Abstract
Regulation of the inflammatory infiltrate is critical to the successful outcome of pneumonia. Alveolar macrophage apoptosis is a feature of pneumococcal infection and aids disease resolution. The host benefits of macrophage apoptosis during the innate response to bacterial infection are however incompletely defined. Since nitric oxide is required for optimal macrophage apoptosis during pneumococcal infection, we have explored the role of macrophage apoptosis in regulating inflammatory responses during pneumococcal pneumonia, using inducible nitric oxide synthase (iNOS)-deficient mice. iNOS-/- mice demonstrated decreased numbers of apoptotic alveolar macrophages as compared to wild-type C57BL/6 mice following pneumococcal challenge. In association with this, iNOS-/- mice demonstrated greater recruitment of neutrophils to the lung and enhanced expression of TNF-α. Pharmacologic inhibition of iNOS produced similar results. Greater pulmonary inflammation was associated with greater levels of early bacteremia, IL-6 production, lung inflammation and mortality within the first 48 h in iNOS-/- mice. Labelled apoptotic alveolar macrophages were phagocytosed by resident macrophages in the lung and intratracheal instillation of exogenous apoptotic macrophages decreased neutrophil recruitment in iNOS-/- mice and decreased TNF-α mRNA in lungs and protein in bronchial alveolar lavage, as well as chemokines and cytokines including IL-6. These changes were associated with a lower probability of mice becoming bacteremic. This demonstrates the potential of apoptotic macrophages to downregulate the inflammatory response and for the first time in vivo demonstrates that clearance of apoptotic macrophages decreases neutrophil recruitment and invasive bacterial disease during pneumonia.
Keywords: Monocytes/Macrophages, Bacterial, Apoptosis, Lung, Inflammation
Introduction
Macrophages play a critical function during bacterial infection by co-ordinating the innate immune response (1) and are long-lived tissue cells with a low incidence of constitutive apoptosis (2). Modulation of macrophage lifespan is, however, an important mechanism for regulation of macrophage function. Although multiple pathogens induce macrophage apoptosis as a mechanism of immune evasion, the existence of host benefits from macrophage apoptosis has been more controversial (3). Nevertheless, it has become apparent that macrophage apoptosis can represent a host response that contributes to bacterial killing of chronic intracellular pathogens (4) and organisms that do not persist for prolonged periods intracellularly but are killed efficiently after phagocytosis (5). However, the functional consequences of host-induced macrophage apoptosis are incompletely characterised.
Infection with the Gram-positive diplococcus Streptococcus pneumoniae represents the most frequent cause of community-acquired pneumonia (6). Although anti-pneumococcal antibody is critical in determining the outcome of infection (7), the importance of innate responses is increasingly recognised (8). Tissue macrophages, including alveolar macrophages (AM)3, are critical to the innate response, phagocytosing bacteria and co-ordinating the innate response to infection (1). AM depletion results in a reduction in the number of pneumococci that are required to trigger neutrophil recruitment to the lung (9). During established pneumonia AM also contribute to resolution of the inflammatory response but are no longer critical for bacterial clearance since neutrophils become the major cell phagocytosing bacteria (10).
Host-mediated macrophage apoptosis is a feature of pneumococcal infection and its inhibition in vitro decreases pneumococcal clearance (5). In murine models decreased AM apoptosis is associated with greater rates of invasive pneumococcal disease (9). We have previously identified a key role for NO production in the activation of the apoptotic cascade during pneumococcal disease (11). Apoptosis is believed to contribute to resolution of inflammation in the lung and pneumococcal pneumonia has been regarded as the paradigm for a host-response that involves pulmonary inflammation with subsequent complete resolution without lung injury (12). The role of neutrophil apoptosis is well established but AM apoptosis may also facilitate resolution of the inflammatory response in the lung. Macrophages produce anti-inflammatory cytokines after phagocytosing apoptotic bodies (13), although the pattern of cytokines produced is modified in the presence of Toll-like receptor ligation (14). It remains unclear whether macrophage apoptosis influences the inflammatory response during pneumonia. In view of the important roles of macrophages and of host-mediated apoptosis in host defence against S. pneumoniae, pneumococcal infection represents a relevant model with which to investigate the effects of macrophage apoptosis on regulation of the inflammatory response. Since we have identified a critical role for NO in regulating initiation of macrophage apoptosis during pneumococcal infection we chose to study the effect of decreasing macrophage apoptosis in vivo using the well characterised (iNOS)-deficient mouse (15). On the basis of our prior in vitro findings we predicted that these mice could have decreased AM apoptosis and this model could then be examined to determine the effects of decreased AM apoptosis, as opposed to NO deficiency, on the regulation of the inflammatory response, using a model of pneumococcal pneumonia.
We demonstrate that iNOS-/- mice have decreased rates of AM apoptosis in vivo and that this is associated with a greater degree of inflammation in the lung. Furthermore, we demonstrate that instillation of apoptotic macrophages into mouse lungs decreases the greater lung inflammation observed in iNOS-/- mice and decreases the likelihood of early invasive pneumococcal disease.
Materials And Methods
Animals
iNOS deficient mice backcrossed onto a C57BL/6 background were obtained from Jackson Laboratories and maintained as a homozygous colony. C57BL/6 mice (Harlan) were used as wild type controls. Female mice were used throughout. For aminoguanidine treatment C57BL/6 mice were treated with 2.5% aminoguanidine in their drinking water, with 5% glucose added to increase palatability, starting 7 days prior to pneumococcal infection (16). Control mice were treated with an equivalent dose of glucose. All animal experiments were conducted in accordance with the Home Office Animals (Scientific Procedures) Act of 1986 and received local ethical committee approval.
In vivo pneumococcal infection model
Infection of mice with 107 CFU type 2 pneumococci (strain D39) or mock infection with PBS was by direct tracheal instillation after anaesthesia with ketamine (100 mg/kg i.p.) and acepromazine (5 mg/kg i.p.) as previously described (9). In survival studies mice showing physical signs of severe illness were culled and their time of death recorded as the time at which they were culled.
Collection of bronchial alveolar lavage, blood and lungs
Mice were killed by overdose of sodium pentabarbitone and exsanguinated by cardiac puncture. Bronchial alveolar lavage (BAL) was performed as described (9). The cell differential was by review of cytospin preparations (9). Viable bacterial counts in lung and blood were obtained as described (17).
Detection of apoptosis
Apoptosis detection was by Annexin V (BD Biosciences)/ToPro 3 (Molecular Probes) staining and flow cytometry or by nuclear morphology on cytospin preparations as described (9). AM were identified by F4/80 fluorescence intensity (anti-mouse F4/80:FITC (CI:A3-1 clone), Serotec) and forward vs. side scatter characteristics and neutrophils by positive Ly6G staining (anti-mouse Ly6G:FITC (1A8 clone), BD Biosciences) (9, 18).
Cytokine/chemokine mRNA detection
Whole lung was harvested and immediately stored in RNAlater (Sigma). Total RNA was extracted from lung tissue by homogenisation into TRIZOL (Invitrogen) following the manufacturers protocol. RNase Protection Assays were performed using 5μg RNA and mCK-2b, mCK-5c and custom probe sets according to the RiboQuant protocol (BD Biosciences). Bands were quantitated using Image J 1.32 software (NIH) and were normalised to GAPDH. To perform TaqMan real-time PCR RNA was DNA digested (Ambion) following the manufacturer’s protocol for routine DNase treatment. cDNAs were synthesised from RNA by reverse transcription using random hexamer primers. Relative expression of TNF-α normalised to β-actin was by TaqMan gene expression assays (Applied Biosystems) for TNF-α (Mm00443258_m1) and β-actin (Mm00607939_s1) following the manufacturer’s protocol.
Cytokine production
Cytokines in BAL were measured using DuoSet ELISA development kits (R&D Systems) for mouse TNF-α, KC (CXCL1), MIP-2 (CXCL2) and IL-6 following the manufacturer’s protocols (19). Limits of detection were 15pg/ml.
Lung Injury
Protein levels in BAL were measured using a BCA protein assay (Pierce), following the manufacturer’s protocol (20).
Histopathology
Unlavaged lungs were fixed via the trachea with 10% buffered formalin at 20 cmH2O, paraffin-embedded sections prepared, sectioned, stained with haematoxylin and eosin and independently evaluated by two pathologists (PGI and SSC) using a BH2 Olympus microscope.
Instillation of apoptotic bodies
Apoptotic AM were generated from AM obtained from BAL of donor C57BL/6 mice. BAL was spun for 5 mins at 1000 x g and cells resuspended in RPMI (Invitrogen) plus 10% heat inactivated FCS (Bioclear), 100μg/ml streptomycin and 100U/ml penicillin (Invitrogen) and incubated at 37°C for 4 h to allow macrophage adherence. AM were washed and labelled with 2μM CellTracker Red (Molecular Probes) for 30 min at 37°C. Apoptosis was induced by exposure to 120mJ/cm2 irradiation (Stratalinker 1800, Stratagene). 12h post UV treatment AM were gently scraped and washed in PBS. After this treatment mean values for AM were 69% AnnexinV+, 8% ToPro3+ and 65% showed loss of Δψm (9, 11). In additional experiments AM were made apoptotic by treatment with 10μM staurosporine for 16 h. Cells were resuspended in PBS at 5x106 cells/ml. 1x104 apoptotic AM were delivered to the lungs by direct intra-tracheal instillation immediately after pneumococcal infection. This number was chosen to provide approximately enough apoptotic cells to restore the numbers of apoptotic cells in the iNOS-/- mice to the level observed in the C57BL/6 mice. 12-48 h post infection mice were killed and BAL performed. To confirm phagocytosis of apoptotic bodies, resident AM of some C57BL/6 mice were labelled with PKH2 green fluorescent phagocytic cell linker compound (Sigma) (21). PKH2 dye (stock 1x10-3M) was diluted 1 in 5 with diluent B and 100μl was administered intravenously. 42 h post PKH2 treatment apoptotic AM were delivered to the lungs by direct intra-tracheal instillation. Lungs were lavaged 30 min post instillation and cytospin preparations made from lung cells. Coverslips were mounted with VectaShield containing DAPI (Vector), and the cells imaged with a DeltaVision Microscope.
