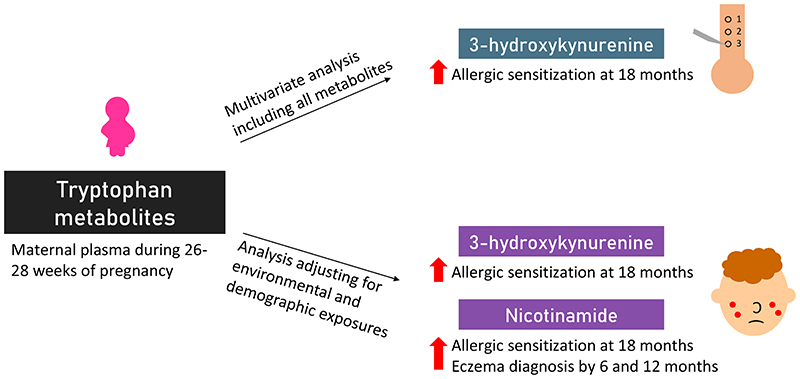
Abstract
Background
Nicotinamide (vitamin B3) is metabolite of tryptophan and a dietary precursor of enzymes involved in many regulatory processes, which may be influence fetal immune development.
Objective
We examined whether maternal plasma concentrations of nicotinamide, tryptophan or nine related tryptophan metabolites during pregnancy were associated with risk of development of infant eczema, wheeze, rhinitis or allergic sensitization.
Methods
In the Growing Up in Singapore Towards Healthy Outcomes (GUSTO) study, we analysed the associations between maternal plasma levels of nicotinamide, tryptophan and tryptophan metabolites at 26-28 weeks gestation and allergic outcomes collected through interviewer-administered questionnaires at multiple timepoints and skin prick testing to egg, milk, peanut and mites at age 18 months. Multivariate analysis was undertaken adjusting for all metabolites measured, and separately adjusting for relevant demographic and environmental exposures. Analyses were also adjusted for multiple comparisons using the false discovery method.
Results
Tryptophan metabolites were evaluated in 976/1247 (78%) women enrolled in GUSTO. In multivariate analysis including all metabolites, maternal plasma 3-hydrokynurenine was associated with increased allergic sensitization at 18 months (AdjRR 2.6, 95% CI 1.3-5.2 for highest quartile) but the association with nicotinamide was not significant (AdjRR 1.8, 95% CI 0.9-3.6). In analysis adjusting for other exposures, both 3-hydrokynurenine and nicotinamide were associated with increased allergic sensitization (AdjRR 2.0, 95% CI 1.1-3.6 for both metabolites). High maternal plasma nicotinamide was associated with increased infant eczema diagnosis at 6 and 12 months, which was not significant when adjusting for all metabolites measured, but was significant when adjusting for relevant environmental and demographic exposures. Other metabolites measured were not associated with allergic sensitisation or eczema, and maternal tryptophan metabolites were not associated with offspring rhinitis and wheeze.
Conclusions and Clinical Relevance
Maternal tryptophan metabolism during pregnancy may influence the development of allergic sensitization and eczema in infants.
Keywords: Nicotinamide, Vitamin B3, Tryptophan metabolism, Kynurenine pathway, Eczema
Graphic abstract.
Introduction
Worldwide, there is a high prevalence of allergic diseases in early life 1. An estimated 22.7% of Singaporean children develop eczema in the first 2 years of life 2. The Developmental Origins of Health and Disease (DOHaD) concept hypothesises that early stimuli, starting from preconception, throughout pregnancy and into early life, may contribute to the early onset of allergic diseases by influencing fetal and neonatal immune regulation 3.
Maternal health, diet and lifestyle during pregnancy have been shown to affect risk for major disease outcomes in the offspring, including eczema and allergic diseases 4-7. Adequate levels of maternal nutrients are important for fetal health, as nutrients essential for the development of the fetal immune system, for instance, can be transferred from the mother to the fetus via the placenta 8,9.
A key nutrient in our diet is vitamin B3 (also known as nicotinamide), the precursor of nicotinamide adenine dinucleotide and nicotinamide adenine dinucleotide phosphate, enzymes that are involved in many regulatory processes 10. Nicotinamide is obtained through diets rich in eggs, nuts, meat, grains and poultry and tryptophan metabolism via the kynurenine pathway 11-15. Although the inflammatory response in allergic diseases is complex, Th2 responses are a major driver. Tryptophan metabolism is key in modulating Th1/Th2 immune responses through production of catabolites and cytokines 16. For instance, 3-hydroxyanthranilic acid and quinolinic acid can alter Th1 cells 16 and tend to increase Th2 activity. The physiological state of an individual affects the tryptophan metabolism rate, where conversion of tryptophan to nicotinamide has been reported to increase from mid to late pregnancy 13. Microbiome and tryptophan metabolites interaction can also play a role in affecting the skin barrier as well as the inflammatory responses 17. High levels of nicotinamide beyond recommended daily intakes have also been linked to adverse outcomes including oxidative cell damage, alteration of DNA methylation and lower sirtuin 1 activity 18, an enzyme involved in skin barrier integrity 19. Besides this, a high quartile of plasma tryptophan has been associated with higher risk of development of inflammatory diseases 20-23. Hence we hypothesised that dysregulation of the tryptophan metabolism pathway and high levels of metabolites may lead to inflammation and allergic disease development.
The Nurses’ Health Study 2 in United States reported an increased risk of eczema in women with the highest levels of total and supplemental nicotinamide consumption as compared to the lowest, and those effects were not continuous across the distribution 24. However, only one study has investigated the association between maternal nicotinamide and allergy in offspring. El-Heis et al. reported that higher maternal plasma levels of nicotinamide and anthranilic acid were associated with a lower risk of eczema development in infants by age of 12 months in the UK Southampton Women’s Survey cohort 25, suggesting involvement of metabolites in the tryptophan metabolism pathway in eczema development.
The relationship between maternal tryptophan metabolites on cord blood cytokines has also not been clearly elucidated although cytokines and chemokines present in blood play a role in the inflammatory pathways influencing allergic disease pathogenesis. In this study, we examined associations between maternal plasma concentrations of nicotinamide and related tryptophan metabolites in the tryptophan pathway during pregnancy and the offspring’s cord blood immune profile and risk of allergic diseases. We focused on the chemokines, monocyte chemoattractant protein-1 (MCP-1) and monokine induced by gamma interferon (MIG) due to their chemoattractant properties as well as role in affecting the T-helper 1 (Th1) and T-helper 2 (Th2) balance. MCP-1 is a C-C chemokine and a strong monocyte chemotactic factor responsible for recruitment of monocytes, T cells and natural killer cells to inflammation sites 26. MIG is an indicator of functional interferon gamma (IFN-γ) signalling pathway and bioactivity and is also key in T cell recruitment27. To the best of our knowledge, this is the first study examining the influences of maternal nicotinamide and tryptophan metabolites levels in pregnancy and their impact on offspring eczema, wheeze, rhinitis and allergic sensitization. Better understanding of their influences and impact on offspring allergy risk can help elucidate supporting evidence for recommendations during pregnancy to lower the risk of allergy development in the offspring.
Methods
Study design and questionnaires
The Growing Up in Singapore Towards healthy Outcomes (GUSTO) study recruited 1247 healthy pregnant mothers. The detailed methodology for the GUSTO study was described by Soh et al. 28. Trained interviewers gathered information on demographic characteristics, family history of allergy, social data and lifestyle factors. We used the modified ISAAC modified questionnaire at ages 3, 6, 9, 12, 15 and 18 months for evaluation of offspring allergic symptoms. Eczema was primarily defined as physician-diagnosed eczema was where the answer to the question: “Has your child ever been diagnosed with eczema?” was ‘yes’. We also used another secondary definition of eczema, defined by having an itchy rash that is coming and going, other than nappy rash and which affected any of the following places: folds of the elbows, behind the knees, in front of the ankles, on the cheeks, or around the neck, ears or eyes. Wheeze with use of nebulizers/inhalers was defined by positive responses to the questions: “Has your child ever wheezed?” and “Has your child ever been prescribed with nebulizer/inhaler treatment?”. Rhinitis was defined by a positive response to the question: “Has your child had running nose, blocked or congested nose, snoring or noisy breathing during sleep or when awake that has lasted for two or more weeks duration?”. Cumulative allergy outcomes by ages 6, 12 and 18 months were classified as “yes” when a subject answered “yes” by the timepoint. Subjects were classified as “no” if the subject answered “no” at all timepoints. Skin prick testing (SPT) was conducted at 18 months for allergens cow’s milk, egg, peanut and house dust mites, Dermatophagoides pteronyssinus (Der p), Dermatophagoides farina (Derp f) (Greer Laboratories, Lenoir, NC, USA) and Blomia tropicalis (Blo t) (developed in-house) 29. Maternal dietary intake during pregnancy was assessed at week 26-28 using 24-h recall food diaries. The detailed methodology was described by Chia et al. 30.
Ethical approval was obtained from the Domain Specific Review Board of Singapore National Healthcare Group (D/2009/021) and the Centralised Institutional Review Board of SingHealth (2018/2767). The conduct of this study was based on the guidelines in the Declaration of Helsinki. Informed consent was obtained from all participants.
Assessment of maternal plasma metabolite levels as well as cord blood cytokines, chemokines and antibodies
Maternal plasma metabolite levels of N1-methylnicotinamide, nicotinamide, trigonelline, 3-hydroxyanthranilic acid, 3-hydroxykynurenine, anthranilic acid, kynurenic acid, kynurenine, quinolic acid, tryptophan, xanthurenic acid at 26-28 weeks gestation were analysed by tandem mass spectrometry (API 4000, AB Sciex). Cord blood MIG, MCP-1 and IFN-γ levels were assayed in plasma using customised Human ProcartaPlex Panels (Thermo Fisher) which uses Luminex xMAP technology in combination with DropArray bead plates (Curiox Biosystems, Singapore). Interleukin-6 (IL-6) was measured using Single molecule array (SiMoA) assays on the SP-X platform (Quanterix Corp., USA). All values were corrected for plate effects using median centering.
