Abstract
Developing sensory circuits exhibit different patterns of spontaneous activity, patterns that are related to the construction and refinement of functional networks. During the development of different sensory modalities, spontaneous activity originates in the immature peripheral sensory structures and in the higher-order central structures, such as the thalamus and cortex. Certainly, the perinatal thalamus exhibits spontaneous calcium waves, a pattern of activity that is fundamental for the formation of sensory maps and for circuit plasticity. Here we will review our current understanding of the maturation of early (including embryonic) patterns of spontaneous activity, and their influence on the assembly of thalamic and cortical sensory networks. Overall, the data currently available suggests similarities between the developmental trajectory of brain activity in experimental models and humans, which in the future may help to improve the early diagnosis of developmental disorders.
Keywords: Spontaneous activity, sensory systems, developing brain
Introduction
The efficiency of information processing in sensory systems relies on the correct organization of their underlying circuits. The assembly of sensory circuits is a result of the dynamic interactions between the unfolding genetic programs and activity-dependent mechanisms (Cang and Feldheim, 2013; Simi and Studer, 2018). Specific profiles of gene expression in each developing neuron establish the first rough arrangement of connections. The neurons of these immature circuits progressively acquire electrical features giving rise to distinct patterns of spontaneous and sensory-driven activity that drive the maturation of functional networks. These patterns of activity participate in a wide range of processes, for instance, regulating cell differentiation, cell migration, apoptosis, axon refinement or synapse formation (Blanquie et al., 2017; Hanson and Landmesser, 2004; Jabaudon, 2017; Katz and Shatz, 1996; Kilb et al., 2011; Rosenberg and Spitzer, 2011; Yamamoto and López Bendito, 2012). The contribution of sensory-driven mechanisms to the establishment of the final neural networks has been characterized well since the pioneering work of Hubel and Wiesel (Hubel and Wiesel, 1963). Spontaneous activity precedes these sensory-driven stimuli and it is thought to configure future connections by providing a template of evoked activity (Ackman and Crair, 2014; McVea et al., 2012; Molnár et al., 2020; Yang et al., 2018). However, the frailty of the developing brain and its inaccessibility during embryogenesis poses a challenge, leaving the functional aspects of early brain formation relatively unexplored.
In this review, we will summarize the main properties of spontaneous activity in sensory systems during development, highlighting recent results, and focusing on the generation of this activity and its transmission to central structures. We center on work carried out on rodents, unless otherwise stated, due to the knowledge yielded by the manipulations permitted in this model. Special emphasis is placed on the interactions between the cortex and thalamus, the latter for long considered a mere relay station and whose access is limited due to its covert location deep in the brain. Importantly, the thalamus handles all sensory modalities except olfaction, conveying ascending sensory signals to the cortex, and it has recently gained attention since spontaneous waves of activity in the embryonic thalamus influence through subplate cells the maturation of cortical columns and sensory maps (Antón-Bolaños et al., 2019).
Spontaneous activity ascending to thalamic and cortical stations
From the sensorimotor system
The perinatal assembly of sensory circuits is assisted by the neuronal activity generated as a result of spontaneous body movements (Figure 1; (Blumberg and Dooley, 2017; Fagard et al., 2018). The nature of these movements puzzled researchers since the German psychologist F. W. Preyer first reported them in 1883 (Preyer, 1937). In the 1960s, V. Hamburger and his group began to unravel the underlying physiology of early body movements by lesioning sensory afferents and supraspinal pathways of chick embryos. They showed that this early motor behavior is generated spontaneously in the spinal cord without any obvious external stimuli (Hamburger and Narayanan, 1969; Hamburger et al., 1966). In 1970, Provine and colleagues extended these results by obtaining extracellular recordings from freely moving chick embryos to directly demonstrate that the burst activity of clusters of neurons in the ventral spinal cord paralleled the spontaneous movement of body parts (Provine et al., 1970). Indeed, Provine’s recordings represented the first direct evidence of spontaneous electrical activity in the developing nervous system.
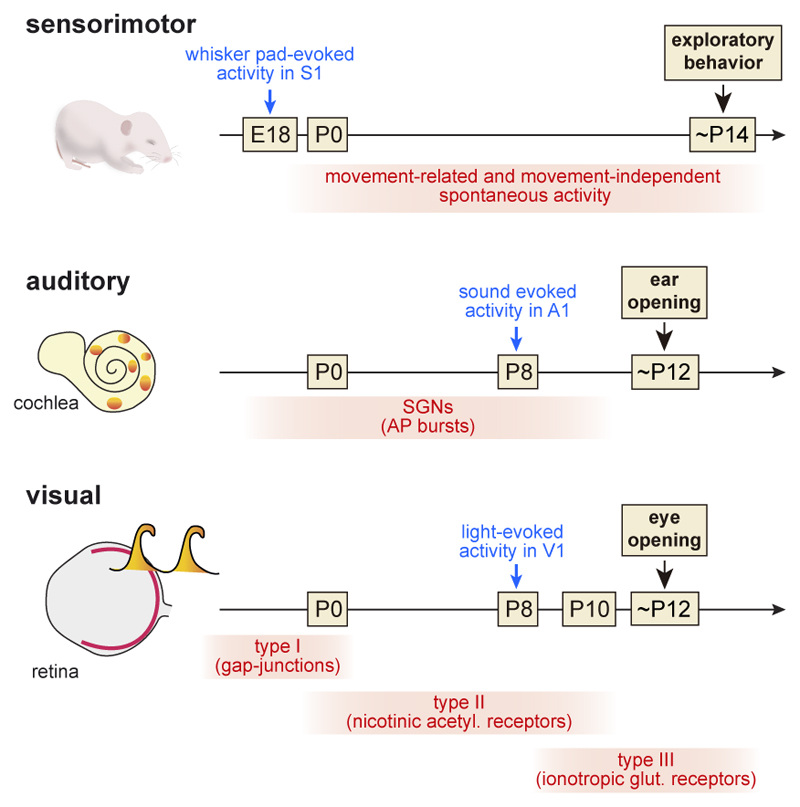
Figure 1. Early manifestations of peripheral spontaneous and evoked activity in developing sensory systems in the mouse.
Developmental progression of peripheral spontaneous (in red) and evoked (in blue) activity in sensorimotor, auditory and visual systems. In the somatosensory system the peripheral activity may be mediated by sensory spontaneous activity or through a sensory-motor feedback. In the auditory system the developing cochlea generates spontaneous activity that is transmitted to SGNs as action potential (AP) bursts. In the visual system the developing retina generates spontaneous waves of activity with a defined developmental profile that is transmitted to the visual thalamus and cortex. E= embryonic age; P= postnatal age; SGN=spiral ganglion neurons.
In rodents, motor commands are generated intrinsically in the spinal cord during perinatal stages without any supraspinal drive (Robinson et al., 2000). Electrical recordings of neonatal rats show that spinal motor circuits activate spontaneously and drive overt limb movements (Inacio et al., 2016). These spinal commands persist until the end of the second postnatal week when the brainstem becomes the main driver of limb movement (Karlsson et al., 2005; Kreider and Blumberg, 2000). An important consequence of the spontaneous body movements is the activation of the matching somatotopic area in the sensory spinal cord (Inacio et al., 2016). This sensory feedback is called reafference and it most likely encodes relevant spatiotemporal features of the self-generated somatosensory stimulus (Blumberg et al., 2013). Since the peripheral-to-central flow of sensory information in the somatosensory pathway is already established by birth (Iwasato and Erzurumlu, 2018), reafference could be part of the activity-dependent mechanisms that shape the organization of the thalamocortical system.
Reafference can be triggered by two types of body movement -local twitches and complex body movements-associated with different behavioral states in neonatal rodents. Twitches are spontaneous limb jerks that dominate during active sleep, whereas long-lasting and complex movements more often occur during waking periods. Although both sleep or waking movements trigger reafferent signals, the ensuing activation of the ascending pathways is state-dependent (Dooley et al., 2019). In sleeping states, twitches evoke responses in the somatosensory thalamus, cortex and motor cortex (Khazipov et al., 2004; McVea et al., 2012), whereas waking movements fail to evoke any supraspinal response (Tiriac et al., 2014; 2012) or they evoke only poorly reliable responses, such as those related to whisker movements (Dooley et al., 2020). This state-dependent gating of movement-related responses seems to be mediated by the external cuneate nucleus of the brainstem. Indeed, there is evidence that the external cuneate nucleus compares the ascending reafferent signal with the corollary discharges provoked by the efferent motor commands (Tiriac and Blumberg, 2016). Before postnatal day (P)11, the external cuneate nucleus cancels out the reafferent signal during waking periods in rats, although it permits its transfer during periods of sleep when reafference is triggered by twitches. In this way, behavioral states control the inflow of information to higher-order brain structures, which in turn might be relevant to shape the developing somatosensory pathway.
Apart from reafferentiation, other signals may be part of the activity-dependent mechanisms that control the development of the somatosensory system. As such, electrical activity in the spinal cord of rodents can also arise independently of any obvious body movement or driven by external mechanical stimulus (Inacio et al., 2016). Irrespective of its driving source, sensory activity in the spinal cord is conveyed to central stations and it triggers cortical responses from early stages of development, even in the embryo (Antón-Bolaños et al., 2019; Mizuno et al., 2018; Yang et al., 2009). However, activity in the primary somatosensory cortex at these stages persists after lesioning the spinal cord (Khazipov et al., 2004) and thus, the exact functional relationship between spinal and higher-order brain structures during development is not yet fully understood.
From the cochlea
Activity in the developing auditory system of rodents is a composite of both sound-evoked responses and sound-independent spontaneous events that occur even before ear opening (Geal-Dor et al., 1993; Meng et al., 2020). While early sound-evoked responses have been only studied in recent years (Meng et al., 2021; Makarov et al., 2021), spontaneous activity has been widely reported, especially in peripheral structures. From embryonic stages, the developing cochlea reveals periodic bursts of spontaneous activity (Kennedy, 2012; Leighton and Lohmann, 2016; Wang and Bergles, 2015), with whole cell recordings from newborn rodents showing that auditory sensory receptors (inner hair cells, IHCs) fire spontaneous bursts of calcium-based action potentials (Johnson et al., 2011; Sendin et al., 2014; Tritsch and Bergles, 2010). Burst activity in IHCs is modulated by inhibitory inputs from the medial superior olive (Glowatzki and Fuchs, 2000; Goutman et al., 2005; Johnson et al., 2011; Marcotti et al., 2004; Roux et al., 2011) and it is primarily triggered by the periodic release of ATP from clusters of supporting cells (Babola et al., 2020b; Tritsch and Bergles, 2010; Tritsch et al., 2007). In close proximity to the IHCs, supporting cells are non-neuronal glia-like cells that belong to a transient structure in the developing cochlea called Kölliker’s organ (Dayaratne et al., 2014). Apart from the activity mediated by supporting cells, IHCs have the intrinsic capacity to fire action potentials with different patterns along the baso-apical axis (Eckrich et al., 2018; Harrus et al., 2018; Johnson et al., 2011; Kros et al., 1998; but see Sendin et al., 2014). In turn, the electrical activity from IHCs excites primary sensory neurons (spiral ganglion neurons) which by P0 fire characteristic intermittent bursts of action potentials (Babola et al., 2020a; Coate et al., 2019; Glowatzki and Fuchs, 2002; Zhang-Hooks et al., 2016).
Spiral ganglion neurons transmit spontaneous activity towards higher order auditory stations during the first postnatal week. In P4-P8 rats, in vivo extracellular recordings reveal spontaneous bursts of action potentials in principal neurons of the medial nucleus of the trapezoid body (a relay nucleus in the brainstem) and in the central nucleus of the inferior colliculus (Tritsch et al., 2010). This electrical activity has a peripheral origin since it is abolished by contralateral cochleotomy. Similar patterns of activity have been recorded in vivo from the auditory nuclei of the mouse brainstem, where neurons shift from bursting activity at P8-P10 to a more continuous non-bursting discharge from P12 (Sonntag et al., 2009). Therefore, cochlear spontaneous activity may play a fundamental role in the development of the networks and tonotopic maps in the ascending structures of the auditory system.
The spatial activity in the inferior colliculus and auditory cortex in rodents has recently been studied by combining mesoscale calcium imaging with high spatial resolution two-photon microscopy. In the inferior colliculus there are discrete, bilateral stationary bands of spontaneous activity oriented within prospective isofrequency domains (Babola et al., 2020a). Bilateral bands are present as early as P1 and their frequency increases until P11-P12. These bands originate in the cochlea and they are correlated with activity in the topographically aligned auditory cortex (Babola et al., 2018). This degree of precision in auditory transmission was unexpected at these early stages given the immaturity of the synaptic contacts, suggesting the existence of mechanisms that enhance synaptic reliability (Dietz et al., 2012; Zhang-Hooks et al., 2016). Together, these results demonstrate that, before ear opening, the cochlea can establish the dynamics of the activity in most central auditory stations. However, one station that remains to be explored is the developing auditory thalamus, which has not yet been recorded from.
From the retina
The immature retina of mice exhibits complex patterns of sensory-independent activity before eye-opening (~P12-P13), and even before rod- and cone-mediated light responses can be recorded in the cortex (~P8; (Ackman et al., 2012; Shen and Colonnese, 2016)). The early patterns of retinal activity contribute to relevant aspects of visual pathway assembly, such as eye-specific segregation, fine retinotopy and the organization of receptive fields. There have been many publications on these topics and in recent years, they have been the subject of thorough reviews (we direct the reader to the works of (Ackman and Crair, 2014; Arroyo and Feller, 2016; Blankenship and Feller, 2010; Cang and Feldheim, 2013; Thompson et al., 2017). As such, here we will only sketch out the main characteristics of spontaneous retinal activity, highlighting aspects relevant to embryonic stages and the transmission of electrical signals to higher order stations.
