Abstract
The Agouti Viable Yellow (Avy) allele is an insertional mutation in the mouse genome caused by a variably methylated intracisternal A-particle (VM-IAP) retrotransposon. Avy expressivity is sensitive to a range of early-life chemical exposures and nutritional interventions, suggesting that environmental perturbations can have long-lasting effects on the methylome. However, the extent to which VM-IAP elements are environmentally labile with phenotypic implications is unknown. Using a recently identified repertoire of VM-IAPs, we assess the epigenetic effects of different environmental contexts. A longitudinal aging analysis indicates that VM-IAPs are stable across the murine lifespan, with only small increases in DNA methylation detected for a subset of loci. No significant effects are observed following maternal exposure to the endocrine-disruptor bisphenol A, to an obesogenic diet, or to methyl-donor supplementation. A genetic mouse model of abnormal folate metabolism exhibits shifted VM-IAP methylation levels and altered VM-IAP-associated gene expression, yet these effects are likely largely driven by differential targeting by polymorphic KRAB zinc-finger proteins. We conclude that epigenetic variability at retrotransposons is not predictive of environmental susceptibility.
Almost half of the mouse genome is made up of transposable elements, the vast majority of which are repressed by DNA methylation and repressive histone modifications1,2. The Agouti Viable Yellow (Avy) metastable epiallele is a notable exception, where an endogenous retrovirus (ERV) of the intracisternal A-particle (IAP) class exhibits varying degrees of DNA methylation across genetically identical individuals3. When unmethylated, the IAP provides a promoter for the downstream coat color gene Agouti, resulting in mice ranging in coat color from yellow to mottled to pseudoagouti3,4.
The Avy mouse model has been extensively studied in the field of environmental epigenetics5. Maternal exposure to a range of environmental conditions causes a shift in offspring coat color distributions, often with a corresponding change in methylation levels at the Avy IAP. Dietary supplementation of dams with methyl-donors and cofactors (e.g., folate, vitamin B12, choline chloride, and anhydrous betaine) leads to a shift in offspring coat color towards pseudoagouti6–8. Other pseudoagouti-shifting environmental perturbations include dietary genistein, ethanol, ionizing radiation, and dibutyl phthalates9–12. Conversely, perinatal exposures to heavy metal (e.g. lead) or to bisphenol A (BPA) were reported to shift offspring coat color towards yellow9,13, although a later study was unable to replicate the BPA effect14. From this, the Avy mouse model is described as an epigenetic biosensor of environmental alterations15,16. Two other metastable epialleles, AxinFu and CabpIAP, were analyzed in the contexts of methyl-donor supplementation and BPA exposure, respectively, and shown to be similarly labile9,17.
We recently carried out a screen for metastable epialleles in the mouse genome (C57BL/6J strain) and identified a set of variably methylated IAPs (VM-IAPs) displaying Avy-like properties18,19. These alleles can be utilized in an isogenic context to assess the extent to which variable methylation at retrotransposons confers environmental sensitivity and to determine whether metastable epialleles in general can act as biosensors of environmental change. The assessment of multiple VM-IAPs within and between individuals provides a more predictive and robust analysis of these topics, with the potential to determine whether VM-IAPs can act as mediators between environmental stimuli and phenotypic outcomes.
Epigenetic instability across generations is a defining feature of VM-IAPs, whereby offspring born to the same inbred parents exhibit a range of DNA methylation levels18. Moreover, VM-IAP methylation levels are consistent across tissues within a single mouse, suggesting they are established prior to tissue differentiation and stably sustained thereafter. However, it is unknown whether VM-IAP methylation levels change throughout the murine lifespan. This factor must be considered when designing and interpreting environmental manipulation experiments and is of particular interest in view of the so-called “DNA methylation clocks”, which utilize DNA methylation at discrete genomic sites to predict age in humans20–22 and mice23–26.
In this study, we investigate the stability of VM-IAP methylation states in different contexts. We demonstrate that VM-IAP methylation levels are stable throughout the murine lifespan despite their characteristic instability after passage through the germline. In addition, we show that maternal exposure to BPA, to an obesogenic diet, or to dietary methyl-donors does not have a profound effect on VM-IAP methylation levels, contrasting with previous studies on classical metastable epialleles. Finally, we assess VM-IAP methylation in a genetic mouse model that recapitulates folate deficiency in humans and find that a subset of VM-IAPs display shifted methylation levels and altered neighboring gene expression. However, rather than reflecting susceptibility to abnormal folate metabolism, we suggest that these effects are largely driven by differential targeting of VM-IAPs by strain-specific KRAB zinc-finger proteins (KRAB-ZFPs).
Results
VM-IAP methylation is stable across the murine lifespan
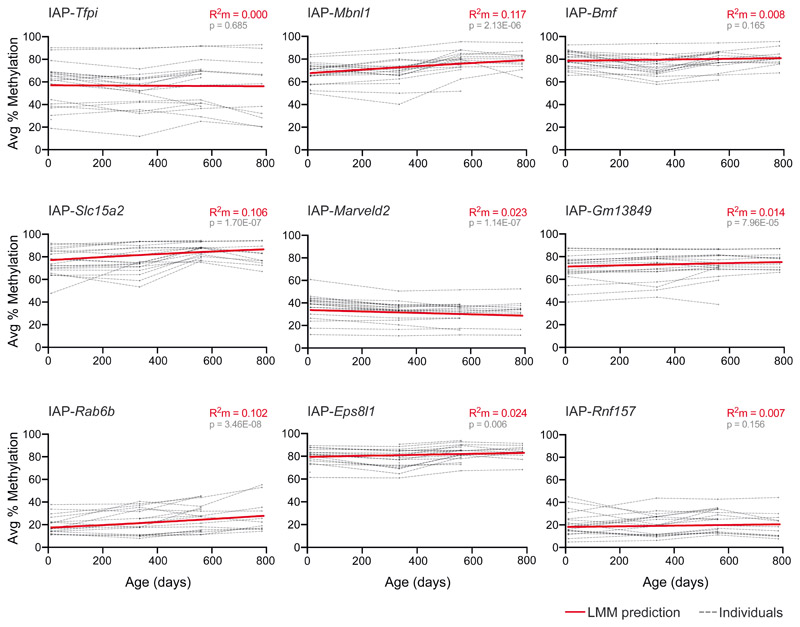
We developed a longitudinal aging study tracking VM-IAP methylation levels in vivo in 20 individual (10 male and 10 female) C57BL/6J mice. Given the inter-individual methylation variation characteristic of VM-IAPs, we assessed the same individuals over time rather than taking an age-sorted cohort-based approach. Ear samples were taken at regular intervals starting 10 days after birth until death. DNA methylation was quantified at the four or five most distal CpGs of the 5’ long terminal repeat (LTR) of VM-IAPs using bisulfite pyrosequencing. Linear mixed-effects models (LMMs) for each VM-IAP, which included age as a fixed effect and mouse and litter as random intercept effects, revealed that age does not have a marked effect on VM-IAP methylation levels (Fig. 1; Supplementary Table 1). Although statistically significant age-related changes in DNA methylation were detected at five out of nine VM-IAPs, these were associated with small effect sizes (as measured by marginal R2 values) that are unlikely to have biological significance (Fig. 1; Supplementary Table 1). More noteworthy was the conservation of the rank order in DNA methylation levels across individuals at each VM-IAP, indicating that individual-specific VM-IAP methylation states are maintained over the murine lifespan. Thus, age does not confound investigations on the susceptibility of VM-IAP methylation to environmental exposures and VM-IAP methylation cannot be used to predict age.
Fig. 1. VM-IAP methylation levels are stable throughout the murine lifespan.
DNA methylation was quantified in ear samples collected from C57BL/6J mice at 10 days, 48 weeks, 80 weeks, and 112 weeks of age using bisulfite pyrosequencing (n = 10 males and 10 females). Linear mixed-effects models (LMMs) were built to assess the effect of age on VM-IAP methylation level. Each VM-IAP model was fitted using the lmer() function from the lme4 R package. Age was included as a fixed effect; litter and mouse ID were included as random intercept effects. Marginal R squared (R2m) values were calculated to quantify the proportion of variance explained by age. t-test P values were calculated with the lmerTest package in R using the Satterthwaite approximation for degrees of freedom71 and interpreted using a Bonferroni-adjusted α value of 0.0056. Each dotted line represents VM-IAP methylation of one individual mouse over time. DNA methylation percentages represent the average percentage methylation at the four or five most distal CpGs of the VM-IAP 5’ LTRs. The solid red line is the LMM prediction. See also Supplementary Table 1.
VM-IAPs are unresponsive to maternal exposure to BPA
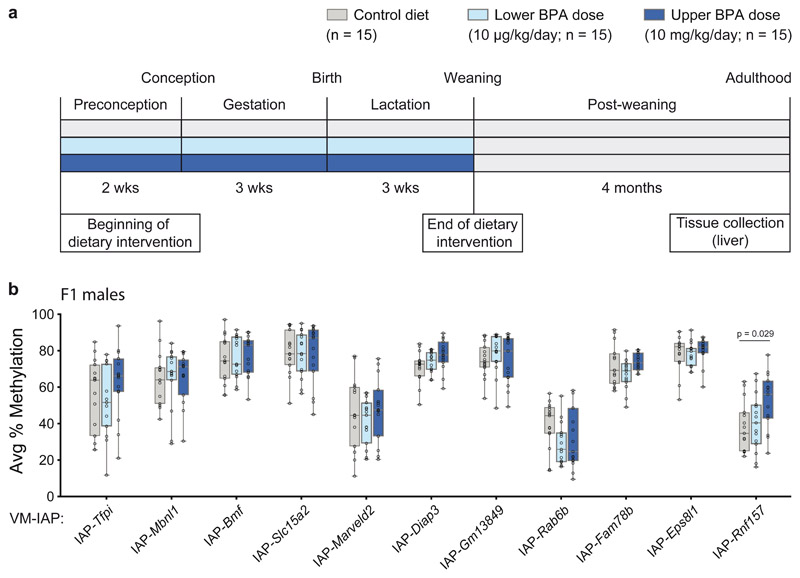
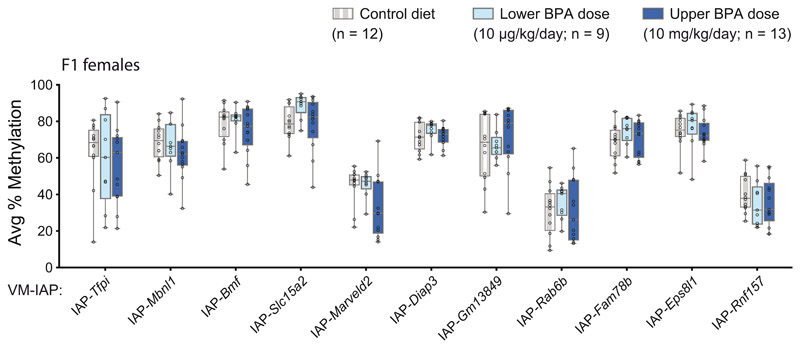
BPA is a ubiquitous endocrine disrupting chemical found in polycarbonate plastics and epoxy resins. Human exposure to BPA is linked to metabolic diseases such as type 2 diabetes27–29, emphasizing the value of developing mouse models to better understand the health implications of this compound. We tested whether maternal exposure to dietary BPA induces epigenetic dysregulation at VM-IAPs using a previously characterized mouse model of maternal BPA exposure30. The model is associated with male offspring-specific multigenerational (F1, F2) metabolic phenotypes, including increased body fat, glucose intolerance, impaired insulin secretion, and pancreatic islet inflammation31,32. C57BL/6J F0 females were fed either a control or BPA-supplemented diet two weeks prior to mating, throughout pregnancy, and during lactation (Fig. 2a). Two different BPA doses representative of human exposure levels were administered: a lower dose of 10 μg/kg/day and an upper dose of 10 mg/kg/day30. The latter dose matches the dose used in previous studies in Avy mice9,14.
