Abstract
Purpose
FRAX® is calibrated using population-specific fracture and mortality data. The need for FRAX to accommodate ethnic diversity within a country is uncertain. We addressed this question using the population-based Manitoba Bone Mineral Density (BMD) Program registry and self-reported ethnicity.
Methods
The study population was women age 40 years or older with baseline FRAX assessments (Canadian and other ethnic calculators), fracture outcomes and self-reported ethnicity (White N=68,907 [referent], Asian N=1,910, Black N=356). Adjusted hazard ratios (HR) with 95 % confidence intervals (CI) for time to MOF and hip fracture were estimated. We examined candidate variables from DXA that might contribute to ethnic differences including skeletal size, hip axis length (HAL), trabecular bone score (TBS), and estimated body composition.
Results
Adjusted for baseline risk using the Canadian FRAX tool with BMD, Asian compared with White women were at much lower risk for MOF (HR 0.46, 95% CI 0.35–0.59) and hip fracture (0.16, 95% CI 0.08–0.34). Black women were also at lower MOF risk (HR 0.58, 95% CI 0.32–1.00); there were no hip fractures. The US ethnic-specific FRAX calculators accounted for most of the between-ethnicity differences in MOF risk (86% for Asian, 92% for Black) but only partially accounted for lower hip fracture risk in Asian women (40%). The candidate variables explained only a minority of the effect of ethnicity. Gradient of risk in analyses was similar (p-interactions ethnicity*FRAX non-significant).
Conclusions
We identified significant ethnic differences in performance of the Canadian FRAX tool with fracture probability overestimated among Asian and Black women. The US Ethnic calculators helped to address this discrepancy for MOF risk assessment, but not for hip fracture risk among Asian women.
Keywords: Osteoporosis, Fractures, Ethnicity, FRAX, Dual-energy x-ray absorptiometry
Introduction
Accurate assessment of osteoporotic fracture risk is required to optimize therapeutic decision making. Fracture risk algorithms that combine clinical risk factors and bone mineral density (BMD) are now widely used in clinical practice to target high-risk individuals for treatment (1). The fracture risk assessment tool (FRAX®) was developed to predict an individual’s 10-year probability of major osteoporotic fracture (MOF; a composite of hip, humerus, forearm and clinical vertebral fractures) and hip fracture from readily assessed clinical risk factors (2).
The FRAX tool is calibrated to the target population using fracture and mortality data. Currently there are over 60 countries with FRAX tools worldwide. The need to further accommodate ethnic diversity within a country is uncertain (3, 4). Currently, only two countries have ethnic-specific FRAX calculators, Singapore (Chinese, Malay, Indian) and the United States (White, Black, Asian, Hispanic). The US calculators assumed correction factors relative to White: for Blacks, 0.43 for women and 0.53 for men; for Asians, 0.50 for women and 0.64 for men; and for Hispanic: 0.53 for women and 0.58 for men (3). The UK QFracture risk calculator considers nine ethnicity categories (5).
One study from Sweden suggested that immigrants to that country had fracture rates that were overestimated using the Swedish FRAX tool (6). Like many countries, Canada has an ethnoculturally diverse population with a large number of immigrants and visible minorities which has been increasing in size. In 2016, 21.9% of the Canadian population were foreign-born immigrants (top three India, China and Philippines) and 22.3% of the Canadian population belonged to a visible minority group (three largest South Asian, Chinese and Black) (7). Whether a single Canadian FRAX tool calibrated to the population at large is applicable to all ethnic subgroups is uncertain. To address this question, we used the population-based Manitoba Bone Mineral Density Program database, a large clinical registry of patients with BMD data for the Province of Manitoba, Canada, which collects limited information on self-reported ethnicity.
Methods
Study Population
The Canadian Province of Manitoba, population 1.2 million in 2016, has an ethnically diverse population similar to the rest of Canada, with 18.3% immigrants and 17.5% from a visible minority (7). Health services are provided to virtually all residents through a public healthcare system. DXA-based BMD testing has been managed as an integrated clinical program since 1997; criteria for testing have been published and include screening at age 65 years for women and in men and younger women with additional risk factors (8). The program maintains a database of all DXA results which can be linked with other provincial population-based computerized health databases through an anonymous personal identifier. The DXA database has completeness and accuracy in excess of 99% (9).
The study population consisted of all women age 40 years or older undergoing baseline DXA assessment from January 1996 to March 2018, with at least one year of coverage prior to the baseline assessment in order to assess covariates. We excluded those not registered for health care in Manitoba, without 365 days of coverage before DXA assessment, without any coverage after DXA assessment, or with missing baseline measurements required for FRAX. For those with more than one qualifying examination, only the first was included. The study was approved by the Health Research Ethics Board for the University of Manitoba.
Self-reported ethnicity
Performing DXA requires selection of ethnicity from a limited range of choices (White, Asian, Black, Hispanic, Other; GE/Lunar Healthcare, Madison WI) and this potentially affects the age/sex-adjusted Z-score, but does not alter the actual BMD value. In order for the DXA technologist to select an appropriate DXA reference database at the time of BMD testing, individuals are asked to self-report ethnicity using this list. We used these values to define ethnicity in the present analysis: Asian was limited to East and Southeast (top three in Manitoba: Philippines, China, Hong Kong), and excluded South (India) and West (Middle East) (10). We excluded from analysis those reporting ethnicity as Hispanic (N=10, 0.01%) or “Other” (N=20, 0.3%). No additional details are collected from the individual in terms of country of birth, ancestral country of origin, or duration of residency in Canada.
Bone Mineral Density Measurements and Fracture Probability
Hip DXA scans were performed and analyzed in accordance with manufacturer recommendations. Femoral neck T-scores (number of SDs above or below young adult mean BMD) were calculated from NHANES III white female reference values for all subjects (11). The program’s quality assurance is under strict supervision by a medical physicist (8). The six cross-calibrated instruments used for this study (3 Prodigy and 3 iDXA, GE/Lunar Healthcare, Madison WI; between-scanner femur neck differences <0.1 T-score) exhibited stable long-term performance (coefficient of variation <0.5%). All reporting physicians and supervising technologists are required to maintain DXA certification with the International Society for Clinical Densitometry (ISCD).
Ten-year probability of a major osteoporotic fracture was calculated using the World Health Organization fracture risk assessment tool, Canadian version (FRAX® Desktop Multi-Patient Entry, version 3.7) (12, 13). Briefly, age, body mass index (BMI), femoral neck BMD and other data required for calculating fracture probability with FRAX were assessed from measurements (height and weight) and information collected directly from subjects through the intake questionnaire which is reviewed at the time of DXA scanning (14). Questionnaire information was supplemented with population-based healthcare data (hospital discharge abstracts, medical claims diagnoses, province-wide retail pharmacy database) as previously described, thereby ensuring complete information in virtually all subjects (15–17). The Canadian FRAX tool was calibrated using nationwide hip fracture and mortality data as previously described, which would include individuals born in Canada and immigrants (13). Predictions agree closely with observed fracture risk in our population (18, 19). In addition to using the Canadian FRAX tool, we also used the US Asian and US Black tools, because they are possible alternatives for cohort members of self-reported Asian and Black ethnicity (3, 4).
