Abstract
Inhalation therapy is a hallmark of modern respiratory medicine. Over recent years, computational fluid-particle dynamics (CFPD) simulations of respiratory airflows and aerosol deposition in the lungs have rapidly developed into an increasingly mature research field in the biomedical engineering realm, owing, among others, to tremendous advances in computational capabilities and available resources. Despite such progress, the intrinsic anatomical and physiological complexity of the lungs prevents the straightforward implementation of ‘brute force’ simulation strategies applied across the entire pulmonary tract. Here, we discuss how knowledge gathered from recent in silico studies can be purposefully leveraged to design efficient hybrid multiscale lung models and explore quantitatively via computational fluid-particle dynamics inhalation therapy outcomes. In contrast to the efforts geared toward patient-specific applications, we argue instead that such in silico strategies hold tremendous promise for broad inter-subject variability studies that can help foster the development of clinically efficient inhalation therapies across large human patient populations.
Keywords: Multiscale, Numerical simulations, CFD, Lungs, Inhalation therapy, Drug delivery
Introduction
Inhalation therapy represents a cornerstone of modern respiratory medicine and serves as the first line of delivering medication (e.g., corticosteroid, bronchodilators, antibiotics) in respiratory diseases [1,2], including asthma, chronic obstructive pulmonary disease (COPD), emphysema, and cystic fibrosis. Therapeutic aerosols also embody promising avenues for systemic drug delivery, gene therapy, and vaccination [3,4]. Yet, delivering aerosols efficiently to the lungs, or its regions (e.g., lung lobes), for localized treatment, remains challenging. Despite its centuries-old use, the efficacy of inhalation therapy remains persistently low and is typically evaluated by assessing the fraction of deposited aerosols in the lungs past the oropharyngeal region, relative to the initial dose inhaled (e.g., total number of aerosols). That is, lung deposition fractions [5,6] are typically well below 50% using commercially available inhalation devices, a number that is even more dismal in obstructive and restrictive airway diseases as well as in younger, pediatric populations [7–9].
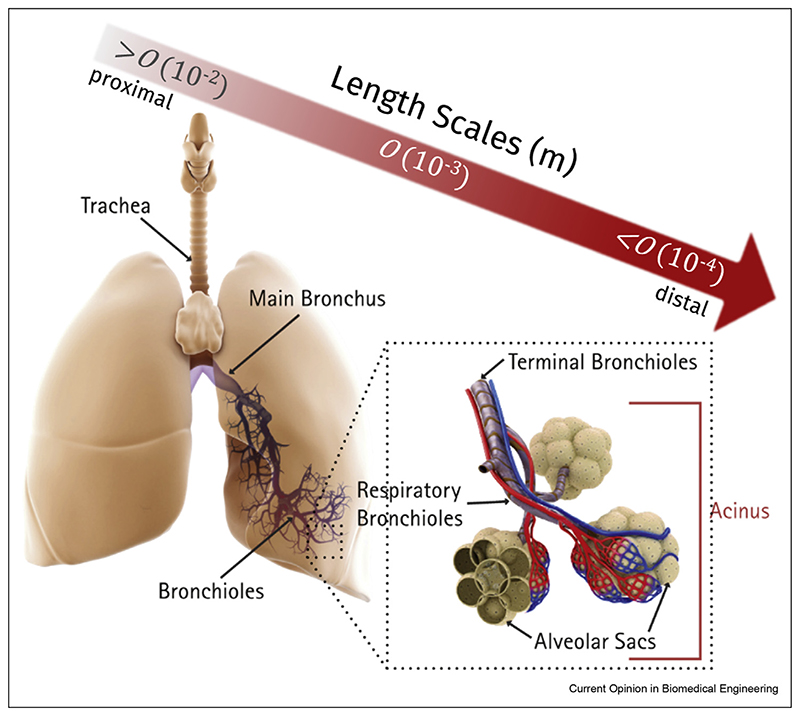
Given the current spatial and temporal limitations of clinical imaging modalities for in vivo human aerosol deposition assessment [10,11] (e.g., gamma scintigraphy), numerical modeling approaches have gained tremendous traction over past decades to characterize aerosol deposition outcomes [12]; such in silico tools have been pivotal in uncovering the mechanistic underpinnings of inhaled aerosol transport and determining local deposition concentrations. Moreover, they have proven useful in evaluating existing inhalers and designing new ones, as well as exploring new inhalation delivery strategies (e.g., controlled particle condensational growth [13,14]). Yet, the accurate, let alone realistic, prediction of aerosol deposition in the lungs remains a hugely complex endeavor owing among others to the intrinsically multiscale characteristics of the respiratory organ (Figure 1). Not only do anatomical length scales span over three orders of magnitude across the respiratory tract, that is, from centimeters in the trachea down to ∼100 μm alveolar cavities in the acinar regions, its surface area available for gas exchange [15] and thus aerosol delivery is ∼100 m2 with >106 m of pathways and >108 alveoli. In turn, ‘brute force’ approaches involving fully resolved numerical simulations of complete lungs are still beyond reach [12,16], even allowing for quickly rising computational capabilities. In the present opinion piece, we discuss how knowledge gathered from recent in silico studies can be leveraged to design efficient hybrid multiscale lung models and explore inhalation therapy outcomes. Specifically, we argue that such in silico strategies can be successfully applied for broad inter-subject variability studies and, thereby, tackle the delivery of clinically efficient inhalation therapies across diverse large populations.
