Abstract
Prostate cancer is one of the leading causes of cancer-related death in men. The identification of new therapeutics to selectively target prostate cancer cells is therefore vital. Recently, the rotenoids rotenone (1) and deguelin (2) were reported to selectively kill prostate cancer cells and the inhibition of mitochondrial complex I was established as essential to their mechanism of action. However, these hydrophobic rotenoids readily cross the blood brain barrier and induce symptoms characteristic of Parkinson’s disease in animals. Since hydroxylated derivatives of 1 and 2 are more hydrophilic and less likely to readily cross the blood brain barrier, 29 natural and unnatural hydroxylated derivatives of 1 and 2 were synthesized for evaluation. The inhibitory potency (IC50) of each derivative against complex I was measured and its hydrophobicity (Slog10P) predicted. Amorphigenin (3), dalpanol (4), dihydroamorphigenin (5), and amorphigenol (6) were selected and evaluated in cell-based assays using C4-2 and C4-2B prostate cancer cells alongside control PNT2 prostate cells. These rotenoids inhibit complex I in cells, decrease oxygen consumption, and selectively inhibit the proliferation of prostate cancer cells, leaving control cells unaffected. The greatest selectivity and anti-proliferative effects were observed with 3 and 5. The data highlight these molecules as promising therapeutic candidates for further evaluation in prostate cancer models.
Prostate cancer is one of the leading causes of cancer-related death in men.1,2 Prostate tumors generally respond well to androgen deprivation therapy and shrink, but then almost invariably become androgen insensitive, regrow autonomously, and metastasize, often to bone.3,4 The identification of new therapeutic agents to selectively target prostate cancer cells is therefore vital. Recently, the natural rotenoids rotenone (1) and especially deguelin (2) (Figure 1) were shown to selectively kill phosphatase and tensin homologue deleted on chromosome 10 (PTEN)-null prostate cancer cells.5 The inhibition of mitochondrial complex I (NADH:ubiquinone oxidoreductase) was established as essential to their mechanism of action and selective toxicity.5 This finding further highlights the therapeutic potential of 1 and 2 6–11 and is consistent with their being canonical ubiquinone-binding site inhibitors of complex I.12–15 Complex I is a major entry point for electrons into the mitochondrial respiratory chain and an integral contributor to oxidative phosphorylation (OxPhos)16 that is a target for other potential anticancer compounds, such as metformin17,18 and IACS-01075919 from the MD Anderson Cancer Center. It oxidizes the NADH produced by the citric acid cycle and other metabolic pathways, transfers the electrons to ubiquinone to sustain the reduction of molecular oxygen through complexes III and IV, and transports protons across the mitochondrial inner membrane to maintain the proton motive force that drives ATP synthesis and metabolite transport.16 In cells, 1 and 2 inhibit complex I and disrupt OxPhos, leading to a decrease in mitochondrial ATP production, hydrolysis of ATP to maintain the mitochondrial membrane potential, and ultimately cell death by apoptosis.5 Selective toxicity towards prostate cancer cells arises because these cells require greater than normal amounts of ATP to sustain unchecked growth, following loss of the tumor suppressor PTEN early in tumorigenesis, and this ATP is produced most efficiently by OxPhos.5 PTEN-null prostate cancer cells are therefore highly dependent on OxPhos and especially vulnerable to inhibitors of complex I, while PTEN-positive normal prostate cells remain relatively tolerant of them.5
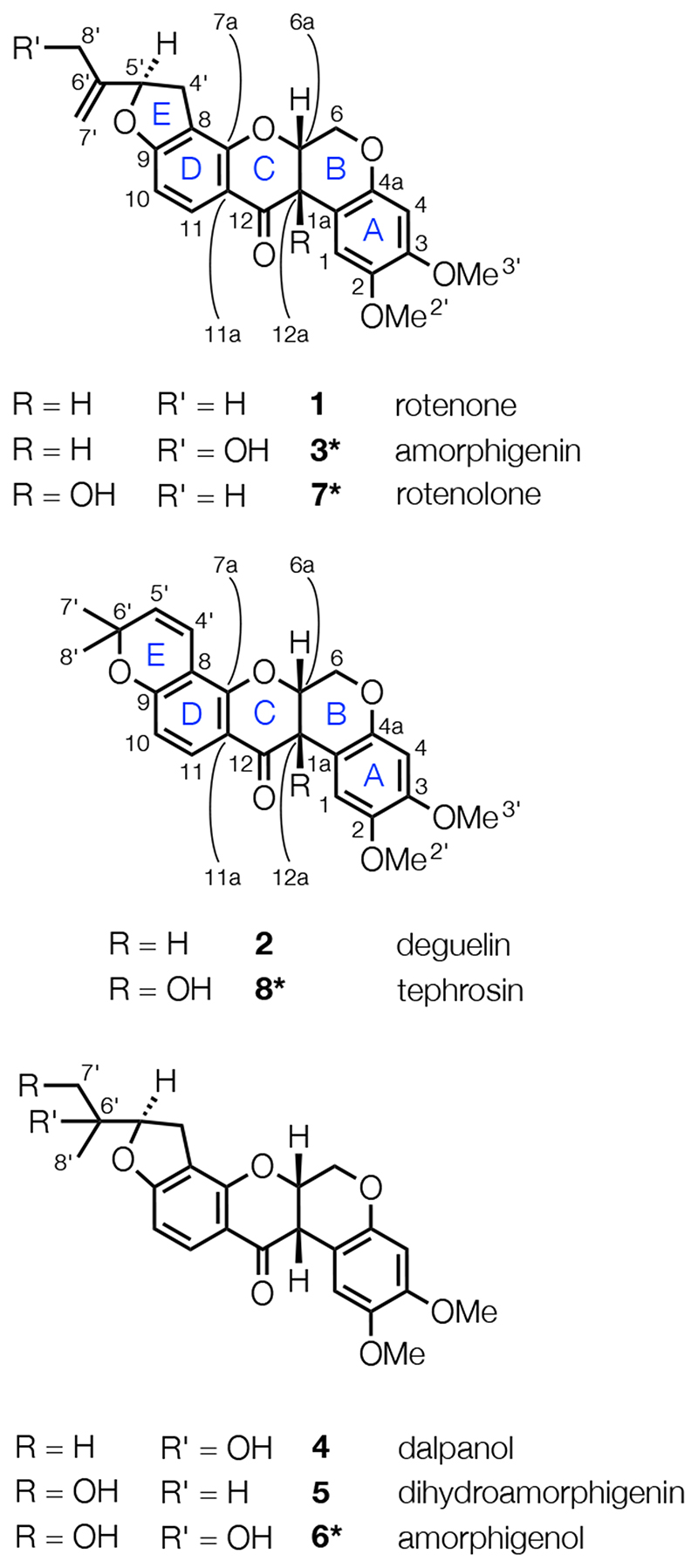
Figure 1.
The structures of rotenoids 1-8 with ring-labels and atom numbers. Molecules marked * are both plant secondary metabolites and products of detoxifying metabolism in mammals, fish, and insects.31,32
While rotenone (1) and particularly deguelin (2) thus appear as promising therapeutic agents, their neurotoxicity must be properly considered. Both 1 and 2 cross the blood-brain barrier (BBB) in rats and mice, cause neuronal damage when administered in doses of 3 and 6 mg kg-1 day-1, respectively, and induce symptoms characteristic of Parkinson’s disease.20 It is clear that 2 is less neurotoxic than 1 because it is more susceptible to oxidation and so metabolized and cleared more rapidly,20 but it is not clear whether 2 is suitable for long-term systemic administration.21 Importantly, recent work5 suggests that rotenoids related to 1 and 2 that inhibit complex I should also selectively kill prostate cancer cells. The pool of potential rotenoid therapeutic agents can therefore be expanded to include those with potentially lower neurotoxicity profiles.
To a first approximation, rotenone (1) and deguelin (2) readily cross the BBB because they are hydrophobic.22,23 Accordingly, hydroxylated rotenoids such as amorphigenin (3),24,25 dalpanol (4),26,27 dihydroamorphigenin (5),28 and amorphigenol (6) (Figure 1),29–32 which by comparison are more hydrophilic than 1 and 2, would not be expected to readily cross the BBB. Hydroxylated metabolites of 1 and 2 were analyzed for in the rat model of Parkinson’s disease, but only rotenolone (7) and tephrosin (8) (Figure 1) were detected in the brains of treated animals.20 Hydroxylated rotenoids that are more hydrophilic than 7 and 8, such as 3 and 6, could thus constitute potentially promising therapeutic agents for the treatment of prostate cancer.
However, although hydrophobicity is an important factor in determining whether a molecule can cross the BBB,22,23 the model outlined above is simplistic. Many additional factors can influence whether a molecule can cross the BBB, including molecular weight, volume, topological molecular polar surface area, solvent accessible surface area, and the number of hydrogen bond donors and acceptors.33,34 Molecules can also be actively expelled from the brain by various efflux transporters, such as P-glycoprotein, and their accumulation thereby prevented.34 Moreover, mice administered with 100-900 mg kg-1 of plant extracts rich in dalpanol (4) were reported to suffer severe neurological impairments,35 although histopathological analysis of the brain tissue of treated mice did not show any differences relative to those of control mice. Given these complicating factors, it is therefore important that the neurotoxicity profile of any rotenoid of therapeutic interest is established through carefully designed animal studies. A recent example of a detailed pharmacological evaluation of rotenone (1) provides a framework that can be applied to the analysis of related rotenoids.36
No comprehensive study has been made of the inhibitory properties of hydroxylated rotenoids against complex I or their ability to selectively target prostate cancer cells, though a number of reports have been made on the cytotoxicity of certain hydroxylated rotenoids.37–44 Thus, a library of 29 hydroxylated rotenoids was prepared from rotenone (1) and deguelin (2) to identify inhibitors of complex I that might be better suited to development as therapeutics than the parent compounds. The inhibitory effect of each hydroxylated rotenoid on complex I was determined and its hydrophobicity predicted. Amorphigenin (3), dalpanol (4), dihydroamorphigenin (5), and amorphigenol (6) (Figure 1) were selected for evaluation in cell-based assays using C4-2 and C4-2B bone-metastasized prostate cancer cells and control PNT2 prostate cells. The ability of each compound to reach and inhibit complex I in cells, its antiproliferative activity, and its selective toxicity towards prostate cancer cells over healthy prostate cells was then established. Each compound reaches and inhibits complex I in cells, selectively inhibits the proliferation of prostate cancer cells, and leaves healthy prostate cells unaffected. Further, the antiproliferative activities and inhibitory potencies are directly correlated. Finally, 3 and 5 are highlighted as highly active potential therapeutic candidates worthy of detailed evaluation in more sophisticated cell-based assays or animal models of prostate cancer.
Results and Discussion
Preparation of a Library of Hydroxylated Rotenoids
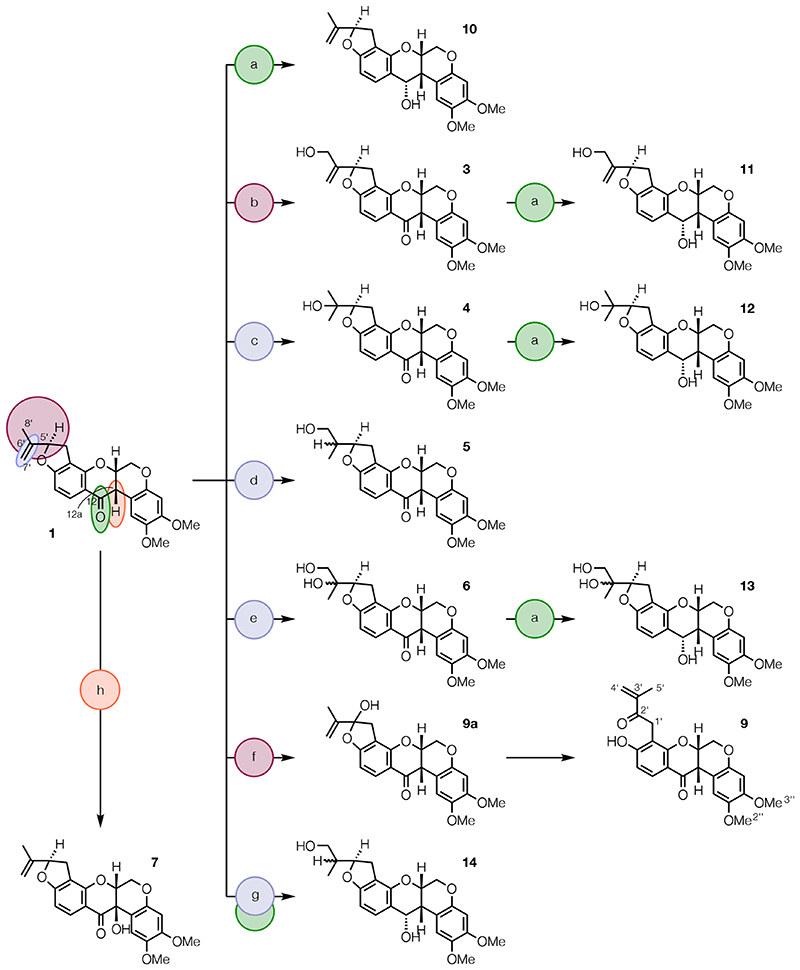
To prepare a library of hydroxylated derivatives of rotenone (1) and deguelin (2), redox reactions were applied to the C-12 carbonyl groups and the C-12a benzylic sites of 1 and 2, the Δ6′(7′) olefinic unit and the C-5′ and C-8′ allylic sites of 1, and the Δ4′(5′) olefinic unit of 2 (Schemes 1-4). Allylic oxidation of 1 at C-5′ with excess SeO2 in dry 1,4-dioxane gave keto-phenol 9 via hemiacetal 9a (Scheme 1).45 Allylic oxidation of 1 at C-8′, using N-(phenylseleno)phthalimide and H2O in the presence of catalytic camphorsulfonic acid followed by oxidation of the resulting β-hydroxyselenide with H2O2 in pyridine and elimination of the tertiary selenoxide, afforded amorphigenin (3).46 Markovnikov hydration of the Δ6′(7′) double bond, using an oxymercuration-demercuration sequence in which 1 was treated with Hg(OAc)2 in aqueous THF followed by work-up with saturated NaCl solution and rapid demurcuration with NaBH4 under weakly basic conditions, gave dalpanol (4).27 Anti-Markovnikov hydration, using an iridium-catalyzed hydroboration-oxidation sequence in which 1 was treated with pinacolborane in the presence of catalytic [Ir(COD)Cl]2 and bis(diphenylphosphino)ethane followed by oxidation with H2O2 under weakly basic conditions, provided dihydroamorphigenin (5).47 Amorphigenol (6) was prepared from 1 as described previously.48 Stereocontrolled reduction of the C-12-carbonyl group of 1 with NaBH4 in MeOH afforded alcohol 10,49 while Étard-like hydroxylation at C-12a of 1 using K2Cr2O7 in aqueous AcOH provided rotenolone (7).50 The diastereoselectivity of the last two reactions probably results from attack on the butterfly-wing conformer of 1,49,51 with hydride delivery (as in the conversion of 1 into 10) and the pericyclic Étard-like reaction (as in the conversion of 1 into 7) taking place on the more accessible convex face of the molecule. Reduction of the C-12 carbonyl groups of 3, 4, and 6 with NaBH4 gave diols 11 and 12, and triol 13, respectively (Scheme 1). Finally, diol 14 was obtained by concomitant hydroboration of the Δ6′(7′) double bond and reduction of the C-12 carbonyl group of 1, using BH3.SMe2, followed by oxidation of the intermediate borane with H2O2 under strongly basic conditions.
scheme 1. Synthesis of Derivatives of Rotenone (1)a .
aReagents and conditions: (a) NaBH4, MeOH, 0 °C to rt, 2 h, 88-92%; (b) i. N-PSP, H2O, CSA, CH2Cl2, rt, 72 h; ii. H2O2, pyridine 0 °C to rt, 2 h, 33%; (c) i. Hg(OAc)2, THF, H2O, rt, 18 h; ii. NaCl then NaHCO3, NaBH4, rt, 0.5 min, 48%; (d) i. [Ir(COD)Cl]2, DPPE, HBPin, CH2Cl2, rt, 20 h; ii. H2O2, NaHCO3, THF, H2O, rt, 20 h, 14%; (e) OsO4, NMO, citric acid, acetone, H2O (see ref 48); (f) SeO2, 4 Å MS, 1,4-dioxane, 80 °C, 3 h, 21%; (g) i. BH3 .SMe2, THF, 0 °C to rt, 1.5 h; ii. H2O2, NaOH, THF, H2O, 0 °C to rt, 18 h, 80%; (h) K2Cr2O7, AcOH, H2O, 60 °C, 0.5 h then rt, 18 h, 82%. Abbreviations: MS, molecular sieves; N-PSP, N-(phenylseleno)phthalimide; CSA, (+)-β-camphorsulfonic acid; COD, 1,5-cyclooctadiene; DPPE, 1,2-bis(diphenylphosphino)ethane; HBPin, pinacolborane; NMO, N-methylmorpholine N-oxide.
scheme 4. Synthesis of Derivatives of Deguelin (2)a .
aReagents and conditions: (a) m-CPBA, TsOH, CHCl3, 0 °C, 2 h, 62-69%; (b) NaBH4, MeOH, 0 °C to rt, 2 h, 90-92%; (c) K2Cr2O7, AcOH, H2O (see ref 54); (d) OsO4, NMO, citric acid, acetone, H2O, rt, 28 h, 78%; (e) i. PhSeCl, CH2Cl2; ii. H2O2, THF (see ref 54). Abbreviations: m-CPBA, meta-chloroperoxybenzoic acid; TsOH, tosic acid, NMO, N-methylmorpholine N-oxide.
