Abstract
Vesicle rocketing has been used as a model system for understanding the dynamics of the membrane-associated F-actin cytoskeleton, but in many experimental systems is induced by persistent, non-physiological stimuli. Localised changes in the concentration of phosphatidylinositol 4,5-bisphosphate (PI (4,5)P2) in membranes stimulate the recruitment of actin-remodelling proteins to their sites of action, regulate their activity and favour vesicle rocketing. The calcium and anionic phospholipid-binding protein annexin A2 is necessary for macropinocytic rocketing and has been shown to bind both PI(4,5)P2 and the barbed-ends of F-actin filaments. Here we show that annexin A2 localises to the comet tails which form constitutively in fibroblasts from patients with Lowe Syndrome. These fibroblasts are deficient in OCRL1, a phosphatidylinositol polyphosphate 5-phosphatase with specificity for PI(4,5)P2. We show that upon depletion of annexin A2 from these cells vesicle rocketing is reduced, and that this is also dependent upon PI (4,5)P2 formation. Annexin A2 co-localised with comet-tails induced by pervanadate and hyperosmotic shock in a basophilic cell line, and in an epithelial cell line upon activation of PKC. In vitro annexin A2 promoted comet formation in a bead-rocketing assay and was sufficient to link F-actin filaments to PI(4,5)P2 containing vesicles. These observations are consistent with a role for annexin A2 as an actin nucleator on PI (4,5)P2-enriched membranes.
Keywords: Annexin; Actin; Phosphoinositide; PI(4,5)P2; Rocketing; Lowe syndrome
1. Introduction
The trafficking of membranous vesicles from one sub-cellular location to another requires many proteins and the specific sorting, modification and degradation of numerous lipids. Certain lipids, by virtue of their shapes or charge can deform planar membranes, facilitating their tubulation and vacuolation. Recruitment of a variety of proteins to these sites stabilizes and enhances this tubulation and ultimately leads to topological closure of the vesicle neck and the de novo formation of an endosome. In some instances this is associated with local depolymerisation of the underlying cortical actin cytoskeleton and its re-assignment in an endosome-associated conformation (for review see [1–3]). Such structures have a range of forms with varying degrees of symmetry. Under certain circumstances the actin adopts an elongated, arborescent form (sometimes called a comet tail), which propels the vesicle through the cytoplasm, the fast-growing ‘barbed’ ends of the branches supporting the vesicle somewhat like a balloon caught in a tree.
Work performed on the superficially similar rockets induced by the bacterial pathogens Listeria monocytogenes, Shigella flexneri and Rickettsiae has revealed that motility is dependent upon ARP2/3 activation by Wasp-family proteins (such as VASP, N-Wasp, Scar-1), and also requires actin-binding proteins such as profilin, filamin, ezrin, paxillin, α-actinin, gelsolin, zyxin, vinculin and Mena [4–9]. Different subsets of proteins are recruited to the comet tails of different bacteria, resulting in variability in the structures of the actin comets generated and the resultant speed of bacterial movement [8].
Evidence from experimental systems examining actin-mediated propulsion (rocketing) of vesicles has been slower to accumulate, but there is growing evidence that vesicle rocketing has a significant physiological role at the plasma membrane, the recycling endosome and the Golgi complex [10–11]. In each case there seems to be a requirement for the local production and turn-over of phosphatidylinositol 4, 5-bisphosphate (PI(4,5)P2), produced by the action of phosphatidylinositol 4-phosphate 5-kinases (PI5 kinases) on phosphatidylinositol 4-phosphate (PI4P) [12]. Over-expression of PI5 kinases or down regulation of phosphatidylinositol 5-phophatases with specificity for PI(4,5)P2 such as OCRL1 and synaptojanin results in enrichment of PI(4,5)P2 in the plasma membrane and Golgi, with rocketing initiating from both of these sites [13]. The specificity for particular species of phosphoinositides was shown most clearly in experiments in which actin rockets were generated in Xenopus egg extracts. Although vesicles containing any poly-phosphorylated PIs would induce actin polymerisation in such extracts (especially when supplemented with GTPγS or sodium orthovanadate) only those containing PI(4,5)P2 or PI(3,4,5)P3 were able to induce rocket formation [14]. PI(4,5)P2 has the potential to increase actin association with membranes by several routes. It stimulates the activity of factors such as WASP and SCAR [15] which initiate actin polymerisation by activation of the ARP2/3 complex [16,17], promotes filament elongation by uncapping barbed-ends, reduces filament severing by inhibiting the activity of cofilin and gelsolin [18,19] and causes the dissociation of actin from profilin, which is key to the addition of monomeric actin to the growing barbed-end [20]. PI(4,5)P2 also alters the membrane affinity of alpha-actinin, vinculin, ezrin, radixin and moesin which act at the interface between the actin cytoskeleton and the membrane.
Annexin A2 is a member of a large family of calcium-binding proteins which associate with and organise anionic phospholipids, often preferentially binding to membranes rich in cholesterol (for review see [21, 22]). We and others have shown that annexin A2 has a particularly high affinity for PI(4,5)P2 [23–25] and is necessary for the motility of macropinocytic rockets [26, 27]. Most recently we have also shown that annexin A2 can regulate actin assembly, both by binding G-actin in the cytoplasm and by capping the barbed ends of F-actin filaments [28]. In this paper we demonstrate the association of annexin A2 with rocketing vesicles from a number of different sources and demonstrate in vitro that annexin A2 enhances actin-mediated rocketing in a Scar-based bead assay and is sufficient to link actin filaments to vesicles containing anionic phospholipids.
2. Materials and methods
2.1. Protein preparation
Actin was purified from rabbit muscle. Arp2/3 complex was purified from human platelets as described in Kaiser et al. 1986. Gelsolin (Sigma) seeds were made according to Blanchion et al. [29]. pET cofilin (rat) (gift from Dr. N Zebda), pGEX-2T scar WA (human) (gift from Prof. L. Machesky) and pET-3d-CapZ-α1/β2 (mouse) (gift from Prof. J Cooper) were bacterially expressed and purified. Recombinant profilin was purified as in Federov et al. [30]). pYeAxII (annexin A2-rat) (a kind gift from Dr. J. Ayala-Sanmartin, Paris) was expressed and purified from yeast.
