Abstract
Background
Some psychiatric disorders have been associated with increased risk of miscarriage. However, there is a lack of studies considering a broader spectrum of psychiatric disorders to clarify the role of common as opposed to independent mechanisms.
Aims
To examine the risk of miscarriage among women diagnosed with a broad range of psychiatric conditions.
Method
We studied all registered pregnancies in Norway between 2010 and 2016 (n=593 009). The birth registry was used to capture pregnancies ending in gestational week 12 or later, while the patient and general practitioner databases were used to identify miscarriages and induced abortions prior to 12 gestational weeks. Odds ratios of miscarriage according to 12 psychiatric diagnoses were calculated using logistic regression.
Results
Miscarriage occurred in 16.6% of pregnancies. Miscarriage risk was increased among women with bipolar disorders, adjusted odds ratio 1.35 (95% confidence interval 1.26-1.44); personality disorders, 1.32 (1.12-1.55); ADHD, 1.27 (1.21-1.33); anxiety disorders, 1.25 (1.23-1.28); depressive disorders, 1.25 (1.23-1.27); somatoform disorders, 1.18 (1.07-1.31); and eating disorders, 1.14 (1.08-1.22). The miscarriage risk was further increased among women with more than one psychiatric diagnosis. Our findings were robust to adjustment for other psychiatric diagnoses, chronic somatic disorders, and substance use disorders. After mutual adjustment for co-occurring psychiatric disorders, we also observed a modest increased risk among women with schizophrenia spectrum disorders (1.22; 1.03-1.44).
Conclusions
A wide range of psychiatric disorders were associated with increased risk of miscarriage. The role of shared versus independent mechanisms or treatment of these conditions remains unclear. The heightened risk of miscarriage among women diagnosed with psychiatric disorders highlights the need for awareness and surveillance of this risk group in antenatal care.
Keywords: Miscarriage, psychiatric disorders
Miscarriage risk is strongly associated with advanced maternal age and aneuploidy arising during fertilization or development (1, 2). Despite decades of research, little is known about other factors that contribute to the risk of miscarriage. Stress and psychiatric disorders plausibly play a role, although evidence is limited. An increased risk of miscarriage has been reported among women with a history of eating disorders (3), anxiety or depression (4, 5). The majority of studies of anxiety or depression have looked at these psychiatric disorders after a miscarriage, in order to better understand how experiencing miscarriages might affect a woman’s mental health. They are therefore not looking at the relationship between pre-existing anxiety or depression in relation to the risk of miscarriage. A few studies also indicate that women using medications for bipolar disorders (6, 7) or ADHD (8) during pregnancy may increase the risk of miscarriage, although none of these studies examined the diagnosis themselves in detail.
Most of these existing studies are small and focused on specific outcomes. They also largely relied on retrospective recall of the psychiatric diagnosis after the end of pregnancy. Understanding whether miscarriage risk is increased for these particular versus a broad range of psychiatric conditions is important because the findings have implications for awareness and surveillance of women at risk in antenatal care. It is also important in order to highlight the need to understand the potential role of common as opposed to independent underlying explanations for how they relate to the risk of miscarriage. The Norwegian health registries provide an opportunity to assess the relationship between psychiatric disorders and risk of miscarriage. Using prospective data on all pregnancies resulting in contact with the health-care system during a seven-year period, we examined the risk of miscarriage across a broad range of psychiatric diagnoses.
Method
We identified all registered pregnancies in Norway with an estimated date of conception between January 1st 2010 and December 31st 2016, using three national health registries: the Medical Birth Registry of Norway, the Norwegian Patient Registry and the general practice database (9). The patient registry comprises visits to medical specialists and hospitals, with diagnoses coded according to International Classification of Diseases version 10 (ICD-10). Visits to general practitioners are coded according to the International Classification of Primary Care version 2 (ICPC-2). The birth registry includes information on pregnancies ending after 12 gestational weeks (live births, stillbirths, late miscarriages and late induced abortions). The patient registry and the general practice database provides information on all women in contact with health care services during pregnancy, including contacts during first trimester. We linked information from the three health registries (birth registry, specialist care and the general practice database) using unique personal identification numbers.
