Abstract
Objective
To assess the associations of several blood immune biomarkers with the future risks of amyotrophic lateral sclerosis and Parkinson’s disease in a prospective cohort study with 20 years of follow-up.
Methods
The Swedish Apolipoprotein-related Mortality RISk (AMORIS) Study is a longitudinal cohort study including 812,073 participants with repeated blood biomarker measurements between 1985 and 1996 and a follow-up until 2011. Using Cox model, we first estimated hazard ratios of amyotrophic lateral sclerosis and Parkinson’s disease in relation to leukocytes, immunoglobulin G, haptoglobin, and uric acid. We further described the temporal changes of these biomarkers during the 20 years prior to the diagnosis of these diseases.
Results
A total of 585 incident cases of amyotrophic lateral sclerosis and 3,769 incident cases of Parkinson’s disease were identified during the follow-up. Increasing concentrations of leukocytes, haptoglobin, and uric acid were associated with a lower risk of Parkinson’s disease. No statistically significant association was however noted between the studied biomarkers and amyotrophic lateral sclerosis. Parkinson’s disease patients appeared to have lower levels of leukocytes and haptoglobin between 20 and 10 years before diagnosis and lower levels of uric acid during the 20 years before diagnosis, compared to controls, although statistically significant differences were only noted during parts of the respective time intervals after multivariable adjustment. No clear differences were noted between patients with amyotrophic lateral sclerosis and controls.
Interpretation
If verified in studies of independent populations, our findings may suggest a different role of systemic inflammation on the risk of Parkinson’s disease compared to amyotrophic lateral sclerosis.
Introduction
It is believed that neuroinflammation is a downstream event of neuronal damage, which can act as a two-sided sword, in neurodegenerative diseases. On the one hand it helps the neurons to recover from the injuries and increases their survival, whereas on the other hand prolonged inflammation can amplify the damage to the neurons1. In addition to the well-established immune activation in the central nervous system, evidence has also started to accumulate for a potential role of peripheral immune responses during the course of neurodegenerative diseases2,3.
There is an active interplay between the central nervous system and the peripheral immunity. But it is difficult to investigate directly neuroinflammation in a human setting, especially before the clinical onset of neurodegenerative diseases. As a result, understanding the temporal pattern of peripheral immune biomarker profiles before the diagnosis of neurodegenerative diseases might be the only way to shed light on whether alterations in immune responses precede or succeed human neurodegeneration. Longitudinal cohort studies with prospective and repeated measurements of peripheral inflammatory biomarkers and the possibility of identifying future diagnoses of neurodegenerative disease might be the only venue to achieve such understanding.
Amyotrophic lateral sclerosis (ALS) and Parkinson’s disease (PD) are two of the most common neurodegenerative diseases and share substantial similarity in disease mechanisms such as mitochondrial dysfunction, misfolded and aggregated proteins, ubiquitin proteasome pathway, and increased reactive oxygen species4,5. Furthermore, the presence of neuro-inflammatory cascades has been reported in both diseases6. In the present study, we therefore followed a large sample of Swedes to investigate several key blood immune biomarkers during the 20 years before the diagnosis of ALS and PD. Similar or contrasting findings between these two diseases will likely provide better understanding of the disease etiologies for both.
Material and Methods
The AMORIS Study
The Swedish Apolipoprotein-related MOrtality RISk (AMORIS) Study is a longitudinal cohort study that contains laboratory test results of 812,073 individuals residing predominantly in the greater Stockholm area during 1985-1996. The measurements were gathered from healthy individuals that were undergoing general health checkups or referrals from outpatient visits. The cohort includes 49% men and 51% women, and accounted for ~35% of the total population in Stockholm at the time of enrollment. All laboratory tests were conducted on fresh blood samples by the Central Automation Laboratory (CALAB), Stockholm, Sweden, using the same techniques and reagents across all years7. In the present study we focused on participants that had at least one measurement of the biomarkers of interest for the present study (N=659,407) and excluded participants that had their first blood sampling at an age younger than 20 (N=21,612).
Details about the laboratory measurements of the AMORIS participants are provided elsewhere7. In brief, the AMORIS Study includes measurement information on 595 biomarkers including 56 tests that are of relevance for immunology. Because both ALS and PD are relatively rare diseases, we a priori decided to focus on immunological biomarkers that are commonly measured clinically, namely leukocytes, immunoglobulin G (IgG), and C-reactive protein (CRP), to ensure sufficient statistical power in the analysis. Because high sensitive CRP was not commonly measured during the enrollment period of the AMORIS Study, we decided to study haptoglobin instead of CRP in the present study. We therefore gathered data on concentrations of leukocytes (109 cells per liter of blood, 109 cells/L), IgG (g/L), and haptoglobin (g/L). Leukocytes play a major role in the course of infection and in controlling inflammation. IgG is the most abundant antibody protecting the body against bacterial and fungal infections. Haptoglobin is an acute phase reactant and a hemoglobin-binding protein that scavenges free hemoglobin and inhibits its oxidative activity as well as modulates host immune responses. The blood levels of these biomarkers can therefore indicate infection and inflammation as well as the general state of the immune system. As a secondary analysis we also studied four subtypes of leukocytes, namely lymphocytes, monocytes, neutrophils, and eosinophils, which are available for a sub-population of the cohort (Table 1). For all the above biomarkers we retrieved information on the date of blood sampling and fasting status at the time of sampling. For lymphocytes, monocytes, neutrophils, and eosinophils, data are available as either the absolute cell counts or the percentage of total leukocytes. Uric acid was used as a quality control of our analysis because of its suggested association with PD8,9. Although the association of uric acid with the risk of ALS has been less discussed, a potential role of uric acid in ALS prognosis has been suggested10,11.
Table 1. Baseline characteristics of the participants included in the prospective cohort analyses of amyotrophic lateral sclerosis (ALS) and Parkinson’s disease (PD).
| Characteristics | Participants in the cohort analysis of ALS | Participants in the cohort analysis of PD | ||
|---|---|---|---|---|
| No. of participants | Mean (SD) or % | No. of participants | Mean (SD) or % | |
| Age at 1st blood sampling, mean (SD), years | 606,421 | 45.93 (15.14) | 587,724 | 50.64 (14.90) |
| Sex, % | ||||
| Male | 310,804 | 51.25 | 300,268 | 51.09 |
| Female | 295,617 | 48.75 | 287,456 | 48.91 |
| Country of birth, % | ||||
| Sweden | 520,478 | 85.83 | 506,484 | 86.18 |
| Other Nordic country | 35,105 | 5.79 | 33,056 | 5.62 |
| Other | 49,564 | 8.17 | 46,933 | 7.99 |
| Unknown | 1,274 | 0.21 | 1,251 | 0.21 |
| Socio-economic status, % | ||||
| Low | 259,794 | 42.84 | 253,763 | 43.18 |
| High | 263,946 | 43.53 | 257,680 | 43.84 |
| Unclassified/missing | 82,681 | 13.63 | 76,281 | 12.98 |
| Inflammatory biomarkers, mean (SD) | ||||
| Leukocyte, 109 cells/L | 201,347 | 6.63 (2.48) | 192,341 | 6.60 (2.27) |
| Immunoglobulin-G, g/L | 132,418 | 11.54 (2.99) | 127,455 | 11.53 (2.94) |
| Haptoglobin, g/L | 401,773 | 1.07 (0.34) | 388,741 | 1.07 (0.34) |
| Uric acid, μMol/L | 491,740 | 289.95 (74.47) | 475,360 | 289.15 (73.78) |
SD: standard deviation
Prospective cohort analysis
We first performed a prospective cohort analysis. Using the individually unique Swedish personal identification numbers, the cohort participants were followed from their first blood sampling until date of diagnosis for the outcome of interest (i.e., ALS or PD), death, migration out of Sweden, or December 31st 2011, whichever occurred first.
