Abstract
The global epidemic of obesity has heightened the need to understand the mechanisms that underpin its pathogenesis. Clinical observations in patients with Cushing’s syndrome have highlighted the link between cortisol and central obesity. However, whilst circulating cortisol levels are normal or reduced in obesity, local regeneration of cortisol, from inactive cortisone, by 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) has been postulated as a pathogenic mechanism. Whilst levels of expression of 11β-HSD1 in adipose tissue in human obesity are debated in the literature, global inhibition of 11β-HSD1 improves insulin sensitivity. We have determined the effects of significant weight loss upon cortisol metabolism and adipose tissue 11β-HSD1 expression following 10 weeks ingestion of a very low calorie diet in 12 obese patients (6 male, BMI 35.9±0.9kg/m2, mean±SE).
All patients achieved significant weight loss (14.1±1.3% of initial body weight). Total fat mass fell from 41.8±1.9 to 32.0±1.7kg, p<0.0001. In addition, fat free mass decreased (64.4±3.4 to 58.9±2.9kg, p<0.0001) and systolic blood pressure (BP) and total cholesterol also fell (systolic BP: 135±5 to 121±5mmHg, p<0.01: total cholesterol: 5.4±0.2 to 4.8±0.2mmol/l, p<0.05). Serum cortisol:cortisone ratio increased following weight loss (p<0.01). 11β-HSD1 mRNA expression in isolated adipocytes increased 3.4 fold (p<0.05).
Decreased 11β-HSD1 activity and expression in obesity may act as a compensatory mechanism to enhance insulin sensitivity through a reduction in tissue specific cortisol concentrations. Inhibition of 11β-HSD1 may therefore be a novel, therapeutic strategy for insulin sensitization.
Keywords: Obesity, 11β-hydroxysteroid dehydrogenase, weight loss, adipose tissue
Introduction
The role of cortisol metabolism in the pathogenesis of obesity is still unclear. There are striking phenotypic similarities between patients with circulating cortisol excess, Cushing’s syndrome, who develop reversible central obesity, and those patients with simple obesity who have normal (or slightly reduced) circulating cortisol levels (1). The HPA axis is not normal in obesity. The metabolic clearance rate for cortisol is increased with a secondary increase in cortisol secretion driven by ACTH (2;3). It remains unclear as to whether these observations are a cause or a consequence of the obese phenotype. Their reversibility with weight loss has not been studied.
Within adipose tissue, the enzyme 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) interconverts inactive glucocorticoid cortisone (E) and cortisol (F). In vivo it is the reductase activity that is believed to pre-dominate, generating cortisol in an autocrine / paracrine manner within the adipocyte microenvironment. Cortisol promotes preadipocyte differentiation and inhibition of 11β-HSD1 prevents cortisone induced preadipocyte differentiation (4). Furthermore, mice over-expressing 11β-HSD1 under the adipocyte specific aP2 promoter develop central obesity as a result of increased adipocyte size (5). However, in human obesity, generation of cortisol from an oral dose of cortisone acetate (believed to largely reflect hepatic 11β-HSD1 activity) is impaired (6–8). The excretion of the urinary tetrahydro-metabolites of cortisol and cortisone (THF+alloTHF:THE ratio), in the setting of a normal urinary free cortisol and cortisone excretion is also believed to reflect global 11β-HSD1 activity (9). Results have been more variable. Some studies have described decreased ratios consistent with decreased 11β-HSD1 reductase activity with increasing BMI in simple obesity (7;8). Other studies have failed to show this relationship (1;10–12) and indeed positive correlations have also been described (6;13;14). The explanation for this discrepancy is not clear. A reduction in hepatic 11β-HSD1 expression may not extend to expression in adipose tissue and a theory has evolved suggesting adipose tissue specific over-expression of 11β-HSD1 in human obesity (8;15;16). We have been unable to endorse this; in our studies, preadipocyte 11β-HSD1 expression was shown to be lowest in the most obese patients and we have hypothesized that this may have an important effect to reduce cortisol availability to the glucocorticoid receptor (GR) and enhance preadipocyte proliferation (17). The global inhibition of 11β-HSD1 in human obesity, and therefore the lack of ability to regenerate cortisol within tissues, is likely to be responsible for the observed increased cortisol metabolic clearance rate and cortisol secretion rates.
The aim of this study was to perform a detailed characterization of the global and adipose tissue specific expression and activity of 11β-HSD1 with significant weight loss in human obesity.
Patients and methods
The study had the approval of the local research ethics committee and all patients gave their full, informed written consent. 12 obese patients (6 male) were recruited from local advertisement (median age 49 years (range 23-58), mean BMI 35.9±0.9kg/m2). Patients had no significant past medical history and were on no regular medications. Fasting screening bloods were drawn at 09.00hr and all patients had normal blood counts, glucose, renal, liver and thyroid function. Patients with impaired fasting glucose (>6.0mmol/l) or diabetes mellitus were excluded from the study. All patients had normal resting electrocardiograms.
Once enrolled into the study all patients had further fasting bloods drawn at 09.00hr for measurement of total cholesterol, triglycerides, cortisol, cortisone, glucose and insulin. Measurements of BMI, waist (measured supine, at the level of the umbilicus) and hip (at the level of the greater trochanter) circumference and blood pressure (average of three readings, measured supine after 10 minutes rest using Dynamap®, Critikon, Tampa, USA) were also taken. In addition, all patients performed a 24-hour urine collection for corticosteroid metabolite analysis using gas chromatography / mass spectrometry as previously described (9). Measurement of the ratio of total cortisol metabolites (Fm = F + THF (tetrahydrocortisol) + 5αTHF + α-cortol + β-cortol + 20αDHF (dihydrocortisol) + 20βDHF + 6βOH-F): total cortisone metabolites (Em = E + THE + α-cortolone + β-cortolone + 20αDHE + 20βDHE) provides an assessment of the global set point of cortisol to cortisone conversion. More specifically, the ratio of tetrahydro-metabolites of cortisol (THF + 5αTHF) to those of cortisone (THE) provides a reflection of 11β-HSD1 activity providing that the ratio of urinary free cortisol (UFF) to cortisone (UFE) (reflecting renal 11β-HSD2 activity) is unaltered. The activities of 5α and 5β-reductases can be inferred from measurement of the ratio THF:5αTHF.
