Abstract
Aims
To explore the glucose-overload hypothesis of artefactual gestational diabetes (GDM) diagnosis in shorter women during oral glucose tolerance testing (OGTT), by investigating associations between height and maternal glycemia; and GDM and pregnancy complications in height-groups.
Methods
Women from GUSTO (n=1100, 2009-2010) and NUH (n=4068, 2017-2018) cohorts underwent a two and three time-point mid-gestation 75g 2-hour OGTT, respectively. GDM-related complications (hypertensive disorders of pregnancy, preterm delivery, emergency cesarean section, neonatal intensive care unit admission, macrosomia, birthweight) were compared within shorter and taller groups, dichotomized by ethnic-specific median height.
Results
Using WHO-1999 criteria, 18.8% (GUSTO) to 22.9% (NUH) of women were diagnosed with GDM-1999; and by WHO-2013 criteria, 21.9% (NUH) had GDM-2013. Each 5-cm height increment was inversely associated with GDM-1999 (adjusted odds ratio [aOR, 95%CI]=0.81 [0.76-0.87], 2h-glycemia (adjusted β [95%CI]=-0.171mmol/L [-0.208,-0.135]) and 1h-glycemia (aβ=-0.160mmol/L [-0.207,-0.112]). The inverse association between height and 2h-glycemia was most marked in “Other” ethnicities (Eurasians/Caucasians/mixed/other Asians) and Indians, followed by Chinese, then Malays. Compared with non-GDM, GDM-1999 was associated with preterm delivery (aOR=1.76 [1.19-2.61]) and higher birthweight (aβ=57.16g [20.95,93.38]) only among taller but not shorter women.
Conclusions
Only taller women had an increased odds of GDM-related pregnancy complications. An artefactual GDM diagnosis due to glucose-overload among shorter women is plausible.
Keywords: Blood glucose, Cohort study, Gestational diabetes mellitus, Glucose tolerance test, Pregnancy complications, Standing height
Introduction
Gestational diabetes mellitus (GDM), defined as glucose intolerance first diagnosed in pregnancy[1], affects approximately 16% of pregnancies worldwide, with the highest prevalence of 27% in the South-East Asian region as compared to 21% in the North American and the Caribbean regions in 2019[2]. These population differences could be due to genetic, social and environmental factors[3]. Several known risk factors for GDM include increasing maternal age, ethnicity, obesity, poor diet, sedentary lifestyle, family history of diabetes, excessive gestational weight gain, and previous history of GDM[1,3,4].
Standing height has been inversely associated with increased risks of GDM[5] and type 2 diabetes[6,7]. Adult height per se is unlikely to have a causal effect on the development of glucose intolerance. Earlier hypothesis suggests that height and metabolic risk are both determined by a complex interplay of genetic traits and environmental factors[8,9], thus, height likely acts as a proxy marker that reflects these underlying exposures.
On average, Western populations have a taller stature than Asian populations[10]. Whether this is predominantly due to genetic or environmental influences remains unclear. Apart from shared early life etiologies for both shorter stature and GDM, a plausible alternative explanation for higher GDM risks among shorter women is an artefact (false-positive) secondary to glucose overload from the fixed load 75-g oral glucose tolerance test (OGTT)[11]. If so, a disproportionately higher proportion of shorter women would be misdiagnosed as “GDM” and may not truly have impaired glucose regulation. Whether this phenomenon contributes to the high incidence of GDM among Asian women, who are generally of shorter height, is unknown. If confirmed, it would prompt reconsiderations of methodologies used to diagnose GDM, interpretation of results and management of GDM in populations of shorter stature in order to avoid over-intervention and unnecessary strain on healthcare resources. While there is limited clinical harm in over-diagnosing shorter women with false-positive diagnosis of GDM since treatment in the form of self-blood glucose monitoring, diet and lifestyle changes are not in themselves harmful; this could impact healthcare delivery resources and burden patients unnecessarily.
Published evidence suggested that shorter maternal height and GDM could each independently increase risks of pregnancy and neonatal complications[12,13]. We postulated that shorter women misdiagnosed as “GDM” due to glucose overload during OGTT would not be expected to have additional risks associated with GDM over and above those associated with shorter stature; and this has not been studied previously.
Our first hypothesis was that shorter women would have increased odds of being labelled “GDM” because of an OGTT post-challenge artefact. Our second hypothesis was that being shorter and labelled with “GDM” would not be associated with an increased risk of GDM-related pregnancy complications compared with women of similar height without GDM. Thus, within an Asian setting we aimed to: (i) examine the association of height and maternal glycemia; and (ii) compare the odds of pregnancy complications in GDM and non-GDM cases in different height groups.
Methods
Study populations
Data was obtained from two cohorts: the Growing Up in Singapore Towards healthy Outcomes (GUSTO) mother-offspring cohort study[14] and the National University Hospital (NUH) cohort.
Briefly, GUSTO is a prospective mother-offspring cohort designed to examine early-life developmental pathways to non-communicable diseases. Pregnant women attending a first trimester dating ultrasound scan (<14 weeks) at the KK Women’s and Children’s
Hospital (KKH) and NUH were enrolled in 2009–2010 (n=1,247). Eligibility was restricted to the three major ethnic groups in Singapore: Chinese, Indian and Malay, and excluded women with type 1 diabetes, and those receiving chemotherapy or psychotropic drugs. Ethical approval was obtained from the National Health Care Group Domain Specific Review Board (reference D/09/021) and the SingHealth Centralized Institutional Review Board (reference 2009/280/D). Written informed consent was obtained from all participants.
A separate NUH-only cohort encompassed all women who received antenatal care and delivered in the hospital in 2017–2018 (n=5,500), a period when universal OGTT screening for GDM was already being practiced. This cohort comprised of Chinese, Indian, Malay and “Other” (Eurasians, White Caucasians, mixed Asians, other Asians) ethnicities. Use of this anonymized dataset extracted from clinical records was ethically approved with waiver of consent granted by the National Health Care Group Domain Specific Review Board (reference 2019/01172).
As shown in the study flowchart (Supplementary Figure S1), only singleton pregnancies were included, and women from the GUSTO and NUH cohorts who did not have an OGTT (known pre-existing Type 1 and Type 2 diabetes mellitus or declined OGTT) were excluded. A total of 1,100 and 4,068 women from the GUSTO and NUH cohorts, respectively, with valid information on height, ethnicity, maternal age, booking weight and glucose concentrations measured during an OGTT were analyzed.
