Abstract
Over the past years, advanced in vitro pulmonary platforms have witnessed exciting developments that are pushing beyond traditional preclinical cell culture methods. Here, we discuss ongoing efforts in bridging the gap between in vivo and in vitro interfaces and identify some of the bioengineering challenges that lie ahead in delivering new generations of human-relevant in vitro pulmonary platforms. Notably, in vitro strategies using foremost lung-on-chips and biocompatible “soft” membranes have focused on platforms that emphasize phenotypical endpoints recapitulating key physiological and cellular functions. We review some of the most recent in vitro studies underlining seminal therapeutic screens and translational applications and open our discussion to promising avenues of pulmonary therapeutic exploration focusing on liposomes. Undeniably, there still remains a recognized trade-off between the physiological and biological complexity of these novel in vitro lung models and their ability to deliver assays with throughput capabilities. The upcoming years are thus anticipated to see further developments in broadening the applicability of such in vitro systems and accelerating therapeutic exploration for drug discovery and translational medicine in treating respiratory disorders.
Keywords: preclinical models, in vitro, lung-on-chips, lung diseases, inhalation therapy, aerosols
Introduction
In recent years, preclinical research has witnessed substantial progress in delivering human-relevant in vitro models of the lungs (Figure 1). Such advances have been widely echoed by voices from the respiratory community at large, spanning academic [1–5] to pharmaceutical research [6–8]. Broadly speaking, there are two leading yet intertwined factors at the forefront of such developments. First, and most significantly perhaps, respiratory diseases embody a worldwide healthcare burden associated with high morbidity and mortality [9,10]. Alone, chronic obstructive pulmonary disease (COPD) stands as one of the major leading causes of death [11–14], with over 3M deaths a year and 200M patients suffering from moderate to severe forms of it. The heterogeneous nature of COPD, an umbrella term relating diverse chronic respiratory disorders, is such that it is typically described but not defined; not only is COPD greatly underdiagnosed it is often diagnosed late [10]. With dire prognoses, there are to date still no curative treatments to reduce the progression of the disease, suppress airway inflammation or restore functional parenchyma lost in emphysematous lungs [15,16]. In turn, significant efforts have focused on strategies of disease prevention given the enduring lack of therapeutic treatment options in COPD [12,17]. Parallel to this, the severity of bacterial respiratory tract infections (e.g. staphylococci, P. aeruginosa) is known to initiate cell and tissue damage as well as the chronic formation of biofilms on the lung surface [18,19]. Notoriously, pneumonia stands as the global leading cause of death in children under the age of five [20]. All the while, bacterial infections are increasingly worsened by antimicrobial resistance to available antibiotics [21]. The dire reality of respiratory afflictions has only been further exacerbated with the outbreak of the current COVID-19 worldwide pandemic that can lead to acute respiratory distress syndrome (ARDS) in severe patients [22–24] as well as the perplexing manifestations of long-term respiratory morbidity following recovery from the SARS-CoV-2 virus [25–27]. Altogether, the overwhelming need for a broadened therapeutic arsenal cannot be sufficiently stressed in tackling the plethora of respiratory conditions.
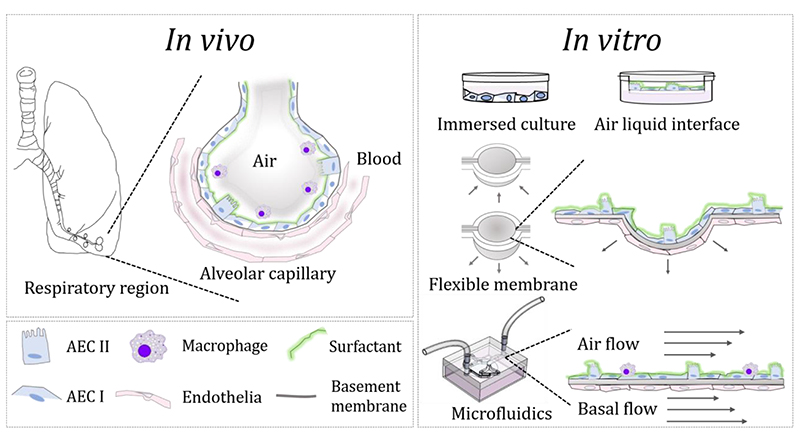
Figure 1.
Bridging the gap between in vivo and in vitro interfaces in the lungs: Left: schematic of the respiratory region (i.e. pulmonary acinus) exemplifying the alveolar-capillary barrier and its cellular make-up. Right: Overview of preclinical in vitro models for pulmonary research spanning traditional assays including culture plates under immersed conditions and air-liquid interface (ALI) based assays to advanced in vitro models, featuring “soft” membrane-based assays and microfluidic lung-on-chips.
In this sobering context, pulmonary medicine has witnessed significantly fewer drugs approved in the past decades [4]: a situation that coincides with less therapeutic candidates and a higher failure rate relative to other areas in medicine (e.g. cardiovascular and neurological diseases). One cause for such shortcomings lies in the challenging hurdles faced with animal experiments as to how these faithfully characterize human diseases; some questioning altogether the translational impact of in vivo findings [28]. Many respiratory drugs have demonstrated good performance in animal models but subsequently failed at the level of safety and/or efficacy in clinical trials, underscoring a call for better predictive human models [29]. Prevalent animal models (e.g. rodents) are limited by underlying differences with humans that constitute essential barriers to new drug development [3,4]. This includes important discrepancies in both innate and adaptive immunity between mice and humans [30], as well as differences in genomic responses highlighted in mouse models of human inflammatory diseases [31]. Turning an eye to anatomical differences, mice obtain their bronchial blood supply from the pulmonary rather than the systemic circulation; their respiratory tract is void of respiratory bronchioles such that their terminal bronchioles open directly into alveolar ducts [32]. Furthermore, the size of the largest intrapulmonary airways of murine lungs are comparable to small airways in humans, underlining major physiological and structural differences compared to human lungs [33]. Taken together, these prominent differences are acknowledged to result in 80% of failure on drug efficacy in human trials leveraging molecules previously screened in rodent lungs [34]. The thrust for advocating in vitro models has thus grown as a response to such hurdles in optimizing research in respiratory diseases [4].
In what follows we review recent progress pertaining to advanced human-relevant in vitro pulmonary models that are pushing beyond traditional preclinical cell culture methods (Figure 1). We discuss ongoing efforts in bridging the gap between in vivo and in vitro interfaces and identify some of the bioengineering challenges that lie ahead in delivering new generations of in vitro pulmonary platforms. As the breadth of the respiratory organ both in scale and complexity imposes an essentially “compartmental” approach [8,35] to emulate some but not all of its vast biological (e.g. cellular and immunological makeup) and physiological (e.g. anatomy, morphology, respiratory airflows) characteristics [36], the in vitro quest to recreate exhaustively whole-organ functions is largely elusive. Indeed, the methodologies pursued to mimic the lungs in vitro [2,37] are distinct from other techniques based for example on leveraging whole-lung architectures of preserved acellular extracellular matrix (ECM) scaffolds [38–40]. Instead, in vitro strategies have focused principally on “small scale” models that pinpoint to a narrow window inside the lungs and deliver foremost phenotypical endpoints (Figure 2) recapitulating key physiological and cellular functions [41]; a philosophy that adheres somewhat more closely to the common aphorism “all models are wrong but some are useful”. Here, we dedicate the bulk of this review to in vitro bioengineering efforts pertaining to the respiratory regions of the lungs , i.e. the pulmonary acinus and parenchymal tissues [42–44] that are most relevant in ultimately addressing respiratory diseases such as, but not limited to, COPD, emphysema, cystic fibrosis, bacterial infections and now COVID-19. We review some of the most recent advanced in vitro studies underlining preclinical pulmonary research on therapeutic applications including for example anti-inflammatory actions. Lastly, we open our discussion to promising avenues of pulmonary therapeutic exploration focusing on liposomes and where preclinical in vitro research may accelerate new respiratory therapies.
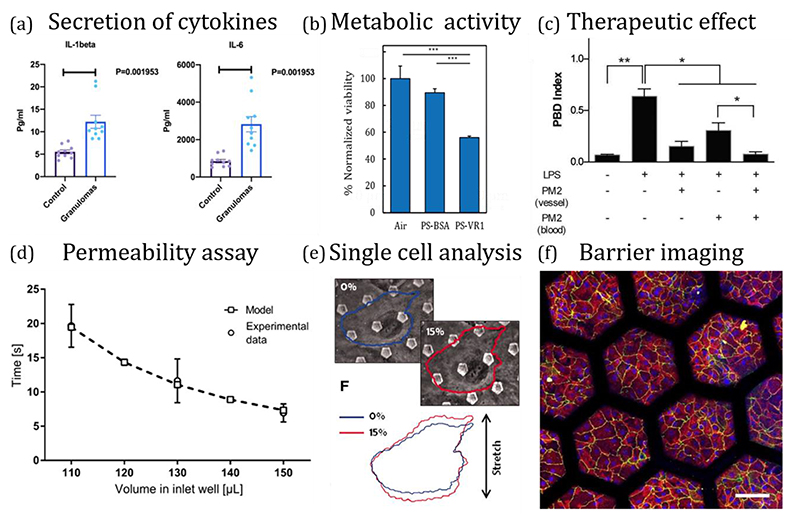
Figure 2.
Examples of in vitro phenotypical endpoints using advanced in vitro lung models. (a) Monitoring secretion of cytokines in a biochip of pulmonary sarcoidosis model [230]. (b) Measurement of metabolic activity following deposition of PM-like particles in airway-on-chip platforms [148]. (c) Assessment of therapeutics in an alveolus-on-a-chip model of intravascular thrombosis [173]. (d) Permeability assay in a breathing alveolus-on-chip model [77]. (e) Single cell distortion as a function of applied force in a stretching alveolar-capillary chip [83]. (f) Imaging tight junction (TJ) and adherent junction markers in a lung-on-a-chip with an array of stretchable alveoli-like membranes [125].
2. State-of-the-art: in vitro lung models
In vitro cell-based assays are widely used for preclinical studies, despite criticism concerning their inability to model accurately complex interactions of human cells in vivo [45]. Traditionally, submerged airway epithelial cell cultures under immersed conditions have long acted as a gold standard (Figure 1); such platforms have been considered as cell systems that mimic in vitro key events known to occur in vivo. Yet, the lack of realism exhibited with submerged cellular assays contrasts starkly with the in situ lung environment as the cellular makeup in airways evolves at an air-liquid interface (ALI) [42–44]. This situation has thus frequently led to misinterpretations and false conclusions [36,46]. Hence, the adoption of transwell inserts with cell cultures grown on porous membranes [1,2,47,48], spanning typical pore sizes ~0.4 to 3 μm that allow the transport of nutrients, growth factors and cell signaling molecules (e.g., cytokines, chemokines) [49], has attempted to bridge some of this gap and design in vitro assays where polarized epithelial airway cells are directly exposed to air on the apical side of the membrane (Figure 1). A number of recent reviews has extensively discussed the breadth of ALI-based in vitro models [37,45,50–57], including amongst other the type (e.g. human alveolar epithelial cells, etc.) and the origin (e.g. immortalized or primary, cancerous or normal with/out transformation, etc.) of the lung cell population as well as the relevance of integrating co-cultures to recapitulate the rich diversity in the cellular makeup (e.g. epithelium, dendritic cells, fibroblasts, endothelium, alveolar macrophages, etc.). Notably, ALI-based assays have been shown to contribute for instance to well-differentiated epithelial cellular populations [58], with stronger monolayer integrity and increased secretion of surfactant [59,60].
2.1. Recreating the alveolar barrier in vitro
The alveolar epithelium is built of two predominant cell types: namely, type I (AEC I) and type II (AEC II) alveolar epithelial cells (Figure 1). AEC I are squamous cells that form the cobblestone-like structure in the deep respiratory regions, making up over 90% of the alveolar surface [61,62]. Meanwhile, AEC II are progenitors to AEC I and secrete pulmonary surfactant (PS) containing 80% (by weight) phospholipids, including 50% 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 10% phosphatidyl-glycerol (PG), 10% other neutral lipids (particularly cholesterol) and 10% surfactant protein-A to –D [63]. Not only is PS essential to reduce surface tension at the ALI and prevent collapse of the airspace at resting lung pressures [36,64], it contributes to innate defense mechanisms [36] and plays a role in maintaining alveolar structure [65]. The integrity of the alveolar epithelium is also known to correlate with barrier quality via the complex of belt-like proteins of the tight junctions (TJ) [66]; the best characterized TJs being occludin and zonula occludens (ZO)-1. As the first line of defense lies at the alveolar airway barrier, exposure to irritants triggers a pro-inflammatory response of AECs and alveolar macrophages (AM) via release of intracellular effector molecules (e.g. interleukins). This cascade results in the activation of an innate immune response, including neutrophil recruitment from blood capillaries, increased phagocytosis and additional infiltration of AM [32,67]. Notoriously, lung inflammation leads to increased epithelial permeability as TJs are disrupted [32], defective phagocytosis [68,69] and elevated oxidative stress [10,70], amongst other.
Hence, one critical characteristic concerns the establishment in vitro of confluent epithelial layers with barrier functionality; a property that is typically quantified using permeability assays (e.g. apparent permeation flux Japp or apparent permeability coefficient Papp) and transepithelial electrical resistance (TEER) measurements, with units spanning ~100 to ~3’000 Ω·cm2 depending on the epithelial cell origin (e.g. A549, hAELVI, primary AECs, etc.). For therapeutic endpoints (Figure 2), recapitulating functional in vitro barrier properties is most pertinent in the context of lung absorption and disposition of inhaled drugs [71,72], as the lung epithelium constitutes the rate-limiting transport barrier for inhaled drugs and molecules [56,73,74]. In particular, the combination of co-cultures (e.g. AECs and endothelial cells) grown respectively on the apical and basal sides of a porous membrane has been shown to increase barrier tightness [75]. While exposure assays under submerged cell cultures have been traditionally conducted with liquid suspensions instilled directly onto the cellular model, the transition to ALI-based setups has led to more realistic exposure conditions. Indeed, the deposition of inhaled particulate matter (PM), e.g. via direct spraying [76–79], has underlined the importance of aerosolization, either liquid (e.g. nebulizer) or solid (e.g. dry powders), for various therapeutic and pharmacokinetic endpoints [80,81] (Figure 2); a point we will return to in our discussion below. For example, mimicking mechanisms of PM uptake at an ALI is not only critical but also known to significantly vary between mono- and co-cultures featuring immune cells, with preferential uptake by the latter relative to the airway epithelium itself [59,78,82].
2.2. Lung-on-chips
Following the advent of ALI-based assays, organ-on-chips have arguably catalyzed the biggest leap in novel in vitro lung designs (Figure 1); a field first epitomized over a decade ago with the seminal lung-on-chip model emulating the alveolar-capillary barrier (ACB), separating epithelial and endothelial monolayers cultured respectively on the apical and basal sides of a porous membrane “sandwiched” inside a flexible straight airway channel [83]. Hence, much of the technical developments of lung-on-chips leveraging various microfabrication techniques (e.g. photo- and soft-lithography, etc.) arose initially from efforts underlining cytotoxicity endpoints [7,36,84,85] (Figure 2). Unlike traditional in vitro setups (i.e. transwell inserts) that are largely static when considering the exchange and/or collection of media (e.g. analytics of inflammation), microfluidic systems offer the integration of continuous perfusion (e.g. culture media, drugs, etc.), and thus replicating relevant fluid-induced shear stresses, from either the basal (i.e. fluid) and/or apical (i.e. air) side of the membrane [86].
