Summary
Background
A range of anti-modified protein antibodies (AMPAs) are associated with rheumatoid arthritis. We aimed to assess the relationship between AMPA profiles and radiographic progression in patients with new-onset rheumatoid arthritis.
Methods
In this cohort study, we obtained samples and data from the Scottish Early Rheumatoid Arthritis (SERA) inception cohort and biobank, which recruited patients with new-onset rheumatoid arthritis or undifferentiated arthritis who had at least one swollen joint from 20 hospitals across Scotland. AMPAs in plasma samples were measured by ELISAs at baseline. Paired radiographs of the hands and feet were taken at baseline and at 1 year and were scored with the Sharp-van der Heijde (SvH) method. We calculated differences in radiographic progression using estimated marginal mean changes between baseline and 1 year, with the baseline values of radiographic variables, rheumatoid factor, sex, age at recruitment, symptom duration, and Disease Activity Score 28 with C-reactive protein included as covariates.
Findings
Between March 1, 2011, and April, 30, 2015, 1073 patients were recruited to the SERA study. 362 patients with rheumatoid arthritis were included in our study and had their AMPA profiles determined. Patients were grouped into four main autoantibody profiles by reactivities to post-translational modifications: single positivity for anti-citrullinated peptide antibodies (ACPAs; 73 [20%]); double positivity for ACPAs and anti-acetylated peptide antibodies (AAPAs; 45 [12%]); triple positivity for ACPAs, AAPAs, and anti-carbamylated peptide antibodies (151 [42%]); and AMPA negativity (74 [20%]). 19 (5%) patients were in one of the minor autoantibody groups. Of the 233 patients with both antibody data and radiographs of sufficient quality, triple-positive patients had more radiographic progression between baseline and 12 months (estimated mean change in total SvH score 1·8, 95% CI 0·9–2·6, SE 0·4) than did single-positive patients (0·5, 0·1–1·0, 0·2; estimated mean difference in the total change in SvH score 1·2, 95% CI 0·1–2·4, SE 0·5). There was no difference in radiographic progression between single positive patients and AMPA negative patients (estimated mean change in total SvH score 0·7, 95% CI 0·1–1·4, SE 0·3; estimated mean difference in the total change in SvH score −0·2, 95% CI −1·1 to 0·7, SE 0·4).
Interpretation
This study suggests that the optimal prediction of future rates of radiographic progression in patients with rheumatoid arthritis will require an assessment of autoantibodies against multiple post-translationally modified proteins or peptides.
Funding
The EU FP7 HEALTH programme, the Scottish Translational Medicine Research Collaboration, and the Chief Scientist Office Scotland.
Introduction
Rheumatoid arthritis is a chronic, inflammatory polyarthritis that causes pain and disability, and reduces life expectancy, to the detriment of the patient and wider society.1 Autoantibodies, including rheumatoid factor and anti-citrullinated peptide antibodies (ACPAs), inform both diagnosis and prognosis in rheumatoid arthritis.2–5
Immune dysfunction in rheumatoid arthritis also leads to the generation of antibodies against proteins that have undergone other post-translational modifications, including carbamylation6,7 and acetylation.8,9 Multiple different anti-modified protein antibodies (AMPAs) can be present in individual patients with rheumatoid arthritis, although the role of these combinations in diagnosis and prognosis is uncertain.
Citrullination of self-proteins occurs in the lung, periodontal tissue, and joint via protein-arginine deiminase enzymes, which convert arginine residues to citrulline. Smoking, in combination with certain HLA-DR haplotypes, has been implicated in the development of ACPAs and the pathogenesis of rheumatoid arthritis.10 ACPAs often pre-date the onset of clinical disease, and the presence of autoantibodies can identify a pre-arthritic state.11 Furthermore, patients with rheumatoid arthritis and ACPAs are more likely to have erosive progression and joint damage than are seronegative patients.4,12
Homocitrullination (or carbamylation) occurs when cyanate ions react with lysine residues to form homocitrulline in a non-enzymatic reaction. Whether carbamylation has a physiological role is unclear, but urea is a source of cyanate ions and therefore carbamylation can occur more often in uraemic states. The presence of antibodies against carbamylated proteins in patients with rheumatoid arthritis has been associated with a worse prognosis, an increased risk of relapse, and increased radiographic progression compared with ACPA seronegativity.6
Acetylation is a complex post-translational modification that can occur irreversibly at the N-terminus of proteins but can also occur when the activated acetyl group from acetyl-coenzyme A is consumed at lysine residues. This modification can be affected by inflammatory and metabolic states, and it is potentially reversible. Lysine acetylation has been extensively studied at the genome-wide level, with the interplay of histone acetyltransferases and histone deacetylases being crucial in the epigenetic control of gene expression. Other roles of amino acid acetylation are less well defined.
Recent work has shown that mice can develop a broad, cross-reactive AMPA response when immunised with a peptide expressing only one post-translational modification.13 Immunisation with acetylated peptides generated autoantibodies against citrullinated, carbamylated, and acetylated peptides, suggesting that a broad AMPA response could be generated not only by breaking tolerance to citrullinated peptides, but also by breaking tolerance to acetylated peptides.
Different AMPAs might have distinct and complementary roles in the aetiopathogenesis of rheumatoid arthritis. Thus far, scarce data are available on the importance of the presence of AMPAs, either acting in isolation or in combination, against citrullinated, carbamylated, and acetylated peptides in patients with rheumatoid arthritis. In our study, we aimed to examine the effect of AMPA combinations on erosive, radiographic progression in patients with new-onset rheumatoid arthritis.
Methods
Study design and participants
In this cohort study, we obtained samples and data from the Scottish Early Rheumatoid Arthritis (SERA) inception cohort and biobank.14 The SERA study is a prospectively acquired, population-wide inception cohort of 1073 patients with newly diagnosed (median time from diagnosis to SERA referral 21 days [IQR 8–57]) rheumatoid arthritis or undifferentiated arthritis recruited from 20 hospitals across Scotland. Patients with at least one swollen joint were invited to participate. Patients were excluded if their joint swelling could have been explained by an alternative diagnosis (eg, psoriatic arthritis) or if they were infected with blood-borne viruses. All patients selected for our study had rheumatoid arthritis, had started methotrexate treatment within 6 weeks of their baseline visit, had attended an assessment appointment at month 12, and were enrolled in a sub-study to predict their response to methotrexate. The study was approved by the West of Scotland Research Ethics Committee 4 (reference 10/S0704/20) and all participants gave written informed consent.
