Abstract
In eukaryotic cells, half of all proteins function as subunits within multi-protein complexes. Imbalanced synthesis of subunits leads to unassembled intermediates that must be degraded to minimize cellular toxicity. Here, we found that excess PSMC5, a subunit of the proteasome base, was targeted for degradation by the HERC1 ubiquitin ligase in mammalian cells. HERC1 identified unassembled PSMC5 via its cognate assembly chaperone PAAF1. Because PAAF1 only dissociates after assembly, HERC1 could also engage later assembly intermediates such as the PSMC4-PSMC5-PAAF1 complex. A missense mutant of HERC1 that causes neurodegeneration in mice was impaired in the recognition and ubiquitination of the PSMC5-PAAF1 complex. Thus, proteasome assembly factors can serve as adaptors for ubiquitin ligases to facilitate elimination of unassembled intermediates and maintain protein homeostasis.
Many of the cell’s multi-subunit complexes, such as ribosomes and proteasomes, are exceptionally abundant and contain a large number of subunits. Their maturation can involve dozens or more assembly factors that participate in a multi-step pathway (1–3). The different subunits cannot be produced at a precise desired stoichiometry owing to the inherent noisiness of transcription and translation (4, 5). For highly abundant complexes, even subtle imbalances in subunit synthesis (6) can produce an appreciable number of assembly intermediates awaiting the next component. Furthermore, cellular stress and various disease states, most notably cancer, can exaggerate subunit imbalances because of altered or dysregulated gene expression (6–8). As exemplified by the thalassemias (9) or aneuploidy (10–13), excessive or chronic subunit imbalance can have detrimental gain-of-function consequences. How cells detect stalled orphaned assembly intermediates for selective elimination is not well understood.
Identification of degraded candidate orphan proteins
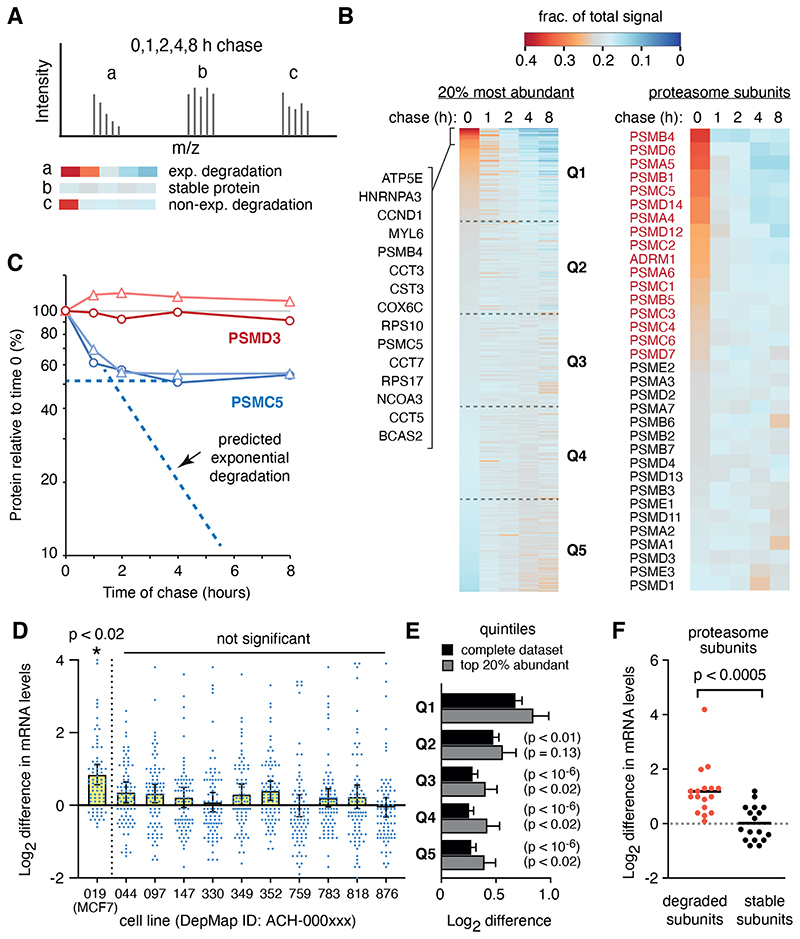
To find prominent orphan proteins caused by imbalanced gene expression, we identified rapidly degraded proteins in the aneuploid breast cancer cell line MCF7. We pulse-labelled nascent proteins for 1 hour with the methionine analog azido-homoalanine (AHA) followed by a chase with methionine for up to 8 hours. The AHA-containing proteins across all time points were captured by click chemistry (14) and analyzed by quantitative proteomics (Fig. 1A, fig. S1, table S1). Focusing on the cell’s ~450 most highly translated proteins (from 2262 total identified proteins), we found that a minor subset of nascent proteins decreased abruptly in the first hour (Fig. 1B). Notably, 14 of the top 15 such proteins were subunits of multi-protein complexes.
Fig 1. Identification of rapidly degraded candidate orphans.
(A) Schematic of how tandem mass tag (TMT) mass spectrometry data from a pulse chase is displayed as a heat map to visualise a protein’s change over time. The relative proportion of the total signal at each time point is displayed in the heat map (red is high, blue is low) for each hypothetical peptide’s spectra. (B) Heat maps as in panel A are shown for the 20% most abundant proteins (left) and for subunits of the proteasome (right). Identities of the first 15 proteins of the left heat map are indicated. Each quintile is indicated (Q1 to Q5). (C) Data from panel B showing examples of non-exponential degradation (PSMC5) and a stable protein (PSMD3). Each of two replicates is shown in different shades. (D) Individual mRNA levels for quintile 1 proteins from panel B (together with their means ± 95% CIs) are shown for eleven cell lines derived from HER2- negative and estrogen receptor-positive breast cancers. Each mRNA level is expressed as the log2 difference from that mRNA’s average level across all 1379 cell lines in the DepMap database. Only MCF7 cells showed a significant (p<0.02) elevation of this set of mRNAs, which was also significantly different (p<0.02) from the levels in all the other breast cancer cell lines. (E) Plot as in panel D comparing the mRNA levels (mean ± SEM) for quintiles 1 through 5 in MCF7 cells. P values for a two-tailed t-test comparison to Q1 are indicated. (F) Plot as in panel D for the degraded and stable subunits of the proteasome shown in panel B, using the same color-coding as in panel B.