In recent years, the breadth of in vitro applications featuring microfluidic-based technologies has drawn pertinent reviews discussing opportunities for disease modeling, drug discovery and translational endpoints that span nearly all body organs [5,8,87–95]. In the most recent debate, the growing maturity of human-relevant organ-on-chips has raised an overarching question as to whether preclinical in vitro research is ready to bypass altogether in vivo animal validation studies [96], concurrently underlining the call for alternatives to animal experiments in the respiratory community [97]. This latter point also ensues from ethical and political considerations in line with the application of the ‘3Rs principles’ (Refinement, Reduction, Replacement) toward the highest standards for humane experimentation on animals.
3. The quest for advanced in vitro lung platforms
Despite impressive progress and the integration of human-relevant lung cells, the majority of lung-on-chip platforms still overwhelmingly feature cells (i.e. mono- or co-cultures) grown on planar 2D substrates, oftentimes employing the same or similar commercial porous membranes used in transwell inserts (Figure 1). Such planar cell cultures still come short of mimicking the intrinsic physiological 3D acinar microenvironment evocative of the deep lungs [61,98,99]. This has largely followed as microfabrication techniques leveraging extruded 2D planar geometries from soft-lithography with hard elastomeric such as poly(dimethylsiloxane) (PDMS) molds do not deliver biomimetic 3D lung microenvironments but rather integrate only some of the critical physiological and mechanical cues characteristic of the deep lungs [36,100]. We note that in the discussions that follow we do not include developments of in vitro organoids focusing on multicellular 3D cultures of stem cells and tumor cells with e.g. self-assembly capabilities that lie beyond the scope of our review but are the subject of extensive reviews elsewhere [94,95,101,102]. While bioengineering progress in the fields of angiogenesis and vascularization has successfully recreated fully-endothelialized in vitro lumens in 3D [103–107], to the best of our knowledge the same is not yet available when envisioning fully-epithelialized 3D lumens of the deep lung airways. There is thus a need to move beyond the state-of-art and comprehensively recapitulate underlying traits of the 3D pulmonary acinar microenvironment (Figure 3). In the sub-sections below, we introduce these leading traits and discuss some of the most recent advancements in delivering advanced in vitro pulmonary platforms.
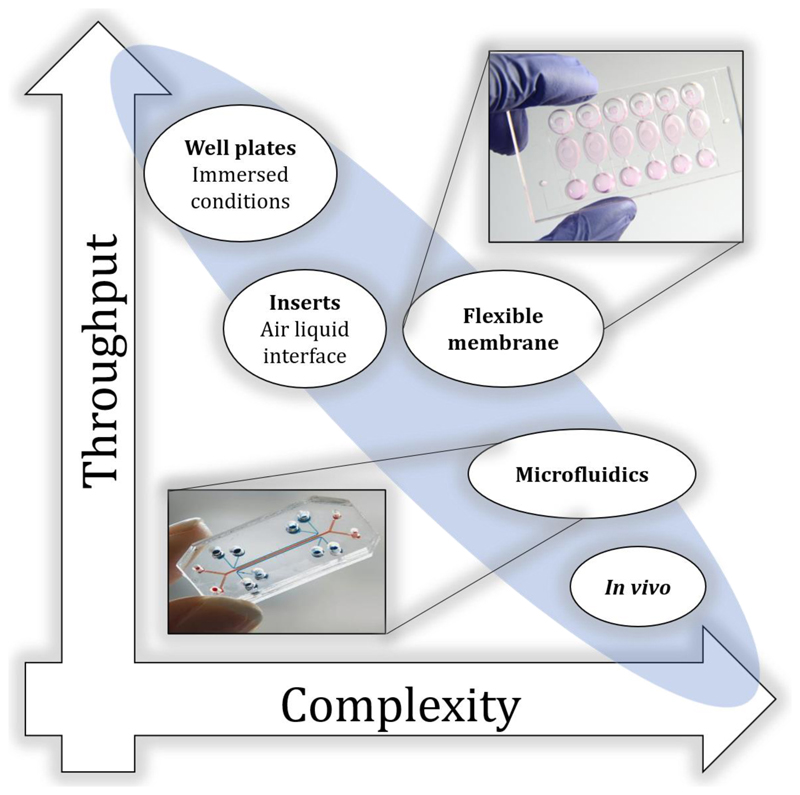
Figure 3.
In vitro approaches currently exhibit a trade-off between throughput capacity and physiological and biological complexity. The feasibility for high-throughput data collection decreases as the in vitro model design mimics physiological and biological characteristics of the lungs in the effort to recapitulate more closely in vivo models, and humans in particular.
3.1. The 3D pulmonary acinar morphology
The pulmonary acinus embodies the branched complex of alveolated airways that first appear at the level of the respiratory bronchioles [98,108], approximately beyond the 15th bifurcating generation of the respiratory tract. Encompassing over 90% of total lung volume with a vast surface area ~100 m2 in an average human adult [62], the pulmonary acinus is designed for efficient oxygen and carbon-dioxide transfer across a very thin alveolar-capillary barrier (~0.5-1 μm). Alveolar cavities form a densely-packed sleeve around the acinar ducts with typical dimensions of ~100-200 μm, where adjacent alveoli are separated by the thin inter-alveolar septum (i.e. alveolar wall) [42,99]. While the shapes of alveoli are intrinsically heterogeneous, the pulmonary acinus has often been compared to honeycomb-like structures comprising hollow polyhedral-shaped cavities [109]. Here, incorporating morphological elements of the true scale 3D acinar airway topology (e.g. alveolar cavities) is crucial towards recapitulating in full 3D cellular functions at the ALI and across the ACB in vitro. In contrast to cellular monolayers grown on 2D substrates both with traditional assays and the majority of existing lung-on-chips, complex interactions between cells inside 3D topologies are known to influence cell properties, protein secretion and gene expression amongst other [110].
A number of microfluidic models have highlighted anatomically-inspired designs of alveolated airway channels [111,112], culminating most recently in models reconstituting bifurcating acinar airway trees with functional epithelial barriers at an ALI [113]. Such structures are somewhat reminiscent of extruded 2D patterns of respiratory bronchioles with alveoli surrounding the main central airways but come short of exhibiting densely arranged alveolar cavities with shared intra-alveolar septa; a limitation of the leading microfabrication and molding techniques currently employed. By and large, most lung-on-chips have generally focused on designs of individual isolated airway channels that forego the detailed acinar topology [5,7,36,114]. Undeniably, the branching 3D space-filling nature of the gas-exchange region remains technically challenging to recreate in vitro. Unlike bioprinting approaches that are increasingly used in a number of tissue engineering disciplines [115,116], the intricate anatomy of the acinar airways remains widely prohibitive to print directly the relevant cellular makeup in morphologically-faithful scaffolds [36]. Seminal 3D printing efforts in the field have been largely limited to depositing monolayer sheets reminiscent of transwell inserts [117]. Whether future solutions will revolve around advanced 3D printing techniques to recapitulate acinar designs in vitro, a space-filling topology becomes most relevant when attempting to bridge the gap with the anticipated inhaled aerosol dose deposited on the 3D airway lumen [36,86,118]. This latter point holds direct ties with the transport and fate of inhaled aerosols in the lungs (see discussion in section 3.4).
3.2. The extra-cellular matrix (ECM)
The ECM serves as the 3D scaffold of all tissues and body organs. In addition to providing a physical scaffolding, the ECM plays a key role in morphogenesis, differentiation and homeostasis [119]. Notably, the integration of an in situ-like ECM is a key to mimic both cell-cell and cell-ECM contacts [48] and extends for example into remodeling phenomena in diseased conditions such as emphysema [120,121]. Amongst other, the ECM allows the transport of nutrients, metabolites and oxygen gradients [122]; critical properties in ultimately assessing drug effectiveness. Among the constituents of the ECM that include elastin and laminin, collagen is the most abundant protein (i.e. ~30% of the total protein mass of the tissue). It regulates cell adhesion, provides tensile strength, and supports chemotaxis and cell migration [121,123]. While collagen-rich hydrogel scaffolds have gained accrued use in various tissue engineering applications including grafts [115,116] (e.g. skin, cardiovascular, bone, etc.), current ALI-based in vitro lung models have instead been mostly limited to coating ECM proteins (e.g. collagen layer) onto the apical and basal sides of the porous membranes acting as the cell substrate.
Recalling that the epithelial and endothelial cell layers are separated in vivo by a very thin basement membrane (~50 nm) critical for efficient gas exchange from air into capillary blood (Figure 1), the total mean thickness of the ACB is a mere ~1 μm [49]. In contrast, current ALI-based assays make most often use of commercially-available polycarbonate (PC) and polyethylene terephthalate (PET) membranes that are typically ~10-20 μm thick and thus result in additional resistance to respiratory gas and macromolecular exchange; note that custom-designs of artificial membranes using for example PDMS can be even thicker (~50-100 μm) [124]. Recent developments in biomaterials are now pushing the limits to achieve thinner constructs but the discrepancy with the in situ environment still confines in vitro approaches to accurately model transport properties across the ACB [72]. Of significance, a novel design has featured a stretchable and biodegradable membrane formed by drop-casting a collagen-elastin solution onto a gold mesh [125], where it spreads and is maintained by the action of surface tension alone. With a thickness of just a few microns the dried solution is suspended on the hexagon-shaped mesh. Primary human alveolar epithelial cells were then co-cultured with primary human lung endothelial cells and demonstrated in vivo-like ACB functions (Figure 2f), thereby setting an exciting new precedent.
3.3. Mechanical strains of the acinar lumen
Acinar wall distensions are an intrinsic property of breathing motions of the lung parenchyma [108]. Such movements induce constant cyclic strains recognized to stimulate maturation of the airway epithelium [126]. The mechanotransduction signaling pathways involved with such strains have been the subject of recent reviews [49,124,127] and are linked to cellular phenotypes and function such as surfactant secretion, epithelial barrier integrity and immune response amongst other [77,83,128]. For example, cytokine secretions have been shown to increase with the inclusion of mechanical strains relative to static conditions [128]. Furthermore, mechanical strains play a tangible role in enhancing PM transport across the ACB [83]; a point most recently underlined as static in vitro assays may underestimate cellular uptake and transbarrier transport of nanoparticles in the lungs [129]. Physiological breathing strains lie typically in the range of ε~5-10% (i.e. linear strains) for quiet breathing conditions [108] but may be significantly increased under increased physical activity or alternatively in diseased lung conditions such as in ARDS (~15-20%) [124]. In the latter cases, large strains can open tight junctions and disrupt barrier integrity across the alveolar epithelium by activation of intracellular signaling pathways [66,130].
Ideally, breathing motions should be recapitulated via mechanical strains applied across an entire 3D airway lumen. Instead, lung-on-chip models (Figure 1) have been widely limited to stretching cells on flat porous membranes or curved 2D surfaces using for example diaphragm-like actuation mechanisms [77,125,128,131]. Nevertheless, the types of mechanical strains reproduced with existing microfluidic models [124] are broad (i.e. unidirectional, bi-directional and even three-dimensional) but still contrast with the idea of recapitulating a confluent epithelial barrier across a 3D airway lumen. Developments in delivering more advanced membrane technologies are now seeing a significant push [77,125,129,132]. For example, combining a micro film (thermo)forming and ion track technology Baptista et al. have introduced curved track-etched membranes, exemplifying spherical geometries reminiscent of acinar structures [133]. Typically, these and other “soft” membranes can be integrated into relatively large arrays supporting the prospect of large throughput assays at an ALI [86], but are however restricted to geometrical constructs that largely forego the intrinsic lung anatomy (i.e. bifurcating airway tree, etc.). In comparison, lung-on-chips featuring more complex airway networks (Table 1) suffer in the lack of throughput capacity (Figure 3). Even the more advanced porous membrane technologies exhibit a stiffness (i.e. Young’s modulus in the range of 1 MPa) that is several orders of magnitude larger than the nominal elastic modulus reported for both healthy alveolar tissue (~1-5 kPa) and under pathological conditions (~15-20 kPa) such as idiopathic pulmonary fibrosis [49]. Moreover, the need for rather complex actuation systems (e.g. vacuum pressure pumps) in conjunction with advanced microfabrication techniques to produce such membranes are still hampering a wider spread use of such setups across the respiratory research community.
Table 1. Overview of advanced in vitro pulmonary platforms, based on their underlying technology (category), physical cues, cellular makeup and type of exposure assay.
| Category | Physical cues | Exposure assay | Cellular makeup | Ref |
|---|---|---|---|---|
| Stretchable membranes |
-mechanical stretching -ALI |
Instillation | -Bronchial epithelial 16HBE14o - Primary human lung microvascular endothelial cells (VeraVec) -Primary human alveolar epithelial cells (hAEpCs) |
[77] |
| -Bronchial epithelial 16HBE14o- cells -primary human pulmonary alveolar epithelial cells (pHPAEC) - primary human umbilical vein endothelial cells (pHUVEC) |
[128] | |||
| -mechanical stretching -ALI |
Instillation | - VeraVec - hAEpCs |
[125] | |
| -mechanical stretching |
N/A | - A549 | [132] | |
| -mechanical stretching -ALI |
Nebulized aerosols |
-A549 -16HBE14o- cells |
[129,231] | |
| N/A | [232] | |||
| Microfluidic isolated channels |
-mechanical stretching -fluid flow |
Instillation | -Human pulmonary microvascular endothelial cells -Alveolar epithelial cells (NCI-H441) |
[172] |
| -fluid flow -ALI |
Instillation | Human Umbilical Vein Endothelial Cells from pooled donors (HUVECs) Human Lung Microvascular Endothelial Cells (hMVECh, CC-2527) Primary alveolar epithelial cells (type I and II cells) |
[173] | |
| -fluid flow -ALI |
Instillation | -Primary human airway epithelial cells (hAECs) - Human umbilical vein endothelial cells -Primary neutrophils |
[174] | |
| -fluid flow -ALI |
Instillation | -hAECs -hMVECs, HUVECs -Primary neutrophils |
[176] | |
| -air Flow | Instillation | - | [233] | |
| -mechanical stretching -ALI -fluids flow |
Instillation | - Human pulmonary microvascular endothelial cells -Alveolar epithelial cells: NCI H441 A549 E10 -Primary neutrophils |
[83] | |
| -fluids flow -AIL |
Instillation | -Primary tracheo-bronchial epithelial cells (AE) -Human lung microvascular endothelial cells - Primary human lung fibroblasts |
[234] | |
| -fluids flow -air flow |
Dry aerosol |
- Primary human small airway epithelial cells |
[235] | |
| -mechanical stretching -ALI -fluid flow |
Instillation | - Human primary airway epithelial cells - Primary human primary alveolar epithelial cells -human lung microvascular endothelial cells - H1975 NSCLC tumor cells |
[236] | |
| -fluid flow | N/A | -A549 cells | [237] | |
| N/A | Instillation | - HUVECs -A549 -HFL1 |
[238] | |
| 3D Microfluidic channels |
N/A | Instillation | -primary human bronchial epithelial (HBEC) - Human lung microvascular endothelial cells (LMVEC) -normal pulmonary fibroblasts (NPF) - primary polymorphonuclear cells (PMNs) |
[239] |
| -fluid flow | Instillation | - Immortalized human alveolar epithelial cells (HPAEpiCs) - Primary human umbilical vein endothelial cells |
[240] | |
| Acinar tree | -wall strains -fluid flow |
Instillation | N/A | [137] |
| -ALI | Nebulized droplets |
-hAELVi cells -THP-1 |
[113] | |
| Airway tree | -ALI | Dry Aerosol |
-Normal Human Bronchial Epithelial (NHBE) cells |
[148] |
3.4. Physiological respiratory airflows and inhaled aerosol transport
Inhalation airflows are a cornerstone of aerosol transport phenomena in the lungs and result from transpulmonary pressure-differences at the origin of parenchymal wall distensions [108]. In the deep respiratory regions, the interplay between the acinar anatomy (e.g. alveolar cavities, see section 3.1) and oscillatory wall strains (see section 3.3) gives rise to 3D airflows showcasing amongst other intricate topologies that evolve with increasing generation depth along the acinar tree [35,134–136]. Quantitative flow visualization studies using micro particle imaging velocimetry (μPIV) in microfluidic models of alveolated trees have supported the existence of slow yet complex vortical flows inside alveolar cavities, with a gradual crossover to radial-like flow streamlines in the deeper acinar generations [137]. In such models, breathing motions were matched to physiologically-realistic strains via thin deformable PDMS walls separating acinar ducts and alveoli from external actuating chambers.