Main exposures and outcomes
In GUSTO, maternal standing height was measured at 26-28 weeks’ gestation in duplicate using a manually-read portable stadiometer (SECA 213). In the NUH cohort, maternal height was measured at the first antenatal clinic visit using the hospital ultrasonic stadiometer (Avatech) that provides digital readings. A two and three time-point 75 g mid-gestation OGTT was conducted in the GUSTO and NUH cohorts, respectively. Fasting plasma glucose (FPG) was measured after an overnight fast during a morning visit. Two diagnostic criteria were applied retrospectively to define GDM for this specific study: (1) GDM-1999: World Health Organization (WHO) 1999 criteria of FPG ≥7.0 mmol/L and/or 2-h post-challenge plasma glucose (2hPG) ≥7.8 mmol/L[15], (2) GDM-2013: WHO 2013 criteria of FPG ≥5.1 mmol/L and/or 1-h post-challenge plasma glucose (1hPG) ≥10.0 mmol/L and/or 2hPG ≥8.5 mmol/L. During pregnancy, GDM-1999 and GDM-2013 were used in the GUSTO and NUH cohort, respectively, to diagnose GDM.
The following GDM-related maternal/neonatal complications, chosen based on published literature [16] were studied: (1) hypertensive disorders of pregnancy (including pre-eclampsia and pregnancy-induced hypertension), (2) preterm delivery (<37 completed weeks’ gestation), (3) emergency cesarean section, (4) admission to neonatal intensive care unit (NICU), (5) macrosomia ≥4.0 kg, (6) low birthweight (<2.5 kg), and (7) birthweight as a continuous variable.
Covariates
In GUSTO, standardized questionnaires were administered to collect participant characteristics (age, ethnicity, parity, previous cesarean section). Clinical information of the index pregnancy was extracted from medical records. Maternal booking weight was recorded at the first antenatal visit (≤14 weeks’ gestation). Gestational age was calculated using the Hadlock crown-rump length curves measured by ultrasound scan at 7-12 weeks[17]. For the NUH cohort, all data were obtained anonymously from databases populated by information available in medical records.
Statistical analysis
To analyze the association between height and maternal glycemia, multiple logistic and linear regression was used to model categorical (GDM) and continuous (FPG, 1hPG, 2hPG) outcome variables, respectively, with height as an exposure variable. Odds ratios (OR) or beta coefficients (β) and their corresponding 95% CI are reported for each 5-cm height increment. All models were adjusted for maternal age, booking weight and ethnicity. The interaction between height and ethnicity on maternal glycemia was explored. A two-sided P value<0.05 was considered statistically significant.
Next, we explored the associations of GDM-1999 with pregnancy complications in combined cohorts following stratification into shorter and taller groups. The median maternal height of each ethnic group was used to categorize women into shorter and taller groups (median height [interquartile range, cm] of Chinese, n=2,229: 159.0 [155.2-163.0]; Malay, n=1,426: 157.0 [153.0-161.0]; Indian, n=854: 158.0 [154.6-162.0]; Caucasian, n=72: 166.0 [162.0-170.0]; Eurasian, n=6: 161.5 [156.0-163.0]; Others, n=581: 157.0 [154.0-162.0]). Multiple logistic regression was used to examine the odds of complications for each height group using different models according to the outcomes; covariates used for adjustment are detailed at the bottom of each table. Missing values were not imputed and only cases with complete datasets were used.
The group without GDM-1999 was used as the reference group across each height strata. Similar analyses were conducted for GDM-2013 using the NUH cohort. Additionally, the association between FPG and 2hPG as continuous variables with each pregnancy complication within each height strata was examined. To account for multiplicity, a two-sided P value<0.007 (0.05/7 outcomes) was considered statistically significant.
Sensitivity analyses were performed excluding those with elevated FPG (i.e. not due to glucose overload and therefore genuine GDM) and categorizing GDM-1999 or GDM-2013 solely based on an elevated 1hPG or 2hPG within each height strata.
All analyses were performed using Stata 15 software (College Station, TX: StataCorp LLC) and forest plots generated using the R package “forestplot”[18].
Results
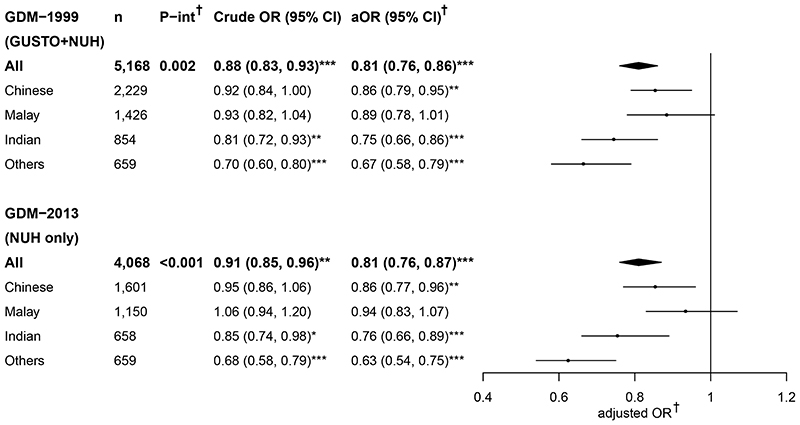
Characteristics of women across cohorts (the GUSTO, NUH and combined cohorts; Table 1) and in ethnic or GDM groups (Supplementary Tables S1-S2) are described. Increasing height was associated with reduced odds of GDM-1999 and GDM-2013, with differences being more apparent following adjustment for age, weight and ethnicity: a 19% reduction in GDM odds per 5-cm height increment among all women across cohorts (Figure 1). Significant interactions between ethnicity and height on GDM-1999 were found in the combined cohorts and on GDM-2013 in the NUH cohort. The inverse association was most marked in the “Other” ethnic group, with a 37% reduction in GDM-2013 odds for every 5-cm height increment. An odds reduction of 24-25% and 14% in Indians and Chinese, respectively, were observed for GDM-1999 and GDM-2013 per 5-cm height increment across cohorts; while a non-significant trend of a reduction in the odds of GDM was observed in Malays.
