Abstract
Aim
Mutations in the ABCC8 gene encoding the SUR1 subunit of the pancreatic KATP channel cause permanent (PNDM) and transient neonatal diabetes mellitus (TNDM). We reviewed the existing literature, extended the number of cases and explored genotype-phenotype correlations.
Methods
Mutations were identified by sequencing in patients diagnosed with diabetes before 6 months without a KCNJ11 mutation.
Results
We identified ABCC8 mutations in an additional 9 probands (including 5 novel mutations L135P, R306H, R1314H, L438F and M1290V), bringing the total of reported families to 48. Both dominant and recessive mutations were observed with recessive inheritance more common in PNDM than TNDM (9 vs 1; p <0.01). The remainder of the PNDM probands (n=12) had de novo mutations. 17/25 children with TNDM inherited their heterozygous mutation from a parent. Nine of these parents had permanent diabetes (median age at diagnosis 27.5 years, range 13 - 35). Recurrent mutations of residues R1183 and R1380 were found only in TNDM probands and dominant mutations causing PNDM clustered within exons 2-5.
Conclusions
ABCC8 mutations cause PNDM, TNDM or permanent diabetes diagnosed outside the neonatal period. There is some evidence that the location of the mutation is correlated with the clinical phenotype.
Keywords: ABCC8, sulfonylurea receptor-1, SUR1, Permanent Neonatal Diabetes, Transient Neonatal Diabetes, sulphonylureas, ATP-sensitive potassium ion channel
Introduction
Diabetes diagnosed within the first six months of life is likely to have a genetic aetiology [1, 2]. Transient neonatal diabetes mellitus (TNDM) is usually diagnosed in the first week, remits by a median age of 12 weeks and relapses in adolescence or early adulthood in up to half of patients [3]. In almost 70% of cases TNDM is associated with an abnormality of the imprinted region 6q24 [3]. Heterozygous gain-of-function mutations in KCNJ11, encoding the Kir6.2 subunit of the ATP-sensitive potassium channel (KATP), are found in some of the remaining cases [4–6]. Permanent neonatal diabetes mellitus (PNDM) has, until recently, required insulin treatment for life. Heterozygous activating mutations in KCNJ11 are the most common cause, accounting for 30-50% of cases [5, 7–10]. Most patients with activating Kir6.2 mutations respond to treatment with high dose sulphonylureas [11]. Transfer from insulin to sulphonylurea tablets for these patients, not only improves quality of life but improves glycaemic control [11, 12].
Heterozygous activating mutations in the ABCC8 gene encoding SUR1, the regulatory subunit of the KATP channel protein, have recently been identified as a cause of PNDM in infants diagnosed under six months of age [13–17] and also TNDM [13, 18]. It is clear from this body of work that mutations in the ABCC8 gene are a common cause of both PNDM and TNDM. In addition, some family members with an activating ABCC8 mutation have permanent diabetes diagnosed at a range of ages that may present as type 2 diabetes. This article aims to bring together the recent genetic and clinical work on SUR1 diabetes.
Role of the K ATP channel in insulin secretion
K ATP channels regulate electrical activity of the plasma membrane in many tissues and cell types including pancreatic beta-cells, heart, brain, kidney, skeletal and smooth muscle [reviewed in 19]. In these tissues membrane depolarisation is linked to stimulatory signals through control of potassium ion flux. Different isoforms of the pore forming and regulatory subunits are found particular to the cell type. The K ATP channel of the pancreatic beta cell links the sensing of glucose metabolism with membrane electrical activity in order to stimulate insulin secretion, a link that was established more than 20 years ago [20].
The channel is comprised of four inwardly rectifying potassium ion pore-forming subunits (Kir6.2) and four high affinity sulphonylurea receptor subunits (SUR1) in an intra-membrane hetero-octomeric complex [21, 22]. The circulating blood glucose level is detected by the KATP channel through changes in the balance of cytosolic nucleotides (ATP and Mg-ADP) within the beta cell. Inhibition and therefore closure of the channel is associated with an increase in glucose levels and is induced by ATP binding to the Kir6.2 subunit [23]. This leads to beta cell membrane depolarisation and opening of the voltage-gated calcium ion channels. The subsequent calcium ion influx initiates beta-cell electrical activity and triggers a cascade of events that result in the secretion of insulin [24].
The SUR1 subunit provides a regulatory control for the KATP channel. Magnesium nucleotides that bind to SUR1 subunits activate the channel thus increasing the probability of an open channel conformation [23, 25]. Channel activity is dependent upon the ratio of adenine nucleotides ATP and Mg-ATP (as well as their hydrolysed derivatives) binding to the channel subunits with their antagonistic functions. The regulatory action of SUR1 on the open state of the channel acts through the cooperative nature of nucleotide binding at the domains NBD1 and NBD2 of ATP and Mg-ADP (or Mg-ATP with subsequent hydrolysis) respectively [26]. Sulphonylurea drugs interrupt this cooperative binding by removing the bound Mg-ADP at NBD2, destabilising any bound ATP at NBD1 and facilitating channel closure [26]. The ability of sulphonylureas to bypass metabolic stimulation and close K ATP channels, hence allowing the patients’ own endogenous insulin to be released, has been widely exploited in the treatment of type 2 diabetes [27].
ABCC8 as a candidate gene for neonatal diabetes
The crucial role of the KATP channel in insulin secretion and the regulatory function of SUR1 suggested that ABCC8 gene was a good candidate in which to identify mutations that could lead to the potential disruption of glucose homeostasis. The human ABCC8 gene that encodes the SUR1 protein is located on chromosome 11p15.1 and consists of 39 exons (accession number NM_000352.2). It spans over 100Kb of genomic DNA and is adjacent to the KCNJ11 gene encoding Kir6.2. The SUR1 protein is predicted, through membrane topology studies, to contain three transmembrane domains (TMD0, TMD1 and TMD2) linked by the cytosolic linker region (L0) and two nucleotide binding domains (NBD1 and NBD2). Two such domains each contain six transmembrane helices and the third, sited at the hydrophobic N-terminus and important for interactions with Kir6.2, contains five transmembrane helices [28]. Each of the nucleotide binding domains contain sequence motifs called Walker A and Walker B which are essential for binding the phosphate groups of nucleotides [29].
Hyperinsulinemia is characterised by oversecretion of insulin despite hypoglycaemia. Recessively inherited loss-of-function mutations in the ABCC8 gene are the most common cause of congenital hyperinsulinemic hypoglycaemia [30, 31]. Inactivating mutations in KCNJ11 also cause HI although they are rarer than ABCC8 mutations [32–34]. Transgenic mice that overexpress Kir6.2 developed the opposite phenotype of severe hyperglycaemia, hypoinsulinemia and ketoacidosis within 2 days [35]. Activating mutations in KCNJ11 are now known to cause both PNDM and TNDM in humans [6, 7]. ABCC8 was therefore an excellent candidate gene for neonatal diabetes and during the past year several reports have described activating mutations in the ABCC8 gene in patients with PNDM or TNDM [13–18].