The Swedish Patient Register was used to identify incident cases of ALS and PD. The Swedish National Board of Health and Welfare started collecting data regarding hospital discharge records from 1964/1965 and the Patient Register became nationwide since 1987. From 2001, the register also collects >80% of all hospital-based specialist outpatient visits in Sweden. Records of the register include the discharge diagnoses and outpatient diagnoses coded by the Swedish revisions of the International Classification of Disease (ICD) codes. Individuals with a newly diagnosed ALS or PD were identified from all hospital visits with ALS or PD as a discharge diagnosis. ALS diagnosis was based on ICD-8 code 348,00 prior to 1987, ICD-9 code 335C during 1987–1996, and ICD-10 code G12.2 since 1997. PD diagnosis was based on ICD-8 code 342 prior to 1987, ICD-9 code 332A during 1987–1996, and ICD-10 code G20 since 1997. For both conditions the first available date for the diagnosis was defined as the date of diagnosis. Deaths and migrations were identified from the Swedish Causes of Death Register and Total Population Register.
Based on previous validation studies, the positive predictive value of register-based definitions is likely high for ALS (91% for any diagnosis)12 and satisfactory for PD (83% for main diagnosis and 71% for any diagnosis)13, compared to standard clinical evaluation. Previous studies have also suggested that identifying ALS and PD patients through inpatient care might have a delay from first symptoms onset of around one year12 and 7.5 years13. As outpatient care was not recorded in the Patient Register during 1985-2000 and the diagnostic delay using outpatient care records (2001-2011) was presumably shorter, we excluded the first year after a participant’s first blood sampling from the analysis of ALS and the first five years after a participant’s first blood sampling from the analysis of PD. As a result, individuals that had already received a diagnosis of ALS or PD (N=29 ALS cases and N=591 PD cases), emigrated out of Sweden (N=29,206 in ALS analysis and N=36,092 in PD analysis), or died (N=2,139 in ALS analysis and N=13,388 in PD analysis) before the redefined cohort entry (one year after the first blood sampling in the cohort analysis of ALS and five years after the first blood sampling in the cohort analysis of PD) were excluded from the analyses. Because of these different exclusion criteria, we had a final cohort of 606,421 for the analysis of ALS (74.7% of the entire AMORIS study) and 587,724 for the analysis of PD (72.4%). The AMORIS Study was also cross-linked to the Swedish Censuses in 1970, 1980, 1985, and 1990 to obtain information regarding country of birth (Sweden, other Nordic countries, other, or unknown) and socio-economic status (low, high, or unclassified or missing) at the time of recruitment. We adjusted for socio-economic status because it has been associated with the level of inflammatory markers14 and potentially also PD and ALS15,16. Socio-economic status was originally classified as unskilled worker, skilled worker, lower employee, intermediate employee, higher employee, self-employed, and farmer in the AMORIS Study, and we classified unskilled worker, skilled worker, and lower employee as low socio-economic status whereas intermediate employee, higher employee, self-employed, and farmer as high socio-economic status.
Nested case-control studies
In order to assess the temporal pattern of the studied biomarkers prior to ALS and PD diagnosis, we conducted further two nested case-control studies within the above cohort study. Cases for the nested case-control studies consisted of all individuals diagnosed with ALS or PD during the follow-up. We defined the date of diagnosis as index date for the cases and randomly selected 25 controls per case using incidence density sampling. For example, when an individual was diagnosed with ALS, we randomly selected 25 individuals from the remaining participants of the cohort that were still at follow-up, i.e., without an ALS diagnosis, death, or migration out of Sweden, as controls for the specific case. The controls were individually matched to the case by sex, year of birth, and calendar period of enrollment to the AMORIS Study. We then defined the date of diagnosis for the case as the index date for the selected controls. In this analysis, we took into account all blood measurements concerning the biomarker of interest, i.e., all measurements available in the AMORIS Study before the index date for both cases and controls.
Statistical analysis
In the cohort analysis, the exposure of interest was the first available measurement for each biomarker, which was studied as a potential risk factor for ALS and PD. We focused on the first measurement because the measurements performed later in time were closer to the diagnosis of ALS or PD and might therefore more likely be secondary to the disease. All biomarkers were first modeled as continuous variables to assess the association of one standard deviation increase in a specific biomarker with the risks of ALS and PD. Furthermore, all variables were categorized with cut-points at the quintiles (five categories) to assess the variation of the risks of ALS and PD across categories of each specific biomarker. Because the study participants were enrolled at various lag times after birth and because only individuals who survived (and without ALS or PD) until enrolment were included in the present study, all survival models were fitted with delayed entry at the actual time of cohort entry to avoid potential immortal time bias. We used Cox models to calculate the hazard ratios (HRs) and their 95% confidence intervals (CIs), using attained age as the underlying time scale and date of birth as the time origin. All models were further adjusted for sex, fasting status (over-night fasting vs. no over-night fasting), age at time of first blood sampling, country of birth, and socio-economic status. In a sensitivity analysis, we adjusted the studied biomarkers for one another to assess the impact of correlation between these biomarkers on the studied associations. Smoking has a known inverse relationship with PD17 and has recently been suggested to be causally related to the risk of ALS18,19. Furthermore, smoking has also been suggested to influence the levels of different inflammatory markers including white blood cells20. Therefore, in another sensitivity analysis, we further adjusted for smoking status.
Information on smoking was gathered from different sources in the AMORIS cohort, including a number of cohort studies and the Swedish Medical Birth Register7. In total, 269,938 individuals (64% female) were found to have information on smoking available for this analysis. Finally, because lipid metabolism is increasingly recognized as playing an important role in ALS and PD21–24 and uric acid is known to modulate lipid metabolism25, we performed another sensitivity analysis to further adjust the associations of uric acid with ALS and PD with total cholesterol and triglycerides. The assumption of proportional hazards was examined using the χ2 test based on Schoenfeld residuals. In order to assess the potential interaction of the studied biomarkers with sex on the studied associations, we stratified all analyses by sex.
In the nested case-control studies, we studied not only the first measurement of each biomarker of interest, but all the subsequent ones that were performed before the index date of the cases and controls. We hypothesized that patients with ALS and PD might demonstrate different temporal trends of the studied immune biomarkers before diagnosis, compared to individuals not developing such diseases. We first plotted the mean concentrations of different biomarkers by time to index date using locally weighted scatterplot smoothing, to visualize the difference in levels of each biomarker during the 20 years before index date between cases and controls. We studied 20 years because the number of measurements became much smaller more than 20 years before index date. We then compared the mean level of a specific biomarker during each 2-year period of the 20 years before index date between the cases and controls using conditional logistic regression models. In these models, we used each matched case and control set as a stratum and adjusted for fasting status.
This study complied with the Declaration of Helsinki and was approved by the Regional Ethical Review Board in Stockholm (Karolinska Institutet), Sweden. All analyses were performed using Stata software, version 14 (StataCorp, College Station, TX).
Results
Prospective cohort analysis
During follow-up, 585 individuals received an ALS diagnosis with an incidence rate of 4.96 per 100,000 person-years. The mean age at first blood sampling was 53.2 and the mean age at diagnosis was 67.0 for these ALS cases (Supplementary Table 1). During follow-up, 3,769 individuals received a diagnosis of PD, leading to an incidence rate of 42.9 per 100,000 person-years. The PD cases were on average 62.5 years at their first blood sampling and 72.8 years at diagnosis (Supplementary Table 1).
