Abstract
Heterozygous mutations in GCK result in a persistent, mildly raised glucose from birth, but it is usually diagnosed in adulthood as maturity-onset diabetes of the young (MODY), where hyperglycemia is often an incidental finding. The hyperglycemia of GCK-MODY is benign and does not require treatment, but is important to be aware of, particularly in females where it has implications for managing pregnancy. We present three cases of neonatal hyperglycemia resulting from a heterozygous mutation in GCK, illustrating its clinical presentation and evolution in early life. In summary, as with adults, neonatal hyperglycemia is an incidental finding, does not require treatment and has no adverse consequences for health. Neonates and their parents should be referred for genetic testing to confirm the diagnosis, avoid a label of diabetes and enable pregnancy counselling for females found to be affected.
Keywords: Glucokinase, monogenic diabetes of the young, GCK-MODY, hyperglycemia, neonatal diabetes
Introduction
Heterozygous inactivating mutations in the gene encoding the glucose sensing enzyme glucokinase, GCK, are common (1 in 1,000 population prevalence1) and result in partial glucokinase deficiency2. This causes mild hyperglycemia (tightly regulated between 5.5-8 mmol/L3), often diagnosed incidentally in adulthood as maturity-onset diabetes of the young (MODY)1,4. The degree of hyperglycemia is uniform irrespective of mutation type5, and treatment of hyperglycemia is not required6, as it does not result in the micro- or macrovascular complications seen with type 1 and type 2 diabetes7. However, it does have implications for women of reproductive age, since fetuses who have not inherited the mutation from their mother secrete higher levels of insulin in response to maternal hyperglycemia, so are at risk of macrosomia and its associated pregnancy complications8,9. GCK-MODY is frequently diagnosed in pregnancy, either because of routine screening for gestational diabetes or a history of fetal macrosomia10.
Neonatal diabetes (NDM) is diagnosed in the first six months of life and affects approximately 1 in 100,000 live births11. It can be permanent (PNDM), requiring lifelong treatment, or transient (TNDM), where diabetes remits but typically returns in childhood or early adulthood12. The clinical presentation of NDM is usually severe, with marked hyperglycemia (frequently >30 mmol/L) and ketoacidosis13, and treatment is required to maintain euglycemia.
NDM may be isolated or diagnosed in association with other conditions or congenital anomalies. NDM has a known genetic etiology in almost 90% of cases, with a mutation in one of 28 known disease-causing genes affecting glucose metabolism, insulin secretion or pancreatic development14–21. Obtaining a genetic diagnosis is important, since prognosis and approach to monitoring and treatment are dependent on the underlying genetic cause22–25.
Bi-allelic inactivating mutations in GCK result in complete glucokinase deficiency and are an infrequent cause of PNDM, accounting for ~3% of cases with a confirmed genetic diagnosis14, but an important differential diagnosis of hyperglycemia in infants with very low birth weight (typically <-3 standard deviations below the mean for sex and gestational age due to substantially reduced fetal insulin-mediated growth)26. This contrasts with heterozygous inactivating mutations in GCK which cause GCK-MODY, where the degree of fetal insulin secretion and its effect on birth weight is determined by the parent-of-origin of the mutation8. Birth weight is reduced by approximately 500 g when the mother is unaffected and the fetus has inherited the mutation from the father, whereas birth weight is typically in the normal range when inherited from a mother with GCK-MODY8,9.
Family studies in GCK-MODY pedigrees27 and genetic screening for suspected GCK-MODY28 have shown that hyperglycemia is present from birth in affected individuals. However, it is rare for it to be recognised in neonates and considered as a cause of neonatal hyperglycemia in the absence of a known family history. Here, we present three cases of neonatal hyperglycemia who did not have a family history of GCK-MODY and were referred for genetic testing for NDM. We describe how GCK-MODY can present in newborns and emphasise the importance of considering it in cases of asymptomatic, mild neonatal hyperglycemia not requiring treatment.