Methods
Patients with diabetes diagnosed in the first six months of life, in which 6q24 abnormalities and KCNJ11 mutations had been excluded were recruited. Requests for referrals were made to the International Society of Paediatric and Adolescent Diabetes (ISPAD) rare diabetes collection. The study was conducted in accordance with the Declaration of Helsinki as revised in 2000. Informed consent was obtained from all participating patients with parental consent given on behalf of children.
Genetic Analysis
Genomic DNA was extracted from peripheral lymphocytes using standard procedures. In patients with a TNDM phenotype analysis of the 6q24 locus was undertaken using previously described methods to detect duplications, uniparental disomy (UPD) and methylation abnormalities [3, 36]. In patients where no 6q24 abnormality was identified or those with permanent diabetes, the single exon of KCNJ11 was amplified in three overlapping fragments as previously described [5]. The 39 exons of ABCC8 were analysed in all patients where no KCNJ11 mutation was identified. The ABCC8 gene was amplified in 38 fragments using previously described primers [14]. PCR products were sequenced using standard methods on an ABI 3100 or ABI 3730 (Applied Biosystems, Warrington, UK). Sequences were compared to the published sequence (NM_000352.2 including the alternatively spliced residue in exon 17; L78208, L78224) using Mutation Surveyor v2.61 (Softgenetics, Pennsylvania, USA). Mutations were tested for co-segregation with diabetes in other family members and in 200 normal chromosomes. Where possible, family relationships were confirmed using a panel of six microsatellite markers on chromosome 11p [5].
Clinical characteristics were obtained from the patient’s hospital records with assistance from their physician. Results are presented as median (range) and comparative statistics used the Mann-Whitney U test and Chi square statistic with Yates correction. Birth weight centiles were calculated using the method described by Cole [37].
Results
ABCC8 mutations causing diabetes mellitus
ABCC8 mutations have been reported previously in 39 index cases with neonatal diabetes [13–18]. In this article we describe a further 9 families (see Figure 1) bringing the total number to 48 (see Table1). These include 21 probands with PNDM, 25 with TNDM and two patients who have not had a period of remission without treatment but are receiving reduced doses of insulin (see Table 1).
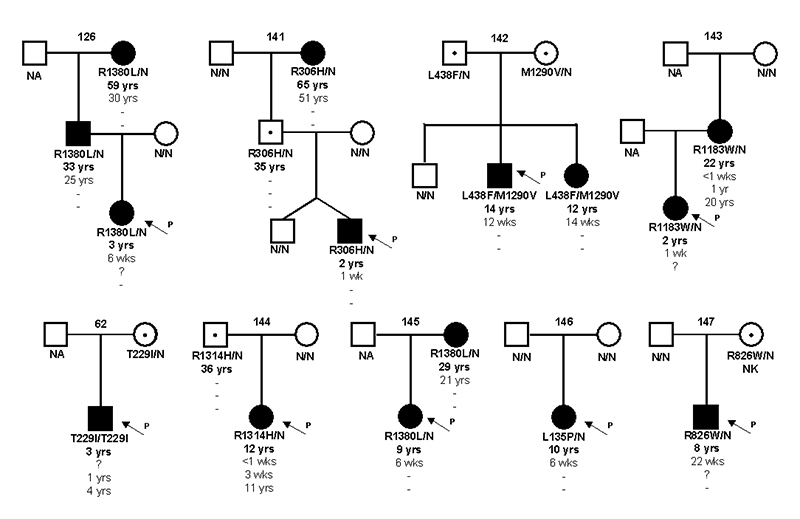
Figure 1.
Partial pedigrees for newly identified families showing inheritance of ABCC8 mutations. Circles represent females and squares males. Filled symbols represent diabetes. Genotype is shown underneath each symbol with residue number and amino acid change for the mutation carriers, N/N denotes no mutation identified. For the mutation carriers their current age, the age of initial diagnosis, the age of remission and the age of relapse is given. A dash represents a non-event,? indicates age at time of diagnosis or remission is not known. NA denotes not available for genetic testing and NK signifies that clinical details were not known. An arrow points to the proband in each family.
Table 1. ABCC8 mutations identified in probands with neonatal diabetes.
| ISPAD Number |
Mutation (protein effect) |
Nucleotide change |
Zygosity | Phenotype | Country of origin |
Age at Diagnosis (wks) |
Birth weight g (Centile) |
Neurological features | SU Transfer |
Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 123 | V86A | c.257T>C | Heterozygous | PNDM | Slovakia | 8 | 2900 (9) | Yes | Ellard et al. 2007; Stanik et al. 2006 |
|
| 124 | V86G | c.257T>G | Heterozygous | PNDM | Canada | 5 | 2900 (13) | Yes | Ellard et al. 2007 | |
| 68 | F132L | c.394T>C | Heterozygous | PNDM | Netherlands | 13 | 2200 (<1) | Developmental and motor delay, hypotonia and epilepsy |
Ellard et al. 2007; Proks et al. 2006 |
|
| 125 | F132L | c.394T>C | Heterozygous | PNDM | Australia | 26 | 2440 (9) | Developmental and motor delay | Ellard et al. 2007 | |
| 82 | F132V | c.394T>G | Heterozygous | PNDM | Poland | 20 | NA | Ellard et al. 2007 | ||
| 146 | L135P | c.404T>C | Heterozygous | PNDM | Netherlands | 6 | N/N | Developmental and motor delay | ||
| 46 | D209E | c.627C>A | Heterozygous | PNDM | USA | 5 | 2720 (13) | Yes | Ellard et al. 2007 | |
| 133 | D209E | c.627C>A | Heterozygous | TNDM | Australia | 3 | 2460 (9) | Yes | Flanagan et al. 2007 | |
| 134 | Q211K | c.631C>A | Heterozygous | PNDM | Chile | 16 | 2400 (3) | Yes | Ellard et al. 2007 | |