In order to provide a more accurate reflection of hepatic 11β-HSD1 activity, all patients underwent a cortisol generation curve as previously described (18). Briefly, 1mg dexamethasone was taken orally at 24.00hr the night preceding the test. At 08.45hr the patients were cannulated in an antecubital fossa vein, bloods drawn at 09.00 and then an oral dose of cortisone acetate (25mg) given. The subsequent generation of cortisol in the serum was then followed by sampling at 30, 60, 90, 12, 150, 180 and 240 minutes.
Body composition analysis was performed using dual energy X-ray absorptiometry (DEXA) with a total body scanner (Lunar DPX-L, Lunar radiation Corp., Madison, WI). Coefficients of variation for multiple scans were less than 3%. Regional fat mass (trunk and leg) was analyzed as previously described (18).
In addition, all patients had a subcutaneous buttock biopsy performed under local anesthetic, in order to obtain approximately 1-2g of adipose tissue. The sample was divided in to two parts. Half was used for adipocyte isolation and subsequent total RNA extraction and preadipocyte culture and the remaining half was snap frozen in liquid nitrogen and stored at -70°C for whole tissue total RNA extraction (see below). All samples were processed within 30 minutes of the biopsy being taken.
After all investigations had been completed, patients were entered on to a weight loss program using a total meal replacement, VLCD (Lipotrim, Howard Foundation, Cambridge, UK). This diet provides 425 (female) and 559 (male) kcal/day. The median duration of dietary intervention was 10 weeks (range 8-14). Following significant weight loss (>10% initial body weight), all subjects returned to a normal diet, and once re-feeding had been commenced for at least 1 week, all the investigation described above were repeated. Investigations were not repeated sooner so as to avoid the confounding effect that the stress of the hypocaloric diet may have had upon the HPA axis
Adipocyte isolation and pre-adipocyte culture
Adipocytes and preadipocytes were isolated as previously reported (19;20). Briefly, adipose tissue biopsies were washed in PBS containing 50,000 units/l of penicillin and 50,000μg/l streptomycin (GibcoBRL, Paisley, UK). The tissue was then prepared and digested with collagenase class 1 [2 mg/ml] (Worthington Biochemical Co-operation, Reading, U.K.) in 1× Hank’s balanced salt solution (Gibco) for 45 minutes at 37°C. Samples were centrifuged at 90g for 1 minute and the intact adipocyte layer removed and RNA extracted as described below. Samples were then centrifuged at 90g for 5 minutes, the pellet containing pre-adipocytes was removed and cells washed with Dulbecco’s MEM/Nutrient Mixture F-12 (Gibco) containing 15% fetal calf serum (Gibco) and seeded on 96 well plates (Costar, UK). Cells were left overnight and washed the following day with 1× Hank’s balanced salt solution. Proliferation assays were performed on days 1, 4 and 7 of culture (see below).
RNA extraction and reverse transcription
Total RNA was extracted using a single step extraction method (Tri reagent, Sigma, UK [adipocytes], or Genelute total mammalian RNA extraction kit, Sigma, UK [whole adipose tissue]). RNA integrity was assessed by electrophoresis on 1% agarose gels and quantity determined spectrophotometrically at OD260. 1μg of total RNA was initially denatured by heating to 70°C for 5 minutes. 30 units of avian myeloblastososis virus, 200ng of random primers, 20U ribonuclease inhibitor and 40nmol deoxy-NTPs with 5× reaction buffer were added to the RNA and the reverse transcriptase reaction carried out at 37°C for 1 hour. The reaction was terminated by heating the cDNA to 95°C for 5 minutes.
Real-time PCR (RT-PCR)
11β-HSD1 mRNA levels were analyzed using an ABI 7700 sequence detection system (Perkin-Elmer, Biosystems, Warrington, UK), which employs TaqMan chemistry for highly accurate quantification of mRNA levels as previously described (21). RT-PCR was performed in 25μl volumes on 96-well plates, in reaction buffer containing TaqMan universal PCR master mix, 3mM Mn(Oac)2, 200μM dNTPs, 1.25U AmpliTaq Gold polymerase, 1.25U AmpErase UNG, 100-200nmol TaqMan probe, 900nmol primers and 25–50ng cDNA. All reactions were multiplexed with the housekeeping gene (18S), provided as a pre-optimized control probe (Perkin-Elmer) enabling data to be expressed in relation to an internal reference to allow for differences in reverse transcription efficiency. Data were obtained as ct values according to the manufacturer’s guidelines (the cycle number at which logarithmic PCR plots cross a calculated threshold line) and used to determine Δct values (Δct = ct of the target gene minus ct of the housekeeping gene). Fold changes in expression were calculated according to the transformation: Fold increase = 2-difference in Δct. All target gene probes were labelled with the fluorescent label FAM, and the housekeeping gene with the fluorescent label VIC. Reactions were as follows: 50 C for 2 min, 95 C for 10 min, and then 44 cycles of 95 C for 15 sec and 60 C for 1 min.
To exclude potential bias owing to averaging data that had been transformed through the equation 2-ct, all statistics were performed at the ct stage.
Oligonucleotide primers and a taqman probe for 11β-HSD1 were as follows: Forward AGGAAAGCTCATGGGAGGACTAG, reverse ATGGTGAATATCATCATGAAAAAGATTC and probe CATGCTCATTCTCAACCACATCACCAACA.
Preadipocyte proliferation
Preadipocyte proliferation was assessed using a commercially available colorimetric assay for determining the number of viable cells (Promega, UK) as per the manufacturers guidelines with appropriate controls (no cells). Cells were incubated with reagents for 1 hour at 37°C, in a humidified 5% CO2 atmosphere. The absorbance at 490nm reflects the number of living cells and was measured using a 96 well plate reader. Readings were performed in triplicate and the mean no cell control reading was subtracted from the mean of the cell containing wells.