Figure 1.
The lungs embody a highly complex organ with a vast dichotomously bifurcating asymmetric tree structure that spans over three orders of magnitude in anatomical length scales, that is, from centimeters in the trachea down to ~100 μm alveolar cavities in the depths of the acinar regions. Its surface area available for gas exchange, and consequently aerosol delivery, is approximately 100 m2 for an average human adult (i.e., the size of a tennis court), with >106 m of pathways and nearly half a billion alveoli.
Lessons learned from in silico studies: The extra-thoracic and conducting airways
Respiratory transport is highly dynamic, covering a wide spectrum of airflow regimes, that is, from transitional to turbulent in the extra-thoracic airways [17,18] down to quasi-steady, creeping flows in the acinar depths [19]. Notably, during a complete breathing cycle, respiratory flowrates are transient such that the particle transport characteristics (e.g., Stokes number) are modulated despite a nominal inhalation rate [20,21]. These latter considerations necessitate the choice of turbulence models, especially in the extra-thoracic and upper airways, when implementing computational fluid-particle dynamics (CFPD) simulations that perform reliably under time-varying flow conditions, as well as during transitions between flow regimes (Figure 2), for example, laminar, transitional, and turbulent flows. For the first time, the SimInhale benchmark case [22,23] consistently addressed the performance of Reynolds-averaged Naviere–Stokes (RANS) simulations and large eddy simulation (LES), respectively, in capturing airflow and aerosol deposition characteristics in the extra-thoracic and upper tracheobronchial (TB) airways (Figure 3). Briefly, a number of independent research teams were solicited to simulate identical cases using both types of models implemented in their own custom numerical codes. The results were compared against in vitro flow (e.g., particle image velocimetry) and deposition measurements (positron emission tomography [PET]) in corresponding 3D manufactured airway casts. It was concluded that while RANS can perform acceptably when simulation conditions are properly set up, LES results exhibit considerably less variability and are generally in better agreement with in vitro measurements. Nevertheless, even in LES, the results were observed to be sensitive to grid quality, design, and resolution; grid resolution requirements were overall found to be higher than what is often reported in the literature and depend on the specific simulation conditions [23] (e.g., anatomy, flowrate, etc.). Thus, a good practice is to undertake a grid independence study in any new case and to avoid relying on rules of thumb to forego going through this latter step.
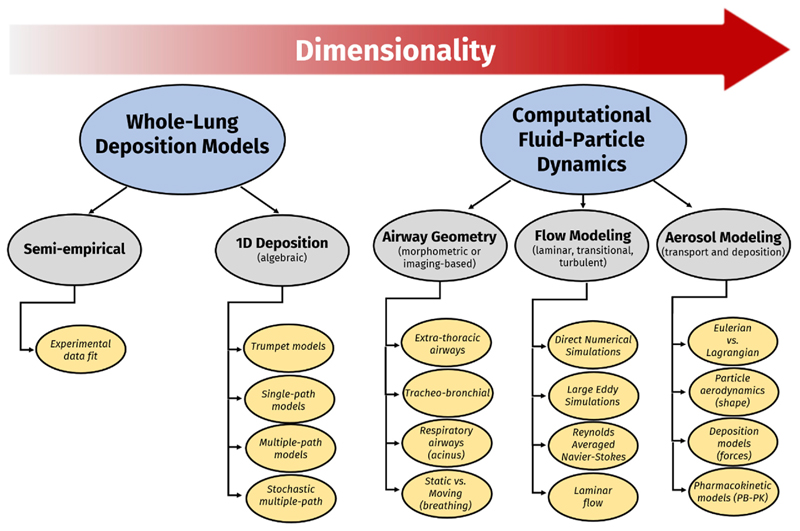
Figure 2.