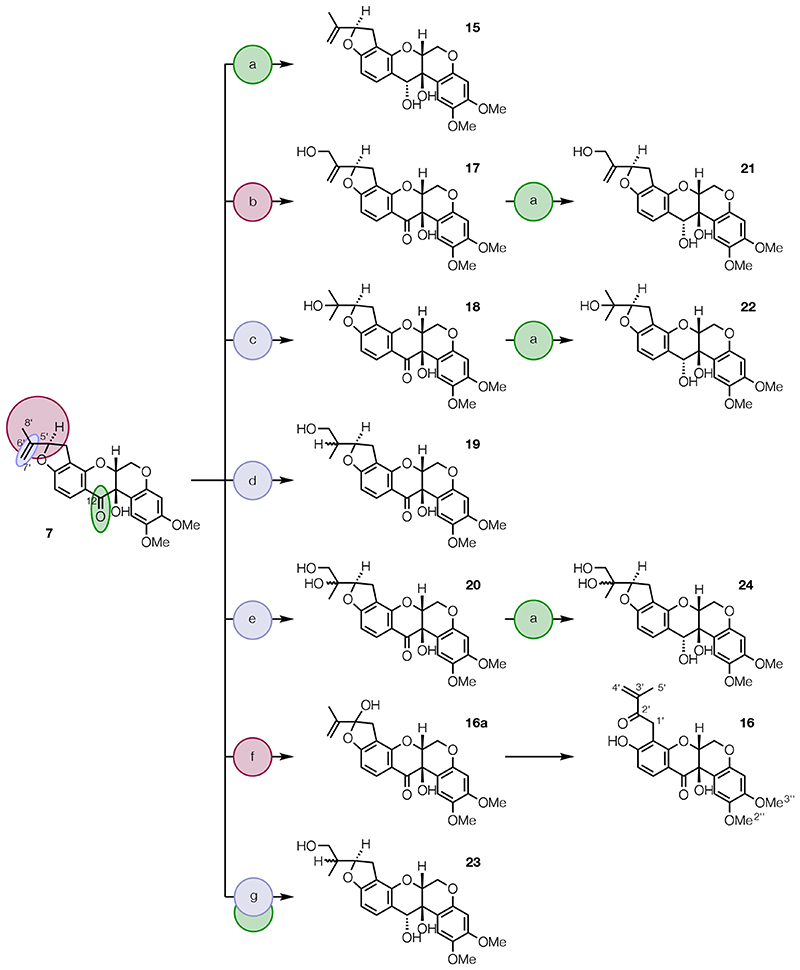
The library of compounds was expanded by derivatization of rotenolone (7) (Scheme 2) using the methods described above. Stereocontrolled reduction of the C-12 carbonyl group of 7 with NaBH4 gave diol 15.50 Regioselective allylic oxidation at C-5′ and C-8′ of 7 gave keto-phenol 16, via hemiacetal 16a, and 12a-hydroxyamorphigenin (17), respectively. Markovnikov and anti-Markovnikov hydration of the Δ6′(7′) double bond of 7 afforded 12a-hydroxydalpanol (18) and 12a-hydroxydihydroamorphigenin (19), respectively. 12a-Hydroxyamorphigenin (20) was obtained by dihydroxylation of 7 using catalytic OsO4 and stoichiometric NMO in the presence of pyridine.45 Reduction of 17, 18, and 20 with NaBH4 gave triols 21 and 22, and tetraol 24, respectively. Finally, concomitant hydroboration of the Δ6′(7′) double bond and reduction of the C-12 carbonyl group of 7 followed by oxidation of the intermediate borane afforded triol 23. Reduction of ketophenols 9 (Scheme 1) and 16 (Scheme 2) was not attempted as this would have led to further rotenonic acid-like molecules, which would likely be inactive.52,53
scheme 2. Synthesis of Derivatives of Rotenolone (7)a .
aReagents and conditions: (a) NaBH4, MeOH, 0 °C to rt, 2 h, 82-90%; (b) i. N-PSP, H2O, CSA, CH2Cl2, rt, 72 h; ii. H2O2, Al2O3, THF, 0 °C to rt, 6.5 h, 21%; (c) i. Hg(OAc)2, THF, H2O, rt, 18 h; ii. NaOH, NaBH4, rt, 0.5 min, 51%; (d) i. [Ir(COD)Cl]2, DPPE, HBPin, CH2Cl2, rt, 20 h; ii. H2O2, NaHCO3, THF, H2O, 0 °C to rt, 20 h, 12%; (e) OsO4, NMO, pyridine, acetone, H2O, rt, 42 h, 49%; (f) SeO2, 4 Å MS, 1,4-dioxane, 80 °C, 3 h, 20%; (g) i. BH3 .SMe2, THF, 0 °C to rt, 1.5 h; ii. H2O2, NaOH, THF, H2O, 0 °C to rt, 18 h, 87%.
Abbreviations: MS, molecular sieves; N-PSP, N-(phenylseleno)phthalimide; CSA, (+)-βcamphorsulfonic acid; COD, 1,5-cyclooctadiene; DPPE, 1,2-bis(diphenylphosphino)ethane; HBPin, pinacolborane; NMO, N-methylmorpholine N-oxide.
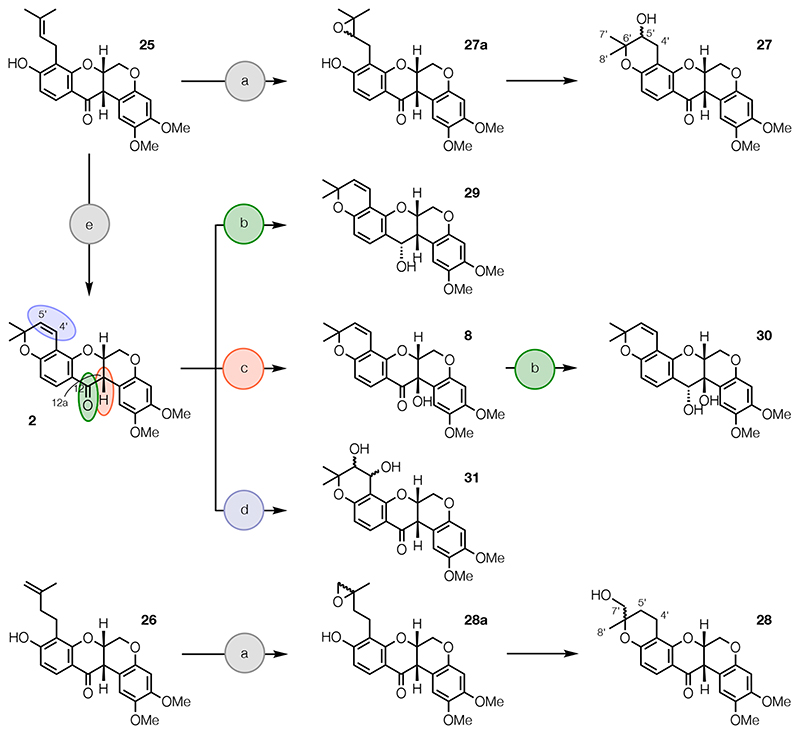
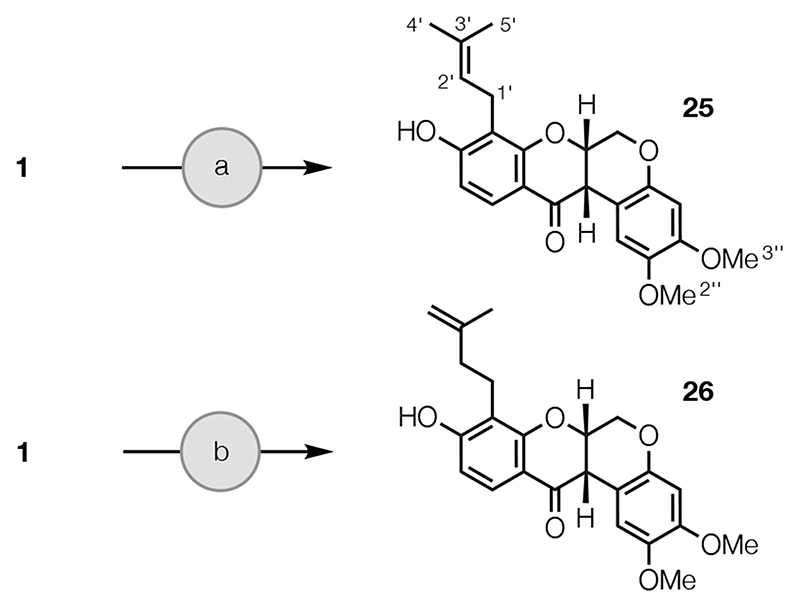
Next, rot-2′-enonic acid (25) was prepared from rotenone (1) as described previously (Scheme 3).54 The isomeric rot-3′-enonic acid (26) was also prepared from 1 (Scheme 3) by ring-opening with BBr3,55,56 to give 4′-bromorot-2′-enonic acid (see Experimental Section), and reaction with activated zinc powder and NH4Cl in aqueous THF.
scheme 3. Synthesis of Isomeric Rotenonic Acidsa .
aReagents and conditions: (a) i. HBr, AcOH; ii. Zn, NH4Cl, THF, H2O (see ref 54); (b) BBr3, CH2Cl2, −20 °C, 0.5 h, 60%; ii. Zn, NH4Cl, THF, H2O, rt, 0.5 h, 79%.
Finally, hydroxylated derivatives of deguelin (2), which was synthesized from 25 as described previously,54 were prepared (Scheme 4). Epoxidation and acid-catalyzed cyclization reactions of the isomeric rotenonic acids 25 and 26, using m-CPBA and tosic acid,57 yielded alcohols 27 and 28, via epoxides 27a and 28a, respectively. Reduction of the C-12 carbonyl group of 2 with NaBH4 gave alcohol 29 and hydroxylation at C-12a of 2 with K2Cr2O7 afforded tephrosin (8), as described previously.54 Reduction of the C-12 carbonyl group of 8 with NaBH4 afforded diol 30, while dihydroxylation of the Δ4′(5′) double bond of 2 using catalytic OsO4 and stoichiometric NMO in the presence of citric acid gave diol 31.58
In summary, by applying chemo-, regio-, and stereoselective redox reactions to rotenone (1), deguelin (2), and their derivatives, a library of 29 hydroxylated rotenoids (23 derivatives of 1 and six derivatives of 2) was assembled without the use of protecting groups.
Measurement of IC50 Values and Calculation of Slog10P Values for Each Derivative
Each derivative was tested for its inhibitory effect on complex I activity by using a bovine heart mitochondrial membrane assay for NADH:O2 oxidoreduction through complexes I, III, and IV. The established bovine model for the human enzyme was used due to ease of access to material. Half-maximal inhibitory concentrations (IC50 values) were calculated for all inhibitors with IC50 < 10 μM (Table 1). Rotenone (1) and deguelin (2) were included as positive controls and were the most potent, with IC50 values of 6.9 and 10.6 nM, respectively. Importantly, 23 out of the 29 hydroxylated derivatives (compounds 3-15, 17, 19, 23, and 25-31) were found to inhibit complex I with IC50 < 10 μM. Further, where direct comparisons can be made, for compounds 1, 2, 7, and 8 for example, the IC50 values agree well with those determined previously.39 It should be noted that compounds 5, 6, 13, 14, 19, 20, 23, 24, 27, and 28 were tested as mixtures of two C-5′ or C-6′-diastereoisomers (epimers) due to difficulties encountered in their separation, while compound 31 was tested as a mixture of two cis-4′,5′-diastereoisomers. IC50 values reported for these compounds are therefore reflective of their diastereoisomeric composition (see Experimental Section). It is well-established that the activity of rotenoids on complex I is strongly dependent on the stereochemistry of the cis-6a,12a BC ring-junction (Figure 1),53,59,60 which is conserved within our library, and less dependent on the stereochemistry of the (remote) C-5′ substituent in the E-ring.59,60 Individual C-5′ and, by extension, C-6′-diastereoisomers are therefore expected to have similar inhibitory activities on complex I.
Table 1. Experimentally Measured IC50 Values and Computationally Predicted Slog10P Values for the Rotenoids Tested.
| compound | IC50 (nM)a | 95 % CI | Slog10P |
|---|---|---|---|
| 1 | 6.9 | 6.5-7.4 | 3.70 |
| 2 | 10.6 | 10.0-11.1 | 4.01 |
| 3 | 130 | 124-136 | 2.68 |
| 4 | 652 | 598-711 | 2.90 |
| 5b | 228 | 220-237 | 2.76 |
| 6b | 1800 | 1620-2000 | 1.87 |
| 7 | 137 | 127-148 | 3.12 |
| 8 | 77.5 | 71.6-83.8 | 3.42 |
| 9 | 2570 | 2460-2690 | 3.02 |
| 10 | 11.4 | 10.3-12.6 | 3.65 |
| 11 | 458 | 437-480 | 2.62 |
| 12 | 750 | 700-802 | 2.84 |
| 13b | 4570 | 4400-4730 | 1.82 |
| 14b | 460 | 425-498 | 2.70 |
| 15 | 125 | 119-131 | 3.06 |
| 16 | >10000 | n/a | 2.44 |
| 17 | 3810 | 3520-4120 | 2.09 |
| 18 | >10000 | n/a | 2.31 |
| 19b | 3580 | 2620-5000 | 2.17 |
| 20b | >10000 | n/a | 1.29 |
| 21 | >10000 | n/a | 2.04 |
| 22 | >10000 | n/a | 2.26 |
| 23b | 2930 | 2770-3090 | 2.12 |
| 24b | >10000 | n/a | 1.23 |
| 25 | 1090 | 1050-1120 | 4.04 |
| 26 | 572 | 540-607 | 4.04 |
| 27c | 850 | 787-922 | 2.90 |
| 28b | 195 | 179-213 | 2.90 |
| 29 | 14.6 | 13.8-15.4 | 3.95 |
| 30 | 135 | 128-142 | 3.37 |
| 31d | 5310 | 4970-5680 | 2.48 |
The error for each IC50 value is reported as a 95% confidence interval (CI).