Only a subset of subunits that comprise the proteasome, chaperonin, and ribosome displayed non-exponential degradation, where a proportion of the protein was degraded rapidly and the remainder was comparatively stable (Fig. 1B, 1C, fig. S2). By contrast, degradation of proteins that were not stable subunits of complexes, or the loss of proteins because of secretion, followed exponential decay (fig. S2C). These findings could not be explained by AHA-induced protein misfolding (table S2, S3) (15) or imbalanced subunit production caused by methionine starvation during AHA labelling (table S4) (16).
Instead, the collection of mRNAs for degraded proteins was elevated more highly in MCF7 cells than in any of ten other similar breast cancer cell lines (Fig. 1D) (17). The degree of elevation for these mRNAs was also greater than for the set of mRNAs coding for the stable proteins (Fig. 1E). The mRNAs for degraded subunits of the proteasome and ribosome were overrepresented around 1.5-fold to 2-fold in MCF7 cells, but not other breast cancer cell lines, relative to the mRNAs for the stable subunits of the respective complex (Fig. 1F, fig. S3). This degree of excess mRNA roughly matched the ~30-50% of their encoded proteins that were rapidly degraded.
Thus, subunits of multi-protein complexes are major targets for quality control in cancer cells because a population of these proteins evidently become orphaned when they are expressed inappropriately at higher levels than their assembly partners.
HERC1 interacts with and ubiquitinates nascent PSMC5
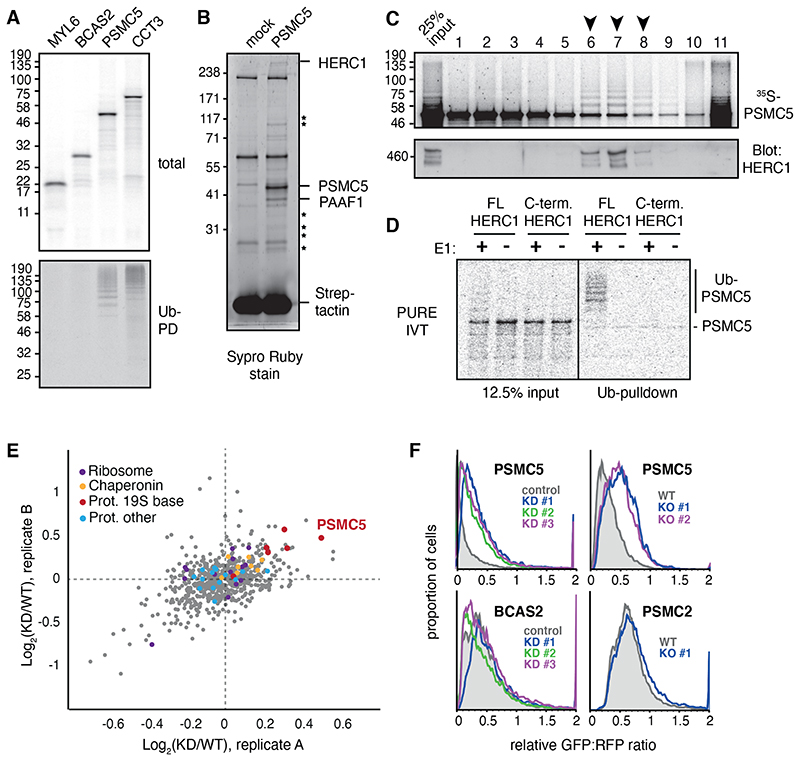
To identify the quality control pathway(s) that facilitate orphan degradation, we analyzed individual subunits of four diverse multi-protein complexes identified as orphans in our proteomics analysis of MCF7 cells. Each epitope-tagged subunit was synthesized in a reticulocyte-based cell free translation extract with the aim of finding at least one whose recognition for quality control was recapitulated in this system. Two subunits, PSMC5 of the proteasome and CCT3 of the cytosolic chaperonin, were ubiquitinated (Fig. 1A). Both complexes are abundant in reticulocytes (18, 19), explaining why factors needed for their quality control are present in this system. Conversely, subunits of the spliceosome and myosin, which do not have an appreciable role in reticulocyte biology, were poorly recognized.
Fig 2. HERC1 mediates nascent PSMC5 degradation.
(A) Subunits of four different multimeric complexes were translated in RRL containing 35S-methionine and His-tagged ubiquitin (Ub). Ubiquitinated translation products were isolated by Ni-NTA pulldown under denaturing conditions. (B) PSMC5 containing a twin-Strep tag (TST) was translated in RRL, affinity purified under native conditions using immobilized Streptactin, and the products visualized by SYPRO Ruby stain. Mock indicates a parallel reaction lacking mRNA. The indicated PSMC5-specific interaction partners were identified by mass spectrometry, with stars denoting other 19S subunits of the proteasome. (C) 35S-methionine labelled PSMC5 translated in in RRL was separated on a 5-25% sucrose gradient. Fractions were analysed by autoradiography for PSMC5, and by immunoblot for HERC1. Arrows indicate fractions where ubiquitinated PSMC5 co-factionates with HERC1. On this gradient, ~300 kD protein typically migrates in fractions 3 to 5, and a ~500 kD protein in fractions 5 to 7. (D) 35S-labeled PSMC5 in complex with PAAF1 was produced by translation in the PURE system (see fig. S5A). The complex was mixed with E1, E2, His-Ub, ATP, and either full length or truncated recombinant HERC1 (see fig. S5B). Ubiquitinated products were isolated by Ni-NTA pulldown under denaturing conditions and visualized by autoradiography. (E) MCF7 cells were pretreated with nontargeting (WT) or HERC1-targeting (KD) siRNAs and metabolically labeled for 1 hour with AHA. The labeled proteins were selectively recovered via click chemistry, and the recovered proteins were analyzed by quantitative mass spectrometry. The top 20% most abundant proteins from two biological replicates are plotted by the KD/WT ratio, with subunits of the proteasome, ribosome, and chaperonin highlighted. (F) Flow cytometry analysis of GFP-tagged reporter proteins compared to an internal RFP control. HERC1 was knocked down (KD) by 3 separate siRNAs or knocked out (KO) using CRISPR.
