Abstract
Although fibroblasts play an essential part during the wound healing response, the mechanisms by which they mediate tissue remodelling and contraction are still unclear. Using live cell and matrix imaging within 3D free-floating fibroblast-populated collagen lattices as a model for tissue contraction, we compared the behaviour of a range of fibroblasts with low and high contraction abilities and analysed the effect of the broad spectrum MMP-inhibitor GM6001 on cell behaviour and matrix contraction. We identified two mechanisms underlying matrix contraction, one via direct cell-mediated contractile activity, the second through matrix degradation. These appear to be linked to cell morphology and regulated by the collagen concentration within the matrix. Cells with a rounded morphology proliferated in the matrix but did not remodel it efficiently, resulting in a poor ability to contract matrices. Cells with an elongated morphology showed higher levels of protrusive activity, leading to efficient matrix remodelling and contraction. GM6001 inhibited week-long matrix contraction to various extents with the different cell lines. However, quantitative analysis of the cell protrusive activity showed that GM6001 consistently decreased cell dynamics in 3D by about 20%, and this was correlated with a significant reduction in early matrix contraction. Overall our results suggest that although fibroblast-mediated matrix contraction depends on both cell dynamics and MMP-mediated matrix degradation, the efficiency of GM6001 treatment in preventing contraction might be linked to a direct effect on cell dynamics.
Keywords: Cell dynamics, Matrix contraction, MMP inhibitor
Introduction
Tissue contraction is a fundamental part of a number of important biological processes, such as tissue morphogenesis and wound healing, and a wide range of debilitating pathologies are associated with abnormal contraction and scarring, from a reduction in visual function and blindness following ocular surgery to painful contractures in Dupuytren’s disease or burn injuries. However, although resident cells such as fibroblasts are believed to play in key role in controlling this process, the mechanisms by which they remodel their environment remain unclear. Identifying such mechanisms would be of immense value to predict and modulate tissue contraction and scarring in clinical settings.
During the wound healing response, fibroblasts are activated and recruited to the wound site to repair the tissue damage by remodelling the matrix (Grinnell, 1994). The free-floating fibroblast–populated collagen matrix is a well-known in vitro model to study tissue contraction (Bell et al., 1979; Grinnell, 2003). Within such a pseudo-physiological 3D environment, stimulated cells start to remodel the collagen network as they spread within the matrix. They attach to neighbouring collagen fibres through cycles of extension and retraction, applying force to the matrix through the contacts and promoting structural reorganization of the collagen architecture (Tomasek et al., 2002). We have shown that during early contraction, this dynamic cellular activity generates a net contraction force, or intrinsic cellular force, that is an important parameter underlying the cells’ ability to contract collagen matrices (Dahlmann-Noor et al., 2007). However, during the different stages of tissue remodelling, fibroblasts also express multiple soluble or membrane bound components such as collagen receptors, growth factors, extracellular matrix (ECM) proteins and more particularly proteases, such as matrix metalloproteinases (MMPs) (Finlay et al., 2000; Ignotz and Massague, 1986; Ravanti and Kahari, 2000). MMPs are a family of 25 endopeptidases, largely targeting ECM components. They have been shown to play important roles in the wound healing response (Ravanti and Kahari, 2000; Sternlicht and Werb, 2001; Wong et al., 2002), with a frequent association between impaired MMP activity and reduced wound healing and matrix remodelling (Bullard et al., 1999; Page-McCaw et al., 2007; Romer et al., 1996). Consequently, a number of studies have focused on utilizing MMP inhibitors in order to modulate the effects of post-operative scarring (Daniels et al., 2003; Wong et al., 2005). However, the exact mechanisms by which broad-spectrum MMP inhibitors deliver a decrease in contraction are not clear. Although MMP inhibition does not affect the contractile force generated by fibroblasts (Phillips et al., 2003), it has been shown to affect cell phenotype and dynamics (Sabeh et al., 2004; Wolf et al., 2003). In particular, cancer cells can switch from protease-led to acto-myosin based motility following MMP inhibition (Sanz-Moreno et al., 2008), and the same proteases can directly influence fibroblast motility (Sabeh et al., 2009a). As the cell dynamics as we measured them (i.e. protrusive activity) traditionally underlie fibroblast motility (Tomasek et al., 2002), we speculated that fibroblasts could appropriate the traditional cell motile machinery, combining cell dynamic activity and MMP-mediated matrix degradation, to contract and remodel the extracellular matrix in the context of tissue contraction. We describe a detailed analysis of cell behaviour during matrix contraction in presence or absence of MMP inhibitor GM6001, comparing various types of fibroblast lines with different abilities to contract collagen matrices. We demonstrate that fibroblasts, like tumour cells, use both active protrusive behaviour and local degradation to achieve collagen matrix contraction. Furthermore, our work suggests that the effect of broad-spectrum MMP inhibition on matrix contraction is unexpectedly linked to an effect on cell dynamics.
Material and Methods
Cell culture and reagents
Human Tenon’s capsule (HTF5003 and HTF7071, from 2 different donors) and scleral (HSF) fibroblasts were isolated as previously described from donor tissue in accordance with the tenets of the declaration of Helsinki and local ethics approval (Daniels and Khaw, 2000). Human dermal fibroblasts (HDF) were a gift from Robert Brown (UCL Tissue Repair & Engineering Centre). Mouse fibroblast NIH3T3 cell line was a gift from Richard Treisman (Cancer Research UK). The cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS, Sigma), 2 mM L-glutamine, 100 IU/ml penicillin, 100 mg/ml streptomycin (Invitrogen) at 37° C with 5% CO2. HTF, HSF and HDF were used between the passages 3 and 12 and 3T3 fibroblasts between passages 5 and 15. For live imaging, DMEM was replaced by Leibovitz L-15 medium with L-glutamine and without phenol-red (Invitrogen). Broad spectrum MMP inhibitor GM6001 (Millipore) was prepared from lyophilized powder as 20 mM stock solution in dimethyl sulfoxide (DMSO).
Collagen contraction assay
Three-dimensional fibroblast-populated collagen gels (1.5mg/ml of collagen type I, First link (UK) Ltd.) were prepared as previously described (Dahlmann-Noor et al., 2007), using the following cell concentrations: 0.5x105 (HTF), 2.6x104 (HDF), 14x105 (HSF) and 1.5x105 cells/ml (NIH3T3), to achieve comparable contraction curves for all cell type, with a plateau around day 5-7. MMP inhibitor GM6001 was added to both the matrix mix and the medium at the required concentration (usually 100 μM). 0.7 and 1.1 mg/ml collagen gels were obtained from 1.1 mg/ml and 1.6 mg/ml collagen solutions, respectively prepared by dilution of the standard stock solution (2.1mg/ml of collagen) in 0.6% of acetic acid, while 2.2 mg/ml and 3.0 mg/ml gels were obtained using a 4.5 mg/ml stock solution in 0.6% of acetic acid made from lyophilized collagen (First link (UK) Ltd). Macroscopic gel contraction was monitored daily by digital photography of the gels, and gel area quantified using Image J software (http://rsb.info.nih.gov/ij/). Relative changes in gel area are reported as a percent of the original area (contraction %). Cell numbers were determined on parallel sets of gels, following treatment with 0.05% collagenase D (Roche) for 20 minutes at 37°C with vigorous shaking. The cells were pelleted by centrifugation at 1800 rpm for 10 minutes, resuspended in PBS (containing Ca+2 and Mg+2) and counted using a hemocytometer. Cell viability was determined using Trypan Blue staining exclusion (Sigma). Contraction/cell (contractibility) was calculated by dividing the macroscopic gel contraction value by the average number of cells per gel (obtained from at least triplicate values) at each time point.
