Abstract
Objective
Transient neonatal diabetes mellitus (TNDM) is diagnosed in the first 6 months of life with remission in infancy or early childhood. For approximately 50% of patients their diabetes will relapse in later life. The majority of cases result from anomalies of the imprinted region on chromosome 6q24 and 14 patients have been reported with KATP channel gene mutations.
Research Design and Methods
We determined the 6q24 status in 97 patients with TNDM. In patients where no abnormality was identified the KCNJ11 gene and/or ABCC8 gene which encode the Kir6.2 and SUR1 subunits of the pancreatic beta-cell ATP sensitive potassium (KATP) channel were sequenced.
Results
KATP channel mutations were found in 25/97 (26%) TNDM probands (12 KCNJ11, 13 ABCC8) while 69/97 (71%) had chromosome 6q24 abnormalities. The phenotype associated with KCNJ11 and ABCC8 mutations was similar, but markedly different from 6q24 patients who had a lower birth weight, and were diagnosed and remitted earlier (all p <0.001). KATP channel mutations were identified in 26 additional family members, 17 had diabetes. Of the 42 diabetic patients, 91% diagnosed before 6 months remitted, but those diagnosed after 6 months had permanent diabetes (p<0.0001).
Conclusion
KATP channel mutations account for 89% of patients with non-6q24 TNDM and result in a discrete clinical subtype which includes biphasic diabetes that can be treated with sulphonylureas. Remitting neonatal diabetes was observed in 2/3 mutation carriers and permanent diabetes occurred after 6 months of age in subjects without an initial diagnosis of neonatal diabetes.
Transient neonatal diabetes mellitus (TNDM) is a clinically defined subgroup affecting approximately 50% of children with neonatal diabetes. TNDM is differentiated from Permanent Neonatal Diabetes Mellitus (PNDM) as the diabetes remits in infancy or early childhood. Relapse in childhood or adolescence occurs in up to 50% of cases (1).
There has been considerable progress in defining the genetic aetiology of TNDM. The major breakthrough was establishing that the majority of cases result from anomalies of the imprinted region on chromosome 6q24 which encodes the ZAC and HYMAI genes (2, 3). Three types of abnormalities have been identified to date which result in the over-expression of the paternal allele: paternally inherited duplication of chromosome 6q24, paternal uniparental isodisomy of chromosome 6 or a methylation defect.
Recent studies have identified 14 patients with TNDM resulting from mutations in the KCNJ11 and ABCC8 genes that encode the Kir6.2 and SUR1 subunits of the potassium ATP channel (KATP channel) in the pancreatic beta cell (4–6, 8). Heterozygous activating mutations in the KCNJ11 gene are the commonest cause of PNDM but have also been described in five patients with TNDM (4–6). Functional work illustrated that TNDM mutations were functionally less severe in vitro, than mutations resulting in PNDM (4). Activating heterozygous ABCC8 mutations have recently been reported in three patients with PNDM and 7 with TNDM (7, 8). Mutations in either gene encoding the subunits of the KATP channel cause diabetes by reducing the ability of the channel to close in response to increased ATP and reduced ADP concentrations. This altered sensitivity of the channel to the ratio of ATP to ADP results in increased potassium efflux from the cell and hyperpolarisation of the membrane thus reducing insulin secretion from the beta cell. The mechanism by which KATP channel mutations result in a remitting/relapsing diabetic phenotype is not known.
The prevalence of KATP channel mutations in TNDM and the associated clinical characteristics are uncertain. The initial descriptions suggest that the onset of diabetes and remission is later in TNDM patients with KATP channel mutations (1, 4). Since the original criteria for TNDM are based on a series of patients with 6q24 diabetes (diabetes diagnosed within 6 weeks of birth with remission by 18 months)(9), this definition may exclude some cases with KATP channel mutations. We and others have shown that Type 1 diabetes is very rare before 6 months of age and suggests a genetic aetiology for most patients diagnosed before 6 months (10, 11). Transient neonatal diabetes might therefore be redefined as diabetes diagnosed before 6 months of age that later remits.
We investigated the genetic aetiology of 97 patients whose diabetes was diagnosed within the first six months of life but entered remission before 5 years. Our aim was to assess the prevalence of 6q24 abnormalities and KATP channel mutations within this cohort and to investigate the clinical features of mutation carriers.
Research Design and Methods
We studied 97 patients with diabetes diagnosed in the first six months of life, whose diabetes remitted before the age of 5 years, 64 have been previously reported (1, 3, 4, 12–15). The patients were referred from 14 different countries across 4 continents and were either recruited following a request for referrals to the International Society of Paediatric and Adolescent Diabetes (ISPAD) rare diabetes collection or following referral to the Wessex Regional Genetics Laboratory for 6q24 abnormality testing. The study was conducted in accordance with the Declaration of Helsinki as revised in 2000. Informed consent was obtained from all patients with parental consent given on behalf of children.
Genetic Analysis
Genomic DNA was extracted from peripheral lymphocytes using standard procedures. In all patients analysis of the 6q24 locus was undertaken using previously described methods to detect duplications, UPD and methylation abnormalities (1, 16). In patients where no 6q24 abnormality was identified the single exon of KCNJ11 was amplified in three overlapping fragments as previously described (17). The 39 exons of ABCC8 were analysed in all patients where no KCNJ11 mutation was identified. The ABCC8 gene was amplified in 38 fragments using previously described primers (7). PCR products were sequenced using standard methods on an ABI 3100 or ABI 3730 (Applied Biosystems, Warrington, UK). Sequences were compared to the published sequences ((KCNJ11 - NM_000525.3) (ABCC8 - NM_000352.2)) using Staden Analysis or Mutation Surveyor v.2.61. Mutations were tested for co-segregation with diabetes in other family members and in 200 normal chromosomes from UK Caucasians. Where possible, family relationships were confirmed using a panel of six microsatellite markers on chromosome 11p (17).