Ex-vivo AM pneumococcal infection
C57BL/6 and iNOS deficient mice were killed with an overdose of sodium pentabarbitone and bronchial alveolar lavage (BAL) was performed as described using RPMI (Invitrogen) plus 10% heat inactivated FCS (Bioclear), 100μg/ml streptomycin and 100U/ml penicillin (Invitrogen) (9). BAL was spun for 5 mins at 1000 x g and cells resuspended in RPMI plus 10% heat inactivated FCS without antibiotics and incubated at 37°C for at least 4 h to allow macrophage adherence. Infection was with type 1 pneumococci opsonized with serum from mice immunised with pneumovax vaccine at a MOI of 10 (5). Killing was assessed by analysing colony counts in supernatants at 4 and 20 h post infection (22). Apoptosis was assessed at 20 h post infection by nuclear morphology after DAPI staining as described (22).
Statistics
Results are recorded as mean and SEM. Survival was calculated by Kaplan-Meier followed by log rank analysis. Parametric or non-parametric testing was performed with the indicated tests using Prism 4.0 software (GraphPad Inc.). Significance was defined as p<0.05.
Results
Alveolar macrophage apoptosis is reduced in iNOS-/- mice during pneumococcal infection
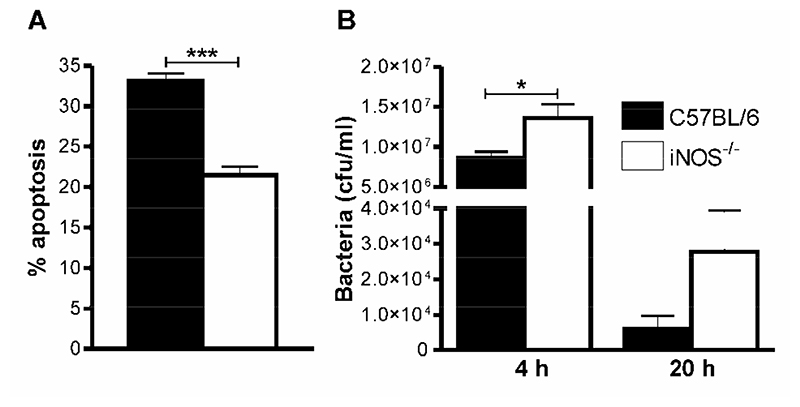
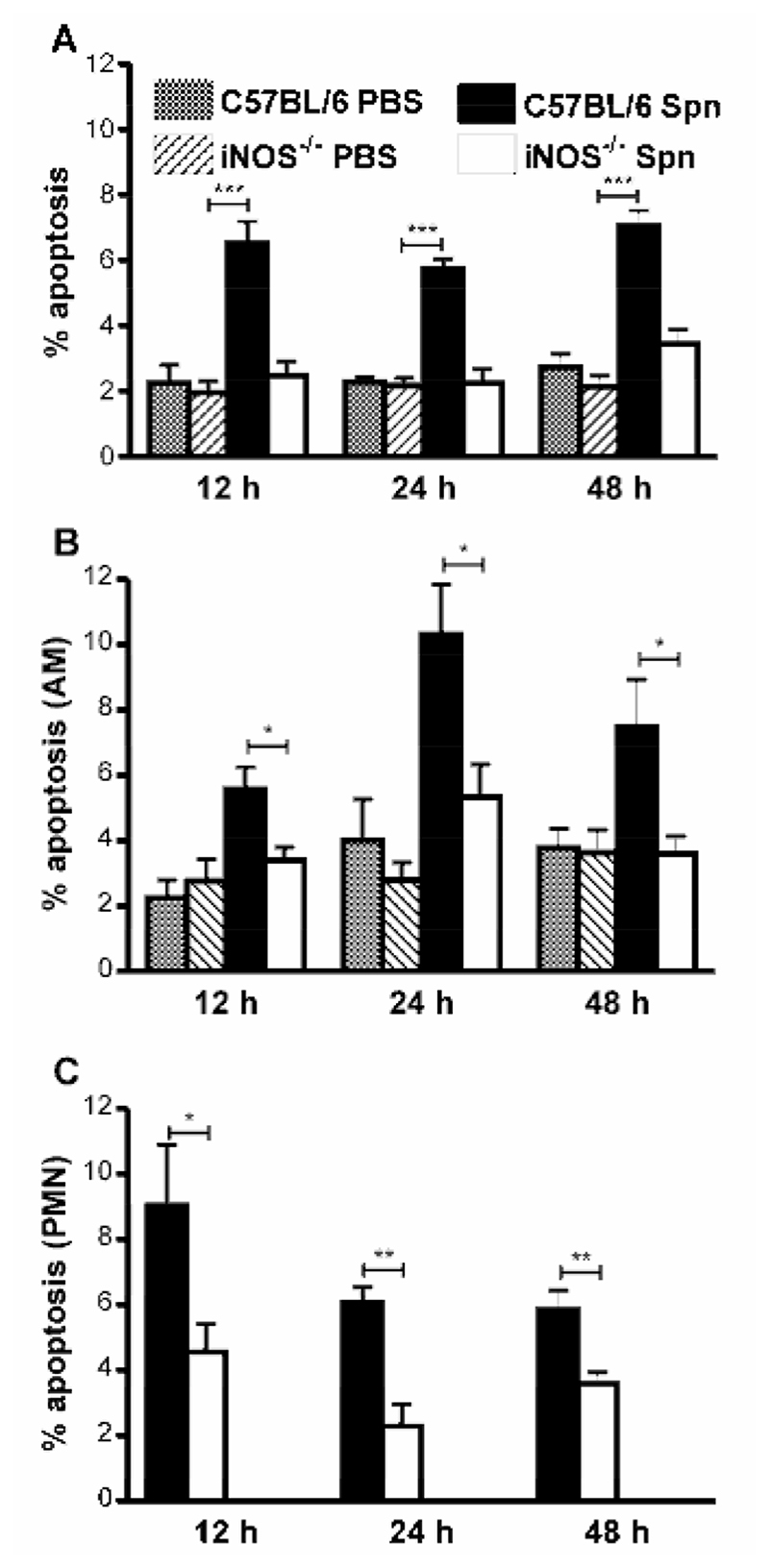
Since NO contributes to apoptosis of human macrophages during pneumococcal infection in vitro (11), but important differences exist between rodent and human macrophages with regard to the level of NO produced (23), we first confirmed that NO also played a role in apoptosis in murine AM when cultured ex vivo and challenged with pneumococci. As shown murine AM from iNOS-/- mice demonstrated lower levels of apoptosis (Figure 1A) and decreased killing of bacteria (Figure 1B) as compared to wild-type cells, thus confirming a similar phenotype to that previously found in human macrophages (11). We next examined levels of macrophage apoptosis in vivo in iNOS-/- mice (15). Pneumococcal-infected C57BL/6 mice had significantly greater percentages of apoptotic cells in BAL in comparison to mock-infected mice (Figure 2A). Pneumococcal-infected iNOS-/- mice, however, had significantly fewer apoptotic cells in BAL than did pneumococcal-infected C57BL/6 mice (Figure 2A). Since the BAL fluid from the pneumococcal infected mice contained significant numbers of neutrophils at each time-point we determined the level of apoptosis in the macrophage population by flow cytometry (9). There were greater percentages of apoptotic macrophages in pneumococcal-infected C57BL/6 mice than in mock-infected mice, but the levels of apoptotic macrophages were significantly decreased in iNOS-/- mice as opposed to C57BL/6 mice (Figure 2B). When apoptosis was measured in neutrophils we also found evidence of decreased neutrophil apoptosis in iNOS-/- mice as opposed to C57BL/6 mice after pneumococcal infection (Figure 2C).
Figure 1. iNOS deficiency is associated with a reduction in AM apoptosis and bacterial killing cells after pneumococcal challenge ex vivo.
A) Percentage apoptotic cells in cultures of alveolar macrophages (AM) isolated from wild type (C57BL/6) and iNOS deficient mice (iNOS-/-) 20 h after in vitro challenge with opsonized type 2 pneumococci (Spn), n=6. B) Concentration of bacteria in culture supernatants of C57BL/6 and iNOS-/- mice 4 and 20 h after in vitro challenge with opsonized type 2 pneumococci (Spn), n=3. Data is derived from triplicate points and is representative of two independent experiments. Mean+SEM, *p<0.05, *** p<0.001, t-test,  C57BL/6,
C57BL/6,  iNOS-/-.
iNOS-/-.
Figure 2. iNOS deficiency is associated with reduction in apoptotic cells in bronchial lavage fluid after pneumococcal infection.
A) The percentage of apoptotic events (apoptotic cells and bodies) in cytospins of bronchial alveolar lavage (BAL) from wild type controls (C57BL/6) and iNOS deficient mice (iNOS-/-) at the indicated time points after instillation of 107 CFU type 2 pneumococci (Spn) or PBS Percentage B) macrophage (AM) or C) neutrophil (PMN) apoptosis (Annexin V-PE+/ToPro 3-, flow cytometry) in BAL from the same experiments as A). 12h; n=6-8, 24 h; n=3-9, 48 h; n=5-16. Mean+SEM, * p<0.05, ** p<0.01, *** p<0.001, t- test,  C57BL/6. PBS,
C57BL/6. PBS,  iNOS-/- PBS,
iNOS-/- PBS,  C57BL/6 Spn,
C57BL/6 Spn,  iNOS-/- Spn.
iNOS-/- Spn.