Statistical analysis
All analyses were performed using SPSS for Windows version 26.0 (SPSS Inc, Chicago, IL, USA). Descriptive statistics for numerical variables were presented as mean (SD) when normality and homogeneity assumptions were satisfied, otherwise median (IQR) were presented and n (%) for categorical variables. Maternal plasma metabolite levels were divided into quartiles. A multivariate analysis was conducted where all metabolites were included in the model, noting that this reduced statistical power as not all participants had complete data. Predictors for infant eczema, rash, wheeze with use of nebulizers/inhalers and rhinitis by ages 6, 12 and 18 months and allergic sensitization at 18 months were assessed using univariate and multivariate Poisson regression. Further multivariate analyses were performed for each metabolite with p<0.05 in univariate Poisson regression, adjusting for demographic and relevant covariates. Differences in the cord blood immune profile between subjects with each allergic outcome and controls were evaluated using Mann Whitney U test. Pearson correlation coefficient was used to investigate the relationship between plasma nicotinamide and 3-hydroxykynurenine concentration with the mother’s dietary, vitamin and supplement intake. Type 1 error for multiple comparisons were adjusted using Benjamini-Hochberg procedure with false discovery rate at 0.20.
Results
Population characteristics
We included in the study 976 mother-offspring pairs with maternal metabolites analysed. The mothers’ median age at delivery was 30.9 years (IQR 27.4-34.6, Table 1). The majority of mothers were of Chinese ethnicity [532 (54.5%)], had at least 12 years of education [668 (69.4%)] and no history of allergy [580 (61.1%)]. Of the 976 infants; 507 (51.9%) were male and by 6, 12 and 18 months, 74 (9.1%), 107 (14.0%) and 157 (22.5%) developed eczema respectively; 197 (23.8%), 253 (32.3%) and 374 (50.1%) developed itchy rash respectively; 26 (3.1%), 69 (9.6%) and 97 (14.6%) developed wheeze with the use of nebulizers/inhalers respectively; 230 (27.5%), 329 (42.6%) and 390 (52.6%) developed rhinitis respectively and 105 (14.0%) developed allergic sensitization at 18 months.
Table 1. Characteristics of the study population.
| n | Median (IQR), Mean (SD) or n (%) | |
|---|---|---|
| Maternal age at child’s birth (years) | 976 | 30.9(27.4-34.6) |
| Ethnicity | 976 | |
| Chinese | 532 | 54.5% |
| Indian | 183 | 18.8% |
| Malay | 261 | 26.7% |
| Education | 963 | |
| ≥ 12 years of education | 668 | 69.4% |
| < 12 years of education | 295 | 30.6% |
| Maternal history of allergy (eczema, rhinitis and/or asthma) | 949 | |
| Yes | 369 | 38.9% |
| No | 580 | 61.1% |
| Parity | 976 | |
| Parous | 562 | 57.6% |
| Nulliparous | 414 | 42.4% |
| Household income | 913 | |
| 0-999 | 17 | 1.9% |
| 1000-1999 | 127 | 13.9% |
| 2000-3999 | 282 | 30.9% |
| 4000-5999 | 231 | 25.3% |
| >=6000 | 256 | 28.0% |
| Smoking status | 971 | |
| Current or ever smoker | 130 | 13.4% |
| Non smoker | 841 | 86.6% |
| Caesarean delivery | 976 | |
| Yes | 288 | 29.5% |
| No | 688 | 70.5% |
| Supplements | 879 | 2(1-3) |
| Maternal serum metabolite concentrations in late pregnancy | ||
| N1-methylnicotinamide (nmol/L) | 976 | 257.50(195.00-329.00) |
| Nicotinamide (nmol/L) | 976 | 184.00 (124.00-252.00) |
| Trigonelline (μmol/L) | 976 | 0.14(0.09-0.27) |
| 3-Hydroxyanthranilic acid (nmol/L) | 976 | 69.75(59.70-82.20) |
| 3-Hydroxykynurenine (nmol/L) | 976 | 46.15(36.73-58.90) |
| Anthranilic acid (nmol/L) | 976 | 10.60(8.78-12.70) |
| Kynurenic acid (nmol/L) | 976 | 16.90(13.70-21.60) |
| Kynurenine (μmol/L) | 976 | 1.02 (0.87-1.16) |
| Quinolinic acid (nmol/L) | 976 | 369.00 (316.00-431.75) |
| Tryptophan (μmol/L) | 976 | 46.60(41.00-51.88) |
| Xanthurenic acid (nmol/L) | 976 | 10.30(6.80-15.08) |
| Infant Sex | 976 | |
| Male | 507 | 51.9% |
| Female | 469 | 48.1% |
| Birth weight (kg) | 930 | 3.1 (0.4) |
| Use of antibiotics | 727 | |
| Yes | 335 | 46.1% |
| No | 392 | 53.9% |
| Type of milk feed | 910 | |
| Mainly formula feed | 407 | 44.7% |
| Mixed feeding | 394 | 43.3% |
| Mainly breastfeeding | 109 | 12.0% |
| Eczema by 6 months | 817 | |
| Yes | 74 | 9.1% |
| No | 743 | 90.9% |
| Eczema by 12 months | 765 | |
| Yes | 107 | 14.0% |
| No | 658 | 86.0% |
| Eczema by 18 months | 699 | |
| Yes | 157 | 22.5% |
| No | 542 | 77.5% |
| Itchy rash by 6 months | 829 | |
| Yes | 197 | 23.8% |
| No | 632 | 76.2% |
| Itchy rash by 12 months | 783 | |
| Yes | 253 | 32.3% |
| No | 530 | 67.7% |
| Itchy rash by 18 months | 747 | |
| Yes | 374 | 50.1% |
| No | 373 | 49.9% |
| Wheeze by 6 months | 828 | |
| Yes | 26 | 3.1% |
| No | 802 | 96.9% |
| Wheeze by 12 months | 720 | |
| Yes | 69 | 9.6% |
| No | 651 | 90.4% |
| Wheeze by 18 months | 666 | |
| Yes | 97 | 14.6% |
| No | 569 | 85.4% |
| Rhinitis by 6 months | 835 | |
| Yes | 230 | 27.5% |
| No | 605 | 72.5% |
| Rhinitis by 12 months | 772 | |
| Yes | 329 | 42.6% |
| No | 443 | 57.4% |
| Rhinitis by 18 months | 742 | |
| Yes | 390 | 52.6% |
| No | 352 | 47.4% |
| SPT by 18 months | 749 | |
| Positive | 105 | 14.0% |
| Negative | 644 | 86.0% |
Analysis of maternal plasma metabolites at 26-28 weeks pregnancy with allergic outcomes
In multivariate analysis where all metabolites were included (Tables 2-6), the highest quartile of 3-hydroxykynurenine was associated with increased risk of allergic sensitization at 18 months (AdjRR=2.6, 95% CI=1.3-5.2, p=0.008, Table 6) after adjusting for confounders. There was a trend of increasing risk of allergic sensitization development at 18 months with increasing quartiles of 3-hydroxykynurenine (p=0.003). There were no associations between metabolites and eczema, rash, wheeze with the use of nebulizers/inhalers and rhinitis outcomes in the first 18 months of life (Tables 2-5).
Table 2. Multivariate associations between all maternal plasma metabolites and eczema development by 6, 12 and 18 months in the offspring.
| 6 months (n=782) | 12 months (n=732) | 18 months (n=667) | ||||
|---|---|---|---|---|---|---|
| RR (95% CI) | p-value * | RR (95% CI) | p-value * | RR (95% CI) | p-value * | |
| N1-methylnicotinamide | 0.102 | 0.167 | 0.423 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 0.88(0.44 – 1.76) | 0.714 | 1.1(0.6 – 2.1) | 0.704 | 1.1(0.7 – 1.8) | 0.708 |