No statistically significant association was noted between any of the studied biomarkers and the risk of ALS when using the biomarkers as continuous variables (Table 2). A potential inverse association was suggested for uric acid and ALS risk overall (HR: 0.93; 95%CI: 0.84-1.04). There was a statistically significant inverse association of leukocytes (HR: 0.86, 95%CI: 0.80-0.93), haptoglobin (HR: 0.94, 95%CI: 0.90-0.97), and uric acid (HR: 0.93, 95%CI: 0.89-0.97) with the risk of PD. These results were in general similar for men and women. When using the biomarkers as categorical variables, we found again no clear association between these biomarkers and ALS risk, whereas a clear dose-response relationship of increasing concentrations of leukocytes, haptoglobin, and uric acid with a decreasing risk of PD (Table 3).
Table 2. Hazard ratios (HRs) and 95% confidence intervals (CIs) of amyotrophic lateral sclerosis (ALS) and Parkinson’s disease (PD) per standard deviation increase in the concentrations of leukocyte, immunoglobulin-G, haptoglobin, and uric acid, after adjustment for age, sex, country of birth, socio-economic status, and fasting status, a prospective cohort analysis*.
| Cohort analysis of ALS | |||
| Overall | Person-years | No. of outcomes | HR (95% CI) |
| Leukocyte, 109 cells/L | 2,936,273 | 187 | 0.98 (0.83-1.17) |
| Immunoglobulin-G, g/L | 2,497,291 | 152 | 1.04 (0.89-1.22) |
| Haptoglobin, g/L | 7,519,941 | 448 | 1.06 (0.97-1.16) |
| Uric acid, μMol/L | 8,668,903 | 500 | 0.93 (0.84-1.04) |
| Men | Person-years | No. of outcomes | HR (95% CI) |
| Leukocyte, 109 cells/L | 1,304,355 | 114 | 0.90 (0.70-1.15) |
| Immunoglobulin-G, g/L | 1,313,322 | 89 | 0.93 (0.74-1.17) |
| Haptoglobin, g/L | 4,103,416 | 290 | 1.09 (0.99-1.21) |
| Uric acid, μMol/L | 4,702,220 | 321 | 0.93 (0.82-1.06) |
| Women | Person-years | No. of outcomes | HR (95% CI) |
| Leukocyte, 109 cells/L | 1,631,918 | 73 | 1.05 (0.92-1.20) |
| Immunoglobulin-G, g/L | 1,183,968 | 63 | 1.15 (0.93-1.43) |
| Haptoglobin, g/L | 3,416,524 | 158 | 0.98 (0.83-1.17) |
| Uric acid, μMol/L | 3,966,682 | 179 | 0.94 (0.78-1.15) |
| Cohort analysis of PD | |||
| Overall | Person-years | No. of outcomes | HR (95% CI) |
| Leukocyte, 109 cells/L | 2,268,953 | 1,179 | 0.86 (0.80-0.93) |
| Immunoglobulin-G, g/L | 2,021,191 | 1,055 | 1.03 (0.97-1.09) |
| Haptoglobin, g/L | 6,045,357 | 2,828 | 0.94 (0.90-0.97) |
| Uric acid, μMol/L | 6,896,873 | 3,172 | 0.93 (0.89-0.97) |
| Men | Person-years | No. of outcomes | HR (95% CI) |
| Leukocyte, 109 cells/L | 1,003,050 | 712 | 0.87 (0.79-0.95) |
| Immunoglobulin-G, g/L | 1,063,381 | 673 | 1.08 (1.01-1.16) |
| Haptoglobin, g/L | 3,292,029 | 1,892 | 0.93 (0.89-0.98) |
| Uric acid, μMol/L | 3,734,259 | 2,112 | 0.94 (0.89-0.98) |
| Women | Person-years | No. of outcomes | HR (95% CI) |
| Leukocyte, 109 cells/L | 1,265,902 | 467 | 0.86 (0.76-0.96) |
| Immunoglobulin-G, g/L | 957,810 | 382 | 0.94 (0.85-1.04) |
| Haptoglobin, g/L | 2,753,328 | 936 | 0.95 (0.89-1.02) |
| Uric acid, μMol/L | 3,162,614 | 1,060 | 0.92 (0.85-0.99) |
Bolded HRs are statistically significant with a P value of <0.05.
Table 3. Hazard ratios (HRs) and 95% confidence intervals (CIs) of amyotrophic lateral sclerosis (ALS) and Parkinson’s disease (PD) in relation to the concentrations of leukocyte, immunoglobulin-G, haptoglobin, and uric acid, after adjustment for age, sex, country of birth, socio-economic status, and fasting status, a prospective cohort analysis*.
| Biomarker analysed | Cohort analysis of ALS | Cohort analysis of PD | ||
|---|---|---|---|---|
| Leukocyte | No. of outcomes | HR (95% CI) | No. of outcomes | HR (95% CI) |
| Below 1st quintile | 39 | Ref. | 300 | Ref. |
| Between 1st and 2nd quintile | 36 | 0.99 (0.63-1.56) | 248 | 0.87 (0.73-1.03) |
| Between 2nd and 3rd quintile | 44 | 1.21 (0.79-1.87) | 252 | 0.86 (0.73-1.02) |
| Between 3rd and 4th quintile | 38 | 1.12 (0.72-1.76) | 220 | 0.85 (0.71-1.01) |
| Above 4th quintile | 30 | 0.99 (0.61-1.60) | 159 | 0.64 (0.53-0.78) |
| Immunoglobulin-G | ||||
| Below 1st quintile | 39 | Ref. | 255 | Ref. |
| Between 1st and 2nd quintile | 42 | 0.83 (0.53-1.28) | 315 | 0.97 (0.82-1.14) |
| Between 2nd and 3rd quintile | 17 | 0.80 (0.45-1.41) | 138 | 1.01 (0.82-1.24) |
| Between 3rd and 4th quintile | 20 | 1.20 (0.70-2.06) | 107 | 1.01 (0.81-1.27) |
| Above 4th quintile | 35 | 1.08 (0.68-1.70) | 240 | 1.17 (0.98-1.39) |
| Haptoglobin | ||||
| Below 1st quintile | 92 | Ref. | 634 | Ref. |
| Between 1st and 2nd quintile | 117 | 1.03 (0.78-1.35) | 776 | 0.96 (0.86-1.06) |
| Between 2nd and 3rd quintile | 65 | 1.05 (0.77-1.45) | 417 | 0.92 (0.81-1.04) |
| Between 3rd and 4th quintile | 86 | 1.07 (0.80-1.44) | 519 | 0.87 (0.77-0.98) |
| Above 4th quintile | 88 | 1.16 (0.86-1.56) | 482 | 0.85 (0.75-0.95) |
| Uric acid | ||||
| Below 1st quintile | 78 | Ref. | 430 | Ref. |
| Between 1st and 2nd quintile | 90 | 0.93 (0.69-1.27) | 554 | 0.93 (0.82-1.06) |
| Between 2nd and 3rd quintile | 106 | 0.92 (0.67-1.26) | 712 | 0.95 (0.83-1.07) |
| Between 3rd and 4th quintile | 113 | 0.90 (0.65-1.23) | 720 | 0.85 (0.75-0.97) |
| Above 4th quintile | 113 | 0.86 (0.62-1.19) | 756 | 0.83 (0.73-0.95) |
Bolded HRs are statistically significant with a P value of <0.05.