Procedures
For the SERA study, detailed demographic, clinical, laboratory, and radiographic data, and biological material were collected.14 Testing techniques and intervals have been described elsewhere.14 Treatment was at the discretion of the treating rheumatologist and was recorded in a web-based portal by research nurses every 6 months. AMPAs were measured at baseline by use of an ELISA in plasma samples. Modified peptides were coated on microtitre plates as previously described7 and we followed the standard protocol for the Orgentec Diagnostika ELISA (Mainz, Germany). Briefly, we coated 0·5 μg/mL of either citrullinated (GRVYAT-Citrulline-SSAVR), carbamylated (GRVYAT-HCit-SSAVR), or acetylated (GRVYAT-Acetylated lysine-SSAVR and GRVYAT-Acetylated ornithine-SSAVR) biotinylated vimentin peptide onto the streptavidin pre-coated cavities of a standard microtitre plate (Thermo Scientific, Roskilde, Denmark) and blocked with phosphate-buffered saline (PBS) supplemented with 1% bovine serum albumin. Patient samples were diluted 1:100 in PBS containing 0·05% Tween 20 and 1% albumin, and incubated for 30 min. Unbound antibodies were washed out with PBS containing 0·05% Tween 20. Bound autoantibodies were detected with horseradish peroxidase-conjugated anti-human IgG (Dianova, Hamburg, Germany) and visualised with 3,3’,5,5’-tetramethylbenzidine as the substrate for the peroxidase. Optical density was measured with a standard microtitre plate reader (Tecan, Männedorf, Germany). The optimal ELISA cutoff values for antibody positivity were determined by comparing the mean antibody reactivity plus 3 SDs of positive and negative serum samples of 200 patients with rheumatoid arthritis (fulfilling 1987 classification criteria from the American College of Rheumatology for rheumatoid arthritis) with that from 121 outpatients with other rheumatic diseases (both patient groups were from the Specialized Practice Rheumatology and Clinical Immunology, Dresden, Germany) and 300 healthy controls (from the Transfusion Center of Johannes-Gutenberg University Mainz, Mainz, Germany). On the basis of these results for the carbamylated, acetylated lysine, and acetylated ornithine assays, a cutoff of more than 25 arbitrary units (AU) was used to define antibody positivity. Rheumatoid factor IgM, anti-modified citrullinated vimentin IgG, and anti-cyclic citrullinated peptide (CCP) IgG were detected by use of commercially available ELISA kits (Orgentec Diagnostika, Mainz, Germany) and a cutoff of 20 AU or more was used to define antibody positivity according to the manufacturer’s instructions.
Standard curves were established by use of patient serum from the Specialized Practice Rheumatology and Clinical Immunology, Dresden, Germany. The reference range was defined in 300 healthy volunteers as the mean antibody reactivity plus three SDs.
Paired radiographs of the hands and feet were taken at baseline and at 1 year. The x-ray images were scored by one assessor, blinded to the order of the radiographs, by use of the Sharp-van der Heijde (SvH) method (Imaging Rheumatology International). Scoring methods have been described elsewhere.15 Briefly, the total SvH score is the sum of the total erosion score and the total joint space narrowing score. Before scoring, as assessments of reliability, the inter-reader (between the reader who scored radiographs for this study and a second reader not involved in this study) intraclass correlation coefficient for status scores had been determined to be 0·97 and the smallest detectable change for change in scores was determined to be 1·75.
Outcomes
The primary outcome was the estimated mean change in SvH total score between baseline and 1 year. Secondary outcomes were the estimated change in SvH erosion and joint space narrowing score between baseline and 1 year. Outcomes were assessed in those patients who were triply positive for ACPAs, anti-acetylated peptide antibodies (AAPAs), and anti-carbamylated peptide antibodies, singly positive for ACPAs, and AMPA-negative, and had usable x-ray images.
Statistical analysis
The SvH erosion, joint space narrowing, and total progression scores followed a negative binomial distribution. To allow for the high number of cases in which there was no change in score, a zero-inflated negative binomial model was fitted to the data by use of the pscl R package16 for each of the progression scores, with the baseline value of the measured variable, rheumatoid factor, sex, age at recruitment, symptom duration, and baseline disease activity score for 28 joints with C-reactive protein (DAS28-CRP) included as covariates. Missing DAS28-CRP values were imputed from the corresponding DAS28 with erythrocyte sedimentation rate (DAS28-ESR) by use of linear regression. Any patients with a negative change in progression were given a score of zero. Estimated marginal mean changes in total SvH progression, erosion score, and joint space narrowing were calculated according to AMPA profile by use of the R emmeans package.17 Comparisons were done between triple positive (citrullinated, carbamylated, and acetylated), single positive (citrullinated only), and AMPA-negative patients to investigate whether the presence of antibodies against citrullinated peptides alone was associated with a worse radiographic prognosis or whether these antibodies were only associated with a worse prognosis if other AMPAs were also present. CIs across all comparisons were adjusted by use of the Tukey method18 included in the R emmeans package. Rapid radiographic progression was defined as a change in the SvH score of 5 or more units from baseline. Non-parametric tests were used to measure differences in clinical characteristics. The Mann-Whitney-Wilcoxon U test was used to compare the means of two independent samples. Categorical variables were compared by use of the Egon Pearson N-1 corrected χ2 test for proportions. R, version 4.0.0, was used for all statistical analyses.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. JSN, FRM, IBM, and DP had access to the totality of the raw data. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
1073 patients were recruited to the SERA study between March 1, 2011, and April 30, 2015. 362 patients with rheumatoid arthritis were included in our study and had their AMPA profiles measured (appendix p 1). Of these, 345 had radiographs taken at baseline and at 12 months, of whom 109 were excluded because they had at least one radiograph that was of insufficient quality for scoring. Three patients were also excluded from the analysis because they had surgery on one or more individual joints. As a result, 233 patients had paired radiographs of hands and feet that were available and of sufficient quality for scoring.
Baseline characteristics for the whole patient population can be found in the appendix (pp 2–3). 244 (67%) of the 362 patients were women, the mean age at recruitment was 58·2 years (SD 13·4), and the mean duration of symptoms at recruitment was 14·4 months (SD 31·1).
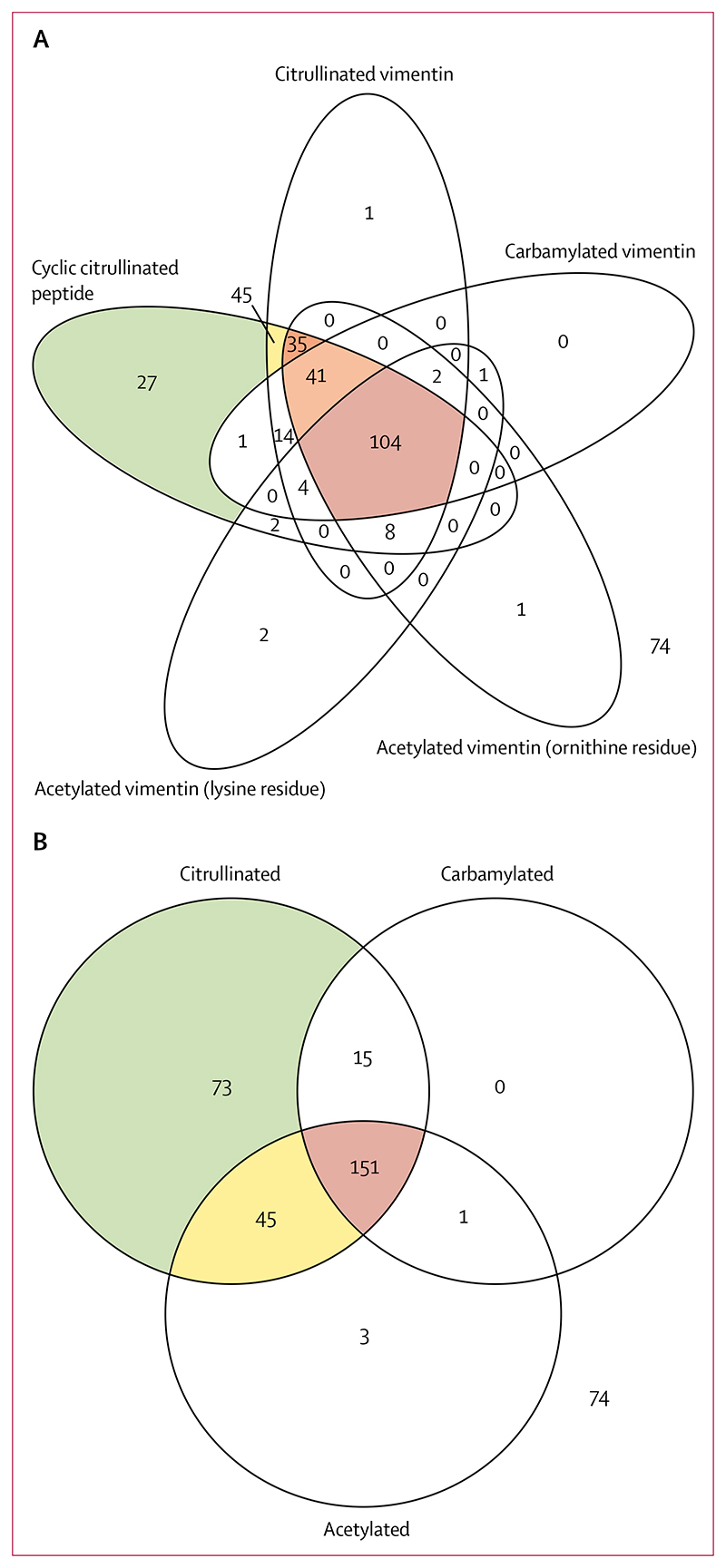
Six main autoantibody groupings were identified in the 362 patients with rheumatoid arthritis: single-positivity for CCP (n=27), double-positivity for CCP and citrullinated vimentin (n=45), triple-positivity for CCP, citrullinated vimentin, and vimentin acetylated at ornithine residues (n=35), quadruple-positivity for CCP, citrullinated vimentin, vimentin acetylated at ornithine residues, and carbamylated vimentin (n=41), those with all five autoantibodies (CCP, citrullinated vimentin, vimentin acetylated at ornithine residues, vimentin acetylated at lysine residues, and carbamylated vimentin; n=104), and those negative for AMPA (n=74; figure 1A). 36 patients had other antibody profiles.