Microscopy and image analysis
Collagen gels were cast as above (HDF, HTF5003 and HTF7071), or with a lower cell density (20000 cells/ml for 3T3 and HSF and 7500 cells/ml for HTF5003) to allow us to study single cell activity (no difference in cell behaviour was detectable with the lower cell density- not shown). For live cell imaging, the gels were transferred to a pre-heated microscope stage (Zeiss Axiovert 100/Biorad confocal laser scanning microscope or Zeiss Axiovert 100M coupled to a computer driven acquisition station, Improvision/Perkin Elmer) at 37°C, and allowed to stabilize for another 30 minutes before imaging was started.
The collagen matrix was imaged by confocal reflection microscopy on live or fixed gels as previously described (Brightman et al., 2000). Briefly, long working distance objectives (Olympus LCP Plan FI 60x, NA 0.70, infinity corrected, or Zeiss 63X/0.75 plan neo fluar with correction collar) were fitted to a Zeiss Axiovert S100/Biorad Radiance 2000 confocal microscope. Specimens were illuminated at 488nm, and the 488nm reflected light was detected by a photomultiplier tube (PMT) through a polarisation filter to image the matrix fibres. Simultaneous reflection and DIC/transmission images were acquired in parallel and individual cell full volume was acquired using 0.5-3 μm steps every 15 minutes for 15 hours. For cell behaviour analysis only, phase/DIC images were recorded on an Zeiss Axiovert 100M inverted microscope, using the objectives above or a Zeiss plan neo fluar 20X/0.50, with a z-stack acquired every 15 minutes for a minimum of 4 hours using an ORCA-ER camera coupled to an Openlab-driven image acquisition system software (Improvision/Perkin Elmer UK). Cell area and changes in cell shape (circularity) were quantified using the standard Image J tools. Cell protrusive activity was quantitated using the dynamic index (DI), which quantifies the remodelled area of the cell (i.e. protrusion plus retraction) as a function of the cell area (Dahlmann-Noor et al., 2007). Briefly, the acquired z-stacks were imported into ImageJ or Openlab software and compressed into single images using the maximum intensity projection setting. The outline of individual cells was manually traced to allow accurate image thresholding. Cell areas were superimposed from one time point to the next and areas of retraction and protrusion were calculated. The cell dynamic activity, or “dynamic index” (DI) was calculated for each time frame as:
The resulting DI value for each cell was calculated as the average of all values recorded for a minimum of 3 hours (i.e. minimum 12 individual calculations, most often 20 or more). All analyses were performed “blind”, with individual movie names coded.
Cell migration in 2D
HTF were plated at low confluency (40000 cell/ml) and incubated overnight in presence of 10% FBS or 10% FBS with 100 μM GM6001. The cells were transferred to a microscope stage equipped with an environmental chamber at 37°C. Phase images were recorded every 10 minutes for a minimum of 4 hours using a Zeiss plan neo fluar 10X/0.30 objective fitted to a Zeiss Axiovert 100M/Openlab station described above. The images were imported into Openlab where the outline of individual cells at each time point was traced and the centroid position was tracked. The data was imported into ImageJ to calculate motility parameters (velocity and directionality) using the “chemotaxis tool” plugin, and the dynamic index using our customized macro as above.
Statistical analysis
Statistical analysis was performed using the Student’s t test to establish significant differences (probability P displayed where appropriate). Graphs display mean ± SEM.
Results
A specific combination of local fibre rearrangement, matrix degradation and cell proliferation underlies fibroblast matrix contraction signature
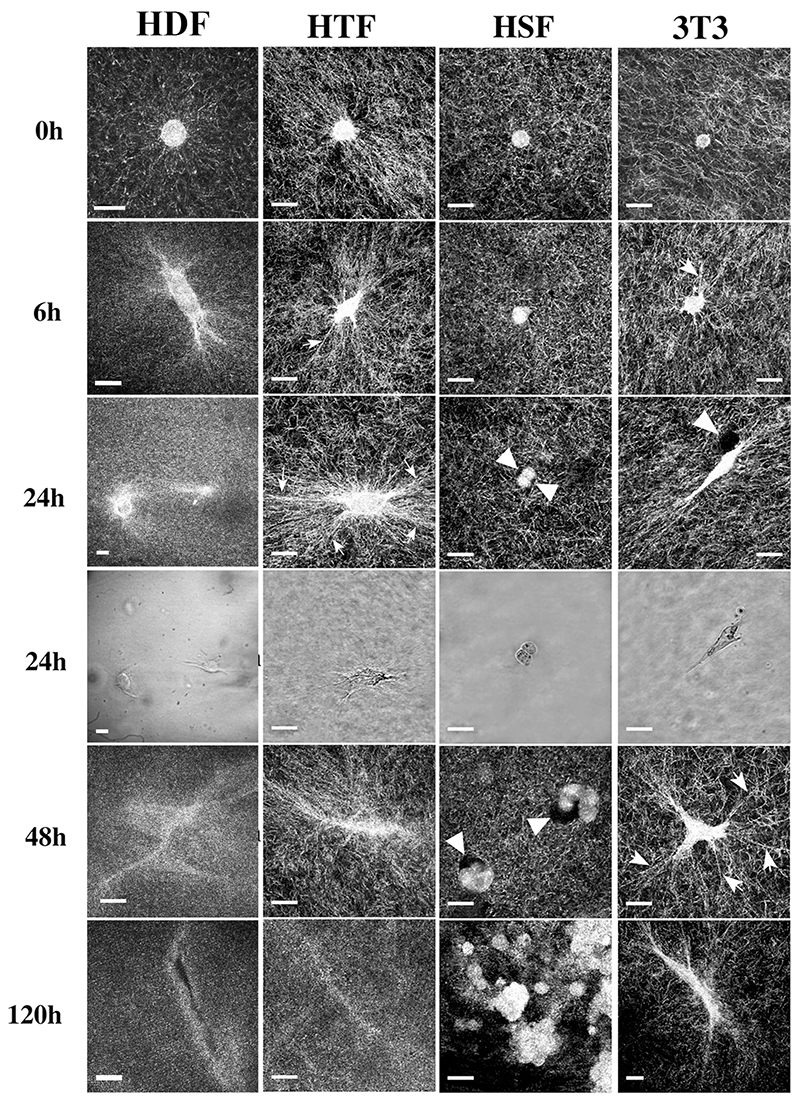
We have previously shown that early gel contraction is linked to the cells’ dynamic activity within the matrix, which in turn generates a net contraction force (Dahlmann-Noor et al., 2007). Here, we used confocal reflection microscopy to analyse in detail cell behaviour and matrix remodelling during collagen gel contraction, comparing fibroblasts of different origins and abilities to contract collagen matrices in presence of 10% serum: highly contractile primary human Tenon’s capsule (2 lines from different donors, HTF5003 and HTF7071) and dermal fibroblasts (HDF), poorly contractile primary human scleral fibroblasts (HSF), and moderately contractile mouse NIH3T3 fibroblasts (3T3). While HDF, HTF (both lines behaving similarly) and 3T3 spread well within the 3D matrix, forming numerous extensions, HSF remained largely ball-shaped (Fig. 1). Essentially two behaviours were observed with respect to the remodelling of the matrix. First, although none of the cells significantly translocated within the gels, cell-matrix interactions induced an alignment of the collagen fibres emanating radially from the cell body and tip of the largest extensions (Fig. 1, arrows). This behaviour was particularly pronounced with HTF, which showed fibre alignment as early as 30 minutes after cell seeding (t 0h, Fig. 1) and dramatic reorganisation of the matrix within 24 hours, but virtually absent with HSF. Fibre alignment was dependent on the presence of serum, as no alignment was visible after 24 hours, even for the HTF, in matrices incubated in serum-free medium (Supplementary Fig. S1A). The second form of matrix remodelling observed was degradation, either through a general fading of the matrix network (in HDF, as well as at later times for HTF and 3T3, Fig. 1), or in the form of pericellular voids merging into almost complete matrix disintegration (mostly in HSF, Fig. 1, arrowheads). The different behavioural interactions of the cells with the matrix were associated with a different organisation of the filamentous actin cytoskeleton. While all 3 highly contractile cells (HDF, HTF and 3T3) displayed some form of intracellular bundles and enhanced staining at the tip of the cell extensions, HSF showed only a dense cortical F-actin staining (Supplementary Fig. S1B).
