Abstract
Adenosine triphosphate (ATP), the cellular energy currency, is essential for life. The ability to provide a constant supply of ATP is therefore crucial for the construction of artificial cells in synthetic biology. Here, we describe the bottom-up assembly and characterization of a minimal respiratory system that uses NADH as a fuel to produce ATP from ADP and inorganic phosphate, and is thus capable of sustaining both upstream metabolic processes that rely on NAD+, and downstream energydemanding processes that are powered by ATP hydrolysis. A detergent-mediated approach was used to co-reconstitute respiratory mitochondrial complex I and an F-type ATP synthase into nanosized liposomes. Addition of the alternative oxidase to the resulting proteoliposomes produced a minimal artificial “organelle” that reproduces the energy-converting catalytic reactions of the mitochondrial respiratory chain: NADH oxidation, ubiquinone cycling, oxygen reduction, proton pumping, and ATP synthesis. As a proof-of-principle, we demonstrate that our nanovesicles are capable of using an NAD+-linked substrate to drive cell-free protein expression. Our nanovesicles are both efficient and durable and may be applied to sustain artificial cells in future work.
Keywords: synthetic biology, artificial cell, respiratory chain, co-reconstitution, complex I, ATP synthase, alternative oxidase
The bottom-up construction of artificial cells from their individual components is a major goal of synthetic biology.1–7 Artificial cells need to fulfill all the basic characteristics of biological cells, including compartmentalization, energy conversion, the replication of genetic information, and protein synthesis.6 The compartmentalized energy handling systems in eukaryotic cells are mitochondria: they use oxidative phosphorylation to extract the redox potential energy from biological “fuels” to regenerate the ATP pool. NADH (nicotinamide adenine dinucleotide) is a biological hydrogen carrier, or fuel, which is essential to all life.8,9 It is produced from NAD+ by many different catabolic processes and is consumed in mitochondria by respiratory complex I (mito-CI), regenerating the NAD+ pool. Complex I oxidizes NADH, reduces ubiquinone, and pumps four protons across the inner mitochondrial membrane for every two-electron redox reaction.10 The electrons are then transferred from ubiquinol to oxygen, through complexes III and IV via cytochrome c. Complexes III and IV pump an additional six protons across the membrane per two electrons, and thereby respiration produces the electrochemical proton motive force (Δp) that powers the universal F1F0 ATP synthase to regenerate the cellular ATP pool.11–13
Incorporating the complete set of molecules required for in vitro transcription−translation into giant unilamellar vesicles to demonstrate compartmentalized protein synthesis has contributed an important step toward building synthetic cells.14–16 However, a limitation of simple microsized vesicular “bioreactors” is that their metabolic reactions quickly run out of power in the absence of a system to regenerate ATP.7 One way to create an efficient ATP-regenerating system is to coreconstitute a fuel-driven proton pumping enzyme with an ATP synthase into nanovesicles.17,18 Racker and Stoeckenius first demonstrated the principle in 1974 when they used bacteriorhodopsin from Halobacter halobium, a light-driven proton pump, to power ATP synthesis in phospholipid vesicles, also called liposomes.19 In 2016, von Ballmoos et al. co-reconstituted both the quinol bo3 oxidase from Escherichia coli and cytochrome c oxidase from Rhodobacter sphaeroides with the F1F0 ATP synthase from E. coli (Ec-F1F0) into liposomes, to drive ATP synthesis using either ubiquinol or reduced cytochrome c as fuels.20 In 2017, Belevich et al. coreconstituted bacterial CI and Ec-F1F0 to study proton pumping by CI,21 but CI catalysis was coupled to recycling of soluble decyubiquinol by bo 3 oxidase in the bulk solution (separate from the proteoliposomes), and CI-driven ATP synthesis was not explored. Recently, in the first example of a microvesicle bioreactor that contains a nanovesicle system for ATP regeneration, liposomes containing bacteriorhodopsin from Halobacterium salinarum and F1F0 ATP synthase from Bacillus PS3 were incorporated inside giant unilamellar vesicles to power light-driven ATP synthesis coupled to intravesicular protein synthesis.22
An important requirement for integrating an ATP-regenerating system into a synthetic cell with complex metabolism and behavior is to build it into the cellular metabolic network, by connecting it to both upstream and downstream metabolic pathways. The system described above22 uses light to drive ATP synthesis as the basis for an artificial photosynthetic cell, but is otherwise metabolically isolated. Similarly, the arginine degradation pathway that has been exploited recently to synthesize ATP in a vesicular bioreactor by conversion of arginine to ornithine is otherwise isolated from standard biochemical energy-producing pathways.23 Here, we focus on the important metabolic redox couple NAD+/NADH. The reduction of NAD+ to NADH is used to oxidize nutrients in catabolic processes such as glycolysis, fermentation, the tricarboxylic acid cycle and the β-oxidation of fatty acids.24 Then, NADH oxidation drives formation of Δp in mitochondria and powers ATP synthesis through oxidative phosphorylation. Because NAD+/NADH is an essential substrate for so many different metabolic pathways and fuel sources, coupling NAD+ recycling to ATP regeneration is a major goal of synthetic biology.7,18,25
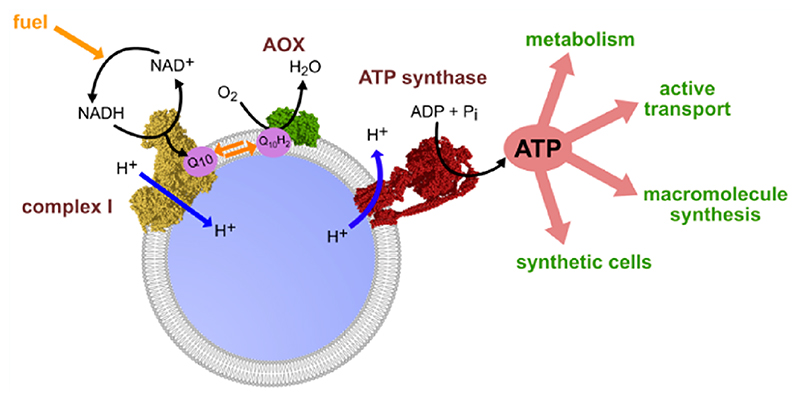
In this study, we have established methods to co-reconstitute mammalian mito-CI with Ec-F1F0 in ubiquinone-10-(Q10)-containing liposomes, together with the alternative oxidase from Trypanosoma brucei brucei (AOX), which uses oxygen to keep the ubiquinone pool oxidized (Figure 1A). The system is fueled by NADH:O2 oxidoreduction, and delivers both NAD+ and ATP regeneration. We provide a comprehensive characterization of the properties and capabilities of our system, then link it to both upstream and downstream pathways to demonstrate how it can be used in synthetic biology to drive key processes within artificial cells.