| 127 | D212I | c.635A>T | Heterozygous | TNDM | Germany | 4 | 2390 (16) | Muscle hypotonia up to age of 8 months | Flanagan et al. 2007 | |
| 49 | D212N | c.634G>A | Heterozygous | TNDM | Greece | 4 | 2800 (12) | Flanagan et al. 2007 | ||
| - | L213R | c.638T>G | Heterozygous | PNDM | N/A | 17 | 3065 (22) | Developmental and motor delay, dyspraxia | Yes | Babenko et al. 2006 |
| - | L225P | c.674T>C | Heterozygous | PNDM | US | 6 | 2100(<1) | Yes | Masia et al. 2007 | |
| 122 | L225P | c.674T>C | Heterozygous | PNDM | Germany | 4 | 2500 (11) | Ellard et al. 2007 | ||
| 141 | R306H | c.917G>A | Heterozygous |
*Not classified |
UK | 1 | 1290 (9) | Developmental delay | ||
| 24 | V324M | c.970G>A | Heterozygous | TNDM | Spain | 1 | N/A | Flanagan et al. 2007 | ||
| - | C435R | c.1303T>C | Heterozygous | TNDM | N/A | 4 | 3040 (25) | Minor visual and spatial dyspraxia | Babenko et al. 2006 | |
| 128 | L451P | c.1352T>C | Heterozygous | TNDM | Austria | 7 | 3030 (20) | Developmental delay | Flanagan et al. 2007 | |
| - | L582V | c. 1744C>G | Heterozygous | TNDM | N/A | 10 | 3570 (67) | Babenko et al. 2006 | ||
| - | L582V | c. 1744C>G | Heterozygous | TNDM | N/A | 2 | 3350 (50) | Slow ideation | Babenko et al. 2006 | |
| 8 | R826W | c.2476C>T | Heterozygous | TNDM | UK | 7 | N/A | Flanagan et al. 2007 | ||
| 147 | R826W | c.2476C>T | Heterozygous | TNDM | Poland | <1 | N/A | |||
| - | H1024Y† | c.1370C>T | Heterozygous | TNDM | N/A | 3 | 3400 (55) | Yes | Babenko et al. 2006 | |
| 71 | R1183Q | c.3548G>A | Heterozygous | TNDM | UK | 1 | 1960 (<1) | Flanagan et al. 2007 | ||
| - | R1183Q† | C.3548G>A | Heterozygous | TNDM | N/A | <1 | 1830 (8) | Babenko et al. 2006 | ||
| 143 | R1183W | c.3547C>T | Heterozygous | TNDM | Germany | 1 | 1340 (20) | |||
| 132 | R1183W | c.3547C>T | Heterozygous | TNDM | UK | 8 | 2600 (2) | Flanagan et al. 2007 | ||
| 131 | R1183W | c.3547C>T | Heterozygous | TNDM | India | 9 | 2800 (6) | Episodes of tonic posturing and seizures prior to insulin therapy |
Flanagan et al. 2007 | |
| 130 | R1183W | c.3547C>T | Heterozygous | TNDM | Germany | 6 | 2550 (4) | Flanagan et al. 2007 | ||
| 26 | R1183W | c.3547C>T | Heterozygous | TNDM | Czech Republic |
1 | 2400 (68) | Flanagan et al. 2007 | ||
| 144 | R1314H | c.3941G>A | Heterozygous | TNDM | USA | <1 | N/A | Yes | ||
| 72 | R1380C | c.4138C>T | Heterozygous | TNDM | USA | 8 | 3400 (23) | Flanagan et al. 2007 | ||
| - | R1380C† | c.4138C>T | Heterozygous | TNDM | N/A | <1 | 2050 (<3) | Minor dystonia | Babenko et al. 2006 | |
| - | R1380C† | c.4138C>T | Heterozygous | TNDM | N/A | 8 | 2330 (<3) | Yes | Babenko et al. 2006 | |
| 65 | R1380H | c.4139G>A | Heterozygous | TNDM | UK | 1 | 1360 (32) | Flanagan et al. 2007 | ||
| 145 | R1380L | c.4139G>T | Heterozygous |
*Not classified |
Romania | 6 | 2700 (6) | Developmental delay with EEG abnormalities | ||
| 126 | R1380L | c.4139G>T | Heterozygous | TNDM | Canada | 6 | 2800 (4) | Developmental delay, seizures and dystonia | ||
| - | I1425V† | c.4273A>G | Heterozygous | PNDM | N/A | 4 | 3080 (25) | Yes | Babenko et al. 2006 | |
| 62 | T229I | c.686C>T | Homozygous | TNDM | UK | N/A | N/A | |||
| 117 | E382K | c.1144G>A | Homozygous | PNDM | Turkey | 8 | 2700 (4) | Ellard et al. 2007 | ||
| 118 | A1185E | c.3554C>A | Homozygous | PNDM | Turkey | <1 | 4200 (95) | Muscle weakness and seizures | Ellard et al. 2007 | |
| 116 | N72S | c.215A>G | Mosaic | PNDM | UK | 5 | 3870 (74) | Ellard et al. 2007 | ||
| 47 | P45L + G1401R |
[c.134C>T] + [c.4201G>A] |
Compound heterozygous |
PNDM | UK | 6 | 2520 (18) | Yes | Ellard et al. 2007 | |
| 119 | E208K + Y263D |
[c.622G>A] + [c.787T>G] |
Compound heterozygous |
PNDM | Canada | 13 | 2950 (28) | Developmental delay | Yes | Ellard et al. 2007 |
| 120 | T229I + V1523L |
[c.686C>T] + [c.4567G>T] |
Compound heterozygous |
PNDM | Canada | 4 | NA | Yes | Ellard et al. 2007 | |
| 142 | L438F + M1290V |
c.1312C>T/c.3 868A>G |
Compound heterozygous |
PNDM | Chile | 12 | 3050 (43) | |||
| 78 | P207S + c.536del4 |
[c.619C>T] + [c.536 539del ATGG] |
Compound heterozygous |
PNDM | Germany | 8 | 3290 (29) | Ellard et al. 2007 | ||
| 121 | [E1327K; V1523A] +T1043Qf sX74 |
[c.3979G>A; 4568C>T] + [c.3127_3129d elACCinsCAG CCAGGACCT G] |
Compound heterozygous |
PNDM | USA | 1 | 2380 (<1) | Ellard et al. 2007 |
Amino acid numbering has been altered to include the alternatively spliced residue in exon 17 (L78208, L78224).
ISPAD probands 141 and 145 were not classified as having either TNDM or PNDM since they have not stopped insulin treatment but are receiving reduced doses of insulin dose (current dose 0.4 and 0.18U/kg/day respectively).