The coupling between respiratory inhalation flows and the relevant transport mechanisms of inhaled PM are known to determine local deposition outcomes in the lungs [35,108,138]. Notably, the mechanistic transport determinants for a given airborne particle (i.e. aerodynamic size, shape, etc.) include foremost convection via breathing (i.e. viscous drag), sedimentation and Brownian diffusion (for equivalent diameters approx. <1 μm) in the deep lung regions [108,139–141]. As these transport mechanisms operate within an intricate 3D airway network covering a breadth of length scales within a space-filling volume, combined with a wide range of gravitational orientations (e.g. apical vs. basal lung lobes), PM deposition patterns in the deep lungs are known to be complex and spatially heterogeneous [35,118,136,142]. Here, microfluidic acinar airway models have enabled seminal quantitative in vitro studies of aerosol (PM) deposition patterns under physiological breathing conditions providing for the first time, temporally-resolved tracking of airborne particle flight within alveolar cavities [143].
Undeniably, ALI-based efforts have moved away from traditional instillation assays directly on cell cultures and instead delivered directly aerosols via techniques including (i) direct spraying, (ii) cloud settling or sedimentation, and (iii) impingement or direct spraying, as recently reviewed [72]. Yet, current in vitro gold standards including now “soft” membrane-based ALI models (Table 1) still come short of replicating the true inhaled “airborne journey” of aerosols in the deep lungs resulting from aerosol transport and deposition [86]. This includes importantly accounting for the dispersion and loss of inhaled aerosols as they are “screened” along the respiratory tract [35,144–147]. Most recently, individual lung-on-chip models mimicking bifurcating airway trees at real scale with an airway epithelium differentiated at the ALI were integrated within a larger 3D printed anatomically-realistic physical airway tree model [148]. The in situ-like inhalation exposure setup was shown to replicate the anticipated mechanistic journey of airborne PM under physiological inhalation airflows in a multiscale structure of the human airways. Such efforts are not only advancing in vitro-in silico correlations of PM deposition but importantly, they support the prospect of realistic in vitro PM exposure assays to emulate more faithfully local 3D deposition outcomes characteristic of in vivo inhalation in humans.
4. Therapeutic applications in advanced preclinical in vitro platforms
4.1. Inhalation therapy
Aerosol therapy via inhalation is a hallmark of respiratory medicine and a cornerstone for treating lung diseases topically, including the use of e.g. β2-agonists, corticosteroids, antibiotics and mucolytics [149–152]. Concurrently, with a vast surface area and direct access to the entire circulation across the thin ACB, the lungs are an ideal port of entry for systemic delivery, gene therapy and vaccines amongst [153–157]. Advantages of aerosol inhalation include on the one hand the delivery of small molecules with rapid action, low metabolism and high bioavailability, while on the other hand macromolecules can be delivered by avoiding invasive intravenous or intramuscular injections [158,159]. Compared with oral administration, inhalation modalities bypass the toxicity of the digestive system, which in many cases degrades the drug and, in most cases, achieve a similar or superior effect with a much lower dosages [160]. Yet, pulmonary delivery systems still exhibit various drawbacks. For example, the small number of excipients approved for inhalation therapy leads to an increase in failure rates of many new formulations, given requirements for isotonicity, sterility, pH restrictions (ranging between 3 and 8.5), biocompatibility and good aerosolization properties [161]. Importantly, and despite its widespread use, inhalation therapy still suffers from low if not dismal deposition efficiencies with the use of common inhalers [138,162], in particular in younger populations [163,164].
To overcome these pharmaceutical and regulatory hurdles, as well as explore opportunities for new therapeutic treatments (Table 2), advanced pulmonary in vitro platforms are accelerating our understanding of the challenges associated with both cellular (e.g. uptake, translocation, modulating inflammatory mediators, etc.) and non-cellular aspects (e.g. respiratory flow transport, aerodynamic aerosol properties, etc.) of the lungs. In conjunction with our earlier discussion (see section 3.4), the prospect of realistic exposure assays holds promise to recapitulate the anticipated deposition efficiencies of inhalation therapy and ultimately explore strategies for improved targeted drug delivery to the lungs. This includes for example selecting aerosol sizes and shape [141,165] and is most critical when seeking to localize deposition towards improved topical delivery (e.g. diseased airways, tumor or nodule). Indeed, large quantities of inhaled drugs are commonly lost during inhalation and/or lead to superfluous deposition in undesired airways or lung lobes, thus undermining the greater potential of inhalation therapy [166,167]. Moreover, reducing lung toxicities and associated side effects is still challenging when considering for example inhaled chemotherapies [168–170]. Although important progress has been unfolding [72,86,171], delivering in vitro models with strong human in vivo correlation remains a major challenge in advancing therapeutic endpoints (Figure 2).
Table 2. Currently available therapeutic applications using advanced in vitro lung-on-chip platforms.
| Disease | Mechanism of action | drug | Ref |
|---|---|---|---|
| Pulmonary edema | Promotes angiogenesis, remodeling, and repair of the vascular system |
Angiopoietin 1 (Ang-1) co-administered with IL-2 |
[172] |
| Ion channel inhibitor | TRPV4 channel blocker GSK2193874 |
[172] | |
| Pulmonary thrombosis |
antithrombotic and anti-inflammatory therapeutic |
Parmodulin-2 (PM2) | [173] |
| COPD + Asthma (HRV induced asthmatic response) |
anti-inflammatory | Bromodomain Containing Protein 4 (BRD4) inhibitor Tofacitinib/ Dexamethasone |
[174] |
| Viral-induced asthma exacerbation |
Navarixin (MK-7123) | [176,241] |
4.2. From advanced in vitro models to therapeutic applications
Perhaps the best known work on therapeutic applications with lung-on-chips is that of Huh et al. [172] who tested with a lung-on-chip a pharmacological agent, i.e. Angiopoietin 1 (Ang-1), to prevent IL-2 induced vascular leakage in a pulmonary edema model. Notably, Ang-1 was shown when co-administered with the cytokine IL-2 to stabilize the endothelial junction and halt entirely vascular leakage. Furthermore, the drug prevented the formation of paracellular gaps despite the presence of cyclic mechanical strains. The authors went on to examine whether the pharmaceutical inhibition of TRPV4 channels with a newly-developed pharmacological agent (GSK2193874, GlaxoSmithKline) could prevent the exacerbation of IL-2-induced permeability owing to cyclic mechanical strains; such strains are known to activate TRPV4 ion channels and the stimulation of these channels can increase alveolar-capillary leakage. When administered intravascularly through the basal channel of the in vitro model, the compound inhibited leakage, underlining the translational value as a prospective treatment option for patients with pulmonary edema who are being mechanically ventilated. In parallel, Jain et al. [173] leveraged the same lung-on-chip model to explore human thrombotic responses to a new therapeutic protease activated receptor-1 inhibitor, i.e. Parmodulin-2 (PM2), that exhibited cyto-protective and antithrombotic properties. Specifically, the authors observed that PM2 treatment of the endothelium significantly decreased thrombotic formation.
Another novel lung-on-chip approach was recently used to detect the effect of several anti-inflammatory drugs on the pulmonary endothelium and epithelium using primary cells isolated from healthy individuals or people with COPD [174]. When an experimental compound, i.e. Bromodomain Containing Protein 4 (BRD4) inhibitor, was tested on the inflamed cells the authors found significant suppression of neutrophil adhesion due to a reduction in the expression of adhesion molecules (E-selectin), vascular cell adhesion protein-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1). BRD4 inhibitor downregulated the expression of cytokine genes (e.g. IL-6 and -8) and significantly decreased the secretion of cytokines and neutrophil chemokine (GM-CSF). Furthermore, the in vitro airway was exposed to IL-13 to produce asthmatic changes in the epithelium, followed by the administration of high doses of tofacitinib (a potent inhibitor of JAK1, -2 and -3) which suppressed goblet cell hyperplasia and decreased secretion of G-CSF and GM-CSF. In contrast, dexamethasone was found to be ineffective using the same protocol. These in vitro results were consistent with the clinical observation that pediatric asthmatic patients often fail to respond to inhalation therapy with dexamethasone [175]. Finally, Nawroth et al. [176] used a commercially-available chip (Emulate) to create a micro-engineered model of fully-differentiated human mucociliary airway epithelium. Stimulation with IL-13 induced a Th2-type asthmatic phenotype and by infecting the cells with live human rhinovirus 16 (HRV16) the authors reproduced clinical features of viral-induced asthma exacerbation. Treatment with MK-7123, a CXCR2 antagonist, reduced the proportion of transmigrating neutrophils, by reducing neutrophil adhesion to the endothelium.
5. Opportunities for respiratory therapeutics with inhaled liposomes
In recent years, the challenges and opportunities facing the drug discovery process have given rise to vibrant discussions in delivering novel inhaled drugs [6,72,154,158,169,177], with therapeutic endpoints geared at respiratory diseases such as COPD, tuberculosis, idiopathic pulmonary fibrosis (IPF), cystic fibrosis, bacterial, fungal and viral lung infections and chemotherapies amongst other. The breadth of new and repurposed inhaled pharmaceutical compounds (e.g. anti-inflammatory corticosterioids, antibacterial agents, antibiotics, long-acting beta agonists, long-acting muscarinic receptor antagonists, etc.) has been the subject of extensive discussions available therein. In this context, we focus here on one exciting therapeutic avenue where advanced in vitro lung platforms can offer tangible paths in preclinical respiratory research, namely with inhaled liposomes and lipid-based therapies (Figure 4). Liposomes, first described as nano-sized lipid vesicles [178,179], consist of concentric phospholipid bilayers that can be enriched with cholesterol, other lipids and biopolymers, thereby forming both hydrophilic and hydrophobic domains [180]. By leveraging such unique structures, lipophilic compounds can be encapsulated inside the lipid bilayer with hydrophilic compounds simultaneously loaded inside the aqueous core, thus enabling to encapsulate a wide range of therapeutics [181]. In the years since, advances in liposomal technology have accelerated applications across various pharmaceuticals areas [182,183].
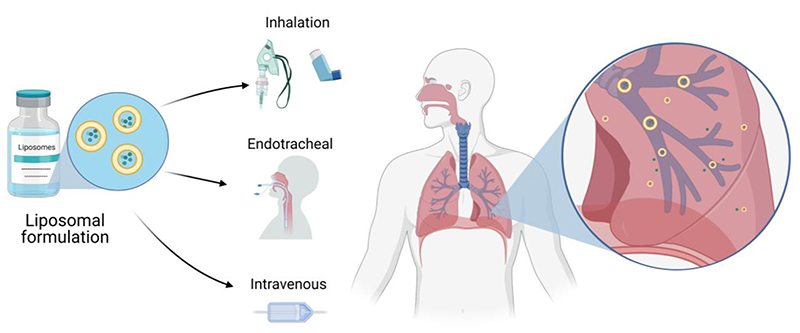
Figure 4.
Liposome-based therapies for treatment of respiratory diseases including method of administration.
5.1. Liposomes for lung therapies
Liposomes have attracted significant attention in pulmonary delivery (Table 3) with the ability to entrap drugs inside vehicles that have a lipid composition similar to PS [184–186]. As the therapeutic efficacy of inhaled liposomes is affected by the ability to overcome the lungs’ biological barriers (e.g. ALI, AMs, etc.) [160], liposomal formulations with high similarity to PS are considered pharmacologically favorable with respect to toxicity and antigenicity [187,188]. While liposomes are used to reduce systemic toxicity from the encapsulated drugs, variations in types and ratios of phospholipids can be leveraged to modulate immune responses [189,190] whereas the liposomal surface-charge (zeta potential) and inclusion of other immune stimulatory factors play a role in immune activation [191,192]. Concurrently, the particle composition, size, morphology, surface characteristics, surface targeting moieties also affect the liposome performance [193–195]. As the biodistribution of particles is strongly influenced by their diameter, we recall as a rule of thumb that inhaled particles with aerodynamic diameters below ~2-3 μm are acknowledged to deposit preferentially in the deep respiratory regions [108,138].