Biochemical assays
Cortisol was assayed using a chemiluminescent immunoassay (Bayer Advia Centaur, Bayer Diagnostics, Newbury, UK) with inter-assay coefficients of variation of 10.2% at 76nmoI/l, 7.7% at 528nmol/l and 7.4% at 882nmol/l. Cortisone was assayed after extraction from serum followed by radioimmunoassay (RIA) of the extract with 125I-Cortisone and Sac-Cel® (IDS ltd., Tyne and Weir, UK) second antibody separation. The coefficient of variation for 10 consecutive assays was less than 15% for values between 50 and 100nmol/l and less than 10% for values over 100nmol/l. Serum insulin was measured using an immunoenzymometric assay with no significant cross-reactivity with pro-insulin(s), calibrated against IRP 66/304 (Medgenix Insulin-EASIA, Biosource UK). Inter-assay coefficients of variation were less than 10% over the range 95-1038pmol/l.
Urea, creatinine and electrolytes, cholesterol and triglycerides were measured using standard laboratory methods (Roche Modular system, Roche Ltd, Lewes, UK). Glucose was assayed using the hexokinase method (Instrumentation Laboratory, Warrington, UK) with inter-assay coefficients of variation of 2.0% at 4.7mmol/l and 1.7% at 33.4mmol/l.
Insulin sensitivity was derived from fasting glucose and insulin data, using the homeostasis model assessment (HOMA) mathematical model for specific insulin assays based on the method previously described (22). (HOMA-R values greater than 1 suggesting insulin resistance).
Statistical analysis
The data are presented as mean±S.E. unless otherwise stated. For comparison of outcomes before and after weight loss, paired t tests have been used. Where multiple comparisons are made, repeated measure ANOVA is used. For real-time PCR data, statistical analysis has been performed on Δct values, rather than fold changes.
Results
Body composition
Treatment with a VLCD resulted in significant weight loss in all subjects. Mean BMI fell from 35.9±0.9 to 30.7±0.7kg/m2, p<0.0001). There were significant reductions in total and regional fat mass as well as smaller reductions in total and regional lean mass. The mean reduction in total fat mass was 9.7±1.5kg and mean reduction in total lean mass was 5.6±0.7kg (table 1). In addition, alterations in body fat distribution were observed. Trunk:leg fat mass ratio fell (1.60±0.15 to 1.34±0.11, p<0.05) indicative of selective central fat loss. Additionally the WHR also fell though this did not reach statistical significance (table 1).
Table 1.
Body composition analysis (total and regional fat and lean mass as measured by DEXA) and anthropometric measurements in 12 obese individuals before and after therapeutic intervention using a very low calorie diet
| Before weight loss | After weight loss | p | |
|---|---|---|---|
| BMI (kg/m2) | 35.9±0.9 | 30.7±0.7 | <0.0001 |
| Total fat mass (kg) | 41.8±1.9 | 32.0±1.7 | <0.0001 |
| Trunk fat mass (kg) | 21.4±1.3 | 15.4±0.7 | <0.001 |
| Trunk:leg fat ratio | 1.60±0.15 | 1.34±0.11 | <0.05 |
| Total lean mass (kg) | 64.4±3.4 | 58.9±2.9 | <0.0001 |
| Trunk lean mass (kg) | 30.6±1.5 | 29.2±1.5 | <0.01 |
| Waist circumference (cm) | 114.0±3.8 | 98.82.7 | <0.0001 |
| Waist:hip ratio | 0.93±0.03 | 0.91±0.03 | 0.18 |
Metabolic profile
Despite significant alterations in body composition, fasting glucose did not change before and after weight loss. However, fasting insulin levels decreased significantly (p<0.01) and insulin resistance decreased as measured by glucose:insulin ratio (p<0.05) and HOMA analysis (p<0.01). Fasting total cholesterol fell, although triglyceride concentrations remained unaltered (table 2).
Table 2.
Fasting metabolic profiles and HOMA-R analysis of insulin sensitivity in 12 obese individuals before and after therapeutic intervention using a very low calorie diet (HOMA-R values greater than 1 suggesting insulin resistance).
| Before weight loss | After weight loss | p | |
|---|---|---|---|
| Glucose (mmol/l) | 5.2±0.1 | 5.2±0.1 | 0.94 |
| Insulin (pmol/l) | 102.9±18.1 | 62.0±10.3 | <0.01 |
| Glucose :insulin ratio | 0.06±0.006 | 0.11±0.02 | <0.05 |
| HOMA-R analysis | 3.3±0.5 | 2.1±0.4 | <0.01 |
| Total cholesterol (mmol/l) | 5.4±0.2 | 4.8±0.2 | <0.05 |
| Triglycerides (mmol/l) | 1.3±0.1 | 1.1±0.1 | 0.21 |
Blood pressure
Systolic blood pressure fell from 135±5 to 121±5mmHg (p<0.01) following weight loss. Although diastolic blood pressure decreased, this failed to reach statistical significance (76±3 vs.72±1mmHg, p=0.20).
Corticosteroid metabolism
Circulating cortisol and cortisone concentrations did not change with weight loss (09:00h cortisol 287±24 vs. 355±36nmol/l, p=0.1; 09:00h cortisone 60±3 vs. 60±5nmol/l, p=0.7). However, 09:00h cortisol:cortisone ratio increased, indicative of a shift in set-point towards cortisol generation consistent with increased 11β-HSD1 activity (table 3). Urinary corticosteroid metabolites analysis using GC/MS demonstrated a significant decrease in total 24-hour production of cortisone metabolites, again consistent with increased 11β-HSD1 reductase activity. In addition we observed a borderline significant decrease in 24h urinary cortisol secretion rate (p=0.06) tetrahydrocortisone (p=0.06). Other corticosteroid metabolites did not change with weight loss (table 3).
Table 3.