Fig. 2. VM-IAP methylation is unresponsive to maternal exposure to the endocrine disruptor BPA.
a, Exposure scheme. F0 dams were fed either a control diet (7% corn oil, grey) or one of two BPA-supplemented diets two weeks prior to mating, throughout pregnancy and lactation (lower BPA dose: 10 μg/kg/day, light blue; upper BPA dose: 10 mg/kg/day, dark blue). Adult F1 male liver tissue was collected from one mouse per litter. b, Comparison of the average percent CpG methylation at eleven VM-IAPs in F1 males across exposure groups (n = 15 males per group; Welch’s ANOVA; adjusted P value for IAP-Rnf157 calculated by Dunnett’s T3 post hoc two-tailed test). Data points represent the average of the four or five most distal CpGs of the VM-IAP 5’ LTRs. Box-plot elements: center line, median; box limits, 25th and 75th percentiles; whiskers, maximum and minimum; all data points shown.
We assessed DNA methylation levels at 11 VM-IAPs in liver samples collected from adult F1 mice exposed to maternal dietary BPA. Both males and females were examined due to the sex specificity of phenotypes in this model31,32. None of the VM-IAPs tested in F1 females revealed a significant difference in methylation levels following maternal BPA exposure, regardless of dose (Extended Data Fig. 1). Similarly, VM-IAPs were unaffected in F1 males, with the exception of IAP-Rnf157, which showed a modest but significant increase in average DNA methylation in mice born to dams exposed to the upper BPA dose compared to control mice (Fig. 2b, P < 0.05). These results indicate that DNA methylation at most metastable epialleles tested to date is not affected by maternal exposure to BPA.
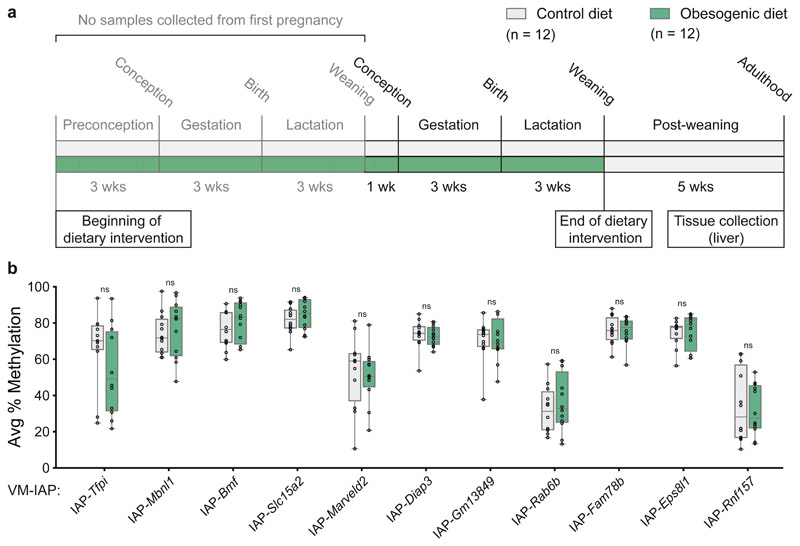
A maternal obesogenic diet has no effect on VM-IAP methylation
Considering that obesity-inducing diets are associated with widespread epigenetic alterations33–35, we examined whether maternal exposure to an obesogenic diet influences VM-IAP methylation levels using a well-established mouse model36. Multiparous C57BL/6J females were fed either a standard chow diet or a calorie-rich obesogenic diet (see Methods) ten weeks before mating, throughout pregnancy, and during lactation (Fig. 3a). F1 offspring born to obese dams display a wide range of cardiovascular and metabolic abnormalities36–38, yet no significant differences in VM-IAP methylation levels were detected between dietary groups in adult F1 male liver samples (Fig. 3b). This finding further indicates that VM-IAP methylation levels are not sensitive to environmental change.
Fig. 3. Maternal exposure to an obesogenic diet has no effect on VM-IAP methylation levels.
a, Exposure scheme. F0 dams were fed either a control standard chow (grey) or an obesogenic diet (green; see methods) ten weeks prior to mating, throughout pregnancy, and during lactation. Adult F1 male liver tissue was collected from one mouse per litter. b, Comparison of the average percent CpG methylation at eleven VM-IAPs between dietary groups shows no significant differences (n = 12 males per group; Welch’s t-tests). Data points represent the average of the four or five most distal CpGs of the VM-IAP 5’ LTRs. Box-plot elements: center line, median; box limits, 25th and 75th percentiles; whiskers, maximum and minimum; all data points shown.
VM-IAP methylation is altered in the Mtrrgt mouse model
One-carbon metabolism, which encompasses the folate and methionine cycles, is required for the transfer of methyl groups destined for substrates such as DNA, RNA, and histones39. Normal metabolic progression involves the enzyme methionine synthase (MTR) to exclusively transfer a methyl-group from 5-methyltetrahydrofolate to homocysteine to form methionine and tetrahydrofolate40. In mammals, MTR activity is maintained by methionine synthase reductase (MTRR) through the reductive methylation of its vitamin B12 cofactor41. A hypomorphic mutation in Mtrr (Mtrrgt) in mice42,43 causes phenotypes commonly associated with dietary folate deficiency in humans44, including increased plasma total homocysteine concentrations42,43, macrocytic anemia45, congenital abnormalities, and locus-specific epigenetic perturbation43. Therefore, the Mtrrgt mouse model provides a powerful genetic model to study abnormal folate metabolism46.
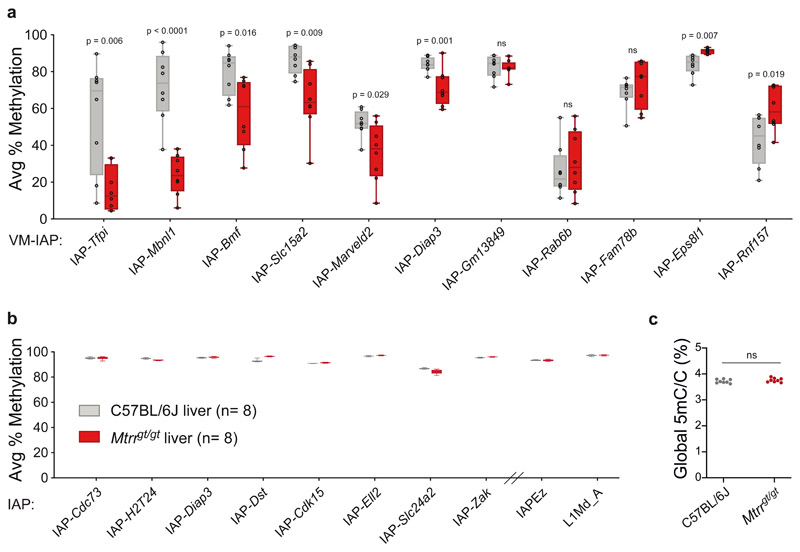
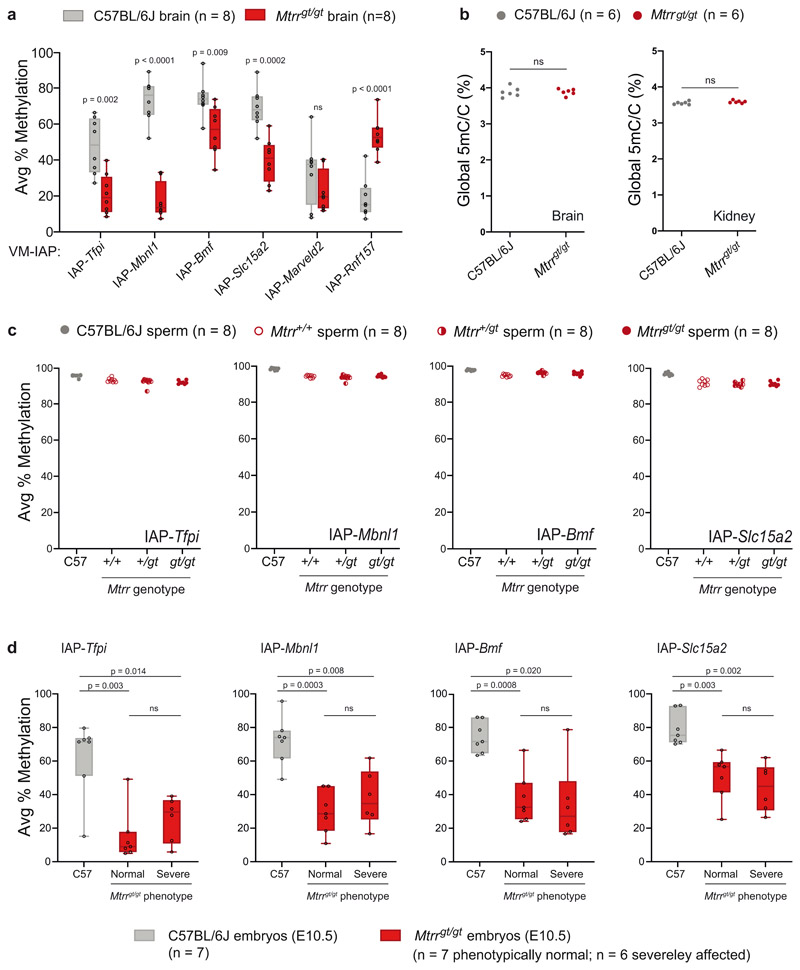
To investigate the effect of abnormal folate metabolism on VM-IAP methylation, we compared C57BL/6J control and homozygous Mtrrgt/gt adult male livers. In contrast to the dietary intervention models above, we found significant differences in DNA methylation at most VM-IAPs (Fig. 4a). The majority showed a decrease in methylation in Mtrrgt/gt samples compared to controls, with two loci (IAP-Eps8l1 and IAP-Rnf157) showing an increase in methylation (Fig. 4a). This is in agreement with previous reports showing locus-specific CpG hypo- and hypermethylation in Mtrrgt/gt tissue43. The magnitude of methylation change was also locus-specific, with IAP-Tfpi, IAP-Mbnl1, IAP-Bmf, and IAP-Slc15a2 showing the most pronounced shifts. Of note, IAP-Tfpi and IAP-Mbnl1 have identical sequences, suggesting that the locus-specificity of the effects is driven by DNA sequence rather than genomic context. With the exception of IAP-Eps8l1, which was hypermethylated in all mutant individuals, inter-individual variation in methylation was conserved in Mtrrgt/gt livers. In addition, the consistency in VM-IAP methylation levels observed across tissues within a C57BL/6J individual held true for Mtrrgt/gt mice, as VM-IAP methylation levels in Mtrrgt/gt brain were comparable to those in Mtrrgt/gt liver (Extended Data Fig. 2a). This result suggests that altered VM-IAP methylation levels in Mtrrgt/gt mice were established prior to tissue differentiation.