Clinical characteristics were obtained from the patient’s hospital records with assistance from their physician. Clinical characteristics are presented as median (range) and comparative statistics predominantly used the Mann-Whitney U test.
Results
Prevalence of different genetic aetiologies
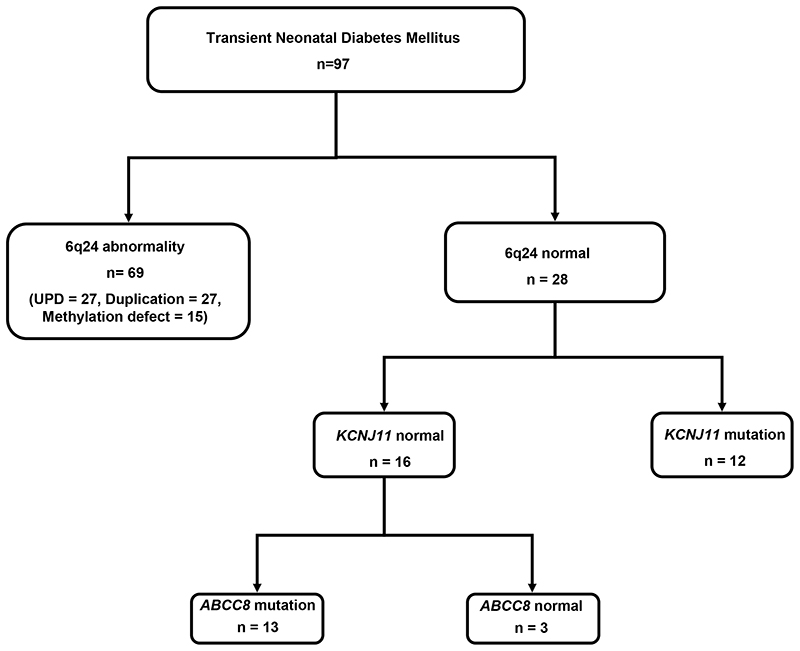
We have determined the genetic aetiology in 94 out of 97 (97%) probands diagnosed with diabetes before the age of 6 months whose diabetes had subsequently entered remission (figure 1). The majority of patients (69/97) had an abnormality at the 6q24 locus. Of these patients, 27 (39%) had paternal uniparental isodisomy, 27 (39%) had a duplication of the paternal allele and 15 (22%) had a methylation defect. For the 28 remaining patients where no 6q24 aberration was identified, sequencing of KCNJ11 and ABCC8 demonstrated that 25 probands were heterozygous for KATP channel mutations.
Figure 1. Number of patients diagnosed with transient neonatal diabetes and results of genetic testing.
Mutation characteristics
KCNJ11 mutations
Twelve of the probands had a heterozygous mutation in the KCNJ11 gene; six of these patients (with 5 different mutations) have been reported previously (4, 12). We found 8 different KCNJ11 mutations; R34C (c.100C>T), G53R (c.157G>C), G53S (c.157G>A), E179A (c.536A>C), I182V (c.544A>G), E227K (c.679G>A), E229K (c.685G>A) and R365H (c.1094G>A). Two mutations were identified in more than one proband; E227K (n=2) and E229K (n=4). The mutations R34C, E179A and R365H are novel. We did not observe these mutations in 200 normal chromosomes and all mutations affect residues that are conserved in human, mouse, rat and dog (http://genome.ucsc.edu).
ABCC8 mutations
Ten different ABCC8 gene mutations were identified in 13 probands: D209E (c.627C>A), D212N (c.634G>A), D212I (c.634 G>A 635A>T), V324M (c.970G>A), L451P (c.1352T>C), R826W (c.2476C>T), R1183W (c.3547C>T), R1183Q (c>3548G>A), R1380C (c.4138C>T) and R1380H (c.4139G>A). All were identified in one proband with the exception of R1183W which was identified in 4 probands. All mutations were novel except for R1380C and R1183Q (8). We did not observe these mutations in 200 normal chromosomes and all affect residues that are conserved in human, mouse, rat and dog (http://genome.ucsc.edu).
Inheritance of mutations
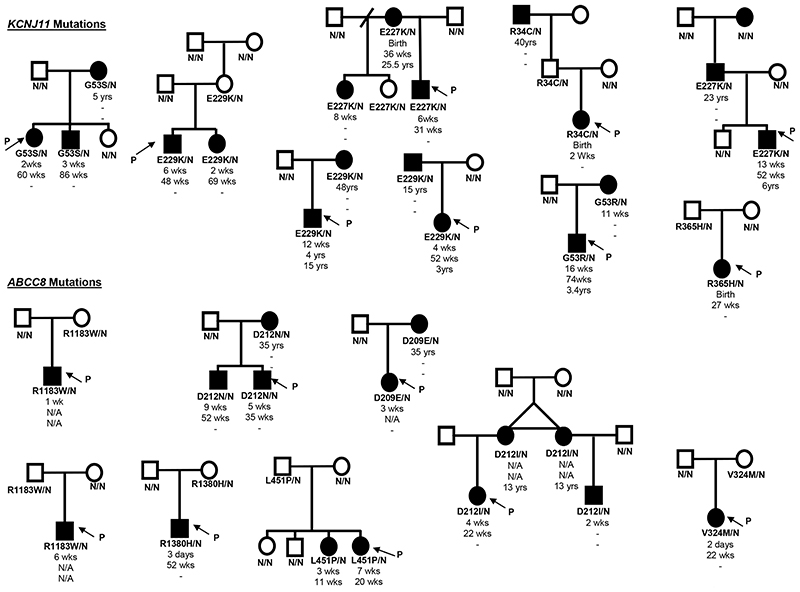
Seven (28%) probands had de novo mutations as confirmed by the absence of the mutation in the unaffected parents (n = 3 KCNJ11, n = 4 ABCC8). Microsatellite analysis was used to verify the family relationships in all but one case with an R1380C ABCC8 mutation, where parental DNA was unavailable. In the remaining 17 probands the mutation had been inherited from a parent (partial pedigrees are shown in figure 2). In these families 17/26 parents or siblings with the KATP channel mutation had diabetes, in contrast only one family member without the mutation was affected (p<0.001). However there was not complete co-segregation of the mutations with diabetes as in 8 probands the mutation was inherited from a non-diabetic parent. Neonatal diabetes (diagnosed in the first 6 months) was described in 5 siblings and 4 parents. Two mothers (identical twins, figure 2), were both reported to have had diabetes in the neonatal period, although information regarding their ages at diagnosis and remission were not available. Non-penetrance was seen in families with 8 different mutations suggesting it was not a feature of specific mutations.
