Abstract
Lung and bladder cancers are mostly incurable due to early development of drug resistance and metastatic dissemination. Hence, better therapies that tackle these two processes are urgently needed to improve clinical outcome. We have identified RSK4 as a promoter of drug resistance and metastasis in lung and bladder cancer cells. Silencing this kinase, either through RNA interference or CRISPR, sensitised tumor cells to chemotherapy and hindered metastasis in vitro and in vivo in a tailvein injection model. Drug screening revealed several floxacin antibiotics as potent RSK4 activation inhibitors and trovafloxacin reproduced all effects of RSK4 silencing in vitro and in/ex vivo using lung cancer xenograft and genetically-engineered mouse models and bladder tumour explants. Through X-ray structure determination and Markov transient and Deuterium exchange analyses, we identified the allosteric binding site and revealed how this compound blocks RSK4 kinase activation through binding to an allosteric site and mimicking a kinase auto-inhibitory mechanism involving the RSK4’s hydrophobic motif. Last, we show that patients undergoing chemotherapy and adhering to prophylactic levofloxacin in the large placebo-controlled randomised phase 3 SIGNIFICANT Trial had significantly (p =0.048) increased long-term overall survival times. Hence, we suggest that RSK4 inhibition may represent an effective therapeutic strategy for treating lung and bladder cancer.
Introduction
Lung and bladder cancers are two smoking-related malignancies that are mostly incurable due to the early development of drug-resistant metastatic disease. Hence, a better understanding of the molecular mechanisms regulating these processes will help develop therapeutic strategies against these malignancies.
The p90 Ribosomal Protein S6 kinases (RPS6KAs, aka RSKs) are dual kinase domain protein kinases involved in multiple processes including protein translation, cell growth, and migration (1). Four isoforms exist in humans (RSK1-4) of which RSK1 and 2 are most studied in cancer. Their biological functions are assumed to overlap with RSK3 and 4 because these proteins have a high degree of sequence identity, especially in their N-terminal kinase domain that mediates substrate phosphorylation. However, our previous work challenged this notion. Indeed, RSK1 differed from other isoforms in its regulation of lung cancer cell invasion as silencing of this kinase promoted metastasis in vitro (8). This suggested that the use of pan-RSK inhibitors may not be optimal for anticancer treatments (2). Moreover, RSK4 differs from other RSKs in its activation mechanism, with this isoform only requiring ERK whereas RSK1-3 need both ERK and PDK1 (1). Therefore, we hypothesise that RSK4 has divergent functions and activation patterns from other RSK isoforms.
The role of RSK4 in cancers is controversial due to conflicting experimental data. A tumour suppressor function of RSK4 was suggested in MDA-231 breast cancer cells where RSK4 overexpression leads to cell cycle arrest, reduced clonogenic growth in vitro, and reduced tumour growth and dissemination in vivo (3). In colon carcinoma cell lines, RSK4 overexpression triggered senescence (4) consistent with large RNAi screens where RSK4 downregulation prevented p53-induced senescence (5). In addition, in breast, colon and renal carcinoma, RSK4 mRNA expression was downregulated in tumour compared to normal tissues (1). In contrast, several studies in the same or different cancers suggest a tumour-promoting function for RSK4. Thus, over-expression of RSK4 enhanced whereas knockdown impaired cell migration and sunitinib resistance in renal and melanoma cell lines (6). Furthermore, RSK4 is over-expressed in breast and renal cancer at either the protein or mRNA levels (1) and in renal cancers this associated with poor patient prognosis (7). Moreover, RSK4 overexpression promoted, and silencing reduced, invasiveness of renal cell adenocarcinoma cells in vitro. One plausible explanation for these conflicting data is that RSK4 may act as a tumour suppressor or promoter in a context- or disease-dependent manner.Apart from our study demonstrating that RSK4 knockdown prevented migration and invasion of lung adenocarcinoma cells (8), the role of this kinase in lung and bladder cancer has not previously been investigated.
Here, we investigate the role of RSK4 in mediating lung and bladder cancer cell invasion as well as sensitization to chemotherapy both in vitro and in vivo through genetic manipulation (silencing and overexpression) and identify potential mechanisms to explain the effects seen. We also, screen known drugs to identify drugs or drug families which could be re-purposed as RSK4 inhibitors and crystalise part of RSK4 and undertake Markov transient and deuterium exchange analyses to assess their mechanism of action. We further test whether one of these drugs reproduces our genetic silencing data in vitro and in vivo and search for cancer patient trials that have previously used members of the drug family of interest. One such trial is found and re-analysed for whether the re-purposed drug in combination with chemotherapy could prolong survival of lung and bladder plus other cancer patients.
Results
siRNA screening reveals RSK4 as a regulator of chemosensitivity in lung and bladder cancer cells
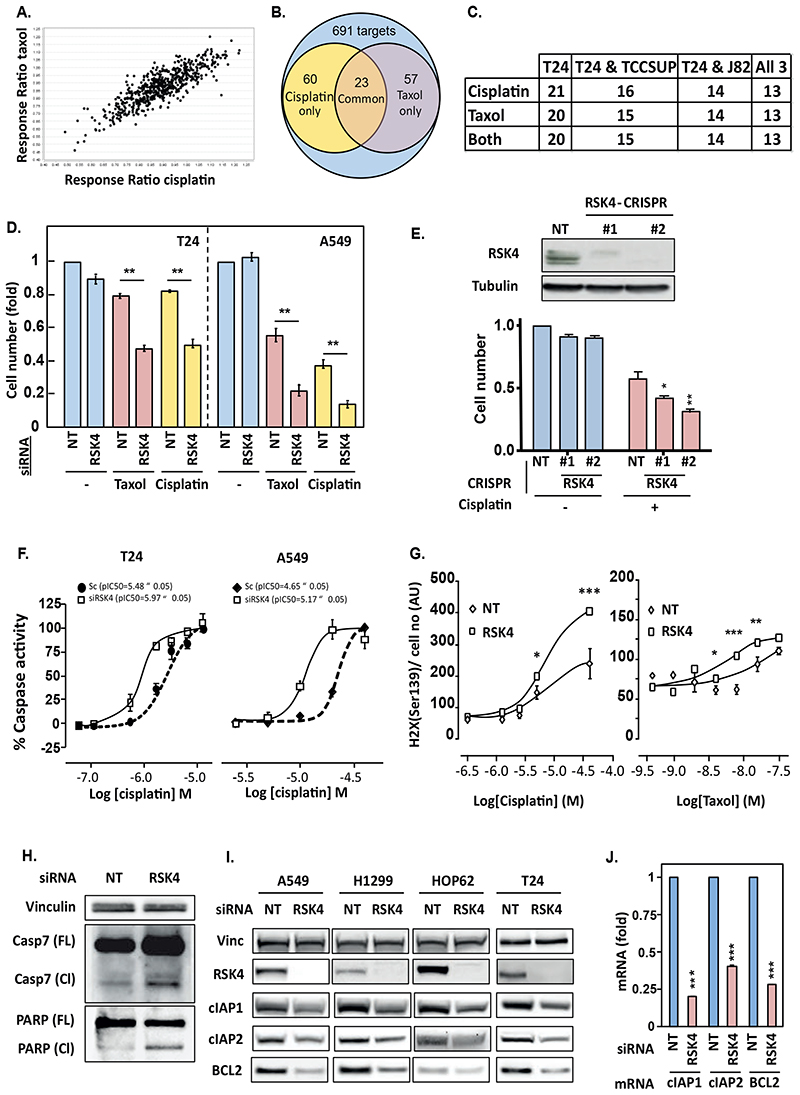
To identify common regulators of chemo response in lung and bladder cancer, we performed a kinome siRNA screen in T24 bladder cancer cells treated with or without taxol or cisplatin and compared this to our prior screen in lung cancer cells (9). Four individual siRNA sequences targeting each kinase were used and hits selected for validation based on at least 2 sequences similarly modulating drug sensitivity (fig S1A-C). Amongst kinases found to control drug sensitivity (Fig 1A), 23 regulated the response to both cisplatin and taxol (Fig 1B, fig. S1C), and 13 of these were cross-validated in 2 additional bladder cancer cell lines, TCCSUP and J82 (Fig 1C, fig. S1D). Cross-correlating these results with those previously-published for A549 lung adenocarcinoma cells (9) revealed RSK4 (aka RPS6KA6) as a potential common target between lung and bladder cancer. Indeed, we confirmed that silencing RSK4 sensitised both bladder and lung cancer cells to taxol and cisplatin (Fig 1D). This was reproduced in additional lung cancer cell lines (fig S2A) independently from the modality of RSK4 downregulation as two A549 clones in which RSK4 was knocked-out using CRISPR technology showed similar drug sensitisation (Fig 1E). This was associated with potentiation of apoptosis and increased DNA damage as assessed by caspase 3/7 activation (Fig 1F) and elevated phospho-H2AX nuclear foci (Fig 1G), respectively. However, RSK4-silenced cells did not show decreased cell viability in the absence of drug (Fig 1D), despite increased total caspase 7 expression, as well as cleavage of caspase 7 and PARP (Fig 1H, fig. S2B), suggesting that RSK4-knockdown only primes the apoptotic process. Conversely, RSK4 overexpression made A549 cells chemoresistant (fig S2C), with decreased background caspase activation and PARP cleavage (fig S2D). The increased caspase 3/7 activity in RSK4-downregulated cells was rescued by RSK4 overexpression (fig S2E). In multiple NSCLC and T24 cell lines, sensitisation to chemotherapy was associated with decreased expression of the anti-apoptotic proteins BCL2, cIAP1 and cIAP2 that we previously showed promote chemo-resistance in lung cancer (Fig 1I, fig. S2F) (10). This decrease occurred at the transcriptional level (Fig 1J). Similar decreases in cIAP1 and cIAP2, but not BCL2, occurred in A549 RSK4-CRISPR clones (fig S2G), which may indicate that cIAP changes are more relevant to chemosensitisation in this biological context. Moreover, cIAP2 is likely the major mediator of the RSK4-induced chemoresistance as only cIAP2 mRNA and protein expression were increased by RSK4 overexpression in A549 cells (fig S2H-I) and cIAP2 but not cIAP1 rescued chemosensitisation following RSK4 silencing (fig S2J). In short, RSK4 downregulation promotes cell death in several lung and bladder cancer cell lines treated with two clinically relevant chemotherapeutic compounds.
Figure 1. RSK4 downregulation sensitises lung and bladder cancer cell lines to chemotherapy.
(A-C) A kinome siRNA screen reveals modulators of taxol and cisplatin response in T24 bladder cancer cells. (A) Dot plot of ratio of cell number change in response to drug treatment for cells silenced for individual targets over those transfected with non-targeting (NT) sequences. (B) Venn diagram of targets modulating response to cisplatin with or without taxol. (C) Number of validated targets in T24, TCCSUP, and J82 bladder cancer cells out of the 23 screen hits. (D) Effect of siRNA-mediated silencing in T24 or A549 or (E) CRISPR-mediated knock-out of RSK4 in A549 cells on cell survival to cisplatin (T24-3.5 μM and A549-12.5 μM) or taxol (T24-40 nM and A549-58 nM). (E-upper) Lysates from RSK4 CRISPR clones (#1,#2) analysed by Western blotting (WB) for RSK4 knock-out efficiency. (E) #1 and #2 refer to two separate clones of RSK4-CRISPR cells. (F) RSK4 silencing effect on drug-induced caspase-3/7 activation and (G) DNA damage in T24 and A549 cells. Cells transfected with RSK4-siRNA for 48h were treated with cisplatin or taxol and substrate-based caspase activity or γH2AX expression measured. (H) RSK4 silencing effect on baseline apoptotic pathway activity in A549 cells. (I) RSK4 downregulation effect on BCL2, cIAP1, and cIAP2 expression. (J) A549 cells treated with siRNA for 48h analysed by RT-qPCR. (A, D and E) Cell number assessed by Crystal violet staining and normalised to the corresponding control condition. (A) Data are average of four individual siRNA sequences per target. (D-J) Data representative of n=3. (D-G and J) Data are mean ± SEM of n=4. (D, H, I) Tubulin or vinculin was used as loading control.