Figure 1. Serum stimulation promotes local matrix deformation and degradation during contraction.
Cells were seeded in collagen matrices in medium containing 10% FBS, and images of fixed (HTF5003, 3T3) or live (HDF, HSF) gels were obtained using confocal reflection microscopy at different times after matrix polymerisation. HTF and 3T3 induced a rapid marked pericellular alignment of the collagen fibres (arrows), while matrix degradation could be seen either in the form of voids around the cell (HSF, arrowheads) or as a general fading of the matrix (t24hr for HDF, t120 hours in HTF and 3T3). Phase images background was filtered for clarity using Photoshop. Bars, 20 μm. Images shown are representative of 3 independent experiments.
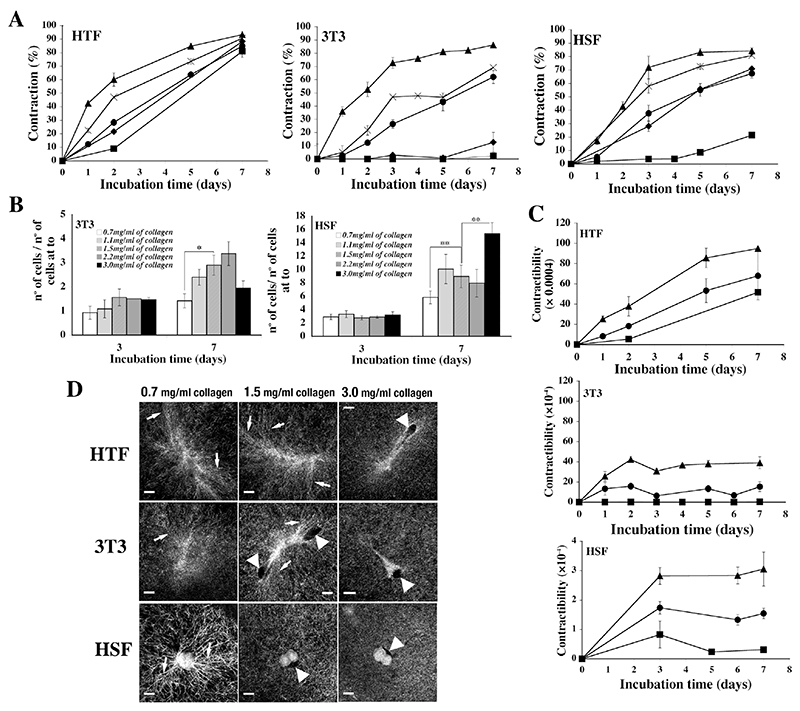
Previous work suggested that matrix density could influence cell behaviour (Zhu et al., 2001). To explore whether some of the behaviours above were matrix-density dependent, we analysed 3 representative fibroblast lines with high, medium and low gel contraction abilities (respectively HTF5003, 3T3 and HSF) in matrices of collagen concentrations ranging from 0.7-3 mg/ml. For comparative purposes, cell seeding numbers in the gels were adjusted for each cell line so that matrix contraction kinetics in standard conditions (1.5 mg/ml collagen) were similar for all 3 cell lines (Fig. 2A, circles). As expected, lattice contraction was roughly inversely correlated to the collagen concentration for all 3 cell types (Fig. 2A). On the other hand, while the HTF did not divide in the matrix irrespective of the collagen concentration (not shown), 3T3 and HSF proliferation was broadly proportional to the collagen concentration (although a decrease in cell numbers for 3T3 at 3 mg/ml collagen suggests an optimal collagen concentration for cell division; Fig. 2B). However, the cells overall retained a consistent contractibility profile (contraction/cell) irrespective of the collagen concentration (short rise leading to a plateau for 3T3 and HSF, sustained increase for HTF), with contractibility levels inversely proportional to the collagen concentration (Fig. 2C). Lower density matrices promoted the formation of early pericellular fibres alignment around HSF while, conversely, high collagen concentrations reduced cell spreading, prevented fibre alignment, and induced the appearance of significant pericellular voids for 3T3 and HTF (Fig. 2D). Interestingly, despite these changes in behaviour, there were no significant changes to the actin cytoskeleton organisation (data not show).
Figure 2. Collagen concentration modulates matrix contraction.
A) Fibroblast contraction profiles in gels with different collagen concentration (0.7 –triangles, 1.1 – crosses, 1.5 – circles, 2.2 - diamonds and 3.0 mg/ml of collagen, squares). Shown are means ± SEM from 2 (HTF5003), 5 (3T3) and 4 (HSF) sets of triplicate matrices. B) Cell proliferation in gels of different collagen concentration. Cells were counted after gel degradation at different time points during contraction and normalized to cell numbers at time 0, showing an increase in cell division in high collagen concentration. HTF did not divide in the gels at any concentration (not shown). Shown is mean ± SEM from 4 sets of triplicate matrices. Statistical significance: **, p < 0.001; *, p<0.05. C) Contractibility (contraction/cell) profiles in gels of different collagen concentration is inversely proportional to the collagen concentration. Shown are means and SEMs from 2 (HTF5003) and 4 (3T3 and HSF) sets of triplicate matrices. D) Cell-matrix interaction after 2 days in presence of 10% of serum in gels with different collagen concentrations visualized using reflection confocal microscopy. Low collagen concentration (0.7mg/ml) induces pericellular fibre alignment for HSF (arrows), while higher collagen concentration induces the formation of holes in the matrix around HTF fibroblasts (arrowheads) and decreases the ability of 3T3 and HTF to induce fibre alignment. Bars, 15 μm.
