Abstract
Context
Glucocorticoid (GC) excess is characterized by central obesity, insulin resistance and in some cases, type 2 diabetes. However, the impact of GC upon insulin signaling in human adipose tissue has not been fully explored.
Objective
We have examined the effect of GC upon insulin signaling in both human subcutaneous primary pre-adipocyte cultures and a novel human immortalized subcutaneous adipocyte cell line (Chub-S7) and contrasted this with observations in primary cultures of human skeletal muscle
Design and Setting
An in vitro study characterizing the impact of GC upon insulin signalling in human tissues.
Patients
Biopsy specimens were from healthy volunteers who gave their full and informed written consent.
Interventions
Combinations of treatments including GC, RU38486 and wortmannin were used.
Main outcome measures
Insulin signaling cascade gene and protein expression and insulin stimulated glucose uptake.
Results
In human adipocytes, pre-treatment with GC induced a dose (1.0 [control]; 1.2±0.1 [50nM]; 2.2±0.2 [250nM], p<0.01 vs. control; 3.4±0.2 [1000nM], p<0.001 vs. control) and time (1.0 [1hr]; 3.2±2.0 [6hr]; 9.1±5.9 [24hr], p<0.05 vs. 1hr; 4.5±2.2 [48hr]) dependent increase in insulin stimulated PKB/akt phosphorylation. In addition, whilst IRS1 protein expression did not change, IRS1 tyrosine phosphorylation increased. Furthermore, GC induced IRS2 mRNA expression (2.8-fold, p<0.05) and increased insulin stimulated glucose uptake (1.0 [control] 1.8±0.1 [insulin] vs. 2.8±0.2 [insulin+GC], p<0.05). In contrast, in primary cultures of human muscle, GC decreased insulin stimulated glucose uptake (1.0 [control] 1.9±0.2 [insulin] vs. GC 1.3±0.1 insulin+GC), p<0.05)
Conclusions
We have demonstrated tissue specific regulation of insulin signaling by GC. Within subcutaneous adipose tissue, GC augment insulin signaling yet in muscle cause insulin resistance. We propose that enhanced insulin action in adipose tissue increases adipocyte differentiation thereby contributing to GC induced obesity.
Keywords: Glucocorticoids, insulin signaling, obesity, cortisol, adipocyte
Introduction
The link between increased central adiposity and insulin resistance is established (1). In addition, increased cardiovascular morbidity and mortality as a consequence of intraabdominal adipose deposition (as opposed to subcutaneous) are well described (2). Consequently, there is a need to identify factors that regulate adipose tissue distribution. Patients with glucocorticoid (GC) excess (both endogenous and exogenous), Cushing’s syndrome develop a phenotype characterised by hypertension, central obesity and profound insulin resistance, emphasizing the potent effects of GC upon adipose tissue. Furthermore, the metabolic consequence of GC excess leads to significantly increased morbidity and mortality, principally through cardiovascular disease. The prevalence of the use of oral GC may be as high as 2.5% of the population (3) and therefore the magnitude of the health burden from their adverse effects can not be underestimated.
The mechanisms by which GC cause obesity and insulin resistance remain unclear. The development of GC induced obesity is complex. GC administration leads to increased food intake (4), without changes in energy expenditure (5). In addition, GCs have multiple effects upon the adipocyte, inducing lipolysis in mature adipocytes through induction of hormone sensitive lipase resulting in increased glycerol release (6). However, in contrast, GC are essential for pre-adipocyte differentiation into mature adipocytes that have the ability to store triglyceride and here GC and insulin act synergistically (7). GC excess results in preferential accumulation of intra-abdominal adipose tissue; increased glucocorticoid receptor (GR) expression (8) and increased expression of the pre-receptor modulating enzyme 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) (9) are putative mechanisms.
Following binding of insulin to the cell surface insulin receptor and the subsequent tyrosine autophosphorylation, a complex intra-cellular signaling cascade is initiated. Whilst activation of the Ras/Raf MAPkinase pathway is crucial in the proliferative actions of insulin (10), the majority of its ‘metabolic’ actions occur via the insulin receptor substrate (IRS), PI3kinase pathway which ultimately leads to activation of PKB/akt through phosphorylation at the S473 and Th308 positions (11). The functional effects of PKB/akt activation are well characterized and include inhibition of glycogen synthase kinase 3 (12), inhibition of apoptosis (13) and GLUT4 translocation to the cell membrane with consequent glucose uptake (14).
GC induce whole body insulin resistance as measured by hyperinsulinaemic euglycaemic clamps in both rodents and humans (15), largely reflecting the effect of GCs upon muscle. The specific interactions between GCs and the insulin signalling cascade in adipose tissue has only been investigated in a relatively small number of studies, and almost all have used rodent models and rodent cell lines (16–19). Extrapolating directly from rodents to man must be done with caution and we have therefore performed a detailed characterization of the activation of the insulin signaling cascade and investigated the impact of glucocorticoids (both endogenous and synthetic) in human tissues. We have used a novel human preadipocyte cell line (chub-s7) that retains the ability to differentiate into mature adipocytes and store lipid. We have endorsed our findings in primary cultures of human adipose tissue and compared them with the effects of GCs upon differentiated human skeletal myocytes as a well characterized model of GC induced insulin resistance.
Research Design and Methods
Chub-S7 cell line
The chub-S7 cell line is a transformed human subcutaneous pre-adipocyte cell line derived from an obese female. In cell culture, they retain the ability to proliferate and upon confluence, in chemically defined media, differentiate into mature adipocytes expressing well characterized markers of adipocyte differentiation as well as lipid droplets (20). Proliferating cells were cultured in Dulbecco’s MEM/Nutrient Mixture F-, DMEM-F12 (Sigma, Poole, UK) with 10% FCS and seeded into 12 well plates and grown until confluent. In some experiments, cells were differentiated in DMEM-F12 media with Biotin 33μM, Pantothenate 17μM, T3 0.2nM, Insulin 167nM, Cortisol 1μM and Rosiglitazone 1μM for 14 days. During the experimental protocol, prior to treatment all cells (both undifferentiated and differentiated) were cultured in media (DMEM-F12) without additives for 24h.