Schematic overview of aerosol deposition prediction models available, that is, from traditional whole-lung deposition models (with either semi-empirical models or algebraic 1D models) to more recent CFPD simulations that deliver 3D spatial–temporal information on both respiratory airflows and aerosol transport dynamics.
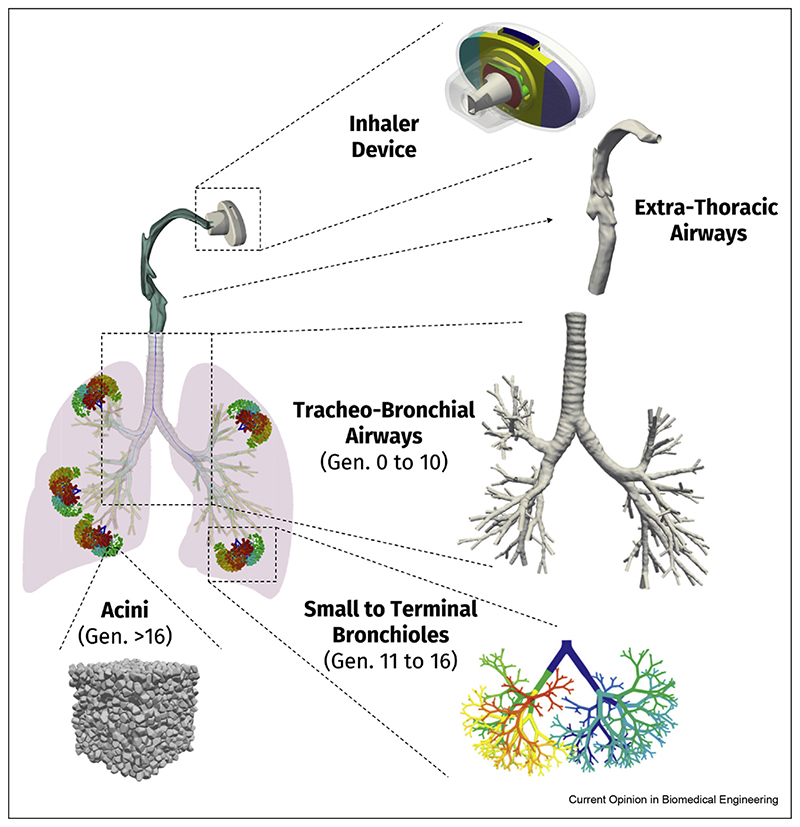
Figure 3.
Schematic embodiment of the in silico multiscale CFPD simulation strategy, whereby different compartments of the lungs (i.e., extra-thoracic, conducting trachea-bronchial, conducting mid/small bronchi, acinus) are independently modeled, but simulated in combination, with various degrees of computational efforts invested in each depending on the required outcomes sought (e.g., regional aerosol deposition fraction, etc.).
Recent studies have underlined that the predicted respiratory airflows and deposition distributions in the upper TB tree are sensitive to the choice of outlet boundary conditions [24–26], a general conundrum with in silico modeling approaches of respiratory transport phenomena in the lungs. Indeed, in the absence of complete patient-specific ventilation quantifications, a common practice is, thus, to impose uniform outlet pressures at the terminal sections of the bronchi. Yet, such strategy can significantly impact regional deposition estimates. Therefore, imposing correct mass fractions, or alternatively, pressure levels, at the outlets of an upper airway CFPD model must be propagated from the alveolar regions upstream back to the distal ends of the 3D upper airway model. Several methods in recent years have been proposed to tackle this boundary condition challenge using either reduced dimensional models [25,27] (e.g., typically based on impedance or resistive models) or instead using direct imaging methods [24,28]. Furthermore, the lack of proper distal airway geometries complicates the simulation of deposition over complete breathing cycles because inhaled aerosols that distally escape the TB model during the inhalation phase and exit into the deeper lungs cannot be tracked. As this aerosol particle distribution will undergo further filtering and deposition in the deeper lung regions, the ensuing particle distribution re-entering the upper bronchial segments during the exhalation phase is anticipated to be modified.