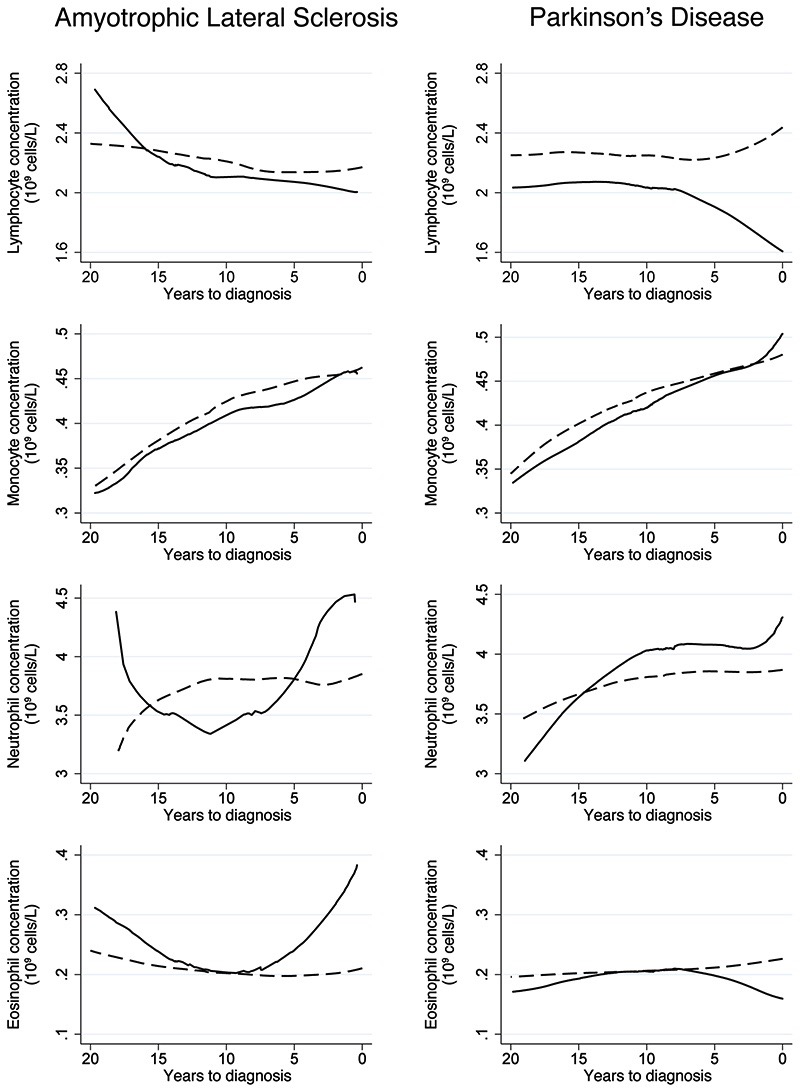
Among the different immune cells, lymphocytes showed a clear inverse association with the risk of PD while an increasing number of eosinophils was associated with an increased risk of ALS (Table 4). Table 5 demonstrates the associations of the studied biomarkers with ALS or PD after mutual adjustment of the biomarkers for one another or additional adjustment for smoking. We found largely similar results after such additional adjustments as in the main analyses, apart from the association of haptoglobin with ALS after further adjustment for leukocytes (HR: 1.26; 95%CI: 1.06-1.49). The associations of uric acid with ALS and PD did not change greatly after further adjustment for total cholesterol (HR: 0.93; 95%CI: 0.83-1.03 for ALS and HR: 0.96, 95%CI: 0.92-1.00 for PD) or triglycerides (HR: 0.93; 95%CI: 0.83-1.04 for ALS and HR: 0.96, 95%CI: 0.92-1.00 for PD).
Table 4. Hazard ratios (HRs) and 95% confidence intervals (CIs) of Parkinson’s disease (PD) per standard deviation increase in the concentrations of specific leukocytes, after adjustment for age, sex, country of birth, socio-economic status, and fasting status a prospective cohort analysis*.
| Cohort analysis of ALS | |||
| Overall | Person-years | No. of outcomes | HR (95% CI) |
| Lymphocyte, 109 cells/L | 771,683 | 53 | 1.04 (0.88-1.23) |
| Monocyte, 109 cells/L | 762,037 | 52 | 0.96 (0.71-1.31) |
| Neutrophil, 109 cells/L | 329,136 | 25 | 0.93 (0.60-1.44) |
| Eosinophil, 109 cells/L | 709,283 | 49 | 1.18 (1.01-1.39) |
| Men | Person-years | No. of outcomes | HR (95% CI) |
| Lymphocyte, 109 cells/L | 320,934 | 26 | 1.00 (0.66-1.52) |
| Monocyte, 109 cells/L | 316,660 | 25 | 0.96 (0.62-1.49) |
| Neutrophil, 109 cells/L | 130,764 | 9 | 0.86 (0.38-1.97) |
| Eosinophil, 109 cells/L | 295,661 | 24 | 0.93 (0.60-1.42) |
| Women | Person-years | No. of outcomes | HR (95% CI) |
| Lymphocyte, 109 cells/L | 450,749 | 27 | 1.06 (0.89-1.26) |
| Monocyte, 109 cells/L | 445,377 | 27 | 0.98 (0.63-1.52) |
| Neutrophil, 109 cells/L | 198,371 | 16 | 0.96 (0.57-1.62) |
| Eosinophil, 109 cells/L | 413,622 | 25 | 1.32 (1.12-1.55) |
| Cohort analysis of PD | |||
| Overall | Person-years | No. of outcomes | HR (95% CI) |
| Lymphocyte, 109 cells/L | 598,755 | 306 | 0.74 (0.59-0.94) |
| Monocyte, 109 cells/L | 590,977 | 297 | 0.97 (0.85-1.09) |
| Neutrophil, 109 cells/L | 242,248 | 152 | 1.00 (0.84-1.19) |
| Eosinophil, 109 cells/L | 549,074 | 284 | 0.97 (0.86-1.10) |
| Men | Person-years | No. of outcomes | HR (95% CI) |
| Lymphocyte, 109 cells/L | 248,078 | 181 | 0.72 (0.54-0.98) |
| Monocyte, 109 cells/L | 244,656 | 174 | 0.95 (0.81-1.11) |
| Neutrophil, 109 cells/L | 95,603 | 87 | 0.88 (0.68-1.13) |
| Eosinophil, 109 cells/L | 228,092 | 170 | 0.94 (0.80-1.10) |
| Women | Person-years | No. of outcomes | HR (95% CI) |
| Lymphocyte, 109 cells/L | 350,676 | 125 | 0.78 (0.54-1.11) |
| Monocyte, 109 cells/L | 346,320 | 123 | 0.99 (0.81-1.21) |
| Neutrophil, 109 cells/L | 146,644 | 65 | 1.14 (0.91-1.43) |
| Eosinophil, 109 cells/L | 320,981 | 114 | 1.02 (0.84-1.24) |
Bolded HRs are statistically significant with a P value of <0.05.
Table 5. Hazard ratios (HRs) and 95% confidence intervals (CIs) of amyotrophic lateral sclerosis (ALS) and Parkinson’s disease (PD) per standard deviation increase in the concentrations of leukocyte, immunoglobulin-G, haptoglobin, and uric acid, after adjustment for age, sex, country of birth, socio-economic status, fasting status, other biomarkers, or smoking, a prospective cohort analysis*.
| Cohort analysis of ALS | Leukocyte | Immunoglobulin-G | Haptoglobin | Uric acid |
| Further adjusted for leukocyte | - | 1.07 (0.78-1.46) | 1.26 (1.06-1.49) | 1.15 (0.92-1.43) |
| Further adjusted for immunoglobulin-G | 0.93 (0.64-1.35) | - | 0.97 (0.84-1.14) | 0.82 (0.67-1.02) |
| Further adjusted for haptoglobin | 0.94 (0.76-1.17) | 1.03 (0.88-1.22) | - | 0.92 (0.82-1.04) |
| Further adjusted for uric acid | 0.97 (0.80-1.17) | 1.04 (0.89-1.22) | 1.07 (0.98-1.18) | - |
| Further adjusted for smoking | 1.04 (0.83-1.30) | 1.12 (0.80-1.56) | 1.15 (0.95-1.40) | 1.00 (0.79-1.25) |
| Cohort analysis of PD | Leukocyte | Immunoglobulin-G | Haptoglobin | Uric acid |
| Further adjusted for leukocyte | - | 0.97 (0.85-1.09) | 0.99 (0.91-1.07) | 0.87 (0.80-0.94) |
| Further adjusted for immunoglobulin-G | 0.91 (0.80-1.04) | - | 0.94 (0.89-1.00) | 0.98 (0.91-1.06) |
| Further adjusted for haptoglobin | 0.90 (0.82-0.98) | 1.02 (0.96-1.08) | - | 0.95 (0.91-1.00) |
| Further adjusted for uric acid | 0.88 (0.82-0.92) | 1.03 (0.97-1.10) | 0.95 (0.91-0.99) | - |
| Further adjusted for smoking | 0.82 (0.70-0.95) | 1.10 (0.98-1.23) | 0.98 (0.91-1.05) | 0.94 (0.87-1.01) |
Bolded HRs are statistically significant with a P value of <0.05.