Human preadipocytes
Findings from the experiments using the chub-s7 cell line were endorsed using primary cultures of human adipose tissue. Human subcutaneous (sc) pre-adipocytes were isolated as previously reported (9) from non-obese patients undergoing elective total abdominal hysterectomy (n=5, mean BMI 26.6±1.3kg/m2, mean age 40±3years) without evidence of malignancy. Patients with diabetes and those who had taken GC therapy within the last 12 months were excluded from the study. All subjects were on no regular medications. Informed, written consent was obtained in all cases and the study had the approval of the local research ethics committee. Briefly, sc adipose tissue was washed in PBS containing 50,000 units of penicillin and 50,000μg streptomycin (Invitrogen, Paisley, UK). The tissue was then prepared and digested with collagenase class 1 [2 mg/ml] (Worthington Biochemical Co-operation, Reading, U.K.) in 1× Hank’s balanced salt solution (Invitrogen) for 45 minutes at 37°C. Samples were then centrifuged at 90g for 5 minutes, the pellet containing pre-adipocytes was removed and cells washed with DMEM-F12 containing 15% fetal calf serum and seeded on 12 well plates (Corning, Schiphol-Rijk, The Netherlands). Cells were left overnight and washed the following day with 1× Hank’s balanced salt solution. Cells were then either cultured to confluence in DMEM F-12 medium containing 15% fetal calf serum or differentiated to mature adipocytes in chemically defined media without thiazolidinedione supplementation as previously described (21). Preparations containing greater than 5% endothelial cell contamination were discarded. Prior to treatment, cells were incubated in serum free media without additives for 24h (for specific treatment times and doses see results).
Human Myoblasts
Primary human myoblasts were obtained from PromoCell (Heidelberg, Germany). Myoblasts were cultured to confluence, as per the manufactures guidelines using the supplied media. Once confluent, media was changed to a chemically defined media (PromoCell, Germany) and cells differentiated into myotubes for 14 days. Following differentiation, cells were incubated with serum free media for 24-hours prior to treatment (for specific treatment times and doses see results).
In all cell culture experiments investigating insulin signaling cascade protein phosphorylation, media was spiked with human insulin (0.1μg/ml, Sigma, UK) for the final 15-minutes of the treatment period. In experiments using the GR antagonist, RU38486, cells were pre-treated with RU38486 (10μM) for 10-minutes before adding dexamethasone (Dex; 1μM). When used, wortmannin (100nM) was added 15-minutes before the addition of insulin to the cell culture media. All treatments and reagents were supplied by Sigma, Poole, UK unless otherwise stated.
RNA extraction and RT
Total RNA was extracted using the Tri-Reagent system. RNA integrity was assessed by electrophoresis on 1% agarose gel. Concentration was determined spectrophotometrically at OD260. One microgram of total RNA was initially denatured with 200ng of random primers in a volume of 10μl. Twenty units of avian myeloblastosis virus, 20 U ribonuclease inhibitor, 1μM deoxy-NTPs and 5 x reaction buffer were added to the volume of 20μl. The reverse transcription reaction was carried out at 37°C for 1hr. The reaction was terminated by heating the cDNA to 95°C for 5min.
Real-Time PCR
PKB/akt1, PKB/akt2, IRS1, IRS2, FABP4 (fatty acid binding protein 4) and G3PDH (glycerol-3-phosphate dehydrogenase) mRNA levels were determined using an ABI 7500 sequence detection system (Perkin-Elmer Applied Biosystems, Warrington, UK). Reactions were performed in 25μl volumes on 96 well plates in reaction buffer containing 2 x TaqMan Universal PCR Master mix (Applied Biosystems, Foster City, CA, USA). Probes and primers for IRS1, IRS2, PKB/akt1, PKB/akt2, FABP4 and G3PDH were supplied by applied biosystems ‘assay on demand’ (Applied Biosystems). All reactions were normalised against the house keeping gene 18S rRNA, provided as a preoptimized control probe. Data were expressed as ct values (ct=cycle number at which logarithmic PCR plots cross a calculated threshold line) and used to determine Δct values (Δct = (ct of the target gene) – (ct of the housekeeping gene) with high Δct values reflecting low mRNA expression levels. Fold changes were calculated using transformation [fold increase = 2-difference in ΔCT].
Protein extraction and immunoblotting
Cells were scraped into 100μl of RIPA buffer (50mM Tris pH 7.4, 1% NP40, 0.25% sodium deoxycholate, 150mM NaCl, 1mM EDTA, 1mM PMSF and protease inhibitor cocktail (Roche, Lewes, UK) and incubated at -80°C for 10 min, incubated on ice for 30min and centrifuged at 4°C for 10min at 14,000 rpm. The supernatant containing the soluble proteins was transferred to a fresh tube and total protein concentration determined by a commercially available protein assay (Bio-Rad Laboratories Inc., Hercules, CA.)
Fifteen μg of protein sample was resolved on a 12.5% SDS PAGE gel. Proteins were transferred onto nitrocellulose membrane, Hybond ECL (GE Healthcare, Chalfont St Giles, UK). Primary (anti PKB/akt, IRS 1 and IRS 2, Upstate, Dundee, UK, anti phosphoIRS (serine 312), Biosource, Nivelles, Belgium and anti phosphoPKB/akt (serine 473), R&D Systems, Abingdon, UK) and secondary antibodies (Dako, Glostrop, UK) were used at a dilution of 1/1000. Membranes were re-probed for β-Actin and primary and secondary antibodies used at a dilution of 1/5000 (Abcam plc, Cambridge, UK). Bands were quantified with Genesnap by Syngene (Cambridge, UK).
Glucose transport assay
Glucose uptake activity was analyzed by measuring the uptake of 2-deoxy-D-[3H] glucose as described previously (22). After treatment, cells were washed 3 times with Krebs-Ringer-Hepes (KRP) buffer and incubated with 0.9mL KRP buffer at 37°C for 30 min. Insulin (0.5ng/ml) was then added, and the cells incubated at 37°C for 15-minutes. Glucose uptake was initiated by the addition of 0.1mL KRP buffer and 37MBq/L 2-deoxy-D-[3H] glucose (GE Healthcare, Chalfont St Giles, UK) and 0.1 mmol/L glucose as final concentrations. After 15 minutes, glucose uptake was terminated by washing the cells 3 times with cold PBS. Cells were lysed and radioactivity retained by the cell lysates determined by scintillation counting.
11β-hydroxysteroid dehydrogenase type 1 assay
Briefly, intact cells were incubated with 100nM cortisone and tritiated tracer for 16 hours. Steroids were then extracted using dichloromethane, separated using a mobile phase consisting of ethanol and chloroform (8:92) by thin layer chromatography and scanned using a Bioscan 3000 image analyser (Lablogic, UK.). Protein levels were assayed using a commercially available kit (Bio-Rad, CA) and activity expressed as pmol cortisol generated/mg of protein/h.