2.2. Immunogold electronmicroscopy
F-actin filaments were polymerised by adding 10× F-buffer (100 mM HEPES, pH7, 20 mM MgCl2, 500 mM KCl). Recombinant annexin A2 was added to a final concentration of 2 mM in the presence of 50 μM calcium and incubated for 30 min at 4 °C. HH7 anti-annexin A2 antibody (a kind gift from Prof. V. Gerke, Münster) was added at a final concentration of 1 mg/ml and incubated for 4 h at 4 °C. A 10 nm immunogold-labelled goat anti-mouse secondary antibody (Aurion; The Netherlands) was added at a final concentration of 1 mg/ ml. F-actin was pelleted by centrifugation at 90 000 ×g for 30 min and filaments were then processed as described by Zhao and Craig [31]. Images were captured with a JEOL 1010 electron microscope.
2.3. Loading of cells with PI(4,5)P2-TMR, rocket induction and subsequent fixation
PI(4,5)P2-TMR (Invitrogen) was loaded into rat basophilic leukaemia cells (RBLs) using a Shuttle PIP™ carrier-1 (Invitrogen) according to the manufacturer's instructions. Briefly, 40% confluent RBL cells grown on glass coverslips (Matek) were incubated with 50 μl growth media (DMEM+10%FCS) containing 3 μl of Shuttle-PIP/TMR-PI(4,5) P2 complexes. Complexes were allowed to form by incubating 2 μl of 1 μg/μl TMR-PI(4,5)P2 with 3 μl 0.5 mM Shuttle PIP stock for 5 min at 20 °C. In order to induce rocketing vesicles, cells were exposed to 150 mM sucrose, 10 nM PMA in HBSS for 15 min as described previously [26]. Cells were washed twice with pre-warmed HBSS, and fixed for 30 min with pre-warmed 4% paraformaldehyde in DMEM, 2 mM EGTA and for a subsequent 3 h in 4% paraformaldehyde in PBS at 4 °C.
2.4. Bead assays
Polystyrene beads (diameter 1 μm, Polysciences) were coated with GST-Scar WA proteins according to the manufacturer's protocol. Protein binding was confirmed by measurement of the protein concentration in the supernatant before and after coupling. Before use, the coated beads were washed, resuspended and stored in 10 mM HEPES, pH7.0, 50 mM KCl, 5 mg/ml BSA, 5% glycerol and 0.01% NaN3. 1% of bead suspension was added to motility buffer (10 mM HEPES pH7.4, 50 mM KCl, 1 mM ATP, 7 mM DTT, 5 mM DACo (sigma), 7.5 μM actin (10% rhodamine labelled), 3.5 μM cofilin, 100 nM Arp2/3 and 50 nM capZ). The reactions were stopped by addition of phalloidicin (final concentration 5 mM) to the mixture at indicated time points, and filaments were mounted between a slide and a coverslip. Images were captured on an Improvision system and analysed using Metamorph.
2.5. Annexin A2 depletion
Annexin A2 was depleted from Lowe's fibroblasts using a human-specific single siRNA oligonucleotide pair (target sequence: AAG TGC ATA TGG GTC TGT CAA) transfected using Fugene HD (Roche, Mannhein, Germany) reagent as per manufacturer’s instructions. A pair of oligonucleotides with specificity for GFP was used as a control.
2.6. Immunofluorescence
Cells were washed twice in cold PBS, fixed in 3.7% paraformaldehyde, stained and imaged on a Leica SP2 AOBS confocal microscope. Annexin A2 was stained using the monoclonal antibody HH7. Actinwas imaged using Alexa fluor (Invitrogen, Oregon, USA). Lowe’s fibroblasts were classified as containing > 100 comets (essentially uncountable), < 100 comets or comet-free. Collagen I was labelled using Alexa Fluor 545 carboxylic acid, succinimidyl ester (Invitrogen, Oregon, USA).
3. Results
3.1. Annexin A2, PI(4,5)P2 and actin colocalise in the tails of macropinocytic rockets
3.1.1. In rat basophils
Rat basophilic leukaemia cells (RBLs) were grown on a collagen and fibronectin extracellular matrix and treated with hyperosmotic shock (HOS: 150 mM sucrose) and sodium pervanadate (200 μM) to induce macropinocytic rocketing. Hyperosmotic shock has been shown to induce the stress response pathway, MAPK kinase signalling and PI(4,5)P2 formation through activation of PI(4)P-5 kinase beta [32] and treatment with sodium pervanadate, an inhibitor of phosphotyrosine phosphatases, results in sustained phosphorylation of proteins on tyrosine, phenocopying constitutive extracellular signalling. The cells were fixed and stained for actin. Fig. 1A shows a series of sections through these cells, starting near the bottom of the cells. Macropinocytic rockets were particularly prevalent near points of cell-cell contact, suggesting a role for physiologic stimuli in the initiation of the rocketing process. The cup-like shape of the actin is evident from some of the larger rockets visible in the sections distal to the coverslip. When the cells were grown on collagen which had been fluorescently labelled it was possible to see internalisation of collagen into a proportion of the rocketing vesicles, showing that the cells may sample the extracellular matrix or perhaps internalise ligand-bound cell surface receptors during this process (Fig. 1B). This result lends weight to the idea that actin is involved in the endocytic trafficking of integrins [33]. Annexin A2 colocalised with actin on the rocket tails along their entire length (Fig. 2A), in agreement with previous results from our laboratory [24].
Fig. 1. HOS and pervanadate induce macropinocytic rocketing in RBL cells.
(A) Sections through the cells, from the bottom to the midline, reveal formation of actin rockets predominantly at the points of cell contact. Blue arrow indicates the cup-shaped region at the tip of a large vesicle. Scale bar=35 μm. (B) When the extracellular matrix was labelled with a fluorescent marker (red) it was seen to be internalised when cells underwent rocketing. Arrows show vesicular structures labelled with TRITC-collagen I (red) at the end of comet tails. Scale bar in A=30 μm, in B top row=30 μm, bottom row=8 μm.
Fig. 2. Annexin A2 colocalises with F-actin and PI(4,5)P2 in rockets induced by HOS and pervanadate.
