Summary
Drosophila melanogaster is an established model for neuroscience research with relevance in biology and medicine. Until recently, research on the Drosophila brain was hindered by the lack of a complete and uniform nomenclature. Recognizing this, Ito et al. (2014) produced an authoritative nomenclature for the adult insect brain, using Drosophila as the reference. Here, we extend this nomenclature to the adult thoracic and abdominal neuromeres, the ventral nerve cord (VNC), to provide an anatomical description of this major component of the Drosophila nervous system. The VNC is the locus for the reception and integration of sensory information and involved in generating most of the locomotor actions that underlie fly behaviors. The aim is to create a nomenclature, definitions, and spatial boundaries for the Drosophila VNC that are consistent with other insects. The work establishes an anatomical framework that provides a powerful tool for analyzing the functional organization of the VNC.
Introduction
Insects, and Drosophila melanogaster in particular, have made huge contributions to neuroscience research (Bellen et al., 2010). The powerful genetic tools and high-resolution neuroanatomy available in flies (Jenett et al., 2012; Scheffer and Meinertzhagen, 2019) and the large number of research groups working on this model will ensure that the fly will remain a powerful tool for analyzing the function and development of complex nervous systems. Here we focus on the organization of an often-overlooked part of the Drosophila nervous system, the ventral nerve cord (VNC). The VNC is the insect analog of the vertebrate spinal cord and a significant part of the fly nervous system. The VNC is the locus for the reception and integration of sensory information and is involved in generating most of the locomotor actions that underlie fly behaviors such as walking (Bidaye et al., 2014; Mamiya et al., 2018; Mendes et al., 2013; Tuthill and Wilson, 2016; Wosnitza et al., 2013), grooming (Seeds et al., 2014), jumping (Card and Dickinson, 2008), flying (Dickinson and Muijres, 2016), courtship (Clyne and Miesenböck, 2008), and copulation (Crickmore and Vosshall, 2013; Pavlou et al., 2016). The VNC is, however, not a passive executive center receiving descending signals from the brain; it also sends significant major ascending projections to it (Tsubouchi et al., 2017). While the VNC in Drosophila is a complex fusion of all of the sub-gnathal neuromeres, it has a relatively simple and highly ordered structure. From external morphology, it is possible to recognize its constituent segmental neuromeres, the larger of which are the three thoracic ones, with the smaller, merged abdominal neuromeres protruding from the posterior end (Figure 1).
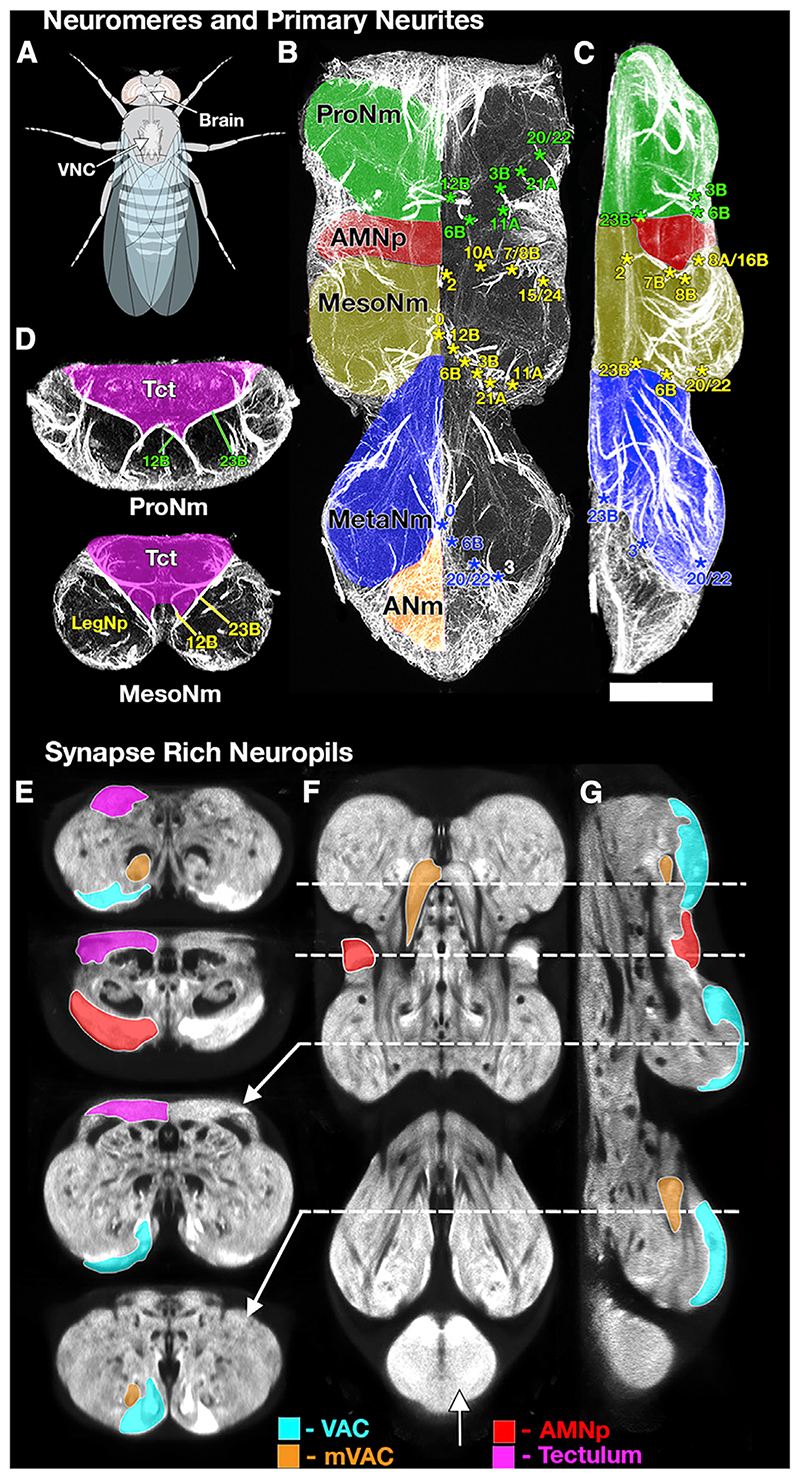
Figure 1. Selected Sections through an Adult VNC Illustrating the Tools Used to Define the Major Structures of the VNC.
(A) Schematic of Drosophila illustrating the position of the VNC with respect to the body and brain.
(B–D) Neuroglian immunostaining showing neuromeres and Primary Neurite bundles in horizontal (B), lateral (C), and transverse (D) sections to reveal the tracts of the primary neurites of the postembryonic neuronal lineages. The pattern of labeled pathways is highly stereotyped; each pathway corresponds to the primary neurites of neurons derived from a single neuroblast. These tracts provide a robust basis for identifying the key structures of the VNC such as the following: (B and C) the neuromere boundaries (ProNm [green], MesoNm [yellow], MetaNm [blue], and ANm [red]) and (D) the tectulum (magenta— Tct). The numbers refer to specific hemilineage primary neurite bundles, with the color indicating their neuromere of origin.