Serum cortisol:cortisone ratios and urinary corticosteroid metabolites in 12 obese individuals before and after therapeutic intervention using a very low calorie diet. Total urinary F metabolites (Fm) = F + THF + 5αTHF + α-cortol + β-cortol + 20αDHF + 20βDHF + 6βOH-F. Total urinary E metabolites (Em) = E + THE + α-cortolone + β-cortolone + 20αDHE + 20βDHE. (E = cortisone, F = cortisol, THF = tetrahydrocortisol, THE = tetrahydrocortisone, DHE = dihydrocortisone, DHF = dihydrocortisol, UFE = urinary free cortisone, UFF = urinary free cortisol).
| Before weight loss | After weight loss | p | |
|---|---|---|---|
| 09.00h serum F:E ratio | 5.0±0.4 | 5.7±0.4 | <0.01 |
| 24h cortisol secretion rate (μg/24hr) | 5352±624 | 3794±558 | 0.06 |
| Total urinary F metabolites (Fm) (μg/24hr) | 2306±315 | 1727±343 | 0.2 |
| Total urinary E metabolites (Em) (μg/24hr) | 3046±325 | 2066±290 | <0.05 |
| Total Fm:Em urinary metabolites | 0.75±0.04 | 0.84±0.14 | 0.7 |
| THF (μg/24hr) | 625±100 | 575±91 | 0.8 |
| AlloTHF (μg/24hr) | 961±200 | 685±212 | 0.2 |
| THE (μg/24hr) | 1647±215 | 1099±175 | 0.06 |
| THF + 5αTHF : THE ratio | 1.0±0.08 | 1.1±0.2 | 0.6 |
| THF : 5αTHF | 1.0±0.2 | 1.2±0.2 | 0.1 |
| UFF : UFE | 0.9±0.05 | 0.7±0.07 | 0.1 |
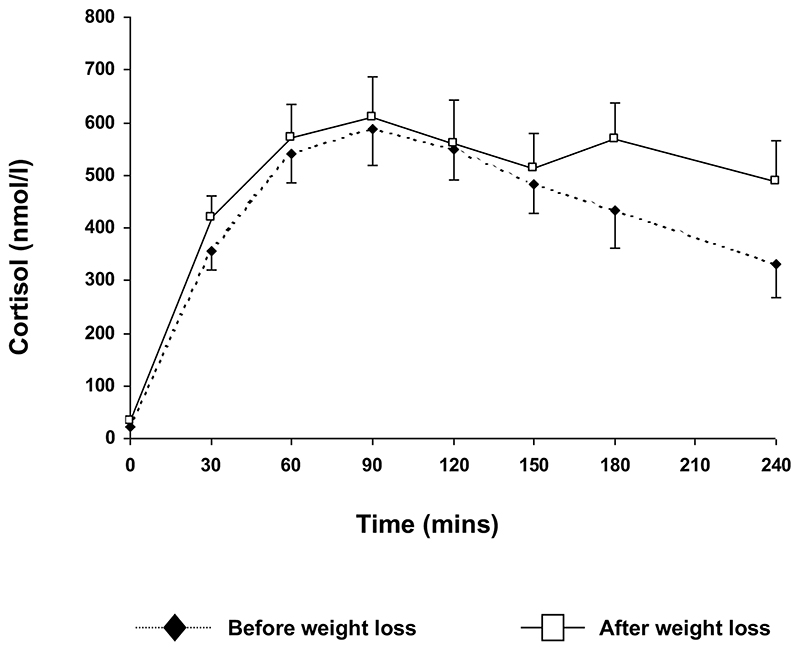
Using repeated measures ANOVA, there was no significant difference in cortisol generation from an oral dose of cortisone acetate at any time point during the test. Area under the curve for cortisol generation was also similar before and after weight loss. (AUC cortisol: 105.5±11.7 vs. 121.5±14.0μmoll-1.min, p=0.26, before vs. after) (figure 1).
Figure 1.
Cortisol generation curves before and after significant weight loss in 12 obese subjects. After overnight dexamethasone suppression (1mg), generation of cortisol in the serum following oral cortisone acetate (25mg) is measured by repeated serum sampling. Cortisol generation does not differ before or after weight loss at any time point.
Adipose tissue expression of 11β-HSD1 and preadipocyte proliferation
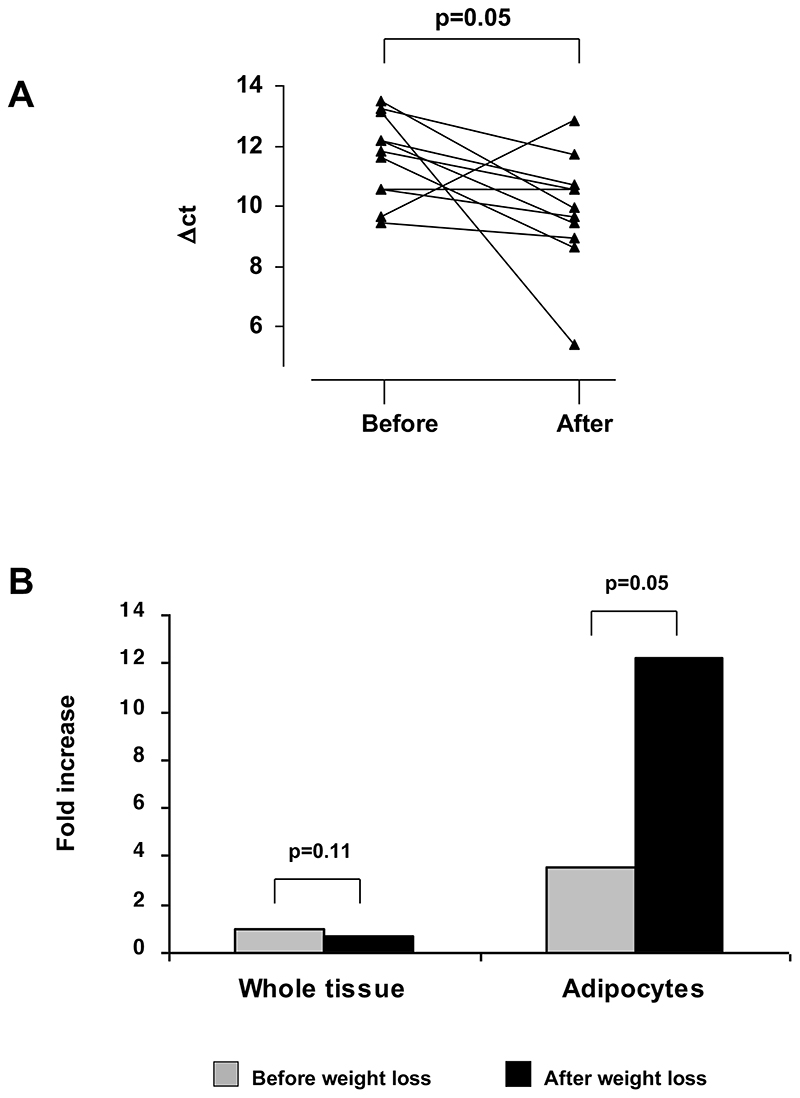
Successful RNA extraction and reverse transcription was performed in 11 patients. Whole adipose tissue expression of 11β-HSD1 did not change with weight loss (13.5±0.2 vs. 14.1±0.3 Δct, p=0.11, before vs. after). However, 11β-HSD1 expression increased in 10 of the 11 patients. Adipocyte 11β-HSD1 expression increased 3.4 fold (11.6±0.4 vs. 9.9±0.6 Δct, p<0.05, before vs. after) (figure 2).