Focusing on newly made PSMC5, we observed that its immunoprecipitation under non-denaturing conditions co-precipitated ubiquitin ligase activity (fig. S4A). Large scale affinity purification of nascent PSMC5 followed by mass spectrometry identified the HECT-domain protein HERC1 as the sole E3 ubiquitin ligase (Fig. 2B; table S5). This interaction was verified by co-immunoprecipitation and immunoblotting (fig. S4B). Furthermore, HERC1 co-fractionated selectively with the ubiquitinated subpopulation of PSMC5 when separated by size by sedimentation through a sucrose gradient (Fig. 1C).
The other interaction partners of nascent PSMC5 suggested that it was engaged in a heterogeneous set of assembly intermediates consistent with PSMC5’s broad distribution across the sucrose gradient. The most abundant interaction partner was PSMC5’s dedicated chaperone PAAF1 (fig. S4B) (20–23). Other subunits of the 19S base were co-purified at lower levels, consistent with partial assembly of PSMC5 with endogenous base subunits present in the lysate (table S5). Assembly beyond this step was evidently less efficient, with incomplete recovery of 19S lid subunits and essentially no recovery of 20S core subunits.
Engagement of PSMC5 with the 20S core would be impaired by the C-terminal epitope tag, thereby ensuring that nearly all nascent PSMC5 would stall at earlier assembly intermediates. Based on staining intensity of the products (Fig. 1B) and sucrose gradient distribution (Fig. 1C), the most abundant complex was the PSMC5-PAAF1 assembly intermediate. Thus, this unassembled product might be at least one quality control target of HERC1.
PSMC5 synthesized in a translation system reconstituted from purified recombinant E. coli translation factors (known as the PURE system) was entirely insoluble unless a chaperone, such as recombinant PAAF1, was included during translation (fig. S5A). The PSMC5-PAAF1 heterodimer from the peak fractions of the gradient could be ubiquitinated by full length recombinant HERC1 at physiologic concentrations, but not by a C-terminal domain that retains full E3 ligase activity (Fig. 2D, fig. S5B). Thus, HERC1 can recognize the unassembled PSMC5-PAAF1 complex and ubiquitinate PSMC5.
HERC1 facilitates degradation of PSMC5 in cells
Pulse-chase experiments using 35S-methionine showed that roughly half of newly synthesized PSMC5 was degraded shortly after synthesis in MCF7 cells (fig. S6A). PSMC5 degradation was blunted in cells knocked out for HERC1 (fig. S6B). Proteomic analysis of nascent proteins synthesized during one hour in untreated versus HERC1 knockdown (KD) cells revealed that PSMC5 was over-represented (~1.4-fold) in KD cells (Fig. 2E, table S6). Relatively few other proteins were over-represented similarly, indicating that HERC1 does not generally influence protein synthesis or degradation. For example, chaperonin subunits, ribosomal proteins, and most subunits of the proteasome were unaffected (Fig. 1E). In contrast, 5 of 6 ATPase subunits (including PSMC5) of the 19S proteasome base were overrepresented in the KD sample, suggesting this class of proteins was selectively influenced by HERC1.
Although the burden of surplus PSMC5 and other subunits is exaggerated in MCF7 cells, ~10-20% imbalanced production can occur in normal cells owing to gene expression noise (4, 5). Indeed, 19S ATPase subunits were modestly (by ~21%) but significantly stabilized upon HERC1 KD in the non-cancer breast epithelial cell line MCF10a (fig. S7, table S7). Subunits of the cytosolic chaperonin (CCTs), which are not targets for HERC1, were not changed in HERC1 KD cells. Thus, the problem of subunit imbalance, while enhanced by genomic dysregulation in cancer cells, is nonetheless appreciable in non-cancer cells.
These proteomic observations were examined in focused assays using fluorescent protein reporters. Reporter translation produces two fluorescent proteins: GFP that is fused to a protein of interest, and RFP. The GFP:RFP ratio provides a quantitative assessment of any changes to the GFP-tagged protein’s stability (24). Overexpressed N-terminally tagged PSMC5 was mostly degraded, but stabilized by knockdown or knockout of HERC1 in MCF7 cells (Fig. 2F, fig. S8).
Furthermore, degradation of excess subunits of other complexes or a subunit of the 20S proteasome core was not affected by HERC1 knockdown (Fig. 2F, fig. S9A). Surprisingly, other 19S ATPase ring members that were impacted by HERC1 knockdown in the proteomics experiment were neither degraded as effectively as PSMC5 nor impacted strongly by HERC1 knockout when tested by exogenous overexpression in the reporter assay (Fig. 2F, fig. S9B). This suggested that the effect of HERC1 on non-PSMC5 base subunits could be indirect, an idea consistent with relatively poor interaction of most of these subunits with HERC1 in vitro despite their considerable sequence and structural homology to each other (fig. S10A).