| 3rd | 0.71(0.34 – 1.50) | 0.370 | 0.92(0.49 – 1.73) | 0.788 | 0.82(0.48 – 1.39) | 0.451 |
| Highest | 0.58(0.25 – 1.34) | 0.202 | 0.65(0.32 – 1.34) | 0.245 | 0.88(0.50 – 1.57) | 0.672 |
| Nicotinamide | 0.004 | 0.019 | 0.136 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 1.8(0.8 – 4.2) | 0.192 | 1.7(0.9 – 3.4) | 0.123 | 1.1(0.7 – 1.9) | 0.683 |
| 3rd | 2.3(1.0 – 5.4) | 0.055 | 1.6(0.8 – 3.3) | 0.177 | 1.1(0.6 – 1.9) | 0.735 |
| Highest | 3.2(1.4 – 7.6) | 0.008 | 2.2(1.1 – 4.6) | 0.029 | 1.4(0.8 – 2.4) | 0.244 |
| Trigonelline | 0.858 | 0.352 | 0.649 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 0.81(0.41 – 1.59) | 0.532 | 0.92(0.53 – 1.61) | 0.781 | 0.95(0.59 – 1.50) | 0.812 |
| 3rd | 0.80(0.40 – 1.58) | 0.522 | 0.83(0.47 – 1.48) | 0.525 | 1.0(0.6 – 1.6) | 0.948 |
| Highest | 0.94(0.44 – 2.02) | 0.880 | 0.82(0.43 – 1.58) | 0.548 | 0.86(0.50 – 1.45) | 0.565 |
| 3-Hydroxyanthranilic acid | 0.512 | 0.264 | 0.032 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 1.2(0.5 – 2.5) | 0.702 | 0.93(0.48 – 1.81) | 0.822 | 1.2(0.7 – 2.0) | 0.504 |
| 3rd | 1.3(0.6 – 2.8) | 0.489 | 1.6(0.8 – 3.0) | 0.160 | 1.6(0.9 – 2.8) | 0.086 |
| Highest | 1.4(0.6 – 3.4) | 0.432 | 1.4(0.7 – 3.0) | 0.358 | 1.9(1.1 – 3.6) | 0.031 |
| 3-Hydroxykynurenine | 0.023 | 0.135 | 0.387 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 1.8(0.8 – 4.0) | 0.167 | 1.7(0.9 – 3.3) | 0.094 | 1.6(1.0 – 2.7) | 0.068 |
| 3rd | 2.7(1.2 – 6.1) | 0.017 | 1.9(0.9 – 3.8) | 0.076 | 1.5(0.9 – 2.7) | 0.118 |
| Highest | 2.9(1.2 – 7.0) | 0.020 | 1.8(0.9 – 3.9) | 0.123 | 1.5(0.8 – 2.7) | 0.192 |
| Anthranilic acid | 0.305 | 0.098 | 0.152 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 0.71(0.36 – 1.41) | 0.334 | 0.72(0.40 – 1.31) | 0.285 | 0.72(0.45 – 1.16) | 0.178 |
| 3rd | 0.70(0.34 – 1.44) | 0.333 | 0.71(0.38 – 1.31) | 0.272 | 0.72(0.44 – 1.17) | 0.179 |
| Highest | 0.72(0.33 – 1.56) | 0.402 | 0.56(0.28 – 1.12) | 0.103 | 0.68(0.39 – 1.16) | 0.155 |
| Kynurenic acid | 0.682 | 0.699 | 0.361 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 1.2(0.5 – 2.6) | 0.734 | 1.2(0.6 – 2.4) | 0.628 | 0.94(0.55 – 1.64) | 0.838 |
| 3rd | 1.0(0.4 – 2.7) | 0.956 | 1.2(0.6 – 2.8) | 0.590 | 0.84(0.45 – 1.59) | 0.595 |
| Highest | 0.81(0.27 – 2.42) | 0.702 | 1.2(0.5 – 3.0) | 0.702 | 0.79(0.38 – 1.64) | 0.524 |
| Kynurenine | 0.080 | 0.067 | 0.338 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 0.87(0.42 – 1.80) | 0.713 | 1.0(0.6 – 1.9) | 0.891 | 0.96(0.58 – 1.58) | 0.878 |
| 3rd | 0.72(0.32 – 1.62) | 0.423 | 0.87(0.44 – 1.70) | 0.675 | 0.84(0.48 – 1.46) | 0.530 |
| Highest | 0.36(0.13 – 1.01) | 0.053 | 0.44(0.19 – 1.04) | 0.061 | 0.71(0.36 – 1.40) | 0.321 |
| Quinolinic acid | 0.362 | 0.643 | 0.597 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 0.59(0.27 – 1.29) | 0.187 | 0.73(0.39 – 1.38) | 0.329 | 0.63(0.38 – 1.07) | 0.086 |
| 3rd | 0.77(0.34 – 1.72) | 0.518 | 0.76(0.39 – 1.49) | 0.418 | 0.77(0.45 – 1.33) | 0.352 |
| Highest | 1.3(0.6 – 3.2) | 0.520 | 1.1(0.5 – 2.4) | 0.741 | 0.70(0.37 – 1.33) | 0.280 |
| Tryptophan | 0.475 | 0.179 | 0.153 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 1.2(0.5 – 2.6) | 0.711 | 1.3(0.7 – 2.5) | 0.468 | 1.6(0.9 – 2.8) | 0.085 |
| 3rd | 1.2(0.5 – 2.7) | 0.642 | 1.5(0.8 – 3.0) | 0.235 | 1.6(0.9 – 2.8) | 0.107 |
| Highest | 1.3(0.6 – 3.0) | 0.547 | 1.5(0.7 – 3.0) | 0.325 | 1.6(0.9 – 3.0) | 0.115 |
| Xanthurenic acid | 0.680 | 0.602 | 0.894 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 0.47(0.21 – 1.08) | 0.075 | 0.62(0.31 – 1.26) | 0.187 | 0.93(0.54 – 1.61) | 0.803 |
| 3rd | 0.60(0.25 – 1.44) | 0.257 | 0.76(0.36 – 1.61) | 0.474 | 0.91(0.49 – 1.71) | 0.771 |
| Highest | 0.72(0.27 – 1.94) | 0.514 | 0.70(0.29 – 1.66) | 0.413 | 1.0(0.5 – 2.0) | 0.997 |
RR = 1.0 is the reference category.
p values of trend of metabolites in italics
Benjamini-Hochberg correction with false discovery rate at 0.20 and n=132 was applied. No associations were significant after correction.
Adjusted for ethnicity, maternal education level, maternal history of allergy, parity, infant sex and type of milk feed
Table 6. Multivariate associations between all maternal plasma metabolites and positive SPT at 18 months in the offspring.
| 18 months (n=716) | ||
|---|---|---|
| RR (95% CI) | p-value * | |
| N1-methylnicotinamide | 0.878 | |
| Lowest | 1.0 | |
| 2nd | 0.97(0.53 – 1.76) | 0.913 |
| 3rd | 0.85(0.44 – 1.64) | 0.630 |
| Highest | 1.1(0.6 – 2.2) | 0.715 |
| Nicotinamide | 0.089 | |
| Lowest | 1.0 | |
| 2nd | 1.6(0.8 – 3.0) | 0.155 |
| 3rd | 1.6(0.8 – 3.1) | 0.162 |
| Highest | 1.8(0.9 – 3.6) | 0.088 |
| Trigonelline | 0.477 | |
| Lowest | 1.0 | |
| 2nd | 1.1(0.7 – 2.0) | 0.633 |
| 3rd | 1.1(0.6 – 1.9) | 0.801 |
| Highest | 0.81(0.41 – 1.58) | 0.530 |
| 3-Hydroxyanthranilic acid | 0.183 | |
| Lowest | 1.0 | |
| 2nd | 0.80(0.43 – 1.48) | 0.477 |
| 3rd | 0.96(0.52 – 1.75) | 0.880 |
| Highest | 0.58(0.28 – 1.21) | 0.147 |
| 3-Hydroxykynurenine | 0.003 | |
| Lowest | 1.0 | |
| 2nd | 1.2(0.6 – 2.3) | 0.625 |
| 3rd | 2.0(1.0 – 3.7) | 0.042 |
| Highest | 2.6(1.3 – 5.2) | 0.008 |
| Anthranilic acid | 0.713 | |
| Lowest | 1.0 | |
| 2nd | 1.0(0.6 – 1.8) | 0.990 |
| 3rd | 0.61(0.31 – 1.19) | 0.149 |
| Highest | 0.95(0.50 – 1.8) | 0.873 |
| Kynurenic acid | 0.774 | |
| Lowest | 1.0 | |
| 2nd | 1.0(0.5 – 2.0) | 0.921 |
| 3rd | 1.0(0.5 – 2.2) | 0.921 |
| Highest | 1.2(0.5 – 2.8) | 0.693 |
| Kynurenine | 0.936 | |
| Lowest | 1.0 | |
| 2nd | 1.3(0.7 – 2.3) | 0.448 |
| 3rd | 1.1(0.5 – 2.2) | 0.792 |
| Highest | 1.1(0.5 – 2.6) | 0.744 |
| Quinolinic acid | 0.270 | |
| Lowest | 1.0 | |
| 2nd | 0.72(0.39 – 1.31) | 0.276 |
| 3rd | 0.68(0.35 – 1.32) | 0.250 |
| Highest | 0.61(0.29 – 1.31) | 0.207 |
| Tryptophan | 0.895 | |
| Lowest | 1.0 | |
| 2nd | 1.0(0.6 – 1.9) | 0.882 |
| 3rd | 1.0(0.5 – 1.9) | 0.985 |
| Highest | 1.0(0.5 – 2.1) | 0.905 |
| Xanthurenic acid | 0.718 | |
| Lowest | 1.0 | |
| 2nd | 0.89(0.45 – 1.78) | 0.749 |
| 3rd | 1.3(0.6 – 2.7) | 0.502 |
| Highest | 1.1(0.4 – 2.6) | 0.870 |
RR = 1.0 is the reference category.
p values of trend of metabolites in italics
Significant p-value after Benjamini-Hochberg correction with false discovery rate at 0.20 and n=44 in bold
Adjusted for ethnicity, smoking status, type of milk feed, caesarean delivery and infant sex
Table 5. Multivariate associations between all maternal plasma metabolites and rhinitis by 6, 12 and 18 months in the offspring.