(E–G) Brp-SNAP labeling (Bogovic et al., 2019) revealing the fine structure of the neuropil shown in transverse (E), horizontal (F), and lateral (G) sections. The bruchpilot (Brp) staining reveals characteristic regions of neuropil with high-density staining indicating synapse-rich neuropils. These synapse-rich neuropils can be used to define and segment specific neuropils such as the VAC (cyan), mVAC (orange), AMNp (red), and those of the tectulum (magenta, neck neuropil, wing neuropil, and haltere neuropil). The planes of the sections are indicated by the dotted lines. See also Video S1. A list ofthe abbreviations is given in Table 1. Scale 50 μm.
As with all arthropods, the neuronal cell bodies of the VNC form an outer cortex with neurons projecting processes centrally to form a dense fibrous central neuropil. The neuropil is stereotyped and highly ordered with functional segregation evident even at the level of the gross anatomy. The VNC is clearly subdivided in the dorso-ventral plane: ventral regions of the thoracic neuropils are innervated by neurons associated with the legs (Merritt and Murphey, 1992), whereas the dorsal neuropils are innervated by neurons associated with the wings and flight (Leise, 1991; Milde et al., 1989; Strausfeld, 1992) with intermediate regions serving to link legs and wing control (Namiki et al., 2018) (Figure 1). At a more detailed level, the neuropils exhibit a fine-grade functional order with modality-specific (Murphey et al., 1989a) and somatotopic (Murphey et al., 1989b) segregation of sensory afferent projections and myotopic organization of motor neuron dendrites (Baek and Mann, 2009; Brierley et al., 2012).
This functional organization of the neuropil provides a rigid anatomical framework against which it is possible to infer the function of neurons simply based on their anatomy. This framework is powerfully informative and an essential tool to analyze how neurons control complex behaviors such as flying, courtship, and walking. Given the fundamental importance of this anatomical order, it is vital that this anatomical framework is robust, with a shared knowledge base to allow researchers to confidently and accurately place neurons within this framework. To achieve this requires a systematic and consistent nomenclature and an anatomical template that precisely defines key anatomical structures, their boundaries, and the terms used to describe them. Recognizing the need for such consistent and robust anatomical framework, a consortium of neurobiologists studying arthropod brains (the insect brain name working group [IBNWG]), was established and produced a comprehensive hierarchical nomenclature system for the insect brain, using Drosophila melanogaster as the reference framework (Ito et al., 2014). This effort focused specifically on the brain and the gnathal regions of insects. In this work, we extend the development of a consistent nomenclature and anatomy to the Drosophila VNC.
Our work builds on previous descriptions of the Drosophila VNC (Power, 1948; Miller and Demerec, 1950; Merritt and Murphey, 1992; Boerner and Duch, 2010). It is also informed by the descriptions of the thoracic and abdominal ganglia of other insects such as grasshopper (Tyrer and Gregory, 1982) and stick insect (Kittmann et al., 1991). These comparative studies also point to clear evolutionary conservation of the basic elements of the Drosophila VNC. While these studies, plus many others, have created a rich catalog of anatomical detail, the inconsistent approach to nomenclature and definitions across the field has created ambiguity and confusion. The aim of the Drosophila adult VNC working group (DAVWG) was to create a nomenclature, definitions, and spatial boundaries for the key anatomical entities of the Drosophila VNC that are consistent with the nomenclature used to describe the VNC in other insects.
Results
Organization of the Working Group
The initial phase of work followed a similar format to that adopted by the original Insect Brain Name Working Group (IBNWG) to create the nomenclature for the Drosophila brain (Ito et al., 2014). We gathered researchers with expertise in the anatomy, development, and physiology of the VNC, hereafter referred to as the Drosophila Anatomy of the Ventral nerve cord Working Group (DAVWG) for a workshop at the Janelia Research Campus in October 2013. We discussed a document listing all of the named regions found in the published literature and from the existing Drosophila anatomy ontology (Costa et al., 2013), as well as representative anatomical images assembled by authors Court and Shepherd. After systematic review and debate, the participants compiled a working proposal for wider comment. Iterative revisions resulted in the current nomenclature described here.
Establishing the Anatomical Framework
Establishment of a systematic nomenclature requires a clear morphological and spatial definition of all the structures to be named and a standard naming scheme. The neuropil regions of the VNC are typically regarded as being “unstructured” or “tangled,” or having a fine, granular appearance in sections with different regions distinguished only by general spatial terms (Merritt and Murphey, 1992). Despite this, fixed landmarks such as longitudinal tracts and commissures can be used to define the structure and organization of different volumes of VNC neuropil (Shepherd et al., 2016).
Developmental origin provides an alternative organizational principle for defining the substructure of the neuropil. Neurons arise from neuroblasts whose first division results in A and B daughter cells. These undergo self-renewing divisions to produce clonal populations referred to as hemilineages. The neurons from a hemilineage tend to share properties, such as neurotransmitter identity and projection pattern—and even function (Harris et al., 2015; Lacin et al., 2019; Shepherd et al., 2019). Shepherd et al. (2016) used the primary projections of neuronal hemilineages to provide an organizational principle for defining the substructure of the neuropil. Although these landmarks may not always correspond to the underlying functional organization, they provide a consistent means of structurally defining neuropil regions.
To provide an initial framework for establishing distinct boundaries within the VNC, we used confocal datasets that reveal various salient features, including tracts and neuropil. The antineuroglian antibody (Iwai et al., 1997) (Figures 1B-1D) was used to reveal the primary projections of clonally related neurons in neuroblast (NB) hemilineages (Shepherd et al., 2016). The detailed structure and textural details of the neuropil were based on VNCs labeled to visualize neuropils according to the density of active-zone-specific proteins using anti-Drosophila N-cad-herin (Shepherd et al., 2016), anti-nc82 (bruchpilot [brp]) (Wagh et al., 2006), or brp-SNAP (Kohl et al., 2014) (Figures 1E-1G and Video S1). For most figures, we have used the high-resolution female VNC template produced by Bogovic et al., 2019, which provides the highest level of resolution and detail currently available. This template can be found at https://www.janelia.org/open-science/jrc-2018-brain-templates. These labels all reveal the fine details of texture and structure in the VNC neuropil, making it possible to distinguish between neuropils that are poor in synapses, such as regions occupied by axons; primary neurites; and glial processes and synapse-rich regions, such as the primary sensory neuropils and the dorsal neuropils associated with the neck, wings, and halteres (Figures 1E-1G). An anti-alpha tubulin antibody (data not shown) was used to reveal fibrous structures such as longitudinal tracts and commissures (Boerner and Duch, 2010). Other images obtained with these labeling methods are available on the Virtual Fly Brain (https://github.com/VirtualFlyBrain/DrosAdultVNSdomains/tree/master/Court2017/template).
