Abstract
Hypoxia and hypoxia-inducible factors (HIF-1α and HIF-2α) modulate innate immune responses in the setting of systemic inflammatory responses and sepsis. The HIF-prolyl hydroxylase enzymes, PHD1, PHD2 and PHD3 regulate the mammalian adaptive response to hypoxia, however, their significance in the innate immune response has not been elucidated. We demonstrate here that genetic loss of PHD3 specifically shortens the survival of mice subjected to various models of abdominal sepsis. In vivo, plasma levels of pro-inflammatory cytokines were enhanced, and recruitment of macrophages to internal organs was increased in septic PHD3-deficient mice, altogether indicating enhanced innate immune functions. Reciprocal bone marrow transplantation in sublethally irradiated mice revealed that the enhanced susceptibility of PHD3-deficient mice to sepsis-related lethality was specifically caused by loss of PHD3 in myeloid cells. Several in vitro assays revealed enhanced cytokine-production, migration, phagocytic capacity, and pro-inflammatory activation of PHD3-deficient macrophages. Increased pro-inflammatory activity of PHD3-deficient macrophages occurred concomitantly with enhanced HIF-1α protein stabilization and increased NF-κB activity, and interference with the expression of HIF-1α or NF-κB blunted their pro-inflammatory phenotype. It is concluded that loss or impairment of PHD3 enzyme function aggravates the innate immune response to septic stimuli via enhanced macrophage pro-inflammatory activity.
Introduction
Sepsis is defined as a systemic inflammatory response syndrome occurring in the presence of microbial pathogens. In clinical practice, sepsis is hallmarked by symptoms of fever, tachycardia, and leukocytosis followed by hypotension, oliguria and metabolic acidosis, altogether indicating functional impairment of vital organs (1). Sepsis is associated with fatal outcomes in 25-30% (2), and septic complications arising from systemic spread of abdominal pathogens represent a leading cause for patient mortality in surgical intensive care units (3). On the cellular level, sepsis is provoked by an overwhelming inflammatory response, initiated by the innate immune system. Specifically, activated macrophages trigger a potentially fatal immune reaction via excessive secretion of proinflammatory cytokines (4).
Sepsis leads to generalized tissue hypoxia due to inadequate perfusion, hypoxemia and altered cellular metabolism (5, 6). Hypoxia, in turn, represents a major stimulus for macrophages to mount an innate immune response (6, 7). This process importantly relies on increased stabilization of hypoxia inducible transcription factors, HIF-1α and HIF-2α (7–9). Macrophage bactericidal activity, motility and tissue-invasion are significantly impaired in mice specifically lacking HIF-1α in myeloid cells (10, 11). As a consequence, these animals are less susceptible to bacterial lipopolysaccharide- (LPS-) induced mortality (8). According to recent experimental evidence, HIF-2α likewise modulates macrophage proinflammatory activity during acute and tumor inflammation (7).
Prolyl hydroxylase domain-containing enzymes (PHD1, 2 and 3) regulate the stability of HIF-1α and HIF-2α in an oxygen-dependent manner. In the presence of oxygen, PHD enzymes catalyze the hydroxylation of two proline residues within the oxygen dependent degradation domain (ODD) of HIF-1α and HIF-2α (12), thus preventing HIF transcription factor dimerization, nuclear translocation and subsequent activation of hypoxia-adaptive target genes (12–14). Recent studies applying genetic loss of function approaches indicated that PHD enzymes carry out specific and non-redundant in vivo functions, and are therefore implicated in pathophysiologic responses to disease conditions characterized by hypoxia. Loss of PHD1 specifically alters the mitochondrial energy metabolism of skeletal muscle (15) or liver cells (16), thus increasing their hypoxic survival. Furthermore, loss of PHD1 increases the intestinal barrier function and is protective against experimental colitis (2, 17). PHD2 exerts a major physiologic function during placentation and cardiac development (18, 19). Of pathophysiologic relevance, haplodeficiency of PHD2 has been shown to normalize the vasculature of expanding solid tumors, resulting in improved tumor oxygenation, and delaying tumor cell intravasation and distant metastasis (20). PHD3 has been assigned a physiologic function in normal development of the sympathoadrenal system (21). PHD2 and PHD3 appear to be cooperatively involved in the development of hepatic steatosis and dilatative cardiomyopathy, which occurs as an effect of excessive HIF-1α stabilization (22). On the other hand, PHD3 has been shown to act as a suppressor of solid cancer growth independently of its effects on HIF stabilization, but via direct involvement in the canonical pathway activating the master regulator of innate immunity, nuclear factor-κB (NF-κB) (23).
Given the implications of hypoxia and hypoxia inducible factors in the innate immune response, we hypothesized that the PHD enzymes might exert important functions in systemic inflammation and sepsis. Here, we delineate a novel function for the PHD3 oxygen sensor in macrophages of the innate immune system. We demonstrate that loss of PHD3 aggravates pro-inflammatory functions of macrophages upon polymicrobial- or LPS-challenge. These changes are relevant in murine models of abdominal sepsis, where loss of PHD3 triggers an overwhelming innate immune response, leading to premature organ dysfunction and lethality. These findings extend our current insight into cell-specific functions of HIF-prolyl hydroxylases, and have potential clinical implications for the management of systemic inflammatory response syndrome and sepsis.
Results
Loss of PHD3 aggravates sepsis-related disease symptoms and lethality
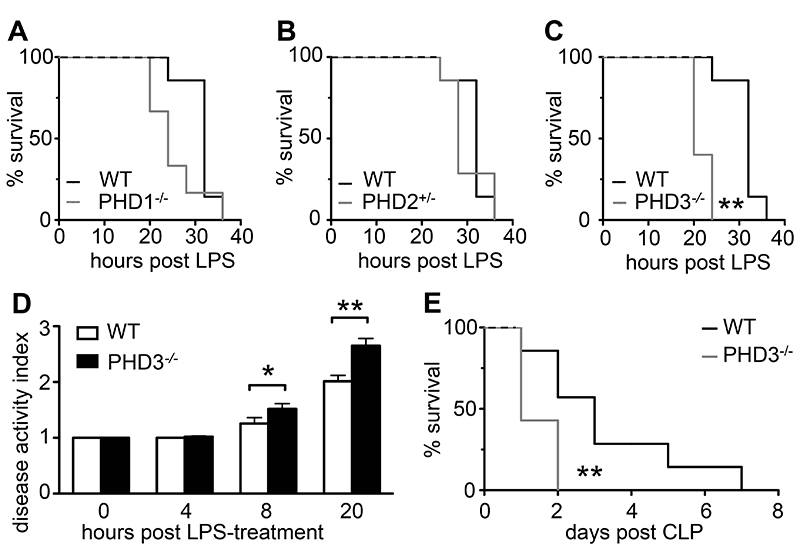
In order to investigate the function of HIF prolyl-hydroxylase enzymes in generalized abdominal sepsis, mice deficient for PHD1, PHD2 or PHD3 and wild type (WT) littermates were subjected to intraperitoneal injection of bacterial lipopolysaccharide (LPS). In non-genetically altered WT mice, 15 mg/kg LPS expectedly induced a marked systemic inflammatory response, leading to death within 36 hours of treatment (Figure 1, A–C). Septic survival of PHD1-deficient (PHD1-/-) or PHD2-haplodeficient (PHD2+/-) mice did not change significantly compared to that of their WT counterparts (Figure 1, A and B). By contrast, survival of homozygous PHD3-deficient mice (PHD3-/-) was significantly impaired compared to that of WT mice subjected to endotoxemic shock (Figure 1C). This phenotype was maintained when WT or PHD3-/- mice were treated with lower concentrations of LPS (12 mg/kg), which induced lethality in 100% of PHD3-/- mice, but only in 67% of WT mice (Supplementary Figure 1A).
Figure 1. Loss of PHD3-/- aggravates sepsis-associated symptoms and lethality.
(A-C) Survival curves of mice suffering LPS-induced sepsis (15 mg/kg, i.p.), revealing unaffected survival of PHD1-/- or PHD2+/- mice, but impaired survival of PHD3-/- mice. (D) Disease activity index, indicating increased clinical sepsis symptoms in PHD3-/- mice subjected to LPS-induced sepsis. (E) Survival curves of mice subjected to caecal lication and puncture, revealing impaired survival of PHD3-/- mice suffering polymicrobial abdominal sepsis. Bars in (C) represent mean ± SEM; *P < 0.05, ** P < 0.01, n = 8 in all groups. Results are representative of two independent experiments.
To further determine whether loss of PHD3 accelerated the course of LPS-induced endotoxemia, clinical disease symptoms were monitored every 4 hours, and scored according to a disease activity index reflecting body temperature, posture and alertness (24). Indeed, clinical disease symptoms were significantly aggravated in PHD3-/- compared to WT mice when measured after 8 or more hours following LPS challenge (Figure 1D).
As LPS-induced endotoxemic shock only partly reproduces human abdominal sepsis, we additionally investigated whether PHD3-deficiency accelerated the susceptibility to polymicrobial peritonitis. Indeed, survival of PHD3-/- mice was strikingly shortened compared to WT animals when subjected to polymicrobial abdominal sepsis, induced by large bowel perforation (caecal ligation and puncture, CLP; Figure 1E).
Thus, loss of PHD3 specifically enhanced the susceptibility of mice to sepsis-induced disease symptoms and death in two alternative models mimicking human abdominal sepsis.