MMP inhibition affects matrix contraction through alterations in cell dynamics
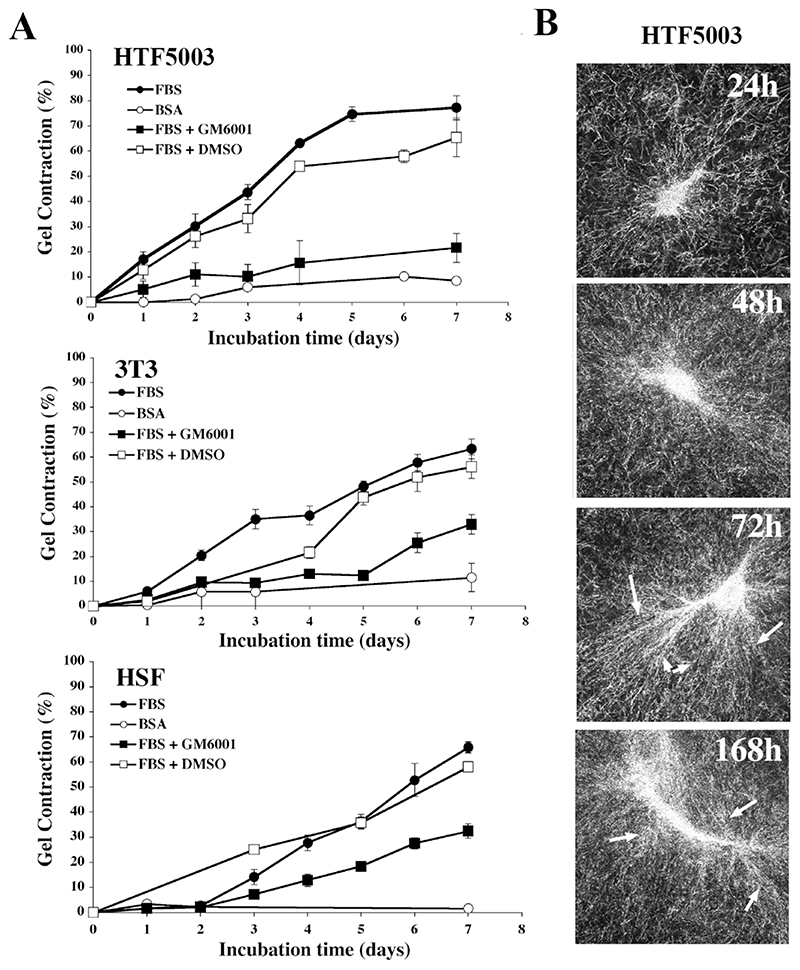
Our analysis of the cell and matrix behaviour during contraction identified some form of matrix degradation and remodelling, largely appearing after 24-48 hrs (Fig. 1). We thus examined the effects of the broad-spectrum MMP inhibitor GM6001 on matrix contraction and cell behaviour. Overall, GM6001 was poorly efficient at preventing fibroblast-mediated matrix contraction at concentrations normally sufficient to inhibit most metalloproteinases (Supplementary Fig. S2A). Consistent with previous work (Daniels et al., 2003; Wong et al., 2004; Wong et al., 2005), a concentration of 100 μM was necessary to achieve maximal inhibition for all cell types, and was adopted for all further experiments (Supplementary Fig. 2A and S2C). Despite being rather high, such concentration was not toxic for the cells in collagen gels, as we could not detect any significant decrease in cell numbers/viability in presence of GM6001 compared to cells in regular medium, even after a week, using a standard cell proliferation/viability assay (Alamar Blue assay, Fig.S2B), nor could we detect a significant amount of dead cells in the gels upon direct cell counting in the presence of Trypan Blue (Fig S2A). No apoptosis could be detected in the gels either, even after 7 days (Tunnel assay, not shown). There was a distinctive, although not significant, trend for reduced cell numbers in the gels in the presence of 100 μM GM6001, most pronounced for the cells with high proliferative capacity (HSF and 3T3, FigS2A), suggesting that a high concentration of GM6001 could affect cell proliferation. This was however not sufficient to account for the strong effect of GM6001 on cell-mediated matrix contraction in the cell lines that did not proliferate significantly in the gels such as HTF and HDF. Following treatment with the highest GM6001 concentration (100 μM), the total soluble MMP activity measured after 3 and 7 days was significantly decreased, but not completely abolished, as measured both by fluorimetric assay and zymography (Supplementary Fig. S3A and S3B). Surprisingly the treatment significantly increased MT1-MMP/MMP14 activity (as well as total membrane bound MMP activity- not shown) in HTF (Supplementary Fig. S3C).
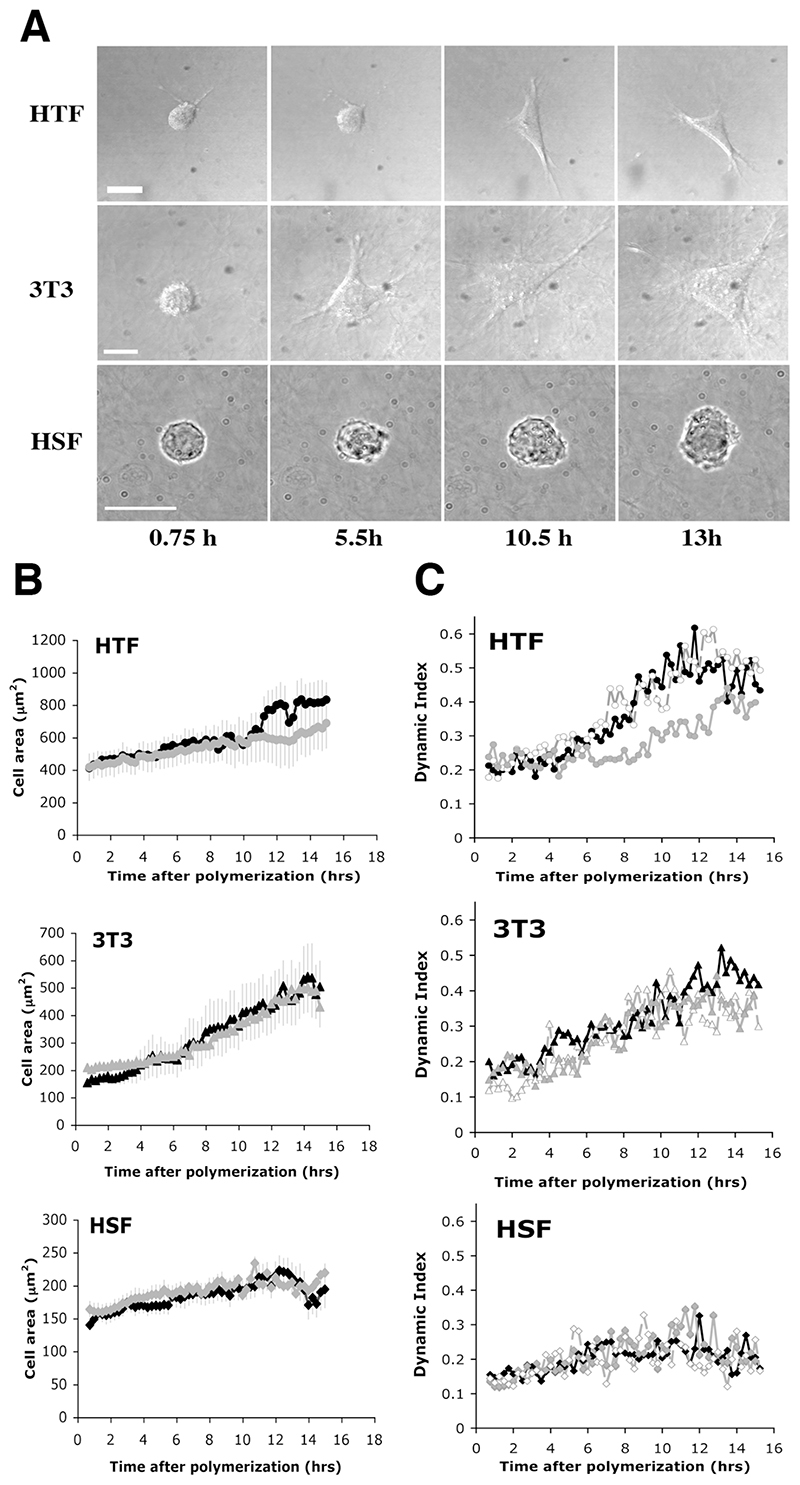
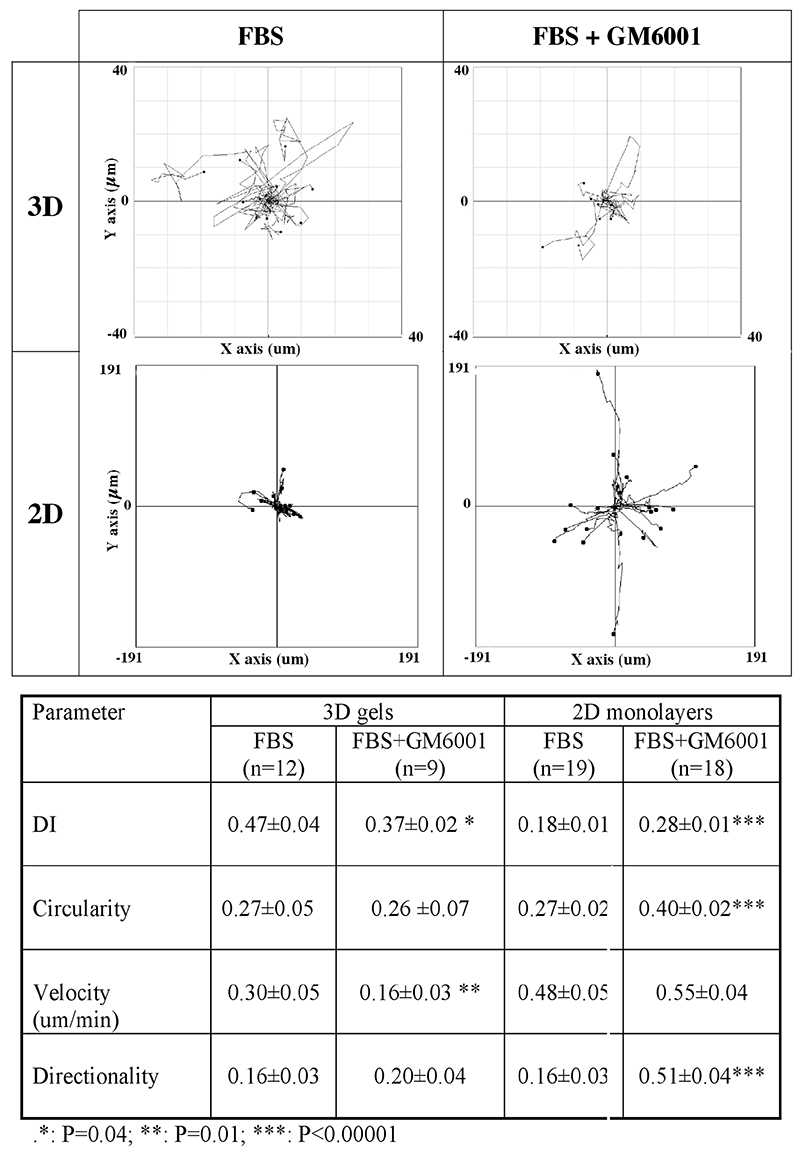
Only HTF5003 were dramatically and consistently impaired by GM6001 treatment in their ability to contract collagen matrices (by 70% at day 7), while all other cell lines, including 3T3 and HSF, though significantly delayed, still contracted the matrices after 7 days by at least half as much as in the control (Fig. 3A and Supplementary Fig. S2A and S2C). GM6001 effect on the contraction was also more pronounced in the early stages of the contraction, despite the drug being replaced in the medium every 2 days. Confocal analysis of matrices following treatment with GM6001 showed, as expected, a decrease in matrix degradation, particularly in the form of the persistence of a distinct fibre pattern in HTF as opposed to the haze seen in the control. Surprisingly, they also show a reduced fibre alignment in the pericellular region at early time points (Fig. 3B), suggesting that the cell dynamics have been affected. We analysed the effect of GM6001 treatment on the dynamic behaviour of the cells in the matrix, using our custom designed parameter, the Dynamic Index (DI) (Dahlmann-Noor et al., 2007). The DI measures the