Pregnancy outcomes and identification of unique pregnancies
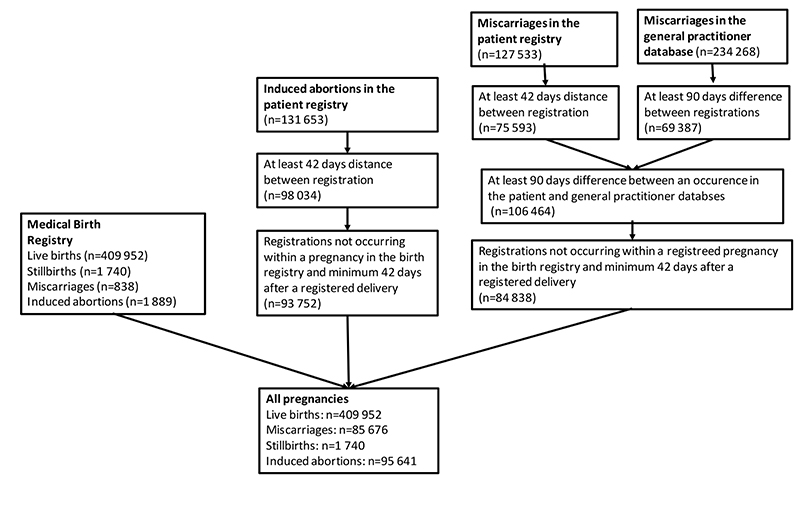
We identified live births and fetal deaths after 12 gestational weeks from the birth registry. A fetal death at 20 gestational weeks or later or with a birthweight of 500 grams or more was considered a stillbirth, while a fetal death before 20 gestational weeks with a birthweight less than 500 grams was considered a miscarriage. This distinction of stillbirth is in accordance with the American National Stillbirth Society and the National Institutes of Health. We included the following ICD-10 codes to capture miscarriages in the patient registry: hydatidiform mole (O01); blighted ovum and non-hydatidiform mole (O02.0); missed abortion (O02.1); other specified abnormal products of conception (O02.8); abnormal product of conception, unspecified (O02.9); spontaneous abortion (O03); and threatened abortion (O02.0). Induced abortion was defined by the following ICD-10 codes in the patient registry: medical abortion (O04), other abortion (O05) and unspecified abortion (O06).
We have expanded our definition of miscarriage used in a previous study to include events reported in the general practitioner database (2). Bleeding in pregnancy (ICPC-2 code W03) and spontaneous abortion (W82) were defined as first trimester miscarriages if they were not followed by a registration in the birth registry or the patient registry. Pregnancies in the patient registry and general practitioner database are not registered with unique pregnancy IDs, which could lead to inadvertent counting of the same pregnancy multiple times. We minimized this error by requiring a minimum of 6 weeks (42 days) between two successive records in the patient registry, and a minimum of 3 months (90 days) between two successive records in the general practitioner database. These cut-offs were chosen by inspecting the distribution of times between registrations in the two registers. We used a longer interval in the general practitioner database because women have more follow-up visits with their general practitioners after a miscarriage. We also required that a record of a miscarriage or induced abortion in the patient registry or general practitioner database be at least 6 weeks (patient registry) or 3 months (general practitioner database) after a registered delivery to the woman in the birth registry. Finally, we excluded any miscarriage or induced abortion that occurred within the gestational period of a registered pregnancy to the same woman in the birth registry.
In the case of multiple fetuses, the outcome was regarded as a live birth if all deliveries resulted in live births, as a miscarriage if there was at least one miscarriage but no stillbirth, and as a stillbirth if at least one of the deliveries resulted in a stillbirth but none in a miscarriage. A multiple birth could result in both a miscarriage and a stillbirth if there was a discrepancy in the birthweight between the fetuses.
Psychiatric disorders
We identified diagnostic codes for 12 psychiatric disorder groups using information available in the patient registry and general practice database. The 12 diagnostic groups were schizophrenia spectrum disorders, bipolar spectrum disorders, depressive disorders, anxiety disorders, somatoform disorders, eating disorders, intellectual disability, autism spectrum disorders, attention-deficit/hyperactivity disorder (ADHD), conduct disorder, personality disorders, and unspecified psychiatric disorders. The diagnostic codes to classify the various conditions are shown in Supplementary Table 1.
We required a minimum of two registrations of the administrative code(s) used to define the particular psychiatric diagnosis to classify women as diagnosed with the psychiatric disorder to avoid coding errors. To ensure that woman had been diagnosed with the condition prior to pregnancy, we required that at least one registration should be before the estimated date of the last menstrual period, defined as the date of delivery minus the gestational age in days. For miscarriages and induced abortions identified in the patient registry and general practice database (in which gestational week was not available), we assumed a gestational age of 12 weeks to ensure that the condition would have been diagnosed prior to pregnancy, assuming that the pregnancy would have been registered in the birth registry if the gestational age was more than 12 weeks.
