Abstract
Purpose of review
Substantial progress has been made recently on the development of new therapeutics for cryptosporidiosis, an infection by the protozoan parasite Cryptosporidium that is associated with diarrhea, malnutrition, growth stunting, cognitive deficits, and oral vaccine failure in children living in low-resource settings.
Recent findings
Various drug discovery approaches have generated promising lead candidates. The repurposed antimycobacterial drug clofazimine was tested in Malawian HIV patients with cryptosporidiosis but was ineffective. Target-based screens identified inhibitors of lysyl-tRNA synthetase, phenylalanyl-tRNA synthetase, methionyl-tRNA synthetase, and calcium-dependent protein kinase 1. Phenotypic screens led to discovery of a phosphatidylinositol 4-kinase inhibitor, the piperazine MMV665917, and the benzoxaborole AN7973. The relationship between pharmacokinetic properties and in-vivo efficacy is gradually emerging. A pathway to clinical trials, regulatory approval, and introduction has been proposed but additional work is needed to strengthen the route.
Summary
Several lead compounds with potent activity in animal models and a favorable safety profile have been identified. A sustained effort will be required to advance at least one to clinical proof-of-concept studies. The demonstrated risk of resistance indicates multiple candidates should be advanced as potential components of a combination therapy.
Keywords: cryptosporidiosis, Cryptosporidium, diarrhea, drug development, low-resource settings
INTRODUCTION
Infection by the protozoan intestinal parasite Cryptosporidium spp. is a leading cause of diarrheal disease morbidity and mortality [1,2]. Recent estimates suggest Cryptosporidium spp. is responsible for approximately 7.6 million cases and between 48 000 and 202 000 deaths annually among young children in low-resource settings [3,4]. Beyond acute morbidity and mortality, both symptomatic and asymptomatic infections are associated with long-term sequelae including growth stunting, malnutrition, and cognitive development deficits [5,6]. Sporadic outbreaks from contaminated water supplies are common even in high-income countries [7]. Cryptosporidium hominis and anthroponotic Cryptosporidium parvum are most commonly identified in humans; person-to-person is the predominant mode of transmission [8], although zoonotic species are occasionally found in humans.
Despite the substantial disease burden caused by Cryptosporidium, treatment options remain limited. The only treatment approved by a stringent regulatory authority (the US Food and Drug Administration) is nitazoxanide, a drug that is efficacious in otherwise healthy adults but has marginal efficacy in malnourished children and is no better than placebo in immunocompromised HIV-positive patients [9]. Another limitation is that nitazoxanide is not approved for children younger than 12 months, the most vulnerable patient population. In the absence of highly effective specific therapy and a simple, point-of-care diagnostic test [10], in most low-resource settings, cryptosporidiosis is treated symptomatically with either oral or intravenous rehydration. A target product profile and use-cases of an ideal anticryptosporidial therapeutic have been proposed [11,12▪▪].
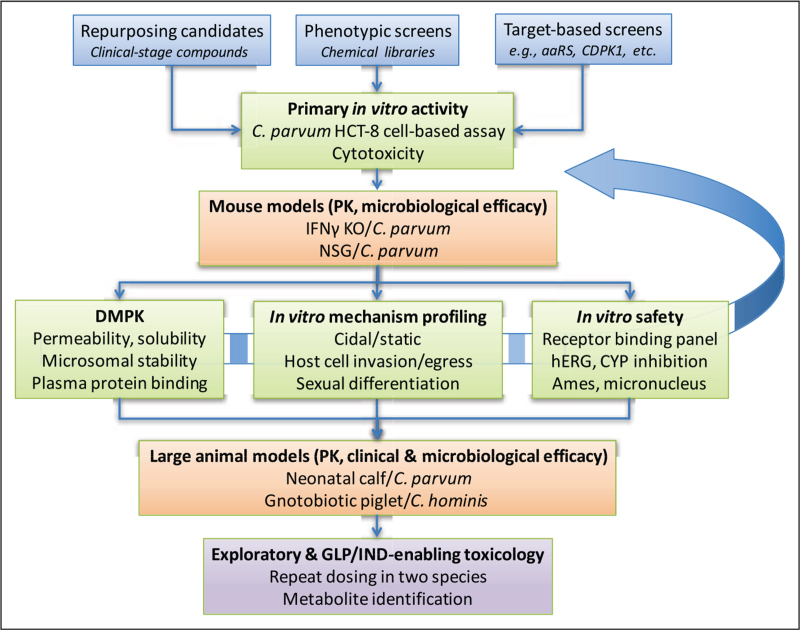
As Cryptosporidium emerged as a major contributor to the global diarrheal disease burden in the past decade, a number of academic and industrial groups have advanced promising new chemical entities (NCEs) through a rigorous screening cascade (Fig. 1). The past 2 years have seen substantial progress with development of many NCEs for cryptosporidiosis, thus the purpose of this review is to provide an overview of these projects.
FIGURE 1.