Statistical analysis
Where data were normally distributed, unpaired students t-test to compare single treatments to control. If normality tests failed then non-parametric tests were used. One way ANOVA on ranks, was to compare multiple treatments, doses or times (SigmaStat 3.1, Systat Software, Inc. Point Richmond, CA). Statistical analysis on real-time PCR data was performed on mean Δct values and not fold changes.
Results
Chub-S7 cells characterization – comparison with primary sc adipocytes
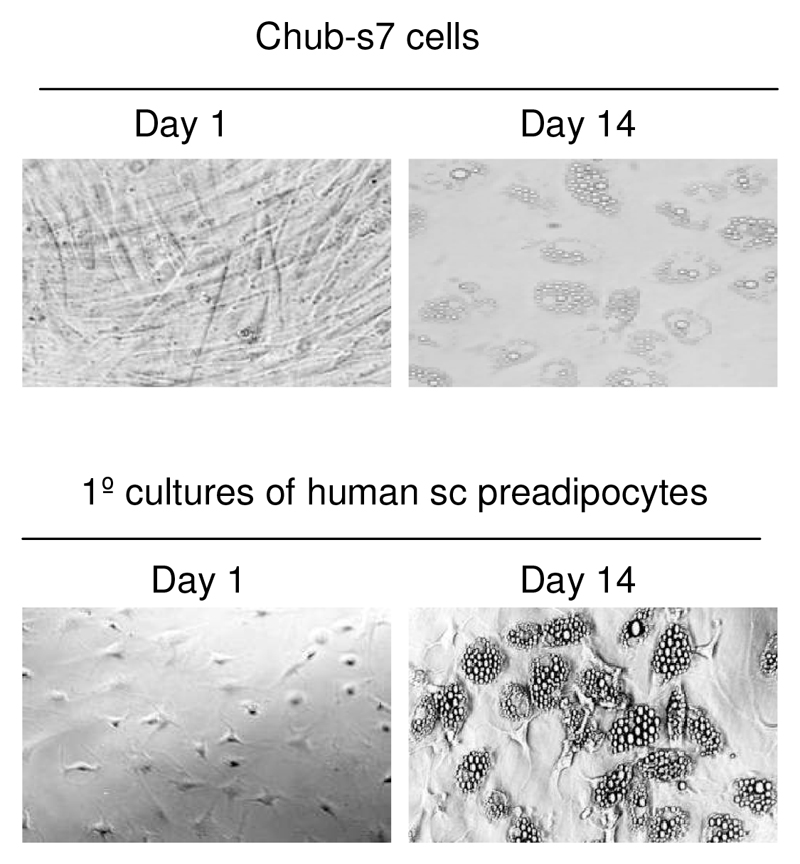
Chub-S7 cells have been characterized in detail previously (20;23). We have carried out our own ‘in-house’ characterization of their ability to differentiate and serve as a model for human adipocyte biology. Following differentiation in chemically defined media, lipid droplets were clearly visible in both chub-S7 cells and primary cultures of human sc preadipocytes (figure 1). Using real-time PCR, mRNA expression of G3PDH increased 153-fold in primary cultures of sc cells and 1554-fold in chub-S7 cells. Similarly, FABP4 increased 19-fold in primary cultures of sc cells and 7960-fold in chub-S7 cells. GR mRNA expression did not change significantly in either cell type across differentiation (data not shown).
Figure 1.
Chub-S7 cells and primary cultures of human sc preadipocytes, differentiate in chemical defined media (14 days) and acquire a mature adipocyte phenotype with clearly visible lipid droplets.
Insulin signalling in differentiated Chub-S7 cells
Insulin stimulated PKB/akt phosphorylation in differentiated chub-S7 cells in a dose dependent manner as measured by semi-quantitative densitometry of Western blotting relative to internal control, β-actin (1.0 [0.1ng/ml], 2.5±0.9 [0.5ng/ml], 3.1±1.0 [1ng/ml]) (figure 2A).
Figure 2.
Insulin stimulation (15 minutes) causes a dose dependent increase in PKB/akt phosphorylation in differentiated chub-S7 cells (A). Dexamethasone pre-treatment (1μM, 48hrs), does not change IRS1 (B) or PKB/akt protein expression (C), but enhances insulin stimulated phosphorylation (B and C). (C=control, D=dexamethasone, representative Western blots are shown with the quantification relative to β-actin of n=3-5 experiments shown below, * p<0.05 vs. control)
Dex pre-treatment (1μM, 48h) in differentiated chub-s7 cells did not regulate total IRS1 or PKB/akt protein expression (figure 2B and C). Interestingly, IRS2 protein expression increased (1.0 [control] vs. 2.22±0.42 [Dex], p<0.05). However, Dex increased both insulin stimulated IRS1 phosphorylation (1.0 [control] vs. 2.5±0.84 [Dex], p<0.05) and PKB/akt phosphorylation (1.0 [control] vs. 6.3±2.4 [Dex], p<0.05). Furthermore, Dex induction of insulin stimulated PKB/akt phosphorylation was blocked by both the GR antagonist, RU38486 1.6±0.6 [Dex+RU38486], p<0.05 vs. Dex.) and by the PI3 kinase inhibitor, wortmannin (1.1±0.7 [Dex+wortmannin], p<0.05 vs. Dex) (figure 3A and B). Wortmanin treatment alone abolished insulin stimulated PKB/akt phosphorylation; treatment with RU38486 alone was with out effect (data not shown)
Figure 3.
Dexamethasone (D) pre-treatment (1μM, 48h) increases insulin stimulated (0.5ng/mL, 15 minutes) PKB/akt phosphorylation in both undifferentiated (A) and differentiated chub-S7 cells (B) and in primary cultures of differentiated subcutaneous human pre-adipocytes (C). In chub-S7 cells, these effects are blocked by co-incubation with the GR antagonist, RU38486 (R, 10μM) or the PI3 kinase inhibitor, wortmannin (W, 100nM)). Data are presented as the mean±se of n=3-5 experiments and quantified relative to β-actin. Representative western blots and light microscopic images are shown inserted (* p<0.05 vs. control (C), † p<0.05 vs. dexamethasone).
Insulin signalling in undifferentiated Chub-S7 cells
To determine whether the differentiation status plays a role in determining the response to GC pre-treatment similar experiments were performed in undifferentiated chub-S7 cells (figure 3A). Replicating our findings in differentiated cells, Dex pre-treatment (1μM, 48h) increased insulin stimulated PKB/akt phosphorylation (undifferentiated chub-S7: 1.0 [control] vs. 5.5±1.5 [Dex], p<0.05; (figure 3A) without altering PKB/akt protein expression (data not shown). This was blocked by both the GR antagonist, RU38486 (2.7±0.9 [Dex+RU38486], p<0.05 vs. Dex.) and by the PI3 kinase inhibitor, wortmannin (0.6±0.4 [Dex+wortmannin], p<0.05 vs. Dex.).
