Abstract
Indoor air purifiers are increasingly marketed for their health benefits, but their cardiovascular effects remain unclear. We systematically reviewed and meta-analysed randomized controlled trials (RCTs) on the cardiovascular effects of indoor air purification interventions in humans of all ages. We searched Embase, Medline, PubMed, and Web of Science from inception to 22 August 2020. Fourteen cross-over RCTs (18 publications) were included. Systolic blood pressure (SBP) was significantly reduced after intervention (-2.28 [95% CI: -3.92, -0.64] mmHg). There were tendencies of reductions in diastolic blood pressure (-0.35 [-1.52, 0.83] mmHg), pulse pressure (PP) (-0.86 [-2.07, 0.34] mmHg), C-reactive protein (-0.23 [-0.63, 0.18] mg/L), and improvement in reactive hyperaemia index (RHI) (0.10 [-0.04, 0.24]) after indoor air purification, although the effects were not statistically significant. However, when restricting the analyses to RCTs using physical-type purifiers only, significant improvements in PP (-1.56 [-2.98, -0.15] mmHg) and RHI (0.13 [0.01, 0.25]) were observed. This study found potential evidence on the short-term cardiovascular benefits of using indoor air purifiers, especially for SBP, PP and RHI. However, under the Grading of Recommendations Assessment, Development and Evaluation framework, the overall certainty of evidence was very low, which discourage unsubstantiated claims on the cardiovascular benefits of air purifiers. We have also identified several key methodological limitations, including small sample size, short duration of intervention, and the lack of wash-out period. Further RCTs with larger sample size and longer follow-up duration are needed to clarify the cardiovascular benefits of air purification interventions.
Keywords: Air purification, Cardiovascular health, Indoor air, Meta-analysis, Randomized controlled trial
1. Introduction
Air pollution is the leading environmental risk factor for ill health globally, estimated to account for 4.9 (Health Effects Institute., 2019) to 8.8 (Lelieveld et al., 2020) million deaths (largely from cardiovascular disease) per year. As people spend most of their time indoors, there have been widespread health concerns about indoor air pollution, particularly fine particulate matter (PM2.5) (Klepeis et al., 2001). Apart from tobacco smoke, the primary sources of indoor PM2.5 include domestic combustion of solid fuels (e.g., coal and wood) for cooking and heating in rural areas (Bruce et al., 2015) and the infiltration of ambient particulates in urban areas (Branco et al., 2014; Guo et al., 2013; Habre et al., 2013; Tong et al., 2016). In poor ventilation conditions, indoor PM2.5 levels can build up to several times higher than outdoor levels even in the absence of solid fuel combustion (Ramachandran et al., 2003).
Ample evidence suggests that exposure to PM2.5 is associated with excess risks of cardiovascular disease (CVD) and mortality (Newby et al., 2015; Rajagopalan et al., 2018). The postulated mechanisms include elevated blood pressure (BP), endothelial function impairment and systemic inflammation (Pope III et al., 2016). Correspondingly, there is a range of well-established biomarkers used in epidemiological assessments, including pulse pressure (PP), reactive hyperemia index (RHI), C-reactive protein (CRP), interleukin-6 (IL-6), and fibrinogen (Newby et al., 2015). It has been increasingly suggested that a modest reduction of ambient PM2.5 exposure at a population level can result in substantial public health benefits, but this is based predominantly on observational studies instead of gold standard randomized controlled trials (RCTs) (Health Effects Institute., 2019; Wei et al., 2019). At the same time, the use of indoor air purifiers against PM2.5 has received growing attention (Eggleston et al., 2005; Rajagopalan et al., 2018) and they are increasingly marketed as a health commodity, especially in populations where strong policy interventions against air pollution levels are not available, but the cardiovascular benefits of such interventions remain unclear.
In an earlier meta-analysis of intervention studies on the effects of using air purifiers on systolic (SBP) and diastolic (DBP) blood pressure, Walzer et al. reported a significant reduction in SBP (-3.94 [95% CI: -7.00, -0.89] mmHg) but a non-significant effect on DBP (-0.95 [-2.81, 0.91] mmHg) in the intervention group (Walzer et al., 2020). However, this meta-analysis included both RCTs and non-randomized studies, and an older and non-specific risk-of-bias assessment framework was used. Two other recent qualitative reviews described a broader range of studies involving other CVD biomarkers, but they did not employ a systematic evidence quality assessment framework and no meta-analysis was conducted (Allen and Barn, 2020; Cheek et al., 2021). In order to more comprehensively and critically assess the cardiovascular effects of reducing PM2.5 exposure through indoor air purification, we conducted a systematic review and meta-analysis following the well-established GRADE (Grading of Recommendations Assessment, Development and Evaluation) framework to synthesize and evaluate the RCT evidence on the effects of air purification intervention on SBP and DBP as primary outcomes and other cardiovascular biomarkers (e.g. PP, RHI, CRP) as secondary outcomes in humans of all ages.
2. Material and methods
2.1. Protocols and search strategy
We searched Ovid Embase, Ovid Medline, PubMed, and Web of Science for published RCTs assessing the effects of indoor air purification interventions on cardiovascular health, between database inception and Aug 22, 2020. Pilot search terms related to the concept of ‘air purifier/ cleaner’ were determined from the top ten relevant studies identified in Google Scholar and tested in the literature databases before being adopted in the final search strategy. A wide range of search terms was used for the concept of cardiovascular health (e.g., cardiovascular diseases/ disorders/ effects, hypertension, heart diseases, atherosclerosis, biomarkers) (see Appendix A in the Supplementary file). To limit our results to RCTs, we used the pre-formulated highly sensitive search filters (database-specific) available in the Cochrane Handbook for Systematic Review of Interventions (JPT and Sally, 2011). Bibliographic references of all articles included after the screening of titles and abstracts were checked for additional studies. We also searched for unpublished trials registered in ClinicalTrials.gov using the terms “air filter” and “air purifier”.
This study has been registered at the Open Science Forum (https://doi.org/10.17605/OSF.IO/F2R9M), and this report follows the Preferred Reporting Items of Systematic Reviews and Meta-analysis (PRISMA) guidelines (Liberati et al., 2009).
2.2. Eligibility criteria and study selection
The inclusion criteria were as follows: 1) study design: RCT; 2) intervention: any type of air cleaner/ purifier/ purification device that was used in indoor environments, including household, office, school, etc; 3) participants: humans, with no limitation on age or medical history; 4) outcomes: SBP and DBP as primary outcomes and any health outcomes related to cardiovascular health as secondary outcomes (for exploratory investigation); 5) full-length peer-reviewed studies; 6) language: English. All articles were first screened for title and abstract, then reviewed in full-text by two independent reviewers (XX and KHC) to evaluate their relevance, and any disagreement was forwarded to KFH.
2.3. Data extraction
XX and KHC independently extracted the data based on the double data entry requirement (JPT and Sally, 2011). Extracted data included citation, participant characteristics, study design, region, intervention details (e.g., type of air purifier, setting, washout period, duration, etc.), and information on air pollutants (PM2.5 concentrations in control and intervention groups, and the reduction efficiency). Where possible, the means and standard deviations (SDs) of the reported health outcomes measured post-intervention and post-control periods, and the mean differences (and corresponding SDs) between arms were extracted. If such information was not reported, SDs were calculated from standard errors, 95% CIs or ranges. If the study only reported geometric means, we converted the data to arithmetic means using an established method (Higgins et al., 2008). For studies that only reported percentage changes of CVD outcomes associated with the intervention, the mean differences between the intervention and control arms were estimated as the product of the baseline values and estimated percentage change. If the results were published in figures only, Web Plot Digitizer was used for data extraction (Rohatgi, 2020).
2.4. Methods for Meta-analyses
Meta-analyses were conducted for the outcomes that were comparably reported in four studies or more. Mean differences with 95% CI (post-intervention minus post-control values) were pooled using inverse-variance weighting, as such data were reported by most of the studies. Where different units of measure have been reported across studies, standardized mean difference was used. The heterogeneity across studies was assessed by I2 statistic (Ioannidis et al., 2007). When I2 < 25%, fixed-effect model was applied; otherwise, random-effect model was used (Mantel and Haenszel, 1959). For outcomes reported in more than six studies, subgroup analyses were conducted to compare the pooled-estimates by baseline PM2.5 levels (≤25 vs. >25 μg/m3), intervention-PM2.5 levels (≥10 vs. >10 μg/m3), baseline blood pressure (SBP<120 vs. SBP≥120 mmHg), study setting (at home vs. at school), type of air purifier (physical-type vs. electrostatic or ionization), intervention duration (≤7 vs. >7 days), health condition of participants (healthy subjects only vs. mixed), level of risk of bias (with low risk or some concerns vs. high risk), and the location (China vs. others) of the study to help explore the heterogeneity (Borenstein et al., 2011). Leave-one-out analyses were performed to test the robustness of the pooled estimates. Funnel plots and Egger’s regression were used to evaluate the risk of publication bias (Peters et al., 2006). All meta-analyses were conducted using the ‘metafor’ package in R version 3.5.3 (DerSimonian and Laird, 1986; R, 2018).
2.5. Risk of bias assessment
The risk-of-bias of the individual studies for each outcome was assessed using the Cochrane Risk-of-Bias Version 2 (RoB2) tool (Sterne et al., 2019). The RoB2 tool includes five domains relevant to the major sources of bias in RCTs, including risk of bias arising from (i) the randomization process (Domain 1), (ii) deviations from the intended interventions (Domain 2), (iii) missing outcome data (Domain 3), (iv) measurement of the outcomes (Domain 4), and (v) selective reporting (Domain 5). Each domain was assessed following standardized guidelines and determined to have “low risk of bias”, “some concerns”, or “high risk of bias”. Each domain consists of a series of detailed signalling questions, which are well-suited criteria for a systematic assessment of risk of bias in RCTs (https://www.riskofbias.info/welcome). For example, if the participants had the risk of being aware of their assigned intervention group during the study, Domain 2 would be determined as having “some concerns”. Finally, based on a summary of the domain-level judgements, an overall risk-of-bias judgement with three final levels (i.e., low, some concerns, high) can be determined (see Appendix B in the Supplementary file). Any disagreement in the risk of bias assessment between XX and KHC was forwarded to KFH and resolved by discussion.