Spontaneous activity in the peripheral visual system was first recorded in vitro using a preparation of the immature retina of newborn rabbits (Masland, 1977) and it was subsequently studied in vivo, using glass electrodes through the lens of intact rat fetuses (Galli and Maffei, 1988). In general, a remarkable property of the spontaneous activity in the immature mammalian retina is the similar profile of activation of neighboring primary sensory neurons, retinal ganglion cells (RGCs). This correlated pattern has a spatiotemporal structure resembling wave-like propagating events (Meister et al., 1991). Retinal waves have been demonstrated in mice in vivo by recording the calcium signal from RGC projections that reach the superior colliculus (Ackman et al., 2012). Based mostly on mouse data, these retinal waves have been classified into three different sequential stages defined by their molecular mechanisms and biophysical properties, and defining different developmental periods: stage I, II, and III (Figure 1). In mice, stage I waves start to occur in embryos around embryonic day (E)17 and they last until ~P1. Stage II waves are mostly postnatal, beginning at ~P1 and lasting until ~P10, whereas stage III waves begin at ~P10 and last until around eye opening. The transition between these stages is progressive and therefore, two wave types may overlap at any one time (Blankenship and Feller, 2010; Torborg and Feller, 2005).
Regarding their triggers and means of propagation, each type of retinal wave relies on different signaling pathways and cell types. Stage I events are stationary waves that occur independent of fast synaptic transmission, yet they seem to depend on gap junctions and adenosine signaling (Bansal et al., 2000; Kähne et al., 2019; Syed et al., 2004). Stage II events are large, slow, propagating waves that depend on cholinergic signaling (Ford et al., 2012; R. O. Wong et al., 1993). Finally, stage III events are fast, focused waves that depend on glutamate release from bipolar cells and the activation of ionotropic glutamate receptors (Blankenship et al., 2009; Maccione et al., 2014). Most research has focused on these latter two stages, probably due to the fact that the first stage mainly unfolds in embryos, which hampers its study. The early onset of stage I retinal waves suggests that they may have an impact on the initial assembly of visual circuits, probably in processes related to the configuration of gross topographic maps (Simon and O’Leary, 1992). Therefore, efforts should be made to study embryonic retinal activity if we wish to understand both the normal and pathological early development of the circuits underlying visual information processing.
Spontaneous retinal activity during embryonic life has been addressed in a few reports, and there is only limited data from mouse models despite them representing the main arena to study retinal activity during development. In fact, the first evidence of spontaneous activity in embryos came from the chick visual system, where large excitatory events were seen to propagate through the RGC layer (Catsicas et al., 1998; W. T. Wong et al., 1998). In these embryos, highly patterned activity spans from E13 to E18, just before hatching, and it seems to be mediated by gap junctions. Although many features of this activity are common to the stage I retinal waves of mice, the wave patterns in the immature avian retina are stable during development and not subdivided into stages. Stage I waves seem to be poorly defined, even in mice, since they are usually recognized as the activity evident prior to the well-defined stage II waves (Bansal et al., 2000). Despite their vague definition, stage I waves have some properties that seem to be consistent: they emerge mostly during embryonic development and they involve small clusters of RGCs that are activated in waves mediated by gap junctions. To some extent consistent with this description, the initial activity in the developing retina of rabbit embryos resembles the dynamics and pharmacology of stage I waves in mice, although the propagating profile differs (Syed et al., 2004). Thus, it seems clear that stage I patterns need to be better studied to achieve a comprehensive classification and unravel their underlying circuits and molecular mechanisms. Moreover, it would be valuable to investigate: i) if they are transmitted to higher order stations; ii) to what extent they affect the development of central visual structures in the brain; and iii) what are the consequences of silencing or perturbing stage I retinal activity.
By contrast, stage II and III retinal waves have been studied more extensively, not only analyzing the underlying circuits but also, their transmission and influence on higher-order stations in the visual system. After the pioneering recordings of retinal waves, accumulated evidence demonstrates their transmission through the visual pathway up to the visual thalamus (Mooney et al., 1996; Weliky and Katz, 1999) and visual cortex (Colonnese and Khazipov, 2010; Hanganu et al., 2006). These results were recently extended in vivo, faithfully reflecting the spatiotemporal structure of retinal waves throughout the visual pathway. By expressing genetically encoded calcium indicators in RGCs, the pattern of spontaneous activity in the retinal projection could be studied in conjunction with the responses in their postsynaptic targets and higher-order structures (Ackman et al., 2012). Experiments in early postnatal mice (P3-P9) showed that retinal waves drive wave-like activity in the visual thalamus, superior colliculus, and the primary and secondary visual cortex. The reliable arrival of the ascending signals to the cortex is secured by their amplification mediated by the corticothalamic loop (Murata and Colonnese, 2016). In fact, the retina is the main driver of activity in the primary visual thalamus and cortex during stage II, evidence of the important role fulfilled by retinal waves in dendrite refinement and map formation in higher visual areas (Burbridge et al., 2014; McLaughlin et al., 2003; Siegel et al., 2012). By the end of the second postnatal week, synaptic transmission of spontaneous activity in the thalamocortical connection of mice becomes less consistent, while its transfer along the retinotectal and retinogeniculate connection remains reliable (Gribizis et al., 2019). By P10 in mice, and in parallel to the drop in thalamocortical reliability, the intermittent and correlated pattern of spontaneous activity in the primary visual cortex begins to shift towards the more mature pattern, characterized by continued and decorrelated ongoing activity (Colonnese et al., 2010; Rochefort et al., 2009; Siegel et al., 2012).
In addition to spontaneous activity, immature retinas exhibit light-evoked activity before eye-opening (Akerman et al., 2002; Rochefort et al., 2011). Light passes through the closed eyelids and reaches the intrinsically photosensitive retinal ganglion cells (ipRGCs), as well as rods and cones. In mice, ipRGCs become functional photoreceptors after birth, mostly overlapping with the stage II retinal waves (Tu et al., 2005). On the other hand, rods and cones can only drive RGC activity after the first postnatal week, overlapping with late stage II and stage III retinal waves (Shen and Colonnese, 2016). Interestingly, light-evoked activity in the retina seems to interact with spontaneous retinal waves to refine the eye-specific segregation of the retinogeniculate projection (Renna et al., 2011; Tiriac et al., 2018). Future studies should interrogate the mechanisms underlying the interaction of spontaneous and evoked activity, and the contribution of this interaction to other aspects of visual pathway development. Apart from morphological and functional analyses, experimental manipulations to examine the development of the visual system require additional behavioral studies. Assessing visual performance in this way will provide missing information regarding the ethological relevance of developmental defects, such as abnormal eye-specific segregation in image-forming centers in the brain.
Spontaneous activity in the developing sensory thalamus
The developing sensory thalamus
All sensory modalities except olfaction relay through the thalamus before reaching their corresponding cortices. Despite its pivotal location, the specific features of thalamic development in relation to the formation of sensory circuits have yet to be thoroughly addressed. Its apparent basic function as a relay station, along with technical difficulties to reach deep brain structures, have precluded assessing the thalamic contribution to circuit assembly. However, the thalamus is not merely a transmitter of peripheral inputs but also, the input-output diversity of mammalian thalamic neurons enables them to form complex circuits that could encode broad contextual information (Rikhye et al., 2018).
Based on the nature of their dominant input, thalamic sensory nuclei have classically been classified as first-order or higher-order. First-order nuclei are mainly driven by subcortical sources carrying sensory information, whereas higher-order nuclei receive their predominant input from layer 5 cortical cells (Halassa and Sherman, 2019; Sherman, 2017). The principal first-order nuclei are divided into the ventral posteromedial nucleus (VPM) that receives input from the trigeminal pathway, the ventral medial geniculate nucleus (MGv) that receives auditory information mainly from the inferior colliculus, and the dorso-lateral geniculate nucleus (dLGN) that receives direct input from the retina (Figure 2). All these first-order nuclei contact with their corresponding primary cortical areas, and subsequently, sensory information reaches secondary cortical areas through corticocortical and transthalamic routes. The transthalamic loop begins with neurons from layer 5 of the primary cortices projecting their axons towards matched higher-order thalamic nuclei, namely the lateral posterior (LP) nucleus for visual information, the posteromedial (POm) nucleus for somatosensory information and dorsal medial geniculate (MGd) nucleus for auditory information. In turn, thalamic cells from the higher-order nuclei project back to layer 4 neurons of the secondary cortical areas.
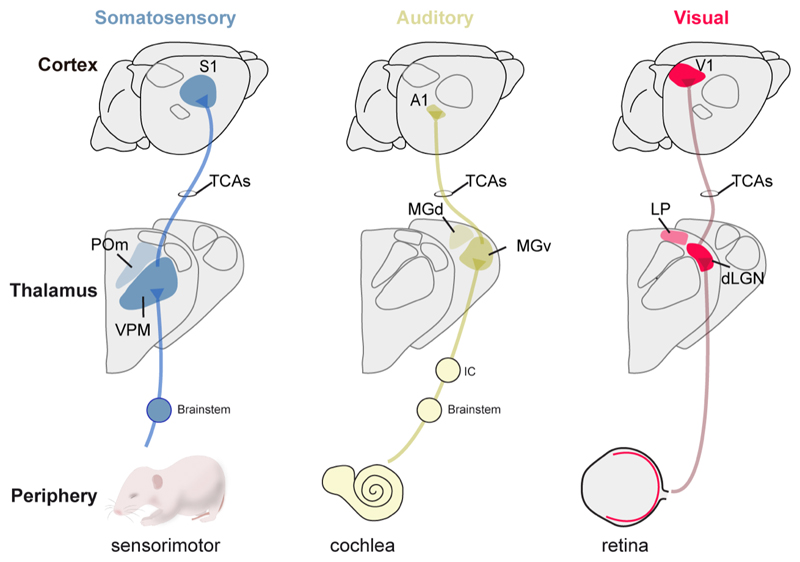
Figure 2. Connectivity from the sensory periphery to the thalamus and cortex.
Schema representing the three major inputs from the peripheral organs to thalamic sensory nuclei and primary sensory cortical areas. First-order sensory nuclei (VPM, dLGN and MGv) receive direct input from peripheral pathways. High-order nuclei (POm, LP and MGd) receive feedback input from sensory cortices. TCAs= thalamocortical axons, POm= postero-medial nucleus, VPM= ventral posteromedial nucleus, MGd= dorsal medial geniculate, MGv= ventral medial geniculate nucleus, LP= lateral posterior nucleus, dLGN= dorso-lateral geniculate nucleus.
The thalamus is the first station where most sensory routes converge, increasing the complexity when analyzing their developmental pathways. The segregation of sensory thalamic nuclei during development requires a precise program that is controlled by activity-driven and genetic mechanisms. Although much is known about the genetic basis of thalamic cell specification (Gezelius and López Bendito, 2017; Nakagawa and Shimogori, 2012; Shi et al., 2017; Song et al., 2015) and thalamocortical axon sorting (Garel and López Bendito, 2014; Leyva-Diáz et al., 2014; López Bendito, 2018), many uncertainties still persist regarding the role of intrinsic and extrinsic activity on thalamic parcellation. Thalamic circuits become organized through a gradual process whereby the neuronal networks of each sensory modality are assembled in segregated nuclei but also, preserving sufficient flexibility to allow intra-modal and cross-modal plasticity triggered by sensory deprivation or malfunction (Izraeli et al., 2002; Moreno-Juan et al., 2017).
Neuronal activity in the nascent sensory thalamus evolves through distinct stages in rodents (Figure 3A). The first stage spans through mid- and late-embryonic life, up to the first postnatal days, and it is defined by endogenous activity that is initially dominated by scant and uncorrelated activity (~E12-E14), this deriving into large correlated events from ~E14-E15 until birth. Shortly after birth the next stage begins, with a characteristic rhythmic pattern engaging cortical territories, mainly triggered spontaneously from the peripheral organs. Close to the onset of active sensory processing, around the second postnatal week for most sensory modalities in rodents, these patterns change to a more mature, decorrelated activity, as occurs in other brain stations, although this stage has yet to be studied in detail.
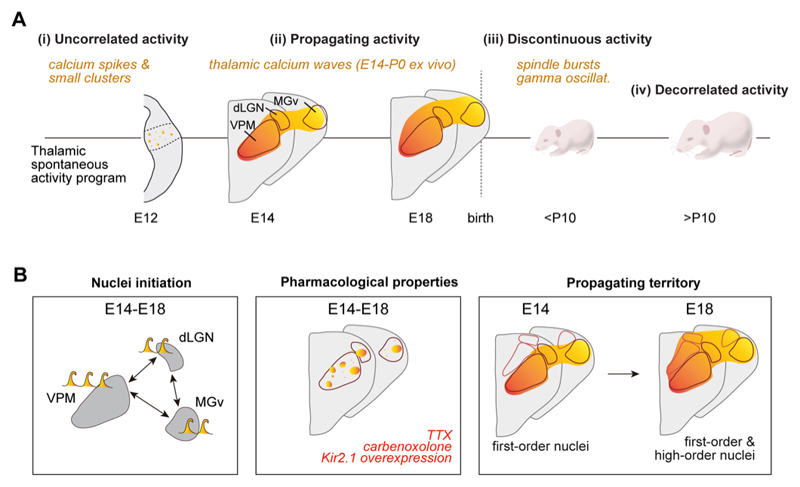
Figure 3. Developmental properties of thalamic spontaneous activity.
(A) The pattern of thalamic spontaneous activity is modified during embryonic and early postnatal development. (B) Properties of embryonic thalamic waves from ex vivo experiments. Left: spontaneous waves can be originated in any of the principal nuclei, but most frequently in the VPM. Middle: initially, waves spread to cover the three principal first-order nuclei while at perinatal stages the activity extends to high-order nuclei. Right: voltage-dependent Na-channel blocker TTX, gap-junction blocker carbonoxolone, or overexpression of the inward rectifier Kir2.1 channel blocks correlated spontaneous activity and only asynchronous activity and small clusters are left in the thalamus. Color gradient represents time course of activation. E= embryonic age; P= postnatal age.