PAAF1 is required for recognition of PSMC5 by HERC1
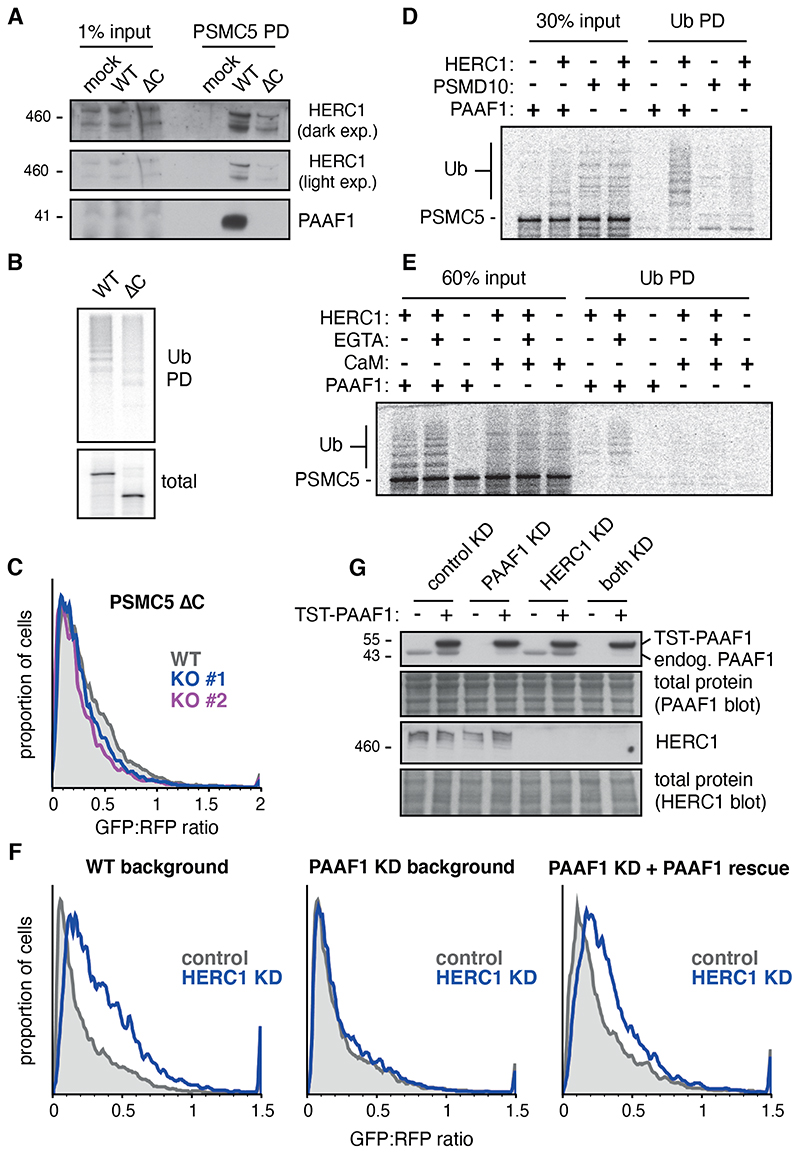
To understand why overexpressed PSMC5, but not other base subunits, was degraded via HERC1, we investigated the mechanism of HERC1 target selection. Although each subunit of the 19S ATPase ring is similar, their C-terminal domains engage different assembly factors (20–23, 25). Recognizing that our reconstituted ubiquitination experiment (Fig. 1D) used the PSMC5-PAAF1 complex as a substrate (fig. S5), we tested whether PAAF1 plays a role in HERC1 recognition. Indeed, in vitro translated PSMC5 lacking the C-terminal domain (PSMC5ΔC) did not bind PAAF1, showed reduced HERC1 binding, and showed reduced ubiquitination compared to full length PSMC5 (Fig. 3A and 3B).
Fig 3. The assembly chaperone PAAF1 participates in HERC1 target selection.
(A) Full length PSMC5 (WT) and PSMC5 lacking its C-terminal 86 residues (ΔC) were translated in RRL and affinity purified under native conditions via a C-terminal twin-Strep tag (TST). The input and purified samples were blotted for HERC1 and PAAF1. SYPRO-Ruby staining (fig. S10B) was used to verify equal recovery of the translation products. (B) Full length and truncated PSMC5 were translated in RRL in the presence of 35S-methionine and His-tagged ubiquitin (Ub). Ubiquitinated products were isolated by Ni-NTA pulldown. (C) Flow cytometry analysis of GFP-tagged C-terminally truncated PSMC5 in WT and HERC1 knockout MCF7 cells. (D) 35S-labeled PSMC5 in complex with PAAF1 or PSMD10 was prepared by translation in the PURE system. The complexes were mixed with E1, E2, His-Ub, ATP, and full length recombinant HERC1. Ubiquitinated products were isolated by Ni-NTA pulldown under denaturing conditions. (E) 35S-labeled PSMC5 in complex with PAAF1 or calmodulin (CaM) was prepared and mixed with ubiquitination components, as in D. Where indicated, EGTA was used to dissociate CaM from PSMC5. Ubiquitinated products were isolated by Ni-NTA pulldown under denaturing conditions. (F) Flow cytometry analysis of GFP-tagged PSMC5 in MCF7 cells. HERC1 and PAAF1 were knocked down separately or simultaneously by single siRNA oligonucleotides. TST-PAAF1 with siRNA-resistant silent mutations was reintroduced where indicated (PAAF1 rescue). (G) Knockdown of endogenous PAAF1 and HERC1, as well as rescue with TST-PAAF1, was verified by immunoblot for the experiment in panel F.
Although overexpressed PSMC5ΔC was degraded in MCF7 cells, its degradation did not depend on HERC1 (Fig. 3C). This suggested that HERC1 either recognizes the C-terminus, recognizes PAAF1, or that non-native interaction partners of PSMC5ΔC (fig. S10B) obscure HERC1 access. To distinguish between these possibilities, we tested PSMC5 ubiquitination when it is complexed with another 19S assembly chaperone (PSMD10), the non-physiologic chaperone-like protein calmodulin (26), or uncomplexed with any factor. PSMC10 ordinarily chaperones the C-terminal domain of PSMC4 but can engage PSMC5 in the purified translation reaction due to the homology between their C-terminal domains. Calmodulin, by contrast, has broad Ca2+-dependent binding activity. Both proteins precluded nascent PSMC5 aggregation sufficiently well in the PURE system to provide substrate complexes for HERC1 ubiquitination assays.