Methods
Out of 2,203 individuals identified as having hyperglycemia in the first six months of life and referred to Exeter Genomics Laboratory for genetic testing of NDM, we identified three individuals with heterozygous inactivating mutations in GCK (NM_000162.3). The GCK mutations were identified either by PCR amplification followed by Sanger sequencing (primers available on request) or by analysis of all the known NDM genes using an in-house targeted next generation sequencing (tNGS) assay as previously described29. Variant classification was performed according to the ACMG international guidelines for variant interpretation30. There was no known history of a genetic cause for diabetes in their families. Their parents were genotyped once a mutation in GCK was identified in the child.
Clinical data (Table 1) was collected from the cases’ health records. Birth weight standard deviation scores (SDS) were calculated using the INTERGROWTH-21st standards31 and most recent available weight and height SDS were calculated using the WHO Child Growth Standards or Reference32,33. Informed consent was received from their parents and ethics approval for research was provided by the North Wales Research Ethics Committee.
Table 1. Clinical characteristics of cases of neonatal hyperglycemia secondary to heterozygous mutations in GCK.
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Sex | Male | Female | Female |
| Country of origin | Germany | Ukraine | Greece |
| Ethnic ancestry | Northern European | Eastern European | Greek |
| Gestational age at birth | 39 weeks | 37 weeks | 38 weeks |
| Birth weight and SDS for gestational age and sex a | 3.9 kg (1.51 SDS) | 3.1 kg (0.77 SDS) | 2.6 kg (−0.80 SDS) |
| Blood glucose at presentation | 8 mmol/L | 6 mmol/L | 5.5 mmol/L |
| Age hyperglycaemia first identified | 4 months | 5 months | 24 hours |
| Age of referral for genetic testing | 6 months | 6 years and 7 months | 1 month |
| HbA1c at referral | 47.5 mmol/mol | 49.7 mmol/mol | - |
| C-peptide at referral | 190 pmol/L | 257 pmol/L | 536 pmol/L |
| Autoantibody screen at referral | Negative | Negative | - |
| Treatment history | Nil | Nil | Nil |
| Most recent height and SDS for age and sex b | 1.16 m (0.11 SDS) | 1.27 m (1.6 SDS) | 76 cm (−1.31 SDS) |
| Most recent weight and SDS for age and sex b | 22.5 kg (0.03 SDS) | 27.0 kg (1.4 SDS) | 10.2 kg (0.13 SDS) |
| Most recent HbA1c (age measured) | 43.2 mmol/mol (6 years old) | 38.7 mmol/mol (11 years old) | - |
Cases
Case 1
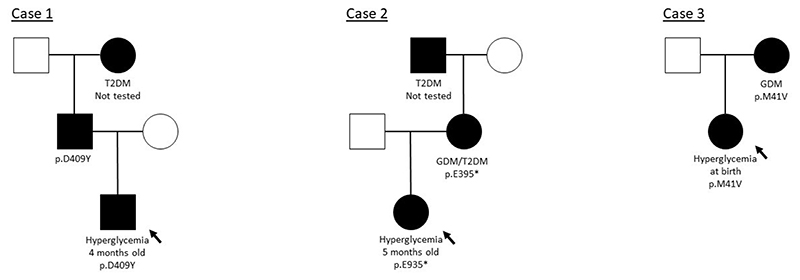
A healthy male infant of Northern European ancestry was born at 39 weeks gestation to non-consanguineous parents weighing 3.9 kg (1.51 SDS). Pregnancy and delivery were uneventful, but he was admitted to hospital with signs suggestive of bowel obstruction at four months of age. He was diagnosed with Hirschsprung’s disease and underwent surgery. His blood glucose prior to surgery was 8 mmol/L and varied between 11 and 16 mmol/L during surgery. He did not require insulin treatment. At six months of age his case was reviewed by a Pediatric Endocrinologist who established that his paternal grandmother had a history of type 2 diabetes but there was no other significant family history. His HbA1c was 47.5 mmol/mol, C-peptide was 190 pmol/L and an autoantibody screen was negative. He was referred for genetic testing for NDM. He first underwent Sanger sequencing of the ABCC8, KCNJ11 and INS genes and was screened for a methylation defect at the 6q24 locus. The results of these tests were negative, so targeted next generation sequencing (tNGS) of a panel of 28 known NDM genes29 was performed and a heterozygous missense variant in GCK (c.1225G>T (p.D409Y)) was identified (Figure 1). This variant was classified as likely pathogenic. The proband’s father, who was not known to have diabetes, was heterozygous for the same GCK mutation. A sample from the maternal grandmother was not available for testing. The proband’s most recent clinical assessment at 6 years of age found him to be fit and well and his hyperglycemia remained untreated.