| 6 months (n=675) | 12 months (n=659) | 18 months (n=651) | ||||
|---|---|---|---|---|---|---|
| RR (95% CI) | p-value * | RR (95% CI) | p-value * | RR (95% CI) | p-value * | |
| N1-methylnicotinamide | 0.305 | 0.561 | 0.943 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 1.0(0.6 – 1.6) | 0.989 | 1.1(0.8 – 1.6) | 0.562 | 1.2(0.8 – 1.6) | 0.396 |
| 3rd | 1.1(0.7 – 1.7) | 0.741 | 1.1(0.7 – 1.6) | 0.679 | 1.1(0.8 – 1.5) | 0.721 |
| Highest | 1.3(0.8 – 2.1) | 0.356 | 1.2(0.7 – 1.8) | 0.522 | 1.1(0.7 – 1.6) | 0.749 |
| Nicotinamide | 0.198 | 0.091 | 0.209 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 0.83(0.55 – 1.24) | 0.358 | 0.83(0.58 – 1.18) | 0.303 | 0.83(0.60 – 1.15) | 0.272 |
| 3rd | 0.74(0.48 – 1.15) | 0.182 | 0.82(0.57 – 1.18) | 0.278 | 0.88(0.63 – 1.23) | 0.456 |
| Highest | 0.77(0.48 – 1.23) | 0.277 | 0.70(0.47 – 1.05) | 0.088 | 0.76(0.52 – 1.11) | 0.155 |
| Trigonelline | 0.539 | 0.962 | 0.691 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 1.0(0.7 – 1.5) | 0.992 | 0.92(0.65 – 1.31) | 0.650 | 0.92(0.67 – 1.27) | 0.620 |
| 3rd | 1.1(0.7 – 1.7) | 0.725 | 1.0(0.7 – 1.5) | 0.847 | 0.92(0.66 – 1.29) | 0.640 |
| Highest | 1.2(0.8 – 2.0) | 0.376 | 1.0(0.7 – 1.5) | 0.980 | 0.97(0.68 – 1.39) | 0.858 |
| 3-Hydroxyanthranilic acid | 0.562 | 0.796 | 0.791 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 1.0(0.7 – 1.5) | 0.987 | 1.1(0.8 – 1.6) | 0.560 | 1.0(0.8 – 1.5) | 0.785 |
| 3rd | 0.83(0.51 – 1.34) | 0.445 | 1.1(0.7 – 1.6) | 0.725 | 0.97(0.67 – 1.41) | 0.890 |
| Highest | 0.90(0.53 – 1.55) | 0.706 | 1.1(0.7 – 1.7) | 0.802 | 0.95(0.63 – 1.43) | 0.797 |
| 3-Hydroxykynurenine | 0.529 | 0.757 | 0.357 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 1.0(0.7 – 1.6) | 0.923 | 1.0(0.7 – 1.5) | 0.995 | 1.0(0.7 – 1.4) | 0.933 |
| 3rd | 1.0(0.6 – 1.6) | 0.990 | 0.99(0.66 – 1.48) | 0.955 | 1.1(0.8 – 1.6) | 0.641 |
| Highest | 1.3(0.8 – 2.1) | 0.357 | 1.2(0.8 – 1.8) | 0.505 | 1.2(0.8 – 1.9) | 0.294 |
| Anthranilic acid | 0.872 | 0.092 | 0.276 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 0.97(0.63 – 1.50) | 0.893 | 0.81(0.57 – 1.15) | 0.242 | 0.94(0.68 – 1.29) | 0.688 |
| 3rd | 0.94(0.58 – 1.51) | 0.789 | 0.69(0.47 – 1.02) | 0.061 | 0.81(0.57 – 1.16) | 0.250 |
| Highest | 1.1(0.7 – 1.8) | 0.692 | 0.74(0.50 – 1.11) | 0.143 | 0.85(0.59 – 1.23) | 0.402 |
| Kynurenic acid | 0.574 | 0.530 | 0.495 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 0.76(0.47 – 1.22) | 0.253 | 0.87(0.59 – 1.30) | 0.506 | 0.93(0.64 – 1.33) | 0.679 |
| 3rd | 0.65(0.37 – 1.15) | 0.142 | 0.80(0.50 – 1.27) | 0.343 | 0.84(0.55 – 1.28) | 0.420 |
| Highest | 1.2(0.7 – 2.3) | 0.483 | 1.2(0.7 – 1.9) | 0.585 | 1.2(0.7 – 1.9) | 0.554 |
| Kynurenine | 0.917 | 0.803 | 0.707 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 0.87(0.54 – 1.42) | 0.584 | 0.94(0.64 – 1.40) | 0.763 | 0.84(0.59 – 1.20) | 0.335 |
| 3rd | 1.1(0.7 – 1.8) | 0.763 | 0.95(0.63 – 1.45) | 0.824 | 0.90(0.61 – 1.32) | 0.576 |
| Highest | 0.88(0.48 – 1.64) | 0.694 | 0.94(0.57 – 1.55) | 0.822 | 0.91(0.57 – 1.45) | 0.681 |
| Quinolinic acid | 0.690 | 0.860 | 0.845 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 0.82(0.51 – 1.31) | 0.400 | 0.87(0.59 – 1.29) | 0.492 | 0.88(0.61 – 1.26) | 0.492 |
| 3rd | 0.99(0.60 – 1.62) | 0.952 | 0.99(0.65 – 1.51) | 0.973 | 1.0(0.7 – 1.5) | 0.831 |
| Highest | 0.84(0.47 – 1.50) | 0.552 | 0.89(0.54 – 1.47) | 0.645 | 0.90(0.57 – 1.43) | 0.664 |
| Tryptophan | 0.704 | 0.242 | 0.761 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 1.1(0.7 – 1.7) | 0.621 | 1.0(0.7 – 1.5) | 0.904 | 0.97(0.69 – 1.37) | 0.858 |
| 3rd | 0.96(0.60 – 1.53) | 0.847 | 1.3(0.9 – 1.9) | 0.232 | 1.2(0.8 – 1.7) | 0.339 |
| Highest | 0.97(0.58 – 1.65) | 0.922 | 1.2(0.8 – 1.9) | 0.360 | 1.0(0.7 – 1.5) | 0.991 |
| Xanthurenic acid | 0.699 | 0.488 | 0.641 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 1.1(0.7 – 1.7) | 0.781 | 1.1(0.8 – 1.7) | 0.517 | 1.0(0.7 – 1.5) | 0.791 |
| 3rd | 1.0(0.6 – 1.8) | 0.911 | 1.1(0.7 – 1.7) | 0.787 | 1.1(0.7 – 1.7) | 0.670 |
| Highest | 0.86(0.45 – 1.62) | 0.635 | 0.86(0.50 – 1.48) | 0.591 | 0.90(0.55 – 1.47) | 0.667 |
RR = 1.0 is the reference category.
p values of trend of metabolites in italics
Benjamini-Hochberg correction with false discovery rate at 0.20 and n=132 was applied. No associations were significant after correction.
Adjusted for maternal age, ethnicity, maternal history of allergy, antibiotic used and infant sex
As inclusion of all metabolites in the same model reduced statistical power, we conducted further analysis by conducting multivariate analysis for each metabolite which was significant in univariate analyses. In multivariate analysis, the highest quartile of nicotinamide remained associated with increased risks of eczema development by ages 6 and 12 months (AdjRR=2.6, 95% CI=1.2-5.7, p=0.013 and AdjRR=2.3, 95% CI=1.2-4.3, p=0.011, respectively) as compared to the lowest quartile. There was a trend of increasing risks of eczema development by ages 6 and 12 months with increasing quartiles of nicotinamide (p-trend=0.008 and 0.016, respectively, Supplementary Table 1 and 2). There were no associations between 3-hydroxyanthranilic acid, 3-hydroxykynurenine and tryptophan with eczema in the first 18 months of life after adjusting for confounders.
The highest quartile of nicotinamide remained significantly associated with increased risks of itchy rash development by age 6 months (AdjRR=1.8, 95% CI=1.2-2.7, p=0.008) as compared to the lowest quartile. There was a trend of increasing risk of itchy rash development by ages 6 and 12 months with increasing quartiles of nicotinamide (p-trend=0.003 and 0.011, respectively, Supplementary Table 3 and 4). There were no associations between trigonelline, 3-hydroxykynurenine and kynurenic acid with rash in the first 18 months of life after adjusting for confounders.
There were no associations between N1-methylnicotinamide and trigonelline with wheeze in the first 18 months of life after adjusting for confounders (Supplementary Table 5 and 6). There were also no associations between nicotinamide and kynurenic acid with rhinitis in the first 18 months of life after adjusting for confounders (Supplementary Table 7 and 8).
The highest quartile of nicotinamide and 3-hydroxykynurenine remained significantly associated with increased risks of allergic sensitization development at age 18 months (AdjRR=2.0, 95% CI=1.1-3.6, p=0.027 and AdjRR=2.0, 95% CI=1.1-3.6, p=0.022 respectively) as compared to the lowest quartile. There was a trend of increasing risks of allergic sensitization development at age 18 months with increasing quartiles of nicotinamide and 3-hydrokynurenine (p-trend=0.043 and 0.008, respectively, Supplementary Table 9 and 10).
Differences in cord blood cytokines and chemokines between subjects with allergic conditions and controls
We next analysed differences in MCP-1, MIG, IL-6 and IFN-γ levels in offspring with allergic conditions and controls. MIG levels were higher in offspring with wheeze by 18 months as compared to controls [median (IQR) 14.29 (10.08-20.2) vs 12.30 (7.89-17.32) pg/ml, p=0.016, Supplementary Table 13]. Similarly, MIG levels were higher in offspring with rhinitis by 12 [median 13.51 (9.02-18.70) vs 12.01 (7.81-16.61) pg/ml, p=0.006, Supplementary Table 14] and 18 months [median 13.45 (9.01-18.80) vs 12.00 (7.86-16.15) pg/ml, p=0.002]. In addition, MCP-1 levels were higher in offspring with rhinitis by 12 [median 108.14 (73.31-162.46) vs 93.23 (65.54-131.0) pg/ml, p=0.007) and 18 months [median 104.76 (74.44-152.18) vs 92.35 (64.33-136.91) pg/ml, p=0.014]. There were no significant differences in cord blood levels of these cytokines and chemokines between offspring with eczema, itchy rash and allergic sensitization with controls (Supplementary Tables 11, 12 and 15).
Correlation analysis between nicotinamide and 3-hydroxykynurenine with diet during pregnancy
To examine if nicotinamide and 3-hydroxykynurenine were associated with the mother’s dietary, vitamin and supplement intake, we carried out correlation analyses between these metabolites and each of the 38 food groups recorded in the 24-hour dietary recall, vitamin and supplement intake. There were positive correlations between nicotinamide levels and healthy red meat (r=0.075, p=0.020), porridge (r=0.095, p=0.003) and supplements (r=0.097, p=0.004, Supplementary Table 16). Positive correlation between 3-hydroxykynurenine and legumes (r=0.092, p=0.004) was observed.
Discussion
In this study, we observed that highest quartile of maternal plasma levels of 3-hydroxykynurenine at 26-28 weeks pregnancy was associated with increased risk of allergic sensitization at age 18 months as compared to the lowest quartile. In addition, high maternal plasma nicotinamide at 26-28 weeks pregnancy was associated with increased risks of eczema development in the offspring by 6 and 12 months of life. Increasing quartiles of nicotinamide were associated with increased risks of eczema by 6 and 12 months as well as allergic sensitization at 18 months. No associations were observed between tryptophan metabolites and risks of wheeze and rhinitis development in the offspring.
These results suggest that 3-hydroxykynurenine and nicotinamide may influence the risk of early life allergic sensitization and eczema, possibly through Th2 immune differentiation. Conversely, most instances of wheeze and rhinitis in early life are recognized to be non-allergic and result from viral infections in early life 31,32; in the COAST cohort of high-risk children with a parental history of respiratory allergy, 90% of wheezing illness in the first 3 years of life were attributed to viral causes 33. The GUSTO cohort also reported higher viral detection from children within a month of a rhinitis episode 34. We also observed higher levels of MIG in the cord blood of offspring with wheeze and rhinitis compared to controls and cord blood MCP-1 levels were higher in offspring with rhinitis. These results suggest that MIG and MCP-1 may affect susceptibility to respiratory viral infections. Preterm infants with respiratory distress syndrome and/or chronic lung disease were reported to have higher cord blood MCP-1 levels as compared to controls 35. Higher MCP-1 and MIG levels were also associated with higher mortality in patients with severe acute respiratory syndrome 36.