total area of dynamic activity (i.e. protrusion plus retraction) as a function of the cell area, thus giving an accurate representation of how much of the cell area is being remodelled over a given period of time (see Material and Methods). Fig. 4A shows a representative view of the different fibroblast morphology in control collagen matrices in presence of 10% serum at early time points (corresponding Supplementary movies 1a, 1b, and 1c). Cell spreading was associated with the extension and retraction of multiple pseudopods for 3T3 and HTF, resulting in an increase in cell area towards a maximum value around 15 hours post-seeding. HSF displayed continuing protrusive activity as well, but the minute protrusions generated resulted in poor spreading. Although HTF spreading appeared delayed in the presence of GM6001, the drug did not significantly affect cell spreading during the first 15 hrs in any of the 3 cell lines analysed (Fig. 4B). However, GM6001 treatment significantly affected the HTF dynamic activity during spreading (Fig. 4C; average DI over the last 6 hours: 0.49±0.05 for FBS versus 0.37±0.02 for GM6001 treated cells, P= 0.02). It did not have a significant effect on the 3T3 or HSF during that period. To confirm whether the effect of the MMP inhibitor on the HTF dynamic index was a general effect on the cell dynamics or a specific effect due to the cells being within a 3D environment, we compared the effect of GM6001 on the behaviour of the HTF in monolayers or in 3D gels after the cells had reached full spreading (i.e. 9 hrs post seeding). Overnight treatment of HTF in monolayers with 100 μM GM6001 in presence of 10% of serum resulted in a major decrease in cell area (8220±942 μm2 for 19 control cells; 2726±217 μm2 for 20 treated cells), and an increase in circularity (Fig. 5). Both cell protrusive activity (DI) and persistence were also increased, leading to an overall increase in cell motility (Fig. 5 and Supplementary movies 2a and 2b). By contrast, both the dynamic index and the velocity were significantly decreased in collagen gels, whilst neither the cell shape, nor the directionality, were altered, resulting in very little cell movement. As this suggested that the effect of the GM6001 treatment on cell dynamics was specific to cell behaviour in 3D rather than a peculiar reaction of HTF5003, we analysed the early 3D dynamic behaviour of all our cell lines in presence of GM6001, as well as its effect on their ability to contract collagen matrices (Table 1). With the exception of the poorly contractile rounded HSF, all cell lines analysed displayed a reduced dynamic index in presence of GM6001 (by about 20%, as measured within the first 24 hours) and a significant decreased in matrix contraction at 24 hrs (Table 1) and 48 hrs (not shown).
Figure 3. MMP inhibition affects matrix remodelling and gel contraction. Fibroblasts seeded in gels were incubated for 7 days in the presence of 10% FBS with or without 100 μM of MMP inhibitor GM6001.
A) Matrix contraction in presence of 10% FBS (filled circles), 10% FBS plus 100 μM GM6001 (filled squares), serum free medium with 0.7% BSA (empty circles) and 10% FBS plus 0.5% DMSO (empty squares). Shown is mean ± SEM, from 4 (HTF, HSF) and 6 (3T3) sets of triplicate matrices. B) MMP inhibition affects cell-matrix interaction. HTF seeded in collagen gels in presence of 10% serum plus 100 μM GM6001 showed delayed pericellular collagen fibre alignment (arrows) and better preservation of the fibre network (compare to Fig. 1). Scale bar, 20 μm.