RBLs were induced to rocket using hyperosmotic stress (HOS) and sodium pervanadate (200 μM). (A) Annexin A2 was observed to colocalise with actin on the tails of comets, particularly those at the periphery of cells. Scale bar=12 μm. (B) TMR-labelled PI(4,5)P2 (red) localised to the tips (top panels) and the in some cases also the actin tails (green) (lower panels) of comets. Scale bar in A=5 μm). (C) Annexin A2 partially colocalised with actin and PI(4,5)P2 (localised using the PH domain of PLCδ-fused to GFP) in the tails of HOS/pervanadate induced rockets. Scale bar=5 μm.
RBL cells were then pre-loaded with TMR-PI(4,5)P2, a fluorescent analogue of PI(4,5)P2, and macropinocytic rocketing was again induced using hyperosmotic shock and pervanadate. The cells were fixed and stained for actin using fluorescent phalloidin. PI(4,5)P2 was present on the tips of many of the rockets, in some cases in a slightly concave distribution, suggesting that it had become localised to one face of the rocketing vesicle. We also observed a partial localisation of TMR-PI(4,5)P2 within the rocket tails, suggesting that PI(4,5)P2 may be shed from macropinosomes as they move through the cell (Fig. 2B, C). We were also able to localise PI(4,5)P2 in these cells using the PH domain of PLCδ fused to GFP. Once again we saw partial colocalisation of annexin A2, actin and PI(4,5)P2 on comet tails when the cells were induced to rocket (Fig. 2C)
3.1.2. In Lowe-syndrome fibroblasts
Actin-based vesicular rocketing has been described in skin fibroblasts derived from a patient with oculocerebral disease of Lowe [34]. Individuals with this condition show a complex syndrome involving congenital cataracts, mental retardation and renal tubular dysfunction [35]. The disease is X-linked and was found to be due to a number of mutations in a PI(4,5)P2-5 phosphatase now known as OCRL1 [36]. OCRL1 is predominantly localised to the trans-Golgi network [37] and extracts from kidney epithelial cells from patients with mutations in this disease typically show less than 10% 5-phosphatase activity. Loss of OCRL1 activity leads to accumulation of PI(4,5)P2 [38]. We hypothesised that such cells may be useful as a model for studying the role of annexin A2 in PI(4,5)P2-mediated vesicle rocketing, and accordingly examined Lowe Syndrome fibroblasts (LS-fibroblasts) from two patients designated (LoweB and Lowe2) and showed that both contained significant numbers of actin-rockets. We observed no significant differences in the behaviour of LoweB and Lowe2 fibroblasts and all results shown are LoweB cells for consistency. Very few rockets were observed in normal human skin fibroblasts (Supplementary Fig. 1). Addition of 10 nM PMA to LS-fibroblasts resulted in an increase in the width of the actin tails, but no obvious increase in their abundance. A minority of cells contained large comet tails that were reminiscent of those observed in RBL cells (Fig. 3A). The majority of cells, however, contained clouds of tiny comets (Fig. 3B). The rockets failed to colocalise with the trans-Golgi network-specific marker TGN46 or Rab11 (a marker for the late-recycling endosome) (data not shown) so it remains to be established whether they are derived from the plasma-membrane or Golgi apparatus. Using indirect immunofluorescence we were able to localise annexin A2 to these comet tails, where it revealed a discontinuous pattern of staining along the tail, a characteristic that has not been observed in other systems (Fig. 3C).
Fig. 3. Annexin A2 is associated with rockets in Lowe’s fibroblasts.
Lowe’s fibroblasts were fixed and stained for F-actin. In a small proportion of cells we observed large comet tails (A) scale bar=15 μm. In the majority of cells however we identified a large population of tiny rockets which clustered together in a perinuclear zone (B) Scale bar=5 μm. Co-staining of the cells with Annexin A2 revealed that it associated with these rockets in a discontinuous, punctate fashion. (C) scale bar=25 μm, close-ups scale bar=3 μm.
To further examine whether elevation of PI(4,5)P2 is responsible for the increase in rocketing in LS-fibroblasts, and not loss of the OCRL1 protein per se, we treated the cells with 20 mM butan-1-ol. Primary alcohols compete with phosphatidylcholine in the reaction forming phosphatidic acid (PA) leading to less activation of PI(4P)-5 kinase and reduced production of PI(4,5)P2 [39]. Pretreatment of LoweB cells with 20 mM butan-1-ol for 5 min resulted in a 50% reduction in the number of cells exhibiting actin rockets.We observed no inhibition of rocketing, however, when the cells were treated with butan-2-ol which does not affect PI(4,5)P2 synthesis (Fig. 4A).
Fig. 4. PI(4,5)P2 and calcium are necessary for actin-based rocketing in Lowe’s fibroblasts.
(A) Lowe’s fibroblasts or control skin fibroblasts were treated with nothing, 2-butanol (2-but) or 1-butanol (1-but) for 20 min before fixing and staining for actin. Cells were classified as containing > 100, <100, large rockets or no rockets. Large numbers of rockets were observed in Lowe’s fibroblasts but not in control fibroblasts, and rocketing was inhibited by the addition of the primary alcohol butan-1-ol, but not by the secondary alcohol butan-2-ol. (B) Lowe’s fibroblasts treated for 20 min with BAPTA-AM (50 μM) fail to generate internal rockets, but external ‘filopod-like’ extensions, appear on their apical surface. (a) Section close to the base of the cell showing stress fibres but no internal rockets. Scalebar=50 μm. (b) Apical surface of the cell, showing filopod-like extensions. (c) Shallow view of a 3D reconstruction of the cell showing the long apical processes. (d) Apical surface of a cell showing filopods, blue boxes represent close-ups scale bar=50 μm (e and f) of filopods with club-like actin structures at their tips, which may be associated with vesicles. Scale bare=8 μm. (C) Treatment with BAPTA-AM inhibits rocking in Lowe’s fibroblasts (results represent mean and SEM of 3 independent experiments at least 100 cells were examined in each case.) ** Represent P values <0.01.