Insulin signalling in primary cultures of human adipocytes
Endorsing our findings in chub-S7 cells, Dex pre-treatment increased insulin stimulated PKB/akt phosphorylation in primary cultures of differentiated subcutaneous adipocytes (figure 3C) (1.0 [control] vs. 1.6±0.2 [Dex], p<0.05) (figure 3C). However, whilst RU38486 decreased Dex induction of insulin stimulated PKB/akt phosphorylation, this failed to reach statistical significance (1.3±0.2 [Dex+RU38486], p=0.2 vs. Dex.), but the effect of Dex was completely abolished by wortmannin (0.7±0.2 [Dex+wortmannin], p<0.05 vs. Dex.).
Time and dose dependency
In order to determine whether the observations with Dex could be extrapolated to endogenous GCs, dose and time course experiments were performed upon differentiated chub-s7 cells using cortisol (figure 4A and B). Cortisol pre-treatment increased insulin stimulated PKB/akt phosphorylation in both a dose (48hr treatment: 1.0 [control]; 1.2±0.1 [50nM]; 2.2±0.2 [250nM], p<0.01 vs. control; 3.4±0.2 [1000nM], p<0.001 vs. control) and time dependent manner (1μM cortisol: 1.0 [1hr]; 3.2±2.0 [6hr]; 9.1±5.9 [24hr], p<0.05 vs. 1hr; 4.5±2.2 [48hr]). In both experiments there were no changes in PKB/akt protein expression.
Figure 4.
Cortisol induces a dose (A) and time (B) dependent increase in insulin stimulated PKB/akt phosphorylation (white bars) without alteration in total PKB/akt expression (black bars) in human differentiated chub-S7 cells. Data are presented as the mean±se of n=5 experiments performed in triplicate. Representative western blots are shown inserted (*p<0.05, ** p<0.01, † p<0.001 vs. control).
Chub S7 cells express 11β-HSD1 mRNA and have demonstrable enzyme activity (24). Consistent with the role of 11β-HSD1 in human adipose tissue to locally activate GC (cortisone ➔ cortisol), cortisone pre-treatment (1μM, 24h) enhanced insulin stimulated PKB/akt phosphorylation (figure 5) (1.0 [control] vs. 1.69±0.09 [cortisone], p<0.05). Co-incubation with glycerrhetinic acid (GE) (5μM) (an inhibitor of 11β-HSD1 thus preventing the activation of cortisone to cortisol) completely abolished this observation (1.0 [control] vs. 1.12±0.14 [cortisone+GE], p=ns). Parallel, 11β-HSD1 activity studies demonstrated complete inhibition of 11β-HSD1 activity following incubation with GE (21.4±4.4 (control) vs. 0.0 pmol cortisol/mg protein/h (GE))
Figure 5.
Both cortisol and cortisone (1μM, 24hrs) pre-treatment enhance insulin stimulated PKB/akt phosphorylation. The effect of cortisone is completely blocked by the 11β-HSD1 inhibitor, glycerrhetinic acid (GE) which prevents the activation of cortisone to cortisol. Data are presented as the mean±se of n=5 experiments performed in triplicate. Representative western blots are shown inserted (*p<0.05, ** p<0.01 vs. control).
mRNA expression
Using real-time PCR, IRS1, IRS2, PKB/akt1 and 2 were all expressed in differentiated chub-s7 cells. Dex treatment (1μM, 48h) induced IRS2 expression in differentiated chub-s7 cells (IRS-2: 2.8-fold 14.7±0.6 [control]; 13.2±0.3 [Dex], p<0.05). Similarly, the endogenous GC, cortisol induced IRS-2 expression in differentiated cells in a dose dependent manner (14.1±0.2 [control], 13.5±0.3, 1.2-fold [50nM]; 13.1±0.3, 2.2-fold, [250nM]; 12.2±0.3, 3.4-fold [1000nM], p<0.05 vs. control). However, IRS1 expression did not change significantly. GC pre-treatment did not change PKB/akt1 or PKB/akt2 mRNA expression levels. Complete mRNA expression data in response to GC treatment and also co-incubation with RU38486 are shown in table 1.
The observed changes in response to GC pre-treatment could not be attributed to changes in differentiation status of the cells. Using real-time PCR, markers of differentiation increased in comparison with undifferentiated cells (FABP4 6x106-fold induction, mean Δct±se 21.8±0.4 [undifferentiated] vs. 4.5±0.5 [differentiated], p<0.001; G3PDH 446-fold induction, 23.1±0.1 [undifferentiated] vs. 14.3±1.0 [differentiated], p<0.01). However, in differentiated cells, following the 24hr wash-out period and subsequent treatment with Dex (1μM, 48h), FABP4 and G3PDH mRNA expression did not change significantly.
Insulin signalling in primary cultures of human myocytes
In order to determine a tissue specificity of response we have performed a small number of studies in primary cultures of skeletal myocytes, In contrast to our findings in adipocytes (primary cultures and chub-S7 cells), Dex (1μM, 48h) had no effect upon PKB/akt phosphorylation or total PKB/akt protein expression in primary cultures of differentiated human skeletal myocytes (1.0 [control], 1.1±0.3 [Dex], 1.0±0.3 [Dex+RU38486]). However, co-incubation with wortmannin significantly decreased insulin stimulated PKB/akt phosphorylation (0.6±0.1 [Dex+wortmannin], p<0.01 vs. Dex.)
Dex induced both IRS-1 (2.6-fold, 14.4±0.6 [control]; 13.0±0.6 [Dex], p<0.05), and IRS-2 mRNA expression (4.3-fold, 16.7±0.3 [control]; 14.6±0.2 [Dex], p<0.05) in differentiated skeletal myocytes. There were no significant changes in PKB/akt mRNA expression (data not shown).