Additional covariates
We considered candidate variables that might account for between-ethnicity differences. These included previous osteoporosis treatment (identified as 6 months or greater dispensed quantity of an osteoporosis medication or systemic estrogen product in the prior year from the province-wide retail pharmacy system) (20); a comorbidity index (number of ambulatory diagnostic groups from the Johns Hopkins Adjusted Clinical Group® [ACG®] Case-Mix System, version 11) (21); diagnosed diabetes from hospital and medical claims (22–24); area of residence (urban versus rural) and income (lower versus upper) defined from neighborhood level data (25, 26); DXA-derived geometric measures of femur neck area (cm2); total hip area; (cm2) and hip axis length (HAL, mm) (27–30); lumbar spine trabecular bone score (TBS, unitless) (31, 32); and DXA-estimated total body lean mass and fat mass divided by height squared to provide a size-adjusted index (kg/m2) (33). In the subset of those undergoing assessment since September 1, 2012 we also collected number of self-reported falls in the preceding year as previously described (34); the falls data are reported cross-sectionally since the numbers and follow up were insufficient for assessment of between-ethnicity differences in fracture outcomes.
Fracture Outcomes
Manitoba Health records were assessed for the presence of fracture diagnostic codes following the BMD assessment. Fractures that were not associated with trauma codes were assessed through a combination of hospital discharge abstracts (diagnoses and procedures coded using the International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] prior to 2004 and International Classification of Diseases, Tenth Revision, Canadian Enhancements [ICD-10-CA] thereafter) and physician billing claims (coded using ICD-9-CM). The primary analysis was based upon incident non-traumatic hip, clinical vertebral, forearm, and humerus fracture diagnostic codes (collectively designated “major osteoporotic” fractures) using previously validated algorithms (35, 36). We required that hip and forearm fractures codes be associated with site-specific fracture reduction, fixation or casting codes to enhance specificity for an acute fracture event. Secondary analyses examine hip fracture alone. To minimize potential misclassification of prior incident fractures, we conservatively required that there be no hospitalization or physician visit(s) with the same fracture type in the six months preceding an incident fracture diagnosis.
Statistical Analysis
Statistical analyses were performed with Statistica (Version 13.0, StatSoft Inc, Tulsa, OK). Descriptive statistics for demographic and baseline characteristics are presented as mean ± SD for continuous variables or number (%) for categorical variables. Time to incident fracture following the DXA scan (index date) was estimated using Cox proportional hazards regression. Observations were censored for death (Vitals Statistics), migration out of province (Manitoba Health registry file), or end of follow up (March 31, 2018). The primary analysis examined incident MOF as the outcome of interest. Cox regression analysis was performed to examine the effect of ethnicity (referent: White) after adjustment for FRAX probability and then considering other candidate variables that might account for between-ethnicity differences on fracture incidence. Proportionality of hazards was confirmed by testing scaled Schoenfeld residuals versus time. All FRAX scores were log-transformed due to a skewed distribution. The base model (Model 1) was based upon the Canadian FRAX tool. A sensitivity analysis was conducted, in which the following covariates were added to Model 1: osteoporosis treatment, comorbidity index, diabetes, area of residence and income. We then examined the potential usefulness of candidate approaches for between-ethnicity differences: using the US ethnic calculators for Asian and Black women (Model 2), or adjusting for femur neck area (Model 3), total hip area (Model 4), HAL (Model 5), lumbar spine TBS (Model 6), or estimated total body lean mass and fat mass index (Model 7). In addition to reporting adjusted HRs to assess the amount of attenuation from each candidate variable, we estimated the percent of the ethnicity effect (i.e., percent change in model χ2 for the ethnicity term) explained by including the candidate variable in the model. Ethnicity-stratified analyses were also conducted to examine the gradient of risk for each SD increase in FRAX score to identify interactions between ethnicity and FRAX output. Finally, the cumulative fracture probability to 10 years was estimated in the presence of competing mortality. Observed versus predicted fracture probability was computed, stratified by ethnicity.
Results
Baseline characteristics summarized in Table 1 showed significant differences in all measures. The final study population comprised 68,907 White women (mean age 64.7 ± 10.9 years), 1,910 Asian women (mean age 62.8 ± 9.9 years) and 356 Black women (mean age 63.0 ± 10.6 years). Previous fracture was greatest among White women (20.1%), followed by Asian (11.7%) and Black (8.7%) women. A similar ordering was seen for fracture probability from the Canadian FRAX tool when calculated without BMD. Femoral neck T-score was significantly lower in Asian women (mean -1.8 ± 0.9) compared with White women (-1.4 ± 1.0) followed by Black women (-0.6 ± 1.1). When fracture probability was calculated with BMD, results for Asian women were similar to White women, and remained significantly lower for Black women. Femur neck area, total hip area and HAL were greatest for White women, lowest for Asian women and intermediate for Black women. Lumbar spine TBS was slightly lower for Asian women. Total body lean mass index and fat mass index were greatest for Black women, least for Asian women, and intermediate for White women. Asian women reported significantly fewer falls in the prior year than White women (13.% vs 22.8%, p<0.001); falls were less common among Black women (17.9%) but this was not statistically significant.
Table 1. Baseline characteristics of the study population stratified by self-reported ethnicity.