Loss of PHD3 enhances septic organ damage
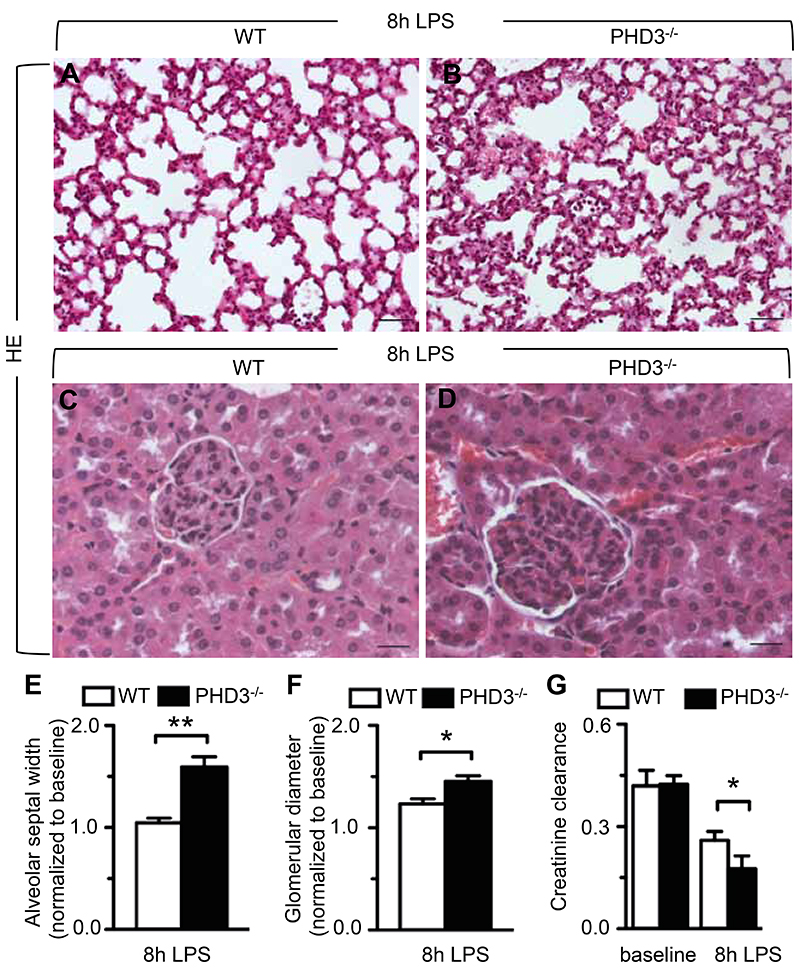
In order to investigate whether accelerated septic death of PHD3-/- mice coincided with enhanced organ damage, internal organs were subjected to histopathological analysis 8 hours after LPS-treatment, when clinical symptoms of systemic inflammatory response became apparent (Figure 1D). Histological assessment of septic lungs revealed that alveolar septa were significantly thicker in LPS-treated PHD3-/- mice than in WT littermates (Figure 2, A and B). Indeed, histomorphometry revealed that thickness of alveolar septa was significantly increased in PHD3-/- compared to WT animals following 8 hours of LPS-treatment, indicating that PHD3-/- mice suffered more severe interstitial pulmonary edema (Figure 2E).
Figure 2. Increased septic organ damage in PHD3-/- mice.
(A-D) Representative histology of lungs (A, B) and kidneys (C, D) from mice subjected to LPS-induced sepsis, revealing more pronounced swelling of lung alveolar septi and kidney glomeruli in PHD3-/- mice (B, D) than in WT animals (A, C). (E, F) Histomorphometric quantification of alveolar septal width (E) and glomerular diameter (F) in septic lungs and kidneys, respectively. (G) Assessment of creatinine clearance, revealing enhanced septic impairment of kidney function in PHD3-/- mice. Bars represent mean ± SEM; * P < 0.05, ** P < 0.01, n = 5. Scale bars = 40 μm in (A, B) and 20 μm in (C, D).
In accordance with these findings, histological assessment of kidneys from LPS-treated animals revealed more extensive glomerular damage in PHD3-/- than in WT mice (Figure 2, C and D). At 8 hours after LPS challenge, glomerular diameter was markedly enhanced in both WT and PHD3-/- mice compared to baseline conditions, however, the extent of glomerular swelling was significantly pronounced in PHD3-/- mice (Figure 2F). In order to assess whether the observed difference in glomerular damage was functionally relevant, we measured serum creatinine levels, and applied a modified Cockroft-Gault formula to determine creatine clearance. LPS-treatment impaired kidney function in both WT and in PHD3-/- mice, however, the impairment of creatinine clearance was significantly enhanced in PHD3-/- animals (Figure 2G).
Taken together, accelerated septic lethality of PHD3-/- mice was accompanied by enhanced inflammatory damage and interstitial edema of vital organs.
Enhanced activation of the innate immune response in septic PHD3-/- mice
As septic shock and -organ dysfunction are boosted by the innate immune response, we sought to determine whether systemic production of pro-inflammatory cytokines and accumulation of innate immune cells were pathologically enhanced in LPS-treated PHD3-/- mice.
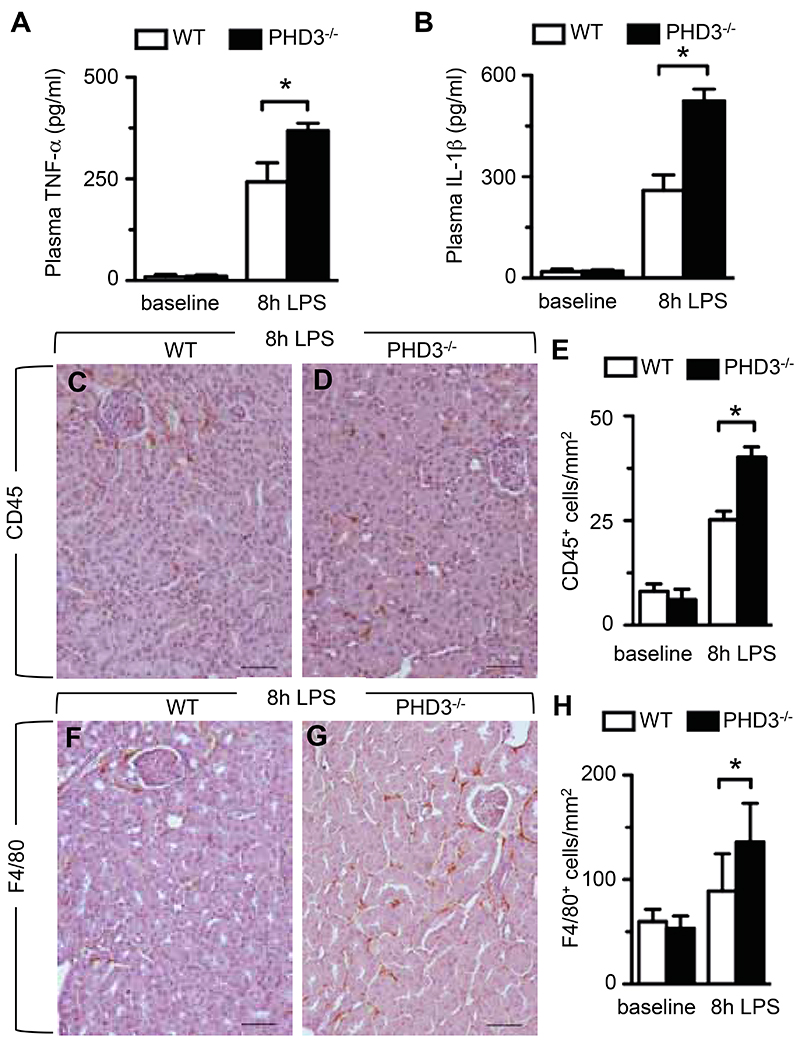
ELISA assays were performed to determine the abundance of pro-inflammatory cytokines in the blood of septic mice, 8 hours after LPS administration. Expectedly, LPS-challenge markedly increased serum levels of TNF-α and IL1-β, but this increase was significantly more pronounced in septic PHD3-/-- than in WT animals (Figure 3, A and B). Serum IL-6 levels were under the detection limit at baseline healthy conditions, but significantly increased in LPS-challenged PHD3-/- mice (serum IL-6 concentrations, pg/ml: 3021 ± 88 in septic WT mice vs 3144 ± 104 in septic PHD3-/- mice; P < 0.01, n = 6). Overall, LPS-induced release of pro-inflammatory cytokines was significantly enhanced in LPS-treated mutant mice compared to their WT counterparts.
Figure 3. Enhanced innate immune response in LPS-treated PHD3-/- mice.
(A, B) ELISA-based assessment of TNF-α (A) and IL-1β (B) in peripheral blood of baseline healthy or LPS-treated mice, revealing enhanced concentrations of pro-inflammatory cytokines in septic PHD3-/- animals. (C-E) Representative CD45-immunostaining (C, D) and histomorphometric quantification analysis (E), revealing enhanced accumulation of CD45-positive leucocytes in kidneys of septic PHD3-/- mice. (F-H) Representative F4/80-immunostaining (F, G) and histomorphometric quantification analysis (H), revealing enhanced accumulation of F4/80-positive macrophages in kidneys of septic PHD3-/- mice. Bars represent mean ± SEM; * P < 0.05, ** P < 0.01, n = 6. Scale bar = 50 μm in (C, D, F, G).
In an initial attempt to determine whether increased cytokine-levels in LPS-challenged PHD3-/- mice were due to enhanced recruitment of innate immune cells, we applied immunohistochemistry and morphometric quantification to assess the accumulation of leukocytes and tissue-infiltrating macrophages in septic organs. Indeed, staining for the leukocyte common antigen CD45 (25) revealed that LPS-treatment induced a substantial accumulation of leukocytes in internal organs such as the kidney, and that this increase was significantly enhanced in organs of septic PHD3-/- mice (Figure 3, C–E). Staining for the macrophage-specific F4/80 antigen (26) revealed that the accumulation of macrophages was likewise significantly increased in kidneys from LPS-injected PHD3-/- mice compared to their WT littermates (Figure 3, F–H). Comparable results were obtained upon immunohistochemical analysis of other internal organs such as the lung or the liver (data not shown).
