Abstract
Macrophages are key in orchestrating immune responses to micro-environmental stimuli, sensed by a complex set of surface receptors. The human cell line THP-1 has a monocytic phenotype, including the ability to differentiate into macrophages, providing a tractable, standardised surrogate for human monocyte-derived macrophages. Here we assessed the expression of 49 surface markers including Fc, complement, C-type lectin and scavenger receptors; TIMs; Siglecs; and co-stimulatory molecules by flow cytometry on both THP-1 monocytes and macrophages and following macrophage activation with seven standard conditioning/polarizing stimuli. Of the 34 surface markers detected on macrophages, 18 altered expression levels on activation. From these, expression of 9 surface markers were consistently altered by all conditioning regimens, while 9 were specific to individual polarizing stimuli. This study provides a resource for the study of macrophages and highlights that macrophage polarization states share much in common and the differences do not easily fit a simple classification system.
Keywords: Monocyte, macrophage, cell surface, flow cytometry, THP-1, polarization
1. Introduction
Monocytes and macrophages play a key role in innate immune responses, from pathogen recognition, phagocytosis, signalling via cytokines and chemokines to tissue repair and remodelling [1]. Resident tissue macrophages sense injury or infection and recruit circulating leukocytes. Infiltrating monocytes/macrophages respond to micro-environmental signals by engaging various activation programs and mature into sub-populations that restore tissue homeostasis by destroying microorganisms or promoting healing. The known range of macrophage activation states, and the cues that induce polarization, continue to expand, but two broad types of macrophage activation in vitro have been characterized in detail: classical or M1 macrophages and alternative or M2 macrophages. M1 activated macrophages are induced by a combination of IFN-γ and pro-inflammatory stimuli (such as LPS or TNF-α) and have anti-microbial and cytotoxic properties, whereas M2 activated macrophages are anti-inflammatory and reparative. M2 macrophages have been further sub-divided, using IL-4 to induce M2a macrophages, immune complexes and IL-1R agonists for M2b and IL-10, TGF-β or glucocorticoids for M2c [2]. However this classification system is now considered to be an oversimplification of a spectrum of activation states and responses observed in vivo [3]. Studies in mice have demonstrated a huge degree of diversity in tissue macrophages under both physiological conditions and disease contexts, which are likely to be similar in man. Macrophage phenotypes not addressed by the M1/M2 classification system have also been delineated. For example Mhem phenotype macrophages are induced by heme or hemoglobin, resulting in enhanced iron handling and increased expression of CD163, a scavenger receptor for hemoglobin and HO-1 [4]. Mhem macrophages are found in areas of plaque hemorrhage [4, 5] and the relative abundance of these macrophage subsets is a better indicator of plaque progression and stability than the total number of lesion macrophages [6].
Whilst the stimuli used to study macrophage polarization in vitro have been well defined, to date no single specific phenotypic markers have been identified to characterize macrophage subtypes. Instead changes in groups of markers have been demonstrated, mostly in mice, which do not necessarily translate directly to humans [7]. Work in primary human macrophages is hampered by difficulties in obtaining primary material, including isolating sufficient quantities for experimentation and variability between donors. The human monocytic cell line THP-1 is widely used for the study of both monocytes and macrophages. THP-1 macrophages provide several advantages over tissue resident or peripheral blood mononuclear cell (PBMC) derived macrophages: ease of access, long-term storage, faster growth rates, relative homogeneity with the same genetic background and the absence of contaminating cells [8]. Published studies have analyzed responses to polarizing stimulation by THP-1 macrophages using transcriptomic or proteomic analyses [9, 10]. However, the range of phenotypic surface markers characterizing THP-1 macrophages has not been reported in detail. This would be invaluable to predict the responses of THP-1 cells to external stimuli and whether they are a representative surrogate for the more complex in vivo primary human macrophages.
To address this gap in knowledge and as a baseline to compare with tissue and blood monocyte derived macrophages, this study used flow cytometry to examine the surface expression of a wide range of important functional markers on both monocyte and macrophage THP-1 cells. These included Sialic acid-binding immunoglobulin-type lectins (Siglecs) which distinguish self from non-self; complement and Fc-gamma receptors that recognize opsonized pathogens; scavenger receptors that clear cell debris; T cell immunoglobulin and mucin domain containing receptors (TIM) that bind phosphatidylserine on apoptotic cells; C-type lectin receptors which have a range of functions including cell adhesion, immune response to pathogens and apoptosis; co-stimulatory molecules important in antigen presentation and commonly used as markers of monocytes and macrophages. Changes in expression of these markers resulting from culturing THP-1 macrophages with widely used polarizing stimuli (LPS, IFN-γ, IL-4, IL-1β, immune complex, IL-10 and hemoglobin:haptoglobin complex) were also determined. The findings allow the examination of an intricate system of changes within a homogeneous cell line and a basis for predicting the suitability of THP-1 cells as a surrogate for primary human monocytes/macrophages. They also provide an extensive resource for investigators interested in studying the immune receptors or conditioning regimens presented for the study of monocyte and macrophage biology.
2. Materials and Methods
2.1. Cells
The THP-1 cells were purchased from Sigma Aldrich and maintained in a humidified incubator at 37°C with 5% CO2 in RPMI 1640 (Gibco) supplemented with 20% heat-inactivated FCS (VWR) and 100 units/mL penicillin, 100 μg/mL streptomycin and 292 μg/mL L-glutamine (Gibco). Cells were seeded at 5 x 105 cells/mL in 5 mL into a 75 cm3 tissue culture flask, then differentiated into (M0) macrophage-like cells by stimulation with PMA 1 ng/mL (Sigma Aldrich) for four days followed by 48 hr without PMA. To alter the phenotype, macrophages were stimulated after 24 hrs rest without PMA for 24 hrs with LPS (10 ng/mL; Sigma Aldrich), LPS and IFN-γ (20 ng/mL; Miltenyi Biotec), IL-4 (20 ng/mL; Miltenyi Biotec), IL-1β (20 ng/mL; Miltenyi Biotec) and immune complex (rabbit Anti-OVA 100 μg/mL bound OVA 10 μg/mL; Sigma Aldrich), IL-10 (20 ng/mL; Miltenyi Biotec) or hemoglobin:haptoglobin complex (1:1, 100 nM; Sigma Aldrich).
2.2. Flow Cytometry (Staining)
THP-1 monocytes were washed with ice-cold HBSS, 2 mM EDTA, 5% FCS. THP-1 macrophages were lifted from the plastic with ice-cold HBSS, 2 mM EDTA, 5% FCS and gentle scraping. Non-specific staining was blocked using Fc block (Human TruStain FcX; BioLegend) incubated at room temperature for 10 minutes. Cells were then split between master-mixes of antibody and isotype panels and incubated for 30 mins at 4°C in the dark. Panel 1: Dectin 1 VioBright FITC; CD69 Brilliant Violet (BV)650; CD207 VioBlue; CLEC12A PerCP-Cy5.5; CLEC4A PE-Vio770; KLRG1 PE-Vio615; Dectin 2 PE; CD45 VioGreen. Panel 2: ASGPR1 VioBright FITC; HLA-DR BV650; CLEC9A VioBlue; CD1a PerCP-Cy5.5; CLEC13A PE-Vio770; CD303 PE/Dazzle 594; CLEC10A PE; CD45 VioGreen. Panel 3: CD329 VioBright FITC; CD14 BV650; CD328 VioBlue; Siglec 8 PerCP; CD169 PE-Vio770; CD33 PE-CF594; Siglec 10 PE; CD45 VioGreen. Panel 4: CD327 AF488; CD11b VioBlue; CD16 PerCP-Vio770; CD32 PE-Cy7; CD64 PE/Dazzle 594; CLEC4D PE; CD45 VioGreen. Panel 5: CD204 VioBright FITC; TIM-3 BV650; CD59 BV421; CD36 PerCP; CD55 PerCP-Cy5.5; TIM-1 PE-Vio770; CD163 PE/Dazzle 594; TIM-4 PE; CD45 VioGreen. Panel 6: SIRPα FITC; CD86 BV650; CD80 BV421; CD209 PerCP-Cy5.5; SIRPβ PE-Vio770; CD206 PE/Dazzle 594; CD205 PE; CD45 VioGreen. Panel 7: CD93 FITC; CD11c BV650; Mertk BV421; CD35 PerCPVio770; CD170 PE-Vio770; CD13 PE/Dazzle 594; CD299 PE; CD45 VioGreen. Further details of isotype controls and antibody clones and sources are given in the MiFlowCyt Section of the Appendices (A.1). After staining, cells were washed and fixed for analysis (Cytofix; BD Biosciences). Cells were acquired on a BD LSRFortessa within 48 hrs and a minimum of 20,000 CD45+ cells (mean 94,000) were acquired for each test.