HERC1 did not effectively ubiquitinate either the PSMC5-PSMD10 complex or the PSMC5-calmodulin complex (Fig. 3D and 3E). EGTA-mediated release of calmodulin to fully expose PSMC5 to HERC1 also did not permit ubiquitination. Thus, neither uncomplexed PSMC5 nor PSMC5 complexed with PSMD10 or calmodulin were recognized by HERC1, illustrating a crucial role for PAAF1 in recognition. Indeed, HERC1 depletion failed to stabilize PSMC5 in cells depleted of PAAF1 (Fig. 3F). Thus, efficient PSMC5 recognition by HERC1 relies on PSMC5’s chaperone PAAF1. PSMC5 produced in the absence of PAAF1 is presumably misfolded (consistent with its aggregation in the PURE system), triggering PSMC5 degradation by a HERC1-independent pathway. This further indicates that HERC1 is not simply recognizing PSMC5 misfolding, but rather a PAAF1-containing putative assembly intermediate.
HERC1 interacts with a PSMC4-containing assembly intermediate
PAAF1 remains bound to the C-terminal domain of PSMC5 throughout its assembly with other ATPase subunits of the 19S base, dissociating concomitant with C-terminal insertion into the 20S core particle (21, 23). Hence, assembly intermediates downstream of the initial PSMC5-PAAF1 complex are potential targets for HERC1, possibly explaining why other base subunits are affected by HERC1 KD in the context of PSMC5 excess. To test this idea, we determined if the PSMC5-PSMC4 assembly intermediate is targeted by HERC1 via retained PAAF1.
In vitro translated PSMC4 interacted equally well with co-expressed PSMC5 and PSMC5ΔC (Fig. 4A top panel), verifying that each nascent subunit is assembly-competent. Affinity purification of PSMC4 co-precipitated PAAF1 and HERC1 only from PSMC5-containing reactions, but not from reactions containing PSMC5ΔC or lacking PSMC5 (Fig. 4A, bottom panel). Thus, PSMC4 associates with HERC1 via the PSMC5-PAAF1 complex. In accordance with HERC1 recruitment, the PSMC5-PSMC4 intermediate remains vulnerable to ubiquitination (fig. S11). Thus, assembly of PSMC5 with PSMC4 does not protect PSMC5 from ubiquitination, presumably because retained PAAF1 is still able to recruit HERC1 to the complex.
Fig 4. HERC1 recognises multiple assembly intermediates via PAAF1.
(A)FLAG-tagged full length or C-terminally truncated (ΔC) PSMC5 was co-translated with a twin-Strep-tagged (TST) partner ATPase (PSMC4) or distal ring member (PSMC2) in RRL. Complexes were affinity purified via the TST, and blotted for TST, FLAG, HERC1, PAAF1, and PSMC5 as indicated. TST-PSMC5 was translated and purified directly as a positive control. Reactions containing only one ring member included mRNA for an irrelevant protein (the cytosolic domain of Sec61β) to ensure equal rates of synthesis. The relative amounts of the different samples loaded for the two sets of blots is indicated. (B) Flow cytometry analysis of GFP-tagged PSMC4 in WT and HERC1 knockout MCF7 cells, with and without co-transfection of non-fluorescent PSMC5.
Although overexpressed fluorescent PSMC4 is mostly degraded independently of HERC1, co-expression with non-fluorescent PSMC5 imparts partial HERC1-dependence to PSMC4 degradation (Fig. 4B). This provides in vivo support for HERC1 targeting PSMC4 via recognition of the associated PSMC5-PAAF1 complex. These observations provide an explanation for partial PSMC4 stabilization in MCF7 cells knocked down for HERC1 (Fig. 1E). Although we have not examined other assembly intermediates, it is plausible that later PAAF1-containing complexes are also potential targets for HERC1.
Why does HERC1 not interfere with normal assembly? One model is kinetic competition (27, 28), with assembly occurring faster than HERC1 recognition. This mechanism is plausible because HERC1 is ~10-fold less abundant than PAAF1 (29, 30). Boosting HERC1 levels using CRISPR-activation (31) of the endogenous HERC1 promoter led to promiscuous degradation of endogenous nascent PSMC5 and PSMC4 (fig. S12), supporting a competition-based mechanism.
Disease-causing HERC1 mutant is deficient in PSMC5 recognition
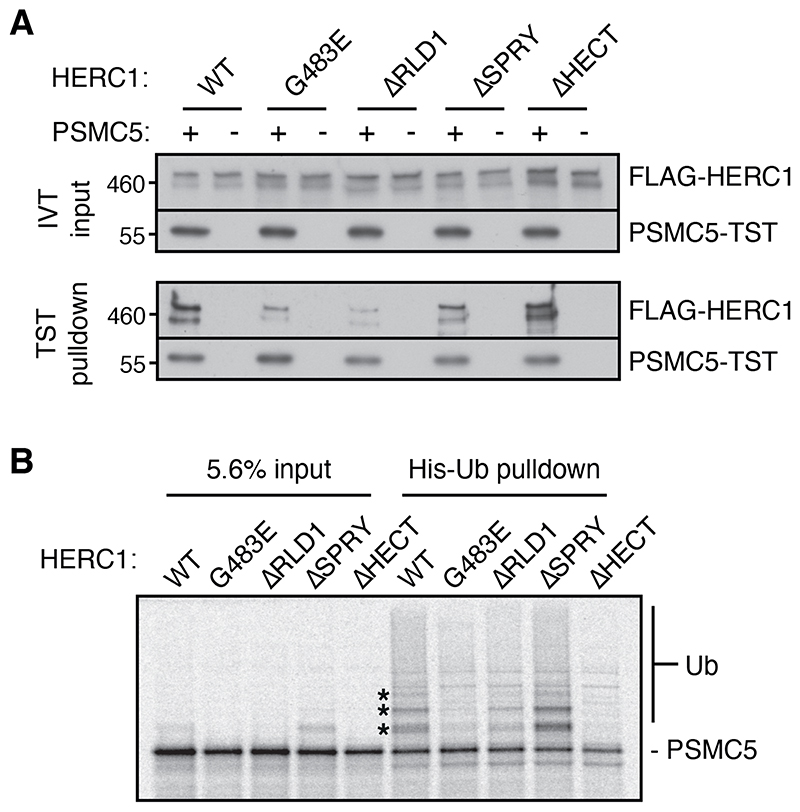
Co-immunoprecipitation experiments using a series of domain deletion constructs expressed in cultured cells showed that the first RCC1-like domain (RLD1) in HERC1 is important for interaction with PSMC5 (fig. S13). This finding was illuminating because the recessive neurodegeneration-causing tambaleante allele in mouse was mapped to a G483E missense mutation in RLD1 of HERC1. Biochemical analysis of purified HERC1 variants (fig. S14) showed that the ΔRLD1 deletion mutant and G483E point mutant were impaired in their interaction with nascent PSMC5, whereas other deletion mutants were not (Fig. 5A, fig. S15A).
