Abstract
Antihypertensive drugs (AHTs) are associated with lowered risks of neurodegenerative diseases and stroke. However, the relative risks associated with different AHT classes are unclear. Using an electronic health records network, we compared rates of these disorders over a 2 year period in propensity score matched cohorts of people taking calcium channel blockers (CCBs) compared to those taking other AHT classes. CCBs were associated with a higher incidence of all disorders compared to renin-angiotensin system agents, and a higher incidence of dementia and cerebrovascular disease compared to diuretics. CCBs were associated with a lower incidence of movement disorders and cerebrovascular disease than with β-blockers. The data show that AHT classes confer differential risks of neurodegenerative and cerebrovascular diagnoses.
Antihypertensive drugs (AHTs) have been associated with lowered risks for developing dementia,1,2 Parkinson’s disease,3 and stroke.4 However, the overall picture remains unclear. Relevant issues include concerns over residual confounding and lack of matching for blood pressure and other factors which may impact the risk of these disorders.
There is also uncertainty as to the diagnostic specificity of the associations and, importantly, whether one AHT class differs from another. We addressed these two issues by studying patients who were free of any of the disorders, and who were then prescribed a CCB or one of the other major AHT classes (diuretics, renin-angiotensin [RAS] agents, or β-blockers). CCBs were used as the reference AHT class based on their potential therapeutic use for neuropsychiatric disorders.5
Method
Our study followed STROBE guidelines. We used the TriNetX Analytics network, part of TriNetX (www.trinetx.com), a global federated cloud-based network providing access to electronic medical records from multiple healthcare organisations. Details have been described elsewhere.6,7 Briefly, the network allows patient cohorts to be created based on defined inclusion and exclusion criteria. Two cohorts can then be compared for other characteristics and outcomes. There is a built-in capability to propensity score match cohorts for any variables of interest;8 TriNetX uses greedy nearest neighbour matching with a caliper distance of 0.1 to produce 1:1 matching. TriNetX has a waiver from the Western Institutional Review Board since only aggregated counts and statistical summaries of de-identified information are used
We excluded patients younger than 50 years old. We also excluded anyone with a history of any of the diagnoses of interest (ICD-10 codes shown in Supplementary Table 1), or with diagnoses which may be prodromal to these conditions (mild cognitive impairment (MCI); delirium; REM sleep behaviour disorder; transient ischaemic attacks).
From the eligible population (~34 million patients), we created cohorts of people receiving their first prescription of each AHT class. The exposure and outcome period was 2 years; exposure was proxied by requiring prescriptions for the assigned AHT class separated by at least 2 years. As predicted based on clinical AHT guidelines, the initial cohorts were not matched for factors such as age, sex, race, or blood pressure (Supplementary Table 2), and also differed in some other variables which could contribute to confounding. Hence, we used propensity score matching to produce cohorts matched for age, sex, race, blood pressure and body mass index, as well as for a range of prior diagnoses and treatments that are risk factors for neurodegeneration or stroke (Supplementary Table 1). A variable with a standard difference between groups of less than 0.1 is considered well matched.8
The outcomes of interest were a first diagnosis of dementia, movement disorder, or cerebrovascular disease. We also measured dementia subtypes, Parkinson’s disease, stroke, and cerebral haemorrhage. Additionally, we measured 12 negative control outcomes; these help identify residual confounding.9 Cohort comparisons were made using odds ratios (OR) and 95% confidence intervals.
Results
Propensity score matching successfully produced cohorts matched for the wide range of demographic factors, prior diagnoses, and exposures, noted above. The main findings are shown in Figure 1. The cohort characteristics and detailed results are provided in Supplementary Table 3.
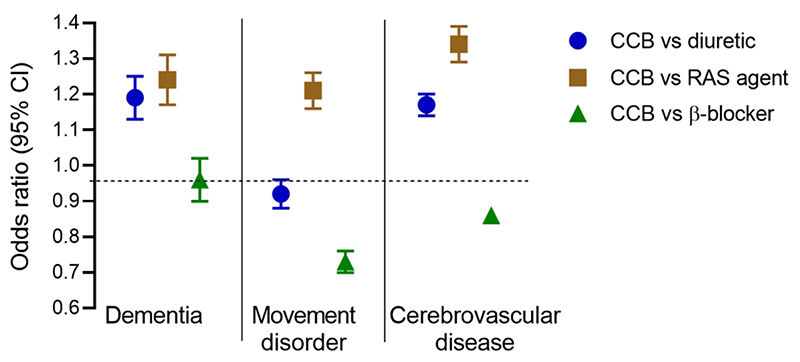
Figure 1.