Effect of iNOS deficiency on neutrophil numbers in BAL
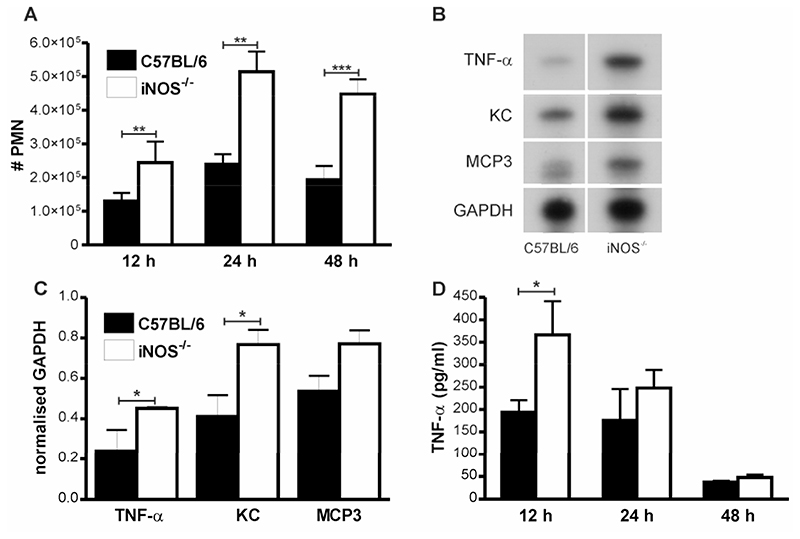
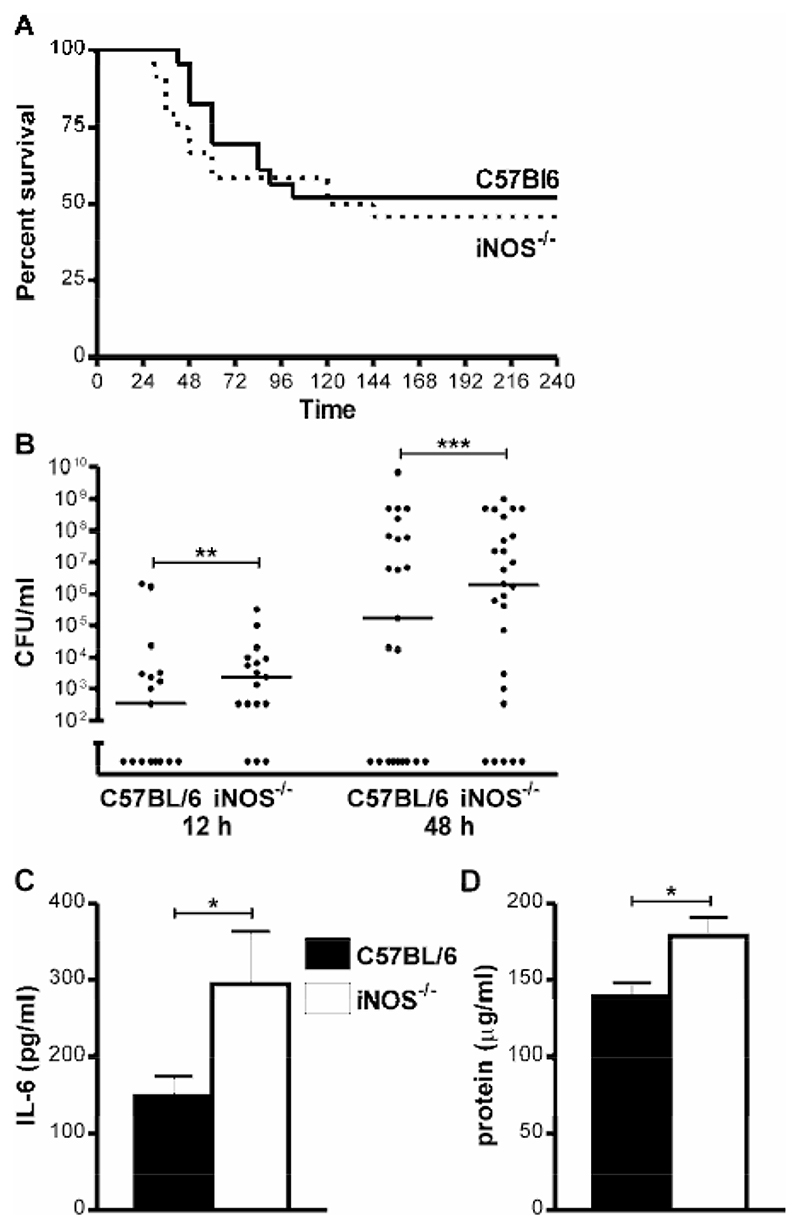
There was a significant increase in the number of neutrophils in BAL from iNOS-/- mice as compared to C57BL/6 mice 24-48 h post-infection (Figure 3A). Although there was evidence of increased neutrophil viability in iNOS-/- mice (reduced PMN apoptosis (Figure 2C) and no increase in necrosis, data not shown), increased recruitment was also likely to contribute to increased neutrophil numbers. The iNOS-/- mice had no significant increase in bacteria in the lung at 12-48 h (Table I), excluding the possibility that a significantly greater bacterial load was the stimulus for neutrophil recruitment. Similarly peripheral blood neutrophil counts were similar between C57BL/6 mice and iNOS-/- mice suggesting an intrinsic difference in neutrophil numbers did not explain the differences in numbers of BAL neutrophils after infection, data not shown.
Figure 3. Increased numbers of neutrophils in the bronchial alveolar lavage from lungs of iNOS-/- mice after pneumococcal infection.
A) Numbers of neutrophils (#PMN) in bronchial alveolar lavage (BAL) from wild type controls (C57BL/6) and iNOS deficient mice (iNOS-/-) at the indicated time points after intratracheal instillation of 107 CFU type 2 pneumococci. B) Representative RNase protection assay of RNA from whole lung from wild type controls (C57BL/6) and iNOS deficient mice (iNOS-/-) 12 h post instillation of 107 CFU type 2 pneumococci. C) Levels of TNF-α, KC and monocyte chemotactic protein-3 (MCP3) mRNA as quantified by densitometry and normalised to GAPDH, n=4. D) TNF-α concentration in BAL in the same experiments as A) 12h; n=11-13, 24 h; n= 8-14, 48 h; n=13-16. Mean+SEM, * p<0.05, **p<0.01, ***p<0.001, t-test,  C57BL/6,
C57BL/6,  iNOS-/-.
iNOS-/-.
Table I. Microbiological outcomes.
| 12 h | 48 h | |
|---|---|---|
| C57BL/6 | 1.8x105(1.5x105-3.1x105), 100%, n=17 | 2.0x104(5.0x102-3.0x107), 81%, n=21 |
| iNOS-/- | 2 0x105(1.2x105-2.8x105)a 100%b n=17 | 1.7x106(8.3x103-2.5x107), 87%, n=23 |
Median (interquartile range)
% with detectible bacteria.
Neutrophil recruitment in pneumococcal infection demonstrates important differences as compared to other stimuli such as LPS with a prominent upstream role for TNF-α (24). We performed RNAse Protection assays on lungs 12h after infection to identify cytokines and chemokines that were upregulated in iNOS-/- mice and were able to demonstrate significant upregulation of TNF-α message (Figure 3B-C). We also observed upregulation of mRNA for the chemokines KC and MCP3, (Figure 3B-C), chemokines known to be associated with neutrophil recruitment in pneumococcal infection (24) and in iNOS deficiency (25). Measurement of cytokines in BAL revealed iNOS-/- mice had a significant increase in TNF-α production relative to C57BL/6 mice 12 h post infection and a non-significant trend towards greater production at 24 h following pneumococcal infection (Figure 3D). Levels of TNF-α in PBS treated mice of both strains were below the level of detection. KC values in BAL fluid 12 h after infection from iNOS-/- vs. C57BL/6 mice were 803±28.6 vs. 578.8±96.58, pg/ml, p<0.05, n=4.
Pharmacologic inhibition of iNOS modulates macrophage apoptosis and neutrophil recruitment
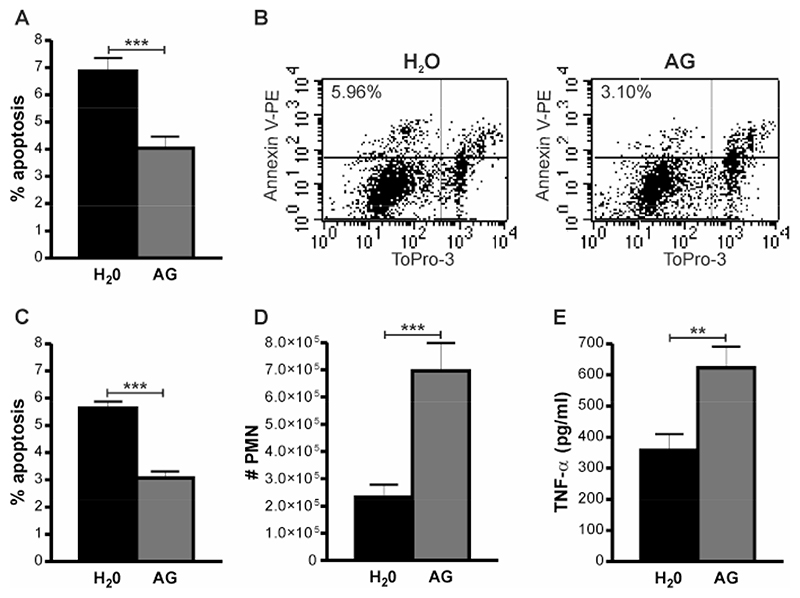
To investigate whether these findings were related to decreased production of NO and/or one of its reaction products or whether they resulted from some non-specific effect associated with iNOS deficiency, for example increased levels of a co-factor or metabolite required for the functional activity of iNOS, we inhibited iNOS activity pharamacologically. Inhibition with aminoguanidine, a specific inhibitor of iNOS (26), replicated the findings in iNOS-/- mice with decreased total or macrophage apoptosis and increased neutrophil numbers or TNF-α levels in BAL all observed (Figure 4 A-E).
Figure 4. iNOS inhibition reduces apoptosis and increases pulmonary inflammation after pneumococcal infection.
A) The percentage of apoptotic events (apoptotic cells and bodies) in cytospins of bronchial alveolar lavage (BAL) from C57BL/6 mice 24 h after instillation of 107 CFU type 2 pneumococci (Spn), in the absence (H2O) or presence of aminoguanidine (AG) treatment. B) Representative dot plots to show the percentage macrophage apoptosis (Annexin V-PE+/ToPro 3-cells as determined by flow cytometry), C) Percentage apoptotic macrophages, D) number of recruited neutrophils (#PMN) and E) TNF-α concentration in BAL in the same experiments as A), n=8. Mean+SEM, * p<0.05, ***p<0.001, t-test,  H2O,
H2O,  AG.