Covariates
We had information on maternal age at the time of conception, gravidity, year of conception, substance use disorders, and diagnoses of somatic conditions. Diagnoses of substance use disorders and somatic diseases were obtained from the patient and general practitioner databases. We included autoimmune diseases (systemic lupus erythematosus, type 1 diabetes, celiac disease, multiple sclerosis, rheumatoid arthritis/ankylosing spondylitis, ulcerative colitis, psoriasis, Addison disease, Crohn´s disease, haemolytic anemia), endocrinological diseases (Cushing syndrome, hypothyroidism, hyperthyroidism, hyperaldosteronism, hyperparathyroidism), cardiometabolic diseases (atherosclerosis, hypertensive disorders, type 2 diabetes), allergic diseases (asthma, allergic rhinitis, atopic dermatitis), neurological diseases (epilepsy, migraine) and reproductive diseases (polycystic ovary syndrome (PCOS), and endometriosis). The various ICD-10 codes (specialist health care services) and ICPC-2 codes (primary health care services) used to capture these disorders are listed in Supplementary Table 2.
Statistical analysis
We used logistic regression to calculate odds ratios (OR) of miscarriage according to psychiatric disorders. We attempted to estimate relative risks using log-binomial regression; however, the model failed to converge for several of the psychiatric diagnoses. We therefore present results using logistic regression for all disorders for consistency. We also estimated risk differences (RD) as an absolute measure of the difference in the risk of miscarriage, using linear regression. Because some women experienced more than one pregnancy during the time period, we used cluster variance estimation to calculate 95% confidence intervals. We have previously shown that miscarriage risk increases in a non-linear fashion with maternal age (2), so we adjusted for woman’s age at the start of pregnancy by including age as both a linear and squared term. We adjusted for the competing risk of induced abortion by adding 20% of induced abortions to the comparison group in the analysis, together with all live and stillbirths. Our basis for this adjustment is described in detail in the supplementary methods (SA1) in the appendix.
We conducted stratified analyses according to whether the woman had previously been pregnant, and whether the miscarriage was registered in the specialist health-care services or only in primary health-care services. We also conducted sensitivity analyses further adjusting for year of conception, restricting to psychiatric disorders registered in the specialist health-care services, and excluding hydatidiform mole from the definition of miscarriage. To examine the independent relationship of the various diagnoses with miscarriage risk, we included all 12 psychiatric diagnoses simultaneously in the model. As an indicator of psychiatric multimorbidity, we also examined the risk of miscarriage according to the total number of different psychiatric diagnoses a woman had been diagnosed with prior to pregnancy, categorized as none (reference), one, two, and three or more diagnoses. This did not include additional registrations of administrative codes used to define the same diagnosis. All analyses were conducted using STATA version 15 (Statacorp, Texas).
Ethics of Research
The study was approved by the Committee for Medical and Health Research Ethics of South/East Norway. The use of the national health registries for health-related research does not require consent according to Norwegian legislation.
Results
We identified a total of 593 009 pregnancies with last menstrual period between January 1st 2010 and December 31st 2016 (Figure 1). Of these pregnancies, 409 952 (69.1%) ended in a live birth, 1 740 (0.3%) in stillbirth, 95 641 (16.1%) in induced abortion, and 85 676 (14.4%) in miscarriage. Miscarriage risk estimated as a proportion of miscarriages plus live and stillbirths was 17.2%, which was reduced to 16.6% after adjustment for the competing risk of induced abortions.
Figure 1. Illustration of identification of unique pregnancies across health registries.