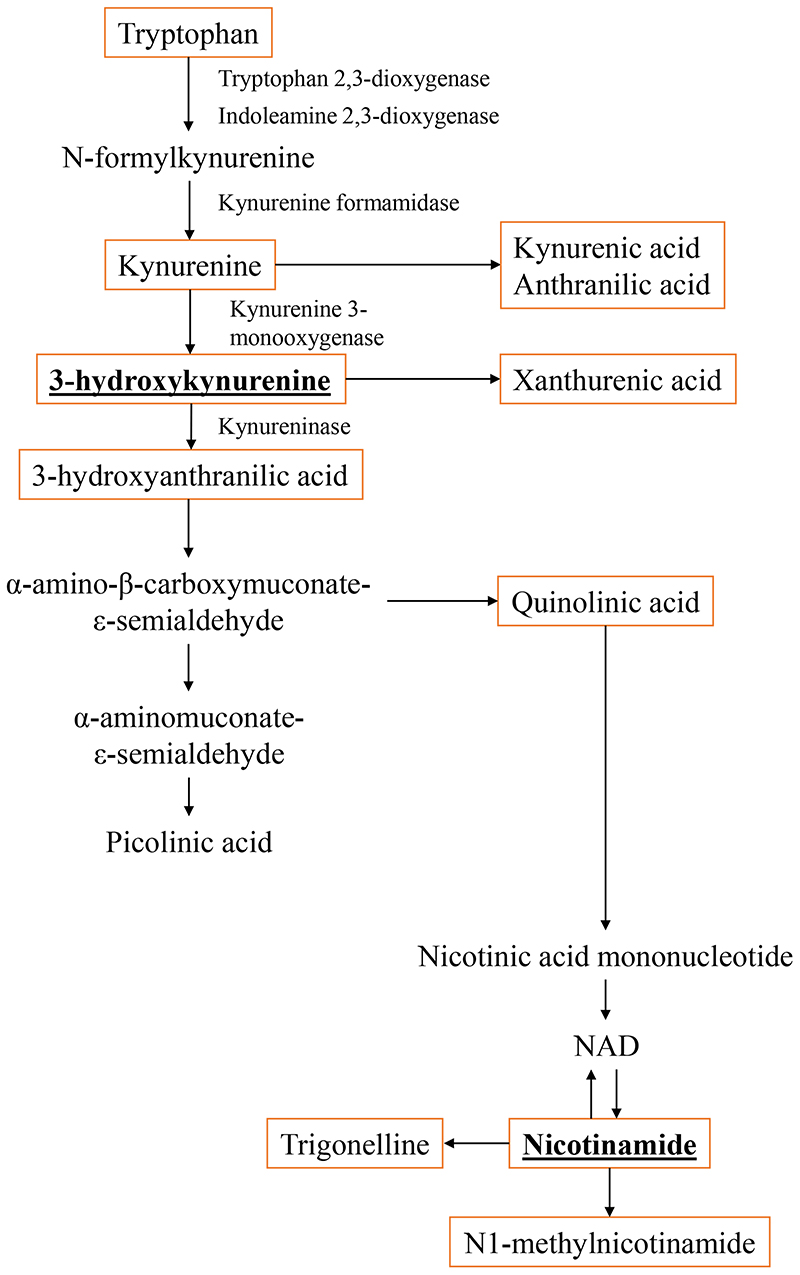
Tryptophan, a source of 3-hydrokynurenine and nicotinamide, is broken down by the enzymes tryptophan 2,3-dioxygenase in the liver and rate-limiting indolamine 2,3-dioxygenase-1 and -2 in other parts of the body to kynurenine derivatives (Figure 1) 13,37. Perturbation to the tryptophan-kynurenine pathway is linked to inflammation and immune activation 38. Tryptophan metabolites have been shown to preferentially induce Th1 cell apoptosis as compared to Th2 16. 3-hydroxykynurenine is a known generator of reactive oxidative species which can induce cell apoptosis 39. Excess reactive oxidative species production and decreased antioxidant response are linked to higher susceptibility to allergic sensitization through altering dendritic cell functions and inducing Th2 differentiation 40. 3-hydrokynurenine also reduced Th1 cytokine IFN-γ and promoted Th2 cytokine IL-4 and IL-13 production in stimulated invariant natural killer T cells 41.
Figure 1.
Metabolism of tryptophan via the kynurenine pathway. Metabolites highlighted in orange boxes were analysed in this study. High levels of maternal plasma 3-hydroxykynurenine and nicotinamide were associated with increased risks of allergic sensitization and eczema in infants.
In the tryptophan-kynurenine pathway, 3-hydrokynurenine is catabolised to 3-hydroxyanthranilic acid by kynureninase. We postulate that 3-hydrokynurenine may be an important point and bottleneck in the tryptophan metabolism pathway where high levels of 3-hydrokynurenine drives the generation of 3-hydroxyanthranilic acid which is quickly broken down to subsequent metabolites and nicotinamide.
Nicotinamide has been shown to reduce Th1 cytokines such as IL-12, hence promoting a Th2 immune response 42. While there are studies suggesting that topical nicotinamide application may protect against eczema through reduced transepidermal water loss 43, anti-inflammatory and antioxidant actions 44, effects of systemic nicotinamide on eczema development remain non-conclusive. Nicotinamide deficiency is commonly associated with pellagra, characterized by an eczema skin reaction 45, while data from the Nurses’ Health Study 2 suggested that women in the highest quartile of total and supplemental nicotinamide consumption had higher risks of eczema 24. Hence we postulate that the relationship between nicotinamide levels and allergic outcomes may be non-linear and follow a U-shaped curve. While small increases in nicotinamide levels may protect against eczema development and allergic sensitization, deficiency or excessive nicotinamide may exhibit an opposite effect. In our study, we observed that the highest quartile of maternal plasma nicotinamide was linked to higher risk of eczema development in early life, opposite to that reported by the UK Southampton Women Survey; this could be due to the higher plasma nicotinamide levels in the GUSTO study compared to the UK study 25 (median 184.0 nmol/l versus 140.2 nmol/l). This may also indicate dietary or microbiome differences or a greater dysregulation of the tryptophan metabolism pathway, resulting in higher levels of nicotinamide.
Besides Th2 immune responses (“inside out” hypothesis), skin barrier dysfunction has recently been proposed to lead to eczema development and allergic sensitization (“outside in” hypothesis). Filaggrin gene mutations may lead to compromised skin epithelium that results in eczema and allergic sensitization and subsequently result in the induction of Th2 responses 46,47 However, this may only explain the occurrence of eczema in a subset of patients as Cai et al. detected filaggrin gene mutations in 25% of Singaporean Chinese with eczema 48. Filaggrin mutations were also not associated with allergic sensitization at age 4 years in the PIAMA cohort 49. Hence it is likely that immune dysregulation plays a greater role in the pathogenesis of eczema and allergic sensitization.
A main source of nicotinamide in diet is meat, which is supported by the correlation between nicotinamide and red meat in this study. Evidence from other studies have demonstrated links between maternal consumption of meat and eczema development in offspring; for example, the Osaka Maternal and Child Health Study showed that higher maternal consumption of meat was associated with higher risk of eczema in infants aged 3 – 4 months 50. Nicotinamide can also be obtained from supplements and we too observed a positive correlation between nicotinamide and use of supplement in our study. The positive association between 3-hydroxykynurenine and legumes is likely because legumes contain high amounts of tryptophan which is subsequently broken down to 3-hydroxykynurenine 51.
Strengths of the study include the objective assessment of maternal plasma metabolites and cord blood cytokines and chemokines profile by laboratory analysis. In addition, there was regular follow up of the offspring with interviewer administered questionnaires at multiple timepoints in early life. A limitation of the study is that the maternal metabolite and cord blood cytokine measurements were only undertaken at single time points and that a limited panel is tested. Hence, further investigation into the role of 3-hydroxykynurenine and nicotinamide in offspring eczema development and allergic sensitization is needed. A further limitation is that the dietary information obtained by 24-hour recall may not be representative of the general diet of the mothers during pregnancy; multiple exploratory analyses of the dietary data were undertaken and the weak associations found need to be interpreted with caution.
Conclusion
In this study, we demonstrated that high maternal plasma concentrations of nicotinamide in late gestation were associated with a higher risk of eczema development and allergic sensitization in infants in early life. Our results highlighted the importance of the tryptophan metabolism pathway on infant eczema development and allergic sensitization.
Supplementary Material
Key messages.
High maternal late pregnancy plasma 3-hydroxykynurenine and nicotinamide concentrations were associated with offspring allergic sensitization.
High maternal late pregnancy plasma nicotinamide concentrations were also associated with offspring infantile eczema.
Maternal plasma tryptophan metabolite concentrations were not associated with offspring rhinitis and wheeze.
Table 3. Multivariate associations between all maternal plasma metabolites and itchy rash by 6, 12 and 18 months in the offspring.