Incidence of dementia, movement disorders and cerebrovascular disease during a 2 year exposure to CCBs compared to diuretics (circles; 231,764 in each cohort), RAS agents (squares; 181,495 in each cohort), or β-blockers (triangles; 234,015 in each cohort). Results are shown as odds ratios with 95% confidence intervals. See Supplementary Table 3 for full details of each cohort, and results for subtypes of dementia, and for Parkinson’s disease, stroke, and cerebral haemorrhage.Figure 1. Incidence of dementia, movement disorders and cerebrovascular disease during a 2 year exposure to CCBs compared to diuretics (circles; 231,764 in each cohort), RAS agents (squares; 181,495 in each cohort), or β-blockers (triangles; 234,015 in each cohort). Results are shown as odds ratios with 95% confidence intervals. See Supplementary Table 3 for full details of each cohort, and results for subtypes of dementia, and for Parkinson’s disease, stroke, and cerebral haemorrhage.
CCBs vs diuretics
CCBs were associated with higher rates of dementia (OR=1.19 [1.13-1.26]) and cerebrovascular disease (OR=1.17 [1.14-1.21]) as well as with dementia subtypes, MCI, stroke, and cerebral haemorrhage. Movement disorders were less common with CCBs than diuretics (OR=0.92 [0.88-0.96]) but Parkinson’s disease was not (OR=1.01 [0.91-1.13]). The mean OR for the negative control outcomes was lower in the CCB group (OR 0.89 [0.84-0.93]).
CCBs vs RAS agents
Compared to RAS agents CCBs were associated with increases in all three diagnostic categories: dementia (OR=1.24 [1.17-1.32]), movement disorders, (OR=1.21 [1.16-1.28]) and cerebrovascular disease (OR=1.34 [1.29-1.28]); ORs for Alzheimer’s disease and Parkinson’s disease showed similar trends (Supplementary Table 4). Negative control outcomes were not different between groups (OR=1.04 [0.97-1.11]).
CCBs vs β-blockers
CCBs were associated with a lower incidence of movement disorders (OR=0.73 [0.70-0.76]) including Parkinson’s disease (OR=0.73 [0.66-0.81]), as well as cerebrovascular disease (OR=0.86 [0.84-0.89]). There was no difference in dementia between the groups (OR=0.96 [0.90-1.01]), and a marginal increase in negative control outcomes (OR=1.06 [1.00-1.13]).
Discussion
Using a federated electronic health records network, we examined rates of dementia, movement disorders, and cerebrovascular disease, in people free of these conditions at baseline who were then exposed to CCBs or other AHT classes for the first time over a two year period. The size of the cohorts, and the use of propensity score matching and negative control outcomes, suggest that our results are relatively robust.
The association of AHTs with reduced risk of these disorders is well established.1–4 The present results strengthen the evidence that not all AHT classes are the same in this respect, and also show that their benefits differ across the various disorders measured. Since cohorts were matched at baseline for blood pressure, and remained so during the two year period, the results are not merely due to differences in control of hypertension.
Regarding the comparisons between AHTs, there was no evidence that CCBs have particular benefits, as we had initially anticipated.5 Indeed, the incidence of dementia and cerebrovascular disease was greater with CCBs than with RAS agents or diuretics. Instead, it was RAS agents that were associated with a lower incidence of all outcomes, extending the evidence that they may be neuroprotective, perhaps through effects on central angiotensin receptors.10
The only clear benefits of CCBs were in comparison to β-blockers for risk of movement disorders and cerebrovascular disease. The association of β-blockers with Parkinson’s disease has been controversial, with a recent review concluding that much of the reported association is probably due to reverse causation (β-blockers are used to treat tremor) and confounded by differential rates of smoking.11 However, our data cannot readily be explained in this way, since all patients at baseline were free of any movement disorder, including tremor, and cohorts were matched for rates of nicotine dependence. We confirmed earlier findings that CCBs are more effective than β-blockers in the prevention of stroke,4 likely due to the fact that CCBs decrease blood pressure variability whereas β-blockers increase it.
The negative control outcomes showed no difference between CCBs and RAS agents, reducing the likelihood of residual confounding. In contrast, their incidence was lower in users of CCBs compared to diuretics, and equivocally higher in users of CCBs compared to β-blockers. These differences may reflect overall health, or healthcare usage, within each cohort. Either way, differences of similar magnitude and direction that are seen for outcomes of interest are likely to be non-specific correlates. Equally, where outcomes of interest are in the opposite direction to the negative control outcomes (e.g. the higher rate of dementia seen with CCBs versus diuretics), the findings are arguably of greater significance.
Despite its size and methodological strengths, our study has limitations. Most importantly, residual confounding can never be eliminated from an observational study. We did not control for concurrent medication use during the outcome period. It is possible that subjects stopped and restarted treatment during the exposure period. Neither do we know about dosage, nor whether compliance was the same between AHT classes, although the fact that blood pressure during the outcome period remained similar between cohorts is reassuring.