Table 1. Participant characteristics for the GUSTO and NUH cohorts, and both cohorts combined.
| Characteristics | Combined GUSTO+NUH | GUSTO | NUH | P* |
|---|---|---|---|---|
| (n=5,168) | (n=1,100) | (n=4,068) | ||
| Age (year), mean (SD) | 32.8 (4.7) | 31.2 (5.1) | 33.3 (4.4) | <0.001 |
| Ethnicity, n (%) | <0.001 | |||
| Chinese | 2,229 (43.1) | 628 (57.1) | 1,601 (39.3) | |
| Malay | 1,426 (27.6) | 276 (25.1) | 1,150 (28.3) | |
| Indian | 854 (16.5) | 196 (17.8) | 658 (16.2) | |
| Others | 659 (12.8) | - | 659 (16.2) | |
| Height (cm), mean (SD) | 158.4 (6.1) | 158.3 (5.6) | 158.4 (6.2) | 0.521 |
| Early pregnancy weight (kg), mean (SD) | 61.6 (12.7) | 59.3 (12.4) | 62.3 (12.7) | <0.001 |
| Early pregnancy BMI (kg/m2), mean (SD) | 24.6 (4.9) | 23.6 (4.7) | 24.8 (4.9) | <0.001 |
| Glucose measures (mmol/L), mean (SD) | ||||
| FPG | 4.4 (0.5) | 4.4 (0.5) | 4.4 (0.5) | 0.993 |
| 1hPG† | - | - | 8.2 (1.8) | |
| 2hPG | 6.7 (1.6) | 6.5 (1.5) | 6.8 (1.6) | <0.001 |
| GDM, n (%) | ||||
| WHO 1999 criteria | 1,140 (22.1) | 207 (18.8) | 933 (22.9) | 0.003 |
| WHO 2013 criteria | - | - | 889 (21.9) | |
| GDM-related complications, n (%) | ||||
| Hypertensive disorders of pregnancy | 184 (3.6) | 68 (6.2) | 116 (2.9) | <0.001 |
| Preterm delivery (<37 weeks) | 330 (6.4) | 81 (7.4) | 249 (6.1) | 0.133 |
| Emergency cesarean section | 727 (14.1) | 106 (9.6) | 621 (15.3) | <0.001 |
| NICU admission | 334 (6.5) | 72 (6.6) | 262 (6.4) | 0.900 |
| Macrosomia (≥4 kg) | 85 (1.7) | 19 (1.7) | 66 (1.6) | 0.808 |
| Low birthweight (<2.5 kg) | 433 (8.4) | 94 (8.6) | 339 (8.3) | 0.821 |
Only available in the NUH cohort with oral glucose tolerance test at three time-point.
Significant difference between GUSTO and NUH cohorts using independent samples t-test for continuous data and Chi-square test for categorical data.
1hPG, 1-h plasma glucose; 2hPG, 2-h plasma glucose; FPG, fasting plasma glucose; GDM, gestational diabetes mellitus; NICU, neonatal intensive care units; SD, standard deviation
Figure 1. Crude and adjusted odds ratio of GDM associated with height (per 5-cm increment) among all women and when stratified by ethnic groups.
†Adjusted for maternal age, booking weight (and ethnicity for “All” women analyses).
P-interaction denotes the P value for the interaction effects between height and ethnicity on GDM.
*P<0.05; **P<0.01; ***P<0.001.
aOR, adjusted odds ratio; GDM-1999, gestational diabetes diagnosed by WHO 1999 criteria;
GDM-2013, gestational diabetes diagnosed by WHO 2013 criteria; OR, odds ratio; P-int, P-interaction
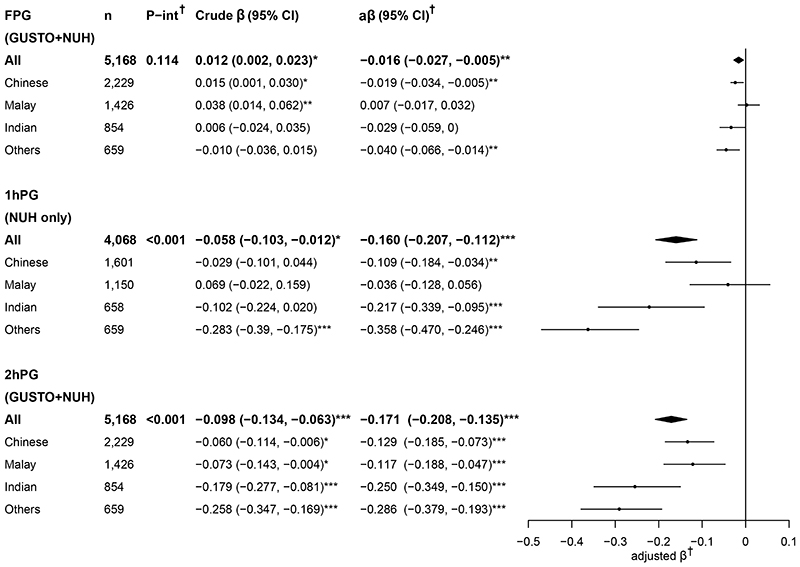
When glycemia was analyzed as a continuous variable (Figure 2), increasing height was associated with decreasing post-challenge glycemia – both 1hPG and 2hPG, in adjusted analyses in all ethnic groups except in Malays where 1hPG showed no association with height. The magnitude of reduction in post-challenge glycemia with every 5-cm height increment was largest in women of “Other” ethnicity, followed by Indians, then Chinese and lastly Malays. The association between height and FPG was less consistent, with demonstrations of a positive or no association in unadjusted analysis, but a more negative association after adjusting for age, weight and ethnicity across ethnic groups. Analyses performed for each cohort separately showed similar results (Supplementary Figures S2-S3).
Figure 2. Crude and adjusted beta coefficients of plasma glucose (mmol/L) associated with height (per-5cm increment) among all women and when stratified by ethnic groups.
†Adjusted for maternal age, booking weight (and ethnicity for “All” women analyses).
P-interaction denotes the P value for the interaction effects between height and ethnicity on plasma glucose.
*P<0.05; **P<0.01; ***P<0.001.
1hPG, 1-h plasma glucose; 2hPG, 2-h plasma glucose; aβ, adjusted beta coefficient; FPG, fasting plasma glucose; P-int, P-interaction
With regard to pregnancy complications, overall in combined cohorts each 5-cm increase in maternal height was associated with reduced odds of emergency cesarean section (aOR=0.81, 95% CI: 0.75-0.87) after adjusting for maternal age, ethnicity, body mass index (BMI), parity, and previous cesarean section. There was no association between each 5-cm increase in maternal height and hypertensive disorders of pregnancy (aOR= 0.94, 0.83-1.07) or preterm delivery (aOR=0.90, 0.82-1.00) following adjustment for maternal age, ethnicity, BMI, and parity.
Next, the combined cohorts were stratified into shorter and taller groups by ethnic-specific median heights to assess associations of GDM with pregnancy complications (Table 2). In all women and within each ethnic group, the odds of GDM were in general lower among taller women than among shorter women (Supplementary Table S3). Despite showing lower odds of GDM overall, taller women diagnosed with GDM-1999 were more likely to deliver preterm (aOR=1.76, 1.19-2.61) and have newborns with greater birthweight (aβ=57.16 g, 95% CI: 20.95-93.38) compared with taller women without GDM in adjusted analyses. Meanwhile the associations of GDM-1999 with preterm delivery (aOR=1.39, 1.00-1.93) and birthweight (aβ=31.72 g, 0.10-63.33) were weaker among shorter women. No differences were observed in the odds of hypertensive disorders of pregnancy or emergency cesarean section with GDM-1999 in either shorter or taller women. Similar trends were observed with GDM-2013 criteria.