Fig. 4. A mouse model of abnormal folate metabolism exhibits altered VM-IAP methylation.
a, Box plots comparing the average percentage of DNA methylation at eleven VM-IAPs between C57BL/6J (n = 8, grey) and Mtrrgt/gt (n = 8, red) liver samples (center line, median; box limits, 25th and 75th percentiles; whiskers, maximum and minimum). Reported values represent the average of the four or five most distal CpGs of the VM-IAP 5’ LTRs. P values were calculated by two-tailed Welch’s t-tests (ns indicates P > 0.05). b, Box plots comparing the average percentage of DNA methylation at non-variable and highly methylated IAPs between C57BL/6J (n = 8, grey) and Mtrrgt/gt (n = 8, red) liver samples (center line, median; box limits, 25th and 75th percentiles; whiskers, maximum and minimum). Reported values represent the average of the four or five most distal CpGs of the 5’ LTRs. The primers for the bulk IAPEz and L1Md_A assays target thousands of conserved elements. c, Global DNA methylation levels are equivalent between C57BL/6J (n = 8, grey circles) and Mtrrgt/gt (n = 8, red circles) liver samples (unpaired two-tailed Student’s t-test; P value = 0.278; ns indicates P > 0.05). Global 5-methyl-cytosine (5mC) content was determined by liquid chromatography-tandem mass spectrometry and expressed as a percentage relative to total cytosine (C) in the genome.
We next quantified DNA methylation at non-variable IAPs in Mtrrgt/gt tissues. Eight individual IAPs known to be highly methylated in C57BL/6J mice remained highly methylated in Mtrrgt/gt liver samples, indicating that the Mtrrgt mutation does not exert a general effect on IAPs (Fig. 4b). This was verified by a bulk assay capturing more than 1,000 distinct IAP elements (IAPEz; Fig. 4b). LINE1 retrotransposons also exhibited comparable methylation levels between C57BL/6J and Mtrrgt/gt mice (Fig. 4b). Furthermore, global 5-methylcytosine levels in liver, kidney, and brain DNA samples quantified by mass spectrometry were equivalent in C57BL/6J and Mtrrgt/gt samples. This result differs from our previous study43 since a more accurate technique was used here47,48 and further demonstrates that altered VM-IAP methylation in Mtrrgt/gt individuals represents a locus-specific effect rather than a genome-wide phenomenon (Fig. 4c; Extended Data Fig. 2b).
As with other repeat elements in the mouse genome, VM-IAPs become fully methylated in the male germline18,49. We assessed VM-IAP methylation levels in mature sperm collected from control and Mtrrgt/gt adult males and found that VM-IAP hypermethylation was maintained in Mtrrgt/gt sperm (Extended Data Fig. 2c). This finding emphasizes that the regulatory mechanisms underlying transposable element methylation in the germline are distinct from the soma and likely reflects the greater evolutionary cost of activating potentially mutagenic mobile elements in the germline.
VM-IAP-associated genes are dysregulated in Mtrrgt/gt mice
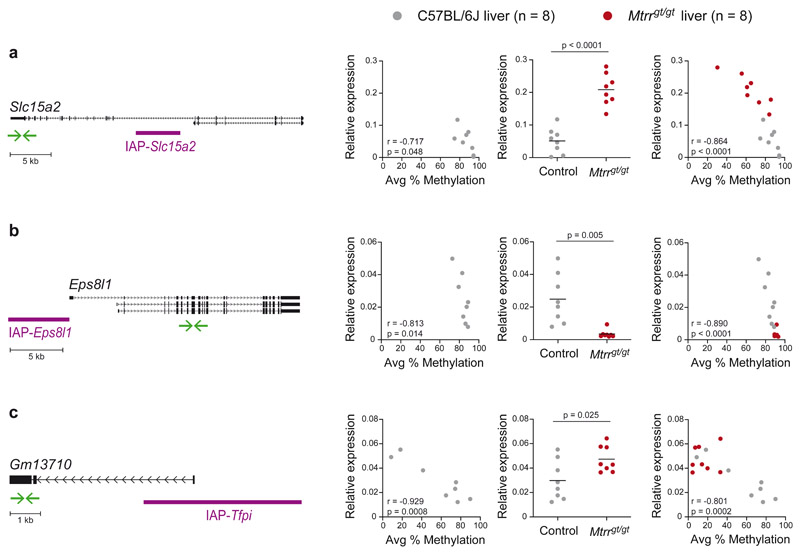
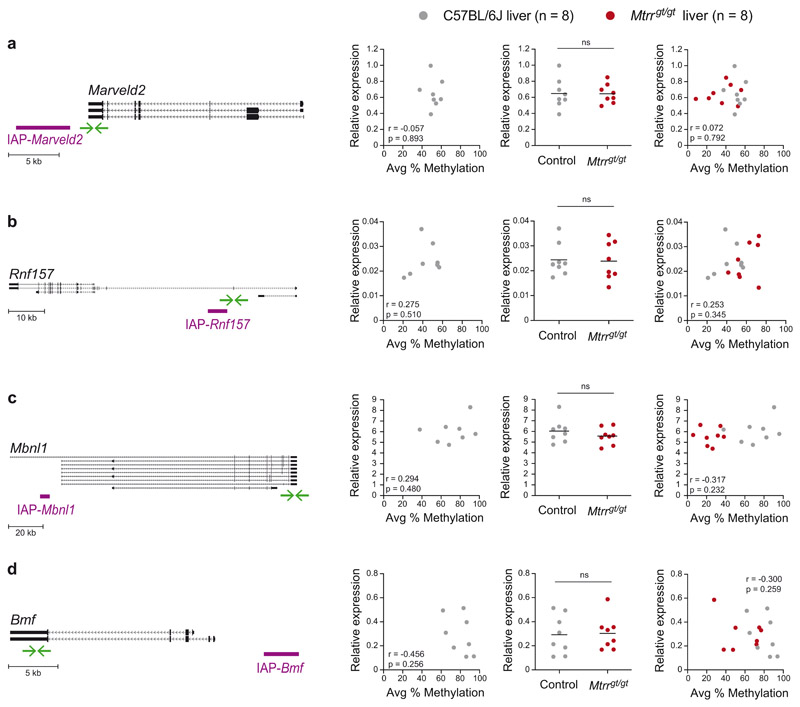
VM-IAPs can act as alternative promoters to adjacent genes in a manner reminiscent of the Avy locus, with DNA methylation at the VM-IAP LTR inversely correlated with gene expression3,18. Replicating our previously published data, we found that Slc15a2, Eps8l1, and Gm13710 expression levels in C57BL/6J liver are inversely correlated with IAP-Slc15a2, IAP-Eps8l1, and IAP-Tfpi methylation levels, respectively (Fig. 5a-c; left-hand graphs). We reasoned that altered DNA methylation at these regions in Mtrrgt/gt mice may influence nearby gene expression. Indeed, all three genes were significantly differentially expressed between control and Mtrrgt/gt liver samples (Fig. 5a-c; center graphs). In addition, combining the datasets from both C57BL/6J control and Mtrrgt/gt liver samples resulted in stronger correlations between DNA methylation and gene expression for these loci (Fig. 5a-c; right-hand graphs). We note that Mtrrgt/gt livers exhibiting ~80% methylation at IAP-Slac15a2 showed higher expression levels than C57BL/6J livers with the same methylation level, suggesting additional mechanisms influence Slc15a2 regulation in Mtrrgt/gt mice. No significant changes in expression were detected in Mtrrgt/gt liver samples for VM-IAP-neighboring genes whose expression was not correlated with VM-IAP methylation in C57BL/6J mice, despite altered VM-IAP methylation in Mtrrgt/gt individuals (Extended Data Fig. 3a-d). These results demonstrate that VM-IAPs can mediate changes in gene expression caused by distant genetic perturbations at the Mtrr locus.
Fig. 5. VM-IAP-associated gene expression is altered in Mtrrgt/gt mice.
Left-hand graphs assess the correlation between VM-IAP methylation and adjacent Slc15a2 (a), Eps8l1 (b), and Gm13710 (c) gene expression in C57BL/6J livers (n = 8, r: Pearson’s correlation coefficient; p: two-tailed P value associated with r). Centre graphs show qRT-PCR expression data of VM-IAP-neighboring genes Slc15a2 (a), Eps8l1 (b), and Gm13710 (c) in C57BL/6J (n = 8, grey circles) and Mtrrgt/gt (n = 8, red circles) liver. P values were calculated by two-tailed Welch’s t-tests (means shown as black lines). Right-hand graphs incorporate both control and Mtrrgt/gt data and assess the correlation between gene expression and VM-IAP methylation (n = 16, r: Pearson’s correlation coefficient; p: two-tailed P value associated with r). Relative expression was normalized to Hprt1 expression and calculated using the ΔCt method. Gene Diagrams of VM-IAPs in relation to their neighboring gene are depicted on the far left. Gene transcripts, extracted from the University of California, Santa Cruz (UCSC) Genome Browser72, are shown in black and VM-IAPs in purple. Green arrows represent the location of qRT-PCR primers. Diagrams are drawn to scale. See also Extended Data Figure 3.
VM-IAP methylation is independent of Mtrrgt/gt phenotype
A wide spectrum of developmental phenotypes is evident in Mtrrgt/gt conceptuses at embryonic day (E) 10.5, from phenotypically normal to severely affected embryos (i.e., display at least one congenital malformation)43. In addition, the Mtrrgt mouse line displays transgenerational epigenetic inheritance: a maternal grandparent that is a carrier of the Mtrrgt mutation can give rise to wildtype F2-F4 offspring with developmental phenotypes at E10.5 that are similar to those observed in Mtrrgt/gt conceptuses43. Phenotypic inheritance in the absence of the original Mtrr mutation has been linked to epigenetic instability43.
To probe the relationship between the previously characterized embryonic phenotypes associated with the Mtrrgt mouse line and the altered VM-IAP methylation states identified here, we assessed VM-IAP methylation in phenotypically normal and severely affected Mtrrgt/gt E10.5 embryos at E10.5 generated from Mtrrgt/gt intercrosses. All Mtrrgt/gt embryos regardless of phenotypic severity exhibited significantly decreased DNA methylation levels compared to C57BL/6J embryos at E10.5 at the four tested VM-IAPs (Extended Data Fig. 2d). These data were consistent with data from adult tissues with regards to degree and directionality of the methylation change (Fig. 4a; Extended Data Fig. 2d). Furthermore, no significant difference in methylation levels was observed between phenotypically normal and severely affected Mtrrgt/gt embryos at E10.5 (Extended Data Fig. 2d), indicating that the mechanism driving the effect on VM-IAP methylation is distinct from the process leading to variable phenotypic penetrance during development.