Table 3. Overview of liposomal applications for lung disease treatments.
| Application | Formulation | Development stage |
Administration | Ref |
|---|---|---|---|---|
| Mycobacterium avium complex (MAC) lung disease |
DPP C :Cholosterol liposomal amikacin, 300 nm |
FDA approved | Inhalation | [196–199] |
| Lung cancer | PEG-modified liposomal Irinotecan, formulated by utilizing sucrose octasulfate gradient, 110 nm |
Now in Phase III clinical trial for lung cancer |
Intravenous | [205] |
| PEG-modified liposomal cisplatin, 110 nm |
Phase III clinical trial |
Intravenous | [206–208] | |
| Dual-ligand anti-CA IX antibody and CPP33 liposomal Triptolide (TPL), 137 nm |
Pre-clinical | Endotracheal | [209] | |
| Lung inflammation |
IgG-modified liposomal dexamethasone, 103 nm |
Pre-clinical | Intravenous | [202] |
| Cystic fibrosis (CF) |
PEG-modified liposomal nanoparticle encoding mRNA of CFTR protein |
Phase I/II clinical trial |
Inhalation | [214] |
| Cationic GL67A liposomes with plasmid DNA encoding CFTR protein |
Phase I clinical trial |
Inhalation | [223,224] | |
| Epithelial targeted liposome with ENaC siRNA, 200 nm |
Pre-clinical | Inhalation | [242] | |
| Neonatal SP-B deficiency |
Cationic liposomes with SP- B mRNA |
Pre-clinical | Intravenous | [215] |
| Chronic obstructive pulmonary disease (COPD) |
Self-assembling ionizable lipid nanoparticle with mRNA for a1-antitrypsin (AAT) |
Pre-clinical | Intravenous | [216,243] |
| Asthma | Liposomes encapsulating salbutamol sulfate |
Pre-clinical | Inhalation | [203,204] |
Liposomes are already in clinical use for treating several lung disorders. For example, Amikacin liposome suspension is an FDA-approved antibacterial formulation administered via inhalation for treating pulmonary infections such as Mycobacterium avium complex (MAC) lung disease [196,197]. These liposomes are composed of DPPC and cholesterol at a 2:1 weight ratio with a reported diameter of 300 nm to allow effective diffusion through 500-1’000 μm of human airway mucus [198]. Notably, the amikacin concentration retained has been found higher in airways and lung tissue after 24 h compared to non-liposomal inhaled amikacin [199]. Preclinical studies have shown that encapsulated liposomal glucocorticoids can be used for treating inflammatory lung disorders and decrease glucocorticoids side effects [200,201]. For example, encapsulated dexamethasone that was administered intravenously to treat lung inflammation demonstrated local release into the lungs and inhibited most parameters of lung inflammation as effectively as free dexamethasone [202]. Encapsulation into liposomes also extended the effect of anti-asthmatic medications (e.g. Salbutamol sulfate, a selective β2-adrenergic receptor agonist) [203,204].
In parallel, liposomal drugs are used in cancer treatments due to their ability to accumulate in tumors and to reduce side effects. An Irinotecan liposomal formulation is undergoing a Phase III trial for treating small-cell lung cancer. This formulation was already approved by the FDA in the treatment of pancreatic cancer [205]. Recently, liposomal cisplatin has been evaluated for treating non-small cell lung cancer via intravenous administration [206,207]. Cisplatin liposomes with 110 nm in diameter was shown to reduce cisplatin toxicity and enhance its tumor targeting after intravenous injection [208]. Possible future applications may be the inhalation of such liposomes for direct treatment of lung tumors and metastases and would call for new in vitro lung platforms as preclinical screening assays. Indeed, lung-specific targeting could be enhanced via modified liposomes (e.g. antibody conjugation) that induce uptake of the liposomes by certain cell populations. Notably, effective targeted delivery of lung cancer drugs was demonstrated using dual-ligand modified liposomes where antibody and tumor lineage-homing cell-penetrating peptides were used to enhance tumor-specific targeting and increase tumor cell penetration [209].
5.2. Lipid-based pulmonary gene therapy and vaccination
The SARS-CoV-2 (COVID-19) pandemic has brought lipid-based delivery into the spotlight with the recently FDA-approved COVID-19 lipid-based nanoparticle mRNA vaccines [210]. The lipid nanoparticle envelopes the strands of mRNA and helps them evade biological degradation and reduce immunogenicity [211,212]. mRNA delivery followed by protein translation into functional form within a targeted cell’s cytoplasm enables protein replacement therapy. With such tremendous progress, there is an urgent need in addressing genetic diseases where endogenous proteins are defected or missing. Examples of potential diseases include cystic fibrosis (CF), neonatal surfactant protein B (SP-B) deficiency, and multifactorial diseases such as COPD [213]. In CF, inhalable mRNA lipid formulations are being evaluated clinically to test lung function improvement [214]. In SP-B deficiency, cationic mRNA liposomes restored 72% of the wild-type SP-B expression and improved survival in mice, when administered intratracheally [215]. In parallel, mRNA delivery shows hope also for treating COPD, where mRNA of a1-antitrypsin (AAT) lipid nanoparticles demonstrated increased AAT levels and lung function in vivo [216].
Gene replacement can also be achieved by encapsulating plasmid DNA. This approach is being evaluated for treating lung cancer [217] and inflammatory diseases [218]. Inhaled gene-expressing plasmids therapy gives hope to chronic airway inflammatory disease such as allergic asthma, where a combination of mucus penetrating nanoparticle demonstrated anti-inflammatory and anti-fibrotic effects [219,220]. The potential for gene therapy to correct the underlying cause of CF disease by expressing the missing CFTR (cystic fibrosis transmembrane conductance regulator) gene is promising [221]. These approaches are reaching the clinic: for example, CF complementary DNA (cDNA) based treatments [222] were reported in patients who received cationic liposomes GL67A therapy, delivered by inhalation [223]. GL67A cationic lipid mixture is currently the preferred option in CF aerosol gene delivery with highest levels of expression [222,224].
Despite clinical successes, there is a growing urgency for developing additional therapeutic strategies for treating people with rare and complex lung impairments where preclinical in vitro pulmonary research holds a promising role [225]. Small interfering RNA (siRNA) is a gene silencing mechanism that holds great promise for downregulating malignant genes in the lungs [226]. Lipid-siRNA nanoparticles targeted to the liver disorders have been approved by the FDA to treat hereditary TTR-mediated amyloidosis (hATTR), a condition caused by extracellular deposits of misfolded transthyretin protein (TTR) [227]. Concurrently, siRNA is being tested for treating lung disorders including COPD [214]. In allergic asthma, dexamethasone delivered concomitantly with siRNA demonstrated reduction of airway inflammation in vitro [228]. siRNA developed to target NF-kB and related pathways, reduce inflammation as well as target genes involved in mucus hypersecretion, are now promising approaches [229].
6. Conclusions
Over the past years we have witnessed exciting developments with lung-on-chip platforms including notably biocompatible “soft” membrane-based technologies (Table 1). These new systems testify to the significant progress witnessed in delivering novel bioengineering solutions for preclinical in vitro respiratory research, in contrast to more traditional preclinical in vitro cell cultures (Figure 1). Undeniably, only a very limited number of studies to date has explored new therapeutic applications using advanced in vitro lung-on-chip platforms. On the one hand, the current state-of-the-art attests to the relative infancy of this field altogether. On the other hand, most of the recent efforts witnessed have largely focused on first establishing these novel bioengineered platforms, including the technology itself and the integration of a human-relevant pulmonary cellular makeup. This is widely the case for example with the newest “soft” membrane-based assays that have yet to be leveraged in conducting therapeutic screens. There still remains a recognized trade-off between the physiological and biological complexity of these novel in vitro lung models and their ability to deliver assays with considerable, if not high, throughput capabilities for screening assays (Figure 3). Bridging this gap is undeniably still a challenge in conjunction with strengthening the in vivo relevance of such studies. Yet, the few seminal pulmonary in vitro studies available have delivered striking preclinical results of significant value and interest to the respiratory research community and beyond (Table 2). The upcoming years are thus anticipated to see further developments in broadening the applicability of such in vitro systems and accelerating therapeutic screens for drug discovery and translational applications in treating respiratory disorders. Here, we have stressed one, among several, exciting avenue with liposome-based respiratory therapies (Table 3).
Acknowledgement
This work was supported by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement No 677772).
Footnotes
Conflict of interest
The authors declare no conflict of interest
References
- [1].Hittinger M, Juntke J, Kletting S, Schneider-Daum N, de Souza Carvalho C, Lehr CM. Preclinical safety and efficacy models for pulmonary drug delivery of antimicrobials with focus on in vitro models. Adv Drug Deliv Rev. 2015;85:44–56. doi: 10.1016/j.addr.2014.10.011. [DOI] [PubMed] [Google Scholar]
- [2].Hittinger M, Schneider-Daum N, Lehr CM. Cell and tissue-based in vitro models for improving the development of oral inhalation drug products. Eur J Pharm Biopharm. 2017;118:73–78. doi: 10.1016/j.ejpb.2017.02.019. [DOI] [PubMed] [Google Scholar]
- [3].Prakash YS, Halayko AJ, Gosens R, Panettieri RA, Camoretti-Mercado B, Penn RB, Aiyar R, Ammit A, Berkman N, Bond R, Brown R, et al. An official American thoracic society research statement: Current challenges facing research and therapeutic advances in airway remodeling. Am J Respir Crit Care Med. 2017;195:e4–e19. doi: 10.1164/rccm.201611-2248ST. [DOI] [PubMed] [Google Scholar]
- [4].Barnes PJ, Bonini S, Seeger W, Belvisi MG, Ward B, Holmes A. Barriers to new drug development in respiratory disease. Eur Respir J. 2015;45:1197–1207. doi: 10.1183/09031936.00007915. [DOI] [PubMed] [Google Scholar]
- [5].Benam KH, Dauth S, Hassell B, Herland A, Jain A, Jang K-J, Karalis K, Kim HJ, MacQueen L, Mahmoodian R, Musah S, et al. Engineered In Vitro Disease Models. Annu Rev Pathol Mech Dis. 2015;10:195–262. doi: 10.1146/annurev-pathol-012414-040418. [DOI] [PubMed] [Google Scholar]
- [6].Strong P, Ito K, Murray J, Rapeport G. Current approaches to the discovery of novel inhaled medicines. Drug Discov Today. 2018;23:1705–1717. doi: 10.1016/j.drudis.2018.05.017. [DOI] [PubMed] [Google Scholar]
- [7].Ainslie GR, Davis M, Ewart L, Lieberman LA, Rowlands DJ, Thorley AJ, Yoder G, Ryan AM. Microphysiological lung models to evaluate the safety of new pharmaceutical modalities: a biopharmaceutical perspective. Lab Chip. 2019 doi: 10.1039/c9lc00492k. [DOI] [PubMed] [Google Scholar]
- [8].Ma C, Peng Y, Li H, Chen W. Organ-on-a-Chip: A New Paradigm for Drug Development. Trends Pharmacol Sci. 2021;42:119–133. doi: 10.1016/j.tips.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wisnivesky J, De-Torres JP. The global burden of pulmonary diseases: Most prevalent problems and opportunities for improvement. Ann Glob Heal. 2019;85:1–2. doi: 10.5334/aogh.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Barnes PJ, Burney PGJ, Silverman EK, Celli BR, Vestbo J, Wedzicha JA, Wouters EFM. Chronic obstructive pulmonary disease. Nat Rev Dis Prim. 2015;1:1–22. doi: 10.1038/nrdp.2015.76. [DOI] [PubMed] [Google Scholar]
- [11].Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet. 2017;389:1931–1940. doi: 10.1016/S0140-6736(17)31222-9. [DOI] [PubMed] [Google Scholar]
- [12].Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Chen R, Decramer M, Fabbri LM, Frith P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. Am J Respir Crit Care Med. 2017;195:557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- [13].Agustí A, Hogg JC. Update on the pathogenesis of chronic obstructive pulmonary disease. N Engl J Med. 2019;381:1248–1256. doi: 10.1056/NEJMra1900475. [DOI] [PubMed] [Google Scholar]
- [14].Celli BR, Decramer M, Wedzicha JA, Wilson KC, Agustí A, Criner GJ, MacNee W, Make BJ, Rennard SI, Stockley RA, Vogelmeier C, et al. An Official American Thoracic Society/European Respiratory Society Statement: Research questions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;191:e4–e27. doi: 10.1164/rccm.201501-0044ST. [DOI] [PubMed] [Google Scholar]
- [15].Barnes PJ, Stockley RA. COPD: current therapeutic interventions and future approaches. Eur Respir J. 2005;25:1084–1106. doi: 10.1183/09031936.05.00139104. [DOI] [PubMed] [Google Scholar]
- [16].Fehrenbach H. Animal models of pulmonary emphysema: a stereologist’s perspective. 2006;15:136–147. doi: 10.1183/09059180.00010104. [DOI] [Google Scholar]
- [17].Vardavas CI, Kyriakos CN, Fernández E, Bamidis P, Siddiqi K, Chavannes NH, Van Der Kleij RMJJ, Parker G, Radu-Loghin C, Ward B, Berkouk K. H2020 funding for respiratory research: Scaling up for the prevention and treatment of lung diseases. Eur Respir J. 2019;54 doi: 10.1183/13993003.01417-2019. [DOI] [PubMed] [Google Scholar]
- [18].Quinton LJ, Mizgerd JP. Dynamics of lung defense in pneumonia: Resistance, resilience, and remodeling. Annu Rev Physiol. 2015;77:407–430. doi: 10.1146/annurev-physiol-021014-071937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Boisvert AA, Cheng MP, Sheppard DC, Nguyen D. Microbial biofilms in pulmonary and critical care diseases. Ann Am Thorac Soc. 2016;13:1615–1623. doi: 10.1513/AnnalsATS.201603-194FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, Lawn JE, Cousens S, Mathers C, Black RE. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388:3027–3035. doi: 10.1016/S0140-6736(16)31593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Montefusco-Pereira CV, Carvalho-Wodarz C de S, Seeger J, Kloft C, Michelet R, Lehr CM. Decoding (patho-)physiology of the lung by advanced in vitro models for developing novel anti-infectives therapies. Drug Discov Today. 2020;26:148–163. doi: 10.1016/j.drudis.2020.10.016. [DOI] [PubMed] [Google Scholar]
