The past years have witnessed concerted efforts across the respiratory community to establish more relevant in vitro preclinical tools for pulmonary research [1–3]. A strong incentive for such endeavors stems from the need to deliver novel therapies for respiratory diseases; a point sharply underlined at the 2014 European Respiratory Society Presidential Summit [4]. Very few new classes of safe and effective drugs have been introduced over the past half century. In contrast to other diseases areas (e.g. cardiovascular, neurological), pulmonary medicine has witnessed substantially fewer drugs approved; a situation coinciding with comparatively less drug candidates and a higher failure rate. Such facts are concurrent to the observation that pulmonary diseases represent a vast and growing healthcare and financial burden worldwide, associated with high morbidity and mortality [5]. Yet, there has been little impact in therapies treating for example chronic obstructive pulmonary disease (COPD), the fourth leading cause of death globally [4]. In parallel, asthma continues to be among the most prevalent worldwide diseases, underscoring the need for effective treatments in pediatric [6] and severe asthmatic populations [7]. Meanwhile, infectious diseases such as tuberculosis (TB) represent an increasing risk due to lack of effective therapies resulting from low efficacy and high toxicity in the face of multidrug-resistant TB [8,9]. The same is true for pulmonary infections by biofilm-forming bacteria (as a consequence of cystic fibrosis), when the increasing occurrence of antimicrobial resistance (AMR) will require novel anti-infective therapies, complementary to established antibiotics [10]. With a dire need to advance available pulmonary therapies, this short editorial underlines bioengineering opportunities for leveraging microfluidic-based lung-on-chips in devising novel human relevant in vitro models of respiratory disorders and ultimately help accelerate pulmonary preclinical research.
1. Lung-on-Chips as Attractive in vitro Platforms
The efforts surrounding the momentum for improved in vitro models are closely linked to growing discussions on alternatives to in vivo animal experiments [11]. On the one hand, this follows from ethical and political concerns in line with the application of the “3Rs principles” (Refinement, Reduction, Replacement) towards the highest standards for humane experimentation on animals. Alternatively, progress in establishing in vitro models comes as a response to the major hurdles faced with animal experiments regarding the extent to which these shed light on human diseases, with some arguing whether findings have a tangible translational impact [12]. Many new drugs have demonstrated good performance in animal models of asthma but failed at the level of safety or efficacy trials in humans, underlining a call for better predictive models [13]. In particular, differences in physiology and pharmacology between animals and humans constitute underlying barriers towards new drug development; a consequence to divergences in airway cell and innate immune responses to injury between humans and prevalent animal models [14].
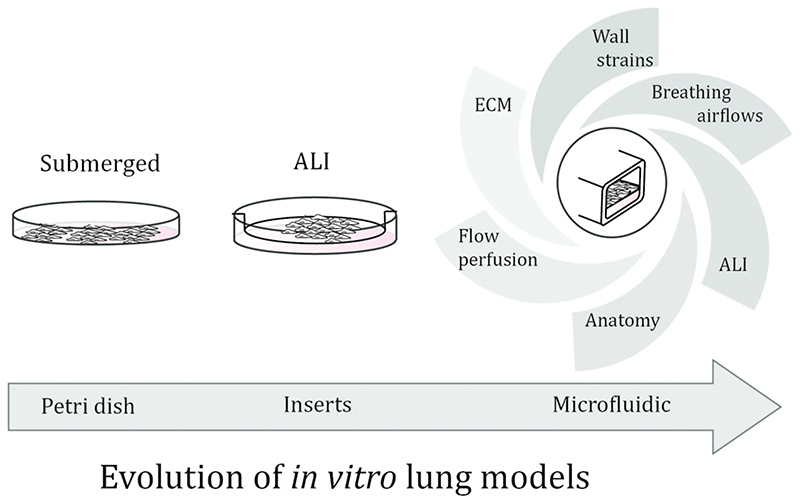