AG.
iNOS-/- mice demonstrate enhanced levels of lung inflammation and bacteremia
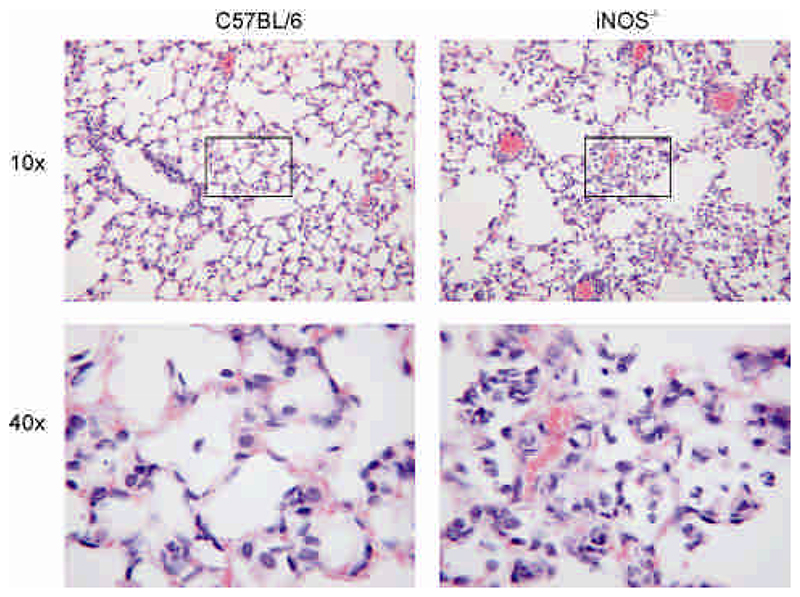
The increased numbers of neutrophils and pro-inflammatory cytokines in the lung in iNOS-/- mice were associated with enhanced early mortality in the first 42h after infection, 22.0% iNOS-/- vs. 4.9% C57BL/6 mice, p<0.05, Fisher’s Exact Test, but this was not associated with a significant decrease in clearance of bacteria from the lung, Table I. However, by 10 days there was no difference in overall mortality in the two groups of mice 54% iNOS-/- vs. 48% C57BL/6 mice, Figure 5A. We have previously shown that decreased levels of macrophage apoptosis are associated with invasive pneumococcal disease (5, 9) and in keeping with this finding iNOS-/- mice had >1 log higher bacterial colony counts in the blood at 12-48 h after infection, Figure 5B. IL-6 production is a marker of sepsis-related mortality and poor outcomes in models of pneumococcal disease (27) and increased IL-6 production in the lungs of pneumococcal-infected mice was apparent 48 h after infection (Figure 5C). In both strains the levels of IL-6 were below the limit of detection after PBS treatment. iNOS-/- mice also demonstrated increased levels of protein leak into the BAL, in keeping with greater lung inflammation (Figure 5D). In keeping with these findings lung histology performed on mouse lungs also illustrated a greater degree of neutrophilic lung inflammation in the iNOS-/- mice in association with a greater degree of disruption to the alveolar units, suggesting greater epithelial cell injury (Figure 6).
Figure 5. Increased lung inflammation in iNOS-/- mice after pneumococcal infection.
A) Survival of wild type iNOS sufficient mice (C57BL/6) and iNOS deficient (iNOS-/-) mice after intratracheal instillation of 107 CFU type 2 pneumococci, n=23-24 per group. B) Concentration of bacteria in blood of C57BL/6 and iNOS-/- mice 12 and 48 h after intratracheal instillation of 107 CFU type 2 pneumococci. The percentage of C57BL/6 vs. iNOS-/- mice with bacteremia at 12 h was 53 vs. 82% and at 48 h was 61 vs. 81%. C-D) Concentration of C) IL-6 and D) protein in bronchial alveolar lavage (BAL) 48 h after intratracheal instillation of 107 CFU type 2 pneumococci n=8-16. Mean+SEM, * p<0.05, **p<0.01, ***p<0.001, t-test,  C57BL/6,
C57BL/6,  iNOS-/-.
iNOS-/-.
Figure 6. Histologic appearance of increased lung inflammation in iNOS-/- mice after pneumococcal infection.
Representative appearances of lung sections stained with haematoxylin and eosin from C57BL/6 and iNOS deficient mice (iNOS-/-) 48 h after intratracheal instillation of 107 CFU type 2 pneumococci as viewed by 10x and 40x objective. Images were obtained from one of four mice in each group reviewed by two independent pathologists.
Macrophages in iNOS-/- mice contain fewer apoptotic bodies
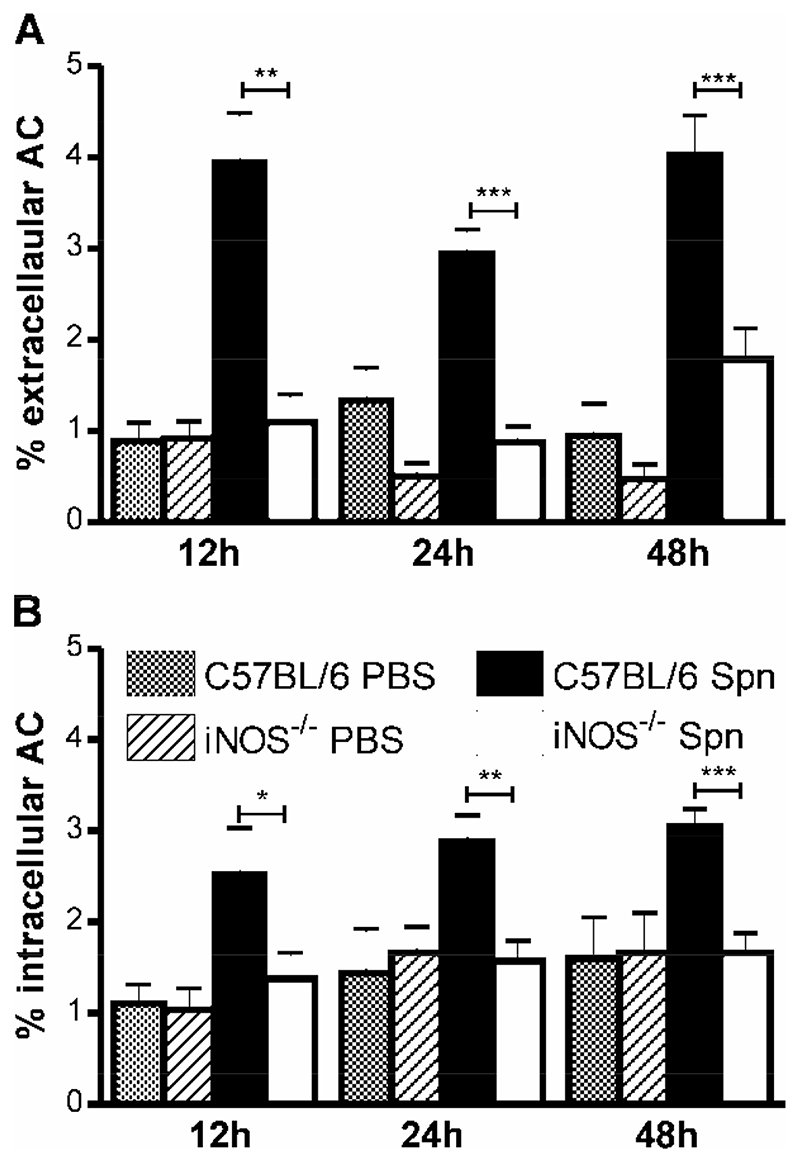
Since there are fewer apoptotic cells in the lungs of iNOS-/- mice we addressed whether this translated into a difference in the number of apoptotic bodies being phagocytosed in vivo. The phagocytic capacity of AM for apoptotic cells in vitro is low in comparison to the capacity to ingest opsonized particles (18) or the capacity of peritoneal macrophages to phagocytose apoptotic cells (28). Nevertheless macrophages with internalized apoptotic cells are observed in BAL from mice with pneumococcal infection (9) We estimated the percentage of extracellular apoptotic cells per cytospin, i.e. apoptotic cells that had not been phagocytosed by macropahges (Figure 7A), and the percentage of intracellular apoptotic cells (Figure 7B). Although, over all the time points studied, only a relatively low percentage of macrophages (C57BL/6 4.4±0.3%; iNOS-/- 5.7±0.5%), contained apoptotic cells these apoptotic cells accounted for a significant percentage of the total apoptotic events (C57BL/6 44±2.4%; iNOS-/- 55.7±3.5%), arguing in favour of a relatively efficient clearance mechanism for apoptotic cells in vivo during pneumococcal infection. As illustrated there were a lower percentage of intracellular apoptotic bodies in iNOS-/- mice at all time points (Figure 7B).
Figure 7. Macrophages phagocytose apoptotic cells during pneumococcal infection.
A) The percentage of extracellular apoptotic cells (AC) and B) intracellular AC in cytospins of bronchial alveolar lavage (BAL) from wild type controls (C57BL/6) and iNOS deficient mice (iNOS-/-) at the indicated time points after instillation of 107 CFU type 2 pneumococci (Spn) or PBS, n=4-16. Mean+SEM, **p<0.01, ***p<0.001, t-test,  C57BL/6 PBS,
C57BL/6 PBS,  iNOS-/- PBS,
iNOS-/- PBS,  C57BL/6 Spn,
C57BL/6 Spn,  iNOS-/- Spn.
iNOS-/- Spn.