Taken together, enhanced septic organ dysfunction and lethality coincided with increased pro-inflammatory cytokine release and accumulation of innate immune cells in internal organs of septic PHD3-/- mice.
Accelerated sepsis-related death in PHD3-/- mice is macrophage-dependent
Macrophages of the innate immune system are essential regulators of inflammation, and their function is critically affected by hypoxia (27). We therefore speculated that increased susceptibility to sepsis progression in PHD3-/- mice is attributable to altered macrophage function. The following experiments were performed to address this hypothesis.
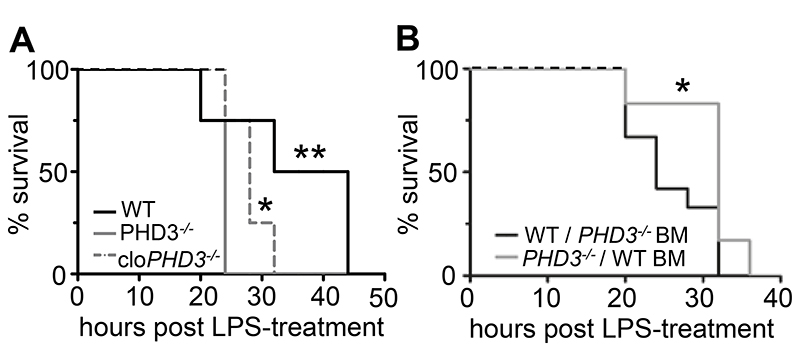
Firstly, animals were treated with clodronate liposomes to specifically induce apoptosis of macrophages (28). Immunohistochemical staining of spleens for the macrophage-marker F4/80 revealed that internal organs were markedly and efficiently depleted of macrophages after 24 hours of clodronate liposome treatment, whereas control-treatment with PBS-liposomes had no such effect (Supplementary Figure 1, B–C). Macrophage depletion was similarly effective in WT and PHD3-/- mice (not shown). Interestingly, clodronate-liposome-mediated elimination of macrophages significantly prolonged the survival of LPS-injected PHD3-/- mice (Figure 4A). By contrast, macrophage-depletion did not affect the survival of septic WT mice (not shown). Thus, depletion of functional macrophages partly but significantly reduced the phenotype of enhanced septic lethality in PHD3-/- mice.
Figure 4. Enhanced lethality of septic mice is specifically due to loss of PHD3 in macrophages.
(A) Survival curves of macrophage-depleted mice upon LPS-induced sepsis, revealing significantly enhanced survival of macrophage-depleted PHD3-/- (cloPHD3-/-). * P < 0.05 for PHD3-/- vs cloPHD3-/-; ** P < 0.01 for WT vs PHD3-/-, n = 8. (B) Survival curves of sublethally irradiated and bone-marrow-transplanted mice upon LPS-induced sepsis, revealing impaired survival of WT mice carrying PHD3-/- bone-marrow (WT / PHD3-/- BM) compared to PHD3-/- mice carrying WT bone-marrow (PHD3-/- / WT BM). * P < 0.05, ** P < 0.01, n = 9.
Secondly, to assess whether enhanced septic lethality was truly attributed to loss of PHD3-function in bone marrow-derived innate immune cells, mice were bone marrow depleted by sublethal irradiation. Subsequently, bone marrow transplantation (BMT) was performed in order to obtain chimeric WT mice reconstituted with PHD3-/- bone marrow-derived leukocytes (WT / PHD3-/- BM), or PHD3-/- mice harboring genetically unaltered WT bone marrow (PHD3-/- / WT BM). Strikingly, the presence of WT bone marrow reverted the phenotype of accelerated septic lethality in PHD3-/- mice, whereas implantation of PHD3-/- bone marrow into WT mice induced accelerated septic lethality. Indeed, survival of LPS-exposed WT / PHD3-/- BM mice was significantly shorter compared to that of equally treated PHD3-/- / WT BM mice (Figure 4B). WT mice implanted with syngenic WT bone marrow were used as a control, and displayed septic survival comparable to that of PHD3-/- / WT BM mice. Survival of PHD3-/- mice transplanted with PHD3-/- bone marrow did not significantly differ from that of WT / PHD3 -/- BM mice (not shown).
This series of experiments indicated that enhanced sepsis progression in PHD3-/- mice was likely due to altered functions of innate immune cells belonging to the monocyte/macrophage lineage.
Enhanced pro-inflammatory macrophage activity upon loss of PHD3
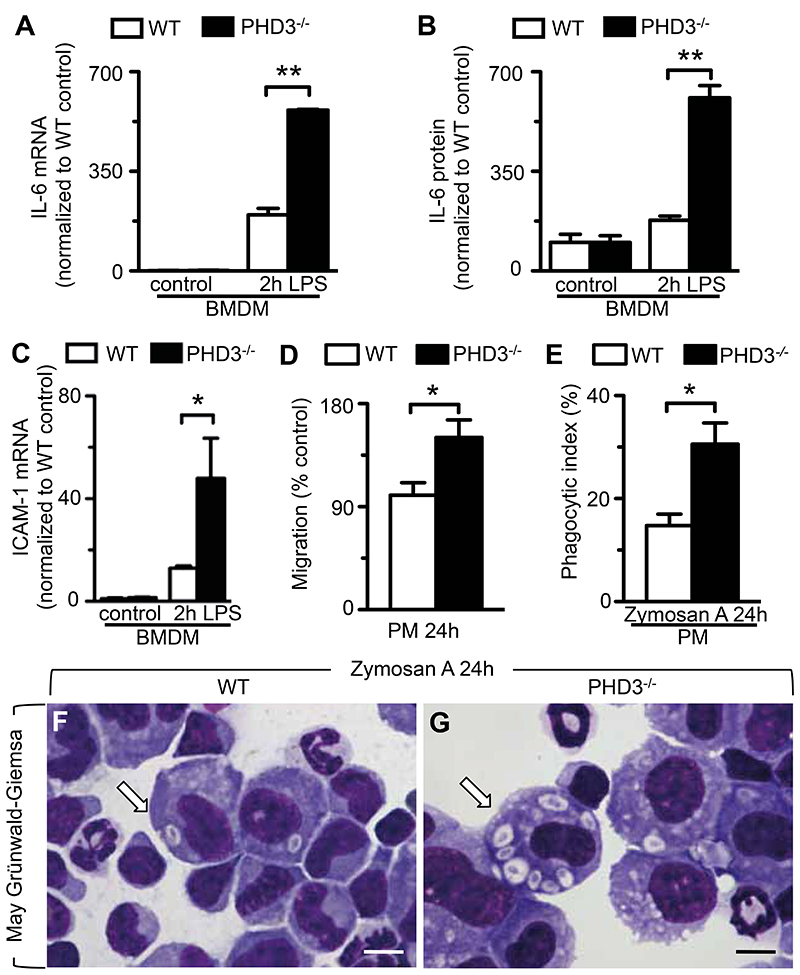
We next sought to determine in further detail whether loss of PHD3 enhanced various pro-inflammatory macrophage functions. To test whether PHD3-deficiency stimulated macrophage expression of pro-inflammatory cytokines, bone marrow-derived macrophages (BMDM) were isolated from WT and PHD3-/- mice (Supplemental Note 1), and subjected to LPS-treatment in vitro. qRT-PCR analysis revealed that IL-6 transcript expression was expectedly up-regulated in both WT and PHD3-/- macrophages 2 hours after LPS-challenge, however, IL-6 expression was 3-fold higher in LPS-treated PHD3-/-- than in WT BMDM (Figure 5A). Consistently, ELISA analysis of supernatants revealed that IL-6 cytokine production was markedly increased in LPS-treated PHD3-/- BMDM (Figure 5B). Similar experiments were performed applying peritoneal macrophages isolated from WT and PHD3-/- mice, and yielded comparable results (not shown).
Figure 5. Enhanced pro-inflammatory functions in PHD3-deficient macrophages.
(A, B) qRT-PCR (A) and ELISA analysis (B), revealing increased expression of IL-6 mRNA transcript and -protein, respectively, in LPS-treated PHD3-/- BMDMs. (C) qRT-PCR analysis, revealing enhanced expression of ICAM-1 mRNA trancripts in LPS-treated PHD3-/- BMDMs. (D) Transwell migration assay, revealing enhanced migration potential of PHD3-/- peritoneal macrophages (PM) compared to control (WT) cells. (E-G) Quantification analysis (E) and representative pictures (F, G) from phagocytosis assays applying peritoneal macrophages (PM), revealing increased ingestion of Zymosan particles (white arrows) by PHD3-/- macrophages (G) compared to WT cells (F) cells. Bars represent mean ± SEM; * P < 0.05, ** P < 0.01, n = 6. Scale bar = 10 μm in (F, G).
We further assessed whether PHD3-deficiency affected macrophage migration, and screened for the expression of inter-cellular adhesion molecule 1 (ICAM-1), a surface glycoprotein that is crucial for the transmigration of macrophages into inflamed tissues (29), in BMDM derived from WT or PHD3-/- mice. Indeed, ICAM-1 transcript expression was significantly increased by 3.7-fold in PHD3-/- BMDM compared to WT cells upon LPS-challenge (Figure 5C), suggesting enhanced migration potential. As a functional readout of macrophage migration, we performed in vitro migration assays applying peritoneal macrophages (30) harvested from WT or PHD3-/- mice. Indeed, transwell migration of these cells was significantly increased upon loss of PHD3 (Figure 5D).