2.3. Flow Cytometry (Analysis)
Flow cytometric data were analyzed by FlowJoX software, details can be seen in A.1. Monocytes and macrophages were gated by forward scatter – area (FSC-A) and side scatter – area (SSC-A), then single cells by SSC-A and side scatter-width (SSC-W). GeoMean was calculated for the single cells, then normalized by subtracting the isotype GeoMean value for the matching cell type. Markers were deemed to be expressed if 2 out of the 4 samples tested had a normalized GeoMean greater than 100.
2.3. Statistics
Results are presented as median ± interquartile range. Statistical significance was tested using Mann-Whitney tests for all comparisons. Graphing and statistical analyses were performed using Prism 5 (GraphPad Software, Inc.).
3. Results
3.1. THP-1 monocytes: surface marker expression
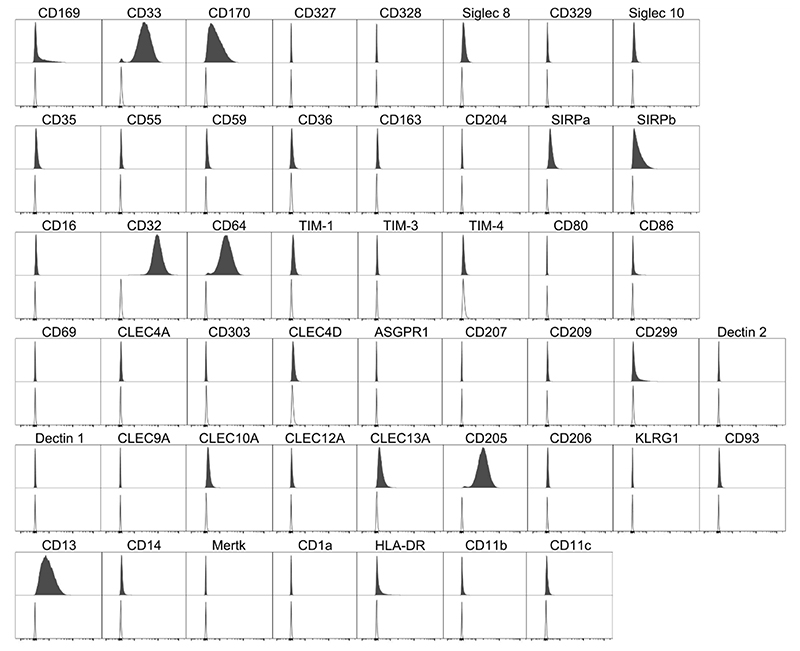
The expression of a wide range of monocyte/macrophage phenotypic and functional markers was assessed. These included those already well known to be expressed on the THP-1 cell surface, such as CD13 and CD14, and some that were previously believed not to be expressed, such as CD1a [11–13]. Figure 1 shows the histogram plots for each marker analyzed on THP-1 monocytes, expression assessed by comparison with matched isotype control. Of the Siglecs tested, THP-1 monocytes expressed CD169 (Siglec 1), CD33 (Sigelec 3) and CD170 (Siglec 5) but not CD327 (Siglec 6), CD328 (Siglec 7), Siglec 8, CD329 (Siglec 9) or Siglec 10. THP-1 monocytes did not express the complement receptors CD35, CD55 and CD59, nor the scavenger receptors CD36, CD163 and CD204. The Fcγ receptors CD32 and CD64 were expressed by THP-1 monocytes, but CD16 was not. Of the TIM receptors tested, THP-1 monocytes expressed TIM-1, but not TIM-3 or TIM-4. Numerous C-type lectin receptors were assessed and THP-1 monocytes showed the presence of only CD299, CLEC10A, CLEC13A and CD205 on the surface. SIRPα and SIRPβ were also expressed, along with CD13 and HLA-DR. THP-1 monocytes did not demonstrate surface expression of CD80, CD86, CD11b, CD11c, CD14, Mertk or CD1a (Figure 1).
Figure 1. Cell Marker Expression on THP-1 Monocytes.
Filled histogram target antibody stained, open histogram matched isotype control. Histograms are representative of n = 4.
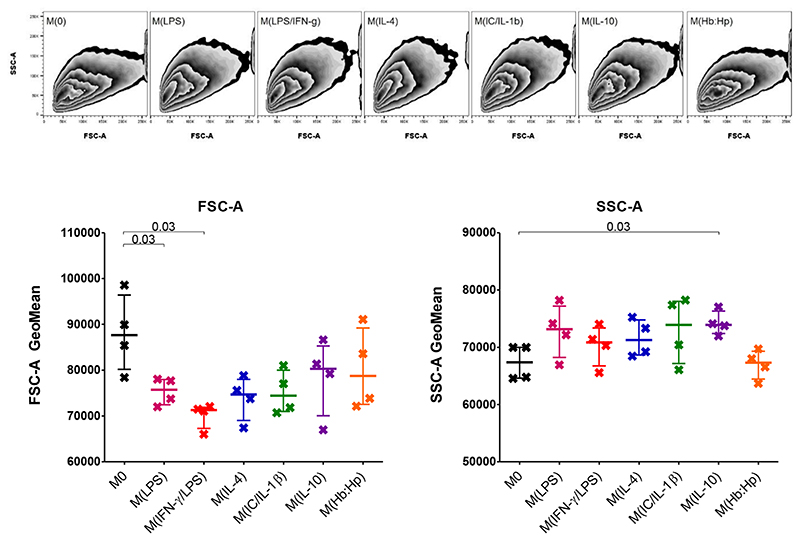
3.2. Differentiation changes THP-1 size and granularity
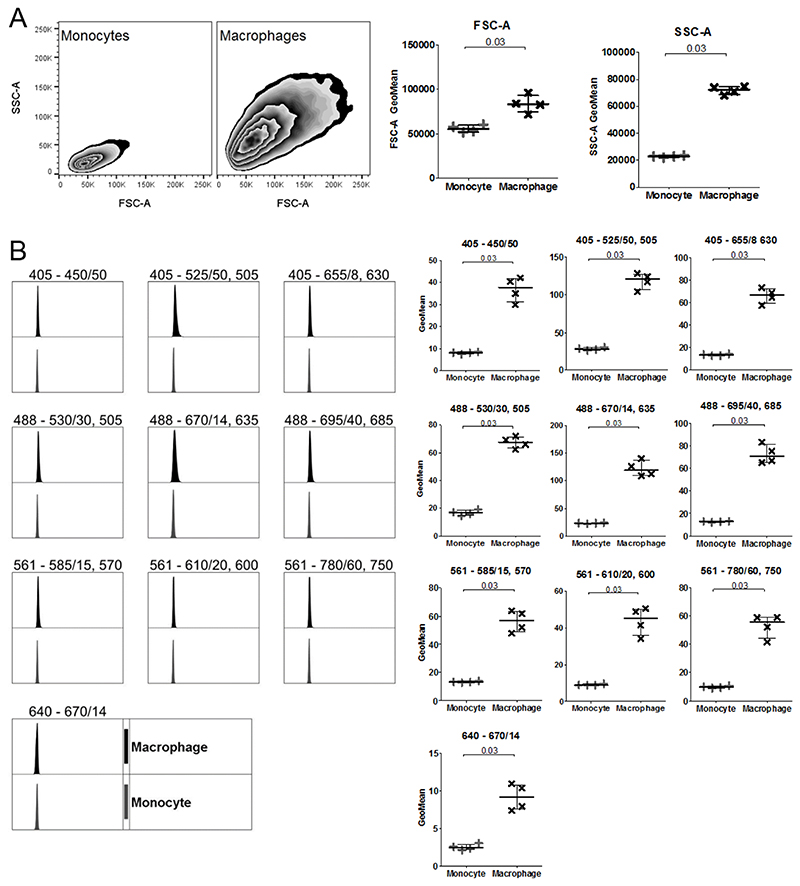
Differentiation from monocyte to macrophage is associated with an increase in size, granularity and auto-fluorescence [14]. To check that the PMA-mediated differentiation of THP-1 monocytes to macrophages was successful, the FSC-A and SSC-A of THP-1 monocytes and macrophages were compared, (Figure 2). In line with other [12], there were significant increases in both size (FSC-A) and granularity (SSC-A) and a slight increase in auto-fluorescence in every channel recorded.