Table 2. Association of GDM and pregnancy complications in two different strata of height (shorter and taller stratified by ethnic-specific median height).
| GDM-1999 | GDM-2013 | ||||||
|---|---|---|---|---|---|---|---|
| Complications | Group | Events/Total | aOR (95% CI) | P | Events/Total | aOR (95% CI) | P |
| Hypertensive disorders of pregnancya | Shorter; no GDM | 77/2,036 | Ref. | 101/1,609 | Ref. | ||
| Shorter; GDM | 32/653 | 1.21 (0.78, 1.88) | 0.388 | 42/494 | 1.04 (0.68, 1.61) | 0.841 | |
| Taller; no GDM | 57/1,989 | Ref. | 81/1,566 | Ref. | |||
| Taller; GDM | 18/487 | 1.07 (0.62, 1.87) | 0.800 | 36/393 | 1.54 (0.96, 2.46) | 0.075 | |
| Preterm deliverya | Shorter; no GDM | 134/2,036 | Ref. | 101/1,610 | Ref. | ||
| Shorter; GDM | 60/653 | 1.39 (1.00, 1.93) | 0.047 | 49/495 | 1.56 (1.08, 2.26) | 0.017 | |
| Taller; no GDM | 96/1,988 | Ref. | 70/1,566 | Ref. | |||
| Taller; GDM | 40/487 | 1.76 (1.19, 2.61) | 0.004* | 29/394 | 1.69 (1.06, 2.68) | 0.026 | |
| Emergency cesarean sectionb | Shorter; no GDM | 314/2,033 | Ref. | 101/1,609 | Ref. | ||
| Shorter; GDM | 131/652 | 1.19 (0.94, 1.52) | 0.149 | 42/494 | 1.02 (0.78, 1.35) | 0.870 | |
| Taller; no GDM | 215/1,986 | Ref. | 81/1,566 | Ref. | |||
| Taller; GDM | 66/487 | 1.21 (0.88, 1.65) | 0.239 | 36/393 | 1.35 (0.97, 1.88) | 0.080 | |
| NICU admissionc | Shorter; no GDM | 132/2,036 | Ref. | 101/1,609 | Ref. | ||
| Shorter; GDM | 49/651 | 0.94 (0.63, 1.40) | 0.769 | 42/494 | 1.04 (0.68, 1.61) | 0.841 | |
| Taller; no GDM | 132/2,036 | Ref. | 81/1,566 | Ref. | |||
| Taller; GDM | 49/651 | 0.98 (0.62, 1.55) | 0.920 | 36/393 | 1.54 (0.96, 2.46) | 0.075 | |
| Macrosomiad | Shorter; no GDM | 24/2,036 | Ref. | 16/1,609 | Ref. | ||
| Shorter; with GDM | 4/651 | 0.56 (0.19, 1.63) | 0.286 | 4/494 | 0.80 (0.26, 2.49) | 0.702 | |
| Taller; no GDM | 40/1,988 | Ref. | 33/1,566 | Ref. | |||
| Taller; GDM | 17/486 | 1.85 (1.02, 3.36) | 0.044 | 13/393 | 1.97 (0.97, 3.98) | 0.060 | |
| Low birthweightd | Shorter; no GDM | 202/2,036 | Ref. | 157/1,609 | Ref. | ||
| Shorter; GDM | 74/651 | 0.99 (0.69, 1.42) | 0.945 | 60/494 | 1.00 (0.67, 1.50) | 0.987 | |
| Taller; no GDM | 124/1,988 | Ref. | 94/1,566 | Ref. | |||
| Taller; GDM | 33/486 | 0.66 (0.39, 1.10) | 0.108 | 28/393 | 0.71 (0.40, 1.25) | 0.231 | |
| n | aβ (95% CI) | P | n | aβ (95% CI) | P | ||
| Birthweight (continuous outcome; g)d | Shorter; no GDM | 2,036 | Ref. | 1,609 | Ref. | ||
| Shorter; GDM | 651 | 31.72 (0.10, 63.33) | 0.049 | 494 | 8.56 (-27.53, 44.66) | 0.642 | |
| Taller; no GDM | 1,988 | Ref. | 1,566 | Ref. | |||
| Taller; GDM | 486 | 57.16 (20.95, 93.38) | 0.002* | 393 | 42.03 (1.65, 82.41) | 0.041 |
Adjusted odds ratio or beta coefficients for GDM compared with no GDM.
Shorter and taller group stratified by ethnic-specific median maternal height.
Model 1: Adjusted for age, ethnicity, BMI, and parity
Model 2: Model 1 adjusted additionally for previous cesarean section
Model 3: Model 1 adjusted additionally for low birthweight <2.5 kg and gestational age
Model 4: Model 1 adjusted additionally for gestational age
A two-sided P value<0.007 (0.05/7 outcomes) was considered statistically significant to account for multiplicity.
aOR, adjusted odds ratio; aβ, adjusted beta coefficient; GDM-1999, gestational diabetes diagnosed by WHO 1999 criteria; GDM-2013, gestational diabetes diagnosed by WHO 2013 criteria; NICU, neonatal intensive care units
Further analyses were conducted using FPG alone (Table 3), which better reflects basal metabolic/glycemic state, and to dissociate this from the potential glucose overload effect, which would be reflected in “GDM” diagnosis and post-challenge glycemia. The association of glycemic level as a continuous variable and pregnancy complications within each height group was assessed. Increased odds of hypertensive disorders of pregnancy with increasing FPG (aOR=1.47, 1.24-1.74) were observed only among taller but not shorter women. Increased odds of preterm delivery (aOR=1.22, 1.04-1.43) and emergency cesarean section (aOR=1.17, 1.03-1.33) with increasing FPG were also observed in only taller women but the associations did not reach the pre-specified significance level of 0.007 taking multiple testing into account. To contrast FPG findings with those of post-challenge glycemia, which would include both appropriately and potentially artefactually-elevated (from glucose overload) measures, we examined the associations of complications with 2hPG (Table 3). Increased odds were observed only for the complication of preterm delivery among taller (aOR=1.33, 1.12-1.57) but less so in shorter women (aOR=1.15, 1.01-1.32). Similarly, there was a trend of increased odds of emergency cesarean section among taller (aOR=1.17, 1.02-1.33) but not shorter women (aOR=1.07, 0.97-1.19).