2.6. Certainty of evidence assessment
The certainty of the body of evidence for each health outcome was assessed using the GRADE framework (Higgins and Thomas, 2019). The assessment was based on outcome-specific groups of studies instead of a judgement for each individual RCT. According to the GRADE guidelines, the certainty of evidence was categorised into four levels (i.e., high, moderate, low or very low). The initial certainty of a body of evidence for RCT starts at the “high” level (i.e., high confidence between true and estimated effect), and then it could be downgraded for five reasons — risk of bias, imprecision, inconsistency, indirectness, and publication bias (see Appendix C in the Supplementary file) — as they could cover most issues that bear on the certainty of evidence (Balshem et al., 2011).
3. Results
3.1. Overview of included studies
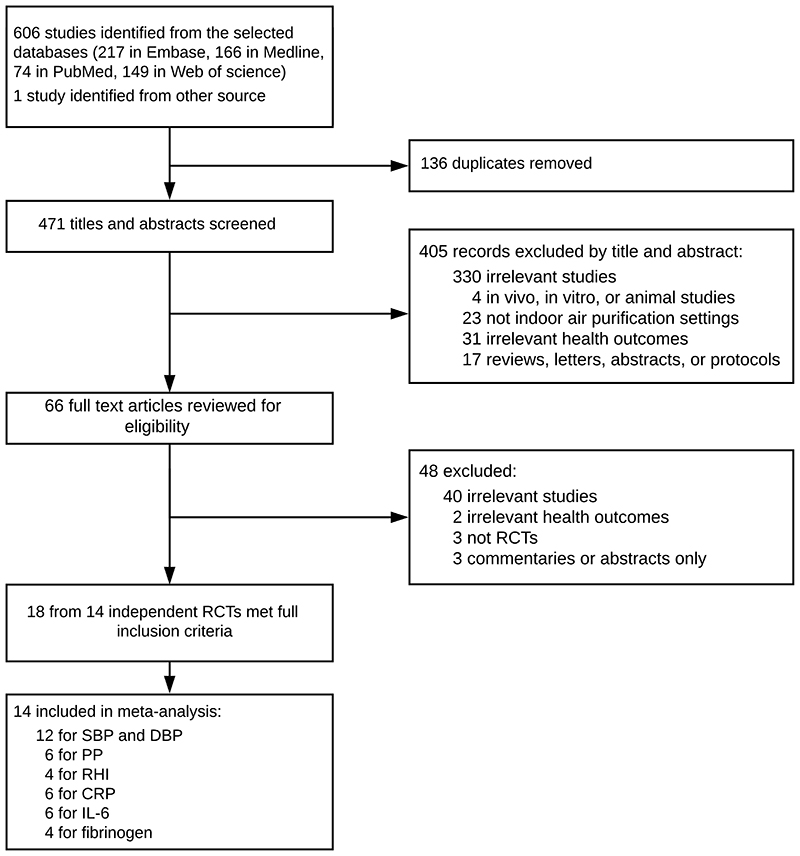
A total of 607 studies were identified. After removing 136 duplicates, the titles and abstracts of 471 studies were screened (Figure 1). Sixty-six studies underwent full-text review, after which 48 studies were excluded (40 irrelevant studies, two without cardiovascular outcomes, three non-RCTs, and three commentaries or abstracts). Ultimately, 18 articles from 14 independent RCTs published during 2008–2020 were included (Allen et al., 2011; Bräuner et al., 2008; Chen et al., 2015, 2016, 2018; Chuang et al., 2017; Cui et al., 2018; Dong et al., 2019; Kajbafzadeh et al., 2015; Karottki et al., 2013; Li et al., 2017; Liu et al., 2018, 2020a, 2020b; Morishita et al., 2018; Padró-Martínez et al., 2015; Shao et al., 2017; Weichenthal et al., 2013; Table 1). Twelve RCTs provided comparable estimates for SBP and DBP (Allen et al., 2011; Bräuner et al., 2008; Chen et al., 2015; Chuang et al., 2017; Cui et al., 2018; Dong et al., 2019; Li et al. 2017; Liu et al., 2020b; Morishita et al., 2018; Padró-Martínez et al., 2015; Shao et al., 2017; Weichenthal et al., 2013), six for pulse pressure (PP) (Chen et al., 2015; Cui et al., 2018; Dong et al., 2019; Li et al. 2017; Morishita et al., 2018; Padró-Martínez et al., 2015), four for RHI (Allen et al., 2011; Bräuner et al., 2008; Kajbafzadeh et al., 2015; Weichenthal et al., 2013), six for CRP (Allen et al., 2011; Bräuner et al., 2008; Chen et al., 2015; Chuang et al., 2017; Kajbafzadeh et al., 2015; Shao et al., 2017), six for IL-6 (Allen et al., 2011; Bräuner et al., 2008; Chen et al., 2015; Cui et al., 2018; Kajbafzadeh et al., 2015; Shao et al., 2017), and four for fibrinogen (Bräuner et al., 2008; Chen et al., 2015; Chuang et al., 2017; Shao et al., 2017). Most trials also examined a range of other cardiovascular outcomes, such as pulse wave velocity (PWV) and other cytokines, but these were not reported in sufficient number of studies for meta-analysis.
Figure 1. Study selection for the systematic review.
CRP, C-reactive protein; DBP, diastolic blood pressure; IL-6, interleukin-6; RCTs, randomized controlled trials; RHI, reactive hyperaemia index; SBP, systolic blood pressure.
Table 1. Main characteristics of the 14 indoor air purifier interventions (18 publications) included in this review.
| Study | N | Population characteristics | Intervention description | Baseline PM2.5 | Particle removing efficiency (%) | Cardiovascular functions and biomarkers measured | Main findings |
|---|---|---|---|---|---|---|---|
| Allen et al. 2011, British Columbia, Canada | 45 | Aged 20-63 years (mean 43), with 53% females; 3 subjects with hypertension or asthma; no smoker. | HEPA air purifier for 7+7 days without a washout period; two air purifiers in living room and bedroom, separately. All households were located in woodsmoke-impacted community, and 51% participants reported using a wood stove at home. | 11.2 μg/m3 | 59 | RHI, SBP, DBP, CRP, IL-6, urinary MDA, 8-isoprostane, band cell counts. | Air purification was significantly associated with the improvement in RHI and decrease in CRP. Decreases in PM2.5 was associated with reduced band cell counts. |
| Bräuner et al. 2008, Copenhagen, Denmark | 41 | Aged 60-75 years (median 67), with 49% females; all subjects were healthy adults; no smoker. | HEPA air purifier for 2+2 days without a washout period; two air purifiers in living room and bedroom, separately. All households were located within 350 m to major roads. | 12.6 μg/m3 | 63 | RHI, SBP, DBP, hemoglobin, red blood cell count, fibrinogen, platelet count, coagulation factors, CRP, IL-6, TNF-α, plasma amyloid A, plasma-selectin, 8-isoprostane. | Using air purifier significantly improved RHI among healthy elderly citizens, none of the biomarkers was significantly changed. |
| Chen et al. 2015, 2016, Shanghai, Chinaa | 35 | Mean age=23 ± 2 years, with 71.4% females; all subjects were healthy college students; no smoker. | Physical-type air purifier for 2+2 days with a washout period of 14 days; one air purifier was placed in the dormitory. | 96.2 μg/m3 | 57 | SBP, DBP, PP, CRP, IL-6, IL-1β, fibrinogen, P-selectin, monocyte chemoattractant protein-1, TNF-α, soluble CD40 ligand, myeloperoxidase, plasminogen activator inhibitor-1, tissue plasminogen activator, D-Dimer, endothelin-1, angiotensin-converting enzyme, DNA methylation. | Using air purifier was significantly associated with decreases in blood pressure, monocyte chemoattractant protein-1, IL-1β, myeloperoxidase, soluble CD40 ligand, and with increased DNA methylation in repetitive elements and all measured genes. |
| Chuang et al. 2017, Taipei, China | 200 | Aged 28-61 years (mean 43.3), with 50% females; all subjects were healthy homemakers; no smoker. | Window-mounted air conditioner equipped with low efficiency filter for 1+1 years without a washout period; air filters were installed in living and dining room, bedroom, and guest room. | 21.4 μg/m3 | 43 | SBP, DBP, CRP, 8-hydroxy-2-deoxyguanosine, fibrinogen. | Using air purifier was significantly associated with lower blood pressure and 8-hydroxy-2-deoxyguanosine levels. CRP and fibrinogen approached statistical significance. |
| Cui et al. 2018, Shanghai, China | 70 | Aged 19-26 years (mean 22), with 59% females; all subjects were healthy college students; no smoker. | HEPA air purifier for 13+13 hours with a washout period of 14 days; one air purifier was placed in the dormitory. | 33.2 μg/m3 | 70 | SBP, DBP, PP, IL-6, soluble CD62P, von Willebrand factor, PWV, urinary MDA, AI, HR. | Using air purifier could significantly improve small airway mechanics. Von Willebrand factor was also significantly lowered after true filtration, indicating reduced risk for thrombosis. |
| Dong et al. 2019 and Liu et al. 2020a, Beijing Chinaa | 44 | Aged 11-14 years (mean 12), with 45% females; all subjects were healthy middle school students; no smoker. | Ionization air purifier for 5+5 days with a washout period of 60 days; one air purifier was placed in the classroom. | 72.5 μg/m3 | 44 | SBP, DBP, PP, HR, HRV (HF, LF, SDNN, LF/HF), ST-segment elevation, EBC MDA, metabolomics analysis. | HRV was negatively altered. |
| Kajbafzadeh et al. 2015, British Columbia, Canada | 68 | Aged 19-72 years (mean 43.8), with 53% females; all subjects were healthy adults and living in traffic- and woodsmoke-impacted areas; no smoker. | HEPA air purifier for 7+7 days without a washout period; two air cleaners in living room and bedroom, separately. | 7.1 μg/m3 | 40 | RHI, CRP, IL-6, band cell count. | No significant difference was found. However, PM2.5 reduction was significantly associated with lower CRP among people living in the traffic-impacted locations. |