Finally, in order to assess effects of PHD3-deficiency on macrophage phagocytic activity, experimental peritonitis was induced by intra-peritoneal injection of the yeast-derived β-glucan Zymosan A, and phagocytic index was determined via assessment of Zymosan A-particles and apoptotic bodies ingested into phagocytosing peritoneal macrophages in vivo (31). Strikingly, macrophages from PHD3-/- animals showed significantly enhanced phagocytic capacity (Figure 5, E–G) compared to WT animals when assayed after 24 hours following intra-peritoneal instillation of Zymosan A. Of note, loss of PHD3 likewise enhanced the ingestion of apoptotic cells (efferocytosis, a key function of macrophages (32)) by peritoneal macrophages (Supplementary Figure 1D).
Taken together, loss of PHD3 strikingly enhanced pro-inflammatory cytokine production, as well as migrative and phagocytic- potential of macrophages.
Altered macrophage maturation and polarization in PHD3-/- mice
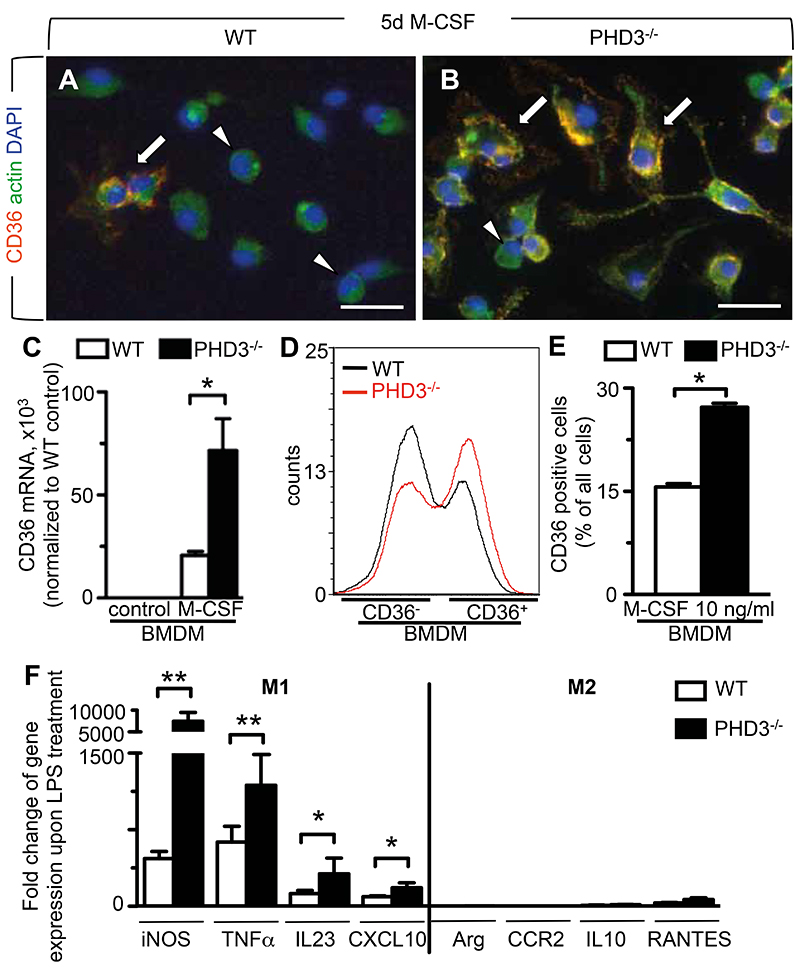
We likewise sought to determine whether the absence of PHD3 stimulated the maturation of macrophages from monocytes, which represents a key initiatory step of the innate immune response during septicemia (33). Mononuclear cells were harvested from the bone marrow of WT and PHD3-/- mice, and their differentiation into macrophages initiated by the addition of macrophage-colony stimulating factor (M-CSF) in vitro. By fluorescence microscopy, PHD3-/- cells more rapidly altered their morphology towards a macrophage-like phenotype with increased cytoplasmic dimensions, occasional presence of pseudopodia and surface expression of the macrophage marker CD36 (Figure 6B). By contrast, more round-shaped and smaller cells devoid of CD36-expression were observed in differentiating cultures of WT cells after 5 days of M-CSF treatment (Figure 6, A and B). For indirect quantification of macrophage maturation in differentiating cultures of WT and PHD3-/- bone marrow cells, we applied qRT-PCR and FACS analysis to screen for the expression of the macrophage marker CD36 (34). By qRT-PCR, mRNA transcript expression of CD36 was almost undetectable in undifferentiated cultures of WT or PHD3-/- cells (Figure 6C). However, upon 5 days of M-CSF treatment, CD36 transcript expression was strikingly increased by 3-fold in PHD3-/- compared to WT cells (Figure 6C). Consistently, FACS analysis revealed that the fraction of CD36-expressing cells was significantly higher in M-CSF-treated cultures of PHD3-/- bone marrow cells than in corresponding WT cultures (Figure 6D-E). Similar results were obtained when screening for the expression of the macrophage marker F4/80 (not shown; Supplemental Note 2).
Figure 6. Enhanced macrophage differentiation and M1-polarization upon loss of PHD3.
(A, B) Representative immunocytochemistry for CD36 (red), actin (green) and DAPI (blue). Increased proportions of roundish cells devoid of CD36-expression (arrowheads) are present in differentiating cultures of WT macrophages (A), while the occurrence of CD36-positive cells with macrophage-like morphology (arrows) is enhanced in differentiating cultures of PHD3-/- macrophages (B). (C) qRT-PCR analysis, revealing increased mRNA transcript expression of the macrophage marker CD36 mRNA in M-CSF-treated bone-marrow cells isolated from PHD3-/- mice. (D-E) Representative histogram (D) and quantification (E) of FACS analyses, revealing increased fractions of CD36+ cells in differentiating cultures of PHD3-/- bone marrow cells treated with 10ng/ml M-CSF for 5 days. (F) qRT-PCR analysis, revealing increased mRNA transcript expression of M1 differentiation markers (iNOS, TNFα, IL23, CXCL10), but not M2-differentiation markers (Arg=Arginase, CCR2, IL10, RANTES) in LPS-treated PHD3-/- BMDMs. Expression values were normalized to expression in non-LPS-treated cells. Bars represent mean ± SEM; * P < 0.05, ** P < 0.01, n = 6. Scale bars = 20 μm in (A, B).
To further establish whether absence of PHD3 affected the M1 (microbicidal) versus M2 (immunomodulatory) polarization of macrophages in case of septicemia, we assessed the expression of M1- and M2-associated genes in BMDM isolated from WT or PHD3-/- mice. At baseline culture conditions, the expression of M1- and M2-associated genes was comparable in WT and PHD3-/- BMDM (not shown). LPS-treatment in vitro expectedly up-regulated the expression of M1-, but not of M2-associated genes in BMDM of both genotypes (Figure 6F). However, upregulation of M1 genes was significantly increased by 2-14 fold in LPS-challenged PHD3-/- BMDM compared to similarly treated WT cells. As expected, no such differences were observed in the expression of genes specific for the M2 phenotype (Figure 6F).
Taken together, these findings indicate that loss of PHD3 promoted the maturation of macrophages from monocytes, and boosted pro-inflammatory M1-polarization of macrophages upon LPS-exposure.
Downstream effectors of PHD3 in macrophage-mediated inflammation
We finally aimed to delineate some of the molecular mechanisms, that act downstream of PHD3 to increase macrophage pro-inflammatory responses and clinical sepsis progression. Focus was placed on the roles of NF-κB and hypoxia inducible factors (HIFs), as these are well-described PHD3 targets with a key regulatory function in innate immunity (35, 36).
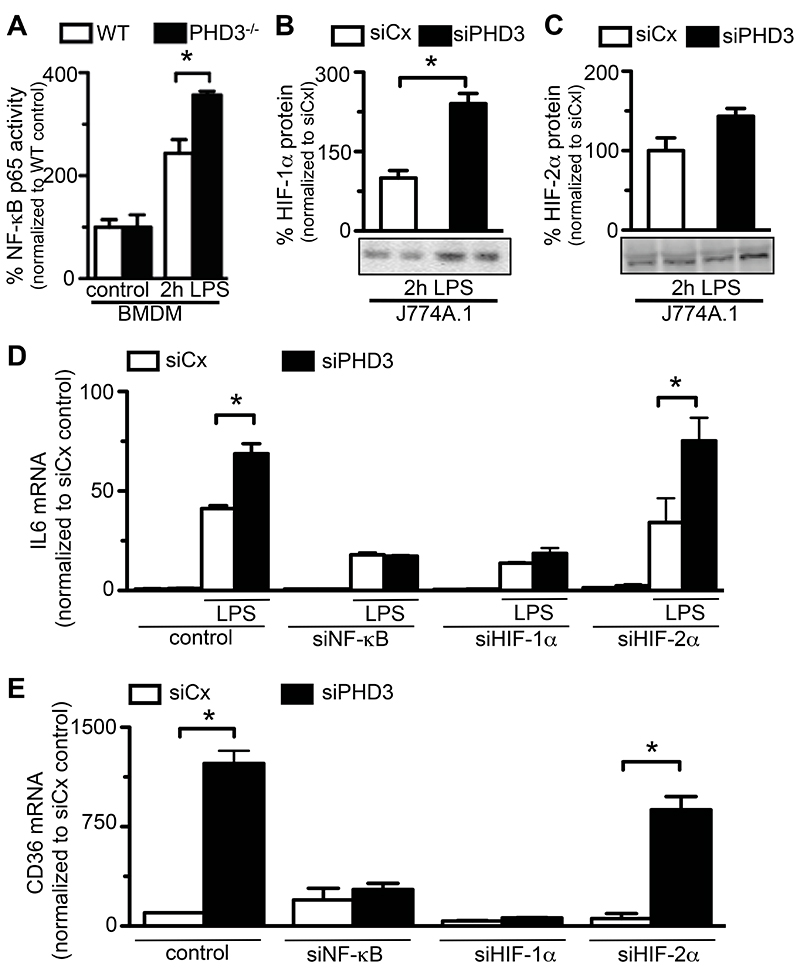
NF-κB activity assays were performed on primary BMDM isolated from WT or PHD3-/- mice, and revealed comparable NF-κB activity in WT and PHD3-/- macrophages at baseline culture conditions (Figure 7A). LPS-treatment caused a marked increase of NF-κB activity in both WT and PHD3-/- macrophages; however, LPS-induced activation of NF-κB-p65 was significantly more pronounced in macrophages lacking functional PHD3 (Figure 7A). Interestingly, further analysis revealed that interference with PHD3 expression did not affect the expression of the NF-κB p50 subunit (Supplementary Figure 1E).