Figure 2.
(A) 11β-HSD1 expression in human adipocytes before and after weight loss in 11 individuals as measured using real-time PCR (Perkin–Elmer UK) (data expressed as Δct, high values representing low expression). (B) Mean whole adipose tissue 11β-HSD1 expression does not alter with weight loss. However, mean adipocyte specific expression increases 3.4 fold.
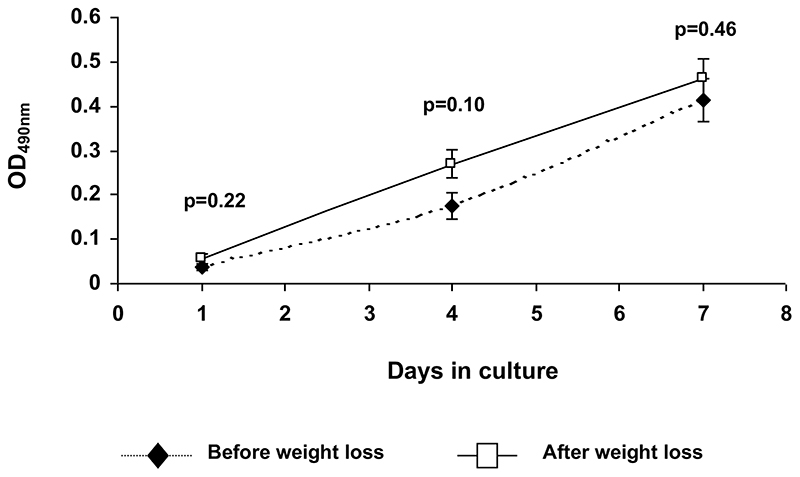
Isolated preadipocytes from subcutaneous buttock biopsies proliferated rapidly in serum containing media. However, there were no significant differences in cell number at any time point before and after weight loss (figure 3). In addition, there was no difference in the number of cell divisions observed over the 7 day period of culture 3.7±0.3 vs. 3.4±0.4, p=0.53, before vs. after).
Figure 3.
Preadipocyte cell number estimation using colourimetric cell proliferation assay (CellTitre96 One Solution Proliferation assay ™, Promega, UK) in subcutaneous buttock biopsies from obese individuals before and after weight loss. Cell number estimations were performed on days 1, 4 and 7 of culture in serum containing media. OD490nm reflects the number of intact viable cells. Cell number does not differ at any time point.
Discussion
In this study, we have characterized cortisol metabolism in obese patients before and after significant weight loss. In addition to the improvements in metabolic profile and blood pressure, we have presented evidence for increased activity and expression of 11β-HSD1 in adipose tissue. Serum cortisol:cortisone ratio increased, total urinary cortisone metabolites decreased, and there was a borderline significant decrease in 24hr cortisol secretion rate and tetrahydrocortisone production. These results are all indicative of a shift in set-point of 11β-HSD1 activity towards the generation of cortisol. Finally at a tissue specific level, adipocyte mRNA expression increased following weight loss. However, we have been unable to show changes in cortisol generation from oral cortisone acetate. This may reflect unchanged hepatic 11β-HSD1 expression and may also explain the lack of change in the urinary THF+alloTHF:THE ratio. In order to allow for the effects of acute stress due to the hypocaloric diet, investigations were performed one week after re-feeding. The aim of the study was to determine the effects of weight loss per se outside the context of acute stress. However, it remains possible that the effects of stress may persist for longer than one week, although we observed no significant difference in 09.00h cortisol before and after weight loss. In addition, we must be cautious not to over-interpret data, this study was designed to characterize changes that occur with weight loss, rather that to compare lean with obese subjects.
The role of 11β-HSD1 in adipocyte biology and in human obesity is still unclear. It is more highly expressed in omental compared with subcutaneous preadipocytes (20). We have postulated that in preadipocytes it may act to limit proliferation (17) and in adipocytes to promote differentiation (4). To support this, in vitro experiments have shown that inhibition of 11β-HSD1 with glycerrhetinic acid blocks the pro-differentiative and anti-proliferative actions of cortisone (4;17). Further evidence for its differentiative role is derived from mice over-expressing 11β-HSD1 under the aP2 promoter that develop obesity as a result of increased adipocyte size due to a tissue specific increase in corticosterone concentrations (5). In this study we were unable to demonstrate significant alterations in preadipocyte proliferation rate following weight loss.
Much of the current literature has discussed the abnormalities of cortisol metabolism as possible causative factors. However, it is equally possible that they arise as a consequence of the obese phenotype. Detailed studies of cortisol metabolism before and after weight loss have not been performed, but circulating levels do not appear to change (23). Cortisol binding globulin (CBG) decreases with weight loss (24). Although not measured in this study, a decrease in CBG would cause a decrease in the cortisol:cortisone ratio, cortisone being essentially unbound to CBG. Alterations in CBG are therefore unlikely to explain the observed changes in circulating glucocorticoids.
Although this study was not designed to determine levels of 11β-HSD1 in obesity, the global inhibition of 11β-HSD1 that has previously been reported in obesity may represent a compensatory mechanism whereby tissue specific cortisol concentrations are reduced in an attempt to improve insulin sensitivity in obese, insulin resistant individuals (7). With significant weight loss this regulatory mechanism is no longer required. Evidence for the role of 11β-HSD1 in the control of insulin sensitivity comes from a wide variety of rodent and clinical studies. Healthy, non-obese, volunteers treated with carbenoxolone to inhibit 11β-HSD activity, display improved insulin sensitivity (25). In addition, in glucose intolerant individuals, 11β-HSD1 activity as measured by cortisol generation curves is impaired (26). One interpretation of this data is that down regulation of 11β-HSD1 activity may represent a mechanism to try and improve insulin sensitivity in a glucose intolerant individual. This hypothesis is also supported by rodent models. The 11β-HSD1 knock-out mouse displays relative insulin sensitivity (27) and in other models of rodent obesity, there is a compensatory decrease in hepatic (28) and adipose tissue (29) 11β-HSD1 expression.