A number of encouraging results from recent studies can be leveraged to reduce the computational cost of simulations targeting the upper airways. For example, Koullapis et al. [29] have shown that regional deposition patterns in the upper TB tree, when expressed as segmental deposition efficiencies, are largely independent of the characteristics of the extra-thoracic, mouth-throat (MT) region. In other words, the general morphological features of the MTregion determine the amount of airborne aerosol filtering occurring in the extra-thoracic regions, and thereby, the particle size distribution crossing distally into the main TB tree (Figure 3). By contrast, regional deposition efficiencies of aerosols across the upper TB region are specific to a particular TB tree morphometry [30] and are, hence, only weakly dependent on the variations in incoming airflows from the trachea. This finding suggests that simulations of regional TB deposition can be conducted either using canonical in silico MT models (e.g., representing small, medium, and large extra-thoracic volumes) or using previously stored airflow fields and particle distributions. Such strategy offers considerable computational savings in the context of in silico population studies using large sample sizes, a point we will return to further below.
Tackling whole-lung in silico modeling
In the deeper acinar regions past the conducting TB tree (Figure 1) that capture over 90% of total lung volume, in silico CFPD simulations of the alveolated airways have focused foremost on the geometrical models using, for example, space-filling acinar networks [31–34] (e.g., polyhedral-based alveolar cavities). This is mostly a consequence of the absence of any direct imaging-based reconstructed anatomies of human acinar parenchyma (some limited micro-CT datasets from rodents are available). These deep lung simulations typically feature wall-displacement boundary conditions imposed on the alveolar walls to mimic physiological breathing conditions, and thus, resolve transient aerosol transport during multiple breathing cycles. Yet, such approaches have largely consisted of so-called ‘bottom-up’ strategies entirely void of the proximal, conducting regions, whereby information on the aerosol distribution crossing through the TB and entering the acinar region tract is, in effect, absent.
To resolve such shortcomings, recent advances have included efforts to couple conductive upper airway trees to breathing alveolar models, and thus, develop complete-lung CFPD models [12,35]. For example, Kolanjiyil and Kleinstreuer [36] recently documented a numerical methodology for predicting particle deposition in a model of the entire respiratory tract (Figure 3) that includes (i) subject-specific upper airways from nose/mouth to generation 3, (ii) airway generations 4 through 21 represented as adjustable triple bifurcation units, and (iii) spherical alveoli introduced with increasing density from generations 16 to 21. Finally, their lung model ends with a double bifurcation unit that represents the terminal alveolar sacs (generations 22–23). We note that some of the shortcomings of the aforementioned study have included the use of planar triple-bifurcation airway models and implementing simulations limited to steady inhalation conditions only. Moreover, a somewhat simplified acinar model with alveoli of constant size and shape located along rigid alveolar ducts was implemented, as the authors hypothesized that the dual-path configuration could accurately capture deposition in the left and right lungs.
In parallel to the abovementioned efforts, a group led by Worth Longest and collaborators published in recent years a series of studies on the development of the stochastic individual pathway (SIP) model [37,38]. In this approach, individual continuous pathways beyond the third TB bifurcation within an MT to generation 3 geometry are stochastically generated and extend into each regional lobe of the lungs through the terminal bronchioles (generation 15). Their concept consists in simulating a sufficient number of such stochastically generated pathways until aerosol deposition results converge toward an ensemble average. Interestingly, the authors found that a single SIP model in the lower-left lobe can provide a representative average of total lung deposition, and afterward, integrated the SIP model with computational fluid dynamics-based (CFD-based) estimates of deposition in the alveolar regions in an effort to validate in silico predictions with in vivo deposition data [35]. Their predicted total lung deposition agreed with a relative error of 6% for the case of monodisperse aerosols in the 1–7 μm size range. In the case of polydisperse aerosols released from a dry powder inhaler (DPI) and a soft mist inhaler, CFPD predictions produced an average relative error <10% for each inhaler. A drawback of the SIP model, however, lies in the constant symmetric outflow conditions imposed at each bifurcation level (generation 4 through 15), an assumption valid only when airflows in the parent branches are fully developed and axisymmetric.