Figure 2. Partial pedigrees of families showing inheritance of KCNJ11 or ABCC8 mutations.
Squares represent male family members, and circles represent females. Filled symbols denote patients with diabetes. Genotype is shown underneath each symbol, residue number and amino acid change are given for the mutation carriers, N/N denotes no mutation identified. Directly below the genotype is the age of initial diagnosis for the mutation carriers, followed by the age of remission and the age of relapse, a dash represents a non-event, N/A denotes information not available. An arrow with the letter P points to the proband in each family.
Clinical characteristics of probands with KCNJ11 or ABCC8 mutations
Diabetes
The characteristics of diabetes in the probands with KCNJ11 or ABCC8 mutations are shown in Table 1. There was no significant difference in clinical characteristics between these two groups. Reduced birth weight was a consistent feature of both KCNJ11 and ABCC8 mutations (25th vs. 11th centile, p= 0.26). Diabetes was most frequently diagnosed between 0-8 weeks (86%) with all cases diagnosed before 17 weeks. None of the patients with an ABCC8 mutation had relapsed at the time of this study, however this may be due to the younger age of these patients compared to those with KCNJ11 mutations.
Table 1.
Clinical characteristics of probands, grouped by genetic aetiology. Comparison of clinical characteristics of patients with a KCNJ11 mutation and ABCC8 mutation and comparison of probands with a KATP channel mutation (combined results) to patients with a 6q24 abnormality. Results given in median, range in brackets, (* (1). Differences between groups were calculated using Mann-Whitney U and Chi-squared. Centile birth weights were calculated according to UK growth charts (28) as the majority of patients were of UK white origin.
| Characteristic | Genetic aetiology unknown (n = 3) | Comparison of KCNJ11 and ABCC8 mutation carriers (P -Value) | Comparison of KCNJ11 and ABCC8 combined data and 6q24 patients (P - Value) | ||||
|---|---|---|---|---|---|---|---|
| Sex (% Males) | 100% | 58% | 54% | 0.33 | 56% | 53% | 0.94 |
| Age at diagnosis (weeks) | 3 (1-4) | 5 (0-16) | 4 (0-9) | 0.53 | 4 (0 - 16) | 0 (0 - 4) | <0.001 |
| Age at remission (weeks) | 8 (17-208) | 45 (2-208) | 22 (7-52) | 0.14 | 35 (2 - 208) | 13 (5-60) | <0.001 |
| Age when entering study (years) | 14 (1-31) | 7.5 (0.84-17) | 5 (0.84-16) | - | 6 (0.84 -17) | 12 (1- 36) | - |
| Number where diabetes has relapsed | 2 | 4 | 0 | - | 4 | 7 | - |
| Age at relapse (years) | 11 (5-17) | 4.7 (3-15) | - | - | 4.7 (3-15) | 16 (4 - 25) | 0.073 |
| Gestation (weeks) | 38 (30-40) | 38 (30-40) | 39 (30-41) | 0.17 | 38 (30-41) | 40 (36-42) | 0.059 |
| Birth weight (g) | 1620 (1100-3373) | 2570 (1535-3570) | 2575 (1360-3400) | 0.73 | 2570 (1360-3570) | 1950 (1600-2670) | <0.001 |
| Centile birth weight | 6 (<1st -35) | 25 (<1st - 89) | 11 (<1st - 32) | 0.26 | 12 (<1st -89) | <1st (<1st - 21) | <0.001 |
Neurological features
In addition to diabetes, neurological features were identified in 4 probands (16%), and were associated with both KCNJ11 and ABCC8 mutations. Two probands and one family member with a KCNJ11 mutation had neurological features. The first proband with a E229K KCNJ11 mutation has reported speech delay and was diagnosed with Autistic Spectrum Disorder at the age of 3 years. Interestingly the affected sibling and unaffected mother who both carry the same mutation have no reported speech problems, although the sibling is currently 2 years of age and still in the early stages of language development. The E229K mutation has been identified in 5 affected patients from 3 further families with no reports of speech or developmental delay in any of the mutation carriers. Mild learning difficulties were observed in a second proband and his mother who both have the G53R KCNJ11 mutation. This mutation has not been identified in any other families to date therefore it is difficult to conclude whether these complications are a feature of the mutation or whether environmental and/or other genetic factors are involved.
Neurological features were also reported in three patients with an ABCC8 mutation. Muscle weakness was identified in two cousins with a D212I mutation. The proband had muscle hypotonia until the age of 8 months and her cousin has been diagnosed with motor developmental delay although no muscle weakness was reported in either of their affected mothers. The most severe neurological phenotype was identified in a proband with a de novo ABCC8 mutation (R1183W). This patient had one episode of tonic posturing with right facial involvement following admission to hospital with diabetic ketoacidosis. He subsequently had two episodes of generalised seizures shortly after diagnosis of diabetes. No further seizures have been reported since insulin therapy was started and it is likely that these seizures were a result of the severe hyperglycaemia rather than as part of the DEND syndrome (18). Neurological features have not been reported in any of the 5 other patients with the R1183W mutation.