| Characteristic | White women | Asian women | Black women | p-value |
|---|---|---|---|---|
| N= | 68,907 | 1,910 | 366 | |
| Age (years) | 64.7 ± 10.9 | 62.8 ± 9.9 a | 63.0 ± 10.6 b | <0.001 |
| BMI (kg/m2) | 27.6 ± 6.1 | 24.8 ± 4.1 a | 28.8 ± 6.0 b | <0.001 |
| Prior fracture | 13,817 (20.1) | 224 (11.7) a | 31 (8.7) b | <0.001 |
| Femoral neck T-score | -1.4 ± 1.0 | -1.8 ± 0.9 a | -0.6 ± 1.1 b | <0.001 |
| Canadian FRAX MOF percent (without BMD) | 11.6 ± 8.8 | 10.0 ± 6.9 a | 9.0 ± 6.6 b | <0.001 |
| Canadian FRAX hip percent (without BMD) | 3.4 ± 5.2 | 2.6 ± 4 a | 2.2 ± 3.4 b | <0.001 |
| Canadian FRAX MOF percent (with BMD) | 10.4 ± 7.3 | 10.2 ± 7.0 | 7.0 ± 4.3 b | <0.001 |
| Canadian FRAX hip percent (with BMD) | 2.4 ± 3.9 | 2.5 ± 3.6 | 0.9 ± 1.5 b | <0.001 |
| US-ethnic FRAX MOF percent (without BMD) | N/A | 6.5 ± 4.6 a | 4.7 ± 3.3 b | <0.001 |
| US-ethnic FRAX hip percent (without BMD) | N/A | 1.7 ± 2.7 a | 1.1 ± 1.7 b | <0.001 |
| US-ethnic FRAX MOF percent (with BMD) | N/A | 6.7 ± 4.7 a | 3.6 ± 2.1 b | <0.001 |
| US-ethnic FRAX hip percent (with BMD) | N/A | 1.6 ± 2.5 a | 0.5 ± 0.7 b | <0.001 |
| Rural residence (vs urban) | 23,568 (34.2) | 192 (10.1) a | 18 (5.1) b | <0.001 |
| Lower income (vs higher) | 23,974 (34.8) | 689 (36.1) | 145 (40.7) b | 0.034 |
| Comorbidity score | 4.9 ± 2.7 | 4.6 ± 2.5 a | 5.2 ± 2.7 b | <0.001 |
| Diabetes | 7,424 (10.8) | 393 (20.6) a | 92 (25.8) b | <0.001 |
| Osteoporosis treatment | 8,933 (13) | 143 (7.5) a | 18 (5.1) b | <0.001 |
| Femur neck area (cm2) | 4.8 ± 0.4 | 4.5 ± 0.3 a | 4.6 ± 0.3 b | <0.001 |
| Total hip area (cm2) | 31.8 ± 2.4 | 28.2 ± 2.0 a | 30.3 ± 2.2 b | <0.001 |
| HAL (mm) | 105.1 ± 6.4 | 96.6 ± 5.4 a | 100 ± 6.2 b | <0.001 |
| Lumbar spine TBS L1-4 | 1.270 ± 0.123 | 1.262 ± 0.104 a | 1.280 ± 0.127 | 0.021 |
| Total lean mass index (kg/m2) | 15.4 ± 2 | 14.8 ± 1.5 a | 16.2 ± 2.0 b | <0.001 |
| Total fat mass index (kg/m2) | 10.9 ± 4.3 | 8.8 ± 2.8 a | 11.4 ± 4.2 | <0.001 |
| One or more falls in the prior year | 3,765 (22.8) | 116 (13.9) a | 24 (17.9) | <0.001 |
Data expressed as mean (SD) or N (percent). Major osteoporotic fracture (MOF) and hip fracture probability computed without and with bone mineral density (BMD). Hip axis length (HAL). Trabecular bone score (TBS). p-value by analysis of variance (ANOVA).
Post hoc P <0.05, Asian vs White.
Post hoc P <0.05, Black vs White.
Limited to September 1 2012 and later.
Observation time was greatest for White women (8.8 ± 5.1 years), shortest for Asian women (6.5 ± 5.0 years) and intermediate for Black women (7.1 ± 5.0 years). Numbers of fractures observed and fracture rates are summarized in Table 2. Unadjusted incident MOF rates were significantly greater (p<0.001) for White women (11.4 per 1,000 person years) compared with Black women (4.3) and Asian women (5.0). A similar pattern was seen for incident hip fractures, again significantly greater (p<0.001) for White women (3.6 per 1,000 person years) compared with Black women (0.0 with no hip fracture events observed) and Asian women (0.6).
Table 2. Unadjusted incident fracture rates (per 1,000 person-years) with 95% confidence interval (CI) and number of fractures observed according to self-reported ethnicity.
| White women Rate per 1,000 person-years (95%CI) | Asian women Rate per 1,000 person-years (95%CI) | Black women Rate per 1,000 person-years (95%CI) | |
|---|---|---|---|
| Incident major osteoporotic fracture | 11.4 (11.2-11.7) N=6,897 |
5.0 (3.8-6.2) a
N=62 |
4.3 (1.8-6.9)b
N=11 |
| Incident hip fracture | 3.6 (3.5-3.8) N=2,192 |
0.6 (0.2-1.0)a
N=7 |
0.0 (0.0-1.3)b
N=0 |
p-value <0.001, Asian vs White.
p-value <0.001, Black vs White.
Table 3 shows the HRs for incident fracture adjusted for baseline risk estimated using the Canadian FRAX tool. For the base model (Model 1) that adjusted for baseline risk using the Canadian FRAX tool (referent White women), Asian women were at much lower risk for MOF (HR 0.52, 95% CI 0.41 – 0.66 FRAX without BMD, 0.47, 95% CI 0.37 – 0.60 FRAX with BMD) and incident hip fracture (HR 0.23, 95% CI 0.11 – 0.46 FRAX without BMD, 0.017, 95% CI 0.08 – 0.34 FRAX with BMD). Black women were also at significantly lower MOF risk (HR 0.48, 95% CI 0.28 – 0.83 without BMD, 0.57, 95% CI 0.33 – 0.99 FRAX with BMD). These results were essentially identical after adjustment for osteoporosis treatment, comorbidity score, diagnosed diabetes, area of residence and income level; therefore, these variables were not included in subsequent models.
Table 3. Hazard ratio (HR) with 95% confidence interval (CI) per standard deviation (SD) increase in FRAX score for incident fracture according to self-reported ethnicity.