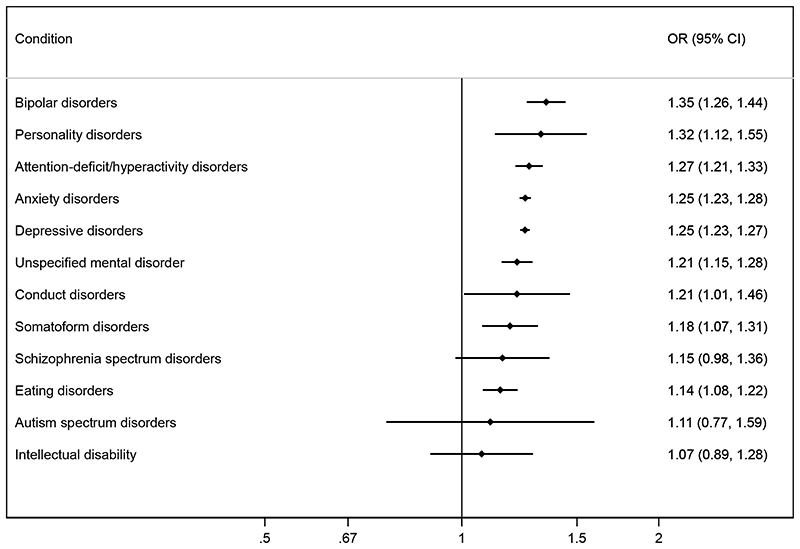
Twenty-two percent of women had a diagnosis of one or more psychiatric conditions, with depressive disorders (12.1%) and anxiety disorders (8.3%) being by far the most common (Table 1). The proportion of pregnant women diagnosed with other psychiatric disorders ranged from 0.02% for autism spectrum disorders to 1.5% for ADHD (Table 1). Women with psychiatric disorders had an increased risk of miscarriage (Figure 2). The absolute increase in miscarriage risk ranged from 2% to 5% on a baseline rate of nearly 17% (Supplementary Figure 1). Odds ratios for specific diagnostic groups were 1.35 for bipolar disorders (95% confidence interval 1.26-1.44); 1.32 for personality disorders (1.12-1.55); 1.27 for ADHD (1.21-1.33); 1.25 for anxiety disorders (1.23-1.28); 1.25 for depressive disorders (1.23-1.27); 1.18 for somatoform disorders (1.07-1.31); and 1.14 for eating disorders (1.08-1.22) (Figure 2). Women’s age was an important confounder, as shown by the stronger associations in unadjusted analysis (Supplementary Figure 2). Further adjustment for year of conception, to account for the amount of follow-up information available, did not changes the associations (Supplementary Figure 3).
Table 1. Distribution of psychiatric conditions in 593,009 pregnancies in Norway 2010-2016.
| Psychiatric disorders | Frequency | % |
|---|---|---|
| Schizophrenia spectrum disorders | 631 | 0.11 |
| Bipolar disorder | 3554 | 0.6 |
| Depressive disorders | 71551 | 12.1 |
| Anxiety disorders | 49472 | 8.3 |
| Somatoform disorders | 1644 | 0.3 |
| Eating disorders | 5131 | 0.9 |
| Intellectual disability | 548 | 0.1 |
| Autism spectrum disorders | 142 | 0.02 |
| Attention-deficit/hyperactivity disorder | 8796 | 1.5 |
| Personality disorders | 687 | 0.1 |
| Conduct disorder | 608 | 0.1 |
| Unspecified mental disorder | 6496 | 1.1 |
| Any psychiatric disorder | 131151 | 22.1 |
Figure 2. Adjusted* odds ratios (OR) for miscarriage according to pre-existing psychiatric disorders.
*Adjusted for the woman’s age at the start of pregnancy as a linear and squared term.
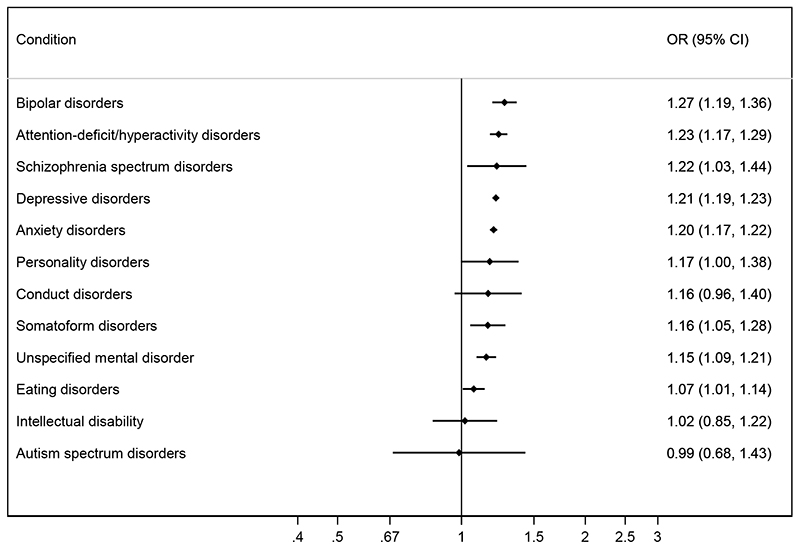
Five percent of all pregnancies were to women with more than one psychiatric diagnosis. The most common combination was anxiety and depression diagnoses (2% of all pregnancies). When we adjusted the risk of miscarriage for each diagnosis for the co-occurrence of other psychiatric diagnoses, the risks of miscarriage were slightly attenuated (Figure 3). The only exception was schizophrenia spectrum disorders, where we observed a strengthening of the association, with an adjusted OR of 1.22 (1.03-1.44). We also looked at the risk of miscarriage according to the number of psychiatric diagnoses as an indicator of psychiatric multimorbidity. The adjusted OR of miscarriage with one was 1.27 (1.25-1.30); with two, 1.45 (1.40-1.51); and with three or more diagnoses, 1.51 (1.31-1.73).