The clinical characteristics of the probands with KATP channel mutations were markedly different when compared to subjects with 6q24 abnormalities (Table 1). Patients with a 6q24 abnormality presented with a more severe clinical phenotype as shown by their lower centile birthweight, <1st vs 12th (p <0.001) and earlier age of diagnosis, 0 weeks vs 4 weeks (p < 0.001). For patients with a KATP channel mutation, the initial episode of diabetes was longer with diabetes remitting significantly later than in patients with a 6q24 abnormality, 35 wks vs 13 wks (p < 0.001). There is a trend towards an earlier age of relapse in patients with a KATP channel mutation (p = 0.07) and this may become more evident as the patients are studied over a longer period of time.
Clinical characteristics of the KATP channel mutations vary according to the age of diagnosis of initial diabetes
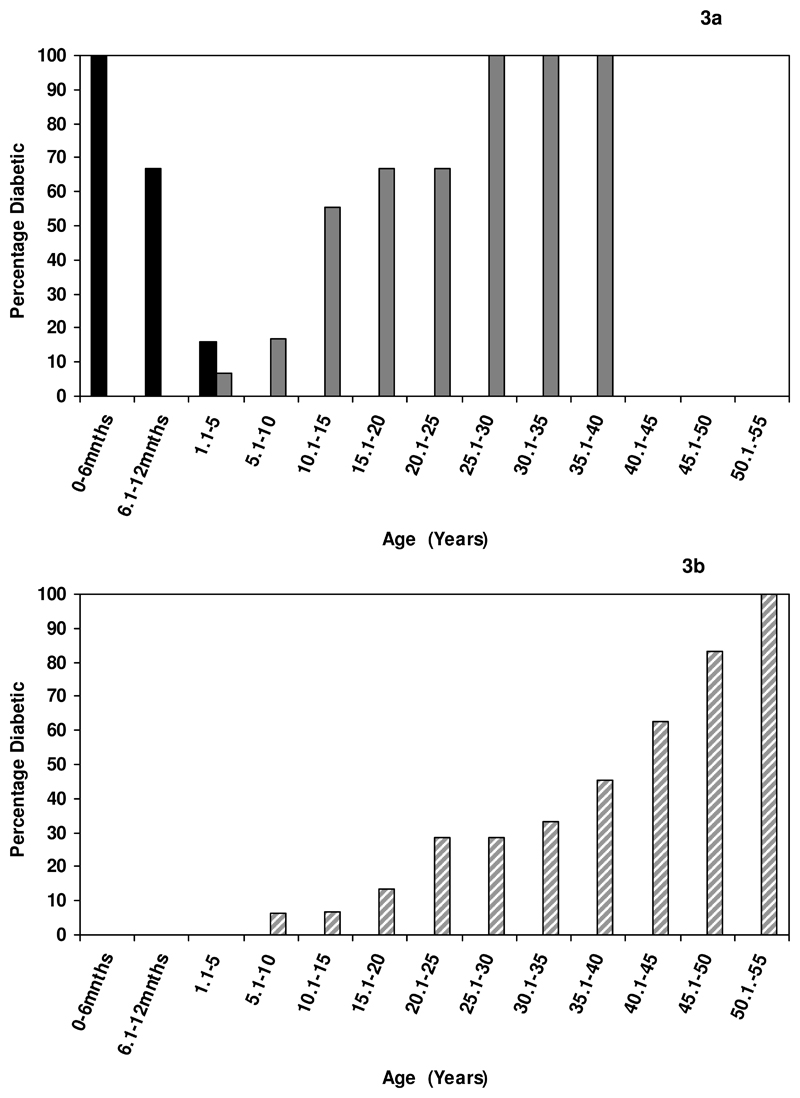
The characteristics of the diabetes varied according to the age at which it was first diagnosed (see table 2). Remission of diabetes occurred in 32/35 (91%) patients diagnosed before 6 months but none of those diagnosed after 6 months (P <0.001) (table 2 and figure 3). The median age at remission was 35 weeks (range 2 -208). The three patients diagnosed before 6 months who had not entered remission were 1, 2 and 43 years of age at mutation screening.
Table 2.
Comparison of clinical and biochemical characteristics of patients with a KATP channel mutation diagnosed before six months of age with patients whose diabetes was not diagnosed before 6 months, and the number of each mutation identified within each group. Results are in median, range given in brackets. Differences between groups were calculated using Mann-Whitney U and Chi-squared. Centile birth weights were calculated according to UK growth charts (28) as the majority of patients were of UK white origin.
| Characteristic | Mutation carriers diagnosed with diabetes within 6 months (n=35) | Mutation carriers who did not have diabetes diagnosed within the first 6 months (n= 16) | P -Value |
|---|---|---|---|
| % Males | 51% | 44% | 0.75 |
| Number of probands | 25 | 0 | |
| Age when entering study (years) | 6 (0.8 -43) | 42 (5 - 56) | - |
| Number ever diagnosed with diabetes | 35 | 7 | 1*10-6 |
| Age at diagnosis (weeks) | 4 (0-17) | 1196 (260 - >2496) | 3.7*10-5 |
| Number where diabetes has remitted | 32 | 0 /7 | 3.7*10-10 |
| Age at remission (weeks) | 35 (2-208) | - | - |
| Number where diabetes has relapsed | 7 | - | - |
| Age at relapse (years) | 13 (3 - 25.5) | - | - |
| Birth weight (g) | 2695 (1360-3570) | 2810 (907-3090) | 0.9 |
| Gestation (weeks) | 39 (30-42) | 38 (34-40) | 0.74 |
| Centile birth weight | 18 (<1st -89) | 15 (<1st - 79) | 0.94 |
| KCNJ11Mutations: | |||
| R34C | 1 | 2 | |
| G53R | 2 | 0 | |
| G53S | 2 | 1 | |
| E179A | 1 | 0 | |
| I182V | 1 | 0 | |
| E227K | 4 | 2 | |
| E229K | 5 | 3 | |
| R365H | 1 | 1 | |
| ABCC8 Mutations: | |||
| D209E | 1 | 1 | |
| D212N | 2 | 1 | |
| D212I | 4 | 0 | |
| V324M | 1 | 1 | |
| L451P | 2 | 1 | |
| R826W | 1 | 0 | |
| R1183W | 4 | 2 | |
| R1183Q | 1 | 0 | |
| R1380C | 1 | 0 | |
| R1380H | 1 | 1 |
Figure 3.