Figure 2. Comparison of flow cytometric cell features between THP-1 Monocytes and Macrophages.
A Size (FSC-A) and granularity (SSC-A) of monocytes and macrophages. B Unstained monocytes (grey; +) and macrophages (black; x) shows autofluorescence within each cytometer channel. Histograms and zebra plots are representative of n = 4. GeoMean was normalised by subtracting the unstained value from the isotype control. Median with IQR; Mann-Whitney tests.
3.3. THP-1 macrophage surface marker expression
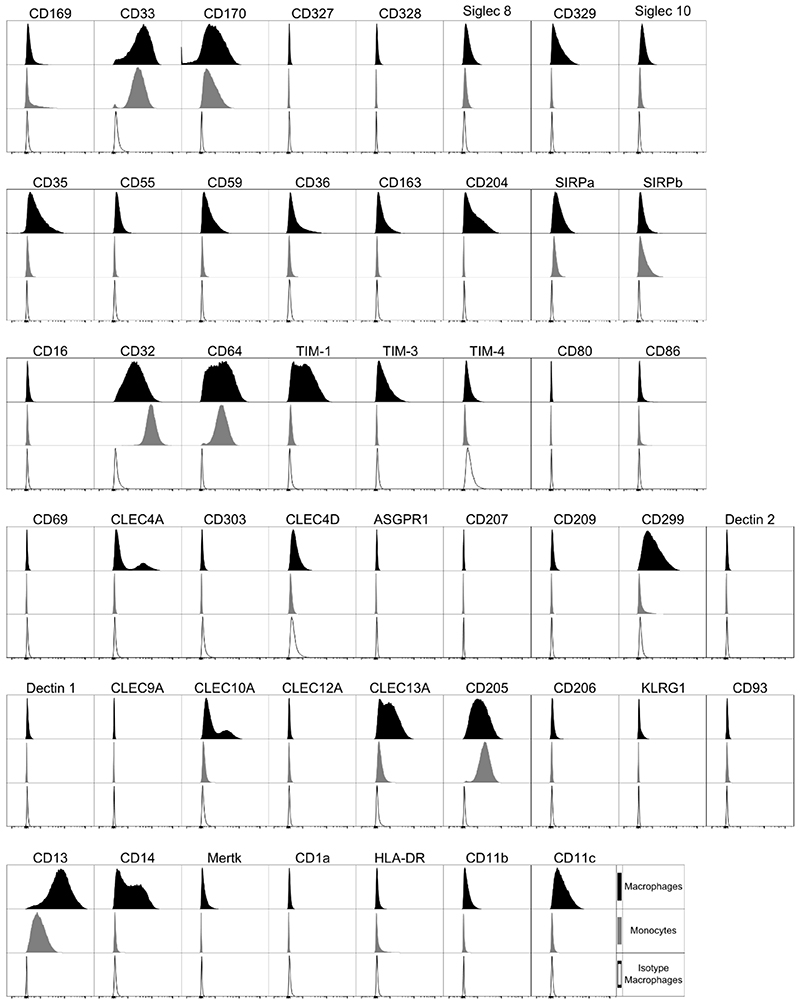
The surface markers expressed by PMA differentiated macrophages that had been rested for 48 hrs (to ensure any stimulation induced had returned to baseline), were analyzed (Figure 3). Like the monocytes from which they were differentiated, THP-1 macrophages demonstrated specific positivity for CD169, CD33, CD170 as well as Siglec 8, CD329 and Siglec 10. In contrast to the monocytes, THP-1 macrophages exhibited expression of CD35, CD55 and CD59, along with the scavenger receptors CD36, CD163 and CD204, reflecting a more mature nature. The THP-1 macrophages, similarly to their monocytic equivalent, expressed CD32 and CD64, but not CD16. THP-1 macrophages expressed TIM-1, but not TIM-4, on their surface, but, unlike monocytes, also expressed TIM-3. Macrophages demonstrated expression of a greater abundance of C-type lectin receptors than monocytes, expressing CLEC4A, CLEC4D, CD299, CLEC10A, CLEC13A, CD205 and KLRG1. As with monocytes, macrophages expressed SIRPα, SIRPβ and CD13, but not CD80 and CD1a. Unlike monocytes, they also expressed CD11b, CD11c, CD14, CD86 and Mertk, with an absence of HLA-DR (Figure 3).
Figure 3. Cell Marker Expression on THP-1 Macrophages.
PMA differentiated THP-1 macrophages. Black filled histogram target antibody stained macrophages, grey filled histogram target antibody stained monocytes, open histogram matched isotype control macrophages. Histograms are representative of n = 4.
3.4. Comparison of the level of surface marker expression by THP-1 monocytes versus THP-1 macrophages
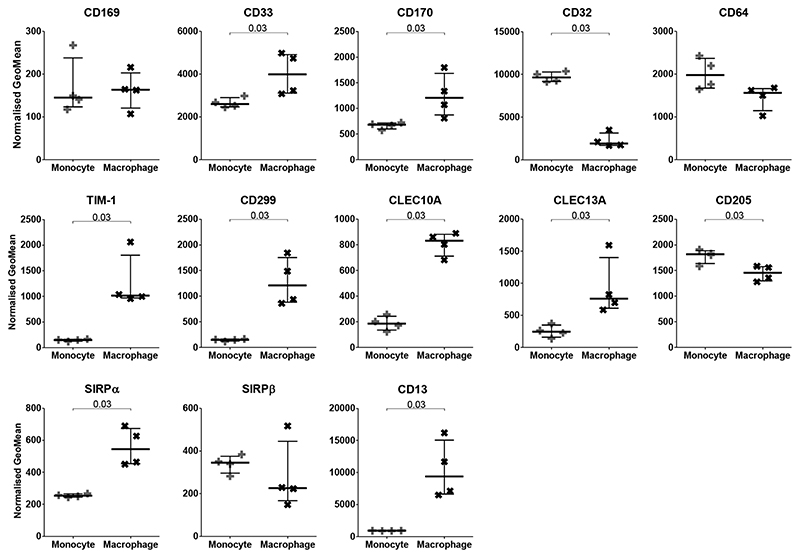
Of the markers expressed by both monocytes and macrophages, macrophages displayed significantly greater expression of CD33, CD170, TIM-1, CD299, CLEC10A, CLEC13A, SIRPα and CD13, but significantly lower expression of CD32 and CD205 (Figure 4). There was no difference in the expression of CD169, CD64 and SIRPβ following PMA induced differentiation into macrophages.