Glucose transport in differentiated chub-S7 cells and human myocytes
To investigate the functional impact of the changes in the insulin signaling cascade, 2-deoxy-D-[3H] glucose uptake was measured (figure 6). In differentiated chub-s7 cells, insulin stimulated 2-deoxy-D-[3H] glucose uptake in a dose (data expressed relative to non-insulin stimulated control±s.e., [insulin dose, ng/ml]: 1.0 [control], 1.7±0.2 [0.1], 1.9±0.2 [0.5] and 1.9±0.2 [1.0]) and time (2.5±0.2 [5min], 7.9±0.2 [10min], 13.4±0.1 [20min], 17.3±0.1 [40min], and 2.7±0.2 [60min]) dependent manner. Wortmannin abolished insulin stimulated glucose transport (1.1±0.1 [insulin 1.0ng/ml + wortmannin]. In both undifferentiated and differentiated cells, Dex increased insulin stimulated glucose uptake into chub-S7 cells (undifferentiated: 1.0 [control], 1.6±0.1 [insulin, 0.5ng/ml] vs. 2.1±0.03 [Dex, 1μM, 48h, insulin 0.5ng/ml], p<0.05; differentiated: 1.0 [control], 1.8±0.1 [insulin] vs. 2.8±0.2 [Dex + insulin], p<0.05). In contrast, in primary cultures of human skeletal muscle, dexamethasone decreased insulin stimulated glucose uptake (1.0 [control], 1.9±0.2 [insulin] vs. 1.3±0.1 [Dex + insulin], p<0.05).
Figure 6.
Insulin stimulated 2-deoxy-D-[3H] glucose uptake in undifferentiated and differentiated chub-S7 cells and skeletal myocytes. Data are expressed as the fold increase in glucose uptake with basal unstimulated uptake set as 1 (white bars). In all cell systems, insulin treatment significantly increased glucose uptake (grey bars). Dexamethasone (1μM, 48h) pre-treatment increased 2-deoxy-D-[3H] glucose uptake in undifferentiated and differentiated chub-S7 cells whilst decreasing uptake in human skeletal muscle cells (black bars). Data are presented as the mean±se of n=3-5 experiments performed in triplicate (*p<0.05 vs. control).
Discussion
It is a widely held belief that GCs induce insulin resistance in all tissues including adipose. However, whilst there is little doubt that globally they induce insulin resistance, tissue specificity of response has not been fully addressed. Although most studies would postulate inhibition of insulin signaling by GC in adipocytes, the data and the mechanism are not clear. Importantly, studies have invariably focussed upon rodent models and cell lines and we would argue that simple extrapolation to human studies can not be made. We have performed studies in a novel human cells line which resembles primary culture systems in its ability to acquire a mature adipocyte phenotype.
In differentiated 3T3L1 cells, GC have been shown to induce insulin resistance through decreased IRS-1 protein expression (16;17). However IRS2 expression and phosphorylation are also increased following GC treatment, whilst PKB/akt phosphorylation remains unchanged. In addition recent kinome analysis has suggested that nongenomic actions of GC in 3T3L1 cells may be important in inducing insulin resistance (25). The net effect of these changes is a decrease in insulin stimulated glucose uptake (17). In primary cultures of rat adipocytes, GC decrease expression of IRS1, PI3 kinase and PKB/akt, although interestingly IRS2 expression is increased (19). In addition, IRS2 phosphorylation is increased following GC treatment in isolated rat hepatocytes (26). There is only a single published study in human adipose tissue that has utilized isolated and not cultured differentiated adipocytes. GC decreased IRS1 expression, decreased insulin stimulated glucose uptake, but had no effect upon PKB/akt phosphorylation in omental adipocytes. GC were without effect in subcutaneous cells (18). In our study, acute exposure to GC (up to 48h) increased insulin stimulated IRS1 and PKB/akt phosphorylation and subsequent glucose uptake study in both undifferentiated and differentiated human subcutaneous adipocytes (both primary cultures and cell line); effects that are mediated by the GR, with insulin acting through PI3 kinase activation. The discrepancies between our observations and the previously published report may be a reflection of the different stage of adipocyte differentiation; proliferating pre-adipocytes, isolated and differentiated in culture versus isolated intact mature adipocytes. The active, highly regulated process of adipocyte differentiation is dependent upon insulin (and glucocorticoids) and therefore once adipocytes are fully differentiated insulin signaling (and its regulation) may differ substantially. The net effect upon insulin stimulated glucose uptake in adipose tissue as a whole remains unclear. This may offer an explanation as to the lack of response in the previously published study. It is possible that this could represent a novel mechanism of GC induced obesity, sensitizing adipose tissue to the effects of insulin and thus enhancing both insulin and GC stimulated adipocyte differentiation. In addition, GC mediated insulin sensitization can promote insulin induced lipid storage through induction of sterol regulatory binding protein 1c (SREBP1c) (27). However, the fact that these cells are cultured ex-vivo always needs to be considered when interpreting the data
The data in skeletal muscle are more clear; insulin receptor auto-phosphorylation and IRS1 expression and / or phosphorylation are decreased (28–30). In addition, PI3kinase activity is decreased (31;32) and this has been attributed to increased expression of the p85alpha regulatory subunit that impairs activation of the p110 catalytic subunit, leading to decreased IRS1 phosphorylation (31). In agreement with the published literature (33), in our study, GC induced insulin resistance as evidenced by decreased insulin stimulated glucose uptake in primary cultures of human muscle.
Species specificity of response within adipose tissue may be important and could underpin some of the differences between published data and our observations. In contrast to humans, rodents treated with GC lose rather than gain weight (34;35). Whilst this is initially through loss of muscle mass there is subsequently progressive loss of adipose tissue (36) although the mechanisms underpinning this process are not clear; a marked contrast to clinical observations in humans. It is possible that this may reflect true insulin resistance within adipose tissue (as well as in other organs), decreasing adipocyte differentiation and lipid accumulation in contrast to our postulated hypothesis of GC mediated insulin sensitization in human adipose tissue.
Tissue specificity of response to GC is not a new concept. In rodents, GC decrease IRS1 and PKB/akt phosphorylation in liver, but not in muscle (29;30). Furthermore, key metabolic enzymes may also be differentially regulated. In liver, GC increase the expression of phosphoenolpyruvate carboxykinase (PEPCK), the rate limiting step in gluconeogenesis, whilst decreasing expression in adipose tissue (37). In our cell systems IRS2 expression increased in response to GC treatment and it is possible that this may represent the mechanism by which GC exert their effect upon insulin signaling in human adipose tissue. Evidence to support the role of IRS proteins as determinants of tissue specific insulin sensitivity is provided by IR, IRS1 and 2 knockout mice. Lack of IRS1 appears to be more crucial in determining insulin resistance in skeletal muscle whilst IRS2 is more important in liver and adipose tissue (38;39) although the detailed mechanisms underpinning this observation are not known. Interestingly, we also observed an increase in IRS1 and IRS2 expression following GC treatment in skeletal muscle cells. However, this did not translate to down-stream activation of PKB/akt and indeed, insulin stimulated glucose uptake was decreased in contrast to our observations in adipocytes and therefore the significance of this observation in muscle is not clear. Phosphorylation status of the IRS proteins as markers of their activation was not examined in this study, and this may be important in explaining the discrepancy between IRS mRNA expression and insulin signaling cascade activation.