Instillation of apoptotic macrophages into the lung corrects markers of greater lung inflammation in iNOS-/- mice
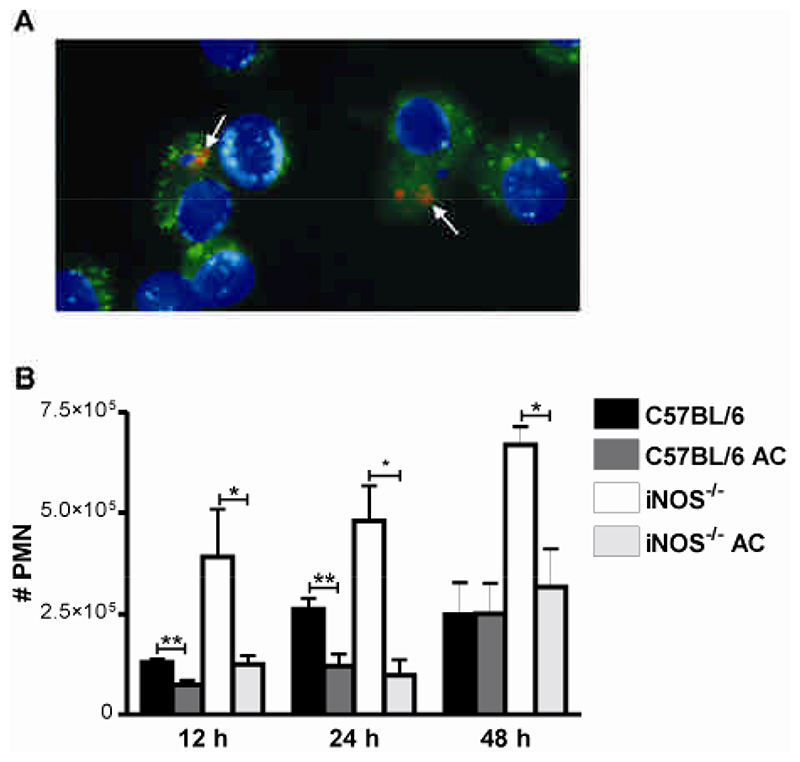
Since clearance of apoptotic cells can downregulate pro-inflammatory cytokines (13) we tested whether exogenous apoptotic macrophages could decrease the pulmonary inflammation observed during pneumococcal infection. UV treatment induces high levels of macrophage apoptosis (5) and efficiently induced AM apoptosis. A suspension of apoptotic AM had no residual antimicrobial effect as there was no difference between bacterial colony counts in the lung 24 h after infection in the presence or absence of these cells: median and interquartile range for C57BL/6 mice in the presence of apoptotic cells 1.6x106 CFU (0.5 103-1.3 x108) vs 1 x105 CFU (8.3 x 103-8.3x107) in the absence of apoptotic cells, n=11, p=0.81, Mann Whitney. Instilled Cell Tracker Red labelled apoptotic AM were phagocytosed by resident AM labelled with the green fluorescent dye, PKH2 (Figure 8A). The greater numbers of recruited neutrophils in the lungs of iNOS-/- mice could be the direct result of NO deficiency increasing production of pro-inflammatory cytokines (29, 30). However, instillation of apoptotic AM decreased inflammation in the lung (Figure 8B). There were decreased numbers of recruited neutrophils in the lung 12-48 h after instillation of apoptotic macrophages in iNOS-/- mice and to a lesser extent in C57BL/6 mice (Figure 8B). In the iNOS-/-mice instillation of apoptotic AM reduced the numbers of neutrophils in the lung to a level comparable to the number that were recruited into C57BL/6 mice not instilled with apoptotic AM. Results were identical regardless of whether AM were derived from iNOS-/- or C57BL/6 mice and in preliminary data if AM were made apoptotic by staurosporine treatment as opposed to UV exposure (data not shown). Therefore decreased numbers of apoptotic macrophages contribute to the increased lung inflammation during pneumococcal infection of iNOS-/- mice and instillation of additional apoptotic macrophages reverses the pro-inflammatory phenotype associated with NO deficiency following pulmonary challenge with pneumococci.
Figure 8. Instillation of apoptotic macrophages into lungs reduces neutrophil numbers in bronchial alveolar lavage after pneumococcal infection.
A) Resident alveolar macrophages (AM) of C57BL/6 mice were labelled in vivo with PKH2 green fluorescent phagocytic cell linker compound. AM from donor mice were labelled in vitro with CellTracker Red and apoptosis induced by UV irradiation. Apoptotic AM were delivered by intra-tracheal instillation. Lungs were lavaged 30 min post instillation and cytospin preparations made from lung cells. AM nuclei were counterstained with DAPI. Arrows indicate red apoptotic donor cells located in green resident AM. B) Numbers of neutrophils (#PMN) in BAL from wild type controls (C57BL/6) iNOS deficient mice (iNOS-/-) at the indicated time points after instillation of 107 CFU type 2 pneumococci in the absence or presence of apoptotic AM (AC), n=3-7. Mean+SEM, * p<0.05, ** p<0.01, t-test,  C57BL/6,
C57BL/6,  C57BL/6 AC,
C57BL/6 AC,  iNOS-/-,
iNOS-/-,  iNOS-/- AC.
iNOS-/- AC.
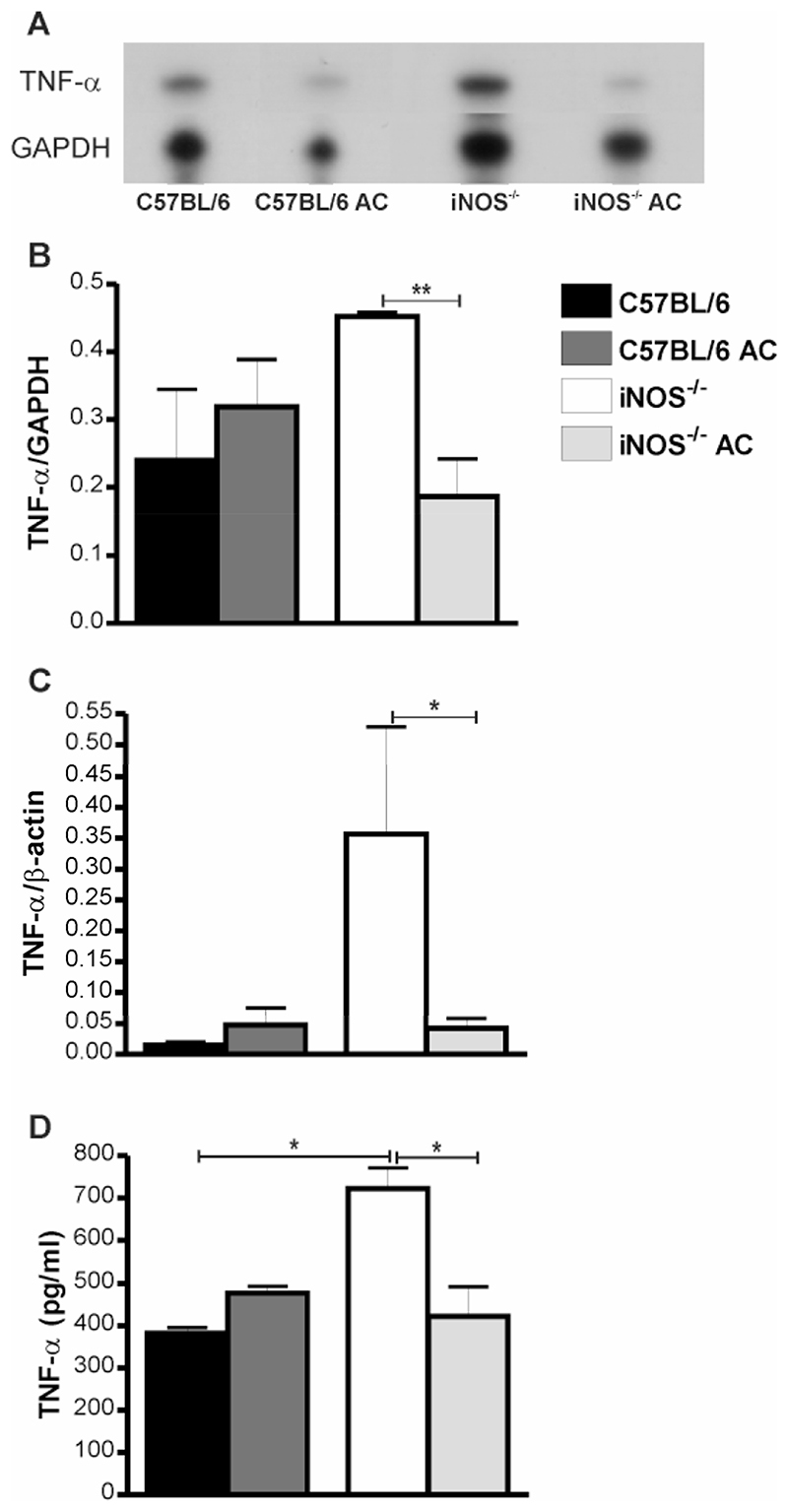
Effect of apoptotic cells on TNF-α expression in iNOS-/- mice
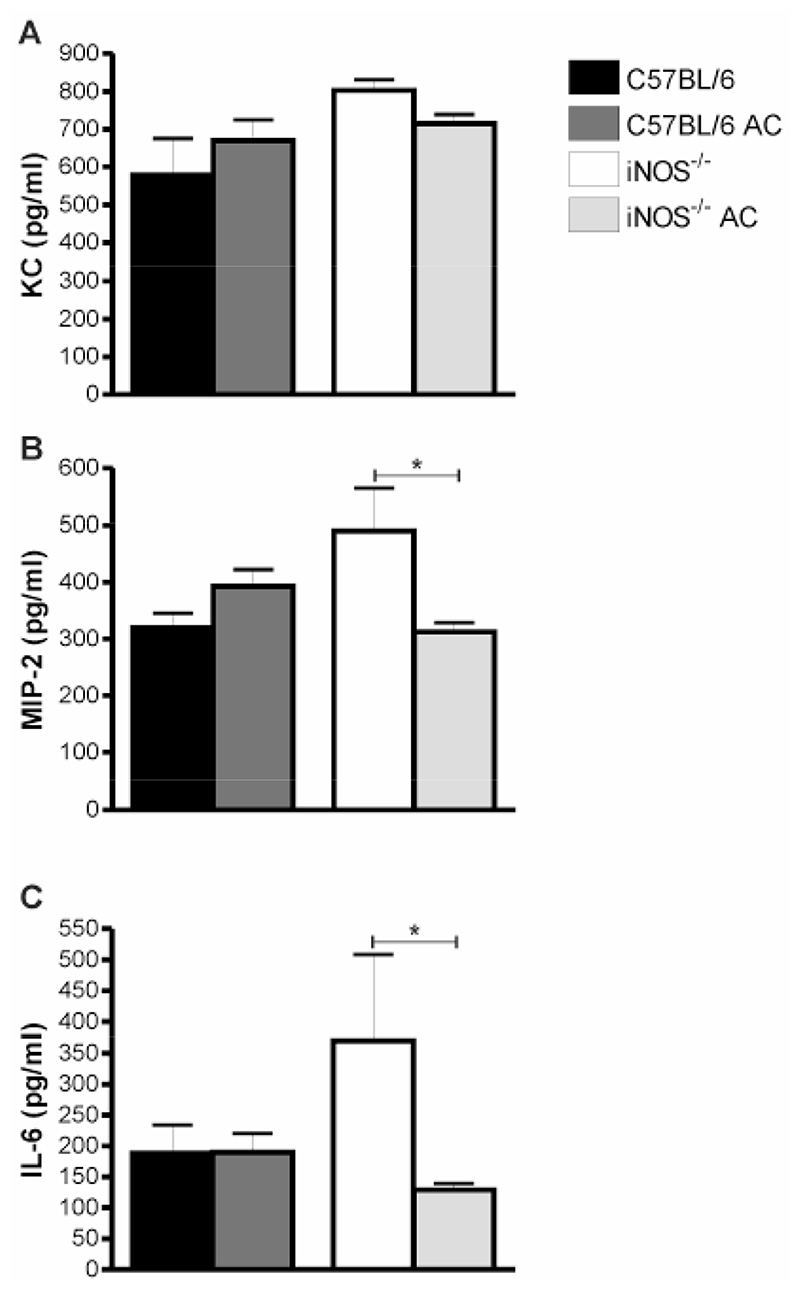
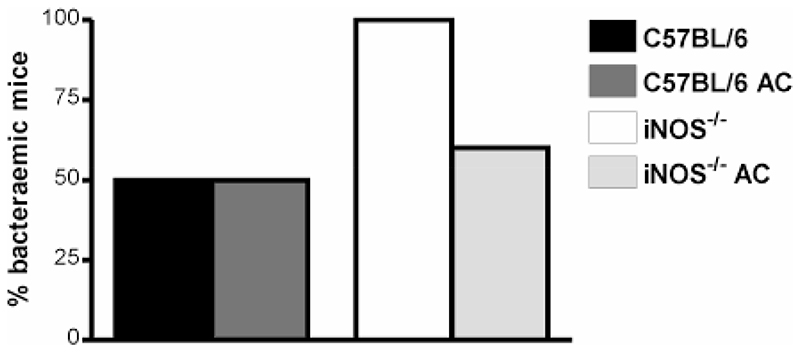
In keeping with the pivotal role of TNF-α expression in recruitment and activation of neutrophils during pneumococcal pneumonia (24) we found that TNF-α mRNA expression in the lung was reduced by instillation of apoptotic cells in iNOS-/- mice, Figure 9A-B. Similar findings were observed by qRT-PCR, Figure 9C. This was confirmed by significant reduction in TNF-α expression by ELISA, following instillation of apoptotic cells to iNOS-/- mice, Figure 9D. These alterations were persistent for up to 48 h after infection (data not shown). Further screen of lung RNAse protection assays and BAL ELISAs showed other changes in early cytokine/chemokine expression such as changes in IL-1 isoforms, MCP3 (data not shown), KC (Figure 10A) and MIP-2 (Figure 10B), but these differences were not as marked as for TNF-α expression. IL-6, however showed a marked reduction in expression after instillation of apoptotic cells (Figure 10C). Further support for the beneficial effect of phagocytosis of apoptotic macrophages in iNOS-/- mice infected with pneumococci comes from the observation that reduction in the number of neutrophils and cytokines such as TNF-α and IL-6 expression was associated with a decreased likelihood of mice developing invasive pneumococcal disease after instillation of apoptotic cells, Figure 11.