Screening cascade for cryptosporidiosis therapeutic candidates. Starting compounds can be identified from collections of clinical-stage compounds, phenotypic screening of chemical libraries, or screens of previously characterized or rationally selected targets. Regardless of the source, compounds are first screened for activity in a standardized cell-based assay such as C. parvum infection of HCT-8 cells. Hits are counterscreened for cytotoxic effects and typically a 20-fold or higher margin is required for progression. The next step is characterizing both PK and microbiological efficacy as determined by reduction in oocyst shedding in a mouse model. Compounds with in-vivo activity are then characterized for several parameters in parallel, including DMPK properties, in-vitro safety assays, and further in-vitro profiling, such as for activity at specific stages of the parasite lifecycle. This may involve an iterative process of repeated medicinal chemistry optimization and retesting with in-vitro and in-vivo assays (curved arrow). Only compounds with confirmed drug-like properties are tested in expensive and limited-capacity large animal models for pharmacokinetics and clinical (reduction in diarrheal stool output) and microbiological efficacy. Only compounds with demonstrated large animal efficacy would be advanced to exploratory (non-GLP) toxicology studies to de-risk and then GLP toxicology to enable IND-filing and clinical testing. aaRS, aminoacyl-tRNA synthetase; CDPK1, calcium dependent protein kinase 1; CYP, cytochrome P450; DMPK, drug metabolism and pharmacokinetics; GLP, Good Laboratory Practices; hERG, human Ether-a-go-go-Related Gene; IFNγ KO, interferon gamma knockout; IND, Investigational New Drug application; NSG, NOD SCID gamma; PK, pharmacokinetics.
Box 1.
no caption available
PIPELINE OF EMERGING CRYPTOSPORIDIOSIS THERAPEUTICS
In this review, we discuss recent advancements around seven candidates in development as cryptosporidiosis-specific therapeutics. These compounds emerged from either a target-based approach of a known or validated target in other pathogens, or a phenotypic whole-cell screen against Cryptosporidium parasites with a curated compound library (Table 1).
Table 1.
Cryptosporidiosis therapeutic candidates
| In-vitro activity | In-vivo efficacy | Pharmacokinetic profile | |||||||||||||||
| Lead compound | Development phase | Target | C. parvum (Iowa) EC50 (μmol/l) | C. hominis (TU502) EC50 (μmol/l) | Enzymatic IC50 (μmol/l) | IFNγ KO mouse | NSG mouse | Dairy calf | GB piglet | Human | Dose | C max | AUC0–last | F% | T1/2 | Vdss | Comments |
| Nitazoxanide | Launched | CpPFOR; possible modulation of host interferons | 2.84 ± 1.90 | 2.82 ± 0.45 | NA | No | No | No | Efficacious at 100 mg b.i.d. for 10 days | Yes in immunocompetent individuals | 100 mg single dose (oral suspension, children 1–3 years) | 3.11 μg/ml | 11.7 μg·h/l | NR | 1.03–1.6 h | NR | All reported parameters are based on the metabolite, tizoxanide; tizoxanide is highly protein bound (>99%) |
| Clofazimine | Clinical (phase IIa) | Unknown | 0.0149 ± 0.0085 | 0.341 ± 0.302 | NA | Efficacious at 10 mg/kg q.d. | No | Weakly active at 30 mg/kg (unpublished) | ND | No | 100 mg Lamprene (day 5; parts A and B in clinical trial) | 280.7 ng/ml (part A); 514.1 ng/ml (part B) | 6863 ng·h/ml (part A); 11 298 ng·h/ml (part B) | NR | 336.5 h (part A); 535.5 h (part B) | NR | Low oral bioavailability; high lipophilicity and permeability in GI tract; clinical trial data showed about two-fold less plasma exposure in participants without diarrhea (part B); less than 2% of the cumulative CFZ doses was recovered in stool in both groups over the 5 days of stool collection |
| KDU731; CpPI4K-SD Lead | Preclinical | CpPI(4)K | 0.063 ± 0.028 | 0.130 ± 0.074 | 0.025 ± 0.004 | Efficacious at 10 mg/kg q.d. | ND | Efficacious at 5 mg/kg b.i.d. | ND | ND | KDU731: 2.3 mg/kg (oral, mouse); 5 mg/kg (i.v., mouse); 5 mg/kg (oral, calf) | 406 nmol/l (oral, mouse); 228 nmol/l (calf) | 2306 nmol/l·h (oral, mouse); 1909 nmol/l·h (calf) | 37% (oral, mouse) | 2.47 h (oral, mouse) | 1.12 l/kg (i.v., mouse) | No correlation between efficacy and plasma exposure |
| BKI-1708 | Preclinical | CpCDPK1 | 0.41 | NR | 0.0007 | Efficacious at 8 mg/kg b.i.d. (BKI-1708); many others | Efficacious at 10 mg/kg b.i.d. (BKI-1553); others | Efficacious at 5 mg/kg b.i.d. (BKI-1369); others | Efficacious at 10 mg/kg b.i.d. (BKI-1369) | ND | BKI-1708: 10 mg/kg (oral, mouse) | 2.9 μM | 247.1 μmol·min/l | NR | 42.6 min | NR | Varied PK/PD across three series scaffolds; GI exposure necessary for efficacy; no correlation with plasma exposure |