Figure 4. Comparison of Cell Marker Expression between THP-1 Monocytes and Macrophages.
Normalized GeoMeans of markers present on both monocytes (+) and macrophages (x) compared. GeoMean was normalised by subtracting the matched isotype control value. N=4; Median with IQR; Mann-Whitney tests.
3.5. THP-1 macrophage extracellular marker expression after phenotype biasing
The expression of many macrophage markers is known to change in response to stimuli, generally associated with an alteration of role, notably, for example, inflammatory or anti-inflammatory. We chose stimuli to reflect commonly studied macrophage phenotypes [2]. We also investigated modifications resulting from exposure to hemoglobin:haptoglobin complexes, which are associated with the development of a macrophage phenotype important in the progression of atherosclerosis in vivo [4].
It was determined whether addition of polarizing stimuli resulted in a change of morphology (Figure 5). The addition of LPS or LPS/IFN-γ produced a decrease in the FSC-A of the macrophages, indicating a decrease in cell size. The SSC-A, indicating granularity, was increased by the addition of IL-10.
Figure 5. Cell size and granularity of THP-1 Macrophages after 24 Hours Phenotype Biasing.
Size (FSC-A) and granularity (SSC-A) of phenotype biased macrophages, zebra plots representative of n = 4. Median with IQR; Mann-Whitney tests.
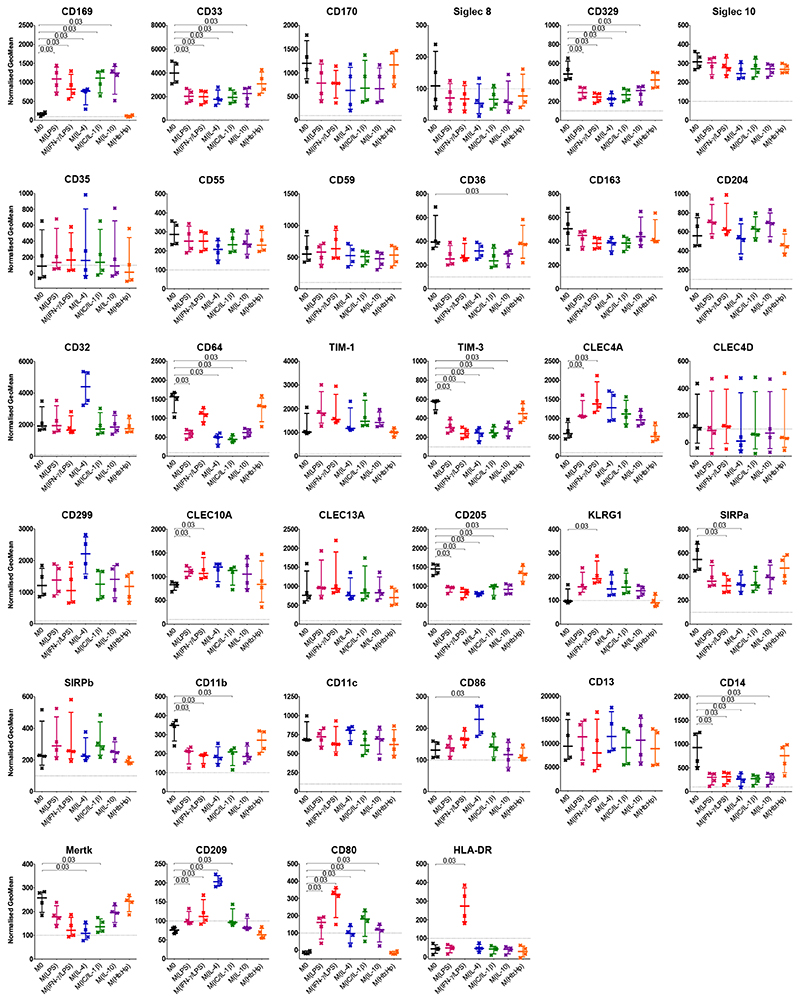
In response to these stimuli, the expression of many markers altered (Figure 6). However, there was no statistically significant change of expression of CD170, Siglec 8, Siglec 10, CD35, CD55, CD59, CD163, CD204, CD32, TIM-1, CLEC4D, CD299, CLEC13A, SIRPβ, CD11c, or CD13 following 24 hr culturing with any of the stimuli tested compared with M0. When comparing the polarizing stimuli (except hemoglobin:haptoglobin complexes, which did not alter the expression of any marker tested) an unexpected pattern of changes emerged. Of the 18 markers that changed in a statistically significant fashion with any of the conditioning regimens, nine altered expression specifically in response to one or two of the regimens (CD36, CLEC4A, CLEC10A, KLRG1, SIRPα, CD86, Mertk, HLA-DR and CD64). The other nine, however, changed expression compared to M0 in the same direction and similar magnitude irrespective of the stimuli. The markers CD33, CD329, TIM-3, CD205, CD11b and CD14 all decreased in expression relative to M0 and CD169, CD209 and CD80 all increased expression relative to M0. CD64 is somewhat different in that all the stimuli except IFN-γ/LPS resulted in a decrease in expression compared to M0.
Figure 6. Cell Marker Expression on THP-1 Macrophages after 24 Hours Phenotype Biasing.
PMA differentiated THP-1 macrophages after phenotype biasing for 24 hours. No stimuli (M0) black, LPS red, LPS and IFN-γ pink, IL-4 blue, IL-1β and immune complex green, IL-10 purple, hemoglobin:haptoglobin orange. GeoMean was normalised by subtracting the matched isotype control value. N=4; Median with IQR; Mann-Whitney tests.
The addition of LPS to THP-1 macrophages resulted in a statistically significant increase in the expression of CD169, CLEC4A and CLEC10A and a decrease in CD33, CD329, CD64, TIM-3, CD205, CD11b and CD14. Addition of IFN-γ in combination with LPS resulted in the same alterations in expression as LPS induced, except in the case of CD64, which did not significantly change compared to no treatment. KLRG1 increased significantly and SIRPα demonstrated decreased expression. IL-4 addition to THP-1 cultures led to an increase in CD169 and CD86. A significant decrease in expression of CD33, CD329, CD64, TIM-3, CD205, SIRPα, CD14 and Mertk was elicited by the addition of IL-4. Immune complexes in combination with IL-1β also increased the expression of CD169 and decreased CD33, CD329, CD64, TIM-3, CD205, CD11b, CD14 and Mertk expression. When added to macrophages, IL-10 increased CD169 expression and decreased that of CD33, CD329, CD36, CD64, TIM-3, CD205 and CD14.
The C-type lectin receptor CD209 was deemed not to be expressed by THP-1 macrophages at initial observation. However, the addition of LPS, LPS/IFN-γ or IL-4 resulted in expression of CD209 above the pre-defined threshold. The co-stimulatory molecule CD80 showed no detectable expression by THP-1 macrophages, but LPS, LPS/IFN-γ, IL-4, IC/IL-1β and IL-10 all increased expression to above the pre-determined threshold. Similarly, HLA-DR expression was not detectable by resting macrophages, but addition of IFN-γ and LPS resulted in a significant increase.