RSK4 controls the migration and invasion of lung and bladder cancer cells
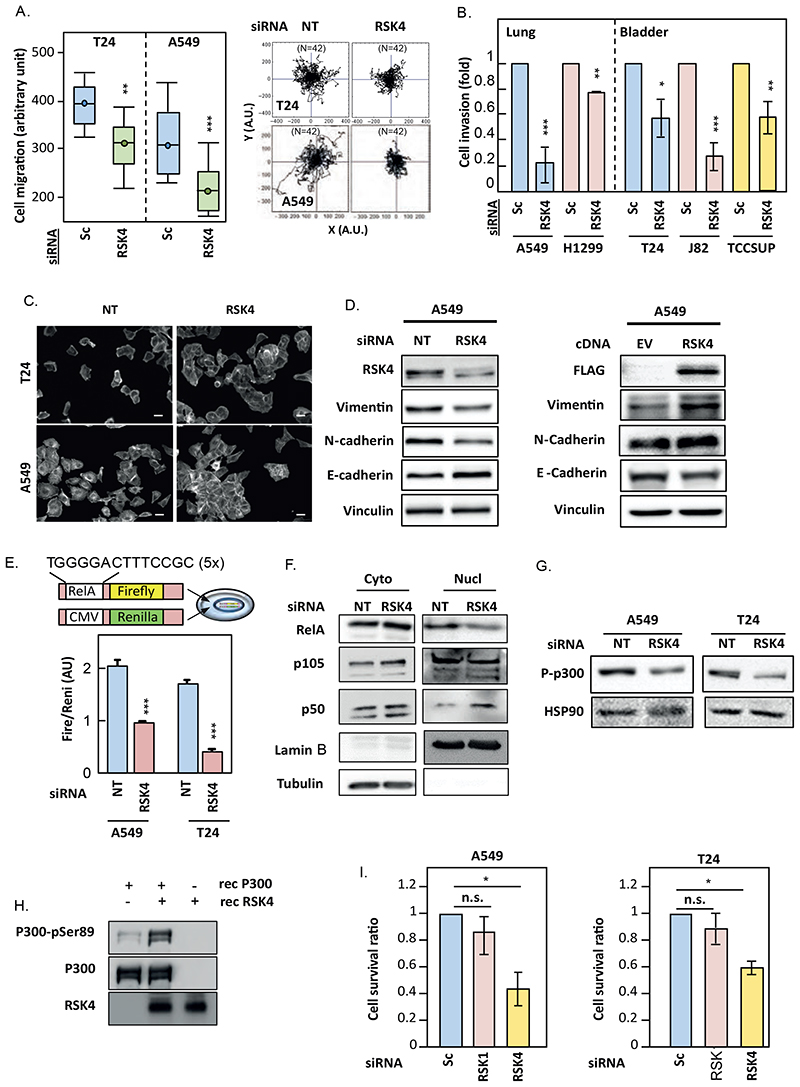
We previously published that RSK4 silencing decreased the migration and invasion of A549 cells (8). Similarly, RSK4 knockdown hindered the migration and invasion of bladder and lung cancer cell lines (Fig 2A, 2B, fig. S3A), whereas RSK4 overexpression promoted migration of A549 cells (fig S3B). Microscopic image analysis of RSK4-silenced cells revealed increased cell clustering and roundness (Fig. 2C, fig. S3C), suggesting a reversal of epithelial-mesenchymal transition (EMT). In agreement, analysis of lysates from RSK4-silenced and control cells for EMT markers revealed decreased vimentin and N-cadherin and increased epithelial marker E-cadherin (Fig. 2D -left, fig. S3D-E).
Figure 2. RSK4 downregulation inhibits the pro-metastatic program.
(A-G) A549 and T24 cells transfected with/without RSK4, RSK1 and non-targeting (NT) siRNAs or RSK4 and empty-vector (EV) cDNAs were analysed 48h or 72h later. (A) Random-walk assays determined cell speed (left) and single-cell trajectories (right). (B) Cells were covered with collagen-I and overlaid with EGF for chemotaxis. 48h later, cells were stained with Cytox Green before confocal imaging to determine the extent of invasion. Data are mean ± SEM of 36 fields-of-view/condition. (C) Cells were stained for actin before fluorescent microscopy. Images are representative of 36 fields-of-view/condition. Scale bar: 10μm. (D) Cell lysates were analysed by SDS-PAGE/WB. Vinculin was used as loading controls. (E) Cells transfected with a RelA-Firefly luciferase reporter construct and a Renilla luciferase control vector were then transfected with siRNAs and lysates and analysed for Firefly luciferase activity normalised to the Renilla luciferase control. (F) cytoplasmic (Cyto) and nuclear (Nuc) fractions were analysed by SDS-PAGE/WB. Ubulin and Lamin B were used as loading controls. (G) Cell lysates were analysed by SDS-PAGE/WB. HSP90 was used as a loading control. (H) Recombinant p300 (1μg) was mixed with or without recombinant RSK4 (200ng) and γATP for 5 min prior to SDS-PAGE/WB analysis. (I) A549 and T24 cells transfected with/without RSK4, RSK1 and non-targeting (NT) siRNAs were split equally 48h later between adherent and ultra-low adhesion plates for 24h prior to re-adhesion. Cell numbers were determined by Crystal violet staining and ratio of suspension-treated to only-adherent cultures represented as mean ± SEM of n=4. Data are representative of at least biological triplicates. Statistics: Student’s t-test (B, I and E) and ANOVA (A) with n.s.; not significant, *; p<0.05, **; p<0.01, ***; p<0.001.
Conversely, RSK4 overexpression had the opposite effect (Fig. 2D -right, fig. S3F). The decreased cell migration observed after siRNA-mediated RSK4 silencing was reproduced in RSK4-CRISPR A549 cells and rescued by RSK4 overexpression, demonstrating the RSK4-selectivity of this phenotype (fig. S3G). EMT is partly controlled by NFκB (11) and reduced expression of EMT markers after RSK4 downregulation associated with decreased NFκB activity (Fig. 2E, fig. S3H) and decreased RELA nuclear localisation (Fig. 2F). This suggested that NFκB activity is controlled by RSK4. RELA nuclear accumulation is negatively regulated through acetylation on K122 and K123 by the acetyl-transferase p300 (12). Figure 2G shows that Ser89 phosphorylation of p300, an event that inhibits p300’s acetyltransferase activity (13), was decreased in RSK4-silenced A549 and T24 cells. Consequently, RSK4 may regulate the activity of the NFκB pathway by direct phosphorylation of p300, as recombinant RSK4 phosphorylates p300 on Ser89 in vitro (Fig. 2H). The role of NFkB in RSK4’s effects on EMT markers and cell migration were confirmed by active-RELA overexpression reverting the changes in EMT markers and decreased cell migration in RSK4-downregulated A549 cells (fig. S4A-B). In contrast, RSK4-induced chemo-resistance was not mediated by RELA, as active-RELA overexpression failed to prevent drug sensitisation or decreased cIAP2 expression in RSK4-downregulated cells (fig. S4C-E). Although changes in cell migration and the actin cytoskeleton play important roles in cellular invasion, resistance to anoikis is crucial to metastatic dissemination, as this maximises the survival of circulating cancer cells (14). Figure 2I shows that unlike RSK1-silenced or non-targeted cells, RSK4-downregulated A549 and T24 cells underwent cell death when placed in suspension for 18h. Taken together, our data suggest that RSK4 silencing impairs the metastatic program of lung and bladder cancer cells.
RSK4 is overexpressed in lung cancer and correlates with decreased survival in patients with adenocarcinoma
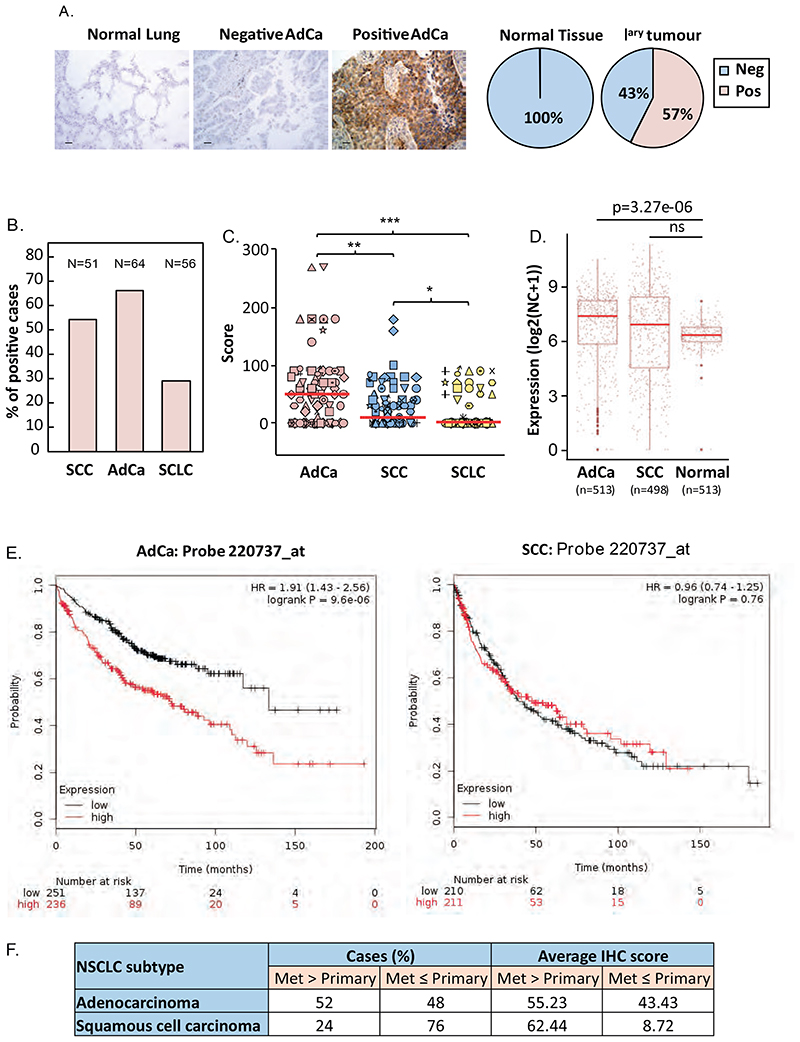
To assess the potential clinical relevance of our findings, we analysed tissue microarrays of normal and lung cancer tissue samples for RSK4 protein expression. Staining optimisation using paraffin-embedded A549 cells silenced or not for RSK4 demonstrated that immunoreactivity depended on RSK4 expression, therefore confirming the antibody’s specificity (fig. S5A), similarly to that observed in Western blotting (fig. S5B). Figure 3A shows that whereas RSK4 was undetectable in all normal lung specimens, 57% of primary lung cancers (n=97) were positive for this kinase. Western blotting of NSCLC and normal lung epithelial cell lines showed similar differences, suggesting that RSK4 expression in our cell lines was representative of that seen in the clinic (fig. S5C). Stratifying our clinical samples for lung cancer subtypes showed that the frequency and intensity of RSK4 expression was highest in adenocarcinoma (AdCa), decreased in squamous cell (SCC) NSCLC and lowest in small cell lung cancers (SCLC) (Fig. 3B-C). Similar differences were observed at mRNA level in TCGA datasets where lung AdCa, but not SCCs showed a significant (p=3.27e-06) increase in RSK4 expression over normal lung (Fig. 3D). To test whether RSK4 was important in lung AdCa, we analysed publically available lung cancer survival data. When all lung cancer subtypes were considered, high RSK4 mRNA expression associated with poorer prognosis (fig. S5D). However, when subtypes were separated, high-expressing AdCa showed significant decreased overall survival (OS) (p= 9.6 10-6) whereas survival of patients with SCC was unaffected (Fig. 3E). This association between RSK4 expression and OS existed despite RSK4 expression being far lower than other RSK isoforms across all lung cancers or AdCa alone (fig. S5E). Amongst other RSKs, only RSK3 showed increased mRNA expression in AdCa compared to normal lung samples, whereas RSK2 showed decreased and RSK1 unchanged expression (fig. S5F). Nevertheless, a negative association between RSK expression and survival was only observed for RSK4 as similar analysis for other RSK isoforms showed that high expression either did not impact (RSK1) or was associated with improved OS (RSK2 and RSK3) in patients with AdCa (fig. S5G). Similar to the case of lung AdCa, analysis of bladder cancer samples showed lower RSK4 mRNA expression as compared to other RSKs (fig. S6A), and RSK4 overexpresion in cancer as compared to normal bladder samples (fig. S6B). Last, consistent with our in vitro data showing that RSK4 mediates the invasiveness of lung AdCa cells, we found that 52% of AdCa metastatic lesions expressed more RSK4 protein than their corresponding primary tumours, whereas this number fell to 24% in SCC (Fig. 3F). These results are also consistent with the difference in correlation between OS and RSK4 expression in patients with lung AdCa and SCC. Taken together, our data suggest that RSK4 over-expression as a predictor of survival might be most relevant in lung AdCa.
Figure 3. RSK4 is overexpressed in lung cancer and correlates with poor survival in patients with lung adenocarcinoma.