Spontaneous thalamic activity during embryonic thalamocortical wiring
Thalamocortical axons grow in a topographical manner under the control of axon guidance cues and intermediate target cells, a process in which spontaneous thalamic activity has also been implicated (Antón-Bolaños et al., 2018). At mid-gestation in the mouse, there is scattered and uncorrelated spontaneous activity in the thalamus, evident as calcium transients. This activity starts in all the principal thalamic nuclei and it seems to play a role in development. For example, the frequency of calcium transients modulates the growth rate of thalamocortical axons by controlling the expression of the Robo1 and Dcc genes, which act as brakes and accelerators of axonal extension, respectively (Castillo-Paterna et al., 2015; Mire et al., 2012). In addition, in vitro and in vivo studies demonstrated that thalamocortical axon branching is modulated by spontaneous activity. Indeed, inhibiting synaptic transmission in the thalamocortical connection reduces axon branching (Antón-Bolaños et al., 2018; Herrmann and Shatz, 1995; Martini et al., 2018; Uesaka et al., 2007) and manipulating spontaneous activity in the thalamus modifies the branching complexity of thalamocortical projections (Antón-Bolaños et al., 2019; Moreno-Juan et al., 2017).
At late gestation in rodents, synchronous activity is the dominant pattern in the thalamus. Ex vivo thalamic recordings identify an organized pattern of spontaneous activity that involves propagating calcium waves (Moreno-Juan et al., 2017). From E14, waves are generated in all first-order nuclei, although most activity comes from the VPM (Figure 3B). Irrespective of their origin, these waves consistently propagate to the adjacent nuclei although within these nuclei, the sites of origin are randomly distributed, suggesting that pacemaker sub-regions do not exist. In slices, waves appear at a mean frequency of ~0.2 events per minute and they last for ~8 seconds, with a mean wave front speed of ~150 μm per second. The mechanisms that underlie the activation of thalamic waves are not fully established, although their abolition by TTX and carbenoxolone (Figure 3B) suggests they are initiated or propagated by activating voltage-dependent sodium channels, and that they might be mediated by gap-junctions. After birth, the pattern of wave-like activity is less well correlated, a switch that occurs at P0 for the primary somatosensory and auditory nuclei, and at P2 for the primary visual nucleus. Before this developmental switch, wave-like activity in the thalamus begins to spread towards higher-order nuclei (Figure 3B), although it remains to be determined what is the timeline of higher-order nuclei recruitment.
Thalamic waves are relevant to control the size of cortical areas. When the frequency of spontaneous thalamic waves in mice increases after bilateral eye enucleation at E14 (the embBE model), the area of the presumptive visual cortex shrinks as the somatosensory representation of the whisker system expands (Moreno-Juan et al., 2017). In addition, disrupting prenatal thalamic calcium waves in the auditory nucleus by overexpressing the inward rectifying ion channel Kir2.1 (AudKir mouse model) alters the pattern of thalamic activity in the VPM, triggering an expansion of the primary somatosensory cortex. By analyzing the transcriptional changes in VPM cells from both models (embBE and AudKir), the expression of Rorβ was correlated with the extent of cortical expansion and with the complexity of the axonal arborization of thalamocortical projections. This novel mechanism of communication among thalamic sensory nuclei seems to underlie early plasticity through the control of gene expression, establishing the thalamus as a key regulator of cross-modal cortical readjustments before sensory onset.
The thalamic influence on cortical organization seems to be orchestrated by a group of neurons located in the subplate zone, below the cortical plate (Molnár et al., 2020). Subplate cells are born during the second week of gestation in rodents, and most of them undergo programmed cell death after birth (Hanganu et al., 2002; Kanold and Luhmann, 2010; Zhao et al., 2009). In sharp contrast to other cells in the developing cortex (except for cells in the marginal zone), subplate cells are electrophysiologically mature, and they form an extensive network of local clusters coupled by gap junctions and chemical synapses (Dupont et al., 2006; Singh et al., 2019). These networks receive input from thalamocortical axons that relay to developing L4 neurons, other subplate cells and the cortical plate (Barkat et al 2011; Friauf et al., 1990; Hanganu et al., 2002; Herrmann et al., 1994; Viswanathan et al., 2012; Zhao et al., 2009). In mouse embryos, the stimulation of a small ensemble of neighboring neurons in the somatosensory thalamus first evokes a large and confined response in the matching subplate, which then spreads radially across the cortical plate remaining as wide as the initial subplate activation (Figure 4A; (Antón-Bolaños et al., 2019; Higashi et al., 2002). This radial activation may represent a prenatal sketch of the columnar organization of cortical circuits.
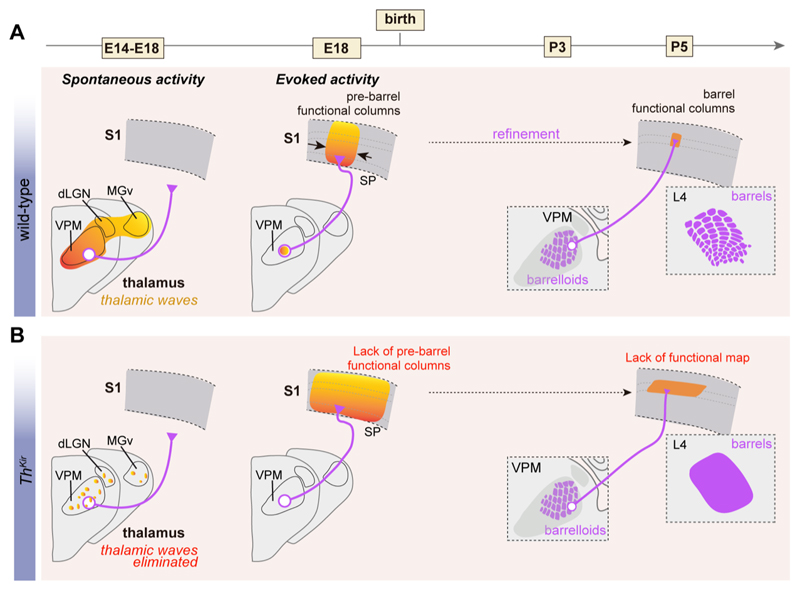
Figure 4. Role of thalamic spontaneous activity on the formation of cortical maps.
(A) Under normal conditions, embryonic thalamic waves emerge around E14 at the time thalamocortical axons (purple) navigate to the cortex. Small VPM stimulations before and soon after birth reveal that S1 cortical responses are fairly columnar. Around P4, similar VPM stimulations trigger columnar responses restricted to L4 as thalamocortical axons already target this layer. (B) In the absence of thalamic waves, with only sparse and asynchronous events in the thalamus, thalamocortical axonal navigation appears spared, however restricted thalamic VPM activation elicits a widespread cortical activity at early stages. At later stages, VPM activation leads to a L4-restricted but not columnar cortical response. Barrels do not form in Thkir mice. Color gradient represents time course of activation. E= embryonic age; P= postnatal age, VPM= ventral posteromedial nucleus, MGv= ventral medial geniculate nucleus, dLGN= dorso-lateral geniculate nucleus, SP= subplate.
Embryonic thalamic waves are also involved in-the functional organization of cortical columns. Indeed, the suppression of thalamic waves in mice alters the correct formation of anatomical and functional columns in the cortex. By overexpressing Kir2.1 in all sensory nuclei of the prenatal thalamus (ThKir mouse model), large propagating waves are replaced by a scattered activation of small clusters of neurons (Figure 4B). In the absence of thalamic waves, spatially restricted thalamic stimulation consistently elicits broad cortical responses that occasionally spread to adjacent cortical areas. This response aligns with in vivo data revealing an increase in the area covered by spontaneous activity in the ThKir cortex, probably mediated by enhanced mGlu5 receptor expression by cortical cells. In accordance with these results, stimulation of neighboring receptive fields of the whisker pad at P3-P4 evokes large and overlapping responses in the primary somatosensory cortex of ThKir mice, whereas similar stimulation provokes adjacent and discrete responses in control mice (Figure 4A-B). The loss of spatial definition in the somatosensory pathway of ThKir mice is explained by an abnormal overexpansion and lack of cluster organization of thalamocortical axons in the cortex. However, the causal relationship between the increased levels of mGluR5 expression and the lack of clustering in cortical layer 4 of thalamic axons in the loss of spatial definition are currently unknown. Together these data reveal that the emergence of cortical columns and the somatotopic map in S1 relies on the thalamic regulation of cortical excitability through embryonic patterns of spontaneous thalamic activity (Antón-Bolaños et al., 2019).
Spontaneous activity in the thalamus after thalamic waves
Prenatal rodent thalamic cells exhibit immature electrical properties that gradually shift towards a more mature profile as development proceeds. This maturation occurs smoothly during the first two postnatal weeks, with a steep shift around P5. As a result, relay cells from the dLGN fire sharp action potentials before the end of the first postnatal week (Antón-Bolaños et al., 2019; Lo et al., 2002; MacLeod et al., 1997).
After birth, spontaneous activity in the rodent thalamus has a discontinuous nature. Bouts of activity in the somatosensory and visual thalamus exhibit stereotypic patterns that can be subdivided into three categories: early gamma oscillations (30-50 Hz), spindle bursts (8-20 Hz), or a consecutive combination of both (Khazipov et al., 2013; Murata and Colonnese, 2016; Yang et al., 2013). Mainly triggered by spontaneous inputs ascending from sense organs, oscillatory bouts could also be elicited by external stimulation, but they do not depend on it. In paired recordings, the oscillating bouts from the thalamus can be seen to engage the cortical counterpart in a coherent manner. Lesioning sense organs abolishes most thalamic and cortical activity but pure spindle bursts remain, suggesting that this specific pattern could be triggered from and starts to be shaped in the thalamus (Murata and Colonnese, 2016; Yang et al., 2013). Similar results were found for somatosensory-evoked responses, showing that the thalamic barreloids are synchronized with matched barrels in S1 through early gamma oscillations, thereby promoting the correct topographic wiring of the thalamocortical projection (Minlebaev et al., 2011; Yang et al., 2013). In turn, it seems that there is corticothalamic feedback modulation in newborn rats, as cortical inactivation affects the frequency spectrum and spiking rate of spontaneous and evoked events in the thalamus (Murata and Colonnese, 2016; Yang et al., 2013).
After the first postnatal week, the initial intermittent activity in the thalamus of rodents progressively switches to a more mature pattern characterized by firing continuity and behavioral state dependence (Murata and Colonnese, 2018). This thalamic transition seems to be critical for the parallel process observed in the cortex. Silencing thalamic activity at these stages suppresses the cortical firing rate and alters the modulation of cortical activity by behavioral states. Among the factors that may account for the thalamic developmental switch are the changes in retinal input, or the gradual integration of inhibitory and neuromodulatory components into thalamic networks (Colonnese, 2014; Demas et al., 2003; Sokhadze et al., 2019). However, the maturation of thalamic patterns of activity must rely on network changes that are not yet known.
Spontaneous activity in the developing sensory cortices
The basic structure of sensory cortices: cortical columns
Back in 1938, Rafael Lorente de Nó depicted an afferent axon reaching a vertical strip of cortex that was comprised of many cell types, stating that “all the elements of the cortex are represented in it [the vertical strip], and therefore it may be called an elementary unit, in which, theoretically, the whole process of the transmission of impulses from the afferent fiber to the efferent axon may be accomplished” (Fulton, 1938). In this way, Lorente de Nó defined the functional architecture of the cerebral cortex as an ensemble of stereotypic, radial modules of neurons, a concept that developed into the “columnar” hypothesis and that has become the most extended paradigm for research into cortical function (Mountcastle, 1997).
Despite the tremendous resonance of the columnar paradigm, the precise definition of a cortical column remains elusive due to the multiple interpretations of this term (DeFelipe et al., 2012). In general, a cortical column refers to a vertical cylinder of variable diameter that spans all layers of the cortex. In this cylinder or column, afferent information is processed by local neurons through stereotypic radial microcircuits that in turn transfer the information to other columns or extra-cortical regions. Thus, the functional organization of the cerebral cortex could be described as an interconnected array of columnar microcircuits of relatively constant size (Mountcastle, 2003; Rakic, 1995). However, columns do not constitute solid modules with watertight borders but rather, they interdigitate with neighboring columns and are part of larger distributed networks (Rockland, 2010). In many sensorimotor areas, columns act as basic computational modules, and they constitute the building blocks of anatomical and functional maps. Columns vary in shape and size across areas and species. For example, the V1 ocular dominance columns in cats, ferrets or primates are elongated slab-like domains, while S1 barrels in rodents are more cylindrical. Columns are not even a sine qua non feature of the cortex, although their appearance may be associated with ethologically relevant functions. The somatosensory cortex of the mouse has a faithful representation of the snout in the column-like barrels that correspond to the whiskers, on which they rely strongly for survival. However, there is no evidence of feature-specific functional columns in the auditory (Bandyopadhyay et al., 2010; Rothschild et al., 2010) or visual cortex of rodents, where neurons with specific orientation tuning are dispersed in V1 in a salt-and-pepper manner (Ohki and Reid, 2007). By contrast, highly visual primates have well-defined orientation columns that are readily identified anatomically (Blasdel and Salama, 1986) and functionally (Bonhoeffer and Grinvald, 1991).