Tested as a mixture of two C-6′-diastereoisomers (epimers).
Tested as a mixture of two C-5′-diastereoisomers (epimers).
Tested as a mixture of two cis-4′,5′-diastereoisomers.
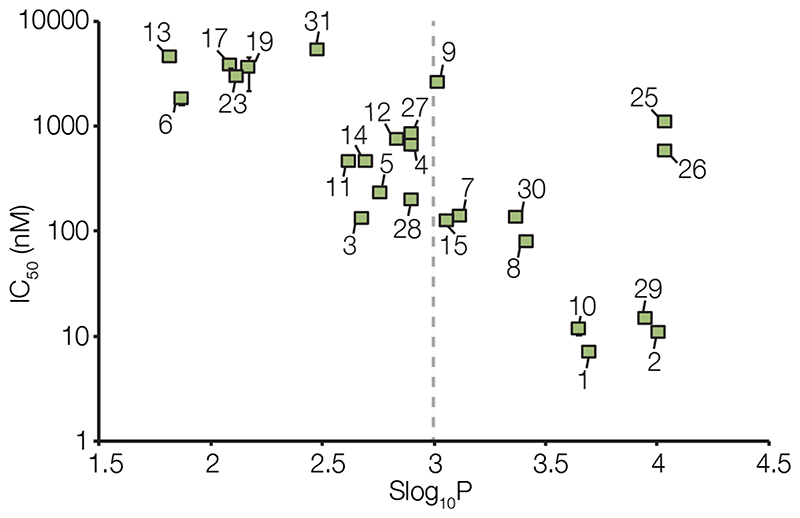
Next, the octanol-water partition coefficient (Slog10P) of each derivative was predicted using the Molecular Operating Environment (MOE) software package (version 2012.10, Chemical Computing Group) (Table 1). This parameter describes the hydrophobicity of a molecule and indicates the probability of it crossing the BBB by passive diffusion.22,23 The high Slog10P values for rotenone (1) and deguelin (2) of 3.70 and 4.01, respectively, were calculated for comparison, showing that they are among the most hydrophobic molecules in the library. Comparison of the IC50 and Slog10P data revealed a clear correlation between the hydrophobicity and inhibitory activity of the rotenoids tested, and the plot of IC50 vs Slog10P (Figure 2) shows that the more hydrophobic rotenoids are better complex I inhibitors. This is consistent with a binding model in which rotenoids bind in (or close to) the ubiquinone headgroup-binding site.61,62 This binding site is accessed through a channel that leads out into the membrane,63–66 and since rotenoids, as ubiquinone-binding site inhibitors, must partition into the lipid membrane to enter this channel, it follows that the more hydrophobic rotenoids are also the more potent inhibitors.
Figure 2.
Dependence of IC50 values for complex I in bovine mitochondrial membranes on Slog10P for each rotenoid tested. IC50 values were determined from dose response curves measured in triplicate. The best-fit values are shown alongside error bars showing 95% confidence intervals. The dotted line at Slog10P = 3 represents the cut-off value used to select rotenoids for evaluation in cell-based assays.
Selection of Hydroxylated Rotenoids for Evaluation in Cell-Based Assays of Prostate Cancer
Of the hydroxylated metabolites of rotenone (1) and deguelin (2), only rotenolone (7) and tephrosin (8), which have Slog10P values of 3.12 and 3.42, respectively, are known to cross the BBB in mice and rats.20 Thus, only molecules with Slog10P < 3 were considered for further evaluation, as they are expected to cross the BBB less readily than 7 and 8 based on a simple hydrophobicity model (see above for a discussion of additional determinants of BBB penetration). Fourteen of the 23 inhibitors with IC50 < 10 μM (3-6, 11-14, 17, 18, 23, 27, 28, and 31) have Slog10P < 3. From these 14 compounds, the natural hydroxylated rotenoids amorphigenin (3), dalpanol (4), dihydroamorphigenin (5), and amorphigenol (6) were selected for evaluation in cell-based assays using PTEN-null prostate cancer cells and PTEN-positive control prostate cells. Compounds 3-6 have inhibitory potencies ranging from 130-1800 nM (Table 1) and were chosen to establish whether a correlation exists between potency and anti-proliferative effect. In addition, compounds 3-6 share a conserved core structure (the ABCD ring-system shown in Figure 1) and differ only in their E-ring substituents, so differences in their activities must rest on these structural differences alone.
Evaluation of Hydroxylated Rotenoids in Cell-Based Assays of Prostate Cancer
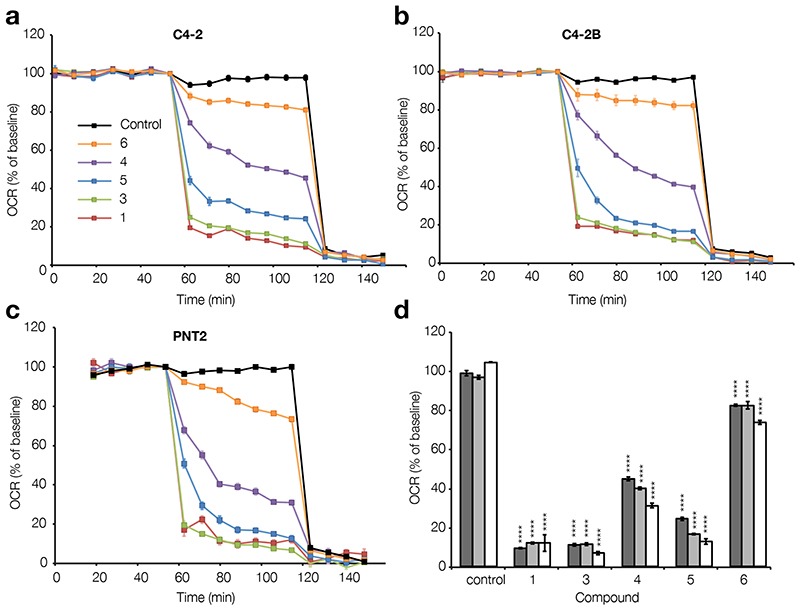
To establish whether amorphigenin (3), dalpanol (4), dihydroamorphigenin (5), and amorphigenol (6) reach and inhibit complex I in cells, oxygen consumption rate (OCR) assays were performed using a Seahorse XF96 instrument in which C4-2, C4-2B, and PNT2 cells were treated with each compound (1 μM). C4-2 and C4-2B cells were chosen as PTEN-null prostate cancer cells representative of advanced metastatic disease (C4-2B cells derive from C4-2 cells and therefore represent an even more invasive form of cancer). PTEN-positive PNT2 cells were selected as cells representative of normal prostate cells. All cell lines were also treated with rotenone (1) (1 μM) as a positive control. The results show that 3-6 all reach and inhibit complex I, and that the reduction in the oxygen consumption rates of all cells, independent of type, correlated with the inhibitory strength of the compound added (Figure 3a-d). Thus, cells were most affected (and most rapidly affected) by 3 (IC50 130 nM), followed by 5 (IC50 228 nM), 4 (IC50 652 nM), and then 6 (IC50 1800 nM). Interestingly, 3 was almost as effective as 1 at inhibiting the oxygen consumption of all cells, even though it is a 19-fold weaker inhibitor of complex I in membranes (Table 1), while 5 also exerted a powerful effect.
Figure 3.
Antimycin-sensitive baselined oxygen consumption rates of a) C4-2, b) C4-2B, and c) PNT2 cells measured in a Seahorse XF96 instrument. Rotenone (1), amorphigenin (3), dalpanol (4), dihydroamorphigenin (5), and amorphigenol (6) were added at 1 μM after 1 hour of basal rate measurement followed by the addition of 2 μM of rotenone and 2 μM of antimycin after 2 hours. Black; control, orange; 6, purple; 4, blue; 5, green; 3, red; 1. d) Antimycin-sentitive baselined oxygen consumption rates after 1 hour of treatment. Dark grey; C4-2, light grey; C4-2B, white; PNT2. **** P ≤ 0.0001. Error bars show S.E.M, n = 6 for treated groups, n = 16 for controls.
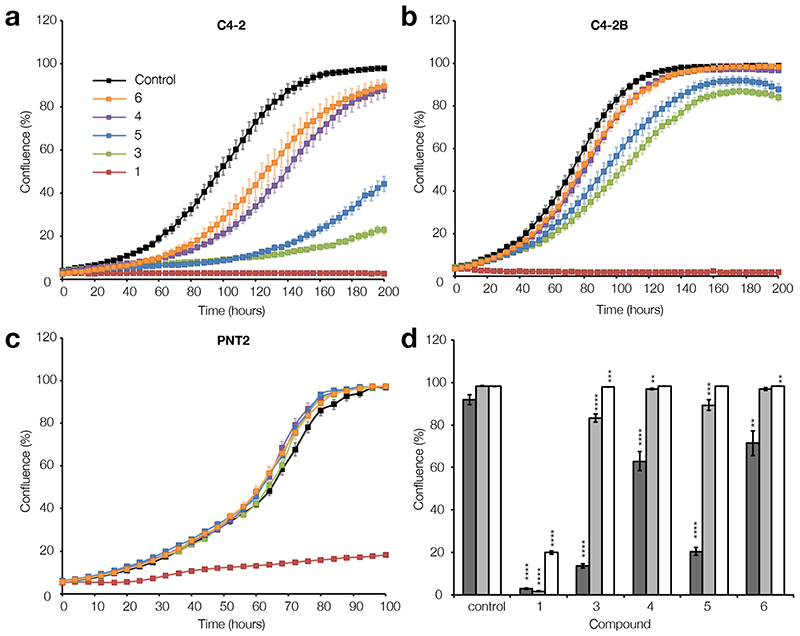
To determine whether the inhibition of complex I by amorphigenin (3), dalpanol (4), dihydroamorphigenin (5), and amorphigenol (6) leads to selective anti-proliferative activity in prostate cancer cells, C4-2, C4-2B, and PNT2 cells were treated with each compound (1 μM) and with rotenone (1) (1 μM) as a positive control. The results (Figure 4a-d) show that 3-6 inhibit the proliferation of the C4-2 and C4-2B prostate cancer cells, but leave the healthy PNT2 prostate cells relatively unaffected. The antiproliferative activity of each derivative against C4-2 and C4-2B was found to correlate directly with its inhibitory activity against complex I. Thus, C4-2 and C4-2B cells treated with 3 (IC50 130 nM) proliferated slowest, followed by those treated with 5 (IC50 228 nM), 4 (IC50 652 nM), and finally 6 (IC50 1800 nM). C4-2 cells were particularly affected by treatment with 3 and 5 (Figure 4a), while, encouragingly, C4-2B cells also responded to 3 and 5, but not 4 and 6 (Figure 4b). Importantly, PNT2 cells treated with 3-6 proliferated at the same rate as untreated cells (Figure 4c). C4-2 and C4-2B cells treated with 1 did not proliferate (Figure 4a-b), however, treated PNT2 cells retained their ability to proliferate, albeit at a substantially reduced rate (Figure 4c).
Figure 4.
Cell growth curves for a) C4-2, b) C4-2B, and c) PNT2 cells in the presence of 1 μM of rotenone (1), amorphigenin (3), dalpanol (4), dihydroamorphigenin (5), and amorphigenol (6). Black; control, orange; 6, purple; 4, blue; 5, green; 3, red; 1. d) Confluence (the percentage surface area of each well covered by adherent cells) after 150 hours of rotenoid treatment. Error bars show S.E.M, n = 8 for all groups. Dark grey; C4-2, light grey; C4-2B, white; PNT2. ** P ≤ 0.01, *** P ≤ 0.001,**** P ≤ 0.0001.
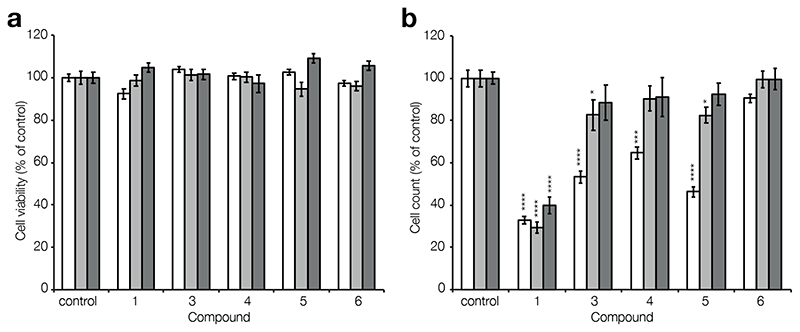
Finally, to confirm that the poor cell growth in the presence of rotenoids 3-6 was caused by inhibited cell proliferation rather than loss of cell viability, cells were treated for 48 hours with each compound (1 μM) before their viability was tested and cell counts were measured (Figure 5) using the acridine orange assay and a chemometec NC-3000. The data show that while rotenone (1) affected cell counts in all cells, and the cell count was lower for all compounds with C4-2 cells, no effect was seen on cell viability with any of the compounds tested. In addition, amorphigenin (3) and dihydroamorphigenin (5) had marginal effects on cell count for C4-2B cells. Therefore, under the growth conditions used (RPMI 1640 containing 11 mM glucose), inhibition of complex I leads primarily to the inhibition of cell proliferation. In contrast, a recent study reports ~70% loss of cell viability of PTEN-null cells in an MTT assay after 24 hours with deguelin (2) (0.5 μM) at the same glucose concentration.5 Inhibitors of the respiratory chain, including 1, have been shown to slow down the rate of the MTT to formazan reaction.67 Interestingly, the complex I inhibitor IACS-01079 has been reported to inhibit both cancer cell proliferation and viability as assayed using Annexin V flow cytometry.19
Figure 5.
a) Cell viability, and b) cell count following 48 hours of treatment with 1 μM of rotenone (1), amorphigenin (3), dalpanol (4), dihydroamorphigenin (5), and amorphigenol (6). White, C4-2; light grey, C4-2B; dark grey, PNT2. Error bars show S.E.M, n = 4 for treated groups, n = 10 for control. * P ≤ 0.1, *** P ≤ 0.001,**** P ≤ 0.0001.
A previous study revealed that 3 and 4 are non-selectively cytotoxic towards a variety human cancer cell types, including A-549 lung, HCT-8 intestinal, RPMI-7951 skin, TE671 brain, and KB cervical cancer cells.37 Half-maximal effective concentrations (ED50 values) determined for all cell types were consistently lower for 3 (0.01-0.05 μg mL-1) than for 4 (0.48-4.83 μg mL-1),37 in agreement with our data showing that in every assay 3 is more active than 4 (Figures 3-5). The cytotoxicity of several hydroxylated rotenoids towards mouse lymphoma L5178Y cells has also been reported, with IC50 values ranging from 0.2-0.9 μM, as measured using the MTT assay,43 suggesting that these cells are as sensitive to treatment with rotenoids as the PTEN-null prostate cancer cells used in our study. Most interestingly, a number of hydroxylated rotenoids, including rotenolone (7) and tephrosin (8) (Figure 1), have been shown to be selectively cytotoxic towards LNCaP prostate cancer cells, with ED50 values ranging from 0.3-0.7 μM.40 The mechanism of action of these compounds was not investigated, but references were made to studies on the involvement of complex I. Taken together, our results show that the inhibition of complex I is essential to the mechanism of action of 3-6, that prostate cancer cells display a greater dependence on OxPhos than healthy prostate cells, and that this dependence makes them vulnerable to inhibitors of complex I. The most significant effect of the inhibition of complex I by rotenoids 3-6 on prostate cancer cells occurs by inhibiting proliferation.