Table 3. Association of FPG and 2hPG with pregnancy complications in two different strata of height (shorter and taller stratified by ethnic-specific median height).
| FPG (mmol/L) | Associations with standardized FPG† | 2hPG (mmol/L) | Associations with standardized 2hPG† | |||||
|---|---|---|---|---|---|---|---|---|
| Complications | Group | n | Median (IQR) | aOR (95% CI) | P | Median (IQR) | aOR (95% CI) | P |
| Hypertensive disorders of pregnancya | Shorter | 2,689 | 4.3 (4.0-4.6) | 1.09 (0.93, 1.28) | 0.276 | 6.6 (5.8-7.7) | 1.17 (0.99, 1.39) | 0.069 |
| Taller | 2,476 | 4.3 (4.1-4.6) | 1.47 (1.24, 1.74) | <0.001* | 6.5 (5.6-7.4) | 1.04 (0.82, 1.32) | 0.736 | |
| Preterm deliverya | Shorter | 2,689 | 4.3 (4.0-4.6) | 1.10 (0.97, 1.25) | 0.148 | 6.6 (5.8-7.7) | 1.15 (1.01, 1.32) | 0.039 |
| Taller | 2,475 | 4.3 (4.1-4.6) | 1.22 (1.04, 1.43) | 0.014 | 6.5 (5.6-7.4) | 1.33 (1.12, 1.57) | 0.001* | |
| Emergency cesarean sectionb | Shorter | 2,685 | 4.3 (4.0-4.6) | 1.02 (0.93, 1.13) | 0.651 | 6.6 (5.8-7.7) | 1.07 (0.97, 1.19) | 0.170 |
| Taller | 2,473 | 4.3 (4.1-4.6) | 1.17 (1.03, 1.33) | 0.014 | 6.5 (5.6-7.4) | 1.17 (1.02, 1.33) | 0.020 | |
| NICU admissionc | Shorter | 2,687 | 4.3 (4.0-4.6) | 1.14 (1.00, 1.30) | 0.048 | 6.6 (5.8-7.7) | 1.03 (0.88, 1.20) | 0.718 |
| Taller | 2,474 | 4.3 (4.1-4.6) | 1.11 (0.94, 1.31) | 0.213 | 6.5 (5.6-7.4) | 1.00 (0.82, 1.21) | 0.988 | |
| Macrosomiad | Shorter | 2,687 | 4.3 (4.0-4.6) | 1.36 (1.12, 1.65) | 0.002* | 6.6 (5.8-7.7) | 1.07 (0.74, 1.56) | 0.720 |
| Taller | 2,474 | 4.3 (4.1-4.6) | 1.40 (1.11, 1.76) | 0.004* | 6.5 (5.6-7.4) | 1.33 (1.02, 1.74) | 0.036 | |
| Low birthweightd | Shorter | 2,687 | 4.3 (4.0-4.6) | 0.82 (0.69, 0.97) | 0.018 | 6.6 (5.8-7.7) | 0.95 (0.82, 1.10) | 0.474 |
| Taller | 2,474 | 4.3 (4.1-4.6) | 0.81 (0.65, 1.01) | 0.059 | 6.5 (5.6-7.4) | 0.78 (0.63, 0.98) | 0.030 | |
| aβ (95% CI) | P | aβ (95% CI) | P | |||||
| Birthweight (continuous outcome; g)d | Shorter | 2,687 | 4.3 (4.0-4.6) | 57.44 (44.12, 70.76) | <0.001* | 6.6 (5.8-7.7) | 27.20 (13.90, 40.50) | <0.001* |
| Taller | 2,474 | 4.3 (4.1-4.6) | 42.86 (27.39, 58.32) | <0.001* | 6.5 (5.6-7.4) | 34.06 (18.85, 49.26) | <0.001* | |
Adjusted odds ratio or beta coefficients per standard deviation increase in glucose. Shorter and taller group stratified by ethnic-specific median maternal height.
Model 1: Adjusted for age, ethnicity, BMI, and parity
Model 2: Model 1 adjusted additionally for previous cesarean section
Model 3: Model 1 adjusted additionally for low birthweight <2.5 kg and gestational age
Model 4: Model 1 adjusted additionally for gestational age
A two-sided P value<0.007 (0.05/7 outcomes) was considered statistically significant to account for multiplicity.
2hPG, 2-h plasma glucose; aOR, adjusted odds ratio; aβ, adjusted beta coefficient; FPG, fasting plasma glucose; IQR, interquartile range; NICU, neonatal intensive care unit
With respect to neonatal outcomes, both increasing maternal height and FPG was each associated with higher birthweight (aβ [95% CI]: 53.11 [45.03-61.21] g per 5-cm increment and 53.52 [43.35-63.69] g per standard deviation increment in glucose, respectively), after adjusting for maternal age, ethnicity, BMI, parity, and gestational age.
When stratified into height groups, FPG was associated with an increased odds of macrosomia and increasing birthweight in both shorter and taller groups (Table 3). As for 2hPG, a positive association was observed with birthweight in both shorter and taller women, but trends of an association with macrosomia (aOR=1.33, 1.02-1.74) and low birthweight (aOR=0.78, 0.63-0.98) was only observed among taller but not shorter women (macrosomia, aOR=1.07, 0.74-1.56 and low birthweight, aOR=0.95, 0.82-1.10) (Table 3).
There was no significant difference in NICU admissions with GDM or glycemia in either height group.
In sensitivity analyses conducted for GDM-1999 and GDM-2013 (Supplementary Table S4) which excluded GDM cases diagnosed by an elevated FPG (and therefore exclude likely true positives not due to artefactual glucose overload), similar trends and associations between GDM and pregnancy complications were observed in the taller but not shorter group.
Discussion
We observed inverse associations of maternal height with odds of GDM and post-challenge glucose concentrations. There were ethnic differences in the effect size of associations, with “Other” ethnicities (inclusive of non-Asians) showing the greatest reduction with increasing height, followed by Indians, then Chinese, and lastly Malays. There was no clear association between height and FPG. The novelty of our study is that we did not only examine GDM or maternal glycemia in relation to height, but also investigated GDM-associated complications. Interestingly, a GDM/“GDM” diagnosis was associated with increased odds of GDM-related complications including preterm delivery, increased birthweight, and an increased trend of macrosomia, in taller but not shorter women. Shorter women, who are more likely to be labelled as “GDM”, as a group, did not display any increase in GDM-related complications compared with non-GDM cases of similar height. This potentially suggests that the adverse implications of GDM in shorter women may be diluted by false-positive cases misdiagnosed as “GDM” possibly due to the artefact of glucose overload in an OGTT.