Patients with Apparent Cortisone Reductase Deficiency (ACRD) are unable to activate oral cortisone acetate to cortisol (30) and represent the putative human 11β-HSD1 knockout (31–34). We have recently defined the molecular basis for the disease which is caused by intronic mutations within the HSD11B1 gene that decrease transcription in combination with mutations in hexose-6-phosphate dehydrogenase, an enzyme that is believed to provide NADPH for 11β-HSD1 which is essential for reductase activity (35). If our hypothesis is correct, these patients should be relatively insulin sensitive due to decreased tissue specific cortisol concentrations, however such studies have not been performed.
Selective 11β-HSD1 inhibition remains an exciting therapeutic prospect. These drugs are not available as yet for studies in humans. However, recently a novel class of agents has been described (arylsulfonamidothiazoles) which, unlike the liquorice derivatives, have a greater than 200-fold selectivity for inhibition of 11β-HSD1 rather than 11β-HSD2. Rodents treated with these drugs show significant improvements in insulin sensitivity (36). These drugs therefore have considerable potential as adjunctive insulin sensitizers in the treatment of type 2 diabetes mellitus and their use may extend to patients with the metabolic syndrome. Clinical studies, using gold standard techniques including hyperinsulinemic, euglycemic clamps, specifically and carefully designed to test the hypothesis that modulation of 11β-HSD1 activity may impact upon tissue specific insulin sensitivity are now warranted. Whilst the results from this study may point to this role we must be careful not to over-interpret data. The aim of this study was to characterize the changes in 11β-HSD1 activity and expression that occur with weight loss, rather than to investigate the functional consequences that occur with this change in expression profile. The control of insulin sensitivity is a complex multi-factorial process. The degree to which insulin sensitivity may be improved with 11β-HSD1 inhibition in human studies is not known. A further important consideration when interpreting the data from this study is that all fat depots are not identical and contribute differently to mortality and morbidity (15;37). In this study we have used subcutaneous gluteal adipose tissue. Depot specific patterns of gene expression as well as differing patterns of differentiation in cell culture are well described (38;39).
The mechanism that underpins this regulation of 11β-HSD1 with obesity and weight loss is not clear. 11β-HSD1 is regulated by a wide variety of growth factors, hormones and cytokines in a tissue specific manner (40). With the recognition of adipose tissue as an endocrine organ and with the rapidly increasing list of adipokines, it is possible that a factor produced locally within the adipocyte microenvironment may have an important regulatory role. Large population studies have failed to show associations between intronic microsatelite markers and body mass index (41) and in smaller studies, no coding sequence mutations of the HSD11B1 gene have been identified in patients with abdominal obesity (42). The true mechanism that determines 11β-HSD1 activity in human obesity remains to be defined.
This study has broadened our understanding of the role of pre-receptor cortisol metabolism within adipose tissue. Tissue specific cortisol excess has never been demonstrated in simple human obesity. Indeed, with weight loss there may be a rise in adipose tissue cortisol concentrations reflecting increased adipocyte expression of 11β-HSD1. Whilst we, and others have speculated that its role may be to control preadipocyte proliferation and adipocyte differentiation, it may have an important role in determining tissue specific insulin sensitivity.
References
- 1.Fraser R, Ingram MC, Anderson NH, Morrison C, Davies E, Connell JM. Cortisol effects on body mass, blood pressure, and cholesterol in the general population. Hypertension. 1999;33(6):1364–1368. doi: 10.1161/01.hyp.33.6.1364. [DOI] [PubMed] [Google Scholar]
- 2.Dunkleman SS, Fairhurst B, Plager J, Waterhouse C. Cortisol metabolism in obesity. J Clin Endocrinol Metab. 1964;24:832–841. doi: 10.1210/jcem-24-9-832. [DOI] [PubMed] [Google Scholar]
- 3.Marin P, Darin N, Amemiya T, Andersson B, Jern S, Bjorntorp P. Cortisol secretion in relation to body fat distribution in obese premenopausal women. Metabolism. 1992;41(8):882–886. doi: 10.1016/0026-0495(92)90171-6. [DOI] [PubMed] [Google Scholar]
- 4.Bujalska IJ, Kumar S, Hewison M, Stewart PM. Differentiation of adipose stromal cells: the roles of glucocorticoids and 11beta-hydroxysteroid dehydrogenase. Endocrinology. 1999;140(7):3188–3196. doi: 10.1210/endo.140.7.6868. [DOI] [PubMed] [Google Scholar]
- 5.Masuzaki H, Paterson J, Shinyama H, Morton NM, Mullins JJ, Seckl JR, Flier JS. A transgenic model of visceral obesity and the metabolic syndrome. Science. 2001;294(5549):2166–2170. doi: 10.1126/science.1066285. [DOI] [PubMed] [Google Scholar]