Since all of these antibodies are available at low cost through the Developmental Studies Hybridoma Bank created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242, they can be used by future researchers to counterstain their own samples, identify neuropil regions described in this nomenclature, and computationally register them to our standard reference brains.
The Naming Scheme
All of the anatomical data used in this manuscript can be found on the Virtual Fly Brain GitHub repository https://github.com/VirtualFlyBrain/DrosAdultVNSdomains. All of the text definitions of the structures and synonyms considered in the nomenclature can be found on http://purl.obolibrary.org/obo/fbbt
A key principle was to integrate existing terminology into the standard nomenclature we propose here. We made changes only to remove ambiguity. When multiple names for an anatomical entity were used in the literature, we gave preference to the name that was most commonly used based on citations. While we sought to preserve consistency with terms used for earlier developmental stages and in other insects, we avoided the implication of homology. Most of the naming scheme relies on morphological features rather than functional data, which we incorporate in the definitions when known. We also include a look-up table of synonyms, prior terms, and references.
Abbreviations
We adopted a systematic approach when developing abbreviations for each named anatomical entity based on the following principles: (1) We adopted abbreviations that are unique across the whole CNS, avoiding abbreviations already in use for regions in the brain. (2) We created a system in which related entities would be easily recognizable. (3) We tried to be consistent with nomenclature established for the brain (Ito et al., 2014). The reasoning behind each abbreviation change was recorded and embedded in the definition. When referring to the neuromere and related structures, abbreviations were changed from a single letter or number to “Pro,” “Meso,” and “Meta.” This removed confusion with positional abbreviations such as posterior or medial. The use of the single letter “N,” which is used widely (neuromere, neuropil, nerve, neuron), was reserved for “nerve” other larger gross anatomy structures differentiated with additional letters (e.g., “Nm” for neuromere and “Np” for neuropil). The letter “C” was used to identify commissures. In cases where multiple abbreviations already exist in the literature for specific structures, the abbreviation that provided the clearest indication with least likelihood of confusion was selected, and additional abbreviations were captured as synonyms. A list of abbreviations is given in Table 1.
Axis Orientation
The general axis of orientation for the VNC is straightforward. The neuroaxis and the body axis are the same, with the prothoracic neuromere being the most anterior and the abdomen (abdominal ganglionic complex) being the most posterior. In the dorsal/ventral plane, the tectulum is dorsal and the leg nerves ventral. The dorsal/ventral axis is also sometimes referred to as superior/inferior, but dorsal and ventral are the preferred terms. The designation of left and right is assigned as if the sample is viewed from above (dorsal). The orientation in all figures is with anterior up for wholemount, lateral and horizontal views and dorsal up for transverse section views.
Definition of the VNC
The VNC is the region of the central nervous system posterior to the brain. It is connected to the brain by descending and ascending neurons that pass through the neck connective. The Drosophila VNC is a single consolidated ganglion located in the ventral part of the thorax. This ganglion contains all of the thoracic and abdominal neuromeres (Figure 1) and was called the thoracicoabdominal ganglion by Power (1948); see also synonyms in the supplemental section.
Identifying and Defining the Neuropil Structures in the VNC
Many insects have a ladder-like ventral nervous system composed of physically separated segmental neuromeres connected by longitudinal tracts (connectives), but in Drosophila, the thoracic and abdominal neuromeres are fused into a single complex (Niven et al., 2008) located within the thorax (Figure 1A). At the gross anatomical level, the segmental organization of the VNC can be resolved from external morphology. The thoracic neuromeres constitute the bulk of the VNC and are recognizable as three paired enlargements at the anterior of the VNC, corresponding to the prothoracic, mesothoracic, and metathoracic neuromeres (ProNm, MesoNm, and MetaNm, Figures 1B and 1C). At the posterior end is a small, dorsally located mass, the abdominal neuromeres, that is a fusion of all the abdominal neuromeres (ANm, Figure 1B).
Despite the evident external segmental organization, the fusion of multiple neuromeres means that identifying precise neuropil boundaries can be problematic. One of our aims was to define different regions of neuropil and provide landmarks to facilitate consistent identification and nomenclature for future studies. Although the VNC does not have the clearly defined compartmental structure found in the Drosophila central brain, it does have a clear architecture of tracts, commissures, and axon bundles that provide the basis for defining different regions of neuropil. Cell body positions are not a reliable indicator of the segmental organization of the VNC. There are many examples of cell bodies being passively displaced during neuropil expansion at metamorphosis, resulting in somata being drawn across the midline or pulled into adjacent neuromeres (Shepherd et al., 2019).
Neuromere Boundaries
Although the VNC is a fusion of thoracic and abdominal neuromeres, it is possible to define neuromere boundaries using the scaffold of neuronal fibers revealed by neuroglian expression. The neuroglian positive bundles are the tightly fasciculated primary neurites from individual neuronal lineages, where somata from a lineage remain closely associated with each other. Since each neuromere is founded by a specific set of NBs, the lineage derived neuroglian bundles create a neuromere-specific set of markers, creating a robust framework that clearly outlines the neuropil within each neuromere and thus helps to define the neuropilar boundaries between each neuromere (Figures 1B and 1C). The neuroglian label also provides markers for other structures such as the tectulum (Tct [magenta], Figure 1D) and some commissures (Figure 2) (Shepherd et al., 2016).
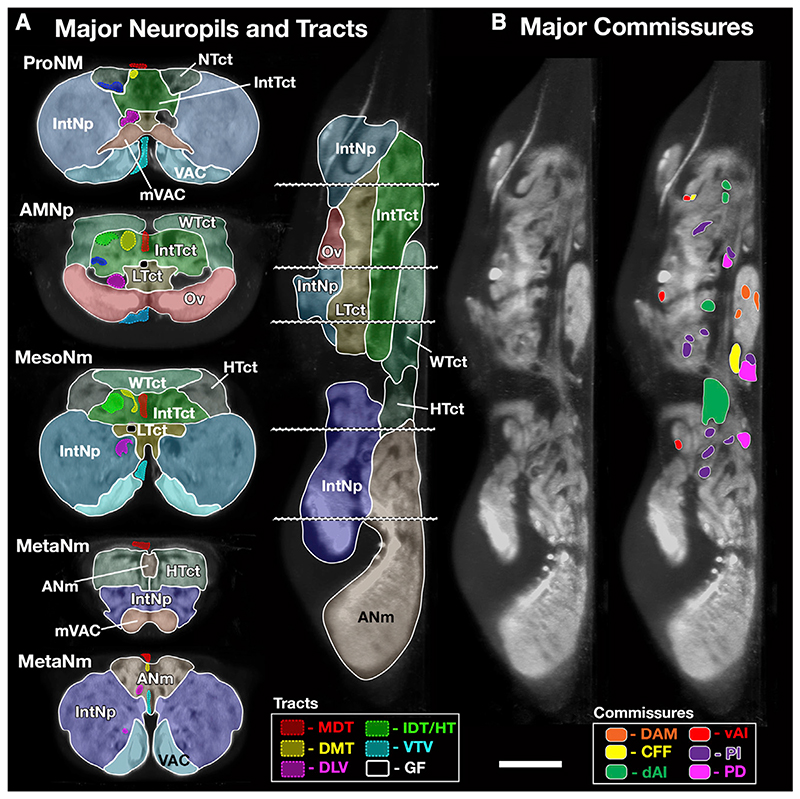
Figure 2. Major Neuropils, Tracts, and Commissures of the VNC.