| Karottki et al. 2013, Copenhagen, Denmark | 48 | Aged 51-81 years (mean 67), with 54% females; 3 subjects with asthma or diabetic, and nearly half of all participants taking vasoactive, statins, or cyclooxygenase inhibitors; no smoker. | Re-circulating custom built units equipped with HEPA filter for 14+14 days without a washout period; two air purifiers in living room and bedroom, separately. All households were located within 1000m from major roads. | 8.0 μg/m3 | 46 | RHI, SBP, DBP, CRP, hemoglobin, leukocytes, lymphocytes, monocytes, granulocytes, CD31, CD62L, CD11b, CD49d, clara cell pneumoprotein 16, surface protein D. | No significant improvement in any measured health outcomes was found. However, RHI was significantly associated with the actual decrease of PM2.5 in the bedroom. |
| Li et al. 2017 and Chen et al. 2018, Shanghai, Chinaa | 55 | Aged 18-22 years (mean 20), with 49% females; all subjects were healthy college students; no smoker. | HEPA air purifier for 9+9 days with a washout period of 12 days; one air cleaner was placed in each dormitory. | 46.8 μg/m3 | 82 | SBP, DBP, PP, IL-1, IL-6, CRP, soluble CD40 ligand, TNF-α, intercellular adhesion molecule-1, insulin, corticotropin-releasing hormone, adrenocorticotropic hormone, urinary 8-hydroxy-2-deoxyguansine, endothelin-1, serum MDA, 8-isoprostane, superoxide dismutase, metabolomics analyses, RNA analyses. | Significant reductions in stress hormone and SBP were observed during true air purification period. Higher PM2.5 exposure was positively associated with the expression (mRNA, protein, or both) of IL-1, IL-6, TNF-α, tolllike receptor-2, tissue factor 3, and endothelin-1. |
| Liu et al. 2020b, Beijing, China | 56 | At least 18 year-old (mean: 22.6), with 59% females; all subjects were healthy college student; no smoker. | Ionization air purifier for 7+7 days with a washout period of 14 days; one air purifier was placed in the dormitory. | 29.5 μg/m3 | 75 | SBP, DBP, AI, PWV, urinary 8-isoprostane and MDA. | No significant difference was observed between the sham and true air purification. |
| Morishita et al. 2018, Midtown Detroit, USA | 40 | With a mean age of 67 ± 8 years, with 38% females; all subjects were low income elderly people and lived near a major state highway and large industrial facilities. 79% had hypertension and 25% had diabetes; no smoker. | 3+3+3 days for sham filter, low efficiency filter and HEPA filter group, respectively; each scenario was separated by 7 days; two air purifiers in living room and bedroom, separately. All households were near a major state highway and large industrial facilities. | 17.5 μg/m3 | 59 | SBP, DBP, PP, HRV (SDNN, LF/HF), AI, PWV. | No significant difference was observed, while beneficial trends were observed for all health outcomes after the HEPA filter intervention. |
| Padró-Martínez et al. 2015, Somerville, USA | 20 | All subjects were at least 40 years old (mean: 53.6), with 80% females; 11 subjects with hypertension and 2 subjects with diabetes; all housed were located less than 200m from the highway; no smoker. | HEPA air purifier for 21+21 days without a washout period; one air purifier was placed in the living room. All households were located within 200m from the highway. | NAb | 42 | SBP, DBP, PP, CRP, IL-6, TNF-α, fibrinogen. | No significant improvement was observed. In contrast, increased IL-6 was observed in the intervention period. |
| Shao et al. 2017 and Liu et al. 2018, Beijing, Chinaa | 35 | 20 COPD patients (66.8±7.9, 5% females) and 15 none-COPD subjects (65.9 ± 6.9, 93% females); 25% subjects had a cardiovascular disease history, and 33% with hypertension; no smoker. | HEPA air purifier for 14+14 days without a washout period; two air purifiers in living room and bedroom, separately. | 60.0 μg/m3 | 60 | SBP, DBP, IL-6, IL-8, CRP, the mean arterial BP, fibrinogen, EBC 8-isoprostane, urinary 8-hydroxy-2-deoxyguanosine, HRV (SDNN, RMSSD, LF, HF, TP). | Using air purifier was significantly associated with reductions in IL-8 among COPD patients. |
| Weichenthal et al. 2013, Southern Manitoba, Canada | 37 | Aged 11-64 (mean 32), with 57% females; 7 subjects with asthma or diabetic, and nearly half of all participants with endothelial dysfunction (RHI<1.67); 64% current smokers and 73% exposed to tobacco smoke at home. | Electrostatic air purifier for 7+7 days with a washout period of 7 days; one air purifier was placed in the living room. | 61.0 μg/m3 | 51 | RHI, SBP, DBP. | Air purifier usage was significantly associated with an decrease in blood pressure. |
Abbreviations: AI, augmentation index; CRP, C-reactive protein; DBP, diastolic blood pressure; EBC, exhaled breath condensate; HEPA, high efficiency particulate air; HF, high frequency; HR, heart rate; HRV, heart rate variability; IL, interleukin; LF, low frequency; MDA, malondialdehyde; NA, not available; PP, pulse pressure; PWV, pulse wave velocity; RHI, reactive hyperaemia index; RMSSD, the square root of the mean of the squared differences between adjacent normal-to-normal intervals; SBP, systolic blood pressure; SDNN, the standard deviation of the normal-to-normal interval; TNF, tumor necrosis factor; 8-isoprostane, 8-epi-prostaglandin F2α.
Two publications from one randomized controlled trial.
This study only reported the concentration of ultrafine particulate matter.
3.1.1. Study design and population
Two RCTs were conducted in the USA (Morishita et al., 2018; Padró-Martínez et al., 2015), three in Canada (Allen et al., 2011; Kajbafzadeh et al., 2015; Weichenthal et al., 2013), two in Denmark (Bräuner et al., 2008; Karottki et al., 2013), and seven in China (including one in Taiwan) (Chen et al., 2015, 2016, 2018; Chuang et al., 2017; Cui et al., 2018; Dong et al., 2019; Li et al., 2017; Liu et al., 2018, 2020a, 2020b; Shao et al., 2017). All RCTs were crossover trials, in which each participant received both the true and sham (e.g., without the internal air filter or the device turned off) air purifiers in a random order. Seven trials separated the true and sham air purifier scenarios by a washout period, ranging from 7 to 60 days (median=14 days) (Chen et al., 2015, 2016, 2018; Cui et al., 2018; Dong et al., 2019; Li et al., 2017; Liu et al., 2020a, 2020b; Morishita et al., 2018; Weichenthal et al., 2013). Two trials involved at least some young participants (<18 years) (Dong et al., 2019; Weichenthal et al., 2013), five included at least some elderly (>65 years) (Bräuner et al., 2008; Kajbafzadeh et al., 2015; Karottki et al., 2013; Liu et al., 2018; Morishita et al., 2018; Shao et al., 2017), and the rest were on adults (18–65 years). Six studies recruited only healthy individuals, and the rest included also individuals with pre-existing health conditions (e.g., asthma, hypertension, or diabetes).
3.1.2. Intervention descriptions
The intervention periods of most RCTs were relatively short, ranging from 13 hours to 21 days (median=7 days; 86% ≤14 days), except for a one-year study in Taipei (Chuang et al., 2017). Nine RCTs were conducted in household environments (Allen et al., 2011; Bräuner et al., 2008; Chuang et al., 2017; Kajbafzadeh et al., 2015; Karottki et al., 2013; Liu et al., 2018; Morishita et al., 2018; Padró-Martínez et al., 2015; Shao et al., 2017; Weichenthal et al., 2013), in which air purifiers were set in the living room, bedroom, and/ or dining room, while five trials were conducted at schools (classrooms or dormitories) (Chen et al., 2015, 2016, 2018; Chuang et al., 2017; Cui et al., 2018; Dong et al., 2019; Li et al., 2017; Liu et al., 2020a, 2020b). Six RCTs were conducted in the residences in close proximity to major sources of outdoor air pollution (e.g., near major roads) (Allen et al., 2011; Bräuner et al., 2008; Kajbafzadeh et al., 2015; Karottki et al., 2013; Morishita et al., 2018; Padró-Martínez et al., 2015). One RCT used electrostatic air purifiers (Weichenthal et al., 2013), two used ionization air purifiers (Liu et al., 2020a, 2020b; Dong et al., 2019), and all the others used physical-type air purifiers (by capturing particles onto the internal air filter, such as high efficiency particulate air [HEPA] filters, when the air is forced through the device).