Despite their many variations and adaptations, columns are a common theme of complex cortical circuits. Yet this complex functional organization prompts the straightforward question: how does columnar circuitry arise during development? The six layers of the mammalian neocortex are generated sequentially by the dorsal telencephalon progenitors in an inside out manner. In the dorsal germinal zone, neuroepithelial cells primarily undergo symmetric divisions to expand the progenitor pool and they undergo asymmetric divisions to give rise to the first neurons, albeit to a lesser extent (Chenn and McConnell, 1995; Götz and Huttner, 2005). Subsequently, these cells transform into radial glial cells that fulfil a key developmental role by both replicating themselves and by directly generating excitatory neurons (Anthony et al., 2004; Malatesta et al., 2003). Calcium waves propagate through the radial glia as early as E12 and they are thought to regulate the production of neurons (Weissman et al., 2004). Radial glia divide at the surface of the ventricle and they have long processes that span the full thickness of the developing cortex, serving to guide the migration of newly born neurons (Rakic, 1972; 1971). This radial migration suggests that the initial synaptic organization of local cortical circuits is constructed by clonally related sister neurons that may represent the basis of the cortical column. Vertically organized clonally related sister neurons initially establish electrical connections, although these later disappear to preferentially form chemical synapses (He et al., 2015; Yu et al., 2009; 2012). However, it is not known how clonally related cells participate in the spontaneous activity evident during development or how this activity influences the formation of functional columns.
Sensory-driven mechanisms are involved in shaping cortical columns, as seen when the plasticity of ocular dominance columns in the cat visual cortex was studied after monocular deprivation during the critical period (Wiesel and Hubel, 1963). However, it was also seen that the columnar arrangement of the functional striatal cortex was already present in visually inexperienced kittens, suggesting that these columns are sculpted before critical periods through mechanisms that are independent of sensory experience (Hubel and Wiesel, 1963). Currently there is a large body of evidence, mostly generated in the visual and somatosensory systems, dictating that the basic columnar organization is established during experience-independent stages in cats and ferrets, and before birth in primates (Crair et al., 1998; Crowley and Katz, 2000; Rakic, 1976). In terms of the mechanisms that govern the early constitution of columnar circuits, spontaneous correlated activity is a solid candidate since its spatial and temporal structure may favor local connectivity. As such, there has been a tremendous drive to understand the role of spontaneous activity in sculpting cortical circuitry (Kanold and Luhmann, 2010; Molnár et al., 2020).
The development of calcium sensitive indicators prompted the study of spontaneous activity at the population level in the developing cerebral cortex. In coronal brain slices, calcium imaging experiments revealed the intermittent gap junction mediated activation of cortical cell ensembles, reminiscent of functional adult columns (Yuste et al., 1992). Tangential sections were also used to describe spontaneous activation of roughly circular domains with a diameter of 75 microns and similar results were drawn from extracellular recordings from intact preparations of the mouse neonatal cortex, where the activity of cortical neurons was synchronized within columns of 100-150 microns in diameter (Figure 5; (Dupont et al., 2006). This patchy pattern of early activation is preserved in vivo, although the diameter of the domains is larger. Electrophysiological recordings in vivo identified spontaneous local synchronized networks of 200-400 microns in the somatosensory cortex of neonatal rats (Yang et al., 2009). Indeed, local responses to single whisker deflection are already observed at P0 and these undergo progressive tuning, evidence of a segregated whisker map as early as P2-P3 (Mitrukhina et al., 2015). At the onset of active whisking (~P14), localized cortical responses to single whisker deflections will undergo further layer-specific and temporal refinement (van der Bourg et al., 2017; 2019; Yang et al., 2009). In vivo calcium imaging further confirms that the diameter of the domains reaches an average of ~300 microns in the presumptive sensory cortices of neonatal rats and mice, whether spontaneous or evoked (Kummer et al., 2016; Nakazawa et al., 2020). In the somatosensory system, these early patches are reminiscent of prospective barrels in shape (Yang et al., 2013), yet by the end of the first postnatal week barrels are clearly defined in rodents, and there is an almost perfect match between patches and barrels (Mizuno et al., 2018). The fact that this organization can be observed on the first postnatal day implies that a primordial columnar organization is established before birth, an organization that could serve as a scaffold for barrels. Indeed, restricted domains of cortical activation can be elicited by thalamic or peripheral stimulation in prenatal mice as early as E18 (Antón-Bolaños et al., 2019). Overall, these data suggest that the developing cortex first establishes templates of the characteristic functional architecture seen at mature stages.
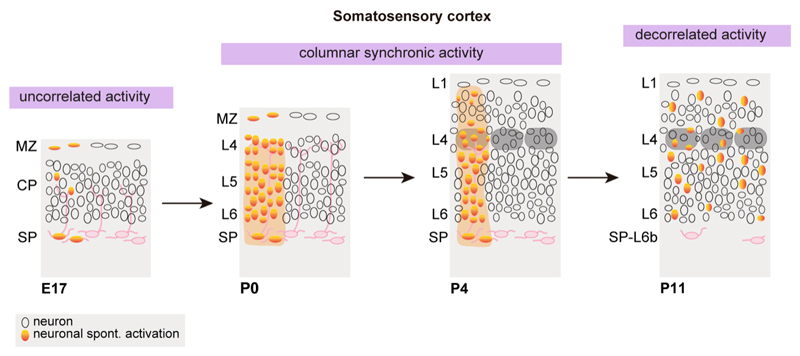
Figure 5. Developmental progression of cortical spontaneous activity.
At embryonic stages, cortical spontaneous activity is uncorrelated and restricted to single cells or small clusters. From birth, cortical spontaneous activity is detected in a columnar fashion that at P4 coincides with the dimension of a barrel. Active whisking is associated with the decorrelation and sparsification of cortical activity. E= embryonic age; P= postnatal age, MZ= marginal zone, CP= cortical plate, SP= subplate.
Correlated spontaneous activity in sensory cortices
In the last days of rodent embryonic life, developing cortical neurons start forming connections and they become progressively more excitable. These new properties induce a switch in the dynamics of spontaneous activity; from a scattered, uncorrelated activation to a more choral and correlated pattern of activity by P0 (Figure 5; (Corlew et al., 2004). As such, the currently accepted hypothesis postulates that correlated activity might strengthen coactive synaptic contacts to shape local circuits (Kerschensteiner, 2014; Winnubst et al., 2015).
Electrophysiological recordings have shown that the dominant pattern of spontaneous activity in the neonatal cortex in vivo involves spindle bursts and gamma oscillations. Spindle bursts are spatially confined rhythmic events oscillating at 5-25 Hz, with a peak frequency in the alpha band, that synchronize local neuronal networks. Spindle bursts last for approximately 1 second, they occur at a frequency of ~5 events per minute and they can be driven by spontaneous peripheral activity (An et al., 2014; Hanganu et al., 2006; Khazipov et al., 2004; Yang et al., 2009). By contrast, gamma oscillations are rapid events that last 0.15-0.3 seconds, with an average spectral frequency of 30-40 Hz, and they occur at a frequency of ~2-6 events per minute (Minlebaev et al., 2011; Yang et al., 2009). In terms of their spatiotemporal profiles, spindle bursts appear to be confined to a columnar network of 200-400 microns in diameter, while gamma oscillations synchronize neuronal activity in a similar column-like pattern of ~200 microns in diameter. Through calcium imaging in cortical slices, two different types of population activity have been described in newborn rats: cortical synchronous plateau assembles and cortical early network oscillations (Allene et al., 2008). However, there is no direct match between the in vitro results from population imaging and in vivo electrophysiological recordings, which differ in the mechanisms that trigger this activity and its spatiotemporal structure. In vivo calcium imaging studies revealed spontaneous events that recruit neuronal ensembles of low or high synchronicity in the superficial layers of V1 in P8-P10 mice (Siegel et al., 2012). While low-synchronicity events are mainly driven by the retina and their frequency increases during development, high-synchronicity events are independent of retinal input and their frequency decreases before eye-opening. We think that combining current technologies of wide-field imaging with high density electrical recordings in vivo will allow the temporal dynamics and spatial spreading of early cortical activity to be fully revealed, at the same time defining the relationship between intracellular calcium changes and electrical activity.
Most patterns of early spontaneous cortical activity rely on subcortical input. Hence, perturbing the ascending signals from the sense organ or thalamus produces abnormalities in cortical firing patterns, which causes defects in the basic organization of cortical networks. The subplate zone plays a key role in this process of primordial cortical organization, serving as an active hub that primes activity in the developing cortex (Yang et al., 2009; Meng et al., 2021). Subplate cells are involved in the generation of patterned cortical activity, such as spindle bursts, becoming a key element laying the foundations for a columnar organization (Tolner et al., 2012; Yang et al., 2009). As mentioned above, subplate cells also relay information from the thalamus, which first evokes large responses in the subplate that then propagate radially to the deep cortical layers and cortical plate (Higashi et al., 2002). This early radial response becomes more restricted as postnatal development proceeds, finally adopting the approximate size of a barrel at P4-P7 in the somatosensory system (Figure 4A; (Antón-Bolaños et al., 2019). Beyond this early columnar organization, the cortical activation driven by the thalamus and mediated by the subplate, exhibits a clear topographic organization that is first established in the embryo. Mesoscale calcium imaging of the cortex in vivo shows that the whisker-to-cortex pathway is not only functional but also spatially segregated at E18 (Antón-Bolaños et al., 2019). Similar topographic organization at the subplate level is found in the developing auditory system of young ferrets before the opening of the ears (Wess et al., 2017). Together, all these data suggest a role for correlated cortical activity in sculpting the connectivity and topography of cortical networks during development (Molnár et al., 2020).
Decorrelation of postnatal spontaneous activity in the cortex
After the discontinuous and correlated pattern observed in early postnatal life, spontaneous activity in the sensory cortices of rodents matures into a state characterized by sparseness and decorrelation (Figure 5; (Colonnese and Phillips, 2018). In mice, this transition occurs by the end of the second postnatal week when the ear canals and eyelids open, and active exploratory behavior begins to stimulate the sensorimotor systems. In the auditory cortex, pairwise correlations of spontaneous activity have been measured from P9, with a decorrelating trend starting before ear opening (Meng et al., 2020). In the barrel cortex, before the onset of active whisking and exploratory behavior, spontaneous activity in the upper layers switches from discontinuous and correlated firing to a continuous pattern of activity with no significant pairwise correlation (Golshani et al., 2009; Mizuno et al., 2018; Nakazawa et al., 2020). This decorrelation is independent of thalamic input and it is evident through the maturation of feedforward inhibition, the decline of transient circuits and the transition from electrical to chemical synaptic coupling between excitatory layer 4 neurons (Marques-Smith et al., 2016; Tuncdemir et al., 2016; Valiullina et al., 2016). Similar results were found in the visual cortex before eye opening, where a decorrelation of neuronal firing and slow oscillations overtake silent periods, increasing continuity (Colonnese et al., 2010; Rochefort et al., 2009; Shen and Colonnese, 2016). As in the barrel system, visual cortex activity also becomes less dependent on peripheral driving during the second postnatal week (Gribizis et al., 2019).
The transition from correlated to decorrelated activity in rodents is a condition for sparse coding, the strategy used for cortical processing in various modalities (Barth and Poulet, 2012; Olshausen and Field, 2004). Sparseness is associated with efficient information processing in mature circuits, although the developing visual and somatosensory pathways seem to be primed for sparse representation of sensory stimuli even before sensory experiences commence. In support of this notion, at the very instant of the onset of sensory experience in mice, when sensory receptors start transducing patterned and non-attenuated stimuli, somatosensory and visual cortical neurons can selectively respond to intrinsic features of sensory stimuli, such as the direction of whisker deflections (van der Bourg et al., 2017) or the orientation, direction and frequency of visual cues (Hagihara et al., 2015; Hoy and Niell, 2015). Subsequently, with somatosensory and visual sensory experience, there is a progressive increase in response selectivity, a decrease in the proportion of responsive neurons and a decorrelation of stimulus-driven activity (Ko et al., 2013; van der Bourg et al., 2017). In the auditory system, the developmental maturation of sparseness occurs at a slightly different pace. After ear opening (~P12), the auditory cortex becomes less selective for sound frequency and the proportion of responsive neurons increases. It also shows a consistent increase in pairwise correlations of both spontaneous and evoked activity (Meng et al., 2020). After P16, all these properties switch and sparseness emerges progressively, a process that has been associated with maturation of the excitatory/inhibitory balance (Liang et al., 2019).
What do we know about spontaneous activity in the developing human neocortex?
The developing human brain exhibits characteristic patterns of spontaneous and immature evoked activity that represent how nascent neural circuits gradually achieve the features of a mature network (André et al., 2010). Scalp recordings from human preterm infants used as a proxy for fetal life reveal a rich repertoire of spontaneous activity transients (SATs) during the third semester of gestation (Vanhatalo and Kaila, 2006). The properties of SATs resemble the correlated activity recorded from rodent models before sensory experience, that is, during the first two postnatal weeks.
SATs involve intermittent bouts of electrical activity in the form of bursts that are followed by long periods of silence. Bouts are characterized by a pattern of mixed slow and rapid oscillations, referred to as delta brushes. They are reminiscent of the mouse spindle bursts and like these, delta brushes can be evoked in different brain regions by external stimuli before sensory experience. Visual stimuli evoke responses in the developing occipital region (Colonnese et al., 2010), auditory stimuli in the mid-temporal region (Chipaux et al., 2013), and stroking the contralateral hand or foot in the mid-central or vertex electrodes, respectively (Milh et al., 2007). Delta brushes present a dominant rhythmic activity, oscillating at 8-25 Hz, and they are nested in slow delta waves of 0.3-1.5 Hz (Khazipov and Luhmann, 2006). In human fetuses, delta brushes appear at 28-30 weeks of gestation, they peak at 32-35 weeks and they then disappear at 38-42 weeks, a process that mirrors the developmental switch observed in rodents around the time of the onset of sensory experience (Whitehead et al., 2017).