Figure 4. MMP inhibition affects cell dynamic behaviour.
Fibroblasts were seeded in gels (20000 cells/ml for 3T3 and HSF and 7500 cells/ml for HTF) in presence of 10% serum with (grey line) or without (black line) 100 μM GM6001, and cell behaviour was recorded for 15 hours, starting 30 min after gel polymerization. A) DIC (HTF, 3T3) and transmission (HSF) sequential images extracted from the 15 hrs time lapse (supporting movies 1a, 1b and 1c respectively) showing the cells’ activity in presence of 10% FBS. Time as indicated. Scale bars, 20μm. B) Early spreading in the matrix in the presence of GM6001. Cell area profile was calculated from the cell area measured on flat projections of phase contrast or DIC images. Show is the mean ± SEM for 7-9 cells for each curve. C) Effect of GM6001 on cell dynamic behaviour. Cell protrusive activity was quantified using the dynamic index in presence of 10% serum (black signs), 10% serum plus 100 μM GM6001 (grey signs) and 10% serum plus DMSO as solvent control (empty signs). Shown is the mean of 5-9 cells for each condition. SEM < 10%. Significant differences in the plateau reached for HTF (11 to 15 hours post-polymerisation, p < 0.0001).
Figure 5. MMP inhibition increases HTF dynamic behaviour in 2D but reduces protrusive activity and velocity in 3D matrices.
HTF in 3D: HTF5003 cells were seeded in collagen gels in presence of 10% FBS with or without 100 μM GM6001 and allowed to spread and settle in the gels for 9 hours before imaging. HTF in 2D: HTF5003 cells were plated in tissue culture dishes and incubated overnight in medium plus 10% FBS without or with GM6001. Images were acquired every 15 and 10 minutes in 3D and 2D respectively, for a minimum of 4 hours using time-lapse phase contrast microscopy. The resulting movies were processed in Image J as described in Material and Methods using the chemotaxis tool and dynamic index plugins. Shown are motility plots centered on the original centroid position for each cell (top) and corresponding cell motility and dynamics parameters.
Table 1. Effect of GM6001 treatment on cell dynamics and early matrix contraction.
| FBS | FBS+GM6001 | |||
|---|---|---|---|---|
| Dynamic index (nb cells analysed) | Gel contraction at day2 (%) (nb experiments) | Dynamic index (nb cells analysed) | Gel contraction at day2 (%) (nb number of experiments) | |
| HTF7071 | 0.48±0.03 (22) | 44±4 (4) | 0.39±0.03 (19) * P=0.01 | 27±4 (4) * P= 0.006 |
| HTF5003 | 0.42±0.03(21) | 17±3 (4) | 0.33±0.02 (28)* P=0.005 | 5±3 (5) * P=0.01 |
| HDF | 0.40±0.02(24) | 42±3 (4) | 0.33±0.02 (18) * P=0.04 | 26±2 (4) * P<0.005 |
| HSF | 0.22±0.02 (7) | 2.3±0.6 (4) | 0.22±0.02 (6) NS | 1.6±0.9 (3) NS |
| 3T3 | 0.42±0.04 (7) | 6±1 (8) | 0.36±0.03 (8) NS | 3±1 (3) * P=0.01 |
probability of a significant difference between the FBS and FBS+GM6001 sets using Student T test. NS, no significant difference.
Discussion
As the main resident cells in connective tissue, fibroblasts participate in the maintenance and repair of a wide range of collagen matrix types within the body (Ross and Odland, 1968). Using the classical free-floating fibroblast-populated collagen lattice as a model for tissue contraction (Bell et al., 1979; Grinnell, 2003), we have previously shown that early matrix contraction is linked to the cells’ dynamic activity within the matrix, which in turn generates a net contraction force (Dahlmann-Noor et al., 2007). Here we further analysed the cell behaviour during contraction and characterized the parameters linked to fibroblast-mediated collagen matrix contraction examining a range of fibroblasts with different abilities to contract collagen matrices.
Overall, we showed that matrix contraction resulted from a combination of two main processes: local active collagen fibre alignment through cellular protrusive activity and matrix degradation. Although some cell lines showed significant proliferation in the gels, they tended to be the fibroblasts with a low ability to contract collagen matrices (3T3, HSF). Indeed, for all the cells analysed, the ability to contract collagen matrices was broadly correlated to the level of cell spreading within the matrix, as well as the cells’ protrusive activity (consistent with our previous work (Dahlmann-Noor et al., 2007)), but inversely related to cell proliferation. As the high proliferating cells (HSF) display low intrinsic cellular force (Dahlmann-Noor et al., 2007), this suggests that cells with low intrinsic force can use proliferation as a means to compensate for individual cell weakness to remodel the matrix. Interestingly, the high proliferative activity of HSF, characterized by the formation of large cell clusters in the matrix, was accompanied by a dramatic pericellular degradative phenotype with large voids within the matrix around the cells. This local collagen remodelling could potentially participate in the activation of proliferation by releasing the spatial restriction round the cells as previously shown for tumour cells (Hotary et al., 2003), which is consistent with the trend in reduced cell proliferation that we observed in presence of GM6001. Thus, for cells with low intrinsic contraction force such as HSF and 3T3, and which rely on high levels of proliferation in the gels to significantly remodel the matrix, such a decrease in cell proliferation could account to some extent for the observed reduction in matrix contraction in presence of GM6001. While collagen density had an effect on the degree of matrix contraction and cell proliferation as previously reported (Bell et al., 1979; Zhu et al., 2001), the overall contractibility profiles (contraction/cell) remained the same for each cell type, suggesting that the contraction force per cell is independent of the matrix density and reflects a cell “matrix contraction” signature. In addition to a specific contractile phenotype, all the fibroblasts analysed here displayed some form of matrix degradation during contraction. Most of this activity took place after 24 hrs, and correlated with a significant increase in expression and activity for both soluble and membrane-bound MMPs during matrix contraction for all cells analysed. This was notable despite the well-known presence of MMP inhibitors in serum (Lee et al., 1999; Ylisirnio et al., 2000), suggesting that fibroblasts produce a large amount of MMP-mediated protease activity during matrix contraction, clearly more than could be inhibited by any inhibitor activity present in the serum. As membrane-bound MMPs such as MT1-MMP and MT2-MMP have been shown to efficiently degrade type I collagen (Sabeh et al., 2010; Sabeh et al., 2009a; Sabeh et al., 2009b) and are poorly inhibited by serum as they are released on the cell membrane as active forms (Li et al., 2008; Sabeh et al., 2010), this could also suggest that a significant proportion of the matrix degradation observed during contraction is linked to localised matrix proteolysis through a membrane-bound MMP.