Figure 1.
Partial pedigrees for cases of GCK-MODY diagnosed where hyperglycemia was first identified in the neonatal period. Filled symbols represent individuals with a genetic diagnosis of GCK-MODY, genotypes are provided under the symbols. The clinical diagnosis is provided under the symbols for individuals affected by diabetes (for the father of Case 1, the affected phenotype is assumed based on genotype). GDM = Gestational Diabetes Mellitus. T2D = Type 2 diabetes. An arrow points to the proband in each family.
Case 2
A healthy female infant of Eastern European ancestry was born at 37 weeks gestation to non-consanguineous parents weighing 3.1 kg (0.77 SDS). Pregnancy and delivery were uneventful. The girl’s blood glucose was measured during a viral illness at four months of age and found to be raised at 6 mmol/L. No treatment was given and her blood glucose was tested intermittently in childhood (7.4 mmol/L at eight months old, 6 mmol/L at three years old and 5.9 mmol/L at four years old) before a specialist review at six years and seven months of age. She was of a normal height and weight (1.6 SDS and 1.4 SDS, respectively), but was noted to have some signs of early pubarche (Tanner Stage 2). At the time her HbA1c was 49.7 mmol/mol, serum C-peptide was 255 pmol/L and an autoantibody screen, including GAD, IA2 and ICA, was negative. Her mother had been diagnosed with gestational diabetes (GDM) in a previous pregnancy at 19 years old and was subsequently diagnosed with type 2 diabetes aged 23 years. The mother was treated with a low carbohydrate diet (including in her pregnancy with the proband) until the age of 26 years, when she was started on metformin and saxagliptin. In addition to her mother’s history of diabetes, the girl’s maternal grandfather had been diagnosed with type 2 diabetes aged 46 years. In view of the history, Sanger sequencing of GCK was performed, which revealed a heterozygous GCK nonsense mutation (c.1183G>T (p.E395*)), which was also present in her mother (Figure 1). A sample from the maternal grandfather was not available for testing. The girl’s most recent HbA1c at the age of 11 years was 38.7 mmol/mol and she was known to be fit and well without treatment of hyperglycemia at 12 years of age. This case has been described briefly in the literature34.
Case 3
A female infant of Greek ancestry was born at 38 weeks gestation to non-consanguineous parents weighing 2.6 kg (−0.80 SDS). Her mother was treated for GDM with insulin in pregnancy. The infant’s blood glucose was measured as a routine screen in the first 24 hours of life and was found to be high; pre-feed blood glucose levels ranged between 5.5 and 10 mmol/L and the highest measured was 12.6 mmol/L. There was no ketonuria and her serum C-peptide was 96 pmol/L pre-feed and 536 pmol/L post-feed. She was noted to have macroglossia, but was otherwise well and her hyperglycemia did not require treatment. She was referred for genetic testing for NDM and underwent screening of the known genes by tNGS and methylation-specific MS-MLPA of the 6q24 locus. A heterozygous novel missense variant was identified in GCK (c.121A>G (p.M41V)) and 6q24 MS-MLPA was negative. The GCK variant was classified as likely pathogenic. The proband had inherited this mutation from her mother, confirming a diagnosis of GCK-MODY in both. At her most recent assessment at the age of 15 months, her hyperglycemia remained untreated and she was well (Table 1).
Discussion
In this series of GCK-MODY diagnosed in cases referred for genetic testing for suspected NDM, we have shown that neonatal hyperglycemia is an incidental finding, does not require treatment and follows a benign course in childhood. Retrospective studies in individuals with a known family history or clinically suspected GCK-MODY have previously confirmed that hyperglycemia can occur from birth27,28. Previous series where genetic screening was performed in individuals with incidental hyperglycemia have identified children who presented as young as one year of age35,36. However, referral for genetic testing with suspected NDM is rare, and these cases confirm the importance of considering GCK-MODY as a cause of neonatal hyperglycaemia.