Arguably, perhaps, the most realistic in silico human acinar airway model to date follows the work of Koshiyama and Wada [39] initially validated using rodents; such algorithm generates heterogeneous acinar structures composed of irregular-sized alveolar cavities assembled into an intricately branched, space-filling ductal tree that captures the morphometrical features of the acinus, including local acinar heterogeneity. For the first time, CFD simulations adapted the original algorithm to match morphometric properties of an average human pulmonary acinus, and thus, deliver high-resolution, spatiale–temporal predictions of local acinar aerosol deposition [40]. In these footsteps, and in view of delivering complete-lung simulations, Koullapis et al. [41] proposed a computationally efficient implementation of a deep lung model consisting of an idealized mid- to small-bronchial tree (approximately generations 10 to 19 of the conducting zone) combined with such heterogeneous acinar structures; here, the Euleriane–Lagrangian approach was used to solve the airflow and aerosol equations. Specifically, the computational cost of the coupled simulation was reduced by taking advantage of the respiratory airflow similarity across the central conducting regions to decompose the bronchial tree into representative subunits. The authors demonstrated resulting deposition estimates in a rather strong agreement with classic deposition predictions using established whole-lung 1D models (Figure 2). Looking forward, integrating such deep lung models with LES simulations in the upper bronchial tree is anticipated to offer the added benefit to emulate more feasibly the effects of disease, including the uneven ventilation due to regional airway constriction in COPD or the destruction and remodeling of alveolar walls in emphysema.
The case for population-based inhalation aerosol predictions
With the advent of reliable CFPD simulation techniques, a notion has surfaced that imaging techniques predicting disease pathophysiology could be useful in generating subject-specific datasets of the lungs in view of tailoring inhaled drug delivery to individual patients [42]. While such personalized medicine approach to inhalation therapy could be attractive, the intrinsic inter-subject variability across lung anatomies is wide-spread across mankind [43], thus underlining the prodigious computational endeavors possibly ahead. Indeed, anatomical variability of the lungs influences aerosol deposition outcomes [44,45] and undeniably needs to be addressed when assessing general inhalation therapy. Rather than following the path of patient-specific CFPD tools, we argue here that the above-mentioned multiscale in silico strategies can be particularly useful to undertake high-fidelity simulations exploring inhalation outcomes for broad classes of human populations based on age [46], sex, and disease status. The appeal of such simulation strategies would foremost lie at the level of pre-clinical in silico studies and suited for exploring drug development and delivery methods, including new inhaler designs [5,47] (Figure 3).
One embodiment of an in silico multiscale simulation strategy would take form in quantitatively mapping how much variability in lung anatomies influences the inhaled aerosol delivery endpoints (e.g., deposition predictions, local drug concentrations, etc.). Indeed, the large anatomical variability in the first upper bronchial generations is anticipated to produce different aerosol distributions entering the various lung lobes, even among healthy individuals inhaling under similar conditions, a point even more exasperated in the presence of respiratory diseases. This follows, as we recall, for example, that details of subject-specific MTanatomy do not affect significantly the fate of those aerosols entering the conducting TB track, having first escaped extra-thoracic (ET) filtering as discussed earlier. Instead, the bulk of computational efforts could be directed toward modeling aspects in TB tree variability (Figure 3). The findings from large-scale, parametric studies would be advisable in understanding, and thus, predicting the distribution (e.g., mean, spread, etc.) in general human lung deposition data. In some sense, the advocated pipeline would be somewhat analogous to conducting pre-clinical studies using established in vitro impactors. With such results in hand, localized distribution characterizations of aerosols obtained from CFPD simulations could be fed, for instance, into existing pharmacokinetic (PK) models, a point that was recently highlighted [12] in delivering both regional lung tissue concentrations of a drug and established PK metrics (e.g., maximum blood concentration, area under the drug concentration curve). An ambitious, yet tangible, outcome would for example lie in improving current 1D model predictions (Figure 2) and expanding existing commercial software to integrate CFPD-based benchmarks, not only for healthy populations but critically for diseased lung cases, as well as pediatric populations.