Nested cases-control studies
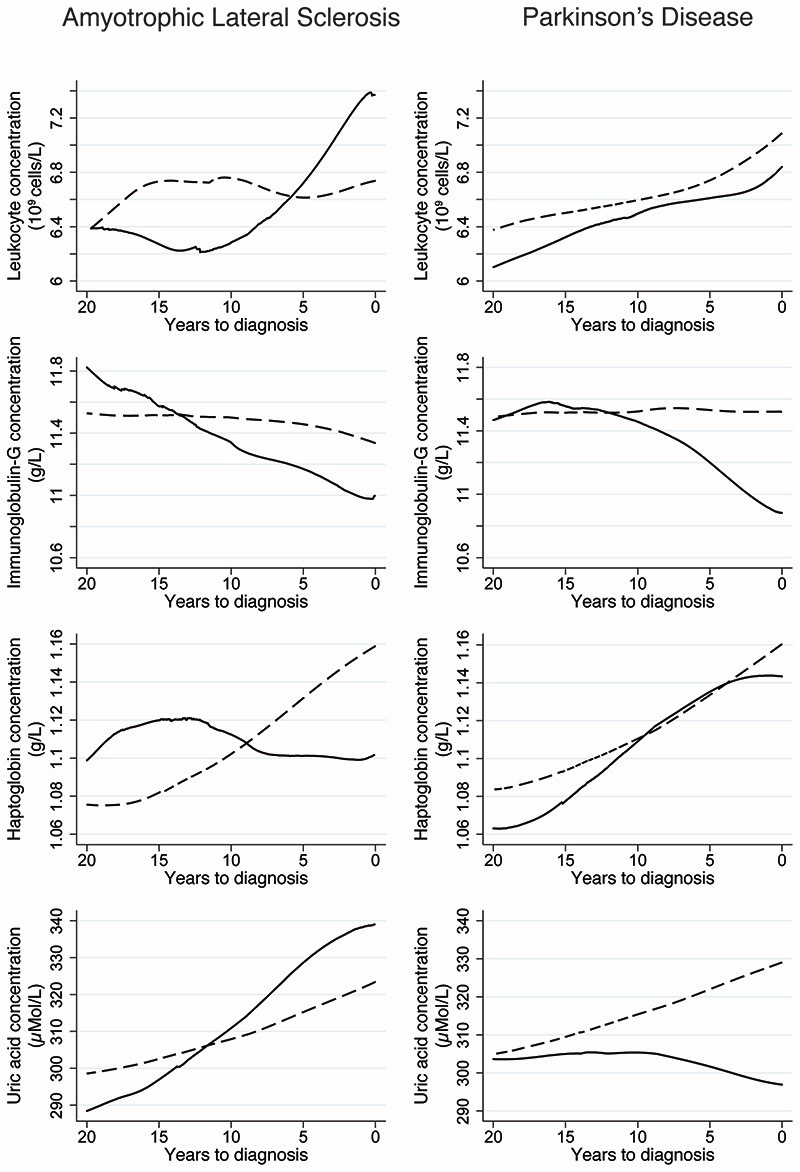
Focusing on the biomarkers of interest, ALS cases had on average slightly more blood samplings compared to their controls before the index date (mean number of blood samplings=2.28 among ALS cases and 2.10 among controls) (Table 6). There were no consistent differences between ALS cases and controls regarding the levels of the studied biomarkers (Fig 1 and Supplementary Table 2), including specific types of leukocytes (Fig 2 and Supplementary Table 3), during the 20 years prior to the index date. In the case of PD, the number of blood samplings was similar between cases and controls (mean number of blood samplings = 2.27 among PD cases and 2.21 among controls) (Table 6). PD patients had lower concentrations of leukocytes and haptoglobin between 20 and 10 years before the index date and uric acid during the 20 years before the index date, compared to controls, although statistically significant differences were only noted during parts of the respective time intervals after multivariable adjustment (Fig 1 and Supplementary Table 2). Compared to controls, PD patients also had quickly declining IgG levels during the 10 years before diagnosis (Fig 1); the difference was however not statistically significant after multivariable adjustment except for 0-2 years before diagnosis (Supplementary Table 2). Among the different cell types examined, a lower concentration of lymphocytes was noted during the 20 years before diagnosis among patients with PD, compared to controls, although the difference was not statistically significant for all the biannual intervals after multivariable adjustment (Fig 2 and Supplementary Table 3).
Table 6. Numbers of individuals with available biomarker measurements for the cases and controls included in the nested case-control studies of amyotrophic lateral sclerosis (ALS) and Parkinson’s disease (PD).
| Biomarker analysed | Total number of individuals with at least one measurement | Number (%) of individuals by number of measurements | Total number of measurements | |||||
|---|---|---|---|---|---|---|---|---|
| 1 measurement | 2 measurements | 3 measurements | 4 measurements | 5 measurements | 6 or more measurements | |||
| Nested case-control study of ALS | ||||||||
| Leukocyte | ||||||||
| Cases | 226 | 123 (54.4%) | 45 (19.9%) | 17 (7.5%) | 9 (4.0%) | 7 (3.1%) | 25 (11.1%) | 546 |
| Controls | 5,621 | 3,209 (57.1%) | 1,093 (19.4%) | 525 (9.3%) | 270 (4.8%) | 147 (2.6%) | 377 (6.7%) | 12,255 |
| Haptoglobin | ||||||||
| Cases | 497 | 251 (50.5%) | 106 (21.3%) | 62 (12.5%) | 38 (7.6%) | 8 (1.6%) | 32 (6.4%) | 1,100 |
| Controls | 12,402 | 6,790 (54.7%) | 2,697 (21.7%) | 1,213 (9.8%) | 657 (5.3%) | 378 (3.0%) | 667 (5.4%) | 25,654 |
| Immunoglobulin-G | ||||||||
| Cases | 178 | 142 (79.8%) | 22 (12.4%) | 7 (3.9%) | 4 (2.2%) | 2 (1.1%) | 1 (0.6%) | 240 |
| Controls | 4,412 | 3,537 (80.2%) | 630 (14.3%) | 155 (3.5%) | 50 (1.1%) | 21 (0.5%) | 19 (0.4%) | 5,698 |
| Uric acid | ||||||||
| Cases | 559 | 257 (46.0%) | 113 (20.2%) | 75 (13.4%) | 45 (8.0%) | 18 (3.2%) | 51 (9.1%) | 1,445 |
| Controls | 13,953 | 6,997 (50.1%) | 3,001 (21.5%) | 1,521 (10.9%) | 820 (5.9%) | 522 (3.7%) | 1,092 (7.8%) | 32,813 |
| Nested case-control study of PD | ||||||||
| Leukocyte | ||||||||
| Cases | 1,682 | 887 (52.7%) | 337 (20.0%) | 159 (9.4%) | 75 (4.4%) | 67 (4.0%) | 157 (9.3%) | 4,150 |
| Controls | 41,639 | 23,037(55.3%) | 7,894 (19.0%) | 3,797 (9.1%) | 2,082 (5.0%) | 1,319 (3.2%) | 3,510 (8.4%) | 97,763 |
| Haptoglobin | ||||||||
| Cases | 3,375 | 1,712 (50.1%) | 747 (22.1%) | 359 (10.6%) | 222 (6.6%) | 121 (3.6%) | 214 (6.3%) | 7,440 |
| Controls | 83,967 | 44,151(52.6%) | 17,857 (21.3%) | 8,774 (10.4%) | 5,064 (6.0%) | 2,992 (3.6%) | 5,129 (6.1%) | 181,967 |
| Immunoglobulin-G | ||||||||
| Cases | 1,329 | 1,018 (76.6%) | 213 (16.0%) | 51 (3.8%) | 23 (1.7%) | 8 (0.6%) | 16 (1.2%) | 1,844 |
| Controls | 32,381 | 25,475(78.7%) | 4,932 (15.2%) | 1,258 (3.9%) | 398 (1.2%) | 133 (0.4%) | 185 (0.6%) | 42,756 |
| Uric acid | ||||||||
| Cases | 3,882 | 1,831 (47.2%) | 820 (21.2%) | 441 (11.4%) | 259 (6.7%) | 172 (4.4%) | 359 (9.2%) | 9,851 |
| Controls | 96,774 | 46,919(48.5%) | 20,467 (21.2%) | 10,459(10.8%) | 6,212 (6.4%) | 3,870 (4.0%) | 8,847 (9.1%) | 241,099 |
Figure 1.