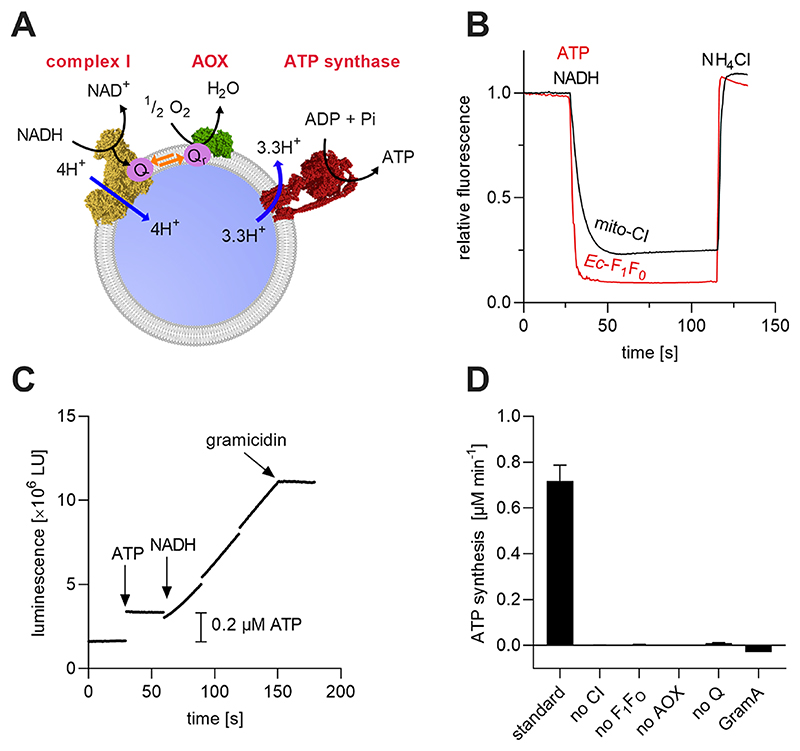
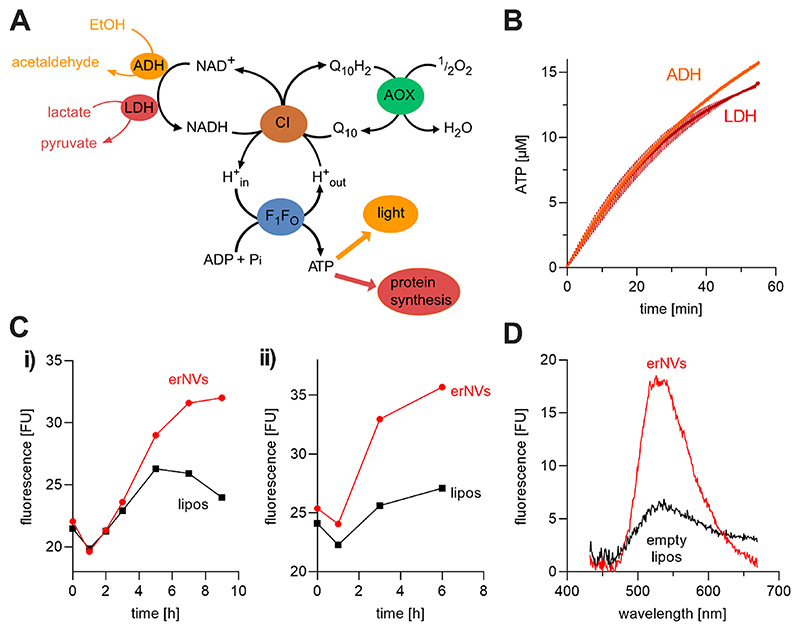
Figure 1. Co-reconstitution of mito-CI, Q10, AOX, and Ec-F1F0 into Q10-liposomes for the production of energy-regenerating nanovesicles (erNVs).
(A) Schematic illustration and reaction sequence of an erNV. CI oxidizes NADH to NAD+, reduces Q10, and pumps protons to form Δp; Q10H2 is reoxidized by AOX to sustain CI turnover, and the Δp is used by Ec-F1F0 to generate ATP. (B) ACMA fluorescence quenching assays. The formation of ΔpH in erNVs from either NADH oxidation by mito-CI or ATP hydrolysis by Ec-F1F0 was detected using the pH-sensitive dye ACMA. The system was equilibrated for 5 min after the addition of ACMA, before the addition of NADH/ATP. (C) ATP synthesis measurements. A typical ATP synthesis measurement on erNVs using the luciferin/luciferase-based luminescence assay. Once the background luminescence was established, a standard aliquot of 0.2 μM ATP was added as an internal standard for quantitation, and NADH-driven ATP synthesis was initiated by addition of 200 μM NADH. (D) Controls showing the specificity of catalysis. No ATP synthesis was detected when any of mito-CI, Ec-F1F0, Q10, or AOX were omitted from the erNVs or when 10 μg/mL of gramicidin A (GramA) were present. Data are mean averages with propagated error (SEM) values from triplicate technical replicates. Unless stated otherwise (panel D), erNVs were prepared using the standard protocol. See Materials and Methods for experimental details.
Production of Energy (ATP) Regenerating Nanovesicles
Ec-F1F0 and complex I from Bos taurus (mito-CI) have both independently been successfully reconstituted into liposomes previously, but by using different methods of reconstitution.20,26–28 Ec-F1F0 was reconstituted by a 1 h protocol involving partial solubilization of preformed liposomes with cholate, followed by removal of the detergent by size-exclusion chromatography on an inexpensive desalting column.20,26 In contrast, mito-CI is typically reconstituted by partial or full solubilization of preformed liposomes with octyl glucoside, followed by slow removal of the detergent by multiple additions of detergent-adsorbing beads (Biobeads), over a total time of up to 6 h.27–30 Clearly, the two protocols differ substantially, and a new, mutually compatible method is required for co-reconstitutions. The monotopic AOX was previously co-reconstituted with mito-CI and Q10 to produce proteoliposomes (PLs) capable of sustained NADH:O2 oxidoreduction,28 but more recently, AOX has been found to associate spontaneously with mature liposomes that already contain mito-CI and Q10.31 This latter approach was adopted here for simplicity.
We began by optimizing the more expedient reconstitution protocol developed for Ec-F1F0 for mito-CI, by mixing partially solubilized preformed liposomes with mito-CI, then using sizeexclusion chromatography on a PD10 column to remove the cholate (Figure S1A,B). The retention (amount retained in the proteoliposomes relative to the amount added at the start of the preparation) and preferred orientation of the mito-CI were lower than for the established Biobeads method (Table S1), but the differences were not substantial enough to convince us to revert to it. Switching to a synthetic lipid mixture of 18:1 cardiolipin (CL), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) and 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) gave 20−40% higher activities than using natural bovine−heart lipid extracts (Table S1). Finally, addition of increasing amounts of AOX to the assay buffer resulted in maximal specific activities of 21.4 μmol NADH min−1 (mg CI)−1, which is equal to a k cat of 357 NADH s−1 CI−1 at a ratio of 600 molecules AOX added per outward-facing CI (Figure S1C). Note that all the enzyme activities we report, as either turnover numbers or specific activities, are for the outwardfacing enzymes; they have been corrected for the protein (mito-CI or Ec-F1F0) concentration and orientation.
The optimized conditions for mito-CI reconstitution were then tested on co-reconstitutions with Ec-F1F0, while AOX was added afterward to result in CI−Q10−AOX−F1F0 PLs, here referred to as energy regenerating nanovesicles (erNVs) (Figure 1A). Successful incorporation of both proton-pumping enzymes was monitored using the fluorescent dye ACMA (9-amino-6-chloro-2-methoxyacridine), a semiquantitative measure for the formation of ΔpH across the liposomal membrane.27,32–34 Briefly, ACMA is added to the external phase of the erNVs and partitions across the membrane. In the presence of a ΔpH (from NADH oxidation by mito-CI or ATP hydrolysis by Ec-F1F0), the ACMA inside the erNV becomes protonated; more ACMA paritions across the membrane, accumulating in the lumen where its increased concentration causes dimerization and fluorescence quenching. The K+-ionophore, valinomycin, and KCl are included to collapse Δψ and ensure that the protom motive force is expressed only as ΔpH. Addition of either 1 mM ATP or 400 μM NADH to erNVs led to rapid ACMA fluorescence quenching, indicating the well-functioning proton-pumping activity of both enzymes and the ability of the membrane to maintain a ΔpH (Figure 1B). To confirm that both enzymes were coincorporated into the same liposomes, a coupled NADH:O2−ATP synthesis assay was performed using a luciferin/luciferase-based ATP detection system (Figure 1C). The background luminescence of a solution containing all reagents and erNVs was monitored for 30 s, and then 0.2 μM ATP was added as an internal standard. Catalysis was initiated by the addition of 200 μM NADH. The rapidly ensuing ATP synthesis was stopped by the addition of 10 μg mL−1 gramicidin, an ionophore and uncoupler, demonstrating the critical need for an intact membrane for functional coupling of the three enzymes. No ATP synthesis was observed in the absence of any of mito-CI, Ec-F1F0, AOX, or ubiquinone (Figure 1D). Together, the results demonstrate successful co-reconstitution of mito-CI, Q10, and Ec-F1F0 into the same well-coupled vesicle.