Parental-zygotic effects at VM-IAPs in the Mtrrgt mouse line
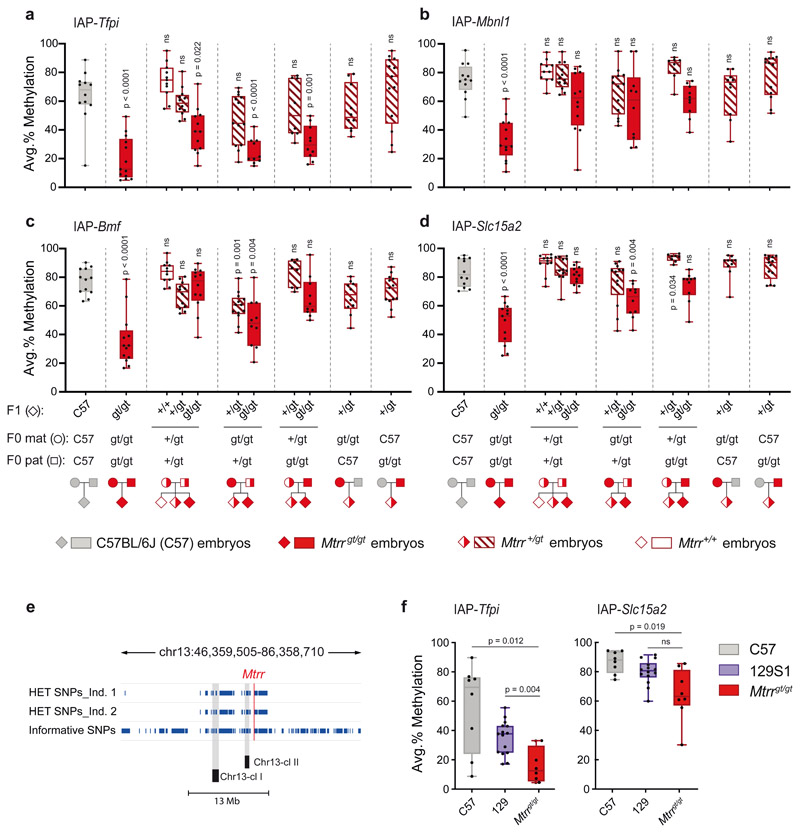
To determine whether an Mtrrgt allele in the parental generation had an effect on the methylation state of VM-IAPs, we analyzed whole Mtrr +/+, Mtrr +/gt, and Mtrrgt/gt embryos at E10.5 generated from heterozygous intercrosses compared to those derived from homozygous mothers and fathers. VM-IAP methylation levels in Mtrr+/+ and Mtrr +/gt embryos from heterozygous intercrosses were comparable to those observed in C57BL/6J embryos (Fig. 6a-d). In fact, altered VM-IAP methylation was largely restricted to homozygous Mtrrgt/gt individuals as shown above (Extended Data Fig. 2d). Notably, the decrease in VM-IAP methylation levels in littermate Mtrrgt/gt embryos from heterozygous Mtrr +/gt parents was not as severe as in Mtrrgt/gt embryos derived from homozygous Mtrrgt/gt parents (Fig. 6a-d). In some cases, the effect on methylation disappeared entirely in Mtrrgt/gt embryos when fewer parental Mtrrgt alleles were present (e.g. IAP-Slc15a2; Fig. 6d). This result indicates that parental Mtrrgt/gt homozygosity is a critical factor driving altered VM-IAP methylation levels in the Mtrrgt mouse model. Reciprocal crosses between homozygous Mtrrgt/gt and heterozygous Mtrr +/gt mice confirmed this finding by demonstrating that both maternal and paternal Mtrrgt/gt homozygosity contribute to VM-IAP hypomethylation in Mtrrgt/gt offspring (albeit to varying degrees depending on the locus examined) (Fig. 6a-d). Maternal Mtrrgt/gt homozygosity tended to have a larger effect than paternal homozygosity, indicative of a more consequential oocyte contribution to the offspring (Fig. 6a-d). However, neither maternal nor paternal Mtrrgt/gt homozygosity alone was sufficient to alter VM-IAP methylation, as reciprocal crosses between Mtrrgt/gt and C57BL/6J mice yielded Mtrr+/gt embryos with normal VM-IAP methylation levels (Fig. 6a-d).
Fig. 6. VM-IAP methylation levels in the Mtrrgt mouse line display parental-zygotic effects and may be driven by polymorphic KRAB-ZFPs.
Methylation levels were quantified at IAP-Tfpi (a), IAP-Mbnl1 (b), IAP-Bmf (c), and IAP-Slc15a2 (d) in embryos at E10.5 generated from distinct parental Mtrr genotypes. Offspring (F1), maternal (F0 mat), and paternal (F0 pat) genotypes are shown below the x-axes (n = 8-13 offspring per group). Average methylation levels were compared across groups by Welch’s ANOVA and adjusted P values were calculated by two-tailed Dunnett’s T3 post hoc comparisons to C57BL/6J (ns indicates P > 0.05). Methylation values represent the average of the four most distal CpGs of the VM-IAP 5’ LTRs. e, The Mtrrgt mutation is adjacent to polymorphic KRAB-ZFP clusters. Informative SNPs between the C57BL/6J and 129P2Ola/Hsd strains in the region surrounding the gene-trap insertion site in the Mtrr gene are shown as navy-blue lines for two Mtrr +/gt individuals. Data were collected using the GigaMUGA genotyping SNP array. Locations of the two KRAB-ZFP clusters on Chromosome 13 (Chr13-cl I and Chr13-cl II) are shown on the diagram (drawn to scale). f, IAP-Tfpi and IAP-Slc15a2 methylation levels in C57BL/6J (C57; n = 8), 129S1/SvlmJ (129S1; n = 15), and Mtrrgt/gt (n = 8) adult genomic DNA. Average methylation levels were compared across groups by Welch’s ANOVA and adjusted P values were calculated by two-tailed Dunnett’s T3 post hoc comparisons to Mtrrgt/gt (ns indicates P > 0.05). Box-plot elements for panels a-d, f: center line, median; box limits, 25th and 75th percentiles; whiskers, maximum and minimum; all data points shown.
The Mtrrgt mutation is adjacent to polymorphic KRAB-ZFPs
The parental-zygotic effects associated with VM-IAP methylation in the Mtrrgt mouse line were reminiscent of inheritance patterns observed for the Zfp57 gene, which exhibits embryonic lethality upon loss of both maternal and zygotic gene copies50. ZFP57 maintains DNA methylation at genomic imprints in early development and is one of hundreds of KRAB-ZFPs encoded by the mouse genome51. Most KRAB-ZFPs are involved in the sequence-specific recognition and epigenetic repression of transposable elements52. Coincidentally, we recently demonstrated that rapidly evolving KRAB-ZFPs are responsible for genetic background-specific DNA methylation levels at VM-IAPs53. This finding, coupled with the parental-zygotic effects describe here, motivated a more careful evaluation of the genetic origins of the Mtrrgt allele.
The Mtrrgt mutation was generated by a gene-trap insertion in the 129P2Ola/Hsd mouse strain that was subsequently backcrossed to the C57BL/6J mouse strain for eight generations43. Due to genetic selection and lack of recombination, a 20-Mb region surrounding the gene-trap insertion site in the Mtrr gene on Chr. 13 retained the 129P2Ola/Hsd sequence in an otherwise C57BL/6J strain54. We hypothesized that altered VM-IAP methylation levels in the Mtrrgt mouse line may be rooted in the strain-specific genes located in the 129P2Ola/Hsd genomic interval surrounding the Mtrrgt allele rather than a downstream effect of the Mtrrgt mutation itself. Indeed, we discovered that two KRAB-ZFP clusters (Chr13-cl-I and Chr13-cl-II) are located within 7 Mb downstream of the Mtrr gene that together contain more than 30 individual KRAB-ZFP genes. We confirmed that both KRAB-ZFP clusters in the Mtrrgt mouse line are derived from the 129P2Ola/Hsd genome using the GigaMUGA genotyping SNP array (Fig. 6e). Thus, Mtrrgt/gt mice are also homozygous carriers of numerous 129P2Ola/Hsd-derived KRAB-ZFP genes that are known to be highly polymorphic between inbred mouse strains55.
As evidenced by their identical sequences, IAP-Tfpi and IAP-Mbnl1 originated in the C57BL/6J genome from the same retrotransposition-competent IAP. In Mtrrgt/gt mice, these VM-IAPs displayed equivalent DNA methylation levels and were the most markedly affected loci in our analysis (Fig. 4a; Supplemental Fig. 2d). Since IAP-Tfpi (but not IAP-Mbnl1) is also present in 129 substrain genomes, we tested IAP-Tfpi methylation levels in wildtype 129S1/SvlmJ mice (the reference strain for 129 substrains). We found that the methylation levels at IAP-Tfpi in 129S1/SvlmJ mice better reflected those in Mtrrgt/gt mice than those in C57BL/6J mice (Fig. 6f)53. IAP-Slc15a2, also present in both the C57BL/6J and 129 genomes but differing in sequence from IAP-Tfpi, showed a similar trend though with a less pronounced effect in relation to Mtrrgt/gt mice (Fig. 6f). Considering the abundance and functional redundancy characteristic of the KRAB-ZFP transcription factor family55, it is possible that other strain-specific KRAB-ZFPs outside of Chr13-cl-I and II target these (and potentially other) VM-IAPs in a pure 129 genetic background. Alternatively, abnormal folate deficiency caused by the Mtrrgt mutation may compound the strain effects, resulting in more striking methylation differences between C57BL/6J and Mtrrgt/gt mice. Based on these data, we theorize that altered VM-IAP methylation levels in Mtrrgt/gt mice largely result from the sequence-specific targeting of VM-IAPs by polymorphic KRAB-ZFP(s) neighboring the Mtrrgt allele.
VM-IAPs are unaffected by maternal methyl-donor supplementation
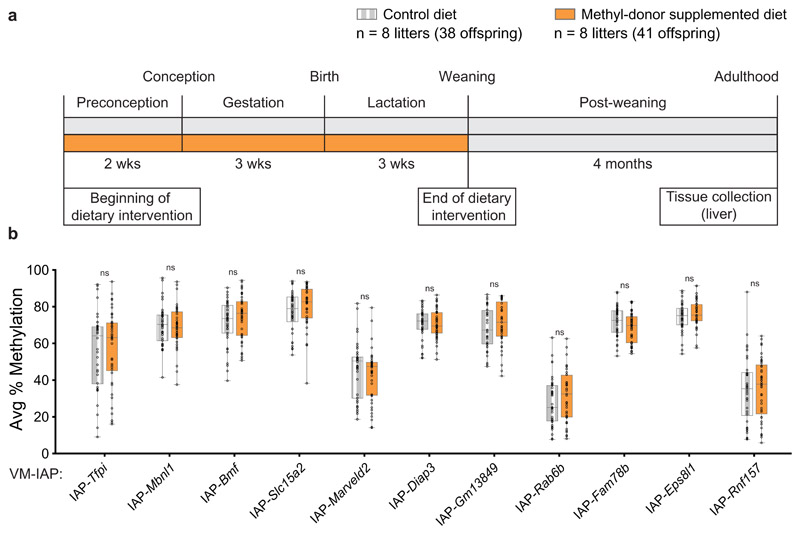
Having formulated an alternative genetically rooted hypothesis for altered VM-IAP methylation levels in Mtrrgt/gt mice, it was important to explore how dietary manipulation of one-carbon methyl-donors alone affected VM-IAP methylation. We chose maternal methyl-donor supplementation over insufficiency because diet-induced folate-deficient mice do not recapitulate the phenotypes observed in folate-deficient humans unless they are challenged with genetic insufficiency43–45. In addition, maternal dietary supplementation with methyl-donors influences DNA methylation at the Avy metastable epiallele8.