- 6.Rask E, Walker BR, Soderberg S, Livingstone DE, Eliasson M, Johnson O, Andrew R, Olsson T. Tissue-specific changes in peripheral cortisol metabolism in obese women: increased adipose 11beta-hydroxysteroid dehydrogenase type 1 activity. J Clin Endocrinol Metab. 2002;87(7):3330–3336. doi: 10.1210/jcem.87.7.8661. [DOI] [PubMed] [Google Scholar]
- 7.Stewart PM, Boulton A, Kumar S, Clark PM, Shackleton CH. Cortisol metabolism in human obesity: impaired cortisone-->cortisol conversion in subjects with central adiposity. J Clin Endocrinol Metab. 1999;84(3):1022–1027. doi: 10.1210/jcem.84.3.5538. [DOI] [PubMed] [Google Scholar]
- 8.Rask E, Olsson T, Soderberg S, Andrew R, Livingstone DE, Johnson O, Walker BR. Tissue-Specific Dysregulation of Cortisol Metabolism in Human Obesity. J Clin Endocrinol Metab. 2001;86(3):1418–1421. doi: 10.1210/jcem.86.3.7453. [DOI] [PubMed] [Google Scholar]
- 9.Palermo M, Shackleton CH, Mantero F, Stewart PM. Urinary free cortisone and the assessment of 11 beta-hydroxysteroid dehydrogenase activity in man. Clin Endocrinol (Oxf) 1996;45(5):605–611. doi: 10.1046/j.1365-2265.1996.00853.x. [DOI] [PubMed] [Google Scholar]
- 10.Csabi GY, Juricskay S, Molnar D. Urinary cortisol to cortisone metabolites in hypertensive obese children. J Endocrinol Invest. 2000;23(7):435–439. doi: 10.1007/BF03343752. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds RM, Walker BR, Syddall HE, Andrew R, Wood PJ, Whorwood CB, Phillips DI. Altered control of cortisol secretion in adult men with low birth weight and cardiovascular risk factors. J Clin Endocrinol Metab. 2001;86(1):245–250. doi: 10.1210/jcem.86.1.7145. [DOI] [PubMed] [Google Scholar]
- 12.Andrew R, Gale CR, Walker BR, Seckl JR, Martyn CN. Glucocorticoid metabolism and the Metabolic Syndrome: associations in an elderly cohort. Exp Clin Endocrinol Diabetes. 2002;110(6):284–290. doi: 10.1055/s-2002-34591. [DOI] [PubMed] [Google Scholar]
- 13.Andrew R, Phillips DI, Walker BR. Obesity and gender influence cortisol secretion and metabolism in man. J Clin Endocrinol Metab. 1998;83(5):1806–1809. doi: 10.1210/jcem.83.5.4951. [DOI] [PubMed] [Google Scholar]
- 14.Tiosano D, Eisentein I, Militianu D, Chrousos GP, Hochberg Z. 11 beta-Hydroxysteroid dehydrogenase activity in hypothalamic obesity. J Clin Endocrinol Metab. 2003;88(1):379–384. doi: 10.1210/jc.2002-020511. [DOI] [PubMed] [Google Scholar]
- 15.Casassus P, Fontbonne A, Thibult N, Ducimetiere P, Richard JL, Claude JR, Warnet JM, Rosselin G, Eschwege E. Upper-body fat distribution: a hyperinsulinemia-independent predictor of coronary heart disease mortality. The Paris Prospective Study. Arterioscler Thromb. 1992;12(12):1387–1392. doi: 10.1161/01.atv.12.12.1387. [DOI] [PubMed] [Google Scholar]
- 16.Paulmyer-Lacroix O, Boullu S, Oliver C, Alessi MC, Grino M. Expression of the mRNA coding for 11beta-hydroxysteroid dehydrogenase type 1 in adipose tissue from obese patients: an in situ hybridization study. J Clin Endocrinol Metab. 2002;87(6):2701–2705. doi: 10.1210/jcem.87.6.8614. [DOI] [PubMed] [Google Scholar]
- 17.Tomlinson JW, Sinha B, Bujalska I, Hewison M, Stewart PM. Expression of 11beta-hydroxysteroid dehydrogenase type 1 in adipose tissue is not increased in human obesity. J Clin Endocrinol Metab. 2002;87(12):5630–5635. doi: 10.1210/jc.2002-020687. [DOI] [PubMed] [Google Scholar]
- 18.Stewart PM, Boulton A, Kumar S, Clark PM, Shackleton CH. Cortisol metabolism in human obesity: impaired cortisone-->cortisol conversion in subjects with central adiposity. Journal of Clinical Endocrinology & Metabolism. 1999;84(3):1022–1027. doi: 10.1210/jcem.84.3.5538. [DOI] [PubMed] [Google Scholar]
- 19.Zhang HH, Kumar S, Barnett AH, Eggo MC. Ceiling culture of mature human adipocytes: use in studies of adipocyte functions. J Endocrinol. 2000;164(2):119–128. doi: 10.1677/joe.0.1640119. [DOI] [PubMed] [Google Scholar]
- 20.Bujalska IJ, Kumar S, Stewart PM. Does central obesity reflect “Cushing’s disease of the omentum”? Lancet. 1997;349(9060):1210–1213. doi: 10.1016/S0140-6736(96)11222-8. [DOI] [PubMed] [Google Scholar]
- 21.McTernan CL, Draper N, Nicholson H, Chalder SM, Driver P, Hewison M, Kilby MD, Stewart PM. Reduced placental 11beta-hydroxysteroid dehydrogenase type 2 mRNA levels in human pregnancies complicated by intrauterine growth restriction: an analysis of possible mechanisms. J Clin Endocrinol Metab. 2001;86(10):4979–4983. doi: 10.1210/jcem.86.10.7893. [DOI] [PubMed] [Google Scholar]
- 22.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 23.Yashkov YI, Vinnitsky LI, Poroykova MV, Vorobyova NT. Some hormonal changes before and after vertical banded gastroplasty for severe obesity. Obes Surg. 2000;10(1):48–53. doi: 10.1381/09608920060674111. [DOI] [PubMed] [Google Scholar]
- 24.Yanovski JA, Yanovski SZ, Gold PW, Chrousos GP. Differences in corticotropin-releasing hormone-stimulated adrenocorticotropin and cortisol before and after weight loss. J Clin Endocrinol Metab. 1997;82(6):1874–1878. doi: 10.1210/jcem.82.6.3998. [DOI] [PubMed] [Google Scholar]
- 25.Walker BR, Connacher AA, Lindsay RM, Webb DJ, Edwards CR. Carbenoxolone increases hepatic insulin sensitivity in man: a novel role for 11-oxosteroid reductase in enhancing glucocorticoid receptor activation. J Clin Endocrinol Metab. 1995;80(11):3155–3159. doi: 10.1210/jcem.80.11.7593419. [DOI] [PubMed] [Google Scholar]