In summary, successive redox reactions were applied to rotenone (1), deguelin (2), and their derivatives to produce a library of 29 hydroxylated rotenoids. The inhibitory potency (IC50) of each hydroxylated rotenoid against complex I was measured using a mitochondrial membrane assay and its hydrophobicity (Slog10P) was predicted computationally. From this library, amorphigenin (3), dalpanol (4), dihydroamorphigenin (5), and amorphigenol (6) were selected for evaluation in cell-based assays using C4-2 and C4-2B prostate cancer cells and control PNT2 prostate cells. Compounds 3-6 reach and inhibit complex I in cells, selectively inhibit the proliferation of prostate cancer cells, and leave control prostate cells unaffected. Further, the clear correlation between the inhibitory potency against complex I and the anti-proliferative activity of 3-6, suggests that complex I inhibition is essential to their mechanism of action. Since the strongest antiproliferative effects were obtained with 3 and 5, these hydroxylated rotenoids, which can be synthesized as described above or extracted from natural sources,25,28,43 are proposed as candidates for detailed evaluation in more sophisticated cell-based assays of prostate cancer and animal models. Finally, the results highlight that prostate cancer cells are more dependent on OxPhos than healthy prostate cells and that they are vulnerable to a range of rotenoid inhibitors of complex I. It is anticipated that this work will stimulate further research into the therapeutic potential of carefully selected rotenoids and related metabolism-based approaches towards the treatment of prostate cancer.
Experimental Section
General Experimental Procedures
Melting points were determined using a Büchi Melting Point B-545 melting point apparatus and are uncorrected. Optical rotations were measured using an Anton-Paar MCP 100 polarimeter. [α]D 20 values are reported in 10-1 deg cm2 g-1 at 598 nm, concentration (c) is given in g (100 mL)-1. Infrared spectra were recorded on a Perkin-Elmer Spectrum One spectrometer with internal referencing as neat films. Absorption maxima (νmax) are reported in wavenumbers (cm-1). 1H NMR spectra were recorded on a Bruker Avance 500 Cryo Ultrashield spectrometer, operating at 500 MHz. Chemical shifts (δ) are quoted in parts per million (ppm) to the nearest 0.01 ppm and are referenced to residual solvent signals, CHCl3 (δ 7.26) or acetone (δ 2.05). Coupling constants (J) are reported in Hertz (Hz) to the nearest 0.5 Hz. Data are reported as follows: chemical shift, integration, multiplicity (br, broad; s, singlet; d, doublet; t, triplet; q, quartet; quint, quintet; m, multiplet; app, apparent; obsc, obscured; or as combinations or these (e.g. dd)), coupling constant(s), and assignment. 13C NMR spectra were recorded on a Bruker Avance 500 Cryo Ultrashield spectrometer, operating at 125 MHz, with broadband proton spin decoupling. Chemical shifts (δ) are quoted in ppm to the nearest 0.1 ppm and are referenced to the solvent signals, CDCl3 (δ 77.16) or acetone-d 6 (δ 29.84). High resolution mass spectra were recorded using a Micromass LCT Premier spectrometer and reported mass values are within the error limits of ± 5 ppm. Analytical TLC was performed using glass plates coated with Sigma Aldrich 60 (F254) silica gel, and compounds were visualized by ultraviolet irradiation followed by staining with ceric ammonium molybdate solution and heating. Flash chromatography was performed with Sigma-Aldrich 60 (230-400 mesh) silica gel. Rotenone (1) (Molekula Fine Chemicals, Darlington, UK, 90-95%) was crystallized from EtOH three times. Deguelin (2), amorphigenol (6), tephrosin (8), and rot-2′-enonic acid (25) were prepared from 1 as described previously.48,54 N-(Phenylseleno)phthalimide (Sigma-Aldrich, technical grade) was crystallized from dry CH2Cl2/n-hexane under N2. m-CPBA (Sigma-Aldrich, Gillingham, UK, < 77%) was crystallized from CH2Cl2. Zinc powder was activated by washing with 2.0 M HCl for 0.5 h, the coagulated metal was collected by filtration, washed in sequence with H2O, EtOH, and Et2O, dried in vacuo, and finely ground using a pestle and mortar. Powdered 4 Å molecular sieves were activated by heating in vacuo at 150 °C overnight. CH2Cl2, nhexane, and MeOH were distilled from CaH2 under N2. THF was distilled from a mixture of CaH2 and LiAlH4 in the presence of triphenylmethane under N2. All other solvents and reagents were used as obtained from commercial suppliers. Moisture sensitive reactions were carried out with freshly distilled dry solvents under N2 in glassware that was oven dried.
General Procedure 1 (GP1)
NaBH4 (10 mol equiv) was added in small portions to a 0.02 M solution/suspension of the relevant rotenoid (1 mol equiv) in dry MeOH under N2 at 0 °C. The mixture was warmed to rt and stirred for a further 2 h or until the reaction had gone to completion as determined by TLC. H2O was added and the mixture was extracted with Et2O. The organic extracts were combined, washed with brine, dried (MgSO4), filtered, and concentrated to afford the product. Products obtained by this method were sufficiently pure such that no further purification was required.
General Procedure 2 (GP2)
BH3 .SMe2 (10 mol equiv) was added dropwise to a 0.04 M solution of the relevant rotenoid (1 mol equiv) in dry THF under N2 at 0 °C. The mixture was stirred at 0 °C for 0.5 h, warmed to rt and stirred for a further 1 h. The mixture was cooled to 0 °C and 3.0 M aqueous NaOH solution (30 mol equiv) was added. H2O2 (30% aqueous solution, 30 mol equiv) was cautiously added dropwise and the mixture was stirred for 18 h while slowly warming to rt. H2O was added and the mixture extracted with Et2O. The organic extracts were combined, washed with brine, dried (MgSO4), filtered, and concentrated to afford the product. Products obtained by this method were sufficiently pure such that no further purification was required.
General Procedure 3 (GP3)
Using a modified version of the procedure described for the synthesis of 9,45 SeO2 (4 mol equiv) was added to a 0.04 M solution/suspension of the relevant rotenoid 1 (1 mol equiv) and powdered 4 Å molecular sieves (equivalent mass to that of the rotenoid) in dry 1,4-dioxane under N2. The mixture was heated at 80 °C for 3 h, cooled to rt, filtered through a pad of Celite, and concentrated. The residue was subjected to flash chromatography to afford the product.
General Procedure 4 (GP4)
Using the procedure described for the synthesis of 27,57 a 0.2 M solution of m-CPBA (2 mol equiv) in dry CHCl3 was added to a 0.04 M solution of the relevant rotenonic acid (1 mol equiv) in dry CHCl3 under N2 at 0 °C. Tosic acid monohydrate (0.5 mol equiv) was then added and the mixture stirred at 0 °C for 2 h. H2O was added and the mixture extracted with CHCl3. The organic phases were combined, washed with saturated aqueous NaHCO3 solution and brine, dried (MgSO4), filtered, and concentrated. The residue was subjected to flash chromatography to afford the product.
(6aS,12aS,5′R)-Amorphigenin (3) was prepared by modifying a literature procedure.46 N-(Phenylseleno)phthalimide (153 mg, 0.508 mmol) was added to a solution of 1 (200 mg, 0.508 mmol), (+)-β-camphorsulfonic acid (12.0 mg, 0.051 mmol) and H2O (183 μL, 10.1 mmol) in CH2Cl2 (10 mL) under N2 in a flask wrapped in aluminium foil. The mixture was stirred at rt for 72 h then concentrated. The yellow solid obtained was cooled to 0 °C and dissolved in pyridine (4.0 mL) before H2O2 (0.5 mL, 30% aqueous solution) was added dropwise over 10 min. The mixture was stirred at 0 °C for 0.5 h, warmed to rt and stirred for 1.5 h. Saturated aqueous NaHCO3 solution (20 mL) was added and the mixture was extracted with CHCl3 (3 x 20 mL). The organic extracts were combined, washed with 3.0 M HCl (3 x 20 mL), H2O (3 x 20 mL) and brine (20 mL), dried (MgSO4), filtered, and concentrated. The residue was subjected to flash chromatography (SiO2, 1:1 hexanes/EtOAc) to give 3 as a pale yellow solid that crystallized from CHCl3/MeOH as colorless needles (68 mg, 33%): mp 196-198 °C (lit.46 mp 196-197 °C);[α]D 20 −127 (c 0.1, CHCl3) [lit.46 [α]D 24 −124 (c 0.2, CHCl3)]; IR νmax 3500-3100, 1671, 1599, 1517, 1455, 1349, 1303, 1234, 1212, 1195, 1087, 817 cm-1; 1H NMR (500 MHz, CDCl3) δ 3.08 (1H, dd, J 9.0, 15.5 Hz, Ha-4′), 3.39 (1H, dd, J 9.0, 15.5 Hz, Hb-4′), 3.76 (3H, s, H-2′), 3.81 (3H, s, H-3′), 3.85 (1H, d, J 4.0 Hz, H-12a), 4.18 (1H, d, J 12.0 Hz, Ha-6), 4.23 (1H, d, 13.5 Hz, Ha-8′), 4.28 (1H, d, 13.5 Hz, Hb-8′), 4.61 (1H, dd, J 3.0, 12.0 Hz, Hb-6), 4.93 (1H, dd, J 3.0, 4.0 Hz, H-6a), 5.26 (1H, s, Ha-7′), 5.28 (1H, s, Hb-7′), 5.39 (1H, t, J 9.0 Hz, H-5′), 6.45 (1H, s, H-4), 6.51 (1H, d, J 9.0 Hz, H-10), 6.76 (1H, s, H-1), 7.84 (1H, d, J 9.0 Hz, H-11); 13C NMR (125 MHz, CDCl3) δ 32.0 (C-4′), 44.8 (C-12a), 56.0 (C-3′), 56.5 (C-2′), 63.1 (C-8′), 66.4 (C-6), 77.4 (C-6a), 85.7 (C-5′), 101.1 (C-4), 104.9 (C-1a), 105.1 (C-10), 110.5 (C-1), 112.9 (C-7′), 113.0 (C-8), 113.7 (C-11a), 130.2 (C-11), 144.0 (C-2), 146.8 (C-6′), 147.5 (C-3), 149.7 (C-4a), 158.1 (C-7a), 167.0 (C-9), 189.1 (C-12); HRESIMS m/z 411.1440 [M + H]+ (calcd for C23H23O7, m/z 411.1444).
(6aS,12aS,5′R)-Dalpanol (4) was prepared by modifying a literature procedure.27 [Caution: Hg(OAc)2 and elemental Hg are highly toxic and must be handled with extreme care – all operations must be carried out in a fume-hood and all Hg-containing waste retained for proper waste disposal]. H2O (2.0 mL) was added dropwise to a suspension of Hg(OAc)2 (178 mg, 0.558 mmol) in THF (2.0 mL) and the bright yellow mixture was stirred at rt for 10 min. 1 (200 mg, 0.508 mmol) was added and the mixture was stirred at rt for 18 h. Brine (20 mL) was added followed by CHCl3 (20 mL) and the two phases were thoroughly mixed. The organic layer was separated, dried (MgSO4), filtered, and concentrated. The solid obtained was dissolved in THF (2.0 mL) and H2O (1.5 mL). Saturated aqueous NaHCO3 solution (0.5 mL) was added followed by NaBH4 (19.0 mg, 0.508 mmol) and the mixture was stirred vigorously for 0.5 min as elemental Hg precipitated and coated the walls of the flask. H2O (20 mL) was added followed by CHCl3 (20 mL) and the two phases were thoroughly mixed. The biphasic mixture was carefully decanted and the organic layer was separated. The aqueous phase was extracted with CHCl3 (2 x 20 mL) and the organic phases were combined, washed with brine (20 mL), dried (MgSO4), filtered, and concentrated. The residue was subjected to flash chromatography (SiO2, 1:1 hexanes/EtOAc) to give 4 as a white solid that crystallized from MeOH/H2O as very fine colorless needles (128 mg, 48%): mp 208-210 °C (lit.27 mp 189-191 °C, benzene); [α]D 20 −102 (c 0.1, CHCl3) [lit.27 [α]D 24 −122 (c 2.0, CHCl3)]; IR νmax 3500-3100, 1678, 1641, 1608, 1514, 1462, 1440, 1348, 1269, 1235, 1212, 1198, 1182, 1095, 1081, 817 cm-1; 1H NMR (500 MHz, CDCl3) δ 1.22 (3H, s, H-7′), 1.35 (3H, s, H-8′), 1.76 (1H, br s, OH-6′), 3.09 (1H, dd, J 9.0, 15.0 Hz, Ha-4′), 3.14 (1H, dd, J 9.0, 15.0 Hz, Hb-4′), 3.76 (3H, s, H-2′), 3.81 (3H, s, H-3′), 3.84 (1H, d, J 4.0 Hz, H-12a), 4.18 (1H, d, J 12.0 Hz, Ha-6), 4.62 (1H, dd, J 3.0, 12.0 Hz, Hb-6), 4.67 (1H, t, J 9.0 Hz, H-5′), 4.93 (1H, dd, J 3.0, 4.0 Hz, H-6a), 6.45 (1H, s, H-4), 6.49 (1H, d, J 8.5 Hz, H-10), 6.76 (1H, s, H-1), 7.82 (1H, d, J 8.5 Hz, H-11); 13C NMR (125 MHz, CDCl3) δ 24.1 (C-7′), 26.4 (C-8′), 27.5 (C-4′), 44.8 (C-12a), 56.0 (C-3′), 56.5 (C-2′), 66.4 (C-6), 71.8 (C-6a), 72.3 (C-6′), 91.6 (C-5′), 101.1 (C-4), 104.9 (C-1a), 104.9 (C-10), 110.4 (C-1), 113.6 (C-8), 113.8 (C-11a), 130.0 (C-11), 144.0 (C-2), 147.5 (C-3), 149.6 (C-4a), 158.1 (C-7a), 167.3 (C-9), 189.2 (C-12); HRESIMS m/z 413.1584 [M + H]+ (calcd for C23H25O7, m/z 413.1595).