Human cell-based assays are widely used for preclinical drug development and include profiling compounds in high-throughput in vitro studies [4]. Nevertheless, a significant criticism faced with current “gold standard” in vitro assays using petri dishes or transwell inserts (Fig. 1) lies in their inability to recapitulate with sufficient accuracy complex interactions between various human cell types and tissues in vivo [15]. In turn, the challenge to develop more pertinent in vitro models has been a driving force behind the advent of lung-on-chips, and more widely organ-on-chip systems [16–18]. One of the hallmarks of lung-on-chips lies in recapitulating more accurately biological functionality of the human airway barrier at the air-liquid interface (ALI), using e.g. biopsy-driven primary cells. This comes hand in hand with the integration of critical physiological cues (Fig. 1) that are beyond reach with traditional assays [17,19–22]. Broadly speaking, lung-on-chips hold the ability to (i) reproduce morphometric traits of the lung anatomy (e.g. bifurcating trees, alveolated airways) potentially at true scale; (ii) recapitulate stretching motions mimicking cyclic breathing and thereby cellular strains in airways and within the extra-cellular matrix (ECM); (iii) incorporate respiratory airflows along the epithelium as well as (iv) provide flow perfusion associated with physiological shear stresses upon the endothelium (e.g. using setups that feature basal flows of nutrient media beneath a porous membrane).
Figure 1.
Schematic of preclinical in vitro research tools in respiratory medicine. Traditional assays include the use of petri dishes where cell cultures are grown under submerged conditions. The introduction of filter inserts permits cell cultures (e.g. epithelium, co-cultures, etc.) at the air-liquid interface (ALI), as well as growing endothelial cells on the basal side of the membrane. With the introduction of lung-on-chip platforms, ALI-based cultures can now include an array of physiological cues more closely in line with the innate pulmonary milieu.
2. From Toxicity Assays to Disease Models
The main efforts to date witnessed with microfluidic-based organ-on-chips have focused on toxicity models [21,23–27]. In respiratory research, this follows the seminal work of Huh et al. [28]. The premise of such assays lies in triggering an inflammatory response of an initially healthy airway barrier model (i.e. using mono- or co-cultures of lung cells) upon exposure to foreign threats via the inhalation route [29,30]. Exposure assays include most prominently inhaled particulate matter (PM), noxious gases, viruses and bacteria. Quantitative assessment is then conducted via characterization of well-established biological endpoints including cytokine secretion (e.g. interleukins), cell viability and gene expression, as well as measurements of lung tissue barrier properties using permeability assays (e.g. apparent permeability coefficient) and Trans-Epithelial Electrical Resistance (TEER). In parallel, there have been recent discussions on leveraging organ-on-chips to model various cancers [31,32]; in practice yet, few have tackled lung specific cancers. A recent organ-on-chip explored the potential of modelling orthotopic lung cancer growth in helping explain the resistance to therapy in patients [33].