| AN7973 | Discovery (late lead) | CpCPSF3 (putative) | 0.13–0.43 | 0.63 | NA | Efficacious at 10 mg/kg q.d. | Efficacious at 10 mg/kg q.d. | Efficacious at 10 mg/kg q.d. | ND | ND | 10 mg/kg (oral, mouse); 5 mg/kg (oral, calf) | 8.63 μg/ml (mouse); 3.57 μg/mL (calf) | 92.7 μg·h/ml (mouse); 190 μg·h/ml (calf) | 37% (mouse) | 6.6 h (mouse); 31 h (calf) | NR | Half life ∼5× greater in calves than mice; high concentrations found in feces |
| Compound 2093 | Discovery (late lead) | CpMetRS | 0.006–0.029 | 0.015 | 0.0009 ± 0.0004 | Efficacious at 25 mg/kg b.i.d. | Efficacious at 50 mg/kg b.i.d. | Initial efficacy, then resistance observed in calf model at 15 mg/kg b.i.d. | ND | ND | 50 mg/kg (oral, mouse) | 5.8 μM | 1863 μmol·min/l | NR | NR | NR | Calf PK and efficacy studies: plasma and fecal levels >3× EC90 for over 24 h |
| MMV665917 | Discovery (late lead) | Unknown | 1.9–2.3 | 4.1 | NA | Efficacious at 30 mg/kg b.i.d. | Efficacious at 30 mg/kg b.i.d. | Efficacious at 22 mg/kg q.d. | Efficacious at 20 mg/kg b.i.d. | ND | 55 mg/kg (oral, mouse); 22 mg/kg (oral, calf); 10 and 20 mg/kg (oral, piglet) | NR | NR | NR | NR | NR | PK in healthy mice: high fecal and plasma concentrations with sustained exposure; PK from infected calf model: sustained fecal and serum concentrations >3× EC90; PK from infected piglet model: plasma exposure remained >3× EC90; gut contents showed concentrations > EC90 60 h after treatment ended |
| Compound 5 | Discovery (late lead) | CpKRS | 1.3 | 6.0 | 0.13 | Efficacious at 20 mg/kg q.d. | Efficacious at 20 mg/kg q.d. | ND | ND | ND | 10 mg/kg (oral, mouse); 3 mg/kg (i.v., mouse) | 5.4 μg/mL (oral) | 1300–3000 μg·min/ml (oral) | 100% | 2.5 h (i.v.) | 1 l/kg (i.v.) | Very high oral bioavailability |
| BRD7929 | Discovery (lead Op) | CpPheRS | 0.008–0.073 | 0.010 | 0.060 (ChPheRS) | ND | Efficacious at 10 mg/kg q.d. | ND | ND | ND | 1 mg/kg (oral, mouse); 0.6 mg/kg (i.v., mouse) | NR | NR | 80% | 32 h (i.v.) | 29 l/kg | BRD7929 has high oral bioavailability, volume of distribution, and solubility; compounds in series with higher bioavailability had better efficacy, possibly because of permeability |
AUC0–last, area under the curve; time-averaged concentration of drug in plasma; Cmax, maximum or peak serum (plasma) concentration of a drug after a single dose; F%, oral bioavailability; GB piglet, gnotobiotic piglet model (C. hominis); T1/2, half-life in plasma; Vdss, volume of distribution at steady state.
Candidates from target-based screens
Although target-based therapeutic screening has historically been perceived to be challenging in Cryptosporidium as it appropriates many essential metabolites from host cells, a number of promising candidates have emerged from target-based screens. One class is inhibitors of aminoacyl-tRNA synthetases (aaRSs) – enzymes that charge amino acids for polypeptide synthesis, and thus tend to be essential for growth. Three will be considered in detail below, along with calcium-dependent protein kinase 1 (CDPK1).
Lysyl-tRNA synthetase
Lysyl-tRNA synthetase (KRS) was originally identified as the target of cladosporin in Plasmodium falciparum. On the basis of structural homology, P. falciparum KRS (PfKRS) inhibitors were tested on C. parvum KRS (CpKRS) and a consortium of investigators led by University of Dundee recently published a comprehensive study of early lead compounds (subsequently referred to in this review as ‘leads’) targeting both enzymes [13]. A potent chromone compound was reported with excellent drug-like properties (Table 1). Because of toxicity observed in rodents, this early lead was deprioritized; however, it provided important validation of KRS as a drug target. Subsequent chemical refinement has identified further optimized leads with greater potency and less toxicity that will be tested in the neonatal calf efficacy model (B. Baragaña, personal communication). The early lead chromone has high solubility and oral bioavailability (Table 1), which likely contribute to its high systemic exposure. However, a later lead compound retained efficacy in mouse models with relatively lower systemic exposure. The basis for this disconnect is under investigation. Molecular modeling comparing PfKRS and CpKRS with human KRS suggested a potential mechanism for selectivity, which would reduce risks of on-target toxicity.