Acknowledgements
This work was supported by the European Research Council (ERC) under the European Union’s Horizon 2020 Research and Innovation Program (grant agreement No 677772). The authors acknowledge COST Action MP1404 SimInhale ‘Simulation and pharmaceutical technologies for advanced patient-tailored inhaled medicines,’ supported by the European Cooperation in Science and Technology (COST).
Footnotes
Conflict of interest Statement
Nothing declared.
References
Papers of particular interest, published within the period of review, have been highlighted as:
-
*
of special interest
-
**
of outstanding interest
- 1.Stein SW, Thiel CG. The history of therapeutic aerosols: a chronological review. J Aerosol Med Pulm Drug Deliv. 2016;29:jamp.2016.1297. doi: 10.1089/jamp.2016.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holguin F, et al. management of severe asthma: a European respiratory society/American thoracic society guideline. Early view task force report. Eur Respir J. 2019 doi: 10.1183/13993003.00588-2019.1900588. [DOI] [Google Scholar]
- 3.Patton JS, Byron PR. Inhaling medicines: delivering drugs to the body through the lungs. Nat Rev Drug Discov. 2007;6:67–74. doi: 10.1038/nrd2153. [DOI] [PubMed] [Google Scholar]
- 4.Laube BL. The expanding role of aerosols in systemic drug delivery, gene therapy, and vaccination. Transl Respir Med. 2014;2 doi: 10.1186/2213-0802-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi J, et al. Differences in particle deposition between members of imaging-based asthma clusters. J Aerosol Med Pulm Drug Deliv. 2019;32:213–223. doi: 10.1089/jamp.2018.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Boer AH, et al. Dry powder inhalation: past, present and future. Expert Opin Drug Deliv. 2017;14:499–512. doi: 10.1080/17425247.2016.1224846. [DOI] [PubMed] [Google Scholar]
- 7.Das P, Nof E, Amirav I, Kassinos SC, Sznitman J. Targeting inhaled aerosol delivery to upper airways in children: insight from computational fluid dynamics (CFD) PLoS One. 2018;13:1–20. doi: 10.1371/journal.pone.0207711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiBlasi RM. Clinical controversies in aerosol therapy for infants and children. Respir Care. 2015;60:894–914. doi: 10.4187/respcare.04137. [DOI] [PubMed] [Google Scholar]
- 9.Amirav I, Newhouse MT. Deposition of small particles in the developing lung. Paediatr Respir Rev. 2012;13:73–78. doi: 10.1016/j.prrv.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Fleming J, et al. Controlled, parametric, individualized, 2-D and 3-D imaging measurements of aerosol deposition in the respiratory tract of asthmatic human subjects for model validation. J Aerosol Med Pulm Drug Deliv. 2015;28:432–451. doi: 10.1089/jamp.2014.1191. [DOI] [PubMed] [Google Scholar]
- 11.Alcoforado L, et al. Anatomically based analysis of radio-aerosol distribution in pulmonary scintigraphy: a feasibility study in asthmatics. J Aerosol Med Pulm Drug Deliv. 2018;31:jamp.2017.1403. doi: 10.1089/jamp.2017.1403. [*A leading effort in the implementation of in vivo gamma scintigraphy measurements in human asthmatic patients] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Longest PW, et al. Use of computational fluid dynamics deposition modeling in respiratory drug delivery. Expert Opin Drug Deliv. 2019;16:7–26. doi: 10.1080/17425247.2019.1551875. [**An extensive review of advances in CFD simulations for broad applications to inhalation therapy] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golshahi L, et al. The use of condensational growth methods for efficient drug delivery to the lungs during noninvasive ventilation high flow therapy. Pharm Res. 2013;30:2917–2930. doi: 10.1007/s11095-013-1123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haddrell AE, et al. Pulmonary aerosol delivery and the importance of growth dynamics. Ther Deliv. 2017;8:1051–1061. doi: 10.4155/tde-2017-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsia CCW, Hyde DM, Weibel ER. Lung structure and the intrinsic challenges of gas exchange. Comp Physiol. 2016;6:827–895. doi: 10.1002/cphy.c150028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Islam MS, Saha SC, Sauret E, Gemci T, Gu YT. Pulmonary aerosol transport and deposition analysis in upper 17 generations of the human respiratory tract. J Aerosol Sci. 2017;108:29–43. [Google Scholar]