| Incident MOF from FRAX without BMD | Incident MOF from FRAX with BMD | Incident HIP from FRAX without BMD | Incident HIP from FRAX with BMD | |
|---|---|---|---|---|
| Model 1: Canadian FRAX, unadjusted | HR per SD | HR per SD | HR per SD | HR per SD |
| White women (referent) | 1 (REF) | 1 (REF) | 1 (REF) | 1 (REF) |
| Asian women | 0.51 (0.40-0.65) | 0.46 (0.35-0.59) | 0.22 (0.11-0.47) | 0.16 (0.08-0.34) |
| Black women | 0.49 (0.27-0.88) | 0.58 (0.32-1.00) | - | - |
| p-value for ethnicity | <0.001 | <0.001 | <0.001 | <0.001 |
|
| ||||
| Model 1 sensitivity: Canadian FRAX, adjusted for osteoporosis treatment, comorbidities, diabetes, residence, income | HR per SD | HR per SD | HR per SD | HR per SD |
| White women (referent) | 1 (REF) | 1 (REF) | 1 (REF) | 1 (REF) |
| Asian women | 0.49 (0.38-0.63) | 0.44 (0.34-0.57) | 0.21 (0.10-0.45) | 0.16 (0.07-0.33) |
| Black women | 0.45 (0.25-0.82) | 0.54 (0.30-0.97) | - | - |
| p-value for ethnicity | <0.001 | <0.001 | <0.001 | <0.001 |
|
| ||||
| Model 2: US ethnic calculators, unadjusted | HR per SD | HR per SD | HR per SD | HR per SD |
| White women (referent) | 1 (REF) | 1 (REF) | 1 (REF) | 1 (REF) |
| Asian women | 0.76 (0.59-0.97) | 0.73 (0.57-0.93) | 0.33 (0.16-0.7) | 0.24 (0.12-0.51) |
| Black women | 0.89 (0.49-1.60) | 1.20 (0.66-2.18) | - | - |
| p-value for ethnicity | 0.089 | 0.037 | 0.004 | <0.001 |
|
| ||||
| Model 3: Canadian FRAX, adjusted for Femur Neck Area | HR per SD | HR per SD | HR per SD | HR per SD |
| White women (referent) | 1 (REF) | 1 (REF) | 1 (REF) | 1 (REF) |
| Asian women | 0.55 (0.43-0.71) | 0.48 (0.38-0.62) | 0.25 (0.12-0.53) | 0.18 (0.08-0.37) |
| Black women | 0.51 (0.28-0.92) | 0.60 (0.33-1.09) | - | - |
| p-value for ethnicity | <0.001 | <0.001 | <0.001 | <0.001 |
|
| ||||
| Model 4: Canadian FRAX, adjusted for Total Hip Area | HR per SD | HR per SD | HR per SD | HR per SD |
| White women (referent) | 1 (REF) | 1 (REF) | 1 (REF) | 1 (REF) |
| Asian women | 0.59 (0.46-0.76) | 0.54 (0.42-0.7) | 0.29 (0.14-0.61) | 0.22 (0.11-0.47) |
| Black women | 0.52 (0.29-0.93) | 0.63 (0.35-1.13) | - | - |
| p-value for ethnicity | <0.001 | <0.001 | 0.001 | <0.001 |
|
| ||||
| Model 5: Canadian FRAX, adjusted for HAL | HR per SD | HR per SD | HR per SD | HR per SD |
| White women (referent) | 1 (REF) | 1 (REF) | 1 (REF) | 1 (REF) |
| Asian women | 0.52 (0.39-0.70) | 0.48 (0.36-0.64) | 0.35 (0.16-0.73) | 0.27 (0.13-0.57) |
| Black women | 0.55 (0.30-1.02) | 0.67 (0.36-1.25) | - | - |
| p-value for ethnicity | <0.001 | <0.001 | 0.005 | <0.001 |
|
| ||||
| Model 6: Canadian FRAX, adjusted for TBS | HR per SD | HR per SD | HR per SD | HR per SD |
| White women (referent) | 1 (REF) | 1 (REF) | 1 (REF) | 1 (REF) |
| Asian women | 0.47 (0.34-0.64) | 0.42 (0.31-0.58) | 0.35 (0.17-0.74) | 0.26 (0.12-0.54) |
| Black women | 0.51 (0.27-0.99) | 0.62 (0.32-1.18) | - | - |
| p-value for ethnicity | <0.001 | <0.001 | 0.006 | <0.001 |
|
| ||||
| Model 7: Canadian FRAX, adjusted for Lean mass and Fat mass Index | HR per SD | HR per SD | HR per SD | HR per SD |
| White women (referent) | 1 (REF) | 1 (REF) | 1 (REF) | 1 (REF) |
| Asian women | 0.47 (0.36-0.61) | 0.42 (0.32-0.55) | 0.25 (0.12-0.53) | 0.18 (0.09-0.39) |
| Black women | 0.47 (0.25-0.88) | 0.57 (0.31-1.07) | - | - |
| p-value for ethnicity | <0.001 | <0.001 | <0.001 | <0.001 |
Results from Cox regression models. Major osteoporotic fracture (MOF) and HIP fracture probability computed without and with femoral neck bone mineral density (BMD), FRAX tools. Black ethnicity not included in the hip fracture models as there were no observed events.
When the FRAX probabilities for MOF and hip fracture were calculated using US ethnic calculators and entered in Model 2, there was considerable attenuation in the HRs for MOF risk though it was still significantly reduced for Asian women (HR 0.73, 95% CI 0.62 – 0.99 FRAX without BMD, 0.75, 95% CI 0.59 – 0.95 FRAX with BMD) but was not significant for Black women (HR 0.88, 95% CI 0.51 – 1.51 FRAX without BMD, 1.17, 95% CI 0.68 – 2.03 FRAX with BMD). Hip fracture risk was still considerably lower for Asian women even using the US Asian calculator (HR 0.34, 95% CI 0.17 – 0.68 FRAX without BMD, 0.25, (95% CI 0.13 – 0.50 FRAX with BMD). Adjustment for femur neck area (Model 3), total hip area (Model 4), lumbar spine TBS (Model 6) and body lean mass index plus fat mass index (Model 7) did not significantly attenuate the effect of ethnicity in the models. HAL (Model 5) attenuated the effect of Black ethnicity on MOF (adjusted HR 0.71, 95% CI 0.37 – 1.36) but not Asian women ethnicity (HR 0.47, 95% CI 0.34 – 0.65).
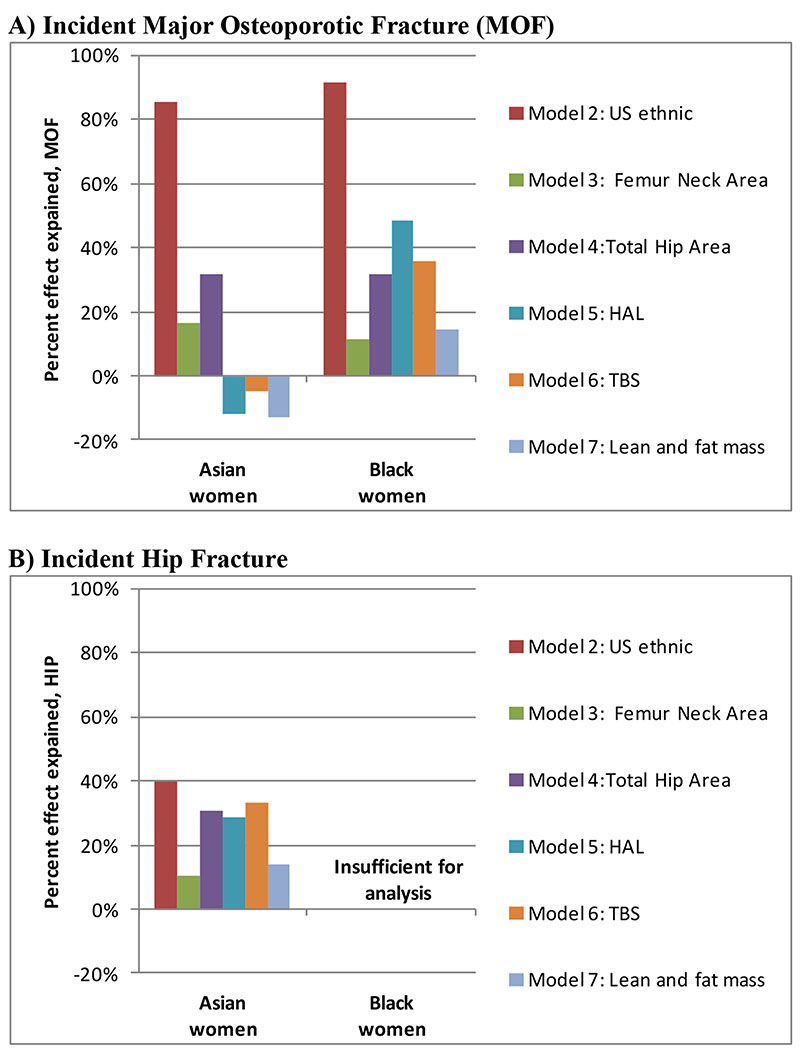
The percent of ethnicity effect explained from the different candidate adjustments is shown Figure 1. For MOF risk assessed with BMD, the US ethnic calculators accounted for the majority of the between-ethnicity differences (86% for Asian, 92% for Black). The effect of Asian ethnicity was partially but incompletely explained by total hip area (32%); all other candidate variables showed low explanatory potential (<20%). HAL explained almost half (48%) of the effect of Black ethnicity with TBS (36%) and total hip area (32%) explaining a slightly smaller amount; all other candidate variables showed low explanatory potential (<20%). For hip fracture risk, all candidate variables explained only a minority of the effect of Asian ethnicity, greatest for the US ethnic calculator (40%), followed by TBS (33%), total hip area (31%) and HAL (28%) (insufficient numbers for analysis of hip fractures in Black women). Findings were similar for FRAX without BMD (data not shown).