Optimization of ATP Synthesis
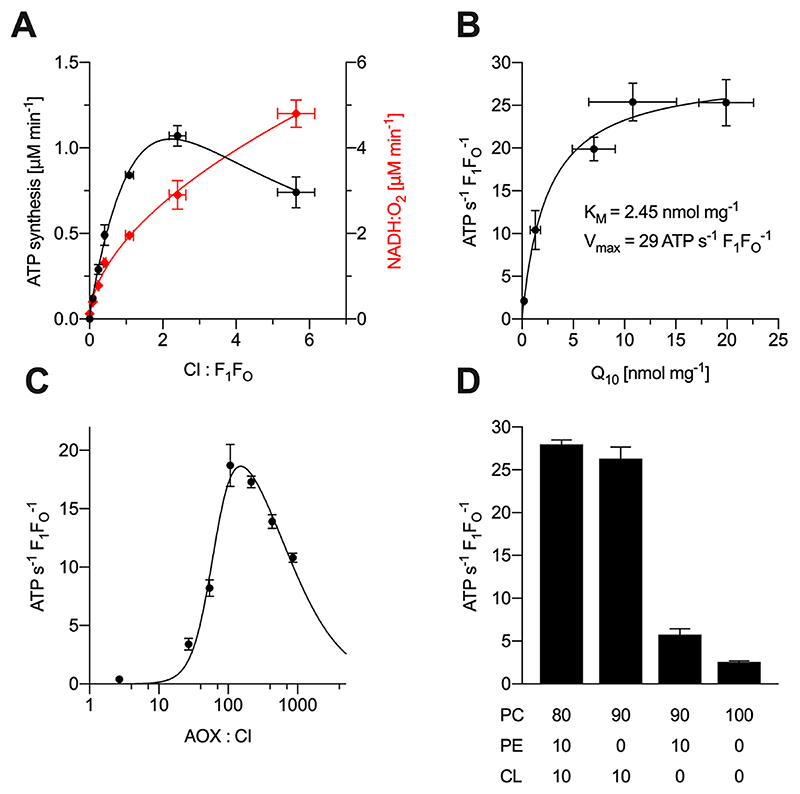
Next, different constituents of the PL system were titrated to maximize the rate of ATP synthesis (Figure 2). First, the amount of mito-CI was varied, while the concentrations of Ec-F1F0 and AOX added were held constant (Figure 2A). Maximum ATP synthesis was observed at a molar ratio of 2:1 CI:F1F0, while the rate of NADH oxidation increased continually: at low CI:F1F0, the amount of CI is insufficient to support substantial ATP synthesis; whereas at too high CI:F1F0, Ec-F1F0 may become insufficient or the membrane integrity might be compromised, increasing the proton leak. The specific NADH:O2 oxidoreduction activity of the erNVs at optimal ATP synthesis activity, measured at 20 °C in the assay solution used for ATP synthesis (see Materials and Methods for details) was 2.87 ± 0.1 μmol NADH min−1 (mg CI)−1 (n = 3) corresponding to a k cat of 48 NADH s−1 CI−1. This value is much smaller than the published k cat value for CI in CI−Q10−AOX PLs28 (measured at 32 °C) of 310 NADH s−1 CI−1 because the amount of AOX added was much lower here. Increasing the amount of AOX (to 510 AOX molecules for each outward-facing CI) and the temperature (to 32 °C) raised the specific activity to 20 ± 1 μmol NADH min−1 (mg CI)−1 (n = 3), equal to a k cat of 330 NADH s−1 CI−1 and matching the published value28 (Table S2). Figure 2B shows how NADH-coupled ATP synthesis depends on the concentration of Q10 in the vesicular membrane with the K M value of 2.45 nmol Q10 (mg phospholipids)−1 within the range of previously reported K M values for NADH:O2 oxidoreduction (0.48 and 3.94 nmol mg−1).28,35 The k cat value of 29 ATP s−1 for Ec-F1F0 is lower than values obtained for the bo 3 oxidase/Ec-F1F0 system (40−60 s−1)20 but higher than reported values for the photosynthetic systems (4−10 s−1).22,36 Varying the amount of AOX added to the CI−Q10−F1F0 PLs (Figure 2C) gave a similar response to CI−Q10 PLs (Figure S1C), with maximal ATP synthesis at 165 molecules of AOX for each outwardfacing CI. The apparent inhibitory effect of high AOX concentrations is ascribed to membrane uncoupling by the n-dodecyl β-D-maltoside (DDM) that was added with the AOX, disabling ATP synthesis. Finally, the lipid composition was varied because specific lipid requirements have been described for most respiratory complexes, including CI.37,38 Synthetic lipids containing two oleoyl groups (18:1, Δ9-cis) were chosen for their low phase-transition temperatures,39 and all combinations contained DOPC as their main scaffolding lipid. Maximal ATP synthesis activities were observed for erNVs containing 10%:10%:80% (w/w) of CL:DOPE:DOPC (Figure 2D). This combination of lipids mimics the mitochondrial inner membrane, which typically contains 10−15% CL and around 30% PE.40 The activity decreased to 94% of the maximal observed value with a 10:0:90 ratio, and to 20% for 0:10:90 erNVs (with respect to the 10:10:80 erNVs). Only residual activities (9%) were observed for DOPC-only liposomes. In contrast, maximal activities were observed in DOPC-only liposomes for co-reconstituted E. coli quinol bo 3 oxidase and Ec-F1F0, and substituting DOPC with DOPE or CL decreased the activity,41,42 suggesting that the effects observed here stem from CI. The dependence of mito-CI activity on CL has been reported previously,37,43 but systematic studies on the effects of the lipid composition for the enzyme reconstituted into proteoliposomes have not been described. Molecular simulations have suggested that CL binds dynamically to CI and induces conformational changes to modulate the accessibility of Q10,44 but no experimental support for this mechanism has been presented so far.
Figure 2. Variation of key components during the formation of erNVs. Rates are reported for catalysis only by outward facing Ec-F1F0.
Data are mean averages with propagated error (SEM) values from triplicate technical replicates. Unless denoted otherwise, erNVs were prepared using the standard protocol. The ratios in panels A and C are molar ratios. See Materials and Methods for experimental details. (A) Variation of the mito-CI concentration. Different amounts of mito-CI (0, 14, 28, 56, 112, 224 μg) were co-reconstituted with 29 μg Ec-F1F0 using the standard protocol, and rates of ATP synthesis (black) and NADH oxidation (red) determined. (B) Dependence of the rate of ATP synthesis on ubiquinone-10 concentration. Liposomes containing 0, 2.5, 10, 15, or 20 nmol Q10 (mg lipid)−1 were prepared and mito-CI and Ec-F1F0 were co-reconstituted using the standard protocol. The final Q10 concentrations shown were determined using HPLC coupled to an electrochemical detector as described in Materials and Methods. (C) Dependence of ATP synthase turnover on the concentration of AOX added. AOX was added in different amounts (0, 0.04, 0.36, 0.72, 1.44, 2.89, 5.77, and 11.55 μg mL−1) to 2.5 μL preformed PLs containing 143 μg mL−1 outward-facing mito-CI and 128 μg mL−1 outward-facing Ec-F1F0. (D) Dependence of the rate of ATP synthesis on lipid composition. Liposomes containing different phospholipid compositions (% (w/w); see inset table below figure) were prepared and mito-CI and Ec-F1F0 co-reconstituted using the standard protocol.
The final optimized protocol used for the preparation of erNVs is termed the standard protocol and is detailed in Materials and Methods.
Biophysical Characterization of the Energy-Regenerating Nanovesicles
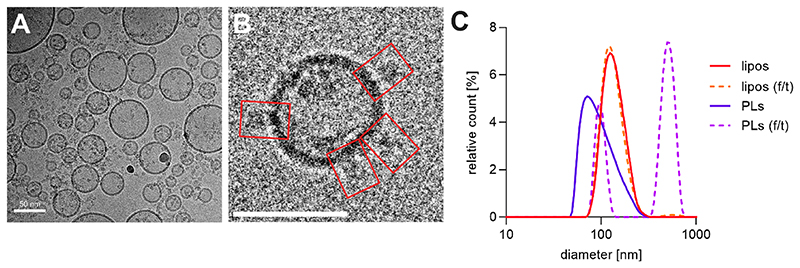
Next, the erNVs were characterized in detail for their physical properties. Figure 3A, B show example data recorded using cryogenic electron microscopy (cryoEM), confirming the unilamellar nature of the erNVs, and Figure S2 reveals their size distribution. The vesicles have diameters between 20 and 300 nm, with around 50% of them in the range of 40−80 nm, and 85% below 120 nm. However, cryoEM allows evaluation of only a small number of vesicles, and ice thickness limitations may lead to the preferential sampling of smaller vesicles. Therefore, we also performed dynamic light scattering (DLS) measurements. Co-reconstitution with mito-CI and Ec-F1F0 at a molar ratio of 2:1 almost halved the mode diameter of the preformed liposomes from 130 ± 10 to 78 ± 9 nm, and doubled their dispersity (from 0.07 ± 0.02 to 0.14 ± 0.03). The overall size distributions were similar for both DLS and cryoEM, although more large vesicles were detected in DLS (Figure S2), and the mode diameter of the volume-weighted distribution determined by DLS, 78 nm, was larger than the peak in the distribution determined by cryoEM (around 60 nm). The difference likely originates from a combination of the effects of the cryoEM ice thickness and the fact that DLS measures the Stokes radius (which includes the hydration shell).45 Freeze−thawing of erNVs led to a polymodal distribution with two distinct peaks, a minor peak at 97 ± 3 nm, and a major peak at 516 ± 12 nm, but interestingly, the same effects did not result from freeze−thawing empty liposomes, suggesting a protein-mediated aggregation process.