3.1.3. Impact of intervention on PM2.5
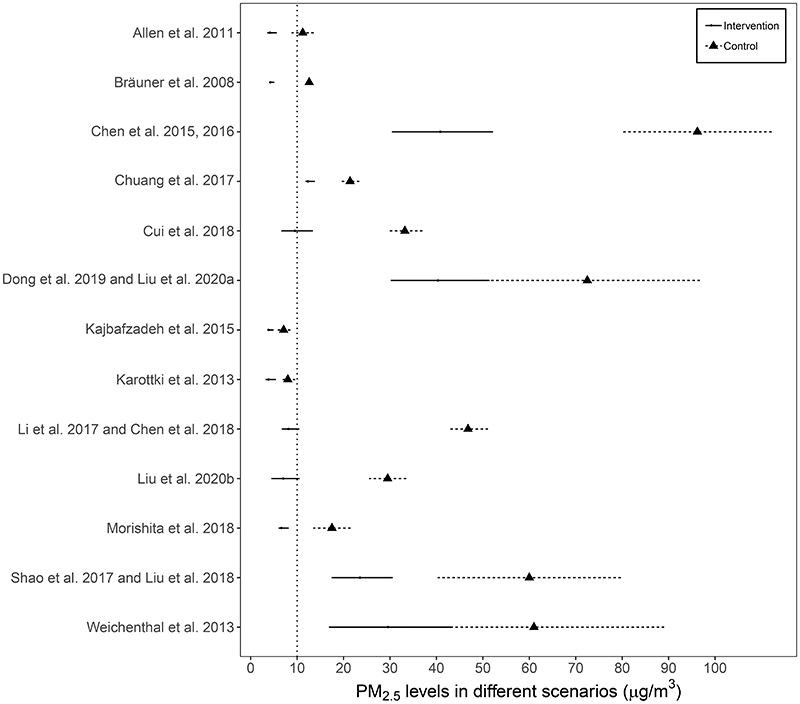
Figure 2 shows the PM2.5 levels during the period with true and sham air purifications in 13 RCTs that reported PM2.5 concentrations (the remaining study only monitored ultrafine particle concentration (i.e., PM1.0; Padró-Martínez et al., 2015). High heterogeneity existed in PM2.5 levels of the control scenarios across studies, ranging from 7.1 to 96.2 μg/m3 (mean=36.7 μg/m3), with two trials (Kajbafzadeh et al., 2015; Karottki et al., 2013) recorded PM2.5 levels below the WHO Air Quality Guidelines level of 10 μg/m3 in the control scenarios. All interventions demonstrated acceptable particle removal efficiency (mean: 56%, range: 40%-82%), with six reaching ≤10 μg/m3 (Allen et al., 2011; Bräuner et al., 2008; Chen et al., 2018; Cui et al., 2018; Li et al., 2017; Liu et al., 2020b; Morishita et al., 2018).
Figure 2. Indoor PM2.5 levels during the period with true and sham air purifications for 13 indoor air purification interventions.
a One study did not report the PM2.5 concentration; solid or dotted horizontal lines represents 95% confidence intervals for group-specific mean estimates; the vertical dotted line indicates the WHO Air Quality Guidelines value for annual mean PM2.5 of 10 μg/m3.
3.2. Risk of bias
The risk of bias judgements and corresponding details for each domain of each included study are presented in Appendix D, and the outcome-specific RoB2 judgements for each domain of all included studies are summarized in Appendix E in the supplementary file. Overall, a sizable proportion of the studies identified suffered from high risk of bias across all outcomes: BP (6/12), CRP (4/9), IL-6 (3/7), PP (1/6), and RHI (3/5).
The dropout rate ranged from 0% (in five RCTs) (Chen et al., 2015, 2016; Chuang et al., 2017; Liu et al., 2018, 2020b; Morishita et al., 2018; Shao et al., 2017) to 20% (overall mean=6.5%). As most of the studies involved relatively short-term interventions, participants were asked to stay in the air-filtered areas, keep the windows closed, and/ or avoid cooking or cleaning as long as possible. Participants in nine RCTs spent on average 85% of their time indoors (range: 74%–100%), indicating a high compliance (Allen et al., 2011; Chen et al., 2015, 2016, 2018; Cui et al., 2018; Dong et al., 2019; Kajbafzadeh et al., 2015; Karottki et al., 2013; Li et al., 2017; Liu et al., 2018, 2020a; Padró-Martínez et al., 2015; Shao et al., 2017). Eleven RCTs clearly described their double-blinding procedures, while three RCTs only reported the blinding of participants (Allen et al., 2011; Kajbafzadeh et al., 2015; Shao et al., 2017). In particular, twelve RCTs used a sham filter in the purifiers for the control periods, whereas the two RCTs with ionization air purifiers had the devices switched off (Dong et al., 2019; Liu et al., 2020a; two publication from one RCT) or the internal power supply wire severed (Liu et al., 2020a), which might have impaired the concealment leading to bias.
3.3. Cardiovascular effects
3.3.1. Effect on blood pressure
In this review, a total of 13 RCTs measured the changes in BP associated with the interventions (Allen et al., 2011; Bräuner et al., 2008; Chen et al., 2015; Chuang et al., 2017; Cui et al., 2018; Dong et al., 2019; Karottki et al., 2013; Li et al. 2017; Liu et al., 2020b; Morishita et al., 2018; Padró-Martínez et al., 2015; Shao et al., 2017; Weichenthal et al., 2013). As one study only reported the median (5th and 95th percentiles) for SBP (120 [100, 150] mmHg in the sham-filter group vs. 120 [100, 140] mmHg in the true-filter group) and DBP (80 [60, 90] mmHg vs. 77.5 [60, 90] mmHg) (Karottki et al., 2013), and with no validated method to accurately estimate mean and SD using these data (McGrath et al., 2020; Wan et al., 2014), it was excluded from the meta-analysis.
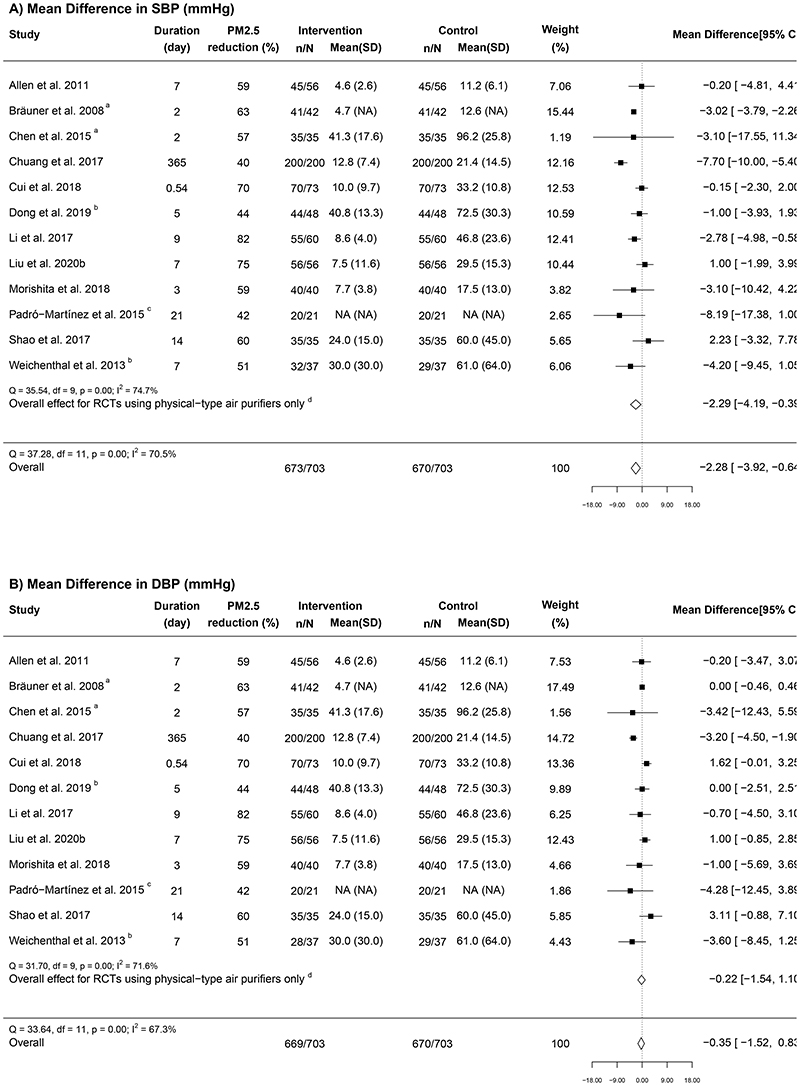
Figures 3A and 3B show the meta-analysis results for BP (12 RCTs). Overall, indoor air purifier intervention was associated with a significant pooled mean difference of -2.28 (95% CI: -3.92, -0.64) mmHg in SBP and a non-significant reduction of -0.35 (-1.52, 0.83) mmHg in DBP. Substantial heterogeneity was found for SBP (I2 = 70.5%) and DBP (I2 = 67.3%). The leave-one-out sensitivity analysis demonstrated the robustness of the result for SBP (Appendix F in the Supplementary file).
Figure 3. Forest plots of the mean difference in (A) systolic blood pressure and (B) diastolic blood pressure in relation to indoor air purifier interventions.
n = the number of participants being recruited initially; N = the number of participants completing the intervention; “Mean (SD)” represents the mean and standard deviation of PM2.5 for each group (unit, μg/m3); a Geometric means were converted to arithmetic means; b RCTs that used electrostatic or ionization air purifiers; c One study did not report the PM2.5 concentration; d The overall results from RCTs using physical-type air purifiers only.
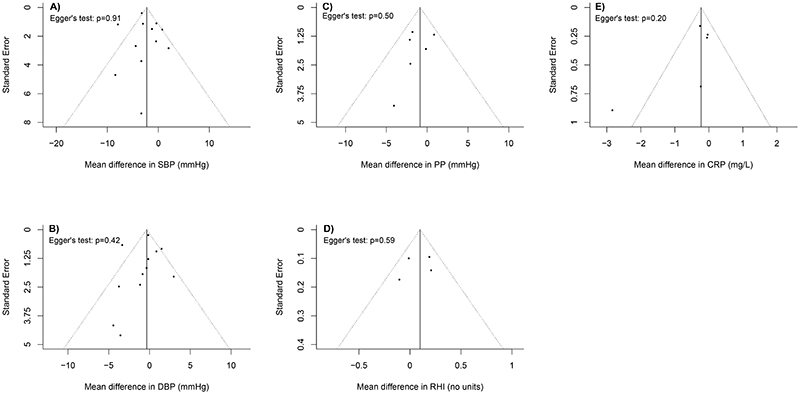
Although the subgroup analyses found no statistically significant differences for BP reduction in relation to the pre-specified characteristics, there was a tendency of greater reduction in SBP in studies conducted among participants with higher baseline SBP (i.e., ≥120mm Hg; -3.92 [-7.09, -0.75] mmHg vs. -1.04 [-2.41, 0.33] mmHg, P = 0.07) or those carried out in household environments (-3.48 [-6.06, -0.89] mmHg vs. -0.92 [-2.34, 0.51] mmHg at schools), P = 0.091) (Appendix G Table S1 in the Supplementary file). Similarly, somewhat stronger reductions were observed for both SBP and DBP in the trials using physical-type air purifiers, conducted in environments with lower PM2.5 levels (≤25 μg/m3), with longer intervention durations (over seven days), or with low risk of bias. The Egger’s test and funnel plots suggested no sign of publication bias (P = 0.91 for SBP and P = 0.42 for DBP; Figures 4A and 4B).