Phenylalanyl-tRNA synthetase
Like KRS, Phenylalanyl-tRNA synthetase (PheRS) was also initially validated as a drug target for P. falciparum and then subsequently explored as a target for Cryptosporidium by a consortium of academic and industrial collaborators. In an effort led by the Broad Institute, a curated set of compounds with previously established antimicrobial activity and known mechanisms of action (MoA) was screened, and a bicyclic azetidine with potent activity against C. parvum in-vitro (Table 1) was identified [14▪]. Homology with PfPheRS was beneficial in rapidly establishing a structure--activity relationship for CpPheRS inhibitors and identifying a range of compounds with varying pharmacokinetic and physiochemical properties, including differences in oral bioavailability, volume of distribution, metabolic half-life, and solubility. Several compounds with a range of properties were then tested in-vivo using the NOD SCID gamma (NSG) mouse model and a direct relationship between higher bioavailability and greater in-vivo activity was demonstrated. As a confirmation of the MoA of bicyclic azetidines on PheRS, CRISPR-Cas9 was used to introduce a resistance-conferring mutation found in PfPheRS into CpPheRS, which afforded resistance to BRD7929, a representative compound.
Methionyl-tRNA synthetase
Exploration of Methionyl-tRNA synthetase (MetRS) inhibitors for cryptosporidiosis arose from a program at the University of Washington investigating this enzyme as a target for other pathogens including Trypanosoma brucei. Crystal structures of the TbMetRS bound to inhibitors were leveraged to identify the homologous binding sites in C. parvum and C. hominis MetRS, which retain 76% identity to T. brucei. Several TbMetRS inhibitors were shown to potently inhibit CpMetRS in-vitro and suppress oocyst shedding in both the IFNγ KO and NSG mouse models [15▪]. Pharmacokinetic analysis of CpMetRS inhibitors found the compounds with highest activity in mouse models had both high systemic exposure (Table 1) and high levels in feces (>10 μmol/l), therefore, it was not possible to discern whether systemic or intestinal luminal exposure is more important for activity with this compound class. In the neonatal calf efficacy model [16], lead compound 2093 initially strongly suppressed oocyst shedding and diarrheal symptoms within the first 4 days postinfection, but then diarrhea and oocyst shedding rebounded in two of three animals. Subsequent sequencing of fecal samples revealed the acquisition of either a D243E or T246I mutation in CpMetRS. Structural modeling indicated these mutations disrupted compound binding to CpMetRS. In-vitro studies with recombinant enzymes containing these mutations were more than 170-fold less sensitive to inhibition by compound 2093, and C. parvum parasites engineered with either mutation via CRISPR-Cas9 were found to be 613-fold (D243E) or 128-fold (T246I) less sensitive to compound 2093. These results demonstrate that resistance to CpMetRS inhibition arose rapidly in-vivo and necessitate caution for future development and introduction of cryptosporidiosis therapeutics.
Calcium-dependent protein kinase 1
Calcium-dependent protein kinases (CDPKs) are an attractive drug target for apicomplexan diseases as they are essential and have no analogous proteins in mammals [17]. Bumped kinase inhibitors (BKIs) are ATP-competitive inhibitors of CDPKs and named for a structural bump that prevents binding in kinases with larger ‘gatekeeper’ residues in the ATP-binding pocket [18]. Researchers at the University of Washington discovered that a selection of Toxoplasma gondii CDPK1 (TgCDPK1) BKI compounds also inhibit CpCDPK1 [19]. Medicinal chemistry efforts over the past decade have resulted in several hundred BKI compounds displaying a range of pharmacokinetic/pharmacodynamic properties and antiparasitic activity [20], with the majority represented by three scaffolds: pyrrolopyrimidine (PrP), pyrazolopyrimidine, and 5-aminopyrazole-4-carboximide. Early leads showed good efficacy in mouse, calf, and gnotobiotic piglet models of cryptosporidiosis, though this series of compounds (subsequently referred to in this review as ‘series’) has faced challenges related to cardiovascular toxicity, teratogenicity, and varying efficacy because of the differing pharmacokinetic/pharmacodynamic parameters across scaffolds [21,22].
Due to ongoing toxicity liabilities in the pyrazolopyrimidine and PrP scaffolds [23], the most promising candidates have come from the 5-aminopyrazole-4-carboximide scaffold. A recent report identified two 5-aminopyrazole-4-carboximide -scaffold preclinical leads (BKI-1708 and BKI-1770) with good in-vitro potency against both the CpCDPK1 enzyme and cells, minimal human Ether-a-go-go Related Gene (hERG) activity, and good efficacy and safety in a mouse model of cryptosporidiosis (Table 1) [24]. Within the 5-aminopyrazole-4-carboximide scaffold series, high solubility but not high plasma or fecal exposure correlated with in-vivo efficacy. Both BKI-1708 and BKI-1770 show good in-vivo efficacy and alleviate many of the toxicity and safety liabilities of the BKI series, and warrant further study as preclinical candidates.