Figure 3. Lamellarity and size distribution of CI−Q10−F1F0 PLs.
(A) Selected micrograph ofco-reconstituted PLs recorded by cryoEM. (B) Single CI−Q10−F1F0 PL; red rectangles depict densities protruding from the membrane. Scale bar = 50 nm. (C) Dynamic light scattering measurements. The averaged volume-weighted size distributions from three preparations are shown. Preformed liposomes were formed by extrusion through a 100 nm polycarbonate membrane (red) and after a cycle of freezing the sample in liquid nitrogen and thawing at 20 °C (orange, dashed line); the same liposomes are shown after co-reconstitution of mito-CI and Ec−F1F0 (purple) and after freezing−thawing (fuchsia, dashed line). erNVs were prepared using the standard protocol. See Materials and Methods for experimental details.
A limitation of many of the ATP-generating vesicles reported so far is that the proton-pumping enzymes are reconstituted in mixed orientations: a particular challenge when several enzymes, all with their individual requirements, are being simultaneously reconstituted. Generally, membrane proteins with large hydrophilic domains insert with their hydrophilic domains predominantly on the outside of the liposome, as has been observed for both Ec-F1F0 46 and mito-CI from Yarrowia lipolytica.27 A similar selectivity has been reported for cytochrome c oxidase20 and bacteriorhodopsin,20,47 while quinol bo 3 oxidase was found to insert only with ~30% in the correct orientation for pumping protons into the liposomal lumen.48 We note that the orientation of bacteriorhodopsin can be optimized by changing the charge of the liposomal membrane or using fusion-domains to guide insertion.49,50 Here, to determine the protein orientations we measured NADH:APAD+ oxidoreductase activity from the flavin-site in mito-CI or ATP hydrolysis by Ec-F1F0 in the presence and absence of alamethicin,28,35 which allows NADH and ATP to access the vesicular lumen and also activate the inward-facing enzymes.51 We found that in erNVs both mito-CI and Ec-F1F0 inserted into the vesicular membrane with their hydrophilic domains predominantly on the outside, in the required orientation for coupled activity, 72 ± 5% for mito-CI and 73 ± 20% for Ec-F1F0 (Table S2). Importantly, the substrates for both CI and F1F0 are membrane impermeable, so any inward facing proteins are catalytically silent, providing an advantage over ATP-regenerating systems that rely on bacteriorhodopsin or on a proton pump with a membranesoluble substrate, such as the bo 3 oxidase.
Our co-reconstitution protocol retains a high proportion of the phospholipids (65 ± 13%), ATP synthase (69 ± 25%), and catalytic activities (NADH:O2, 72 ± 5%; ATP hydrolysis, 73 ± 20%) present at the start, but lower proportions of the ubiquinone (40 ± 11%) and mito-CI (31 ± 9%) (see Table S2). Generally, less activity was retained in co-reconstitutions than for individual reconstitutions (see Table S2), perhaps because of the complex detergent mixture that results from combining the purification buffers. In summary, each erNV was estimated to contain on average 12 molecules of correctly orientated mito-CI and 13 of Ec-F1F0, plus 970 molecules of Q10. Note that we used the volume-weighted mode diameter (78 nm) determined by DLS for these calculations; using the smaller value determined by cryoEM (~60 nm) would decrease the number of molecules per vesicle (see Materials and Methods for details of the calculations).
Demonstration and Applications in Synthetic Biology
For application of our erNVs in synthetic biology, an important parameter is their efficiency at coupling NADH oxidation to ATP production. To calculate efficiencies, the rate of NADH:O2 oxidoreduction (v NADH) was combined with the known stoichiometries of 4 H+ per NADH for mito-CI and 3 ATP per 10 H+ (1.2 ATP per NADH) to define the theoretical limit for Ec-F1F0 NADH-driven ATP synthesis (v ATP), for comparison with the measured value. The highest efficiencies (~50%, the percentage of protons used to produce ATP rather than wasted in leaking back across the membrane) were observed for CI:F1F0 molar ratios from 0.25:1 to 2:1 (Figure 4A) showing that the erNVs are able to maintain a high Δp without excessive proton leak, similar to vesicles prepared from the native membranes from mammalian or bacterial cells, with reported efficiencies of 20−40% for bovine submitochondrial particles and 60−80% for Paracoccus denitrif icans sub-bacterial vesicles.52 Interestingly, despite the erNVs showing excellent coupling between NADH oxidation and ATP synthesis, they show no respiratory control ratio for NADH oxidation (RCR, the ratio of the coupled and uncoupled rates53 = 1.01 ± 0.02, n = 3).
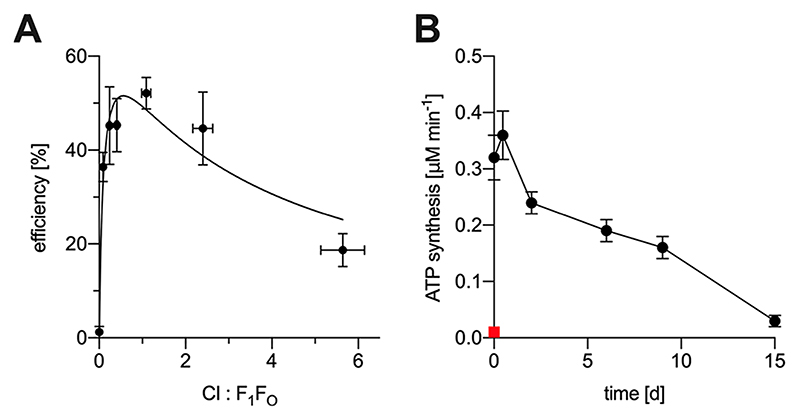
Figure 4. Efficiency and long-term storage of erNVs. (A) Efficiency of the system in using NADH to produce ATP, relative to the theoretical limit of perfect coupling.
Rates for NADH:O2 oxidoreduction (vNADH) and NADH-driven ATP synthesis (v ATP) (μM min−1) were used to calculate the efficiency (from the data in Figure 2A) by using the known stoichiometries of 4 H+ per NADH for mito-CI and 3 ATP per 10 H+ for Ec-F1F0 to define the theoretical limit of 1.2 ATP per NADH. (B) Effects of long-term storage of erNVs at 4 °C on their activity. NADH-driven ATP synthesis by a sample of CI−Q10−F1F0 PLs was measured over time, following the addition of AOX, as described in Materials and Methods, with the PLs stored at 4 °C between measurements. The red square represents a fresh sample that was frozen in liquid N2 and thawed at 20 °C. Data are mean averages with propagated error (SEM) values from triplicate technical replicates. erNVs were prepared using the standard protocol.
For applications in synthetic biology, it is important to know if the activity of the erNVs diminishes during storage. Generally, liposomes can be stored for several weeks at 4 °C without loss of structural integrity,54–57 and Figure 4B shows that NADH-coupled ATP synthesis by erNVs also persists well during storage at 4 °C for 15 days. No loss of activity was detected within the first 24 h, followed by a gradual decrease to about 50% of the initial activity after 9 days and 10% after 15 days. The longevity is remarkable, considering that both mito-CI and Ec-F1F0 are complex multiprotein enzymes, and it underpins the relevance of the system to synthetic biology applications. Freeze−thawing of the vesicles caused loss of activity, probably due to vesicle aggregation (Figure 3C), precluding long-term storage at −80 °C.
Finally, ATP synthesis by the co-reconstituted vesicles was driven by different fuels through NAD+ cycling, and used to drive luciferase-coupled light production or cell-free protein synthesis, in proof-of-principle demonstrations of how the system can be integrated into both upstream and downstream pathways and complex metabolic networks. In principle, erNVs can be coupled to any upstream NAD+-reducing reaction. Here, we coupled NAD+ reduction to either the oxidation of ethanol to acetaldehyde by alcohol dehydrogenase (ADH) or the oxidation of lactate to pyruvate by lactate dehydrogenase (LDH) (Figure 5A). The NADH produced was used to fuel our synthetic respiratory system, and NAD+ was returned to maintain the enzymatic cascade, thus recruiting NAD+/NADH cycling as an intermediate metabolic step.58,59 Downstream coupling requires the erNVs to be coupled to any ATP hydrolyzing pathway. Here, we coupled our system to ATP/ luciferase-driven light production (see above) and thus demonstrated continuous ATP production by our five-enzyme cascade over more than 60 min, with similar efficiencies for both ethanol/ADH and lactate/LDH (Figure 5B). This sustained ATP production further supports integration of our system into more complex and demanding downstream pathways, as required in synthetic cells. To demonstrate this capability, we used erNVs and lactate/LDH to fuel cell-free synthesis of a superecliptic pH-sensitive GFP variant (SEpHluorin).60 Prolonged SEpHluorin synthesis was observed when erNVs were added to the reaction mixture due to continuous ATP (and NAD+) regeneration, in comparison to control experiments with empty liposomes in which the ATP (starting concentration 1.2 mM) was depleted after around 6 h (Figure 5C and D).