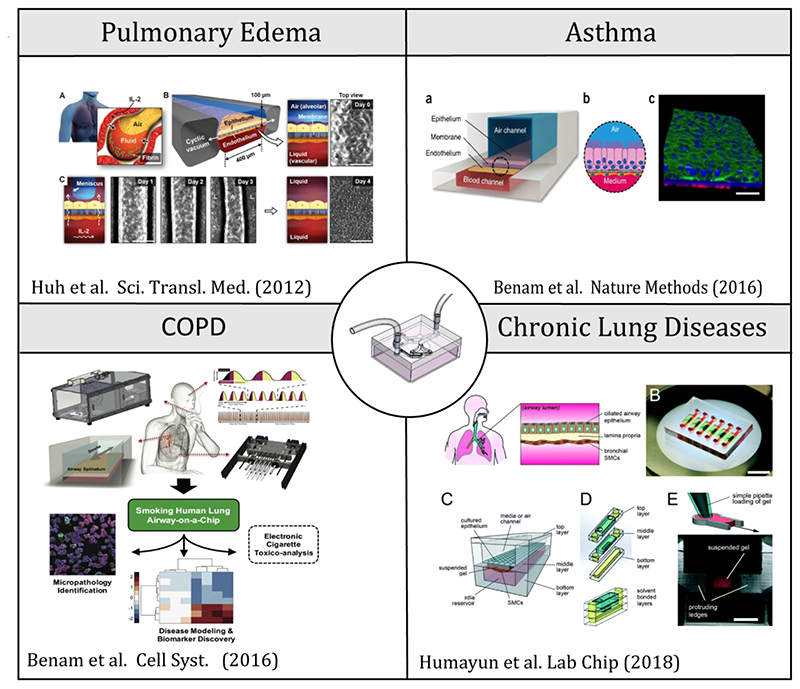
One of the strongholds of lung-on-chips is their potential as research tools to deliver much more realistic, in situ-like, human inhalation assays [20], thereby mimicking physiological and mechanistic determinants surrounding the journey of airborne aerosols to deposition at the ALI. This represents an important departure from traditional in vitro assays, in particular those conducted with liquid instillations on cell cultures under submerged conditions or via direct spraying at an ALI (Fig. 1). Recalling that in vivo exposure models comprise animals such as rodents whose anatomy differs quite significantly from humans [4], the prospect of more realistic in situ-like assays becomes even more critical when aiming to reproduce in vivo deposition outcomes (e.g. localized concentration, hot spots, etc.) in human airways [34–37]. Despite progress and recent discussions [38,39], few lung-on-chip studies have gone beyond cytotoxicity assays and instead been geared at investigating respiratory diseases (e.g. using patient cells) or conversely eliciting a respiratory disease state. Noticeable examples (Fig. 2) include an asthma model [27], pulmonary edema [40] and a COPD model [41]. Recently, an airway-on-chip study has explored epithelial and smooth muscle cell interactions in the pathogenesis of chronic lung diseases [42].
Figure 2.
Recent examples of lung-on-chips modeling respiratory diseases include an asthma model [27], pulmonary edema [40] and a COPD model [41], as well as most an airway-on-chip study on epithelial and smooth muscle cell interactions in the pathogenesis of chronic lung diseases [42]. By and large, most microfluidic in vitro designs still consist of simple, straight channels featuring an alveolar capillary barrier (ACB) model made of co-cultures of epithelial and endothelial cells grown above and below a porous membrane, respectively.
3. Expert Opinion
Voiced concern on the lack of therapies for respiratory disorders is calling for accrued efforts in establishing improved in vitro disease models of COPD, idiopathic pulmonary fibrosis (IPF), lung infections, acute lung injury (ALI) and pulmonary hypertension amongst other. Ultimately no single in vitro platform is likely to reflect the overall complexity of such diseases, in particular chronic ones. Yet the prospect of advanced lung-on-chips that successfully integrate relevant physiological cues of the human pulmonary milieu is extremely promising to explore specific clinical phenotypes, and thereby identify clinically-relevant endpoints. In this context, in vitro models are still widely seen as complimentary to in vivo animal experiments, but in most instances they do not yet offer viable alternative approaches on their own [11]. Whether lung-on-chips can ultimately reverse this situation remains an open question. Due to their complex designs, characteristics and functions, lung-on-chips are yet to establish themselves as standardized preclinical tools at the hands of end users within respiratory research and medicine [43].
In the case of pulmonary infections (e.g. pneumonia, TB), there is a significant need to advance in vitro models of pathogen-host interactions, whereby a major challenge lies in faithfully recapitulating interactions between recruited cells, as well as local airway cells, and a given pathogen [44]. To date, few lung-on-chip studies on lung infections are available [25,27]; these have been mostly based on simulating conditions using e.g. bacterial wall–derived components that stimulate cytokine secretion (e.g. lipopolysaccharides). Looking ahead, however, future lungon-chips should aim to model chronic infections by biofilm-forming bacteria, ideally allowing the repeated administration of anti-infectives [45]. With an aim towards clinical relevance in patient populations, exploring new therapeutic approaches (e.g. pathoblockers) calls for purposeful and standardized endpoints with meaningful readouts not only for antibacterial efficacy but also regarding pathophysiological changes at the host end [46].