Inheritance of mutations
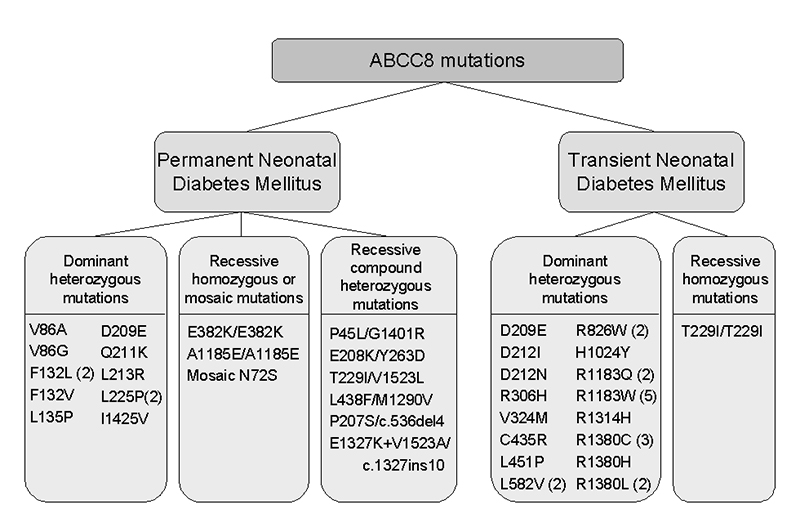
The modes of inheritance of mutations in probands with PNDM and TNDM are illustrated in Figure 2. Dominantly acting heterozygous mutations account for 79% (38/48) of the index cases. Recessive inheritance was more common in PNDM than in TNDM cases (9 vs. 1; Chi square p <0.01). Spontaneous mutations accounted for 57% (12/21) of PNDM and 32% (8/25) of TNDM probands (family relationships were confirmed where possible by microsatellite marker analysis). The remaining 16 TNDM probands showed dominant inheritance of a heterozygous mutation from a parent. In contrast, no families have been described with dominantly inherited PNDM due to an ABCC8 mutation.
Figure 2.
A diagram illustrating the inheritance of ABCC8 mutations in probands with permanent and transient forms of neonatal diabetes. A figure in brackets indicates the number of probands identified with that mutation where this is more than one.
Characteristics of mutations
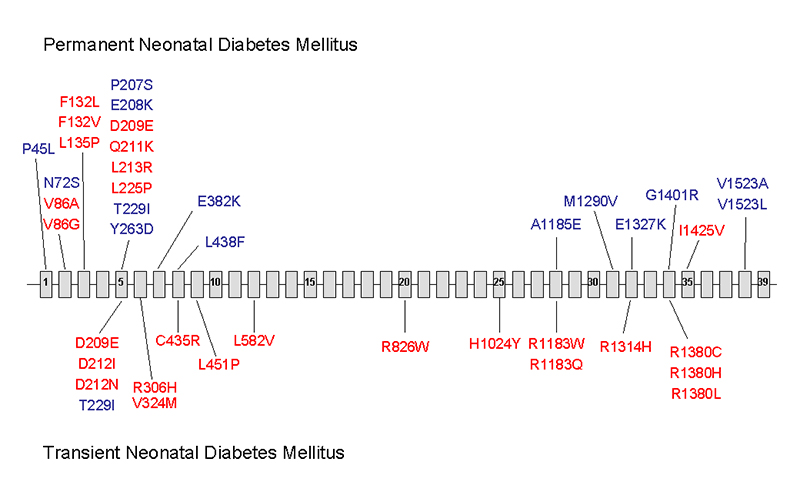
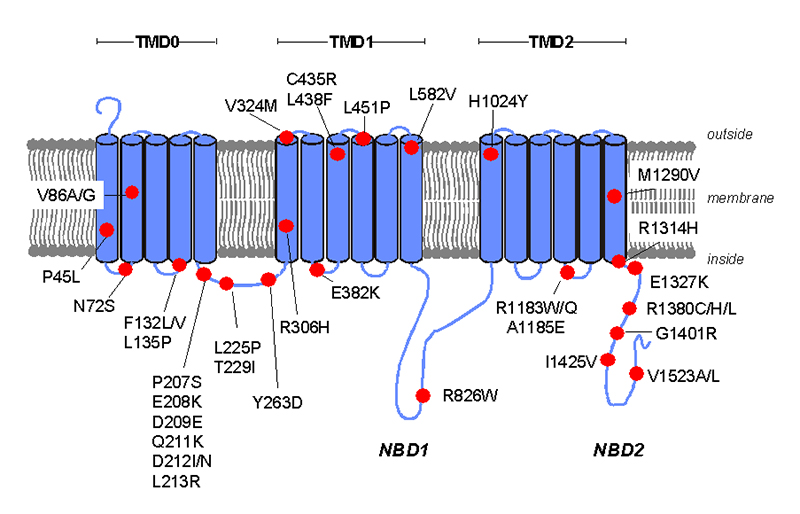
A total of 39 different missense and 2 frameshift ABCC8 mutations have been identified in patients with diabetes. None of these mutations have been reported as loss-of-function mutations causing hyperinsulinism. The majority of mutations result in missense amino acid substitutions distributed across the gene (Figure 3). Nearly half (18) of these missense mutations are located in exons 2-6. A particular mutation hotspot is exon 5 where 10 different mutations have been identified. These mutations affect residues 207 to 263 which lie within the cytosolic linker (L0) between transmembrane domains TMD0 and TMD1 (Figure 4).
Figure 3.
The location of missense mutations causing neonatal diabetes within the coding sequence of ABCC8. The mutations above the line indicate those associated with permanent neonatal diabetes (PNDM) and those below are mutations associated with transient neonatal diabetes. Recessively inherited mutations are in blue with dominantly inherited mutations in red.
Figure 4.
A schematic of the membrane topologies of SUR1 showing the location of the ABCC8 missense mutations causing neonatal diabetes. The transmembrane domains are indicated by TMD0, TMD1 and TMD2. The nucleotide binding domains are indicated by NBD1 and NBD2 and the cytosolic linker L0 is between TMD0 and TMD1.
Five novel mutations were identified; L135P (c.404T>C), R306H (c.917G>A), R1314H (c.3941G>A), L438F (c.1312C>T) and M1290V (c.3868A>G). The substituted amino acids are conserved across vertebrates including human, mouse, rat and dog and were not observed in at least 200 normal chromosomes.
Five residues are sites for different amino acid substitutions; V86A/G, F132L/V, D212I/N, R1183Q/W and R1380C/H/L. Nine mutations were observed in more than one proband; R1183W (c.3547C>T) was identified in five probands, R1380C (c.4138C>T) in three probands and the remainder; F132L (c.394T>C), D209E (c.627C>A), T229I (c.686C>T), L582V (c.1744C>G), R826W (c.2476C>T), R1183Q (c.3548G>A) and R1380L (c.4139G>T) were each observed in two probands. The recurrent mutations at codons R1183 and R1380 result in a base substitution within a CpG dinucleotide (c.4138-4139 or c.3547-3548). CpG dinucleotides are well known sites prone to mutation through the deamination of methylated cytosine bases.
Mutations in probands with PNDM
PNDM was caused either by a de novo dominantly acting heterozygous mutation (n=12) or recessive inheritance of mutations from unaffected parents with heterozygous mutations (n=9). We report two new families with PNDM; a novel, de novo L135P mutation (ISPAD146) and one with recessive inheritance (ISPAD142). Most (9/10) dominantly acting missense mutations in patients with PNDM were found in exons 2, 3 and 5 (Figure 3) which encode the TMD0 and cytosolic linker L0 regions of SUR1. Recessively inherited missense mutations were more widely distributed throughout exons 1-8 and 28-38. Mutations affecting residues V86 (n=2) or F132 (n=3) were found only in probands with PNDM.