Figure 9. Instillation of apoptotic macrophages into lungs of iNOS-/- mice reduces TNF-α levels in lung and bronchial alveolar lavage after pneumococcal infection.
A) Representative RNase protection assay of RNA from whole lung from wild type controls (C57BL/6) and iNOS deficient mice (iNOS-/-) 12 h post instillation of 107 CFU type 2 pneumococci in the absence or presence of apoptotic alveolar macrophages (AC). B) Levels of TNF-α mRNA as quantified by densitometry and normalised to GAPDH, n=3-4. C) Levels of TNF-α mRNA as quantified by RT-PCR and normalised to β-actin, in the same mice as B). D) Concentration of TNF-α in bronchial alveolar lavage (BAL) 12 h after instillation of 107 CFU type 2 pneumococci with and without AC, n=3-4. Mean+SEM, * p<0.05, t-test,  C57BL/6,
C57BL/6,  C57BL/6 AC,
C57BL/6 AC,  iNOS-/-,
iNOS-/-,  iNOS-/- AC.
iNOS-/- AC.
Figure 10. Instillation of apoptotic macrophages into lungs of iNOS-/- mice reduces proinflammatory cytokine levels in bronchial alveolar lavage after pneumococcal infection.
Concentration of A) MIP-2, B) KC in bronchial alveolar lavage (BAL) 12 h after or C) IL-6 24 h after instillation of 107 CFU type 2 pneumococci with and without AC. n=3-4. Mean+SEM, * p<0.05, t-test,  C57BL/6,
C57BL/6,  C57BL/6 AC,
C57BL/6 AC,  iNOS-/-,
iNOS-/-,  iNOS-/- AC.
iNOS-/- AC.
Figure 11. Instillation of apoptotic macrophages into lungs of iNOS-/- mice reduces bacteremia after pneumococcal infection.
Percentage of wild type controls (C57BL/6) and iNOS deficient mice (iNOS-/-) with detectable bacteremia 12 h post instillation of 107 CFU type 2 pneumococci in the absence or presence of apoptotic alveolar macrophages (AC), n=4-5.  C57BL/6,
C57BL/6,  C57BL/6 AC,
C57BL/6 AC,  iNOS-/-,
iNOS-/-,  iNOS-/- AC.
iNOS-/- AC.
Discussion
We demonstrate decreased rates of macrophage apoptosis in the lungs of iNOS-/- and aminoguanidine treated mice during pneumococcal infection. The decrease in macrophage apoptosis in iNOS-/- mice is associated with development of earlier bacteraemia and death, but also with increased markers of inflammation in the lung. Instillation of apoptotic macrophages into the lungs of iNOS-/- mice reversed the features of increased inflammation and reduced the development of invasive bacterial disease.
The potential benefits of host-mediated macrophage apoptosis in the innate response to infection are not fully understood. Apoptosis is a mechanism which removes unwanted cells, thus limiting inflammation and tissue injury (31). It is plausible that macrophage apoptosis during bacterial infection plays a role in downregulating the inflammatory response in addition to its role in enhancing microbial killing (5). The innate response to pneumococci involves pulmonary inflammation (32), induction of host-mediated macrophage apoptosis (9) and a requirement for resolution of the inflammatory response for a successful outcome (12). Since iNOS-/- mice have decreased levels of macrophage apoptosis during pneumococcal infection, we anticipated this model would provide insights into the relationship between regulation of inflammation and induction of macrophage apoptosis.
The roles of NO in murine models of pneumococcal pneumonia have been conflicting reflecting the variety of strains of mice studied, varying doses and strains of bacteria and the use of pharmacologic agents as opposed to genetically modified mice (33–35). Our findings with type 2 pneumococci have been reproduced with type 1 pneumococci, demonstrating that the findings of less macrophage apoptosis and greater neutrophil infiltration and TNF-α expression in the lung were not specific to the strain we used (data not shown). Although we have previously found that NO contributes to macrophage killing of pneumococci (11), we have also demonstrated that during low-dose infection other elements of the host response, including recruited neutrophils can compensate for decreased bacterial killing by AM (9). Clearly NO is only one factor involved in both microbicidal killing and AM apoptosis induction and clearly other factors also contribute. Furthermore NO also contributes to host defense by mechanisms independent of its effects on macrophage function (29, 36). On the basis of our findings of increased inflammation in iNOS-/- mice we cannot exclude a role for NO produced in other cells for the phenotype we observed. However, the removal of one factor involved in host defense resets the AM response, and indeed the total innate host response in the lung, leading to an altered threshold at which the next element in the host response becomes critical to controlling infection. As Kerr and colleagues have clearly illustrated at intermediate doses of pneumococci (albeit in mice of a different genetic background to those we studied) host defense is unable to compensate for NO-deficiency and larger numbers of bacteria are recovered from the lung of iNOS-/- mice (35). In the current study, we have used a high dose of pneumococci and have been unable to document any overall defect in bacterial killing in the lungs but have demonstrated greater lung inflammation, consistent with prior fulminant infection models using NO inhibitors (34). Our inability to demonstrate a clear microbiologic or survival difference in iNOS-/- mice suggests significant redundancy and compensation exists in this aspect of innate immunity in the lung and also that at the high doses we used the compensatory measures are overwhelmed so they are no longer the critical determinant of outcome in the setting of iNOS deficiency.
Nevertheless, decreased macrophage apoptosis, in the setting of decreased NO production, was associated with increased levels of bacteremia, in keeping with our previous observation that inhibition of macrophage apoptosis is associated in particular with increased levels of invasive pneumococcal disease (5, 11). Importantly, instillation of apoptotic AM in iNOS-/- mice reversed many of the features of increased inflammation, even though these mice had the same defects in NO production in macrophages and other cells as the iNOS-/- mice that did not receive apoptotic cells. This suggests that a relative deficiency in numbers of apoptotic cells and their clearance is at least a contributory factor to increased lung inflammation and bacterial tissue invasion. Nevertheless, the pathogenesis of bacteremia is multifactorial and many other factors play a role.
NO has a wide range of actions including cell signalling function (37). The pro-inflammatory effects of NO deficiency in the lung have been demonstrated both by pharmacologic inhibition of iNOS, and also by study of iNOS-/- mice (38). NO modifies levels of the transcription factor NFκB (39) and decreases NFκB binding to the regulatory region of pro-inflammatory cytokine genes such as TNF-α and IL-6 (30). Nevertheless, it remains possible that the effects we observed are mediated by a reaction product of NO not by NO itself but the fact that pharmacologic inhibition of iNOS replicated the phenotype of iNOS-/- mice argued in favour of mediation by NO or a reactive nitrogen species rather than a non-specific result of deletion of the iNOS gene (36).
In addition to its signalling role, NO also plays a role in the regulation of apoptosis (40). Ingestion of apoptotic bodies by macrophages modifies cytokine production following stimulation with microbial products (14). Although phagocytosis of apoptotic bodies in the unstimulated macrophage results in production of anti-inflammatory cytokines such as TGF-β (13), the effect of their ingestion in the context of stimulation of Toll-like receptors in vitro is to shorten the duration of pro-inflammatory cytokine production (14). We instilled apoptotic AM to address whether decreased levels of phagocytosis of apoptotic macrophages, contributes to decreased pulmonary inflammation during pneumococcal infection. Having demonstrated this we conclude that one mechanism by which NO deficiency contributes to lung inflammation is by decreasing macrophage apoptosis and one consequence of this is that macrophages are less likely to phagocytose apoptotic cells and therefore reset their cytokine expression profile. Interestingly, we previously demonstrated that AM depletion also increased neutrophil recruitment during pneumococcal infection in vivo (9). Whilst AM depletion would be anticipated to decrease total NO production it would also decrease the number of AM undergoing apoptosis during infection and the number of AM phagocytosing apoptotic cells. Although absolute NO deficiency or high level AM deficiency is unlikely during bacterial pneumonia our findings raise the possibility that subtle manipulation of NO generation or levels of macrophage apoptosis could exert beneficial effects on the degree of pulmonary inflammation during bacterial pneumonia.