The non-invasive assessment of cortical activity in humans provides valuable prognostic cues that predict to what extent a developing brain will grow normally or if it will be prone to neurodevelopmental malfunction (Iyer et al., 2015b). For example, enhanced spontaneous activity in preterm infants correlates positively with brain growth, suggesting that brains with weaker activity may not develop normally (Benders et al., 2015). Somatosensory evoked responses (SERs) triggered after tactile stimulus are also biomarkers of brain development. In preterm infants, both the slow wave and the higher-frequency components of SERs show developmental changes that may reflect shifts in subplate-cortex interactions (Leikos et al., 2020). Infants with intraventricular hemorrhaging initially display normal SERs, yet those with bilateral hemorrhages later show a developmental loss of the ipsilateral SER (Iyer et al., 2015a; Leikos et al., 2020). Likewise, abnormal responses in the secondary somatosensory cortex can predict abnormal neuromotor development in surviving, extremely low-gestational-age preterm infants (Rahkonen et al., 2013).
The unfolding of spontaneous activity is associated with important biological processes that are related to normal brain development. For instance, when the structural substrate of early spontaneous activity is analyzed, functional connectivity representing primary motor and sensory areas is already present at 31 weeks of gestation, increasing rapidly thereafter (Cao et al., 2017). Likewise, diffusion magnetic resonance imaging (MRI) indicated that a set of interconnected cortical hubs that are typical of the mature brain are already present at 30 weeks of gestation, some features of which are affected by premature birth (Ball et al., 2014; Turk et al., 2019). Combining the temporal sensitivity of electroencephalography with the whole brain spatial specificity of functional MRI, patterns of spontaneous delta brush activity have been associated with significant localized hemodynamic activity in distinct regions of the developing cortex before term birth. The predominant pattern of spontaneous activity in the late preterm period, i.e. posterior-temporal delta brushes, reflects activity in the insular cortices, an area that receives somatotopic afferent signals from all parts of the body and that fulfills essential homeostatic functions (Arichi et al., 2017). Therefore, to ensure normal brain development it is crucial to prevent distortions in the prenatal patterns of spontaneous activity, which could be caused by exposure to external agents like endocrine disrupting chemicals or drugs used in neonatal intensive care units (Malk et al., 2014; Nesan and Kurrasch, 2020).
In conjunction, all these data suggest a parallel developmental trajectory of brain activity between rodent models and humans. Clinical experience from human neonates supports the view that spontaneous activity during the third trimester of human development follows a pattern comparable to that found in basic animal research. Thus, in the future clinicians may benefit from animal research to improve the early diagnosis of developmental disorders and to refine the prediction of clinical outcomes in premature or neurologically impaired neonates.
Conclusions and future perspectives
The capacity of the developing brain for self-assembly resides in the intrinsic knowhow embodied in its building blocks: immature neurons and circuits. In order to proceed with the construction of the brain, these initially autonomous building blocks must communicate to spread the information and instructions as to how to assemble into larger functional structures. Information flows through at least two biological channels: molecular and electrical messages. In the former, communication between cells or groups of cells can be mediated by morphogenic substances, adhesion proteins or diffusible guidance cues. In addition, information can be transmitted using the incipient neuronal wiring that subsequently gives rise to a more responsive and specialized nervous system. Spontaneous but meaningful bioelectric signals that flow through the developing networks could take different, possibly related, forms, such as calcium waves, membrane potential oscillations and action potential bursts. In each developing brain structure, these bioelectrical signals could be triggered by inputs or generated intrinsically, and then conveyed to downstream stations.
Clear results have been obtained when assessing the molecular determinants of circuit assembly, not least due to the possibility of specifically manipulating genes to study the role of a single protein or morphogen in a given developmental process. However, assessing the roles of activity-dependent mechanisms at embryonic and early postnatal stages has proved challenging to neurodevelopmental scientists. Many factors may account for this, not least that clean experimental manipulations are compromised by the diverse sources of synchronic activity, and that sensory-independent and sensory-driven evoked activity concur. Also, comparative analyses among species do not often generate straightforward results, since many assumptions must be included to fit the different models. In other words, the relationship between patterns of activity and brain development can vary in different species, complicating efforts to build a general theory regarding the role of activity-dependent mechanism in brain development. Research in this field has followed four main avenues, namely: i) the identification of different electrical patterns of activity at embryonic and postnatal stages, and those associated with the onset of sensory experience, which has revealed homologies across species, including humans; ii) the identification of the cellular and molecular components that account for spatiotemporal features of these patterns of activity; iii) the identification of the brain circuits that act as temporary or stable sources of spontaneous activity; and iv) the discovery of causal links between different patterns of spontaneous activity and the developmental processes that shape brain circuits.
Some specific questions arise from the literature examined in this review. For example, how does spontaneous and evoked activity interact before the onset of sensory experience? How do peripheral and central patterns of spontaneous activity interact? Is there a hierarchy between them? In the specific case of thalamic waves, in vivo recording of embryonic thalamic waves would be helpful to discern if there is a dominant pattern in the thalamus of intact embryos. In this regard, more insights are needed to reveal the cellular and molecular mechanisms underlying early thalamic activity, either thalamic waves or other patterns of correlated activity. We know that these subcortical functional inputs, together with the subplate, establish the basis for cortical activity by shaping the columnar organization of cortical networks. However, the exact mechanisms by which spontaneous activity orchestrates cortical circuit assembly remain elusive. Another important line of research is to determine whether there is a link between synchronized activity and the clonal origin of cortical organization, since clonally related neurons are preferentially connected during development and they lay the foundations for cortical columns. Finally, spontaneous patterns of cortical activity during development exhibit remarkable homology across species and thus, translational research would benefit from the implementation of similar tools to study human brain development and for the early diagnosis of neurodevelopmental disorders.
In Brief.
In this review, Martini et al. summarize our current understanding of the role of spontaneous activity on the assembly of sensory networks, highlighting the participation of peripheral and central structures and tracing the similarities of developmental activity between experimental models and humans.
Acknowledgments
We thank Mark Sefton for linguistic correction of the manuscript, and the López-Bendito’s laboratory for stimulating discussions.
Funding
Supported by grants from the European Research Council (ERC-2014-CoG-647012) and the Spanish Ministry of Science, Innovation and Universities (PGC2018/096631-B-I00 and Severo Ochoa Grant SEV-2017-0723).
Footnotes
Declaration of Interests
The authors declare no competing interests
References
- Ackman JB, Burbridge TJ, Crair MC. Retinal waves coordinate patterned activity throughout the developing visual system. Nature. 2012;490:219–225. doi: 10.1038/nature11529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackman JB, Crair MC. Role of emergent neural activity in visual map development. Current Opinion in Neurobiology. 2014;24:166–175. doi: 10.1016/j.conb.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerman CJ, Smyth D, Thompson ID. Visual experience before eye-opening and the development of the retinogeniculate pathway. Neuron. 2002;36:869–879. doi: 10.1016/s0896-6273(02)01010-3. [DOI] [PubMed] [Google Scholar]
- Allene C, Cattani A, Ackman JB, Bonifazi P, Aniksztejn L, Ben-Ari Y, Cossart R. Sequential Generation of Two Distinct Synapse-Driven Network Patterns in Developing Neocortex. Journal of Neuroscience. 2008;28:12851–12863. doi: 10.1523/JNEUROSCI.3733-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An S, Kilb W, Luhmann HJ. Sensory-evoked and spontaneous gamma and spindle bursts in neonatal rat motor cortex. Journal of Neuroscience. 2014;34:10870–10883. doi: 10.1523/JNEUROSCI.4539-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André M, Lamblin MD, d’Allest AM, Curzi-Dascalova L, Moussalli-Salefranque F, Nguyen The Tich S, Vecchierini-Blineau MF, Wallois F, Walls-Esquivel E, Plouin P. Electroencephalography in premature and full-term infants. Developmental features and glossary. Neurophysiol Clin. 2010;40:59–124. doi: 10.1016/j.neucli.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Anthony TE, Klein C, Fishell G, Neuron NH. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron. 2004;41:881–890. doi: 10.1016/s0896-6273(04)00140-0. [DOI] [PubMed] [Google Scholar]
- Antón-Bolaños N, Espinosa A, López Bendito G. Developmental interactions between thalamus and cortex: a true love reciprocal story. Current Opinion in Neurobiology. 2018;52:33–41. doi: 10.1016/j.conb.2018.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antón-Bolaños N, Sempere-Ferràndez A, Guillamón-Vivancos T, Martini FJ, Pérez-Saiz L, Gezelius H, Filipchuk A, Valdeolmillos M, López Bendito G. Prenatal activity from thalamic neurons governs the emergence of functional cortical maps in mice. Science. 2019;364:987–990. doi: 10.1126/science.aav7617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arichi T, Whitehead K, Barone G, Pressler R, Padormo F, Edwards AD, Fabrizi L. Localization of spontaneous bursting neuronal activity in the preterm human brain with simultaneous EEG-fMRI. Elife. 2017;6:229. doi: 10.7554/eLife.27814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo DA, Feller MB. Spatiotemporal Features of Retinal Waves Instruct the Wiring of the Visual Circuitry. Front Neural Circuits. 2016;10:54. doi: 10.3389/fncir.2016.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babola TA, Li S, Gribizis A, Lee BJ, Issa JB, Wang HC, Crair MC, Bergles DE. Homeostatic Control of Spontaneous Activity in the Developing Auditory System. Neuron. 2018;99:511–524.:e5. doi: 10.1016/j.neuron.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babola TA, Li S, Wang Z, Kersbergen CJ, Elgoyhen AB, Coate T, Bergles DE. Purinergic signaling controls spontaneous activity in the auditory system throughout early development. bioRxiv. 2020a doi: 10.1101/2020.08.11.246306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babola TA, Li S, Wang Z, Kersbergen CJ, Elgoyhen AB, Coate TM, Bergles DE. Purinergic signaling controls spontaneous activity in the auditory system throughout early development. J Neurosci. 2020b doi: 10.1523/JNEUROSCI.2178-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball G, Aljabar P, Zebari S, Tusor N, Arichi T, Merchant N, Robinson EC, Ogundipe E, Rueckert D, Edwards AD, Counsell SJ. Rich-club organization of the newborn human brain. Proc Natl Acad Sci USA. 2014;111:7456–7461. doi: 10.1073/pnas.1324118111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal A, Singer JH, Hwang BJ, Xu W, Beaudet A, Feller MB. Mice lacking specific nicotinic acetylcholine receptor subunits exhibit dramatically altered spontaneous activity patterns and reveal a limited role for retinal waves in forming ON and OFF circuits in the inner retina. J Neurosci. 20:7672–7681. doi: 10.1523/JNEUROSCI.20-20-07672.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth AL, Poulet JFA. Experimental evidence for sparse firing in the neocortex. Trends in Neurosciences. 2012;35:345–355. doi: 10.1016/j.tins.2012.03.008. [DOI] [PubMed] [Google Scholar]
- Barkat TR, Polley DB, Hensch TK. A critical period for auditory thalamocortical connectivity. Nat Neurosci. 2011;14:1189–1194. doi: 10.1038/nn.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benders MJ, Palmu K, Menache C, Borradori-Tolsa C, Lazeyras F, Sizonenko S, Dubois J, Vanhatalo S, Hüppi PS. Early Brain Activity Relates to Subsequent Brain Growth in Premature Infants. Cerebral Cortex. 2015;25:3014–3024. doi: 10.1093/cercor/bhu097. [DOI] [PubMed] [Google Scholar]