- [22].Fan E, Beitler JR, Brochard L, Calfee CS, Ferguson ND, Slutsky AS, Brodie D. COVID-19-associated acute respiratory distress syndrome: is a different approach to management warranted? Lancet Respir Med. 2020;8:816–821. doi: 10.1016/S2213-2600(20)30304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Grasselli G, Tonetti T, Protti A, Langer T, Girardis M, Bellani G, Laffey J, Carrafiello G, Carsana L, Rizzuto C, Zanella A, et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Respir Med. 2020;8:1201–1208. doi: 10.1016/S2213-2600(20)30370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hasan SS, Capstick T, Ahmed R, Kow CS, Mazhar F, Merchant H a, Zaidi STR. Mortality in COVID-19 patients with acute respiratory distress syndrome and corticosteroids use: a systematic review and meta-analysis. Expert Rev Respir Med. 2020;14:1149–1163. doi: 10.1080/17476348.2020.1804365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Marshall M. The lasting misery of coronavirus long-haulers. Nature. 2020;585:339–341. doi: 10.1038/d41586-020-02598-6. [DOI] [PubMed] [Google Scholar]
- [26].Fraser E. Long term respiratory complications of covid-19. BMJ. 2020;370:m3001. doi: 10.1136/bmj.m3001. [DOI] [PubMed] [Google Scholar]
- [27].Cortinovis M, Perico N, Remuzzi G. Long-term follow-up of recovered patients with COVID-19. Lancet. 2021;397:173–175. doi: 10.1016/S0140-6736(21)00039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bart van der Worp1* MRMH, Howells2 David W, Sena2 3 Emily S, Porritt2 Michelle J, Rewell2 Sarah, O’Collins2 1 Victoria. Can animal models of disease reliably inform human studies? PLoS Med. 2010;7:e1000245. doi: 10.1371/journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Holmes AM, Solari R, Holgate ST. Animal models of asthma: Value, limitations and opportunities for alternative approaches. Drug Discov Today. 2011;16:659–670. doi: 10.1016/j.drudis.2011.05.014. [DOI] [PubMed] [Google Scholar]
- [30].Mestas J, Hughes CCW. Of Mice and Not Men: Differences between Mouse and Human Immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- [31].Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hogg JC, Timens W. The Pathology of Chronic Obstructive Pulmonary Disease. Annu Rev Pathol Mech Dis. 2009;4:435–459. doi: 10.1146/annurev.pathol.4.110807.092145. [DOI] [PubMed] [Google Scholar]
- [33].Fehrenbach H, Wagner C, Wegmann M. Airway remodeling in asthma: what really matters. Cell Tissue Res. 2017 doi: 10.1007/s00441-016-2566-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Miller AJ, Spence JR. In Vitro Models to Study Human Lung Development. Disease and Homeostasis. 2021:246–260. doi: 10.1152/physiol.00041.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Koullapis P, Ollson B, Kassinos SC, Sznitman J. Multiscale in silico lung modeling strategies for aerosol inhalation therapy and drug delivery. Curr Opin Biomed Eng. 2019;11:130–136. doi: 10.1016/j.cobme.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Tenenbaum-Katan J, Artzy-Schnirman A, Fishler R, Korin N, Sznitman J. Biomimetics of the pulmonary environment in vitro: A microfluidics perspective. Biomicrofluidics. 2018;12 doi: 10.1063/1.5023034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Nichols JE, a Niles J, Vega SP, Argueta LB, Eastaway A, Cortiella J. Modeling the lung: Design and development of tissue engineered macro-and micro-physiologic lung models for research use. Exp Biol Med (Maywood) 2014;239:1135–69. doi: 10.1177/1535370214536679. [DOI] [PubMed] [Google Scholar]
- [38].Guyette JP, Gilpin SE, Charest JM, Tapias LF, Ren X, Ott HC. Perfusion decellularization of whole organs. 2014;9:1451–1468. doi: 10.1038/nprot.2014.097. [DOI] [PubMed] [Google Scholar]
- [39].Nichols JE, Niles J, Riddle M, Vargas G, Schilagard T, Ma L, Edward K, La Francesca S, Sakamoto J, Vega S, Ogadegbe M, et al. Production and Assessment of Decellularized Pig and Human Lung Scaffolds. Tissue Eng. 2013;19:2045–2062. doi: 10.1089/ten.tea.2012.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gilpin SE, Wagner DE. Acellular human lung scaffolds to model lung disease and tissue regeneration. Eur Respir Rev. 2018;27:1–10. doi: 10.1183/16000617.0021-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Low LA, Mummery CL, Berridge BR, Austin CP, Tagle D. Organs-on-chips: into the next decade. Nat Rev Drug Discov. 2020 doi: 10.1038/s41573-020-0079-3. [DOI] [PubMed] [Google Scholar]
- [42].Weibel ER. Lung morphometry: the link between structure and function. Cell Tissue Res. 2017;367:413–426. doi: 10.1007/s00441-016-2541-4. [DOI] [PubMed] [Google Scholar]
- [43].Hsia CW, Hyde DM, Weibel ER. Lung Structure and the Intrinsic Challenges of Gas Exchange. Compr Physiol. 2016;6:827–95. doi: 10.1002/cphy.c150028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Weibel ER, Sapoval B, Filoche M. Design of peripheral airways for efficient gas exchange. Respir Physiol Neurobiol. 2005;148:3–21. doi: 10.1016/j.resp.2005.03.005. [DOI] [PubMed] [Google Scholar]
- [45].de Souza Carvalho C, Daum N, Lehr C-M. Carrier interactions with the biological barriers of the lung: Advanced in vitro models and challenges for pulmonary drug delivery. Adv Drug Deliv Rev. 2014;75:129–140. doi: 10.1016/j.addr.2014.05.014. [DOI] [PubMed] [Google Scholar]
- [46].van der Meer AD, van den Berg A. Organs-on-chips: breaking the in vitro impasse. Integr Biol. 2012;4:461. doi: 10.1039/c2ib00176d. [DOI] [PubMed] [Google Scholar]
- [47].Braakhuis HM, Kloet SK, Kezic S, Kuper F, Park MVDZ, Bellmann S, van der Zande M, Le Gac S, Krystek P, Peters RJB, Rietjens IMCM, et al. Progress and future of in vitro models to study translocation of nanoparticles. Arch Toxicol. 2015;89:1469–1495. doi: 10.1007/s00204-015-1518-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Bhowmick R, Gappa-Fahlenkamp H. Cells and Culture Systems Used to Model the Small Airway Epithelium. Lung. 2016:1–10. doi: 10.1007/s00408-016-9875-2. [DOI] [PubMed] [Google Scholar]
- [49].Doryab A, Tas S, Taskin MB, Yang L, Hilgendorff A, Groll J, Wagner DE, Schmid O. Evolution of Bioengineered Lung Models: Recent Advances and Challenges in Tissue Mimicry for Studying the Role of Mechanical Forces in Cell Biology. Adv Funct Mater. 2019;29:1–20. doi: 10.1002/adfm.201903114. [DOI] [Google Scholar]
- [50].Hasan S, Sebo P, Osicka R. A guide to polarized airway epithelial models for studies of host – pathogen interactions. FEBS J. 2018;285:4343–4358. doi: 10.1111/febs.14582. [DOI] [PubMed] [Google Scholar]
- [51].Nahar K, Gupta N, Gauvin R, Absar S, Patel B, Gupta V, Khademhosseini A, Ahsan F. In vitro, in vivo and ex vivo models for studying particle deposition and drug absorption of inhaled pharmaceuticals. Eur J Pharm Sci. 2013;49:805–18. doi: 10.1016/j.ejps.2013.06.004. [DOI] [PubMed] [Google Scholar]
- [52].Krimmer DI, Oliver BGG. What can in vitro models of COPD tell us? Pulm Pharmacol Ther. 2011;24:471–477. doi: 10.1016/j.pupt.2010.12.002. [DOI] [PubMed] [Google Scholar]
- [53].Lacroix G, Koch W, Larsen ST, Loret T, Zanetti F, Constant S, Chortarea S, Rothen-rutishauser B, Hiemstra PS, Frejafon E, Hubert P, et al. Air – Liquid Interface In Vitro Models for Respiratory Toxicology Research. 2018;4:91–106. doi: 10.1089/aivt.2017.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Faber SC, McCullough SD. Through the Looking Glass: In Vitro Models for Inhalation Toxicology and Interindividual Variability in the Airway. Appl Vitr Toxicol. 2018;4:115–128. doi: 10.1089/aivt.2018.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sundarakrishnan A, Chen Y, Black LD, Aldridge BB, Kaplan DL. Engineered cell and tissue models of pulmonary fibrosis. Adv Drug Deliv Rev. 2018;129:78–94. doi: 10.1016/j.addr.2017.12.013. [DOI] [PubMed] [Google Scholar]
- [56].Sporty JL, Horálková L, Ehrhardt C. Expert Opinion on Drug Metabolism & Toxicology In vitro cell culture models for the assessment of pulmonary drug disposition In vitro cell culture models for the assessment of pulmonary. 2008:5255. doi: 10.1517/17425255.4.4.333. [DOI] [PubMed] [Google Scholar]
- [57].Forbes B, Ehrhardt C. Human respiratory epithelial cell culture for drug delivery applications. Eur J Pharm Biopharm. 2005;60:193–205. doi: 10.1016/j.ejpb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- [58].Lehmann AD, Daum N, Bur M, Lehr C-M, Gehr P, Rothen-Rutishauser BM. An in vitro triple cell co-culture model with primary cells mimicking the human alveolar epithelial barrier. Eur J Pharm Biopharm. 2011;77:398–406. doi: 10.1016/j.ejpb.2010.10.014. [DOI] [PubMed] [Google Scholar]
- [59].Rothen-Rutishauser BM, Kiama SG, Gehr P. A three-dimensional cellular model of the human respiratory tract to study the interaction with particles. Am J Respir Cell Mol Biol. 2005;32:281–9. doi: 10.1165/rcmb.2004-0187OC. [DOI] [PubMed] [Google Scholar]
- [60].Kuehn A, Kletting S, de Souza Carvalho-Wodarz C, Repnik U, Griffiths G, Fischer U, Meese E, Huwer H, Wirth D, May T, Schneider-Daum N, et al. Human alveolar epithelial cells expressing tight junctions to model the air-blood barrier. Altex. 2016;33:251–260. doi: 10.14573/altex.1511131. [DOI] [PubMed] [Google Scholar]
- [61].Weibel ER. On the Tricks Alveolar Epithelial Cells Play to Make a Good Lung. Am J Respir Crit Care Med. 2015;191:504–513. doi: 10.1164/rccm.201409-1663OE. [DOI] [PubMed] [Google Scholar]
- [62].Weibel ER. The Pathway for Oxygen: Structure and Function in the Mammalian Respiratory System. Harvard University Press, n.d; [Google Scholar]
- [63].Nkadi PO, Merritt TA, Pillers D-AM. An overview of pulmonary surfactant in the neonate: genetics, metabolism, and the role of surfactant in health and disease. Mol Genet Metab. 2009;97:95–101. doi: 10.1016/j.ymgme.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Whitsett JA, Wert SE, Weaver TE. Diseases of Pulmonary Surfactant Homeostasis. Annu Rev Pathol. 2015;10:371–393. doi: 10.1146/annurev-pathol-012513-104644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Sharafkhaneh A, Hanania NA, Kim V. Pathogenesis of emphysema: From the bench to the bedside. Proc Am Thorac Soc. 2008;5:475–477. doi: 10.1513/pats.200708-126ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Cavanaugh KJ, Cohen TS, Margulies SS. Stretch increases alveolar epithelial permeability to uncharged micromolecules. Am J Physiol - Cell Physiol. 2006;290:1179–1188. doi: 10.1152/ajpcell.00355.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Brusselle GG, Joos GF, Bracke KR. New insights into the immunology of chronic obstructive pulmonary disease. Lancet. 2011;378:1015–1026. doi: 10.1016/S0140-6736(11)60988-4. [DOI] [PubMed] [Google Scholar]
- [68].Taylor AE, Finney-Hayward TK, Quint JK, Thomas CMR, Tudhope SJ, Wedzicha JA, Barnes PJ, Donnelly LE. Defective macrophage phagocytosis of bacteria in COPD. Eur Respir J. 2010;35:1039–1047. doi: 10.1183/09031936.00036709. [DOI] [PubMed] [Google Scholar]
- [69].Donnelly LE, Barnes PJ. Defective phagocytosis in airways disease. Chest. 2012;141:1055–1062. doi: 10.1378/chest.11-2348. [DOI] [PubMed] [Google Scholar]
- [70].Aridgides DS, Mellinger DL, Armstrong DA, Hazlett HF, Dessaint JA, Hampton TH, Atkins GT, Carroll JL, Ashare A. Functional and metabolic impairment in cigarette smoke-exposed macrophages is tied to oxidative stress. Sci Rep. 2019;9:9624. doi: 10.1038/s41598-019-46045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Sakagami M. In vivo, in vitro and ex vivo models to assess pulmonary absorption and disposition of inhaled therapeutics for systemic delivery. Adv Drug Deliv Rev. 2006;58:1030–1060. doi: 10.1016/j.addr.2006.07.012. [DOI] [PubMed] [Google Scholar]
- [72].Sakagami M. In vitro, ex vivo and in vivo methods of lung absorption for inhaled drugs. Adv Drug Deliv Rev. 2020;161-162:63–74. doi: 10.1016/j.addr.2020.07.025. [DOI] [PubMed] [Google Scholar]
- [73].Fernandes CA, Vanbever R, Fernandes CA, Vanbever R. Preclinical models for pulmonary drug delivery Preclinical models for pulmonary drug delivery. 2009:5247. doi: 10.1517/17425240903241788. [DOI] [PubMed] [Google Scholar]
- [74].Patton JS, Brain JD, Davies L a, Fiegel J, Gumbleton M, Kim K-J, Sakagami M, Vanbever R, Ehrhardt C. The Particle has Landed—Characterizing the Fate of Inhaled Pharmaceuticals. J Aerosol Med Pulm Drug Deliv. 2010;23:S-71–S-87. doi: 10.1089/jamp.2010.0836. [DOI] [PubMed] [Google Scholar]
- [75].Costa A, de Souza Carvalho-Wodarz C, Seabra V, Sarmento B, Lehr CM. Triple coculture of human alveolar epithelium, endothelium and macrophages for studying the interaction of nanocarriers with the air-blood barrier. Acta Biomater. 2019;91:235–247. doi: 10.1016/j.actbio.2019.04.037. [DOI] [PubMed] [Google Scholar]
- [76].Röhm M, Carle S, Maigler F, Flamm J, Kramer V, Mavoungou C, Schmid O, Schindowski K. A comprehensive screening platform for aerosolizable protein formulations for intranasal and pulmonary drug delivery. Int J Pharm. 2017;532:537–546. doi: 10.1016/j.ijpharm.2017.09.027. [DOI] [PubMed] [Google Scholar]
- [77].Stucki JD, Hobi N, Galimov A, Stucki AO, Schneider-Daum N, Lehr CM, Huwer H, Frick M, Funke-Chambour M, Geiser T, Guenat OT. Medium throughput breathing human primary cell alveolus-on-chip model. Sci Rep. 2018;8:1–13. doi: 10.1038/s41598-018-32523-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Blank F, Rothen-Rutishauser BM, Schurch S, Gehr P. An optimized in vitro model of the respiratory tract wall to study particle cell interactions. J Aerosol Med Off J Int Soc Aerosols Med. 2006;19:392–405. doi: 10.1089/jam.2006.19.392. [DOI] [PubMed] [Google Scholar]
- [79].Lenz AG, Stoeger T, Cei D, Schmidmeir M, Semren N, Burgstaller G, Lentner B, Eickelberg O, Meiners S, Schmid O. Efficient bioactive delivery of aerosolized drugs to human pulmonary epithelial cells cultured in air-liquid interface conditions. Am J Respir Cell Mol Biol. 2014;51:526–35. doi: 10.1165/rcmb.2013-0479OC. [DOI] [PubMed] [Google Scholar]