Although the clearance of apoptotic cells alters cytokine expression profiles, these data have been generated in vitro using apoptotic neutrophils or lymphocytes (41, 42). This study provides, to our knowledge, the first data on the role of apoptotic macrophages in the regulation of the inflammatory phenotype during a model of pneumonia. Importantly, we have replaced the same cell type as that which is undergoing apoptosis in the pathologic condition we are studying rather than using transformed cell lines. Although our study used UV-treated AM rather than pneumococcal-exposed AM we believe this was reasonable as it increased the yield of apoptotic cells and it excluded the possibility of instilling additional bacteria with the apoptotic cells, a potential confounding effect in prior studies (42). We show that these exogenous apoptotic cells, downregulate pulmonary inflammation during pneumonia. This provides clear evidence that, in the context of pneumonia, macrophage apoptosis helps decrease the production of inflammation which is of potential benefit to the host and raises the possibility that enhancing levels of macrophage apoptosis or the clearance of apoptotic cells could modulate levels of lung inflammation in other pulmonary diseases.
The mechanism by which apoptotic macrophages exert this effect during pneumococcal pneumonia involves decreased TNF-α production. TNF-α production is a critical upstream cytokine in pneumococcal pneumonia which in combination with IL-1 isoforms mediates neutrophil recruitment in murine models of pneumococcal pneumonia via the CXC chemokines
KC and MIP-2 (24). TNF-α production peaks at 12h after infection and contributes to microbiologic outcome and survival (33, 43), while enhanced generation of TNF-α compensates for absence of IL-1 signalling during pneumococcal pneumonia (44). Nevertheless, excessive TNF-α production, via its effects on neutrophil activation, contributes to lung injury in a variety of diseases (45–47) and may worsen disease outcome during septic shock (48, 49). AM are the major source of TNF-α during pneumococcal pneumonia (50), thus it is not surprising that phagocytosis of apoptotic macrophages might alter the expression of this cytokine during pneumococcal pneumonia. Prior studies have shown that macrophage TNF-α expression is altered by phagocytosis of apoptotic cells (13, 51). Downstream consequences of phagocytosis of apoptotic macrophages, such as chemokine expression profiles, might be less obvious, even though there was clearly a sustained effect on neutrophil numbers to 48h, since the experimental design involved the instillation of apoptotic cells at the same time as bacteria. Instillation of iNOS-/- mice with apoptotic AM reduced TNF-α (and other cytokines/chemokines) expression. In addition to its potential to contribute to cytotoxicity, TNF-α expression can cause upregulation of receptors implicated in tissue invasion of pneumococci such as the platelet activating factor (PAF) receptor (52). Macropahge apoptosis can therefore exert two important effects on microbiologic outcome. The direct effect is to enhance macrophage killing of phagocytosed bacteria, as we have observed in vitro (11, 53) and confirmed in low-dose infection models in which AM killing of bacteria is critical to preventing clinical disease (5, 11). A second indirect effect is mediated by the phagocytosis of apoptotic macrophages, and is associated with downregulation of TNF-α. This decreases the development of bacteremia by decreasing inflammation and /or the cytokine mediated expression of receptors required for tissue invasion.
The clinical picture is likely to be complex with an optimal number of apoptotic cells and cytokine profile required. This is illustrated by the observation that restoration of apoptotic numbers in iNOS-/- mice reduced inflammation and invasive disease, but when numbers of apoptotic macrophages were optimal, as seen in the C57BL/6 mice, instillation of additional apoptotic cells had less impact on microbiologic and inflammatory outcomes. While our data supports a beneficial role for AM apoptosis in the lung, apoptosis in other settings may be harmful. Inhibition of lymphocyte apoptosis in sepsis improves disease outcome (54, 55). In the current study the increased levels of bacteremia in iNOS-/- mice did not worsen outcome, in keeping with the observation that iNOS-/- mice are better able to tolerate bacteremia (35). Since NO contributes to lymphocyte apoptosis in a variety of settings (56, 57), these findings may illustrate how modulation of apoptosis in one anatomic location can be harmful and yet in another distinct location can benefit host defence.
In conclusion, we provide evidence, in a model of pneumonia, that phagocytosis of apoptotic AM is associated with reduced TNF-α expression, neutrophil recruitment and invasive pneumococcal disease. These studies further define the role of host-mediated macrophage apoptosis during bacterial infection and highlight its impact on microbiologic and inflammatory outcomes.
Acknowledgements
The authors are grateful to Vanessa Singleton for help with the RNase Protection Assay and Drs Sarah Walmsley and Isobel Gowers for help with the qRT-PCR.
Footnotes
Abbreviations used in this paper: AM – alveolar macrophage, iNOS - inducible nitric oxide synthase, BAL - bronchial alveolar lavage
References
- 1.Underhill DM, Ozinsky A. Phagocytosis of microbes: complexity in action. Annu Rev Immunol. 2002;20:825–852. doi: 10.1146/annurev.immunol.20.103001.114744. [DOI] [PubMed] [Google Scholar]
- 2.Liu H, Perlman H, Pagliari LJ, Pope RM. Constitutively activated Akt-1 is vital for the survival of human monocyte-differentiated macrophages. Role of Mcl-1, independent of nuclear factor (NF)-kappaB, Bad, or caspase activation. J Exp Med. 2001;194:113–126. doi: 10.1084/jem.194.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zychlinsky A, Sansonetti P. Perspectives series: host/pathogen interactions. Apoptosis in bacterial pathogenesis. J Clin Invest. 1997;100:493–495. doi: 10.1172/JCI119557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lammas DA, Stober C, Harvey CJ, Kendrick N, Panchalingam S, Kumararatne DS. ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z(P2X7) receptors. Immunity. 1997;7:433–444. doi: 10.1016/s1074-7613(00)80364-7. [DOI] [PubMed] [Google Scholar]
- 5.Marriott HM, Bingle CD, Read RC, Braley KE, Kroemer G, Hellewell PG, Craig RW, Whyte MK, Dockrell DH. Dynamic changes in Mcl-1 expression regulate macrophage viability or commitment to apoptosis during bacterial clearance. J Clin Invest. 2005;115:359–368. doi: 10.1172/JCI21766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown PD, Lerner SA. Community-acquired pneumonia. Lancet. 1998;352:1295–1302. doi: 10.1016/S0140-6736(98)02239-9. [DOI] [PubMed] [Google Scholar]
- 7.McCool TL, Cate TR, Moy G, Weiser JN. The immune response to pneumococcal proteins during experimental human carriage. J Exp Med. 2002;195:359–365. doi: 10.1084/jem.20011576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kadioglu A, Andrew PW. The innate immune response to pneumococcal lung infection: the untold story. Trends Immunol. 2004;25:143–149. doi: 10.1016/j.it.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Dockrell DH, Marriott HM, Prince LR, Ridger VC, Ince PG, Hellewell PG, Whyte MK. Alveolar macrophage apoptosis contributes to pneumococcal clearance in a resolving model of pulmonary infection. J Immunol. 2003;171:5380–5388. doi: 10.4049/jimmunol.171.10.5380. [DOI] [PubMed] [Google Scholar]
- 10.Knapp S, Leemans JC, Florquin S, Branger J, Maris NA, Pater J, van Rooijen N, van der Poll T. Alveolar macrophages have a protective antiinflammatory role during murine pneumococcal pneumonia. Am J Respir Crit Care Med. 2003;167:171–179. doi: 10.1164/rccm.200207-698OC. [DOI] [PubMed] [Google Scholar]
- 11.Marriott HM, Ali F, Read RC, Mitchell TJ, Whyte MK, Dockrell DH. Nitric oxide levels regulate macrophage commitment to apoptosis or necrosis during pneumococcal infection. Faseb J. 2004;18:1126–1128. doi: 10.1096/fj.03-1450fje. [DOI] [PubMed] [Google Scholar]
- 12.Haslett C. Granulocyte apoptosis and its role in the resolution and control of lung inflammation. Am JRespir Crit Care Med. 1999;160:S5–11. doi: 10.1164/ajrccm.160.supplement_1.4. [DOI] [PubMed] [Google Scholar]
- 13.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lucas M, Stuart LM, Savill J, Lacy-Hulbert A. Apoptotic cells and innate immune stimuli combine to regulate macrophage cytokine secretion. J Immunol. 2003;171:2610–2615. doi: 10.4049/jimmunol.171.5.2610. [DOI] [PubMed] [Google Scholar]
- 15.Wei XQ, Charles IG, Smith A, Ure J, Feng GJ, Huang FP, Xu D, Muller W, Moncada S, Liew FY. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- 16.Tufariello JM, Mi K, Xu J, Manabe YC, Kesavan AK, Drumm J, Tanaka K, Jacobs WR, Jr, Chan J. Deletion of the Mycobacterium tuberculosis Resuscitation-Promoting Factor Rv1009 Gene Results in Delayed Reactivation from Chronic Tuberculosis. Infect Immun. 2006;74:2985–2995. doi: 10.1128/IAI.74.5.2985-2995.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon SB, Irving GR, Lawson RA, Lee ME, Read RC. Intracellular trafficking and killing of Streptococcus pneumoniae by human alveolar macrophages are influenced by opsonins. Infect Immun. 2000;68:2286–2293. doi: 10.1128/iai.68.4.2286-2293.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jennings JH, Linderman DJ, Hu B, Sonstein J, Curtis JL. Monocytes recruited to the lungs of mice during immune inflammation ingest apoptotic cells poorly. Am J Respir Cell Mol Biol. 2005;32:108–117. doi: 10.1165/rcmb.2004-0108OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rowe SJ, Allen L, Ridger VC, Hellewell PG, Whyte MK. Caspase-1-deficient mice have delayed neutrophil apoptosis and a prolonged inflammatory response to lipopolysaccharide-induced acute lung injury. J Immunol. 2002;169:6401–6407. doi: 10.4049/jimmunol.169.11.6401. [DOI] [PubMed] [Google Scholar]