In pregnancies affected by GCK-MODY, as fetal growth depends on the parent-of-origin of the mutation, birth weight combined with parental history can provide useful clues to the underlying diagnosis in an otherwise well infant with hyperglycemia. However, birth weight is not always a reliable indicator, as fetal insulin- and non-insulin-mediated growth will have different effects between different individuals. In Case 3, the infant had a birth weight lower than might be expected for a mother who had GDM requiring insulin. It is possible that maternal insulin treatment contributed to the lower birth weight in this case37. The birth weight for the infant in Case 2 was normal, despite her mother’s history of diabetes diagnosed after screening in a previous pregnancy. Therefore, for a hyperglycemic infant of normal or low birth weight born to a mother with GDM, particularly where maternal hyperglycemia has failed to respond to insulin treatment in pregnancy9,38 and persists postpartum, GCK-MODY is an important consideration. Neonatal hypoglycemia and macrosomia may be evident in the infant who has not inherited the same GCK mutation as their mother9, so a high birth weight and history of neonatal hypoglycemia in siblings may also provide a useful clue to the diagnosis. Where the mutation is inherited from the father, birth weight is reduced by approximately 500 g on average8, but this was not seen in Case 1, and his father (who he had inherited the GCK mutation from) was not known to have diabetes. It is very common for adult males to be unaware of their diagnosis, since the hyperglycemia of GCK-MODY does not cause symptoms or complications, so it may be less clear-cut when the mutation is inherited from the father. However, where there is a strong family history of “type 2 diabetes” without other clinical features or complications and diagnosed at a young age (as was seen in Case 2), this should also raise suspicion for GCK-MODY.
We found that GCK-MODY was a rare diagnosis for infants with neonatal hyperglycemia and without a known family history referred for genetic testing (~0.1%). These three cases also had other clinical conditions or signs which might have prompted the clinicians to consider an underlying genetic aetiology. Hirschsprung’s disease and possibly precocious puberty were present in Case 1 and 2, respectively, but these are not related to hyperglycemia and have not been reported to be typical clinical features for individuals with NDM. The infant in Case 3 had macroglossia, which has been identified as a feature of some cases of 6q24 TNDM39,40, but this is a variable and imprecise sign which has no known relation to GCK-MODY. Overall, the presence of other features emphasises that these children are at risk of other pediatric conditions and these should not be attributed to GCK-MODY.
Hyperglycemia is stable in GCK-MODY, as shown by Case 2 where her glucose was maintained within a tight range when tested at different points in early childhood, and no progression in HbA1c levels in the absence of treatment in Cases 1 and 2. This is consistent with the adult literature, where HbA1c is relatively stable, showing a slight rise in later life which is also seen in individuals without diabetes3. Recent measures of height and weight in the cases reported here were also within the normal range, consistent with that seen in adults with GCK-MODY41.
In summary, although GCK-MODY is more commonly identified in adults as an incidental finding of hyperglycemia1, it can be identified at birth. As blood glucose monitoring becomes more frequent, and if neonatal bloodspot glucose becomes routine42, it may be diagnosed more often in the future. The distinguishing features are a mild hyperglycemia that does not require treatment or cause illness, unlike that seen in other cases of NDM, including complete glucokinase deficiency caused by bi-allelic inactivating mutations. Furthermore, the combination of hyperglycemia with a birth weight within the normal range, particularly where their mother also had GDM, and a strong family history of early-onset, non-progressive “type 2 diabetes” should raise suspicion for GCK-MODY. Genetic diagnosis is useful, since unnecessary treatment and monitoring are avoided. Additionally, for female infants and mothers of probands found to have the mutation, it allows preparation for future pregnancies, where antenatal management differs to typical cases of diabetes in pregnancy1,9.
Acknowledgements
We thank the patients, their families and their clinicians, and the Exeter Genomics Laboratory who performed the genetic testing. The genetic testing was funded by The Wellcome Trust.