The Geomeans for all markers and conditions investigated are presented in Appendices Fig B.1 for reference.
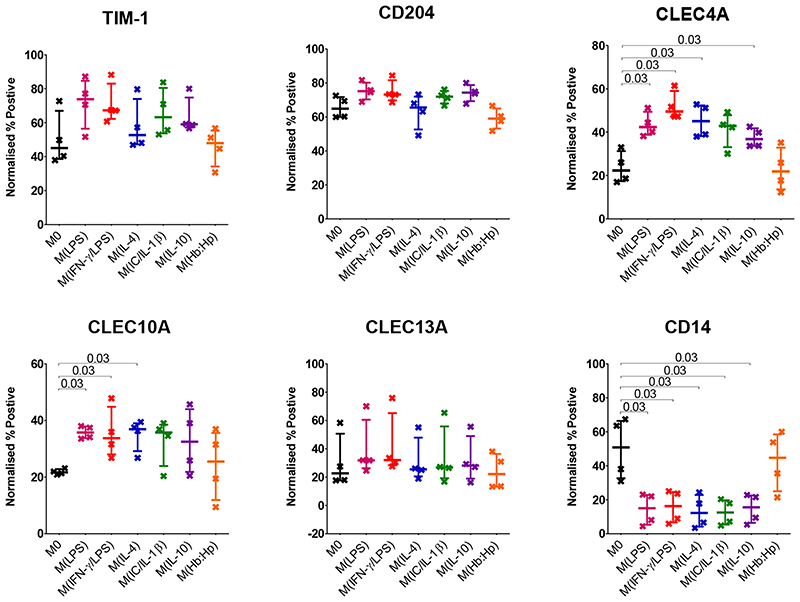
The markers CLEC4A and CLEC10A have a bimodal distribution as shown in Figure 3. The histograms for markers TIM-1, CD204, CLEC13A and CD14 suggest that only a portion of the population express the marker. Figure 7 shows the percentage positive of these markers following phenotype biasing. Similarly to the normalised GeoMean, there is no statistically significant difference in percentage TIM-1 and CLEC13A positive cells following phenotype biasing. Also similar to GeoMean, there is a statistically significant decrease in CD14 percentage positive cells after all phenotype biasing stimuli except hemoglobin: haptoglobin complex. The percentage CLEC10A positive cells increased significantly with LPS, LPS/IFN-γ, and, unlike GeoMean, IL-4. The percentage of cells positive for CLEC4A significantly increased in response to LPS, LPS/IFN-γ, IL-4 and IL-10. The presence of two markers in the same panel allows a comparison of the expression by cells positive for either of both of the markers. CLEC10A and CLEC13A are on the same panel, on average 67% of CLEC10A+ cells are also positive for CLEC13A, and conversely 68% of CLEC13A+ cells are also positive for CLEC10A. TIM-1 and CD204 are also on the same panel, 72% of CD204+ cells are positive for TIM-1, and 83% of TIM-1+ cells are positive for CD204.
Figure 7. Percentage Positive Cell Marker Expression on THP-1 Macrophages after 24 Hours Phenotype Biasing.
PMA differentiated THP-1 macrophages after phenotype biasing for 24 hours. No stimuli (M0) black, LPS red, LPS and IFN-γ pink, IL-4 blue, IL-1β and immune complex green, IL-10 purple, hemoglobin:haptoglobin orange. Percentage positive determined by isotype control then normalised by subtracting the matched isotype control value. N=4; Median with IQR; Mann-Whitney tests.
4. Discussion
Macrophages play an important role in immune responses by sensing and responding to their environment to either drive or resolve inflammation. Key to this function are molecular signatures expressed on their cell surfaces. To provide a resource for the study of macrophage biology and to establish patterns of expression in a homogenous cell type, we assessed the surface expression of a wide range of receptors on the human THP-1 monocytic cell line and their PMA differentiated macrophages, and determined changes in expression following activation by well described conditioning regimens. We found that macrophages, as expected given their physiological role, expressed a greater range of surface markers than monocytes. Much is made in the literature of the difference between M1-like and M2-like phenotypes, but we found all of the conditioning regimens, with the exception of hemoglobin:haptoglobin, tended to effect similar changes, with some exceptions discussed below. These similarities suggest that many of the polarizing stimuli induce common pathways rather than distinct differentiation programs.
Complex mixtures of glycans are expressed on the surfaces of all cells, both host and microbial, and their recognition is important in maintaining homeostasis and fighting disease. Sialic acids are prominent on human cells and are recognized by Siglecs, a series of inhibitory type 1 membrane proteins. We analyzed the expression of CD169 (Siglec 1), CD33 (Siglec 3), CD170 (Siglec 5), CD327 (Siglec 6), CD328 (Siglec 7), Siglec 8, CD329 (Siglec 9) and Siglec 10. We found that CD169 was present on the surface of both THP-1 monocytes and macrophages and that stimulation with all the phenotype altering conditions, except hemoglobin:haptoglobin complexes, significantly increased its expression. CD169 has been shown to be expressed on circulating monocytes and is important in inflammatory conditions such as rheumatoid arthritis and multiple sclerosis, where CD169 expression is higher in patients than healthy controls [15, 16]. Little is known about the effects of phenotype biasing on the expression of CD169 by macrophages [17].
All other Siglecs tested were from the CD33-related Siglec family (CD33, CD170, CD327, CD328, Siglec 8, CD329, Siglec 10). Our study indicated that THP-1 monocytes expressed only CD169, CD33 and CD170. It has been previously shown that monocytes isolated from peripheral blood express CD33, CD170, CD328, CD329 and Siglec 10 but not Siglec 8 [18]. Therefore THP-1 monocytes have a reduced expression profile of Siglecs compared to primary circulating monocytes. This should be considered in studies determining the influences on Siglecs when using THP-1 monocytes as a surrogate to primary cells.
The study presented here showed expression of CD33, CD170, Siglec 8, CD329 and Siglec 10, but not CD327 or CD328 by THP-1 macrophages. The expression of Siglecs on human monocyte derived M-CSF and GM-CSF differentiated macrophages has previously been reported, with CD170, CD328 and CD329 basally expressed and Siglec 8 and Siglec 10 expressed after GM-CSF differentiation [18]. Gene expression by qPCR showed that CD328 and CD329 were induced by M-CSF or GM-CSF driven differentiation, but expression of CD170, Siglec 10 and CD33 decreased during differentiation [19]. CD329 mRNA was unaltered by treatment with LPS/IFN-γ or IL-4; CD328 decreased with LPS /IFN-γ; Siglec 10 increased with IL-4 and CD33 and CD170 decreased with both LPS/IFN-γ and IL-4 [19]. This indicates that THP-1 macrophages resemble macrophages differentiated from circulating blood monocytes with GM-CSF. We found decreased CD33 expression after all phenotype biasing stimuli, except hemoglobin:haptoglobin complexes, in line with Higuchi et al. [19]. However results from THP-1 macrophages contrasted with blood monocyte derived macrophage phenotypes [19], in that CD170, Siglec 8 and Siglec 10 were unaltered and CD329 expression decreased with all phenotype biasing stimuli except hemoglobin:haptoglobin.
The complement receptors we tested, CD35, CD55 and CD59, were not expressed on THP-1 monocytes, but were on THP-1 macrophages, although remained unaltered by the stimuli we used. Few studies have investigated complement receptors on THP-1 cells, but our results are consistent with those showing an increase in receptor expression following differentiation [20]. This reflects the greater role of macrophages as compared to monocytes in clearing complement opsonised pathogens.