Eight probands inherited a heterozygous mutation from each parent. Two are the offspring of consanguineous relationships (parents are first cousins) and are homozygous for a missense mutation (ISPAD117 and118, [14]. Compound heterozygous mutations that cause neonatal diabetes were first described in 5 families by Ellard et al. [14] and a further family is reported here (two affected siblings who are compound heterozygotes for the novel L438F and M1290V mutations; ISPAD142). Compound heterozygous mutations may include two activating mutations or an activating mutation inherited in trans with a loss-of-function mutation [14]. A case of mosaic uniparental disomy in combination with an ABCC8 mutation has also been described in a child with PNDM whose unaffected father was heterozygous for the mutation [14].
Mutations in probands with TNDM and their relatives
We report 5 new families where the proband had transient neonatal diabetes (see Figure 1). They include the novel missense mutation R1315H (ISPAD144), three families with the previously reported mutations R826W, R1183W and R1380L [18] and the first case of a homozygous mutation (T229I) causing TNDM (ISPAD62).
A total of 17 different mutations have now been reported in TNDM and these are located in exons 5-34 (Figure 3). Mutations affecting residues D212 (n=2), L582 (n=2), R826 (n=2), R1183 (n=7) or R1380 (n=6) were found only in probands with TNDM.
The majority of TNDM probands (16/25) inherited a heterozygous mutation from a parent. Nine of the mutation carrier parents had permanent diabetes (median age at diagnosis 27.5 years, range 13 - 35), but only two of these had been diagnosed with hyperglycaemia during the neonatal period. Two probands each had a sibling affected with TNDM [18] and in three families (ISPAD126; [13] there was a history of adult-onset diabetes affecting a grandparent (ABCC8 mutation confirmed in two).
Clinical phenotype
Table 2 shows a comparison of the clinical data for all mutation carriers. Affected probands and family members can be separated into three distinct groups based on age of diagnosis and whether there was a period of remission (or reduced insulin dose). The calculated median birth weight for the PNDM and TNDM cases were the12th and 16th centiles respectively (p=0.69). TNDM cases were diagnosed earlier than PNDM at a median of 3 vs 7 weeks of age (p<0.01). The median age of diagnosis for all cases of diabetes that were diagnosed after 6 months is 30 years of age (range 13-52) and the current median age of the unaffected mutation carrier parents is 36 years (range 25-42).
Table 2. Clinical characteristics of mutation carriers diagnosed with diabetes.
| PNDM†
(n=24) |
TNDM‡
(n=31) |
DM∓
(n=11) |
|
|---|---|---|---|
| Sex (Number of Males) | 12 | 16 | 4 |
| Age at diagnosis | 7 wks (<1-26) | 3 wks (<1-22) | 30 yrs(13-51) |
| Birth weight centile | 12 (<1-95) | 16 (<1-69) | - |
| - Any Neurological features | 8 | 7 | 0 |
| - Developmental delay | 8 | 3 | 0 |
| - Muscle Weakness | 2 | 0 | 0 |
| - Epilepsy | 2 | 0 | 0 |
Permanent Neonatal Diabetes with onset ≤ 6 months age and no remission
Transient Neonatal Diabetes with onset ≤ 6 months age and a period of remission with no treatment
Diabetes diagnosed >6 months of age.
Clinical characteristics are presented as median (range)
Response to sulphonylurea therapy
Eleven patients have previously been reported to have successfully transferred from insulin to sulphonylurea tablets [13, 15, 17, 18]. The patient with DEND syndrome and the F132L mutation was not able to discontinue insulin [16]. An additional 7 patients have now stopped insulin as a result of their molecular genetic diagnosis (Table 1).
Neurological features
Neurological features were reported in 15/48 (31%) probands with 13 different mutations (see Table 1 for details). One patient (ISPAD68) has previously been reported with DEND syndrome [16]. A second patient with the same mutation (F132L) had developmental delay but no epilepsy. No neurological features were reported in a third patient with a different mutation at the same residue (F132V).
Developmental delay was seen in both probands with the R1380L mutation. Further complications suggestive of DEND syndrome were also observed in these patients including abnormalities on electro encephalogram (EEG) in one patient and reported seizures in the second. One of the three patients with the R1380C mutation was reported to have minor dystonia [13]. No neurological features were reported in the patient with the R1380H mutation [18].
One of the six TNDM probands with the R1183W mutation experienced neurological problems prior to starting insulin treatment. He had one episode of tonic posturing with right facial involvement following admission to hospital with diabetic ketoacidosis. He subsequently had two episodes of generalised seizures but no further episodes since insulin therapy was started [18].
Muscle weakness was identified in two cousins with the D212I mutation. The proband had muscle hypotonia until the age of 8 months and her cousin has also been diagnosed with motor developmental delay. No muscle weakness was reported in either of their affected mothers or the three carriers of the D212N mutation [18].
Of the 9 PNDM probands with recessive mutations, two had neurological features (muscle weakness and seizures or learning difficulties) which were not found in their affected siblings. Neurological features were identified in 4/12 probands with dominant mutations.
Genotype-phenotype correlation
Most of the dominantly acting mutations located in exons 2-5 of the ABCC8 gene (V86A/G, F132L/V, L135P, D209E, Q211K, L213R and L225P) cause PNDM. The exceptions are D212I and D212N that result in a remitting/relapsing phenotype with permanent diabetes in later life. Two arginine residues in exons 28 and 34 are hotspots for recurrent mutations (R1183Q/W and R1380C/H/L) found only in patients with TNDM or adult-onset permanent diabetes (n=18).
There are examples of phenotypic variability both between and within families. The heterozygous mutation D209E has been reported as de novo in one proband (current age 6 yrs) with PNDM [14] and in a second family where the proband had TNDM but her mother was diagnosed with diabetes at 35 years of age [18]. New data from the previously reported family ISPAD47 [14] reveals that the proband (now aged 8 years) has permanent diabetes but his younger sister (now aged 7 years) had transient diabetes that remitted at the age of 2 years. Both are compound heterozygotes for the P45L and G1401R mutations.
Discussion
We report ABCC8 mutations in a further 9 probands and present a summary that includes the 39 previously reported families. A spectrum of phenotypes is observed in patients with diabetes caused by ABCC8 mutations as has previously been reported for KCNJ11 mutations [38]. These include DEND syndrome [16], permanent diabetes diagnosed before 6 months of age [13–15, 17], transient neonatal diabetes [13, 18] or permanent diabetes diagnosed in adolescence or adulthood [13, 18]. The latter two phenotypes are usually found within the same family where a proband was referred for testing because of a diagnosis of transient neonatal diabetes and an affected parent (and/or grandparent) may have permanent diabetes. This implies that these KATP channel mutations have a biphasic course and patients diagnosed later may have had a period of hyperglycaemia that was undetected in the neonatal period [18].