Figure 1.
Percent of self-reported ethnicity effect explained from candidate variables (decrease in model χ2 from including the candidate variable). Analysis based upon FRAX with BMD. Insufficient numbers for analysis of hip fractures in Black women.
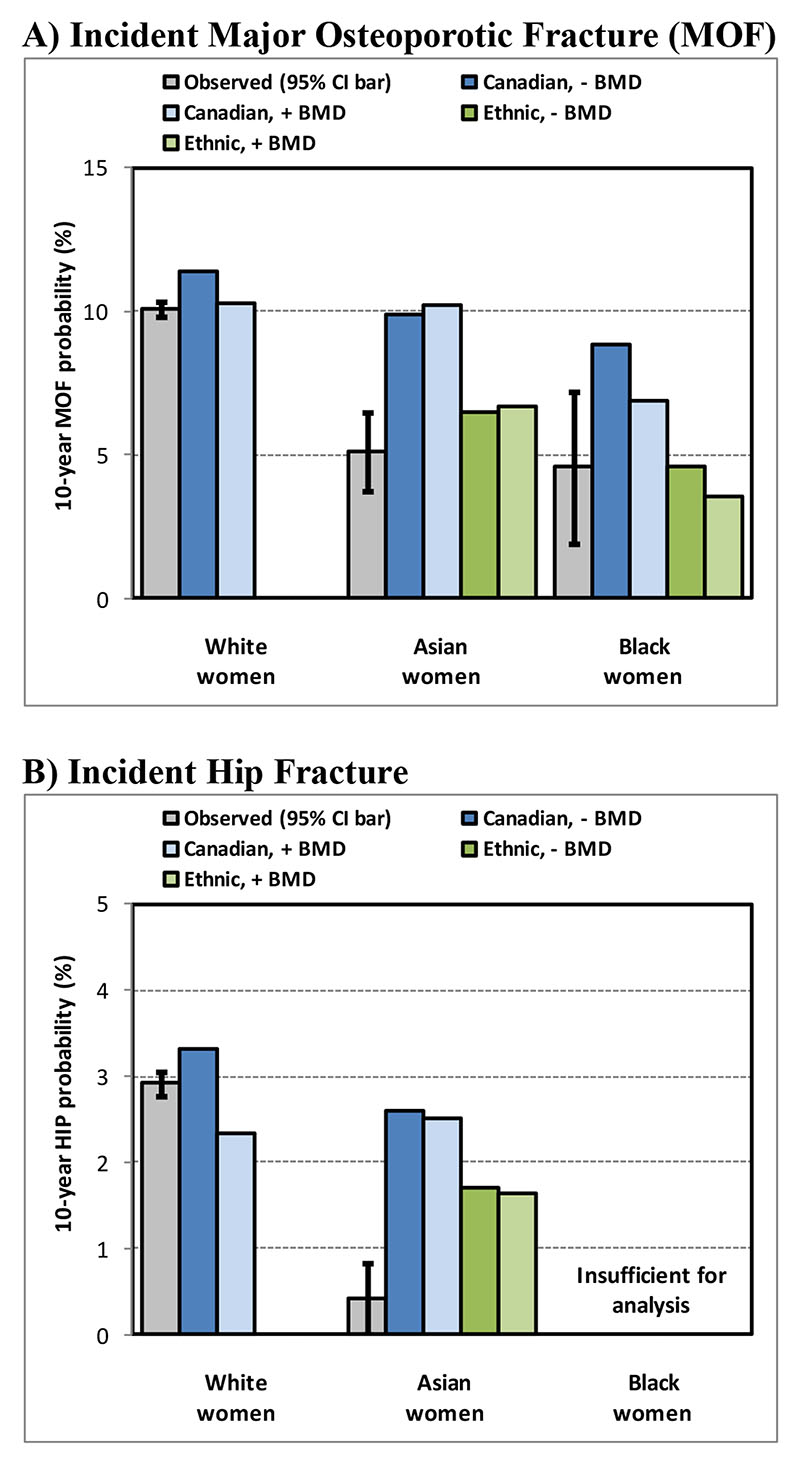
Stratified analyses shown in Table 4 demonstrated that FRAX showed a statistically significant gradient of risk for MOF and hip fracture in both White women and Asian women, whether evaluated without or with BMD. This was not statistically significant for MOF prediction in Black women, but the confidence intervals were wide and the p-interaction (ethnicity*FRAX) was not significant. Calibration plots in Figure 2 demonstrate observed versus predicted 10-year fracture probability. For White women, there was reasonable concordance between observed and predicted MOF and hip fracture events. Consistent with the Cox regression analysis, for Asian women predicted MOF greatly exceeded observed MOF using the Canadian FRAX calculator, but 95% CI limits overlapped when using the US ethnic calculator. For Black women, MOF prediction without BMD exceeded the observed fracture probability, overlapped the 95% CI limit for the Canadian FRAX tool with BMD and most closely agreed with FRAX predictions from the US ethnic calculator. For hip fracture prediction, predictions from the Canadian and US Asian calculators both substantially overestimated observed fracture probability.