Fig 5. Disease-causing HERC1 mutant is deficient in PSMC5 recognition and ubiquitination.
(A) Recombinant purified FLAG-tagged HERC1 proteins (see fig. S14) were added at 2 nM to RRL in vitro translation reactions with or without mRNA for twin-Strep-tagged (TST) PSMC5. PSMC5-TST was affinity purified under native conditions using Streptactin, and inputs and elutions visualised by immunoblotting. (B) 35S-labeled PSMC5 in complex with PAAF1 was produced by translation in the PURE system (as in fig. S5A). The complex was mixed with E1, E2, His-Ub, ATP, and 8nM wild-type or mutant HERC1. Ubiquitinated products were enriched by Ni-NTA pulldown under denaturing conditions. The input and ubiquitin pulldown samples were visualized by autoradiography. The positions of mono-, di-, and tri-ubiquitinated PSMC5 are indicated with asterisks (also visible faintly in the input sample).
Ubiquitination assays using the PSMC5-PAAF1 complex produced in the PURE system showed a marked reduction of PSMC5 ubiquitination by the ΔRLD1 and G483E mutants (Fig. 5B, fig. S15B) despite no impairment of autoubiquitination (fig. S15C). This suggests that G483E is specifically deficient in interaction with- and ubiquitination of PSMC5. This mutant is expressed normally in mouse (32) and cultured cells with comparable biochemical behavior as the wild type protein (fig. S15D, S15E), illustrating that the mutant protein is not grossly misfolded or eliminated by cellular quality control. These results suggest that a deficiency in HERC1-mediated quality control contributes to the disease phenotype of tambaleante mice. Consistent with this idea, the impaired cell types in mice are those that most rely on effective proteostasis (33), especially Purkinje cells, which are frequently lost with perturbed proteostasis (34–36).
Conclusions and perspective
Unassembled subunits have long been appreciated to be quality control targets for one of two reasons: either they misfold in the absence of assembly, or the exposed assembly interface is recognized by quality control factors (37–40). In both cases, exposed hydrophobic surfaces are thought to be the target for quality control. Our findings reveal a qualitatively different mechanism: delayed assembly cues ubiquitin ligase recruitment via the associated assembly factor. A major advantage for the cell of such a kinetic competition mechanism is that recognition does not depend on potentially toxic misfolding or aggregation. Instead, a simple delay would be sufficient to trigger elimination of otherwise normal intermediates. By utilizing an assembly factor to identify incomplete products, a ubiquitin ligase can potentially recognize multiple intermediates along the assembly pathway.
HERC1 is both widely expressed and widely conserved (41, 42). Loss of HERC1 function in mice and humans leads to neurological defects (32, 43–46) associated with deficient proteostasis (33). Our finding that a neurodegeneration-causing missense mutation is strongly impaired in PSMC5 ubiquitination in a purified system implicates this quality control pathway in maintaining proteostasis in vivo. More generally, the insights provided here should motivate systematic searches for assembly factor-dependent ubiquitin ligases that eliminate stalled intermediates of other major cellular complexes. Because orphan proteins and intermediates are especially prominent in cancer cells with aberrant gene expression, mechanisms to eliminate these products may be important for their rapid growth. Intriguingly, HERC1 is upregulated in various cancer cell lines and tumors (42) and its overexpression can provide a selective advantage to tumor growth and metastasis in mouse (47). RNA sequencing of a wide range of cancer-derived cells (17) indicates that different populations of orphans feature in different tumors. Thus, identifying the quality control pathways for other abundant orphans using the approaches defined here may provide useful therapeutic targets.
Supplementary Material
One-Sentence Summary.
Unassembled subunits of the proteasome are targeted for degradation by a factor whose impairment causes neurodegeneration.
Acknowledgements
We thank members of the Hegde lab for productive discussions and comments on this manuscript; L. Figueiredo for help with heatmaps; M. Daly and F. Zhang for help with flow cytometry; and M. Kivlen for preparation of the PURE translation system. Funding was provided by the UK Medical Research Council (MC_ UP_A022_1007 to R.S.H.) and the LMB-AZ BlueSky Fund (BSF27).
Funding
UK Medical Research Council grant MC_ UP_A022_1007 (RSH); LMB-AZ BlueSky project BSF27 (AJN, RSH).
Footnotes
Author contributions: EZ performed nearly all of the experiments. SP performed mass spectrometry and contributed to its analysis. SJ helped with mapping the substrate-binding domains of HERC1. EZ, AJN, and RSH conceived the project. EZ and RSH wrote the manuscript. AJN and RSH provided project supervision.
Competing interests: Authors declare that they have no competing interests.
Data and materials availability
All data are available in the main text or the supplementary materials. Reagents used in this study are available from RSH upon request.