- [80].Hein S, Bur M, Kolb T, Muellinger B, Schaefer UF, Lehr CM. The Pharmaceutical Aerosol Deposition Device on Cell Cultures (PADDOCC) in vitro system: Design and experimental protocol. ATLA Altern to Lab Anim. 2010;38:285–295. doi: 10.1177/026119291003800408. [DOI] [PubMed] [Google Scholar]
- [81].Herzog F, Loza K, Balog S, Clift MJD, Epple M, Gehr P, Petri-Fink A, Rothen-Rutishauser B. Mimicking exposures to acute and lifetime concentrations of inhaled silver nanoparticles by two different in vitro approaches. Beilstein J Nanotechnol. 2014;5:1357–1370. doi: 10.3762/bjnano.5.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Mühlfeld C, Rothen-Rutishauser B, Blank F, Vanhecke D, Ochs M, Gehr P. Interactions of nanoparticles with pulmonary structures and cellular responses. Am J Physiol - Lung Cell Mol Physiol. 2008;294:L817–L829. doi: 10.1152/ajplung.00442.2007. [DOI] [PubMed] [Google Scholar]
- [83].Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting Organ-Level Lung Functions on a Chip. Science (80-) 2010;328:1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Mahto SK, Charwat V, Ertl P, Rothen-Rutishauser B, Rhee SW, Sznitman J. Microfluidic platforms for advanced risk assessments of nanomaterials. Nanotoxicology. 2015;9:381–395. doi: 10.3109/17435390.2014.940402. [DOI] [PubMed] [Google Scholar]
- [85].Benam KH, Königshoff M, Eickelberg O. Breaking the in vitro barrier in respiratory medicine: Engineered microphysiological systems for chronic obstructive pulmonary disease and beyond. Am J Respir Crit Care Med. 2018;197:869–875. doi: 10.1164/rccm.201709-1795PP. [DOI] [PubMed] [Google Scholar]
- [86].Artzy-Schnirman A, Hobi N, Schneider-Daum N, Guenat OT, Lehr C-M, Sznitman J. Advanced in vitro lung-on-chip platforms for inhalation assays: From prospect to pipeline. Eur J Pharm Biopharm. 2019;144:11–17. doi: 10.1016/j.ejpb.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Zhang B, Radisic M. Organ-on-a-chip devices advance to market. Lab Chip. 2017;17:2395–2420. doi: 10.1039/C6LC01554A. [DOI] [PubMed] [Google Scholar]
- [88].Esch EW, Bahinski A, Huh D. Organs-on-chips at the frontiers of drug discovery. Nat Rev Drug Discov. 2015;14:248–260. doi: 10.1038/nrd4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat Biotechnol. 2014;32 doi: 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- [90].Liu D, Zhang H, Fontana F, Hirvonen JT, Santos HA. Current developments and applications of microfluidic technology toward clinical translation of nanomedicines. Adv Drug Deliv Rev. 2017;128:54–83. doi: 10.1016/j.addr.2017.08.003. [DOI] [PubMed] [Google Scholar]
- [91].Mittal R, Woo FW, Castro CS, Cohen MA, Karanxha J, Mittal J, Chhibber T, Jhaveri VM. Organ-on-chip models: Implications in drug discovery and clinical applications. J Cell Physiol. 2019;234:8352–8380. doi: 10.1002/jcp.27729. [DOI] [PubMed] [Google Scholar]
- [92].Kimura H, Sakai Y, Fujii T. Organ/body-on-a-chip based on microfluidic technology for drug discovery. Drug Metab Pharmacokinet. 2018;33:43–48. doi: 10.1016/j.dmpk.2017.11.003. [DOI] [PubMed] [Google Scholar]
- [93].Haddrick M, Simpson PB. Organ-on-a-chip technology: turning its potential for clinical benefit into reality. Drug Discov Today. 2019;24:1217–1223. doi: 10.1016/j.drudis.2019.03.011. [DOI] [PubMed] [Google Scholar]
- [94].Shrestha J, Bazaz SR, Es HA, Azari Y, Thierry B, Warkiani ME. Critical Reviews in Biotechnology Lung-on-a-chip : the future of respiratory disease models and pharmacological studies. Crit Rev Biotechnol. 2020;40:213–230. doi: 10.1080/07388551.2019.1710458. [DOI] [PubMed] [Google Scholar]
- [95].Zhang B, Korolj A, Lai BFL, Radisic M. Advances in organ-on-a-chip engineering. Nat Rev Mater. 2018;3:257–278. doi: 10.1038/s41578-018-0034-7. [DOI] [Google Scholar]
- [96].Ingber DE. Is it Time for Reviewer 3 to Request Human Organ Chip Experiments Instead of Animal Validation Studies ? 3. Are there Viable In Vitro Alternatives to Animal. 2020:1–15.:2002030. doi: 10.1002/advs.202002030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Bonniaud P, Fabre A, Frossard N, Guignabert C, Inman M, Kuebler WM, Maes T, Shi W, Stampfli M, Uhlig S, White E, et al. Optimising experimental research in respiratory diseases: An ERS statement. Eur Respir J. 2018;51 doi: 10.1183/13993003.02133-2017. [DOI] [PubMed] [Google Scholar]
- [98].Haefeli-Bleuer B, Weibel ER. Morphometry of the human pulmonary acinus. Anat Rec. 1988;220:401–414. doi: 10.1002/ar.1092200410. [DOI] [PubMed] [Google Scholar]
- [99].Weibel ER. How to make an alveolus. Eur Respir J. 2008;31:483–5. doi: 10.1183/09031936.00003308. [DOI] [PubMed] [Google Scholar]
- [100].Hiemstra PS, Grootaers G, van der Does AM, Krul CAM, Kooter IM. Human lung epithelial cell cultures for analysis of inhaled toxicants: Lessons learned and future directions. Toxicol Vitr. 2018;47:137–146. doi: 10.1016/j.tiv.2017.11.005. [DOI] [PubMed] [Google Scholar]
- [101].Miller AJ, Dye BR, Ferrer-Torres D, Hill DR, Overeem AW, Shea LD, Spence JR. Generation of lung organoids from human pluripotent stem cells in vitro. Nat Protoc. 2019;14:518–540. doi: 10.1038/s41596-018-0104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Astashkina A, Grainger DW. Critical analysis of 3-D organoid in vitro cell culture models for high-throughput drug candidate toxicity assessments. Adv Drug Deliv Rev. 2014;69-70:1–18. doi: 10.1016/j.addr.2014.02.008. [DOI] [PubMed] [Google Scholar]
- [103].Schöneberg J, De Lorenzi F, Theek B, Blaeser A, Rommel D, Kuehne AJC, Kießling F, Fischer H. Engineering biofunctional in vitro vessel models using a multilayer bioprinting technique. Sci Rep. 2018;8:1–13. doi: 10.1038/s41598-018-28715-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Nguyen HT, Stapleton SC, Yang MT, Cha SS, Choi CK, Galie PA, Chen CS. Biomimetic model to reconstitute angiogenic sprouting morphogenesis in vitro. Proc Natl Acad Sci U S A. 2013;110:6712–6717. doi: 10.1073/pnas.1221526110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Mandrycky C, Hadland B, Zheng Y. 3D curvature-instructed endothelial flow response and tissue vascularization. Sci Adv. 2020;6 doi: 10.1126/sciadv.abb3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Zheng Y, Chen J, Craven M, Choi NW, Totorica S, Diaz-Santana A, Kermani P, Hempstead B, Fischbach-Teschl C, López JA, Stroock AD. In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci U S A. 2012;109:9342–9347. doi: 10.1073/pnas.1201240109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Wang X, Sun Q, Pei J. Microfluidic-based 3D engineered microvascular networks and their applications in vascularized microtumor models. Micromachines. 2018;9:1–26. doi: 10.3390/mi9100493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Sznitman J. Respiratory microflows in the pulmonary acinus. J Biomech. 2013;46:284–98. doi: 10.1016/j.jbiomech.2012.10.028. [DOI] [PubMed] [Google Scholar]
- [109].Fung YC. A model of the lung structure and its validation. J Appl Physiol. 1988;64:2132–2141. doi: 10.1152/jappl.1988.64.5.2132. [DOI] [PubMed] [Google Scholar]
- [110].Duval K, Grover H, Han LH, Mou Y, Pegoraro AF, Fredberg J, Chen Z. Modeling physiological events in 2D vs. 3D cell culture. Physiology. 2017 doi: 10.1152/physiol.00036.2016. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- [111].Tenenbaum-Katan J, Fishler R, Rothen-Rutishauser B, Sznitman J. Biomimetics of fetal alveolar flow phenomena using microfluidics. Biomicrofluidics. 2015;9:014120. doi: 10.1063/1.4908269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Mahto SK, Tenenbaum-Katan J, Greenblum A, Rothen-Rutishauser B, Sznitman J. Microfluidic shear stress-regulated surfactant secretion in alveolar epithelial type II cells in vitro. Am J Physiol Lung Cell Mol Physiol. 2014;306:L672–83. doi: 10.1152/ajplung.00106.2013. [DOI] [PubMed] [Google Scholar]
- [113].Artzy-Schnirman A, Zidan H, Elias-Kirma S, Ben-Porat L, Tenenbaum-Katan J, Carius P, Fishler R, Schneider-Daum N, Lehr C, Sznitman J. Capturing the Onset of Bacterial Pulmonary Infection in Acini-On-Chips. Adv Biosyst. 2019;3:1900026. doi: 10.1002/adbi.201900026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Uhl C, Shi W, Liu Y. Organ-on-Chip Devices Toward Applications in Drug Development and Screening. J Med Device. 2018;12:040801. doi: 10.1115/1.4040272. [DOI] [Google Scholar]
- [115].Duan B. State-of-the-Art Review of 3D Bioprinting for Cardiovascular Tissue Engineering. Ann Biomed Eng. 2017;45:195–209. doi: 10.1007/s10439-016-1607-5. [DOI] [PubMed] [Google Scholar]
- [116].Sears NA, Seshadri DR, Dhavalikar PS, Cosgriff-Hernandez E. A Review of ThreeDimensional Printing in Tissue Engineering. Tissue Eng - Part B Rev. 2016;22:298–310. doi: 10.1089/ten.teb.2015.0464. [DOI] [PubMed] [Google Scholar]
- [117].Horvath L, Yuki U, Jud C, Blank F, Petri-fink A, Horva L, Rothen-Rutishauser B. Engineering an in vitro air-blood barrier by 3D bioprinting. Sci Rep. 2015;5:7974. doi: 10.1038/srep07974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Sun X, Zhang X, Ren X, Sun H, Wu L, Wang C. Multiscale Co-reconstruction of Lung Architectures and Inhalable Materials Spatial Distribution. 2021:1–14.:2003941. doi: 10.1002/advs.202003941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010 doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Kulkarni T, O’Reilly P, Antony VB, Gaggar A, Thannickal VJ. Matrix remodeling in pulmonary fibrosis and emphysema. Am J Respir Cell Mol Biol. 2016;54:751–760. doi: 10.1165/rcmb.2015-0166PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Burgstaller G, Oehrle B, Gerckens M, White ES, Schiller HB, Eickelberg O. The instructive extracellular matrix of the lung: Basic composition and alterations in chronic lung disease. Eur Respir J. 2017;50 doi: 10.1183/13993003.01805-2016. [DOI] [PubMed] [Google Scholar]
- [122].Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng. 2009;103:655–663. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Rozario T, DeSimone DW. The extracellular matrix in development and morphogenesis: A dynamic view. Dev Biol. 2010 doi: 10.1016/j.ydbio.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Guenat OT, Berthiaume F. Incorporating mechanical strain in organs-on-a-chip: Lung and skin. Biomicrofluidics. 2018;12:042207. doi: 10.1063/1.5024895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Zamprogno P, Wüthrich S, Achenbach S, Thoma G, Stucki JD, Hobi N, Schneider-Daum N, Lehr C, Huwer H, Geiser T, Schmid RA, et al. Second-generation lung-on-a-chip with an array of stretchable alveoli made with a biological membrane. Commun Biol (n.d.) :1–10. doi: 10.1038/s42003-021-01695-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Sanchez-Esteban J, Cicchiello LA, Wang Y, Tsai SW, Williams LK, Torday JS, Rubin LP. Mechanical stretch promotes alveolar epithelial type II cell differentiation. J Appl Physiol. 2001;91:589–595. doi: 10.1152/jappl.2001.91.2.589. [DOI] [PubMed] [Google Scholar]
- [127].Thompson CL, Fu S, Knight MM, Thorpe SD. Mechanical Stimulation: A Crucial Element of Organ-on-Chip Models. Front Bioeng Biotechnol. 2020;8:1–18. doi: 10.3389/fbioe.2020.602646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Stucki AO, Stucki JD, Hall SRR, Felder M, Mermoud Y, Schmid RA, Geiser T, Guenat OT. A lung-on-a-chip array with an integrated bio-inspired respiration mechanism. Lab Chip. 2015;15:1302–10. doi: 10.1039/c4lc01252f. [DOI] [PubMed] [Google Scholar]
- [129].Doryab A, Taskin MB, Stahlhut P, Schroeppel A, Orak S, Voss C, Ahluwalia A, Rehberg M, Hilgendorff A, Stöger T, Groll J, et al. A Bioinspired In Vitro Lung Model to Study Particokinetics of Nano-/Microparticles under Cyclic Stretch and AirLiquid Interface Conditions. Front Bioeng Biotechnol. 2021;9:1–18. doi: 10.3389/fbioe.2021.616830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Trepat X, Grabulosa M, Puig F, Maksym GN, Navajas D, Farré R. Viscoelasticity of human alveolar epithelial cells subjected to stretch. Am J Physiol - Lung Cell Mol Physiol. 2004;287 doi: 10.1152/ajplung.00077.2004. [DOI] [PubMed] [Google Scholar]
- [131].Huh D, Leslie DC, Matthews BD, Fraser JP, Jurek S, a Hamilton G, Thorneloe KS, McAlexander MA, Ingber DE. A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Sci Transl Med. 2012;4:159ra147. doi: 10.1126/scitranslmed.3004249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Felder M, Trueeb B, Stucki AO, Borcard S, Stucki JD, Schnyder B, Geiser T, Guenat OT. Impaired wound healing of alveolar lung epithelial cells in a breathing lung-on-a-chip. Front Bioeng Biotechnol. 2019;7:1–5. doi: 10.3389/fbioe.2019.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Baptista D, Teixeira LM, Birgani ZT, van Riet S, Pasman T, Poot A, Stamatialis D, Rottier RJ, Hiemstra PS, Habibović P, van Blitterswijk C, et al. 3D alveolar in vitro model based on epithelialized biomimetically curved culture membranes. Biomaterials. 2021;266 doi: 10.1016/j.biomaterials.2020.120436. [DOI] [PubMed] [Google Scholar]
- [134].Hofemeier P, Koshiyama K, Wada S, Sznitman J. One (sub-)acinus for all: Fate of inhaled aerosols in heterogeneous pulmonary acinar structures. Eur J Pharm Sci. 2018;113:53–63. doi: 10.1016/j.ejps.2017.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Hofemeier P, Sznitman J. The role of anisotropic expansion for pulmonary acinar aerosol deposition. J Biomech. 2016;49:3543–3548. doi: 10.1016/j.jbiomech.2016.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Koullapis PG, Hofemeier P, Sznitman J, Kassinos SC. An efficient computational fluid-particle dynamics method to predict deposition in a simplified approximation of the deep lung. Eur J Pharm Sci. 2018;113:132–144. doi: 10.1016/j.ejps.2017.09.016. [DOI] [PubMed] [Google Scholar]
- [137].Fishler R, Mulligan MK, Sznitman J. Acinus-on-a-chip: a microfluidic platform for pulmonary acinar flows. J Biomech. 2013;46:2817–23. doi: 10.1016/j.jbiomech.2013.08.020. [DOI] [PubMed] [Google Scholar]
- [138].Darquenne C. Aerosol deposition in health and disease. J Aerosol Med Pulm Drug Deliv. 2012;25:140–7. doi: 10.1089/jamp.2011.0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Hofemeier P, Sznitman J. Revisiting pulmonary acinar particle transport: convection, sedimentation, diffusion and their interplay. J Appl Physiol. 2015;118:1375–1385. doi: 10.1152/japplphysiol.01117.2014. [DOI] [PubMed] [Google Scholar]