Table 4. Hazard ratio (HR) with 95% confidence interval (CI) per standard deviation (SD) increase in FRAX score for incident fracture according to self-reported ethnicity.
| Incident MOF from FRAX without BMD | Incident MOF from FRAX with BMD | Incident HIP from FRAX without BMD | Incident HIP from FRAX with BMD | |
|---|---|---|---|---|
| HR per SD | HR per SD | HR per SD | HR per SD | |
| White women (referent) | 1.98 (1.93-2.04) | 2.10 (2.04-2.15) | 3.69 (3.51-3.87) | 4.32 (4.10-4.56) |
| Asian women | 1.55 (1.16-2.07) | 1.73 (1.33-2.26) | 6.42 (2.34-17.6) | 10.7 (3.03-38.0) |
| Black women | 1.89 (0.93-3.86) | 1.78 (0.85-3.70) | - | - |
| p-interaction (ethnicity*FRAX) | 0.439 | 0.534 | 0.251 | 0.139 |
Results from Cox regression models. Major osteoporotic fracture (MOF) and HIP fracture probability computed without and with femoral neck bone mineral density (BMD), Canadian FRAX tool. Black ethnicity not included in the hip fracture models as there were no observed events.
Figure 2.
Concordance between observed (with 95% confidence bars) cumulative fracture probability to 10 years in the presence of competing mortality and mean predicted 10-year fracture probability from Canadian and US ethnic FRAX calculators, without (-) and with (+) bone mineral density (BMD), according to self-reported ethnicity. Insufficient numbers for analysis of hip fractures in Black women.
Supplemental Table1 and Supplemental Table 2 show calibration analyses for the Asian women compared with all available Asian FRAX calculators. Although confidence intervals are wide and often overlapping, for MOF prediction the FRAX tool for China most closely approximated the observed fracture probability (calibration ratios 1.10 without BMD and 1.06 with BMD). For hip fracture prediction none of the tools showed good calibration; the closest was the FRAX tool for Philippines (calibration ratios 0.47 without BMD and 0.46 with BMD).
Discussion
In this large population-based registry with self-reported ethnicity categorized as White, Asian or Black we identified significant between-ethnicity performance differences in the Canadian FRAX tool. Although gradient of risk in stratified analyses was similar (p-interaction ethnicity*FRAX non-significant), there were large calibration differences such that the Canadian FRAX tool substantially overestimated MOF fracture risk in Asian women and Black women, and overestimated hip fracture risk in Asian women (no observed hip fracture events in Black women). Use of the US ethnic calculators for Asian and Black women greatly attenuated the effect of ethnicity on MOF risk and gave results that most closely agreed with the observed fracture probability. Other candidate adjustments for ethnicity had weaker or no effect on MOF risk assessment. None of the candidate adjustments for hip fracture risk assessment in Asian women was satisfactory.
Our findings should probably come as no surprise, since Canada shares many of the same immigration and ethnicity patterns as the US. Ethnic calculators for the US show substantial differences from the US White calculator, and specifically generate much lower risk calculations for Asian and Black women (3, 4). Interestingly, FRAX calculators for Asian countries show much greater variability, with some generating very low and some much higher fracture scores (37). Whether this relates to population differences, quality of data used in model calibration, or a combination of factors is uncertain. Although we do not have specific information on country or origin or duration of time resident in Canada for our subjects, the predominant country of origin for Asian women in Manitoba is from the Philippines (10). It is therefore instructive to note that the fracture risk calculator for the Philippines generates much lower MOF and hip fracture probabilities than the Canadian calculator or even the US Asian calculator.
Our study complements previous work from Sweden suggesting that immigrants retain fracture risk characteristics of their country of origin, such that the Swedish FRAX tool overestimates fracture risk in foreign-born individuals living in Sweden. (6). Our study extends these observations and demonstrates the potential value of ethnic calculators within a country to more accurately reflect fracture risk among minority groups. Many challenges could arise in trying to apply this to other countries, however. High quality fracture data within ethnic groups is needed and may not be available at the population level. The number of ethnic calculators is potentially open ended. Although there are four ethnic calculators for the US and three for Singapore, ethnic diversity within these countries is considerably more complex. Indeed, a single US Asian ethnic calculator exists whereas in Asia there are thirteen FRAX calculators at the present time. A single calculator may not meet the needs of all immigrant groups. Furthermore, this potentially creates much confusion on the part of practitioners who must decide which calculator to use, how to deal with ethnic groups that are not represented or where there is mixed ethnicity, and whether time of residency in the new country gradually attenuates the effect from the foreign country of origin. Without knowing whether individuals had lived their entire life in Canada or date of arrival for immigrants, our study can not answer the important clinical question of whether to use the FRAX tool specific to the country of birth, country of ancestral origin, or the new arrival country.
Limitations to this analysis are acknowledged. The small number of ethnic categories available to us is clearly a gross over-simplification of the complexity and richness of societal diversity, which defies attempts at measurement or enumeration as noted by United Nations (38). Within the broad and imprecise subgroups of White, Asian and Black ethnicity we would expect enormous heterogeneity. Even within a single country, China, there are 56 officially recognized ethnic groups, and Black ethnicity encompasses individuals from Africa, the Americas and the Caribbean. Ethnicity was crudely categorized based upon self-report at the time of BMD testing and cannot be independently verified. Moreover, we do not have any information regarding country of origin or duration of residence in Canada. Our designation of Asian excluded those from South Asia (predominantly India) and West Asian (Middle Eastern countries); therefore, individuals from these regions cannot be identified. This contributes to the lower proportion of Asian women than expected from the nationwide census data, since India, Pakistan and Iran are major sources of immigration to Canada (7). In addition, older individuals (age 65 years and above) make up a small proportion recent immigrants (3.3% in 2006-2011 and 4.6% in 2011-2016 versus 19.1% of the overall Canadian population). Non-White women may be less likely to undergo referral for BMD testing, though this would likely select higher risk women which would be contrary to our findings. Fracture events are identified from administrative data sources, though the definitions used have been validated (35, 36). We did not have sufficient falls data to examine this as an explanatory variable. Our findings are specific to the Canadian FRAX tool and referred women from the Manitoba population. Whether our findings would be applicable in other populations, with different ethnic mix or among men is uncertain.
In conclusion, we identified significant ethnic differences in the performance of Canadian FRAX tool such that fracture probability was substantially overestimated among Asian and Black women. The US ethnic calculators helped to address this discrepancy for MOF risk assessment, but not for hip fracture risk among Asian women. Alternatively, downgrading the Canadian FRAX output by a fixed fraction similar to that used in the US ethnic calculators could be considered for those of Asian or Black ethnicity (3). Independent validation of our findings and comparison of these approaches will be required before developed a general recommendation for the Canadian population.
Supplementary Material
Mini-Abstract.
We identified large between-ethnicity calibration differences in the Canadian FRAX® tool which substantially overestimated major osteoporotic fracture (MOF) risk in Asian women and Black women, and overestimated hip fracture risk in Asian women.
