Abstract
The pulmonary innate response to low-dose bacterial challenge requires functioning alveolar macrophages (AM) but also subsequent macrophage apoptosis. To address the role of reactive oxygen species (ROS) and nitric oxide (NO) in AM apoptosis, sub-clinical Streptococcus pneumoniae infection was established in gp91phox-/- and inducible NO synthase deficient (iNOS-/-) mice. Both AM apoptosis and the number of macrophages containing apoptotic bodies are reduced in iNOS-/- as compared to control or gp91phox-/- mice. iNOS-/- mice recruit neutrophils and generate TNF-α to compensate for impaired AM competence but ROS deficiency has no apparent effect on AM function in this model.
Keywords: Macrophages, Apoptosis, Pneumococci, Mice, Nitric oxide
1. Introduction
Following aspiration of small numbers of bacteria into the distal airways alveolar macrophages play a critical role ingesting and removing bacteria [1]. The fate of these alveolar macrophages has previously been uncertain but we have demonstrated that increased alveolar macrophage apoptosis is a feature of sub-clinical challenge with small inocula of Streptococcus pneumoniae [1]. Alveolar macrophage apoptosis allows both clearance of cells that have phagocytosed bacteria and provides a potential source of pneumococcal antigens for presentation by antigen presenting cells, thus linking the innate and adaptive immune responses [2]. Previous studies have demonstrated a link between pneumococcal phagocytosis and killing and induction of apoptosis, suggesting that microbicidal molecules could contribute to the induction of alveolar macrophage apoptosis [3].
Macrophages, like neutrophils, contain the NADPH oxidase complex [4] and generate reactive oxygen species (ROS) following phagocytosis of bacteria [5], although whether ROS generated through the myeloperoxidase system plays a significant role in host defense in macrophages remains unclear [6]. Macrophage-derived ROS induces alveolar macrophage apoptosis in certain settings [7]. Nevertheless, the exact role of ROS in the innate response to pulmonary challenge with pneumococci is undetermined. The major source of nitric oxide (NO) in macrophages is from inducible nitric oxide synthase (iNOS) and this is believed to be of particular importance in the macrophage during infection with intracellular pathogens [6]. NO can mediate apoptosis via a number of mechanisms [8]. We have recently demonstrated that NO also contributes to macrophage killing of pneumococci [9] and NO deficiency has been associated with decreased clearance of bacteria from the lung in iNOS deficient mice [10].
Since both ROS and NO contribute to induction of apoptosis in a variety of settings [8, 11] we examined the role of each in the induction of alveolar macrophage apoptosis using well characterised mice that either lack gp91phox, a component of the NADPH oxidase system (gp91phox-/- [12]) or iNOS (iNOS-/- [13]). We studied these mice using a low dose sub-clinical infection model in which alveolar macrophages can clear small numbers of bacteria without recruitment of other inflammatory cells [1]. This model is characterised by alveolar macrophage apoptosis and provides an opportunity to investigate the role of both ROS and NO in alveolar macrophage apoptosis and the clearance of apoptotic cells in a setting where the apoptotic cells are derived solely from alveolar macrophages and not epithelial cells or recruited inflammatory cells [1].
2. Materials And Methods
2.1. Animals
iNOS and gp91phox deficient mice backcrossed onto a C57BL/6 background were obtained from Jackson Laboratories (Bar Harbor, Maine, US) and maintained as a homozygous colony. C57BL/6 mice (Harlan, Bicester, UK) were used as wild type controls. Female mice were used throughout. All animal experiments were conducted in accordance with the Home Office Animals (Scientific Procedures) Act of 1986 and received local ethical committee approval.
2.2. Pneumococcal infection model
Infection of mice with 104 (or in selected experiments 107) colony forming units (CFU) type 1 pneumococci (Statens Serum Institut, Copenhagen, Denmark) or mock infection with PBS was by direct tracheal instillation after anaesthesia with ketamine (100 mg/kg i.p.) and acepromazine (5 mg/kg i.p.) as previously described [1].