Figure 5. erNVs couple NAD+-driven metabolism to ATP synthesis and protein synthesis. (A) Schematic pathway of a five-enzyme system used to convert fuel (EtOH or lactate) into energy (ATP).
Either ethanol (EtOH) and alcohol dehydrogenase (ADH) or lactate and lactate dehydrogenase (LDH) were used to feed the erNVs with NADH, and the generated ATP was used to produce light by the luciferin/luciferase system or to drive cell-free protein synthesis. (B) Continuous ATP production by the coupled system. ATP synthesis was initiated by addition of 15 μM NAD+ to a reaction mixture containing, 5 mM MgCl2, 50 μM ADP, 20 mM Tris−PO4 (pH 7.4), 2 μL mL−1 luciferin/luciferase reagent, 1.2 μg/mL AOX, and 3 μL mL−1 CI−Q10−F1F0 PLs. The reaction was initiated either by addition of 0.1% (v/v) (17.1 mM) ethanol and 3 U/mL ADH (from Saccharomyces cerevisiae, Sigma-Aldrich) or 10 mM lactate and 0.2 U/mL LDH (from bovine heart, Sigma-Aldrich), and luminescence was monitored continuously over 60 min. Averaged values and error bars from three technical replicates are shown. (C) Cell-free synthesis of SEpHLuorin. Cell-free protein synthesis was carried out using lactate as fuel, and the fluorescence emission of the reaction mixture was scanned after 0−9 h as described in Materials and Methods. Individual results of two independent biological replicates, (i) and (ii), are shown. The erNVs present in the experiment (red) were replaced with empty liposomes for the control (black), but the compositions of the solutions were otherwise identical. (D) Difference spectra. The change in the fluorescence spectrum after 9 h (relative to t = 0) for samples containing either erNVs (red) or empty, protein-free Q10−liposomes (black). erNVs were prepared using the standard protocol.
Conclusions
In recent years, several synthetic systems have been developed to mimic aspects of oxidative phosphorylation, but they have focused only on either NAD+ regeneration20 or ATP synthesis21 and have not captured the whole process. Our energy-regenerating nanovesicles are the first in vitro example of a synthetic respiratory chain that regenerates NAD+ and ATP, two of the key features of mitochondrial respiration and oxidative phosphorylation. Our system is applicable to future bioenergetics studies on the reversibility, mechanisms, and lipid dependencies of energy-conserving respiratory enzymes, and how their reactions are coupled across energy-transducing membranes. It is the first example of a synthetic system that can oxidize many different metabolic fuels to drive ATP regeneration by NADH/NAD+ cycling and further downstream processes such as cell-free protein expression. The nanosized proteoliposomes we describe are suitable for incorporation into microsized vesicles to drive complex, metabolically integrated synthetic cells.
Materials and Methods
Chemicals
All chemicals were purchased from Sigma-Aldrich unless stated otherwise.
Protein Purifications
Expression and purification of Ec-F1F0 using E. coli strain DK8 and the pBWU13-αHis plasmid,46 overexpression and purification of AOX from T. brucei brucei,31 and preparation of mito-CI (complex I from Bos taurus heart mitochondria)31 were performed as described previously. Concentrations of purified enzymes were determined using the BCA assay.61
Preparation of Liposomes
For the standard preparation protocol, a total of 5 mg synthetic lipids (stock: 25 mg mL−1 in chloroform) at a mass ratio of 8:1:1 DOPC:DOPE:CL were mixed with 50 nmol ubiquinone Q10 (stock: 6.8 mM in chloroform) in a 25 mL round bottomed flask. For optimization of the co-reconstitution protocol, the amount of Q10 and the lipid composition were varied as described in the figure legends. The chloroform was removed by swirling the flask under a stream of nitrogen, then placing it under vacuum in a desiccator for 2 h. The dried film was rehydrated by the addition of 1 mL reconstitution buffer (10 mM MOPS, pH 7.4, 50 mM KCl) to achieve 5 mg lipid mL−1, and then the flask was filled with nitrogen, sealed, and incubated for 1 h at room temperature. To form the liposomes, the lipid−Q10 mixture was resuspended by vigorous mixing (Vortex Genie 2, Scientific Instruments) followed by 11 extrusions through a 100 nm Nucleopore polycarbonate membrane (Whatman).
Protein Co-Reconstitutions To Produce erNVs
Purified mito-CI and Ec-F1F0 were co-reconstituted into preformed liposomes using the following standard protocol. Briefly, 250 μL Q10-containing liposomes (5 mg lipid mL−1) were partially solubilized by addition of 9 μL 20% (w/v) sodium cholate (0.6% final concentration); the solution was mixed by inversion and incubated for 10 min on ice. Then, 112 μg mito-CI (28 mg mL−1) and 29 μg Ec-F1F0 (2.2 mg mL−1) were added yielding a molar ratio of 2:1 CI:F1F0, followed by the additional reconstitution buffer required to give a final volume of 300 μL. To optimize the ratio of CI to F1F0, the amount of CI used for the reconstitution was adjusted as noted in the figure legend. The solution was mixed gently, incubated on ice for 15 min, and then passed over a PD10 desalting column (GEHealthcare) at 4 °C to remove the cholate. PLs were collected by centrifugation at 100,000 × g (4 °C for 45 min), resuspended in 200 μL ice-cold reconstitution buffer, and stored on ice until required.
Characterization of Proteoliposomes and erNVs
Total phospholipid contents were determined using an assay for the quantification of phosphate described previously.28 Total protein contents were determined using the amido black method to minimize interference by phospholipids.62,63 Total Q10 content was measured using a previously published method with modifications.35 1−2 μL of PL sample was added to 100 μL of HPLC-grade ethanol, vortexed for ~30 s, and then the Q10 was reduced by addition of 1 mM KBH4 from a 1 M aqueous stock solution. After 10 min, the precipitated protein was removed by a 2 min spin at 16,300 × g, and samples placed on dry ice until required. For analysis, 50 μL was injected onto a Nucleosil 100−5C18 column attached to an Agilent 1100 series HPLC system equipped with a Thermo Scientific Dionex Ultimate 3000RS Electrochemical Detector (ECD) and eluted in a mobile phase of 70% ethanol, 30% methanol, 0.7% NaClO4, and 0.07% HClO4 flowing at 800 μL min−1. Standard ECD potentials of +1000, −500, and +300 mV were used to detect the Q10H2. The first cell (+1000 mV) conditions the buffer, while the second and third cells allow quantification of Q10 and Q10H2. Because no Q10 is present (it has been reduced chemically prior to injection), we observe only the Q10H2 peak detected using the third cell. Mito-CI content and orientation were determined using the NADH:A-PAD+ oxidoreduction assay and by comparison to standard samples as described previously,28 in the presence and absence of 20 μg mL−1 alamethicin. Ec-F1F0 orientation was determined by measuring ATP hydrolysis as described previously,28 in the presence and absence of 20 μg mL−1 alamethicin. Ec-F1F0 contents were calculated by subtracting the mito-CI content from the total protein content.
Cryogenic Electron Microscopy and Dynamic Light Scattering
Aliquots of PLs (2.5 μL, 122 μg-CI mL−1) were applied to glow-discharged (90 s at 20 mA) Quantifoil R 2/2 300 mesh copper grids. The grids were plunge-frozen in liquid ethane using an FEI Vitrobot Mark IV at 4 °C in 100% humidity, with a blotting force of −6 for 6 s. Images were collected using a Talos Arctica cryo-TEM (transmission electron microscope) (Thermo Fisher Scientific). The micrographs were recorded using a Falcon III detector (Thermo Fisher Scientific) at a nominal magnification of 73,000× in the linear mode with the EPU software.
For DLS measurements, the vesicle size distribution of 40 μL of a standard CI−Q10−F1F0 PL sample was measured in Brand UV cuvettes using a Zetasizer Nano S (Malvern Instruments Ltd.). Technical triplicates were each measured 20 times. The intensity-weighted distributions were converted into volume-weighted distributions to account for over-estimation of large particles,45 using the Zetasizer Software (ver. 7.13).