(A) Major Neuropils and Tracts—segmented VNC shown in transverse and lateral sections illustrating the outlines of the major neuropils and longitudinal tracts described in this study. The tectulum domains are shown in different shades of green, and the leg neuropil domains are shown in shades of blue. To further aid visualization, labeled tracts are only shown in the left half of the transverse sections. The plane of the transverse sections is indicated by dotted lines.
(B) The position of the major commissural pathways shown on a lateral section at the midline of the VNC. Tracts derived from the same larval commissure are shown in the same colors. An unlabeled section is provided to show the detail unhindered by labeling. See also Figure S1 and Video S2. A list of the abbreviations is given in Table 1. Scale 50 μm.
Major Subdivisions of the Thoracic Neuropils
While the neuromeres divide the VNC along the anterior-to-posterior axis, there is also specialization on the dorso-ventral axis with a dorsal region called the tectulum (Tct) and a ventral region called leg neuropil (LegNp) (Figure 1D).
The Tectulum (Tct)
The tectulum (Tct) was described by Power (1948) as a discrete dorsal region of the VNC, overlying the mesothoracic neuromere like a saddle and extending from the posterior prothoracic to the anterior metathoracic neuromeres. The neuroglian positive primary neurites provide boundaries that precisely circumscribe the tectulum to define its boundaries (Figure 1D) (Shepherd et al., 2016). Although Power (1948) defined the tectulum as a single neuropil without sub-divisions, the tectulum can be stratified into three layers in the dorsal ventral plane that the working group renamed as upper, intermediate, and lower tectulum (Figure 2A). The lower and intermediate tectulum show no overt signs of segmental barriers and are considered to lack a segmental organization. The upper tectulum, however, does have some segmental specializations and can be segregated on the basis of the synapse rich neuropils revealed by N-Cadherin/bruchpilot expression into three neuromere specific neuropils: neck, wing, and haltere tectulum for the ProNm, MesoNm, and MetaNm neuromeres, respectively (Figures 1B and 2A; Video S2).
The Leg Neuropil
The ventral portion of each thoracic neuropil outside of the tectulum is the leg neuropil (LegNp, see Supplemental Information for details). Unlike the tectulum, the leg neuropils exhibit clear segmental boundaries and, although each thoracic neuromere is slightly different, they all conform to the same organizational principles (Figure 2A; Video S2). The legNps contain the sensory afferent endings of leg sensory neurons, the leg motor neurons, and local interneurons that control leg movement. The leg neuropils are best described in transverse section and can be partitioned into distinct regions along the dorsoventral axis (Figure 2A; Video S2). The ventralmost layer of leg neuropil, the ventral association center (VAC) (Merritt and Murphey, 1992) is readily distinguishable as synapse rich neuropils (VAC, Figures 1E-1G and 2A; Video S2). The VAC is innervated by sensory afferents from sensory neurons associated with tactile bristles on the leg (Murphey et al., 1989b). Adjacent to the VAC is a paired globular structure, the medial ventral association center (mVAC) (mVAC, Figures 1E-1G and 2A; Video S1). The mVAC is a bilaterally symmetrical neuropil region that can be identified both by its fine textured appearance and as dense synaptic neuropil (Merritt and Murphey, 1992). In Drosophila, the mVAC is innervated by a subset of femoral chordotonal organ (FeCO) sensory neurons which form a “club”-shaped projection that terminates in the mVAC (Phillis et al., 1996). The Drosophila mVAC is homologous to the mVAC described in locusts and other insects that also receive primary sensory afferents for leg chordotonal organs and is known as “auditory neuropil” (Oshinsky and Hoy, 2002; Römer et al., 1988).
The leg neuropil, between the VAC and the tectulum, is called “intermediate neuropil” (IntNp) because it occupies most of the central third of the dorsoventral area in transverse section (IntNp, Figure 2A; Video S2). The IntNp contains the dendritic branches of the leg motorneurons, premotor interneurons (Shepherd et al., 2019), and sensory afferents from leg campaniform sensilla, hair plates, and the “hook” and “claw” projection types from the FeCO (Mamiya et al., 2018). Like the tectulum, the leg neuropils exhibit clear functional segregation: motor neuron dendrites show clear spatial and functional organization (Maniates-Selvin et al., 2020), and the sensory modalities are partitioned into layers, with proprioception in intermediate neuropil and a somatotopic representation of tactile information in the ventralmost zone (Murphey et al., 1989b; Tsubouchi et al., 2017).