(A-C) Normal (n=25) and cancerous (n=183) lung FFPE samples were stained with H&E or a RSK4 antibody followed by 3,3’-Diaminobenzidinedevelopment. (A-left) Examples of RSK4 staining in normal lung and RSK4-negative and -positive adenocarcinoma (AdCa) samples. Scale bar: 30 μm. (A-right and B) Percentage of samples showing positive (pos) or negative (neg) RSK4 staining shown for (A) all primary (Iary) lung cancer samples or (B) lung cancer (LC) subtypes. SCC; squamous cell carcinoma, SCLC; small-cell LC. (C) RSK4 staining semi-quantitative scores for each LC subtype. (D) Comparison of RSK4 mRNA expression between AdCa, SCC, and normal lung samples in TCGA RNA-Seq data. Statistics: t-test; ns, not significant. (E) Overall survival of patients with AdCa or SCC with high or low tumour RSK4 mRNA expression. Cut-off: median value. (F) Comparison of post-mortem matched primary and metastatic AdCa (n=26) and SCC (n=20) lesions for RSK4 IHC staining scores. Percentage of cases where metastatic lesions expressed more (Met>Primary) or less (Met≤Primary) RSK4 than the primary site. Average IHC scores are shown. Statistics: (C) ANOVA; ***; p<0.001, **; p<0.005 and *; p<0.05.
RSK4 silencing impairs tumour progression and maximises therapy response in vivo
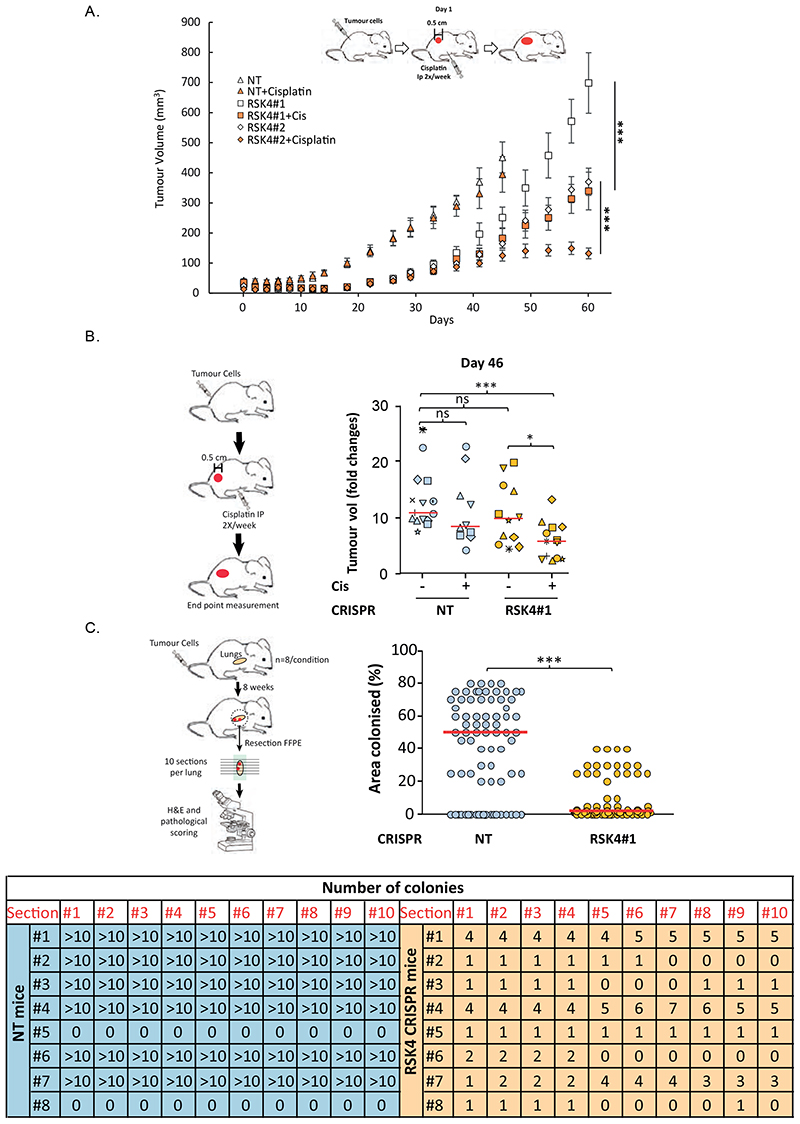
We next assessed the effects of RSK4 silencing on tumour growth in vivo using A549 non-targeting or RSK4-CRISPR cells injected subcutaneously into nude mice. Tumour growth was noticeably delayed for both RSK4-CRISPR clones tested as compared to their non-targeted counterpart (Fig. 4A). Moreover, potentiation of cisplatin response was seen for RSK4-CRISPR as compared to non-targeted tumours (Fig. 4A-B). Because RSK4 silencing imparied cell invasion and anoikis in vitro, we also assessed metastasis in vivo. RSK4-CRISPR or non-targeted A549 cells were injected into the tail vein of Severe Combined Immunodeficiency (SCID) mice and their lungs histologically examined 2 months later. This revealed a decreased ability of RSK4-CRISPR cells to colonise the lungs of injected mice (Fig 4C, fig. S7). Hence, RSK4 downregulation hinders lung adenocarcinoma tumour growth and dissemination in vivo.
Figure 4. RSK4 downregulation impairs growth of lung adenocarinoma xenografts, sensitises to chemotherapy, and reduces metastatic potential of lung cancer cells.
(A-B) Non-targeting (NT) or RSK4 CRISPR A549 cells were injected subcutaneously in nude mice and tumour growth rate (A) or fold volume change at Day-46 following treatment cycle with/without cisplatin (B) monitored. (C) A459 NT or RSK4 CRISPR cell clone #1 were injected through the tail vein of SCID mice. Animals were sacrificed 2 months later and their lungs paraffin-embedded. 10 sections (each separated by 60μm) were H&E stained and numbers of (lower), and lung area colonised by (upper) tumour nodules quantified. Upper: dots represent individual lung sections. Statistics: ANOVA (A-B) and Student’s t-test (C). n.s.; not significant, *; p<0.05, **; p<0.01, ***; p<0.001.
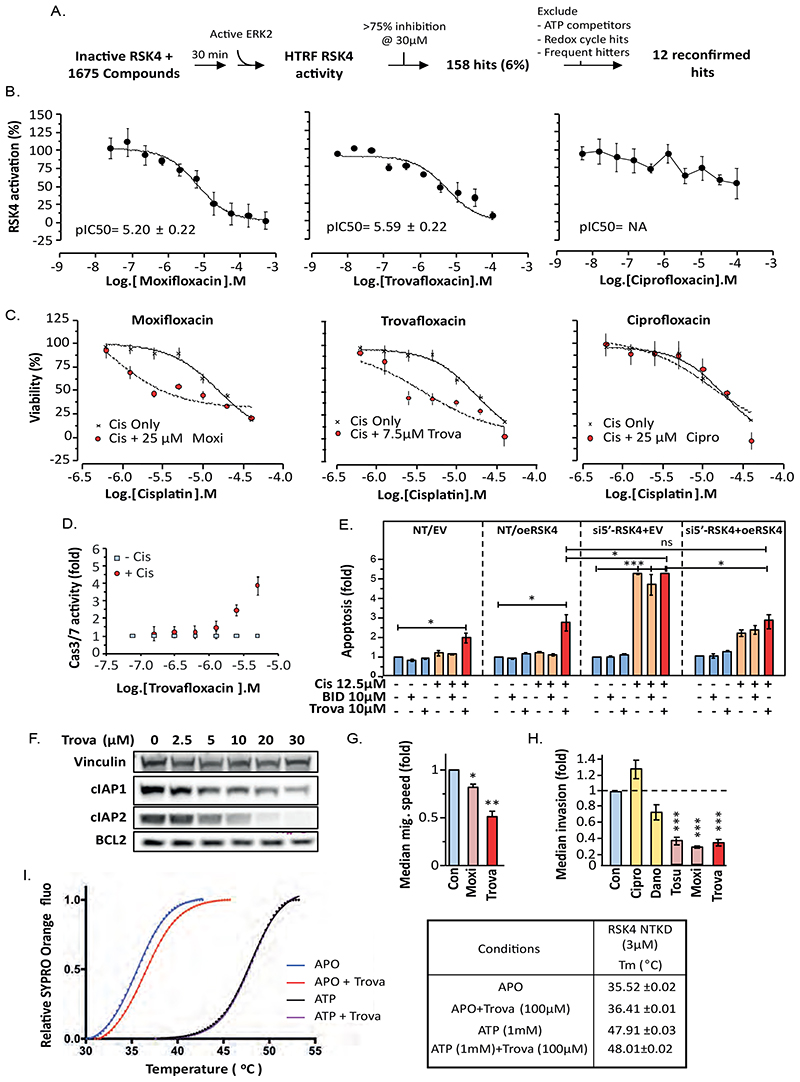
Small compound library screening identifies moxifloxacin as a RSK4 activation inhibitor
Our data suggested that RSK4 inhibition may be clinically beneficial in the treatment of lung adenocarcinoma. Although pan-RSK inhibitors exist (15) (16), we previously published that RSK1 downregulation promotes lung cancer invasiveness (8). Here, we reproduce this finding by treating A549 cells with the pan-RSK inhibitor SL-0101 (fig. S8A). Moreover, contrary to the results obtained for RSK4, RSK1 silencing in bladder and lung cancer cells induced resistance to taxol and cisplatin (fig. S8B) and increased cIAP2 expression (fig. S8C). Last, unlike RSK4, RSK1 was well expressed in normal lung epithelial cells as aforementioned. Hence, inhibiting this kinase as part of therapeutic targeting of RSK4 may yield distinct toxicity and loss of anti-cancer effects. Consequently, we wished to identify selective RSK4 inhibitors. We therefore developed a high-throughput Homogeneous Time-Resolved Fluorescence (HTRF)-based assay to identify molecules interfering with RSK4 activation by ERK as, unlike other RSKs, RSK4 does not require PDK1 (1). Because bacterially-produced recombinant RSK4 showed high basal activity in vitro, we first inactivated it through dephosphorylation with the phosphatase PP1MA before incubation with test compounds and subsequent re-activation by ERK2 (Fig. 5A, fig. S8D). This favoured the selection of molecules likely to be non-ATP competitive. We screened 1675 molecules and discovered twelve compounds showing >75% inhibition of RSK4 activation at 30 μM, amongst which was the antibiotic, moxifloxacin (Fig. 5B). This molecule acted as a micromolar (IC50=6.2 μM) non-ATP competitive reversible inhibitor of RSK4 activation (fig. S8E-F) and did not interfere with ERK activity in contrast to the well documented ATP-competitive pan-RSK inhibitor, BI-D1870 (fig. S8G). Increasing the concentration of RSK4 by 10 fold did not modify the efficiency of moxifloxacin, suggesting it does not act by degrading RSK4 (fig. S8E). Time-dependence inhibition studies showed a linear relationship with product formation in the presence of moxifloxacin or BI- D1870 (fig. S8H), indicating that moxifloxacin is not a slow binder and that mechanism of action studies could be performed using classical steady-state analysis. Michaelis Menten kinetics confirmed that in the presence of sub-maximal inhibition by BI-D1870, RSK4 maintained ATP Vmax but showed a trend to increased Km in agreement with the ATP competitive nature of this inhibitor (fig. S8J-K). In contrast, when incubated with moxifloxacin, RSK4 maintained Km and displayed reduced Vmax (α = 1), confirming ATP non-competitive binding equally to the Enzyme and Enzyme-Substrate state of the enzyme (fig. S8I-K). Moreover, Moxifloxacin showed a preference for preventing RSK4 activation rather than inhibiting fully-active RSK4 and further favoured RSK4 over RSK1 (fig. S8L), making this compound a useful chemical starting point for future synthesis of RSK4-selective inhibitors.
Figure 5. Trovafloxacin, an inhibitor of RSK4 activation, reproduces the biological effects of RSK4 silencing.
(A) Diagram of the drug screening process. (B) Moxifloxacin, trovafloxacinand ciprofloxacin differ in their ability to inhibit activation of RSK4 by ERK2 in HTRF assays. (C-D) A549 cells incubated with the indicated concentration of floxacin for 1h were treated with cisplatin (12.5 μM) for 72 h (C) or 24 h (D). (C) Cell viability determined using Alamar Blue. (D) Caspase activity determined using a substrate-based fluorigenic assay. (E) Relation of cisplatin sensitisation by trovafloxacin in A549 cells to RSK4 expression. NT; non-targeting siRNA, EV; empty-vector DNA, oeRSK4; overexpressed RSK4, si5’UTR-RSK4; 5’UTR RSK4 mRNA-directed siRNA. (F) Lysates from A549 cells treated with or swithout trovafloxacin (Trova) for 24 h were analysed by SDS-PAGE/WB. Vinculin used as loading control. (G) Random cell migration or (H) 3D collagen-I invasion in A549 cells treated with indicated inhibitors (10 μM). Dano; danofloxacin, Tosu; tosufloxacin tosylate, Moxi; moxifloxacin. (I) Trovafloxacin binding tos inactive (dephosphorylated, APO) and not fully-active (ATP) RSK4-NTKD. Tm-shift assay performed over the indicated temperature range (left) and parameters recorded (right). (B-H) Results representative of at least three independent repeats. Data are mean (B-D and F) or median (G-H) of n=4 ± SEM. Statistics: ANOVA. *; p≤0.05, **; p≤0.01, ***; p≤0.001.