Figure 1. Autoantibody profiles.
362 patients with rheumatoid arthritis had autoantibodies to post-translationally modified peptides measured. (A) The autoantibody profiles. Six main groups emerged in which the numbers within each group represented more than 5% of the total sample. Relevant segments of the Venn diagram are coloured. (B) Summarised autoantibody profiles. Venn segments are coloured when more than 5% of the population lies within a segment and this results in four groupings: single-positivity (green segment); double-positivity (yellow segment); triple-positivity (red segment); and negative for anti-modified peptide autoantibodies (outside the circles).
These patterns were simplified by combining patients who were positive for antibodies against CCP, citrullinated vimentin, or both into an ACPA-positive group, and combining those who were positive for antibodies against acetylated ornithine, acetylated lysine, or both into an AAPA-positive group. This method generated four major groupings in the whole cohort: single ACPA positivity (n=73 [20%]; from herein referred to as single-positive); double-positivity for ACPAs and AAPAs (n=45 [12%]; from herein referred to as double-positive); triple-positivity for ACPAs, AAPAs, and anti-carbamylated peptide antibodies (n=151 [42%]; from herein referred to as triple-positive); and AMPA-negative patients (n=74 [20%]; figure 1B). 19 (5%) patients were in one of the minor autoantibody groups (figure 1B). 284 (78%) of the 362 patients were positive for antibodies to citrullinated peptides and 78 (22%) were negative for these antibodies. Only 15 (4%) patients had antibodies against citrullinated and carbamylated peptides alone.
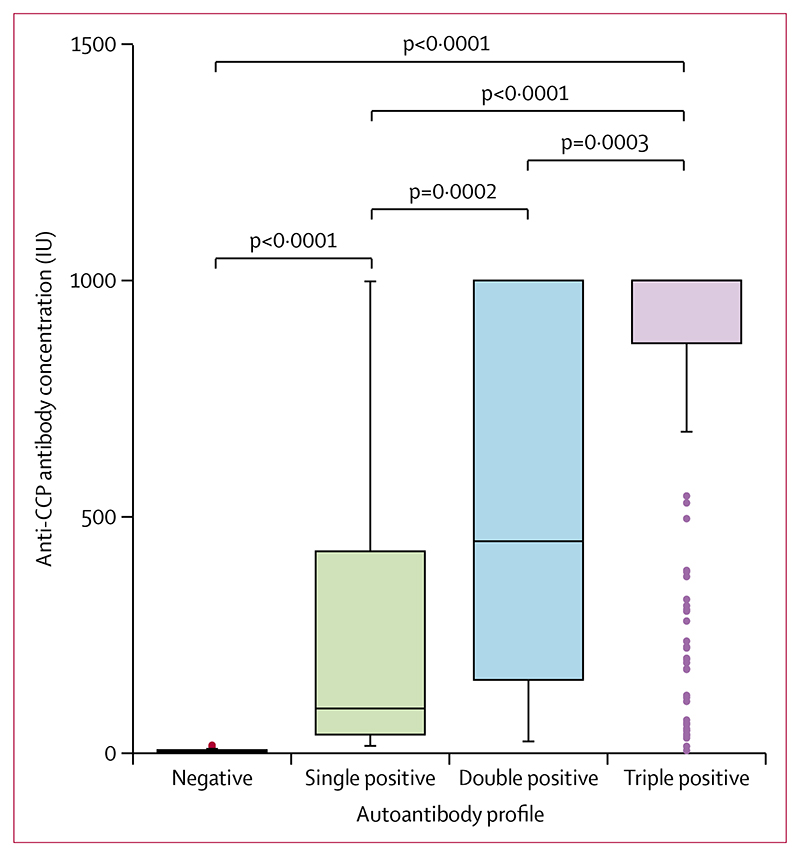
Median concentrations of anti-CCP were higher in patients with more AMPA classes than in patients with fewer AMPA classes (figure 2). For example, patients who were triple-positive had higher median anti-CCP concentrations (1000 IU [IQR 867–1000]) than did patients who were double-positive (449 IU [156–1000]) and patients who were single-positive (96 IU [41–428]; figure 2).
Figure 2. Anti-CCP antibody concentrations in patients with rheumatoid arthritis.
In the boxplots, the box represents the IQR, the line within the box represent the median, and the whiskers represent ± 1·5 × IQR. The dots represent the values more than ± 1·5 × IQR. CCP=cyclic citrullinated peptide.
To evaluate whether autoantibody status was associated with variation in the risk of radiographic progression, we combined the data from the AMPA assays with radiographic progression data that had been derived from baseline and 12-month hand and foot radiographs. The 233 patients who had radiographs of sufficient quality had similar baseline characteristics (appendix pp 2–3) and similar differences in anti-CCP concentrations between the AMPA groups (appendix p 6) to the total population of 362 patients. Baseline characteristics of the radiographic cohort are outlined in table 1. AMPA reactivity groups were analysed if they made up more than 5% of the total population (n=233), totalling 221 people analysed. Age at recruitment, mean disease duration, the Health Assessment Questionnaire Disability Index (HAQ-DI) score, the Hospital Anxiety and Depression Scale (HADS) score, the EQ-5D score, and disease activity, when measured by DAS28-ESR or the DAS28-CRP, did not differ between patients who were single-positive and those who were triple-positive (table 1). Furthermore, there was no difference in smoking status (table 1) or in baseline values for laboratory tests (appendix p 5) between triple-positive and single-positive patients.