| 6 months (n=792) | 12 months (n=747) | 18 months (n=707) | ||||
|---|---|---|---|---|---|---|
| RR (95% CI) | p-value * | RR (95% CI) | p-value * | RR (95% CI) | p-value * | |
| N1-methylnicotinamide | 0.359 | 0.685 | 0.643 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 0.88(0.57 – 1.36) | 0.563 | 0.98(0.67 – 1.45) | 0.926 | 1.1(0.8 – 1.5) | 0.489 |
| 3rd | 0.81(0.51 – 1.28) | 0.369 | 0.92(0.62 – 1.38) | 0.684 | 0.93(0.66 – 1.30) | 0.660 |
| Highest | 0.79(0.48 – 1.30) | 0.358 | 0.92(0.59 – 1.42) | 0.693 | 0.94(0.65 – 1.36) | 0.757 |
| Nicotinamide | 0.002 | 0.011 | 0.042 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 1.1(0.7 – 1.8) | 0.605 | 0.94(0.63 – 1.43) | 0.782 | 1.0(0.7 – 1.4) | 0.985 |
| 3rd | 1.5(0.9 – 2.4) | 0.102 | 1.3(0.9 – 1.9) | 0.199 | 1.1(0.8 – 1.5) | 0.567 |
| Highest | 2.0(1.2 – 3.2) | 0.006 | 1.6(1.0 – 2.4) | 0.037 | 1.4(1.0 – 1.9) | 0.074 |
| Trigonelline | 0.433 | 0.684 | 0.587 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 1.1(0.7 – 1.6) | 0.725 | 1.2(0.8 – 1.7) | 0.373 | 1.1(0.8 – 1.5) | 0.627 |
| 3rd | 1.0(0.7 – 1.5) | 0.982 | 1.1(0.7 – 1.5) | 0.784 | 1.1(0.8 – 1.5) | 0.416 |
| Highest | 0.82(0.51 – 1.32) | 0.414 | 0.94(0.62 – 1.43) | 0.774 | 1.1(0.8 – 1.5) | 0.577 |
| 3-Hydroxyanthranilic acid | 0.352 | 0.768 | 0.540 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 0.79(0.50 – 1.24) | 0.299 | 0.87(0.59 – 1.29) | 0.490 | 0.94(0.68 – 1.29) | 0.703 |
| 3rd | 0.93(0.59 – 1.47) | 0.761 | 0.99(0.66 – 1.49) | 0.973 | 0.98(0.70 – 1.37) | 0.913 |
| Highest | 0.72(0.43 – 1.23) | 0.229 | 0.89(0.56 – 1.41) | 0.606 | 0.89(0.61 – 1.30) | 0.540 |
| 3-Hydroxykynurenine | 0.044 | 0.147 | 0.752 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 1.4(0.9 – 2.2) | 0.193 | 1.2(0.8 – 1.8) | 0.308 | 1.1(0.8 – 1.5) | 0.641 |
| 3rd | 1.8(1.1 – 2.8) | 0.017 | 1.5(1.0 – 2.2) | 0.072 | 1.2(0.8 – 1.7) | 0.330 |
| Highest | 1.6(1.0 – 2.7) | 0.071 | 1.4(0.9 – 2.2) | 0.190 | 1.1(0.8 – 1.6) | 0.614 |
| Anthranilic acid | 0.780 | 0.738 | 0.395 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 0.86(0.55 – 1.32) | 0.484 | 0.85(0.58 – 1.25) | 0.407 | 0.76(0.55 – 1.04) | 0.085 |
| 3rd | 0.79(0.50 – 1.27) | 0.333 | 0.76(0.50 – 1.14) | 0.182 | 0.84(0.61 – 1.16) | 0.302 |
| Highest | 0.95(0.59 – 1.52) | 0.818 | 0.96(0.64 – 1.45) | 0.849 | 0.85(0.60 – 1.19) | 0.334 |
| Kynurenic acid | 0.413 | 0.844 | 0.851 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 1.1(0.7 – 1.8) | 0.670 | 1.0(0.7 – 1.6) | 0.849 | 0.86(0.61 – 1.22) | 0.395 |
| 3rd | 1.4(0.8 – 2.5) | 0.209 | 1.2(0.7 – 1.9) | 0.487 | 0.97(0.66 – 1.42) | 0.861 |
| Highest | 1.4(0.7 – 2.6) | 0.336 | 1.1(0.6 – 1.9) | 0.728 | 0.99(0.63 – 1.55) | 0.965 |
| Kynurenine | 0.435 | 0.438 | 0.562 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 0.92(0.58 – 1.45) | 0.711 | 0.95(0.64 – 1.42) | 0.803 | 0.86(0.62 – 1.19) | 0.361 |
| 3rd | 0.99(0.60 – 1.62) | 0.951 | 1.0(0.7 – 1.6) | 0.921 | 0.87(0.61 – 1.26) | 0.466 |
| Highest | 0.75(0.41 – 1.36) | 0.346 | 0.78(0.46 – 1.32) | 0.348 | 0.83(0.54 – 1.28) | 0.403 |
| Quinolinic acid | 0.292 | 0.536 | 0.339 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 0.93(0.58 – 1.48) | 0.755 | 0.83(0.55 – 1.25) | 0.368 | 0.89(0.64 – 1.25) | 0.509 |
| 3rd | 1.1(0.7 – 1.9) | 0.682 | 0.97(0.62 – 1.52) | 0.908 | 1.2(0.9 – 1.8) | 0.269 |
| Highest | 1.3(0.7 – 2.2) | 0.435 | 1.1(0.7 – 1.8) | 0.743 | 1.1(0.7 – 1.7) | 0.649 |
| Tryptophan | 0.208 | 0.363 | 0.921 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 1.0(0.6 – 1.5) | 0.983 | 1.0(0.7 – 1.5) | 0.992 | 1.2(0.9 – 1.7) | 0.241 |
| 3rd | 0.84(0.53 – 1.36) | 0.480 | 0.94(0.62 – 1.42) | 0.758 | 1.1(0.8 – 1.6) | 0.571 |
| Highest | 0.74(0.45 – 1.22) | 0.238 | 0.82(0.53 – 1.28) | 0.381 | 1.1(0.7 – 1.5) | 0.695 |
| Xanthurenic acid | 0.776 | 0.881 | 0.709 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 0.97(0.61 – 1.55) | 0.896 | 0.93(0.62 – 1.40) | 0.735 | 1.1(0.8 – 1.5) | 0.609 |
| 3rd | 0.83(0.49 – 1.42) | 0.500 | 0.88(0.55 – 1.40) | 0.578 | 1.0(0.7 – 1.5) | 0.989 |
| Highest | 0.91(0.49 – 1.68) | 0.759 | 0.95(0.55 – 1.64) | 0.849 | 1.1(0.7 – 1.7) | 0.762 |
RR = 1.0 is the reference category.
p values of trend of metabolites in italics
Benjamini-Hochberg correction with false discovery rate at 0.20 and n=132 was applied. No associations were significant after correction.
Adjusted for ethnicity, maternal history of allergy, parity and type of milk feed
Table 4. Multivariate associations between all maternal plasma metabolites and wheeze by 6, 12 and 18 months in the offspring.
| 6 months (n=694) | 12 months (n=667) | 18 months (n=646) | ||||
|---|---|---|---|---|---|---|
| RR (95% CI) | p-value * | RR (95% CI) | p-value * | RR (95% CI) | p-value * | |
| N1-methylnicotinamide | 0.748 | 0.688 | 0.952 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 0.37(0.08 – 1.78) | 0.215 | 0.81(0.35 – 1.87) | 0.627 | 0.92(0.46 – 1.84) | 0.804 |
| 3rd | 0.22(0.04 – 1.23) | 0.085 | 0.60(0.25 – 1.41) | 0.241 | 0.93(0.46 – 1.88) | 0.839 |
| Highest | 0.88(0.18 – 4.27) | 0.870 | 0.89(0.35 – 2.29) | 0.813 | 0.94(0.42 – 2.11) | 0.884 |
| Nicotinamide | 0.783 | 0.459 | 0.398 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 1.3(0.3 – 5.2) | 0.748 | 0.70(0.33 – 1.53) | 0.373 | 0.69(0.35 – 1.35) | 0.279 |
| 3rd | 0.48(0.09 – 2.66) | 0.400 | 0.86(0.40 – 1.84) | 0.691 | 0.68(0.35 – 1.33) | 0.263 |
| Highest | 0.69(0.12 – 3.86) | 0.670 | 0.69(0.28 – 1.68) | 0.412 | 0.74(0.36 – 1.52) | 0.411 |
| Trigonelline | 0.161 | 0.417 | 0.251 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 1.9(0.5 – 7.8) | 0.350 | 0.91(0.43 – 1.93) | 0.803 | 0.80(0.43 – 1.49) | 0.482 |
| 3rd | 1.0(0.2 – 4.4) | 0.965 | 0.90(0.41 – 1.96) | 0.793 | 0.79(0.41 – 1.52) | 0.482 |
| Highest | 0.13(0.01 – 1.67) | 0.118 | 0.53(0.20 – 1.39) | 0.197 | 0.55(0.25 – 1.20) | 0.131 |
| 3-Hydroxyanthranilic acid | 0.799 | 0.533 | 0.612 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 1.5(0.3 – 7.7) | 0.608 | 1.8(0.8 – 4.2) | 0.162 | 1.7(0.8 – 3.5) | 0.132 |
| 3rd | 0.56(0.08 – 4.14) | 0.572 | 1.1(0.4 – 2.9) | 0.904 | 0.83(0.36 – 1.92) | 0.663 |
| Highest | 2.6(0.4 – 16.6) | 0.323 | 2.0(0.7 – 5.7) | 0.212 | 1.7(0.7 – 4.0) | 0.229 |
| 3-Hydroxykynurenine | 0.365 | 0.369 | 0.091 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 1.4(0.3 – 7.3) | 0.705 | 0.88(0.36 – 2.14) | 0.783 | 1.1(0.5 – 2.2) | 0.891 |
| 3rd | 1.1(0.2 – 6.9) | 0.938 | 1.6(0.7 – 3.8) | 0.254 | 1.5(0.7 – 3.2) | 0.243 |
| Highest | 2.1(0.4 – 11.7) | 0.409 | 1.4(0.5 – 3.8) | 0.467 | 1.9(0.9 – 4.2) | 0.113 |
| Anthranilic acid | 0.616 | 0.781 | 0.778 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 1.1(0.3 – 4.7) | 0.857 | 0.88(0.39 – 1.98) | 0.755 | 0.81(0.39 – 1.66) | 0.562 |
| 3rd | 0.43(0.07 – 2.79) | 0.378 | 1.0(0.4 – 2.4) | 0.991 | 1.2(0.6 – 2.5) | 0.556 |
| Highest | 0.57(0.11 – 3.00) | 0.503 | 1.0(0.4 – 2.5) | 0.924 | 0.97(0.46 – 2.06) | 0.932 |
| Kynurenic acid | 0.530 | 0.864 | 0.866 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 0.43(0.06 – 2.96) | 0.388 | 0.63(0.26 – 1.55) | 0.317 | 0.68(0.32 – 1.45) | 0.314 |
| 3rd | 0.52(0.07 – 4.19) | 0.542 | 0.53(0.19 – 1.44) | 0.211 | 0.60(0.27 – 1.34) | 0.212 |
| Highest | 1.2(0.2 – 9.9) | 0.841 | 1.2(0.4 – 3.2) | 0.781 | 0.91(0.38 – 2.19) | 0.826 |
| Kynurenine | 0.883 | 0.179 | 0.403 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 0.83(0.14 – 4.87) | 0.840 | 0.53(0.22 – 1.29) | 0.161 | 0.75(0.36 – 1.55) | 0.432 |
| 3rd | 0.95(0.16 – 5.64) | 0.956 | 0.51(0.21 – 1.28) | 0.154 | 0.63(0.29 – 1.37) | 0.244 |
| Highest | 1.3(0.1 – 11.4) | 0.824 | 0.38(0.12 – 1.16) | 0.089 | 0.66(0.26 – 1.65) | 0.372 |
| Quinolinic acid | 0.809 | 0.890 | 0.481 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 0.37(0.06 – 2.40) | 0.296 | 0.51(0.20 – 1.31) | 0.163 | 0.75(0.36 – 1.56) | 0.439 |
| 3rd | 0.87(0.16 – 4.79) | 0.872 | 0.99(0.40 – 2.44) | 0.986 | 0.98(0.46 – 2.11) | 0.963 |
| Highest | 0.44(0.06 – 3.46) | 0.436 | 0.84(0.28 – 2.51) | 0.753 | 0.69(0.27 – 1.77) | 0.443 |
| Tryptophan | 0.349 | 0.666 | 0.154 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 1.1(0.2 – 6.2) | 0.891 | 1.5(0.7 – 3.3) | 0.340 | 1.3(0.6 – 2.6) | 0.528 |
| 3rd | 3.1(0.6 – 16.0) | 0.181 | 1.5(0.6 – 3.7) | 0.350 | 1.5(0.7 – 3.3) | 0.281 |
| Highest | 2.0(0.3 – 12.5) | 0.473 | 1.4(0.5 – 3.8) | 0.532 | 2.0(0.9 – 4.5) | 0.103 |
| Xanthurenic acid | 0.779 | 0.886 | 0.904 | |||
| Lowest | 1.0 | 1.0 | 1.0 | |||
| 2nd | 4.4(0.6 – 31.1) | 0.142 | 1.8(0.8 – 4.4) | 0.182 | 1.7(0.8 – 3.8) | 0.158 |
| 3rd | 4.0(0.5 – 32.2) | 0.197 | 2.1(0.8 – 5.4) | 0.146 | 2.0(0.8 – 4.7) | 0.117 |
| Highest | 0.98(0.07 – 13.48) | 0.985 | 0.90(0.27 – 3.03) | 0.870 | 1.0(0.4 – 2.9) | 0.934 |
RR = 1.0 is the reference category.