Not unlike the challenges faced with in vivo animal models, though, the relevance and success of lung-on-chip models will lie in their validity to mirror major hallmarks of respiratory conditions, including foremost chronic progressive and irreversible changes in the lungs. Such endeavors are extremely challenging, in particular when etiological factors (e.g. occupational exposure, cigarette smoke) and natural history of the disease are poorly understood (e.g. COPD, IPF) or conversely in the absence of unique molecular signatures (e.g. ARDS). Nevertheless, and in contrast to human tissue in vivo, the relatively simple cell composition of such platforms offers advantages. The use of primary (and immune) cells originating from different donors holds the potential to explore disease diversity across populations, towards personalized medicine applications, and as such a path towards understanding basic mechanisms of disease initiation. As a final remark, we briefly recall that in vitro studies should be distinguished between investigations aimed at determining underlying mechanisms of injury or disease and those focused on the mechanisms of resolution, in particular when concerned with therapeutic action. In the latter case, one open research avenue with lung-on-chips lies for example in administering target therapeutics in the hope of observing symptoms returning to normal conditions following the elicitation of a disease state.
Acknowledgement
This work was supported by the German Israel Foundation (GIF, grant agreement no. I-1348-409.10/2016) and the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement No 677772).
Footnotes
Conflict of Interest
CM Lehr is co-founder, scientific advisor and shareholder of PharmBioTec GmbH, Saarbrücken, Germany.
References
- [1].Hittinger M, Juntke J, Kletting S, Schneider-Daum N, de Souza Carvalho C, Lehr CM. Preclinical safety and efficacy models for pulmonary drug delivery of antimicrobials with focus on in vitro models. Adv Drug Deliv Rev. 2015;85:44–56. doi: 10.1016/j.addr.2014.10.011. [DOI] [PubMed] [Google Scholar]
- [2].Hittinger M, Schneider-Daum N, Lehr CM. Cell and tissue-based in vitro models for improving the development of oral inhalation drug products. Eur J Pharm Biopharm. 2017;118:73–78. doi: 10.1016/j.ejpb.2017.02.019. [DOI] [PubMed] [Google Scholar]
- [3].Prakash YS, Halayko AJ, Gosens R, Panettieri RA, Camoretti-Mercado B, Penn RB, Aiyar R, Ammit A, Berkman N, Bond R, et al. An official American thoracic society research statement: Current challenges facing research and therapeutic advances in airway remodeling. Am J Respir Crit Care Med. 2017;195:e4–e19. doi: 10.1164/rccm.201611-2248ST. [DOI] [PubMed] [Google Scholar]
- [4].Barnes PJ, Bonini S, Seeger W, Belvisi MG, Ward B, Holmes A. Barriers to new drug development in respiratory disease. Eur Respir J. 2015;45:1197–1207. doi: 10.1183/09031936.00007915. [DOI] [PubMed] [Google Scholar]
- [5].Wisnivesky J, De-Torres JP. The global burden of pulmonary diseases: Most prevalent problems and opportunities for improvement. Ann Glob Heal. 2019;85:1–2. doi: 10.5334/aogh.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kwok PCL, Chan H-K. Delivery of inhalation drugs to children for asthma and other respiratory diseases. Adv Drug Deliv Rev. 2014;73:83–8. doi: 10.1016/j.addr.2013.11.007. [DOI] [PubMed] [Google Scholar]