Given that annexin A2 is a calcium-binding protein, and that elevation of the cytoplasmic calcium concentration invariably promotes annexin A2 association with membranes and actin, we examined the effect of sequestering cytoplasmic calcium on rocket formation. The calcium chelator BAPTA-AM almost completely inhibited internal comet formation, in agreement with our previous results [23] (Fig. 4C). Interestingly, we saw the formation of large numbers of extended actin-structures on the surface of a proportion of these cells in response to calcium chelation (Fig. 4B), suggesting that the dendritic actin-polymerisation previously directed towards plasma-membrane invagination and migration of the rockets was instead promoting filopod-like evagination of the membrane. We did not observe a dramatic affect of BAPTA-AM on the actin skeleton of normal skin fibroblasts (Supplementary Fig. 2) and did not observe these novel ‘filopod-like’ extensions. This observation will be the subject of further investigation. Partial depletion of annexin A2 from LS fibroblasts using siRNA (confirmed by Western blotting: Fig 5A) resulted in a reduction in the number of rockets (Fig. 5B, C) showing that, as in other cell types, annexin A2 has an important role in rocket formation in this system.
Fig. 5. Annexin A2 is involved in the actin-mediated rocketing observed in Lowe’s fibroblasts.
A) Western blot showing partial depletion of annexin2 from Lowe’s fibroblasts using siRNA. B) Complete knockdown of Annexin A2 was only apparent in a minority of cells. Close examination of the cytoplasm reveal punctate actin in Annexin A2-depleted cells, but actin rockets in the cells in which Annexin A2 is present. (C) Histogram showing inhibition of actin-rocketing in Lowe’s fibroblasts depleted of Annexin A2. Cells were classified as having greater than 100, less than a hundred or no visible rockets. We also examined the cells for the presence of large rockets. At least 100 cells were counted in 3 independent experiments. siRNA treated cells showed a significant reduction in the number of rockets (P<0.001). Scale bars= 50 μm or 6 μm in close-ups.
3.1.3. In an epithelial cell line
Comet-based vesicular trafficking from the Golgi to the apical membrane has also been described in polarized epithelial Madine-Darby canine kidney cells (MDCK) [10]. In this study, overexpression of PI(4)P 5-kinase-alpha or treatment with PMA induced wholesale rocket formation in approximately 25% of cells. In this system the cells become full of tiny rockets (of similar dimensions to those seen in the LS fibroblasts). When we used PMA to induce rocket formation in MDCK cells we observed formation not only of tiny rockets, but also very large, stubby rockets, which appeared at the periphery of clusters of cells (Fig. 6A). Using immunofluorescence we were able to localise annexin A2 throughout the comet tail (it was less clear whether annexin A2 localised to the tiny rockets due to the high background of cytoplasmic staining). Butan-1-ol but not Butan-2-ol inhibited the production of PMA-induced rockets in these cells indicating that PA or PI(4,5)P2 is also necessary for rocketing in this cell line (Fig. 6B, C). Loading of the cells with the calcium chelator BAPTA-AM did not inhibit rocket formation (data not shown) suggesting that, in contrast to RBL cells, rocketing is not dependent upon calcium levels under these conditions.
Fig. 6. Annexin A2 is present on actin rockets induced by PMA in MDCK cells.
(A) Actin rockets were induced in MDCK cells using 10 μM PMA. The cells were fixed and stained for Annexin A2 and actin. Rocket formation predominantly occurred in cells at the periphery of small colonies. These stubby rockets co-stained strongly for Annexin A2. (B) butan-1-ol but not butan-2-ol completely abrogated rocketing suggesting that PI(4,5)P2 production down-stream of PKA activation was necessary for comet formation. Scale bars=80 μm. (C) Close-ups showing the actin cytoskeleton. Yellow arrows indicate rocket tails. Scale bars=20 μm.
3.2. Annexin A2 promotes actin-tail formation in vitro
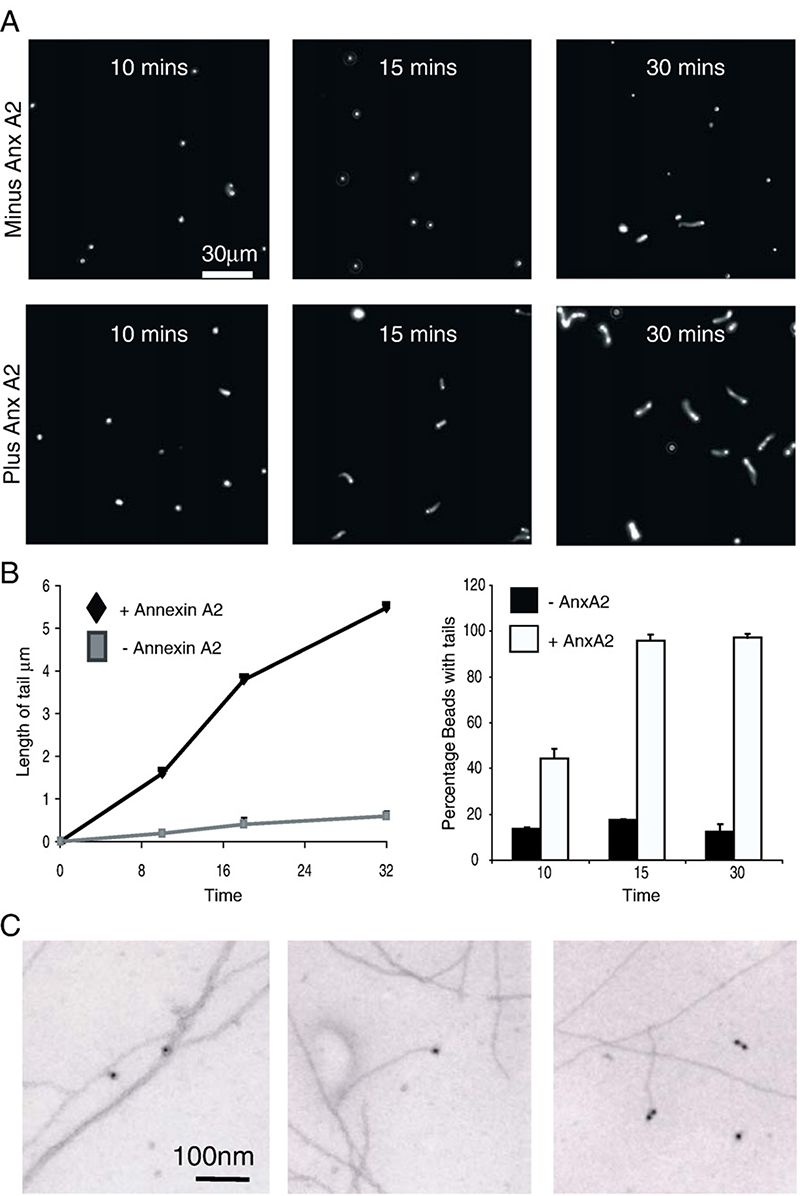
Actin tails have been reconstituted in vitro by many labs with no apparent requirement for Annexin A2 [40,41]). To determine whether annexin A2 exerts an influence on any of the parameters of actin tail formation, such as the kinetics of tail elongation, we used an established assay system including Arp2/3, actin, cofilin and polystyrene beads coated with the Arp2/3-activating (VCA) domain of Scar, to which annexin A2 was added. Initial experiments not only revealed efficient actin tail formation in the absence of annexin A2, but also that annexin A2 was unable to functionally substitute for any of the protein constituents of this assay (data not shown). We therefore examined the role of one of the major buffer components, namely methylcellulose, which has been shown to be critically dependent for rapid actin assembly by favouring spontaneous nucleation [40]. In the absence of methylcellulose, functionalised beads with actin tails were virtually non-existent, though following a 30 minute incubation there was an increase in the number of beads with actin clouds. Addition of recombinant annexin A2 to the assay mix lacking methylcellulose restored the formation and elongation of actin tails in a dose-dependent manner (Fig 7A). During a 15–30 minute incubation period and in the absence of annexin A2, the number of beads with associated actin was virtually constant at ~ 10%, whereas in the presence of annexin A2 almost 100% of beads had associated actin tails within 15 min (Fig 7B). The mean length of these actin tails increased with linear rate kinetics to an average of approximately 6 μm after 30 min. These results show that annexin A2 can functionally substitute for methylcellulose, suggesting that it may have a role in facilitating nucleation of filament growth in this system.