Figure 4. Funnel plots showing publication bias for the included studies by each outcome.
Note: (A) SBP, systolic blood pressure; (B) DBP, diastolic blood pressure; (C) PP, pulse pressure; (D) RHI, reactive hyperaemia index; (E) CRP, C-reactive protein, as the standard error for CRP in Shao et al. 2017 was relatively large, the data point for Shao et al. 2017 was not presented in the plot.
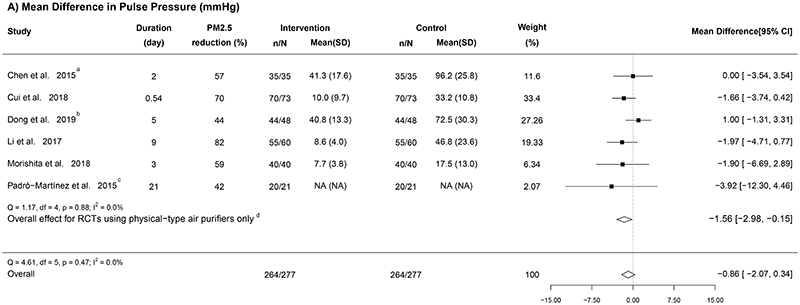
3.3.2. Effect on pulse pressure
Six RCTs measured the variations of PP, and all were included in our meta-analysis (Figure 5A) (Chen et al., 2015; Cui et al., 2018; Dong et al., 2019; Li et al. 2017; Morishita et al., 2018; Padró-Martínez et al., 2015). With low heterogeneity (I2 = 0%), the fixed-effect model yielded a pooled estimate of -0.86 (95% CI: -2.07, 0.34) mmHg. In the leave-one-out analysis (Appendix F in the Supplementary file), after removing the only RCT using ionization air purifier (i.e., restricting the analysis to physical-type air purifiers only) (Dong et al., 2019), a statistically significant reduction in PP was observed (-1.56 [-2.98, -0.15] mmHg; Figure 5A).
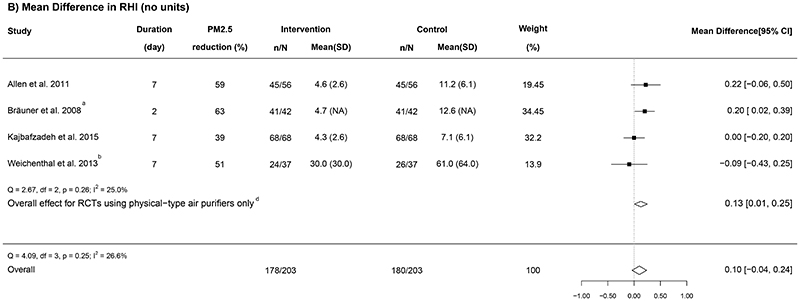
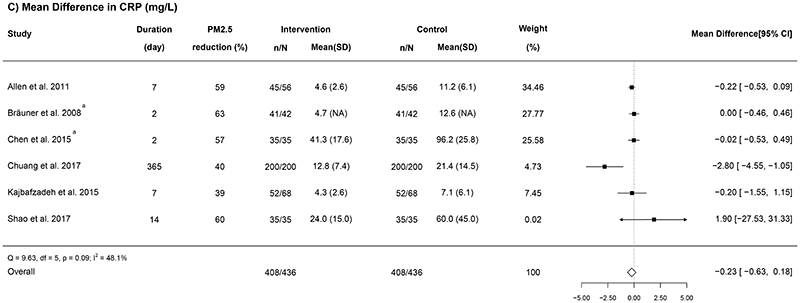
Figure 5. Forest plots of the mean difference in (A) pulse pressure, (B) reactive hyperaemia index, and (C) C-reactive protein in relation to indoor air purifier interventions.
n = the number of participants being recruited initially; N = the number of participants completing the intervention; “Mean (SD)” represents the mean and standard deviation of PM2.5 for each group (unit, μg/m3); a Geometric means were converted to arithmetic means; b RCTs that used electrostatic or ionization air purifiers; c One study did not report the PM2.5 concentration; d The overall results from RCTs using physical-type air purifiers only.
Although the subgroup analysis showed no significant differences for the selected characteristics (Appendix G Table S2 in the Supplementary file), the subgroups that observed somewhat stronger reductions in PP were the same as those for BP (e.g., RCTs conducted in household environments or those with longer intervention durations). Besides, the Egger’s test for funnel plot asymmetry was non-significant (P = 0.50; Figure 4C).
3.3.3. Effects on vascular function indicators
Eight RCTs measured vascular function using a range of indicators (Allen et al., 2011; Bräuner et al., 2008; Cui et al., 2018; Kajbafzadeh et al., 2015; Karottki et al., 2013; Liu et al., 2020b; Morishita et al., 2018; Weichenthal et al., 2013). Three RCTs measured both augmentation index (AI) and PWV (Cui et al., 2018; Liu et al., 2020b; Morishita et al., 2018), two biomarkers of arterial stiffness (Milan et al., 2019; Nichols and Singh, 2002), and they found no statistically significant effects.
Five RCTs measured RHI (Allen et al., 2011; Bräuner et al., 2008; Kajbafzadeh et al., 2015; Karottki et al., 2013; Weichenthal et al., 2013), an indicator for vascular endothelial dysfunction, and they reported inconsistent results. Because one RCT only reported the median and the 5th and 95th percentiles of RHI (Karottki et al., 2013), we conducted a meta-analysis on the other four RCTs (Figure 5B). Overall, there was a marginally non-significant improvement of 0.10 (-0.04 to 0.24) associated with air purification (I2 = 26.6%). In the leave-one-out analysis (Appendix F in the Supplementary file), the removal of one RCT that used electrostatic air purifier (Weichenthal et al., 2013) led to a statistically significant improvement in RHI (0.13 [0.01, 0.25]). No statistical evidence for publication bias was found (P = 0.59; Figure 4D).
3.3.4. Effects on the autonomic nervous system
Only four RCTs reported the effects of indoor air filtration interventions on the autonomic nervous system (Cui et al., 2018; Dong et al., 2019; Morishita et al., 2018; Shao et al., 2017), which precluded a meta-analysis. Two of them examined the changes in heart rate (HR): one observed a non-significant reduction of 1.47 (-3.72, 0.79) min-1 (Cui et al., 2018), and one found a significantly higher HR (mean ± SD: 92 ±12 min-1 in the true-filter group vs. 91±13 min-1 in the sham-filter group, P < 0.001) (Dong et al., 2019). Besides, three trials examined heart rate variability (HRV), but the indicators used were heterogeneous (e.g., high frequency [HF], low frequency [LF], LF/HF, the square root of the mean of the squared differences between adjacent normal-to-normal intervals [RMSSD], and the standard deviation of the normal-to-normal interval [SDNN]) (Dong et al., 2019; Morishita et al., 2018; Shao et al., 2017). One RCT that used ionization air purifiers reported significantly lower HRV indices during the true air purification period (P < 0.001) (Dong et al., 2019), whilst one observed non-significant negative effects (Shao et al., 2017) and another reported a non-significant improvement (Morishita et al., 2018).
3.3.5. Effects on blood biomarkers
Various blood biomarkers were measured across the RCTs included in this review, including CRP (nine trials: Allen et al., 2011; Bräuner et al., 2008; Chen et al., 2015; Chuang et al., 2017; Kajbafzadeh et al., 2015; Karottki et al., 2013; Li et al., 2017; Padró-Martínez et al., 2015; Shao et al., 2017), IL-6 (eight trials: Allen et al., 2011; Bräuner et al., 2008; Chen et al., 2015; Cui et al., 2018; Kajbafzadeh et al., 2015; Li et al., 2017; Padró-Martínez et al., 2015; Shao et al., 2017), fibrinogen (five trials: Bräuner et al., 2008; Chen et al., 2015; Chuang et al., 2017; Padró-Martínez et al., 2015; Shao et al., 2017), malondialdehyde (MDA) (five trials: Allen et al., 2011; Cui et al., 2018; Dong et al., 2019; Li et al., 2017; Liu et al., 2020b), and 8-epi–prostaglandin F2α (8-isoprostane) (five trials: Allen et al., 2011; Bräuner et al., 2008; Li et al., 2017; Liu et al., 2020b; Shao et al., 2017).
Of the nine trials that measured CRP, three provided insufficient or incompatible data to be transformed to means and SDs (e.g., only reported the median [5th percentile, 95th percentile] or percentage change only) (Karottki et al., 2013; Li et al., 2017; Padró-Martínez et al., 2015), so only the remaining six RCTs were meta-analysed. Overall, a non-significant pooled reduction was found (-0.23 [-0.63, 0.18] mg/L; I2 = 48.1%; Figure 5C). The subgroup analysis suggested that RCTs with longer intervention duration (over seven days) tended to have a larger reduction in CRP (-2.78 [-4.53, -1.03] mg/L vs. -0.13 [-0.35, 0.1] mg/L, P = 0.003; see Appendix G Table S2 in the Supplementary file). No statistical evidence for publication bias was suggested by the Egger’s test (P = 0.20; Figure 4E).