It is notable that the results are observed after only two years’ exposure. Given that neurodegenerative and cerebrovascular disorders have a pathogenesis thought to begin at least a decade before diagnosis, this suggests that AHTs differ in their ability to retard the disease process soon before it manifests clinically, rather than (or as well as) having a direct causal role. Longer-term exposures and outcomes would be of interest. They are more difficult to assess, since cohort sizes become much smaller, but we find comparable results for 4 years’ AHT exposure, except for a lower incidence of dementia with CCBs than with β-blockers (data not shown).
The results extend the evidence that AHT classes are associated with differential risks of neurodegenerative and cerebrovascular disease. Future research should explore risk differences between drugs within an AHT class, and examine the mechanisms by which AHTs affect the brain and its disorders.
Supplementary Material
Acknowledgements
We thank Daniel Prieto Alhambra, Michele Hu, Paul Leeson, Steve Lethbridge, Stephan Palm, Peter Rothwell and Jennifer Stacey for support and advice.
Funding
L.C. is funded by the Wellcome Trust Oxford Clinical Doctoral Programme. P.J.H. is supported by the National Institute for Health Research (NIHR) Oxford Health Biomedical Research Centre (grant BRC-1215-20005). The views expressed are those of the authors and not necessarily those of the National Health Service, NIHR, or the Department of Health.
Footnotes
Declaration of interest. P.J.H. and L.C. were granted unrestricted access to the TriNetX Analytics network for the purposes of research relevant to psychiatry, and with no constraints on the analyses performed nor the decision to publish. S.L. is an employee of TriNetX Inc.
Author contributions. P.J.H. and L.C. designed the study. P.J.H. conducted the analyses, assisted by S.L. P.J.H. wrote the paper, with input from L.C. and S.L. All authors revised and approved the submission.
Data availability statement
Access to TriNetX’s de-identified patient data is available for the purpose of health care research with an approved user license.
References
- 1.Rouch L, Cestac P, Hanon O, Cool C, Helmer C, Bouhanick B, et al. Antihypertensive drugs, prevention of cognitive decline and dementia: a systematic review of observational studies, randomized controlled trials and meta-analyses, with discussion of potential mechanisms. CNS Drugs. 2015;29:113–30. doi: 10.1007/s40263-015-0230-6. [DOI] [PubMed] [Google Scholar]
- 2.Ding J, Davis-Plourde KL, Sedaghat S, Tully PJ, Wang W, Phillips C, et al. Antihypertensive medications and risk for incident dementia and Alzheimer’s disease: a meta-analysis of individual participant data from prospective cohort studies. Lancet Neurol. 2020;19:61–70. doi: 10.1016/S1474-4422(19)30393-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mullapudi A, Gudala K, Boya CS, Bansal D. Risk of Parkinson’s disease in the users of antihypertensive agents: An evidence from the meta-analysis of observational studies. J Neurodegener Dis. 2016:5780809. doi: 10.1155/2016/5780809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mukete BN, Cassidy M, Ferdinand KC, Le Jemtel TH. Long-term anti-hypertensive therapy and stroke prevention: A meta-analysis. Am J Cardiovasc Drugs. 2015;15:243–57. doi: 10.1007/s40256-015-0129-0. [DOI] [PubMed] [Google Scholar]
- 5.Harrison PJ, Tunbridge EM, Dolphin AC, Hall J. Voltage-gated calcium channel blockers for psychiatric disorders: genomic reappraisal. Br J Psychiatry. 2020;216:250–3. doi: 10.1192/bjp.2019.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrison PJ, Luciano S, Colbourne L. Rates of delirium associated with calcium channel blockers compared to diuretics, renin-angiotensin system agents and beta-blockers: an electronic health records network study. J Psychopharmacol. 2020;34:848–855. doi: 10.1177/0269881120936501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taquet M, Luciano S, Geddes JR, Harrison PJ. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. doi: 10.1016/S2215-0366(20)30462-4. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haukoos JS, Lewis RJ. The propensity score. JAMA. 2015;314:1637–1638. doi: 10.1001/jama.2015.13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipsitch M, Tchetgen Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology. 2010;21:383–388. doi: 10.1097/EDE.0b013e3181d61eeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson LaD, Eldahshan W, Fagan SC, Ergul A. Within the brain: the renin angiotensin system. Int J Mol Sci. 2018;19:876. doi: 10.3390/ijms19030876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hopfner F, Höglinger GU, Kuhlenbäumer G, Pottegard A, Wood M, Chritensen K, et al. β-adrenoceptors and the risk of Parkinson’s disease. Lancet Neurol. 2020;19:247–254. doi: 10.1016/S1474-4422(19)30400-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Access to TriNetX’s de-identified patient data is available for the purpose of health care research with an approved user license.