Trovafloxacin reproduces the effects of RSK4 silencing in vitro
Following our results with moxifloxacin, we screened other floxacin family members in our RSK4 HTRF-activation assay and identified both additional inhibitors (trovafloxacin, marbofloxacin, rufloxacin, tosufloxacin) as well as inactive analogues (ciprofloxacin, danofloxacin, and piromidic acid) (Fig. 5B, fig. S9A). As all these compounds are active antibiotics, we assumed that their RSK4-targeting activity was independent of their known clinical indication. However, no clear structure-activity relationship was readily identifiable (fig. S10). Crucially, only the compounds active against RSK4 sensitised A549 cells to cisplatin (Fig. 5C, fig. S11A). In particular, trovafloxacin showed superior abilities to moxifloxacin in this assay with 7.5 μM of this compound reducing the IC50 for cisplatin (Fig. 5C). This relationship was synergistic (fig. S11B) and the potentiation of cisplatin or taxol effects maximal when combined with ≈10 μM trovafloxacin (fig. S11B-C). This correlated with promotion of caspase activation in response to drug treatment in A549 cells (Fig. 5D), an effect dependent on the expression of RSK4. Indeed, RSK4 silencing using a 5’UTR-targeting siRNA prevented potentiation of caspase activation by trovafloxacin over that obtained with cisplatin alone whereas simultaneous RSK4 overexpression rescued this difference (Fig. 5E). In contrast, the pan-RSK inhibitor BI-D1870 failed to promote cisplatin-induced caspase activation (Fig. 5E). As with RSK4 silencing, increased apoptosis in response to trovafloxacin associated with decreased expression of BCL2 and cIAP1/2, an effect not seen with either ciprofloxacin (non-RSK4 inhibitor) or BI-D1870 (Fig 5F, fig. S11D). Moreover, trovafloxacin treatment reproduced the inhibition of cell migration/invasiveness (Fig. 5G-H, fig. S11E-F) and EMT reversal (fig. S11G) previously observed with RSK4 silencing in A549 cells. Other RSK4-targeting floxacins (moxifloxacin, tosufloxacin) shared this ability, whereas non RSK4-targeting compounds (ciprofloxacin, danofloxacin) did not impair cell invasiveness (Fig. 5H). In addition, differential scanning fluorimetry assays demonstrated that trovafloxacin bound the inactive/dephosphorylated, but not the fully-active, RSK4 N-terminal kinase domain (RSK4-NTKD) (Fig. 5I), supporting the selectivity profile previously seen for moxifloxacin. In contrast, ciprofloxacin failed to bind RSK4 in the same assay (fig. S11H), explaining why this compound fails to inhibit this kinase. Last, we tested 10 μM trovafloxacin, corresponding to the IC50 of this drug on recombinant RSK4, against a panel of 140 kinases in an in-vitro activity screen. No other kinase was substantially inhibited by trovafloxacin at this concentration (Supplementary Excel data file). In short, trovafloxacin appears to be a reasonably-selective allosteric RSK4-NTKD activation inhibitor.
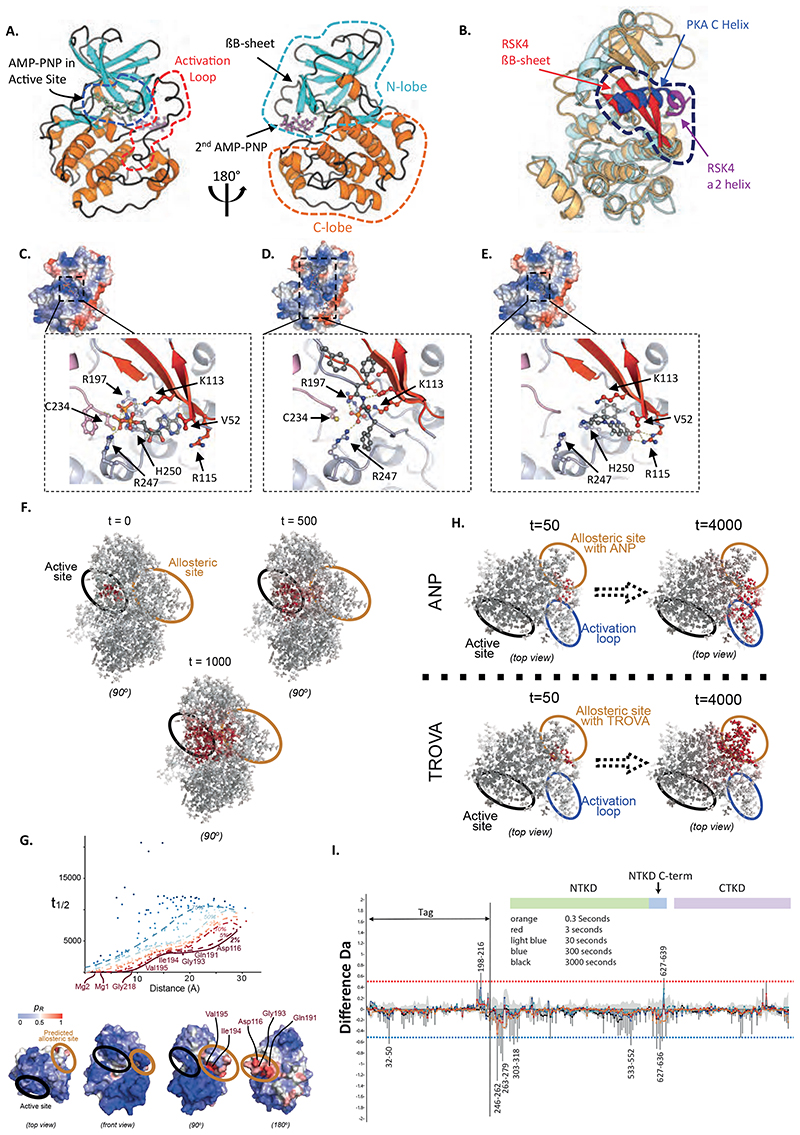
RSK4-NTKD crystal structures
To obtain structural and mechanistic information on RSK4 and elucidate how it might be allosterically regulated, we solved the crystal structures of the phosphorylated (pS232) and dephosphorylated RSK4-NTKD, as well as the S232E phospho-mimetic mutant (table S1, fig. S12A-C). All three structures display a classic bilobal protein kinase fold (Fig. 6A), with the activation loop, which runs between the C- and N-lobes, well-ordered and visible in dephosphorylated and pS232 RSK4-NTKD structures. The overall folding of these NTKD versions is identical, with an RMSD of 0.35 Å between RSK4 NTKD chain A and RSK4 NTKD S232E chain A, and 0.33 Å between RSK4 NTKD chain A and RSK4 NTKD pS232 chain A.The two molecules in the asymmetric unit of the RSK4 NTKD pS232 are in the same orientation seen for the dephosphorylated protein, with only 2.6 Å translation of chain B in respect to its relative position in RSK4 NTKD when using chain A as reference. During the refinement stage of RSK4 NTKD pS232, we found a well-defined electron density on the activation loop of chain A in which it was possible to model the phosphate of phosphorylated S232 (fig. S12A). The phosphorylation is oriented inward and points towards D197, part of the HRD motif, and, at the same time, the side chain of D197 moves ~1.3 Å closer to the activation loop compared to its relative position on the dephosphorylated structure.
Figure 6. RSK4-NTKD structure and trovafloxacin binding site prediction.
(A) Structure of dephosphorylated RSK4-NTKD. (B) Overlay of dephosphorylated RSK4-NTKD and PKA structures showing the characteristic βB-sheet and α2-helix of RSK4-NTKD as opposed to the C-helix of classical AGC kinases. (C-E) Docking of second AMP-PNP (C), the phospho-HM peptide (D) and trovafloxacin (E) to the RSK4-NTKD. Inserts: view of interaction residues. Colours: βB-sheet; red, activation; teal, H-bonds; yellow dotted lines. AMP-PNP, trovafloxacin and hydrophobic motif FXXF-pS-F residues coloured by element. Key binding site residues shown in ball-and-stick. (F-G) Identification of an allosteric hotspot in RSK4 through Markov transient analysis. (F) Propagation of a random walk on the protein graph, originating at the active site showingactive site and a hotspot where trovafloxacin and AMP-PNP bind. The evolution of the probability distribution (shown at t=0, 500, 1000 sec; atoms coloured from white to red with increasing probability) is shown relative to the trovafloxacin binding site and the upper and lower lobes of RSK4. (G) Top: From the RSK4 structure and location of its active site, the pathway is extracted through statistical quantile regression of the Markov transient times of the random walk, t1/2, against distance. Low quantile scores indicate residues highly connected to the active site (relative to their distance). Bottom: Quantile score (pR) of each residue mapped onto RSK4’s surface (different views). Residues below the 2% quantile connect the active (black ellipse) and the allosteric (green ellipse) sites. (H) The propagation of the random walk originating from AMP-PNP or trovafloxacin binding at the allosteric site exhibits different communication pathways. (I) Deuterium exchange mass-spectrometry for RSK4 incubated with or without trovafloxacin. Difference plot obtained by subtracting the incorporation of deuterium in the absence versus in the presence of trovafloxacin. Colours: Experimental error; light grey, other colours represent different D2O incubation times. Upper half of graph; more exposed regions, lower half; protected regions. RSK4 amino acid numbers are given for regions showing significant protection or exposure as indicated by being above or below the dotted red and blue lines (98% confidence limit).
In all three structures the top part of the C-helix, present in classical protein kinase topology (17), is disordered with the bottom part forming a helix that swings away from its classical position in PKA by up to 12 Å (Fig. 6B). In this new position, the C-helix is not involved in any catalytic activities. In its place lies the βB-sheet formed by β1-β5-β10 (residues: 52-56, 109-115, 220-222). The βB-sheet has been previously seen in both MSK1- and RSK2-NTKD crystal structures (18, 19), suggesting that this motif may characterise AGC family members with two kinase domains on the same polypeptide chain. Furthermore, in agreement with the RSK2-NTKD structure (18), our data suggest that the βB-sheet is a structural feature of both inactive and active states. This was confirmed by far UV CD spectra of the three proteins, which are characteristic of a mixed αβ secondary structure content and suggest the absence of major conformational changes between inactive and active states (fig. S12A, fig. S12D). This absence may facilitate RSK4 activation, as it would lower the energy barrier to reach the active state and is consistent with the high autophosphorylation property of RSK4 and with evidences that basal cellular kinase activity of ERK1/2 can already activate RSK4 to 30-50 % (20). The three structures have similar active site organisation and AMP-PNP coordination (fig. S12C). Specifically, the N1 and N6 of the AMP-PNP adenine ring form hydrogen bonds with the backbone of L155 and of D153, as well as hydrophobic interactions with L205, A103, V87 and L79. The position of the α- and β-phosphate groups is stabilized by interactions with the side chains of the conserved residues K105, N203, K221, T215 and F84. The γ-phosphate interacts with K200, which is indispensable for the formation of enzymatic intermediate during catalysis. The AMP-PNP phosphates are in an optimal alignment for transferring the γ-phosphate to the substrate (17) in all three structures. The major difference in the active sites as compared to a typical protein kinase is the contribution of residues from the βB-sheet motif, particularly K221. The residue K221 extends down from β10 of the βB-sheet, and inserts directly into the active site, interacting with D216 (part of the DFG motif) and with the β-phosphate of the AMP-PNP and the three together should coordinate a Mg ++ ion (due to resolutions limits, we have not been able to confidently place Mg ++ ions in the structure), which help the correct positioning required for ATP binding and catalysis (17). Furthermore, K221 produces an interaction network similar to that of K72 in PKA, known to be important in catalysis. This arrangement is almost identical to the active site of RSK2 NTKD (18) (fig. S12C), where mutations of K216, equivalent of K221 in RSK4, caused impaired RSK2 NTKD activity (18). Taken together, these structural data strongly confirm the importance of K221 for RSK4 function.