Out of the eight RCTs that assessed IL-6, six with comparable data were meta-analysed (Appendix H in the Supplementary file) (Allen et al., 2011; Bräuner et al., 2008; Chen et al., 2015; Cui et al., 2018; Kajbafzadeh et al., 2015; Shao et al., 2017), showing a non-significant pooled estimate of 0.04 [-0.32, 0.40] pg/ml (I2 = 0%). For the remaining two RCTs, one conducted among young healthy college students in China reported a non-significant reduction (in modelled percentage changes) associated with the intervention (Li et al., 2017), whereas one conducted in households near a highway in the USA reported significantly lower IL-6 levels during the sham air filtration period (-49.6 [-93.3, -5.9] %) (Padró-Martínez et al., 2015).
For fibrinogen, four of the five RCTs were meta-analysed (Appendix H in the Supplementary file) (Bräuner et al., 2008; Chen et al., 2015; Chuang et al., 2017; Shao et al., 2017), yielding a non-significant pooled standardized mean difference of -0.11 (-0.27, 0.05; I2 = 0%). One RCT was excluded from the meta-analysis due to incompatible data, in which a non-significant reduction was observed (Padró-Martínez et al., 2015).
The concentrations of MDA and 8-isoprostane were measured using different bio-samples in the relevant RCTs. Four RCTs measured urinary levels (three for MDA [Allen et al., 2011; Cui et al., 2018; Li et al., 2017] and three for 8-isoprostane [Allen et al., 2011; Bräuner et al., 2008; Liu et al., 2020b]), and two RCTs assessed the concentrations in exhaled breath condensate (one for MDA [Dong et al., 2019] and one for 8-isoprostane [Shao et al., 2017]). However, they failed to find any significant associations. Besides, one RCT measured serum-level MDA and 8-isoprostane, reporting significant reductions associated with air purification (Li et al., 2017).
3.4. Certainty of evidence
Table 2 presents a summary of findings for the certainty of evidence for each health outcome meta-analysed. As many studies were assessed as having “some concerns” or “high” risk of bias based on the RoB2 tool, the risk-of-bias certainty assessment was “serious” for all outcomes. Serious indirectness (significant heterogeneity in population) and imprecision (wide 95% CIs and small sample size) across studies were the main reasons for downgrading the certainty of evidence for each health outcome. Egger’s tests and funnel plots found no evidence of publication bias for any health outcomes examined, suggesting unsuspected publication bias. In summary, the certainty of evidence across the indoor air purification RCTs were assessed as “very low” for all health outcomes, implying that more rigorous studies are very likely to change the estimated effects.
Table 2. Summary of findings for the certainty of evidence for each health outcome.
| Health outcome | No. of study | Certainty assessment | Overall quality | ||||
|---|---|---|---|---|---|---|---|
| Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | |||
| SBP and DBP | 12 | Serious | Serious | Serious | Not serious | None | Very low |
| PP | 6 | Serious | None | Serious | Serious | None | Very low |
| RHI | 4 | Serious | Not serious | Serious | Serious | None | Very low |
| CRP | 6 | Serious | Serious | Serious | Serious | None | Very low |
| IL-6 | 6 | Serious | None | Serious | Serious | None | Very low |
| Fibrinogen | 4 | Serious | None | Not serious | Serious | None | Very low |
Abbreviations: CRP, C-reactive protein; DBP, diastolic blood pressure; IL, interleukin; PP, pulse pressure; RHI, reactive hyperaemia index; SBP, systolic blood pressure.
3.5. Description for ongoing RCTs
The search in ClinicalTrails.gov identified three relevant ongoing RCTs registered in 2017–2020 (Appendix I in the Supplementary file). All three RCTs employ HEPA air purifiers and recruit middle-aged adults or elderly non-smokers, with one adopted a longer-term (12 months) parallel design examining endothelial function and cognitive impairment, and the rest assessing short-term effects (~30 days) with a crossover design on BP, HRV and biomarkers, but only one of them has planned for a washout period.
4. Discussion
To the best of our knowledge, this is the first systematic review and meta-analysis to comprehensively and quantitatively evaluate the cardiovascular effects of indoor air purification interventions in RCTs assessing BP, PP, RHI, CRP, IL-6 and fibrinogen. The 14 crossover RCTs involving more than 700 participants from four countries show that air purification interventions consistently lead to a substantial reduction of indoor PM2.5 levels (mean=56%) and lower SBP (by ~2.5 mmHg). There is also suggestive evidence on reduced PP and increased RHI in RCTs using physical-type air purifiers only. However, the overall certainty of evidence remains low due to a range of study limitations identified, warranting larger and more robust studies to clarify the cardiovascular benefits of air purification interventions.
4.1. Blood pressure
Elevated BP is the second leading risk factor of premature death globally and is considered a major pathway linking air pollution to CVD (Newby et al., 2015). In air purification RCTs, BP has been the most widely studied cardiovascular outcome. Compared to an earlier review of nine crossover trials and one before-and-after study (Walzer et al., 2020), we included exclusively RCT evidence (with four more trials included) (Allen et al., 2011; Dong et al., 2019; Liu et al., 2020b; Weichenthal et al., 2013) and excluded an under-powered RCT that reported inappropriate effect estimate for BP (Karottki et al., 2013). Using a more updated risk-of-bias assessment tool tailored for crossover RCTs, we highlighted the key methodological concerns in the literature more clearly than the previous review (3/12 vs 8/10 studies judged as having low risk-of-bias) and concluded a “very low” certainty of evidence on the benefits of air purifier intervention on BP. Furthermore, we found considerably smaller overall reductions in SBP (-2.28 [95% CI: -3.92, -0.64] vs -3.94 [-7.00, -0.89] mmHg) and DBP (-0.35 [-1.52, 0.83] vs -0.95 [-2.81, 0.91] mmHg). The difference may be attributed to the inclusion of a before-and-after study in the previous review, which reported more extreme reductions in SBP (-15.1 [-23.4, -6.85] mmHg) and DBP (-5.00 [-12.54, 2.54] mmHg) that may be biased due to the lack of randomization (Lin et al., 2011). In addition, the previous meta-analysis appeared to have inappropriately combined geometric means with arithmetic means directly (Walzer et al., 2020; Higgins et al. 2008), whereas we have transformed the data into comparable forms for the meta-analysis.
From the subgroup analyses, there is indicative evidence showing a slightly greater reduction in SBP in trials involving individuals with higher baseline SBP (≥120 mmHg), conducted at home (vs. schools), using physical-type air purifiers only, recorded lower baseline PM2.5 levels (≤25 μg/m3), and with lower risk of bias. Although such differences did not reach statistical significance, possibly due to the limited power, they are well expected. First, hypertensive individuals tend to be more vulnerable to the adverse effect of PM2.5 (Auchincloss et al., 2008). Second, the RCTs conducted in classrooms or dormitories usually set one air purifier in each room for three to eight participants, which might have weakened the effects in reducing personal PM2.5 exposure. Third, pervious study reported that electrostatic or ionization air purifiers could produce ozone, a highly reactive gas associated with increased cardiovascular risks (Srebot et al., 2009), during their electric charging process (Michael et al., 2008). This may explain the greater effect size observed in the RCTs using physical-type air purifiers. Fourth, given the supra-linear association between PM2.5 and cardiovascular disease, the same proportional reduction of PM2.5 levels in low pollution settings should result in greater benefits (Pope III et al., 2018). Fifth, studies with high risk of bias have various issues (e.g. deviation from intended intervention) that might have weakened the interventions. These offer important insight into more efficient design of future RCTs with greater power to detect modest short-term effects.
4.2. Other cardiovascular biomarkers
Among other cardiovascular biomarkers, PP, endothelial dysfunction, and inflammatory biomarkers (e.g. CRP, IL-6, fibrinogen) are well-established predictors of cardiovascular disease risk (Bourdrel et al., 2017; Winston et al., 2013). Similar to the conclusion drawn on BP, the existing RCT evidence on the effects of air purifier interventions on the above biomarkers remains inconclusive.
Exposure to PM2.5 has been shown to be associated with higher PP and HRV and lower endothelial function (Auchincloss et al., 2008; Niu et al., 2020; Schlesinger, 2007). However, our review found no significant benefits in the overall analysis on PP, but the effect estimate became marginally statistically significant when restricting the analysis to RCTs using physical-type air purifiers only. Similarly, a significant improvement was also observed in RHI among the RCTs using physical-type air purifiers only. In addition, only the RCT using ionization air purifier reported significantly lower HRV (Dong et al., 2019), which may reflect an adverse cardiovascular effect of the ozone produced by electrostatic and ionization air purifiers.
Previous in vivo and in vitro experimental studies have consistently shown negative impact of PM2.5 exposure on systemic inflammation (Münzel et al., 2017), and similar findings have been reported in human experiments on short-term concentrated PM2.5 exposure challenge (Pope III et al., 2016). Although we found non-significant changes in CRP, IL-6 and fibrinogen (biomarkers of inflammation) after indoor air filtration interventions, it should be noted that this is based on the limited number of studies identified and there are moderate to high risk of bias in most RCTs. In addition to the lack of power, the short duration and variability in PM2.5 reduction of intervention might have resulted in modest effects that can be easily masked by noise.
A wide range of other cardiovascular biomarkers (e.g. MDA, 8-isoprostane) have been investigated previously, but the evidence remains scattered and no reliable meta-analysis can be performed. Although some of the signals could serve as indications for further investigation, no reliable conclusion can be drawn from the totality of evidence given the small sample sizes and lack of consistency across studies. Considering the overall quality of the evidence on the more frequently investigated health outcomes, interpretation on the less well-studied biomarkers must be even more cautious.