- [140].Fishler R, Ostrovski Y, Lu CY, Sznitman J. Streamline crossing: An essential mechanism for aerosol dispersion in the pulmonary acinus. J Biomech. 2017;50:222–227. doi: 10.1016/j.jbiomech.2016.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Shachar-Berman L, Ostrovski Y, Koshiyama K, Wada S, Kassinos SC, Sznitman J. Targeting inhaled fibers to the pulmonary acinus: Opportunities for augmented delivery from in silico simulations. Eur J Pharm Sci. 2019;137:105003. doi: 10.1016/j.ejps.2019.105003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Yang L, Gradl R, Dierolf M, Möller W, Kutschke D, Feuchtinger A, Hehn L, Donnelley M, Günther B, Achterhold K, Walch A, et al. Multimodal Precision Imaging of Pulmonary Nanoparticle Delivery in Mice: Dynamics of Application. Spatial Distribution, and Dosimetry, Small. 2019:1904112. doi: 10.1002/smll.201904112. [DOI] [PubMed] [Google Scholar]
- [143].Fishler R, Hofemeier P, Etzion Y, Dubowski Y, Sznitman J. Particle dynamics and deposition in true-scale pulmonary acinar models. Sci Rep. 2015;5:14071. doi: 10.1038/srep14071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Ostrovski Y, Dorfman S, Mezhericher M, Kassinos S, Sznitman J. Targeted Drug Delivery to Upper Airways Using a Pulsed Aerosol Bolus and Inhaled Volume Tracking Method. Flow, Turbul Combust. 2018:1–15. doi: 10.1007/s10494-018-9927-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Longest PW, Holbrook LT. In silico models of aerosol delivery to the respiratory tract — Development and applications. Adv Drug Deliv Rev. 2012;64:296–311. doi: 10.1016/j.addr.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Tian G, Longest PW, Li X, Hindle M. Targeting aerosol deposition to and within the lung airways using excipient enhanced growth. J Aerosol Med Pulm Drug Deliv. 2013;26:248–265. doi: 10.1089/jamp.2012.0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Das P, Nof E, Amirav I, Kassinos SC, Sznitman J. Targeting inhaled aerosol delivery to upper airways in children: Insight from computational fluid dynamics (CFD) PLoS One. 2018;13:1–20. doi: 10.1371/journal.pone.0207711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Elias-Kirma S, Artzy-Schnirman A, Das P, Heller-Algazi M, Korin N, Sznitman J. In situ-like aerosol inhalation exposure for cytotoxicity assessment using airway-on-chips platforms. Front Bioeng Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].P. Kwok CL, Chan H-K. Delivery of inhalation drugs to children for asthma and other respiratory diseases. Adv Drug Deliv Rev. 2014;73:83–8. doi: 10.1016/j.addr.2013.11.007. [DOI] [PubMed] [Google Scholar]
- [150].Stein SW, Thiel CG. The History of Therapeutic Aerosols: A Chronological Review. J Aerosol Med Pulm Drug Deliv. 2017;30:20–41. doi: 10.1089/jamp.2016.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Dolovich MB, Dhand R. Aerosol drug delivery: developments in device design and clinical use. Lancet. 2011;377:1032–45. doi: 10.1016/S0140-6736(10)60926-9. [DOI] [PubMed] [Google Scholar]
- [152].Labiris NR, Dolovich MB. Pulmonary drug delivery Part II: The role of inhalant delivery devices and drug formulations in therapeutic effectiveness of aerosolized medications. 2003 doi: 10.1046/j.1365-2125.2003.01893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Laube BL. The expanding role of aerosols in systemic drug delivery, gene therapy, and vaccination. Respir Care. 2005;50:1161–1176. doi: 10.1186/2213-0802-2-3. [DOI] [PubMed] [Google Scholar]
- [154].Laube BL. The expanding role of aerosols in systemic drug delivery, gene therapy, and vaccination. Transl Respir Med. 2014;2 doi: 10.1186/2213-0802-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Laube BL. Aerosolized Medications for Gene and Peptide Therapy. Respir Care. 2015;60:806–824. doi: 10.4187/respcare.03554. [DOI] [PubMed] [Google Scholar]
- [156].Scheuch G, Kohlhaeufl MJ, Brand P, Siekmeier R. Clinical perspectives on pulmonary systemic and macromolecular delivery. Adv Drug Deliv Rev. 2006;58:996–1008. doi: 10.1016/j.addr.2006.07.009. [DOI] [PubMed] [Google Scholar]
- [157].Rajapaksa AE, Ho JJ, Qi A, Bischof R, Nguyen T-H, Tate M, Piedrafita D, McIntosh MP, Yeo LY, Meeusen E, Coppel RL, et al. Effective pulmonary delivery of an aerosolized plasmid DNA vaccine via surface acoustic wave nebulization. Respir Res. 2014;15:60. doi: 10.1186/1465-9921-15-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Patton JS, Byron PR. Inhaling medicines : delivering drugs to the body through the lungs. Nat Rev Drug Discov. 2007;6:67–74. doi: 10.1038/nrd2153. [DOI] [PubMed] [Google Scholar]
- [159].Birchall J. Pulmonary delivery of nucleic acids. Expert Opin Drug Deliv. 2007;4:575–578. doi: 10.1517/17425247.4.6.575. [DOI] [PubMed] [Google Scholar]
- [160].Labiris NR, Dolovich MB. Pulmonary drug delivery . Part I: Physiological factors affecting therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003;56:588–599. doi: 10.1046/j.1365-2125.2003.01892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Moreno-Sastre M, Pastor M, Salomon CJ, Esquisabel A, Pedraz JL. Pulmonary drug delivery: A review on nanocarriers for antibacterial chemotherapy. J Antimicrob Chemother. 2015;70:2945–2955. doi: 10.1093/jac/dkv192. [DOI] [PubMed] [Google Scholar]
- [162].Rau JL. The inhalation of drugs: advantages and problems. Respir Care. 2005;50:367–382. [PubMed] [Google Scholar]
- [163].Ari A, Fink JB. Aerosol therapy in children: challenges and solutions. Expert Rev Respir Med. 2013;7:665–72. doi: 10.1586/17476348.2013.847369. [DOI] [PubMed] [Google Scholar]
- [164].Ari A, Fink JB. Guidelines for aerosol devices in infants, children and adults: which to choose, why and how to achieve effective aerosol therapy. Expert Rev Respir Med. 2011;5:561–72. doi: 10.1586/ers.11.49. [DOI] [PubMed] [Google Scholar]
- [165].Shachar-berman L, Bhardwaj S, Ostrovski Y, Das P, Koullapis P, Kassinos S, Sznitman J. In silico optimization of fiber-shaped aerosols in inhalation therapy for augmented targeting and deposition across the respiratory tract. Pharmaceutics. 2020;12:1–12. doi: 10.3390/pharmaceutics12030230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Zarogoulidis P, Chatzaki E, Porpodis K, Domvri K, Hohenforst-Schmidt W, Goldberg EP, Karamanos N, Zarogoulidis K. Inhaled chemotherapy in lung cancer: Future concept of nanomedicine. Int J Nanomedicine. 2012;7:1551–1572. doi: 10.2147/IJN.S29997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Ostrovski Y, Dorfman S, Poh W, Chye Joachim Loo S, Sznitman J. Focused targeting of inhaled magnetic aerosols in reconstructed in vitro airway models. J Biomech. 2021;118:110279. doi: 10.1016/j.jbiomech.2021.110279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Mangal S, Gao W, Li T, Zhou QT. Pulmonary delivery of nanoparticle chemotherapy for the treatment of lung cancers: Challenges and opportunities. Acta Pharmacol Sin. 2017;38:782–797. doi: 10.1038/aps.2017.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Velkov T, Rahim NA, Zhou QT, Chan H-K, Li J. Inhaled anti-infective chemotherapy for respiratory tract infections: Successes, challenges and the road ahead. Adv Drug Deliv Rev. 2014 doi: 10.1016/j.addr.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Poh W, Ab Rahman N, Ostrovski Y, Sznitman J, Pethe K, Loo SCJ. Active pulmonary targeting against tuberculosis (TB) via triple-encapsulation of Q203, bedaquiline and superparamagnetic iron oxides (SPIOs) in nanoparticle aggregates. Drug Deliv. 2019;26:1039–1048. doi: 10.1080/10717544.2019.1676841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Artzy-Schnirman A, Lehr CM, Sznitman J. Advancing human in vitro pulmonary disease models in preclinical research: opportunities for lung-on-chips. Expert Opin Drug Deliv. 2020;17:621–625. doi: 10.1080/17425247.2020.1738380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Huh D, Leslie DC, Matthews BD, Fraser JP, Jurek S, Hamilton GA, Thorneloe KS, McAlexander MA, Ingber DE. A Human Disease Model of Drug Toxicity-Induced Pulmonary Edema in a Lung-on-a-Chip Microdevice. Sci Transl Med. 2012;4:159ra147. doi: 10.1126/scitranslmed.3004249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Jain A, Barrile R, van der Meer AD, Mammoto A, Mammoto T, De Ceunynck K, Aisiku O, Otieno MA, Louden CS, Hamilton GA, Flaumenhaft R, et al. Primary Human Lung Alveolus-on-a-chip Model of Intravascular Thrombosis for Assessment of Therapeutics. Clin Pharmacol Ther. 2017:02115. doi: 10.1002/cpt.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Benam KH, Villenave R, Lucchesi C, Varone A, Hubeau C, Lee H-H, Alves SE, Salmon M, Ferrante TC, Weaver JC, Bahinski A, et al. Small airway-on-a-chip enables analysis of human lung inflammation and drug responses in vitro. Nat Methods. 2016;13:151–157. doi: 10.1038/nmeth.3697. [DOI] [PubMed] [Google Scholar]
- [175].Bush A, Hedlin G, Carlsen KH, de Benedictis F, Lodrup-Carlsen K, Wilson N. Severe childhood asthma: a common international approach? Lancet. 2008;372:1019–1021. doi: 10.1016/S0140-6736(08)61422-1. [DOI] [PubMed] [Google Scholar]
- [176].Nawroth JC, Lucchesi C, Cheng D, Shukla A, Ngyuen J, Shroff T, Varone A, Karalis K, Lee HH, Alves S, Hamilton GA, et al. A microengineered airway lung chip models key features of viral-induced exacerbation of asthma. Am J Respir Cell Mol Biol. 2020 doi: 10.1165/RCMB.2020-0010MA. [DOI] [PubMed] [Google Scholar]
- [177].Sardeli C, Zarogoulidis P, Kosmidis C, Amaniti A. Inhaled chemotherapy adverse effects : mechanisms and protection methods. 2020;8 doi: 10.2217/lmt-2019-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomedicine. 2015;10:975–999. doi: 10.2147/IJN.S68861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Choudhury A. Liposome: a carrier for effective drug delivery. J Appl Pharm Res. 2020;8:22–28. doi: 10.18231/j.joapr.2019.v.8.i.1.003. [DOI] [Google Scholar]
- [180].Mozafari MR. Liposomes: an overview of manufacturing techniques. Cell Mol Biol Lett. 2005;10:711–719. [PubMed] [Google Scholar]
- [181].Lombardo D, Calandra P, Barreca D, Magazù S, Kiselev MA. Soft Interaction in Liposome Nanocarriers for Therapeutic Drug Delivery. Nanomaterials. 2016;6:125. doi: 10.3390/nano6070125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Daraee H, Etemadi A, Kouhi M, Alimirzalu S, Akbarzadeh A. Application of liposomes in medicine and drug delivery,Cells Artif Nanomedicine. Biotechnol. 2016;44:381–391. doi: 10.3109/21691401.2014.953633. [DOI] [PubMed] [Google Scholar]
- [183].Egorov E, Pieters C, Korach-Rechtman H, Shklover J, Schroeder A. Robotics, microfluidics, nanotechnology and AI in the synthesis and evaluation of liposomes and polymeric drug delivery systems. Drug Deliv Transl Res. 2021 doi: 10.1007/s13346-021-00929-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Justo OR, Moraes ÂM, Justo OR, Moraes M. Incorporation of Antibiotics in Liposomes Designed for Tuberculosis Therapy by Inhalation Incorporation of Antibiotics in Liposomes Designed ˆ. 2008:7544. doi: 10.1080/713840401. [DOI] [Google Scholar]
- [185].Rudokas M, Najlah M, Alhnan MA, Elhissi A. Liposome Delivery Systems for Inhalation : A Critical Review Highlighting Formulation Issues and Anticancer Applications. 2016;25:60–72. doi: 10.1159/000445116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Mehta PP, Ghoshal D, Pawar AP, Kadam SS, Dhapte-pawar VS. Journal of Drug Delivery Science and Technology Recent advances in inhalable liposomes for treatment of pulmonary diseases : Concept to clinical stance. J Drug Deliv Sci Technol. 2020;56:101509. doi: 10.1016/j.jddst.2020.101509. [DOI] [Google Scholar]
- [187].Engelhard VH, Powers GA, Moore LC, Holterman MJ, Correa-Freire MC. Cytotoxic T lymphocyte recognition of HLA-A/B antigens introduced into EL4 cells by cell-liposome fusion. J Immunol (Baltimore, Md. 1950) 1984;132:76–80. [PubMed] [Google Scholar]
- [188].Bassetti M, Vena A, Russo A, Peghin M. Inhaled Liposomal Antimicrobial Delivery in Lung Infections. Drugs. 2020;80:1309–1318. doi: 10.1007/s40265-020-01359-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Shade CW. Liposomes as Advanced Delivery Systems for Nutraceuticals. Integr Med A Clin J. 2016;15:33–36. [PMC free article] [PubMed] [Google Scholar]
- [190].Babai I, Barenholz Y, Zakay-Rones Z, Greenbaum E, Samira S, Hayon I, Rochman M, Kedar E. A novel liposomal influenza vaccine (INFLUSOME-VAC) containing hemagglutinin-neuraminidase and IL-2 or GM-CSF induces protective antineuraminidase antibodies cross-reacting with a wide spectrum of influenza A viral strains. Vaccine. 2001;20:505–515. doi: 10.1016/s0264-410x(01)00326-7. [DOI] [PubMed] [Google Scholar]
- [191].Badiee A, Khamesipour A, Samiei A, Soroush D, Heravi V, Tavassoti M, Barkhordari F, Mc WR, Mahboudi F, Jaafari MR. Experimental Parasitology The role of liposome size on the type of immune response induced in BALB / c mice against leishmaniasis : rgp63 as a model antigen. Exp Parasitol. 2012;132:403–409. doi: 10.1016/j.exppara.2012.09.001. [DOI] [PubMed] [Google Scholar]
- [192].Veldhuizen EJ, Haagsman HP. Role of pulmonary surfactant components in surface film formation and dynamics. Biochim Biophys Acta. 2000;1467:255–270. doi: 10.1016/S0005-2736(00)00256-X. [DOI] [PubMed] [Google Scholar]
- [193].Lai SK, Wang YY, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliv Rev. 2009;61:158–171. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Morgan LE, Jaramillo AM, Shenoy SK, Raclawska D, Emezienna NA, Richardson VL, Hara N, Harder AQ, NeeDell JC, Hennessy CE, El-Batal HM, et al. Disulfide disruption reverses mucus dysfunction in allergic airway disease. Nat Commun. 2021;12 doi: 10.1038/s41467-020-20499-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Kim YC, Hsueh HT, Kim N, Rodriguez J, Leo KT, Rao D, West NE, Hanes J, Suk JS. Strategy to Enhance Dendritic Cell-Mediated DNA Vaccination in the Lung. Adv Ther. 2020;3 doi: 10.1002/adtp.202000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].About ARIKAYCE | ARIKAYCE (amikacin liposome inhalation suspension), (n.d.)