2.3. Collection of bronchial alveolar lavage, blood and lungs
Mice were killed by overdose of sodium pentabarbitone and exsanguinated by cardiac puncture. Bronchial alveolar lavage (BAL) was performed as described [1]. The cell differential was determined by review of cytospin preparations, with differentials determined by analysing the number of each cell type in a total population of 300 cells and then determining the percentage for that cell type [1]. Viable bacterial counts in lung were obtained as described [14].
2.4. Detection of apoptosis
Apoptosis detection was by nuclear morphology on cytospin preparations as described [1]. 300 cells were analysed per cytospin. The identity of cells as macrophages was confirmed by identifying F4/80 positivity [1, 15]. Clearance of apoptotic bodies was estimated by determining the number of alveolar macrophages with internalised apoptotic bodies per 300 cells in Diff-Quik stained cytospins.
2.5. Cytokine production
TNF-α in BAL was measured using an eBioscience mouse TNF-α ELISA Ready-SET-Go reagent set (Insight Biotechnology Limited, Wembley, UK) following the manufacturer’s protocols. Limit of detection was 15pg/ml.
2.6. Statistics
Results are recorded as mean and SEM. Statistical testing was performed using ANOVA with Bonferroni’s Multiple Comparison Test by Prism 4.0 software (GraphPad Inc.). Differences in microbiologic outcome were assessed using the Mann-Whitney test. Significance was defined as p<0.05
3. Results
3.1. gp91phox-/- and iNOS-/- mice clear a low dose pneumococcal challenge from the lung
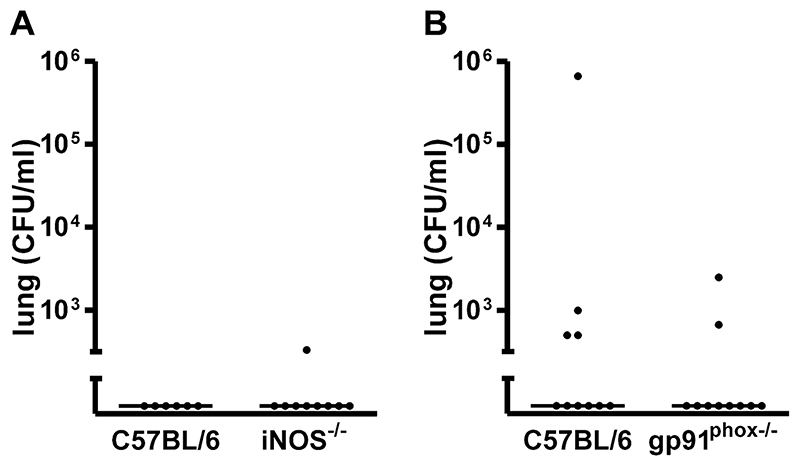
Instillation of a small inoculum of type 1 pneumococci results in clearance of bacteria from the lung over a 24 h period without demonstrable recruitment of neutrophils or other inflammatory cells at any time point in C57BL/6 mice [1]. When these experiments were repeated with either iNOS-/- or gp91phox-/- mice bacterial clearance was also similar to C57BL/6 mice, confirming both models remained models of sub-clinical resolving infection, Figure 1. Mice remained well without signs of illness and blood cultures showed no evidence of bacteremia in the majority of mice (data not shown). Examination of cytospins from bronchial alveolar lavage showed no evidence of recruitment of neutrophils or other inflammatory cells (data not shown).
Figure 1. No difference in bacteria in lungs from iNOS or gp91phox deficient mice after low dose pneumococcal infection.
Bacteria in lung homogenates 24 hours after intratracheal instillation of 104 CFU type 1 pneumococci in A) wild type controls (C57BL/6, n=6) and iNOS deficient mice(iNOS-/-, n=9 and B) C57BL/6 mice, n=10 and gp91phox deficient (gp91phox-/-, n=10) both from 3 independent experiments.