- 26.Andrews RC, Herlihy O, Livingstone DE, Andrew R, Walker BR. Abnormal cortisol metabolism and tissue sensitivity to cortisol in patients with glucose intolerance. J Clin Endocrinol Metab. 2002;87(12):5587–5593. doi: 10.1210/jc.2002-020048. [DOI] [PubMed] [Google Scholar]
- 27.Kotelevtsev Y, Holmes MC, Burchell A, Houston PM, Schmoll D, Jamieson P, Best R, Brown R, Edwards CR, Seckl JR, Mullins JJ. 11beta-hydroxysteroid dehydrogenase type 1 knockout mice show attenuated glucocorticoid-inducible responses and resist hyperglycemia on obesity or stress. Proc Natl Acad Sci U S A. 1997;94(26):14924–14929. doi: 10.1073/pnas.94.26.14924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Nakagawa Y, Wang Y, et al. Leptin activation of corticosterone production in hepatocytes may contribute to the reversal of obesity and hyperglycemia in leptin-deficient ob/ob mice. Diabetes. 2003;52(6):1409–1416. doi: 10.2337/diabetes.52.6.1409. [DOI] [PubMed] [Google Scholar]
- 29.Morton NM, Patterson JM, Masuzaki H, Holmes MC, Staels B, Fievet C, Walker BR, Flier JS, Mullins JJ, Seckl JR. Reduced intra-adipose glucocorticoid regeneration: A novel adaptive response to, and therapy for, the metabolic syndrome. Endocrine Abstracts. 2003;5:S23. (Abstract) [Google Scholar]
- 30.Jamieson A, Wallace AM, Andrew R, et al. Apparent cortisone reductase deficiency: a functional defect in 11beta-hydroxysteroid dehydrogenase type 1. J Clin Endocrinol Metab. 1999;84(10):3570–3574. doi: 10.1210/jcem.84.10.6031. [DOI] [PubMed] [Google Scholar]
- 31.Laing I, Adams JE, Wood PJ, Taylor NF, Ray DW. Cortisone reductase deficiency (11 beta-hydroxysteroid dehydrogenase type 1) deficiency presenting with features of late onset congenital adrenal hyperplasia. Endocrine Abstracts. 2002;3:P264. (Abstract) [Google Scholar]
- 32.Biason-Lauber A, Suter SL, Shackleton CH, Zachmann M. Apparent cortisone reductase deficiency: a rare cause of hyperandrogenemia and hypercortisolism. Horm Res. 2000;53(5):260–266. doi: 10.1159/000023577. [DOI] [PubMed] [Google Scholar]
- 33.Nordenstrom A, Marcus C, Axelson M, Wedell A, Ritzen EM. Failure of cortisone acetate treatment in congenital adrenal hyperplasia because of defective 11beta-hydroxysteroid dehydrogenase reductase activity. J Clin Endocrinol Metab. 1999;84(4):1210–1213. doi: 10.1210/jcem.84.4.5584. [DOI] [PubMed] [Google Scholar]
- 34.Phillipov G, Palermo M, Shackleton CH. Apparent cortisone reductase deficiency: a unique form of hypercortisolism. J Clin Endocrinol Metab. 1996;81(11):3855–3860. doi: 10.1210/jcem.81.11.8923828. [DOI] [PubMed] [Google Scholar]
- 35.Draper N, Walker EA, Bujalska IJ, Tomlinson JW, Chalder SM, Arlt W, Lavery GG, Bedendo O, Ray DW, Laing I, Malunowicz E, et al. Mutations in the genes encoding 11beta-hydroxysteroid dehydrogenase type 1 and hexose-6-phosphate dehydrogenase interact to cause cortisone reductase deficiency. Nat Genet. 2003;34(4):434–439. doi: 10.1038/ng1214. [DOI] [PubMed] [Google Scholar]
- 36.Barf T, Vallgarda J, Emond R, et al. Arylsulfonamidothiazoles as a New Class of Potential Antidiabetic Drugs Discovery of Potent and Selective Inhibitors of the 11beta-Hydroxysteroid Dehydrogenase Type 1. J Med Chem. 2002;45(18):3813–3815. doi: 10.1021/jm025530f. [DOI] [PubMed] [Google Scholar]
- 37.Fontbonne A, Thibult N, Eschwege E, Ducimetiere P. Body fat distribution and coronary heart disease mortality in subjects with impaired glucose tolerance or diabetes mellitus: the Paris Prospective Study, 15-year follow-up. Diabetologia. 1992;35(5):464–468. doi: 10.1007/BF02342445. [DOI] [PubMed] [Google Scholar]
- 38.Montague CT, Prins JB, Sanders L, Zhang J, Sewter CP, Digby J, Byrne CD, O’Rahilly S. Depot-related gene expression in human subcutaneous and omental adipocytes. Diabetes. 1998;47(9):1384–1391. doi: 10.2337/diabetes.47.9.1384. [DOI] [PubMed] [Google Scholar]
- 39.Hauner H, Entenmann G. Regional variation of adipose differentiation in cultured stromal-vascular cells from the abdominal and femoral adipose tissue of obese women. Int J Obes. 1991;15(2):121–126. [PubMed] [Google Scholar]
- 40.Tomlinson JW, Moore J, Cooper MS, Bujalska I, Shahmanesh M, Burt C, Strain A, Hewison M, Stewart PM. Regulation of expression of 11beta-hydroxysteroid dehydrogenase type 1 in adipose tissue: tissue-specific induction by cytokines. Endocrinology. 2001;142(5):1982–1989. doi: 10.1210/endo.142.5.8168. [DOI] [PubMed] [Google Scholar]
- 41.Draper N, Echwald SM, Lavery GG, et al. Association studies between microsatellite markers within the gene encoding human 11beta-hydroxysteroid dehydrogenase type 1 and body mass index, waist to hip ratio, and glucocorticoid metabolism. J Clin Endocrinol Metab. 2002;87(11):4984–4990. doi: 10.1210/jc.2001-011375. [DOI] [PubMed] [Google Scholar]
- 42.Caramelli E, Strippoli P, Di Giacomi T, Tietz C, Carinci P, Pasquali R. Lack of mutations of type 1 11beta-hydroxysteroid dehydrogenase gene in patients with abdominal obesity. Endocr Res. 2001;27(1-2):47–61. doi: 10.1081/erc-100107169. [DOI] [PubMed] [Google Scholar]