Table 1. Baseline characteristics in the radiographic cohort.
| Negative (n=47) |
Single-positive (n=48) |
Double-positive (n=29) |
Triple-positive (n=97) |
p-value (triple-positive vs single-positive) |
|
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Sex | |||||
| Female | 34 (72%) | 36 (75%) | 21 (72%) | 57 (59%) | 0·056* |
| Male | 13 (28%) | 12 (25%) | 8 (28%) | 40 (41%) | .. |
| Ethnicity | |||||
| White | 47 (100%) | 48 (100%) | 28 (97%) | 97 (100%) | .. |
| Indian subcontinent | 0 | 0 | 1 (3%) | 0 | .. |
| Age at recruitment, years | 63·7 (12·4) | 56·6 (13·7) | 54·4 (15·0) | 57·8 (12·6) | 0·61 |
| Smoking status | |||||
| Ever smoked | 22 (47%) | 33 (69%) | 17 (59%) | 67 (69%) | .. |
| Never smoked | 25 (53%) | 15 (31%) | 12 (41%) | 30 (31%) | 0·97* |
| Alcohol intake, units per week | 5·1 (7·5) | 5·1 (11·3) | 4·9 (7·2) | 7·1 (15·7) | 0·29 |
| Disease characteristics | |||||
| Disease duration, months | 14·1 (41·1) | 12·0 (13·5) | 15·3 (27·5) | 13·3 (19·2) | 0·61 |
| Swollen joints (0-28) | 7·5 (5·6) | 6·7 (5·8) | 5·2 (3·6) | 7·0 (4·8) | 0·33 |
| Tender joints (0-28) | 12·1 (6·8) | 9·6 (7·3) | 8·4 (7·3) | 10·2 (7·3) | 0·66 |
| Erythrocyte sedimentation rate, mm/h | 33·2 (26·3) | 27·5 (20·9) | 37·2 (27·8) | 31·8 (22·6) | 0·37 |
| Patients with complete data | 39 | 40 | 27 | 84 | .. |
| C-reactive protein blood concentration, mg/L |
24·7 (28·6) | 14·8 (12·0) | 29·6 (36·7) | 24·6 (38·1) | 0·84 |
| Patients with complete data | 41 | 43 | 25 | 83 | .. |
| HAQ-DI score (0-3) | 1·3 (0·8) | 1·1 (0·8) | 1·2 (0·8) | 1·1 (0·8) | 0·86 |
| Patients with complete data | 47 | 48 | 29 | 96 | .. |
| HADS score (0-42) | 12·5 (7·4) | 13·1 (7·8) | 11·8 (8·7) | 11·7 (7·3) | 0·36 |
| Depression (0-21) | 6·3 (3·9) | 5·7 (3·7) | 5·4 (4·2) | 5·3 (3·7) | 0·44 |
| Anxiety (0-21) | 6·2 (4·2) | 7·3 (4·7) | 6·3 (4·9) | 6·4 (4·4) | 0·30 |
| Patients with complete data | 46 | 47 | 29 | 96 | .. |
| EQ-5D score | 0·45 (0·30) | 0·46 (0·36) | 0·52 (0·36) | 0·52 (0·31) | 0·42 |
| Patients with complete data | 47 | 48 | 29 | 96 | .. |
| PtGA VAS (0-100mm) | 51·1 (26·0) | 46·3 (24·7) | 48·9 (24·5) | 49·9 (27·1) | 0·42 |
| Rheumatoid factor | |||||
| High positive (>60 IU) | 3 (6%) | 21 (44%) | 13 (45%) | 43 (44%) | .. |
| Low positive (>20 IU) | 7 (15%) | 5 (10%) | 4 (14) | 25 (26%) | .. |
| Negative | 37 (79%) | 22 (46%) | 12 (41%) | 29 (30%) | 0·059* |
| Cyclic citrullinated peptide | |||||
| High positive (>60 AU) | 0 | 34 (71%) | 27 (93%) | 95 (98%) | .. |
| Low positive (>20 AU) | 0 | 14 (29%) | 2 (7%) | 2 (2%) | <0·0001* |
| Negative | 47 (100%) | 0 | 0 | 0 | .. |
| DAS28-ESR (0·0-9·4) | 5·4 (1·2) | 4·8 (1·4) | 4·9 (1·5) | 5·2 (1·4) | 0·16 |
| Patients with complete data | 39 | 40 | 27 | 84 | .. |
| DAS28-CRP (0·0-9·4) | 5·0 (1·5) | 4·5 (1·2) | 4·5 (1·8) | 4·7 (1·7) | 0·23 |
| Patients with complete data | 41 | 43 | 25 | 83 | .. |
| Radiographic scores | |||||
| Erosion score (0-280) | 3·9 (6·6) | 2·3 (4·1) | 2·1 (3·8) | 4·0 (8·5) | 0·55 |
| Joint space narrowing score (0-168) | 4·7 (5·0) | 3·25 (6·2) | 2·5 (4·7) | 3·9 (7·9) | 0·66 |
| Total SvH score (0-448) | 8·6 (10·7) | 5·6 (9·3) | 4·6 (7·4) | 7·9 (15·5) | 0·86 |
Data are n (%) or mean (SD), unless otherwise stated. The Mann-Whitney-Wilcoxon U test was used to calculate p-values for the difference between the single-positive group and the triple-positive group. p-values less than 0·05 were considered significant. For the swollen joint count, a score of 0 represents no swollen joints and a score of 28 represents swelling in all joints assessed. For the tender joint count, a score of 0 represents no tender joints and a score of 28 represents tenderness in all joints assessed. A HAQ-DI score of 0 indicates no disability and a score of 3 indicates severe disability. The score range for the HADS is 0–42, where the cutoff is 15 or more for a diagnosis of psychosocial distress. For both the anxiety and depression components of the HAD, the score range is 0–21, where the cutoff of 8 or more suggests the presence of anxiety or depression. For PtGA VAS, a score of 0 represents the lowest disease activity and a score of 100 represents the worst disease activity. For DAS28-ESR and DAS28-CRP, a score of more than 5·1 indicates high disease activity, a score of 3·2–5·1 indicates moderate disease activity, a score of 2·6–3·2 indicates low disease activity, and a score less than 2·6 indicates remission. The erosion score per joint ranges from 0 to 10. A total score of 0 represents no erosion of the hands and feet. A total score of 280 represents maximal total erosion. The joint space narrowing score per joint ranges from 0 to 4. A total score of 0 represents no joint space narrowing in the hands and feet. A total score of 168 represents maximal total joint space narrowing. The total SvH score is the sum of the total erosion score and the total joint space narrowing score. ACPAs=anti-citrullinated peptide antibodies. DAS28-CRP=Disease activity score of 28 joints with C-reactive protein.DAS28-ESR=Disease activity score of 28 joints with erythrocyte sedimentation rate. HADS=Hospital Anxiety and Depression Scale. HAQ-DI=Health Assessment Questionnaire Disability Index. PtGA VAS=patient global assessment of disease activity visual analog scale. SvH=Sharp-van der Heijde.