Recessive ABCC8 mutations were first reported as a cause of diabetes by Ellard et al. [14]. Although there are no reports of recessive KCNJ11 mutations, such mutations are not uncommon in ABCC8, accounting for 19% of all probands. All except one has PNDM. De novo ABCC8 mutations explain 42% of neonatal diabetes cases but are more common in PNDM (57%) than TNDM (32%). This pattern is also seen for de novo KCNJ11 mutations; 84% in PNDM [39] vs 29% in TNDM (Flanagan 2007). The remainder are dominantly inherited heterozygous mutations found exclusively in patients with TNDM or permanent diabetes diagnosed outside the neonatal period. The mode of inheritance is important since it determines the risk of diabetes in future siblings, offspring, parents and the wider family.
The clinical phenotype for TNDM vs PNDM mutations is similar as measured by birth weight (a surrogate for insulin secretion in utero) which was reduced in both groups (16th vs 12th centiles respectively; p=0.69). Probands with TNDM were diagnosed earlier 3 vs 7 weeks of age (p<0.01) and neurological features were present in a minority (31%) of index cases.
Many patients with SUR1 subunit mutations can transfer from insulin to sulphonylurea treatment and at least 18 patients are known to have achieved successful transfer. There are no reports of patients with DEND syndrome caused by either ABCC8 or KCNJ11 mutations who have been able to stop insulin treatment. No data is yet available on the level of glycaemic control on sulphonylureas compared to insulin for patients with SUR1 mutations. A number of ABCC8 mutation carriers who are not known to have diabetes have been identified [13, 18, this study] but not all have undergone a formal OGTT. Annual blood glucose monitoring is recommended for this group who may benefit from sulphonylurea treatment if/when they develop diabetes.
A genotype-phenotype relationship for SUR1 mutations is suggested by the clustering of dominantly acting mutations in exons 2-5 that cause PNDM and the recurrent mutations of the arginine residues at codons 1183 and 1380 that only cause TNDM or permanent diabetes diagnosed outside the neonatal period. Furthermore, different mutations at the same residue (V86A/G, F132L/V, D212I/N, R1183Q/W and R1380C/H/L) cause either PNDM (V86, F132) or biphasic TNDM (D212, R1183, R1380), suggesting a different pathological mechanism.
The N-terminal transmembrane domain (TMD0) and cytosolic linker (L0) form allosteric interactions with Kir6.2 that are essential for normal intrinsic activity and increase channel density in the membrane [25]. Residues in the L0 region confer constitutive channel activity and together with TMD0 have been implicated in the intrinsic gating properties of the KATP channel [40, 41]. The cluster of NDM causing mutations in the first five exons of the ABCC8 gene that encode these regions might cause diabetes by increasing the open stability of the channel through interaction with the Kir6.2 subunit as demonstrated for the F132L mutation [16]. The phenotypic differences between different mutations in this region might provide evidence to suggest which residues interact most closely with the Kir6.2 subunits. The dominant heterozygous mutations causing a PNDM phenotype could therefore highlight residues that directly influence gating whilst the recessively inherited or TNDM causing mutations in the same region may identify residues with less influence.
The most common mutations in patients with TNDM/permanent diabetes diagnosed in adolescence or adulthood affect the arginine residues R1183 and R1380. These residues are implicated in nucleotide binding, since R1183 is located at a position involved in the joining of transmembrane domain 2 (TMD2) to nucleotide binding domain 2 (NBD2) and R1380 is located within NBD2. NBD2 is important for the regulation of channel activity by preferentially binding magnesium nucleotides Mg-ATP or Mg-ADP [26, 41] and in the 3D structure R1380 lies adjacent to the ATP binding site of NBD1 [42]. Loss-of-function mutations in these regions of ABCC8 can prevent binding of ATP and abolish responsiveness of SUR1 to MgADP leading to congenital hyperinsulinaemic hypoglycaemia [43]. However activating mutations in these NBD regions have now been reported (e.g. R826W, R1380C/H/L, V1523A/L) that could act to enhance the stimulatory effect of magnesium nucleotide binding or reduce the hydrolysis rate of Mg-ATP [16].
Neurological features in addition to neonatal diabetes were identified in 31% of probands with an ABCC8 mutation. They include developmental delay, muscle weakness and epilepsy, but were not strongly associated with mutation position in the gene or consistent between carriers of the same mutation. KATP channels are found in many other tissues but the expression pattern of ABCC8 restricts the pairing of the SUR1 protein subunits with Kir6.2 to the beta cell and brain [19]. The neurological complications observed in probands with ABCC8 mutations could result from disrupted neuronal signalling and/or glucose homeostasis. Seizures were observed in three of the probands although they may have occurred secondary to severe hyperglycaemia. In support of this, none of the patients with onset of diabetes outside the neonatal period exhibited neurological features.
There is some evidence that activating mutations causing recessively inherited neonatal diabetes may have a milder functional effect compared to dominant SUR1 or Kir6.2 mutations [14], but some overlap was observed between the functional effects of the mutations present in unaffected heterozygotes and those in patients with neonatal diabetes. We report the first patient with TNDM and a homozygous ABCC8 mutation (T229I). This mutation has previously been found in a child with PNDM who is a compound heterozygote for T229I and V1523L. Functional studies of heterozygous mutant channels suggested that V1523L has a greater effect on ATP sensitivity than T229I [14] and is consistent with the more severe PNDM phenotype caused by the T229I/V1523L genotype.
Although it is not possible to predict with confidence the clinical phenotype from the position of an ABCC8 mutation, we have shown that there are some emerging patterns of specific mutations associated with clinical subtypes. The phenotypic variability observed both between and within some families with the same ABCC8 mutation means that genotype-phenotype correlations are not absolute. Functional data has only been published for 8/39 ABCC8 missense mutations to date (F132L, [16]; I1425V and H1024Y, [13]; mutations (P207S, T229I, A1185E and V1523L, [14]; L225P, [15]). Further studies of additional mutations may help to explain the mechanisms underlying these apparent genotype-phenotype correlations and will further our understanding of beta-cell KATP channel biology. The discovery that ABCC8 mutations account for a significant number of KCNJ11-negative cases of neonatal diabetes strengthens the view that interactions between Kir6.2 and SUR1 are crucial for correct channel function and thus the appropriate secretion of insulin.
Acknowledgements
The authors would like to thank all of the families for participating in this study and the many referring clinicians. We also thank Professor Frances Ashcroft for her ongoing collaboration with functional analysis of mutations. For technical assistance we thank Mr Andrew Parrish. We acknowledge funding from the Sir Graham Wilkins studentship to Sarah Flanagan. The study was funded by the Wellcome Trust; A.T. Hattersley is a Wellcome Trust Research Leave fellow.