Candidates from phenotypic screening efforts
Another approach for finding new cryptosporidiosis therapeutics is phenotypic screening. Phenotypic screens are less biased and not dependent on a known target, and several emerging therapeutics for cryptosporidiosis were discovered from screening compound collections with known activity in other pathogens against Cryptosporidium spp. [25]. Phenotypic screens may identify compounds with novel targets or pathways, with the caveat that target identification and MoA studies are often needed after active compounds are discovered. There have been a number of additional phenotypic screens for Cryptosporidium drug discovery [25,26], though the compounds discussed in this review are the most advanced.
Phosphatidylinositol 4-kinase 4 inhibitors
Pyrazolopyridine inhibitors of Phosphatidylinositol 4-kinase 4 (PI(4)K) (a validated malaria drug target) were discovered from a phenotypic screen of 6220 parasite actives. Lead compound KDU731 was shown to have potent in-vitro activity against C. parvum and C. hominis, excellent efficacy in mouse and calf models of cryptosporidiosis (Table 1) and demonstrated safety in various in-vitro tests and a rat toxicology study [27]. Further in-vitro activity profiling showed that a related analog, KDU691, was parasiticidal at its EC90, and its MoA was impediment of merozoite formation [28], likely because of impairment of lipid membrane processing from inhibition of CpPI(4)K. Interestingly, pharmacokinetics of KDU731 in C. parvum-infected calves showed limited systemic exposure, indicating that it may not be required for clinical efficacy in this series.
MMV665917
A phenotypic high-content imaging screen of the Medicines for Malaria Venture (MMV) Malaria Box against C. parvum conducted by researchers at the University of Vermont discovered MMV665917, a novel piperazine-based compound that is specific for Cryptosporidium parasites and blood-stage Plasmodium spp. [29]. Although the molecular target is unknown, in-vitro time of action assays indicate it may affect the transition between asexual and sexual life stages [28,30]. Excitingly, this molecule shows efficacy in both the IFNγ KO and NSG mouse models of cryptosporidiosis, as well as in the gnotobiotic piglet and dairy calf models [31,32]. MMV665917 pharmacokinetic studies in uninfected neonatal calves showed high exposures in both the serum and feces (Table 1), though it is unclear if exposure in both compartments is necessary for in-vivo efficacy. The compound was well tolerated in animals, and no significant organ toxicity was observed; however, MMV665917 partially inhibits hERG, indicating a potential for cardiac toxicity. It is possible that medicinal chemistry efforts can reduce the hERG inhibition potential of this scaffold while retaining the excellent anticryptosporidial activity, and the identification of the molecular target could greatly aid in these efforts.
AN7973
Another molecule that has emerged from a phenotypic screen of a focused collection of malaria active compounds is the benzoxaborole AN7973. Similar to MMV665917, AN7973 does not have a confirmed target or MoA but it has potent in-vitro activity against both C. parvum and C. hominis, and shows efficacy in both the IFNγ KO and NSG mouse models as well as the calf model of cryptosporidiosis [28,33]. In pharmacokinetic studies of AN7973 in mice and dairy calves, the compound displayed high plasma exposure, a long half-life (Table 1), high fecal exposure, and was well tolerated in rodents and calves. The putative target is Cleavage and Polyadenylation Specific Factor 3 (CPSF3) based on the inhibition of PfCPSF3 and TgCPSF3 by related benzoxaborole compounds and the shared catalytic core homology of this enzyme between these apicomplexan parasites [34], though more work is needed to confirm this as the target of AN7973 and related compounds in Cryptosporidium.
Pharmacokinetic/pharmacodynamic drivers of in-vivo efficacy
To understand the ideal pharmacokinetic profile for an anti-Cryptosporidium drug, researchers have explored various approaches including empirical observations of systemic and intestinal exposure, physiologically based pharmacokinetic modeling [35], and consideration of efflux pumps [36]. Although a broadly applicable profile remains elusive, in some cases, such as the BKIs and PI(4)K inhibitors, compound concentrations in intestinal epithelial cells seem to drive efficacy. In contrast, with PheRS inhibitors, systemic concentrations are most closely correlated with efficacy. Further work is needed to determine whether more broad observations can be made, or whether drivers are specific to individual targets, MoAs, or chemical series. Having clearly defined physiochemical and pharmacokinetic properties that drive in-vivo and clinical efficacy for cryptosporidiosis therapeutics would help to prioritize emerging scaffolds and allow for efficient allocation of resources within drug discovery programs.