A unique feature of dephosphorylated and pS232 RSK4-NTKD structures is the presence of a second AMP-PNP molecule which could be modelled with full occupancy (Fig. 6C). This AMP-PNP contacts a symmetry equivalent molecule and is important for crystal lattice formation. It is located in a surface area absent in classical AGC kinases, created through structural rearrangement caused by formation of the βB-sheet and the displacement of the C-helix. The AMP-PNP molecule in the secondary site is stabilised by a network of hydrogen bonds with water molecules and the side chains of residues C234, H250, Q81, K86, R247, D225 and K113. C234 and H250 together with the β- and γ-phosphates coordinate in a “Zing Finger”-like way a Zn++ ion. This tetrahedral coordination clearly distinguishes this Zn++ ion from a Mg++ ion. This coordination is identical in the dephosphorylated and pS232 RSK4-NTKD structures. We speculated that this unique surface is a “hot spot” (21) and represents a promising target for drug design.
RSK4 hydrophobic motif interaction site
Many AGC kinases possess a hydrophobic motif (HM), located C-terminal of the kinase domain, that is phosphorylated to achieve maximal activity (22). In classic AGC kinases the HM wraps around the N-lobe, where a phosphate-binding pocket is localized, and stabilizes the active conformation of the C helix (23) (24). For RSK1-3, phosphorylation of the HM - found within the linker region - acts to recruit PDK1, which then phosphorylates the NTKD activation loop, resulting in activation of these RSKs. However, because RSK4 activation is PDK1-independent (20), we investigated the role of the HM in this process. Due to the presence of the βB-sheet the phosphate-binding pocket of RSK4-NTKD is rotated ≈50° clockwise compared to that of other AGC kinases. Moreover, this pocket is where we located the second AMP-PNP molecule (Fig. 6C). We used this information to guide a restrained docking simulation (25) using our RSK4-NTKD structure and a fully flexible phosphorylated peptide encompassing the HM sequence (NAHQLFKGF-pS-FVATSIAEE). HADDOCK (25) produced two clusters of solutions which represents 98.5 % of the water-refined models the software generated and the best one had a score of -189.0 +/- 18.2, a cluster size of 190 with RMSD from the overall lowest-energy structure of 1.5 +/- 1.2 Å and a Z score of -1. The most striking feature of the model generated by HADDOCK (26) is the position of the pS389 that occupies the same area of the γ-phosphate of the second AMP-PNP molecule, supporting our hypothesis that the latter is mimicking the HM (Fig. 6D). Specifically, the pHM position is mainly stabilised by a large network of hydrophobic interaction, while the pSer 389 forms H-bonds with E54, K113, C234 and R247. Taken together, our bioinformatics studies identified a potential phosphate-binding pocket in RSK4-NTKD.
Mathematical models based on the RSK4-NTKD structure predict an allosteric binding site
To obtain structural information about trovafloxacin binding to RSK4 we tried soaking and co-crystallisation experiments using both RSK4-NTKD and RSK4-NTKD S232E. Resulting crystals either did not contain trovafloxacin or failed to diffract. Consequently, as our wet-lab experiments suggested that trovafloxacin is an allosteric inhibitor, we adopted Markov Transient Analysis (MTA). This atomistic graph-theoretical approach can identify allosteric hotspots on the RSK4-NTKD without any a priori knowledge other than the location of the active site. MTA quantifies the speed at which a random walk diffuses on the atomistic protein graph constructed from interatomic strong and weak chemical interactions by computing the half-time t1/2 for each atom (27). We applied MTA to our RSK4-NTKD structure to establish the specific pattern of propagation of perturbations originating from the active site and diffusing into the rest of the protein (Fig. 6F). Using quantile regression (28) (29) on the computed t1/2, MTA identified a statistically significant hotspot (residues with ≤5% significance level) that corresponded to the second AMP-PNP binding site (Fig. 6G). The identified allosteric site exhibited a direct communication pathway with the active site (Fig. 6F), as confirmed in more detail through exhaustive computational alanine screening (fig. S13A-B). Each residue was individually alanized computationally to identify mutations that perturbed the communication between the active and obtained allosteric sites. Significant residues (one-sided, Z > 1.64, p < 0.05, red) under alanisation (fig. S14A) formed one of two different pathways connecting the active and allosteric sites (fig. S14B). Taken together, our data suggest that the second AMP-PNP binding site represents an allosteric site on the RSK4-NTKD.
Trovafloxacin docking and impact on Markov transient propagation
We performed molecular docking of Trovafloxacin using AutoDock Vina (30) around the site where the second AMP-PNP molecule sits in our crystal structures. This suggested that trovafloxacin bound to that site with a favourable energy of −8.1 kcal/mol (Fig. 6E), which corresponded well with our in vitro inhibition data. We noticed that trovafloxacin and the allosteric inhibitor positive controls sarafloxacin and tosufloxacin tosylate had a similar overall architecture and were predicted to bind RSK4 in a similar way (fig. S13C). In contrast, the non-inhibitor negative controls ciprofloxacin and clinafloxacin, which have a more liner structure, occupied a slightly different site on RSK4 (fig. S13D). This may be connected to the predicted 1.5 order of magnitude difference in the binding energy between RSK4 allosteric inhibitors (−8.3 and −8.4 kcal/mol for sarafloxacin and tosufloxacin tosylate, respectively) and non-inhibitors (−6.6 and −6.7 kcal/mol for ciprofloxacin and clinafloxacin, respectively). Comparison of the trovafloxacin docking model with the RSK4-NTKD X-ray structure showed that the naphthyridine and phenyl moieties of trovafloxacin occupied the same cavity where the adenine ring of the second AMP-PNP molecule sits, whereas the azabicyclo group pointed outwards and occupied the same area as the ribose and α-phosphate of the AMP-PNP (Fig. 6C and E). To further validate this binding pose, we repeated the molecular docking using alternative software, GLIDE (31). The top-ranked pose, nearly identical to the one obtained using AutoDock Vina (fig. S13E), had a binding free energy (MM-GBSA ΔGbind) of -31.93 kcal/mol.
To assess the impact of trovafloxacin binding to the second AMP-PNP site, we again employed MTA to test the reverse propagation of perturbations originating from the allosteric site into the rest of the protein in the presence or absence of AMP-PNP and our inhibitor. Despite the close similarity of the binding for the two molecules, MTA revealed a marked change in the communication pathways originating from the trovafloxacin-binding site as compared to those originating from the AMP-PNP-binding site (Fig. 6H). In particular, MTA revealed enhanced communication from the trovafloxacin site to the active site, whereas propagation from the AMP-PNP site displayed direct communication with the activation loop and lower lobe of RSK4 (fig. S13F). These data suggest that the binding of trovafloxacin would impact the active site, providing a rational for the activity of this compound on RSK4 activation.
Determination of trovafloxacin’s binding site on RSK4 through hydrogen/deuterium exchange
To gain further insight into the mechanism of action of trovafloxacin, we carried out hydrogen/deuterium exchange studies with RSK4 in the presence or absence of the drug. In this analysis, the protein is exposed to deuteriated water (D2O), which allows hydrogens on the protein backbone amides to be exchanged for deuterium. The exchange is stopped at various times by reducing the pH, the protein is digested with pepsin, and the deuterium content of peptides determined by mass spectrometry (32). Because the rate of exchange is dependent on the tertiary structure (degree of hydrogen bonding) and solvent accessibility of amide hydrogens, such studies can yield information about binding or protein conformation, which are revealed by changes in the kinetics of deuterium incorporation into specific amino acids/peptides (33) (34).
Figure 6I shows a difference plot in daltons of each RSK4 peptide induced by the presence of trovafloxacin, averaged over all replicates and time points. Three regions, each covered by peptic peptides (fig. S14A), showed a reduction in exchange. One of these regions (533-552) was towards the NTKD’s C-terminus, whereas a second was adjacent to this in the NTKD kinase C-terminal region (627-636). A third region (246-279) N-terminal to the NTKD also showed reduced uptake of deuterium, but was outside of any predicted domain. The butterfly plot indicates that all three sites showing reduced uptake are in regions of sequence that are more exposed to bulk solvent. From the uptake kinetics these sites are predicted to be unstructured loops rather than α-helices or β-sheets (fig. S14B). The positions of protection suggest that trovafloxacin is not binding directly in the active or nucleotide binding sites of RSK4, but instead has an allosteric effect, inducing a conformational change in the overall structure of RSK4. The overlap between the docking data, the Markov transient analysis and the deuterium exchange results suggest that the 627-636 stretch in our tagged protein (aa 398-407 on RSK4) corresponds to the region of trovafloxacin binding, whereas the other protected sites undergo subsequent conformational changes as a result of the allosteric nature of this region. Other minor differences observed were close to the background error of the system and therefore were considered non-significant.
Trovafloxacin and the role of the HM in RSK4 activation
Our data suggest that both trovafloxacin and the HM interact with the second AMP-PNP binding site on the RSK4-NTKD (Fig. 6C-E). As MTA predicts this region is an allosteric site, we hypothesized that the HM may affect the autophosphorylation rate of RSK4-NTKD. To test this hypothesis, the RSK4-NTKD was incubated in presence of saturating amount of ATP/Mg2+ alone or with the HM synthetic peptides HP (NAHQLFKGFSFVATSIAEE) or pHP (NAHQLFKGF-pS-FVATSIAEE) corresponding to residues 380-398 of RSK4 (fig. S15). RSK4-NTKD autophosphorylation was measured over time using a phospho-Ser232-specific antibody. Incubation with pHP promoted RSK4-NTKD autophosphorylation compared to that of the HP or the RSK4-NTKD alone (fig. S15). Taken together, our data suggest that the phosphorylated HM binds RSK4-NTKD to increase its autophosphorylation rate. This explains how trovafloxacin inhibits RSK4 activation through preventing HM docking to the phosphate-binding pocket of the kinase.
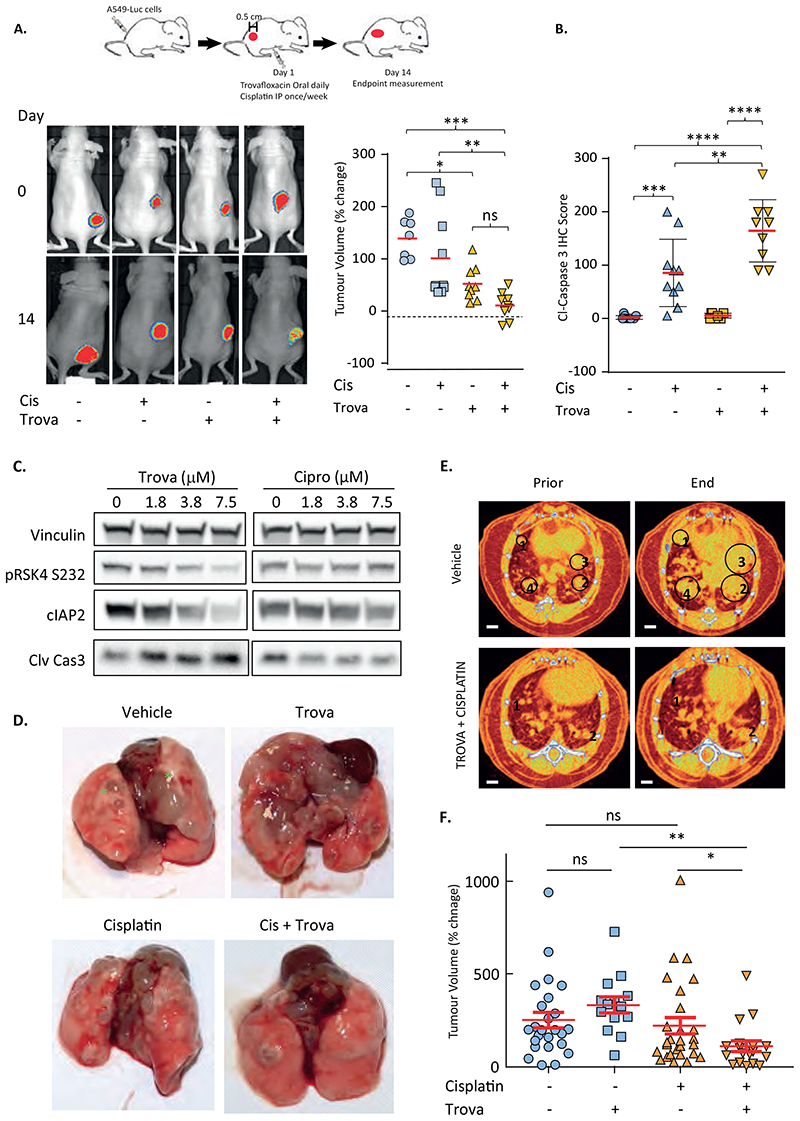
Trovafloxacin inhibits tumour growth and sensitises tumours to drug treatment in vivo
Having shown how trovofloxacin might mechanistically impair RSK4 activation and that it reproduces the biological effects of RSK4 downregulation in vitro, we assessed the activity of this drug in vivo. Luciferase-encoding A549 cells (A549-Luc) were injected subcutaneously into nude mice. Once tumours became palpable, the mice were randomised to receive or not daily oral gavage of trovafloxacin (30 mg/kg) and weekly intraperitoneal injections of cisplatin (5 mg/kg) for 14 days. Bioluminescent whole-animal imaging performed on the first and last days of treatment after injection of luciferin (Fig. 7A, fig. S16A) shows that, as for RSK4 CRISPR cells, administration of trovafloxacin significantly (p<0.05) decreased tumour growth compared to vehicle-only treatment and the effect of the trovafloxacin/cisplatin combination was superior to that of cisplatin alone (p<0.01). This potentiation of cisplatin response was consistent with the statistically significant increase in caspase 3 cleavage observed in tumour samples from animals treated with the drug combination as compared to trovafloxacin (p<0.001) or cisplatin alone (p<0.01) (Fig. 7B, fig. S16B). The ability of trovafloxacin to potentiate response to cisplatin was further confirmed in the KRASV12/TP53-/- (KP)-driven genetically-engineered mouse model where spontaneous lung adenocarcinoma tumours are triggered through adenoviral Cre-recombinase delivery (35) (Fig. 7C-F). This was consistent with trovafloxacin-induced molecular changes in cells isolated from KP tumours, such as decreased cIAP2 expression and increased caspase 3 cleavage, similar to those seen in A549 cells (Fig. 7C, fig. S16C), whereas the RSK4 non-targeting compounds ciprofoxacin failed to elicit such changes. The trovafloxacin/cisplatin combination was associated with decreased tumour number and volume as assessed by macroscopic observation (Fig. 7D) and by μCT scanning (Fig. 7E-F, fig. S16D, movie S1 and S2). For bladder cancer, as we lacked a genetically engineered mouse model, we instead employed a recently described human cancer explant system (36). We demonstrated that the cisplatin/trovafloxacin combination was beneficial in 3 out of 4 patient-derived bladder cancer explants, with synergistic effects observed in two and additive effects in one (fig. S16E). The last patient’s tumour showed extreme sensitivity to cisplatin alone so the added benefit of trovafloxacin could not be assessed. Collectively, our results suggest that administration of RSK4-targeting floxacins may provide clinical benefit in patients with lung adenocarcinoma and bladder cancer.