(6aS,12aS,5′R,6′R)- and (6aS,12aS,5′R,6′S)-6′,7′-Dihydroamorphigenin (5). 1 (200 mg, 0.508 mmol) was added to a solution of [Ir(COD)Cl]2 (3.4 mg, 0.005 mmol), 1,2-bis(diphenylphosphino)ethane (4.0 mg, 0.010 mmol), and pinacol borane (295 μL, 2.030 mmol) in dry CH2Cl2 (6.0 mL) under N2. The mixture was stirred at rt for 20 h. MeOH (1 mL) was then added and the mixture was concentrated. The resulting yellow oil was dissolved in THF (6.0 mL) and NaHCO3 (21 mg, 0.254 mmol) was added followed by H2O2 (1.73 mL, 30% aqueous solution, 15.2 mmol), which was cautiously added dropwise, and the mixture stirred at rt for 20 h. H2O (20 mL) was added and the mixture was extracted with CHCl3 (3 x 20 mL). The organic extracts were combined, washed with brine (3 x 20 mL), dried (MgSO4), filtered, and concentrated. The oily residue was subjected to flash chromatography (SiO2, 1:1 hexanes/EtOAc) to give the mixture of diastereoisomers 5 as a white solid (28 mg, 14%, dr 74:26 unassigned): IR νmax 3500-3200, 1682, 1609, 1514, 1454, 1438, 1348, 1308, 1238, 1212, 1190, 1090, 1074, 1005, 974, 829, 818 cm-1; NMR data for the major diastereoisomer: δ H (500 MHz, CDCl3) 0.99 (3H, d, J 7.0 Hz, H-8′), 2.00-2.18 (1H, m obsc, H-6′), 2.95 (1H, dd, J 8.5, 16.0 Hz, Ha-4′), 3.24 (1H, dd, J 8.5, 16.0 Hz, Hb-4′), 3.66-3.74 (2H, m obsc, Ha-7′ and Hb-7′), 3.76 (3H, s, H-2′), 3.81 (3H, s, H-3"), 3.84 (1H, d, J 4.0 Hz, H-12a), 4.18 (1H, d, J 12.0 Hz, Ha-6), 4.62 (1H, dd, J 3.0, 12.0 Hz, Hb-6), 5.00 (dd, J 4.5, 8.5 Hz, H-5′), 4.93 (1H, ddd, J 1.0, 3.0, 4.0 Hz, H-6a), 6.45 (1H, s, H-4), 6.46 (1H, d, J 8.5 Hz, H-10), 6.77 (1H, s, H-1), 7.82 (1H, d, J 8.5 Hz, H-11); δ C (125 MHz, CDCl3) 10.9 (C-8′), 29.8 (C-4′), 40.6 (C-6′), 44.8 (C-12a), 56.0 (C-3′), 56.5 (C-2′), 65.2 (C-7′), 66.4 (C-6), 72.4 (C-6a), 86.7 (C-5′), 101.1 (C-4), 104.9 (C-1a), 105.0 (C-10), 110.5 (C-1), 113.2 (C-11a), 113.4 (C-8), 130.1 (C-11), 144.0 (C-2), 147.5 (C-4a), 149.6 (C-3), 158.1 (C-7a), 167.6 (C-9), 189.1 (C-12); NMR data for the minor diastereoisomer: δ H (500 MHz, CDCl3) 0.99 (3H, d, J 7.0 Hz, H-8′), 2.00-2.18 (1H, m obsc, H-6′), 2.91 (1H, dd, J 8.5, 16.0 Hz, Ha-4′), 3.27 (1H, dd, J 8.5, 16.0 Hz, Hb-4′), 3.66-3.74 (2H, m obsc, Ha-7′ and Hb-7′), 3.76 (3H, s, H-2′), 3.81 (3H, s, H-3′), 3.84 (1H, d, J 4.0 Hz, H-12a), 4.18 (1H, d, J 12.0 Hz, Ha-6), 4.62 (1H, dd, J 3.0, 12.0 Hz, Hb-6), 4.78 (dd, J 4.5, 8.5 Hz, H-5′), 4.93 (1H, ddd, J 1.0, 3.0, 4.0 Hz, H-6a), 6.45 (1H, s, H-4), 6.47 (1H, d, J 8.5 Hz, H-10), 6.77 (1H, s, H-1), 7.83 (1H, d, J 8.5 Hz, H-11); δ C (125 MHz, CDCl3) 12.7 (C-8′), 30.7 (C-4′), 41.1 (C-6′), 44.8 (C-12a), 56.0 (C-3′), 56.5 (C-2′), 65.2 (C-7′), 65.9 (C-6), 72.4 (C-6a), 88.9 (C-5′), 101.1 (C-4), 104.9 (C-1a), 105.1 (C-10), 110.5 (C-1), 113.2 (C-11a), 113.5 (C-8), 130.1 (C-11), 144.0 (C-2), 147.5 (C-4a), 149.6 (C-3), 158.1 (C-7a), 167.1 (C-9), 189.1 (C-12); HRESIMS m/z 435.1403 [M + Na]+ (calcd for C23H24O7Na, m/z 435.1414).
(6aR,12aR,5′R)-Rotenolone (7) was prepared by modifying a literature procedure.50 A solution of K2Cr2O7 (2.8 g, 9.45 mmol) in H2O (40 mL) was added dropwise over 10 min to a solution of 1 (4.0 g, 10.15 mmol) in AcOH (80 mL) at 60 °C. The mixture was stirred for 0.5 h then cooled to rt and stirred for a further 18 h. The dark green mixture was poured onto crushed ice (400 mL) and the resulting suspension was stirred for 1 h while an off-white precipitate formed. The precipitate was collected by filtration, washed thoroughly with H2O (200 mL), dried in vacuo, and purified by flash chromatography (SiO2, 2:1 hexanes/EtOAc) to give 7 as a white solid (3.42 g, 82%): [α]D 20 −176 (c 0.1, CHCl3) [lit.50 [α]D 16 −189 (c 2.0, CHCl3)]; IR νmax 3600-3300, 1673, 1607, 1508, 1455, 1331, 1258, 1216, 1154, 1085, 1023, 905, 816 cm-1; 1H NMR (500 MHz, CDCl3) δ 1.76 (3H, s, H-8′), 2.93 (1H, dd, J 8.0, 16.0 Hz, Ha-4′), 3.29 (1H, dd, J 8.0, 16.0 Hz, Hb-4′), 3.72 (3H, s, H-2′), 3.82 (3H, s, H-3′), 4.47 (1H, s, OH-12a), 4.49 (1H, d, J 11.5 Hz, Ha-6), 4.58 (1H, dd, J 1.0, 2.5 Hz, H-6a), 4.59 (1H, dd, J 2.5, 11.5 Hz, Hb-6), 4.93 (1H, s, Ha-7′), 5.06 (1H, s, Hb-7′), 5.23 (1H, app t, J 8.0 Hz, H-5′), 6.48 (1H, s, H-4), 6.53 (1H, d, J 8.5 Hz, H-10), 6.55 (1H, s, H-1), 7.82 (1H, d, J 8.5 Hz, H-11); 13C NMR (125 MHz, CDCl3) δ 17.2 (C-8′), 31.3 (C-4′), 56.0 (C-3′), 56.5 (C-2′), 64.0 (C-6), 67.7 (C-12a), 76.2 (C-6a), 88.1 (C-5′), 101.2 (C-4), 105.5 (C-10), 108.9 (C-1a), 109.4 (C-1), 111.9 (C-11a), 112.9 (C-7′), 113.3 (C-8), 130.2 (C-11), 143.0 (C-6′), 144.1 (C-2), 148.5 (C-4a), 151.2 (C-3), 157.8 (C-7a), 168.2 (C-9), 191.2 (C-12); HRESIMS m/z 433.1241 [M + Na]+ (calcd for C23H22O7Na, m/z 433.1258).
(6aS,12aS)-2′-Oxorot-3′-enonic Acid (9).45 Prepared from 1 (200 mg, 0.508 mmol) by GP3 and obtained as a yellow solid (44 mg, 21%): [α]D 20 +34 (c 0.1, CHCl3) [lit.45 [α]D 25 +38 (c 0.1, CHCl3)]; IR νmax 3400-3100, 1675, 1579, 1547, 1513, 1444, 1347, 1286, 1259, 1216, 1196, 1051, 817 cm-1; 1H NMR (500 MHz, CDCl3) δ 1.86 (3H, s, H-5′), 3.75 (3H, s, H-2″), 3.81 (3H, s, H-3″), 3.84 (1H, d, J 4.0 Hz, H-12a), 3.87 (1H, d, J 13.5 Hz, Ha-1′), 4.22 (1H, d, J 12.0 Hz, Ha-6), 4.23 (1H, d, J 13.5 Hz, Hb-1′), 4.64 (1H, dd, J 3.5, 12.0 Hz, Hb-6), 4.95 (1H, ddd, J 1.0, 3.5, 4.0 Hz, H-6a), 6.00 (1H, s, Ha-4′), 6.42 (1H, s, H-4), 6.59 (1H, s, Hb-4′), 6.63 (1H, d, J 8.5 Hz, H-10), 6.74 (1H, s, H-1), 7.81 (1H, d, J 8.5 Hz, H-11), 8.97 (1H, s, OH-9); 13C NMR (125 MHz, CDCl3) δ 17.5 (C-5′), 32.3 (C-1′), 44.4 (C-12a), 56.1 (C-3″), 56.4 (C-2″), 66.5 (C-6), 72.6 (C-6a), 100.9 (C-4), 104.7 (C-1a), 108.5 (C-8), 110.4 (C-1), 112.6 (C-10), 112.8 (C-11a), 128.7 (C-11), 130.3 (C-4′), 143.7 (C-3′), 144.1 (C-2), 147.5 (C-4a), 149.7 (C-3), 159.1 (C-7a), 163.9 (C-9), 189.4 (C-12), 203.7 (C-2′); HRESIMS m/z 433.1239 [M + Na]+ (calcd for C23H22O7Na, m/z 433.1258).
(6aS,12S,12aR,5′R)-12-Deoxo-12-hydroxyrotenone (10).49 Prepared from 1 (1.0 g, 2.54 mmol) by GP1 and obtained as a white solid (921 mg, 92%): [α]D 20 −150 (c 0.1, acetone); IR νmax 3600-3300, 1619, 1512, 1479, 1463, 1217, 1194, 1131, 1091, 1037, 799 cm-1; 1H NMR (500 MHz, acetone-d 6) δ 1.76 (3H, s, H-8′), 2.88 (1H, dd, J 9.0, 15.5 Hz, Ha-4′), 3.22 (1H, dd, J 9.0, 15.5 Hz, Hb-4′), 3.44 (1H, app t, J 4.5 Hz, H-12a), 3.70 (3H, s, H-2′), 3.74 (3H, s, H-3′), 4.17 (1H, dd, J 3.0, 10.0 Hz, Ha-6), 4.48 (1H, d, J 4.0 Hz, OH-12), 4.61 (1H, app t, J 10.0 Hz, Hb-6), 4.81 (1H, ddd, J 3.0, 4.5, 10.0 Hz, H-6a), 4.88 (1H, s, Ha-7′), 5.06 (1H, s, Hb-7′), 5.12 (1H, dd, J 4.0, 4.5 Hz, H-12), 5.16 (1H, app t, J 9.0 Hz, H-5′), 6.32 (1H, d, J 8.0 Hz, H-10), 6.37 (1H, s, H-4), 7.10 (1H, d, J 8.0 Hz, H-11), 7.17 (1H, s, H-1); 13C NMR (125 MHz, acetone-d 6) δ 17.4 (C-8′), 32.7 (C-4′), 38.5 (C-12a), 55.9 (C-3′), 56.8 (C-2′), 66.5 (C-6), 67.6 (C-12), 71.2 (C-6a), 86.8 (C-5′), 101.3 (C-4), 102.3 (C-10), 111.5 (C-7′), 111.6 (C-1a), 112.8 (C-8), 114.6 (C-1), 117.6 (C-11a), 129.6 (C-11), 144.4 (C-2), 145.5 (C-6′), 150.2 (C-3), 150.3 (C-4a), 150.6 (C-7a), 161.9 (C-9); HRESIMS m/z 419.1449 [M + Na]+ (calcd for C20H24O6Na, m/z 419.1465).
(6aS,12S,12aR,5′R)-12-Deoxo-12-hydroxyamorphigenin (11). Prepared from 3 (20 mg, 0.048 mmol) by GP1 and obtained as a white solid (17.7 mg, 88%): [α]D 20 −164 (c 0.1, acetone); IR νmax 3600-3300, 1620, 1515, 1465, 1220, 1194, 1131, 1093, 1039, 984, 787 cm-1; 1H NMR (500 MHz, acetone-d 6) δ 3.00 (1H, dd, J 8.5, 15.5 Hz, Ha-4′), 3.28 (1H, dd, J 8.5, 15.5 Hz, Hb-4′), 3.44 (1H, app t, J 4.5 Hz, H-12a), 3.70 (3H, s, H-2′), 3.74 (3H, s, H-3′), 4.17 (1H, J 3.0, 9.5 Hz, Ha-6), 4.18 (1H, d, J 14.0 Hz, Ha-8′), 4.19 (1H, d, J 14.0 Hz, Hb-8′), 4.50 (1H, d, J 4.0 Hz, OH-12), 4.59 (1H, app t, J 9.5 Hz, Hb-6), 4.80 (1H, ddd, J 3.0, 4.5, 9.5 Hz, H-6a), 5.16 (1H, dd, J 4.0, 4.5 Hz, H-12), 5.17 (1H, s, Ha-7′), 5.19 (1H, s, Hb-7′), 5.30 (1H, app t, J 8.5 Hz, H-5′), 6.33 (1H, d, J 8.0 Hz, H-10), 6.37 (1H, s, H-4), 7.10 (1H, d, J 8.0 Hz, H-11), 7.17 (1H, s, H-1); 13C NMR (125 MHz, acetone-d 6) δ 33.3 (C-4′), 38.5 (C-12a), 55.9 (C-3′), 56.8 (C-2′), 62.3 (C-8′), 66.5 (C-6), 67.6 (C-12), 71.2 (C-6a), 84.6 (C-5′), 101.3 (C-4), 102.3 (C-10), 109.6 (C-7′), 111.6 (C-1a), 112.9 (C-8), 114.6 (C-1), 117.7 (C-11a), 129.6 (C-11), 144.4 (C-2), 150.2 (C-6′), 150.2 (C-3), 150.3 (C-4a), 150.6 (C-7a), 161.8 (C-9); HRESIMS m/z 435.1402 [M + Na]+ (calcd for C23H24O7Na, m/z 435.1414).
(6aS,12S,12aR,5′R)-12-Deoxo-12-hydroxydalpanol (12).27 Prepared from 4 (20 mg, 0.049 mmol) by GP1 and obtained as a white solid (18.5 mg, 92%): [α]D 20 −120 (c 0.1, acetone); IR νmax 3600-3300, 1617, 1518, 1505, 1483, 1467, 1240, 1220, 1192, 1131, 1078, 1039, 956, 789 cm-1; 1H NMR (500 MHz, acetone-d 6) δ 1.20 (3H, s, H-7′), 1.24 (3H, s, H-8′), 2.98 (1H, dd, J 8.5, 13.5 Hz, Ha-4′), 3.10 (1H, dd, J 8.5, 13.5 Hz, Hb-4′), 3.43 (1H, app t, J 5.0 Hz, H-12a), 3.70 (3H, s, H-2′), 3.74 (3H, s, H-3′), 4.17 (1H, dd, J 4.0, 10.0 Hz, Ha-6), 4.44 (1H, s, OH-12), 4.59 (1H, app t, J 8.5 Hz, H-5′), 4.60 (1H, app t, J 8.5 Hz, Hb-6), 4.80 (1H, ddd, J 4.0, 5.0, 10.0 Hz, H-6a), 5.11 (1H, dd, J 4.0, 5.0 Hz, H-12), 6.26 (1H, d, J 8.0 Hz, H-10), 6.37 (1H, s, H-4), 7.05 (1H, d, J 8.0 Hz, H-11), 7.16 (1H, s, H-1); 13C NMR (125 MHz, acetone-d 6) δ 25.6 (C-7′), 26.0 (C-8′), 28.5 (C-4′), 38.6 (C-12a), 55.9 (C-3′), 56.8 (C-2′), 66.5 (C-6), 67.6 (C-12), 71.1 (C-6a), 71.5 (C-6′), 90.9 (C-5′), 101.3 (C-4), 102.2 (C-10), 111.7 (C-1a), 113.7 (C-8), 114.6 (C-1), 117.3 (C-11a), 129.3 (C-11), 144.4 (C-2), 150.2 (C-4a), 150.3 (C-7a), 150.5 (C-3), 162.2 (C-9); HRESIMS m/z 437.1557 [M + Na]+ (calcd for C23H26O7Na, m/z 437.1571).