Our methyl-donor supplementation exposure scheme matched that of the other dietary models in this study and that known to influence Avy6: C57BL/6J females were fed either a control diet or a methyl-donor-supplemented diet for two weeks prior to mating, throughout pregnancy, and during lactation (Fig. 7a). VM-IAP methylation levels were quantified in liver samples collected from adult offspring and no significant differences were detected between control and methyl-donor-supplemented groups (Fig. 7b). Altogether, our data demonstrate that metastable epialleles are more resistant to environmental changes in early life than previously suggested15.
Fig. 7. VM-IAP methylation is unaffected by maternal dietary methyl-donor supplementation.
a, Exposure scheme. F0 dams were fed either a control (grey) or a methyl-donor supplemented diet (orange) two weeks prior to mating, throughout pregnancy, and during lactation. Adult F1 male liver tissue was collected from all offspring. b, Comparison of the average percentage of CpG methylation at 11 VM-IAPs between dietary groups shows no significant differences (n = 8 litters per group; Welch’s t-tests on litter averages; all offspring methylation levels shown on the graph). Data points represent the average of the four or five most distal CpGs of the VM-IAP 5’ LTRs. Samples sizes are shown in the legend. Box-plot elements: center line, median; box limits, 25th and 75th percentiles; whiskers, maximum and minimum; all data points shown.
Discussion
The Avy mouse model is described as an epigenetic biosensor of environmental compromise3,16. The identification of additional metastable epialleles genome-wide allows for the interrogation of multiple loci simultaneously within a single individual, enabling a more holistic assessment of whether innate epigenetic instability predisposes a region to environmental vulnerability, and if so, whether this has functional consequences. Here, we assessed VM-IAP methylation states throughout aging and in four different mouse models of environmental compromise, all of which are associated with multigenerational effects with implications for human disease risk. In contrast to previous work on individual classical loci, we found here that VM-IAP methylation levels are largely stable across the murine lifespan and in response to environmental change.
Maternal dietary supplementation of BPA or methyl-donors was previously documented to influence DNA methylation at metastable epialleles8,9,17. Despite the similarities in dosage and duration between previous studies and our own, we found no BPA-associated effect on VM-IAP methylation levels bar a modest increase at IAP-Rnf157 in male offspring of dams exposed to the upper BPA dose. In this regard our findings are more consistent with a later study showing that Avy coat color is unaffected by maternal exposure to BPA14.
In view of the direct link between dietary intake of methyl-donors, such as folic acid, and the deposition of methyl groups on DNA through one-carbon metabolism44, we chose dietary methyl-donor supplementation as the most likely intervention to influence DNA methylation at VM-IAPs. The absence of methylation changes in our analysis challenges the notion that variably methylated retrotransposons are more readily responsive to fluctuating environments. In the case of Avy, dietary methyl-donor supplementation following the same exposure scheme used in the present study resulted in DNA hypermethylation of the IAP upstream of the Agouti gene along with a shift in Agouti-controlled coat color6,8. However, provision of the same diet to wild-derived deer mice, which do not harbor a variably methylated retrotransposons near the Agouti gene, also results in altered Agouti-controlled coat color56. Thus, it is possible that the increase in DNA methylation at the Avy IAP is a secondary effect caused by downregulation of Agouti transcription following methyl-donor supplementation. Moreover, methyl-donor supplementation leads to a substantial increase in DNA methylation at the AxinFu metastable epiallele in tail but not liver tissue17. Given that AxinFu is associated with tail morphology phenotypes, this finding supports the possibility that increased availability of methyl-donors influences DNA methylation at a minority of metastable epialleles in an indirect transcriptionally driven manner.
There is compelling evidence that the methylation status of the Avy IAP in the oocyte is instructive for that of the offspring. In fact, a recent study successfully manipulated Avy offspring coat color by artificially editing DNA methylation at the Avy locus in individual oocytes followed by in vitro fertilisation57. Therefore, oocyte VM-IAP methylation may also be important for offspring VM-IAP methylation. Since de novo methylation of the female germline occurs in the growing oocyte58, the window of dietary exposure implemented by us and others has the potential to influence both the establishment and maintenance of oocyte DNA methylation. Nevertheless, future studies in which environmental insults are specifically targeted to growing oocytes will shed light on whether offspring VM-IAP methylation can be environmentally modulated during oocyte development in the previous generation.
Unlike the dietary models investigated in this study, the Mtrrgt/gt mouse line uses a genetic approach to incur metabolic disruption that is similar to dietary folate deficiency in humans. We observed considerable shifts in VM-IAP methylation levels and neighboring gene expression in Mtrrgt/gt individuals, indicating that VM-IAPs are capable of mediating transcriptional changes in response to genetic alterations at a distant locus. The contrast between the effects caused by the Mtrrgt allele and the stability of VM-IAP methylation following maternal methyl-donor supplementation, paired with our discovery that the Mtrrgt locus contains a cluster of 129-derived KRAB-ZFPs in an otherwise C57BL/6J background, suggests that strain-specific KRAB-ZFPs are predominantly responsible for altered VM-IAP methylation and associated misexpression in the Mtrrgt mouse line. It is unclear whether Mtrr-adjacent KRAB-ZFPs play a role in the establishment of Mtrr gt-associated embryonic phenotypes.
Our working model for the emergence of inter-individual methylation variation in mammals implicates a mechanistic role for the low-affinity recognition of transposable elements by KRAB-ZFPs53. KRAB-ZFPs are rapidly evolving in the mouse due to recent waves of ERV activity55, resulting in repetitive KRAB-ZFP clusters that are highly polymorphic between inbred mouse strains59. We recently reported that the KRAB-ZFP cluster on Chr. 4 (Chr4-cl) is responsible for the strain-specific hypermethylation of a subset of VM-IAPs53. The identification of Chr13-cl-I and II as additional candidate VM-IAP-modifying KRAB-ZFP clusters supports our model and is in line with previous studies demonstrating that KRAB-ZFPs in these clusters modulate a range of ERV-associated strain-specific phenotypes60–63.
We note that the size and directionality of the methylation changes detected in Mtrrgt/gt mice varies across VM-IAPs, leaving open the possibility that abnormal folate metabolism caused by mutating the Mtrr gene itself might account for some of the observed changes. Further experiments on other genetic models that disrupt one-carbon metabolism will help to determine the extent that metabolic disruption influences VM-IAP methylation. The generation of gap-free reference sequences for the Chr13-cl clusters across multiple mouse strains will similarly address this hypothesis.
In this study we showed that epigenetic variability is a poor predictor of environmental vulnerability, rendering metastable epialleles largely inadequate as biosensors of environmental compromise. Furthermore, we highlight the importance of carefully considering genetic background effects when studying epigenetic processes in congenic mouse strains. Our findings establish the Mtrrgt mouse line as a model to elucidate the evolutionary and mechanistic underpinnings of variable epigenetic states and associated phenotypes.
Online Methods
Mice
Mouse work was regulated under the Animals (Scientific Procedures) Act 1986 Amendment Regulations 2012 following ethical review by the University of Cambridge Animal Welfare and Ethical Review Body (Home Office project license numbers: PC213320E, PC6CEFE59, P5FDF0206) or with the approval of the University of Pennsylvania Institutional Animal Care and Use Committee. All mice were housed in temperature and humidity-controlled conditions under a 12-h light-dark cycle. Unless otherwise stated, mice were fed a standard chow diet ad libitum.
Aged mice
Ten C57BL/6J males and 10 C57BL/6J females from five different litters were ear notched at 10 days, 48 weeks, 80 weeks, and 112 weeks. With the exception of one mouse, lifespan ranged from 20 to 31 months, with an average of 26 months. Mice died of natural causes or were humanely culled for health reasons.
BPA-exposed mice
Experimental conditions for BPA exposure were performed as previously described31. C57BL/6J females were randomly assigned one of three modified AIN 93G diets: control (7% corn oil, Harlan Teklad), lower BPA dose (10 μg/kg/day, Harlan Teklad), or upper BPA dose (10 mg/kg/day, Harlan Teklad). Dietary intervention began two weeks prior to mating and continued throughout pregnancy and lactation. All F1 pups were weaned onto the control diet. F1 adult livers were dissected from one mouse per litter, with reported sample sizes representing the number of litters.
Diet-induced obese mice
Experimental conditions for the generation of diet-induced mice were previously described36. C57BL/6J females were randomly assigned one of two diets: standard RM1 chow (7% simple sugars, 3% fat, 50% polysaccharide, 15% protein [w/w], 10.74 kJ/g; Special Dietary Services, Witham, UK) or a semi-synthetic energy-rich highly palatable obesogenic diet (10% simple sugars, 20% animal lard, 28% polysaccharide, 23% protein [w/w], 28.43 kJ/g; Special Dietary Services, Witham, UK) supplemented with sweetened condensed milk (16% fat, 33% simple sugars, 15% protein, 13.7 kJ/g; Nestle, UK) and fortified with mineral and vitamin mix AIN93G. Dietary intervention began three weeks prior to a first pregnancy that was performed to prove fertility and establish good maternal care. One week after weaning of pups from the first pregnancy, dams were mated for the experimental pregnancy. Dams were maintained on the same experimental diet through both pregnancies and lactation. All F1 pups were weaned onto the standard RM1 chow diet. F1 adult livers were dissected from one mouse per litter, with reported sample sizes representing the number of litters.
Methyl-donor supplemented mice
C57BL/6J females were randomly assigned one of two diets: standard RM3(E) chow (with (per kg of diet) 1.6 g choline, 2.73 mg folic acid, 26.8 μg vitamin B12, 3.4 g methionine, 51.3 mg zinc; Special Dietary Services, Witham, UK) or a methyl-donor supplemented diet (RM3(E) supplemented with (per kg of diet) 15 g choline, 15 g betaine, 15 mg folic acid, 1.5 mg vitamin B12, 7.5 g L-methionine, 150 mg zinc; Special Diet Services, Witham, UK). The composition of the methyl-donor supplemented diet is consistent with the high-concentration 3SZM diet used by Wolff and colleagues6. Dietary intervention began two weeks prior to mating and continued throughout pregnancy and lactation. All F1 pups were weaned onto standard RM3(E) chow (see above). F1 adult livers were dissected from all offspring and statistics were carried out on litter averages.