Fig. 7. Annexin A2 promotes actin tail formation in vitro.
(A) Annexin A2 promotes actin comet-tail formation on scar-VCA coated polystyrene beads. By 30 min in the presence of Annexin A2 (4 μM) all beads have a prominent actin tail, whereas in the absence of Annexin A2 only a minority of beads have succeeded in nucleating tail formation (representative fields of view, 5% rhodamine-labelled Mg-ATP actin). (B) Histogram quantifying the data in (A) (accumulated from 25 fields of view – approximately 300 beads). Representative experiment of 3 repeats. ** data significant to P<0.001. Also: growth of actin filament comet-tails on scar-VCA coated beads as a function of time. Measurements of actin tail-length were calculated using Metamorph (at least 200 tails at each time point). Annexin A2 was added at 4 μM, at lower concentrations there was no significant effect (data not shown). Control beads were incubated with 4 μM BSA in the same buffer. We noted a significant increase in the percentage of beads with tails at all time points. (P<0.001). (C) Annexin A2 localises to the ends of F-actin filaments. Purified recombinant Annexin A2 was added to pre-formed F-actin filaments, and was immuno-labelled using an anti-Annexin A2 antibody and a secondary anti-mouse antibody conjugated to 10 nM gold. The sample was then shadowed and prepared for electron microscopy.
3.3. Annexin A2 is localised to F-actin filament ends
Although we have previously ascertained through biochemical means that annexin A2 blocks polymerisation at the barbed-ends of F-actin filaments, which is highly suggestive that it physically associates with the ends of actin filaments, we wanted to obtain further direct evidence for this. Recombinant annexin A2 was incubated with preformed F-actin filaments and they were prepared for immunoelectron microscopy. Annexin A2 (labelled by immuno-gold) is clearly seen at the ends of actin filaments (Fig. 7C). Careful observation of many images revealed that all gold particles were associated with filament ends and none was found on the sides of actin filaments or between bundles of filaments in the absence of a filament end terminating at that point in the filament bundle; suggesting that annexin A2 has a specific affinity for the free ends in agreement with our previous observations. We did not see annexin A2 on the end of every filament, which may be an artefact of sample preparation, or may reflect specific binding of annexin A2 to the fast-growing, barbed end of the filament and not to the slow-growing, pointed end, as was suggested by our previous biochemical analyses [28].
3.4. Annexin A2 is sufficient to link F-actin filaments to PS and PI(4,5)P2 containing vesicles
In light of the dual functions (both lipid and F-actin interactions) of annexin A2 we wanted to explore whether annexin A2 was sufficient to link F-actin filaments to anionic phospholipids in membranes. We incubated pre-formed F-actin filaments (stabilized and labelled by TRITC-phalloidin) with vesicles containing 10% phosphatidylserine (PS) and 90% phosphatidylcholine (PC). We did not see any obvious association between the vesicles or the filaments (Fig. 8, top left panel). When annexin A2 was added in the presence of 50 μM calcium, we observed aggregation of the F-actin with the vesicles (remaining panels), which was not present when the vesicles were solely composed of PC (not shown). We then examined the effect of annexin A2 on the association of F-actin with PI(4,5)P2 containing vesicles (2.5% PI(4,5)P2) and 97.5% PC (Fig. 8B). We observed an effect of annexin A2 in the presence (top panels) or the absence (lower panels) of calcium; though the aggregates of F-actin around the vesicles appeared smaller. These data suggest that annexin A2 is sufficient to link F-actin to vesicular membranes.
Fig. 8. Annexin A2 is sufficient to link F-actin filaments to vesicles charged with anionic phospholipids.
(A) Liposomes were formed containing 10% PS and 90% PC. They were added to a preformed F-actin filaments which were stabilized and labelled with FITC-phalloidin in a buffer containing 50 μM calcium. There was no obvious association of filaments with vesicles (top left panel). When Annexin A2 was added (4 μM) however, the F-actin filaments associated with the vesicles, forming large aggregates. (B) In another experiment Annexin A2 was added to vesicles containing 2.5% PI(4,5)P2 and 97.5% PC. Annexin A2 was able to associate the F-actin filaments to the vesicles to induce the formation of actin-vesicle aggregates (left hand panels). In this case the vesicles were labelled internally with rhodamine-dextran, and the vesicles themselves can be seen in the panels on the right. Aggregation occurred in the presence (top) or absence (bottom) of 50 μM calcium. Scale bar=50 μm.