(6aS,12S,12aR,5′R,6′R)- and (6aS,12S,12aR,5′R,6′S)-12-Deoxo-12-hydroxyamorphigenol (13). Prepared from 6 (20.0 mg, 0.047 mmol) by GP1, the mixture of diastereoisomers 13 was obtained as a white solid (17.9 mg, 89%, dr 63:37 unassigned): IR νmax 3600-3200, 1621, 1513, 1464, 1261, 1218, 1195, 1132, 1094, 1038, 987, 787 cm-1; NMR data for the major diastereoisomer: δ H (500 MHz, acetone-d 6) 1.15 (3H, s, H-8′), 2.98 (1H, dd, J 6.5, 15.5 Hz, Ha-4′), 3.17 (1H, dd, J 6.5, 15.5 Hz, Hb-4′), 3.43 (1H, app t, J 6.5 Hz, H-12a), 3.57 (1H, dd, J 5.5, 11.0 Hz, Ha-7′), 3.68 (1H, dd, J 5.5, 11.0 Hz, Hb-7′), 3.70 (3H, s, H-2′), 3.74 (3H, s, H-3′), 4.16 (1H, dd, J 3.0, 10.0 Hz, Ha-6), 4.44 (1H, d, J 4.0 Hz, OH-12), 4.60 (1H, app t, J 10.0 Hz, Hb-6), 4.81 (1H, m obsc, H-6a), 4.83 (1H, app t, J 6.0 Hz, H-5′), 5.12 (1H, dd, J 4.0, 6.5 Hz, H-12), 6.27 (1H, d, J 8.0 Hz, H-10), 6.37 (1H, s, H-4), 7.06 (1H, d, J 8.0 Hz, H-11), 7.16 (1H, s, H-1); δ C (125 MHz, acetone-d 6) 19.7 (C-8′), 28.0 (C-4′), 38.6 (C-12a), 55.9 (C-3′), 56.8 (C-2′), 66.5 (C-6), 67.6 (C-12), 68.1 (C-7′), 71.1 (C-6a), 73.8 (C-6′), 86.7 (C-5′), 101.4 (C-4), 102.3 (C-10), 111.7 (C-1a), 113.7 (C-8), 114.6 (C-1), 117.3 (C-11a), 129.3 (C-11), 144.4 (C-2), 150.2 (C-3), 150.3 (C-4a), 150.5 (C-7a), 162.1 (C-9); NMR data for the minor diastereoisomer: δ H (500 MHz, acetone-d 6) 1.16 (3H, s, H-8′), 2.98 (1H, dd, J 6.5, 15.5 Hz, Ha-4′), 3.18 (1H, dd, J 6.5, 15.5 Hz, Hb-4′), 3.43 (1H, app t, J 6.5 Hz, H-12a), 3.49 (1H, dd, J 5.5, 11.0 Hz, Ha-7′), 3.65 (1H, dd, J 5.5, 11.0 Hz, Hb-7′), 3.70 (3H, s, H-2′), 3.74 (3H, s, H-3′), 4.16 (1H, dd, J 3.0, 10.0 Hz, Ha- 6), 4.42 (1H, d, J 4.0 Hz, OH-12), 4.60 (1H, app t, J 10.0 Hz, Hb-6), 4.80 (1H, m obsc, H-6a), 4.83 (1H, app t, J 6.0 Hz, H-5′), 5.12 (1H, dd, J 4.0, 7.0 Hz, H-12), 6.25 (1H, d, J 8.0 Hz, H-10), 6.37 (1H, s, H-4), 7.04 (1H, d, J 8.0 Hz, H-11), 7.15 (1H, s, H-1); δ C (125 MHz, acetone-d 6) 20.8 (C-8′), 28.0 (C-4′), 38.6 (C-12a), 55.9 (C-3′), 56.8 (C-2′), 66.5 (C-6), 67.6 (C-12), 67.9 (C-7′), 71.1 (C-6a), 74.0 (C-6′), 87.8 (C-5′), 101.4 (C-4), 102.3 (C-10), 111.7 (C-1a), 113.7 (C-8), 114.6 (C-1), 117.3 (C-11a), 129.3 (C-11), 144.4 (C-2), 150.2 (C-3), 150.3 (C-4a), 150.5 (C-7a), 162.1 (C-9); HRESIMS m/z 453.1505 [M + Na]+ (calcd for C23H26O8Na, m/z 453.1520).
(6aS,12S,12aR,5′R,6′R)- and (6aS,12S,12aR,5′R,6′S)-6′,7′-Dihydro-12-deoxo-12-hydroxyamorphigenin (14). Prepared from 1 (200 mg, 0.508 mmol) by GP2, the mixture of diastereoisomers 14 was obtained as a white solid (168 mg, 80%, dr 53:47 unassigned): IR νmax 3600-3300, 1620, 1508, 1463, 1261, 1218, 1194, 1130, 1089, 1037, 985, 786 cm-1; NMR data for the major diastereoisomer: δ H (500 MHz, acetone-d 6) 0.98 (3H, d, J 8.0 Hz, H-8′), 1.90 (1H, app quint, J 6.5, H-6′), 2.91 (1H, dd, J 9.0, 15.5 Hz, Ha-4′), 3.12 (1H, dd, J 9.0, 15.5 Hz, Hb-4′), 3.43 (1H, app t, J 6.0 Hz, H-12a), 3.52-3.57 (1H, m, Ha-7′), 3.59-3.64 (1H, m obsc, Hb-7′), 3.70 (3H, s, H-2′), 3.74 (3H, s, H-3′), 4.17 (1H, dd, J 3.0, 9.5 Hz, Ha-6), 4.42 (1H, d, J 4.5 Hz, OH-12), 4.60 (1H, app t, J 9.5 Hz, Hb-6), 4.79 (1H, m obsc, H-5′), 4.82 (1H, m obsc, H-6a), 5.11 (1H, dd, J 4.5, 5.0 Hz, H-12), 6.27 (1H, d, J 8.0 Hz, H-10), 6.37 (1H, s, H-4), 7.06 (1H, d, J 8.0 Hz, H-11), 7.15 (1H, s, H-1); δ C (125 MHz, acetone-d 6) 12.0 (C-8′), 31.3 (C-4′), 38.6 (C-12a), 42.2 (C-6′), 55.9 (C-3′), 56.8 (C-2′), 64.8 (C-7′), 66.5 (C-6), 67.6 (C-12), 71.1 (C-6a), 85.8 (C-5′), 101.3 (C-4), 102.2 (C-10), 111.7 (C-1a), 113.5 (C-8), 114.6 (C-1), 117.3 (C-11a), 129.5 (C-11), 144.4 (C-2), 150.2 (C-3), 150.3 (C-4a), 150.6 (C-7a), 162.2 (C-9); NMR data for the minor diastereoisomer: δ H (500 MHz, acetone-d 6) 0.97 (3H, d, J 8.0 Hz, H-8′), 2.04 (1H, app quint, J 6.5, H-6′), 2.86 (1H, dd, J 9.0, 15.5 Hz, Ha-4′), 3.08 (1H, dd, J 9.0, 15.5 Hz, Hb-4′), 3.43 (1H, app t, J 6.0 Hz, H-12a), 3.52-3.57 (1H, m, Ha-7′), 3.59-3.64 (1H, m obsc, Hb-7′), 3.70 (3H, s, H-2′), 3.74 (3H, s, H-3′), 4.17 (1H, dd, J 3.0, 9.5 Hz, Ha-6), 4.43 (1H, d, J 4.5 Hz, OH-12), 4.60 (1H, app t, J 9.5 Hz, Hb-6), 4.72 (1H, dd, J 8.0, 9.0 Hz, H-5′), 4.82 (1H, ddd, J 3.0, 5.0, 9.5 Hz, H-6a), 5.11 (1H, dd, J 4.5, 5.0 Hz, H-12), 6.29 (1H, d, J 8.0 Hz, H-10), 6.37 (1H, s, H-4), 7.06 (1H, d, J 8.0 Hz, H-11), 7.16 (1H, s, H-1); δ C (125 MHz, acetone-d 6) 12.4 (C-8′), 30.5 (C-4′), 38.6 (C-12a), 41.8 (C-6′), 55.9 (C-3′), 56.8 (C-2′), 64.8 (C-7′), 66.5 (C-6), 67.5 (C-12), 71.1 (C-6a), 86.5 (C-5′), 101.3 (C-4), 102.3 (C-10), 111.7 (C-1a), 113.3 (C-8), 114.6 (C-1), 117.4 (C-11a), 129.5 (C-11), 144.4 (C-2), 150.2 (C-3), 150.5 (C-4a), 150.6 (C-7a), 162.0 (C-9); HRESIMS m/z 437.1554 [M + Na]+ (calcd for C23H26O7Na, m/z 437.1571).
(6aR,12R,12aS,5′R)-12-Deoxo-12-hydroxyrotenolone (15).50 Prepared from 7 (200 mg, 0.489 mmol) by GP1 and obtained as a white solid that crystallized from MeOH/H2O as colorless needles (173 mg, 86%): mp 126-127 °C (lit.50 mp 125-126 °C); [α]D 20 −143 (c 0.1, CHCl3) [lit.50 [α]D 20 −152 (c 2.0, CHCl3)]; IR νmax 3600-3300, 1620, 1508, 1479, 1464, 1265, 1219, 1195, 1137, 1099, 1061, 1040, 849 cm-1; 1H NMR (500 MHz, CDCl3) δ 1.78 (3H, s, H-8′), 2.14 (1H, d, J 2.5 Hz, OH-12), 2.35 (1H, s, OH-12a), 2.95 (1H, dd, J 8.5, 16.0 Hz, Ha-4′), 3.26 (1H, dd, J 8.5, 16.0 Hz, Hb-4′), 3.84 (6H, s, H-2′ and H-3′), 4.35 (1H, dd, J 4.0, 10.5 Hz, Ha-6), 4.61 (1H, dd, J 4.0, 9.5 Hz, H-6a), 4.68 (1H, dd, J 9.5, 10.5 Hz, Hb-6), 4.84 (1H, app s, H-12), 4.91 (1H, s, Ha-7′), 5.08 (1H, s, Hb-7′), 5.18 (1H, app t, J 8.5 Hz, H-5′), 6.41 (1H, s, H-4), 6.47 (1H, d, J 8.0 Hz, H-10), 7.13 (1H, d, J 8.0 Hz, H-11), 7.24 (1H, s, H-1); 13C NMR (125 MHz, CDCl3) δ 17.4 (C-8′), 32.1 (C-4′), 56.0 (C-2′), 56.6 (C-3′), 65.0 (C-6), 68.6 (C-12a), 72.0 (C-12), 75.2 (C-6a), 86.8 (C-5′), 100.4 (C-4), 103.3 (C-10), 108.9 (C-1), 112.2 (C-7′), 112.3 (C-1a), 112.8 (C-8), 113.1 (C-11a), 129.7 (C-11), 143.9 (C-3), 144.1 (C-6′), 148.7 (C-7a), 149.4 (C-4a), 150.7 (C-2), 161.9 (C-9); HRESIMS m/z 435.1404 [M + Na]+ (calcd for C23H24O7Na, m/z 435.1414).
(6aR,12aR)-2′-Oxorot-3′-enolonic Acid (16).45 Prepared from 7 (200 mg, 0.488 mmol) by GP3 and obtained as a yellow solid (42 mg, 20%): [α]D 20 +24 (c 0.1, CHCl3) [lit.45 [α]D 25 +33 (c 0.1, CHCl3)]; IR νmax 3500-3100, 1672, 1598, 1508, 1446, 1332, 1272, 1200, 1083, 1049, 816 cm-1; 1H NMR (500 MHz, CDCl3) δ 1.85 (3H, s, H-5′), 3.71 (3H, s, H-2″), 3.78 (1H, d, J 14.0 Hz, Ha-1′), 3.81 (3H, s, H-3″), 4.24 (1H, d, J 14.0 Hz, Hb-1′), 4.44 (1H, s, OH-12a), 4.53 (1H, d, J 12.0 Hz, Ha-6), 4.60 (1H, dd, J 1.0, 2.5 Hz, H-6a), 4.64 (1H, dd, J 2.5, 12.0 Hz, Hb-6), 6.00 (1H, s, Ha-4′), 6.44 (1H, s, H-4), 6.51 (1H, s, H-1), 6.55 (1H, s, Hb-4′), 6.65 (1H, d, J 8.5 Hz, H-10), 7.79 (1H, d, J 8.5 Hz, H-11) 9.13 (1H, s, OH); 13C NMR (125 MHz, CDCl3) δ 17.4 (C-5′), 32.1 (C-1′), 56.1 (C-3″), 56.4 (C-2″), 64.0 (C-6), 67.4 (C-12a), 76.3 (C-6a), 101.0 (C-4), 108.5 (C-1a), 108.7 (C-8), 109.3 (C-1), 111.1 (C-11a), 113.1 (C-10), 128.7 (C-11), 130.5 (C-4′), 143.6 (C-3′), 144.2 (C-2), 148.4 (C-4a), 151.3 (C-3), 158.8 (C-7a), 164.7 (C-9), 191.4 (C-12), 203.7 (C-2′); HRESIMS m/z 449.1213 [M + Na]+ (calcd for C23H22O8Na, m/z 449.1207).
(6aR,12aR,5′R)-12a-Hydroxyamorphigenin (17). N-(Phenylseleno)phthalimide (147 mg, 0.488 mmol) was added to solution of 7 (200 mg, 0.488 mmol), (+)-βcamphorsulfonic acid (11.0 mg, 0.049 mmol), and H2O (176 μL, 9.76 mmol) in CH2Cl2 (10 mL) under N2 in a flask wrapped in aluminium foil. The mixture was stirred at rt for 72 h and was then concentrated. The yellow solid obtained was cooled to 0 °C and dissolved in THF (4 mL) before H2O2 (0.5 mL, 30% aqueous solution) was added dropwise over 10 min, followed by basic Al2O3 (400 mg). The suspension was stirred at 0 °C for a further 0.5 h then warmed to rt and stirred for an additional 6 h. Saturated aqueous NaHCO3 solution (20 mL) was added followed by Et2O (20 mL) and the two phases were mixed vigorously for 10 min. The organic layer was separated, washed with H2O (3 x 20 mL) and brine (20 mL), dried (MgSO4), filtered, and concentrated. The residue was purified by flash chromatography (SiO2, 1:1 hexanes/EtOAc) to give 17 as a yellow solid. The solid was dissolved in CHCl3 and activated charcoal was added. The suspension was then filtered and concentrated to give a white solid that crystallized from MeOH/H2O as colorless needles (44 mg, 21%): mp 89-91 °C (lit.68 mp 92-95 °C); [α]D 20 −173 (c 0.1, CHCl3) [lit.68 [α]D 20 −175 (c 1.8, CHCl3)]; IR vmax 3500-3200, 2924, 1672, 1607, 1509, 1455, 1258, 1216, 1196, 1154, 1085, 1025, 816 cm-1; 1H NMR (500 MHz, CDCl3) δ 3.05 (1H, dd, J 9.0, 16.0 Hz, Ha-4′), 3.36 (1H, dd, J 9.0, 16.0 Hz, Hb-4′), 3.72 (3H, s, H-2′), 3.82 (3H, s, H-3′), 4.24 (1H, d, J 14.0 Hz, Ha-8′), 4.28 (1H, d, J 14.0 Hz, Hb-4′), 4.46 (1H, s, OH-12a), 4.48 (1H, dd, J 1.0, 11.5 Hz, Ha-6), 4.58 (1H, dd, J 1.0, 2.5 Hz, H-6a), 4.59 (1H, dd, J 2.5, 11.5 Hz, Hb-6), 5.25 (1H, s, Ha-7′), 5.28 (1H, s, Hb-7′), 5.39 (1H, app t, J 9.0 Hz, H-5′), 6.48 (1H, s, H-4), 6.53 (1H, d, J 8.5 Hz, H-10), 6.54 (1H, s, H-1), 7.83 (1H, d, J 8.5 Hz, H-11); 13C NMR (125 MHz, CDCl3) δ 31.8 (C-4′), 56.0 (C-3′), 56.5 (C-2′), 63.1 (C-8′), 64.0 (C-6), 67.7 (C-12a), 76.2 (C-6a), 85.8 (C-5′), 101.2 (C-4), 105.5 (C-10), 108.8 (C-1a), 109.4 (C-1), 112.1 (C-11a), 113.0 (C-7′), 113.3 (C-8), 130.3 (C-11), 144.1 (C-2), 146.6 (C-6′), 148.5 (C-4a), 151.3 (C-3), 157.8 (C-7a), 167.7 (C-9), 191.2 (C-12); HRESIMS m/z 449.1210 [M + Na]+ (calcd for C23H22O8Na, m/z 449.1207).