- [7].Maglione M, Poeta M, Santamaria F. New drugs for pediatric asthma. Front Pediatr. 2019;6:1–7. doi: 10.3389/fped.2018.00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tiberi S, Muñoz-Torrico M, Duarte R, Dalcolmo M, D’Ambrosio L, Migliori GB. New drugs and perspectives for new anti-tuberculosis regimens. Rev Port Pneumol (English Ed. 2018;24:86–98. doi: 10.1016/j.rppnen.2017.10.009. [DOI] [PubMed] [Google Scholar]
- [9].Dooley KE, Hanna D, Mave V, Eisenach K, Savic RM. Advancing the development of new tuberculosis treatment regimens: The essential role of translational and clinical pharmacology and microbiology. PLoS Med. 2019;16:1–14. doi: 10.1371/journal.pmed.1002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ho DK, Nichols BLB, Edgar KJ, Murgia X, Loretz B, Lehr CM. Challenges and strategies in drug delivery systems for treatment of pulmonary infections. Eur J Pharm Biopharm. 2019;144:110–124. doi: 10.1016/j.ejpb.2019.09.002. [DOI] [PubMed] [Google Scholar]
- [11].Bonniaud P, Fabre A, Frossard N, Guignabert C, Inman M, Kuebler WM, Maes T, Shi W, Stampfli M, Uhlig S, White E, et al. Optimising experimental research in respiratory diseases: An ERS statement. Eur Respir J. 2018;51 doi: 10.1183/13993003.02133-2017. [DOI] [PubMed] [Google Scholar]
- [12].Van Der Worp HB, Howells DW, Sena ES, Porritt MJ, Rewell S, Collins O, Macleod MR. Can Animal Models of Disease Reliably Inform Human Studies? 2010;7 doi: 10.1371/journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Holmes AM, Solari R, Holgate ST. Animal models of asthma: Value, limitations and opportunities for alternative approaches. Drug Discov Today. 2011;16:659–670. doi: 10.1016/j.drudis.2011.05.014. [DOI] [PubMed] [Google Scholar]
- [14].Mestas J, Hughes CCW. Of Mice and Not Men: Differences between Mouse and Human Immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- [15].de Souza Carvalho C, Daum N, Lehr C-M. Carrier interactions with the biological barriers of the lung: Advanced in vitro models and challenges for pulmonary drug delivery. Adv Drug Deliv Rev. 2014;75:129–140. doi: 10.1016/j.addr.2014.05.014. [DOI] [PubMed] [Google Scholar]
- [16].Zhang B, Korolj A, Lai BFL, Radisic M. Advances in organ-on-a-chip engineering. Nat Rev Mater. 2018;3:257–278. doi: 10.1038/s41578-018-0034-7. [DOI] [Google Scholar]
- [17].Huh D, Torisawa Y, a Hamilton G, Kim HJ, Ingber DE. Microengineered physiological biomimicry: organs-on-chips. Lab Chip. 2012;12:2156–64. doi: 10.1039/c2lc40089h. [DOI] [PubMed] [Google Scholar]
- [18].Huh D, Kim HJ, Fraser JP, Shea DE, Khan M, Bahinski A, a Hamilton G, Ingber DE. Microfabrication of human organs-on-chips. Nat Protoc. 2013;8:2135–57. doi: 10.1038/nprot.2013.137. [DOI] [PubMed] [Google Scholar]
- [19].Tenenbaum-Katan J, Artzy-Schnirman A, Fishler R, Korin N, Sznitman J. Biomimetics of the pulmonary environment in vitro: A microfluidics perspective. Biomicrofluidics. 2018;12:042209. doi: 10.1063/1.5023034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Artzy-Schnirman A, Hobi N, Schneider-Daum N, Guenat OT, Lehr C-M, Sznitman J. Advanced in vitro lung-on-chip platforms for inhalation assays: From prospect to pipeline. Eur J Pharm Biopharm. 2019;144:11–17. doi: 10.1016/j.ejpb.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ainslie GR, Davis M, Ewart L, Lieberman LA, Rowlands DJ, Thorley AJ, Yoder G, Ryan AM. Microphysiological lung models to evaluate the safety of new pharmaceutical modalities: a biopharmaceutical perspective. Lab Chip. 2019 doi: 10.1039/c9lc00492k. [DOI] [PubMed] [Google Scholar]