CLINICAL TRIAL OF CLOFAZIMINE AND LESSONS FOR FUTURE PROOF-OF-CONCEPT STUDIES
A repurposing candidate that emerged from a phenotypic screen, clofazimine (CFZ) [37], was quickly advanced into a clinical trial to assess its safety and efficacy in HIV-infected patients with cryptosporidiosis [38▪]. This phase 2a randomized, double-blind, placebo-controlled study had two parts: part A had primary outcomes of safety, pharmacokinetics, and reduction in Cryptosporidium oocyst shedding; part B was an open-labeled study comparing the pharmacokinetics of matched HIV-positive individuals without cryptosporidiosis (or diarrhea). The study faced many unexpected challenges with study initiation, population, implementation, and cultural issues [39▪▪], though the sponsors were able to find solutions to successfully reach the endpoint. The study found treatment with CFZ had no significant impact on Cryptosporidium oocyst shedding, diarrheal episodes, stool weight, or consistency scores. Of note, the part A participants (with diarrhea) had about two-fold less plasma exposure of CFZ as compared with part B participants (no diarrhea), though no conclusions could be made whether this impacted the lack of efficacy. Although the results of the study do not support the efficacy of CFZ for treatment of cryptosporidiosis in a severely immunocompromised HIV population, this trial served to lay essential groundwork for future human studies to assess the efficacy of potential new anticryptosporidials [40].
In the past decade, increased awareness and funding toward finding a Cryptosporidium-specific therapeutic has resulted in a reasonably diverse pipeline; however, there is still a lack of a clinical proof of concept for a drug that is equivalent or superior to nitazoxanide. Specific use-case scenarios may also dictate the best clinical path for emerging therapeutics for cryptosporidiosis [12▪▪]. Other practical considerations of treatment settings include the need for simple and affordable point-of-care diagnostics as well as reliable access to cryptosporidiosis treatments, that is, wherever children with diarrhea receive medical care and at nutritional rehabilitation and HIV treatment centers. These requirements are the most difficult to achieve in low-resource settings where the burden of Cryptosporidium infection is the highest. Accessible diagnostics and clearly defined diagnosis criteria are essential for deciding how to treat patients with new anticryptosporidials [41], especially as there is a lack of guidance around empiric treatment or mass drug administration campaigns.
Another possible solution for deconvoluting the clinical path for emerging treatments for cryptosporidiosis could be a controlled human infection model (CHIM). It is feasible that the clinical pathway for anticryptosporidial NCEs may diverge between the different target populations and use cases, and therefore, these studies could serve as a small-scale first pass to determine proof-of-concept efficacy of NCEs before a large financial investment in specific studies involving immunocompromised patients or young children. Although there is interest and precedence of CHIM for cryptosporidiosis [42], the most recent studies were conducted nearly two decades ago, and new regulatory requirements present unique challenges that complicate the re-establishment of this model [43▪].
To further complicate matters, the spontaneous resistance to CpMetRS inhibitor 2093 in the neonatal calf efficacy model [16] is extremely alarming and necessitates thoughtful design of future clinical trials and eventual implementation of new treatments. More studies are needed, ideally in early stages of development, to determine the frequency at which resistance mutations arise for this drug and others in the pipeline. Combination therapies and regimens are essential in combating other diseases with a large global health burden (e.g. malaria, HIV, tuberculosis), and the rollout of an effective therapy for cryptosporidiosis may quickly be rendered useless or exacerbate the burden of Cryptosporidium infection. Of note, there are currently no studies looking at any emerging therapeutics in combination.
CONCLUSION
The field of drug discovery for Cryptosporidium has made great strides in a short amount of time through both targeted approaches and phenotypic high-throughput screens; however, there is likely to be attrition of compounds as they progress through the later stages of development. The evolution of the clinical path for new anticryptosporidial compounds must allow for advancement of promising compounds for key target populations and use-case scenarios while balancing the risks that may push away support from the pharmaceutical industry. The CRYPTOFAZ study was invaluable for lessons learned and capacity building efforts for future trials in resource-limited settings. Finally, implementation strategies of new drugs are critical. Even the best drug for treating Cryptosporidium infection will have limited impact if it cannot be effectively implemented with accessible and accurate diagnostics, and monotherapy strategies may be problematic if spontaneous resistance selection is observed with other compounds.
Acknowledgements
We thank Beatriz Baragaña for sharing unpublished results.
Financial support and sponsorship
R.K.M.C. was supported by a grant to PATH from the UK Government. M.S.L. was supported by grants to The Scripps Research Institute from the Wellcome Trust (HIT NTD) and the Bill & Melinda Gates Foundation (OPP1208899, OPP1156296, OPP1107194).