Tracts and Commissures
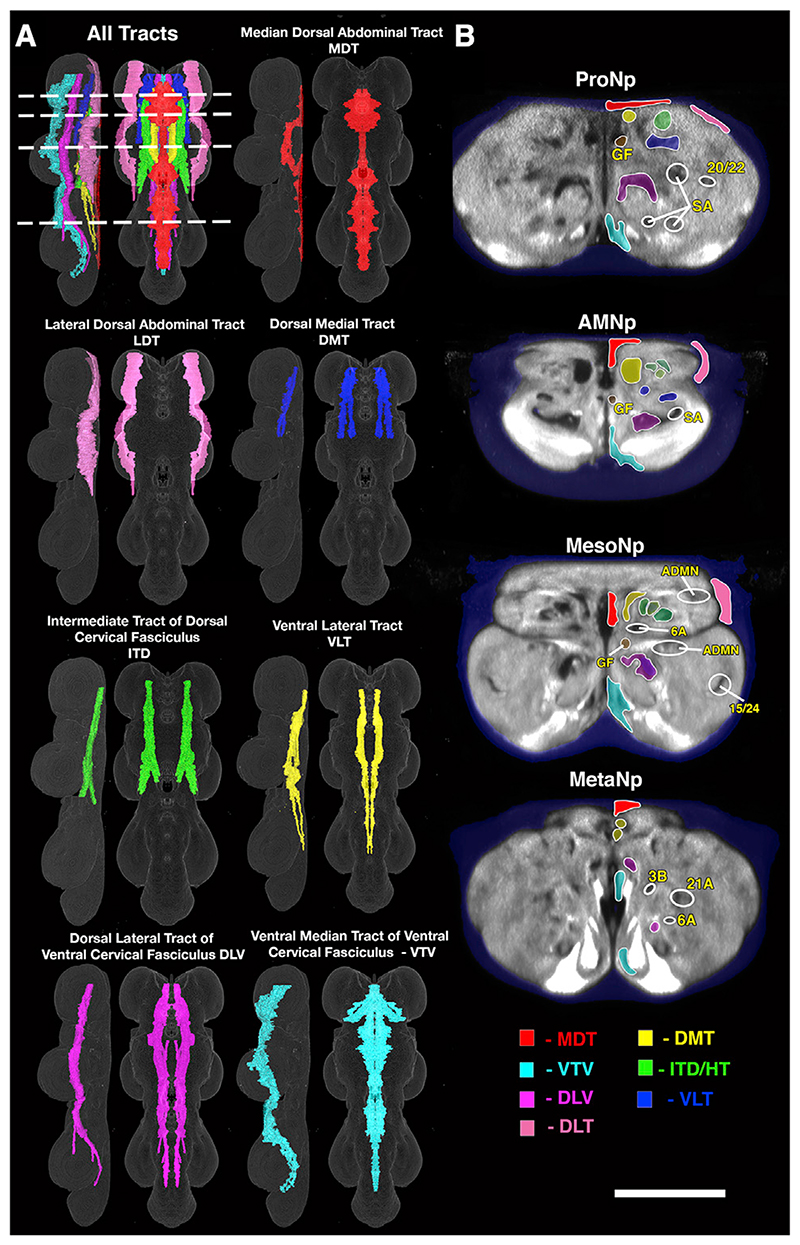
Building on studies of orthopterous insect ganglia such as the grasshopper (Tyrer and Gregory, 1982), Merritt and Murphey, (1992) and Boerner and Duch (2010) described the stereotyped patterns of longitudinal tracts and commissures in the adult Drosophila VNC (Figures 2A, 3, and S1; Video S3). Here we have reviewed these studies and nomenclatures and extended them by providing high resolution volumes for these structures. The nomenclature for the commissures has been redesigned to create a new consistent naming system that reflects the developmental origins of each adult commissure. Truman et al., (2004) showed that the larval VNC has just five commissures per neuromere and that the postembryonic neuronal lineages that cross the midline do so via a specific and invariant commissure (Truman et al., 2004). The five larval commissures split into additional pathways during metamorphosis due to the expansion and extension of the neuropil, so the adult fly has more commissures than the larva (Figures 3 and S1). Using lineage-based markers, Shepherd et al. (2016) linked the larval commissures to their adult counterpart (Power, 1948; Merritt and Murphey, 1992). These lineage-based definitions underlie the proposed nomenclature. Unlike the commissures, the longitudinal tracts were fully described by Power (1948) and Merritt and Murphey (1992) with a largely consistent and widely accepted nomenclature that we have retained.
Figure 3. Major Longitudinal Tracts of the VNC.
(A) The major tracts of the VNC shown as rendered volumes from lateral and dorsal perspectives.
(B) Transverse section views of the tracts at selected points in the VNC. The areas outlined by white circles identify other key structures (GF, giant fiber; ADMN, sensory afferents entering from the ADMN; SA, sensory afferents entering from the leg nerve; the numbers refer to hemilineage-derived axon fascicles). The planes of section are indicated by dotted lines in (A). See also Video S3. A list of the abbreviations is given in Table 1. Scale (A), 100 μm; (B), 50 μm.
Discussion
With this nomenclature, we address two primary issues required to create a clearer understanding of the VNC structure and to facilitate dialog and data exchange among neuroscience researchers. The first was to establish a common anatomical framework to precisely define and describe, textually and spatially, the anatomical organization of the VNC. The second was to create a clear and consistent naming scheme for each anatomical entity. The detailed VNC map we provide is essential for integrating past and future work into a common space, thereby contributing to new lines of investigation. In addition, our effort will inform researchers working with other insects, providing them with a template that can be adapted to their own model organism. Although the nomenclature developed in this project will serve as an initial standard, we acknowledge that to remain useful it must be maintained as a “living” process and evolve as our understanding of the VNC structure and function grows. Future revisions and additions will be required, and there are regions of the neuropil that will benefit from further analysis to provide a clearer breakdown of the substructure. Most notably, the thoracic IntNp, which, although extremely important, still remains a broadly defined region that lacks detailed spatial information, particularly in relation to the spatial organization of sensory neurons and motor neuron dendrites. Such additions and improvements will be handled via the existing online system for posting anatomy ontology suggestions located at https://github.com/FlyBase/Drosophila-anatomy-developmental-ontology/issues and maintained by VirtualFlyBrain.org.
Unlike the brain, the VNC in insects demonstrates significant diversity in its gross organization and structure (Niven et al., 2008). However, there is, a large anatomical literature for several insect groups that exhibit markedly different VNC structures (e.g., grasshoppers, crickets, and moths) that often use the same terms as used for Drosophila. The differences among the VNCs of different insects are likely to be largely superficial and simply reflect the pattern of ganglionic fusion. While this fusion does create some anatomical confusion, the basic pattern of tracts and commissures is preserved throughout the insects. Considering the conservation of lineages, tracts, and commissures, insects do exhibit remarkably similar CNS structures despite the distortions imposed by ganglionic fusion. Consequently, it is important not only to have a consistent nomenclature to benefit Drosophila researchers but also to develop a nomenclature that can be used as broadly as possible across the insects to create a consistent cross-species terminology. While this would require some work to confirm homology rather than rely on inference from similar structure, extension of a consistent nomenclature to other insects would provide a framework to explore cross-species homologies in the VNC, the evolution of neuronal networks, and the deep evolutionary conservation of the nervous system.
Star⋆Methods
Key Resources Table.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-neuroglian | Developmental Studies Hybridoma Bank (Iwai et al., 1997) | Cat.no. BP 104 anti-neuroglian RRID:AB_528402 |
| Anti-Drosophila N-cadherin | Developmental Studies Hybridoma Bank | Cat. no. DN-Ex 8 RRID:AB_528121 |
| Deposited Data | ||
| All datasets and segmented domains | https://github.com/VirtualFlyBrain/DrosAdultVNSdomains/tree/master/Court2017/template | This Paper |
| Experimental Models: Organisms/Strains | ||
| Drosophila melanogaster brp-SNAP transgene | Kohl etal., 2014 | Dmel\brpSNAPf-tag |
| Software and Algorithms | ||
| ITK-SNAP | Yushkevich et al., 2006 | http://www.itksnap.org/pmwiki/pmwiki.php RRID:SCR_002010 |
| Fluorender | Scientific Computing and Imaging Institute, University of Utah | https://www.sci.utah.edu/software/fluorender.html RRID:SCR_014303 |
| FIJI | Schindelin et al., 2012 | https://fiji.sc RRID:SCR_002285 |
| Adobe Premiere | Adobe.com | N/A |
Resource Availability
Lead Contact
Further information and requests for data and resources should be directed to and will be fulfilled by the Lead Contact, David Shepherd (d.shepherd@bangor.ac.uk).