- 20.Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, Pearse D, Tuder RM, Garcia JG. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med. 2004;169:1245–1251. doi: 10.1164/rccm.200309-1258OC. [DOI] [PubMed] [Google Scholar]
- 21.Jennings JH, Linderman DJ, Hu B, Sonstein J, Curtis JL. Monocytes recruited to the lungs of mice during immune inflammation ingest apoptotic cells poorly. Am J Respir Cell Mol Biol. 2004 doi: 10.1165/rcmb.2004-0108OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dockrell DH, Lee M, Lynch DH, Read RC. Immune-mediated phagocytosis and killing of Streptococcus pneumoniae are associated with direct and bystander macrophage apoptosis. J Infect Dis. 2001;184:713–722. doi: 10.1086/323084. [DOI] [PubMed] [Google Scholar]
- 23.Fang FC, Vazquez-Torres A. Nitric oxide production by human macrophages: there’s NO doubt about it. Am J Physiol Lung Cell Mol Physiol. 2002;282:L941–943. doi: 10.1152/ajplung.00017.2002. [DOI] [PubMed] [Google Scholar]
- 24.Jones MR, Simms BT, Lupa MM, Kogan MS, Mizgerd JP. Lung NF-kappaB activation and neutrophil recruitment require IL-1 and TNF receptor signaling during pneumococcal pneumonia. J Immunol. 2005;175:7530–7535. doi: 10.4049/jimmunol.175.11.7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Speyer CL, Neff TA, Warner RL, Guo RF, Sarma JV, Riedemann NC, Murphy ME, Murphy HS, Ward PA. Regulatory effects of iNOS on acute lung inflammatory responses in mice. Am J Pathol. 2003;163:2319–2328. doi: 10.1016/S0002-9440(10)63588-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hongo D, Bryson JS, Kaplan AM, Cohen DA. Endogenous nitric oxide protects against T cell-dependent lethality during graft-versus-host disease and idiopathic pneumonia syndrome. J Immunol. 2004;173:1744–1756. doi: 10.4049/jimmunol.173.3.1744. [DOI] [PubMed] [Google Scholar]
- 27.Clatworthy MR, Smith KG. FcgammaRIIb balances efficient pathogen clearance and the cytokine-mediated consequences of sepsis. J Exp Med. 2004;199:717–723. doi: 10.1084/jem.20032197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu B, Jennings JH, Sonstein J, Floros J, Todt JC, Polak T, Curtis JL. Resident murine alveolar and peritoneal macrophages differ in adhesion of apoptotic thymocytes. Am J Respir Cell Mol Biol. 2004;30:687–693. doi: 10.1165/rcmb.2003-0255OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 30.Walley KR, McDonald TE, Higashimoto Y, Hayashi S. Modulation of proinflammatory cytokines by nitric oxide in murine acute lung injury. Am J Respir Crit Care Med. 1999;160:698–704. doi: 10.1164/ajrccm.160.2.9809081. [DOI] [PubMed] [Google Scholar]
- 31.Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;407:784–788. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- 32.Tuomanen EI, Austrian R, Masure HR. Pathogenesis of pneumococcal infection. N Engl J Med. 1995;332:1280–1284. doi: 10.1056/NEJM199505113321907. [DOI] [PubMed] [Google Scholar]
- 33.Bergeron Y, Ouellet N, Deslauriers AM, Simard M, Olivier M, Bergeron MG. Cytokine kinetics and other host factors in response to pneumococcal pulmonary infection in mice. Infect Immun. 1998;66:912–922. doi: 10.1128/iai.66.3.912-922.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bergeron Y, Ouellet N, Simard M, Olivier M, Bergeron MG. Immunomodulation of pneumococcal pulmonary infection with N(G)-monomethyl-L-arginine. Antimicrob Agents Chemother. 1999;43:2283–2290. doi: 10.1128/aac.43.9.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kerr AR, Wei XQ, Andrew PW, Mitchell TJ. Nitric oxide exerts distinct effects in local and systemic infections with Streptococcus pneumoniae. Microb Pathog. 2004;36:303–310. doi: 10.1016/j.micpath.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Fang FC. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nature Reviews Microbiology. 2004;2:820–832. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- 37.Nathan C. Specificity of a third kind: reactive oxygen and nitrogen intermediates in cell signaling. J Clin Invest. 2003;111:769–778. doi: 10.1172/JCI18174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Vliet A, Eiserich JP, Cross CE. Nitric oxide: a pro-inflammatory mediator in lung disease? Respir Res. 2000;1:67–72. doi: 10.1186/rr14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marshall HE, Stamler JS. Exhaled nitric oxide (NO), NO synthase activity, and regulation of nuclear factor (NF)-kappaB. Am J Respir Cell Mol Biol. 1999;21:296–297. doi: 10.1165/ajrcmb.21.3.f164. [DOI] [PubMed] [Google Scholar]
- 40.Brune B, von Knethen A, Sandau KB. Nitric oxide (NO): an effector of apoptosis. Cell Death Differ. 1999;6:969–975. doi: 10.1038/sj.cdd.4400582. [DOI] [PubMed] [Google Scholar]
- 41.Hu B, Sonstein J, Christensen PJ, Punturieri A, Curtis JL. Deficient in vitro and in vivo phagocytosis of apoptotic T cells by resident murine alveolar macrophages. J Immunol. 2000;165:2124–2133. doi: 10.4049/jimmunol.165.4.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng L, He M, Long M, Blomgran R, Stendahl O. Pathogen-induced apoptotic neutrophils express heat shock proteins and elicit activation of human macrophages. J Immunol. 2004;173:6319–6326. doi: 10.4049/jimmunol.173.10.6319. [DOI] [PubMed] [Google Scholar]
- 43.van der Poll T, Keogh CV, Buurman WA, Lowry SF. Passive immunization against tumor necrosis factor-alpha impairs host defense during pneumococcal pneumonia in mice. Am J Respir Crit Care Med. 1997;155:603–608. doi: 10.1164/ajrccm.155.2.9032201. [DOI] [PubMed] [Google Scholar]
- 44.Rijneveld AW, Florquin S, Branger J, Speelman P, VanDeventer SJ, van der Poll T. TNF-alpha compensates for the impaired host defense of IL-1 type I receptor-deficient mice during pneumococcal pneumonia. J Immunol. 2001;167:5240–5246. doi: 10.4049/jimmunol.167.9.5240. [DOI] [PubMed] [Google Scholar]
- 45.Windsor AC, Walsh CJ, Mullen PG, Cook DJ, Fisher BJ, Blocher CR, Leeper-Woodford SK, Sugerman HJ, Fowler AA., 3rd Tumor necrosis factor-alpha blockade prevents neutrophil CD18 receptor upregulation and attenuates acute lung injury in porcine sepsis without inhibition of neutrophil oxygen radical generation. J Clin Invest. 1993;91:1459–1468. doi: 10.1172/JCI116351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wright TW, Pryhuber GS, Chess PR, Wang Z, Notter RH, Gigliotti F. TNF receptor signaling contributes to chemokine secretion, inflammation, and respiratory deficits during Pneumocystis pneumonia. J Immunol. 2004;172:2511–2521. doi: 10.4049/jimmunol.172.4.2511. [DOI] [PubMed] [Google Scholar]
- 47.Hildebrandt GC, Olkiewicz KM, Corrion LA, Chang Y, Clouthier SG, Liu C, Cooke KR. Donor-derived TNF-alpha regulates pulmonary chemokine expression and the development of idiopathic pneumonia syndrome after allogeneic bone marrow transplantation. Blood. 2004;104:586–593. doi: 10.1182/blood-2003-12-4259. [DOI] [PubMed] [Google Scholar]
- 48.Tracey KJ, Fong Y, Hesse DG, Manogue KR, Lee AT, Kuo GC, Lowry SF, Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 49.Kurahashi K, Kajikawa O, Sawa T, Ohara M, Gropper MA, Frank DW, Martin TR, Wiener-Kronish JP. Pathogenesis of septic shock in Pseudomonas aeruginosa pneumonia. J Clin Invest. 1999;104:743–750. doi: 10.1172/JCI7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kirby AC, Raynes JG, Kaye PM. The role played by tumor necrosis factor during localized and systemic infection with Streptococcus pneumoniae. J Infect Dis. 2005;191:1538–1547. doi: 10.1086/429296. [DOI] [PubMed] [Google Scholar]
- 51.Fadok VA, Bratton DL, Guthrie L, Henson PM. Differential effects of apoptotic versus lysed cells on macrophage production of cytokines: role of proteases. J Immunol. 2001;166:6847–6854. doi: 10.4049/jimmunol.166.11.6847. [DOI] [PubMed] [Google Scholar]
- 52.Cundell DR, Gerard NP, Gerard C, Idanpaan-Heikkila I, Tuomanen EI. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature. 1995;377:435–438. doi: 10.1038/377435a0. [DOI] [PubMed] [Google Scholar]
- 53.Ali F, Lee ME, Iannelli F, Pozzi G, Mitchell TJ, Read RC, Dockrell DH. Streptococcus pneumoniae-Associated Human Macrophage Apoptosis after Bacterial Internalization via Complement and Fcgamma Receptors Correlates with Intracellular Bacterial Load. J Infect Dis. 2003;188:1119–1131. doi: 10.1086/378675. [DOI] [PubMed] [Google Scholar]
- 54.Hotchkiss RS, Tinsley KW, Swanson PE, Chang KC, Cobb JP, Buchman TG, Korsmeyer SJ, Karl IE. Prevention of lymphocyte cell death in sepsis improves survival in mice. Proc Natl Acad Sci U S A. 1999;96:14541–14546. doi: 10.1073/pnas.96.25.14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hotchkiss RS, Chang KC, Swanson PE, Tinsley KW, Hui JJ, Klender P, Xanthoudakis S, Roy S, Black C, Grimm E, Aspiotis R, et al. Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nat Immunol. 2000;1:496–501. doi: 10.1038/82741. [DOI] [PubMed] [Google Scholar]
- 56.Fehsel K, Kroncke KD, Meyer KL, Huber H, Wahn V, Kolb-Bachofen V. Nitric oxide induces apoptosis in mouse thymocytes. J Immunol. 1995;155:2858–2865. [PubMed] [Google Scholar]
- 57.Tarrant TK, Silver PB, Wahlsten JL, Rizzo LV, Chan CC, Wiggert B, Caspi RR. Interleukin 12 protects from a T helper type 1-mediated autoimmune disease, experimental autoimmune uveitis, through a mechanism involving interferon gamma, nitric oxide, and apoptosis. J Exp Med. 1999;189:219–230. doi: 10.1084/jem.189.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]