Mtrrgt mice
The Mtrrgt mouse line was generated by a gene-trap insertion into the Mtrr locus as previously described43. The C57BL/6J mice (The Jackson Laboratory, Stock No. 000664) and 129S1/SvlmJ mice (The Jackson Laboratory, Stock No. 002448) compared to Mtrrgt/gt mice in this study were maintained in-house but separately from the Mtrrgt mouse line. The Mtrrgt/gt mice used to generate the data in Figures 4, 5, 6f, and Extended Figures 2a, 2b, 2d, 3 were derived from Mtrrgt/gt intercrosses. Mice were fed standard RM3(E) chow (see above; Special Diet Services, Witham, UK) ad libitum from weaning onward. Liver, whole brains, and sperm were collected from fertile males. Eight males (from 3-7 separate litters) were assessed per tissue type per genotype. Brain and liver tissues were collected from different mice.
Mtrrgt genotyping and phenotyping
DNA samples were obtained from yolk sac or ear tissue for PCR genotyping as previously described43. Noon of the day that the copulatory plug was detected was determined as embryonic day (E) 0.5. Implantation sites were dissected at E10.5 in cold 1× phosphate buffered saline. Each conceptus was individually scored for phenotypes as previously described in detail64. Briefly, phenotypically normal conceptuses were absent of congenital malformations, with crown-rump length within two SDs of the mean length of C57BL/6J embryos, and with 30-40 somite pairs. Embryos were classified as growth restricted or growth enhanced if their crown-rump length was more than two SD from the mean C57BL/6J embryo crown-rump length at E10.5 but were otherwise phenotypically normal (i.e., normal somite pair count and no congenital malformations). Severely affected conceptuses displayed one or more congenital malformation as determined by gross analysis (e.g., placental phenotypes, neural tube closure defects, pericardial edema, reversed heart looping, overall abnormal morphology, etc.) and while appreciating the occurrence of developmental delay (by somite counts: <30 somite pairs) and what is normal for that developmental stage. Conceptuses with hemorrhages were also classified as severely affected. For each phenotype or genotype, 6-13 embryos from 2-5 litters were assessed. For information about the embryos used in this study, including genotype, phenotype, sex, somite pair count, and crown rump length as a determinant of growth phenotype, refer to Supplementary Table 2.
SNP analysis of Mtrr locus
The SNP analysis of the Mtrrgt locus was carried out using the GigaMUGA genotyping SNP array (Neogen Inc.). Four Mtrr +/gt ear samples were sent to Neogen Inc. for DNA extraction followed by microarray hybridization. Informative heterozygous SNPs between C57BL/6J and 129P2Ola/Hsd were filtered using the filter() function in the dplyr (v.0.8.5) R package 65 and visualized in the Integrative Genomics Viewer66. Unfiltered SNP calls are listed in Supplementary Data 1.
Sperm collection
Sperm was isolated from the cauda epididymis and vas deferens from adult fertile males as previously described54,67, with the following modifications. Sperm were released at 37°C in Donners Medium (25 mM NaHCO3, 20 mg/ml BSA, 1 mM sodium pyruvate and 0.53 % vol/vol sodium dl-lactate in Donners stock: 135 mM NaCl, 5 mM KCl, 1 mM MgSO4, 2 mM CaCl2 and 30 mM HEPES) for 20 min and centrifuged at 500× g for 10 min at room temperature. The supernatant was transferred and centrifuged at 3,000 rpm for 15 min at 4°C. After discarding most of the supernatant, sperm were centrifuged at 3,000 rpm for 5 min at 4°C, and again at 12,000× g for 1 min after a final supernatant removal. Samples were stored at -80°C.
DNA extraction and bisulfite conversion
Liver, brain, ear, and embryonic tissues were RNase A-treated at 37°C for 60 min and digested overnight Proteinase K in lysis buffer (10 mM EDTA, 150 mM NaCl, 10 mM Tris-HCl pH 8, 0.1% SDS) at 55°C. gDNA was isolated using a standard phenol-chloroform extraction and ethanol precipitation. Sperm gDNA was extracted in the same way, except that the Proteinase K digestion was carried out in Solution A (75 mM NaCl pH 8; 25 mM EDTA) and Solution B (10 mM Tris-HCl pH 8; 10 mM EDTA; 1% SDS; 80 mM DTT). Liver gDNA used in the BPA exposure study was extracted and purified using the QIAamp DNA Mini Kit (QIAGEN, cat. no. 51304) following the manufacturer’s protocol. DNA was bisulfite converted using the Imprint® DNA Modification Kit (Sigma, cat. no. MOD50) according to the manufacturer’s instructions. The Two-Step Modification Procedure was used to maximize yield.
DNA methylation analysis by bisulfite pyrosequencing
CpG-site specific methylation was quantified via bisulfite pyrosequencing. VM-IAP and IAP genomic coordinates from genome assembly mm10 are listed in Supplementary Table 3. Bisulfite pyrosequencing assays were designed using PyroMark Assay Design SW 2.0 software (QIAGEN). PCR and sequencing primers are listed in Supplementary Table 4. PCR was carried out on bisulfite converted DNA using biotinylated reverse primers and HotStarTaq DNA Polymerase (QIAGEN, cat. no. 203207). The PCR conditions were (1) 95°C for 5 min; (2) 94°C for 30 s, 56°C for 30 s, 72°C for 55 s, 40 cycles; (3) 72°C for 5 min. Each reaction was run in triplicate. 10-20 μl of the PCR product was bound to Streptavidin Sepharose High Performance beads (GE healthcare, cat no. 17-5113-01) by shaking at room temperature in binding buffer (10 mM Tris-HCl pH 7.6, 2 M NaCl, 1 mM EDTA, 0.1% Tween-20) for at least 5 min at 1,400 rpm. The beads and attached biotinylated strands were purified by sequential washing in 70% ethanol, denaturation solution (0.2 M NaOH), and wash buffer (10 mM Tris-acetate, pH 7.6) using the PyroMark Q96 Vacuum Workstation (QIAGEN). The purified product was resuspended in annealing buffer (20 mM Tris-acetate pH 7.6, 2 mM magnesium acetate) and incubated at 85°C for 4 min with the sequencing primer. Following a 5 min incubation at room temperature, bisulfite pyrosequencing was carried out on the PyroMark Q96 MD pyrosequencer (QIAGEN) using PyroMark Gold Q96 Reagents (QIAGEN, cat. no. 972804). Percentage methylation at each CpG site was quantified using Pyro Q-CpG 1.0.9 software (Biotage). Methylation levels of the assayed CpGs were averaged for each individual at each locus.
Mass spectrometry
C57BL/6J and Mtrrgt/gt liver, brain, and kidney DNA was degraded into individual nucleosides using DNA Degradase Plus (Zymo, cat. no. E2020) according to the manufacturer’s instructions. 100-200 ng of degraded DNA was sent to the Mass Spectrometry Facility at Babraham Institute, Cambridge, UK where global cytosine (C) and 5-methylcytosine (5mC) levels were determined by liquid chromatography-tandem mass spectrometry as previously described68. All samples and standards were spiked with stable-isotope-labelled internal standards: 2’-deoxycytidine-13C1 15N2 (Santa Cruz), 5-(methyl-2H3)-2’-deoxycytidine (Santa Cruz), and 5-(hydroxymethyl-2H3)-2’-deoxycytidine (Toronto Research Chemicals). Global 5mC levels are reported as percentages relative to C. Samples were not pooled in this analysis.
RNA extraction and cDNA synthesis
Total RNA was extracted from 20-30 mg of C57BL/6J and Mtrrgt/gt liver tissue using the AllPrep DNA/RNA Mini Kit (QIAGEN, cat. no. 80204). 50% ethanol was used in the precipitation step to maximize RNA yield, as per the manufacturer’s recommendation. A DNase digestion was performed on the RNeasy spin column membrane using the RNase-Free DNase Set (QIAGEN, cat. no. 79254). RNA integrity was assessed by running aliquots of RNA samples on an agarose gel and assessing the presence of 28S and 18S rRNA bands. cDNA synthesis was carried out on 5 μg RNA using the RevertAid H Minus First Strand cDNA Synthesis kit (Thermo Scientific, cat. no. K1632).
Quantitative reverse transcription PCR (qRT-PCR)
qRT-PCR primers were designed using primer3 software and are listed in Supplementary Table 5. A DNA dilution series was performed for each primer pair to assess amplification efficiency. All qRT-PCR reactions were carried out on the LightCycler 480 Instrument (Roche) using Brilliant III Ultra-Fast SYBR® Green QPCR Master Mix (Agilent, cat. no. 600882). The PCR conditions consisted of pre-incubation at 95°C for 5 min followed by 45 cycles of 95°C for 10 s, 60°C for 10 s, 72°C for 10 s. A melting curve analysis of 65°C to 95°C was performed after amplification to assess product specificity. Minus RT controls were run for each sample and no-template controls were run for each primer pair. All reactions were performed in triplicate. Relative expression was normalized to Hprt1 expression (which did not differ between groups) and calculated using the ΔCt method.
Statistics
Unless otherwise stated, statistical analyses were carried out using GraphPad Prism 8 software. Welch’s t-tests were applied when comparing methylation or expression levels between two groups. Comparisons of three or more groups were analyzed using Welch’s ANOVA followed by Tamhane T2 or Dunnett’s T3 post hoc tests, depending on whether all means were compared or each mean was compared to controls, respectively. Correlations between expression and methylation levels were tested by generating Pearson’s correlation coefficients and two-tailed P values.
Linear Mixed-effects models
REML-fitted linear mixed-effects models (LMMs) were used to assess the effect of age on VM-IAP methylation levels, allowing us to account for the non-independence of littermates and of data points collected from the same mouse across time. The analysis was carried out in R (v.3.6.1) using the lmer() function in the lme4 package (v.1.1-23)69. The methylation levels of the first four CpGs at the VM-IAP LTRs were averaged for each individual at each time point. For IAP-Rnf157 and IAP-Rab6b, only CpGs 3 and 4 were averaged due to technical difficulties. All percentage methylation data were logit transformed prior to analysis. For each VM-IAP, a LLM was fitted with age as a fixed effect and litter and mouse ID as random intercept effects. Sex and mouse cage were explored as possible fixed and random effects, respectively, but both were excluded from the final models as doing so reduced the Akaike and Bayesian information criteria (AIC and BIC).
To evaluate effect sizes (i.e. the proportion of variance explained by age), marginal R2 values ranging from 0 to 1 were generated using the r2beta() function from the r2glmm (v.0.1.2) package in R, applying the Nakagawa and Schielzeth method70. t-test P values were calculated with the lmerTest (v.3.1-2) package in R (v.3.6.1) using the Satterthwaite approximation for degrees of freedom71. To account for multiple testing, a Bonferroni-adjusted α value of 0.0056 was used to interpret P values. Parameter estimates, standard errors, t values, marginal R2 values, and P values are reported in Supplementary Table 1.
Extended Data
Extended Data Fig. 1. VM-IAP methylation in F1 females is unresponsive to maternal exposure to the endocrine disruptor BPA.