- Blankenship AG, Feller MB. Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat Rev Neurosci. 2010;11:18–29. doi: 10.1038/nrn2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship AG, Ford KJ, Johnson J, Seal RP, Edwards RH, Copenhagen DR, Feller MB. Synaptic and Extrasynaptic Factors Governing Glutamatergic Retinal Waves. Neuron. 2009;62:230–241. doi: 10.1016/j.neuron.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanquie O, Yang J-W, Kilb W, Sharopov S, Sinning A, Luhmann HJ. Electrical activity controls area-specific expression of neuronal apoptosis in the mouse developing cerebral cortex. Elife. 2017;6 doi: 10.7554/eLife.27696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasdel GG, Salama G. Voltage-sensitive dyes reveal a modular organization in monkey striate cortex. Nature. 1986;321:579–585. doi: 10.1038/321579a0. [DOI] [PubMed] [Google Scholar]
- Blumberg MS, Coleman CM, Gerth AI, McMurray B. Spatiotemporal structure of REM sleep twitching reveals developmental origins of motor synergies. Curr Biol. 2013;23:2100–2109. doi: 10.1016/j.cub.2013.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS, Dooley JC. Phantom Limbs, Neuroprosthetics, and the Developmental Origins of Embodiment. Trends in Neurosciences. 2017;40:603–612. doi: 10.1016/j.tins.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonhoeffer T, Grinvald A. Iso-orientation domains in cat visual cortex are arranged in pinwheel-like patterns. Nature. 1991;353:429–431. doi: 10.1038/353429a0. [DOI] [PubMed] [Google Scholar]
- Burbridge TJ, Xu H-P, Ackman JB, Ge X, Zhang Y, Ye M-J, Zhou ZJ, Xu J, Contractor A, Crair MC. Visual circuit development requires patterned activity mediated by retinal acetylcholine receptors. Neuron. 2014;84:1049–1064. doi: 10.1016/j.neuron.2014.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang J, Feldheim DA. Developmental mechanisms of topographic map formation and alignment. Annu Rev Neurosci. 2013;36:51–77. doi: 10.1146/annurev-neuro-062012-170341. [DOI] [PubMed] [Google Scholar]
- Cao M, He Y, Dai Z, Liao X, Jeon T, Ouyang M, Chalak L, Bi Y, Rollins N, Dong Q, Huang H. Early Development of Functional Network Segregation Revealed by Connectomic Analysis of the Preterm Human Brain. Cereb Cortex. 2017;27:1949–1963. doi: 10.1093/cercor/bhw038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Paterna M, Moreno-Juan V, Filipchuk A, Rodriguez Malmierca L, Susín R, López Bendito G. DCC functions as an accelerator of thalamocortical axonal growth downstream of spontaneous thalamic activity. EMBO Rep. 2015;16:851–862. doi: 10.15252/embr.201439882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catsicas M, Bonness V, Becker D, Mobbs P. Spontaneous Ca2+ transients and their transmission in the developing chick retina. Current Biology. 1998;8:283–288. doi: 10.1016/s0960-9822(98)70110-1. [DOI] [PubMed] [Google Scholar]
- Chenn A, McConnell SK. Cleavage orientation and the asymmetric inheritance of Notch1 immunoreactivity in mammalian neurogenesis. Cell. 1995;82:631–641. doi: 10.1016/0092-8674(95)90035-7. [DOI] [PubMed] [Google Scholar]
- Chipaux M, Colonnese MT, Mauguen A, Fellous L, Mokhtari M, Lezcano O, Milh M, Dulac O, Chiron C, Khazipov R, Kaminska A. Auditory stimuli mimicking ambient sounds drive temporal “delta-brushes” in premature infants. PLoS ONE. 2013;8:e79028. doi: 10.1371/journal.pone.0079028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coate TM, Scott MK, Gurjar M. Current concepts in cochlear ribbon synapse formation. Synapse. 2019;73:e22087. doi: 10.1002/syn.22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonnese MT. Rapid developmental emergence of stable depolarization during wakefulness by inhibitory balancing of cortical network excitability. Journal of Neuroscience. 2014;34:5477–5485. doi: 10.1523/JNEUROSCI.3659-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonnese MT, Kaminska A, Minlebaev M, Milh M, Bloem B, Lescure S, Moriette G, Chiron C, Ben-Ari Y, Khazipov R. A conserved switch in sensory processing prepares developing neocortex for vision. Neuron. 2010;67:480–498. doi: 10.1016/j.neuron.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonnese MT, Khazipov R. “Slow activity transients” in infant rat visual cortex: a spreading synchronous oscillation patterned by retinal waves. Journal of Neuroscience. 2010;30:4325–4337. doi: 10.1523/JNEUROSCI.4995-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonnese MT, Phillips MA. Thalamocortical function in developing sensory circuits. Current Opinion in Neurobiology. 2018;52:72–79. doi: 10.1016/j.conb.2018.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlew R, Bosma MM, Moody WJ. Spontaneous, synchronous electrical activity in neonatal mouse cortical neurones. The Journal of Physiology. 2004;560:377–390. doi: 10.1113/jphysiol.2004.071621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crair MC, Gillespie DC, Stryker MP. The role of visual experience in the development of columns in cat visual cortex. Science. 1998;279:566–570. doi: 10.1126/science.279.5350.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley JC, Katz LC. Early development of ocular dominance columns. Science. 2000;290:1321–1324. doi: 10.1126/science.290.5495.1321. [DOI] [PubMed] [Google Scholar]
- Dayaratne MWN, Vlajkovic SM, Lipski J, Thorne PR. Kölliker’s organ and the development of spontaneous activity in the auditory system: Implications for hearing dysfunction. Biomed Res Int. 2014:367939. doi: 10.1155/2014/367939. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J, Markram H, Rockland KS. The neocortical column. Front Neuroanat. 2012;6:22. doi: 10.3389/fnana.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demas J, Eglen SJ, Wong ROL. Developmental loss of synchronous spontaneous activity in the mouse retina is independent of visual experience. J Neurosci. 2003;23:2851–2860. doi: 10.1523/jneurosci.23-07-02851.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz B, Jovanovic S, Wielsch B, Nerlich J, Rübsamen R, Milenkovic I. Purinergic modulation of neuronal activity in developing auditory brainstem. J Neurosci. 2012;32:10699–10712. doi: 10.1523/JNEUROSCI.0372-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley JC, Glanz RM, Sokoloff G, Blumberg MS. Self-Generated Whisker Movements Drive State-Dependent Sensory Input to Developing Barrel Cortex. Current Biology. 2020;30:1–18. doi: 10.1016/j.cub.2020.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley JC, Sokoloff G, Blumberg MS. Behavioral states modulate sensory processing in early development. Curr Sleep Medicine Rep. 2019;5:112–117. doi: 10.1007/s40675-019-00144-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont E, Hanganu IL, Kilb W, Hirsch S, Luhmann HJ. Rapid developmental switch in the mechanisms driving early cortical columnar networks. Nature. 2006;439:79–83. doi: 10.1038/nature04264. [DOI] [PubMed] [Google Scholar]
- Eckrich T, Blum K, Milenkovic I, Engel J. Fast Ca2+ Transients of Inner Hair Cells Arise Coupled and Uncoupled to Ca2+ Waves of Inner Supporting Cells in the Developing Mouse Cochlea. Front Mol Neurosci. 2018;11:264. doi: 10.3389/fnmol.2018.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagard J, Esseily R, Jacquey L, O’Regan K, Somogyi E. Fetal Origin of Sensorimotor Behavior. Front Neurorobot. 2018;12:23. doi: 10.3389/fnbot.2018.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford KJ, Félix AL, Feller MB. Cellular mechanisms underlying spatiotemporal features of cholinergic retinal waves. J Neurosci. 2012;32:850–863. doi: 10.1523/JNEUROSCI.5309-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friauf E, McConnell SK, Shatz CJ. Functional synaptic circuits in the subplate during fetal and early postnatal development of cat visual cortex. Journal of Neuroscience. 1990;10:2601–2613. doi: 10.1523/JNEUROSCI.10-08-02601.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton JF. Physiology of the nervous system. London: Oxford University Press; 1938. [Google Scholar]
- Galli L, Maffei L. Spontaneous impulse activity of rat retinal ganglion cells in prenatal life. Science. 1988;242:90–91. doi: 10.1126/science.3175637. [DOI] [PubMed] [Google Scholar]
- Garel S, López Bendito G. Inputs from the thalamocortical system on axon pathfinding mechanisms. Current Opinion in Neurobiology. 2014;27:143–150. doi: 10.1016/j.conb.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Geal-Dor M, Freeman S, Li G, Sohmer H. Development of hearing in neonatal rats: Air and bone conducted ABR thresholds. Hear Res. 69:236–242. doi: 10.1016/0378-5955(93)90113-F. [DOI] [PubMed] [Google Scholar]
- Gezelius H, López Bendito G. Thalamic neuronal specification and early circuit formation. Devel Neurobio. 2017;77:830–843. doi: 10.1002/dneu.22460. [DOI] [PubMed] [Google Scholar]
- Glowatzki E, Fuchs PA. Transmitter release at the hair cell ribbon synapse. Nat Neurosci. 2002;5:147–154. doi: 10.1038/nn796. [DOI] [PubMed] [Google Scholar]
- Glowatzki E, Fuchs PA. Cholinergic synaptic inhibition of inner hair cells in the neonatal mammalian cochlea. Science. 2000;288:2366–2368. doi: 10.1126/science.288.5475.2366. [DOI] [PubMed] [Google Scholar]
- Golshani P, Goncalves JT, Khoshkhoo S, Mostany R, Smirnakis S, Portera-Cailliau C. Internally Mediated Developmental Desynchronization of Neocortical Network Activity. J Neurosci. 2009;29:10890–10899. doi: 10.1523/JNEUROSCI.2012-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goutman JD, Fuchs PA, Glowatzki E. Facilitating efferent inhibition of inner hair cells in the cochlea of the neonatal rat. J Physiol (Lond) 2005;566:49–59. doi: 10.1113/jphysiol.2005.087460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- Gribizis A, Ge X, Daigle TL, Ackman JB, Zeng H, Lee D, Crair MC. Visual Cortex Gains Independence from Peripheral Drive before Eye Opening. Neuron. 2019 doi: 10.1016/j.neuron.2019.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagihara KM, Murakami T, Yoshida T, Tagawa Y, Ohki K. Neuronal activity is not required for the initial formation and maturation of visual selectivity. Nat Neurosci. 2015;18:1780–1788. doi: 10.1038/nn.4155. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Sherman SM. Thalamocortical Circuit Motifs: A General Framework. Neuron. 2019;103:762–770. doi: 10.1016/j.neuron.2019.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V, Narayanan CH. Effects of the deafferentation of the trigeminal area on the motility of the chick embryo. J Exp Zool. 1969;170:411–426. doi: 10.1002/jez.1401700404. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Wenger E, Oppenheim R. Motility in the chick embryo in the absence of sensory input. Journal of Experimental Zoology. 1966;162:133–159. doi: 10.1002/jez.1401620202. [DOI] [Google Scholar]
- Hanganu IL, Ben-Ari Y, Khazipov R. Retinal waves trigger spindle bursts in the neonatal rat visual cortex. Journal of Neuroscience. 2006;26:6728–6736. doi: 10.1523/JNEUROSCI.0752-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanganu IL, Kilb W, Luhmann HJ. Functional synaptic projections onto subplate neurons in neonatal rat somatosensory cortex. J Neurosci. 2002;22:7165–7176. doi: 10.1523/JNEUROSCI.22-16-07165.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MG, Landmesser LT. Normal patterns of spontaneous activity are required for correct motor axon guidance and the expression of specific guidance molecules. Neuron. 2004;43:687–701. doi: 10.1016/j.neuron.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Harrus A-G, Ceccato J-C, Sendin G, Bourien J, Puel J-L, Nouvian R. Spiking Pattern of the Mouse Developing Inner Hair Cells Is Mostly Invariant Along the Tonotopic Axis. Front Cell Neurosci. 2018;12:407. doi: 10.3389/fncel.2018.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Li Z, Ge S, Yu Y-C, Shi S-H. Inside-Out Radial Migration Facilitates Lineage-Dependent Neocortical Microcircuit Assembly. Neuron. 2015;86:1159–1166. doi: 10.1016/j.neuron.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann K, Antonini A, Shatz CJ. Ultrastructural Evidence for Synaptic Interactions between Thalamocortical Axons and Subplate Neurons. European Journal of Neuroscience. 1994;6:1729–1742. doi: 10.1111/j.1460-9568.1994.tb00565.x. [DOI] [PubMed] [Google Scholar]
- Herrmann K, Shatz CJ. Blockade of action potential activity alters initial arborization of thalamic axons within cortical layer 4. Proc Natl Acad Sci USA. 1995;92:11244–11248. doi: 10.1073/pnas.92.24.11244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi S, Molnár Z, Kurotani T, Toyama K. Prenatal development of neural excitation in rat thalamocortical projections studied by optical recording. Neuroscience. 2002;115:1231–1246. doi: 10.1016/S0306-4522(02)00418-9. [DOI] [PubMed] [Google Scholar]
- Hoy JL, Niell CM. Layer-Specific Refinement of Visual Cortex Function after Eye Opening in the Awake Mouse. Journal of Neuroscience. 2015;35:3370–3383. doi: 10.1523/JNEUROSCI.3174-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields of cells in striate cortex of very young, visually inexperienced kittens. Journal of Neurophysiology. 1963;26:994–1002. doi: 10.1152/jn.1963.26.6.994. [DOI] [PubMed] [Google Scholar]
- Inacio AR, Nasretdinov A, Lebedeva J, Khazipov R. Sensory feedback synchronizes motor and sensory neuronal networks in the neonatal rat spinal cord. Nat Commun. 2016;7:13060. doi: 10.1038/ncomms13060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasato T, Erzurumlu RS. Development of tactile sensory circuits in the CNS. Current Opinion in Neurobiology. 2018;53:66–75. doi: 10.1016/j.conb.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer KK, Roberts JA, Hellström-Westas L, Wikström S, Hansen Pupp I, Ley D, Breakspear M, Vanhatalo S. Early Detection of Preterm Intraventricular Hemorrhage From Clinical Electroencephalography. Crit Care Med. 2015a;43:2219–2227. doi: 10.1097/CCM.0000000000001190. [DOI] [PubMed] [Google Scholar]