Figure 3. Adjusted* odds ratios (OR) for miscarriage by pre-existing psychiatric disorders after mutual adjustment for other comorbid psychiatric disorders.
*Adjusted for the woman’s age at the start of pregnancy as a linear and squared term.
A past miscarriage might contribute to psychiatric conditions, so we conducted a sensitivity analysis restricting to first pregnancies (Supplementary Figure 4). The main associations persisted. Excluding hydatidiform mole from the definition of miscarriage did not change results.
About 25% of miscarriages were identified in the general practitioner database with no subsequent record in the patient registry or the birth registry. When we analyzed the general practitioner and specialist health-care sources of data separately, results were similar overall, although risks were more consistently elevated among women whose miscarriage was referred to specialist health-care services (Supplementary Figure 5). Associations were slightly stronger when restricting the evaluation to psychiatric disorders diagnosed in specialist care services (Supplementary Figure 6).
We also conducted an evaluation of the role of somatic chronic conditions. The proportion of women with the different chronic somatic conditions is shown in Supplementary Table 3. Further adjustment for substance use disorders and all the somatic conditions did not explain the increased risk of miscarriage seen among women with psychiatric disorders in the main analysis (Supplementary Figure 7).
Discussion
In this large registry-based study, women with a broad range of psychiatric disorders had an increased risk of miscarriage. Risks were most prominently elevated with bipolar disorders, personality disorders, ADHD, anxiety disorders, depressive disorders, and somatoform disorders. We also observed a weak evidence of an increased risk of miscarriage among women with schizophrenia, which was strengthened after mutual adjustment for co-occurring psychiatric disorders. The risk of miscarriage increased with multiple psychiatric diagnoses.
Our results confirm specific associations with risk of miscarriage reported in previous studies of women with eating disorders (3), bipolar disorder (6, 7, 10), and anxiety or depression (4, 5). A systematic review found evidence for increased miscarriage risk specifically among women with anorexia nervosa (3), and a few small-scale studies support an increased risk with anxiety or depression (4, 5). Some previous studies obtained information on pre-existing psychiatric disorders retrospectively, after the end of pregnancy, which can leave open the possibility of recall bias following a miscarriage. Women with schizophrenia have an increased risk of pregnancy complications (11, 12). The modest increased risk of miscarriage among women with schizophrenia spectrum disorders identified in this study is novel and should be further explored.
It is well documented that women who experience miscarriage (especially recurrent miscarriage) have an increased risk of psychiatric disorders (13, 14). We therefore conducted a sensitivity analysis restricted to women who were pregnant for the first time to address the possibility of reverse causation. The results were overall very similar to our main findings including all pregnancies. While it is plausible that miscarriage can increase the risk of psychiatric illness, this sensitivity analysis shows that such an effect does not contribute to the associations we observe.
A direct effect of psychiatric illness on miscarriage risk is plausible. One potential biological mechanisms is the link between peptides and proteins synthesized in the brain and placental development (15). It has been hypothesized that women with psychiatric conditions such as depression, bipolar disorder, and schizophrenia experience an increased risk of adverse obstetric outcomes through changes in these neurotrophin factors in the brain (16, 17). Another possibility is an effect of stress hormones, which can influence both placental development and the intrauterine environment, perhaps increasing the risk of miscarriage (5). These biological mechanisms may be shared across diverse psychiatric disorders. More specific mechanisms may be at work in conditions such as anorexia nervosa, in which nutritional deficiency may impair the woman’s ability to sustain a pregnancy (18). Prenatal depression has also been associated with placental barrier dysfunction (5).