3.2. iNOS-/- but not gp91phox-/- mice have reduced numbers of apoptotic alveolar macrophages in the lung after a low dose pneumococcal challenge
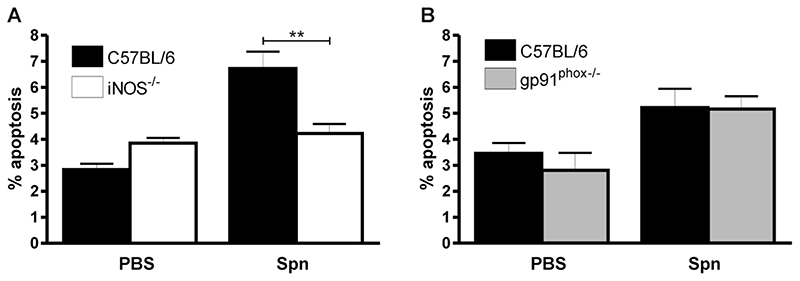
During a low dose challenge increased numbers of apoptotic alveolar macrophages are detected in bronchial alveolar lavage and in lung sections [1]. Since both ROS and NO can contribute to induction of apoptosis [8, 11] we addressed how decreased generation of these molecules might influence levels of observable apoptosis. As shown in Figure 2, low dose infection resulted in increased numbers of apoptotic alveolar macrophages in the bronchial alveolar lavage as compared to mock-infection in C57BL/6 mice. These findings were not altered in gp91phox-/- mice which still demonstrated increased alveolar macrophage apoptosis as compared to mock-infection. However in iNOS-/- mice there was a significant decline in the numbers of apoptotic macrophages detected in the lavage specimen, as compared to the numbers identified in the C57BL/6 mice.
Figure 2. iNOS deficiency is associated with reduction in apoptotic cells in bronchial lavage fluid after pneumococcal infection.
The percentage of apoptotic events (apoptotic cells and bodies) in cytospins of bronchial alveolar lavage 24 hours after instillation of 104 CFU type 1 pneumococci (Spn) or PBS from A) wild type controls (C57BL/6, n=6) and iNOS deficient mice (iNOS-/-, n=9) and B) C57BL/6 mice and gp91phox deficient (gp91phox-/-) (PBS treated C57BL/6 n=11 and gp91phox-/- n=5; Spn treated C57BL/6 n=10 and gp91phox-/- n=10), both from 3 independent experiments. Mean+SEM, ** p<0.01, ANOVA with Bonferroni’s Multiple Comparison Test.
3.3. Consequences of NO deficiency and reduced alveolar macrophage apoptosis in iNOS-/- mice
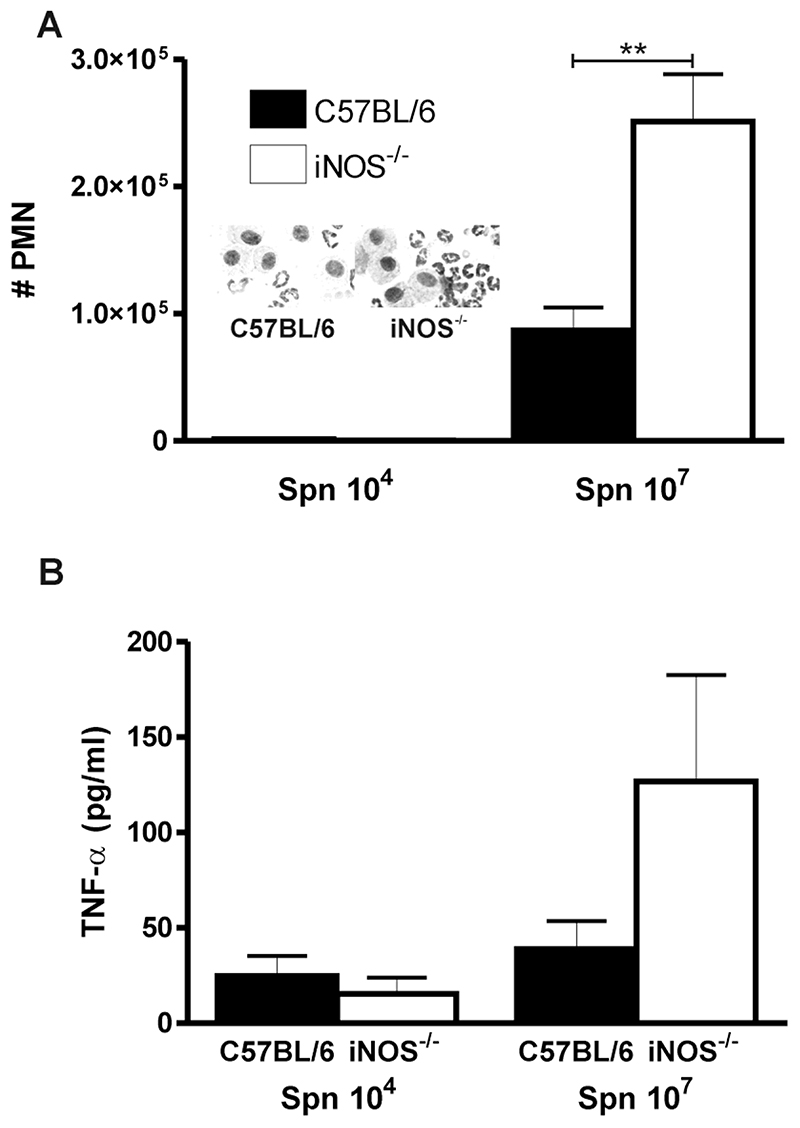