Figure 7. Trovafloxacin enhances cisplatin efficacy in A549 xenografts and KRASV12/TP53-/- (KP)-driven genetic mouse models.
(A-B) Nude mice injected subcutaneously with luciferase-expressing A549 cells treated with or without trovafloxacin and cisplatin once tumours reached 0.5cm in any dimension. (A) Left: representative images from each condition showing luciferase signal at day 0 and 14. Right: Percent tumour volume change at Day 14. Red bar; median. Statistics: Student’s t-test, *; p≤0.05, **; p≤0.01, ***; p≤0.001. (B) IHC of end-point tumours for caspase-3 cleavage. (C) Differential effect of trovafloxacin and ciprofloxacin on RSK4 phosphorylation (pRSK4-S232), cIAP2 expression and cleaved (Clv) caspase-3 in a cancer cell line isolated from KP tumours (D) Representative images of lungs from treated KP animals. Green stars; examples of lung tumour nodules in the vehicle-only condition. (E) Representative μCT scans from vehicle-only and combination-treated KP animals before treatment and at end-point. Individually tractable tumours indicated by numbers and green circles. (F) Percent volume change in individually tractable tumours in KP mice determined by differential analysis of CT-scans before treatment and at end-point. Dots represent individual tumours. Red line: median ± SEM. Statistics: ANOVA; *; p≤0.05, **; p≤0.01, ***; p≤0.005, ****; p≤0.001 and ns; non-significant.
Levofloxacin inhibits RSK4 in vitro and prolongs survival of patients with cancer
Because floxacins are frequently used in the clinic to prevent infections in patients undergoing chemotherapy we wondered whether this could be associated with improved OS. A large placebo-controlled randomised phase 3 study (SIGNIFICANT Trial) (fig. S17) demonstrated that levofloxacin reduced infective complications in patients undergoing chemotherapy in several cancer types, including lung and bladder cancer (37). HTRF assays revealed that levofloxacin inhibited RSK4 activation, resulting in decreased cellular cIAP1 and cIAP2 expression (fig. S18A-B) at concentrations achievable from the dose given in the clinical trial (38, 39). We therefore undertook a retrospective exploratory analysis of 1565 SIGNIFICANT trial patients to investigate whether the use of prophylactic levofloxacin during chemotherapy treatment had an effect on long-term OS. In patients adhering to trial treatment (n=937), OS was significantly longer for those taking levofloxacin compared to placebo (median OS 72 versus 59 months, respectively, HR 0.83; 95% CI 0.69-0.999; p =0.048; fig S18C -left). Analysis of non-adherent patients (n=543) further substantiated this levofloxacin effect by showing no difference in OS (HR=1.11; 95% CI 0.89-1.40; p=0.36; fig S18C -right). Sensitivity analysis showed that excluding patients who died during treatment did not change the conclusions and thus this improvement in OS could not be due to a reduction in infective deaths due to the antibacterial effect of levofloxacin. The hazard ratio was further improved for lung and bladder cancer patients compared to the entire cohort (fig. S18D). Collectively, these results provide intriguing clinical evidence that a RSK4-targeting floxacin might also potentiate the effects of anticancer treatments and improve survival particularly in patients with lung or bladder cancer.
Discussion
Our findings demonstrate the role of RSK4 as a tumour promoter in lung adenocarcinoma and bladder cancer cells. Overexpression of this kinase lead to drug resistance and increased cell migration in lung and bladder cancer cell lines in vitro, and correlated with poorer survival in lung adenocarcinoma patients. Conversely, silencing this kinase using RNA interference or CRISPR-mediated knock-out, sensitised cells to chemotherapeutic drugs, and prevented invasion in vitro and in vivo. Our data were consistent with findings in renal carcinoma (6, 7), but conflicted with the proposed role of RSK4 as a tumour suppressor in breast cancer (3, 4, 40). Several explanations might account for this discrepancy including a tissue-specific role of RSK4 or differential expression of RSK4 splice variants as at least two protein-coding transcripts for RSK4 are reported in Ensembl; RPS6KA6-201 and RPS6KA6-204. Further work is necessary to elucidate these seemingly conflicting tissuespecific data.
Our observations led us to hypothesize that a selective RSK4 inhibitor might represent a valuable therapeutic tool in the treatment of lung and bladder cancer. Indeed, RSK4 protein is overexpressed in the majority of lung malignancies, especially in adenocarcinoma, but undetectable in normal lung and poorly expressed in most other tissues (1). Similarly, bladder cancer samples show increased RSK4 mRNA expression compared to matching normal tissue. Hence, RSK4 inhibitors could exhibit some degree of specificity for these cancers. Moreover, the fact that RSK1, unlike RSK4, is well expressed in a variety of normal human tissues (1) may suggest that a RSK4-selective, rather than pan-RSK, inhibitor would be preferable. In support of this, our previous research showed that RSK1 silencing promoted invasion of lung cancer cells (8), an effect also seen in A549 cells with the pan-RSK inhibitor SL0101 but not the pan-RSK inhibitor BI-D1870 (41). Furthermore, we showed here that RSK1 in contrast to RSK4 silencing rendered lung and bladder cancer cell lines chemo-resistant. S, while silencing RSK2 or RSK3 either led to inconsistent biological outcomes across cell lines or had no impact on cell migration and drug response (8, 9). Thus, it appears crucial that a therapeutically useful RSK4 inhibitor should avoid targeting RSK1 at the least.
Prior crystal structure data for the NTKDs of RSK1 and 2 (18) compared to that shown for RSK4 in the present paper reveal that the active sites for all three kinases are essentially identical. Consequently, the best hope of a RSK4 selective inhibitor might be a molecule that binds allosterically rather than in an ATP competitive fashion. The floxacins were originally developed as antibiotics (42) but our results show that some members of this family appear to selectively inhibit the activation of RSK4 through allosteric binding. This occurs via a second AMP-PNP binding site not conservered in other AGC kinases providing a likely mechanism for RSK4-selectivity. Several questions arise from our observations including whether the antibiotic function of floxacins is necessary for inhibition of RSK4 activation and whether floxacins could be re-purposed for use as an anticancer agent in the clinic?
A clue to answering the first question is provided by our finding that whilst all floxacins are antibtioics, several family members such as ciprofloxacin and danofloxacin fail to impair RSK4 activation. This combined with the information on the RSK4 binding site for active floxacins should enable chemistry to derive new allosteric inhibitors lacking the antibiotic function but nevertheless maintain RSK4 targeting. Loss of floxacin antibiotic function could be clinically advantageous in potential cancer therapy. This is because prolonged use of these agents can cause serious antibiotic-induced complications including severe diarrohea due to clostridium difficile toxin as well as facilitating a rise in bacterial antibiotic resistance (43, 44). It is unknown whether dissociation of antibiotic from RSK4 targeting effects of floxacins will be possible and whether this would remove all unwanted side-effects, but it seems likely it would eliminate the diarrhoea and issues related to bacterial resistance. Intriguingly, results from several studies in cancer patients receiving chemotherapy provide tentative hope that at least one RSK4 targeting floxacin, levofloxacin, is well tolerated (44). Whilst there is good data to show that use of floxacins such as levofloxacin, prevents neutropaenic sepsis and it’s related acute deaths in cancer patients undergoing chemotherapy, there was no evidence to inducate that this might improve longer-term survival. Data presented here suggest that at least for levofloxacin, cancer patients taking this drug as part of the SIGNIGICANT trial do survive longer leading us to speculate that this benefit could be due to inhibition of RSK4. However, other reports show that floxacins may exert distinct effects on cancer cells such as impaired mitochondrial function and oxidative stress responses (45) which may be independent of RSK4 targeting so further work is needed.
So how could RSK4 inihibtion impair cell migration/invasion and enhance chemotherapeutic effects in lung and bladder cancer cells? Prior data in other RSKs across several cancer types have not revealed any clear mechanistic data for RSK4 (46). Here, we have provided some evidence that RSK4 enhances resistance to chemotherapeutic drugs at least in part by increasing cIAP2 protein levels through a transcriptional mechanism. However, how enhanced transcription of its mRNA is achieved is not yet clear although this is not dependent on NFkB. We also provide some evidence that RSK4 phosphorlyates Ser89 of p300 likely leading to increased RELA nuclear localisation and enhanced activity of NFkB thereby increasing EMT and metastatic behaviour.
There are several limitations of our study and some are now discussed. Firstly, we have not provided a formal demonstration of trovafloxacin specificity for inhibiting RSK4 as opposed to RSK1 activation. This would require the development of an in vitro RSK1 activation assay which is complicated by the fact that both ERK2 and PDK1 would be needed and our attempt to develop this have so far failed. However, our preliminary data show that trovafloxacin does not inhibit the activation of RSK1 in cells. Secondly, further research is required to provide definitive proof of endogenous interaction between RSK4 and p300 and to conclusively show how this mediates the EMT changes observed. Thirdly, although our prior research (8) and results shown here demonstrate that RSK1 and RSK4 have opposite biological outputs in regulating invasion and chemo-sensitivity of lung cancer cells, the downstream mediators responsible are currently unclear. Large scale omics analysis assessing the effects of RSK1 and RSK4 silencing and over-expression are required to identify potential discriminating effector pathways. Forthly, some, but not all floxacins, are potent RSK4 activation inhibitors. However, our current data do not provide sufficient structure-activity relationship information to clearly define the reason behind this selectivity. Finally, the re-analysed SIGNIFICANT trial data do not establish that the reason for improved survival with levofloxacin is anything to do with RSK4 inhibition. Indeed, a proof of concept trial is urgently needed to test this hypothesis in patients.
In summary, we have identified RSK4 as a mediator of tumour metastasis and chemotherapy resistance in vitro and in vivo and as a potential therapeutic target in lung and bladder cancer. Our drug screen identified certain floxacins as RSK4 allosteric inhibitors with our structural and in-silico modelling work providing a potential site for their binding. Collectively, our data suggest that RSK4-targeting floxacins such as levofloxacin could be re-purposed for proof-of-concept combination studies with existing chemotherapeutics in patients with lung and bladder cancer.