F0 dams were fed either a control diet (7% corn oil, grey) or one of two BPA-supplemented diets two weeks prior to mating, throughout pregnancy and lactation (lower BPA dose: 10 μg/kg/day, light blue; upper BPA dose: 10 mg/kg/day, dark blue). Adult F1 female liver tissue was collected from one mouse per litter. Comparison of the average percentage of CpG methylation at 11 VM-IAPs in F1 females across exposure groups shows no significant differences (Welch’s ANOVA; n = 12, 9, and 13 females for the control diet, lower BPA dose, and upper BPA dose, respectively). Data points represent the average of the four or five most distal CpGs of the VM-IAP 5’ LTRs. Box-plot elements: centre line, median; box limits, 25th and 75th percentiles; whiskers, maximum and minimum; all data points shown.
Extended Data Fig. 2. Characterisation of VM-IAP methylation levels in the Mtrrgt/gt mouse model.
a, VM-IAP methylation levels are altered in Mtrrgt/gt brain. VM-IAP methylation levels were compared between C57BL/6J (n = 8, grey box plots) and Mtrrgt/gt (n = 8, red box plots) brains. P-values were calculated by two-tailed Welch’s t-tests (ns indicates p > 0.05). b, Global DNA methylation levels are equivalent between C57BL/6J (n = 8, grey circles) and Mtrrgt/gt (n = 8, red circles) brain (left; p-value = 0.147) and kidney (right; p-value = 0.989) samples (unpaired two-tailed Student’s t-test; ns indicates p > 0.05). Global 5-methyl-cytosine (5mC) content was determined by liquid chromatography-tandem mass spectrometry and expressed as a percentage relative to total cytosine in the genome. c, VM-IAP methylation levels are unaffected by the Mtrrgt allele in mature sperm. VM-IAP methylation levels were quantified in sperm collected from the cauda epididymides and vas deferens of C57BL/6J (n = 8, grey circles), Mtrr+/+ (n = 8, hollow red circles), Mtrr+/gt (n = 8, half-filled red circles), and Mtrrgt/gt (n = 8, red circles) adult fertile males. d, VM-IAP methylation states are not associated with phenotypic severity in whole Mtrrgt/gt embryos. VM-IAP methylation levels were compared across C57BL/6J embryos (n = 7, grey circles), phenotypically normal Mtrrgt/gt embryos (n = 7, red circles), and severely affected Mtrrgt/gt embryos (n = 6, red circles) at E10.5 by Welch’s ANOVA. Adjusted p-values were calculated by two-tailed Tamhane T2 post hoc tests (ns indicates p > 0.05). Methylation data in all panels are shown as average percentage of DNA methylation across the four most distal CpGs at VM-IAP 5’ LTRs. Box-plot elements: centre line, median; box limits, 25th and 75th percentiles; whiskers, maximum and minimum; all data points shown.
Extended Data Fig. 3. VM-IAP-neighbouring genes unaffected in Mtrrgt/gt mice.
Left-hand graphs assess the correlation between VM-IAP methylation and adjacent Marveld2 (a), Rnf157 (b), Mbnl1(c), and Bmf (d) gene expression in C57BL/6J livers (n=8, r: Pearson’s correlation coefficient; p: two-tailed p-value associated with r). Centre graphs show qRT-PCR expression data of VM-IAP-neighbouring genes Marveld2 (a), Rnf157 (b), Mbnl1(c), and Bmf (d) in C57BL/6J (n=8, grey circles) and Mtrrgt/gt (n = 8, red circles) liver (two-tailed Welch’s t-tests; ns indicates p > 0.5; means shown as black lines). Right-hand graphs incorporate both control and Mtrrgt/gt data and assess the correlation between gene expression and VM-IAP methylation (n = 16, r: Pearson’s correlation coefficient; p: twotailed p-value associated with r). Diagrams of VM-IAPs in relation to their neighbouring gene are depicted on the far left. Gene transcripts, extracted from the University of California, Santa Cruz (UCSC) Genome Browser72, are shown in black and VM-IAPs in purple. Green arrows represent the location of qRT-PCR primers. Diagrams are drawn to scale.
Supplementary Material
Acknowledgements
Research was funded by grants from the Wellcome Trust (WT095606, 210757/Z/18/Z) and MRC (MR/R009791/1, MR/J00159) to A.C.F.-S., from the Lister Institute for Preventative Medicine to E.D.W., from the NIH (R01 ES 023284) to M.S.B. and R.A.S., and from the MRC (MC_UU_12012/4 and MC_UU_00014/4) and the British Heart Foundation (RG/17/12/33167) to D.S.F.-T. and S.E.O. We are grateful for PhD scholarships from the Cambridge Trust, Downing College, and Pomona College to T.M.B., from the Wellcome Trust to G.E.T.B., and from the European Union’s Horizon 2020 research and innovation programme (under the Marie Skłodowska Curie grant agreement No 812660) to J.L.B. We thank Noah Kessler, Jessica Elmer, Amir Hay, Nozomi Takahashi and other members of the Ferguson-Smith laboratory for valuable discussions. We are grateful to Dr. Matt Castle for contributions to our statistical analyses, Ali Robinson and Christopher Krapp for technical assistance, and Judith Webster and David Oxley from the Babraham Institute Mass Spectrometry Facility for sample processing.
Footnotes
Author contributions
T.M.B. collected and analyzed the data. T.M.B. and J.L.B. performed pyrosequencing assays. T.M.B., J.L.B., G.E.T.B., A.B., and D.N. carried out DNA extractions. T.M.B., G.E.T.B., E.D.W., A.B., and D.S.F.-T. performed somatic tissue dissections. G.E.T.B. collected sperm samples. E.D.W. developed the Mtrrgt model, R.A.S. and M.S.B. developed the BPA exposure model, and S.E.O. developed the diet-induced obesity model. A.C.F.-S. conceived the project. T.M.B., E.D.W., and A.C.F.-S. designed the experiments and interpreted the results. T.M.B., E.D.W., and A.C.F.-S. wrote the manuscript. All authors read and revised the manuscript.
Competing interests
The authors declare no competing interests.
Data availability
The data supporting the findings of this study can be found within the article and its Supplementary Information files.
Code availability
All computational tools have been previously described and no custom computational pipelines were employed in this study.
References
- 1.Smit AFA, Hubley R, Green P. RepeatMasker Open-4.0. 2015 Available at: http://www.repeatmasker.org/
- 2.Slotkin RK, Martienssen R. Transposable elements and the epigenetic regulation of the genome. Nat Rev Genet. 2007;8:272–285. doi: 10.1038/nrg2072. [DOI] [PubMed] [Google Scholar]
- 3.Morgan HD, Sutherland HG, Martin DI, Whitelaw E. Epigenetic inheritance at the agouti locus in the mouse. Nat Genet. 1999;23:314–318. doi: 10.1038/15490. [DOI] [PubMed] [Google Scholar]
- 4.Duhl DMJ, Vrieling H, Miller KA, Wolff GL, Barsh GS. Neomorphic agouti mutations in obese yellow mice. Nat Genet. 1994;8:59–65. doi: 10.1038/ng0994-59. [DOI] [PubMed] [Google Scholar]
- 5.Bertozzi TM, Ferguson-Smith AC. Metastable epialleles and their contribution to epigenetic inheritance in mammals. Semin Cell Dev Biol. 2020;97:93–105. doi: 10.1016/j.semcdb.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Wolff GL, Kodell RL, Moore SR, Cooney CA. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J. 1998;12:949–957. [PubMed] [Google Scholar]
- 7.Cooney CA, Dave AA, Wolff GL. Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J Nutr. 2002;132:2393S–2400S. doi: 10.1093/jn/132.8.2393S. [DOI] [PubMed] [Google Scholar]
- 8.Waterland RA, Jirtle RL. Transposable Elements : Targets for Early Nutritional Effects on Epigenetic Gene Regulation. Mol Cell Biol. 2003;23:5293–5300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. PNAS. 2007;104:13056–13061. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaminen-Ahola N, et al. Maternal Ethanol Consumption Alters the Epigenotype and the Phenotype of Offspring in a Mouse Model. PLoS Genet. 2010;6:e1000811. doi: 10.1371/journal.pgen.1000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernal AJ, et al. Adaptive radiation-induced epigenetic alterations mitigated by antioxidants. FASEB J. 2013;27:665–671. doi: 10.1096/fj.12-220350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neier K, Cheatham D, Bedrosian LD, Dolinoy DC. Perinatal exposures to phthalates and phthalate mixtures result in sex-specific effects on body weight, organ weights and intracisternal A-particle (IAP) DNA methylation in weanling mice. J Dev Orig Health Dis. 2019;10:176–187. doi: 10.1017/S2040174418000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faulk C, Barks A, Liu K, Goodrich JMJ, Dolinoy DCD. Early-life lead exposure results in dose-and sex-specific effects on weight and epigenetic gene regulation in weanling mice. Epigenomics. 2013;5:487–500. doi: 10.2217/epi.13.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenfeld CS, et al. Maternal exposure to bisphenol A and genistein has minimal effect on A(vy)/a offspring coat color but favors birth of agouti over nonagouti mice. Proc Natl Acad Sci U S A. 2013;110:537–42. doi: 10.1073/pnas.1220230110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jirtle RL. The Agouti mouse: A biosensor for environmental epigenomics studies investigating the developmental origins of health and disease. Epigenomics. 2014;6:447–450. doi: 10.2217/epi.14.58. [DOI] [PubMed] [Google Scholar]
- 16.Dolinoy DC. The agouti mouse model: an epigenetic biosensor for nutritional and environmental alterations on the fetal epigenome. Nutr Rev. 2008;66:S7–S11. doi: 10.1111/j.1753-4887.2008.00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waterland RA, et al. Maternal Methyl Supplements Increase Offspring DNA Methylation at Axin Fused. Genesis. 2006;44:401–406. doi: 10.1002/dvg.20230. [DOI] [PubMed] [Google Scholar]
- 18.Kazachenka A, et al. Identification, Characterization, and Heritability of Murine Metastable Epialleles: Implications for Non-genetic Inheritance. Cell. 2018;175:1259–1271. doi: 10.1016/j.cell.2018.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elmer JL, et al. Genomic properties of variably methylated repetitive elements in mouse. Mob DNA. 2021;12:6. doi: 10.1186/s13100-021-00235-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bocklandt S, et al. Epigenetic Predictor of Age. PLoS One. 2011;6:1–6. doi: 10.1371/journal.pone.0014821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hannum G, et al. Genome-wide Methylation Profiles Reveal Quantitative Views of Human Aging Rates. Mol Cell. 2013;49:359–367. doi: 10.1016/j.molcel.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14:1–20. doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petkovich DA, et al. Using DNA Methylation Profiling to Evaluate Biological Age and Longevity Interventions. Cell Metab. 2017;25:954–960. doi: 10.1016/j.cmet.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang T, et al. Epigenetic aging signatures in mice livers are slowed by dwarfism, calorie restriction and rapamycin treatment. Genome Biol. 2017;18:1–11. doi: 10.1186/s13059-017-1186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stubbs TM, et al. Multi-tissue DNA methylation age predictor in mouse. Genome Biol. 2017;18:1–14. doi: 10.1186/s13059-017-1203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meer MV, Podolskiy DI, Tyshkovskiy A, Gladyshev VN. A whole lifespan mouse multi-tissue DNA methylation clock. Elife. 2018;7:e40675. doi: 10.7554/eLife.40675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun Q, et al. Association of Urinary Concentrations of Bisphenol A and Phthalate Metabolites with Risk of Type 2 Diabetes: A Prospective Investigation in the Nurses’ Health Study (NHS) and NHSII Cohorts. Environ Health Perspect. 2014;122:616–623. doi: 10.1289/ehp.1307201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aekplakorn W, Chailurkit L, Ongphiphadhanakul B. Relationship of serum bisphenol A with diabetes in the Thai population, National Health Examination Survey IV, 2009. J Diabetes. 2015;7:240–249. doi: 10.1111/1753-0407.12159. [DOI] [PubMed] [Google Scholar]