- [22].Guenat OT, Berthiaume F. Incorporating mechanical strain in organs-on-a-chip: Lung and skin. Biomicrofluidics. 2018;12:042207. doi: 10.1063/1.5024895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Caplin JD, Granados NG, James MR, Montazami R, Hashemi N. Microfluidic Organ-on-a-Chip Technology for Advancement of Drug Development and Toxicology. Adv Healthc Mater. 2015;4:1426–1450. doi: 10.1002/adhm.201500040. [DOI] [PubMed] [Google Scholar]
- [24].Mahto SK, Charwat V, Ertl P, Rothen-Rutishauser B, Rhee SW, Sznitman J. Microfluidic platforms for advanced risk assessments of nanomaterials. Nanotoxicology. 2015;9:381–395. doi: 10.3109/17435390.2014.940402. [DOI] [PubMed] [Google Scholar]
- [25].Artzy‐Schnirman A, Zidan H, Elias‐Kirma S, Ben‐Porat L, Tenenbaum‐Katan J, Carius P, Fishler R, Schneider‐Daum N, Lehr C, Sznitman J. Capturing the Onset of Bacterial Pulmonary Infection in Acini‐On‐Chips. Adv Biosyst. 2019;3:1900026. doi: 10.1002/adbi.201900026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhang M, Xu C, Jiang L, Qin J. A 3D human lung-on-a-chip model for nanotoxicity testing. Toxicol Res (Camb) 2018;7:1048–1060. doi: 10.1039/c8tx00156a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Benam KH, Villenave R, Lucchesi C, Varone A, Hubeau C, Lee H-H, Alves SE, Salmon M, Ferrante TC, Weaver JC, Bahinski A, et al. Small airway-on-a-chip enables analysis of human lung inflammation and drug responses in vitro. Nat Methods. 2016;13:151–157. doi: 10.1038/nmeth.3697. [DOI] [PubMed] [Google Scholar]
- [28].Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science (80-) 2010;328:1662–8. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Faber SC, McCullough SD. Through the Looking Glass: In Vitro Models for Inhalation Toxicology and Interindividual Variability in the Airway. Appl Vitr Toxicol. 2018;4:115–128. doi: 10.1089/aivt.2018.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hiemstra PS, Grootaers G, van der Does AM, Krul CAM, Kooter IM. Human lung epithelial cell cultures for analysis of inhaled toxicants: Lessons learned and future directions. Toxicol Vitr. 2018;47:137–146. doi: 10.1016/j.tiv.2017.11.005. [DOI] [PubMed] [Google Scholar]
- [31].Phelps AS. Modelling cancer in microfluidic. Nat Rev Cancer. 2019;19 doi: 10.1038/s41568-018-0104-6. [DOI] [PubMed] [Google Scholar]
- [32].Sun W, Luo Z, Lee J, Kim H, Lee K, Tebon P, Feng Y, Dokmeci MR, Sengupta S, Khademhosseini A. Organ-on-a-Chip for Cancer and Immune Organs Modeling. 2019:1–12.:1801363. doi: 10.1002/adhm.201801363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hassell BA, Goyal G, Lee E, Sontheimer-phelps A, Levy O, Chen CS, Ingber DE, Hassell BA, Goyal G, Lee E, Sontheimer-phelps A, et al. Human Organ Chip Models Recapitulate Orthotopic Lung Cancer Growth, Therapeutic Responses, and Tumor Dormancy In Vitro Human Organ Chip Models Recapitulate Orthotopic Lung Cancer Growth, Therapeutic Responses, and Tumor Dormancy In Vitro. CellReports. 2017;21:508–516. doi: 10.1016/j.celrep.2017.09.043. [DOI] [PubMed] [Google Scholar]