p values of trend of metabolites in italics
Benjamini-Hochberg correction with false discovery rate at 0.20 and n=132 was applied. No associations were significant after correction.
Adjusted for maternal age, ethnicity, antibiotic used and infant sex
Acknowledgements
We thank the GUSTO study group and all clinical and home-visit staff involved. The voluntary participation of all subjects is greatly appreciated. We thank Dr Anis Larbi for his help in cord blood cytokine analysis. The GUSTO study group includes Allan Sheppard, Amutha Chinnadurai, Anne Eng Neo Goh, Anne Rifkin-Graboi, Anqi Qiu, Arijit Biswas, Bee Wah Lee, Birit Froukje Philipp Broekman, Boon Long Quah, Chai Kiat Chng, Cheryl Shufen Ngo, Choon Looi Bong, Christiani Jeyakumar Henry, Daniel Yam Thiam Goh, Doris Ngiuk Lan Loh, Fabian Kok Peng Yap, George Seow Heong Yeo, Helen Yu Chen, Hugo P S van Bever, Iliana Magiati, Inez Bik Yun Wong, Ivy Yee-Man Lau, Jeevesh Kapur, Jenny L. Richmond, Jerry Kok Yen Chan, Joanna Dawn Holbrook, Joshua J. Gooley, Keith M. Godfrey, Kenneth Yung Chiang Kwek, Kok Hian Tan, Krishnamoorthy Naiduvaje, Leher Singh, Lin Lin Su, Lourdes Mary Daniel, Lynette Pei-Chi Shek, Marielle V. Fortier, Mark Hanson, Mary Foong-Fong Chong, Mary Rauff, Mei Chien Chua, Michael J. Meaney, Mya Thway Tint, Neerja Karnani, Ngee Lek, Oon Hoe Teoh, P. C. Wong, Peter David Gluckman, Pratibha Keshav Agarwal, Rob Martinus van Dam, Salome A. Rebello, Seang Mei Saw, Shang Chee Chong, Shirong Cai, Shu-E Soh, Sok Bee Lim, Stephen Chin-Ying Hsu, Victor Samuel Rajadurai, Walter Stunkel, Wee Meng Han, Wei Wei Pang, Yap Seng Chong, Yin Bun Cheung, Yiong Huak Chan and Yung Seng Lee.
Funding
This research is supported by the Singapore National Research Foundation under its Translational and Clinical Research (TCR) Flagship Program and administered by the Singapore Ministry of Health’s National Medical Research Council (NMRC), Singapore— NMRC/TCR/004-NUS/2008; NMRC/TCR/012-NUHS/ 2014. Additional funding is provided by the Singapore Institute for Clinical Sciences, Agency for Science Technology and Research (A*STAR), Singapore. K.M Godfrey is supported by the National Institute for Health Research through the NIHR Southampton Biomedical Research Centre and by the European Union’s Seventh Framework Program (FP7/2007–2013), projects EarlyNutrition and ODIN under grant agreement numbers 289346 and 613977.
Abbreviations
- GUSTO
Growing Up in Singapore Towards healthy Outcomes
- IFN-γ
Interferon gamma
- IL-6
Interleukin 6
- MCP-1
Monocyte chemoattractant protein-1
- MIG
Monokine induced by gamma interferon
- Th
T-helper
Footnotes
Conflicts of interest: Godfrey KM has received reimbursement for speaking at conferences sponsored by Nestle and Shek LP has received reimbursement for speaking at conferences sponsored by Danone and Nestle and consulting for Mead Johnson and Nestle. Godfrey KM, Chong YS and Karnani N are part of an academic consortium that has received research funding from Abbot Nutrition, Nestle and Danone. Shek LP has received research funding from Danone.
Author Contributions
Lau HX and Yap QV analysed the data and wrote the manuscript. Chan YH provided statistical advice and intellectual input. El-Heis S and Tan CPT provided intellectual input and wrote the manuscript. Karnani N, Tan KML, Tham EH, Goh AEN, Teoh OH, Tan KH, Eriksson JG, Chong YS, Chong MF-F, Van Bever H, Lee BW, Shek LP contributed to the study design and provided intellectual input. Loo EXL and Godfrey KM conceptualized the study design, contributed to the analysis and wrote the manuscript. All authors critically reviewed the manuscript.
Contributor Information
Hui Xing Lau, Email: lau_hui_xing@sics.a-star.edu.sg.
Sarah El Heis, Email: se@mrc.soton.ac.uk.
Qai Ven Yap, Email: qaiven@nus.edu.sg.
Yiong Huak Chan, Email: medcyh@nus.edu.sg.
Cheryl Pei Ting Tan, Email: paetptc@nus.edu.sg.
Neerja Karnani, Email: neerja_karnani@sics.a-star.edu.sg.
Karen Mei Ling Tan, Email: karen_tan@sics.a-star.edu.sg.
Elizabeth Huiwen Tham, Email: elizabeth_tham@nuhs.edu.sg.
Anne Eng Neo Goh, Email: anne.goh.e.n@singhealth.com.sg.
Oon Hoe Teoh, Email: teoh.oon.hoe@singhealth.com.sg.
Kok Hian Tan, Email: tan.kok.hian@singhealth.com.sg.
Johan Gunnar Eriksson, Email: obgjge@nus.edu.sg.
Yap Seng Chong, Email: obgcys@nus.edu.sg.
Mary Foong-Fong Chong, Email: ephmcff@nus.edu.sg.
Hugo Van Bever, Email: hugo_van_bever@nuhs.edu.sg.
Bee Wah Lee, Email: paeleebw@nus.edu.sg.
Lynette P Shek, Email: lynette_pc_shek@nuhs.edu.sg.
Keith M Godfrey, Email: kmg@mrc.soton.ac.uk.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1.Chad Z. Allergies in children. Paediatr Child Health. 2001;6(8):555–566. doi: 10.1093/pch/6.8.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan TN, Lim DLC, Chong YS, Lee BW, van Bever HPS. Prevalence of eczema symptoms in the second year of life. J Allergy Clin Immunol. 2004;113(2):S295 [Google Scholar]
- 3.Waterland RA, Michels KB. Epigenetic Epidemiology of the Developmental Origins Hypothesis. Annu Rev Nutr. 2007;27(1):363–388. doi: 10.1146/annurev.nutr.27.061406.093705. [DOI] [PubMed] [Google Scholar]
- 4.El-Heis S, Crozier SR, Healy E, et al. Maternal stress and psychological distress preconception: association with offspring atopic eczema at age 12 months. Clin Exp Allergy. 2017;47(6):760–769. doi: 10.1111/cea.12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raherison C, Pénard-Morand C, Moreau D, et al. In utero and childhood exposure to parental tobacco smoke, and allergies in schoolchildren. Respir Med. 2007;101(1):107–117. doi: 10.1016/j.rmed.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Loo EXL, Ong L, Goh A, et al. Effect of Maternal Dietary Patterns during Pregnancy on Self-Reported Allergic Diseases in the First 3 Years of Life: Results from the GUSTO Study. Int Arch Allergy Immunol. 2017;173(2):105–113. doi: 10.1159/000475497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leermakers ETM, Sonnenschein-van der Voort AMM, Heppe DHM, et al. Maternal fish consumption during pregnancy and risks of wheezing and eczema in childhood: The Generation R Study. Eur J Clin Nutr. 2013;67(4):353–359. doi: 10.1038/ejcn.2013.36. [DOI] [PubMed] [Google Scholar]
- 8.Macpherson AJ, de Agüero MG, Ganal-Vonarburg SC. How nutrition and the maternal microbiota shape the neonatal immune system. Nat Rev Immunol. 2017;17(8):508–517. doi: 10.1038/nri.2017.58. [DOI] [PubMed] [Google Scholar]
- 9.Lisa Study Group. Maternal diet during pregnancy in relation to eczema and allergic sensitization in the offspring at 2 y of age. Am J Clin Nutr. 2007;85(2):530–537. doi: 10.1093/ajcn/85.2.530. [DOI] [PubMed] [Google Scholar]
- 10.Sauve AA. NAD+ and Vitamin B3: From Metabolism to Therapies. J Pharmacol Exp Ther. 2008;324(3):883. doi: 10.1124/jpet.107.120758. [DOI] [PubMed] [Google Scholar]
- 11.Bellows L, Moore R. Fact sheet (Colorado State University Extension) Food and Nutrition. 2012. Water-soluble vitamins: B-complex and vitamin C. (9312). [Google Scholar]
- 12.Van der Leek AP, Yanishevsky Y, Kozyrskyj AL. The Kynurenine Pathway As a Novel Link between Allergy and the Gut Microbiome. Front Immunol. 2017;8:1374. doi: 10.3389/fimmu.2017.01374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukuwatari T, Shibata K. Nutritional Aspect of Tryptophan Metabolism. Int J Tryptophan Res. 2013:6s1:IJTR.S11588. doi: 10.4137/IJTR.S11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Floc’h N, Otten W, Merlot E. Tryptophan metabolism, from nutrition to potential therapeutic applications. Amino Acids. 2011;41(5):1195–1205. doi: 10.1007/s00726-010-0752-7. [DOI] [PubMed] [Google Scholar]