Denotes Egon Pearson N-1 corrected χ2 test for proportions.19
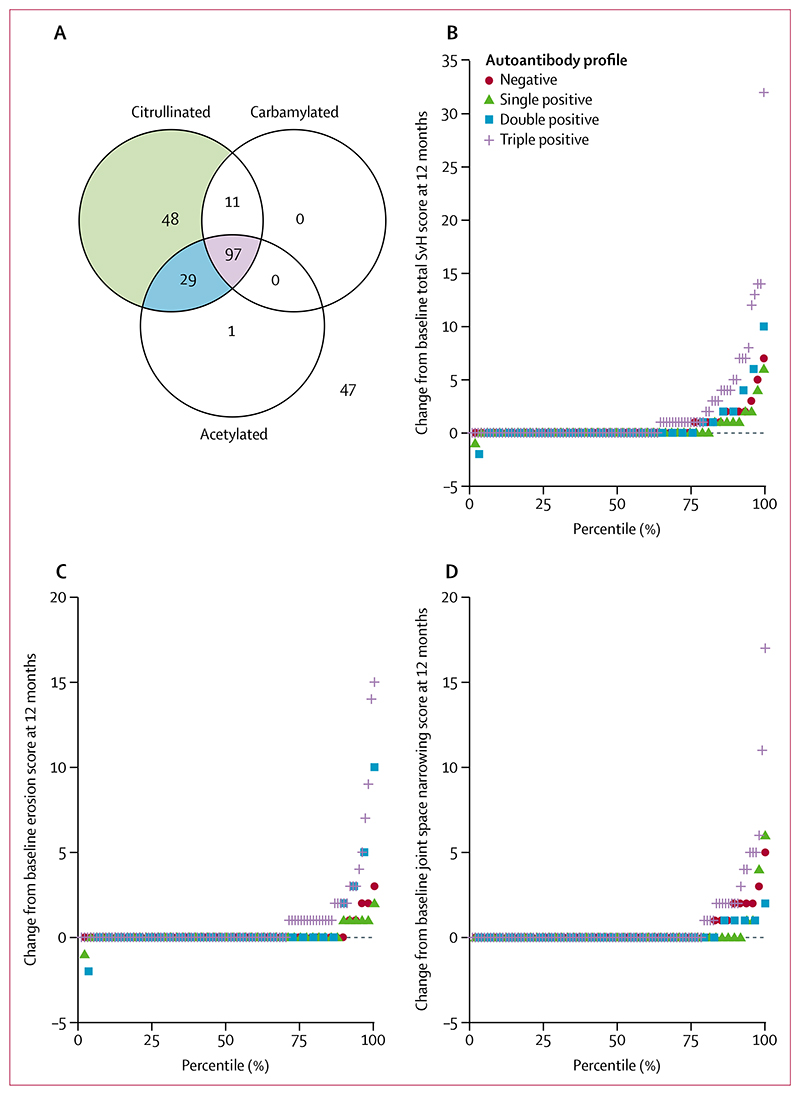
Patients in the radiographic cohort (figure 3A) who were triple-positive had more radiographic progression during the 12 months from baseline (estimated mean change in total SvH score 1·8, 95% CI 0·9–2·6, SE 0·4) than did those who were single-positive (0·5, 0·1–1·0, 0·2; figure 3B; table 2). The estimated mean difference in the total change in SvH score between those who were triple-positive and those who were single-positive was 1·2 (95% CI 0·1–2·4, SE 0·5; table 3). There was no difference in radiographic progression between patients who were single-positive and those who were AMPA-negative (estimated mean change in total SvH score 0·7, 95% CI 0·1–1·4, SE 0·3; estimated mean difference in the total change in SvH score −0·2, 95% CI −1·1 to 0·7, SE 0·4; tables 2, 3). There were no differences in radiographic progression scores between men and women with the same antibody profile (appendix p 4). Triple-positive patients had a larger change in erosion score over 1 year than did single-positive patients; however, there was no difference in erosion score change between single-positive patients and AMPA-negative patients (tables 2, 3). No difference was found in the change in joint space narrowing score between triple-positive patients and single-positive patients, or between single-positive patients and AMPA-negative patients (table 3).
Figure 3. Radiographic progression according to AMPA status.
(A) Four AMPA reactivity groups were identified in patients for whom radiographic data were available. Groups were coloured in the Venn diagram and analysed if they made up more than 5% of the total population (n=233). (B) Change in total SvH score. (C) Change in SvH erosion score. (D) Change in SvH joint space narrowing score. AMPA=anti-modified peptide autoantibodies. SvH=Sharp-van der Heijde.
Table 2. Clinical and radiographic progression over 12 months in the radiographic cohort.
| Negative (n=47) |
Single-positive (n=48) |
Double-positive (n=29) |
Triple-positive (n=97) |
p-value (triple-positive vs single-positive) |
||
|---|---|---|---|---|---|---|
| Change in disease activity over 12 months | ||||||
| DAS28-ESR | -1·8 (1·5) | -1·7 (1·3) | -1·3 (1·9) | -1·5 (1·5) | 0·43 | |
| Patients with complete data | 35 | 37 | 23 | 78 | .. | |
| DAS28-CRP | -2·5 (1·6) | -2·4 (1·3) | -2·0 (2·2) | -2·0 (2·0) | 0·34 | |
| Patients with complete data | 28 | 31 | 16 | 66 | .. | |
| EULAR response over 12 months year using DAS28-ESR* | ||||||
| Good | 13/35 (37%) | 16/37 (43%) | 8/23 (35%) | 29/78 (37%) | .. | |
| Moderate | 14/35 (40%) | 12/37 (32%) | 7/23 (30%) | 22/78 (28%) | .. | |
| No response | 8/35 (23%) | 9/37 (24%) | 8/23 (35%) | 27/78 (35%) | 0.54† | |
| Radiographic progression over 12 months | ||||||
| Change in total SvH score | 0·7 (0·1-1·4, 0·3) | 0·5 (0·1-1·0, 0·2) | 0·8 (0·2-1·4, 0·3) | 1·8 (0·9-2·6, 0·4) | 0·038 | |
| Change in erosion score | 0·3 (0·0-0·6, 0·2) | 0·2 (0·0-0·4, 0·1) | 0·5 (0·0-0·9, 0·2) | 1·1 (0·4-1·7, 0·3) | 0·028 | |
| Change in joint space narrowing score | 0·4 (0·1-0·8, 0·2) | 0·3 (-0·1 to 0·7, 0·2) | 0·1 (0·0-0·2, 0·1) | 0·9 (0·4-1·5, 0·3) | 0·22 | |
| Patients with total SvH score increase ≥1 | 12 (26%) | 9 (19%) | 7 (24%) | 35 (36%) | 0·033† | |
| Patients with rapid radiographic progression (total SvH score increase ≥5) |
2 (4%) | 1 (2%) | 2 (7%) | 11 (11%) | 0·058† | |
Data are mean (SD), n/N (%), n (%), or n (95% CI, SE), unless otherwise stated. The Mann-Whitney-Wilcoxon U test was used to calculate p-values between the single-positive group and the triple-positive group. p-values less than 0·05 were considered significant. A good EULAR response is an improvement in score of more than 1·2 and a month 12 score of 3·2 or less. A moderate response is an improvement of more than 0·6 but 1·2 or less and a present score of 5·1 or more, or an improvement of more than 1·2 and a month 12 score of more than 3·2. A non-response is an improvement of 0·6 or less, or an improvement of more than 0·6 but 1·2 or less, and a month 12 score of more than 5·1. DAS28-CRP=Disease activity score of 28 joints with C-reactive protein. DAS28-ESR=Disease activity score of 28 joints with erythrocyte sedimentation rate. EULAR= European League Against Rheumatism. SvH=Sharp-van der Heijde.
Denominators exclude missing data.