Our prior in vitro studies involving human macrophages suggested that reduced NO production or reduced alveolar macrophage apoptosis would compromise bacterial clearance [9]. In these sub-clinical infection models the first signs of reduced alveolar macrophage competence are the recruitment of neutrophils to compensate for the defect in alveolar macrophage function [1]. We therefore reasoned that by increasing the dose of bacteria we would unmask the impairment in bacterial host defence induced by reduced NO production and decreased alveolar macrophage apoptosis. We increased the infecting inoculum of type 1 pneumococci from 104 to 107 CFU. This increased dose resulted in a resolving model of infection, which differs from the fulminant pneumonia and invasive disease seen when the highly capsulated type 2 pneumococcus is studied [1]. Instead the current model results in an infection in which small numbers of neutrophils are required to compensate for the decreased competency of the alveolar macrophage system. As seen in Figure 3A the number of recruited neutrophils required for clearance was significantly greater for the iNOS-/- mice and the level of a key cytokine involved in host defence against pneumococci, TNF-α□ was significantly greater in bronchial alveolar lavage fluid, Figure 3B. These findings suggested that limitations in alveolar macrophage competence in the face of reduced NO production and alveolar macrophage apoptosis were compensated for by a lower threshold to recruit neutrophils and greater TNF-α □ expression.
Figure 3. Increased numbers of neutrophils in the bronchial alveolar lavage from lungs of iNOS-/- mice after pneumococcal infection.
A) Numbers of neutrophils in bronchial alveolar lavage (BAL) from iNOS deficient mice (iNOS-/-) and wild type controls (C57BL/6) 24 hours after intratracheal instillation of 104 and 107 CFU type 1 pneumococci. (104 CFU Spn treated C57BL/6 n=6 and iNOS-/- n=9; 107 CFU Spn treated C57BL/6 n=6 and iNOS-/- n=8), from 3 independent experiments. Inserts shows representative pictures of cytospins from C57BL/6 and iNOS-/- mice after instillation with 107 CFU type 1 pneumococci. B) TNF-α concentration in BAL in the same experiments as A). Mean+SEM, **p<0.01, ANOVA with Bonferroni’s Multiple Comparison Test.