Mean concentrations of leukocyte, immunoglobulin-G, haptoglobin, and uric acid during the 20 years before the diagnosis of amyotrophic lateral sclerosis and Parkinson’s disease, comparing patients (solid line) to matched controls (dashed line). Locally weighted scatterplot smoothing was used to produce smooth curves for the mean concentrations based on all available measurements during the 20-years period, for both the cases and their matched controls.
Figure 2.
Mean concentrations of lymphocyte, monocyte, neutrophil, and eosinophil during the 20 years before the diagnosis of amyotrophic lateral sclerosis and Parkinson’s disease, comparing patients (solid line) to matched controls (dashed line). Locally weighted scatterplot smoothing was used to produce smooth curves for the mean concentrations based on all available measurements during the 20-years period, for both the cases and their matched controls.
Discussion
In this large cohort study, we investigated three key peripheral immune biomarkers during the 20 years before the clinical diagnosis of two progressive neurodegenerative disorders, namely, ALS and PD. We found no statistically significant association of leukocyte, IgG, and haptoglobin with the future risk of ALS, whereas an increasing concentration of leukocyte or haptoglobin was associated with a decreasing risk of PD. If verified in future studies of independent populations, these findings may suggest a different role of systemic inflammation on the subsequent risks of ALS and PD. The clear inverse association noted between uric acid and future risk of PD demonstrates further the robustness of these findings.
ALS and PD are two of the most common neurodegenerative diseases, and are both primarily presented as sporadic diseases5,17. Mitochondrial dysfunction, misfolded and aggregated proteins, ubiquitin proteasome pathway, and increased reactive oxygen species have all been described as key characteristics for ALS and PD4,5. Studies have also supported the presence of neuro-inflammatory cascades in both disorders, highlighted by the neurotoxic activation of microglia and the infiltration of T cells at sites of neuronal injury6. Yet, it is debated whether the altered immune responses play a causal role or are purely secondary to the neuronal damages in these diseases26,27. In contrast to the proposed pathophysiological similarities, it is interesting to note that ALS and PD do appear to have different risk factors. For example, smoking has been repeatedly shown as a protective factor for PD17, but has been recently suggested to be causally related to a higher risk of ALS18,19. Opposite result patterns have also been shown for lipids18,22–24,28,29. Similar or contrasting findings between ALS and PD, when studying risk factors or disease pathways, might therefore provide improved understanding of the disease etiologies for both.
Studies directed at mapping peripheral adaptive immune profiles in ALS and associating immune alterations to clinical disease phenotypes have been conducted30–32. For example, ALS patients were shown to have altered levels of CD4+, CD8+, and natural killer T cells in the peripheral blood compared to healthy controls, and such alterations were shown to be associated with the progression rate of the disease30,31,33. The present study did however not reveal different associations of specific cell types with the risk of ALS or different concentrations of specific cell types between ALS patients and individuals without ALS during the 20 years before ALS diagnosis. The fact that previous studies compared leukocyte concentrations among clinical patients with ALS to healthy volunteers, whereas the present study examined leukocyte concentrations long before diagnosis of ALS patients to randomly selected population-controls might have attributed to part of the contrasting results pattern. Using cerebrospinal fluid (CSF) samples of ALS patients, it was shown that a pro-inflammatory cytokine cascade was initiated following microglia activation34. However, in another study, ALS patients and controls were not found to differ in terms of IL-6 and TNF-α in either blood or CSF, and no strong correlation was found between the levels of these markers in blood and CSF either35. PD patients have been shown to have increased levels of pro-inflammatory cytokines in blood, CSF, and midbrain36–38. T-cells and their composition in blood have also been related to the disease severity of human PD39,40. Signs of DNA damage and increased levels of apoptosis markers have been described in the peripheral lymphocytes of PD patients41,42 as well as infiltration of T cells into the site of neuronal injury39. A prospective cohort study showed that a high plasma concentration of interleukin-6 was associated with an increased risk of PD; the result was however limited to men43. Animal studies have also suggested that the neuro-inflammatory cascades involved in ALS and PD might differ between males and females44–46, we found however little evidence to suggest gender-specific associations between the studied blood immune biomarkers and the risks of ALS and PD.
Evidence accumulated from human studies is mostly based on diagnosed or deceased patients with ALS or PD so far, whereas little knowledge exists for immune responses before the onset of these diseases. Such studies are inherently difficult in humans because of the lack of large prospective studies with available biological samples or relevant measures, especially concerning the central nervous system. Therefore, taking advantage of a large cohort study with 20 years’ follow-up of more than half a million participants, we examined the dynamic changes of leukocytes, IgG, haptoglobin, and uric acid during the 20 years before the diagnosis of ALS and PD. Patients with PD were found to have a statistically significantly lower level of uric acid during most part of the 20 years before diagnosis, compared to controls. Patients with PD also tended to have lower levels of leukocytes and haptoglobin between 20 and 10 years before diagnosis, compared to controls, the differences were however statistically significant only for part of the time interval after multivariable adjustment. The findings on ALS are less straightforward. Although no statistically significant association was noted between any of the three immune biomarkers studied and the risk of ALS, our results were suggestive of some differences in the levels of these biomarkers in ALS patients during the 20 years before diagnosis, compared to controls. For example, during the 10 years before diagnosis, patients with ALS tended to have lower levels of IgG and haptoglobin whereas higher levels of leukocyte than controls. None of the suggested differences was however statistically significant after multivariable adjustment. The complexity of these findings might be attributable to different factors. First, although this is the biggest study of its kind so far, the relatively small number of ALS cases, in comparison to PD, makes it more difficult to detect statistically significant associations or differences in the analysis of ALS. Second, it is possible that the prodromal non-motor symptoms of ALS, including an altered peripheral immune response, did indeed start around 10 years before diagnosis. Such a possibility is supported by our previous study demonstrating altered lipid and apolipoprotein metabolisms during the 10 years before ALS diagnosis, compared to before28.