References
- 1.Bohnsack KE, Bohnsack MT. Uncovering the assembly pathway of human ribosomes and its emerging links to disease. EMBO J. 2019;38:e100278. doi: 10.15252/embj.2018100278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klinge S, Woolford JL. Ribosome assembly coming into focus. Nat Rev Mol Cell Biol. 2019;20:116–131. doi: 10.1038/s41580-018-0078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rousseau A, Bertolotti A. Regulation of proteasome assembly and activity in health and disease. Nat Rev Mol Cell Biol. 2018;1 doi: 10.1038/s41580-018-0040-z. [DOI] [PubMed] [Google Scholar]
- 4.Raser JM, O’Shea EK. Noise in gene expression: origins, consequences, and control. Science. 2005;309:2010–2013. doi: 10.1126/science.1105891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munsky B, Neuert G, van Oudenaarden A. Using gene expression noise to understand gene regulation. Science. 2012;336:183–187. doi: 10.1126/science.1216379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harper JW, Bennett EJ. Proteome complexity and the forces that drive proteome imbalance. Nature. 2016;537:328–338. doi: 10.1038/nature19947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang Y-C, Amon A. Gene copy number alterations: A cost-benefit analysis. Cell. 2013;152:394–405. doi: 10.1016/j.cell.2012.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Origa R. β-Thalassemia. Genet Med Off J Am Coll Med Genet. 2017;19:609–619. doi: 10.1038/gim.2016.173. [DOI] [PubMed] [Google Scholar]
- 10.Brennan CM, Vaites LP, Wells JN, Santaguida S, Paulo JA, Storchova Z, Harper JW, Marsh JA, Amon A. Protein aggregation mediates stoichiometry of protein complexes in aneuploid cells. Genes Dev. 2019;33:1031–1047. doi: 10.1101/gad.327494.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dephoure N, Hwang S, O’Sullivan C, Dodgson SE, Gygi SP, Amon A, Torres EM. Quantitative proteomic analysis reveals posttranslational responses to aneuploidy in yeast. eLife. 2014;3:e03023. doi: 10.7554/eLife.03023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torres EM, Sokolsky T, Tucker CM, Chan LY, Boselli M, Dunham MJ, Amon A. Effects of Aneuploidy on Cellular Physiology and Cell Division in Haploid Yeast. Science. 2007;317:916–924. doi: 10.1126/science.1142210. [DOI] [PubMed] [Google Scholar]
- 13.Williams BR, Prabhu VR, Hunter KE, Glazier CM, Whittaker CA, Housman DE, Amon A. Aneuploidy affects proliferation and spontaneous immortalization in mammalian cells. Science. 2008;322:703–709. doi: 10.1126/science.1160058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dieterich DC, Link AJ, Graumann J, Tirrell DA, Schuman EM. Selective identification of newly synthesized proteins in mammalian cells using bioorthogonal noncanonical amino acid tagging (BONCAT) Proc Natl Acad Sci U S A. 2006;103:9482–9487. doi: 10.1073/pnas.0601637103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McShane E, Sin C, Zauber H, Wells JN, Donnelly N, Wang X, Hou J, Chen W, Storchova Z, Marsh JA, Valleriani A, et al. Kinetic Analysis of Protein Stability Reveals Age-Dependent Degradation. Cell. 2016;167:803–815.:e21. doi: 10.1016/j.cell.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Thoreen CC, Chantranupong L, Keys HR, Wang T, Gray NS, Sabatini DM. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature. 2012;485:109–113. doi: 10.1038/nature11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghandi M, Huang FW, Jané-Valbuena J, Kryukov GV, Lo CC, McDonald ER, Barretina J, Gelfand ET, Bielski CM, Li H, Hu K, et al. Next-generation characterization of the Cancer Cell Line Encyclopedia. Nature. 2019;569:503–508. doi: 10.1038/s41586-019-1186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cowan NJ. Mammalian cytosolic chaperonin. Methods Enzymol. 1998;290:230–241. doi: 10.1016/s0076-6879(98)90022-2. [DOI] [PubMed] [Google Scholar]
- 19.Driscoll J, Goldberg AL. The proteasome (multicatalytic protease) is a component of the 1500-kDa proteolytic complex which degrades ubiquitin-conjugated proteins. J Biol Chem. 1990;265:4789–4792. [PubMed] [Google Scholar]
- 20.Funakoshi M, Tomko RJ, Kobayashi H, Hochstrasser M. Multiple assembly chaperones govern biogenesis of the proteasome regulatory particle base. Cell. 2009;137:887–899. doi: 10.1016/j.cell.2009.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaneko T, Hamazaki J, Iemura S, Sasaki K, Furuyama K, Natsume T, Tanaka K, Murata S. Assembly Pathway of the Mammalian Proteasome Base Subcomplex Is Mediated by Multiple Specific Chaperones. Cell. 2009;137:914–925. doi: 10.1016/j.cell.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Roelofs J, Park S, Haas W, Tian G, McAllister FE, Huo Y, Lee B-H, Zhang F, Shi Y, Gygi SP, Finley D. Chaperone-mediated pathway of proteasome regulatory particle assembly. Nature. 2009;459:861–865. doi: 10.1038/nature08063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saeki Y, Toh-e A, Kudo T, Kawamura H, Tanaka K. Multiple Proteasome-Interacting Proteins Assist the Assembly of the Yeast 19S Regulatory Particle. Cell. 2009;137:900–913. doi: 10.1016/j.cell.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Itakura E, Zavodszky E, Shao S, Wohlever ML, Keenan RJ, Hegde RS. Ubiquilins Chaperone and Triage Mitochondrial Membrane Proteins for Degradation. Mol Cell. 2016;63:21–33. doi: 10.1016/j.molcel.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park S, Roelofs J, Kim W, Robert J, Schmidt M, Gygi SP, Finley D. Hexameric assembly of the proteasomal ATPases is templated through their C termini. Nature. 2009;459:866–870. doi: 10.1038/nature08065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shao S, Hegde RS. A calmodulin-dependent translocation pathway for small secretory proteins. Cell. 2011;147:1576–1588. doi: 10.1016/j.cell.2011.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shao S, Hegde RS. Target Selection during Protein Quality Control. Trends Biochem Sci. 2015 doi: 10.1016/j.tibs.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Wickner S, Maurizi MR, Gottesman S. Posttranslational quality control: folding, refolding, and degrading proteins. Science. 1999;286:1888–1893. doi: 10.1126/science.286.5446.1888. [DOI] [PubMed] [Google Scholar]