Materials and Methods
Study design
The aim of this study was to highlight the role of RSK4 in invasiveness and chemoresistance of lung and bladder cancer cells and show how targeting this kinase may represent an effective therapeutic strategy for these cancers. We silenced or induced RSK4 expression in lung AdCa and bladder cancer cell lines and studied the molecular mechanisms involved in modulating the above processes using molecular biology, biochemical, and phenotypic analysis. We also screened for and identified RSK4 activation inhibitors that reproduced the molecular and phenotypic changes observed through RSK4 silencing. We validated our findings through varied corresponding mouse or explant models and proposed mechanism of action for the inhibitors based on structural, mathematical, and biochemical approaches. For in vitro or cell culture experiments, at least three independent biological replicates per condition were used for statistical analysis. For animal experiments, the work was performed under UK Home office approved project licenses and in accordance with institutional welfare guidelines. Analysis of Ct scan images to measure changes in tumour volume was done blinded. Animals were treated without knowledge of anticipated outcomes. No data points were removed as outliers.Cohort sizes were selected based on prior work using the same models and corresponding power analyses to provide power of 0.8 and P<0.05. Tumour-bearing animals were randomized before treatment. Animals showing no tumour growth prior to treatment administration were excluded from further analysis. Last, we searched for prior clinical trials using floxacins in cancers including lung and bladder to ask if there was any evidence that use of such antibiotics might extend patient survival. Further methods details are provided in the Supplementary Materials.
Statistical analysis
Statistical testing was performed using Prism (GraphPad) or Excel (Microsoft). Most experiments were analyzed by one-way ANOVA with Tukey’s, Dunnett’s or Benjamini-Hochberg correction multiple comparison or with two-tailed unpaired Student’s t-test. For datasets where the reduced count did not allow normality test, a normal distribution was assumed on the basis of data distribution. For all statistical analyses, significance was accepted at the 95% confidence level (P < 0.05) and significance levels indicated. Error bars in all figures represent the SEM as a measure of the accuracy of the mean values shown unless otherwise stated.
Supplementary Material
One-sentence summary.
RSK4 promotes invasiveness and drug resistance in lung and bladder cancer and inhibition of this kinase shows therapeutic potential.
Acknowledgements
The authors gratefully acknowledge infrastructure support from the Cancer Research UK Imperial Centre, the Imperial Experimental Cancer Medicine Centre and the National Institute for Health Research Imperial Biomedical Research Centre. OEP, MJS, FP thank Cancer Research UK (CRUK) for funding SC, UM and FP, and CTRT for funding RR. MMUA acknowledges CRUK funding (C33269/A11161 and C33269/A20752). SNY and MB acknowledge Engineering and Physical Sciences Research Council (EPSRC) grant EP/N014529/1 supporting the EPSRC Centre for Mathematics of Precision Healthcare. DJP thank the Wellcome Trust Strategic Fund (PS3416). MMUA thanks CDF CRUK funding (A11161). JD is funded by the Francis Crick Institute—which receives its core funding from Cancer Research UK (FC001070), the UK Medical Research Council (FC001070), and the Wellcome Trust (FC001070).
Footnotes
Competing Interests: OEP is scientific adviser for ClyzLabs Limited. JD acted as consultant for AstraZeneca, Bayer, Jubilant, Theras, Vividion and Novartis, and has funded research agreements with BMS, Revolution Medicines and Boehringer Ingelheim. PC is on the Scientific Advisory Board of Mission Therapeutics. SJS, VB and AZ are employed by Curesponse. DJP received lecture fees from ViiV Healthcare, Bayer Healthcare, Falk, BMS, EISAI and Roche, travel expenses from BMS, MSD and Bayer Healthcare, consulting fees for Mina Therapeutics, EISAI, H3B, Roche, Astra Zeneca and DaVolterra. RL is employed by AstraZeneca and a Biosceptre shareholder.
DM/LC/JP/MW/CM/FM/CG/JMS/SO/MM-A/DH/MC/UM/MMUA/FP/SP/YW/LT/MB/SG/GG declare that they have no competing interests.
Data and materials availability
Coordinates for the RSK4 NTKD structures are deposited with the Protein Data Bank: RSK4 NTKD (6G77), RSK4 NTKD pS232 (6G76) and RSK4 NTKD S232E (6G78). Some plasmids used in this study are covered by MTA: RELA, MTA#2020S-1003-1024, Addgene; cIAP1 and 2, MTA#2018S-0908-1058, Addgene.
References
- 1.Lara R, Seckl MJ, Pardo OE. The p90 RSK family members: common functions and isoform specificity. Cancer Res. 2013;73:5301–5308. doi: 10.1158/0008-5472.CAN-12-4448. [DOI] [PubMed] [Google Scholar]
- 2.Romeo Y, Roux PP. Paving the way for targeting RSK in cancer. Expert Opin Ther Targets. 2011;15:5–9. doi: 10.1517/14728222.2010.531014. [DOI] [PubMed] [Google Scholar]
- 3.Thakur A, Sun Y, Bollig A, Wu J, Biliran H, Banerjee S, Sarkar FH, Liao DJ. Anti-invasive and antimetastatic activities of ribosomal protein S6 kinase 4 in breast cancer cells. Clin Cancer Res. 2008;14:4427–4436. doi: 10.1158/1078-0432.CCR-08-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopez-Vicente L, Armengol G, Pons B, Coch L, Argelaguet E, Lleonart M, Hernandez-Losa J, de Torres I, Ramon y Cajal S. Regulation of replicative and stress-induced senescence by RSK4, which is down-regulated in human tumors. Clin Cancer Res. 2009;15:4546–4553. doi: 10.1158/1078-0432.CCR-08-3159. [DOI] [PubMed] [Google Scholar]
- 5.Berns K, Hijmans EM, Mullenders J, Brummelkamp TR, Velds A, Heimerikx M, Kerkhoven RM, Madiredjo M, Nijkamp W, Weigelt B, Agami R, et al. A large-scale RNAi screen in human cells identifies new components of the p53 pathway. Nature. 2004;428:431–437. doi: 10.1038/nature02371. [DOI] [PubMed] [Google Scholar]
- 6.Bender C, Ullrich A. PRKX, TTBK2 and RSK4 expression causes Sunitinib resistance in kidney carcinoma- and melanoma-cell lines. International journal of cancer. 2012;131:E45–55. doi: 10.1002/ijc.26486. [DOI] [PubMed] [Google Scholar]
- 7.Fan L, Li P, Yin Z, Fu G, Liao DJ, Liu Y, Zhu J, Zhang Y, Wang L, Yan Q, Guo Y, et al. Ribosomal s6 protein kinase 4: a prognostic factor for renal cell carcinoma. Br J Cancer. 2013;109:1137–1146. doi: 10.1038/bjc.2013.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lara R, Mauri FA, Taylor H, Derua R, Shia A, Gray C, Nicols A, Shiner RJ, Schofield E, Bates PA, Waelkens E, et al. An siRNA screen identifies RSK1 as a key modulator of lung cancer metastasis. Oncogene. 2011 doi: 10.1038/onc.2011.61. [DOI] [PubMed] [Google Scholar]
- 9.Swanton C, Marani M, Pardo O, Warne PH, Kelly G, Sahai E, Elustondo F, Chang J, Temple J, Ahmed AA, Brenton JD, et al. Regulators of mitotic arrest and ceramide metabolism are determinants of sensitivity to paclitaxel and other chemotherapeutic drugs. Cancer cell. 2007;11:498–512. doi: 10.1016/j.ccr.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Pardo OE, Lesay A, Arcaro A, Lopes R, Ng BL, Warne PH, McNeish IA, Tetley TD, Lemoine NR, Mehmet H, Seckl MJ, et al. Fibroblast growth factor 2-mediated translational control of IAPs blocks mitochondrial release of Smac/DIABLO and apoptosis in small cell lung cancer cells. Mol Cell Biol. 2003;23:7600–7610. doi: 10.1128/MCB.23.21.7600-7610.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonavida B, Baritaki S. The novel role of Yin Yang 1 in the regulation of epithelial to mesenchymal transition in cancer via the dysregulated NF-kappaB/Snail/YY1/RKIP/PTEN Circuitry. Crit Rev Oncog. 2011;16:211–226. doi: 10.1615/critrevoncog.v16.i3-4.50. [DOI] [PubMed] [Google Scholar]
- 12.Kiernan R, Bres V, Ng RW, Coudart MP, El Messaoudi S, Sardet C, Jin DY, Emiliani S, Benkirane M. Post-activation turn-off of NF-kappa B-dependent transcription is regulated by acetylation of p65. J Biol Chem. 2003;278:2758–2766. doi: 10.1074/jbc.M209572200. [DOI] [PubMed] [Google Scholar]
- 13.Yuan LW, Soh JW, Weinstein IB. Inhibition of histone acetyltransferase function of p300 by PKCdelta. Biochimica et biophysica acta. 2002;1592:205–211. doi: 10.1016/s0167-4889(02)00327-0. [DOI] [PubMed] [Google Scholar]
- 14.Simpson CD, Anyiwe K, Schimmer AD. Anoikis resistance and tumor metastasis. Cancer Lett. 2008;272:177–185. doi: 10.1016/j.canlet.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 15.Hilinski MK, Mrozowski RM, Clark DE, Lannigan DA. Analogs of the RSK inhibitor SL0101: optimization of in vitro biological stability. Bioorg Med Chem Lett. 2012;22:3244–3247. doi: 10.1016/j.bmcl.2012.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sapkota GP, Cummings L, Newell FS, Armstrong C, Bain J, Frodin M, Grauert M, Hoffmann M, Schnapp G, Steegmaier M, Cohen P, et al. BI-D1870 is a specific inhibitor of the p90 RSK (ribosomal S6 kinase) isoforms in vitro and in vivo. Biochem J. 2007;401:29–38. doi: 10.1042/BJ20061088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Endicott JA, Noble MEM, Johnson LN. The Structural Basis for Control of Eukaryotic Protein Kinases. Annual Review of Biochemistry. 2012;81:587–613. doi: 10.1146/annurev-biochem-052410-090317. 81. [DOI] [PubMed] [Google Scholar]
- 18.Malakhova M, Kurinov I, Liu K, Zheng D, D’Angelo I, Shim JH, Steinman V, Bode AM, Dong Z. Structural diversity of the active N-terminal kinase domain of p90 ribosomal S6 kinase 2. PLoS One. 2009;4:e8044. doi: 10.1371/journal.pone.0008044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith KJ, Carter PS, Bridges A, Horrocks P, Lewis C, Pettman G, Clarke A, Brown M, Hughes J, Wilkinson M, Bax B, et al. The structure of MSK1 reveals a novel autoinhibitory conformation for a dual kinase protein. Structure. 2004;12:1067–1077. doi: 10.1016/j.str.2004.02.040. [DOI] [PubMed] [Google Scholar]
- 20.Dummler BA, Hauge C, Silber J, Yntema HG, Kruse LS, Kofoed B, Hemmings BA, Alessi DR, Frodin M. Functional characterization of human RSK4, a new 90-kDa ribosomal S6 kinase, reveals constitutive activation in most cell types. Journal of Biological Chemistry. 2005;280:13304–13314. doi: 10.1074/jbc.M408194200. [DOI] [PubMed] [Google Scholar]
- 21.Krantz A. Probing protein surfaces for ‘hot spots’: a new frontier. Trends Biotechnol. 1998;16:198–199. [Google Scholar]
- 22.Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nature reviews. 2010;11:9–22. doi: 10.1038/nrm2822. [DOI] [PubMed] [Google Scholar]
- 23.Yang J, Cron P, Thompson V, Good VM, Hess D, Hemmings BA, Barford D. Molecular mechanism for the regulation of protein kinase B/Akt by hydrophobic motif phosphorylation. Molecular cell. 2002;9:1227–1240. doi: 10.1016/s1097-2765(02)00550-6. [DOI] [PubMed] [Google Scholar]
- 24.Frodin M, Antal TL, Dummler BA, Jensen CJ, Deak M, Gammeltoft S, Biondi RM. A phosphoserine/threonine-binding pocket in AGC kinases and PDK1 mediates activation by hydrophobic motif phosphorylation. Embo Journal. 2002;21:5396–5407. doi: 10.1093/emboj/cdf551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Vries SJ, van Dijk ADJ, Krzeminski M, van Dijk M, Thureau A, Hsu V, Wassenaar T, Bonvin AMJJ. HADDOCK versus HADDOCK: New features and performance of HADDOCK2.0 on the CAPRI targets. Proteins. 2007;69:726–733. doi: 10.1002/prot.21723. [DOI] [PubMed] [Google Scholar]
- 26.van Zundert GCP, Rodrigues JPGLM, Trellet M, Schmitz C, Kastritis PL, Karaca E, Melquiond ASJ, van Dijk M, de Vries SJ, Bonvin AMJJ. The HADDOCK2.2 Web Server: User-Friendly Integrative Modeling of Biomolecular Complexes. Journal of Molecular Biology. 2016;428:720–725. doi: 10.1016/j.jmb.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 27.Amor B, Yaliraki SN, Woscholski R, Barahona M. Uncovering allosteric pathways in caspase-1 using Markov transient analysis and multiscale community detection. Mol Biosyst. 2014;10:2247–2258. doi: 10.1039/c4mb00088a. [DOI] [PubMed] [Google Scholar]
- 28.Koenker R. Quantile Regression. 2005 [Google Scholar]
- 29.Koenker R. Quantreg: Quantile Regression. 2015 R package version 5.19. Available at http://CRAN.R-project.org/package=quantreg.