- 17.Kleinstreuer C, Zhang Z. Airflow and particle transport in the human respiratory system. Annu Rev Fluid Mech. 2010;42:301–334. [Google Scholar]
- 18.Kleinstreuer C, Zhang Z, Donohue JF. Targeted drug-aerosol delivery in the human respiratory system. Annu Rev Biomed Eng. 2008;10:195–220. doi: 10.1146/annurev.bioeng.10.061807.160544. [DOI] [PubMed] [Google Scholar]
- 19.Sznitman J. Respiratory microflows in the pulmonary acinus. J Biomech. 2013;46:284–298. doi: 10.1016/j.jbiomech.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 20.Xi J, April Si X, Dong H, Zhong H. Effects of glottis motion on airflow and energy expenditure in a human upper airway model. Eur J Mech B Fluid. 2018;72:23–37. [Google Scholar]
- 21.Miyawaki S, Hoffman EA, Lin CL. Effect of static vs. dynamic imaging on particle transport in CT-based numerical models of human central airways. J Aerosol Sci. 2016;100:129–139. doi: 10.1016/j.jaerosci.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janke T, Koullapis P, Kassinos SC, Bauer K. PIV measurements of the SimInhale benchmark case. Eur J Pharm Sci. 2019;133:183–189. doi: 10.1016/j.ejps.2019.03.025. [*Benchmark comparisons of CFPD simulations with paticle image velocimetry data in 3D airway casts] [DOI] [PubMed] [Google Scholar]
- 23.Koullapis P, et al. Regional aerosol deposition in the human airways: the SimInhale benchmark case and a critical assessment of in silico methods. Eur J Pharm Sci. 2018;113:77–94. doi: 10.1016/j.ejps.2017.09.003. [**The first extensive benchmark CFPD study in the field from the COST Action SimInhale European network group] [DOI] [PubMed] [Google Scholar]
- 24.Yin Y, Choi J, Hoffman Ea, Tawhai MH, Lin CL. Simulation of pulmonary air flow with a subject-specific boundary condition. J Biomech. 2010;43:2159–2163. doi: 10.1016/j.jbiomech.2010.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Comerford A, Förster C, Wall Wa. Structured tree impedance outflow boundary conditions for 3D lung simulations. J Biomech Eng. 2010;132:081002. doi: 10.1115/1.4001679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asgharian B, Price O. Airflow distribution in the human lung and its influence on particle deposition. Inhal Toxicol. 2006;18:795–801. doi: 10.1080/08958370600748687. [DOI] [PubMed] [Google Scholar]
- 27.Pozin N, et al. A tree-parenchyma coupled model for lung ventilation simulation. Int J Numer Method Biomed Eng. 2017;33:1–30. doi: 10.1002/cnm.2873. [DOI] [PubMed] [Google Scholar]
- 28.De Backer JW, et al. Flow analyses in the lower airways: patient-specific model and boundary conditions. Med Eng Phys. 2008;30:872–879. doi: 10.1016/j.medengphy.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Koullapis PG, Nicolaou L, Kassinos SC. In silico assessment of mouth-throat effects on regional deposition in the upper tracheobronchial airways. J Aerosol Sci. 2018;117:164–188. [Google Scholar]
- 30.Oakes JM, Roth SC, Shadden SC. Airflow simulations in infant, child, and adult pulmonary conducting airways. Ann Biomed Eng. 2018;46:498–512. doi: 10.1007/s10439-017-1971-9. [DOI] [PubMed] [Google Scholar]
- 31.Hofemeier P, Sznitman J. The role of anisotropic expansion for pulmonary acinar aerosol deposition. J Biomech. 2016;49:3543–3548. doi: 10.1016/j.jbiomech.2016.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oakes JM, Hofemeier P, Vignon-Clementel IE, Sznitman J. Aerosols in healthy and emphysematous in silico pulmonary acinar rat models. J Biomech. 2016;49:2213–2220. doi: 10.1016/j.jbiomech.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 33.Hofemeier P, Sznitman J. Revisiting pulmonary acinar particle transport: convection, sedimentation, diffusion and their interplay. J Appl Physiol. 2015;118:1375–1385. doi: 10.1152/japplphysiol.01117.2014. [DOI] [PubMed] [Google Scholar]