The expression of the scavenger receptors CD36, CD163 and CD204 by THP-1 macrophages but not monocytes, as shown here, is consistent with multiple studies. CD36 in particular is often used as a marker of THP-1 differentiation [21]. The results shown here contrast with studies demonstrating CD163 and CD204 as M2 markers (as induced by IL-4) [22, 23]. Moreover CD163 has been shown to be a main receptor upregulated following stimulation by the hemoglobin:haptoglobin complex. Here we showed no statistically significant alteration in CD163 and CD204 expression with any conditioning regimen. This discrepancy might be due to differences in culture times: we used 24hrs, others 72hrs [22].
It is well established that THP-1 monocytes lack CD16, an observation confirmed here, we also confirmed previous reports that THP-1 monocytes expressed CD32 and CD64 [24] and expression is lower after differentiation to macrophages [25]. Fc gamma receptor expression on THP-1 macrophages after phenotype altering stimuli has not previously been reported. However, studies on other human macrophages suggest that CD64 is a M1-like phenotype marker [26]. Our data suggest that the majority of our conditioning stimuli reduce the expression of CD64, while LPS/IFN-γ does not; thus if the results from out-dated M1 and M2 classified inducing stimuli were compared in isolation, it would appear that M1 had increased expression of CD64, when in fact the M2 had decreased expression. The increase in CD32 with the addition of IL-4 is in contrast to studies on macrophages derived from blood monocytes, which indicate that IL-10 but not IL-4 increases its expression [27].
Few studies have investigated TIMs on THP-1 cells. TIM-3 mRNA transcription by THP-1 macrophages has been shown to be induced by plasma from ischemic patients [28]. This study is the first to show the absence of TIM-4 on either THP-1 monocytes or macrophages, and contrasts with human tissue macrophages such as those from spleen [29]. It is also the first to show TIM-1 expression on both THP-1 monocytes and macrophages and that TIM-3 is expressed on THP-1 macrophages with expression decreased by phenotype biasing stimuli, similar to studies using murine macrophages [30].
Of the many C-type lectin receptors we investigated, only CD69, CLEC4A, CD207, CD209, Dectin 1 and CD206 had previously been analyzed for expression by THP-1 cells and our data are broadly consistent with these reports [31–33]. The lack of expression of CD303 (BDCA-2), CLEC4D (Dectin 3), Dectin 2, ASGPR1, CLEC9A (DNGR1), CLEC12A (MICL, CLL-1), or CD93 and the presence of CD299 (L-SIGN), CLEC10A (CD301, MGL), CLEC13A (CD302), CD205 (DEC-205) and KLRG1 (CLEC15A, MAFA) has not previously been reported by other studies of THP-1 cells. The alteration of CD205, CLEC4A and CLEC10A with phenotype biasing stimuli is also previously unreported.
However, our data also contrast with certain other previous reports, albeit often using alternate culture conditions and maturation phenotypes. Cermelli et al. reported CD69 was expressed on THP-1 monocytes [34]. We found CD207 not to be expressed by THP-1, but CD207 was reported from THP-1 cells differentiated into Langerhans-like cells [35]. Similarly Dectin 1 has been repeatedly found to be expressed at the mRNA or protein levels by THP-1 cells in response to fungal stimulation [36, 37]. CD206, also known as mannose receptor 1 (MRC1), which our data suggest is not expressed by THP-1 under any conditions, has been reported to be expressed by THP-1 cells in response to IL-4 [22, 38] or after differentiation to dendritic-like cells [39]. Other studies support a lack of expression of CD206 in both native [40] and polarized cells [41]. Compared to primary human macrophages, mRNA levels for MRC1 are very low [9]. CLEC4A (DCIR) has only previously been shown on tumor associated macrophages isolated from ovarian carcinoma [42], whereas our findings suggest that CLEC4A is associated with an inflammatory phenotype. CLEC10A is well established as an M2-associated marker on macrophages from other sources [43], which contrasts with our data indicating increased expression on LPS and LPS/IFN-γ stimulated THP-1 macrophages. These discrepancies indicate caution should be practiced when considering the use of C-type lectin receptors as macrophage polarization markers on THP-1 cells.
Signal regulatory protein beta (SIRPβ) has not previously been investigated on THP-1 cells, but has been shown to be present on human monocytes [44]. SIRPα is well established as expressed on both THP-1 monocytes and macrophages and our data support this [45]. Conversely, we found that SIRPα expression is decreased by both LPS/IFN-γ and IL-4 stimulation, in contrast to a study suggesting IL-4 increased the expression of SIRPα after 48 hours [46].
Macrophages play a key role in the progression of atherosclerotic lesions [47], as does intralesional hemorrhage which releases hemoglobin to the plaque interior [48]. Hemoglobin complexed with haptoglobin is scavenged by macrophages via CD163 [49, 50], resulting in the secretion of IL-10 [50]. Boyle et al. showed that monocytes differentiated with Hb:Hp complexes result in an atheroprotective macrophage population, which express high levels of CD163 and low levels of HLA-DR [4]. The lack of change in the expression of any markers, in particular CD163 and HLA-DR, in response to culturing with Hb:Hp suggests that this stimulation of THP-1 macrophages is an unrepresentative substitute for human blood monocyte derived macrophages in the study atherosclerosis.
The markers TIM-1, CD204, CLEC4A, CLEC10A, CLEC13A and CD14 displayed heterogeneous expression by macrophages. There are several potential causes of this heterogeneity. It is well established that macrophages have two distinct morphologies that may also be related to differences in marker expression. The macrophages may also be at different stages of either cell cycle or activation. In addition, macrophages are primed to sense a constantly varying environment to which they respond by varying their expression of many proteins, including surface markers.
5. Conclusions
The results presented here should provide a valuable resource for the study of human monocytes and macrophages by documenting the surface molecules expressed by THP-1 cells. Both similarities and differences between THP-1 macrophage and primary human monocyte derived macrophage surface expression are evident. Our findings support the current view that individual markers fail to define polarization phenotypes, such as the out-dated M1 or M2. Moreover these subtypes are a spectrum rather than distinct and are characterised by many similarities as well as differences.
Supplementary Material
Acknowledgements
We would like to acknowledge the assistance of the Iain Fraser Cytometry Centre at the University of Aberdeen. Funding for this project was provided by the Wellcome Trust (094847).
Abbreviations
- BV
Brilliant Violet
- CD
Cluster of Differentiation
- CLEC
C-type lectin receptor
- EDTA
Ethylenediaminetetraacetic acid
- FCS
Fetal calf serum
- FSC-A
Forward Scatter
- HBSS
Hank’s Balanced Salt Solution
- IFN
Interferon
- LPS
Lipopolyscaccharide
- PMA
Phorbol 12-myristate 13-acetate
- Siglec
Sialic acid-binding immunoglobulin-type lectins
- SSC-A
Side Scatter - Area
- SSC-W
Side Scatter - Width
- TIM
T cell immunoglobulin and mucin domain containing
- TNF
Tumor necrosis factor
Footnotes
Authorship Contributions
MAF designed the study, developed methods, performed the experiments, acquired, analyzed and interpreted data and prepared the manuscript.
HJW assisted in developing methods and performing experiments and reviewed study design and manuscript.
LSH assisted in performing experiments.
HC assisted in developing methods.
HMW reviewed study design and manuscript.
MAV and RNB obtained grant funding, reviewed study design, and reviewed the manuscript.
Disclosure of Conflicts of Interest
The authors declare no competing interests.