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013; 382:209–222. [DOI] [PubMed] [Google Scholar]
- 2.Platts-Mills JA, Babji S, Bodhidatta L, et al. MAL-ED Network Investigators. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet Glob Health 2015; 3:e564–e575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sow SO, Muhsen K, Nasrin D, et al. The burden of Cryptosporidium diarrheal disease among children < 24 months of age in moderate/high mortality regions of Sub-Saharan Africa and South Asia, utilizing data from the Global Enteric Multicenter Study (GEMS). PLoS Negl Trop Dis 2016; 10:e0004729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khalil IA, Troeger C, Rao PC, et al. Morbidity, mortality, and long-term consequences associated with diarrhoea from Cryptosporidium infection in children younger than 5 years: a meta-analyses study. Lancet Glob Health 2018; 6:e758–e768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogawski ET, Liu J, Platts-Mills JA, et al. MAL-ED Network Investigators. Use of quantitative molecular diagnostic methods to investigate the effect of enteropathogen infections on linear growth in children in low-resource settings: longitudinal analysis of results from the MAL-ED cohort study. Lancet Glob Health 2018; 6:e1319–e1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guerrant DI, Moore SR, Lima AA, et al. Association of early childhood diarrhea and cryptosporidiosis with impaired physical fitness and cognitive function four-seven years later in a poor urban community in northeast Brazil. Am J Trop Med Hyg 1999; 61:707–713. [DOI] [PubMed] [Google Scholar]
- 7.Widerström M, Schönning C, Lilja M, et al. Large outbreak of Cryptosporidium hominis infection transmitted through the public water supply, Sweden. Emerg Infect Dis 2014; 20:581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang X, Guo Y, Xiao L, Feng Y. Molecular epidemiology of human cryptosporidiosis in low- and middle-income countries. Clin Microbiol Rev 2021; 34:e00087-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amadi B, Mwiya M, Musuku J, et al. Effect of nitazoxanide on morbidity and mortality in Zambian children with cryptosporidiosis: a randomised controlled trial. Lancet 2002; 360:1375–1380. [DOI] [PubMed] [Google Scholar]
- 10.Johansen ØH, Abdissa A, Zangenberg M, et al. Performance and operational feasibility of two diagnostic tests for cryptosporidiosis in children (CRYPTO-POC): a clinical, prospective, diagnostic accuracy study. Lancet Infect Dis 2021; 21:722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huston CD, Spangenberg T, Burrows J, et al. A proposed target product profile and developmental cascade for new cryptosporidiosis treatments. PLoS Negl Trop Dis 2015; 9:e0003987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12▪▪.Ashigbie PG, Shepherd S, Steiner KL, et al. Use-case scenarios for an anti-Cryptosporidium therapeutic. PLoS Negl Trop Dis 2021; 15:e0009057. [DOI] [PMC free article] [PubMed] [Google Scholar]; Outlines specific use-case scenarios that will help determine introduction and implementation of new therapeutics for cryptosporidiosis.
- 13.Baragaña B, Forte B, Choi R, et al. Lysyl-tRNA synthetase as a drug target in malaria and cryptosporidiosis. Proc Natl Acad Sci U S A 2019; 116:7015–7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14▪.Vinayak S, Jumani RS, Miller P, et al. Bicyclic azetidines kill the diarrheal pathogen Cryptosporidium in mice by inhibiting parasite phenylalanyl-tRNA synthetase. Sci Transl Med 2020; 12:eaba8412. [DOI] [PMC free article] [PubMed] [Google Scholar]; Introduction of CpPheRS inhibitors for cryptosporidiosis, including lead compound BRD7929.
- 15▪.Buckner FS, Ranade RM, Gillespie JR, et al. Optimization of methionyl tRNA-synthetase inhibitors for treatment of Cryptosporidium infection. Antimicrob Agents Chemother 2019; 63:e02061-18. [DOI] [PMC free article] [PubMed] [Google Scholar]; Calf study with CpMetRS inhibitor compound 2093 showing rapid emergence of spontaneous resistance mutations.
- 16.Hasan MM, Stebbins EE, Choy RKM, et al. Spontaneous selection of Cryptosporidium drug resistance in a calf model of infection. Antimicrob Agents Chemother 2021; 65:e00023-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ojo KK, Larson ET, Keyloun KR, et al. Toxoplasma gondii calcium-dependent protein kinase 1 is a target for selective kinase inhibitors. Nat Struct Mol Biol 2010; 17:602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Voorhis WC, Doggett JS, Parsons M, et al. Extended-spectrum antiprotozoal bumped kinase inhibitors: a review. Exp Parasitol 2017; 180:71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keyloun KR, Reid MC, Choi R, et al. The gatekeeper residue and beyond: homologous calcium-dependent protein kinases as drug development targets for veterinarian Apicomplexa parasites. Parasitology 2014; 141:1499–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hulverson MA, Vinayak S, Choi R, et al. Bumped-kinase inhibitors for cryptosporidiosis therapy. J Infect Dis 2017; 215:1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi R, Hulverson MA, Huang W, et al. Bumped Kinase Inhibitors as therapy for apicomplexan parasitic diseases: lessons learned. Int J Parasitol 2020; 50:413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee S, Ginese