacromolecules. 2017;18:1373. doi: 10.1021/acs.biomac.7b00100. [DOI] [PubMed] [Google Scholar]
- [43].Trnka TM, Grubbs RH. Acc Chem Res. 2001;34:18. doi: 10.1021/ar000114f. [DOI] [PubMed] [Google Scholar]
- [44].Liu M, van Hensbergen J, Burford RP, Lowe AB. Polymer Chemistry. 2012;3:1647 [Google Scholar]
- [45].Aponte MA, Butler GB. Journal of Polymer Science: Polymer Chemistry Edition. 1984;22:2841 [Google Scholar]
- [46].Lee S-S, Ahn TO. Journal of Applied Polymer Science. 1999;71:1187 [Google Scholar]
- [47].Searle NE. 1948 inv. [Google Scholar]
- [48].Gabriel GJ, Madkour AE, Dabkowski JM, Nelson CF, Nüsslein K, Tew GN. Biomacromolecules. 2008;9:2980. doi: 10.1021/bm800855t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Colak S, Tew GN. Langmuir. 2012;28:666. doi: 10.1021/la203683u. [DOI] [PubMed] [Google Scholar]
- [50].Lienkamp K, Madkour AE, Musante A, Nelson CF, Nusslein K, Tew GN. J Am Chem Soc. 2008;130:9836. doi: 10.1021/ja801662y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lienkamp K, Kins CF, Alfred SF, Madkour AE, Tew GN. J Polym Sci Part A Polym Chem. 2009;47:1266 [Google Scholar]
- [52].Al-Ahmad A, Laird D, Zou P, Tomakidi P, Steinberg T, Lienkamp K. PLoS ONE. 2013;8:e73812. doi: 10.1371/journal.pone.0073812. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.