Estimation of the Number of Proteins and Q10 per erNV
The average numbers of Q10 and protein molecules in each erNV were estimated by calculating the membrane volume of a typical erNV using the volume-weighted mode vesicle diameter (78 nm, from DLS measurements), a membrane thickness of 5 nm, and the published volume of a DOPC molecule (0.993 μL mg−1 or 1296 Å3 per molecule at 22 °C).63 The calculation was simplified by using DOPC as the only membrane phospholipid, neglecting the volume displacement caused by Q10, mito-CI and Ec-F1F0, and assuming a spherical vesicle geometry and homogeneous protein reconstitution. The number of erNVs present was calculated by dividing the total lipid volume (calculated from the measured phospholipid concentration) by the lipid volume per erNV. Finally, the copy numbers of Q10, mito-CI, and Ec-F1F0 per erNV were calculated by dividing the total numbers of retained Q10, mito-CI, and Ec-F1F0 by the number of erNVs (see Table S2 for average retention values).
Enzymatic Activity Assays
All spectrophotometric assays were carried out in reconstitution buffer (10 mM MOPS, pH 7.4, 50 mM KCl) at 32 °C, using a SpectraMax plus 348 96-well plate reader (Molecular Devices) and either a 96-well cell culture plate (Corning Costar) or a quartz cuvette. Rates of NADH:O2 oxidoreduction were measured at 340 and 380 nm (ε 340−380= 4.81 mM−1 cm−1) with 200 μM NADH, 10 μg mL−1 AOX, and PLs at 2 μg outward-facing CI mL−1. Fluorescence quench assays were conducted by preincubating CI−Q10-F1F0 PLs at 3 μg outward facing CI mL−1 with 1 μM 9-amino-6-chloro-2-methoxyacridine (ACMA), 10 μg-AOX mL−1, and 65 nM valinomycin, to convert Δψ to ΔpH, in 1.5 mL reconstitution buffer at 32 °C in a 1.5 mL quartz cuvette. Fluoresence quenches were initiated by addition of either 1 mM Mg-ATP (for Ec-F1F0) or 400 μM NADH (for mito-CI) and monitored at 32 °C using an RF-5301PC Spectrofluorometer (Shimadzu Europe) with excitation at 410 nm and emission at 480 nm. ΔpH was released by the addition of 25 mM NH4 Cl. ATP synthesis was quantified at 20 °C by the luciferin/luciferase system using a Glomax 20/20 luminometer (Promega) in buffer containing 20 mM Tris-PO4 (pH 7.4), 5 mM MgCl2, 50 μM ADP, 20 μL mL−1 luciferase reagent (ATP Bioluminescence Assay Kit CLS-II, Roche), 1.2 μg mL−1 of AOX, and 3 μL mL−1 of CI−Q10−F1F0 PLs. Before measurement, the background luminescence was measured for 30 s, and then the luminescence of 0.2 μM ATP was determined as an internal standard. The amounts of ATP generated were confirmed to remain within the linear detection range of the CLS-II system as described previously.20 ATP synthesis was initiated by addition of 200 μM NADH, monitored continuously for at least 2 min, and stopped by addition of 10 μg mL−1 gramicidin A. Only linear rates of catalysis were used for calculations. The Michaelis-Menten equation was used to calculate V max and K M using Prism 8.0 (GraphPad Software Inc.). Enzyme activities are reported as turnover numbers or specific activities for the outward-facing enzymes; they have been calculated from the measured CI or ATP synthase concentration and corrected for protein orientation.
Cell-Free Protein Synthesis
SEpHluorin (synthesized by GENEWIZ) was cloned into the AOX expression plasmid (a pET15-b derivative) by using NdeI and BamHI to replace the aox gene.35,60 Cell-free protein synthesis was performed according to the manufacturer’s protocol (Cube Biotech) in 0.5 mL of pH 8.0 solution containing 175 μL cell-free E. coli lysate (Cube Biotech), 30 μL 440 ng μL−1 SEpHluorin plasmid, 0.5 mg mL−1 tRNAs from E. coli strain MRE 600 (Roche), 20 U mL−1 bovine heart lactate dehydrogenase, 0.3 U μL−1 RiboLock RNase inhibitor (Thermo Fisher Scientific), 10 μg mL−1 AOX, 20 μL CI−Q10−F1F0 PLs containing 370 μg mL−1 CI (outward facing), 100 mM HEPES, 110 mM K+−acetate, 20 mM lactate, 20 mM Li+ K+−acetyl phosphate, 16 mM Mg2+−acetate, 2% (w/v) PEG8000, 2 mM DTT, 0.8 mM EDTA, 1.5 mM of each of arginine, aspartate, cysteine, methionine, glutamate, and tryptophan, 0.5 mM of each of alanine, asparagine, glutamine, glycine, histidine, isoleucine, leucine, phenylalanine, proline, lysine, serine, threonine, tyrosine, and valine, 1.2 mM ATP, 0.8 mM of each of CTP, GTP, UTP, 0.1 mg mL−1 Ca2+−folinic acid, 75 μM NAD+, and 1× cOmplete EDTA-free protease inhibitor cocktail (Roche). As a control, PLs were replaced with empty, protein-free Q10−liposomes. The solution was dialyzed using a 0.5 mL Slide-A-Lyzer MINI Dialysis Device with a 10 MWCO (Thermo Fisher Scientific) at 30 °C against 8 mL of reaction buffer containing an additional 10 mM Tris-acetate, 60 mM K+-acetate, 50 mM lactic acid, 14 mM Mg2+−acetate, 0.5 mM DTT, and 0.25 mM of each amino acid at pH 8.2. After 0, 1, 2, 3, 5, 7, and 9 h, 20 μL aliquots were frozen in liquid N2. For fluorescence measurements, samples were thawed, diluted in 180 μL 10 mM MOPS (pH 7.4) and 50 mM KCl, centrifuged at 12,000 × g (20 °C for 5 min), and their fluorescence spectra recorded using a Falcon 96-well black/clear flat-bottomed TC-treated imaging microplate and a CLARIOstar (BMG Labtech) multimode plate reader. Fluorescence was excited at 370 nm (bandwidth: 16 nm) and emission scanned from 432−670 nm (bandwidth: 14 nm) using a gain of 1000, 20 flashes per well, and a focal height of 7.5 mm.
Abbreviations
- ACMA
9-amino-6-chloro-2-methoxyacridine
- ADH
alcohol dehydrogenase
- ATP
adenosine triphosphate
- AOX
alternative oxidase
- CL
18:1 cardiolipin
- cryoEM
electron cryomicroscopy
- DDM
n-dodecyl β-D-maltoside
- DOPC
1,2-dioleoyl-sn-glycero-3-phosphocholine
- DOPE
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine
- Ec-F1F0
E. coli ATP synthase
- erNVs
energy-regenerating nanovesicles
- mito-CI
mitochondrial (bovine) complex I
- NADH
nicotinamide adenine dinucleotide
- PL
proteoliposome
- Q10
ubiquinone Q10
Supplementary Material
Acknowledgments
We thank S. Palmer (MBU) for assistance with sample preparation, C. von Ballmoos (University of Bern) for helpful suggestions with the purification of E. coli ATP synthase, and S. Hardwick for assistance with CryoEM.
Funding
This work was supported by the Medical Research Council (MC_UU_00015/2 to J.H.) and by the Swiss National Science Foundation (P2BEP3_181897 to O.B.).
Footnotes
Author Contributions
O.B., J.G.F., and J.H. designed experiments; O.B. and J.G.F. performed experiments; Z.Y. performed cryoEM experiments and analyzed the corresponding data; O.B. and J.G.F. analyzed data; O.B., J.G.F., and J.H. wrote the manuscript; J.H. directed the research; all authors have approved the final version of the manuscript.
Notes
The authors declare no competing financial interest.
Contributor Information
Olivier Biner, MRC Mitochondrial Biology Unit, University of Cambridge, Cambridge CB2 0XY, United Kingdom.
Justin G. Fedor, MRC Mitochondrial Biology Unit, University of Cambridge, Cambridge CB2 0XY, United Kingdom
Zhan Yin, MRC Mitochondrial Biology Unit, University of Cambridge, Cambridge CB2 0XY, United Kingdom.
Judy Hirst, MRC Mitochondrial Biology Unit, University of Cambridge, Cambridge CB2 0XY, United Kingdom.