4. Discussion
In this study we have shown, in a variety of independent systems, that the actin, PI(4,5)P2 and calcium-binding protein annexin A2 is involved in a number of aspects of comet tail formation. We observe that annexin A2 and PI(4,5)P2 co-localise in comets induced by HOS and pervanadate in RBL cells, and show that annexin A2 colocalises to the rockets which form constitutively in skin fibroblasts derived from patients with oculocerebrorenal disease of Lowe. This condition arises due to mutation in OCRL1, a PI(4,5)P2-5-phosphatase, and constitutive rocketing is thought to be due to increased abundance of PI(4,5)P2 on endomembranes [34]. The molecular mechanism by which mutations in OCRL1 contribute to the complex pathology observed in this disease is unknown; OCRL1 has been shown, however, to be involved in the formation of early endosomes [46] and for rab-mediated trafficking from the Golgi [47]. Poorly-regulated transport through the endosomal pathway could explain the elevated concentration of lysosomal components in the blood plasma of patients with this disease [48]. Here we show that depletion of annexin A2 from these cells partially inhibits this aberrant rocketing. We also show that rocketing is calcium dependent and may also be inhibited by a primary alcohol, which blocks PA and subsequently PI(4,5)P2 production. We have shown previously that calcium partially regulates both the actin and PI(4,5)P2 binding capability of annexin A2 [23,28]. Calcium and PI(4,5)P2 regulate the actin-modulatory activity of a number of proteins. In the case of gelsolin for example, calcium and PI(4,5)P2 act in concert to control the capping, nucleating and severing activities mediated by its four actin-binding sites [42–44]. Thus, we cannot be certain that annexin A2 is the only calcium-dependent component of the rocket. Although it is not known exactly what annexin A2 is doing in the context of the growing rocket tail, the fact that annexin A2 is involved in barbed-end capping and is sufficient to link F-actin filaments to PI(4,5)P2 containing vesicles, suggests that annexin A2 acts to cap filaments at the head of the growing rocket and also to bridge between the F-actin tail and its associated vesicle. In this regard it is perhaps distinct from other actin-modulatory proteins, the actin binding of which is usually inhibited by PI(4,5)P2.
We also localised annexin A2 to rockets induced in an epithelial cell line (MDCK cells) induced by PMA. PMA is an agonist of PKC-family members and has been shown to induce actin rockets in both MDCK cells and in Xenopus egg extracts [10,49]. The exact role for PKCs in rocket formation has not been fully elucidated; but it has been suggested that diacylglycerol (formed, in part, by the action of phospholipase C family members on PI(4,5)P2) could recruit PKCs to endosomes and that this, in turn, recruits N-Wasp which promotes actin tail formation [49]. Interestingly, over-expression of OCRL1 in MDCK cells has been shown to inhibit lipid raft associated apical transport [10]. In the same paper the authors showed that inhibition of PA and PI(4,5)P2 production by butan-1-ol also inhibited apical transport. We were also able to inhibit formation of the large rockets using butan-1-ol. Together these results suggest a critical role for PA or PI(4,5)P2 metabolism in both apical transport and comet-tail formation; though we cannot say with certainty that apical transport is dependent upon vesicular rocketing.
We have also shown that annexin A2 facilitates comet tail formation in a bead assay in which there is no requirement for annexin A2 to link the tail to the bead. In this context annexin A2 may facilitate nucleation of new filaments or cap the filaments. It has been suggested that the transition from a dendrite-dominated mode of actin growth to a filopod-like bundled, linear mode of growth is critically dependent upon the amount of filament capping that occurs [45] and that it is enhanced filament capping and branching in the vicinity of the bead that is the driving force for rocketing [40]. We would predict that annexin A2 is involved in this process, given its preference for binding to filament ends. Although a ‘minimal’ set up of six proteins has been defined for actin rocketing in in vitro assays, it is clear that pathological bacteria use a far wider toolbox of actin remodelling proteins to facilitate their migration through the cytoplasm. It seems likely that physiological, vesicular rocketing employs an overlapping but distinct complement of proteins and that annexin A2 plays a crucial role in this context.
Supplementary Material
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.bbamcr.2008.10.007.
Acknowledgements
The authors thank Volker Gerke and Ursula Rescher for the Annexin A2 antibodies and Peter Munro for the electron microscopy advice and technical assistance. This work was supported by a programme grant from the Wellcome Trust (ref: GR072694MF) and the Lowe Syndrome Trust (A.G and T.L).
References
- [1].Lanzetti L. Actin in membrane trafficking. Curr Opin Cell Biol. 2007;19:453–458. doi: 10.1016/j.ceb.2007.04.017. [DOI] [PubMed] [Google Scholar]
- [2].Girao H, Geli MI, Idrissi FZ. Actin in the endocytic pathway: from yeast to mammals. FEBS Lett. 2008;582:2112–2119. doi: 10.1016/j.febslet.2008.04.011. [DOI] [PubMed] [Google Scholar]
- [3].De Matteis MA, Luini A. Exiting the Golgi complex. Nat Rev Mol Cell Biol. 2008;9:273–284. doi: 10.1038/nrm2378. [DOI] [PubMed] [Google Scholar]
- [4].Cameron LA, Giardini PA, Soo FS, Theriot JA. Secrets of actin-based motility revealed by a bacterial pathogen. Nat Rev Mol Cell Biol. 2000;1:110–119. doi: 10.1038/35040061. [DOI] [PubMed] [Google Scholar]