a Representation of the percentage of patients with diabetes at any given age for patients diagnosed before the age of 6 months with subsequent remission of diabetes. The calculation for the percentage number of patients diabetic is based on patients who have reached that given age. Black columns represent the initial diagnosis and grey columns represent the 2nd episode of diabetes (relapse).
b Percentage of patients diabetic at any given age, results are for patients whose initial diagnosis was not before 6 months. Percentages were calculated according to the number of patients who had reached that given age.
All patients were treated with insulin during their initial episode of diabetes and during any subsequent relapse of diabetes except for one proband and his father with a KCNJ11 mutation (E227K), a father with a KCNJ11 mutation (E229K) and a mother with an ABCC8 mutation (D209E) who were treated with sulphonylureas. Two probands and 4 family members have now attempted transfer off insulin and onto sulphonylureas using a similar protocol to that described for KCNJ11 mutations (19). Transfer was successful in 5 cases (83%), but unsuccessful in a mother with a G53R mutation. Her son, who carries the same mutation, has successfully transferred onto sulphonylureas suggesting that the lack of response to sulphonylureas observed is not a feature of the mutation.
Phenotype of patients in whom the genetic aetiology is not defined
We are able to determine the genetic aetiology in all but three of the 97 probands diagnosed with TNDM. Interestingly all three patients had an atypical phenotype. The first patient was born at 30 weeks’ gestation and his transient hyperglycaemia was possibly as a consequence of the premature birth. Early gestational age was not a consistent feature in patients with either a 6q24 anomaly or a KATP channel mutation. The second patient stopped insulin treatment at the age of 4 years but this was recommenced one year later. He also developed epilepsy at the age of 9 years, which has not been reported in any other patients in our series. The third patient has had four episodes of remission and relapse, a unique phenotype which has also not been described in any other patients included in this study. In addition the coding sequence of the glucokinase gene was normal in all three patients. This phenotypic variation suggests further rare distinct aetiologies for TNDM.
Discussion
We have shown that mutations in the KCNJ11 and ABCC8 genes which encode the Kir6.2 and SUR1 subunits of the KATP channel are a major cause of TNDM, accounting for 29% of all cases and 89% of non-6q24 TNDM. As a result, a genetic diagnosis could be made in 97% of patients with neonatal diabetes that remitted. These estimates of the relative prevalence of the three major genetic subgroups are likely to be robust as this study of a consecutive series of 97 TNDM patients is more than twice the size of the largest previous study. In our series, KCNJ11 (n=12) and ABCC8 (n=13) mutations were equally represented, which is in marked contrast to PNDM where there are over 60 KCNJ11 mutations and only 3 ABCC8 mutations reported (7, 8). The only previous comprehensive study is from France which showed that a genetic diagnosis was possible for 33/44 patients with TNDM (8). This is in contrast to our study where the genetic aetiology remains unknown in only 3/97 patients. The reason for this discrepancy is unknown; it could result from differences in the patients studied or in the detection of KATP channel mutations. KCNJ11 mutations have now been identified in a total of 18 patients with TNDM (5, 6, 20). Our study emphasises that both KCNJ11 and ABCC8 mutations are an important cause of TNDM.
Defining mutations
There is strong evidence that the mutations detected in KCNJ11 or ABCC8 are the cause of TNDM in these patients. All of the mutations occur at residues that are conserved across multiple species, alter the coding sequence (an essential requirement for an activating channel mutation), and are not found in normal controls or patients with PNDM. The strongest evidence for an aetiological role is the de novo mutations, as spontaneous mutations are rare, even in genes as large as ABCC8 (8.5x 10-5 (21)). However, de novo mutations are only seen for 4 of the 14 novel mutations (29%) in contrast to PNDM where 84% of cases represent spontaneous mutations (22). For the majority of cases (68%), the mutation is inherited from a parent and although co-segregation of TNDM is rare, all cases of TNDM in the families have inherited the mutation. Fifty percent of parents have diabetes diagnosed before 45 years which is rare in the general population (<0.2%)(calculated from (23)). and is not seen in any of the non-mutation carrier parents. The final evidence that these mutations are pathogenic is that functional studies for all mutations performed to date (G53R, G53S, I182V, E227K and E229K in KCNJ11) have shown altered response to ATP in the presence of magnesium ions (4, 8, 20). The presence of de novo mutations identified in 5 probands strongly supports an aetiological role for the I182V, E229K, R1380C and R1183Q mutations. Therefore these mutations are highly likely to be causative of TNDM even in the absence of complete co-segregation.
Location and function of mutations
We have identified a number of commonly mutated residues in patients with TNDM within both KATP channel genes. KCNJ11 mutations in our TNDM series cluster with residue G53 mutated in 2/12 probands and E227 and E229 residues mutated in 6/12 probands. Structural analysis has shown that G53 lies in a region linking the ATP binding site to the transmembrane domain (4), whereas E227 and E229 lie distant to the ATP biding site at the interface between Kir6.2 subunits and are thought to influence channel gating (20).