- 29.Itzhak DN, Tyanova S, Cox J, Borner GH. Global, quantitative and dynamic mapping of protein subcellular localization. eLife. 2016;5:e16950. doi: 10.7554/eLife.16950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kulak NA, Pichler G, Paron I, Nagaraj N, Mann M. Minimal, encapsulated proteomic-sample processing applied to copy-number estimation in eukaryotic cells. Nat Methods. 2014;11:319–324. doi: 10.1038/nmeth.2834. [DOI] [PubMed] [Google Scholar]
- 31.Konermann S, Brigham MD, Trevino AE, Joung J, Abudayyeh OO, Barcena C, Hsu PD, Habib N, Gootenberg JS, Nishimasu H, Nureki O, et al. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature. 2015;517:583–588. doi: 10.1038/nature14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mashimo T, Hadjebi O, Amair-Pinedo F, Tsurumi T, Langa F, Serikawa T, Sotelo C, Guenet J-L, Rosa JL. Progressive Purkinje Cell Degeneration in tambaleante Mutant Mice Is a Consequence of a Missense Mutation in HERC1 E3 Ubiquitin Ligase. PLOS Genet. 2009;5:e1000784. doi: 10.1371/journal.pgen.1000784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruiz R, Pérez-Villegas EM, Bachiller S, Rosa JL, Armengol JA. HERC 1 Ubiquitin Ligase Mutation Affects Neocortical, CA3 Hippocampal and Spinal Cord Projection Neurons: An Ultrastructural Study. Front Neuroanat. 2016;10 doi: 10.3389/fnana.2016.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 35.Zhao L, Longo-Guess C, Harris BS, Lee J-W, Ackerman SL. Protein accumulation and neurodegeneration in the woozy mutant mouse is caused by disruption of SIL1, a cochaperone of BiP. Nat Genet. 2005;37:974–979. doi: 10.1038/ng1620. [DOI] [PubMed] [Google Scholar]
- 36.Lee JW, Beebe K, Nangle LA, Jang J, Longo-Guess CM, Cook SA, Davisson MT, Sundberg JP, Schimmel P, Ackerman SL. Editing-defective tRNA synthetase causes protein misfolding and neurodegeneration. Nature. 2006;443:50–55. doi: 10.1038/nature05096. [DOI] [PubMed] [Google Scholar]
- 37.Shemorry A, Hwang C-S, Varshavsky A. Control of protein quality and stoichiometries by N-terminal acetylation and the N-end rule pathway. Mol Cell. 2013;50:540–551. doi: 10.1016/j.molcel.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sung M-K, Porras-Yakushi TR, Reitsma JM, Huber FM, Sweredoski MJ, Hoelz A, Hess S, Deshaies RJ. A conserved quality-control pathway that mediates degradation of unassembled ribosomal proteins. eLife. 2016;5 doi: 10.7554/eLife.19105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Y, Anderson DE, Ye Y. The HECT domain ubiquitin ligase HUWE1 targets unassembled soluble proteins for degradation. Cell Discov. 2016;2:16040. doi: 10.1038/celldisc.2016.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yanagitani K, Juszkiewicz S, Hegde RS. UBE2O is a quality control factor for orphans of multi-protein complexes. Science. 2017;357:472–475. doi: 10.1126/science.aan0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.García-Cano J, Martinez-Martinez A, Sala-Gaston J, Pedrazza L, Rosa JL. HERCing: Structural and Functional Relevance of the Large HERC Ubiquitin Ligases. Front Physiol. 2019;10 doi: 10.3389/fphys.2019.01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosa JL, Casaroli-Marano RP, Buckler AJ, Vilaró S, Barbacid M. p619, a giant protein related to the chromosome condensation regulator RCC1, stimulates guanine nucleotide exchange on ARF1 and Rab proteins. EMBO J. 1996;15:4262–4273. [PMC free article] [PubMed] [Google Scholar]
- 43.Aggarwal S, Bhowmik AD, Ramprasad VL, Murugan S, Dalal A. A splice site mutation in HERC1 leads to syndromic intellectual disability with macrocephaly and facial dysmorphism: Further delineation of the phenotypic spectrum. Am J Med Genet A. 2016;170:1868–1873. doi: 10.1002/ajmg.a.37654. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen LS, Schneider T, Rio M, Moutton S, Siquier-Pernet K, Verny F, Boddaert N, Desguerre I, Munich A, Rosa JL, Cormier-Daire V, et al. A nonsense variant in HERC1 is associated with intellectual disability, megalencephaly, thick corpus callosum and cerebellar atrophy. Eur J Hum Genet. 2016;24:455–458. doi: 10.1038/ejhg.2015.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ortega-Recalde O, Beltrán OI, Gálvez JM, Palma-Montero A, Restrepo CM, Mateus HE, Laissue P. Biallelic HERC1 mutations in a syndromic form of overgrowth and intellectual disability. Clin Genet. 2015;88:e1–e3. doi: 10.1111/cge.12634. [DOI] [PubMed] [Google Scholar]
- 46.Utine GE, Taşkiran EZ, Koşukcu C, Karaosmanoglu B, Güleray N, Doğan ÖA, Kiper PÖŞ, Boduroglu K, Alikasifoglu M. HERC1 mutations in idiopathic intellectual disability. Eur J Med Genet. 2017;60:279–283. doi: 10.1016/j.ejmg.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 47.Fasano O, Birnbaum D, Edlund L, Fogh J, Wigler M. New human transforming genes detected by a tumorigenicity assay. Mol Cell Biol. 1984;4:1695–1705. doi: 10.1128/mcb.4.9.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharma A, Mariappan M, Appathurai S, Hegde RS. In vitro dissection of protein translocation into the mammalian endoplasmic reticulum. Methods Mol Biol Clifton NJ. 2010;619:339–363. doi: 10.1007/978-1-60327-412-8_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shao S, Rodrigo-Brenni MC, Kivlen MH, Hegde RS. Mechanistic basis for a molecular triage reaction. Science. 2017;355:298–302. doi: 10.1126/science.aah6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shimizu Y, Ueda T. In: Methods in Molecular Biology. Endo Y, Takai K, Ueda T, editors. Humana Press; Totowa, NJ: 2010. Cell-Free Protein Production: Methods and Protocols; pp. 11–21. [DOI] [Google Scholar]
- 51.Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8:2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol. 2008;26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- 53.DepMap, Broad. DepMap 21Q2 Public. figshare. Dataset. 2021 doi: 10.6084/m9.figshare.14541774.v2. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the main text or the supplementary materials. Reagents used in this study are available from RSH upon request.