Method Details
Anatomical Materials
All images are based on previously published data and described methodologies. The anti-neuroglian antibody (Iwai et al., 1997) was used to reveal the primary projections of neuron hemilineages as described by (Shepherd et al., 2016). The structure of the neuropil was revealed using anti-Drosophila N-cadherin (Developmental Studies Hybridoma Bank; Cat. no. DN-Ex 8 RRID:AB_528121) as described by (Shepherd et al., 2016), anti-nc82 (Developmental Studies Hybridoma Bank; Cat.no. nc82 anti-Bruchpilot RRI-D:AB_2314866) as described by (Wagh et al., 2006) and the brp-SNAP transgene (Kohl et al., 2014) as described by (Bogovic et al., 2019).
Boundary Drawing and 3D rendering
Neuropil regions, tracts and commissures were manually painted using ITK-SNAP (Yushkevich et al., 2006, RRID:SCR_002010, http://www.itksnap.org/pmwiki/pmwiki.php) using the adult female VNC template produced by (Bogovic et al., 2019), (https://www.janelia.org/open-science/jrc-2018-brain-templates). Surface rendered images were generated with Fluorender software (RRID:SCR_014303, https://www.sci.utah.edu/software/fluorender.html). Videos were created with Adobe Premiere from on TIFF stacks created in FIJI (Schindelin et al., 2012) RRID:SCR_002285).
Supplementary Material
Highlights.
A framework defining the anatomy of the adult Drosophila ventral nerve cord (VNC)
A clear and consistent naming scheme for the anatomy of the adult Drosophila VNC
The framework is a tool for integrating past and future work into a common space
Provides a template that can be adapted to other arthropod nervous systems
In Brief.
The ventral nerve cord (VNC) of Drosophila is an important model system for understanding how nervous systems generate locomotion. In this issue of Neuron, Court et al. define the structures of the adult VNC to provide an anatomical framework for analyzing the functional organization of the VNC.
Table 1. List of the Major Structures and Their Abbreviations.
| Major Neuromeres and Neuropils | Longitudinal Tracts |
|---|---|
| Prothoracic neuromere (ProNm), Accessory Mesothoracic neuropil (AMNp), Mesothoracic neuromere (MesoNm), Metathoracic neuromere (MetaNm), Abdominal neuromere (ANm), Tectulum (Tct), Upper tectulum (UTct), Intermediate tectulum (IntTct), Lower tectulum (LTct), Wing tectulum (WTct), Haltere tectulum (HTct), Neck tectulum (NTct), Leg neuropil (LegNp), Intermediate neuropil (IntNp), Ventral Association Centre (VAC), Medial Ventral association centre (mVAC), Intermediate Lateral association centre (iLAC) | Dorsal lateral tract (DLT), Intermediate tract of dorsal cervical fasciculus (ITD), Dorsal lateral tract of ventral cervical fasciculus (DLV), Ventral lateral tract (VLT), Ventral median tract of ventral cervical fasciculus (VTV), Median dorsal abdominal tract (MDT), Ventral cervical fasciculus (VCF), Dorsal cervical fasciculus (DCF), Dorsal median tract (DMT), Ventral ellipse (VE) |
| Commissures | Peripheral Nerves |
| anterior Anterior Ventral Commissure (aAV), posterior Anterior Ventral Commissure (pAV), Anterior Intermediate Commissure (AI), ventral Anterior Intermediate Commissure (vAI), Anterior Intermediate anterior Commissure (AIa), Anterior Intermediate posterior Commissure (AIp), dorsal Anterior Intermediate Commissure (dAI), anterior Posterior Intermediate Commissure (aPI), posterior Posterior Intermediate Commissure (pPI), dorsal PI Commissure (dPI), Posterior Dorsal Commissures (PD), Commissure of Fine Fibers of the Intermediate Tract of the Dorsal Cervical Fasciculus (CFF), Commissure of Prothoracic Neuromeres (CPN), Dorsal Accessory Commissure of the Mesothoracic Neuromeres (DAM), Ventral Ellipse (VE) | Cervical nerve (CvN), Dorsal prothoracic nerve (DProN), Prosternal nerve (PrN), Prothoracic chordotonal nerve (ProCN), Prothoracic accessory nerve (ProAN), Ventral prothoracic nerve (VProN), Prothoracic leg nerve (ProLN), Anterior dorsal mesothoracic nerve (ADMN), Posterior dorsal mesothoracic nerve (PDMN), Mesothoracic accessory nerve (MesoAN), Mesothoracic leg nerve (MesoLN), Dorsal metathoracic nerve (DMetaN), Metathoracic leg nerve (MetaLN), First abdominal nerve (AbN1), Second abdominal nerve (AbN2), Third abdominal nerve (AbN3), Fourth abdominal nerve (AbN4), Abdominal nerve trunk (AbNT) |
| Specific Neurons | Other Structures |
| Giant Fiber (GF), Contralateral haltere interneurons (cHIN) | Femoral chordotonal organ (FeCO), Cervical connective (CvC) |
Acknowledgments
We thank Gerald M. Rubin, Janine Stevens, the Visiting Science and Conference Programs of the Howard Hughes Medical Institute’s Janelia Research Campus for hosting the workshop. This work was initially conceived of as part of the Descending Interneuron Project Team at Janelia. This work was also supported, in part, by grants EP/F500385/1 and BB/F529254/1 for the University of Edinburgh School of Informatics Doctoral Training Centre in Neuroinformatics and Computational Neuroscience (http://www.anc.ed.ac.uk/dtc) from the UK Engineering and Physical Sciences Research Council (EPSRC), UK Biotechnology and Biological Sciences Research Council (BBSRC), and the UK Medical Research Council (MRC). Finally, by the Wellcome Trust as part of the “Virtual Fly Brain: a global informatics hub for Drosophila neurobiology” WT105023MA.
Footnotes
Author Contributions
Conceptualization, W.K., J.W.T., and D.S.; Software, R.C.; Validation, All; Resources, D.S., R.C., and J.B.; Data Curation, R.C., M.C., and J.D.A; Writing— Original Draft, D.S. and R.C.; Writing—Review & Editing, M.C., M.D., R.K.M., A.M.S., J.H.S., T.S., J.C.T., J.W.T., and D.W.W.; Visualization, D.S. and R.C.; Funding Acquisition, All.
Declaration of Interests
The authors declare no competing interests.
Materials Availability
This study did not generate any new reagents.
Data and Code Availability
All anatomical datasets and segmented domains have been deposited at Virtual Fly Brain (https://github.com/VirtualFlyBrain/DrosAdultVNSdomains/tree/master/Court2017/template) and are openly available.