- 34.Khajeh-Hosseini-Dalasm N, Longest PW. Deposition of particles in the alveolar airways: inhalation and breath-hold with pharmaceutical aerosols. J Aerosol Sci. 2015;79:15–30. doi: 10.1016/j.jaerosci.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tian G, Hindle M, Lee S, Longest PW. Validating CFD predictions of pharmaceutical aerosol deposition with in vivo data. Pharm Res. 2015 doi: 10.1007/s11095-015-1695-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolanjiyil AV, Kleinstreuer C. Computational analysis of aerosol-dynamics in a human whole-lung airway model. J Aerosol Sci. 2017;114:301–316. [Google Scholar]
- 37.Longest PW, Tian G, Khajeh-Hosseini-Dalasm N, Hindle M. Validating whole-airway CFD predictions of DPI aerosol deposition at multiple flow rates. J Aerosol Med Pulm Drug Deliv. 2016;29:jamp.2015.1281. doi: 10.1089/jamp.2015.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Longest W, Spence B, Hindle M. Devices for improved deliveryof nebulized pharmaceutical aerosols to the lungs. J Aerosol Med Pulm Drug Deliv. 2019;32:1–23. doi: 10.1089/jamp.2018.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koshiyama K, Wada S. Mathematical model of a heterogeneous pulmonary acinus structure. Comput Biol Med. 2015;62:25–32. doi: 10.1016/j.compbiomed.2015.03.032. [DOI] [PubMed] [Google Scholar]
- 40.Hofemeier P, Koshiyama K, Wada S, Sznitman J. One (sub-)acinus for all: fate of inhaled aerosols in heterogeneous pulmonary acinar structures. Eur J Pharm Sci. 2018;113:53–63. doi: 10.1016/j.ejps.2017.09.033. [*First fully-resolved aerosol deposition simulation in realistic 3D heterogeneous space-filling acinar models] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koullapis PG, Hofemeier P, Sznitman J, Kassinos SC. An efficient computational fluid-particle dynamics method to predict deposition in a simplified approximation of the deep lung. Eur J Pharm Sci. 2018;113:132–144. doi: 10.1016/j.ejps.2017.09.016. [**Implementation of a multiscale CFPD simulation for aerosol deposition spanning the mid/lower bronchi to the pulmonary acinus over complete breathing cycles] [DOI] [PubMed] [Google Scholar]
- 42.Corcoran TE. Imaging in aerosol medicine. Respir Care. 2015;60:850–855. doi: 10.4187/respcare.03537. [DOI] [PubMed] [Google Scholar]
- 43.Dominelli XPB, et al. Sex differences in large conducting airway anatomy. J Appl Physiol. 2019;960 doi: 10.1152/japplphysiol.00440.2018. [*Anatomical data supporting intrinsic anatomical variability between human lung anatomies in the upper airways] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rissler J, et al. Deposition efficiency of inhaled particles (15-5000 nm) related to breathing pattern and lung function: an experimental study in healthy children and adults. Part Fibre Toxicol. 2017;14:10. doi: 10.1186/s12989-017-0190-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jakobsson JKF, et al. Altered deposition of inhaled nanoparticles in subjects with chronic obstructive pulmonary disease. BMC Pulm Med. 2018;18:1–11. doi: 10.1186/s12890-018-0697-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Darquenne C, Prisk GK. The effect of aging on aerosol bolus deposition in the healthy adult lung: a 19-year longitudinal study. J Aerosol Med Pulm Drug Deliv. 2019;32:1–7. doi: 10.1089/jamp.2019.1566. [*First long-term longitudinal study of in vivo aerosol deposition using a bolus inhalation technique across aging] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miyawaki S, Hoffman EA, Wenzel SE, Lin CL. Aerosol deposition predictions in computed tomography-derived skeletons from severe asthmatics: a feasibility study. Clin Biomech. 2019;66:81–87. doi: 10.1016/j.clinbiomech.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]