Acknowledgements
The authors acknowledge the Manitoba Centre for Health Policy for use of data contained in the Population Health Research Data Repository (HIPC 2016/2017-29). The results and conclusions are those of the authors and no official endorsement by the Manitoba Centre for Health Policy, Manitoba Health, Seniors and Active Living, or other data providers is intended or should be inferred. This article has been reviewed and approved by the members of the Manitoba Bone Density Program Committee.
Funding
No funding support was received for this research. SNM is chercheur-boursier des Fonds de Recherche du Québec en Santé. LML is supported by a Tier I Canada Research Chair.
Footnotes
Disclosures
Suzanne Morin: Nothing to declare for the context of this paper, but has received research grants: Amgen.
Eugene McCloskey: Nothing to declare for the context of this paper, but numerous ad hoc consultancies/ speaking honoraria and/or research funding from Amgen, Bayer, General Electric, GSK, Hologic, Lilly, Merck Research Labs, Novartis, Novo Nordisk, Nycomed, Ono, Pfizer, ProStrakan, Roche, Sanofi-Aventis, Servier, Tethys, UBS and Warner-Chilcott
Nicholas Harvey: Nothing to declare for the context of this paper, but has received consultancy/ lecture fees/ honoraria/ grant funding from Alliance for Better Bone Health, Amgen, UCB, MSD, Eli Lilly, Kyowa Kirin, Radius Health, Servier, Shire, Consilient Healthcare and Internis Pharma.
John A. Kanis: Grants from Amgen, Lilly, Radius Health and non-financial support from Medimaps outside the submitted work
William Leslie, Lisa Lix, Helena Johansson: No conflicts of interest.
Contributor Information
William D. Leslie, Email: bleslie@sbgh.mb.ca.
Suzanne N. Morin, Email: suzanne.morin@mcgill.ca.
Lisa M. Lix, Email: Lisa.Lix@umanitoba.ca.
Eugene V. McCloskey, Email: e.v.mccloskey@sheffield.ac.uk.
Helena Johansson, Email: helena@statiq.se.
Nicholas C. Harvey, Email: nch@mrc.soton.ac.uk.
John A. Kanis, Email: w.j.pontefract@shef.ac.uk.
References
- 1.Kanis JA, Harvey NC, Cooper C, Johansson H, Oden A, McCloskey EV, et al. A systematic review of intervention thresholds based on FRAX : A report prepared for the National Osteoporosis Guideline Group and the International Osteoporosis Foundation. Arch Osteoporos. 2016;11(1):25. doi: 10.1007/s11657-016-0278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanis JA. University of Sheffield: 2007. Assessment of osteoporosis at the primary health-care level. Technical Report. Accessible at http://www.shef.ac.uk/FRAX/pdfs/WHO_Technical_Report.pdf. [Google Scholar]
- 3.Cauley JA, El-HajjFuleihan G, Arabi A, Fujiwara S, Ragi-Eis S, Calderon A, et al. Official Positions for FRAX(R) clinical regarding international differences from Joint Official Positions Development Conference of the International Society for Clinical Densitometry and International Osteoporosis Foundation on FRAX(R) J Clin Densitom. 2011;14(3):240–62. doi: 10.1016/j.jocd.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 4.Cauley JA, El-HajjFuleihan G, Luckey MM, Members FPDC. FRAX(R) International Task Force of the 2010 Joint International Society for Clinical Densitometry & International Osteoporosis Foundation Position Development Conference. J Clin Densitom. 2011;14(3):237–9. doi: 10.1016/j.jocd.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 5.Hippisley-Cox J, Coupland C. Derivation and validation of updated QFracture algorithm to predict risk of osteoporotic fracture in primary care in the United Kingdom: prospective open cohort study. BMJ. 2012;344:e3427. doi: 10.1136/bmj.e3427. [DOI] [PubMed] [Google Scholar]
- 6.Johansson H, Oden A, Lorentzon M, McCloskey E, Kanis JA, Harvey NC, et al. Is the Swedish FRAX model appropriate for Swedish immigrants? Osteoporos Int. 2015;26(11):2617–22. doi: 10.1007/s00198-015-3180-4. [DOI] [PubMed] [Google Scholar]
- 7.Statistics Canada. Ottawa: 2016. [last accessed February 16, 2020]. Focus on Geography Series, 2016 Census. Data products, 2016 Census. Statistics Canada Catalogue no. 98-404-X2016001. Ontario.2017 updated April 18, 2019 Available from https://www12.statcan.gc.ca/census-recensement/2016/as-sa/fogs-spg/Facts-CAN-Eng.cfm?TOPIC=7&LANG=Eng&GK=CAN&GC=01. [Google Scholar]
- 8.Leslie WD, Metge C. Establishing a regional bone density program: lessons from the Manitoba experience. J Clin Densitom. 2003;6(3):275–82. doi: 10.1385/jcd:6:3:275. [DOI] [PubMed] [Google Scholar]
- 9.Leslie WD, Caetano PA, Macwilliam LR, Finlayson GS. Construction and validation of a population-based bone densitometry database. J Clin Densitom. 2005;8(1):25–30. doi: 10.1385/jcd:8:1:025. [DOI] [PubMed] [Google Scholar]
- 10.Statistics Canada. Statistics Canada Catalogue no. 98-404-X2016001. Ottawa: [last accessed February 16, 2020]. Focus on Geography Series, Province of Mantioba, 2016 Census. Data products, 2016 Census. Ontario.2017 Available from https://www12.statcan.gc.ca/census-recensement/2016/as-sa/fogs-spg/Facts-pr-eng.cfm?Lang=Eng&GK=PR&GC=46&TOPIC=7updated April 18, 2019. [Google Scholar]
- 11.Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8(5):468–89. doi: 10.1007/s001980050093. [DOI] [PubMed] [Google Scholar]
- 12.Kanis JA, Oden A, Johansson H, Borgstrom F, Strom O, McCloskey E. FRAX and its applications to clinical practice. Bone. 2009;44(5):734–43. doi: 10.1016/j.bone.2009.01.373. [DOI] [PubMed] [Google Scholar]
- 13.Leslie WD, Lix LM, Langsetmo L, Berger C, Goltzman D, Hanley DA, et al. Construction of a FRAX(R) model for the assessment of fracture probability in Canada and implications for treatment. Osteoporos Int. 2011;22(3):817–27. doi: 10.1007/s00198-010-1464-2. [DOI] [PubMed] [Google Scholar]
- 14.Bisson EJ, Finlayson ML, Ekuma O, Marrie RA, Leslie WD. Accuracy of FRAX(R) in people with multiple sclerosis. J Bone Miner Res. 2019 doi: 10.1002/jbmr.3682. [DOI] [PubMed] [Google Scholar]
- 15.Leslie WD, Morin SN, Lix LM, Niraula S, McCloskey EV, Johansson H, et al. Performance of FRAX in Women with Breast Cancer Initiating Aromatase Inhibitor Therapy: A Registry-Based Cohort Study. J Bone Miner Res. 2019;34(8):1428–35. doi: 10.1002/jbmr.3726. [DOI] [PubMed] [Google Scholar]