- [34].Darquenne C, Fleming JS, Katz I, Martin AR, Schroeter J, Usmani OS, Venegas J, Schmid O. Bridging the Gap Between Science and Clinical Efficacy: Physiology, Imaging, and Modeling of Aerosols in the Lung. J Aerosol Med Pulm Drug Deliv. 2016;29:107–126. doi: 10.1089/jamp.2015.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Darquenne C. Aerosol deposition in health and disease. J Aerosol Med Pulm Drug Deliv. 2012;25:140–7. doi: 10.1089/jamp.2011.0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Elias‐Kirma S, Artzy‐Schnirman A, Das P, Heller-Algazi M, Korin N, Sznitman J. In situ-like aerosol inhalation exposure for cytotoxicity assessment using airway-on-chips platforms. Front Bioeng Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Fishler R, Hofemeier P, Etzion Y, Dubowski Y, Sznitman J. Particle dynamics and deposition in true-scale pulmonary acinar models. Sci Rep. 2015;5:14071. doi: 10.1038/srep14071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Viola H, Chang J, Grunwell JR, Hecker L, Tirouvanziam R, Grotberg JB, Takayama S. Microphysiological systems modeling acute respiratory distress syndrome that capture mechanical force-induced injury-inflammation-repair. APL Bioeng. 2019;3:041503. doi: 10.1063/1.5111549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Nawroth JC, Barrile R, Conegliano D, van Riet S, Hiemstra PS, Villenave R. Stem cell-based Lung-on-Chips: The best of both worlds? Adv Drug Deliv Rev. 2019;140:12–32. doi: 10.1016/j.addr.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Huh D, Leslie DC, Matthews BD, Fraser JP, Jurek S, a Hamilton G, Thorneloe KS, McAlexander MA, Ingber DE. A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Sci Transl Med. 2012;4:159ra147. doi: 10.1126/scitranslmed.3004249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Benam KH, Novak R, Nawroth J, Hirano-Kobayashi M, Ferrante TC, Choe Y, Prantil-Baun R, Weaver JC, Bahinski A, Parker KK, Ingber DE. Matched-Comparative Modeling of Normal and Diseased Human Airway Responses Using a Microengineered Breathing Lung Chip. Cell Syst. 2016;3:456–466.:e4. doi: 10.1016/j.cels.2016.10.003. [DOI] [PubMed] [Google Scholar]
- [42].Humayun M, Chow CW, Young EWK. Microfluidic lung airway-on-a-chip with arrayable suspended gels for studying epithelial and smooth muscle cell interactions. Lab Chip. 2018;18:1298–1309. doi: 10.1039/c7lc01357d. [DOI] [PubMed] [Google Scholar]
- [43].Junaid A, Mashaghi A, Hankemeier T, Vulto P. An end-user perspective on Organ-on-a-Chip: Assays and usability aspects. Curr Opin Biomed Eng. 2017;1:15–22. doi: 10.1016/j.cobme.2017.02.002. [DOI] [Google Scholar]
- [44].Frija-Masson J, Martin C, Regard L, Lothe MN, Touqui L, Durand A, Lucas B, Damotte D, Alifano M, Fajac I, Burgel PR. Bacteria-driven peribronchial lymphoid neogenesis in bronchiectasis and cystic fibrosis. Eur Respir J. 2017;49 doi: 10.1183/13993003.01873-2016. [DOI] [PubMed] [Google Scholar]
- [45].Horstmann JC, Boese A, Schneider-Daum N, Carvalho-Wodarz C, Lehr C-M. Altex. in press; 2019. P aeruginosa biofilm infected bronchial epithelium as test-system for anti-infective aerosol formulations. [Google Scholar]
- [46].Montefusco-Pereira CV, Horstmann JC, Ebensen T, Beisswenger C, Bals R, Guzmán CA, Schneider-Daum N, de Souza Carvalho-Wodarz N, Lehr C-M. Establishment of a Pseudomonas aeruginosa infected-3D co-culture of bronchial epithelial cells and macrophages at air-liquid interface for preclinical evaluation of anti-infectives. doi: 10.3791/61069. Submitted (n.d.) [DOI] [PubMed] [Google Scholar]