3.4. Alveolar macrophages from iNOS-/- mice are less likely to contain internalised apoptotic bodies
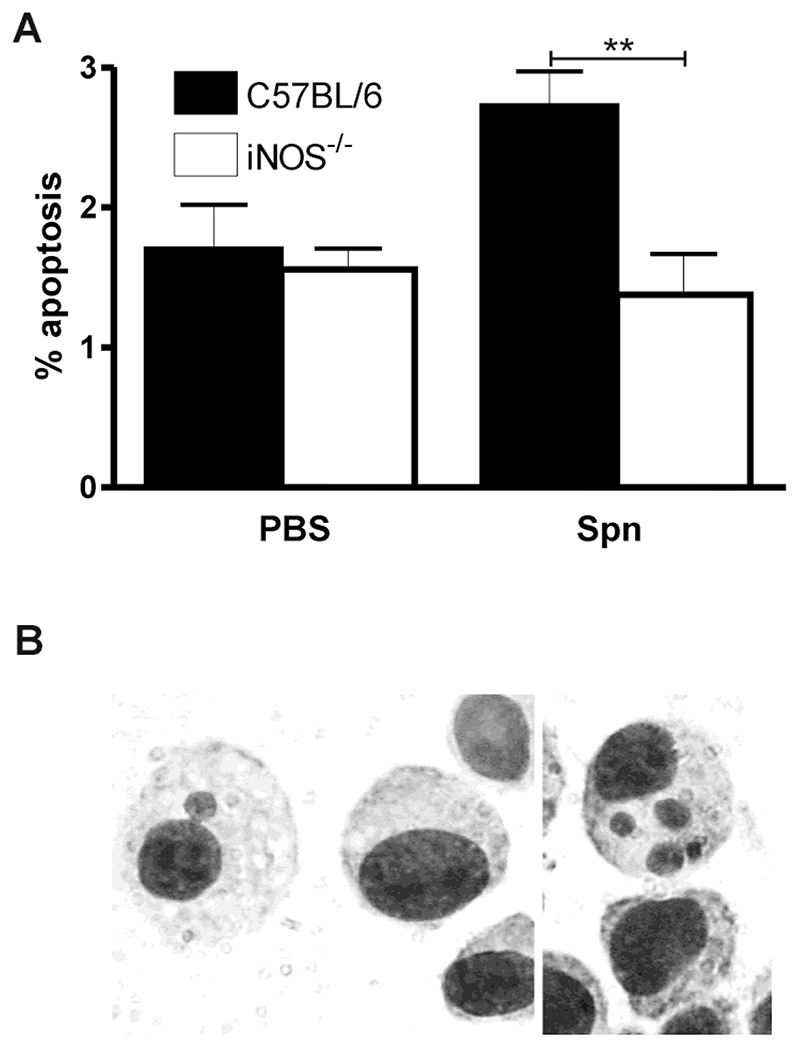
Induction of apoptosis not only has consequences for the cell that undergoes apoptosis but has indirect effects on the lung by resetting the cytokine network and indirectly influencing the inflammatory response. This is because phagocytosis of apoptotic cells modifies the profile of pro-inflammatory cytokines induced [16]. It remains unclear however whether in the lung apoptotic cells are cleared locally or traffic to regional lymph nodes. As shown in Figure 4 an increased percentage of alveolar macrophages had detectable apoptotic cells following infection with pneumococci. The number of alveolar macrophages that contained apoptotic bodies was however significantly reduced in iNOS-/- mice. The cells ingesting these apoptotic bodies all stained with a macrophage marker F4/80 (data not shown).
Figure 4. iNOS deficiency is associated with reduction in apoptotic bodies in macrophages from bronchial lavage fluid after pneumococcal infection.