- [5].Skoble J, Auerbuch V, Goley ED, Welch MD, Portnoy DA. Pivotal role of VASP in Arp2/3 complex-mediated actin nucleation, actin branch-formation, and Listeria monocytogenes motility. J Cell Biol. 2001;155:89–100. doi: 10.1083/jcb.200106061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fehrenbacher K, Huckaba T, Yang HC, Boldogh I, Pon L. Actin comet tails, endosomes and endosymbionts. J Exp Biol. 2003;206:1977–1984. doi: 10.1242/jeb.00240. [DOI] [PubMed] [Google Scholar]
- [7].Lambrechts A, Gevaert K, Cossart P, Vandekerckhove J, Van Troys M. Listeria comet tails: the actin-based motility machinery at work. Trends Cell Biol. 2008;18:220–227. doi: 10.1016/j.tcb.2008.03.001. [DOI] [PubMed] [Google Scholar]
- [8].Gouin E, Gantelet H, Egile C, Lasa I, Ohayon H, Villiers V, Gounon P, Sansonetti PJ, Cossart P. A comparative study of the actin-based motilities of the pathogenic bacteria Listeria monocytogenes, Shigella flexneri and Rickettsia conorii . J Cell Sci. 1999;112:1697–1708. doi: 10.1242/jcs.112.11.1697. [DOI] [PubMed] [Google Scholar]
- [9].Wiesner S, Boujemaa R, Carlier M-F. Actin-based motility of Listeria monocytogenes and Shigella flexneri . Methods Microbiol. 2002;31:245–262. [Google Scholar]
- [10].Guerriero CJ, Weixel KM, Bruns JR, Weisz OA. Phosphatidylinositol 5-kinase stimulates apical biosynthetic delivery via an Arp2/3-dependent mechanism. J Biol Chem. 2006;281:15376–15384. doi: 10.1074/jbc.M601239200. [DOI] [PubMed] [Google Scholar]
- [11].Sandilands E, Cans C, Fincham VJ, Brunton VG, Mellor H, Prendergast GC, Norman JC, Superti-Furga G, Frame MC. RhoB and actin polymerization coordinate Src activation with endosome-mediated delivery to the membrane. Dev Cell. 2004;7:855–869. doi: 10.1016/j.devcel.2004.09.019. [DOI] [PubMed] [Google Scholar]
- [12].Jost M, Simpson F, Kavran JM, Lemmon MA, Schmid SL. Phosphatidylinositol 4,5-bisphosphate is required for endocytic coated vesicle formation. Curr Biol. 1998;8:1399–1402. doi: 10.1016/s0960-9822(98)00022-0. [DOI] [PubMed] [Google Scholar]
- [13].Rozelle AL, Machesky LM, Yamamotoa M, Driessensc MHE, Insallb RH, Rotha MG, Luby-Phelpsa K, Marriottd G, Hallc A, Yin HL. Phosphatidylinositol 4,5-bisphosphate induces actin-based movement of raft-enriched vesicles through WASP-Arp2/3. Curr Biol. 2000;10:311–320. doi: 10.1016/s0960-9822(00)00384-5. [DOI] [PubMed] [Google Scholar]
- [14].Ma L, Cantley LC, Janmey PA, Kirschner MW. Corequirement of specific phosphoinositides and small GTP-binding protein Cdc42 in inducing actin assembly in Xenopus egg extracts. J Cell Biol. 1998;140:1125–1136. doi: 10.1083/jcb.140.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Miki H, Miura K, Takenawa T. N-WASP, A novel actin-depolymerizing protein, regulates the cortical cytoskeletal rearrangement in a PIP2-dependent manner downstream of tyrosine kinases. EMBO J. 1996;15:5326–5335. [PMC free article] [PubMed] [Google Scholar]
- [16].Higgs HN, Pollard TD. Regulation of actin polymerization by Arp2/3 complex and WASp/Scar proteins. J Biol Chem. 1999;274:32531–32534. doi: 10.1074/jbc.274.46.32531. [DOI] [PubMed] [Google Scholar]
- [17].Machesky LM, Insall RH. Scar1 and the related Wiskott-Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Curr Biol. 1998;8:1347–1356. doi: 10.1016/s0960-9822(98)00015-3. [DOI] [PubMed] [Google Scholar]
- [18].Janmey PA, Iida K, Yin HL, Stossel TP. Polyphosphoinositide micelles and polyphosphoinositide-containing vesicles dissociate endogenous gelsolin-actin complexes and promote actin assembly from the fast-growing end of actin filaments blocked by gelsolin. J Biol Chem. 1987;262:12228–12236. [PubMed] [Google Scholar]
- [19].Yonezawa N, Nishida E, Iida K, Yahara I, Sakai H. Inhibition of the interactions of cofilin, destrin, and deoxyribonuclease I with actin by phosphoinositides. J Biol Chem. 1990;265:8382–8386. [PubMed] [Google Scholar]
- [20].Lassing I, Lindberg U. Specific interaction between phosphatidylinositol 4,5-bisphosphate and profilactin. Nature. 1985;314:472–474. doi: 10.1038/314472a0. [DOI] [PubMed] [Google Scholar]
- [21].Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev. 2002;82:331–371. doi: 10.1152/physrev.00030.2001. [DOI] [PubMed] [Google Scholar]
- [22].Gerke V, Creutz CE, Moss SE. Annexins: linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell Biol. 2005;6:449–461. doi: 10.1038/nrm1661. [DOI] [PubMed] [Google Scholar]
- [23].Hayes MJ, Merrifield CJ, Shao D, Ayala-Sanmartin J, Schorey CD, Levine TP, Proust J, Curran J, Bailly M, Moss SE. Annexin A2 binding to phosphatidylinositol 4,5-bisphosphate on endocytic vesicles is regulated by the stress response pathway. J Biol Chem. 2004;279:14157–14164. doi: 10.1074/jbc.M313025200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rescher U, Ruhe D, Ludwig C, Zobiack N, Gerke V. Annexin A2 is a phosphatidylinositol (4,5)-bisphosphate binding protein recruited to actin assembly sites at cellular membranes. J Cell Sci. 2004;117:3473–3480. doi: 10.1242/jcs.01208. [DOI] [PubMed] [Google Scholar]
- [25].Gokhale NA, Abraham A, Digman MA, Gratton E, Cho W. Phosphoinositide specificity of and mechanism of lipid domain formation by annexin A2-p11 heterotetramer. J Biol Chem. 2005;280:42831–42840. doi: 10.1074/jbc.M508129200. [DOI] [PubMed] [Google Scholar]
- [26].Merrifield CJ, Moss SE, Ballestrem C, Imhof BA, Giese G, Wunderlich I, Almer W. Endocytic vesicles move at the tips of actin tails in cultured mast cells. Nat Cell Biol. 1999;1:72–74. doi: 10.1038/9048. [DOI] [PubMed] [Google Scholar]