- 30.Trott O, Olson AJ. Software News and Update AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. Journal of Computational Chemistry. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friesner RA, Murphy RB, Repasky MP, Frye LL, Greenwood JR, Halgren TA, Sanschagrin PC, Mainz DT. Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J Med Chem. 2006;49:6177–6196. doi: 10.1021/jm051256o. [DOI] [PubMed] [Google Scholar]
- 32.Wales TE, Engen JR. Hydrogen exchange mass spectrometry for the analysis of protein dynamics. Mass Spectrom Rev. 2006;25:158–170. doi: 10.1002/mas.20064. [DOI] [PubMed] [Google Scholar]
- 33.Vadas O, Dbouk HA, Shymanets A, Perisic O, Burke JE, Abi Saab WF, Khalil BD, Harteneck C, Bresnick AR, Nurnberg B, Backer JM, et al. Molecular determinants of PI3Kgamma-mediated activation downstream of G-protein-coupled receptors (GPCRs) Proc Natl Acad Sci U S A. 2013;110:18862–18867. doi: 10.1073/pnas.1304801110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung KY, Rasmussen SG, Liu T, Li S, DeVree BT, Chae PS, Calinski D, Kobilka BK, Woods VL, Jr, Sunahara RK. Conformational changes in the G protein Gs induced by the beta2 adrenergic receptor. Nature. 2011;477:611–615. doi: 10.1038/nature10488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DuPage M, Dooley AL, Jacks T. Conditional mouse lung cancer models using adenoviral or lentiviral delivery of Cre recombinase. Nat Protoc. 2009;4:1064–1072. doi: 10.1038/nprot.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ben-Hamo R, Jacob Berger A, Gavert N, Miller M, Pines G, Oren R, Pikarsky E, Benes CH, Neuman T, Zwang Y, Efroni S, et al. Predicting and affecting response to cancer therapy based on pathway-level biomarkers. Nature communications. 2020;11:3296. doi: 10.1038/s41467-020-17090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cullen M, Steven N, Billingham L, Gaunt C, Hastings M, Simmonds P, Stuart N, Rea D, Bower M, Fernando I, Huddart R, et al. Antibacterial prophylaxis after chemotherapy for solid tumors and lymphomas. N Engl J Med. 2005;353:988–998. doi: 10.1056/NEJMoa050078. [DOI] [PubMed] [Google Scholar]
- 38.Cao G, Zhu Y, Xie X, Chen Y, Yu J, Zhang J, Chen Z, Pang L, Zhang Y, Shi Y. Pharmacokinetics and pharmacodynamics of levofloxacin in bronchial mucosa and lung tissue of patients undergoing pulmonary operation. Exp Ther Med. 2020;20:607–616. doi: 10.3892/etm.2020.8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fish DN, Chow AT. The clinical pharmacokinetics of levofloxacin. Clin Pharmacokinet. 1997;32:101–119. doi: 10.2165/00003088-199732020-00002. [DOI] [PubMed] [Google Scholar]
- 40.Zhu J, Li QY, Liu JL, Wei W, Yang HW, Tang W. RSK4 knockdown promotes proliferation, migration and metastasis of human breast adenocarcinoma cells. Oncol Rep. 2015;34:3156–3162. doi: 10.3892/or.2015.4291. [DOI] [PubMed] [Google Scholar]
- 41.Samson SC, Elliott A, Mueller BD, Kim Y, Carney KR, Bergman JP, Blenis J, Mendoza MC. p90 ribosomal S6 kinase (RSK) phosphorylates myosin phosphatase and thereby controls edge dynamics during cell migration. J Biol Chem. 2019;294:10846–10862. doi: 10.1074/jbc.RA119.007431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aldred KJ, Kerns RJ, Osheroff N. Mechanism of quinolone action and resistance. Biochemistry. 2014;53:1565–1574. doi: 10.1021/bi5000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuula LSM, Viljemaa KM, Backman JT, Blom M. Fluoroquinolone-related adverse events resulting in health service use and costs: A systematic review. PLoS One. 2019;14:e0216029. doi: 10.1371/journal.pone.0216029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee SSF, Fulford AE, Quinn MA, Seabrook J, Rajakumar I. Levofloxacin for febrile neutropenia prophylaxis in acute myeloid leukemia patients associated with reduction in hospital admissions. Support Care Cancer. 2018;26:1499–1504. doi: 10.1007/s00520-017-3976-1. [DOI] [PubMed] [Google Scholar]
- 45.Yadav V, Talwar P. Repositioning of fluoroquinolones from antibiotic to anti-cancer agents: An underestimated truth. Biomed Pharmacother. 2019;111:934–946. doi: 10.1016/j.biopha.2018.12.119. [DOI] [PubMed] [Google Scholar]
- 46.Cronin R, Brooke GN, Prischi F. The role of the p90 ribosomal S6 kinase family in prostate cancer progression and therapy resistance. Oncogene. 2021 doi: 10.1038/s41388-021-01810-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swanton C, Marani M, Pardo O, Warne PH, Kelly G, Sahai E, Elustondo F, Chang J, Temple J, Ahmed AA, Brenton JD, et al. Regulators of mitotic arrest and ceramide metabolism are determinants of sensitivity to paclitaxel and other chemotherapeutic drugs. Cancer cell. 2007;11:498–512. doi: 10.1016/j.ccr.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 49.Chen LF, Williams SA, Mu Y, Nakano H, Duerr JM, Buckbinder L, Greene WC. NF-kappaB RelA phosphorylation regulates RelA acetylation. Mol Cell Biol. 2005;25:7966–7975. doi: 10.1128/MCB.25.18.7966-7975.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li X, Yang Y, Ashwell JD. TNF-RII and c-IAP1 mediate ubiquitination and degradation of TRAF2. Nature. 2002;416:345–347. doi: 10.1038/416345a. [DOI] [PubMed] [Google Scholar]
- 51.Herberger B, Puhalla H, Lehnert M, Wrba F, Novak S, Brandstetter A, Gruenberger B, Gruenberger T, Pirker R, Filipits M. Activated mammalian target of rapamycin is an adverse prognostic factor in patients with biliary tract adenocarcinoma. Clin Cancer Res. 2007;13:4795–4799. doi: 10.1158/1078-0432.CCR-07-0738. [DOI] [PubMed] [Google Scholar]
- 52.Bain J, McLauchlan H, Elliott M, Cohen P. The specificities of protein kinase inhibitors: an update. Biochem J. 2003;371:199–204. doi: 10.1042/BJ20021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8:2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee JS, Leem SH, Lee SY, Kim SC, Park ES, Kim SB, Kim SK, Kim YJ, Kim WJ, Chu IS. Expression signature of E2F1 and its associated genes predict superficial to invasive progression of bladder tumors. J Clin Oncol. 2010;28:2660–2667. doi: 10.1200/JCO.2009.25.0977. [DOI] [PubMed] [Google Scholar]
- 55.Sanchez-Carbayo M, Socci ND, Lozano J, Saint F, Cordon-Cardo C. Defining molecular profiles of poor outcome in patients with invasive bladder cancer using oligonucleotide microarrays. J Clin Oncol. 2006;24:778–789. doi: 10.1200/JCO.2005.03.2375. [DOI] [PubMed] [Google Scholar]
- 56.Malakhova M, Kurinov I, Liu K, Zheng D, D’Angelo I, Shim JH, Steinman V, Bode AM, Dong Z. Structural diversity of the active N-terminal kinase domain of p90 ribosomal S6 kinase 2. PLoS One. 2009;4:e8044. doi: 10.1371/journal.pone.0008044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kabsch W. XDS. Acta crystallographica. Section D, Biological crystallography. 2010;66:125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mccoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. Phaser crystallographic software. J Appl Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pannu NS, Murshudov GN, Dodson EJ, Read RJ. Incorporation of prior phase information strengthens maximum-likelihood structure refinement. Acta Crystallogr D. 1998;54:1285–1294. doi: 10.1107/s0907444998004119. [DOI] [PubMed] [Google Scholar]
- 60.Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Vries SJ, van Dijk ADJ, Krzeminski M, van Dijk M, Thureau A, Hsu V, Wassenaar T, Bonvin AMJJ. HADDOCK versus HADDOCK: New features and performance of HADDOCK2.0 on the CAPRI targets. Proteins. 2007;69:726–733. doi: 10.1002/prot.21723. [DOI] [PubMed] [Google Scholar]
- 63.Trott O, Olson AJ. Software News and Update AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. Journal of Computational Chemistry. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meier C, Brookings DC, Ceska TA, Doyle C, Gong HP, McMillan D, Saville GP, Mushtaq A, Knight D, Reich S, Pearl LH, et al. Engineering human MEK-1 for structural studies: A case study of combinatorial domain hunting. Journal of Structural Biology. 2012;177:329–334. doi: 10.1016/j.jsb.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 65.Feinstein WP, Brylinski M. Calculating an optimal box size for ligand docking and virtual screening against experimental and predicted binding pockets. J Cheminformatics. 2015;7 doi: 10.1186/s13321-015-0067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Friesner RA, Murphy RB, Repasky MP, Frye LL, Greenwood JR, Halgren TA, Sanschagrin PC, Mainz DT. Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J Med Chem. 2006;49:6177–6196. doi: 10.1021/jm051256o. [DOI] [PubMed] [Google Scholar]
- 67.Jacobson MP, Pincus DL, Rapp CS, Day TJ, Honig B, Shaw DE, Friesner RA. A hierarchical approach to all-atom protein loop prediction. Proteins. 2004;55:351–367. doi: 10.1002/prot.10613. [DOI] [PubMed] [Google Scholar]
- 68.Whitmore L, Wallace BA. Protein secondary structure analyses from circular dichroism spectroscopy: methods and reference databases. Biopolymers. 2008;89:392–400. doi: 10.1002/bip.20853. [DOI] [PubMed] [Google Scholar]
- 69.Whitmore L, Wallace BA. DICHROWEB, an online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucleic Acids Res. 2004;32:W668–673. doi: 10.1093/nar/gkh371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Silva JC, Denny R, Dorschel CA, Gorenstein M, Kass IJ, Li GZ, McKenna T, Nold MJ, Richardson K, Young P, Geromanos S. Quantitative proteomic analysis by accurate mass retention time pairs. Anal Chem. 2005;77:2187–2200. doi: 10.1021/ac048455k. [DOI] [PubMed] [Google Scholar]
- 71.Ben-Hamo R, Jacob Berger A, Gavert N, Miller M, Pines G, Oren R, Pikarsky E, Benes CH, Neuman T, Zwang Y, Efroni S, et al. Predicting and affecting response to cancer therapy based on pathway-level biomarkers. Nature communications. 2020;11:3296. doi: 10.1038/s41467-020-17090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Valovka T, Verdier F, Cramer R, Zhyvoloup A, Fenton T, Rebholz H, Wang ML, Gzhegotsky M, Lutsyk A, Matsuka G, Filonenko V, et al. Protein kinase C phosphorylates ribosomal protein S6 kinase betaII and regulates its subcellular localization. Mol Cell Biol. 2003;23:852–863. doi: 10.1128/MCB.23.3.852-863.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Romeo Y, Zhang X, Roux PP. Regulation and function of the RSK family of protein kinases. Biochem J. 2012;441:553–569. doi: 10.1042/BJ20110289. [DOI] [PubMed] [Google Scholar]
- 74.Endicott JA, Noble MEM, Johnson LN. The Structural Basis for Control of Eukaryotic Protein Kinases. Annual Review of Biochemistry. 2012;81:587–613. doi: 10.1146/annurev-biochem-052410-090317. 81. [DOI] [PubMed] [Google Scholar]
- 75.Lanczky A, Nagy A, Bottai G, Munkacsy G, Szabo A, Santarpia L, Gyorffy B. miRpower: a web-tool to validate survival-associated miRNAs utilizing expression data from 2178 breast cancer patients. Breast Cancer Res Treat. 2016;160:439–446. doi: 10.1007/s10549-016-4013-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Coordinates for the RSK4 NTKD structures are deposited with the Protein Data Bank: RSK4 NTKD (6G77), RSK4 NTKD pS232 (6G76) and RSK4 NTKD S232E (6G78). Some plasmids used in this study are covered by MTA: RELA, MTA#2020S-1003-1024, Addgene; cIAP1 and 2, MTA#2018S-0908-1058, Addgene.