The underlying mechanisms for the noted associations between leukocytes, haptoglobin, and PD are unknown. Although we agree that peripheral adaptive immunity is involved in PD, we have relatively little knowledge about the detailed peripheral immune phenotypes in PD. For example, a recent study showed that PD patients had lower levels of Th2, Th17 and regulatory T cells in blood, compared to age- and sex-matched healthy controls47. It is therefore tempting to speculate that the lower risk of PD in relation to higher concentration of lymphocytes as shown in our study might be due to the increased levels of these specific T cell subsets. Furthermore, because the concentrations of leukocytes and haptoglobin observed in our study are within the normal ranges, these measurements are unlikely directly useful as predictive biomarkers in the diagnosis of PD. Regardless, further studies are warranted to validate these findings in other populations and assess the potential use of the studied biomarkers in predicting disease progression of patients with PD. Such additional findings could in turn shed light on novel therapeutic targets for PD potentially, although SURE-PD3, the Study of URate Elevation in Parkinson’s disease, was prematurely stopped after an interim analysis, which concluded that long-term treatment with inosine would be unlikely to demonstrate benefit in patients with PD (https://www.ninds.nih.gov/Disorders/Clinical-Trials/Study-Urate-Elevation-Parkinsons-Disease-Phase-3-SURE-PD3).
The main strengths of our study include the population-based design, the large sample size with long and complete follow-up, the prospectively and repeatedly collected information on several key biomarkers for peripheral immune responses prior to the diagnosis of ALS and PD, the possibility to study different subtypes of immune cells, the independent identification of study outcomes, and the novel design to contrast findings between ALS and PD – two neurodegenerative diseases that are known to share pathophysiological similarities. These strengths precluded greatly the concern of selection and information biases as commonly noted in observational studies and provide a unique opportunity to demonstrate the temporal change of peripheral immune responses during two decades before the clinical diagnosis of ALS and PD. Another strength is the fact that all biomarker measurements of the AMORIS Study were conducted on fresh blood samples, by the same laboratory that used the same techniques and reagents during the entire enrolment period. Finally, to assess the robustness of our findings, we used uric acid as a quality control and found that increasing level of uric acid was associated with a decreasing risk of PD.
There are also limitations in the study, including the lack of possibility to clinically verify all diagnoses for ALS and PD, the likely delayed ascertainment of ALS and PD diagnoses using in- and out-patient hospital discharge records, and the possibility for residual confounding. A diagnosis of ALS based on the hospital discharge records of the Swedish Patient Register has been shown to have a high positive predictive value and a diagnostic delay of around one year12.
The diagnosis of PD based on inpatient care records of this register has on the other hand a slightly lower positive predictive value and a longer diagnostic delay13. The validity of PD diagnosis and the delay of ascertainment should however have improved substantially after 2001 when the vast majority of the patients were identified first through outpatient specialist care. To circumstance the concern of delayed ascertainment of ALS and PD, we excluded the first year of follow-up from the cohort analysis for ALS and the first five years of follow-up from the cohort analysis of PD. The fact that, compared to controls, PD patients tended to have lower levels of leukocyte and haptoglobin more than 10 years before PD diagnosis - although not always statistically significant - but not thereafter could however alleviate concerns over potential reverse causality. Further, because of the known link between smoking and PD, we made every effort to collect information on smoking for the AMORIS participants. However, the similar results obtained in the sensitivity analysis with further adjustment for smoking, as in the main analysis, suggested that smoking is not likely a critical confounder for the studied associations. Finally, the number of ALS cases identified during the study is relatively small, compared to PD, leading to a lack of statistical power in the analysis of ALS. This might explain the lack of statistical significance for the association between uric acid and the risk of ALS, although the magnitude of the association was rather similar to that of the association between uric acid and PD. However, the present study is still the biggest of its kind to date to address the associations between peripheral immune biomarkers and the risks of ALS and PD.
In summary, our findings suggest a different role of systemic inflammation on the subsequent risk of PD, compared to ALS. Studies from independent populations are warranted to verify these findings and to further test this hypothesis.
Supplementary Material
Acknowledgements
The authors thank sincerely Ingmar Jungner for his important contribution to the AMORIS study. The study was supported by the European Research Council (ERC) Starting Grant (No. 802091), the Swedish Research Council (grant No. 2015-03170), the Gunnar and Ingmar Jungner Foundation for Laboratory Medicine, and the Karolinska Institutet (Senior Researcher Award and Strategic Research Area in Epidemiology).
Footnotes
Author Contributions
All authors contributed to study design, data analysis, result interpretation, and manuscript drafting of the study. NH and GW were responsible for the acquisition of data.
Potential Conflicts of Interest
Nothing to report.
Data Availability
Researchers can apply for access to data from the AMORIS Study for well-defined research questions that are in line with the overall research agenda for the cohort. The steering committee of AMORIS study can be contacted via https://ki.se/en/imm/amoris.
References
- 1.Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease--a double-edged sword. Neuron. 2002;35:419–432. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- 2.Godbout JP, Chen J, Abraham J, et al. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J. 2015;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- 3.Qin L, Wu X, Block ML, et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 5.Hardiman O, Al-Chalabi A, Chio A, et al. Amyotrophic lateral sclerosis. Nat Rev Dis Primers. 2017;3:17085. doi: 10.1038/nrdp.2017.85. [DOI] [PubMed] [Google Scholar]
- 6.Molteni M, Rossetti C. Neurodegenerative diseases: The immunological perspective. J Neuroimmunol. 2017;313:109–115. doi: 10.1016/j.jneuroim.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Walldius G, Malmstrom H, Jungner I, et al. Cohort Profile: The AMORIS cohort. Int J Epidemiol. 2017;46:1103-1103i. doi: 10.1093/ije/dyw333. [DOI] [PubMed] [Google Scholar]
- 8.Weisskopf MG, O’Reilly E, Chen H, et al. Plasma urate and risk of Parkinson’s disease. Am J Epidemiol. 2007;166:561–567. doi: 10.1093/aje/kwm127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen H, Mosley TH, Alonso A, et al. Plasma urate and Parkinson’s disease in the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol. 2009;169:1064–1069. doi: 10.1093/aje/kwp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paganoni S, Zhang M, Quiroz Zarate A, et al. Uric acid levels predict survival in men with amyotrophic lateral sclerosis. J Neurol. 2012;259:1923–1928. doi: 10.1007/s00415-012-6440-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Reilly EJ, Liu D, Johns DR, et al. Serum urate at trial entry and ALS progression in EMPOWER. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18:120–125. doi: 10.1080/21678421.2016.1214733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Longinetti E, Regodon Wallin A, Samuelsson K, et al. The Swedish motor neuron disease quality registry. Amyotroph Lateral Scler Frontotemporal Degener. 2018;19:528–537. doi: 10.1080/21678421.2018.1497065. [DOI] [PubMed] [Google Scholar]