Figure 7. Role of HIF-1α and NF-κB.
(A) NF-κB activity assay, revealing increased NF-κB activity in LPS-treated BMDMs isolated from PHD3-/- mice. (B, C) Representative immunoblots (bottom) and semi-quantitative analysis (top), revealing increased LPS-induced stabilization of HIF-1α- (B) but not HIF-2α protein levels (C) in J774A.1 macrophages with silenced PHD3 expression (siPHD3). (D) qRT-PCT analysis, revealing that LPS-induced expression of IL-6 transcripts in PHD3-silenced macrophages is significantly reverted by simultaneous knock-down of NF-κB (siNFκB) and HIF-1α (siHIF-1α), but not HIF-2α (siHIF-1α). (E) qRT-PCT analysis, revealing that LPS-induced expression of CD36 transcripts in PHD3-silenced (siPHD3) macrophages is significantly reverted by simultaneous knock-down of NF-κB (síNFκB) and HIF-1α (siHIF-1α), but not HIF-2α (siHIF-1α). Bars represent mean ± SEM, * P < 0.05, ** P < 0.01, n = 6.
Stabilization of HIF-1α and HIF-2α was studied in murine J774A.1 macrophages subjected to siRNA-mediated knock-down of PHD3-expression (Supplemental Note 3). Due to the difficulty of quantifying appropriate amounts of murine HIF-1α protein under baseline conditions, where HIF-1α protein is mostly below the detection limit (8, 37), we assessed mRNA expression levels of the HIF-1α target gene carbonic-anhydrase 9 (CaIX) (38). Indeed, expression of CaIX was 2.3-fold upregulated in PHD3-silenced macrophages compared to control-transfected cells (CaIX mRNA copies/106 copies 18S: 0.9 ± 0.3 in control-transfected cells vs 2.1 ± 1.2 in PHD3-silenced cells; P = 0.03, n = 6). After 2 hours of LPS-exposure, HIF-1α protein levels were detectable by Western blotting, and found to be significantly increased in PHD3-silenced compared to control-transfected macrophages (Figure 7B). HIF-2α protein levels were slightly but not significantly increased in PHD3-silenced cells already at baseline conditions (data not shown). LPS-treatment did not cause a significant further increase of HIF-2α protein levels in PHD3-silenced or control cells (Figure 7C). Taken together, interference with PHD3-expression increased the LPS-dependent induction of both NF-κB activity and HIF-1α stabilization in macrophages.
In order to investigate whether these factors are indeed mediators of the pro-inflammatory phenotype in PHD3-deficient mice, we assessed whether interference with the expression of HIF-1α-, HIF-2α- or the p65 subunit of NF-κB reverted increased cytokine production and differentiation of PHD3-deficient macrophages in vitro. RNAi-transfer resulted in incomplete and selective silencing of HIF-1α-, HIF-2α- or NF-κB-p65 mRNA levels by approximately 80% (Supplemental Note 4). In accordance with the findings outlined above, expression of IL-6 mRNA transcripts was increased by approximately 2-fold in PHD3- silenced cells compared to control-transfected cells following 2-hours of LPS-treatment (Figure 7D). A comparable induction of IL-6 transcript expression was observed in PHD3- and HIF-2α -double-silenced macrophages (Figure 7D). By contrast, simultaneous down-regulation of HIF-1α or NF-κB-p65 dramatically blunted the LPS-induced increase of IL-6 expression in PHD3-silenced cells (Figure 7D). Similarly, knock-down of HIF-1α- or NF-κB-65 expression fully reverted the enhanced maturation of PHD3-silenced J774A.1 cells into macrophages, as indicated by a blunted increase of the macrophage differentiation marker CD36 (Figure 7E). Of note, silencing of HIF-2α expression did not affect enhanced macrophage differentiation from PHD3-deficient bone marrow cells. Altogether, these experiments suggest a prominent role for NF-κB and HIF-1α, but not HIF-2α in mediating the increased inflammatory response that occurs upon macrophage loss of PHD3.
Discussion
Hypoxia and hypoxia inducible factors are crucial modulators of systemic inflammation (7, 35, 39), however, experimental insight into specific in vivo functions of the HIF prolyl-hydroxylase enzymes, PHD1, PHD2 and PHD3, in this context is scarce. All three HIF prolyl-hydroxylases are reportedly expressed in leukocytes and macrophages of the innate immune system (8, 27) altogether suggesting that interference with their expression or enzymatic activity might alter innate immune functions and, therefore, the systemic inflammatory response to bacterial pathogens. Here, we provide in vivo evidence that homozygous loss of PHD3 aggravates the clinical course of abdominal sepsis. This effect appeared to specific for loss of the PHD3 enzyme, since deficiency for PHD1 (PHD1-/-) or PHD2 (PHD2+/-) did not result in a similar phenotype.
Several lines of in vivo evidence from this study indicate that premature lethality of septic PHD3-/- mice was due to innate immune responses, which were hyperactivated upon loss of PHD3 gene function. Firstly, increased lethality of septic PHD3-/- mice occurred concomitantly with enhanced levels of leukocyte-derived cytokines, and with enforced recruitment of activated leukocytes to vital organs such as the lung or the kidney. Since overwhelming production of pro-inflammatory cytokines such as TNF-α and IL-1β represents a key trigger for the systemic inflammatory response to endotoxemia (40–42), an augmented cytokine storm is a likely cause for increased clinical sepsis symptoms in PHD3-/- mice. Likely as a consequence of enforced invasion by activated leukocytes, organs of septic PHD3-/- mice displayed enhanced interstitial edema and parenchymal damage, leading to early organ dysfunction. Secondly, depletion of functional macrophages markedly attenuated the hypersensitivity of PHD3-/- mice to septicemia. Furthermore, replacement of bone marrow (and, therefore, innate immune cells) from PHD3-/- mice with non-genetically altered bone marrow abrogated enhanced lethality of PHD3-/- mice in conditions of abdominal sepsis. Conversely, introduction of PHD3-deficient bone marrow increased sepsis-related lethality of normal wild type mice, altogether indicating that bone marrow-derived macrophages are crucial mediators of the observed phenotype.
Indeed, a series of functional in vitro assays demonstrated that inhibition of the PHD3 gene led to a robust stabilization of diverse pro-inflammatory macrophage functions. For instance, PHD3-/- macrophages displayed increased phagocytic properties, and more readily upregulated their expression of the cell surface protein ICAM-1, which is required for hypoxia-induced adhesion to vascular endothelium and transmigration into inflamed tissues (43, 44). In fact, the intrinsic potential of bone marrow-derived macrophages to migrate in response to LPS-stimuli was significantly enhanced upon loss of PHD3. Further in vitro assays confirmed that differentiation and pro-inflammatory activation of macrophages was augmented in the absence of PHD3.
Given the well-described implications of hypoxia and oxygen sensing in the innate immune response, the notion that immunomodulatory functions could be assigned to HIF-prolyl hydroxylase oxygen sensors is not far-fetched. Macrophage pro-inflammatory functions, in particular, are crucially stimulated by hypoxia, (6, 11, 27, 45, 46). For instance, macrophages produce higher levels of pro-inflammatory cytokines when exposed to hypoxia than under normoxic conditions, an effect that is mediated by HIF-1α (27). HIF-1α is however likewise stabilized upon activation of macrophages by nonhypoxic stimuli such as LPS-treatment (9), and is then indispensable to foster the potential of leukocytes to invade inflamed tissues and to destroy bacterial pathogens, thus limiting their spread from localized sites of infection (8, 10, 11, 45, 47). Due to the relevance of HIF-1α in inflammatory responses, its cell-specific deletion in macrophages is protective against LPS-induced lethality (11) in mice. These findings are consistent with our observation that loss of PHD3, which is suspected to positively regulate HIF-1α-dependent transcriptional signaling in macrophages, promotes LPS-induced lethality.