- 16.Peschken CA, Hitchon CA, Garland A, Bernstein CN, Chen H, Fransoo R, et al. A Population-based Study of Intensive Care Unit Admissions in Rheumatoid Arthritis. J Rheumatol. 2016;43(1):26–33. doi: 10.3899/jrheum.150312. [DOI] [PubMed] [Google Scholar]
- 17.Yang S, Leslie WD, Yan L, Walld R, Roos LL, Morin SN, et al. Objectively Verified Parental Hip Fracture Is an Independent Risk Factor for Fracture: a Linkage Analysis of 478,792 Parents and 261,705 Offspring. J Bone Miner Res. 2016;31(9):1753–9. doi: 10.1002/jbmr.2849. [DOI] [PubMed] [Google Scholar]
- 18.Leslie WD, Lix LM, Johansson H, Oden A, McCloskey E, Kanis JA, et al. Independent clinical validation of a Canadian FRAX tool: fracture prediction and model calibration. J Bone Miner Res. 2010;25(11):2350–8. doi: 10.1002/jbmr.123. [DOI] [PubMed] [Google Scholar]
- 19.Fraser LA, Langsetmo L, Berger C, Ioannidis G, Goltzman D, Adachi JD, et al. Fracture prediction and calibration of a Canadian FRAX(R) tool: a population-based report from CaMos. Osteoporos Int. 2011;22(3):829–37. doi: 10.1007/s00198-010-1465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metge C, Black C, Peterson S, Kozyrskyj AL. The population's use of pharmaceuticals. Med Care. 1999;37(6 Suppl):JS42–JS59. doi: 10.1097/00005650-199906001-00008. [DOI] [PubMed] [Google Scholar]
- 21.Reid RJ, Roos NP, MacWilliam L, Frohlich N, Black C. Assessing population health care need using a claims-based ACG morbidity measure: a validation analysis in the Province of Manitoba. Health Serv Res. 2002;37(5):1345–64. doi: 10.1111/1475-6773.01029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khokhar B, Jette N, Metcalfe A, Cunningham CT, Quan H, Kaplan GG, et al. Systematic review of validated case definitions for diabetes in ICD-9-coded and ICD-10-coded data in adult populations. BMJ Open. 2016;6(8):e009952. doi: 10.1136/bmjopen-2015-009952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blanchard JF, Ludwig S, Wajda A, Dean H, Anderson K, Kendall O, et al. Incidence and prevalence of diabetes in Manitoba, 1986-1991. Diabetes Care. 1996;19(8):807–11. doi: 10.2337/diacare.19.8.807. [DOI] [PubMed] [Google Scholar]
- 24.Fazli GS, Moineddin R, Bierman AS, Booth GL. Ethnic differences in prediabetes incidence among immigrants to Canada: a population-based cohort study. BMC Medicine. 2019;17(1) doi: 10.1186/s12916-019-1337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manitoba Centre for Health Policy. Income quintiles. [Last accessed February 10, 2019];2003 Available from http://mchp-appserv.cpe.umanitoba.ca/viewDefinition.php?definitionID=102882.
- 26.Manitoba Centre for Health Policy. Concept: Regional Health Authority (RHA) Districts and Zones in Manitoba. [Last accessed January 12, 2020];2013 Available from http://mchp-appserv.cpe.umanitoba.ca/viewConcept.php?printer=Y&conceptID=1219.
- 27.Leslie WD, Lix LM, Morin SN, Johansson H, Oden A, McCloskey EV, et al. Hip axis length is a FRAX- and bone density-independent risk factor for hip fracture in women. J Clin Endocrinol Metab. 2015;100(5):2063–70. doi: 10.1210/jc.2014-4390. [DOI] [PubMed] [Google Scholar]
- 28.Leslie WD, Lix LM, Majumdar SR, Morin SN, Johansson H, Oden A, et al. Total Hip Bone Area Affects Fracture Prediction with FRAX(R) in Canadian White Women. J Clin Endocrinol Metab. 2017 doi: 10.1210/jc.2017-01327. [DOI] [PubMed] [Google Scholar]
- 29.Christensen AM, Leslie WD, Baim S. Ancestral differences in femoral neck axis length: possible implications for forensic anthropological analyses. Forensic Sci Int. 2014;236:193 e1–4. doi: 10.1016/j.forsciint.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 30.Clark P, Tesoriero LJ, Morton DJ, Talavera JO, Karlamangla A, Schneider DL, et al. Hip axis length variation: its correlation with anthropometric measurements in women from three ethnic groups. Osteoporos Int. 2008;19(9):1301–6. doi: 10.1007/s00198-008-0572-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hans D, Goertzen AL, Krieg MA, Leslie WD. Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: the Manitoba study. J Bone Miner Res. 2011;26(11):2762–9. doi: 10.1002/jbmr.499. [DOI] [PubMed] [Google Scholar]
- 32.Looker AC, Sarafrazi Isfahani N, Fan B, Shepherd JA. Trabecular bone scores and lumbar spine bone mineral density of US adults: comparison of relationships with demographic and body size variables. Osteoporos Int. 2016;27(8):2467–75. doi: 10.1007/s00198-016-3550-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leslie WD, Orwoll ES, Nielson CM, Morin SN, Majumdar SR, Johansson H, et al. Estimated lean mass and fat mass differentially affect femoral bone density and strength index but are not FRAX independent risk factors for fracture. J Bone Miner Res. 2014;29(11):2511–9. doi: 10.1002/jbmr.2280. [DOI] [PubMed] [Google Scholar]
- 34.Leslie WD, Morin SN, Lix LM, Martineau P, Bryanton M, McCloskey EV, et al. Fracture prediction from self-reported falls in routine clinical practice: a registry-based cohort study. Osteoporos Int. 2019;30(11):2195–203. doi: 10.1007/s00198-019-05106-3. [DOI] [PubMed] [Google Scholar]
- 35.Lix LM, Azimaee M, Osman BA, Caetano P, Morin S, Metge C, et al. Osteoporosis-related fracture case definitions for population-based administrative data. BMC Public Health. 2012;12:301. doi: 10.1186/1471-2458-12-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Epp R, Alhrbi M, Ward L, Leslie WD. Radiological validation of fracture definitions from administrative data. J Bone Miner Res. 2018;33(Supp 1):S275. [Google Scholar]
- 37.Kanis JA, Oden A, McCloskey EV, Johansson H, Wahl DA, Cooper C, et al. A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos Int. 2012;23(9):2239–56. doi: 10.1007/s00198-012-1964-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.United Nations Statistics Division. Ethnicity: A Review of Data Collection and Dissemination. 2003 Available from: https://unstats.un.org/unsd/demographic/sconcerns/popchar/Ethnicitypaper.pdflast visited June 28, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.