- 13.Feldman AL, Johansson AL, Gatz M, et al. Accuracy and sensitivity of Parkinsonian disorder diagnoses in two Swedish national health registers. Neuroepidemiology. 2012;38:186–193. doi: 10.1159/000336356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muscatell KA, Brosso SN, Humphreys KL. Socioeconomic status and inflammation: a meta-analysis. Molecular Psychiatry. 2018 doi: 10.1038/s41380-018-0259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang F, Johansson AL, Pedersen NL, et al. Socioeconomic status in relation to Parkinson’s disease risk and mortality: A population-based prospective study. Medicine (Baltimore) 2016;95:e4337. doi: 10.1097/MD.0000000000004337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts AL, Johnson NJ, Chen JT, et al. Race/ethnicity, socioeconomic status, and ALS mortality in the United States. Neurology. 2016;87:2300–2308. doi: 10.1212/WNL.0000000000003298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ascherio A, Schwarzschild MA. The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol. 2016;15:1257–1272. doi: 10.1016/S1474-4422(16)30230-7. [DOI] [PubMed] [Google Scholar]
- 18.Bandres-Ciga S, Noyce AJ, Hemani G, et al. Shared polygenic risk and causal inferences in amyotrophic lateral sclerosis. Ann Neurol. 2019;85:470–481. doi: 10.1002/ana.25431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhan Y, Fang F. Smoking and amyotrophic lateral sclerosis: A mendelian randomization study. Ann Neurol. 2019;85:482–484. doi: 10.1002/ana.25443. [DOI] [PubMed] [Google Scholar]
- 20.King CC, Piper ME, Gepner AD, et al. Longitudinal Impact of Smoking and Smoking Cessation on Inflammatory Markers of Cardiovascular Disease Risk. Arterioscler Thromb Vasc Biol. 2017;37:374–379. doi: 10.1161/ATVBAHA.116.308728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dupuis L, Pradat PF, Ludolph AC, et al. Energy metabolism in amyotrophic lateral sclerosis. Lancet Neurol. 2011;10:75–82. doi: 10.1016/S1474-4422(10)70224-6. [DOI] [PubMed] [Google Scholar]
- 22.Huang X, Abbott RD, Petrovitch H, et al. Low LDL cholesterol and increased risk of Parkinson’s disease: prospective results from Honolulu-Asia Aging Study. Mov Disord. 2008;23:1013–1018. doi: 10.1002/mds.22013. [DOI] [PubMed] [Google Scholar]
- 23.Huang X, Alonso A, Guo X, et al. Statins, plasma cholesterol, and risk of Parkinson’s disease: a prospective study. Mov Disord. 2015;30:552–559. doi: 10.1002/mds.26152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang X, Chen H, Miller WC, et al. Lower low-density lipoprotein cholesterol levels are associated with Parkinson’s disease. Mov Disord. 2007;22:377–381. doi: 10.1002/mds.21290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lima WG, Martins-Santos ME, Chaves VE. Uric acid as a modulator of glucose and lipid metabolism. Biochimie. 2015;116:17–23. doi: 10.1016/j.biochi.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 26.McCombe PA, Henderson RD. The Role of immune and inflammatory mechanisms in ALS. Curr Mol Med. 2011;11:246–254. doi: 10.2174/156652411795243450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kannarkat GT, Boss JM, Tansey MG. The role of innate and adaptive immunity in Parkinson’s disease. J Parkinsons Dis. 2013;3:493–514. doi: 10.3233/JPD-130250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mariosa D, Hammar N, Malmstrom H, et al. Blood biomarkers of carbohydrate, lipid, and apolipoprotein metabolisms and risk of amyotrophic lateral sclerosis: A more than 20-year follow-up of the Swedish AMORIS cohort. Ann Neurol. 2017;81:718–728. doi: 10.1002/ana.24936. [DOI] [PubMed] [Google Scholar]
- 29.Rozani V, Gurevich T, Giladi N, et al. Higher serum cholesterol and decreased Parkinson’s disease risk: A statin-free cohort study. Mov Disord. 2018;33:1298–1305. doi: 10.1002/mds.27413. [DOI] [PubMed] [Google Scholar]
- 30.Murdock BJ, Bender DE, Kashlan SR, et al. Increased ratio of circulating neutrophils to monocytes in amyotrophic lateral sclerosis. Neurol Neuroimmunol Neuroinflamm. 2016;3:e242. doi: 10.1212/NXI.0000000000000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murdock BJ, Zhou T, Kashlan SR, et al. Correlation of Peripheral Immunity With Rapid Amyotrophic Lateral Sclerosis Progression. JAMA Neurol. 2017;74:1446–1454. doi: 10.1001/jamaneurol.2017.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zondler L, Muller K, Khalaji S, et al. Peripheral monocytes are functionally altered and invade the CNS in ALS patients. Acta Neuropathol. 2016;132:391–411. doi: 10.1007/s00401-016-1548-y. [DOI] [PubMed] [Google Scholar]
- 33.Gustafson MP, Staff NP, Bornschlegl S, et al. Comprehensive immune profiling reveals substantial immune system alterations in a subset of patients with amyotrophic lateral sclerosis. PLoS One. 2017;12:e0182002. doi: 10.1371/journal.pone.0182002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuhle J, Lindberg RL, Regeniter A, et al. Increased levels of inflammatory chemokines in amyotrophic lateral sclerosis. Eur J Neurol. 2009;16:771–774. doi: 10.1111/j.1468-1331.2009.02560.x. [DOI] [PubMed] [Google Scholar]
- 35.Moreau C, Devos D, Brunaud-Danel V, et al. Elevated IL-6 and TNF-alpha levels in patients with ALS: inflammation or hypoxia? Neurology. 2005;65:1958–1960. doi: 10.1212/01.wnl.0000188907.97339.76. [DOI] [PubMed] [Google Scholar]
- 36.Wang Q, Liu Y, Zhou J. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl Neurodegener. 2015;4:19. doi: 10.1186/s40035-015-0042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- 38.Rektor I, Goldemund D, Sheardova K, et al. Vascular pathology in patients with idiopathic Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:24–29. doi: 10.1016/j.parkreldis.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 39.Brochard V, Combadiere B, Prigent A, et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest. 2009;119:182–192. doi: 10.1172/JCI36470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stevens CH, Rowe D, Morel-Kopp MC, et al. Reduced T helper and B lymphocytes in Parkinson’s disease. J Neuroimmunol. 2012;252:95–99. doi: 10.1016/j.jneuroim.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 41.Blandini F, Mangiagalli A, Cosentino M, et al. Peripheral markers of apoptosis in Parkinson’s disease: the effect of dopaminergic drugs. Ann N Y Acad Sci. 2003;1010:675–678. doi: 10.1196/annals.1299.123. [DOI] [PubMed] [Google Scholar]
- 42.Migliore L, Petrozzi L, Lucetti C, et al. Oxidative damage and cytogenetic analysis in leukocytes of Parkinson’s disease patients. Neurology. 2002;58:1809–1815. doi: 10.1212/wnl.58.12.1809. [DOI] [PubMed] [Google Scholar]
- 43.Chen H, O’Reilly EJ, Schwarzschild MA, et al. Peripheral inflammatory biomarkers and risk of Parkinson’s disease. Am J Epidemiol. 2008;167:90–95. doi: 10.1093/aje/kwm260. [DOI] [PubMed] [Google Scholar]
- 44.Cardona AE, Pioro EP, Sasse ME, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- 45.Frutiger K, Lukas TJ, Gorrie G, et al. Gender difference in levels of Cu/Zn superoxide dismutase (SOD1) in cerebrospinal fluid of patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2008;9:184–187. doi: 10.1080/17482960801984358. [DOI] [PubMed] [Google Scholar]
- 46.Naumenko N, Pollari E, Kurronen A, et al. Gender-Specific Mechanism of Synaptic Impairment and Its Prevention by GCSF in a Mouse Model of ALS. Front Cell Neurosci. 2011;5:26. doi: 10.3389/fncel.2011.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kustrimovic N, Comi C, Magistrelli L, et al. Parkinson’s disease patients have a complex phenotypic and functional Th1 bias: cross-sectional studies of CD4+ Th1/Th2/T17 and Treg in drug-naive and drug-treated patients. J Neuroinflammation. 2018;15:205. doi: 10.1186/s12974-018-1248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Researchers can apply for access to data from the AMORIS Study for well-defined research questions that are in line with the overall research agenda for the cohort. The steering committee of AMORIS study can be contacted via https://ki.se/en/imm/amoris.