The prevalence of GDM in Singapore has remained relatively stable over the study period (2009-2010 vs. 2017-2018). The slightly higher incidence of GDM-1999 in the NUH cohort compared with the GUSTO cohort may be partly due to older age, higher BMI and different ethnic mix. Similarly, previous studies have shown that greater height was associated with lower GDM risks among women of various ethnicities[5,11,19,20]. The magnitude of association in our study (OR: 0.79-0.81) was comparable to previous studies on height and GDM risk. For example, the ethnic group with the strongest magnitude of association for GDM risk was Asian/Pacific Islander Americans (OR for each 5-cm increment in height: 0.76), followed by whites (OR: 0.78), Hispanics (OR: 0.83) and non-Hispanic blacks (OR: 0.86)[5]. Different from previous studies, another novelty of our study resides in the further breakdown of the Asian ethnicities, demonstrating a gradient of effect sizes – strongest for Indians, followed by Chinese, then Malays. Such ethnic-variability in the magnitude of associations may be due to the genetically-determined physiological differences. It was reported that the propensity for fat accumulation varies with ethnicity, affecting the development of insulin resistance and diabetes[20–22].
Epidemiological evidence has linked shorter height with several non-communicable diseases including type 2 diabetes and cardiovascular diseases[23,24]. Taller height has been associated with increased insulin sensitivity and lower levels of liver fat[25,26]. Impaired prepubertal growth has been implicated in the development of a shorter stature, hypothesized to be attributable to several pathways during early development such as undernourishment[23,27], deficiency in hormonal factors (e.g. insulin-like growth factor-I)[28,29], and intrauterine growth retardation[30]. Each of these pathways has also been implicated in the development of future cardiometabolic risk[31], of which GDM is an example. Thus, the clear association between height and glycemia may not be surprising. Nonetheless, the most interesting observation here is that this association is most markedly and clearly observed for post-challenge glycemia rather than for FPG. The association between height and FPG was substantially influenced by age, weight and ethnicity, and our demonstrations of a negative association between height and FPG would not be influenced at all by the glucose load.
The method of assessing glucose tolerance using a fixed glucose load in all adults regardless of body size, in a standard OGTT may itself result in bias. It can be supposed that taller individuals may have more muscle mass, which is the major tissue responsible for glucose uptake[32], and thus may have a better glucose profile in response to the OGTT for a given level of insulin sensitivity. Another possible explanation is the dilution effect of increased total body water in taller individuals[11]. These factors could result in disproportionately more women of shorter stature being labelled as “GDM” in whom there is no real impaired glucose regulation, and hence no increased risk of pregnancy complications over non-GDM women of similar height. The possibility of the elevated glycemia being a function of the glucose load and hence represents an artefact generated by glucose overload in the OGTT, cannot be dismissed in light of our findings of no increased GDM-related complication risks among shorter women.
Even though the pregnancy complications we investigated are not specific to the diagnosis of GDM and are multifactorial; still, our results indicate that GDM and 2hPG were associated with preterm delivery in taller women but not shorter ones. Although controversy exists about whether there is an association between GDM and prematurity, our findings are in line with previous studies from China[33], the USA[34], and Austria[35]. Maternal height has also been linked to other neonatal phenotypes such as increased abdominal aortic diameter, which may occur independently of GDM[36].
An elevated FPG cannot be attributed to glucose overload in an OGTT and is reflective of basal metabolic status primarily hepatic glucose production through gluconeogenesis. Yet associations of FPG with hypertensive disorders of pregnancy, and a non-significant trend for preterm delivery and emergency cesarean section only occurring in taller but not shorter women suggest that there is an alternative hypothesis to glucose overload in shorter women. Since taller women demonstrate increased insulin sensitivity compared with shorter ones[26,29], plausibly, a GDM diagnosis in taller women and elevated FPG may be indicative of more severe glucose dysregulation[37] and hence greater pregnancy adversity. It is also possible that management of GDM during pregnancy may have blunted the association between GDM and maternal or neonatal complications, but this would have been expected to occur to the same degree in both shorter and taller women.
The finding that FPG and 2hPG were associated with birthweight and with increasing odds of macrosomia in both shorter and taller women is not surprising since this continuum of effect across the glycemic range is well documented[38]. A positive association between GDM and the rate of macrosomia has been described in taller Finnish women (≥168 cm) but not in shorter women[37]. While it is well-established that GDM increases the risk of macrosomia[39], and that taller women deliver larger babies[40], it has not always been recognized that having GDM in addition to being tall are two independent risk factors that may interact to influence offspring birthweight. The expected association between GDM and macrosomia in our study was not evident among shorter and taller women as the number of macrosomia cases in the cohorts was low, hence this data should be interpreted with caution.
A limitation of this study is the potential biases attributable to unmeasured covariates. We have also not accounted for genetic differences and early life factors that may be associated with GDM development. Furthermore, only a limited number of adverse outcomes were examined as possible indicators of the presence of true GDM pathology. Another limitation is that we could not determine the true positives of GDM among shorter women, and that their results were analyzed as a group; therefore, the adverse effects of true GDM on pregnancy complications would be diluted out by the false-positives.
Notwithstanding these limitations, strengths of our study included a relatively large sample size of multi-ethnic, predominantly Asian pregnant women where the GDM risk is thought to be amongst the highest worldwide. All women underwent universal OGTT and standardized protocols using validated measurements of GDM, which enabled us to explore the possible glucose overload theory in shorter women. Further studies with adequate sampling of different ethnic groups are warranted to confirm our findings.
Conclusion
Shorter height is a strong and independent risk factor predictive of GDM, and ethnic differences in the strength of association were observed. The strongest association is observed for non-Asian and mixed Asian ethnicities living in Singapore, followed by Indians, then Chinese and lastly Malays. In addition to shorter stature being a marker of early life health adversity associated with poorer metabolic outcomes, our finding that height only clearly associated with reductions in post-challenge glycemia and not FPG is supportive of the glucose overload hypothesis in a fixed-load OGTT leading to a disproportionately higher number of shorter women artefactually labelled with “GDM”. This could explain our novel findings on the association of GDM with the pregnancy complications of preterm delivery and increasing birthweight only amongst taller but not shorter women in a multi-ethnic Asian cohort. This raises the need to consider the role of height in OGTT administration, especially in pregnant populations of generally shorter stature. Possible overdiagnosis of GDM in populations of shorter stature has significant implications on healthcare resource as well as patient-burden. However, the longer term effects of an apparent “GDM” diagnosis on offspring of shorter women needs to be investigated and understood before any changes to clinical practice should be considered.