Insulin sensitization of adipose by GC would explain many clinical scenarios. In states of GC excess, there is a predilection for increased intra-abdominal adipose tissue (40), however, whilst the data are less clear, it is probable that there is an associated (albeit less dramatic) increase in abdominal subcutaneous adipose tissue (40). The primary pre-adipocyte cultures in this study were all taken from female patients. Sexually dimorphic expression of GR has been described in adipose tissue with increased expression in female subcutaneous versus omental preadipocytes, but no such changes in men (41). Although there are no published data, it is interesting to speculate that in light of the differential GR expression, GC mediated changes in fat distribution may also be different between sexes. Certainly, following successful treatment of Cushing’s disease in women, subcutaneous fat mass decreases (42). Whilst we are unable to comment specifically about the intra-abdominal depot (our studies were exclusively performed on a sc derived cell line and endorsed in human sc primary cultures), GC mediated increases in insulin sensitivity in sc adipose tissue may predispose to increases in sc fat mass and contribute to the Cushing’s phenotype and health risks (43). Whilst it is plausible that this may have an impact upon insulin sensitivity in distant organs including liver and muscle (44), sc adipose tissue deposition per se may not always be detrimental and indeed may be protective form some of the adverse consequences of obesity (45).
Tissue specific differential regulation of insulin signaling by GC may have therapeutic implications. Local GC availability to bind to the GR is controlled by the isoenzymes of 11β-hydroxysteroid dehydrogenase (11β-HSD1 and 2). In adipose, liver and to a lesser extent in muscle, the type 1 isoform predominates, converting inactive cortisone to active cortisol thus amplifying local GC effects (46). Selectively inhibiting 11β-HSD1, decreases local GC availability and it is exciting to speculate that this may improve insulin sensitivity in muscle, whilst making adipose relatively more insulin resistant. Certainly our observations would endorse this hypothesis. Initial studies (albeit in rodents) confirm global profound insulin sensitization (47), and recently depot specific changes in adipocyte biology have been shown with decreased fat cell size, decreased fatty acid synthesis and evidence of enhanced lipid oxidation (48). However, results from human studies are still awaited. In conclusion, we have characterized the first description of human tissue specific regulation of insulin signaling by acute exposure to GC. As well as offering a putative mechanism for GC induced obesity, therapeutic manipulation of local GC availability may offer a novel therapeutic strategy by enhancing global insulin sensitivity (through its actions in liver and muscle) and inducing adipose tissue specific insulin resistance.
Table 1.
mRNA expression of markers of adipocyte differentiation and key components of the insulin signalling cascade in differentiated Chub-s7 cells following treatment with Dexamethasone (Dex, 1μM, 48h) and the glucocorticoid receptor antagonist, RU38486. Data are expressed as mean Δct values ± s.e.
| Gene | Control | Dex | Dex and RU38486 | Control vs Dex P value |
|---|---|---|---|---|
| FABP4 | 4.1±0.28 | 4.38±0.54 | 4.60±0.31 | ns |
| G3PDH | 13.48±0.90 | 13.80±0.30 | 14.15±0.74 | ns |
| GR | 11.10±1.10 | 11.00±1.00 | 11.50±1.00 | ns |
| IR | 14.4±0.31 | 14.60±0.92 | 14.60±0.82 | ns |
| IRS-1 | 14.36±0.95 | 13.85±0.72 | 13.90±0.49 | ns |
| IRS-2 | 14.70±0.56 | 13.21±0.29 | 14.10±0.40 | p<0.05 |
| PI3K | 13.70±0.31 | 14.30±0.92 | 13.40±0.83 | ns |
| AKT-1 | 12.18±0.76 | 12.19±0.68 | 12.50±0.83 | ns |
| AKT-2 | 14.0±0.81 | 13.75±0.67 | 14.19±0.87 | ns |
| GLUT4 | 18.45±0.88 | 18.25±0.78 | 18.48±0.57 | ns |
Acknowledgments
Funding for this study has been provided by the Wellcome Trust, UK (programme grant to PMS and Clinician Scientist Fellowship to JWT). We would like to thank Dr C Darimont (Nestle, Switzerland) for providing the chub-S7 cells.
Footnotes
Disclosure
L.L.G., I.J.B. and J.W.T. have nothing to declare. P.M.S. has had a consultancy with Pfizer Global R and D.
References
- 1.Carey DG, Jenkins AB, Campbell LV, Freund J, Chisholm DJ. Abdominal fat and insulin resistance in normal and overweight women: Direct measurements reveal a strong relationship in subjects at both low and high risk of NIDDM. Diabetes. 1996;45(5):633–638. doi: 10.2337/diab.45.5.633. [DOI] [PubMed] [Google Scholar]
- 2.Fontbonne A, Thibult N, Eschwege E, Ducimetiere P. Body fat distribution and coronary heart disease mortality in subjects with impaired glucose tolerance or diabetes mellitus: the Paris Prospective Study, 15-year follow-up. Diabetologia. 1992;35(5):464–468. doi: 10.1007/BF02342445. [DOI] [PubMed] [Google Scholar]
- 3.Van Staa TP, Leufkens HG, Abenhaim L, Begaud B, Zhang B, Cooper C. Use of oral corticosteroids in the United Kingdom. QJM. 2000;93(2):105–111. doi: 10.1093/qjmed/93.2.105. [DOI] [PubMed] [Google Scholar]
- 4.Strack AM, Sebastian RJ, Schwartz MW, Dallman MF. Glucocorticoids and insulin: reciprocal signals for energy balance. Am J Physiol. 1995;268(1 Pt 2):R142–R149. doi: 10.1152/ajpregu.1995.268.1.R142. [DOI] [PubMed] [Google Scholar]
- 5.Schneiter P, Tappy L. Kinetics of dexamethasone-induced alterations of glucose metabolism in healthy humans. Am J Physiol. 1998;275(5 Pt 1):E806–E813. doi: 10.1152/ajpendo.1998.275.5.E806. [DOI] [PubMed] [Google Scholar]