There can also be other direct or indirect explanations for the relationship between psychiatric disorders and risk of miscarriage. This can include both socio-economic pathways and lifestyle factors. The risk of psychiatric disorders is strongly related to socio-economic factors (19, 20), but the extent to which such factors might influence miscarriage risk is not well established. We used the E-value (21) to estimate that an unmeasured confounder would have to be associated with a 1.7 fold increased risk of both miscarriage and bipolar disorders (the strongest association in our analysis) to completely explain the relationship. Lifestyle characteristics that are strongly linked to psychiatric disorders (for example smoking and higher body-mass index) (22, 23) may also contribute to explain an increased risk of miscarriage. Differences in lifestyle factors between women with and without psychiatric disorders could therefore be one explanatory pathway for an increased risk of miscarriage.
Our prospective data on diagnosis of both miscarriage and psychiatric conditions provide a strong basis for assessing associations free of self-reporting bias. Coverage across the whole population of Norway provides power and protects against selection bias that might arise within self-selected study populations.
Perhaps the most important limitation of our analysis is the lack of data on medications used for psychiatric conditions. We did not have this information available due to the strict regulations for linkage with the prescription registry. An increased risk of miscarriage has been reported with use of lithium for bipolar disorders (6, 7), various antidepressants and antianxiety medications (24, 25), and medications for ADHD (8, 26). However, if psychiatric illness has a causal effect on risk of miscarriage, it is also possible that treatment reduces the risk of miscarriage. A better understanding of underlying mechanisms would allow clinicians to properly weigh the potential risks of treatment against the risks of untreated disease.
We are likely to have underestimated the proportion of women with a psychiatric condition prior to pregnancy, as our databases capture diagnoses only among women who seek care from the health-care system (27, 28). If this is the case, out estimates of miscarriage risk may be biased towards the null. Furthermore, we did not have information available on the age of diagnosis of the psychiatric disorder, and could therefore not account for the potential chronicity of the condition.
In conclusion, a wide range of psychiatric diagnoses were associated with increased risk of miscarriage. While these associations are biologically plausible, we cannot assess the extent to which treatment for the illness might influence the observed risk. Nevertheless, the findings highlight the importance of identifying and adequately treating psychiatric disorders among women of reproductive age.
Supplementary Material
Funding
This research was supported by the Research Council of Norway through its Centres of Excellence funding scheme, project number 262700. The work was also supported by the Intramural Program of the National Institute of Environmental Health Sciences, NIH (AJW). MCM works at the Medical Research Council (MRC) Integrative Epidemiology Unit at the University of Bristol which receives infrastructure funding from the UK MRC (MC_UU_00011/6). AH was supported by the South-Eastern Norway Regional Health Authority (2018059 and 2020022).
Footnotes
Declaration of interest: None.
Contributors
MCM, AJW, NHM, KAW and SHE contributed to the design of the study. MCM, AH, NHM, KAW, AJW and SHE contributed to the organisation and conduct of the study. SEH was responsible for acquisition of data. MCM analysed the data and drafted the initial version of the manuscript. All authors contributed to the interpretation of the data. All authors have read and approved the final version of the manuscript. MCM will serve as guarantor for the contents of the paper.
Contributor Information
Maria C Magnus, Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway; MRC Integrative Epidemiology Unit at the University of Bristol, Bristol, United Kingdom; and Population Health Sciences, Bristol Medical School, Bristol, United Kingdom.
Alexandra Havdahl, Nic Waals Institute, Lovisenberg Diaconal Hospital, Oslo, Norway; Department of Mental Disorders, Norwegian Institute of Public Health, Oslo, Norway; and MRC Integrative Epidemiology Unit at the University of Bristol, Bristol, United Kingdom.
Nils-Halvdan Morken, Noren, Bergen, Norway; and Department of Obstetrics and Gynecology, Haukeland University Hospital, Bergen, Norway.
Knut-Arne Wensaas, Research Unit for General Practice, NORCE Norwegian Research Centre, Bergen, Norway.
Allen J Wilcox, Epidemiology Branch, National Institute of Environmental Health Sciences, National Institutes of Health, Durham, North Carolina, United States.
Siri E Håberg, Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway.