The ABCC8 mutations are located throughout the SUR1 protein. The residue R1183, which is located at a position involved in joining transmembrane domain 2 (TMD2) to nucleotide binding domain 2 (NBD2), was mutated in 5 probands with 2 different residues tryptophan and glutamine substituting arginine. The second most commonly affected residue was R1380, which is located in nucleotide binding domain 2 (NBD2), and is mutated in 2 probands. There are a cluster of mutations (D209E, D212N and D212I) in the intracellular region that links the transmembrane domain with the gatekeeper module (8).
Functional studies of channels with the TNDM associated KCNJ11 mutations G53S, G53R and I182V showed a moderate reduction in activity in the presence of ATP, which is less marked than the reduction seen with PNDM mutations (4). This suggested clear association of the genotype with functional effect as well as clinical phenotype, but further studies have shown that there is some overlap between the magnitude of the KATP currents in TNDM and PNDM associated mutations (20). Furthermore, the V252A and R201H mutations have been described in both patients with PNDM and TNDM (6, 20) so the genotype/phenotype correlation is not absolute.
A biphasic course for diabetes in KCNJ11 and ABCC8 mutations associated with TNDM
We have shown that patients with KATP channel mutations, when diagnosed within the neonatal period, have similar clinical characteristics irrespective of whether KCNJ11 or ABCC8 is mutated (table 1).
The TNDM probands with KATP channel mutations show a biphasic course where diabetes is diagnosed before 6 months, they then typically go into remission between 6 -12 months and are likely to relapse during adolescence or early adulthood. None of the patients diagnosed with diabetes after 6 months of age had a remission of diabetes even if they had the same mutation as patients who remit. Interestingly birth weight, a reflection of insulin mediated growth and hence insulin secretion in utero, is reduced to a similar extent whether the patient is diagnosed before 6 months or not. These findings imply that the KATP channel mutations have a biphasic course and patients diagnosed later may have had a period of hyperglycaemia that was undetected in the neonatal period. This could explain the apparent phenotypic heterogeneity with the same mutation. Importantly this means that KATP channel mutations can present outside the neonatal period and if the parents in our families had not had a child with TNDM they would have been diagnosed as having Type 1 or Type 2 diabetes. It will be important to test cohorts with a clinical diagnosis of Type 1 diabetes to see how prevalent these mutations are especially as it will have implications for treatment.
The explanation of the biphasic course in both types of KATP channel mutations is not known. A fixed channel defect would be expected to produce a fixed phenotype as seen with KATP channel mutations causing PNDM. The change in glucose tolerance during remission and subsequent relapse seen are likely to reflect either changes in insulin requirements or changes in the number of beta-cells or both. As insulin levels are very low in non-diabetic neonates (24) this would suggest the initial diabetes does not reflect increased insulin requirements at this time although the relapse around puberty may reflect the associated increase in insulin resistance. The biphasic course seen in 6q24 TNDM is still not fully understood even with the creation of a good mouse model (25).
Neurological complications were present in addition to diabetes in 6 patients with an ABCC8 or KCNJ11 mutation. In all cases the affected individual had been diagnosed with diabetes before the age of 6 months. In one family both mutation carriers had learning difficulties. In all other families neurological features did not co-segregate with the mutation, suggesting that neurological phenotype is not a consistent feature of these mutations.
6q24 TNDM differs from TNDM resulting from KATP channel mutations
Our series provides strong evidence that clinical characteristics differ markedly between patients with a KATP channel mutation and those with a chromosome 6q24 anomaly. In addition to diabetes 7 patients with a 6q24 abnormality (30%) had macroglossia and 2 patients had umbilical hernia (9%). These abnormalities were not present in any of the patients with a KATP channel mutation. Patients with KATP TNDM have a higher birth weight (2570 v 1950 grams), are diagnosed later (4 v 0 weeks) and enter remission later (35 v 13 weeks) than patients with 6q24 abnormalities. Although these clinical characteristics can aid in the identification of the cause of TNDM there is overlap in the range of values (table 1). Therefore while clinical features can guide the order in which genetic tests are performed a molecular genetic diagnosis is still required.
Phenotype of TNDM vs. PNDM mutations
There is a clear genotype/phenotype relationship. None of the KATP channel mutations identified within this series have been found in patients with PNDM. Only one report to date has identified R201H, a well characterised PNDM mutation, in a patient with TNDM (6). Between the TNDM and PNDM groups there are no significant differences between the centile birth weights and age of diagnosis ((12th vs 3rd centile) and (4 vs 5 weeks)) (17). Distinction between the two subgroups can be made only by the presence of a period of remission in the TNDM patients. One difference between the groups is the mode of inheritance. In our series a greater number of probands (68%) have a familial mutation, this is in contrast to PNDM where 84% of all mutations occur de novo (22).
Therapeutic implications
Identification of a KATP channel mutation has important implications for the patient’s clinical management. Recent reports have shown that most patients with a KCNJ11 mutation and PNDM are able to transfer from insulin onto sulphonylurea treatment with an improvement in glycaemic control in all cases reported (19, 26, 27). Babenko et al reported two patients with an ABCC8 mutation who were successfully treated with sulphonylureas after their TNDM relapsed (8). In our series 3 of the 14 patients who developed diabetes outside the initial 6 months (either as a relapse or initial diagnosis) were initially treated with sulphonylureas. Since the diagnosis was made, a further 5 patients have successfully transferred onto sulphonylureas from insulin using a similar protocol to that described for the transfer of patients with PNDM and a KCNJ11 mutation (19). For the remaining patients transfer is either in progress or the patients are currently in remission.
In conclusion we have shown that a genetic diagnosis is possible for 97% of patients diagnosed with TNDM. Mutations of the KATP channel genes, ABCC8 and KCNJ11, account for the majority (89%) of non-6q24 transient neonatal diabetes. Diabetes associated with these KATP channel mutations has a biphasic course, although neonatal diabetes that enters remission may not be detected. Patients therefore may present with permanent diabetes diagnosed in childhood or adulthood. In addition not all mutation carriers may be diabetic when the mutation is detected in a proband. The therapeutic implications for those patients found to have a KATP channel mutation highlights the importance of screening all patients with non-6q24 TNDM for mutations in ABCC8 and KCNJ11.