References
- Bacon JP, Strausfeld NJ. The dipteran ‘Giant fibre’ pathway: neurons and signals. J Comp Physiol A. 1986;158:529–548. [Google Scholar]
- Baek M, Mann RS. Lineage and birth date specify motor neuron targeting and dendritic architecture in adult Drosophila. J Neurosci. 2009;29:6904–6916. doi: 10.1523/JNEUROSCI.1585-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellen HJ, Tong C, Tsuda H. 100 years of Drosophila research and its impact on vertebrate neuroscience: a history lesson for the future. Nat Rev Neurosci. 2010;11:514–522. doi: 10.1038/nrn2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidaye SS, Machacek C, Wu Y, Dickson BJ. Neuronal control of Drosophila walking direction. Science. 2014;344:97–101. doi: 10.1126/science.1249964. [DOI] [PubMed] [Google Scholar]
- Bodenstein D. The Postembryonic Development of Drosophila. Wiley; 1950. pp. 275–367. [Google Scholar]
- Boerner J, Duch C. Average shape standard atlas for the adult Drosophila ventral nerve cord. J Comp Neurol. 2010;518:2437–2455. doi: 10.1002/cne.22346. [DOI] [PubMed] [Google Scholar]
- Bogovic J, Otsuna H, Heinrich L, Ito M, Jeter J, Meissner G, Nern A, Colonell J, Malkesman O, Ito K, Saalfeld S. An unbiased template of the Drosophila brain and ventral nerve cord. bioRxiv. 2019 doi: 10.1101/376384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley DJ, Rathore K, VijayRaghavan K, Williams DW. Developmental origins and architecture of Drosophila leg motoneurons. J Comp Neurol. 2012;520:1629–1649. doi: 10.1002/cne.23003. [DOI] [PubMed] [Google Scholar]
- Card G, Dickinson MH. Visually mediated motor planning in the escape response of Drosophila. Curr Biol. 2008;18:1300–1307. doi: 10.1016/j.cub.2008.07.094. [DOI] [PubMed] [Google Scholar]
- Clyne JD, Miesenböck G. Sex-specific control and tuning of the pattern generator for courtship song in Drosophila. Cell. 2008;133:354–363. doi: 10.1016/j.cell.2008.01.050. [DOI] [PubMed] [Google Scholar]
- Costa M, Reeve S, Grumbling G, Osumi-Sutherland D. The Drosophila anatomy ontology. J Biomed Semantics. 2013;4:32. doi: 10.1186/2041-1480-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crickmore MA, Vosshall LB. Opposing dopaminergic and GABAergic neurons control the duration and persistence of copulation in Drosophila. Cell. 2013;155:881–893. doi: 10.1016/j.cell.2013.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson MH, Muijres FT. The aerodynamics and control of free flight manoeuvres in Drosophila. Philos Trans R Soc Lond B Biol Sci. 2016;371:20150388. doi: 10.1098/rstb.2015.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghysen A. The projection of sensory neurons in the central nervous system of Drosophila: choice of the appropriate pathway. Dev Biol. 1980;78:521–541. doi: 10.1016/0012-1606(80)90351-6. [DOI] [PubMed] [Google Scholar]
- Harris RM, Pfeiffer BD, Rubin GM, Truman JW. Neuron hemilineages provide the functional ground plan for the Drosophila ventral nervous system. eLife. 2015;4:e04493. doi: 10.7554/eLife.04493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Shinomiya K, Ito M, Armstrong DJ, Boyan G, Hartenstein V, Harzsch S, Heisenberg M, Homberg U, Jenett A, et al. A Systematic Nomenclature for the Insect Brain. Neuron. 2014;81:755–765. doi: 10.1016/j.neuron.2013.12.017. [DOI] [PubMed] [Google Scholar]
- Iwai Y, Usui T, Hirano S, Steward R, Takeichi M, Uemura T. Axon patterning requires DN-cadherin, a novel neuronal adhesion receptor, in the Drosophila embryonic CNS. Neuron. 1997;19:77–89. doi: 10.1016/s0896-6273(00)80349-9. [DOI] [PubMed] [Google Scholar]
- Jenett A, Rubin GM, Ngo TT, Shepherd D, Murphy C, Dionne H, Pfeiffer BD, Cavallaro A, Hall D, Jeter J, et al. A GAL4-driver line resource for Drosophila neurobiology. Cell Rep. 2012;2:991–1001. doi: 10.1016/j.celrep.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittmann R, Dean J, Schmitz J. An atlas of the thoracic ganglia in the stick insect, Carausius morosus. Philos Trans R Soc Lond B Biol Sci. 1991;331:101–121. [Google Scholar]
- Kohl J, Ng J, Cachero S, Ciabatti E, Dolan M-J, Sutcliffe B, Tozer A, Ruehle S, Krueger D, Frechter S, et al. Ultrafast tissue staining with chemical tags. Proc Natl Acad Sci U S A. 2014;111:E3805–E3814. doi: 10.1073/pnas.1411087111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacin H, Chen HM, Long X, Singer RH, Lee T, Truman JW. Neurotransmitter identity is acquired in a lineage-restricted manner in the Drosophila CNS. eLife. 2019;8:e43701. doi: 10.7554/eLife.43701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leise EM. Evolutionary trends in invertebrate ganglionic structure. Semin Neurosci. 1991;3:369–377. [Google Scholar]
- Lundquist T, Nässel DR. Substance P-, FMRFamide-, and gastrin/cholecystokinin-like immunoreactive neurons in the thoraco-abdominal ganglia of the flies Drosophila and Calliphora. J Comp Neurol. 1990;294:161–178. doi: 10.1002/cne.902940202. [DOI] [PubMed] [Google Scholar]
- Mamiya A, Gurung P, Tuthill JC. Neural Coding of Leg Proprioception in Drosophila. Neuron. 2018;100:636–650.:e6. doi: 10.1016/j.neuron.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniates-Selvin JT, Hildebrand DGC, Graham BJ, Kuan AT, Thomas LA, Nguyen T, Buhmann J, Azevedo AW, Shanny BL, Funke J, et al. Reconstruction of Motor Control Circuits in Adult Drosophila Using Automated Transmission Electron Microscopy. bioRxiv. 2020 doi: 10.1101/2020.01.10.902478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes CS, Bartos I, Akay T, Márka S, Mann RS. Quantification of gait parameters in freely walking wild type and sensory deprived Drosophila melanogaster. eLife. 2013;2:e00231. doi: 10.7554/eLife.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt DJ, Murphey RK. Projections of leg proprioceptors within the CNS of the fly Phormia in relation to the generalized insect ganglion. J Comp Neurol. 1992;322:16–34. doi: 10.1002/cne.903220103. [DOI] [PubMed] [Google Scholar]