The dynamic behaviour of fibroblasts in collagen matrices during early contraction consists of cycles of cell extension and retraction, with the level of activity (Dynamic Index) related to the cells ability to contract collagen gels (Dahlmann-Noor et al., 2007). We confirm here with a larger sample that the DI is predictive of the fibroblasts’ ability to contract collagen matrices. More importantly, we show that GM6001 treatment leads to a decrease of the cell dynamic index by about 20% for all the cells analysed, and that this decrease correlates with a decrease in early matrix contraction. Although some reports have suggested that GM6001 might have an effect on cell dynamics, notably studies on tumour cell movement in vitro (Wolf et al., 2003) and post-injury corneal epithelium spreading in vivo (Kato et al., 2006), our report is the first to quantitatively assess the decrease in protrusive activity in 3D following GM6001 treatment and link it to a decrease in matrix contraction. As a) we have shown previously that an alteration in adhesion can decrease growth factor-mediated protrusive activity to an extent similar to the effect of GM6001 observed in this study (Bailly et al., 1998), and b) cells converting to the ameboid rounded phenotype display reduced adhesion phenotype (Sanz-Moreno et al., 2008; Wolf et al., 2003), this suggests that GM6001 could affects the cells’ dynamic behaviour by modifying cell adhesion. Previous work has shown that treatment with GM6001 altered the MT1-MMP and integrins profile in tumour cells (Wolf et al., 2003). It also increased significantly the amount of MT1-MMP activity in dermal fibroblasts (Townley et al., 2008), whilst at the same time decreasing their ability to contract collagen matrices, similar to the effect on the HTF in the present study and suggesting that the cells may be trying to compensate for the blocking effect of GM6001. Although the increased in MT1-MMP was demonstrated after 48hrs (Townley et al., 2008) or 3 days in our study above, it is possible that GM6001 has an even earlier effect on MT1-MMP activity that would be significant enough to disturb integrin distribution and alter adhesion patterns. Interestingly, as a) GM6001 treatment can convert tumour cells with a mesenchymal phenotype (fibroblast-like shape and fully spread, i.e more similar to the fibroblasts with a high DI in our study) to an amoeboid phenotype (rounded cells with a blebbing-like phenotype; (Hooper et al., 2006; Wolf et al., 2003)), b) the conversion from the elongated to the rounded phenotype has been shown to be dependent on the level of Rac activity, and c) cell protrusive activity is most commonly linked to downstream activation of Rac, it is tempting to suggest that GM6001 could decrease protrusive activity through a downregulation of Rac activity. Indeed, an effect of GM6001 on integrin engagement could result in the observed decrease in protrusive activity through a decrease in Rac activation (DeMali et al., 2003). Although more work is needed to test this hypothesis, our preliminary data indicates that Rac activation is indeed essential for fibroblast-mediated matrix contraction (V. Tovell, manuscript in preparation). Finally, although there are striking similarities between tumour cell motility phenotype in 3D and fibroblast-mediated matrix contraction, it is clear that these represent two different processes. Unlike tumour cells movement, fibroblast-mediated collagen contraction appears linked to adhesion, particularly through integrins, as blocking adhesion prevents contraction (Cooke et al., 2000; Langholz et al., 1995).
Overall, our study indicates that two different mechanisms mediate matrix contraction, one via contractile activity, intrinsic to cell type, and the other through proliferative and degradative activity, which appear to be linked to the different cell morphologies. Cells with a more rounded morphology are less efficient at matrix remodelling, use a proliferation-dependent mechanism to contract the matrix efficiently, and are poorly inhibited by GM6001. Cells with a more elongated morphology actively remodel the matrix and are overall more sensitive to broad-spectrum MMP inhibition. More importantly, we show that the broad-spectrum inhibitor GM6001 directly affects cell protrusive behaviour and that this correlates with a decreased ability to contract matrices. These behaviours bear striking similarities to the morphological changes observed during mesenchymal-amoeboid transition (MAT) in tumour cells (Hooper et al., 2006), raising the interesting possibility that the underlying signal transduction pathways might be similar. Although further work is needed confirm this, we present evidence that, while MMP inhibitors clearly are an appropriate target for the modulation wound healing and tissue contraction (Daniels et al., 2003; Mirastschijski et al., 2004; Scott et al., 1998), further work is needed to understand how they work as, just like for tumour cells, their efficiency might be linked to particular cell phenotypes.
Supplementary Material
Acknowledgments
This work was supported by Medical Research Council grants MRC G0100195, G0100200 and G0801049 (MB and PTK), the Royal Society (MB), the Special Trustees of Moorfields Eye Hospital (MB) and the Moorfields Eye Hospital/UCL Institute of Ophthalmology NIHR Biomedical Research Centre (PTK). Laboratories and imaging facilities were supported by the Wellcome Trust and the MRC. BMM was funded by a grant from the Fundacion Ramon Areces (Spain) and AHDN by a Wellcome Trust Research Training Fellowship for Medical, Dental and Veterinary Graduates.
References
- Bailly M, Yan L, Whitesides GM, Condeelis JS, Segall JE. Regulation of protrusion shape and adhesion to the substratum during chemotactic responses of mammalian carcinoma cells. Exp Cell Res. 1998;241:285–299. doi: 10.1006/excr.1998.4031. [DOI] [PubMed] [Google Scholar]
- Bell E, Ivarsson B, Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci U S A. 1979;76:1274–1278. doi: 10.1073/pnas.76.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brightman AO, Rajwa BP, Sturgis JE, McCallister ME, Robinson JP, Voytik-Harbin SL. Time-lapse confocal reflection microscopy of collagen fibrillogenesis and extracellular matrix assembly in vitro. Biopolymers. 2000;54:222–234. doi: 10.1002/1097-0282(200009)54:3<222::AID-BIP80>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Bullard KM, Lund L, Mudgett JS, Mellin TN, Hunt TK, Murphy B, Ronan J, Werb Z, Banda MJ. Impaired wound contraction in stromelysin-1-deficient mice. Ann Surg. 1999;230:260–265. doi: 10.1097/00000658-199908000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke ME, Sakai T, Mosher DF. Contraction of collagen matrices mediated by alpha2beta1A and alpha(v)beta3 integrins. J Cell Sci. 2000;113(Pt 13):2375–2383. doi: 10.1242/jcs.113.13.2375. [DOI] [PubMed] [Google Scholar]
- Dahlmann-Noor AH, Martin-Martin B, Eastwood M, Khaw PT, Bailly M. Dynamic protrusive cell behaviour generates force and drives early matrix contraction by fibroblasts. Exp Cell Res. 2007;313:4158–4169. doi: 10.1016/j.yexcr.2007.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels JT, Cambrey AD, Occleston NL, Garrett Q, Tarnuzzer RW, Schultz GS, Khaw PT. Matrix metalloproteinase inhibition modulates fibroblast-mediated matrix contraction and collagen production in vitro. Invest Ophthalmol Vis Sci. 2003;44:1104–1110. doi: 10.1167/iovs.02-0412. [DOI] [PubMed] [Google Scholar]