Acknowledgements
The authors would like to thank all of the families for participating in this study. Our thanks go to the many referring clinicians who include Ijaz Ahmad, Jesús Argente, Chandar Batra, Mark Bone, Jill Challener, Ethel Codner, Elizabeth Davis, Dorothy Deiss, Jan Fairchild, Peter Fowlie, Mathias Herr, Sabine Hofer, Mary Jetha, Olga Kordonouri, Michael MacDonald, Kathryn Noyes, Laurent Legault, Derek Sandeman, Annabelle Slingerland, Zdenek Sumnick, Peter Swift, Charalambos Theodoridis, Verena Wagner and Esko Wiltshire. We also thank Mr Andrew Parrish for his technical assistance and Dr Beverley Shields for statistical analysis. We would also like to acknowledge funding from the Sir Graham Wilkins studentship to Sarah Flanagan. The study was funded by the Wellcome Trust and was supported by the European Union (Integrated Project EURODIA LSHM-CT-2006-518153 in the Framework Programme 6 of the European-Community). A.T. Hattersley is a Wellcome Trust Research Leave fellow. A.L. Gloyn is a Diabetes UK RD Lawrence Research Fellow.
Abbreviations
- DEND
Developmental delay, Epilepsy and Neonatal Diabetes
- ISPAD
International Society of Paediatric and Adolescent Diabetes
- KATP
ATP-sensitive Potassium Channel
- Kir6.2
Potassium inwardly rectifying channel 6.2
- PNDM
Permanent Neonatal Diabetes
- SUR1
Sulphonylurea receptor 1
- TNDM
Transient Neonatal Diabetes
References
- 1.Temple IK, Gardner RJ, Mackay DJ, Barber JC, Robinson DO, Shield JP. Transient neonatal diabetes: widening the understanding of the etiopathogenesis of diabetes. Diabetes. 2000;49:1359–1366. doi: 10.2337/diabetes.49.8.1359. [DOI] [PubMed] [Google Scholar]
- 2.Temple IK, Gardner RJ, Robinson DO, Kibirige MS, Ferguson AW, Baum JD, Barber JCK, James RS, Shield JPH. Further evidence for an imprinted gene for neonatal diabetes localised to chromosome 6q22-q23. Hum Molec Genet. 1996;5:1117–1124. doi: 10.1093/hmg/5.8.1117. [DOI] [PubMed] [Google Scholar]
- 3.Gardner RJ, Mackay DJ, Mungall AJ, Polychronakos C, Siebert R, Shield JP, Temple IK, Robinson DO. An imprinted locus associated with transient neonatal diabetes mellitus. Hum Mol Genet. 2000;9:589–596. doi: 10.1093/hmg/9.4.589. [DOI] [PubMed] [Google Scholar]
- 4.Gloyn AL, Reimann F, Girard C, Edghill EL, Proks P, Pearson ER, Temple IK, Mackay DJ, Shield JP, Freedenberg D, Noyes K, et al. Relapsing diabetes can result from moderately activating mutations in KCNJ11. Hum Mol Genet. 2005;14:925–934. doi: 10.1093/hmg/ddi086. [DOI] [PubMed] [Google Scholar]
- 5.Yorifuji T, Nagashima K, Kurokawa K, Kawai M, Oishi M, Akazawa Y, Hosokawa M, Yamada Y, Inagaki N, Nakahata T. The C42R mutation in the Kir6.2 (KCNJ11) gene as a cause of transient neonatal diabetes, childhood diabetes, or later-onset, apparently type 2 diabetes mellitus. J Clin Endocrinol Metab. 2005;90:3174–3178. doi: 10.1210/jc.2005-0096. [DOI] [PubMed] [Google Scholar]
- 6.Colombo C, Delvecchio M, Zecchino C, Faienza MF, Cavallo L, Barbetti F. Transient neonatal diabetes mellitus is associated with a recurrent (R201H) KCNJ11 (KIR6.2) mutation. Diabetologia. 2005;48:2439–2441. doi: 10.1007/s00125-005-1958-1. [DOI] [PubMed] [Google Scholar]
- 7.Proks P, Arnold AL, Bruining J, Girard C, Flanagan SE, Larkin B, Colclough K, Hattersley AT, Ashcroft FM, Ellard S. A heterozygous activating mutation in the sulphonylurea receptor SUR1 (ABCC8) causes neonatal diabetes. Hum Mol Genet. 2006;15:1793–1800. doi: 10.1093/hmg/ddl101. [DOI] [PubMed] [Google Scholar]
- 8.Babenko AP, Polak M, Cave H, Busiah K, Czernichow P, Scharfmann R, Bryan J, Aguilar-Bryan L, Vaxillaire M, Froguel P. Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. N Engl J Med. 2006;355:456–466. doi: 10.1056/NEJMoa055068. [DOI] [PubMed] [Google Scholar]
- 9.Temple IK, Shield JP. Transient neonatal diabetes, a disorder of imprinting. J Med Genet. 2002;39:872–875. doi: 10.1136/jmg.39.12.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edghill EL, Dix RJ, Flanagan SE, Bingley PJ, Hattersley AT, Ellard S, Gillespie KM. HLA Genotyping Supports a Nonautoimmune Etiology in Patients Diagnosed With Diabetes Under the Age of 6 Months. Diabetes. 2006;55:1895–1898. doi: 10.2337/db06-0094. [DOI] [PubMed] [Google Scholar]
- 11.Iafusco D, Stazi MA, Cotichini R, Cotellessa M, Martinucci ME, Mazzella M, Cherubini V, Barbetti F, Martinetti M, Cerutti F, Prisco F. Permanent diabetes mellitus in the first year of life. Diabetologia. 2002;45:798–804. doi: 10.1007/s00125-002-0837-2. [DOI] [PubMed] [Google Scholar]