Denotes Egon Pearson N-1 corrected χ2 test for proportions.19
Table 3. Differences in radiographic progression between autoantibody groups in the radiographic cohort.
| Triple-positive vs single-positive | Triple-positive vs negative | Single-positive vs negative | |
|---|---|---|---|
| Difference in total SvH change between baseline and 12 months | 1·2 (0·1-2·4, 0·5) | 1·0 (-0·2 to 2·3, 0·5) | -0·2 (-1·1 to 0·7, 0·4) |
| Difference in erosion score change between baseline and 12 months | 0·8 (0·1-1·6, 0·3) | 0·8 (-0·1 to 1·6, 0·4) | -0·1 (-0·6 to 0·4, 0·2) |
| Difference in joint space narrowing score change between baseline and 12 months | 0·6 (-0·3 to 1·5, 0·4) | 0·5 (-0·3 to 1·3, 0·3) | -0·1 (-0·7 to 0·5, 0·3) |
Data are mean (95% CI, SE). SvH=Sharp-van der Heijde.
Fewer patients in the single-positive group than in the triple-positive group showed an increase in the total SvH score of 1 or more during the 12 months (9 [19%] of 48 vs 35 [36%] of 97; p=0·033). The difference between these groups in the number of patients with rapid radiographic progression during the 12 months (one [2%] of 48 vs 11 [11%] of 97; p=0·058) did not reach statistical significance.
Higher ACPA concentrations have been associated with increased radiographic progression in patients with rheumatoid arthritis.5 Within each AMPA group, there were no differences in anti-CCP concentrations in patients with radiographic progression compared to those without radiographic progression (appendix p 7).
The change in disease activity score during the 12 months and the proportion of patients classified as good or moderate responders, as measured by the European League Against Rheumatism (EULAR) response criteria, at 12 months were not different between triple-positive patients and single-positive patients, although the number of patients without missing data for these values (141 for DAS28-CRP, and 173 for DAS28-ESR and EULAR response) was less than the number of patients in the full radiographic cohort (n=233; table 2).
Rheumatoid factor was measured to establish whether the relationship between radiographic progression and autoantibody profiles was limited only to patients seropositive for rheumatoid factor. Similar proportions of patients were single-positive and double-positive between the group positive for rheumatoid factor and the group negative for rheumatoid factor (appendix p 8). Most patients in the rheumatoid factor-positive group were triple-positive and, conversely, for those who were negative for rheumatoid factor, more patients were in the AMPA-negative group than were in all other groups (appendix p 8).
Of the patients who were positive for rheumatoid factor, those who were triple-positive had more radiographic progression during the 12 months than did those who were single-positive (appendix pp 9–11). In patients who were negative for rheumatoid factor, there was no difference in the total change in SvH score between triple-positive and single-positive patients (appendix pp 10–11). For each radiographic measure (ie, total SvH change, erosion score change, and joint space narrowing score change), there was no difference between patients positive for rheumatoid factor and those who were negative for rheumatoid factor in any of the analysed autoantibody groups (appendix p 12).
The use of disease-modifying anti-rheumatic drugs (DMARDs) is shown in table 4. At 12 months, a higher proportion of patients in the single-positive group than in the triple-positive group were on conventional synthetic DMARD monotherapy. Furthermore, fewer patients in the single-positive group than in the triple-positive group were being treated with three or more conventional synthetic DMARDs (ie, methotrexate, sulfasalazine, and hydroxychloroquine), although this difference was not statistically significant (table 4). Thus, triple-positive patients did not show more radiographic progression than single-positive patients as a consequence of triple-positive patients receiving less intense therapy.
Table 4. Medication use at 12 months in the radiographic cohort.
| Negative (n=47) |
Single-positive (n=48) |
Double-positive (n=29) |
Triple-positive (n=97) |
p-value (triple-positive vs single-positive) |
|
|---|---|---|---|---|---|
| Methotrexate monotherapy | 12 (26%) | 25 (52%) | 8 (28%) | 31 (32%) | 0·020 |
| Sulfasalazine monotherapy | 3 (6%) | 4 (8%) | 2 (7%) | 12 (12%) | 0·46 |
| Hydroxychloroquine monotherapy | 4 (9%) | 3 (6%) | 0 | 4 (4%) | 0·58 |
| Any csDMARD monotherapy | 19 (40%) | 32 (67%) | 10 (34%) | 47 (48%) | 0·039 |
| Two csDMARDs | 11 (23%) | 14 (29%) | 8 (28%) | 27 (28%) | 0·86 |
| Three or more csDMARDs | 4 (9%) | 2 (4%) | 5 (17%) | 14 (14%) | 0·064 |
| Biologics | 0 | 0 | 0 | 2 (2%) | NA |
| Total taking csDMARDs or biologics at 12 months |
34 (72%) | 48 (100%) | 23 (79%) | 89 (92%) | NA |
| Oral prednisolone at 12 months | 10 (21%) | 12 (25%) | 10 (34%) | 33 (34%) | 0·27 |
| Any intramuscular or intra-articular steroid injection |
23 (49%) | 30 (63%) | 17 (59%) | 55 (57%) | 0·51 |
DMARD and oral prednisolone use is at the 1 year timepoint. Intramuscular or intra-articular steroid use is over the course of 1 year. The Egon Pearson N-1 corrected χ2 test for proportions was used to calculate the differences in proportions between triple-positive and single-positive patients. p-values less than 0·05 were considered significant. csDMARDs=conventional synthetic disease-modifying antirheumatic drugs.
Discussion
To our knowledge, this study is the first to investigate the relationship between multiple autoantibodies to citrullinated, carbamylated, and acetylated peptides and radiographic progression in patients with rheumatoid arthritis. In the SERA cohort, we found four major AMPA groups: single ACPA-positivity, double-positivity for ACPAs and AAPAs, triple-positivity for ACPAs, AAPAs, and anti-carbamylated peptide antibodies, and AMPA-negativity. Our key result shows that triple-positive patients had more radiographic progression during 12 months than did single-positive patients. Furthermore, radiographic progression in the single-positive group was similar to that of patients who were seronegative for all AMPAs, suggesting that the effect on radiographic progression of being ACPA-positive is restricted to those patients who also have other AMPAs.
Different rates of change in radiological variables (total SvH score, erosion score, or joint space narrowing score) between the triple-positive and single-positive groups were not explained by baseline values of the radiological variable of interest, rheumatoid factor, age at recruitment, sex, symptom duration before recruitment, or DAS28-CRP at baseline. The median concentration of anti-CCP was higher in patients who were triple-positive than in those who were single-positive. However, within each autoantibody grouping, we did not see a difference in ACPA concentration between those patients with radiographic progression and those patients without.
Comparator studies of radiographic progression in ACPA-positive patients have shown more radiographic progression in seropositive patients than in seronegative patients.4,12 At face value, our results are at odds with this finding; however, 185 (79%) of the 233 patients in the radiographic cohort were ACPA-positive, so the SERA cohort is similar to other cohorts. When antibodies to carbamylated and acetylated vimentin were measured, we found that radiographic progression was greater in patients who had all three classes of autoantibody than in those who had ACPA only.
Shi and colleagues6 explored the influence of anti-carbamylated peptide antibodies on radiographic progression in patients with rheumatoid arthritis and found that the presence of these antibodies was associated with more severe radiographic progression in those with and without anti-CCP antibodies. In our cohort, only a small proportion of ACPA-negative patients had anti-carbamylated peptide antibodies (1% in our subgroup of the SERA cohort vs 16% in the Leiden Early Arthritic Cohort).6 Intercohort differences could be due to cohort-related factors (eg, disease duration at recruitment) but might be because we measured autoantibodies that are specific to a post-translational modification of a vimentin-derived peptide rather than to a modified, heterogeneous mixture of peptides in fetal calf serum. Furthermore, only a small proportion of ACPA-positive patients had anti-carbamylated peptide antibodies in the absence of anti-acetylated antibodies and therefore we could not draw any conclusions about radiographic progression in this subset of patients. Our results are in keeping with a report from the RETRO study8 in which the largest subgroup of patients had autoantibodies to all three classes of post-translational modification.