M, Beamer G, et al. Therapeutic efficacy of bumped kinase inhibitor 1369 in a pig model of acute diarrhea caused by Cryptosporidium hominis. Antimicrob Agents Chemother 2018; 62:e00147-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hulverson MA, Choi R, Vidadala RSR, et al. Pyrrolopyrimidine bumped kinase inhibitors for the treatment of cryptosporidiosis. ACS Infect Dis 2021; 7:1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang W, Hulverson MA, Choi R, et al. Development of 5-aminopyrazole-4-carboxamide-based bumped-kinase inhibitors for Cryptosporidiosis therapy. J Med Chem 2019; 62:3135–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Love MS, McNamara CW. Phenotypic screening techniques for Cryptosporidium drug discovery. Expert Opin Drug Discov 2021; 16:59–74. [DOI] [PubMed] [Google Scholar]
- 26.Hulverson MA, Choi R, McCloskey MC, et al. Repurposing infectious disease hits as anti-Cryptosporidium leads. ACS Infect Dis 2021; 7:1275–1282. [DOI] [PubMed] [Google Scholar]
- 27.Manjunatha UH, Vinayak S, Zambriski JA, et al. A Cryptosporidium PI(4)K inhibitor is a drug candidate for cryptosporidiosis. Nature 2017; 546:376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Funkhouser-Jones LJ, Ravindran S, Sibley LD. Defining stage-specific activity of potent new inhibitors of Cryptosporidium parvum growth in vitro. mBio 2020; 11: e00052-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jumani RS, Bessoff K, Love MS, et al. A novel piperazine-based drug lead for cryptosporidiosis from the medicines for malaria venture open-access malaria box. Antimicrob Agents Chemother 2018; 62: e01505-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jumani RS, Hasan MM, Stebbins EE, et al. A suite of phenotypic assays to ensure pipeline diversity when prioritizing drug-like Cryptosporidium growth inhibitors. Nat Commun 2019; 10:1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stebbins E, Jumani RS, Klopfer C, et al. Clinical and microbiologic efficacy of the piperazine-based drug lead MMV665917 in the dairy calf cryptosporidiosis model. PLoS Negl Trop Dis 2018; 12:e0006183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee S, Ginese M, Girouard D, et al. Piperazine-derivative MMV665917: an effective drug in the diarrheic piglet model of Cryptosporidium hominis. J Infect Dis 2019; 220:285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lunde CS, Stebbins EE, Jumani RS, et al. Identification of a potent benzoxaborole drug candidate for treating cryptosporidiosis. Nat Commun 2019; 10:2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bellini V, Swale C, Brenier-Pinchart MP, et al. Target identification of an Antimalarial Oxaborole Identifies AN13762 as an alternative chemotype for targeting CPSF3 in apicomplexan parasites. iScience 2020; 23:101871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arnold SLM, Choi R, Hulverson MA, et al. Necessity of bumped kinase inhibitor gastrointestinal exposure in treating Cryptosporidium infection. J Infect Dis 2017; 216:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arnold SLM, Choi R, Hulverson MA, et al. P-glycoprotein-mediated efflux reduces the in vivo efficacy of a therapeutic targeting the gastrointestinal parasite Cryptosporidium. J Infect Dis 2019; 220:1188–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Love MS, Beasley FC, Jumani RS, et al. A high-throughput phenotypic screen identifies clofazimine as a potential treatment for cryptosporidiosis. PLoS Negl Trop Dis 2017; 11:e0005373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38▪.Iroh Tam PY, Arnold SLM, Barrett LK, et al. Clofazimine for treatment of cryptosporidiosis in HIV-infected adults (CRYPTOFAZ): an experimental medicine, randomized, double-blind, placebo-controlled phase 2a trial. Clin Infect Dis 2020; ciaa42.[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]; Clinical trial of clofazimine, the first clinical study of a therapeutic for cryptosporidiosis as the publication of the GEMS and MAL-ED results that highlighted the burden of disease in low-income and middle-income countries.
- 39▪▪.Toto N, Douglas E, Gmeiner M, et al. Conducting clinical trials in sub-Saharan Africa: challenges and lessons learned from the Malawi Cryptosporidium study. Trials 2020; 21:680. [DOI] [PMC free article] [PubMed] [Google Scholar]; Discussion of challenges and lessons learned from the CRYPTOFAZ trial and helpful guidance for future human trials for cryptosporidiosis therapeutics.
- 40.Huston CD. The CRYPTOFAZ trial and lessons learned for anticryptosporidial drug development. Clin Infect Dis 2020; ciaa425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chalmers RM, Alexander C. Defining the diagnosis of cryptosporidiosis. Lancet Infect Dis 2020; 21:589–590. [DOI] [PubMed] [Google Scholar]
- 42.DuPont HL, Chappell CL, Sterling CR, et al. The infectivity of Cryptosporidium parvum in healthy volunteers. N Engl J Med 1995; 332:855–859. [DOI] [PubMed] [Google Scholar]
- 43▪.Jumani RS, Blais J, Tillmann HC, et al. Opportunities and challenges in developing a Cryptosporidium controlled human infection model for testing antiparasitic agents. ACS Infect Dis 2021; 7:959–968. [DOI] [PMC free article] [PubMed] [Google Scholar]; Historical summary of controlled human infection model studies and discussion of prospects for future studies.