- Daniels JT, Khaw PT. Temporal stimulation of corneal fibroblast wound healing activity by differentiating epithelium in vitro. Invest Ophthalmol Vis Sci. 2000;41:3754–3762. [PubMed] [Google Scholar]
- DeMali KA, Wennerberg K, Burridge K. Integrin signaling to the actin cytoskeleton. Curr Opin Cell Biol. 2003;15:572–582. doi: 10.1016/s0955-0674(03)00109-1. [DOI] [PubMed] [Google Scholar]
- DesMarais V, Macaluso F, Condeelis J, Bailly M. Synergistic interaction between the Arp2/3 complex and cofilin drives stimulated lamellipod extension. J Cell Sci. 2004;117:3499–3510. doi: 10.1242/jcs.01211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay GA, Thannickal VJ, Fanburg BL, Paulson KE. Transforming growth factor-beta 1-induced activation of the ERK pathway/activator protein-1 in human lung fibroblasts requires the autocrine induction of basic fibroblast growth factor. J Biol Chem. 2000;275:27650–27656. doi: 10.1074/jbc.M000893200. [DOI] [PubMed] [Google Scholar]
- Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol. 1994;124:401–404. doi: 10.1083/jcb.124.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F. Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol. 2003;13:264–269. doi: 10.1016/s0962-8924(03)00057-6. [DOI] [PubMed] [Google Scholar]
- Hooper S, Marshall JF, Sahai E. Tumor cell migration in three dimensions. Methods Enzymol. 2006;406:625–643. doi: 10.1016/S0076-6879(06)06049-6. [DOI] [PubMed] [Google Scholar]
- Hotary KB, Allen ED, Brooks PC, Datta NS, Long MW, Weiss SJ. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell. 2003;114:33–45. doi: 10.1016/s0092-8674(03)00513-0. [DOI] [PubMed] [Google Scholar]
- Ignotz RA, Massague J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986;261:4337–4345. [PubMed] [Google Scholar]
- Kato T, Saika S, Ohnishi Y. Effects of the matrix metalloproteinase inhibitor GM6001 on the destruction and alteration of epithelial basement membrane during the healing of post-alkali burn in rabbit cornea. Jpn J Ophthalmol. 2006;50:90–95. doi: 10.1007/s10384-005-0287-8. [DOI] [PubMed] [Google Scholar]
- Langholz O, Rockel D, Mauch C, Kozlowska E, Bank I, Krieg T, Eckes B. Collagen and collagenase gene expression in three-dimensional collagen lattices are differentially regulated by alpha 1 beta 1 and alpha 2 beta 1 integrins. J Cell Biol. 1995;131:1903–1915. doi: 10.1083/jcb.131.6.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MA, Palace J, Stabler G, Ford J, Gearing A, Miller K. Serum gelatinase B, TIMP-1 and TIMP-2 levels in multiple sclerosis. A longitudinal clinical and MRI study. Brain. 1999;122(Pt 2):191–197. doi: 10.1093/brain/122.2.191. [DOI] [PubMed] [Google Scholar]
- Li XY, Ota I, Yana I, Sabeh F, Weiss SJ. Molecular dissection of the structural machinery underlying the tissue-invasive activity of membrane type-1 matrix metalloproteinase. Mol Biol Cell. 2008;19:3221–3233. doi: 10.1091/mbc.E08-01-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirastschijski U, Haaksma CJ, Tomasek JJ, Agren MS. Matrix metalloproteinase inhibitor GM 6001 attenuates keratinocyte migration, contraction and myofibroblast formation in skin wounds. Exp Cell Res. 2004;299:465–475. doi: 10.1016/j.yexcr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JA, Vacanti CA, Bonassar LJ. Fibroblasts regulate contractile force independent of MMP activity in 3D-collagen. Biochem Biophys Res Commun. 2003;312:725–732. doi: 10.1016/j.bbrc.2003.10.179. [DOI] [PubMed] [Google Scholar]
- Ravanti L, Kahari VM. Matrix metalloproteinases in wound repair (review) Int J Mol Med. 2000;6:391–407. [PubMed] [Google Scholar]
- Romer J, Bugge TH, Pyke C, Lund LR, Flick MJ, Degen JL, Dano K. Impaired wound healing in mice with a disrupted plasminogen gene. Nat Med. 1996;2:287–292. doi: 10.1038/nm0396-287. [DOI] [PubMed] [Google Scholar]
- Ross R, Odland G. Human wound repair. II. Inflammatory cells, epithelial-mesenchymal interrelations, and fibrogenesis. J Cell Biol. 1968;39:152–168. doi: 10.1083/jcb.39.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeh F, Fox D, Weiss SJ. Membrane-type I matrix metalloproteinasedependent regulation of rheumatoid arthritis synoviocyte function. J Immunol. 2010;184:6396–6406. doi: 10.4049/jimmunol.0904068. [DOI] [PubMed] [Google Scholar]
- Sabeh F, Li XY, Saunders TL, Rowe RG, Weiss SJ. Secreted versus membrane-anchored collagenases: relative roles in fibroblast-dependent collagenolysis and invasion. J Biol Chem. 2009a;284:23001–23011. doi: 10.1074/jbc.M109.002808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeh F, Ota I, Holmbeck K, Birkedal-Hansen H, Soloway P, Balbin M, Lopez-Otin C, Shapiro S, Inada M, Krane S, et al. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J Cell Biol. 2004;167:769–781. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeh F, Shimizu-Hirota R, Weiss SJ. Protease-dependent versus - independent cancer cell invasion programs: three-dimensional amoeboid movement revisited. J Cell Biol. 2009b;185:11–19. doi: 10.1083/jcb.200807195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Moreno V, Gadea G, Ahn J, Paterson H, Marra P, Pinner S, Sahai E, Marshall CJ. Rac activation and inactivation control plasticity of tumor cell movement. Cell. 2008;135:510–523. doi: 10.1016/j.cell.2008.09.043. [DOI] [PubMed] [Google Scholar]
- Scott KA, Wood EJ, Karran EH. A matrix metalloproteinase inhibitor which prevents fibroblast-mediated collagen lattice contraction. FEBS Lett. 1998;441:137–140. doi: 10.1016/s0014-5793(98)01542-7. [DOI] [PubMed] [Google Scholar]
- Shao D, Forge A, Munro PM, Bailly M. Arp2/3 complex-mediated actin polymerisation occurs on specific pre-existing networks in cells and requires spatial restriction to sustain functional lamellipod extension. Cell Motil Cytoskeleton. 2006;63:395–414. doi: 10.1002/cm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- Townley WA, Cambrey AD, Khaw PT, Grobbelaar AO. Matrix metalloproteinase inhibition reduces contraction by dupuytren fibroblasts. J Hand Surg Am. 2008;33:1608–1616. doi: 10.1016/j.jhsa.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Wolf K, Mazo I, Leung H, Engelke K, von Andrian UH, Deryugina EI, Strongin AY, Brocker EB, Friedl P. Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J Cell Biol. 2003;160:267–277. doi: 10.1083/jcb.200209006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TT, Daniels JT, Crowston JG, Khaw PT. MMP inhibition prevents human lens epithelial cell migration and contraction of the lens capsule. Br J Ophthalmol. 2004;88:868–872. doi: 10.1136/bjo.2003.034629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TT, Mead AL, Khaw PT. Prolonged antiscarring effects of ilomastat and MMC after experimental glaucoma filtration surgery. Invest Ophthalmol Vis Sci. 2005;46:2018–2022. doi: 10.1167/iovs.04-0820. [DOI] [PubMed] [Google Scholar]
- Wong TT, Sethi C, Daniels JT, Limb GA, Murphy G, Khaw PT. Matrix metalloproteinases in disease and repair processes in the anterior segment. Surv Ophthalmol. 2002;47:239–256. doi: 10.1016/s0039-6257(02)00287-4. [DOI] [PubMed] [Google Scholar]
- Ylisirnio S, Hoyhtya M, Turpeenniemi-Hujanen T. Serum matrix metalloproteinases -2, -9 and tissue inhibitors of metalloproteinases -1, -2 in lung cancer--TIMP-1 as a prognostic marker. Anticancer Res. 2000;20:1311–1316. [PubMed] [Google Scholar]
- Zhu YK, Umino T, Liu XD, Wang HJ, Romberger DJ, Spurzem JR, Rennard SI. Contraction of fibroblast-containing collagen gels: initial collagen concentration regulates the degree of contraction and cell survival. Vitro Cell Dev Biol Anim. 2001;37:10–16. doi: 10.1290/1071-2690(2001)037<0010:COFCCG>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.