- Iyer KK, Roberts JA, Hellström-Westas L, Wikström S, Hansen Pupp I, Ley D, Vanhatalo S, Breakspear M. Cortical burst dynamics predict clinical outcome early in extremely preterm infants. Brain. 2015b;138:2206–2218. doi: 10.1093/brain/awv129. [DOI] [PubMed] [Google Scholar]
- Izraeli R, Koay G, Lamish M, Heicklen-Klein AJ, Heffner HE, Heffner RS, Wollberg Z. Cross-modal neuroplasticity in neonatally enucleated hamsters: structure, electrophysiology and behaviour. Eur J Neurosci. 2002;15:693–712. doi: 10.1046/j.1460-9568.2002.01902.x. [DOI] [PubMed] [Google Scholar]
- Jabaudon D. Fate and freedom in developing neocortical circuits. Nat Commun. 2017;8:16042. doi: 10.1038/ncomms16042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Eckrich T, Kuhn S, Zampini V, Franz C, Ranatunga KM, Roberts TP, Masetto S, Knipper M, Kros CJ, Marcotti W. Position-dependent patterning of spontaneous action potentials in immature cochlear inner hair cells. Nat Neurosci. 2011;14:711–717. doi: 10.1038/nn.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanold PO, Luhmann HJ. The subplate and early cortical circuits. Annu Rev Neurosci. 2010;33:23–48. doi: 10.1146/annurev-neuro-060909-153244. [DOI] [PubMed] [Google Scholar]
- Karlsson KAE, Gall AJ, Mohns EJ, Seelke AMH, Blumberg MS. The neural substrates of infant sleep in rats. Plos Biol. 2005;3:e143. doi: 10.1371/journal.pbio.0030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kähne M, Rüdiger S, Kihara AH, Lindner B. Gap junctions set the speed and nucleation rate of stage I retinal waves. PLoS Comput Biol. 2019;15:e1006355. doi: 10.1371/journal.pcbi.1006355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy HJ. New developments in understanding the mechanisms and function of spontaneous electrical activity in the developing mammalian auditory system. J Assoc Res Otolaryngol. 2012;13:437–445. doi: 10.1007/s10162-012-0325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschensteiner D. Spontaneous Network Activity and Synaptic Development. The Neuroscientist. 2014;20:272–290. doi: 10.1177/1073858413510044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazipov R, Luhmann HJ. Early patterns of electrical activity in the developing cerebral cortex of humans and rodents. Trends in Neurosciences. 2006;29:414–418. doi: 10.1016/j.tins.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Minlebaev M, Valeeva G. Early gamma oscillations. Neuroscience. 2013;250:240–252. doi: 10.1016/j.neuroscience.2013.07.019. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Sirota A, Leinekugel X, Holmes GL, Ben-Ari Y, Buzsáki G. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature. 2004;432:758–761. doi: 10.1038/nature03132. [DOI] [PubMed] [Google Scholar]
- Kilb W, Kirischuk S, Luhmann HJ. Electrical activity patterns and the functional maturation of the neocortex. European Journal of Neuroscience. 2011;34:1677–1686. doi: 10.1111/j.1460-9568.2011.07878.x. [DOI] [PubMed] [Google Scholar]
- Ko H, Cossell L, Baragli C, Antolik J, Clopath C, Hofer SB, Mrsic-Flogel TD. The emergence of functional microcircuits in visual cortex. Nature. 2013;496:96–100. doi: 10.1038/nature12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreider JC, Blumberg MS. Mesopontine contribution to the expression of active “twitch” sleep in decerebrate week-old rats. Brain Research. 2000;872:149–159. doi: 10.1016/s0006-8993(00)02518-x. [DOI] [PubMed] [Google Scholar]
- Kros CJ, Ruppersberg JP, Rüsch A. Expression of a potassium current in inner hair cells during development of hearing in mice. Nature. 1998;394:281–284. doi: 10.1038/28401. [DOI] [PubMed] [Google Scholar]
- Kummer M, Kirmse K, Zhang C, Haueisen J, Witte OW, Holthoff K. Column-like Ca(2+) clusters in the mouse neonatal neocortex revealed by threedimensional two-photon Ca(2+) imaging in vivo. Neuroimage. 2016;138:64–75. doi: 10.1016/j.neuroimage.2016.05.050. [DOI] [PubMed] [Google Scholar]
- Leighton AH, Lohmann C. The Wiring of Developing Sensory Circuits-From Patterned Spontaneous Activity to Synaptic Plasticity Mechanisms. Front Neural Circuits. 2016;10:71. doi: 10.3389/fncir.2016.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leikos S, Tokariev A, Koolen N, Nevalainen P, Vanhatalo S. Cortical responses to tactile stimuli in preterm infants. Eur J Neurosci. 2020;51:1059–1073. doi: 10.1111/ejn.14613. [DOI] [PubMed] [Google Scholar]
- Leyva-Diáz E, del Toro D, Menal MJ, Cambray S, Susín R, Tessier-Lavigne M, Klein R, Egea J, López Bendito G. FLRT3 is a Robo1-interacting protein that determines Netrin-1 attraction in developing axons. Curr Biol. 2014;24:494–508. doi: 10.1016/j.cub.2014.01.042. [DOI] [PubMed] [Google Scholar]
- Liang F, Li H, Chou X-L, Zhou M, Zhang NK, Xiao Z, Zhang KK, Tao HW, Zhang LI. Sparse representation in awake auditory cortex: Cell-type dependence, synaptic mechanisms, developmental emergence, and modulation. Cerebral Cortex. 2019;29:3796–3812. doi: 10.1093/cercor/bhy260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo F-S, Ziburkus J, Guido W. Synaptic mechanisms regulating the activation of a Ca(2+)-mediated plateau potential in developing relay cells of the LGN. Journal of Neurophysiology. 2002;87:1175–1185. doi: 10.1152/jn.00715.1999. [DOI] [PubMed] [Google Scholar]
- López Bendito G. Development of the Thalamocortical Interactions: Past, Present and Future. Neuroscience. 2018;385:67–74. doi: 10.1016/j.neuroscience.2018.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccione A, Hennig MH, Gandolfo M, Muthmann O, van Coppenhagen J, Eglen SJ, Berdondini L, Sernagor E. Following the ontogeny of retinal waves: pan-retinal recordings of population dynamics in the neonatal mouse. The Journal of Physiology. 2014;592:1545–1563. doi: 10.1113/jphysiol.2013.262840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod N, Turner C, Edgar J. Properties of developing lateral geniculate neurones in the mouse. Int J Dev Neurosci. 1997;15:205–224. doi: 10.1016/s0736-5748(96)00088-3. [DOI] [PubMed] [Google Scholar]
- Makarov R, Sintsov M, Valeeva G, Starikov P, Negrov D, Khazipov R. Bone-conducted Responses in the Neonatal rat Auditory Cortex. 2021 doi: 10.21203/rs.3.rs-200367/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malatesta P, Hack MA, Hartfuss E, Kettenmann H, Klinkert W, Kirchhoff F, Götz M. Neuronal or glial progeny: regional differences in radial glia fate. Neuron. 2003;37:751–764. doi: 10.1016/s0896-6273(03)00116-8. [DOI] [PubMed] [Google Scholar]
- Malk K, Metsäranta M, Vanhatalo S. Drug effects on endogenous brain activity in preterm babies. Brain Dev. 2014;36:116–123. doi: 10.1016/j.braindev.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Marcotti W, Johnson SL, Kros CJ. A transiently expressed SK current sustains and modulates action potential activity in immature mouse inner hair cells. J Physiol (Lond) 2004;560:691–708. doi: 10.1113/jphysiol.2004.072868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques-Smith A, Lyngholm D, Kaufmann A-K, Stacey JA, Hoerder-Suabedissen A, Becker EBE, Wilson MC, Molnár Z, Butt SJB. A Transient Translaminar GABAergic Interneuron Circuit Connects Thalamocortical Recipient Layers in Neonatal Somatosensory Cortex. Neuron. 2016;89:536–549. doi: 10.1016/j.neuron.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini FJ, Moreno-Juan V, Filipchuk A, Valdeolmillos M, López Bendito G. Impact of thalamocortical input on barrel cortex development. Neuroscience. 2018;368:246–255. doi: 10.1016/j.neuroscience.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masland RH. Maturation of function in the developing rabbit retina. J Comp Neurol. 1977;175:275–286. doi: 10.1002/cne.901750303. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Torborg CL, Feller MB, O’Leary DDM. Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron. 2003;40:1147–1160. doi: 10.1523/JNEUROSCI.1527-04.2004. [DOI] [PubMed] [Google Scholar]
- McVea DA, Mohajerani MH, Murphy TH. Voltage-sensitive dye imaging reveals dynamic spatiotemporal properties of cortical activity after spontaneous muscle twitches in the newborn rat. Journal of Neuroscience. 2012;32:10982–10994. doi: 10.1523/JNEUROSCI.1322-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister M, Wong RO, Baylor DA, Shatz CJ. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science. 1991;252:939–943. doi: 10.1126/science.2035024. [DOI] [PubMed] [Google Scholar]
- Meng X, Solarana K, Bowen Z, Liu J, Nagode DA, Sheikh A, Winkowski DE, Kao JPY, Kanold PO. Transient Subgranular Hyperconnectivity to L2/3 and Enhanced Pairwise Correlations during the Critical Period in the Mouse Auditory Cortex. Cerebral Cortex. 2020;30:1914–1930. doi: 10.1093/cercor/bhz213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Mukherjee D, Kao JPY, Kanold PO. Early peripheral activity alters nascent subplate circuits in the auditory cortex. Science advances. 2021;7:eabc9155. doi: 10.1126/sciadv.abc9155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milh M, Kaminska A, Huon C, Lapillonne A, Ben-Ari Y, Khazipov R. Rapid cortical oscillations and early motor activity in premature human neonate. Cerebral Cortex. 2007;17:1582–1594. doi: 10.1093/cercor/bhl069. [DOI] [PubMed] [Google Scholar]
- Minlebaev M, Colonnese M, Tsintsadze T, Sirota A, Khazipov R. Early γ oscillations synchronize developing thalamus and cortex. Science. 2011;334:226–229. doi: 10.1126/science.1210574. [DOI] [PubMed] [Google Scholar]
- Mire E, Mezzera C, Leyva-Diáz E, Paternain AV, Squarzoni P, Bluy L, Castillo-Paterna M, López MJ, Peregrin S, Tessier-Lavigne M, Garel S, et al. Spontaneous activity regulates Robo1 transcription to mediate a switch in thalamocortical axon growth. Nat Neurosci. 2012;15:1134–1143. doi: 10.1038/nn.3160. [DOI] [PubMed] [Google Scholar]
- Mitrukhina O, Suchkov D, Khazipov R, Minlebaev M. Imprecise Whisker Map in the Neonatal Rat Barrel Cortex. Cerebral Cortex. 2015;25:3458–3467. doi: 10.1093/cercor/bhu169. [DOI] [PubMed] [Google Scholar]
- Mizuno H, Ikezoe K, Nakazawa S, Sato T, Kitamura K, Iwasato T. Patchwork-Type Spontaneous Activity in Neonatal Barrel Cortex Layer 4 Transmitted via Thalamocortical Projections. Cell Reports. 2018;22:123–135. doi: 10.1016/j.celrep.2017.12.012. [DOI] [PubMed] [Google Scholar]
- Molnár Z, Luhmann HJ, Kanold PO. Transient cortical circuits match spontaneous and sensory-driven activity during development. Science. 2020;370:eabb2153. doi: 10.1126/science.abb2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney R, Penn AA, Gallego R, Shatz CJ. Thalamic relay of spontaneous retinal activity prior to vision. Neuron. 1996;17:863–874. doi: 10.1016/s0896-6273(00)80218-4. [DOI] [PubMed] [Google Scholar]
- Moreno-Juan V, Filipchuk A, Antón-Bolaños N, Mezzera C, Gezelius H, Andrés B, Rodriguez Malmierca L, Susín R, Schaad O, Iwasato T, Schüle R, et al. Prenatal thalamic waves regulate cortical area size prior to sensory processing. Nat Commun. 2017;8:14172–14. doi: 10.1038/ncomms14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle VB. Introduction. Computation in cortical columns. Cereb Cortex. 2003;13:2–4. doi: 10.1093/cercor/13.1.2. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB. The columnar organization of the neocortex. Brain. 1997;120(Pt 4):701–722. doi: 10.1093/brain/120.4.701. [DOI] [PubMed] [Google Scholar]
- Murata Y, Colonnese MT. Thalamus controls development and expression of arousal states in visual cortex. Journal of Neuroscience. 2018;38:8772–8786. doi: 10.1523/JNEUROSCI.1519-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Colonnese MT. An excitatory cortical feedback loop gates retinal wave transmission in rodent thalamus. Elife. 2016;5 doi: 10.7554/eLife.18816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y, Shimogori T. Diversity of thalamic progenitor cells and postmitotic neurons. Eur J Neurosci. 2012;35:1554–1562. doi: 10.1111/j.1460-9568.2012.08089.x. [DOI] [PubMed] [Google Scholar]
- Nakazawa S, Yoshimura Y, Takagi M, Mizuno H, Iwasato T. Developmental Phase Transitions in Spatial Organization of Spontaneous Activity in Postnatal Barrel Cortex Layer 4. J Neurosci. 2020 doi: 10.1523/JNEUROSCI.1116-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesan D, Kurrasch DM. Gestational Exposure to Common Endocrine Disrupting Chemicals and Their Impact on Neurodevelopment and Behavior. Annu Rev Physiol. 2020;82:177–202. doi: 10.1146/annurev-physiol-021119-034555. [DOI] [PubMed] [Google Scholar]
- Ohki K, Reid RC. Specificity and randomness in the visual cortex. Current Opinion in Neurobiology. 2007;17:401–407. doi: 10.1016/j.conb.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshausen BA, Field DJ. Sparse coding of sensory inputs. Current Opinion in Neurobiology. 2004;14:481–487. doi: 10.1016/j.conb.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Preyer WT. Embryonic Motility and Sensitivity. 1937 doi: 10.2307/1165413. [DOI] [Google Scholar]
- Provine RR, Sharma SC, Sandel TT, Hamburger V. Electrical activity in the spinal cord of the chick embryo, in situ. Proc Natl Acad Sci USA. 1970;65:508–515. doi: 10.1073/pnas.65.3.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahkonen P, Nevalainen P, Lauronen L, Pihko E, Lano A, Vanhatalo S, Pesonen A-K, Heinonen K, Räikkönen K, Valanne L, Autti T, et al. Cortical somatosensory processing measured by magnetoencephalography predicts neurodevelopment in extremely low-gestational-age infants. Pediatr Res. 2013;73:763–771. doi: 10.1038/pr.2013.46. [DOI] [PubMed] [Google Scholar]
- Rakic P. A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends in Neurosciences. 1995;18:383–388. doi: 10.1016/0166-2236(95)93934-p. [DOI] [PubMed] [Google Scholar]
- Rakic P. Prenatal genesis of connections subserving ocular dominance in the rhesus monkey. Nature. 1976;261:467–471. doi: 10.1038/261467a0. [DOI] [PubMed] [Google Scholar]
- Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 1972;145:61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- Rakic P. Guidance of neurons migrating to the fetal monkey neocortex. Brain Research. 1971;33:471–476. doi: 10.1016/0006-8993(71)90119-3. [DOI] [PubMed] [Google Scholar]
- Renna JM, Weng S, Berson DM. Light acts through melanopsin to alter retinal waves and segregation of retinogeniculate afferents. Nat Neurosci. 2011;14:827–829. doi: 10.1038/nn.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikhye RV, Wimmer RD, Halassa MM. Toward an Integrative Theory of Thalamic Function. Annu Rev Neurosci. 2018;41:163–183. doi: 10.1146/annurev-neuro-080317-062144. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Blumberg MS, Lane MS, Kreber LS. Spontaneous motor activity in fetal and infant rats is organized into discrete multilimb bouts. Behavioral Neuroscience. 2000;114:328–336. doi: 10.1037//0735-7044.114.2.328. [DOI] [PubMed] [Google Scholar]
- Rochefort NL, Garaschuk O, Milos R-I, Narushima M, Marandi N, Pichler B, Kovalchuk Y, Konnerth A. Sparsification of neuronal activity in the visual cortex at eye-opening. Proceedings of the National Academy of Sciences. 2009;106:15049–15054. doi: 10.1073/pnas.0907660106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochefort NL, Narushima M, Grienberger C, Marandi N, Hill DN, Konnerth A. Development of Direction Selectivity in Mouse Cortical Neurons. Neuron. 2011;71:425–432. doi: 10.1016/j.neuron.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Rockland KS. Five points on columns. Front Neuroanat. 2010;4:22. doi: 10.3389/fnana.2010.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SS, Spitzer NC. Calcium signaling in neuronal development. Cold Spring Harbor Perspectives in Biology. 2011;3:a004259-13. doi: 10.1101/cshperspect.a004259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux I, Wersinger E, McIntosh JM, Fuchs PA, Glowatzki E. Onset of cholinergic efferent synaptic function in sensory hair cells of the rat cochlea. J Neurosci. 2011;31:15092–15101. doi: 10.1523/JNEUROSCI.2743-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendin G, Bourien J, Rassendren F, Puel J-L, Nouvian R. Spatiotemporal pattern of action potential firing in developing inner hair cells of the mouse cochlea. Proc Natl Acad Sci USA. 2014;111:1999–2004. doi: 10.1073/pnas.1319615111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Colonnese MT. Development of Activity in the Mouse Visual Cortex. Journal of Neuroscience. 2016;36:12259–12275. doi: 10.1523/JNEUROSCI.1903-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM. Functioning of Circuits Connecting Thalamus and Cortex. Compr Physiol. 2017;7:713–739. doi: 10.1002/cphy.c160032. [DOI] [PubMed] [Google Scholar]
- Shi W, Xianyu A, Han Z, Tang X, Li Z, Zhong H, Mao T, Huang K, Shi S-H. Ontogenetic establishment of order-specific nuclear organization in the mammalian thalamus. Nat Neurosci. 2017;20:516–528. doi: 10.1038/nn.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel F, Heimel JA, Peters J, Lohmann C. Peripheral and central inputs shape network dynamics in the developing visual cortex in vivo. Curr Biol. 2012;22:253–258. doi: 10.1016/j.cub.2011.12.026. [DOI] [PubMed] [Google Scholar]
- Simi A, Studer M. Developmental genetic programs and activity-dependent mechanisms instruct neocortical area mapping. Current Opinion in Neurobiology. 2018;53:96–102. doi: 10.1016/j.conb.2018.06.007. [DOI] [PubMed] [Google Scholar]
- Simon DK, O’Leary DD. Development of topographic order in the mammalian retinocollicular projection. J Neurosci. 1992;12:1212–1232. doi: 10.1523/JNEUROSCI.12-04-01212.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MB, White JA, McKimm EJ, Milosevic MM, Antic SD. Mechanisms of Spontaneous Electrical Activity in the Developing Cerebral Cortex-Mouse Subplate Zone. Cereb Cortex. 2019;29:3363–3379. doi: 10.1093/cercor/bhy205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokhadze G, Campbell PW, Guido W. Postnatal development of cholinergic input to the thalamic reticular nucleus of the mouse. Eur J Neurosci. 2019;49:978–989. doi: 10.1111/ejn.13942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Lee B, Pyun D, Guimera J, Son Y, Yoon J, Baek K, Wurst W, Jeong Y. Ascl1 and Helt act combinatorially to specify thalamic neuronal identity by repressing Dlxs activation. Developmental Biology. 2015;398:280–291. doi: 10.1016/j.ydbio.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Sonntag M, Englitz B, Kopp-Scheinpflug C, Rubsamen R. Early Postnatal Development of Spontaneous and Acoustically Evoked Discharge Activity of Principal Cells of the Medial Nucleus of the Trapezoid Body: An In Vivo Study in Mice. Journal of Neuroscience. 2009;29:9510–9520. doi: 10.1523/JNEUROSCI.1377-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed MM, Lee S, Zheng J, Zhou ZJ. Stage-dependent dynamics and modulation of spontaneous waves in the developing rabbit retina. J Physiol (Lond) 2004;560:533–549. doi: 10.1113/jphysiol.2004.066597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A, Gribizis A, Chen C, Crair MC. Science Direct Activity-dependent development of visual receptive fields. Current Opinion in Neurobiology. 2017;42:136–143. doi: 10.1016/j.conb.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiriac A, Blumberg MS. Gating of reafference in the external cuneate nucleus during self-generated movements in wake but not sleep. Elife. 2016;5:123. doi: 10.7554/eLife.18749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiriac A, Del Rio-Bermudez C, Blumberg MS. Self-generated movements with “unexpected” sensory consequences. Current Biology. 2014;24:2136–2141. doi: 10.1016/j.cub.2014.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiriac A, Smith BE, Feller MB. Light Prior to Eye Opening Promotes Retinal Waves and Eye-Specific Segregation. Neuron. 2018;100:1059–1065.:e4. doi: 10.1016/j.neuron.2018.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiriac A, Uitermarkt BD, Fanning AS, Sokoloff G, Blumberg MS. Rapid Whisker Movements in Sleeping Newborn Rats. Current Biology. 2012;22:2075–2080. doi: 10.1016/j.cub.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolner EA, Sheikh A, Yukin AY, Kaila K, Kanold PO. Subplate neurons promote spindle bursts and thalamocortical patterning in the neonatal rat somatosensory cortex. Journal of Neuroscience. 2012;32:692–702. doi: 10.1523/JNEUROSCI.1538-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torborg CL, Feller MB. Spontaneous patterned retinal activity and the refinement of retinal projections. Progress in Neurobiology. 2005;76:213–235. doi: 10.1016/j.pneurobio.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Bergles DE. Developmental regulation of spontaneous activity in the Mammalian cochlea. J Neurosci. 2010;30:1539–1550. doi: 10.1523/JNEUROSCI.3875-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Rodríguez-Contreras A, Crins TTH, Wang HC, Borst JGG, Bergles DE. Calcium action potentials in hair cells pattern auditory neuron activity before hearing onset. Nat Neurosci. 2010;13:1050–1052. doi: 10.1038/nn.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Yi E, Gale JE, Glowatzki E, Bergles DE. The origin of spontaneous activity in the developing auditory system. Nature. 2007;450:50–55. doi: 10.1038/nature06233. [DOI] [PubMed] [Google Scholar]
- Tu DC, Zhang D, Demas J, Slutsky EB, Provencio I, Holy TE, Van Gelder RN. Physiologic diversity and development of intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48:987–999. doi: 10.1016/j.neuron.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Tuncdemir SN, Wamsley B, Stam FJ, Osakada F, Goulding M, Callaway EM, Rudy B, Fishell G. Early Somatostatin Interneuron Connectivity Mediates the Maturation of Deep Layer Cortical Circuits. Neuron. 2016;89:521–535. doi: 10.1016/j.neuron.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk E, van den Heuvel MI, Benders MJ, de Heus R, Franx A, Manning JH, Hect JL, Hernandez-Andrade E, Hassan SS, Romero R, Kahn RS, et al. Functional Connectome of the Fetal Brain. J Neurosci. 2019;39:9716–9724. doi: 10.1523/JNEUROSCI.2891-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uesaka N, Hayano Y, Yamada A, Yamamoto N. Interplay between laminar specificity and activity-dependent mechanisms of thalamocortical axon branching. J Neurosci. 2007;27:5215–5223. doi: 10.1523/JNEUROSCI.4685-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiullina F, Akhmetshina D, Nasretdinov A, Mukhtarov M, Valeeva G, Khazipov R, Rozov A. Developmental Changes in Electrophysiological Properties and a Transition from Electrical to Chemical Coupling between Excitatory Layer 4 Neurons in the Rat Barrel Cortex. Front Neural Circuits. 2016;10:365. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bourg A, Yang J-W, Reyes-Puerta V, Laurenczy B, Wieckhorst M, StUttgen MC, Luhmann HJ, Helmchen F. Layer-Specific Refinement of Sensory Coding in Developing Mouse Barrel Cortex. Cerebral Cortex. 2017;27:4835–4850. doi: 10.1093/cercor/bhw280. [DOI] [PubMed] [Google Scholar]
- van der Bourg A, Yang J-W, StUttgen MC, Reyes-Puerta V, Helmchen F, Luhmann HJ. Temporal refinement of sensory-evoked activity across layers in developing mouse barrel cortex. Eur J Neurosci. 2019;50:2955–2969. doi: 10.1111/ejn.14413. [DOI] [PubMed] [Google Scholar]
- Vanhatalo S, Kaila K. Development of neonatal EEG activity: from phenomenology to physiology. Semin Fetal Neonatal Med. 2006;11:471–478. doi: 10.1016/j.siny.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Viswanathan S, Bandyopadhyay S, Kao JPY, Kanold PO. Changing microcircuits in the subplate of the developing cortex. J Neurosci. 2012;32:1589–1601. doi: 10.1523/JNEUROSCI.4748-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HC, Bergles DE. Spontaneous activity in the developing auditory system. Cell Tissue Res. 2015;361:65–75. doi: 10.1007/s00441-014-2007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman TA, Riquelme PA, Ivic L, Flint AC, Kriegstein AR. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron. 2004;43:647–661. doi: 10.1016/j.neuron.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Weliky M, Katz LC. Correlational structure of spontaneous neuronal activity in the developing lateral geniculate nucleus in vivo. Science. 1999;285:599–604. doi: 10.1126/science.285.5427.599. [DOI] [PubMed] [Google Scholar]
- Wess JM, Isaiah A, Watkins PV, Kanold PO. Subplate neurons are the first cortical neurons to respond to sensory stimuli. Proc Natl Acad Sci USA. 2017;114:12602–12607. doi: 10.1073/pnas.1710793114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead K, Pressler R, Fabrizi L. Characteristics and clinical significance of delta brushes in the EEG of premature infants. Clinical Neurophysiology Practice. 2017;2:12–18. doi: 10.1016/j.cnp.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. Journal of Neurophysiology. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Winnubst J, Cheyne JE, Niculescu D, Lohmann C. Spontaneous Activity Drives Local Synaptic Plasticity In Vivo. Neuron. 2015;87:399–410. doi: 10.1016/j.neuron.2015.06.029. [DOI] [PubMed] [Google Scholar]
- Wong RO, Meister M, Shatz CJ. Transient period of correlated bursting activity during development of the mammalian retina. Neuron. 1993;11:923–938. doi: 10.1016/0896-6273(93)90122-8. [DOI] [PubMed] [Google Scholar]
- Wong WT, Sanes JR, Wong RO. Developmentally regulated spontaneous activity in the embryonic chick retina. Journal of Neuroscience. 1998;18:8839–8852. doi: 10.1523/JNEUROSCI.18-21-08839.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N, López Bendito G. Shaping brain connections through spontaneous neural activity. Eur J Neurosci. 2012;35:1595–1604. doi: 10.1111/j.1460-9568.2012.08101.x. [DOI] [PubMed] [Google Scholar]
- Yang J-W, An S, Sun J-J, Reyes-Puerta V, Kindler J, Berger T, Kilb W, Luhmann HJ. Thalamic network oscillations synchronize ontogenetic columns in the newborn rat barrel cortex. Cereb Cortex. 2013;23:1299–1316. doi: 10.1093/cercor/bhs103. [DOI] [PubMed] [Google Scholar]
- Yang J-W, Hanganu-Opatz IL, Sun J-J, Luhmann HJ. Three patterns of oscillatory activity differentially synchronize developing neocortical networks in vivo. J Neurosci. 2009;29:9011–9025. doi: 10.1523/JNEUROSCI.5646-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J-W, Kilb W, Kirischuk S, Unichenko P, StUttgen MC, Luhmann HJ. Development of the whisker-to-barrel cortex system. Current Opinion in Neurobiology. 2018;53:29–34. doi: 10.1016/j.conb.2018.04.023. [DOI] [PubMed] [Google Scholar]
- Yu Y-C, Bultje RS, Wang X, Shi S-H. Specific synapses develop preferentially among sister excitatory neurons in the neocortex. Nature. 2009;458:501–504. doi: 10.1038/nature07722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y-C, He S, Chen S, Fu Y, Brown KN, Yao X-H, Ma J, Gao KP, Sosinsky GE, Huang K, Shi S-H. Preferential electrical coupling regulates neocortical lineage-dependent microcircuit assembly. Nature. 2012 doi: 10.1038/nature10958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R, Peinado A, Katz LC. Neuronal domains in developing neocortex. Science. 1992;257:665–669. doi: 10.1126/science.1496379. [DOI] [PubMed] [Google Scholar]
- Zhang-Hooks Y, Agarwal A, Mishina M, Bergles DE. NMDA Receptors Enhance Spontaneous Activity and Promote Neuronal Survival in the Developing Cochlea. Neuron. 2016;89:337–350. doi: 10.1016/j.neuron.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Kao JPY, Kanold PO. Functional excitatory microcircuits in neonatal cortex connect thalamus and layer 4. Journal of Neuroscience. 2009;29:15479–15488. doi: 10.1523/JNEUROSCI.4471-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]