Supplementary Material
Funding
This research is supported by the Singapore National Research Foundation under its Translational and Clinical Research (TCR) Flagship Programme and administered by the Singapore Ministry of Health’s National Medical Research Council (NMRC), Singapore - NMRC/TCR/004-NUS/2008; NMRC/TCR/012-NUHS/2014. Additional funding is provided by the Singapore Institute for Clinical Sciences (SICS) – Agency for Science Technology and Research (A*STAR). KMG is supported by the UK Medical Research Council (MC_UU_12011/4), the National Institute for Health Research (NIHR Senior Investigator (NF-SI-0515-10042) and NIHR Southampton Biomedical Research Centre (IS-BRC-1215-20004)), the European Union (Erasmus+ Project ImpENSA 598488-EPP-1-2018-1-DE-EPPKA2-CBHE-JP), British Heart Foundation (RG/15/17/3174) and the US National Institute On Aging of the National Institutes of Health (Award No. U24AG047867). The study sponsor/funder was not involved in the design of the study; the collection, analysis, and interpretation of data; writing the report; and did not impose any restrictions regarding the publication of the report.
Abbreviations
- 1hPG
1-h post-challenge plasma glucose
- 2hPG
2-h post-challenge plasma glucose
- aβ
adjusted beta coefficient
- aOR
adjusted odds ratio
- BMI
body mass index
- FPG
fasting plasma glucose
- GDM
gestational diabetes mellitus
- GUSTO
Growing Up in Singapore Towards healthy Outcomes
- KKH
KK Women’s and Children’s Hospital
- NICU
neonatal intensive care unit
- NUH
National University Hospital
- OGTT
oral glucose tolerance test
- OR
odds ratio
Footnotes
Declaration of interest: KMG has received reimbursement for speaking at conferences sponsored by companies selling nutritional products. YSC, KMG and SYC are part of an academic consortium that has received research funding from Abbott Nutrition, Nestle and Danone. The other authors have no financial or personal conflict of interest to declare.
Contribution to authorship: JGE and SYC contributed to the conception and design of the study. AHYC and SYC wrote the first draft. WLY, SLL, SES, JYB, MTT, SSTH, HG, AR, MK, CG, LTA, LPCS, YSC, KHT, LLS, AB, PDG, FY, YSL, CC, KMG, and SYC contributed to the data collection. AHYC and SYC contributed to the analysis and interpretation of the data. All authors read and approved the final manuscript. AHYC and SYC are guarantors of the paper.
Ethics approval: Ethical approval for the GUSTO study was obtained from the National Health Care Group Domain Specific Review Board (reference D/09/021) and the SingHealth Centralized Institutional Review Board (reference 2009/280/D). Use of anonymized dataset from the NUH was extracted from clinical records, ethically approved with waiver of consent granted by the National Health Care Group Domain Specific Review Board (reference 2019/01172).
References
- [1].American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Diabetes Care. 2016;40:S11–S24. doi: 10.2337/dc17-s005. [DOI] [PubMed] [Google Scholar]
- [2].International Diabetes Federation. IDF Diabetes Atlas. 9th Ed. International Diabetes Federation; 2019. https://diabetesatlas.org/data/en/ [Google Scholar]
- [3].Zhu Y, Zhang C. Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: a Global Perspective. Curr Diab Rep. 2016;16:7. doi: 10.1007/s11892-015-0699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Petry CJ. Gestational diabetes: Risk factors and recent advances in its genetics and treatment. Br J Nutr. 2010;104:775–87. doi: 10.1017/S0007114510001741. [DOI] [PubMed] [Google Scholar]
- [5].Brite J, Shiroma EJ, Bowers K, Yeung E, Laughon SK, Grewal JG, et al. Height and the risk of gestational diabetes: variations by race/ethnicity. Diabetic Medicine. 2013;31:332–40. doi: 10.1111/dme.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rehunen SKJ, Kautiainen H, Eriksson JG, Korhonen PE. Adult height and glucose tolerance: a re-appraisal of the importance of body mass index. Diabetic Medicine. 2017;34:1129–35. doi: 10.1111/dme.13382. [DOI] [PubMed] [Google Scholar]
- [7].Janghorbani M, Momeni F, Dehghani M. Hip circumference, height and risk of type 2 diabetes: systematic review and meta-analysis. Obesity Reviews. 2012;13:1172–81. doi: 10.1111/J1467-789x.2012.01030.x. [DOI] [PubMed] [Google Scholar]
- [8].Nüesch E, Dale C, Palmer TM, White J, Keating BJ, van Iperen EP, et al. Adult height, coronary heart disease and stroke: a multi-locus Mendelian randomization meta-analysis. Int J Epidemiol. 2016;45:1927–37. doi: 10.1093/ije/dyv074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gunnell D, Okasha M, DaveySmith G, Oliver SE, Sandhu J, Holly JMP. Height, Leg Length, and Cancer Risk: A Systematic Review. Epidemiol Rev. 2001;23:313–42. doi: 10.1093/oxfordjournals.epirev.a000809. [DOI] [PubMed] [Google Scholar]
- [10].NCD Risk Factor Collaboration (NCD-RisC) A century of trends in adult human height. ELife. 2016;5:e13410. doi: 10.7554/eLife.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sicree RA, Zimmet PZ, Dunstan DW, Cameron AJ, Welborn TA, Shaw JE. Differences in height explain gender differences in the response to the oral glucose tolerance test-the AusDiab study. Diabetic Medicine. 2008;25:296–302. doi: 10.1111/J1464-5491.2007.02362.x. [DOI] [PubMed] [Google Scholar]
- [12].Reece EA. The fetal and maternal consequences of gestational diabetes mellitus. J Matern-Fetal Neonatal Med. 2010;23:199–203. doi: 10.3109/14767050903550659. [DOI] [PubMed] [Google Scholar]
- [13].Kozuki N, Katz J, Lee AC, Vogel JP, Silveira MF, Sania A, et al. Short maternal stature increases risk of small-for-gestational-age and preterm births in low- and middle-income countries: individual participant data meta-analysis and population attributable fraction. J Nutr. 2015;145:2542–2550. doi: 10.3945/jn.115.216374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Soh S-E, Tint MT, Gluckman PD, Godfrey KM, Rifkin-Graboi A, Chan YH, et al. Cohort profile: Growing Up in Singapore Towards healthy Outcomes (GUSTO) birth cohort study. Int J Epidemiol. 2014;43:1401–9. doi: 10.1093/ije/dyt125. [DOI] [PubMed] [Google Scholar]