Abbreviations
- DEND
Developmental delay, Epilepsy and Neonatal Diabetes
- PNDM
Permanent Neonatal Diabetes
- TNDM
Transient Neonatal Diabetes
- ISPAD
International Society of Paediatric and Adolescent Diabetes
- KATP
ATP-sensitive potassium channel
- NBD
Nucleotide Binding Domain
- L0
SUR1 cytosolic linker region
References
- [1].Edghill EL, Dix RJ, Flanagan SE, Bingley PJ, Hattersley AT, Ellard S, et al. HLA Genotyping Supports a Nonautoimmune Etiology in Patients Diagnosed With Diabetes Under the Age of 6 Months. Diabetes. 2006;55(6):1895–8. doi: 10.2337/db06-0094. [DOI] [PubMed] [Google Scholar]
- [2].Iafusco D, Stazi MA, Cotichini R, Cotellessa M, Martinucci ME, Mazzella M, et al. Permanent diabetes mellitus in the first year of life. Diabetologia. 2002;45(6):798–804. doi: 10.1007/s00125-002-0837-2. [DOI] [PubMed] [Google Scholar]
- [3].Temple IK, Gardner RJ, Mackay DJ, Barber JC, Robinson DO, Shield JP. Transient neonatal diabetes: widening the understanding of the etiopathogenesis of diabetes. Diabetes. 2000;49(8):1359–66. doi: 10.2337/diabetes.49.8.1359. [DOI] [PubMed] [Google Scholar]
- [4].Colombo C, Delvecchio M, Zecchino C, Faienza MF, Cavallo L, Barbetti F. Transient neonatal diabetes mellitus is associated with a recurrent (R201H) KCNJ11 (KIR6.2) mutation. Diabetologia. 2005;48(11):2439–41. doi: 10.1007/s00125-005-1958-1. [DOI] [PubMed] [Google Scholar]
- [5].Flanagan SE, Edghill EL, Gloyn AL, Ellard S, Hattersley AT. Mutations in KCNJ11, which encodes Kir6.2, are a common cause of diabetes diagnosed in the first 6 months of life, with the phenotype determined by genotype. Diabetologia. 2006;49(6):1190–7. doi: 10.1007/s00125-006-0246-z. [DOI] [PubMed] [Google Scholar]
- [6].Gloyn AL, Reimann F, Girard C, Edghill EL, Proks P, Pearson ER, et al. Relapsing diabetes can result from moderately activating mutations in KCNJ11. Hum Mol Genet. 2005;14(7):925–34. doi: 10.1093/hmg/ddi086. [DOI] [PubMed] [Google Scholar]
- [7].Gloyn AL, Pearson ER, Antcliff JF, Proks P, Bruining GJ, Slingerland AS, et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med. 2004;350(18):1838–49. doi: 10.1056/NEJMoa032922. [DOI] [PubMed] [Google Scholar]
- [8].Massa O, Iafusco D, D’Amato E, Gloyn AL, Hattersley AT, Pasquino B, et al. KCNJ11 activating mutations in Italian patients with permanent neonatal diabetes. Hum Mutat. 2005;25(1):22–7. doi: 10.1002/humu.20124. [DOI] [PubMed] [Google Scholar]
- [9].Sagen JV, Raeder H, Hathout E, Shehadeh N, Gudmundsson K, Baevre H, et al. Permanent neonatal diabetes due to mutations in KCNJ11 encoding Kir6.2: patient characteristics and initial response to sulfonylurea therapy. Diabetes. 2004;53(10):2713–8. doi: 10.2337/diabetes.53.10.2713. [DOI] [PubMed] [Google Scholar]
- [10].Vaxillaire M, Populaire C, Busiah K, Cave H, Gloyn AL, Hattersley AT, et al. Kir6.2 mutations are a common cause of permanent neonatal diabetes in a large cohort of French patients. Diabetes. 2004;53(10):2719–22. doi: 10.2337/diabetes.53.10.2719. [DOI] [PubMed] [Google Scholar]
- [11].Pearson ER, Flechtner I, Njolstad PR, Malecki MT, Flanagan SE, Larkin B, et al. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med. 2006;355(5):467–77. doi: 10.1056/NEJMoa061759. [DOI] [PubMed] [Google Scholar]
- [12].Shepherd M. Transforming lives: transferring patients with neonatal diabetes from insulin to sulphonylureas. European Diabetes Nursing. 2006;3(3):137–42. [Google Scholar]
- [13].Babenko AP, Polak M, Cave H, Busiah K, Czernichow P, Scharfmann R, et al. Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. N Engl J Med. 2006;355(5):456–66. doi: 10.1056/NEJMoa055068. [DOI] [PubMed] [Google Scholar]
- [14].Ellard S, Flanagan SE, Girard CA, Patch AM, Harries LW, Parrish A, et al. Permanent neonatal diabetes caused by dominant, recessive or compound heterozygous SUR1 mutations with opposite functional effects. Submitted. 2007 doi: 10.1086/519174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Masia R, Deleon DD, Macmullen C, McKnight H, Stanley CA, Nichols CG. A mutation in the TMD0-L0 region of SUR1 (L225P) causes Permanent Neonatal Diabetes Mellitus (PNDM) Diabetes. 2007 doi: 10.2337/db06-1746. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [16].Proks P, Arnold AL, Bruining J, Girard C, Flanagan SE, Larkin B, et al. A heterozygous activating mutation in the sulphonylurea receptor SUR1 (ABCC8) causes neonatal diabetes. Hum Mol Genet. 2006;15(11):1793–800. doi: 10.1093/hmg/ddl101. [DOI] [PubMed] [Google Scholar]
- [17].Stanik J, Gasperikova D, Paskova M, Barak L, Javorkova J, Jancova E, et al. Prevalence of permanent neonatal diabetes in Slovakia and successful replacement of insulin with sulfonylurea therapy in KCNJ11 and ABCC8 mutation carriers. J Clin Endocrinol Metab. 2007 doi: 10.1210/jc.2006-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Flanagan SE, Patch AM, MacKay DJG, Edghill EL, Gloyn AL, Robinson DO, et al. Mutations in KATP channel genes cause transient neonatal diabetes and permanent diabetes in childhood or adulthood. Diabetes. 2007 doi: 10.2337/db07-0043. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Seino S, Miki T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog Biophys Mol Biol. 2003;81(2):133–76. doi: 10.1016/s0079-6107(02)00053-6. [DOI] [PubMed] [Google Scholar]
- [20].Ashcroft FM, Harrison DE, Ashcroft SJ. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature. 1984;312(5993):446–8. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- [21].Inagaki N, Tohru G, Seino S. Subunit stoichiometry of the pancreatic beta-cell ATP-sensitive K+ channel. FEBS Lett. 1997;409(2):232–6. doi: 10.1016/s0014-5793(97)00488-2. [DOI] [PubMed] [Google Scholar]