Therefore, on the molecular level, it appears likely that HIF-1α is a major effector stimulating pro-inflammatory functions in PHD3-deficient macrophages. In this regard, our protein expression analyses revealed that stabilization of HIF-1α in macrophages is indeed strongly enhanced by the presence of LPS (which is consistent with other reports (8, 9)), but even further increased upon interference with PHD3 expression. By contrast, HIF-2α protein stabilization in macrophages was neither significantly affected by LPS-treatment, nor by knock-down of PHD3 expression. Interestingly, increased pro-inflammatory cytokine expression, as well as enhanced differentiation of PHD3-deficient macrophages, was fully reverted by simultaneous interference with HIF-1α-expression in vitro, but maintained in the absence of HIF-2α. Altogether, these findings suggest that PHD3 is a key regulator of HIF-1α stabilization in macrophages of the innate immune system, and that PHD3-driven stabilization of HIF-1α is a key molecular mechanism underlying enhanced sepsis-related lethality in PHD3-/- mice. Obviously, these results do not exclude a general role for HIF-2α in mediating macrophage-mediated inflammation. Indeed, it has recently been demonstrated that HIF-2α likewise regulates pro-inflammatory cytokine expression and migration of macrophages, and that mice lacking HIF-2α in myeloid cells display increased resistance to LPS-induced endotoxemia (7). According to these evolving insights it is most conceivable that HIF-1α and HIF-2α fulfill differential and non-redundant functions in the physiology of macrophage-mediated inflammation. It is in this regard interesting that expression of HIF-1α and HIF-2α appears to be differentially induced by Th1 cytokines driving pro-inflammatory (M1-) activation and Th2 cytokines driving immunomodulatory (M2-) activation of macrophages, respectively (48). This is in keeping with our finding that increased HIF-1α protein stabilization in PHD3-deficient macrophages occurs concomitantly with enhanced pro-inflammatory- (M1-) activation.
It is however also worthwhile to speculate whether the observed hyperinflammation phenotype in PHD3-/- mice is at least partly mediated independently of classic HIF-pathways. For instance, loss of PHD3 in macrophages might directly affect the transcriptory activity of the master regulator of innate immunity, nuclear factor-κB (NF-κB) (30). It has been demonstrated that HIF-prolyl hydroxylases are repressors of NF-κB activity, likely via their potential to directly hydroxylate the inhibitor of NF-κB (IκB) kinase (IKKß), which is responsible for phosphorylation-dependent degradation of IκB inhibitors, and, therefore, liberation and activation of NF-κB in response to inflammatory stimuli (49, 50). Furthermore, it has been documented that PHD3 can associate with IKKß independently of its hydroxylase function, thereby blocking further interaction between IKKß and the chaperone HsP90, which is required for IKKß phosphorylation and release of NF-κB (23, 51). In our study, we found that PHD3-deficient macrophages displayed significantly enhanced NF-κB activity. This appeared to be functionally relevant, as interference with NF-κB-p65 expression reverted the pro-inflammatory phenotype of PHD3-deficent macrophages. This might appear paradox in the light of previous findings that targeted inhibition of IKKß, which blocks NF-κB activation, likewise causes enhanced susceptibility of mice to endotoxemic shock (52). However, unlike in our model, these effects importantly relied on excess recruitment of neutrophil granulocytes. Indeed, IKKß-deficient mice develop massive granulocytosis, and the depletion of neutrophil granulocytes improves their survival in conditions of sepsis (52). By contrast, anti-inflammatory effects of IKKß were also reported. In particular, myeloid-specific deletion of IKKß can enhance the survival of mice during bacterial inflammation (53), likely via inhibiting classical “M1” activation of macrophages, thus preventing their excess pro-inflammatory activity during infection (53). Consistent with previous reports (43), it is therefore conceivable that pro-inflammatory effects in PHD3-deficient macrophages are at least partly due to HIF-independent induction of NF-κB activity in macrophages.
On the basis of our study, it can be concluded that both HIF-1α and NF-κB are required for boosting of the innate immune response in septic PHD3-/- mice. Recent work from our laboratories (54) has shown that PHD3-deficiency accelerates the resolution of tissue inflammation, specifically by abrogating hypoxia-mediated neutrophil survival. Interestingly, there was no evidence of up-regulation of HIF-1α or NF-κB targets in PHD3-deficient neutrophils (54). Further work will determine whether these differences in myeloid cell behavior reflect differences between monocyte-macrophages and neutrophils, specific responses to hypoxia, the local versus systemic nature of the insults, or distinct roles for PHD3 in the initiation compared to resolution of inflammation.
Regardless the underlying molecular mechanisms, the finding that loss of PHD3 enzyme function augments the macrophage-mediated innate immune response might bear important consequences from a clinical perspective. On the one hand, it should be considered that interference with PHD3 function might lead to unfavorable effects in critically ill patients, a notion that might become of clinical relevance in the prospect of applying pharmaceutical PHD-inhibitors to treat anemia (55) or hypoxic organ damage (56, 57). On the other hand, the finding that inhibition of PHD3 can boost the innate immune defense might offer novel treatment strategies in clinical conditions requiring a strong and immediate activation of the innate immune response, such as overwhelming bacterial infections that occur with resistance to common antibiotic drugs, or in immunocompromized patients.
Methods
Mouse models
The generation of PHD1, 2, or 3 knock-out mice on a mixed 50%Swiss/50%SV129 background has been described elsewhere (16, 20, 21). All mice were bred and maintained in the pathogen-free facility of the Interfacultary Biomedical Research Unit at the University of Heidelberg. All animal experiments were approved by the ethical commission of the local government. To induce endotoxemia, 8- to 10-week-old mice were injected i.p. with 15 mg/kg or 12 mg/kg Escherichia coli LPS serotype 0111:B4 (Sigma-Aldrich, Taufkirchen, Germany). Mice were monitored every 4 hours to assess survival for a period of up to 44 hours. For the assessment clinical symptoms, disease activity was scored as described elsewhere (58). In alternative experiments, blood was collected by cardiac puncture 8 hours after LPS challenge to assess serum creatinine values by routine laboratory testing, and for the measurement of cytokine levels; or for FACS analysis of mature macrophages, where white blood cells were purified via Ficoll gradient (PAA Laboratories, Pasching, Austria).
For cecal ligation and puncture, animals were anesthetized and subjected to midline laparotomy. Subsequently, the cecum was ligated and punctured twice below the ileocecal valve with a 22G needle.
For bone marrow transplantation, 5-week old WT and PHD3-/- mice (C57Bl/6 genetic background) were sublethally irradiated and subsequently received bone marrow from syngeneic animals via intravenous injection. Repopulation of the bone marrow was allowed for 5 weeks, after that sepsis was induced by i.p. injection with 15 mg/kg LPS and follow-up as described.
Macrophage depletion was induced by injection of clodronate (Cl2MDP, Roche Diagnostics GmbH, Mannheim, Germany) (59) one day prior to LPS-injection.
Histology and immunostaining
Internal organs from septic mice were dissected, embedded in paraffin and sectioned at 6-μm thickness. For histological assessment of organ damage, sections were stained with hematoxilin and eosin (HE). For immunostaining of invading leukocytes and resident macrophages, sections were stained for CD45 (BD Pharmingen, San Diego, USA) or F4/80 (AbD Serotec, Düsseldorf, Germany), respectively. Microscopic pictures were taken with a Zeiss Axiostar Plus microscope with Axiocam MRC camera and Axiovision software. All histomorphometrical quantification analyses were performed by two independent investigators, applying 18 microscopic fields. Glomerular clearance was calculated according to a modified Cockroft-Gault formula, CCr = 140 x mass (in kilograms) x 0.85 (if female)/72 x plasma creatinine (in mg/dl).
Measurement of cytokine levels
Mouse TNF-a, IL-1b, IL-6 cytokines were assayed by the Quantikine ELISA kit from R&D Systems (Wiesbaden, Germany).
Phagocytosis assay
Intra-peritoneal Zymosan A (0.1 mg) was used to generate a resolving model of sterile peritonitis (31) with cells harvested by lavage with 2ml PBS at 0-72 hours and total and differential cell counts determined by haemocytometer count and cytospin morphology. Phagocytic index (60) was determined (ratio ingesting cells to non-ingesting cells X average number of zymosan A particles per cell) by for both Zymosan A and apoptotic bodies by morphology.
Cell culture experiments
Bone marrow-derived or peritoneal macrophages of WT and PHD3-/- mice were isolated as described (61). Differentiation of the bone marrow cells was induced by the addition of 50ng/ml M-CSF (unless indicated differently: 10ng/ml; Sigma) in the media for 5 days. Differentiated cells were fixed and stained for May-Grünwald Giemsa according to standard protocols, or harvested for RNA isolation. On the fifth day another set of cells was treated with LPS (2h 1μg/ml, E. coli serotype 0111:B4, Sigma-Aldrich, Taufkirchen, Germany) and applied for RNA isolation. Immunocytochemical- and FACS- stainings were performed on differentiated macrophages applying the CD36 (34) and F4/80 (26) macrophages markers (AbD Serotec, Düsseldorf, Germany) according to standard protocols. Phalloidin-FITC and DAPI staing (DAKO, Hamburg, Germany) were applied to visualize cell shape and nuclei, respectively, on the immonohistochemial slides.
The murine macrophage cell line (J774A.1) was obtained from ATCC (Berlin, Germany). Cells were maintained in DMEM media supplemented with 10% FCS, 1% Penicillin/Streptomycin at 37°C in humidified 5% CO2/95% air. J774A.1 cells were transfected with a siRNA oligonucleotide targeting PHD3, HIF-1, HIF-2α, the p65 subunit of NF-kB, or a control siRNA oligo (sequences: Supplemental Table 1). Transfection was performed using Lipofectamine 2000 (Invitrogen, Darmstadt, Germany).
In Vitro Migration Assay
Migration was assessed in a modified Boyden chamber assay applying cell culture inserts with a polycarbonate-filter (PVP-free, pore size 5 μM, Cell Biolabs, Heidelberg, Germany). 100 μl of cell suspension (5 × 104 cells) was added to the upper wells. Chambers were incubated for 24 hours at 37°C in a 5% CO2 atmosphere. Cells on the lower side of the filter were quantified by dissolving cell bound crystal violet in 10% acetic acid for 5 min and subsequent spectrophotometric analysis at 560 nm. Data was expressed as the percentage compared to random migration.