4.3. Methodological limitations and knowledge gaps
In this review, we identified a range of methodological limitations in the previous RCTs. First, most previous studies were shot-term interventions in small samples, which may underestimate the benefits of reducing PM2.5 exposure. Notably, the largest (n=200) and longest (intervention duration=1 year) RCT (with little risk of bias) reported highly significant effects on SBP (-7.70 [-10.0, -5.40] mmHg), DBP (-3.20 [-4.50, -1.90] mmHg), and CRP (-2.80 [-2.98, -2.62] mg/L) of clinical relevance (Chuang et al., 2017), but these findings are masked in the random-effect models that assigned disproportionately smaller weight to this study. Second, only half of the trials incorporated a wash-out period (and most were relatively short, median=7 days), which is crucial to minimise carry-over effects, a major challenge in crossover RCTs that typically bias the effect estimate towards the null. Third, the included studies were highly heterogeneous. Subgroup analyses were conducted to investigate this, but there was limited power to detect true subgroup-difference. In particular, the report of effect estimates other than mean differences (e.g. median) without supplementary information (e.g. inter-quartile range) in some studies may have introduced additional noise to our meta-analyses, because indirect approximation of means and SDs were required that entails extra uncertainty. In particular, the inconsistent reporting of percentage change in arithmetic and geometric mean across intervention arms without providing the exact group-specific means prevented us from conducting meta-analysis on some biomarkers. Besides, the indirect approximation of means and SDs in some studies might have introduced additional noise to our meta-analyses. Fourth, since most of the studies included were either conducted in China or a primarily-Caucasian population (i.e. Denmark, USA, Canada), there is a lack of evidence from more diverse population with different air pollution exposure patterns. More studies in different populations are needed to assess the potential cardiovascular benefits of air purifiers.
It should also be noted that most of the existing short-term RCTs explored the cardiovascular benefits of using indoor air purifiers under an experimental environment (e.g., by asking the participants to stay in the air-filtered areas as long as possible), which restricted the daily activity patterns of the participants. Therefore, in the real-world settings where people would spend less time in the air-filtered areas, the cardiovascular benefits of using indoor air purifiers might be lower than the estimated effects reported in this review.
5. Conclusions
Based on 18 articles from 14 independent RCTs, this review suggests statistically significant reduction in SBP (although being modest in absolute term) following indoor air purification interventions, with hints of stronger effect from more robust intervention. There is also indicative evidence of reduced PP and increased RHI, particularly in the RCTs using physical-type air purifiers. In contrast, we found no clear changes in levels of CRP, IL-6 and fibrinogen, and there is even less evidence on other cardiovascular biomarkers. According to the Cochrane RoB2 criteria, most published trials suffered from moderate to high risk of bias, contributing to the “very low” overall certainty of evidence under the GRADE framework. Besides, there are a range of methodological limitations in the existing RCTs, particularly small sample size, short intervention duration, and lack of wash-out period. Future high-quality studies with larger sample size, longer intervention period, more robust medium-to-long term endpoints (e.g. carotid intima-media thickness) are urgently needed to clarify the cardiovascular benefits of air purifier interventions, and claims on such benefits should be more cautious before more conclusive evidence.
Supplementary Material
Acknowledgments
KHC acknowledges support from the BHF Centre of Research Excellence, University of Oxford (Grant: RE/18/3/34214).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Author Contribution Statement
XX: Conceptualization, Methodology, Formal analysis, Writing-Original draft preparation, Visualization. KHC: Data curation, Writing-Review & Editing, Visualization. KBHL and HQ: Review & Editing. ZL and SHLY: Software, Validation. KFH: Conceptualization, Review & Editing, Supervision.
Declaration of Completing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Xi Xia, Email: xiaxi@link.cuhk.edu.hk, The Jockey Club School of Public Health and Primary Care, The Chinese University of Hong Kong, Hong Kong SAR.
Ka Hung Chan, Clinical Trial Service Unit and Epidemiological Studies Unit, Nuffield Department of Population Health, University of Oxford, UK; Oxford British Heart Foundation Centre of Research Excellence, University of Oxford, UK.
Kin Bong Hubert Lam, Email: hubert.lam@ndph.ox.ac.uk, Clinical Trial Service Unit and Epidemiological Studies Unit, Nuffield Department of Population Health, University of Oxford, UK.
Hong Qiu, Email: hongqiu@cuhk.edu.hk, Institute of Environment, Energy and Sustainability, The Chinese University of Hong Kong, Hong Kong SAR.
Zhiyuan Li, Email: zhiyuanli@cuhk.edu.hk, Institute of Environment, Energy and Sustainability, The Chinese University of Hong Kong, Hong Kong SAR.
Steve Hung Lam Yim, Email: steveyim@cuhk.edu.hk, Institute of Environment, Energy and Sustainability, The Chinese University of Hong Kong, Hong Kong SAR; The Department of Geography and Resource Management, The Chinese University of Hong Kong, Hong Kong Special Administrative Region; Asian School of the Environment, Nanyang Technological University, Singapore.
Kin-Fai Ho, The Jockey Club School of Public Health and Primary Care, The Chinese University of Hong Kong, Hong Kong SAR.
Availability of data and materials
The full dataset is available from the corresponding authors upon reasonable request.
References
- Allen RW, Carlsten C, Karlen B, Leckie S, Eeden SV, Vedal S, et al. An air filter intervention study of endothelial function among healthy adults in a woodsmoke-impacted community. Am J Respir Crit Care Med. 2011;183:1222–1230. doi: 10.1164/rccm.201010-1572OC. [DOI] [PubMed] [Google Scholar]
- Allen RW, Barn P. Individual-and Household-Level Interventions to Reduce Air Pollution Exposures and Health Risks: a Review of the Recent Literature. Curr Environ Health Rep. 2020;7(4):424–440. doi: 10.1007/s40572-020-00296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auchincloss AH, Roux AVD, Dvonch JT, Brown PL, Barr RG, Daviglus ML, et al. Associations between recent exposure to ambient fine particulate matter and blood pressure in the Multi-ethnic Study of Atherosclerosis (MESA) Environ Health Persp. 2008;116:486–91. doi: 10.1289/ehp.10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Borenstein Michael, Hedges Larry V, Higgins Julian PT, Rothstein Hannah R. Introduction to meta-analysis. John Wiley & Sons; 2011. [Google Scholar]
- Bourdrel T, Bind M-A, Béjot Y, Morel O, Argacha J-F. Cardiovascular effects of air pollution. Arch Cardiovasc Dis. 2017;110:634–642. doi: 10.1016/j.acvd.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco PTBS, Alvim-Ferraz MCM, Martins FG, Sousa SIV. Indoor air quality in urban nurseries at Porto city: Particulate matter assessment. Atmos Environ. 2014;84:133–143. doi: 10.1016/j.atmosenv.2013.11.035. [DOI] [Google Scholar]
- Bräuner EV, Forchhammer L, Møller P, Barregard L, Gunnarsen L, Afshari A, et al. Indoor Particles Affect Vascular Function in the Aged. Am J Resp Crit Care. 2008;177:419–425. doi: 10.1164/rccm.200704-632oc. [DOI] [PubMed] [Google Scholar]
- Bruce N, Pope D, Rehfuess E, Balakrishnan K, Adair-Rohani H, Dora C. WHO indoor air quality guidelines on household fuel combustion: Strategy implications of new evidence on interventions and exposure-risk functions. Atmos Environ. 2015;106:451–457. doi: 10.1016/j.atmosenv.2014.08.064. [DOI] [Google Scholar]
- Cheek E, Guercio V, Shrubsole C, Dimitroulopoulou S. Portable air purification: Review of impacts on indoor air quality and health. Sci Total Environ. 2021;766:142585. doi: 10.1016/j.scitotenv.2020.142585. [DOI] [PubMed] [Google Scholar]
- Chen R, Zhao A, Chen H, Zhao Z, Cai J, Wang C, et al. Cardiopulmonary benefits of reducing indoor particles of outdoor origin: A randomized, double-blind crossover trial of air purifiers. J Am Coll Cardiol. 2015;65:2279–2287. doi: 10.1016/j.jacc.2015.03.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Li H, Cai J, Wang C, Lin Z, Liu C, et al. Fine particulate air pollution and the expression of microRNAs and circulating cytokines relevant to inflammation, coagulation, and vasoconstriction. Envrion Health Perspect. 2018;126:017007. doi: 10.1289/EHP1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Meng X, Zhao A, Wang C, Yang C, Li H, et al. DNA hypomethylation and its mediation in the effects of fine particulate air pollution on cardiovascular biomarkers: A randomized crossover trial. Envrion Int. 2016;94:614–619. doi: 10.1016/j.envint.2016.06.026. [DOI] [PubMed] [Google Scholar]
- Chuang H-C, Ho K-F, Lin L-Y, Chang T-Y, Hong G-B, Ma C-M, et al. Long-term indoor air conditioner filtration and cardiovascular health: A randomized crossover intervention study. Environ Int. 2017;106:91–96. doi: 10.1016/j.envint.2017.06.008. [DOI] [PubMed] [Google Scholar]
- Cui X, Li F, Xiang J, Fang L, Chung MK, Day DB, et al. Cardiopulmonary effects of overnight indoor air filtration in healthy non-smoking adults: A double-blind randomized crossover study. Envrion Int. 2018;114:27–36. doi: 10.1016/j.envint.2018.02.010. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Dong W, Liu S, Chu M, Zhao B, Yang D, Chen C, et al. Different cardiorespiratory effects of indoor air pollution intervention with ionization air purifier: Findings from a randomized, double-blind crossover study among school children in Beijing. Environ Pollut. 2019;254:113054. doi: 10.1016/j.envpol.2019.113054. [DOI] [PubMed] [Google Scholar]
- Eggleston PA. Improving indoor environments: Reducing allergen exposures. J Allergy Clin Immun. 2005;116:122–126. doi: 10.1016/j.jaci.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Guo P, Yokoyama K, Piao F, Sakai K, Khalequzzaman M, Kamijima M, et al. Sick Building Syndrome by Indoor Air Pollution in Dalian, China. Int J Environ Res Pu. 2013;10:1489–1504. doi: 10.3390/ijerph10041489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habre R, Coull B, Moshier E, Godbold J, Grunin A, Nath A, et al. Sources of indoor air pollution in New York City residences of asthmatic children. J Expo Sci Environ Epid. 2013;24:269–278. doi: 10.1038/jes.2013.74. [DOI] [PubMed] [Google Scholar]
- Health Effects Institute. State of Global Air 2019. Special Report. MA: Health Effects Institute; Boston: 2019. [Google Scholar]
- Higgins JP, Thomas J. [accessed: 27 Jan 2021];Cochrane handbook for systematic reviews of interventions. 2019 Available: https://training.cochrane.org/handbook/current.