A) The percentage of macrophages containing apoptotic bodies in cytospins of bronchial alveolar lavage from iNOS deficient mice (iNOS-/-) and wild type controls (C57BL/6) 24 hours after instillation of 104 CFU type 1 pneumococci (Spn) or PBS. (PBS treated C57BL/6 n=11 and iNOS-/- n=18; Spn treated C57BL/6 n=6 and iNOS-/- n=8), from 3 independent experiments. Mean+SEM, ** p<0.01, ANOVA with Bonferroni’s Multiple Comparison Test. B) Representative picture of apoptotic bodies in alveolar macrophages.
4. Discussion
In this study we demonstrate that during low dose pneumococcal challenge alveolar macrophage apoptosis involves NO but not ROS generation. ROS deficiency does not modify clearance of low dose pneumococcal challenge or alter rates of alveolar macrophage apoptosis. In contrast NO is required for optimal clearance by alveolar macrophages and in its absence greater numbers of neutrophils are recruited to compensate for the deficit in alveolar macrophage function.
Predictions for the roles of host defense molecules based on in vitro studies of single cell cultures have the limitation that they fail to take account of the cell’s function as part of a dynamic network of cells. At the other extreme in vivo studies of overwhelming infection make it difficult to determine individual roles since one cell type can compensate for a specific defect in another. In determining the competence of the alveolar macrophage system we have elected to combine observations based on in vitro macrophage culture [3] and those from models of pneumonia in mice with a sub-clinical infection model, in which alveolar macrophages clear bacteria [1]. The sub-clinical infection model has demonstrated that, when alveolar macrophage competence is overwhelmed by increasing the ratio of bacteria to alveolar macrophages, neutrophil recruitment is required to limit bacterial replication. Furthermore, these studies demonstrate that decreased alveolar macrophage apoptosis is associated with impaired bacterial clearance [1].
ROS and NO have both pro- and anti-apoptotic effects and modulate phagocyte survival [7, 17–19]. We found that NO but not ROS contributed to alveolar macrophage apoptosis. NO has many potential pro-apoptotic effects including down-regulation of the anti-apoptotic molecule Bcl-2 and up-regulation of the pro-apoptotic molecule Bax [20]. However, we have found that the anti-apoptotic molecule Mcl-1 is a critical determinant of macrophage apoptosis during exposure to pneumococci and that NO generated during pneumococcal infection leads to down-regulation of Mcl-1, triggering an apoptotic pathway that involves mitochondria and caspase activation [3, 9, 21].
We have confirmed that alveolar macrophages are able to phagocytose apoptotic cells derived from resident alveolar macrophages locally. Our study design used the sub-clinical infection model, in which no recruited cells were present, hence alveolar macrophages were confirmed as the source of the apoptotic cells. It remains to be determined whether these apoptotic cells play any role as a source of pneumococcal antigens for antigen presentation by dendritic cells as has been demonstrated in other settings [2] or whether induction of apoptosis might compromise subsequent antigen presentation as shown for dendritic cells that undergo apoptosis following pneumococcal exposure [22].
Despite demonstrating that NO contributes to alveolar macrophage competence by enhancing apoptosis and prior data suggesting that NO contributes to bacterial clearance in the lung [10] we did not find any defect in clearance of bacteria from the lung following low dose challenge. Increasing the infecting inoculum, in the presence of decreased NO production, however, increases reliance on other aspects of the innate response with greater neutrophil recruitment and production of TNF-α. These adaptations compensate for the macrophage defect, as previously shown following alveolar macrophage depletion [1]. As shown in studies using the highly capsulated and more virulent type 2 pneumococcus [10], when the system is further stressed these compensatory measures are no longer adequate and an overall defect in clearance of bacteria from the lung becomes apparent.
ROS did not contribute to host defense against low doses of pneumococci. Macrophages, like neutrophils, generate reactive oxygen species (ROS) following phagocytosis of bacteria but have negligible myeloperoxidase activity and are less dependent on ROS for pneumococcal killing [5, 23]. Pneumococci, generate H2O2 and bacterial produced ROS can provide a substrate to interact with host-derived NO to generate microbicidal molecules such as reactive nitrogen species [6]. Furthermore, pneumococci have adaptations to survive in the presence of H2O2 potentially limiting its microbicidal potential [24, 25]. In keeping with these observations individuals with chronic granulomatous disease (CGD) (a condition caused by genetic mutations in the NADPH complex which generates superoxide anions) do not have increased susceptibility to the pneumococcus [26].