- 6.Slavin BG, Ong JM, Kern PA. Hormonal regulation of hormone-sensitive lipase activity and mRNA levels in isolated rat adipocytes. J Lipid Res. 1994;35(9):1535–1541. [PubMed] [Google Scholar]
- 7.Hauner H, Schmid P, Pfeiffer EF. Glucocorticoids and insulin promote the differentiation of human adipocyte precursor cells into fat cells. J Clin Endocrinol Metab. 1987;64(4):832–835. doi: 10.1210/jcem-64-4-832. [DOI] [PubMed] [Google Scholar]
- 8.Rebuffe-Scrive M, Bronnegard M, Nilsson A, Eldh J, Gustafsson JA, Bjorntorp P. Steroid hormone receptors in human adipose tissues. J Clin Endocrinol Metab. 1990;71(5):1215–1219. doi: 10.1210/jcem-71-5-1215. [DOI] [PubMed] [Google Scholar]
- 9.Bujalska IJ, Kumar S, Stewart PM. Does central obesity reflect “Cushing’s disease of the omentum”? Lancet. 1997 Aug 26;349:1210–1213. doi: 10.1016/S0140-6736(96)11222-8. [DOI] [PubMed] [Google Scholar]
- 10.Boulton TG, Nye SH, Robbins DJ, Ip NY, Radziejewska E, Morgenbesser SD, DePinho RA, Panayotatos N, Cobb MH, Yancopoulos GD. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991;65(4):663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- 11.Hanada M, Feng J, Hemmings BA. Structure, regulation and function of PKB/AKT--a major therapeutic target. Biochim Biophys Acta. 2004;1697(1-2):3–16. doi: 10.1016/j.bbapap.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Burgering BM, Coffer PJ. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376(6541):599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 13.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91(2):231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 14.Watson RT, Kanzaki M, Pessin JE. Regulated membrane trafficking of the insulin-responsive glucose transporter 4 in adipocytes. Endocr Rev. 2004;25(2):177–204. doi: 10.1210/er.2003-0011. [DOI] [PubMed] [Google Scholar]
- 15.Larsson H, Ahren B. Short-term dexamethasone treatment increases plasma leptin independently of changes in insulin sensitivity in healthy women. J Clin Endocrinol Metab. 1996;81(12):4428–4432. doi: 10.1210/jcem.81.12.8954054. [DOI] [PubMed] [Google Scholar]
- 16.Turnbow MA, Keller SR, Rice KM, Garner CW. Dexamethasone down-regulation of insulin receptor substrate-1 in 3T3-L1 adipocytes. J Biol Chem. 1994;269(4):2516–2520. [PubMed] [Google Scholar]
- 17.Sakoda H, Ogihara T, Anai M, Funaki M, Inukai K, Katagiri H, Fukushima Y, Onishi Y, Ono H, Fujishiro M, Kikuchi M, et al. Dexamethasone-induced insulin resistance in 3T3-L1 adipocytes is due to inhibition of glucose transport rather than insulin signal transduction. Diabetes. 2000;49(10):1700–1708. doi: 10.2337/diabetes.49.10.1700. [DOI] [PubMed] [Google Scholar]
- 18.Lundgren M, Buren J, Ruge T, Myrnas T, Eriksson JW. Glucocorticoids down-regulate glucose uptake capacity and insulin-signaling proteins in omental but not subcutaneous human adipocytes. J Clin Endocrinol Metab. 2004;89(6):2989–2997. doi: 10.1210/jc.2003-031157. [DOI] [PubMed] [Google Scholar]
- 19.Buren J, Liu HX, Jensen J, Eriksson JW. Dexamethasone impairs insulin signalling and glucose transport by depletion of insulin receptor substrate-1, phosphatidylinositol 3-kinase and protein kinase B in primary cultured rat adipocytes. Eur J Endocrinol. 2002;146(3):419–429. doi: 10.1530/eje.0.1460419. [DOI] [PubMed] [Google Scholar]
- 20.Qiao L, Maclean PS, Schaack J, Orlicky DJ, Darimont C, Pagliassotti M, Friedman JE, Shao J. C/EBPalpha regulates human adiponectin gene transcription through an intronic enhancer. Diabetes. 2005;54(6):1744–1754. doi: 10.2337/diabetes.54.6.1744. [DOI] [PubMed] [Google Scholar]
- 21.Bujalska IJ, Kumar S, Hewison M, Stewart PM. Differentiation of adipose stromal cells: the roles of glucocorticoids and 11beta-hydroxysteroid dehydrogenase. Endocrinology. 1999;140(7):3188–3196. doi: 10.1210/endo.140.7.6868. [DOI] [PubMed] [Google Scholar]
- 22.Liu F, Kim J, Li Y, Liu X, Li J, Chen X. An extract of Lagerstroemia speciosa L. has insulin-like glucose uptake-stimulatory and adipocyte differentiation-inhibitory activities in 3T3-L1 cells. J Nutr. 2001;131(9):2242–2247. doi: 10.1093/jn/131.9.2242. [DOI] [PubMed] [Google Scholar]
- 23.Darimont C, Avanti O, Zbinden I, Leone-Vautravers P, Mansourian R, Giusti V, Mace K. Liver X receptor preferentially activates de novo lipogenesis in human preadipocytes. Biochimie. 2005;88:309–318. doi: 10.1016/j.biochi.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Bujalska IJ, Gathercole LL, Tomlinson JW, Darimont C, Stewart PM. 11β-Hydroxysteroid Dehydrogenase Type 1-Specific Inhibitor Prevents Human Adipogenesis; Proceedings of the 88th Annual meeting of the American Endocrine Society; 2006. pp. P2–240. [Google Scholar]
- 25.Lowenberg M, Tuynman J, Scheffer M, Verhaar A, Vermeulen L, van Deventer S, Hommes D, Peppelenbosch M. Kinome analysis reveals nongenomic glucocorticoid receptor-dependent inhibition of insulin signaling. Endocrinology. 2006;147(7):3555–3562. doi: 10.1210/en.2005-1602. [DOI] [PubMed] [Google Scholar]
- 26.Klein HH, Ullmann S, Drenckhan M, Grimmsmann T, Unthan-Fechner K, Probst I. Differential modulation of insulin actions by dexamethasone: studies in primary cultures of adult rat hepatocytes. J Hepatol. 2002;37(4):432–440. doi: 10.1016/s0168-8278(02)00217-9. [DOI] [PubMed] [Google Scholar]