References
- [1].Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization, Front. Biosci. 2008:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- [3].Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014:13–13.:eCollection 2014. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Boyle JJ, Harrington HA, Piper E, Elderfield K, Stark J, Landis RC, Haskard DO. Coronary intraplaque hemorrhage evokes a novel atheroprotective macrophage phenotype. Am J Pathol. 2009;3:1097–1108. doi: 10.2353/ajpath.2009.080431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Boyle JJ, Johns M, Kampfer T, Nguyen AT, Game L, Schaer DJ, Mason JC, Haskard DO. Activating transcription factor 1 directs Mhem atheroprotective macrophages through coordinated iron handling and foam cell protection. Circ Res. 2012;1:20–33. doi: 10.1161/CIRCRESAHA.111.247577. [DOI] [PubMed] [Google Scholar]
- [6].Chinetti-Gbaguidi G, Colin S, Staels B. Macrophage subsets in atherosclerosis. Nat Rev Cardiol. 2015;1:10–17. doi: 10.1038/nrcardio.2014.173. [DOI] [PubMed] [Google Scholar]
- [7].Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;1:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chanput W, Mes JJ, Wichers HJ. THP-1 cell line: an in vitro cell model for immune modulation approach. Int Immunopharmacol. 2014;1:37–45. doi: 10.1016/j.intimp.2014.08.002. [DOI] [PubMed] [Google Scholar]
- [9].Spiller KL, Wrona EA, Romero-Torres S, Pallotta I, Graney PL, Witherel CE, Panicker LM, Feldman RA, Urbanska AM, Santambrogio L, Vunjak-Novakovic G, et al. Differential gene expression in human, murine, and cell line-derived macrophages upon polarization. Exp Cell Res. 2016;1:1–13. doi: 10.1016/j.yexcr.2015.10.017. [DOI] [PubMed] [Google Scholar]
- [10].Meijer K, Weening D, de Vries MP, Priebe MG, Vonk RJ, Roelofsen H. Quantitative proteomics analyses of activation states of human THP-1 macrophages. J Proteomics. 2015:164–172. doi: 10.1016/j.jprot.2015.07.013. [DOI] [PubMed] [Google Scholar]
- [11].Licona-Limon I, Garay-Canales CA, Munoz-Paleta O, Ortega E. CD13 mediates phagocytosis in human monocytic cells. J Leukoc Biol. 2015;1:85–98. doi: 10.1189/jlb.2A0914-458R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Aldo PB, Craveiro V, Guller S, Mor G. Effect of culture conditions on the phenotype of THP-1 monocyte cell line. Am J Reprod Immunol. 2013;1:80–86. doi: 10.1111/aji.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Miyazawa M, Ito Y, Yoshida Y, Sakaguchi H, Suzuki H. Phenotypic alterations and cytokine production in THP-1 cells in response to allergens. Toxicol In Vitro. 2007;3:428–437. doi: 10.1016/j.tiv.2006.10.005. [DOI] [PubMed] [Google Scholar]
- [14].Daigneault M, Preston JA, Marriott HM, Whyte MK, Dockrell DH. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS One. 2010;1:e8668. doi: 10.1371/journal.pone.0008668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Xiong YS, Cheng Y, Lin QS, Wu AL, Yu J, Li C, Sun Y, Zhong RQ, Wu LJ. Increased expression of Siglec-1 on peripheral blood monocytes and its role in mononuclear cell reactivity to autoantigen in rheumatoid arthritis. Rheumatology (Oxford) 2014;2:250–259. doi: 10.1093/rheumatology/ket342. [DOI] [PubMed] [Google Scholar]
- [16].Malhotra S, Castillo J, Bustamante M, Vidal-Jordana A, Castro Z, Montalban X, Comabella M. SIGLEC1 and SIGLEC7 expression in circulating monocytes of patients with multiple sclerosis. Mult Scler. 2013;5:524–531. doi: 10.1177/1352458512458718. [DOI] [PubMed] [Google Scholar]
- [17].Chavez-Galan L, Olleros ML, Vesin D, Garcia I. Much More than M1 and M2 Macrophages, There are also CD169(+) and TCR(+) Macrophages. Front Immunol. 2015:263. doi: 10.3389/fimmu.2015.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lock K, Zhang J, Lu J, Lee SH, Crocker PR. Expression of CD33-related siglecs on human mononuclear phagocytes, monocyte-derived dendritic cells and plasmacytoid dendritic cells. Immunobiology. 2004;1–2:199–207. doi: 10.1016/j.imbio.2004.04.007. [DOI] [PubMed] [Google Scholar]
- [19].Higuchi H, Shoji T, Iijima S, Nishijima K. Constitutively expressed Siglec-9 inhibits LPS-induced CCR7, but enhances IL-4-induced CD200R expression in human macrophages. Biosci Biotechnol Biochem. 2016;6:1141–1148. doi: 10.1080/09168451.2016.1146070. [DOI] [PubMed] [Google Scholar]
- [20].Izban MG, Nowicki BJ, Nowicki S. 1,25-Dihydroxyvitamin D3 promotes a sustained LPS-induced NF-kappaB-dependent expression of CD55 in human monocytic THP-1 cells. PLoS One. 2012;11:e49318. doi: 10.1371/journal.pone.0049318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Maess MB, Sendelbach S, Lorkowski S. Selection of reliable reference genes during THP-1 monocyte differentiation into macrophages. BMC Mol Biol. 2010:90-2199-11-90. doi: 10.1186/1471-2199-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Soldano S, Pizzorni C, Paolino S, Trombetta AC, Montagna P, Brizzolara R, Ruaro B, Sulli A, Cutolo M. Alternatively Activated (M2) Macrophage Phenotype Is Inducible by Endothelin-1 in Cultured Human Macrophages. PLoS One. 2016;11:e0166433. doi: 10.1371/journal.pone.0166433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Goerdt S, Orfanos CE. Other functions, other genes: alternative activation of antigen-presenting cells. Immunity. 1999;2:137–142. doi: 10.1016/s1074-7613(00)80014-x. [DOI] [PubMed] [Google Scholar]
- [24].Fleit HB, Kobasiuk CD. The human monocyte-like cell line THP-1 expresses Fc gamma RI and Fc gamma RII. J Leukoc Biol. 1991;6:556–565. doi: 10.1002/jlb.49.6.556. [DOI] [PubMed] [Google Scholar]
- [25].Auwerx J, Staels B, Van Vaeck F, Ceuppens JL. Changes in IgG Fc receptor expression induced by phorbol 12-myristate 13-acetate treatment of THP-1 monocytic leukemia cells. Leuk Res. 1992;3:317–327. doi: 10.1016/0145-2126(92)90070-n. [DOI] [PubMed] [Google Scholar]
- [26].Tarique AA, Logan J, Thomas E, Holt PG, Sly PD, Fantino E. Phenotypic, functional, and plasticity features of classical and alternatively activated human macrophages. Am J Respir Cell Mol Biol. 2015;5:676–688. doi: 10.1165/rcmb.2015-0012OC. [DOI] [PubMed] [Google Scholar]
- [27].Clavel C, Ceccato L, Anquetil F, Serre G, Sebbag M. Among human macrophages polarised to different phenotypes, the M-CSF-oriented cells present the highest pro-inflammatory response to the rheumatoid arthritis-specific immune complexes containing ACPA. Ann Rheum Dis. 2016;12:2184–2191. doi: 10.1136/annrheumdis-2015-208887. [DOI] [PubMed] [Google Scholar]
- [28].Zhao D, Hou N, Cui M, Liu Y, Liang X, Zhuang X, Zhang Y, Zhang L, Yin D, Gao L, Zhang Y, et al. Increased T cell immunoglobulin and mucin domain 3 positively correlate with systemic IL-17 and TNF-alpha level in the acute phase of ischemic stroke. J Clin Immunol. 2011;4:719–727. doi: 10.1007/s10875-011-9534-6. [DOI] [PubMed] [Google Scholar]