In summary we show that NO but not ROS contribute to alveolar macrophage apoptosis. Decreased alveolar macrophage apoptosis leads to less efficient clearance of pulmonary bacteria which requires enhanced cytokine expression and neutrophil recruitment in resolving infection models to compensate for the defect. As others have previously shown [10], these compensatory measures ultimately break-down, creating an overall deficit in bacterial clearance.
5. Acknowledgements
This work was supported by a Wellcome Trust Senior Clinical Fellowship to DHD, #076945
References
- [1].Dockrell DH, Marriott HM, Prince LR, Ridger VC, Ince PG, Hellewell PG, et al. Alveolar macrophage apoptosis contributes to pneumococcal clearance in a resolving model of pulmonary infection. J Immunol. 2003;171(10):5380–8. doi: 10.4049/jimmunol.171.10.5380. [DOI] [PubMed] [Google Scholar]
- [2].Yrlid U, Wick MJ. Salmonella-induced apoptosis of infected macrophages results in presentation of a bacteria-encoded antigen after uptake by bystander dendritic cells. J Exp Med. 2000;191(4):613–24. doi: 10.1084/jem.191.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dockrell DH, Lee M, Lynch DH, Read RC. Immune-mediated phagocytosis and killing of Streptococcus pneumoniae are associated with direct and bystander macrophage apoptosis. J Infect Dis. 2001;184(6):713–22. doi: 10.1086/323084. [DOI] [PubMed] [Google Scholar]
- [4].Segal AW, Garcia R, Goldstone H, Cross AR, Jones OT. Cytochrome b-245 of neutrophils is also present in human monocytes, macrophages and eosinophils. Biochem J. 1981;196(1):363–7. doi: 10.1042/bj1960363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gee JB, Vassallo CL, Bell P, Kaskin J, Basford RE, Field JB. Catalase-dependent peroxidative metabolism in the alveolar macrophage during phagocytosis. J Clin Invest. 1970;49(6):1280–7. doi: 10.1172/JCI106340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fang FC. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol. 2004;2(10):820–32. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- [7].Shen HM, Zhang Z, Zhang QF, Ong CN. Reactive oxygen species and caspase activation mediate silica-induced apoptosis in alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2001;280(1):L10-7. doi: 10.1152/ajplung.2001.280.1.L10. [DOI] [PubMed] [Google Scholar]
- [8].Brune B, von Knethen A, Sandau KB. Nitric oxide (NO): an effector of apoptosis. Cell Death Differ. 1999;6(10):969–75. doi: 10.1038/sj.cdd.4400582. [DOI] [PubMed] [Google Scholar]
- [9].Marriott HM, Ali F, Read RC, Mitchell TJ, Whyte MK, Dockrell DH. Nitric oxide levels regulate macrophage commitment to apoptosis or necrosis during pneumococcal infection. Faseb J. 2004;18(10):1126–8. doi: 10.1096/fj.03-1450fje. [DOI] [PubMed] [Google Scholar]
- [10].Kerr AR, Wei XQ, Andrew PW, Mitchell TJ. Nitric oxide exerts distinct effects in local and systemic infections with Streptococcus pneumoniae. Microb Pathog. 2004;36(6):303–10. doi: 10.1016/j.micpath.2004.02.001. [DOI] [PubMed] [Google Scholar]
- [11].Buttke TM, Sandstrom PA. Oxidative stress as a mediator of apoptosis. Immunol Today. 1994;15(1):7–10. doi: 10.1016/0167-5699(94)90018-3. [DOI] [PubMed] [Google Scholar]