Cross-reactivity of autoantibodies has been investigated by Juarez and colleagues9 with inhibition studies, which are crucial given the structural similarity of the epitopes from various post-translational modifications. As in our study, Juarez and colleagues9 showed that AAPAs typically occurred concurrently with ACPAs and did not help to further subclassify the ACPA-negative group. Work by Kampstra and colleagues13 has indicated that both ACPAs and anti-carbamylated peptide antibodies react to citrullinated and carbamylated antigens. This work also showed that both antibody classes can recognise acetylated antigens, which is surprising given the difference in structure between acetylated lysine and citrulline or homocitrulline.
Further work is required to assess whether subgroups of patients, defined on the basis of different combinations of AMPAs, differ in other outcome measures. Findings from the RETRO study8 suggest that relapse rate is higher in those patients with rheumatoid arthritis and multiple autoantibody specificities than in those patients without.
An important limitation of this work is that our study only considered autoantibodies to modified vimentin-derived peptides and not those to other proteins, such as fibronectin and alpha-enolase. We suggest that autoantibodies to other modified proteins should be assessed in the future with a view to developing a clinical diagnostic and prognostic test based on AMPA profiles. Furthermore, the findings of this study are restricted to patients treated with conventional synthetic DMARDs (only two patients were on biological DMARDs [biologics] at the 12-month timepoint). Future work should address whether our findings are replicated in other cohorts of patients treated with conventional synthetic DMARDs and in cohorts given biological DMARDs at an earlier stage of disease. The autoantibody data presented herein were generated from samples collected at baseline in patients with rheumatoid arthritis presenting with clinically apparent synovitis. Future work should address the evolution of the serological profile with time, such as in the arthralgia phase before the development of joint swelling and following the introduction of DMARD therapy. This work could provide important insights for clinicians, such as clarifying whether assessing these antibodies at earlier stages (as has already been done for anti-carbamylated peptide antibodies and ACPAs)20 and later stages of disease is useful in predicting future radiographic progression.
Our study adds to the published literature by showing that patients with antibodies to citrullinated, carbamylated, and acetylated peptides have a higher rate of radiographic progression than do those with antibodies to citrullinated peptides alone. Interestingly, there is a group of patients with ACPA positivity only who progress at a rate similar to that of patients negative for ACPAs and for other AMPAs. We believe that further prospective work is required to understand the effects of multiple autoantibody specificities in patients with rheumatoid arthritis and whether such baseline autoantibody status should be used to stratify patients with rheumatoid arthritis in therapeutic trials.
Supplementary Material
Research in context.
Evidence before this study
Patients with rheumatoid arthritis, anti-citrullinated peptide antibodies (ACPAs), and anti-carbamylated peptide antibodies have more rapid radiographic progression than patients with rheumatoid arthritis who are antibody-negative. The 2019 European League Against Rheumatism recommendations for the management of patients with rheumatoid arthritis (informed by systematic literature reviews covering the period 2013-19) highlighted that the presence of rheumatoid factor, ACPAs, or both is a poor prognostic factor. The 2018 guidelines for the management of rheumatoid arthritis in adults from the UK’s National Institute for Health and Care Excellence (informed by a systematic literature review covering the period 1946-2017) highlighted that if anti-cyclic citrullinated peptide antibodies are present, then patients should be advised that they have an increased risk of radiological progression.
Added value of this study
In patients with newly diagnosed rheumatoid arthritis, there were four major groupings of anti-modified protein antibodies (AMPAs). Patients with triple AMPA positivity had increased radiographic progression compared with patients with only ACPA positivity. Those patients with only ACPAs had similar radiographic progression to those who were seronegative for all AMPAs.
Implications of all the available evidence
Data presented in this study suggest that optimal prediction of future rates of radiographic progression will require the assessment of autoantibodies against multiple post-translationally modified proteins and peptides. Future observational and interventional studies, in which radiographic progression is measured as an outcome variable, should consider incorporating a measurement of anti-acetylated peptide antibodies, anti-carbamylated peptide antibodies, and ACPAs, to allow an appropriate description of the cohort or of the different study groups.
Acknowledgements
This research was funded within the FP7 HEALTH programme under the grant agreement FP7-HEALTH-F2-2012-305549 (EuroTEAM).
This work was supported by awards (INF-GU-168) from the Translational Medicine Research Collaboration, a consortium made up of the Universities of Aberdeen, Dundee, Edinburgh, and Glasgow. KR and CDB are funded by the Birmingham National Institute for Health Research Biomedical Research Centre. This work has been supported by the Research into Inflammatory Arthritis Centre Versus Arthritis and the MRC Versus Arthritis Centre for Musculoskeletal Ageing Research. The views expressed in this Article are those of the authors and are not necessarily those of the MRC or Versus Arthritis. The Scottish Early Rheumatoid Arthritis (SERA) inception cohort and biobank was supported by awards (INF-GU-168) from the Translational Medicine Research Collaboration, four associated National Health Service Health Boards (ie, Grampian, Tayside, Lothian, and Greater Glasgow and Clyde), and Pfizer, and from the Chief Scientific Office (Ref ETM-40). We thank Louise Bennett for her assistance with extracting samples from the SERA biobank and Peter Nightingale for his statistical insights. The use of biological and clinical data from the SERA biobank and SERA dataset for the purposes of this study was reviewed by the SERA Access Committee. This Committee has four patient members and they were involved in the development and execution of the SERA Access Policy and reviewed the proposed study, the data and samples required, and the analysis plan.
Footnotes
Contributors
JSN and FRM analysed the data and drafted the manuscript. HB coordinated the antibody assays, interpreted the data, and contributed to the development of the manuscript. CDB contributed to the development of the study and the manuscript. DvdH coordinated the radiographic scoring, interpreted the data, and contributed to the development of the manuscript. AG and CP coordinated the collection of data from participants in the Scottish Early Rheumatoid Arthritis Inception Cohort and Biobank (SERA) and contributed to the development of the manuscript. IBM and DP are lead investigators for the SERA and contributed to the development of the study, data interpretation, and the development of the manuscript. KR conceived and coordinated this study, interpreted the data, and contributed to the development of the manuscript. The Scottish Early Rheumatoid Arthritis Inception Cohort Investigators recruited participants.
Declaration of interests
JSN reports personal fees from Janssen Pharmaceuticals and UCB outside the submitted work. HB reports grants from the FP7 HEALTH programme during the conduct of the study, and is an employee of Orgentec Diagnostika GmbH, which manufactures and sells in vitro diagnostics. CDB reports grants from Roche and GSK, and consultancy fees from Pfizer and GSK outside the submitted work. DvdH reports personal fees from AbbVie, Amgen, Astellas, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Cyxone, Daichii, Eisai, Elly-Lilly, Galapagos, Gilead, GSK, Janssen, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi, Takeda, and UCB Pharma outside the submitted work, and is the director of Imaging Rheumatology. IBM reports personal fees from AbbVie, Celgene, Compugen, Galvani, Lilly, Pfizer, and UCB, and grants from AstraZeneca, Celgene, Compugen, Novartis, Roche, and UCB, outside the submitted work. DP reports grants from Pfizer and the Scottish Government’s Chief Scientist Office during the conduct of the study. KR reports grants from Abbvie and Pfizer, and personal fees from Abbvie, Pfizer, Sanofi, Lilly, Bristol Myers Squibb, UCB, Janssen, and Roche Chugai outside the submitted work. All other authors declare no competing interests.