Funding statement
A.E.H. is a Wellcome Trust funded PhD fellow. E.D.F. is a Diabetes UK RD Lawrence Fellow. S.E.F. is a Sir Henry Dale Fellow, jointly funded by The Wellcome Trust and the Royal Society (Grant Number 105636/Z/14/Z). A.T.H. is a Wellcome Trust Senior Investigator and in receipt of a National Institute of Health Research Senior Investigator Award. For the purpose of Open Access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
Footnotes
Author contributions:
A.E.H., E.D.F., E.G., N.Z., D.H., P.S. and S.E.F. collected data. A.E.H. and E.D.F. wrote and edited the manuscript. E.G., N.Z., D.H., P.S., S.E.F. and A.T.H. reviewed and edited the manuscript.
Conflict of interest disclosure:
The authors declare no conflicts of interest.
Ethics approval statement:
Ethics approval for the Genetic Beta Cell Research Bank was provided by North Wales Research Ethics Committee.
Patient consent statement:
The patients’ guardians provided informed, written consent for their samples to be stored in the Genetic Beta Cell Research Bank and utilise their data for research.
Data availability statement
The data that supports this work is not freely available due to its identifiable nature, but reasonable requests for additional data can be made to the corresponding author.
References
- 1.Chakera AJ, Steele AM, Gloyn AL, et al. Recognition and Management of Individuals With Hyperglycemia Because of a Heterozygous Glucokinase Mutation. Diabetes Care. 2015;38(7):1383–1392. doi: 10.2337/dc14-2769. [DOI] [PubMed] [Google Scholar]
- 2.Gloyn AL. Glucokinase (GCK) mutations in hyper- and hypoglycemia: Maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemia of infancy. Human Mutation. 2003;22(5):353–362. doi: 10.1002/humu.10277. [DOI] [PubMed] [Google Scholar]
- 3.Steele AM, Wensley KJ, Ellard S, et al. Use of HbA1c in the Identification of Patients with Hyperglycaemia Caused by a Glucokinase Mutation: Observational Case Control Studies. PLoS One. 2013;8(6) doi: 10.1371/journal.pone.0065326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vionnet N, Stoffel M, Takeda J, et al. Nonsense mutation in the glucokinase gene causes early-onset non-insulin-dependent diabetes mellitus. Nature. 1992;356(6371):721–722. doi: 10.1038/356721a0. [DOI] [PubMed] [Google Scholar]
- 5.Sturis J, Kurland IJ, Byrne MM, et al. Compensation in pancreatic beta-cell function in subjects with glucokinase mutations. Diabetes. 1994;43(5):718–723. doi: 10.2337/diab.43.5.718. [DOI] [PubMed] [Google Scholar]
- 6.Stride A, Shields B, Gill-Carey O, et al. Cross-sectional and longitudinal studies suggest pharmacological treatment used in patients with glucokinase mutations does not alter glycaemia. Diabetologia. 2014;57(1):54–56. doi: 10.1007/s00125-013-3075-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steele AM, Shields BM, Wensley KJ, Colclough K, Ellard S, Hattersley AT. Prevalence of Vascular Complications Among Patients With Glucokinase Mutations and Prolonged, Mild Hyperglycemia. JAMA. 2014;311(3):279–286. doi: 10.1001/jama.2013.283980. [DOI] [PubMed] [Google Scholar]
- 8.Hattersley AT, Beards F, Ballantyne E, Appleton M, Harvey R, Ellard S. Mutations in the glucokinase gene of the fetus result in reduced birth weight. Nature Genetics. 1998;19(3):268–270. doi: 10.1038/953. [DOI] [PubMed] [Google Scholar]
- 9.Spyer G, Macleod KM, Shepherd M, Ellard S, Hattersley AT. Pregnancy outcome in patients with raised blood glucose due to a heterozygous glucokinase gene mutation. Diabetic Medicine. 2009;26(1):14–18. doi: 10.1111/j.1464-5491.2008.02622.x. [DOI] [PubMed] [Google Scholar]
- 10.Chakera AJ, Spyer G, Vincent N, Ellard S, Hattersley AT, Dunne FP. The 0.1% of the Population With Glucokinase Monogenic Diabetes Can Be Recognized by Clinical Characteristics in Pregnancy: The Atlantic Diabetes in Pregnancy Cohort. Diabetes Care. 2014;37(5):1230–1236. doi: 10.2337/dc13-2248. [DOI] [PubMed] [Google Scholar]