- [12].Pollock JD, Williams DA, Gifford MA, Li LL, Du X, Fisherman J, et al. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9(2):202–9. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- [13].Wei XQ, Charles IG, Smith A, Ure J, Feng GJ, Huang FP, et al. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375(6530):408–11. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- [14].Gordon SB, Irving GR, Lawson RA, Lee ME, Read RC. Intracellular trafficking and killing of Streptococcus pneumoniae by human alveolar macrophages are influenced by opsonins. Infect Immun. 2000;68(4):2286–93. doi: 10.1128/iai.68.4.2286-2293.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jennings JH, Linderman DJ, Hu B, Sonstein J, Curtis JL. Monocytes recruited to the lungs of mice during immune inflammation ingest apoptotic cells poorly. Am J Respir Cell Mol Biol. 2004 doi: 10.1165/rcmb.2004-0108OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101(4):890–8. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Brune B, Gotz C, Messmer UK, Sandau K, Hirvonen MR, Lapetina EG. Superoxide formation and macrophage resistance to nitric oxide-mediated apoptosis. J Biol Chem. 1997;272(11):7253–8. doi: 10.1074/jbc.272.11.7253. [DOI] [PubMed] [Google Scholar]
- [18].Davis DW, Weidner DA, Holian A, McConkey DJ. Nitric oxide-dependent activation of p53 suppresses bleomycin-induced apoptosis in the lung. J Exp Med. 2000;192(6):857–69. doi: 10.1084/jem.192.6.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hortelano S, Castrillo A, Alvarez AM, Bosca L. Contribution of Cyclopentenone Prostaglandins to the Resolution of Inflammation Through the Potentiation of Apoptosis in Activated Macrophages. J Immunol. 2000;165(11):6525–31. doi: 10.4049/jimmunol.165.11.6525. [DOI] [PubMed] [Google Scholar]
- [20].Mannick JB. Immunoregulatory and Antimicrobial Effects of Nitrogen Oxides. Proc Am Thorac Soc. 2006;3(2):161–65. doi: 10.1513/pats.200505-048BG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Marriott HM, Bingle CD, Read RC, Braley KE, Kroemer G, Hellewell PG, et al. Dynamic changes in Mcl-1 expression regulate macrophage viability or commitment to apoptosis during bacterial clearance. J Clin Invest. 2005;115(2):359–68. doi: 10.1172/JCI21766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Colino J, Snapper CM. Opposing signals from pathogen-associated molecular patterns and IL-10 are critical for optimal dendritic cell induction of in vivo humoral immunity to Streptococcus pneumoniae. J Immunol. 2003;171(7):3508–19. doi: 10.4049/jimmunol.171.7.3508. [DOI] [PubMed] [Google Scholar]
- [23].Biggar WD, Buron S, Holmes B. Bactericidal mechanisms in rabbit alveolar macrophages: evidence against peroxidase and hydrogen peroxide bactericidal mechanisms. Infect Immun. 1976;14(1):6–10. doi: 10.1128/iai.14.1.6-10.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Paterson GK, Blue CE, Mitchell TJ. An operon in Streptococcus pneumoniae containing a putative alkylhydroperoxidase D homologue contributes to virulence and the response to oxidative stress. Microb Pathog. 2006;40(4):152–60. doi: 10.1016/j.micpath.2005.12.003. [DOI] [PubMed] [Google Scholar]
- [25].Pericone CD, Park S, Imlay JA, Weiser JN. Factors contributing to hydrogen peroxide resistance in Streptococcus pneumoniae include pyruvate oxidase (SpxB) and avoidance of the toxic effects of the fenton reaction. J Bacteriol. 2003;185(23):6815–25. doi: 10.1128/JB.185.23.6815-6825.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lekstrom-Himes JA, Gallin JI. Immunodeficiency Diseases Caused by Defects in Phagocytes. N Engl J Med. 2000;343(23):1703–14. doi: 10.1056/NEJM200012073432307. [DOI] [PubMed] [Google Scholar]