- [29].Kobayashi N, Karisola P, Pena-Cruz V, Dorfman DM, Jinushi M, Umetsu SE, Butte MJ, Nagumo H, Chernova I, Zhu B, Sharpe AH, et al. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity. 2007;6:927–940. doi: 10.1016/j.immuni.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wang Z, Yin N, Zhang Z, Zhang Y, Zhang G, Chen W. Upregulation of T-cell Immunoglobulin and Mucin-Domain Containing-3 (Tim-3) in Monocytes/Macrophages Associates with Gastric Cancer Progression. Immunol Invest. 2017;2:134–148. doi: 10.1080/08820139.2016.1229790. [DOI] [PubMed] [Google Scholar]
- [31].Hitzler M, Bergert A, Luch A, Peiser M. Evaluation of selected biomarkers for the detection of chemical sensitization in human skin: a comparative study applying THP-1, MUTZ-3 and primary dendritic cells in culture. Toxicol In Vitro. 2013;6:1659–1669. doi: 10.1016/j.tiv.2013.04.009. [DOI] [PubMed] [Google Scholar]
- [32].Puig-Kroger A, Serrano-Gomez D, Caparros E, Dominguez-Soto A, Relloso M, Colmenares M, Martinez-Munoz L, Longo N, Sanchez-Sanchez N, Rincon M, Rivas L, et al. Regulated expression of the pathogen receptor dendritic cell-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing nonintegrin in THP-1 human leukemic cells, monocytes, and macrophages. J Biol Chem. 2004;24:25680–25688. doi: 10.1074/jbc.M311516200. [DOI] [PubMed] [Google Scholar]
- [33].Schafer M, Koppe F, Stenger B, Brochhausen C, Schmidt A, Steinritz D, Thiermann H, Kirkpatrick CJ, Pohl C. Influence of organophosphate poisoning on human dendritic cells. Chem Biol Interact. 2013;3:472–478. doi: 10.1016/j.cbi.2013.08.011. [DOI] [PubMed] [Google Scholar]
- [34].Cermelli C, Orsi CF, Ardizzoni A, Lugli E, Cenacchi V, Cossarizza A, Blasi E. Herpes simplex virus type 1 dysregulates anti-fungal defenses preventing monocyte activation and downregulating toll-like receptor-2. Microbiol Immunol. 2008;12:575–584. doi: 10.1111/j.1348-0421.2008.00074.x. [DOI] [PubMed] [Google Scholar]
- [35].Kumar MM, Adurthi S, Ramachandran S, Mukherjee G, Joy O, Krishnamurthy H, Krishna S, Bafna UD, Uma DK, Jayshree RS. Toll-like receptors 7, 8, and 9 expression and function in primary human cervical cancer Langerhans cells: evidence of anergy. Int J Gynecol Cancer. 2013;1:184–192. doi: 10.1097/IGC.0b013e31827a2003. [DOI] [PubMed] [Google Scholar]
- [36].Du L, Chen X, Duan Z, Liu C, Zeng R, Chen Q, Li M. MiR-146a negatively regulates dectin-1-induced inflammatory responses. Oncotarget. 2017;23:37355–37366. doi: 10.18632/oncotarget.16958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Duan Z, Chen X, Du L, Liu C, Zeng R, Chen Q, Li M. Inflammation Induced by Candida parapsilosis in THP-1 Cells and Human Peripheral Blood Mononuclear Cells (PBMCs) Mycopathologia. 2017 doi: 10.1007/s11046-017-0187-8. [DOI] [PubMed] [Google Scholar]
- [38].Shao LN, Zhu BS, Xing CG, Yang XD, Young W, Cao JP. Effects of autophagy regulation of tumor-associated macrophages on radiosensitivity of colorectal cancer cells. Mol Med Rep. 2016;3:2661–2670. doi: 10.3892/mmr.2016.4820. [DOI] [PubMed] [Google Scholar]
- [39].Berges C, Naujokat C, Tinapp S, Wieczorek H, Hoh A, Sadeghi M, Opelz G, Daniel V. A cell line model for the differentiation of human dendritic cells. Biochem Biophys Res Commun. 2005;3:896–907. doi: 10.1016/j.bbrc.2005.05.171. [DOI] [PubMed] [Google Scholar]
- [40].Neu C, Sedlag A, Bayer C, Forster S, Crauwels P, Niess JH, van Zandbergen G, Frascaroli G, Riedel CU. CD14-dependent monocyte isolation enhances phagocytosis of listeria monocytogenes by proinflammatory, GM-CSF-derived macrophages. PLoS One. 2013;6:e66898. doi: 10.1371/journal.pone.0066898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Dierichs L, Kloubert V, Rink L. Cellular zinc homeostasis modulates polarization of THP-1-derived macrophages. Eur J Nutr. 2017 doi: 10.1007/s00394-017-1491-2. [DOI] [PubMed] [Google Scholar]
- [42].Allavena P, Chieppa M, Bianchi G, Solinas G, Fabbri M, Laskarin G, Mantovani A. Engagement of the mannose receptor by tumoral mucins activates an immune suppressive phenotype in human tumor-associated macrophages. Clin Dev Immunol. 2010:547179. doi: 10.1155/2010/547179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Winkler C, Witte L, Moraw N, Faulenbach C, Muller M, Holz O, Schaumann F, Hohlfeld JM. Impact of endobronchial allergen provocation on macrophage phenotype in asthmatics. BMC Immunol. 2014:12-2172-15-12. doi: 10.1186/1471-2172-15-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Dietrich J, Cella M, Seiffert M, Buhring HJ, Colonna M. Cutting edge: signal-regulatory protein beta 1 is a DAP12-associated activating receptor expressed in myeloid cells. J Immunol. 2000;1:9–12. doi: 10.4049/jimmunol.164.1.9. [DOI] [PubMed] [Google Scholar]
- [45].Ha B, Lv Z, Bian Z, Zhang X, Mishra A, Liu Y. ‘Clustering’ SIRPalpha into the plasma membrane lipid microdomains is required for activated monocytes and macrophages to mediate effective cell surface interactions with CD47. PLoS One. 2013;10:e77615. doi: 10.1371/journal.pone.0077615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zhang Y, Sime W, Juhas M, Sjolander A. Crosstalk between colon cancer cells and macrophages via inflammatory mediators and CD47 promotes tumour cell migration. Eur J Cancer. 2013;15:3320–3334. doi: 10.1016/j.ejca.2013.06.005. [DOI] [PubMed] [Google Scholar]
- [47].Bobryshev YV, Ivanova EA, Chistiakov DA, Nikiforov NG, Orekhov AN. Macrophages and Their Role in Atherosclerosis: Pathophysiology and Transcriptome Analysis. Biomed Res Int. 2016:9582430. doi: 10.1155/2016/9582430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Levy AP, Levy JE, Kalet-Litman S, Miller-Lotan R, Levy NS, Asaf R, Guetta J, Yang C, Purushothaman KR, Fuster V, Moreno PR. Haptoglobin genotype is a determinant of iron, lipid peroxidation, and macrophage accumulation in the atherosclerotic plaque. Arterioscler Thromb Vasc Biol. 2007;1:134–140. doi: 10.1161/01.ATV.0000251020.24399.a2. [DOI] [PubMed] [Google Scholar]
- [49].Fabriek BO, Dijkstra CD, van den Berg TK. The macrophage scavenger receptor CD163. Immunobiology. 2005;2-4:153–160. doi: 10.1016/j.imbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- [50].Philippidis P, Mason JC, Evans BJ, Nadra I, Taylor KM, Haskard DO, Landis RC. Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis: antiinflammatory monocyte-macrophage responses in vitro, in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery. Circ Res. 2004;1:119–126. doi: 10.1161/01.RES.0000109414.78907.F9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.