- 11.Iafusco D, Massa O, Pasquino B, et al. Minimal incidence of neonatal/infancy onset diabetes in Italy is 1:90,000 live births. Acta Diabetologica. 2012;49(5):405–408. doi: 10.1007/s00592-011-0331-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Temple IK, Gardner RJ, Mackay DJ, Barber JC, Robinson DO, Shield JP. Transient neonatal diabetes: widening the understanding of the etiopathogenesis of diabetes. Diabetes. 2000;49(8):1359–1366. doi: 10.2337/diabetes.49.8.1359. [DOI] [PubMed] [Google Scholar]
- 13.Bappal B, Raghupathy P, de Silva V, Khusaiby SMA. Permanent neonatal diabetes mellitus: clinical presentation and epidemiology in Oman. Archives of Disease in Childhood - Fetal and Neonatal Edition. 1999;80(3):F209–F212. doi: 10.1136/fn.80.3.F209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franco ED, Flanagan SE, Houghton JA, et al. The effect of early, comprehensive genomic testing on clinical care in neonatal diabetes: an international cohort study. The Lancet. 2015;386(9997):957–963. doi: 10.1016/S0140-6736(15)60098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flanagan SE, Haapaniemi E, Russell MA, et al. Activating germline mutations in STAT3 cause early-onset multi-organ autoimmune disease. Nature Genetics. 2014;46(8):812–814. doi: 10.1038/ng.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Franco E, Flanagan SE, Yagi T, et al. Dominant ER Stress–Inducing WFS1 Mutations Underlie a Genetic Syndrome of Neonatal/Infancy-Onset Diabetes, Congenital Sensorineural Deafness, and Congenital Cataracts. Diabetes. 2017;66(7):2044–2053. doi: 10.2337/db16-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson MB, De Franco E, Lango-Allen H, et al. Recessively inherited LRBA mutations cause autoimmunity presenting as neonatal diabetes. Diabetes. 2017;66(8):2316–2322. doi: 10.2337/db17-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Franco E, Caswell R, Johnson MB, et al. De Novo Mutations in EIF2B1 Affecting eIF2 Signaling Cause Neonatal/Early-Onset Diabetes and Transient Hepatic Dysfunction. Diabetes. 2020;69(3):477–483. doi: 10.2337/db19-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Franco E, Watson RA, Weninger WJ, et al. A Specific CNOT1 Mutation Results in a Novel Syndrome of Pancreatic Agenesis and Holoprosencephaly through Impaired Pancreatic and Neurological Development. The American Journal of Human Genetics. 2019;104(5):985–989. doi: 10.1016/j.ajhg.2019.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson MB, Franco ED, Greeley SAW, et al. Trisomy 21 Is a Cause of Permanent Neonatal Diabetes That Is Autoimmune but Not HLA Associated. Diabetes. 2019;68(7):1528–1535. doi: 10.2337/db19-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Franco E, Lytrivi M, Ibrahim H, et al. YIPF5 mutations cause neonatal diabetes and microcephaly through endoplasmic reticulum stress. Journal of Clinical Investigation. 2020;130(12):6338–6353. doi: 10.1172/JCI141455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearson ER, Flechtner I, Njølstad PR, et al. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. New England Journal of Medicine. 2006;355(5):467–477. doi: 10.1056/NEJMoa061759. [DOI] [PubMed] [Google Scholar]
- 23.Rafiq M, Flanagan SE, Patch A-M, et al. Effective treatment with oral sulfonylureas in patients with diabetes due to sulfonylurea receptor 1 (SUR1) mutations. Diabetes Care. 2008;31(2):204–209. doi: 10.2337/dc07-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delépine M, Nicolino M, Barrett T, Golamaully M, Mark Lathrop G, Julier C. EIF2AK3, encoding translation initiation factor 2-a kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nature Genetics. 2000;25(4):406–409. doi: 10.1038/78085. [DOI] [PubMed] [Google Scholar]
- 25.Rao A, Kamani N, Filipovich A, et al. Successful bone marrow transplantation for IPEX syndrome after reduced-intensity conditioning. Blood. 2007;109(1):383–385. doi: 10.1182/blood-2006-05-025072. [DOI] [PubMed] [Google Scholar]
- 26.Njølstad PR, Søvik O, Cuesta-Muñoz A, et al. Neonatal Diabetes Mellitus Due to Complete Glucokinase Deficiency. New England Journal of Medicine. 2001;344(21):1588–1592. doi: 10.1056/NEJM200105243442104. [DOI] [PubMed] [Google Scholar]