- 12.Edghill EL, Gloyn AL, Goriely A, Harries LW, Flanagan SE, Rankin J, Hattersley AT, Ellard S. Origin of de novo KCNJ11 mutations and risk of neonatal diabetes for subsequent siblings. J Clin Endocrinol Metab. 2007 doi: 10.1210/jc.2006-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mackay DJ, Temple IK, Shield JP, Robinson DO. Bisulphite sequencing of the transient neonatal diabetes mellitus DMR facilitates a novel diagnostic test but reveals no methylation anomalies in patients of unknown aetiology. Hum Genet. 2005;116:255–261. doi: 10.1007/s00439-004-1236-1. [DOI] [PubMed] [Google Scholar]
- 14.Mackay DJ, Boonen SE, Clayton-Smith J, Goodship J, Hahnemann JM, Kant SG, Njolstad PR, Robin NH, Robinson DO, Siebert R, Shield JP, et al. A maternal hypomethylation syndrome presenting as transient neonatal diabetes mellitus. Hum Genet. 2006;120:262–269. doi: 10.1007/s00439-006-0205-2. [DOI] [PubMed] [Google Scholar]
- 15.Temple IK, James RS, Crolla JA, Sitch FL, Jacobs PA, Howell WM, Betts P, Baum JD, Shield J. An imprinted gene(s) for diabetes? Nat Genet. 1995;9:110–112. doi: 10.1038/ng0295-110. [DOI] [PubMed] [Google Scholar]
- 16.Mackay DJTI, Shield JP, Robinson DO. Bisulphite sequencing of the transient neonatal diabetes mellitus DMR facilitates a novel diagnostic test but reveals no methylation anomalies in patients of unknown aetiology. Human Genetics. 2005;116:255–261. doi: 10.1007/s00439-004-1236-1. [DOI] [PubMed] [Google Scholar]
- 17.Flanagan SE, Edghill EL, Gloyn AL, Ellard S, Hattersley AT. Mutations in KCNJ11, which encodes Kir6.2, are a common cause of diabetes diagnosed in the first 6 months of life, with the phenotype determined by genotype. Diabetologia. 2006;49:1190–1197. doi: 10.1007/s00125-006-0246-z. [DOI] [PubMed] [Google Scholar]
- 18.Hattersley AT, Ashcroft FM. Activating mutations in kir6.2 and neonatal diabetes: new clinical syndromes, new scientific insights, and new therapy. Diabetes. 2005;54:2503–2513. doi: 10.2337/diabetes.54.9.2503. [DOI] [PubMed] [Google Scholar]
- 19.Pearson ER, Flechtner I, Njolstad PR, Malecki MT, Flanagan SE, Larkin B, Ashcroft FM, Klimes I, Codner E, Iotova V, Slingerland AS, et al. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med. 2006;355:467–477. doi: 10.1056/NEJMoa061759. [DOI] [PubMed] [Google Scholar]
- 20.Girard CA, Shimomura K, Proks P, Absalom N, Castano L, Perez de Nanclares G, Ashcroft FM. Functional analysis of six Kir6.2 (KCNJ11) mutations causing neonatal diabetes. Pflugers Arch. 2006;453:323–332. doi: 10.1007/s00424-006-0112-3. [DOI] [PubMed] [Google Scholar]
- 21.Kondrashov AS. Direct Estimates of Human per nucleotide mutation rates at 20 loci causing mendelian disease. Human Mutation. 2002;21:21–27. doi: 10.1002/humu.10147. [DOI] [PubMed] [Google Scholar]
- 22.Slingerland AS, Hattersley AT. Mutations in the Kir6.2 subunit of the KATP channel and permanent neonatal diabetes: new insights and new treatment. Ann Med. 2005;37:186–195. doi: 10.1080/07853890510007287. [DOI] [PubMed] [Google Scholar]
- 23.Owen KR, Stride A, Ellard S, Hattersley AT. Etiological investigation of diabetes in young adults presenting with apparent type 2 diabetes. Diabetes Care. 2003;26:2088–2093. doi: 10.2337/diacare.26.7.2088. [DOI] [PubMed] [Google Scholar]
- 24.Shields BM, Knight B, Shakespeare L, Babrah J, Powell RJ, Clark PM, Hattersley AT. Determinants of insulin concentrations in healthy 1-week-old babies in the community: applications of a bloodspot assay. Early Hum Dev. 2006;82:143–148. doi: 10.1016/j.earlhumdev.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 25.Ma D, Shield JP, Dean W, Leclerc I, Knauf C, Burcelin RR, Rutter GA, Kelsey G. Impaired glucose homeostasis in transgenic mice expressing the human transient neonatal diabetes mellitus locus, TNDM. J Clin Invest. 2004;114:339–348. doi: 10.1172/JCI19876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zung A, Glaser B, Nimri R, Zadik Z. Glibenclamide treatment in permanent neonatal diabetes mellitus due to an activating mutation in Kir6.2. J Clin Endocrinol Metab. 2004;89:5504–5507. doi: 10.1210/jc.2004-1241. [DOI] [PubMed] [Google Scholar]
- 27.Tonini G, Bizzarri C, Bonfanti R, Vanelli M, Cerutti F, Faleschini E, Meschi F, Prisco F, Ciacco E, Cappa M, Torelli C, et al. Sulfonylurea treatment outweighs insulin therapy in short-term metabolic control of patients with permanent neonatal diabetes mellitus due to activating mutations of the KCNJ11 (KIR6.2) gene. Diabetologia. 2006;49:2210–2213. doi: 10.1007/s00125-006-0329-x. [DOI] [PubMed] [Google Scholar]
- 28.Cole TJ, Freeman JV, Preece MA. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med. 1998;17:407–429. [PubMed] [Google Scholar]