- Higgins JP, White IR, Anzures-Cabrera J. Meta-analysis of skewed data: combining results reported on log-transformed or raw scales. Stat Med. 2008;27(29):6072–92. doi: 10.1002/sim.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis JPA, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. Bmj. 2007;335:914–916. doi: 10.1136/bmj.39343.408449.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JPT H, Sally G. Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0; 2011. The Cochrane Collaboration; 2011. [accessed 25 Aug 2020]. Available: https://www.handbook.cochrane.org. [Google Scholar]
- Kajbafzadeh M, Brauer M, Karlen B, Carlsten C, van Eeden S, Allen RW. The impacts of traffic-related and woodsmoke particulate matter on measures of cardiovascular health: a HEPA filter intervention study. Occup Environ Med. 2015;72:394. doi: 10.1136/oemed-2014-102696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karottki DG, Spilak M, Frederiksen M, Gunnarsen L, Brauner EV, Kolarik B, et al. An indoor air filtration study in homes of elderly: cardiovascular and respiratory effects of exposure to particulate matter. Environ Health. 2013;12:116. doi: 10.1186/1476-069x-12-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepeis NE, Nelson WC, Ott WR, Robinson JP, Tsang AM, Switzer P, et al. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. J Expo Sci Environ Epidemiol. 2001;11:231–252. doi: 10.1038/sj.jea.7500165. [DOI] [PubMed] [Google Scholar]
- Lelieveld J, Pozzer A, Pöschl U, Fnais M, Haines A, Münzel T. Loss of life expectancy from air pollution compared to other risk factors: a worldwide perspective. Cardiovasc Res. 2020;116:1910–1917. doi: 10.1093/cvr/cvaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Cai J, Chen R, Zhao Z, Ying Z, Wang L, et al. Particulate matter exposure and stress hormone levels: A randomized, double-blind, crossover trial of air purification. Circulation. 2017;136:618–627. doi: 10.1161/CIRCULATIONAHA.116.026796. [DOI] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. Plos Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L-Y, Chen H-W, Su T-L, Hong G-B, Huang L-C, Chuang K-J. The effects of indoor particle exposure on blood pressure and heart rate among young adults: An air filtration-based intervention study. Atmos Environ. 2011;45:5540–5544. doi: 10.1016/j.atmosenv.2011.05.014. [DOI] [Google Scholar]
- Liu S, Chen J, Zhao Q, Song X, Shao D, Meliefste K, et al. Cardiovascular benefits of short-term indoor air filtration intervention in elderly living in Beijing: An extended analysis of BIAPSY study. Environ Res. 2018;167:632–638. doi: 10.1016/j.envres.2018.08.026. [DOI] [PubMed] [Google Scholar]
- Liu S, Huang Q, Wu Y, Song Y, Dong W, Chu M, et al. Metabolic linkages between indoor negative air ions, particulate matter and cardiorespiratory function: A randomized, double-blind crossover study among children. Envrion Int. 2020a;138:105663. doi: 10.1016/j.envint.2020.105663. [DOI] [PubMed] [Google Scholar]
- Liu W, Huang J, Lin Y, Cai C, Zhao Y, Teng Y, et al. Negative Ions Offset Cardiorespiratory Benefits of PM2.5 Reduction from Residential Use of Negative Ion Air Purifiers. Indoor air 2021. 2020b;31(1):220–228. doi: 10.1111/ina.12728. [DOI] [PubMed] [Google Scholar]
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- McGrath S, Zhao X, Steele R, Thombs BD, Benedetti A. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res. 2020;29(9):2520–2537. doi: 10.1177/0962280219889080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael SW, Jeffrey AS, Richard LC. Ultrafine particle removal and generation by portable air cleaners. Atmos Environ. 2008;42:5003–5014. doi: 10.1016/j.atmosenv.2008.02.011. [DOI] [Google Scholar]
- Milan A, Zocaro G, Leone D, Tosello F, Buraioli I, Schiavone D, et al. Current assessment of pulse wave velocity: comprehensive review of validation studies. J Hypertens. 2019;37(8):1547–1557. doi: 10.1097/HJH.0000000000002081. [DOI] [PubMed] [Google Scholar]
- Morishita M, Adar SD, D’Souza J, Ziemba RA, Bard RL, Spino C, et al. Effect of Portable Air Filtration Systems on Personal Exposure to Fine Particulate Matter and Blood Pressure Among Residents in a Low-Income Senior Facility. Jama Intern Med. 2018;178:1350–1357. doi: 10.1001/jamainternmed.2018.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münzel T, Sørensen M, Gori T, Schmidt FP, Rao X, Brook FR, et al. Environmental stressors and cardio-metabolic disease: Part II-mechanistic insights. Eur Heart J. 2017;38:557–564. doi: 10.1093/eurheartj/ehw294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newby DE, Mannucci PM, Tell GS, Baccarelli AA, Brook RD, Donaldson K, et al. Expert position paper on air pollution and cardiovascular disease. Eur Heart J. 2015;36:83–93. doi: 10.1093/eurheartj/ehu458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols WW, Singh BM. Augmentation index as a measure of peripheral vascular disease state. Curr Opin Cardiol. 2002;17(5):543–51. doi: 10.1097/00001573-200209000-00016. [DOI] [PubMed] [Google Scholar]
- Niu Z, Liu F, Li B, Li N, Yu H, Wang Y, et al. Acute effect of ambient fine particulate matter on heart rate variability: an updated systematic review and meta-analysis of panel studies. Environ Health Prev Med. 2020;25(1):77. doi: 10.1186/s12199-020-00912-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padro-Martinez LT, Owusu E, Reisner E, Zamore W, Simon MC, Mwamburi M, et al. A randomized cross-over air filtration intervention trial for reducing cardiovascular health risks in residents of public housing near a highway. Int J Environ Res Public Health. 2015;12:7814–7838. doi: 10.3390/ijerph120707814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of Two Methods to Detect Publication Bias in Meta-analysis. Jama. 2006;295:676–680. doi: 10.1001/jama.295.6.676. [DOI] [PubMed] [Google Scholar]
- Pope CAP, III, Bhatnagar A, McCracken JP, Abplanalp W, Conklin DJ, O’Toole T. Exposure to fine particulate air pollution is associated with endothelial injury and systemic inflammation. Circ Res. 2016;119:1204–1214. doi: 10.1161/circresaha.116.309279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CAP, III, Cohen AJ, Burnett RT. Cardiovascular Disease and Fine Particulate Matter. Circ Res. 2018;122:1645–1647. doi: 10.1161/circresaha.118.312956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S, Al-Kindi SG, Brook RD. Air Pollution and Cardiovascular Disease JACC State-of-the-Art Review. J Am Coll Cardiol. 2018;72:2054–2070. doi: 10.1016/j.jacc.2018.07.099. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2018. [accessed: 25 Dec 2020]. Available: https://www.R-project.org/ [Google Scholar]
- Ramachandran G, Adgate JL, Pratt GC, Sexton K. Characterizing Indoor and Outdoor 15 Minute Average PM2.5 Concentrations in Urban Neighborhoods. Aerosol Sci Tech. 2003;37:33–45. doi: 10.1080/02786820300889. [DOI] [Google Scholar]
- Rohatgi A. [accessed: 25 Jan 2021];WebPlotDigitalizer: Web based tool to extract data from plots, images, and maps. 2020 Version 4.2. Available: https://automeris.io/WebPlotDigitizer/
- Schlesinger RB. The health impact of common inorganic components of fine particulate matter (PM2.5) in ambient air: a critical review. Inhal Toxicol. 2007;19(10):811–32. doi: 10.1080/08958370701402382. [DOI] [PubMed] [Google Scholar]
- Shao D, Du Y, Liu S, Brunekreef B, Meliefste K, Zhao Q, et al. Cardiorespiratory responses of air filtration: A randomized crossover intervention trial in seniors living in Beijing Beijing Indoor Air Purifier StudY, BIAPSY. Sci Total Environ. 2017;603:541–549. doi: 10.1016/j.scitotenv.2017.06.095. [DOI] [PubMed] [Google Scholar]
- Srebot V, Gianicolo EA, Rainaldi G, Trivella MG, Sicari R. Ozone and cardiovascular injury. Cardiovasc Ultrasound. 2009;7:30. doi: 10.1186/1476-7120-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. Bmj Clin Res Ed. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- Tong Z, Chen Y, Malkawi A, Adamkiewicz G, Spengler JD. Quantifying the impact of traffic-related air pollution on the indoor air quality of a naturally ventilated building. Environ Int. 2016;89:138–146. doi: 10.1016/j.envint.2016.01.016. [DOI] [PubMed] [Google Scholar]
- Walzer D, Gordon T, Thorpe L, Thurston G, Xia Y, Zhong H, et al. Effects of Home Particulate Air Filtration on Blood Pressure: A Systematic Review. Hypertension. 2020;76:44–50. doi: 10.1161/hypertensionaha.119.14456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Wang Y, Di Q, Choirat C, Wang Y, Koutrakis P, et al. Short term exposure to fine particulate matter and hospital admission risks and costs in the Medicare population: time stratified, case crossover study. Bmj. 2019;367:l6258. doi: 10.1136/bmj.l6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichenthal S, Mallach G, Kulka R, Black A, Wheeler A, You H, et al. A randomized double-blind crossover study of indoor air filtration and acute changes in cardiorespiratory health in a First Nations community. Indoor air. 2013;23:175–84. doi: 10.1111/ina.12019. [DOI] [PubMed] [Google Scholar]
- Winston GJ, Palmas W, Lima J, Polak JF, Bertoni AG, Burke G, et al. Pulse pressure and subclinical cardiovascular disease in the multi-ethnic study of atherosclerosis. Am J Hypertens. 2013;26:636–42. doi: 10.1093/ajh/hps092. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The full dataset is available from the corresponding authors upon reasonable request.