- [15].Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO Consultation. Diabetic Medicine. 1998;15:539–53. doi: 10.1002/(sici)1096-9136(199807)15:7<539::aid-dia668>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- [16].Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. New England Journal of Medicine. 2005;352:2477–86. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- [17].Hadlock FP, Shah YP, Kanon DJ, Lindsey Jv. Fetal crown-rump length: reevaluation of relation to menstrual age (5-18 weeks) with high-resolution realtime US. Radiology. 1992;182:501–5. doi: 10.1148/radiology.182.2.1732970. [DOI] [PubMed] [Google Scholar]
- [18].Gordon M, Lumley T. forestplot: Advanced Forest Plot Using “grid” Graphics. R package version 1.10. 2020 https://cran.r-project.org/package=forestplot. [Google Scholar]
- [19].Jang HC, Min HK, Lee HK, Cho NH, Metzger BE. Short stature in Korean women: a contribution to the multifactorial predisposition to gestational diabetes mellitus. Diabetologia. 1998;41:778–83. doi: 10.1007/s001250050987. [DOI] [PubMed] [Google Scholar]
- [20].Branchtein L, Schmidt MI, Matos MCG, Yamashita T, Pousada JMDC, Duncan BB. Short stature and gestational diabetes in Brazil. Diabetologia. 2000;43:848–51. doi: 10.1007/s001250051460. [DOI] [PubMed] [Google Scholar]
- [21].Bertoli S, Leone A, Krakauer NY, Bedogni G, Vanzulli A, Redaelli VI, et al. Association of Body Shape Index (ABSI) with cardio-metabolic risk factors: A cross-sectional study of 6081 Caucasian adults. PLOS ONE. 2017;12:e0185013. doi: 10.1371/journal.pone.0185013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Khoo CM, Sairazi S, Taslim S, Gardner D, Wu Y, Lee J, et al. Ethnicity Modifies the Relationships of Insulin Resistance, Inflammation, and Adiponectin With Obesity in a Multiethnic Asian Population. Diabetes Care. 2011;34:1120–6. doi: 10.2337/dc10-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Asao K, Kao WHL, Baptiste-Roberts K, Bandeen-Roche K, Erlinger TP, Brancati FL. Short Stature and the Risk of Adiposity, Insulin Resistance, and Type 2 Diabetes in Middle Age: The Third National Health and Nutrition Examination Survey (NHANES III), 1988-1994. Diabetes Care. 2006;29:1632–7. doi: 10.2337/dc05-1997. [DOI] [PubMed] [Google Scholar]
- [24].Paajanen TA, Oksala NKJ, Kuukasjärvi P, Karhunen PJ. Short stature is associated with coronary heart disease: A systematic review of the literature and a meta-analysis. European Heart Journal. 2010;31:1802–9. doi: 10.1093/eurheartj/ehq155. [DOI] [PubMed] [Google Scholar]
- [25].Wittenbecher C, Kuxhaus O, Boeing H, Stefan N, Schulze MB. Associations of short stature and components of height with incidence of type 2 diabetes: mediating effects of cardiometabolic risk factors. Diabetologia. 2019;62:2211–21. doi: 10.1007/s00125-019-04978-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Stefan N, Häring H-U, Hu FB, Schulze MB. Divergent associations of height with cardiometabolic disease and cancer: epidemiology, pathophysiology, and global implications. The Lancet Diabetes & Endocrinology. 2016;4:457–67. doi: 10.1016/s2213-8587(15)00474-x. [DOI] [PubMed] [Google Scholar]
- [27].Gunnell D, Smith GD, McConnachie A, Greenwood R, Upton M, Frankel S. Separating in-utero and postnatal influences on later disease. The Lancet. 1999;354:1526–7. doi: 10.1016/s0140-6736(99)02937-2. [DOI] [PubMed] [Google Scholar]
- [28].Sandhu MS, Heald AH, Gibson JM, Cruickshank JK, Dunger DB, Wareham NJ. Circulating concentrations of insulin-like growth factor-I and development of glucose intolerance: a prospective observational study. The Lancet. 2002;359:1740–5. doi: 10.1016/s0140-6736(02)08655-5. [DOI] [PubMed] [Google Scholar]
- [29].Lawlor D, Ebrahim S, DaveySmith G. The Association Between Components of Adult Height and Type II Diabetes and Insulin Resistance: British Women’s Heart and Health Study. Diabetologia. 2002;45:1097106. doi: 10.1007/s00125-002-0887-5. [DOI] [PubMed] [Google Scholar]
- [30].Barker DJP, Godfrey KM, Gluckman PD, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. The Lancet. 1993;341:938–41. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- [31].Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental origins of health and disease: Brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med. 2009;27:358–368. doi: 10.1055/s-0029-1237424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Watkins PJ. In: Diabetic Medicine. 2nd edition. Alberti KGMM, Zimmet P, DeFronzo RA, Keen H, editors. Vol. 15. 1998. Book review: International textbook of diabetes mellitus; p. 979. [DOI] [Google Scholar]
- [33].Yang X, Hsu-Hage B, Zhang H, Zhang C, Zhang Y, Zhang C. Women With Impaired Glucose Tolerance During Pregnancy Have Significantly Poor Pregnancy Outcomes. Diabetes Care. 2002;25:1619–24. doi: 10.2337/diacare.25.9.1619. [DOI] [PubMed] [Google Scholar]
- [34].Hedderson MM, Ferrara A, Sacks DA. Gestational Diabetes Mellitus and Lesser Degrees of Pregnancy Hyperglycemia. Obstetrics & Gynecology. 2003;102:8506. doi: 10.1097/00006250-200310000-00030. [DOI] [PubMed] [Google Scholar]
- [35].Köck K, Köck F, Klein K, Bancher-Todesca D, Helmer H. Diabetes mellitus and the risk of preterm birth with regard to the risk of spontaneous preterm birth. J Matern-Fetal Neonatal Med. 2010;23:1004–8. doi: 10.3109/14767050903551392. [DOI] [PubMed] [Google Scholar]
- [36].Ciccone MM, Scicchitano P, Salerno C, Gesualdo M, Fornarelli F, Zito A, et al. Aorta Structural Alterations in Term Neonates: The Role of Birth and Maternal Characteristics. BioMed Research International. 2013;2013:459168. doi: 10.1155/2013/459168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Masalin S, Laine MK, Kautiainen H, Gissler M, Raina M, Pennanen P, et al. Impact of maternal height and gestational diabetes mellitus on offspring birthweight. Diabetes Research and Clinical Practice. 2019;148:110–8. doi: 10.1016/Jdiabres.2019.01.004. [DOI] [PubMed] [Google Scholar]
- [38].Metzger BE, Lowe LP, Dyer AR, Trimble ER, Sheridan B, Hod M, et al. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: associations with neonatal anthropometrics. Diabetes. 2008;58:453–9. doi: 10.2337/db08-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kc K, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab. 2015;66:14–20. doi: 10.1159/000371628. [DOI] [PubMed] [Google Scholar]
- [40].TrojnerBregar A, Blickstein I, Steblovnik L, Verdenik I, Lucovnik M, Tul N. Do tall women beget larger babies? J Matern Fetal Neonatal Med. 2016;29:1311–3. doi: 10.3109/14767058.2015.1046830. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.