Contributor Information
Jagtar S Nijjar, Department of Medicine, University of Cambridge, Cambridge, UK.
Fraser R Morton, Research into Inflammatory Arthritis Centre Versus Arthritis, Institute of Infection, Immunity and Inflammation, University of Glasgow, Glasgow, UK.
Holger Bang, Orgentec Diagnostika, Mainz, Germany.
Christopher D Buckley, Rheumatology Research Group, Institute for Inflammation and Ageing, College of Medical and Dental Sciences, Queen Elizabeth Hospital, University of Birmingham, Birmingham, UK; Kennedy Institute of Rheumatology, University of Oxford, Oxford, UK.
Désirée van der Heijde, Department of Rheumatology, Leiden University Medical Centre, Leiden, Netherlands.
Ashley Gilmour, Research into Inflammatory Arthritis Centre Versus Arthritis, Institute of Infection, Immunity and Inflammation, University of Glasgow, Glasgow, UK.
Caron Paterson, Research into Inflammatory Arthritis Centre Versus Arthritis, Institute of Infection, Immunity and Inflammation, University of Glasgow, Glasgow, UK.
Iain B McInnes, Research into Inflammatory Arthritis Centre Versus Arthritis, Institute of Infection, Immunity and Inflammation, University of Glasgow, Glasgow, UK.
Duncan Porter, Department of Rheumatology, Gartnavel General Hospital, National Health Service Greater Glasgow and Clyde, Glasgow, UK.
Karim Raza, Research into Inflammatory Arthritis Centre Versus Arthritis and MRC Versus Arthritis Centre for Musculoskeletal Ageing Research, Institute of Inflammation and Ageing, University of Birmingham, Birmingham, UK; Department of Rheumatology, Sandwell and West Birmingham National Health Service Trust, Birmingham, UK.
Scottish Early Rheumatoid Arthritis Inception Cohort Investigators:
Cosimo di Bari, Margaret Duncan, Susan Fraser, Mohini Gray, Lisa Hutton, John Harvie, Vinod Kumar, Mike McMahon, Robin Munro, John Larkin, Neil McKay, John McLaren, Stuart Ralston, David M Reid, Duncan Porter, Ruth Richmond, Gillian Roberts, Sarah Saunders, and Hilary Wilson
Data sharing
Data are available upon reasonable request. Access to data would be subject to approval by the Scottish Early Rheumatoid Arthritis Inception Cohort and Biobank Data Access Committee.
References
- 1.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–19. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 2.van Venrooij WJ, van Beers JJBC, Pruijn GJM. Anti-CCP antibodies: the past, the present and the future. Nat Rev Rheumatol. 2011;7:391–98. doi: 10.1038/nrrheum.2011.76. [DOI] [PubMed] [Google Scholar]
- 3.Bang H, Egerer K, Gauliard A, et al. Mutation and citrullination modifies vimentin to a novel autoantigen for rheumatoid arthritis. Arthritis Rheum. 2007;56:2503–11. doi: 10.1002/art.22817. [DOI] [PubMed] [Google Scholar]
- 4.Mathsson L, Mullazehi M, Wick MC, et al. Antibodies against citrullinated vimentin in rheumatoid arthritis: higher sensitivity and extended prognostic value concerning future radiographic progression as compared with antibodies against cyclic citrullinated peptides. Arthritis Rheum. 2008;58:36–45. doi: 10.1002/art.23188. [DOI] [PubMed] [Google Scholar]
- 5.Raza K, Breese M, Nightingale P, et al. Predictive value of antibodies to cyclic citrullinated peptide in patients with very early inflammatory arthritis. J Rheumatol. 2005;32:231–38. [PMC free article] [PubMed] [Google Scholar]
- 6.Shi J, Knevel R, Suwannalai P, et al. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proc Natl Acad Sci USA. 2011;l08:17372–77. doi: 10.1073/pnas.1114465108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martínez G, Gómez JA, Bang H, et al. Carbamylated vimentin represents a relevant autoantigen in Latin American (Cuban) rheumatoid arthritis patients. Rheumatol Int. 2016;36:781–91. doi: 10.1007/s00296-016-3472-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Figueiredo CP, Bang H, Cobra JF, et al. Antimodified protein antibody response pattern influences the risk for disease relapse in patients with rheumatoid arthritis tapering disease modifying antirheumatic drugs. Ann Rheum Dis. 2017;76:399–407. doi: 10.1136/annrheumdis-2016-209297. [DOI] [PubMed] [Google Scholar]
- 9.Juarez M, Bang H, Hammar F, et al. Identification of novel antiacetylated vimentin antibodies in patients with early inflammatory arthritis. Ann Rheum Dis. 2016;75:1099–107. doi: 10.1136/annrheumdis-2014-206785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makrygiannakis D, Hermansson M, Ulfgren A-K, et al. Smoking increases peptidylarginine deiminase 2 enzyme expression in human lungs and increases citrullination in BAL cells. Ann Rheum Dis. 2008;67:1488–92. doi: 10.1136/ard.2007.075192. [DOI] [PubMed] [Google Scholar]
- 11.Tracy A, Buckley CD, Raza K. Pre-symptomatic autoimmunity in rheumatoid arthritis: when does the disease start? Semin Immunopathol. 2017;39:423–35. doi: 10.1007/s00281-017-0620-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Syversen SW, Goll GL, van der Heijde D, et al. Prediction of radiographic progression in rheumatoid arthritis and the role of antibodies against mutated citrullinated vimentin: results from a 10-year prospective study. Ann Rheum Dis. 2010;69:345–51. doi: 10.1136/ard.2009.113092. [DOI] [PubMed] [Google Scholar]
- 13.Kampstra ASB, Dekkers JS, Volkov M, et al. Different classes of anti-modified protein antibodies are induced on exposure to antigens expressing only one type of modification. Ann Rheum Dis. 2019;78:908–16. doi: 10.1136/annrheumdis-2018-214950. [DOI] [PubMed] [Google Scholar]
- 14.Dale J, Paterson C, Tierney A, et al. The Scottish Early Rheumatoid Arthritis (SERA) study: an inception cohort and biobank. BMC Musculoskelet Disord. 2016;17:461. doi: 10.1186/s12891-016-1318-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol. 2000;27:261–63. [PubMed] [Google Scholar]
- 16.Jackman S. pscl: classes and methods for R developed in the political science computational laboratory. R package version 1.5.2. 2017. https://github.com/atahk/pscl/. accessed April 4, 2020.
- 17.Lenth RV. emmeans: Estimated marginal means, aka least-squares means. R package version 1.4.8. 2020. https://CRAN.R-project.org/package=emmeans. accessed Sept 16, 2020.
- 18.Tukey JW. Exploratory data analysis. Reading, MA: Addison-Wesley Publishing Company; 1977. [Google Scholar]
- 19.Pearson ES. The choice of statistical tests illustrated on the interpretation of data classed in a 2 X 2 table. Biometrika. 1947;34:139–69. doi: 10.1093/biomet/34.1-2.139. [DOI] [PubMed] [Google Scholar]
- 20.Brink M, Verheul MK, Rönnelid J, et al. Anti-carbamylated protein antibodies in the pre-symptomatic phase of rheumatoid arthritis, their relationship with multiple anti-citrulline peptide antibodies and association with radiological damage. Arthritis Res Ther. 2015;17:25–28. doi: 10.1186/s13075-015-0536-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request. Access to data would be subject to approval by the Scottish Early Rheumatoid Arthritis Inception Cohort and Biobank Data Access Committee.