(6aR,12aR,5′R)-12a-Hydroxydalpanol (18). [Caution: Hg(OAc)2 and elemental Hg are highly toxic and must be handled with extreme care – all operations must be carried out in a fume-hood and all Hg-containing waste retained for proper waste disposal]. H2O (2.0 mL) was added to a suspension of Hg(OAc)2 (171 mg, 0.537 mmol) in THF (2.0 mL) and the bright yellow solution was stirred at rt for 10 min. 7 (200 mg, 0.488 mmol) was added and the mixture was stirred at rt for 18 h. The mixture was diluted with THF (2.0 mL) and 3.0 M NaOH solution (2.0 mL) was added followed by NaBH4 (18.5 mg, 0.488 mmol). The mixture was stirred vigorously for 0.5 min as elemental Hg rapidly precipitated and coated the walls of the flask. The reaction was worked up as described for 4 and the residue was subjected to flash chromatography (SiO2, 1:1 hexanes/EtOAc) to give 18 as a white solid that crystallized from MeOH/H2O as colorless needles (106 mg, 51%): mp 202-204 °C (lit.37 mp 206.5-207 °C); [α]D 20 −174 (c 0.1, CHCl3); IR νmax 3600-3300, 2924, 1666, 1611, 1509, 1456, 1337, 1247, 1220, 1192, 1085, 1027, 951, 856, 808 cm-1; 1H NMR (500 MHz, CDCl3) δ 1.21 (3H, s, H-7′), 1.34 (3H, s, H-8′), 1.76 (1H, s, OH-6′), 3.07 (1H, dd, J 9.0, 14.0 Hz, Ha-4′), 3.10 (1H, dd, J 9.0, 14.0 Hz, Hb-4′), 3.72 (3H, s, H-2′), 3.81 (3H, s, H-3′), 4.47 (1H, s, OH-12a), 4.49 (1H, dd, J 1.0, 12.0 Hz, Ha-6), 4.57 (1H, dd, J 1.0, 2.5 Hz, H-6a), 4.60 (1H, dd, J 2.5, 12.0 Hz, Hb-6), 4.68 (1H, app t, J 9.0 Hz, H-5′), 6.48 (1H, s, H-4), 6.51 (1H, d, J 8.5 Hz, H-10), 6.54 (1H, s, H-1), 7.81 (1H, d, J 8.5 Hz, H-11); 13C NMR (125 MHz, CDCl3) δ 24.2 (C-7′), 26.3 (C-8′), 27.4 (C-4′), 56.0 (C-3′), 56.5 (C-2′), 64.0 (C-6), 67.7 (C-12a), 71.8 (C-6′), 76.1 (C-6a), 91.8 (C-5′), 101.2 (C-4), 105.3 (C-10), 108.8 (C-1a), 109.4 (C-1), 112.0 (C-11a), 114.0 (C-8), 130.1 (C-11), 144.1 (C-2), 148.5 (C-4a), 151.2 (C-3), 157.8 (C-7a), 167.9 (C-9), 191.2 (C-12); HRESIMS m/z 451.1367 [M + Na]+ (calcd for C23H24O8Na, m/z 451.1363).
(6aR,12aR,5′R,6′R)- and (6aR,12aR,5′R,6′S)-6′,7′-Dihydro-12a-hydroxyamorphigenin (19). 7 (200 mg, 0.488 mmol) was added to a solution of [Ir(COD)Cl]2 (6.5 mg, 0.010 mmol), 1,2-bis(diphenylphosphino)ethane (7.8 mg, 0.020 mmol), and pinacol borane (283 μL, 1.951 mmol) in dry CH2Cl2 (6.0 mL) under N2. The mixture was stirred at rt for 20 h. MeOH (1.0 mL) was then added and the mixture was concentrated. The yellow oil obtained was dissolved in THF (6.0 mL), NaHCO3 (20 mg, 0.254 mmol) was added followed by H2O2 (1.7 mL, 30% aqueous solution, 15.2 mmol), which was added dropwise over 10 min. The mixture was stirred vigorously at rt for an additional 20 h. The reaction was worked up as described for 5 and the residue was subjected to flash chromatography (SiO2, 1:2 hexanes/EtOAc) to give the mixture of diastereoisomers 19 as a white solid (24 mg, 12%, dr 59:41 unassigned): IR νmax 3600-3300, 2920, 1672, 1607, 1509, 1455, 1259, 1215, 1200, 1085, 1026, 817 cm-1; NMR data for the major diastereoisomer: δ H (500 MHz, CDCl3) 0.98 (3H, d, J 7.0 Hz, H-8′), 2.00 (1H, dq, J 7.0, 9.0 Hz, H-6′), 2.93 (1H, dd, J 8.5, 16.0 Hz, Ha-4′), 3.22 (1H, dd, J 8.5, 16.0 Hz, Hb-4′), 3.67-3.74 (2H, m obsc, Ha-7′ and Hb-7′), 3.73 (3H, s, H-2′), 3.81 (3H, s, H-3′), 4.47 (1H, s, OH-12a), 4.49 (1H, d, J 11.5 Hz, Ha-6), 4.58 (1H, dd, J 1.5, 2.5 Hz, H-6a), 4.60 (1H, dd, J 2.5, 11.5 Hz, Hb-6), 5.00 (1H, app td, J 4.5, 9.0 Hz, H-5′), 6.48 (1H, s, H-4), 6.49 (1H, d, J 8.5 Hz, H-10), 6.54 (1H, s, H-1), 7.80 (1H, d, J 8.5 Hz, H-11); δ C (125 MHz, CDCl3) 10.8 (C-8′), 29.7 (C-4′), 40.6 (C-6′), 56.0 (C-3′), 56.5 (C-2′), 64.0 (C-6), 65.1 (C-7′), 67.7 (C-12a), 76.2 (C-6a), 86.7 (C-5′), 101.2 (C-4), 105.4 (C-10), 108.8 (C-1a), 109.4 (C-1), 111.7 (C-11a), 113.6 (C-8), 130.2 (C-11), 144.1 (C-2), 148.5 (C-4a), 151.3 (C-3), 157.8 (C-7a), 168.3 (C-9), 191.2 (C-12); NMR data for the minor diastereoisomer: δ H (500 MHz, CDCl3) 0.98 (3H, d, J 7.0 Hz, H-8′), 2.08 (1H, dq, J 7.0, 9.0 Hz, H-6′), 2.91 (1H, dd, J 8.5, 16.0 Hz, Ha-4′), 3.21 (1H, dd, J 8.5, 16.0 Hz, Hb-4′), 3.67-3.74 (2H, m obsc, Ha-7′ and Hb-7′), 3.73 (3H, s, H-2′), 3.81 (3H, s, H-3′), 4.47 (1H, s, OH-12a), 4.49 (1H, d, J 11.5 Hz, Ha-6), 4.58 (1H, dd, J 1.5, 2.5 Hz, H-6a), 4.60 (1H, dd, J 2.5, 11.5 Hz, Hb-6), 4.76 (1H, dd, J 4.5, 9.0 Hz, H-5′), 6.48 (1H, s, H-4), 6.49 (1H, d, J 8.5 Hz, H-10), 6.54 (1H, s, H-1), 7.80 (1H, d, J 8.5 Hz, H-11); δ C (125 MHz, CDCl3) 12.6 (C-8′), 30.5 (C-4′), 41.0 (C-6′), 56.0 (C-3′), 56.5 (C-2′), 64.0 (C-6), 65.7 (C-7′), 67.7 (C-12a), 76.2 (C-6a), 88.9 (C-5′), 101.2 (C-4), 105.5 (C-10), 108.8 (C-1a), 109.4 (C-1), 111.8 (C-11a), 113.4 (C-8), 130.2 (C-11), 144.1 (C-2), 148.5 (C-4a), 151.3 (C-3), 157.8 (C-7a), 167.8 (C-9), 191.2 (C-12); HRESIMS m/z 451.1350 [M + Na]+ (calcd for C23H24O8Na, m/z 451.1363).
(6aR,12aR,5′R,6′R)- and (6aR,12aR,5′R,6′S)-12a-Hydroxyamorphigenol (20) were prepared by modifying a literature procedure.45 OsO4 (99 μL of a 2.5 wt% solution in t-BuOH, 0.010 mmol) was added to a solution of 7 (200 mg, 0.488 mmol), N-methylmorpholine N-oxide (71 mg, 0.976 mmol), and pyridine (78 μL, 0.976 mmol) in acetone (8.0 mL) and H2O (0.8 mL). The mixture was stirred at rt for 42 h. EtOAc (20 mL) was added followed by saturated aqueous Na2SO3 solution (20 mL) and the two phases were mixed vigorously for 10 min. The organic layer was separated, washed with 3.0 M HCl (3 x 20 mL), H2O (20 mL) and brine (20 mL), dried (MgSO4), filtered, and concentrated. The residue was subjected to flash chromatography (SiO2, 1:1 hexanes/EtOAc) to give the mixture of diastereoisomers 20 as a white solid (106 mg, 49%, dr 62:38 unassigned): IR νmax 3600-3300, 1672, 1607, 1509, 1455, 1338, 1248, 1216, 1199, 1155, 1085, 1048, 1025, 817, 746 cm-1; NMR data for the major diastereoisomer: δ H (500 MHz, CDCl3) 1.21 (3H, s, H-8′), 3.17 (1H, dd, J 11.0, 17.5 Hz, Ha-4′), 3.19 (1H, dd, J 11.0, 17.5 Hz, Hb-4′), 3.53 (1H, d, J 11.0 Hz, Ha-7′), 3.71 (3H, s, H-2′), 3.74 (1H, d, J 11.0 Hz, Hb-7′), 3.82 (3H, s, H-3′), 4.46 (1H, s, OH-12a), 4.49 (1H, d, J 12.0 Hz, Ha-6), 4.59 (1H, dd, 1.0, 2.5 Hz, H-6a), 4.60 (1H, dd, J 2.5, 12.0 Hz, Hb-6), 4.86 (1H, app t, J 11.0 Hz, H-5′), 6.49 (1H, s, H-4), 6.50 (1H, d, J 8.5 Hz, H-10), 6.53 (1H, s, H-1), 7.80 (1H, d, J 8.5 Hz, H-11); δ C (125 MHz, CDCl3) 19.4 (C-8′), 27.0 (C-4′), 56.0 (C-3′), 56.5 (C-2′), 63.9 (C-6), 67.0 (C-7′), 67.8 (C-12a), 73.5 (C-6′), 76.2 (C-6a), 87.5 (C-5′), 101.2 (C-4), 105.4 (C-10), 108.8 (C-1a), 109.4 (C-1), 112.1 (C-11a), 113.9 (C-8), 130.1 (C-11), 144.1 (C-2), 148.6 (C-4a), 151.3 (C-3), 157.8 (C-7a), 167.7 (C-9), 191.3 (C-12); NMR data for the minor diastereoisomer: δ H (500 MHz, CDCl3) 1.16 (3H, s, H-8′), 3.16 (1H, dd, J 11.0, 17.5 Hz, Ha-4′), 3.20 (1H, dd, J 11.0, 17.5 Hz, Hb-4′), 3.57 (1H, d, J 11.0 Hz, Ha-7′), 3.71 (3H, s, H-2′), 3.79 (1H, d, J 11.0 Hz, Hb-7′), 3.82 (3H, s, H-3′), 4.45 (1H, s, OH-12a), 4.49 (1H, d, J 12.0 Hz, Ha-6), 4.59 (1H, dd, 1.0, 2.5 Hz, H-6a), 4.60 (1H, dd, J 2.5, 12.0 Hz, Hb-6), 4.86 (1H, app t, J 11.0 Hz, H-5′), 6.48 (1H, s, H-4), 6.51 (1H, d, J 8.5 Hz, H-10), 6.53 (1H, s, H-1), 7.81 (1H, d, J 8.5 Hz, H-11); δ C (125 MHz, CDCl3) 19.8 (C-8′), 27.3 (C-4′), 56.0 (C-3′), 56.5 (C-2′), 63.9 (C-6), 67.7 (C-12a), 68.6 (C-7′), 73.1 (C-6′), 76.2 (C-6a), 90.2 (C-5′), 101.2 (C-4), 105.3 (C-10), 108.8 (C-1a), 109.4 (C-1), 112.2 (C-11a), 113.7 (C-8), 130.1 (C-11), 144.1 (C-2), 148.6 (C-4a), 151.3 (C-3), 157.8 (C-7a), 167.4 (C-9), 191.3 (C-12); HRESIMS m/z 467.1316 [M + Na]+ (calcd for C23H24O9Na, m/z 467.1313).
(6aR,12R,12aS,5′R)-12-Deoxo-12,12a-dihydroxyamorphigenin (21).69 Prepared from 17 (18.0 mg, 0.042 mmol) by GP1 and obtained as a white solid (14.8 mg, 82%): [α]D 20 −86 (c 0.1, CHCl3); IR νmax 3600-3300, 1620, 1509, 1466, 1265, 1221, 1194, 1137, 1099, 1036, 734 cm-1; 1H NMR (500 MHz, CDCl3) δ 2.25 (1H, d, J 2.5 Hz, OH-12), 2.51 (1H, s, OH-12a), 3.04 (1H, dd, J 9.0, 15.5 Hz, Ha-4′), 3.32 (1H, dd, J 9.0, 15.5 Hz, Hb-4′), 3.83 (6H, s, H-2′ and H-3′), 4.22 (1H, d, J 14.0 Hz, Ha-8′), 4.27 (1H, d, J 14.0 Hz, Hb-8′), 4.32 (1H, dd, J 4.0, 10.5 Hz, Ha-6), 4.59 (1H, dd, J 4.0, 8.0 Hz, H-6a), 4.66 (1H, dd, J 8.0, 10.5 Hz, Hb-6), 4.84 (1H, app s, H-12), 5.24 (1H, s, Ha-7′), 5.25 (1H, s, Hb-7′), 5.33 (1H, app t, J 9.0 Hz, H-5′), 6.40 (1H, s, H-4), 6.46 (1H, d, J 8.5 Hz, H-10), 7.14 (1H, d, J 8.5 Hz, H-11), 7.24 (1H, s, H-1); 13C NMR (125 MHz, CDCl3) δ 32.6 (C-4′), 56.0 (C-2′), 56.6 (C-3′), 63.2 (C-8′), 64.9 (C-6), 68.5 (C-12a), 72.0 (C-12), 75.2 (C-6a), 84.8 (C-5′), 100.4 (C-4), 103.3 (C-10), 109.0 (C-1), 112.3 (C-1a), 112.6 (C-8), 112.7 (C-7′), 113.6 (C-11a), 129.7 (C-11), 144.1 (C-3), 147.4 (C-6′), 148.7 (C-7a), 149.4 (C-4a), 150.7 (C-2), 161.3 (C-9); HRESIMS m/z 451.1373 [M + Na]+ (calcd for C23H24O8Na, m/z 451.1369.