- Middleton CA, Nongthomba U, Parry K, Sweeney ST, Sparrow JC, Elliott CJ. Neuromuscular organization and aminergic modulation of contractions in the Drosophila ovary. BMC Biol. 2006;4:17. doi: 10.1186/1741-7007-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milde JJ, Seyan HS, Strausfeld NJ. The Neck Motor System of the Fly Calliphora erythrocephala – II. Sensory Organization. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1989;160:225–238. [Google Scholar]
- Miller A, Demerec M. The internal anatomy and histology of the imago of Drosophila melanogaster. Wiley; 1950. p. 420. [Google Scholar]
- Murphey RK, Possidente D, Pollack G, Merritt DJ. Modality-specific axonal projections in the CNS of the flies Phormia and Drosophila. J Comp Neurol. 1989a;290:185–200. doi: 10.1002/cne.902900203. [DOI] [PubMed] [Google Scholar]
- Murphey RK, Possidente DR, Vandervorst P, Ghysen A. Compartments and the topography of leg afferent projections in Drosophila. J Neurosci. 1989b;9:3209–3217. doi: 10.1523/JNEUROSCI.09-09-03209.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namiki S, Dickinson MH, Wong AM, Korff W, Card GM. The functional organization of descending sensory-motor pathways in Drosophila. Elife. 2018;7:e34272. doi: 10.7554/eLife.34272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niven JE, Graham CM, Burrows M. Diversity and evolution of the insect ventral nerve cord. Annu Rev Entomol. 2008;53:253–271. doi: 10.1146/annurev.ento.52.110405.091322. [DOI] [PubMed] [Google Scholar]
- Oshinsky ML, Hoy RR. Physiology of the auditory afferents in an acoustic parasitoid fly. J Neurosci. 2002;22:7254–7263. doi: 10.1523/JNEUROSCI.22-16-07254.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlou HJ, Lin AC, Neville MC, Nojima T, Diao F, Chen BE, White BH, Goodwin SF. Neural circuitry coordinating male copulation. eLife. 2016;5:e20713. doi: 10.7554/eLife.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflüger H, Bräunig P, Hustert R, Burrows M. The organization of mechanosensory neuropiles in locust thoracic ganglia. Phil Trans R Soc London B. 1988;321:1–26. [Google Scholar]
- Phillis R, Statton D, Caruccio P, Murphey RK. Mutations in the 8 kDa dynein light chain gene disrupt sensory axon projections in the Drosophila imaginal CNS. Development. 1996;122:2955–2963. doi: 10.1242/dev.122.10.2955. [DOI] [PubMed] [Google Scholar]
- Power ME. The thoracico-abdominal nervous system of an adult insect, Drosophila melanogaster. J Comp Neurol. 1948;88:347–409. doi: 10.1002/cne.900880303. [DOI] [PubMed] [Google Scholar]
- Römer H, Marquart V, Hardt M. Organization of a sensory neuropile in the auditory pathway of two groups of Orthoptera. J Comp Neurol. 1988;275:201–215. doi: 10.1002/cne.902750204. [DOI] [PubMed] [Google Scholar]
- Scheffer LK, Meinertzhagen IA. The Fly Brain Atlas. Annu Rev Cell Dev Biol. 2019;35:637–653. doi: 10.1146/annurev-cellbio-100818-125444. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeds AM, Ravbar P, Chung P, Hampel S, Midgley FM, Jr, Mensh BD, Simpson JH. A suppression hierarchy among competing motor programs drives sequential grooming in Drosophila. eLife. 2014;3:e02951. doi: 10.7554/eLife.02951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd D, Smith SA. Central projections of persistent larval sensory neurons prefigure adult sensory pathways in the CNS of Drosophila. Development. 1996;122:2375–2384. doi: 10.1242/dev.122.8.2375. [DOI] [PubMed] [Google Scholar]
- Shepherd D, Harris R, Williams DW, Truman JW. Postembryonic lineages of the Drosophila ventral nervous system: Neuroglian expression reveals the adult hemilineage associated fiber tracts in the adult thoracic neuromeres. J Comp Neurol. 2016;524:2677–2695. doi: 10.1002/cne.23988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd D, Sahota V, Court R, Williams DW, Truman JW. Developmental organization of central neurons in the adult Drosophila ventral nervous system. J Comp Neurol. 2019;527:2573–2598. doi: 10.1002/cne.24690. [DOI] [PubMed] [Google Scholar]
- Strausfeld NJ. In: The Head-Neck Sensory Motor System. Vidal PP, editor. Oxford University Press; 1992. The Head-Neck System of the Blowfly Calliphora: 1 Anatomic Organization of Neck Muscles, Motor Neurons, and Multimodal and Visual Inputs; pp. 56–63. [Google Scholar]
- Strausfeld NJ, Seyan HS. Convergence of visual, haltere, and prosternai inputs at neck motor neurons of Calliphora erythrocephala. Cell Tissue Res. 1985;240:601–615. [Google Scholar]
- Truman JW, Schuppe H, Shepherd D, Williams DW. Developmental architecture of adult-specific lineages in the ventral CNS of Drosophila. Development. 2004;131:5167–5184. doi: 10.1242/dev.01371. [DOI] [PubMed] [Google Scholar]
- Tsubouchi A, Yano T, Yokoyama TK, Murtin C, Otsuna H, Ito K. Topological and modality-specific representation of somatosensory information in the fly brain. Science. 2017;358:615–623. doi: 10.1126/science.aan4428. [DOI] [PubMed] [Google Scholar]
- Tuthill JC, Wilson RI. Mechanosensation and Adaptive Motor Control in Insects. Curr Biol. 2016;26:R1022–R1038. doi: 10.1016/j.cub.2016.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrer NM, Gregory GE. A guide to the neuroanatomy of locust suboesophageal and thoracic ganglia. Philos Trans R Soc Lond, B. 1982;297:91–123. [Google Scholar]
- Wagh DA, Rasse TM, Asan E, Hofbauer A, Schwenkert I, Dürrbeck H, Buchner S, Dabauvalle MC, Schmidt M, Qin G, et al. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron. 2006;49:833–844. doi: 10.1016/j.neuron.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Wosnitza A, Bockemühl T, Dübbert M, Scholz H, Büschges A. Inter-leg coordination in the control of walking speed in Drosophila. J Exp Biol. 2013;216:480–491. doi: 10.1242/jeb.078139. [DOI] [PubMed] [Google Scholar]
- Yu JY, Kanai MI, Demir E, Jefferis GS, Dickson BJ. Cellular organization of the neural circuit that drives Drosophila courtship behavior. Curr Biol. 2010;20:1602–1614. doi: 10.1016/j.cub.2010.08.025. [DOI] [PubMed] [Google Scholar]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All anatomical datasets and segmented domains have been deposited at Virtual Fly Brain (https://github.com/VirtualFlyBrain/DrosAdultVNSdomains/tree/master/Court2017/template) and are openly available.