References
- (1).Ding Y, Wu F, Tan C. Synthetic biology: A bridge between artificial and natural cells. Life. 2014;4:1092–1116. doi: 10.3390/life4041092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Brea RJ, Hardy MD, Devaraj NK. Towards self-assembled hybrid artificial cells: Novel bottom-up approaches to functional synthetic membranes. Chem - Eur J. 2015;21:12564–12570. doi: 10.1002/chem.201501229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Buddingh’ BC, van Hest JCM. Artificial cells: Synthetic compartments with life-like functionality and adaptivity. Acc Chem Res. 2017;50:769–777. doi: 10.1021/acs.accounts.6b00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Blain JC, Szostak JW. Progress toward synthetic cells. Annu Rev Biochem. 2014;83:615–640. doi: 10.1146/annurev-biochem-080411-124036. [DOI] [PubMed] [Google Scholar]
- (5).Spoelstra WK, Deshpande S, Dekker C. Tailoring the appearance: What will synthetic cells look like? Curr Opin Biotechnol. 2018;51:47–56. doi: 10.1016/j.copbio.2017.11.005. [DOI] [PubMed] [Google Scholar]
- (6).Miller DM, Gulbis JM. Engineering protocells: Prospects for self-assembly and nanoscale production-lines. Life. 2015;5:1019–1053. doi: 10.3390/life5021019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Sikkema HR, Gaastra BF, Pols T, Poolman B. Cell fuelling and metabolic energy conservation in synthetic cells. ChemBioChem. 2019;20:2581–2592. doi: 10.1002/cbic.201900398. [DOI] [PubMed] [Google Scholar]
- (8).Cantó C, Menzies KJ, Auwerx J. NAD+ metabolism and the control of energy homeostasis: A balancing act between mitochondria and the nucleus. Cell Metab. 2015;22:31–53. doi: 10.1016/j.cmet.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Houtkooper RH, Cantó C, Wanders RJ, Auwerx J. The secret life of NAD+ An old metabolite controlling new metabolic signaling pathways. Endocr Rev. 2010;31:194–223. doi: 10.1210/er.2009-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Hirst J. Mitochondrial complex I. Annu Rev Biochem. 2013;82:551–575. doi: 10.1146/annurev-biochem-070511-103700. [DOI] [PubMed] [Google Scholar]
- (11).von Ballmoos C, Wiedenmann A, Dimroth P. Essentials for ATP synthesis by F1F0 ATP synthases. Annu Rev Biochem. 2009;78:649–672. doi: 10.1146/annurev.biochem.78.081307.104803. [DOI] [PubMed] [Google Scholar]
- (12).Walker JE. The ATP synthase: The understood, the uncertain and the unknown. Biochem Soc Trans. 2013;41:1–16. doi: 10.1042/BST20110773. [DOI] [PubMed] [Google Scholar]
- (13).Kuhlbrandt W. Structure and mechanisms of F-type ATP synthases. Annu Rev Biochem. 2019;88:515–549. doi: 10.1146/annurev-biochem-013118-110903. [DOI] [PubMed] [Google Scholar]
- (14).Soga H, Fujii S, Yomo T, Kato Y, Watanabe H, Matsuura T. In vitro membrane protein synthesis inside cell-sized vesicles reveals the dependence of membrane protein integration on vesicle volume. ACS Synth Biol. 2014;3:372–379. doi: 10.1021/sb400094c. [DOI] [PubMed] [Google Scholar]
- (15).Jia H, Heymann M, Bernhard F, Schwille P, Kai L. Cell-free protein synthesis in micro compartments: Building a minimal cell from biobricks. New Biotechnol. 2017;39:199–205. doi: 10.1016/j.nbt.2017.06.014. [DOI] [PubMed] [Google Scholar]
- (16).Elani Y, Law RV, Ces O. Protein synthesis in artificial cells: Using compartmentalisation for spatial organisation in vesicle bioreactors. Phys Chem Chem Phys. 2015;17:15534–15537. doi: 10.1039/c4cp05933f. [DOI] [PubMed] [Google Scholar]
- (17).Biner O, Schick T, Ganguin AA, von Ballmoos C. Towards a synthetic mitochondrion. Chimia. 2018;72:291–296. doi: 10.2533/chimia.2018.291. [DOI] [PubMed] [Google Scholar]
- (18).Otrin L, Kleineberg C, Caire da Silva L, Landfester K, Ivanov I, Wang M, Bednarz C, Sundmacher K, Vidakovic-Koćh T. Artificial organelles for energy regeneration. Adv Biosys. 2019;3:1800323. doi: 10.1002/adbi.201800323. [DOI] [PubMed] [Google Scholar]
- (19).Racker E, Stoeckenius W. Reconstitution of purple membrane vesicles catalyzing light-driven proton uptake and adenosine triphosphate formation. J Biol Chem. 1974;249:662–663. [PubMed] [Google Scholar]
- (20).von Ballmoos C, Biner O, Nilsson T, Brzezinski P. Mimicking respiratory phosphorylation using purified enzymes. Biochim Biophys Acta, Bioenerg. 2016;1857:321–331. doi: 10.1016/j.bbabio.2015.12.007. [DOI] [PubMed] [Google Scholar]
- (21).Belevich N, von Ballmoos C, Verkhovskaya M. Activation of proton translocation by respiratory complex I. Biochemistry. 2017;56:5691–5697. doi: 10.1021/acs.biochem.7b00727. [DOI] [PubMed] [Google Scholar]
- (22).Berhanu S, Ueda T, Kuruma Y. Artificial photosynthetic cell producing energy for protein synthesis. Nat Commun. 2019;10:1325. doi: 10.1038/s41467-019-09147-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Pols T, Sikkema HR, Gaastra BF, Frallicciardi J, Śmigiel WM, Singh S, Poolman B. A synthetic metabolic network for physicochemical homeostasis. Nat Commun. 2019;10:4239. doi: 10.1038/s41467-019-12287-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Casem ML. In: Case Studies in Cell Biology. Casem ML, editor. Academic Press; Boston: 2016. Chapter 11 - Cell metabolism; pp. 263–281. [Google Scholar]
- (25).Schwille P, Spatz J, Landfester K, Bodenschatz E, Herminghaus S, Sourjik V, Erb TJ, Bastiaens P, Lipowsky R, Hyman A, et al. MaxSynBio: Avenues towards creating cells from the bottom up. Angew Chem, Int Ed. 2018;57:13382–13392. doi: 10.1002/anie.201802288. [DOI] [PubMed] [Google Scholar]
- (26).Ishmukhametov RR, Galkin MA, Vik SB. Ultrafast purification and reconstitution of His-tagged cysteine-less Escherichia coli F1F0 ATP synthase. Biochim Biophys Acta, Bioenerg. 2005;1706:110–116. doi: 10.1016/j.bbabio.2004.09.012. [DOI] [PubMed] [Google Scholar]
- (27).Dröse S, Galkin A, Brandt U. Proton pumping by complex I (NADH:ubiquinone oxidoreductase) from Yarrowia lipolytica reconstituted into proteoliposomes. Biochim Biophys Acta, Bioenerg. 2005;1710:87–95. doi: 10.1016/j.bbabio.2005.10.001. [DOI] [PubMed] [Google Scholar]
- (28).Jones AJ, Blaza JN, Bridges HR, May B, Moore AL, Hirst J. A self-assembled respiratory chain that catalyzes NADH oxidation by ubiquinone-10 cycling between complex I and the alternative oxidase. Angew Chem, Int Ed. 2016;55:728–731. doi: 10.1002/anie.201507332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Roberts PG, Hirst J. The deactive form of respiratory complex I from mammalian mitochondria is a Na+/H+ antiporter. J Biol Chem. 2012;287:34743–34751. doi: 10.1074/jbc.M112.384560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Dröse S, Krack S, Sokolova L, Zwicker K, Barth HD, Morgner N, Heide H, Steger M, Nübel E, Zickermann V, Kerscher S, et al. Functional dissection of the proton pumping modules of mitochondrial complex I. PLoS Biol. 2011;9:e1001128. doi: 10.1371/journal.pbio.1001128. No. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Fedor JG, Hirst J. Mitochondrial super-complexes do not enhance catalysis by quinone channeling. Cell Metab. 2018;28:525–531. doi: 10.1016/j.cmet.2018.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Wielandt AG, Palmgren MG, Fuglsang AT, Gunther-Pomorski T, Justesen BH. Measuring H+ pumping and membrane potential formation in sealed membrane vesicle systems. Methods Mol Biol. 2016;1377:171–180. doi: 10.1007/978-1-4939-3179-8_17. [DOI] [PubMed] [Google Scholar]