Real-time quantitative RT-PCR
The RNA of macrophages exposed to 1 μg/ml LPS (Sigma-Aldrich) for 2 hours was isolated using RNA isolation kit (Qiagen) according to the manufacturer’s instructions. First-strand synthesis and real-time PCR were performed with Fermentas products and a TaqMan Universal SYBR Green Master Mix (Applied Biosystems, Darmstadt, Germany) using specific primers (sequences: Suppl. Table 2). Rates were normalized to the expression level of 18S ribosomal RNA.
Western blot studies
Transfected J774A.1 cells were matured for 3 days with 10 nM PMA (Sigma), treated with LPS (2h 1 μg/ml). Ater harvesting, nuclear protein was isolated for the detection of HIF-1α (Novus Biologicals) and HIF-2α (Novus Biologicals, Cambridge, UK) or for the p50 subunit of NF-κB (Santa Cruz, Heidelberg, Germany). As loading control actin (Santa Cruz, Heidelberg, Germany), or Histone H3 was used (NEB, Frankfurt, Germany). After development with an HRP-conjugated secondary antibody, semiquantitative analysis of the bands was performed applying the ImageJ software (NIH, Bethesda, USA).
NF-κB activity assay
M-CSF-differentiated BMDMs were treated with LPS (2h 1 μg/ml) and harvested. Nuclear protein was isolated for the detection of NF-κB activity with the use of TransAM Transcription Factor ELISA kit specific for p65 subunit of NF-κB (Active Motif, Rixensart, Belgium).
Statistical analysis
All values are represented as mean ± SEM. The significance of experimental differences was evaluated by the Student’s T-test. Survival data were analyzed by the construction of Kaplan-Meier plots and applying of the log-rank test. *P values < 0.05 and ** p < 0.01 were considered significant.
Supplementary Material
Acknowlegdements
This study was supported by the Emmy Noether-Program of the Deutsche Forschungsgemeinschaft (DFG) (Grant 947/2-1 to M. Schneider), and in the framework of the Clinical Research Group KFO 227 by the DFG (Grant 947/4-1 to M. Schneider).
Abbreviations
- BMDM
bone-marrow derived macrophage
- CLP
caecal ligation and puncture
- HIF-1/2α
hypoxia inducible factor 1 or 2-alpha
- IL-1β
interleukin-1 beta
- IL-6
interleukin 6
- LPS
lipopolysaccharide
- PHD3
proly-hydroxylase domain 3
- TNF-α
tumor necrosis factor alpha
- WT
wild type
Footnotes
Potential conflict of interest:
Nothing to report
References
- 1.Russell JA, Singer J, Bernard GR, Wheeler A, Fulkerson W, Hudson L, Schein R, Summer W, Wright P, Walley KR. Changing pattern of organ dysfunction in early human sepsis is related to mortality. Crit Care Med. 2000;28:3405–3411. doi: 10.1097/00003246-200010000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Russell JA. Management of sepsis. N Engl J Med. 2006;355:1699–1713. doi: 10.1056/NEJMra043632. [DOI] [PubMed] [Google Scholar]
- 3.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 4.Brown MA, Jones WK. NF-kappaB action in sepsis: the innate immune system and the heart. Front Biosci. 2004;9:1201–1217. doi: 10.2741/1304. [DOI] [PubMed] [Google Scholar]
- 5.Angus DC, Wax RS. Epidemiology of sepsis: an update. Crit Care Med. 2001;29:S109–116. doi: 10.1097/00003246-200107001-00035. [DOI] [PubMed] [Google Scholar]
- 6.Nizet V, Johnson RS. Interdependence of hypoxic and innate immune responses. Nat Rev Immunol. 2009;9:609–617. doi: 10.1038/nri2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imtiyaz HZ, Williams EP, Hickey MM, Patel SA, Durham AC, Yuan LJ, Hammond R, Gimotty PA, Keith B, Simon MC. Hypoxia-inducible factor 2alpha regulates macrophage function in mouse models of acute and tumor inflammation. J Clin Invest. 2010;120:2699–2714. doi: 10.1172/JCI39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peyssonnaux C, Cejudo-Martin P, Doedens A, Zinkernagel AS, Johnson RS, Nizet V. Cutting edge: Essential role of hypoxia inducible factor-1alpha in development of lipopolysaccharide-induced sepsis. J Immunol. 2007;178:7516–7519. doi: 10.4049/jimmunol.178.12.7516. [DOI] [PubMed] [Google Scholar]
- 9.Frede S, Stockmann C, Freitag P, Fandrey J. Bacterial lipopolysaccharide induces HIF-1 activation in human monocytes via p44/42 MAPK and NF-kappaB. Biochem J. 2006;396:517–527. doi: 10.1042/BJ20051839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peyssonnaux C, Datta V, Cramer T, Doedens A, Theodorakis EA, Gallo RL, Hurtado-Ziola N, Nizet V, Johnson RS. HIF-1alpha expression regulates the bactericidal capacity of phagocytes. J Clin Invest. 2005;115:1806–1815. doi: 10.1172/JCI23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Appelhoff RJ, Tian YM, Raval RR, Turley H, Harris AL, Pugh CW, Ratcliffe PJ, Gleadle JM. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem. 2004;279:38458–38465. doi: 10.1074/jbc.M406026200. [DOI] [PubMed] [Google Scholar]
- 14.Bracken CP, Whitelaw ML, Peet DJ. The hypoxia-inducible factors: key transcriptional regulators of hypoxic responses. Cell Mol Life Sci. 2003;60:1376–1393. doi: 10.1007/s00018-003-2370-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aragones J, Schneider M, Van Geyte K, Fraisl P, Dresselaers T, Mazzone M, Dirkx R, Zacchigna S, Lemieux H, Jeoung NH, et al. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat Genet. 2008;40:170–180. doi: 10.1038/ng.2007.62. [DOI] [PubMed] [Google Scholar]
- 16.Schneider M, Van Geyte K, Fraisl P, Kiss J, Aragones J, Mazzone M, Mairbaurl H, Debock K, Jeoung NH, Mollenhauer M, et al. Loss or Silencing of the PHD1 Prolyl Hydroxylase Protects Livers of Mice Against Ischemia/Reperfusion Injury. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.09.057. [DOI] [PubMed] [Google Scholar]
- 17.Tambuwala MM, Cummins EP, Lenihan CR, Kiss J, Stauch M, Scholz CC, Fraisl P, Lasitschka F, Mollenhauer M, Saunders SP, et al. Loss of prolyl hydroxylase-1 protects against colitis through reduced epithelial cell apoptosis and increased barrier function. Gastroenterology. 2010;139:2093–2101. doi: 10.1053/j.gastro.2010.06.068. [DOI] [PubMed] [Google Scholar]
- 18.Takeda K, Ho VC, Takeda H, Duan LJ, Nagy A, Fong GH. Placental but not heart defects are associated with elevated hypoxia-inducible factor alpha levels in mice lacking prolyl hydroxylase domain protein 2. Mol Cell Biol. 2006;26:8336–8346. doi: 10.1128/MCB.00425-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minamishima YA, Moslehi J, Bardeesy N, Cullen D, Bronson RT, Kaelin WG., Jr Somatic inactivation of the PHD2 prolyl hydroxylase causes polycythemia and congestive heart failure. Blood. 2008;111:3236–3244. doi: 10.1182/blood-2007-10-117812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mazzone M, Dettori D, Leite de Oliveira R, Loges S, Schmidt T, Jonckx B, Tian YM, Lanahan AA, Pollard P, Ruiz de Almodovar C, et al. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell. 2009;136:839–851. doi: 10.1016/j.cell.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bishop T, Gallagher D, Pascual A, Lygate CA, de Bono JP, Nicholls LG, Ortega-Saenz P, Oster H, Wijeyekoon B, Sutherland AI, et al. Abnormal sympathoadrenal development and systemic hypotension in PHD3-/- mice. Mol Cell Biol. 2008;28:3386–3400. doi: 10.1128/MCB.02041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minamishima YA, Moslehi J, Padera RF, Bronson RT, Liao R, Kaelin WG., Jr A feedback loop involving the Phd3 prolyl hydroxylase tunes the mammalian hypoxic response in vivo. Mol Cell Biol. 2009;29:5729–5741. doi: 10.1128/MCB.00331-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xue J, Li X, Jiao S, Wei Y, Wu G, Fang J. Prolyl Hydroxylase-3 Is Down-regulated in Colorectal Cancer Cells and Inhibits IKKbeta Independent of Hydroxylase Activity. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.09.049. [DOI] [PubMed] [Google Scholar]
- 24.Brealey D, Karyampudi S, Jacques TS, Novelli M, Stidwill R, Taylor V, Smolenski RT, Singer M. Mitochondrial dysfunction in a long-term rodent model of sepsis and organ failure. Am J Physiol Regul Integr Comp Physiol. 2004;286:R491–497. doi: 10.1152/ajpregu.00432.2003. [DOI] [PubMed] [Google Scholar]
- 25.Khandoga A, Kessler JS, Hanschen M, Khandoga AG, Burggraf D, Reichel C, Hamann GF, Enders G, Krombach F. Matrix metalloproteinase-9 promotes neutrophil and T cell recruitment and migration in the postischemic liver. J Leukoc Biol. 2006;79:1295–1305. doi: 10.1189/jlb.0805468. [DOI] [PubMed] [Google Scholar]
- 26.McKnight AJ, Macfarlane AJ, Dri P, Turley L, Willis AC, Gordon S. Molecular cloning of F4/80, a murine macrophage-restricted cell surface glycoprotein with homology to the G-protein-linked transmembrane 7 hormone receptor family. J Biol Chem. 1996;271:486–489. doi: 10.1074/jbc.271.1.486. [DOI] [PubMed] [Google Scholar]