- [27].Merrifield CJ, Rescher U, Almers W, Proust J, Gerke V, Sechi AS, Moss SE. Annexin A2 has an essential role in actin-based macropinocytic rocketing. Curr Biol. 2001;11:1136–1141. doi: 10.1016/s0960-9822(01)00321-9. [DOI] [PubMed] [Google Scholar]
- [28].Hayes MJ, Shao D, Bailly M, Moss SE. Regulation of actin dynamics by Annexin A2. EMBO J. 2006;25:1816–1826. doi: 10.1038/sj.emboj.7601078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Blanchoin L, Pollard TD, Mullins RD. Interactions of ADF/cofilin, Arp2/3 complex capping protein and profilin in remodeling of branched actin filament networks. Curr Biol. 2000;10:1273–1282. doi: 10.1016/s0960-9822(00)00749-1. [DOI] [PubMed] [Google Scholar]
- [30].Fedorov AA, Pollard TD, Almo SC. Purification, characterization and crystallization of human platelet profilin expressed in Escherichia coli. J Mol Biol. 1994;241:480–482. doi: 10.1006/jmbi.1994.1522. [DOI] [PubMed] [Google Scholar]
- [31].Zhao FQ, Craig R. Capturing time-resolved changes in molecular structure by negative staining. J Struct Biol. 2003;141:43–52. doi: 10.1016/s1047-8477(02)00546-4. [DOI] [PubMed] [Google Scholar]
- [32].Yamamoto M, Chen MZ, Wang YJ, Sun HQ, Wei Y, Martinez M, Yin HL. Hypertonic stress increases phosphatidylinositol 4,5-bisphosphate levels by activating PIP5KIbeta. J Biol Chem. 2006;281:32630–32638. doi: 10.1074/jbc.M605928200. [DOI] [PubMed] [Google Scholar]
- [33].Del Pozo MA, Balasubramanian N, Alderson NB, Kiosses WM, Grande-Garcia A, Anderson RG, Schwartz MA. Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization. Nat Cell Biol. 2005;7:901–908. doi: 10.1038/ncb1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Allen PG. Actin filament uncapping localizes to ruffling lamellae and rocketing vesicles. Nat Cell Biol. 2003;5:972–979. doi: 10.1038/ncb1059. [DOI] [PubMed] [Google Scholar]
- [35].Lowe CU, Terrey M, MacLachlan EA. Organic-aciduria, decreased renal ammonia production, hydrophthalmos, and mental retardation; a clinical entity. AMA Am J Dis Child. 1952;83:164–184. doi: 10.1001/archpedi.1952.02040060030004. [DOI] [PubMed] [Google Scholar]
- [36].Attree O, Olivos IM, Okabe I, Bailey LC, Nelson DL, Lewis RA, McInnes RR, Nussbaum RL. The Lowe’s oculocerebrorenal syndrome gene encodes a protein highly homologous to inositol polyphosphate-5-phosphatase. Nature. 1992;358:239–242. doi: 10.1038/358239a0. [DOI] [PubMed] [Google Scholar]
- [37].Dressman MA, Olivos-Glander IM, Nussbaum RL, Suchy SF. Ocrl1, a PtdIns(4,5) P(2) 5-phosphatase, is localized to the trans-Golgi network of fibroblasts and epithelial cells. J Histochem Cytochem. 2000;48:179–190. doi: 10.1177/002215540004800203. [DOI] [PubMed] [Google Scholar]
- [38].Zhang X, Hartz PA, Philip E, Racusen LC, Majerus PW. Cell lines from kidney proximal tubules of a patient with Lowe syndrome lack OCRL inositol polyphosphate 5-phosphatase and accumulate phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 1998;273:1574–1582. doi: 10.1074/jbc.273.3.1574. [DOI] [PubMed] [Google Scholar]
- [39].Morris AJ, Frohman MA, Engebrecht J. Measurement of phospholipase D activity. Anal Biochem. 1997;252:1–9. doi: 10.1006/abio.1997.2299. [DOI] [PubMed] [Google Scholar]
- [40].Wiesner S, Helfer E, Didry D, Ducouret G, Lafuma F, Carlier MF, Pantaloni D. A biomimetic motility assay provides insight into the mechanism of actin-based motility. J Cell Biol. 2003;160:387–398. doi: 10.1083/jcb.200207148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Pantaloni D, Le Clainche C, Carlier MF. Mechanism of actin-based motility. Science. 2001;292:1502–1506. doi: 10.1126/science.1059975. [DOI] [PubMed] [Google Scholar]
- [42].Weeds AG, Harris H, Gratzer W, Gooch J. Interactions of pig plasma gelsolin with G-actin. Eur J Biochem. 1986;161:77–84. doi: 10.1111/j.1432-1033.1986.tb10126.x. [DOI] [PubMed] [Google Scholar]
- [43].Lagarrigue E, Maciver SK, Fattoum A, Benyamin Y, Roustan C. Co-operation of domain-binding and calcium-binding sites in the activation of gelsolin. Eur J Biochem. 2003;270:2236–2243. doi: 10.1046/j.1432-1033.2003.03591.x. [DOI] [PubMed] [Google Scholar]
- [44].Janmey PA, Iida K, Yin HL, Stossel TP. Polyphosphoinositide micelles and polyphosphoinositide-containing vesicles dissociate endogenous gelsolin-actin complexes and promote actin assembly from the fast-growing end of actin filaments blocked by gelsolin. J Biol Chem. 1987;262:12228–12236. [PubMed] [Google Scholar]
- [45].Vignjevic D, Peloquin J, Borisy GG. In vitro assembly of filopodia-like bundles. Methods Enzymol. 2006;406:727–739. doi: 10.1016/S0076-6879(06)06057-5. [DOI] [PubMed] [Google Scholar]
- [46].Erdmann KS, Mao Y, McCrea HJ, Zoncu R, Lee S, Paradise S, Modregger J, Biemesderfer D, Toomre D, De Camilli P. A role of the Lowe syndrome protein OCRL in early steps of the endocytic pathway. Dev Cell. 2007;13:377–390. doi: 10.1016/j.devcel.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hyvola N, Diao A, McKenzie E, Skippen A, Cockcroft S, Lowe M. Membrane targeting and activation of the Lowe syndrome protein OCRL1 by rab GTPases. EMBO J. 2006;25:3750–3761. doi: 10.1038/sj.emboj.7601274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ungewickell AJ, Majerus PW. Increased levels of plasma lysosomal enzymes in patients with Lowe syndrome. Proc Natl Acad Sci U S A. 1999;96:13342–13344. doi: 10.1073/pnas.96.23.13342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Taunton J, Rowning BA, Coughlin ML, Wu M, Moon RT, Mitchison TJ, Larabell CA. Actin-dependent propulsion of endosomes and lysosomes by recruitmentof N-wasp. J Cell Biol. 2000;148:519–530. doi: 10.1083/jcb.148.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.