- [197].Shirley M. Amikacin Liposome Inhalation Suspension: A Review in Mycobacterium avium Complex Lung Disease. Drugs. 2019;79:555–562. doi: 10.1007/s40265-019-01095-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Zhang J, Leifer F, Rose S, Chun DY, Thaisz J, Herr T, Nashed M, Joseph J, Perkins WR, DiPetrillo K. Amikacin Liposome Inhalation Suspension (ALIS) Penetrates Non-tuberculous Mycobacterial Biofilms and Enhances Amikacin Uptake Into Macrophages. Front Microbiol. 2018;9:1–12. doi: 10.3389/fmicb.2018.00915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Zhang J, Leifer F, Rose S, Chun DY, Thaisz J, Herr T, Nashed M, Joseph J, Perkins WR, DiPetrillo K. Amikacin liposome inhalation suspension (ALIS) penetrates non-tuberculous mycobacterial biofilms and enhances amikacin uptake into macrophages. Front Microbiol. 2018;9 doi: 10.3389/fmicb.2018.00915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Avnir Y, Ulmansky R, Wasserman V, Even-Chen S, Broyer M, Barenholz Y, Naparstek Y. Amphipathic weak acid glucocorticoid prodrugs remote-loaded into sterically stabilized nanoliposomes evaluated in arthritic rats and in a Beagle dog: A novel approach to treating autoimmune arthritis. Arthritis Rheum. 2008;58:119–129. doi: 10.1002/art.23230. [DOI] [PubMed] [Google Scholar]
- [201].Lühder F. Novel Drug Delivery Systems Tailored for Improved Administration of Glucocorticoids. 2017 doi: 10.3390/ijms18091836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Hegeman MA, Cobelens PM, Kamps J, Hennus MP. dexamethasone attenuates ventilator-induced lung. 2011 doi: 10.1111/j.1476-5381.2011.01314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Honmane S, Hajare A, More H, Osmani RAM, Salunkhe S. Lung delivery of nanoliposomal salbutamol sulfate dry powder inhalation for facilitated asthma therapy. J Liposome Res. 2019;29 doi: 10.1080/08982104.2018.1531022. [DOI] [PubMed] [Google Scholar]
- [204].Chen X, Huang W, Kwan Wong BC, Yin L, Wong YF, Xu M, Yang Z. Liposomes prolong the therapeutic effect of anti-asthmatic medication via pulmonary delivery. Int J Nanomedicine. 2012;7 doi: 10.2147/IJN.S28011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Press D. Onivyde for the therapy of multiple solid tumors. 2016:3001–3007. doi: 10.2147/OTT.S105587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Bulbake U, Doppalapudi S, Kommineni N, Khan W. Liposomal Formulations in Clinical Use : An Updated Review development. 2017:1–33. doi: 10.3390/pharmaceutics9020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Mylonakis N, Athanasiou A, Ziras N, Angel J, Rapti A, Lampaki S, Politis N, Karanikas C, Kosmas C. Phase II study of liposomal cisplatin (Lipoplatin) plus gemcitabine versus cisplatin plus gemcitabine as first line treatment in inoperable (stage IIIB/IV) nonsmall cell lung cancer. Lung Cancer. 2010;68:240–247. doi: 10.1016/j.lungcan.2009.06.017. [DOI] [PubMed] [Google Scholar]
- [208].Golombek SK, May JN, Theek B, Appold L, Drude N, Kiessling F, Lammers T. Tumor targeting via EPR: Strategies to enhance patient responses. Adv Drug Deliv Rev. 2018;130:17–38. doi: 10.1016/j.addr.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Lin C, Zhang X, Chen H, Bian Z, Zhang G, Kashif M. Dual-ligand modified liposomes provide effective local targeted delivery of lung-cancer drug by antibody and tumor lineage-homing cell-penetrating peptide. Drug Deliv. 2018;0:256–266. doi: 10.1080/10717544.2018.1425777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Talebian S, Wallace GG, Schroeder A, Stellacci F, Conde J. Nanotechnology-based disinfectants and sensors for SARS-CoV-2. Nat Nanotechnol. 2020;15:618–621. doi: 10.1038/s41565-020-0751-0. [DOI] [PubMed] [Google Scholar]
- [211].Oliver SE, Gargano JW, Marin M, Wallace M, Curran KG, Chamberland M, McClung N, Campos-Outcalt D, Morgan RL, Mbaeyi S, Romero JR, et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine - United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1922–1924. doi: 10.15585/mmwr.mm6950e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, McCullough MP, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ, et al. An mRNA Vaccine against SARS-CoV-2 — Preliminary Report. N Engl J Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Sahu I, Haque AKMA, Weidensee B, Weinmann P, Kormann MSD. Recent Developments in mRNA-Based Protein Supplementation Therapy to Target Lung Diseases. Mol Ther. 2019;27:803–823. doi: 10.1016/j.ymthe.2019.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].M. Chow YT, Qiu Y, Lam JKW. Inhaled RNA Therapy: From Promise to Reality. Trends Pharmacol Sci. 2020;41 doi: 10.1016/j.tips.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].M. Kormann SD, Hasenpusch G, Aneja MK, Nica G, Flemmer AW, Herber-Jonat S, Huppmann M, Mays LE, Illenyi M, Schams A, Griese M, et al. Expression of therapeutic proteins after delivery of chemically modified mRNA in mice. Nat Biotechnol. 2011;29 doi: 10.1038/nbt.1733. [DOI] [PubMed] [Google Scholar]
- [216].Connolly B, Isaacs C, Cheng L, Asrani KH, Subramanian RR. SERPINA1 mRNA as a Treatment for Alpha-1 Antitrypsin Deficiency. J Nucleic Acids. 2018;2018 doi: 10.1155/2018/8247935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Lara-Guerra H, Roth JA. Gene therapy for lung cancer. Crit Rev Oncog. 2016;21:115–124. doi: 10.1615/CritRevOncog.2016016084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Chen J, Tang Y, Liu Y, Dou Y. Nucleic Acid-Based Therapeutics for Pulmonary Diseases. AAPS PharmSciTech. 2018;19:3670–3680. doi: 10.1208/s12249-018-1183-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].da Silva AL, de Oliveira GP, Kim N, Cruz FF, Kitoko JZ, Blanco NG, Martini SV, Hanes J, Rocco PRM, Suk JS, Morales MM. Nanoparticle-based thymulin gene therapy therapeutically reverses key pathology of experimental allergic asthma. Sci Adv. 2020;6 doi: 10.1126/sciadv.aay7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Mastorakos P, Silva ALD, Chisholm J, Song E, Choi WK, Boyle MP, Morales MM, Hanes J, Suk JS. Highly compacted biodegradable DNA nanoparticles capable of overcoming the mucus barrier for inhaled lung gene therapy. Proc Natl Acad Sci U S A. 2015;112:8720–8725. doi: 10.1073/pnas.1502281112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Koehler DR, Hitt MM, Hu J. Challenges and strategies for cystic fibrosis lung gene therapy. Mol Ther. 2001;4:84–91. doi: 10.1006/mthe.2001.0435. [DOI] [PubMed] [Google Scholar]
- [222].Mention K, Santos L, Harrison PT. Gene and base editing as a therapeutic option for cystic fibrosis-learning from other diseases. Genes (Basel) 2019;10 doi: 10.3390/genes10050387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Alton EWFW, Armstrong DK, Ashby D, Bayfield KJ, Bilton D, Bloomfield EV, Boyd AC, Brand J, Buchan R, Calcedo R. A randomised, double-blind, placebo-controlled trial of repeated nebulisation of non-viral cystic fibrosis transmembrane conductance regulator (CFTR) gene therapy in patients with cystic fibrosis. Effic Mech Eval. 2016;3 doi: 10.3310/eme03050. [DOI] [PubMed] [Google Scholar]
- [224].McLachlan G, Davidson H, Holder E, Davies LA, Pringle IA, Sumner-Jones SG, Baker A, Tennant P, Gordon C, Vrettou C, Blundell R, et al. Pre-clinical evaluation of three non-viral gene transfer agents for cystic fibrosis after aerosol delivery to the ovine lung. Gene Ther. 2011;18 doi: 10.1038/gt.2011.55. [DOI] [PubMed] [Google Scholar]
- [225].Clancy JP, Cotton CU, Donaldson SH, Solomon GM, VanDevanter DR, Boyle MP, Gentzsch M, Nick JA, Illek B, Wallenburg JC, Sorscher EJ, et al. CFTR modulator theratyping: Current status, gaps and future directions. J Cyst Fibros. 2019;18 doi: 10.1016/j.jcf.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].M. Ruigrok JR, Frijlink HW, Hinrichs WLJ. Pulmonary administration of small interfering RNA: The route to go? J Control Release. 2016;235:14–23. doi: 10.1016/j.jconrel.2016.05.054. [DOI] [PubMed] [Google Scholar]
- [227].Hoy SM. Patisiran: First Global Approval. Drugs. 2018;78:1625–1631. doi: 10.1007/s40265-018-0983-6. [DOI] [PubMed] [Google Scholar]
- [228].Choi M, Gu J, Lee M, Rhim T. A new combination therapy for asthma using dualfunction dexamethasone-conjugated polyethylenimine and Vitamin D binding protein siRNA. Gene Ther. 2017;24 doi: 10.1038/gt.2017.83. [DOI] [PubMed] [Google Scholar]
- [229].Mei D, Tan WSD, Tay Y, Mukhopadhyay A, Wong WSF. Therapeutic RNA Strategies for Chronic Obstructive Pulmonary Disease. Trends Pharmacol Sci. 2020;41 doi: 10.1016/j.tips.2020.04.007. [DOI] [PubMed] [Google Scholar]
- [230].Calcagno TM, Zhang C, Tian R, Ebrahimi B, Mirsaeidi M. Novel three-dimensional biochip pulmonary sarcoidosis model. PLoS One. 2021;16:e0245805. doi: 10.1371/journal.pone.0245805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Cei D, Doryab A, Lenz AG, Schröppel A, Mayer P, Burgstaller G, Nossa R, Ahluwalia A, Schmid O. Development of a dynamic in vitro stretch model of the alveolar interface with aerosol delivery. Biotechnol Bioeng. 2020 doi: 10.1002/bit.27600. [DOI] [PubMed] [Google Scholar]
- [232].Doryab A, Taskin MB, Stahlhut P, Schröppel A, Wagner DE, Groll J, Schmid O. A Biomimetic, Copolymeric Membrane for Cell-Stretch Experiments with Pulmonary Epithelial Cells at the Air-Liquid Interface. Adv Funct Mater. 2020 doi: 10.1002/adfm.202004707. [DOI] [Google Scholar]
- [233].Tavana H, Kuo C-H, Lee QY, Mosadegh B, Huh D, Christensen PJ, Grotberg JB, Takayama S. Dynamics of liquid plugs of buffer and surfactant solutions in a microengineered pulmonary airway model. Langmuir. 2010;26:3744–52. doi: 10.1021/la903038a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Sellgren KL, Butala EJ, Gilmour BP, Randell SH, Grego S. A biomimetic multicellular model of the airways using primary human cells. Lab Chip. 2014;14:3349–58. doi: 10.1039/c4lc00552j. [DOI] [PubMed] [Google Scholar]
- [235].Benam KH, Novak R, Nawroth J, Hirano-Kobayashi M, Ferrante TC, Choe Y, Prantil-Baun R, Weaver JC, Bahinski A, Parker KK, Ingber DE. Matched-Comparative Modeling of Normal and Diseased Human Airway Responses Using a Microengineered Breathing Lung Chip. Cell Syst. 2016;3:456–466.:e4. doi: 10.1016/j.cels.2016.10.003. [DOI] [PubMed] [Google Scholar]
- [236].Hassell BA, Goyal G, Lee E, Sontheimer-Phelps A, Levy O, Chen CS, Ingber DE. Erratum: Human Organ Chip Models Recapitulate Orthotopic Lung Cancer Growth, Therapeutic Responses, and Tumor Dormancy In Vitro (Cell Reports (2017) 21(2) (508516) (S2211124717313311) (10.1016/j.celrep.2017.09.043)) Cell Rep. 2017:508–516. doi: 10.1016/j.celrep.2018.06.028. [DOI] [PubMed] [Google Scholar]
- [237].Felder M, Stucki AO, Stucki JD, Geiser T, Guenat OT. The potential of microfluidic lung epithelial wounding: towards in vivo-like alveolar microinjuries. Integr Biol. 2014;6:1132–1140. doi: 10.1039/C4IB00149D. [DOI] [PubMed] [Google Scholar]
- [238].Yang X, Li K, Zhang X, Liu C, Guo B, Wen W, Gao X. Nanofiber membrane supported lung-on-a-chip microdevice for anti-cancer drug testing. Lab Chip. 2018 doi: 10.1039/c7lc01224a. [DOI] [PubMed] [Google Scholar]
- [239].Barkal LJ, Procknow CL, Álvarez-Garciá YR, Niu M, Jiménez-Torres JA, Brockman-Schneider RA, Gern JE, Denlinger LC, Theberge AB, Keller NP, Berthier E, et al. Microbial volatile communication in human organotypic lung models. Nat Commun. 2017 doi: 10.1038/s41467-017-01985-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Zhang M, Xu C, Jiang L, Qin J. A 3D human lung-on-a-chip model for nanotoxicity testing. Toxicol Res (Camb) 2018;7:1048–1060. doi: 10.1039/c8tx00156a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].V. R., L. C., C. D., L. H., N. J., V. A., K. K., A. S., S. M., H. G.A. Severe asthma-on-chip: A novel in vitro platform to model viral-induced exacerbations in asthma. Am J Respir Crit Care Med. 2017 [Google Scholar]
- [242].Manunta MDI, Tagalakis AD, Attwood M, Aldossary AM, Barnes JL, Munye MM, Weng A, Mcanulty RJ, Hart SL. Delivery of ENaC siRNA to epithelial cells mediated by a targeted nanocomplex : a therapeutic strategy for cystic fibrosis. Sci Rep. 2017:1–12. doi: 10.1038/s41598-017-00662-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Ahmad K, Alex GC, Zem H, Now G, Eybye ME, Zhu X, Guadagnin E, White RA, Rice LM, Andrea LF, St S, Jo C, et al. Systemic modified messenger RNA for replacement therapy in alpha 1-antitrypsin deficiency. 2020:1–11. doi: 10.1038/s41598-020-64017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]