- 27.Prisco F, Iafusco D, Franzese A, Sulli N, Barbetti F. MODY 2 presenting as neonatal hyperglycaemia: a need to reshape the definition of “neonatal diabetes”? Diabetologia. 2000;43(10):1331–1332. doi: 10.1007/s001250051531. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Ting TH, Sheng H, et al. Genetic and clinical characteristics of Chinese children with Glucokinase-maturity-onset diabetes of the young (GCK-MODY) BMC Pediatrics. 2018;18 doi: 10.1186/s12887-018-1060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellard S, Lango Allen H, De Franco E, et al. Improved genetic testing for monogenic diabetes using targeted next-generation sequencing. Diabetologia. 2013;56(9):1958–1963. doi: 10.1007/s00125-013-2962-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genetics in Medicine. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villar J, Ismail LC, Victora CG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. The Lancet. 2014;384(9946):857–868. doi: 10.1016/S0140-6736(14)60932-6. [DOI] [PubMed] [Google Scholar]
- 32.de Onis M. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatrica. 2006;95(S450):76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 33.de Onis M. Development of a WHO growth reference for school-aged children and adolescents. Bulletin of the World Health Organanization. 2007;85(09):660–667. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Globa E, Zelinska N, Mackay DJG, et al. Neonatal diabetes in Ukraine: incidence, genetics, clinical phenotype and treatment. Journal of Pediatric Endocrinology and Metabolism. 2015;28(11-12):1279–1286. doi: 10.1515/jpem-2015-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lorini R, Klersy C, d’Annunzio G, et al. Maturity-Onset Diabetes of the Young in Children With Incidental Hyperglycemia:: A multicenter Italian study of 172 families. Diabetes Care. 2009;32(10):1864–1866. doi: 10.2337/dc08-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Codner E, Rocha A, Deng L, et al. Mild fasting hyperglycemia in children: high rate of glucokinase mutations and some risk of developing type 1 diabetes mellitus. Pediatric Diabetes. 2009;10(6):382–388. doi: 10.1111/j.1399-5448.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dickens LT, Letourneau LR, Sanyoura M, Greeley SAW, Philipson LH, Naylor RN. Management and pregnancy outcomes of women with GCK-MODY enrolled in the US Monogenic Diabetes Registry. Acta Diabetologica. 2019;56(4):405–411. doi: 10.1007/s00592-018-1267-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.López Tinoco C, Sánchez Lechuga B, Bacon S, et al. Evaluation of pregnancy outcomes in women with GCK-MODY. Diabetic Medicine. :e14488. doi: 10.1111/dme.14488. Published online December 5, 2020. [DOI] [PubMed] [Google Scholar]
- 39.Das S, Lese CM, Song M, et al. Partial Paternal Uniparental Disomy of Chromosome 6 in an Infant with Neonatal Diabetes, Macroglossia, and Craniofacial Abnormalities. The American Journal of Human Genetics. 2000;67(6):1586–1591. doi: 10.1086/316897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Battin M, Yong C, Phang M, Daaboul J. Transient neonatal diabetes mellitus and macroglossia. Journal of Perinatology. 1996;16(4):288–291. [PubMed] [Google Scholar]
- 41.Velho G, Hattersley AT, Froguel P. Maternal diabetes alters birth weight in glucokinase-deficient (MODY2) kindred but has no influence on adult weight, height, insulin secretion or insulin sensitivity. Diabetologia. 2000;43(8):1060–1063. doi: 10.1007/s001250051490. [DOI] [PubMed] [Google Scholar]
- 42.McDonald TJ, Besser RE, Perry M, et al. Screening for neonatal diabetes at day 5 of life using dried blood spot glucose measurement. Diabetologia. 2017;60(11):2168–2173. doi: 10.1007/s00125-017-4383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that supports this work is not freely available due to its identifiable nature, but reasonable requests for additional data can be made to the corresponding author.