- 29.Ahmadkhaniha R, et al. Association of urinary bisphenol a concentration with type-2 diabetes mellitus. J Environ Heal Sci Eng. 2014;12:64. doi: 10.1186/2052-336X-12-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Susiarjo M, Sasson I, Mesaros C, Bartolomei MS. Bisphenol A Exposure Disrupts Genomic Imprinting in the Mouse. PLoS Genet. 2013;9:e1003401. doi: 10.1371/journal.pgen.1003401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Susiarjo M, et al. Bisphenol A Exposure Disrupts Metabolic Health Across Multiple Generations in the Mouse. Endocrinology. 2015;156:2049–2058. doi: 10.1210/en.2014-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bansal A, et al. Sex- and dose-specific effects of maternal bisphenol A exposure on pancreatic islets of first- and second-generation adult mice offspring. Environ Health Perspect. 2017;125:097022. doi: 10.1289/EHP1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lavebratt C, Almgren M, Ekström TJ. Epigenetic regulation in obesity. Int J Obes. 2012;36:757–765. doi: 10.1038/ijo.2011.178. [DOI] [PubMed] [Google Scholar]
- 34.Radford EJ. Exploring the extent and scope of epigenetic inheritance. Nature Reviews Endocrinology. 2018;14:345–355. doi: 10.1038/s41574-018-0005-5. [DOI] [PubMed] [Google Scholar]
- 35.Andersen E, et al. Preadipocytes from obese humans with type 2 diabetes are epigenetically reprogrammed at genes controlling adipose tissue function. Int J Obes. 2019;43:306–318. doi: 10.1038/s41366-018-0031-3. [DOI] [PubMed] [Google Scholar]
- 36.Samuelsson A-M, et al. Diet-Induced Obesity in Female Mice Leads to Offspring Hyperphagia, Adiposity, Hypertension, and Insulin Resistance. Hypertension. 2008;51:383–392. doi: 10.1161/HYPERTENSIONAHA.107.101477. [DOI] [PubMed] [Google Scholar]
- 37.Loche E, et al. Maternal diet-induced obesity programmes cardiac dysfunction in male mice independently of post-weaning diet. Cardiovasc Res. 2018;114:1372–1384. doi: 10.1093/cvr/cvy082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alfaradhi MZ, et al. Maternal Obesity in Pregnancy Developmentally Programs Adipose Tissue Inflammation in Young, Lean Male Mice Offspring. Endocrinology. 2016;157:4246–4256. doi: 10.1210/en.2016-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friso S, Udali S, De Santis D, Choi S-W. One-carbon metabolism and epigenetics. Mol Aspects Med. 2017;54:28–36. doi: 10.1016/j.mam.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Shane B, Stokstad ELR. Vitamin B12-Folate Interrelationships. Annu Rev Nutr. 1985;5:115–141. doi: 10.1146/annurev.nu.05.070185.000555. [DOI] [PubMed] [Google Scholar]
- 41.Yamada K, Gravel RA, Toraya T, Matthews RG. Human methionine synthase reductase is a molecular chaperone for human methionine synthase. Proc Natl Acad Sci U S A. 2006;103:9476–9481. doi: 10.1073/pnas.0603694103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elmore CL, et al. Metabolic derangement of methionine and folate metabolism in mice deficient in methionine synthase reductase. Mol Genet Metab. 2007;91:85–97. doi: 10.1016/j.ymgme.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Padmanabhan N, et al. Mutation in Folate Metabolism Causes Epigenetic Instability and Transgenerational Effects on Development. Cell. 2013;155:81–93. doi: 10.1016/j.cell.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ducker GS, Rabinowitz JD. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017;25:27–42. doi: 10.1016/j.cmet.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Padmanabhan N, et al. Abnormal folate metabolism causes age-, sex- and parent-of-origin-specific haematological defects in mice. J Physiol. 2018;596:4341–4360. doi: 10.1113/JP276419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Czeizel A, et al. Folate Deficiency and Folic Acid Supplementation: The Prevention of Neural-Tube Defects and Congenital Heart Defects. Nutrients. 2013;5:4760–4775. doi: 10.3390/nu5114760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iwamoto N, Takanashi M, Shimada T, Sasaki J, Hamada A. Comparison of Bevacizumab Quantification Results in Plasma of Non-small Cell Lung Cancer Patients Using Bioanalytical Techniques Between LC-MS/MS, ELISA, and Microfluidic-based Immunoassay. AAPS J. 2019;21 doi: 10.1208/s12248-019-0369-z. [DOI] [PubMed] [Google Scholar]
- 48.Kahl KW, Seither JZ, Reidy LJ. LC-MS-MS vs ELISA: Validation of a Comprehensive Urine Toxicology Screen by LC-MS-MS and a Comparison of 100 Forensic Specimens. J Anal Toxicol. 2019;43:734–745. doi: 10.1093/jat/bkz066. [DOI] [PubMed] [Google Scholar]
- 49.Kobayashi H, et al. Contribution of Intragenic DNA Methylation in Mouse Gametic DNA Methylomes to Establish Oocyte-Specific Heritable Marks. PLoS Genet. 2012;8:e1002440. doi: 10.1371/journal.pgen.1002440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li X, et al. A Maternal-Zygotic Effect Gene, Zfp57, Maintains Both Maternal and Paternal Imprints. Dev Cell. 2008;15:547–557. doi: 10.1016/j.devcel.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strogantsev R, et al. Allele-specific binding of ZFP57 in the epigenetic regulation of imprinted and non-imprinted monoallelic expression. Genome Biol. 2015;16:112. doi: 10.1186/s13059-015-0672-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bruno M, Mahgoub M, Macfarlan TS. The Arms Race Between KRAB–Zinc Finger Proteins and Endogenous Retroelements and Its Impact on Mammals. Annu Rev Genet. 2019;53:393–416. doi: 10.1146/annurev-genet-112618-043717. [DOI] [PubMed] [Google Scholar]
- 53.Bertozzi TM, Elmer JL, Macfarlan TS, Ferguson-Smith AC. KRAB zinc finger protein diversification drives mammalian interindividual methylation variability. Proc Natl Acad Sci U S A. 2020;117:31290–31300. doi: 10.1073/pnas.2017053117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blake GET, et al. Defective folate metabolism causes germline epigenetic instability and distinguishes Hira as a phenotype inheritance biomarker. Nature Communications. 2021 doi: 10.1038/s41467-021-24036-5. In press (ref. NCOMMS-20-19078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wolf G, et al. Krab-zinc finger protein gene expansion in response to active retrotransposons in the murine lineage. Elife. 2020;9:1–22. doi: 10.7554/eLife.56337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shorter KR, et al. Pleiotropic effects of a methyl donor diet in a novel animal model. PLoS One. 2014;9:e104942. doi: 10.1371/journal.pone.0104942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wei Y, et al. DNA methylation analysis and editing in single mammalian oocytes. Proc Natl Acad Sci U S A. 2019;116:9883–9892. doi: 10.1073/pnas.1817703116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tomizawa SI, Nowacka-Woszuk J, Kelsey G. DNA methylation establishment during oocyte growth: Mechanisms and significance. Int J Dev Biol. 2012;56:867–875. doi: 10.1387/ijdb.120152gk. [DOI] [PubMed] [Google Scholar]
- 59.Lilue J, et al. Sixteen diverse laboratory mouse reference genomes define strain-specific haplotypes and novel functional loci. Nat Genet. 2018;50:1574–1583. doi: 10.1038/s41588-018-0223-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kano H, Kurahashi H, Toda T. Genetically regulated epigenetic transcriptional activation of retrotransposon insertion confers mouse dactylaplasia phenotype. PNAS. 2007;104:19034–19039. doi: 10.1073/pnas.0705483104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krebs CJ, et al. Regulator of sex-limitation (Rs1) encodes a pair of KRAB zinc-finger genes that control sexually dimorphic liver gene expression. Genes Dev. 2003;17:2664–2674. doi: 10.1101/gad.1135703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maeda-Smithies N, et al. Ectopic expression of the Stabilin2 gene triggered by an intracisternal A particle (IAP) element in DBA/2J strain of mice. Mamm Genome. 2020;31:2–16. doi: 10.1007/s00335-019-09824-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Plamondon JA, Harris MJ, Mager DL, Gagnier L, Juriloff DM. The clf2 gene has an epigenetic role in the multifactorial etiology of cleft lip and palate in the A/WySn mouse strain. Birth Defects Res Part A - Clin Mol Teratol. 2011;91:716–727. doi: 10.1002/bdra.20788. [DOI] [PubMed] [Google Scholar]
- 64.Padmanabhan N, et al. Multigenerational analysis of sex-specific phenotypic differences at midgestation caused by abnormal folate metabolism. Environ Epigenetics. 2017;3:dvx014. doi: 10.1093/eep/dvx014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wickham H, François R, Henry L, Müller K. dplyr: A Grammar of Data Manipulation. 2019 doi: 10.1007/978-0-387-98141-3. https://dplyr.tidyverse.org/ [DOI]
- 66.Thorvaldsdóttir H, Robinson JT, Mesirov JP. Integrative Genomics Viewer (IGV): High-performance genomics data visualization and exploration. Brief Bioinform. 2013;14:178–192. doi: 10.1093/bib/bbs017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hisano M, et al. Genome-wide chromatin analysis in mature mouse and human spermatozoa. Nat Protoc. 2013;8:2449–2470. doi: 10.1038/nprot.2013.145. [DOI] [PubMed] [Google Scholar]
- 68.Ficz G, et al. FGF Signaling Inhibition in ESCs Drives Rapid Genome-wide Demethylation to the Epigenetic Ground State of Pluripotency. Cell Stem Cell. 2013;13:351–359. doi: 10.1016/j.stem.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 70.Jaeger BC, Edwards LJ, Das K, Sen PK. An R2 statistic for fixed effects in the generalized linear mixed model. J Appl Stat. 2017;44:1086–1105. [Google Scholar]
- 71.Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest Package: Tests in Linear Mixed Effects Models. J Stat Softw. 2017;82:1–26. [Google Scholar]
- 72.Haeussler M, et al. The UCSC Genome Browser database: 2019 update. Nucleic Acids Res. 2019;47:D853–D858. doi: 10.1093/nar/gky1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study can be found within the article and its Supplementary Information files.
All computational tools have been previously described and no custom computational pipelines were employed in this study.