Data availability
Data from the national health registries are available upon application though an online proposal system. Information regarding access can be found on the Directorate of e-health website (https://helsedata.no/no/om-helsedata/).
References
- 1.Garrido-Gimenez C, Alijotas-Reig J. Recurrent miscarriage: causes, evaluation and management. Postgrad Med J. 2015;91(1073):151–62. doi: 10.1136/postgradmedj-2014-132672. [DOI] [PubMed] [Google Scholar]
- 2.Magnus MC, Wilcox AJ, Morken NH, Weinberg CR, Haberg SE. Role of maternal age and pregnancy history in risk of miscarriage: prospective register based study. Bmj. 2019;364:l869. doi: 10.1136/bmj.l869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charbonneau KD, Seabrook JA. Adverse Birth Outcomes Associated with Types of Eating Disorders: A Review. Can J Diet Pract Res. 2019;80(3):131–6. doi: 10.3148/cjdpr-2018-044. [DOI] [PubMed] [Google Scholar]
- 4.Bonari L, Pinto N, Ahn E, Einarson A, Steiner M, Koren G. Perinatal risks of untreated depression during pregnancy. Can J Psychiatry. 2004;49(11):726–35. doi: 10.1177/070674370404901103. [DOI] [PubMed] [Google Scholar]
- 5.Marinescu IP, Foarfa MC, Pirlog MC, Turculeanu A. Prenatal depression and stress - risk factors for placental pathology and spontaneous abortion. Rom J Morphol Embryol. 2014;55(3 Suppl):1155–60. [PubMed] [Google Scholar]
- 6.Diav-Citrin O, Shechtman S, Tahover E, Finkel-Pekarsky V, Arnon J, Kennedy D, et al. Pregnancy outcome following in utero exposure to lithium: a prospective, comparative, observational study. Am J Psychiatry. 2014;171(7):785–94. doi: 10.1176/appi.ajp.2014.12111402. [DOI] [PubMed] [Google Scholar]
- 7.Fornaro M, Maritan E, Ferranti R, Zaninotto L, Miola A, Anastasia A, et al. Lithium Exposure During Pregnancy and the Postpartum Period: A Systematic Review and Meta-Analysis of Safety and Efficacy Outcomes. Am J Psychiatry. 2019:appiajp201919030228. doi: 10.1176/appi.ajp.2019.19030228. [DOI] [PubMed] [Google Scholar]
- 8.Bro SP, Kjaersgaard MI, Parner ET, Sorensen MJ, Olsen J, Bech BH, et al. Adverse pregnancy outcomes after exposure to methylphenidate or atomoxetine during pregnancy. Clin Epidemiol. 2015;7:139–47. doi: 10.2147/CLEP.S72906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bakken IJ, Ariansen AMS, Knudsen GP, Johansen KI, Vollset SE. The Norwegian Patient Registry and the Norwegian Registry for Primary Health Care: Research potential of two nationwide health-care registries. Scand J Public Health. 2020;48(1):49–55. doi: 10.1177/1403494819859737. [DOI] [PubMed] [Google Scholar]
- 10.Di Florio A, Jones L, Forty L, Gordon-Smith K, Craddock N, Jones I. Bipolar disorder, miscarriage, and termination. Bipolar Disord. 2015;17(1):102–5. doi: 10.1111/bdi.12217. [DOI] [PubMed] [Google Scholar]
- 11.Vigod SN, Fung K, Amartey A, Bartsch E, Felemban R, Saunders N, et al. Maternal schizophrenia and adverse birth outcomes: what mediates the risk? Soc Psychiatry Psychiatr Epidemiol. 2019 doi: 10.1007/s00127-019-01814-7. [DOI] [PubMed] [Google Scholar]
- 12.Vigod SN, Kurdyak PA, Dennis CL, Gruneir A, Newman A, Seeman MV, et al. Maternal and newborn outcomes among women with schizophrenia: a retrospective population-based cohort study. Bjog. 2014;121(5):566–74. doi: 10.1111/1471-0528.12567. [DOI] [PubMed] [Google Scholar]
- 13.Jacob L, Polly I, Kalder M, Kostev K. Prevalence of depression, anxiety, and adjustment disorders in women with spontaneous abortion in Germany - A retrospective cohort study. Psychiatry Res. 2017;258:382–6. doi: 10.1016/j.psychres.2017.08.064. [DOI] [PubMed] [Google Scholar]
- 14.Farren J, Mitchell-Jones N, Verbakel JY, Timmerman D, Jalmbrant M, Bourne T. The psychological impact of early pregnancy loss. Hum Reprod Update. 2018;24(6):731–49. doi: 10.1093/humupd/dmy025. [DOI] [PubMed] [Google Scholar]