- [22].Shyng SL, Nichols CG. Octameric stoichiometry of the KATP channel complex. Journal of General Physiology. 1997;110(6):655–64. doi: 10.1085/jgp.110.6.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tucker SJ, Gribble FM, Zhao C, Trapp S, Ashcroft FM. Truncation of Kir6.2produces ATP-sensitive K+ channels in the absence of the sulphonylurea receptor. Nature. 1997;387:179–83. doi: 10.1038/387179a0. [DOI] [PubMed] [Google Scholar]
- [24].Ashcroft FM, Rorsman P. Electrophysiology of the pancreatic β-cell. Progress in Biophysiology and Molecular Biology. 1989;54(2):87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- [25].Babenko AP, Gonzalez G, Aguilar-Bryan L, Bryan J. Sulfonylurea receptors set the maximal open probability ATP sensitivity and plasma membrane density of KATP channels. FEBS Lett. 1999;445(1):131–6. doi: 10.1016/s0014-5793(99)00102-7. [DOI] [PubMed] [Google Scholar]
- [26].Ueda K, Komine J, Matsuo M, Seino S, Amachi T. Cooperative binding of ATP and MgADP in the sulfonylurea receptor is modulated by glibenclamide. Proc Natl Acad Sci U S A. 1999;96(4):1268–72. doi: 10.1073/pnas.96.4.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ashcroft FM, Gribble FM. ATP-sensitive K+ channels and insulin secretion: their role in health and disease. Diabetologia. 1999;42(8):903–19. doi: 10.1007/s001250051247. [DOI] [PubMed] [Google Scholar]
- [28].Tusnady GE, Sarkadi B, Simon I, Varadi A. Membrane topology of human ABC proteins. FEBS Lett. 2006;580(4):1017–22. doi: 10.1016/j.febslet.2005.11.040. [DOI] [PubMed] [Google Scholar]
- [29].Gribble FM, Tucker SJ, Ashcroft FM. The essential role of the Walker A motifs of SUR1 in K-ATP channel activation by Mg-ADP and diazoxide. Embo J. 1997;16(6):1145–52. doi: 10.1093/emboj/16.6.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gloyn AL, Siddiqui J, Ellard S. Mutations in the genes encoding the pancreatic beta-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) in diabetes mellitus and hyperinsulinism. Hum Mutat. 2006;27(3):220–31. doi: 10.1002/humu.20292. [DOI] [PubMed] [Google Scholar]
- [31].Thomas PM, Cote GJ, Wohilk N, Haddad B, Mathew PM, Rabel W, et al. Mutations in the sulphonylurea receptor and familial persistent hyperinsulinemic hypoglycemia of infancy. Science. 1995;268:426–9. doi: 10.1126/science.7716548. [DOI] [PubMed] [Google Scholar]
- [32].Nestorowicz A, Inagaki N, Gonoi T, Schoor KP, Wilson BA, Glaser B, et al. A nonsense mutation in the inward rectifier potassium channel gene Kir6.2, is associated with familial hyperinsulinism. Diabetes. 1997;46(11):1743–8. doi: 10.2337/diab.46.11.1743. [DOI] [PubMed] [Google Scholar]
- [33].Thomas P, Ye Y, Lightner E. Mutation of the pancreatic islet inward rectifier Kir6.2 also leads to familial persistent hyperinsulinemic hypoglycemia of infancy. Hum Mol Genet. 1996;5(11):1809–12. doi: 10.1093/hmg/5.11.1809. [DOI] [PubMed] [Google Scholar]
- [34].Tornovsky S, Crane A, Cosgrove KE, Hussain K, Lavie J, Heyman M, et al. Hyperinsulinism of infancy: novel ABCC8 and KCNJ11 mutations and evidence for additional locus heterogeneity. J Clin Endocrinol Metab. 2004;89(12):6224–34. doi: 10.1210/jc.2004-1233. [DOI] [PubMed] [Google Scholar]
- [35].Koster JC, Marshall BA, Ensor N, Corbett JA, Nichols CG. Targeted overactivity of beta cell K(ATP) channels induces profound neonatal diabetes. Cell. 2000;100(6):645–54. doi: 10.1016/s0092-8674(00)80701-1. [DOI] [PubMed] [Google Scholar]
- [36].Mackay DJ, Temple IK, Shield JP, Robinson DO. Bisulphite sequencing of the transient neonatal diabetes mellitus DMR facilitates a novel diagnostic test but reveals no methylation anomalies in patients of unknown aetiology. Hum Genet. 2005;116(4):255–61. doi: 10.1007/s00439-004-1236-1. [DOI] [PubMed] [Google Scholar]
- [37].Cole TJ, Freeman JV, Preece MA. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med. 1998;17:407–29. [PubMed] [Google Scholar]
- [38].Hattersley AT, Ashcroft FM. Activating mutations in Kir6.2 and neonatal diabetes: new clinical syndromes, new scientific insights and new therapy. Diabetes. 2005;54(9):2503–13. doi: 10.2337/diabetes.54.9.2503. [DOI] [PubMed] [Google Scholar]
- [39].Slingerland AS, Hattersley AT. Mutations in the Kir6.2 subunit of the KATP channel and permanent neonatal diabetes: new insights and new treatment. Ann Med. 2005;37(3):186–95. doi: 10.1080/07853890510007287. [DOI] [PubMed] [Google Scholar]
- [40].Babenko AP, Bryan J. Sur domains that associate with and gate KATP pores define a novel gatekeeper. J Biol Chem. 2003;278(43):41577–80. doi: 10.1074/jbc.C300363200. [DOI] [PubMed] [Google Scholar]
- [41].Fang K, Csanady L, Chan KW. The N-terminal transmembrane domain (TMD0) and a cytosolic linker (L0) of sulphonylurea receptor define the unique intrinsic gating of KATP channels. J Physiol. 2006;576(Pt 2):379–89. doi: 10.1113/jphysiol.2006.112748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Campbell JD, Proks P, Lippiat JD, Sansom MS, Ashcroft FM. Identification of a functionally important negatively charged residue within the second catalytic site of the SUR1 nucleotide-binding domains. Diabetes. 2004;53(Suppl 3):S123–7. doi: 10.2337/diabetes.53.suppl_3.s123. [DOI] [PubMed] [Google Scholar]
- [43].Nichols CG, Shyng SL, Newtorowicz A, Glaser B, IV JPC, Gonzalez G, et al. Adenosine diphosphate as an intracellular regulator of insulin secretion. Science. 1996;272:1785–7. doi: 10.1126/science.272.5269.1785. [DOI] [PubMed] [Google Scholar]