- 27.Kim JB, Sarraf P, Wright M, Yao KM, Mueller E, Solanes G, Lowell BB, Spiegelman BM. Nutritional and insulin regulation of fatty acid synthetase and leptin gene expression through ADD1/SREBP1. J Clin Invest. 1998;101(1):1–9. doi: 10.1172/JCI1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giorgino F, Almahfouz A, Goodyear LJ, Smith RJ. Glucocorticoid regulation of insulin receptor and substrate IRS-1 tyrosine phosphorylation in rat skeletal muscle in vivo. J Clin Invest. 1993;91(5):2020–2030. doi: 10.1172/JCI116424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rojas FA, Hirata AE, Saad MJ. Regulation of insulin receptor substrate-2 tyrosine phosphorylation in animal models of insulin resistance. Endocrine. 2003;21(2):115–122. doi: 10.1385/ENDO:21:2:115. [DOI] [PubMed] [Google Scholar]
- 30.Saad MJ, Folli F, Kahn JA, Kahn CR. Modulation of insulin receptor, insulin receptor substrate-1, and phosphatidylinositol 3-kinase in liver and muscle of dexamethasone-treated rats. J Clin Invest. 1993;92(4):2065–2072. doi: 10.1172/JCI116803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giorgino F, Pedrini MT, Matera L, Smith RJ. Specific increase in p85alpha expression in response to dexamethasone is associated with inhibition of insulin-like growth factor-I stimulated phosphatidylinositol 3-kinase activity in cultured muscle cells. J Biol Chem. 1997;272(11):7455–7463. doi: 10.1074/jbc.272.11.7455. [DOI] [PubMed] [Google Scholar]
- 32.Giorgino F, Smith RJ. Dexamethasone enhances insulin-like growth factor-I effects on skeletal muscle cell proliferation. Role of specific intracellular signaling pathways. J Clin Invest. 1995;96(3):1473–1483. doi: 10.1172/JCI118184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruzzin J, Wagman AS, Jensen J. Glucocorticoid-induced insulin resistance in skeletal muscles: defects in insulin signalling and the effects of a selective glycogen synthase kinase-3 inhibitor. Diabetologia. 2005;48(10):2119–2130. doi: 10.1007/s00125-005-1886-0. [DOI] [PubMed] [Google Scholar]
- 34.Elliott P, Peters RF, White AM. A study of the relationship between glucocorticoid-induced weight loss in rats and the activity of skeletal-muscle and cardiac-muscle ribosomes in vitro. Biochem J. 1971;125(4):106P–107P. doi: 10.1042/bj1250106pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tonolo G, Fraser R, Connell JM, Kenyon CJ. Chronic low-dose infusions of dexamethasone in rats: effects on blood pressure, body weight and plasma atrial natriuretic peptide. J Hypertens. 1988;6(1):25–31. [PubMed] [Google Scholar]
- 36.Huang H, Gazzola C, Pegg GG, Sillence MN. Differential effects of dexamethasone and clenbuterol on rat growth and on beta2-adrenoceptors in lung and skeletal muscle. J Anim Sci. 2000;78(3):604–608. doi: 10.2527/2000.783604x. [DOI] [PubMed] [Google Scholar]
- 37.Olswang Y, Blum B, Cassuto H, Cohen H, Biberman Y, Hanson RW, Reshef L. Glucocorticoids repress transcription of phosphoenolpyruvate carboxykinase (GTP) gene in adipocytes by inhibiting its C/EBP-mediated activation. J Biol Chem. 2003;278(15):12929–12936. doi: 10.1074/jbc.M300263200. [DOI] [PubMed] [Google Scholar]
- 38.Kido Y, Burks DJ, Withers D, Bruning JC, Kahn CR, White MF, Accili D. Tissue-specific insulin resistance in mice with mutations in the insulin receptor, IRS-1, and IRS-2. J Clin Invest. 2000;105(2):199–205. doi: 10.1172/JCI7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Previs SF, Withers DJ, Ren JM, White MF, Shulman GI. Contrasting effects of IRS-1 versus IRS-2 gene disruption on carbohydrate and lipid metabolism in vivo. J Biol Chem. 2000;275(50):38990–38994. doi: 10.1074/jbc.M006490200. [DOI] [PubMed] [Google Scholar]
- 40.Rockall AG, Sohaib SA, Evans D, Kaltsas G, Isidori AM, Monson JP, Besser GM, Grossman AB, Reznek RH. Computed tomography assessment of fat distribution in male and female patients with Cushing’s syndrome. Eur J Endocrinol. 2003;149(6):561–567. doi: 10.1530/eje.0.1490561. [DOI] [PubMed] [Google Scholar]
- 41.Joyner JM, Hutley LJ, Cameron DP. Glucocorticoid receptors in human preadipocytes: regional and gender differences. J Endocrinol. 2000;166(1):145–152. doi: 10.1677/joe.0.1660145. [DOI] [PubMed] [Google Scholar]
- 42.Lonn L, Kvist H, Ernest I, Sjostrom L. Changes in body composition and adipose tissue distribution after treatment of women with Cushing’s syndrome. Metabolism. 1994;43(12):1517–1522. doi: 10.1016/0026-0495(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 43.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D’Agostino RB, Sr, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116(1):39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 44.Dietze D, Koenen M, Rohrig K, Horikoshi H, Hauner H, Eckel J. Impairment of insulin signaling in human skeletal muscle cells by co-culture with human adipocytes. Diabetes. 2002;51(8):2369–2376. doi: 10.2337/diabetes.51.8.2369. [DOI] [PubMed] [Google Scholar]
- 45.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 46.Tomlinson JW, Walker EA, Bujalska IJ, Draper N, Lavery GG, Cooper MS, Hewison M, Stewart PM. 11beta-hydroxysteroid dehydrogenase type 1: a tissue-specific regulator of glucocorticoid response. Endocr Rev. 2004;25(5):831–866. doi: 10.1210/er.2003-0031. [DOI] [PubMed] [Google Scholar]
- 47.Alberts P, Nilsson C, Selen G, Engblom LO, Edling NH, Norling S, Klingstrom G, Larsson C, Forsgren M, Ashkzari M, Nilsson CE, et al. Selective inhibition of 11{beta}-hydroxysteroid dehydrogenase type 1 improves hepatic insulin sensitivity in hyperglycemic mice strains. Endocrinology. 2003;144(11):4755–4762. doi: 10.1210/en.2003-0344. [DOI] [PubMed] [Google Scholar]
- 48.Berthiaume M, Laplante M, Festuccia W, Gelinas Y, Poulin S, Lalonde J, Joanisse DR, Thieringer R, Deshaies Y. Depot-specific modulation of rat intraabdominal adipose tissue lipid metabolism by pharmacological inhibition of 11beta-hydroxysteroid dehydrogenase type 1. Endocrinology. 2007;148(5):2391–2397. doi: 10.1210/en.2006-1199. [DOI] [PubMed] [Google Scholar]