- 15.Hoirisch-Clapauch S, Brenner B, Nardi AE. Adverse obstetric and neonatal outcomes in women with mental disorders. Thromb Res. 2015;135(Suppl 1):S60–3. doi: 10.1016/S0049-3848(15)50446-5. [DOI] [PubMed] [Google Scholar]
- 16.Munkholm K, Vinberg M, Kessing LV. Peripheral blood brain-derived neurotrophic factor in bipolar disorder: a comprehensive systematic review and meta-analysis. Mol Psychiatry. 2016;21(2):216–28. doi: 10.1038/mp.2015.54. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigues-Amorim D, Rivera-Baltanas T, Bessa J, Sousa N, Vallejo-Curto MC, Rodriguez-Jamardo C, et al. The neurobiological hypothesis of neurotrophins in the pathophysiology of schizophrenia: A meta-analysis. J Psychiatr Res. 2018;106:43–53. doi: 10.1016/j.jpsychires.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Triunfo S, Lanzone A. Impact of maternal under nutrition on obstetric outcomes. J Endocrinol Invest. 2015;38(1):31–8. doi: 10.1007/s40618-014-0168-4. [DOI] [PubMed] [Google Scholar]
- 19.Molarius A, Berglund K, Eriksson C, Eriksson HG, Linden-Bostrom M, Nordstrom E, et al. Mental health symptoms in relation to socio-economic conditions and lifestyle factors--a populationbased study in Sweden. BMC Public Health. 2009;9:302. doi: 10.1186/1471-2458-9-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahnquist J, Wamala SP. Economic hardships in adulthood and mental health in Sweden. The Swedish National Public Health Survey 2009. BMC Public Health. 2011;11:788. doi: 10.1186/1471-2458-11-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E Value. Ann Intern Med. 2017;167(4):268–74. doi: 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- 22.Cavalcante MB, Sarno M, Peixoto AB, Araujo Junior E, Barini R. Obesity and recurrent miscarriage: A systematic review and meta-analysis. J Obstet Gynaecol Res. 2019;45(1):30–8. doi: 10.1111/jog.13799. [DOI] [PubMed] [Google Scholar]
- 23.Balsells M, Garcia-Patterson A, Corcoy R. Systematic review and meta-analysis on the association of prepregnancy underweight and miscarriage. Eur J Obstet Gynecol Reprod Biol. 2016;207:73–9. doi: 10.1016/j.ejogrb.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Almeida ND, Basso O, Abrahamowicz M, Gagnon R, Tamblyn R. Risk of Miscarriage in Women Receiving Antidepressants in Early Pregnancy, Correcting for Induced Abortions. Epidemiology. 2016;27(4):538–46. doi: 10.1097/EDE.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 25.Kjaersgaard MI, Parner ET, Vestergaard M, Sorensen MJ, Olsen J, Christensen J, et al. Prenatal antidepressant exposure and risk of spontaneous abortion - a population-based study. PLoS One. 2013;8(8):e72095. doi: 10.1371/journal.pone.0072095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haervig KB, Mortensen LH, Hansen AV, Strandberg-Larsen K. Use of ADHD medication during pregnancy from 1999 to 2010: a Danish register-based study. Pharmacoepidemiol Drug Saf. 2014;23(5):526–33. doi: 10.1002/pds.3600. [DOI] [PubMed] [Google Scholar]
- 27.Alonso J, Angermeyer MC, Bernert S, Bruffaerts R, Brugha TS, Bryson H, et al. Use of mental health services in Europe: results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatr Scand Suppl. 2004;(420):47–54. doi: 10.1111/j.1600-0047.2004.00330.x. [DOI] [PubMed] [Google Scholar]
- 28.Wang PS, Angermeyer M, Borges G, Bruffaerts R, Tat Chiu W, DEG G, et al. Delay and failure in treatment seeking after first onset of mental disorders in the World Health Organization’s World Mental Health Survey Initiative. World Psychiatry. 2007;6(3):177–85. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the national health registries are available upon application though an online proposal system. Information regarding access can be found on the Directorate of e-health website (https://helsedata.no/no/om-helsedata/).