- 27.Acosta-Iborra B, Elorza A, Olazabal IM, Martin-Cofreces NB, Martin-Puig S, Miro M, Calzada MJ, Aragones J, Sanchez-Madrid F, Landazuri MO. Macrophage oxygen sensing modulates antigen presentation and phagocytic functions involving IFN-gamma production through the HIF-1 alpha transcription factor. J Immunol. 2009;182:3155–3164. doi: 10.4049/jimmunol.0801710. [DOI] [PubMed] [Google Scholar]
- 28.van Rooijen N, van Kesteren-Hendrikx E. Clodronate liposomes: perspectives in research and therapeutics. J Liposome Res. 2002;12:81–94. doi: 10.1081/lpr-120004780. [DOI] [PubMed] [Google Scholar]
- 29.Paine R, 3rd, Morris SB, Jin H, Baleeiro CE, Wilcoxen SE. ICAM-1 facilitates alveolar macrophage phagocytic activity through effects on migration over the AEC surface. Am J Physiol Lung Cell Mol Physiol. 2002;283:L180–187. doi: 10.1152/ajplung.00430.2001. [DOI] [PubMed] [Google Scholar]
- 30.Hatada EN, Krappmann D, Scheidereit C. NF-kappaB and the innate immune response. Curr Opin Immunol. 2000;12:52–58. doi: 10.1016/s0952-7915(99)00050-3. [DOI] [PubMed] [Google Scholar]
- 31.Navarro-Xavier RA, Newson J, Silveira VL, Farrow SN, Gilroy DW, Bystrom J. A new strategy for the identification of novel molecules with targeted proresolution of inflammation properties. J Immunol. 2010;184:1516–1525. doi: 10.4049/jimmunol.0902866. [DOI] [PubMed] [Google Scholar]
- 32.Henson PM, Bratton DL, Fadok VA. Apoptotic cell removal. Curr Biol. 2001;11:R795–805. doi: 10.1016/s0960-9822(01)00474-2. [DOI] [PubMed] [Google Scholar]
- 33.Takashiba S, Van Dyke TE, Amar S, Murayama Y, Soskolne AW, Shapira L. Differentiation of monocytes to macrophages primes cells for lipopolysaccharide stimulation via accumulation of cytoplasmic nuclear factor kappaB. Infect Immun. 1999;67:5573–5578. doi: 10.1128/iai.67.11.5573-5578.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oda T, Hirota K, Nishi K, Takabuchi S, Oda S, Yamada H, Arai T, Fukuda K, Kita T, Adachi T, et al. Activation of hypoxia-inducible factor 1 during macrophage differentiation. Am J Physiol Cell Physiol. 2006;291:C104–113. doi: 10.1152/ajpcell.00614.2005. [DOI] [PubMed] [Google Scholar]
- 35.Zinkernagel AS, Johnson RS, Nizet V. Hypoxia inducible factor (HIF) function in innate immunity and infection. J Mol Med. 2007;85:1339–1346. doi: 10.1007/s00109-007-0282-2. [DOI] [PubMed] [Google Scholar]
- 36.Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 37.Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wykoff CC, Beasley NJ, Watson PH, Turner KJ, Pastorek J, Sibtain A, Wilson GD, Turley H, Talks KL, Maxwell PH, et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000;60:7075–7083. [PubMed] [Google Scholar]
- 39.Gale DP, Maxwell PH. The role of HIF in immunity. Int J Biochem Cell Biol. 2010;42:486–494. doi: 10.1016/j.biocel.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 40.Pinsky MR, Vincent JL, Deviere J, Alegre M, Kahn RJ, Dupont E. Serum cytokine levels in human septic shock. Relation to multiple-system organ failure and mortality. Chest. 1993;103:565–575. doi: 10.1378/chest.103.2.565. [DOI] [PubMed] [Google Scholar]
- 41.Casey LC, Balk RA, Bone RC. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann Intern Med. 1993;119:771–778. doi: 10.7326/0003-4819-119-8-199310150-00001. [DOI] [PubMed] [Google Scholar]
- 42.Mei YQ, Ji Q, Liu H, Wang X, Feng J, Long C, Cheng B, Xing Y, Li J, Hu D. Study on the relationship of APACHE III and levels of cytokines in patients with systemic inflammatory response syndrome after coronary artery bypass grafting. Biol Pharm Bull. 2007;30:410–414. doi: 10.1248/bpb.30.410. [DOI] [PubMed] [Google Scholar]
- 43.Winning S, Splettstoesser F, Fandrey J, Frede S. Acute hypoxia induces HIF-independent monocyte adhesion to endothelial cells through increased intercellular adhesion molecule-1 expression: the role of hypoxic inhibition of prolyl hydroxylase activity for the induction of NF-kappa B. J Immunol. 2010;185:1786–1793. doi: 10.4049/jimmunol.0903244. [DOI] [PubMed] [Google Scholar]
- 44.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burke B, Tang N, Corke KP, Tazzyman D, Ameri K, Wells M, Lewis CE. Expression of HIF-1alpha by human macrophages: implications for the use of macrophages in hypoxia-regulated cancer gene therapy. J Pathol. 2002;196:204–212. doi: 10.1002/path.1029. [DOI] [PubMed] [Google Scholar]
- 46.Murdoch C, Muthana M, Lewis CE. Hypoxia regulates macrophage functions in inflammation. J Immunol. 2005;175:6257–6263. doi: 10.4049/jimmunol.175.10.6257. [DOI] [PubMed] [Google Scholar]
- 47.Imtiyaz HZ, Simon MC. Hypoxia-inducible factors as essential regulators of inflammation. Curr Top Microbiol Immunol. 2010;345:105–120. doi: 10.1007/82_2010_74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takeda N, O’Dea EL, Doedens A, Kim JW, Weidemann A, Stockmann C, Asagiri M, Simon MC, Hoffmann A, Johnson RS. Differential activation and antagonistic function of HIF-{alpha} isoforms in macrophages are essential for NO homeostasis. Genes Dev. 2010;24:491–501. doi: 10.1101/gad.1881410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cummins EP, Berra E, Comerford KM, Ginouves A, Fitzgerald KT, Seeballuck F, Godson C, Nielsen JE, Moynagh P, Pouyssegur J, et al. Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activity. Proc Natl Acad Sci U S A. 2006;103:18154–18159. doi: 10.1073/pnas.0602235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silverman N, Maniatis T. NF-kappaB signaling pathways in mammalian and insect innate immunity. Genes Dev. 2001;15:2321–2342. doi: 10.1101/gad.909001. [DOI] [PubMed] [Google Scholar]
- 51.Fang HY, Hughes R, Murdoch C, Coffelt SB, Biswas SK, Harris AL, Johnson RS, Imityaz HZ, Simon MC, Fredlund E, et al. Hypoxia-inducible factors 1 and 2 are important transcriptional effectors in primary macrophages experiencing hypoxia. Blood. 2009;114:844–859. doi: 10.1182/blood-2008-12-195941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Greten FR, Arkan MC, Bollrath J, Hsu LC, Goode J, Miething C, Goktuna SI, Neuenhahn M, Fierer J, Paxian S, et al. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell. 2007;130:918–931. doi: 10.1016/j.cell.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fong CH, Bebien M, Didierlaurent A, Nebauer R, Hussell T, Broide D, Karin M, Lawrence T. An antiinflammatory role for IKKbeta through the inhibition of “classical” macrophage activation. J Exp Med. 2008;205:1269–1276. doi: 10.1084/jem.20080124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walmsley SR, Chilvers ER, Thompson AA, Vaughan K, Marriott HM, Parker LC, Shaw G, Parmar S, Schneider M, Sabroe I, et al. Prolyl hydroxylase 3 (PHD3) is essential for hypoxic regulation of neutrophilic inflammation in humans and mice. J Clin Invest. 2011;121:1053–1063. doi: 10.1172/JCI43273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bernhardt WM, Wiesener MS, Scigalla P, Chou J, Schmieder RE, Gunzler V, Eckardt KU. Inhibition of prolyl hydroxylases increases erythropoietin production in ESRD. J Am Soc Nephrol. 2010;21:2151–2156. doi: 10.1681/ASN.2010010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cummins EP, Seeballuck F, Keely SJ, Mangan NE, Callanan JJ, Fallon PG, Taylor CT. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology. 2008;134:156–165. doi: 10.1053/j.gastro.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 57.Tanaka T, Nangaku M. Drug discovery for overcoming chronic kidney disease (CKD): prolyl-hydroxylase inhibitors to activate hypoxia-inducible factor (HIF) as a novel therapeutic approach in CKD. J Pharmacol Sci. 2009;109:24–31. doi: 10.1254/jphs.08r09fm. [DOI] [PubMed] [Google Scholar]
- 58.van Schaik SM, Abbas AK. Role of T cells in a murine model of Escherichia coli sepsis. Eur J Immunol. 2007;37:3101–3110. doi: 10.1002/eji.200737295. [DOI] [PubMed] [Google Scholar]
- 59.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 60.Fadok VA, Savill JS, Haslett C, Bratton DL, Doherty DE, Campbell PA, Henson PM. Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. J Immunol. 1992;149:4029–4035. [PubMed] [Google Scholar]
- 61.Vujic Spasic M, Kiss J, Herrmann T, Galy B, Martinache S, Stolte J, Grone HJ, Stremmel W, Hentze MW, Muckenthaler MU. Hfe acts in hepatocytes to prevent hemochromatosis. Cell Metab. 2008;7:173–178. doi: 10.1016/j.cmet.2007.11.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.