- (33).Dufour JP, Goffeau A, Tsong TY. Active proton uptake in lipid vesicles reconstituted with the purified yeast plasma membrane ATPase. Fluorescence quenching of 9-amino-6-chloro-2-methoxyacridine. J Biol Chem. 1982;257:9365–9371. [PubMed] [Google Scholar]
- (34).Palmgren MG. Acridine orange as a probe for measuring pH gradients across membranes: Mechanism and limitations. Anal Biochem. 1991;192:316–321. doi: 10.1016/0003-2697(91)90542-2. [DOI] [PubMed] [Google Scholar]
- (35).Fedor JG, Jones AJY, Di Luca A, Kaila VRI, Hirst J. Correlating kinetic and structural data on ubiquinone binding and reduction by respiratory complex I. Proc Natl Acad Sci U S A. 2017;114:12737–12742. doi: 10.1073/pnas.1714074114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Lee KY, Park SJ, Lee KA, Kim SH, Kim H, Meroz Y, Mahadevan L, Jung KH, Ahn TK, Parker KK, et al. Photosynthetic artificial organelles sustain and control ATP-dependent reactions in a protocellular system. Nat Biotechnol. 2018;36:530–535. doi: 10.1038/nbt.4140. [DOI] [PubMed] [Google Scholar]
- (37).Sharpley MS, Shannon RJ, Draghi F, Hirst J. Interactions between phospholipids and NADH:ubiquinone oxidoreductase (complex I) from bovine mitochondria. Biochemistry. 2006;45:241–248. doi: 10.1021/bi051809x. [DOI] [PubMed] [Google Scholar]
- (38).Paradies G, Paradies V, De Benedictis V, Ruggiero FM, Petrosillo G. Functional role of cardiolipin in mitochondrial bioenergetics. Biochim Biophys Acta, Bioenerg. 2014;1837:408–417. doi: 10.1016/j.bbabio.2013.10.006. [DOI] [PubMed] [Google Scholar]
- (39).Caffrey M, Hogan J. LIPIDAT: A database of lipid phase transition temperatures and enthalpy changes. Chem Phys Lipids. 1992;61:100–109. doi: 10.1016/0009-3084(92)90002-7. [DOI] [PubMed] [Google Scholar]
- (40).Horvath SE, Daum G. Lipids of mitochondria. Prog Lipid Res. 2013;52:590–614. doi: 10.1016/j.plipres.2013.07.002. [DOI] [PubMed] [Google Scholar]
- (41).Nilsson T, Lundin CR, Nordlund G, Adelroth P, von Ballmoos C, Brzezinski P. Lipid-mediated protein-protein interactions modulate respiration-driven ATP synthesis. Sci Rep. 2016;6:24113. doi: 10.1038/srep24113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Sjoholm J, Bergstrand J, Nilsson T, Sachl R, von Ballmoos C, Widengren J, Brzezinski P. The lateral distance between a proton pump and ATP synthase determines the ATP-synthesis rate. Sci Rep. 2017;7:2926. doi: 10.1038/s41598-017-02836-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Dröse S, Zwicker K, Brandt U. Full recovery of the NADH:ubiquinone activity of complex I (NADH:ubiquinone oxidoreductase) from Yarrowia lipolytica by the addition of phospholipids. Biochim Biophys Acta, Bioenerg. 2002;1556:65–72. doi: 10.1016/s0005-2728(02)00307-9. [DOI] [PubMed] [Google Scholar]
- (44).Jussupow A, Di Luca A, Kaila VRI. How cardiolipin modulates the dynamics of respiratory complex I. Sci Adv. 2019;5:eaav1850. doi: 10.1126/sciadv.aav1850. No. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Stetefeld J, McKenna SA, Patel TR. Dynamic Light Scattering: a Practical Guide and Applications in Biomedical Sciences. Biophys Rev. 2016;8:409–427. doi: 10.1007/s12551-016-0218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Wiedenmann A, Dimroth P, von Ballmoos C. Δψ and ΔpH are equivalent driving forces for proton transport through isolated F0 complexes of ATP synthases. Biochim Biophys Acta, Bioenerg. 2008;1777:1301–1310. doi: 10.1016/j.bbabio.2008.06.008. [DOI] [PubMed] [Google Scholar]
- (47).Rigaud JL, Paternostre MT, Bluzat A. Mechanisms of membrane protein insertion into liposomes during reconstitution procedures involving the use of detergents. 2. Incorporation of the light-driven proton pump bacteriorhodopsin. Biochemistry. 1988;27:2677–2688. doi: 10.1021/bi00408a007. [DOI] [PubMed] [Google Scholar]
- (48).Berg J, Block S, Höök F, Brzezinski P. Single proteoliposomes with E. coli quinol oxidase: Proton pumping without transmembrane leaks. Isr J Chem. 2017;57:437–445. [Google Scholar]
- (49).Ritzmann N, Thoma J, Hirschi S, Kalbermatter D, Fotiadis D, Muller DJ. Fusion domains guide the oriented insertion of light-driven proton pumps into liposomes. Biophys J. 2017;113:1181–1186. doi: 10.1016/j.bpj.2017.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Tunuguntla R, Bangar M, Kim K, Stroeve P, Ajo-Franklin CM, Noy A. Lipid bilayer composition can influence the orientation of proteorhodopsin in artificial membranes. Biophys J. 2013;105:1388–1396. doi: 10.1016/j.bpj.2013.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Gostimskaya IS, Grivennikova VG, Zharova TV, Bakeeva LE, Vinogradov AD. In situ assay of the intramitochondrial enzymes: Use of alamethicin for permeabilization of mitochondria. Anal Biochem. 2003;313:46–52. doi: 10.1016/s0003-2697(02)00534-1. [DOI] [PubMed] [Google Scholar]
- (52).Jones AJ, Blaza JN, Varghese F, Hirst J. Respiratory complex I in Bos taurus and Paracoccus denitrificans pumps four protons across the membrane for every NADH oxidized. J Biol Chem. 2017;292:4987–4995. doi: 10.1074/jbc.M116.771899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Muppidi K, Pumerantz AS, Wang J, Betageri G. Development and stability studies of novel liposomal vancomycin formulations. ISRN Pharm. 2012;2012:636743. doi: 10.5402/2012/636743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Grit M, Crommelin DJ. Chemical stability of liposomes: Implications for their physical stability. Chem Phys Lipids. 1993;64:3–18. doi: 10.1016/0009-3084(93)90053-6. [DOI] [PubMed] [Google Scholar]
- (55).Armengol X, Estelrich J. Physical stability of different liposome compositions obtained by extrusion method. J Microencapsulation. 1995;12:525–535. doi: 10.3109/02652049509006783. [DOI] [PubMed] [Google Scholar]
- (56).Rideau E, Dimova R, Schwille P, Wurm FR, Landfester K. Liposomes and Polymersomes: A Comparative Review Towards Cell Mimicking. Chem Soc Rev. 2018;47:8572–8610. doi: 10.1039/c8cs00162f. [DOI] [PubMed] [Google Scholar]
- (57).Edenberg HJ, McClintick JN. Alcohol dehydrogenases, aldehyde dehydrogenases, and alcohol use disorders: A critical review. Alcohol: Clin Exp Res. 2018;42:2281–2297. doi: 10.1111/acer.13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Leskovac V, Trivic S, Pericin D. The three zinc-containing alcohol dehydrogenases from baker’s yeast, Saccharomyces cerevisiae . FEMS Yeast Res. 2002;2:481–494. doi: 10.1111/j.1567-1364.2002.tb00116.x. [DOI] [PubMed] [Google Scholar]
- (59).Shen Y, Rosendale M, Campbell RE, Perrais D. pHuji, a pH-sensitive red fluorescent protein for imaging of exo- and endocytosis. J Cell Biol. 2014;207:419–432. doi: 10.1083/jcb.201404107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- (61).Kaplan RS, Pedersen PL. Determination of microgram quantities of protein in the presence of milligram levels of lipid with amido black. Anal Biochem. 1985;150:97–104. doi: 10.1016/0003-2697(85)90445-2. [DOI] [PubMed] [Google Scholar]
- (62).Kessler RJ, Fanestil DD. Interference by lipids in the determination of protein using bicinchoninic acid. Anal Biochem. 1986;159:138–142. doi: 10.1016/0003-2697(86)90318-0. [DOI] [PubMed] [Google Scholar]
- (63).Nagle JF, Tristram-Nagle S. Structure of lipid bilayers. Biochim Biophys Acta, Rev Biomembr. 2000;1469:159–195. doi: 10.1016/s0304-4157(00)00016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.