- 15.Badawy AAB. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int J Tryptophan Res. 2017;10:1178646917691938. doi: 10.1177/1178646917691938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fallarino F, Grohmann U, Vacca C, et al. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9(10):1069–1077. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- 17.Lee S-Y, Lee E, Park YM, Hong S-J. Microbiome in the Gut-Skin Axis in Atopic Dermatitis. Allergy Asthma Immunol Res. 2018;10(4):354–362. doi: 10.4168/aair.2018.10.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang ES, Song SB. Possible Adverse Effects of High-Dose Nicotinamide: Mechanisms and Safety Assessment. Biomolecules. 2020;10(5) doi: 10.3390/biom10050687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ming M, Zhao B, Shea CR, et al. Loss of sirtuin 1 (SIRT1) disrupts skin barrier integrity and sensitizes mice to epicutaneous allergen challenge. J Allergy Clin Immunol. 2015;135(4):936–945.:e934. doi: 10.1016/j.jaci.2014.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu E, Papandreou C, Ruiz-Canela M, et al. Association of Tryptophan Metabolites with Incident Type 2 Diabetes in the PREDIMED Trial: A Case–Cohort Study. Clin Chem. 2018;64(8):1211–1220. doi: 10.1373/clinchem.2018.288720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rebnord EW, Strand E, Midttun Ø, et al. The kynurenine:tryptophan ratio as a predictor of incident type 2 diabetes mellitus in individuals with coronary artery disease. Diabetologia. 2017;60(9):1712–1721. doi: 10.1007/s00125-017-4329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murr C, Grammer TB, Kleber ME, Meinitzer A, März W, Fuchs D. Low serum tryptophan predicts higher mortality in cardiovascular disease. Eur J Clin Invest. 2015;45(3):247–254. doi: 10.1111/eci.12402. [DOI] [PubMed] [Google Scholar]
- 23.Sulo G, Vollset SE, Nygård O, et al. Neopterin and kynurenine;tryptophan ratio as predictors of coronary events in older adults, the Hordaland Health Study. Int J Cardiol. 2013;168(2):1435–1440. doi: 10.1016/j.ijcard.2012.12.090. [DOI] [PubMed] [Google Scholar]
- 24.Drucker AM, Li W-Q, Park MK, Li T, Qureshi AA, Cho E. Niacin intake and incident adultonset atopic dermatitis in women. J Allergy Clin Immunol. 2017;139(6):2020–2022.:e2022. doi: 10.1016/j.jaci.2016.12.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Heis S, Crozier SR, Robinson SM, et al. Higher maternal serum concentrations of nicotinamide and related metabolites in late pregnancy are associated with a lower risk of offspring atopic eczema at age 12 months. Clin Exp Allergy. 2016;46(10):1337–1343. doi: 10.1111/cea.12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte Chemoattractant Protein-1 (MCP-1): An Overview. J Interferon Cytokine Res. 2009;29(6):313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berthoud TK, Dunachie SJ, Todryk S, Hill AVS, Fletcher HA. MIG (CXCL9) is a more sensitive measure than IFN-γ of vaccine induced T-cell responses in volunteers receiving investigated malaria vaccines. J Immunol Methods. 2009;340(1):33–41. doi: 10.1016/j.jim.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soh S-E, Tint MT, Gluckman PD, et al. Cohort Profile: Growing Up in Singapore Towards healthy Outcomes (GUSTO) birth cohort study. Int J Epidemiol. 2014;43(5):1401–1409. doi: 10.1093/ije/dyt125. [DOI] [PubMed] [Google Scholar]
- 29.Yi F, Chew F, Jimenez S, Chua K, Lee B. Culture of Biomia tropicalis and IgE Immunoblot Characterization of Its Allergenicity. Asian Pac J Allergy Immunol. 1999;17(3):189. [PubMed] [Google Scholar]
- 30.Chia A-R, de Seymour JV, Colega M, et al. A vegetable, fruit, and white rice dietary pattern during pregnancy is associated with a lower risk of preterm birth and larger birth size in a multiethnic Asian cohort: the Growing Up in Singapore Towards healthy Outcomes (GUSTO) cohort study. Am J Clin Nutr. 2016;104(5):1416–1423. doi: 10.3945/ajcn.116.133892. [DOI] [PubMed] [Google Scholar]
- 31.Giavina-Bianchi P, Aun MV, Takejima P, Kalil J, Agondi RC. United airway disease: current perspectives. J Asthma Allergy. 2016;9:93–100. doi: 10.2147/JAA.S81541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rimmer J, Ruhno JW. 6: Rhinitis and asthma: united airway disease. Med J Aust. 2006;185(10):565–571. doi: 10.5694/j.1326-5377.2006.tb00693.x. [DOI] [PubMed] [Google Scholar]
- 33.Jackson DJ, Gangnon RE, Evans MD, et al. Wheezing Rhinovirus Illnesses in Early Life Predict Asthma Development in High-Risk Children. Am J Respir Crit Care Med. 2008;178(7):667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hardjojo A, Goh A, Shek LPC, et al. Rhinitis in the first 18 months of life: Exploring the role of respiratory viruses. Pediatr Allergy Immunol. 2015;26(1):25–33. doi: 10.1111/pai.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Otsubo Y, Hashimoto K, Kanbe T, Sumi M, Moriuchi H. Association of cord blood chemokines and other biomarkers with neonatal complications following intrauterine inflammation. PLOS ONE. 2017;12(5):e0175082. doi: 10.1371/journal.pone.0175082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang K-J, Su I-J, Theron M, et al. An interferon-γ-related cytokine storm in SARS patients. J Med Virol. 2005;75(2):185–194. doi: 10.1002/jmv.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harden JL, Lewis SM, Lish SR, et al. The tryptophan metabolism enzyme L-kynureninase is a novel inflammatory factor in psoriasis and other inflammatory diseases. J Allergy Clin Immunol. 2016;137(6):1830–1840. doi: 10.1016/j.jaci.2015.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y, Guillemin GJ. Kynurenine Pathway Metabolites in Humans: Disease and Healthy States. Int J Tryptophan Res. 2009;2:IJTR.S2097. doi: 10.4137/ijtr.s2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okuda S, Nishiyama N, Saito H, Katsuki H. 3-Hydroxykynurenine, an Endogenous Oxidative Stress Generator, Causes Neuronal Cell Death with Apoptotic Features and Region Selectivity. J Neurochem. 1998;70(1):299–307. doi: 10.1046/j.1471-4159.1998.70010299.x. [DOI] [PubMed] [Google Scholar]
- 40.van Rijt LS, Utsch L, Lutter R, van Ree R. Oxidative Stress: Promoter of Allergic Sensitization to Protease Allergens? Int J Mol Sci. 2017;18(6) doi: 10.3390/ijms18061112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Molano A, Illarionov PA, Besra GS, Putterman C, Porcelli SA. Modulation of invariant natural killer T cell cytokine responses by indoleamine 2,3 -dioxygenase. Immunol Lett. 2008;117(1):81–90. doi: 10.1016/j.imlet.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krętowski A, Myśliwiec J, Szelachowska M, Kinalski M, Kinalska I. Nicotinamide inhibits enhanced in vitro production of interleukin-12 and tumour necrosis factor-α in peripheral whole blood of people at high risk of developing Type 1 diabetes and people with newly diagnosed Type 1 diabetes. Diabetes Res Clin Pract. 2000;47(2):81–86. doi: 10.1016/s0168-8227(99)00122-9. [DOI] [PubMed] [Google Scholar]
- 43.Soma Y, Kashima M, Imaizumi A, Takahama H, Kawakami T, Mizoguchi M. Moisturizing effects of topical nicotinamide on atopic dry skin. Int J Dermatol. 2005;44(3):197–202. doi: 10.1111/j.1365-4632.2004.02375.x. [DOI] [PubMed] [Google Scholar]
- 44.Djokic-Gallagher J, Rosher P, Hart V, Walker J. Steroid-sparing effects and acceptability of a new skin gel containing the anti-inflammatory medicinal substance-nicotinamide. Clin Cosmet Investig Dermatol. 2019;12:545–552. doi: 10.2147/CCID.S210444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meyer-Ficca M, Kirkland JB. Niacin. AdvNutr. 2016;7(3):556–558. doi: 10.3945/an.115.011239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silverberg NB, Silverberg JI. Inside out or outside in: does atopic dermatitis disrupt barrier function or does disruption of barrier function trigger atopic dermatitis? Cutis. 2015;96(6):359–361. [PubMed] [Google Scholar]
- 47.van den Oord RAHM, Sheikh A. Filaggrin gene defects and risk of developing allergic sensitisation and allergic disorders: systematic review and meta-analysis. BMJ. 2009;339:b2433. doi: 10.1136/bmj.b2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cai SCS, Chen H, Koh WP, et al. Filaggrin mutations are associated with recurrent skin infection in Singaporean Chinese patients with atopic dermatitis. Br J Dermatol. 2012;166(1):200–203. doi: 10.1111/j.1365-2133.2011.10541.x. [DOI] [PubMed] [Google Scholar]
- 49.Schuttelaar MLA, Kerkhof M, Jonkman MF, et al. Filaggrin mutations in the onset of eczema, sensitization, asthma, hay fever and the interaction with cat exposure. Allergy. 2009;64(12):1758–1765. doi: 10.1111/j.1398-9995.2009.02080.x. [DOI] [PubMed] [Google Scholar]
- 50.Saito K, Yokoyama T, Miyake Y, et al. Maternal meat and fat consumption during pregnancy and suspected atopic eczema in Japanese infants aged 3–4 months: The Osaka Maternal and Child Health Study. Pediatr Allergy Immunol. 2010;21(1-Part-I):38–46. doi: 10.1111/j.1399-3038.2009.00897.x. [DOI] [PubMed] [Google Scholar]
- 51.Shimizu F, Ishii Y, Ogawa M, et al. Plasma Levels of Tryptophan Metabolites in Healthy Young and Old Men and Women, and Patients of Type 2 Diabetes Mellitus (T2DM) Obesity: Open Access. 2018;4:1–9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.