(6aR,12R,12aS,5′R)-12-Deoxo-12,12a-dihydroxydalpanol (22). Prepared from 18 (22.0 mg, 0.051 mmol) by GP1 and obtained as a colorless oil that solidified upon standing at rt to a white solid (19.9 mg, 90%): [α]D 20 −134 (c 0.1, CHCl3); IR νmax 3500-3200, 1620, 1508, 1481, 1464, 1266, 1220, 1194, 1138, 1098, 1063, 1038, 968 cm-1; 1H NMR (500 MHz, CDCl3) δ 1.20 (3H, s, H-7′), 1.33 (3H, s, Ha-8′), 1.94 (1H, s, OH-6′), 2.37 (1H, d, J 2.0 Hz, OH-12), 2.67 (1H, s, OH-12a), 3.03 (1H, dd, J 9.5, 17.0 Hz, Ha-4′), 3.06 (1H, dd, J 9.5, 17.0 Hz, Hb-4′), 3.81 (3H, s, H-2′), 3.82 (3H, s, H-3′), 4.34 (1H, dd, J 4.0, 10.5 Hz, Ha-6), 4.51 (1H, dd, J 4.0, 9.0 Hz, H-6a), 4.52 (1H, m obsc, H-5′), 4.65 (1H, dd, J 9.0, 10.5 Hz, Hb-6), 4.82 (1H, app s, H-12), 6.38 (1H, s, H-4), 6.42 (1H, d, J 8.0 Hz, H-10), 7.11 (1H, d, J 8.0 Hz, H-11), 7.24 (1H, s, H-1); 13C NMR (125 MHz, CDCl3) δ 24.0 (C-7′), 26.4 (C-8′), 28.1 (C-4′), 56.0 (C-2′), 56.6 (C-3′), 64.9 (C-6), 68.4 (C-12a), 72.0 (C-6′), 72.1 (C-12), 75.2 (C-6a), 90.4 (C-5′), 100.4 (C-4), 103.0 (C-10), 109.2 (C-1), 112.4 (C-1a), 113.3 (C-8), 113.5 (C-11a), 129.4 (C-11), 144.0 (C-3), 148.7 (C-7a), 149.4 (C-4a), 150.6 (C-2), 161.5 (C-9); HRESIMS m/z 435.1502 [M + Na]+ (calcd for C23H26O8Na, m/z 453.1520).
(6aR,12R,12aS,5′R,6′R)- and (6aR,12R,12aS,5′R,6′S)-6′,7′-Dihydro-12-deoxo-12,12a-dihydroxyamorphigenin (23). Prepared from 7 (200 mg, 0.488 mmol) by GP2, the mixture of diastereoisomers 23 was obtained as a white solid (182 mg, 87%, dr 57:43 unassigned): IR νmax 3600-3300, 1619, 1512, 1464, 1281, 1218, 1193, 1130, 1090, 985, 870, 786 cm-1; NMR data for the major diastereoisomer: δ H (500 MHz, CDCl3) 0.99 (3H, d, J 7.0 Hz, H-8′), 2.02 (1H, m, H-6′), 2.32 (1H, s, OH-12), 2.63 (1H, s, OH-12a), 2.92 (1H, dd, J 9.0, 15.0 Hz, Ha-4′), 3.16 (1H, dd, J 9.0, 15.0 Hz, Hb-4′), 3.64 (1H, dd, J 4.5, 11.0 Hz, Ha-7′), 3.74 (1H, dd, J 4.5, 11.0 Hz, Hb-7′), 3.82 (6H, s, H-2′ and H-3′), 4.34 (1H, dd, J 4.0, 10.5 Hz, Ha-6), 4.60 (1H, dd, J 4.0, 9.0 Hz, H-6a), 4.68 (1H, dd, J 9.0, 10.5 Hz, Hb-6), 4.83 (1H, app s, H-12), 4.88 (1H, app td, J 4.5, 9.0 Hz, H-5′), 6.39 (1H, s, H-4), 6.41 (1H, d, J 8.0 Hz, H-10), 7.11 (1H, d, J 8.0 Hz, H-11), 7.23 (1H, s, H-1); δ C (125 MHz, CDCl3) 11.2 (C-8′), 30.1 (C-4′), 40.3 (C-6′), 56.0 (C-2′), 56.6 (C-3′), 64.9 (C-6), 65.4 (C-7′), 68.5 (C-12a), 72.0 (C-12), 75.2 (C-6a), 85.7 (C-5′), 100.3 (C-4), 103.1 (C-10), 109.0 (C-1), 112.4 (C-1a), 113.0 (C-8), 113.2 (C-11a), 129.6 (C-11), 144.0 (C-3), 148.7 (C-7a), 149.4 (C-4a), 150.6 (C-2), 161.7 (C-9); NMR data for the minor diastereoisomer: δ H (500 MHz, CDCl3) 0.95 (3H, d, J 7.0 Hz, H-8′), 2.02 (1H, m, H-6′), 2.32 (1H, s, OH-12), 2.63 (1H, s, OH-12a), 2.90 (1H, dd, J 9.0, 15.0 Hz, Ha-4′), 3.18 (1H, dd, J 9.0, 15.0 Hz, Hb-4′), 3.66 (1H, dd, J 4.5, 11.0 Hz, Ha-7′), 3.72 (1H, dd, J 4.5, 11.0 Hz, Hb-7′), 3.82 (6H, s, H-2′ and H-3′), 4.34 (1H, dd, J 4.0, 10.5 Hz, Ha-6), 4.60 (1H, dd, J 4.0, 9.0 Hz, H-6a), 4.63 (1H, dd, J 4.5, 9.0 Hz, H-5′), 4.69 (1H, dd, J 9.0, 10.5 Hz, Hb-6), 4.83 (1H, app s, H-12), 6.39 (1H, s, H-4), 6.41 (1H, d, J 8.0 Hz, H-10), 7.12 (1H, d, J 8.0 Hz, H-11), 7.23 (1H, s, H-1); δ C (125 MHz, CDCl3) 12.9 (C-8′), 31.6 (C-4′), 41.1 (C-6′), 56.0 (C-2′), 56.6 (C-3′), 64.9 (C-6), 65.4 (C-7′), 68.5 (C-12a), 72.0 (C-12), 75.2 (C-6a), 88.4 (C-5′), 100.3 (C-4), 103.2 (C-10), 109.0 (C-1), 112.4 (C-1a), 112.7 (C-8), 113.5 (C-11a), 129.6 (C-11), 144.0 (C-3), 148.7 (C-7a), 149.4 (C-4a), 150.6 (C-2), 161.2 (C-9); HRESIMS m/z 453.1524 [M + Na]+ (calcd for C23H26O8Na, m/z 453.1520).
(6aR,12R,12aS,5′R,6′R)- and (6aR,12R,12aS,5′R,6′S)-12-Deoxo-12,12a-dihydroxyamorphigenol (24). Prepared from 20 (20.0 mg, 0.045 mmol) by GP1, the mixture of diastereoisomers 24 was obtained as a white solid (18.1 mg, 90%, dr 62:38 unassigned): IR νmax 3600-3300, 1621, 1515, 1479, 1219, 1195, 1132, 1094, 1039, 801 cm-1; NMR data for the major diastereoisomer: δ H (500 MHz, CDCl3) 1.22 (3H, s, H-8′), 2.39 (1H, s, OH-12), 2.48 (1H, s, OH-12a), 3.13 (1H, dd, J 9.0, 15.5 Hz, Ha-4′), 3.15 (1H, dd, J 9.0, 15.5 Hz, Hb-4′), 3.52 (1H, d, J 13.0 Hz, Ha-7′), 3.75 (1H, d, J 13.0 Hz, Hb-7′), 3.83 (6H, s, H-2′ and H-3′), 4.36 (1H, dd, J 3.5, 10.5 Hz, Ha-6), 4.62 (1H, dd, J 3.5, 9.0 Hz, H-6a), 4.68 (1H, dd, J 9.0, 10.5 Hz, Hb-6), 4.77 (app t, J 9.0 Hz, H-5′), 4.84 (1H, app s, H-12), 6.41 (1H, s, H-4), 6.43 (1H, d, J 8.0 Hz, H-10), 7.12 (1H, d, J 8.0 Hz, H-11), 7.26 (1H, s, H-1); δ C (125 MHz, CDCl3) 19.8 (C-8′), 27.8 (C-4′), 56.0 (C-2′), 56.6 (C-3′), 64.9 (C-6), 67.0 (C-7′), 68.5 (C-12a), 72.0 (C-12), 73.7 (C-6′), 75.2 (C-6a), 87.0 (C-5′), 100.4 (C-4), 103.1 (C-10), 109.0 (C-1), 112.3 (C-1a), 113.1 (C-8), 113.6 (C-11a), 129.6 (C-11), 144.1 (C-3), 148.8 (C-7a), 149.4 (C-4a), 150.7 (C-2), 161.3 (C-9); NMR data for the minor diastereoisomer: δ H (500 MHz, CDCl3) 1.15 (3H, s, H-8′), 2.39 (1H, s, OH-12), 2.58 (1H, s, OH-12a), 3.12 (1H, dd, J 9.0, 15.5 Hz, Ha-4′), 3.22 (1H, dd, J 9.0, 15.5 Hz, Hb-4′), 3.54 (1H, d, J 13.0 Hz, Ha-7′), 3.77 (1H, d, J 13.0 Hz, Hb-7′), 3.83 (6H, s, H-2′ and H-3′), 4.36 (1H, dd, J 3.5, 10.5 Hz, Ha-6), 4.62 (1H, dd, J 3.5, 9.0 Hz, H-6a), 4.68 (1H, dd, J 9.0, 10.5 Hz, Hb-6), 4.78 (app t, J 9.0 Hz, H-5′), 4.84 (1H, app s, H-12), 6.41 (1H, s, H-4), 6.43 (1H, d, J 8.0 Hz, H-10), 7.12 (1H, d, J 8.0 Hz, H-11), 7.25 (1H, s, H-1); δ C (125 MHz, CDCl3) 19.8 (C-8′), 28.0 (C-4′), 56.0 (C-2′), 56.6 (C-3′), 64.9 (C-6), 68.4 (C-12a), 68.7 (C-7′), 72.0 (C-12), 73.1 (C-6′), 75.2 (C-6a), 89.1 (C-5′), 100.4 (C-4), 103.2 (C-10), 109.0 (C-1), 112.2 (C-1a), 112.9 (C-8), 113.9 (C-11a), 129.5 (C-11), 144.1 (C-3), 148.8 (C-7a), 149.4 (C-4a), 150.7 (C-2), 161.3 (C-9); HRESIMS m/z 469.1451 [M + Na]+ (calcd for C23H26O9Na, m/z 469.1469).
(6aS,12aS)-4′-Bromorot-2′-enonic Acid was prepared by adapting several literature procedures.55,56 The following procedure gave consistently reproducible results: BBr3 (2.54 mL of a 1.0 M solution in CH2Cl2, 2.54 mmol) was added dropwise over 10 min to a solution of 1 (1.00 g, 2.54 mmol) in dry CH2Cl2 (6.0 mL) under N2 at −20 °C. The mixture was stirred at −20 °C for a further 20 min before the reaction was quenched with H2O (20 mL), quickly warmed to rt, and extracted with EtOAc (20 mL). The organic layer was separated, washed with H2O (3 x 20 mL) and brine (20 mL), dried (MgSO4), filtered, and concentrated. The yellow oily residue was dissolved in MeOH (14 mL) and the solution set aside at 4 °C overnight to give 4′-bromorot-2′-enonic acid as a white powdery solid that was collected by filtration and dried in vacuo. The solid crystallized from CH2Cl2/MeOH at 0 °C as large colorless needles (0.718 g, 60%): mp 152-154 °C (lit.70 mp 152-154 °C); [α]D 20 +22 (c 0.1, CHCl3) [lit.70 [α]D 20 +27 (c 0.7, CHCl3)]; IR νmax 3200-3000, 1662, 1595, 1513, 1453, 1442, 1350, 1276, 1214, 1199, 1098, 882, 814 cm-1; 1H NMR (500 MHz, CDCl3) δ 1.93 (3H, s, H-5′), 3.37 (1H, dd, J 7.5, 15.0 Hz, Ha-1′), 3.42 (1H, dd J 7.5, 15.0 Hz, Hb-1′), 3.75 (3H, s, H-2″), 3.80 (3H, s, H-3″), 3.83 (1H, d, J 4.0 Hz, H-12a), 3.95 (2H, s, H-4′), 4.18 (1H, d, J 12.0 Hz, Ha-6), 4.62 (1H, dd, J 3.5, 12.0 Hz, Hb-6), 4.91 (1H, dd, J 3.5, 4.0 Hz, H-6a), 5.63 (1H, t, J 7.5 Hz, H-2′), 5.93 (1H, s, OH-9), 6.45 (1H, s, H-4), 6.52 (1H, d, J 8.5 Hz, H-10), 6.76 (1H, s, H-1), 7.74 (1H, d, J 8.5 Hz, H-11); 13C NMR (125 MHz, CDCl3) δ 14.9 (C-5′), 22.5 (C-1′), 41.5 (C-4′), 44.4 (C-12a), 56.0 (C-3″), 56.5 (C-2″), 66.4 (C-6), 72.4 (C-6a), 101.1 (C-4), 104.7 (C-1a), 110.6 (C-1), 110.8 (C-10), 113.3 (C-8), 113.9 (C-11a), 127.5 (C-11), 128.0 (C-2′), 133.4 (C-3′), 143.9 (C-2), 147.7 (C-3), 149.6 (C-4a), 160.4 (C-7a), 161.0 (C-9), 189.9 (C-12); HRESIMS m/z 475.0739 [M + H]+ (calcd for C23H24O6 79Br, m/z 475.0756) and m/z 477.039 [M + H]+ (calcd for C23H24O6 81Br, m/z 477.0736).
(6aS,12aS)-Rot-3′-enonic Acid (26). Zinc powder (219 mg, 3.368 mmol, activated as described above) was added to a solution of 4′-bromorot-2′-enonic acid (400 mg, 0.842 mmol) and NH4Cl (180, mg, 3.368 mmol) in THF (12 mL) and H2O (2.0 mL) and the suspension was stirred vigorously at rt for 0.5 h. The mixture was filtered through a pad of Celite and the inorganic residues were washed with EtOAc (80 mL). The combined filtrates were washed with H2O (3 x 80 mL) and brine (80 mL), dried (MgSO4), filtered, and concentrated to afford 26 as a pale yellow solid that crystallized from MeOH/H2O as small colorless prisms (264 mg, 79%): mp 177-178 °C (lit.71 176-178 °C); [α]D 20 +69 (c 0.1, CHCl3); IR νmax 3600-3300, 1657, 1604, 1594, 1515, 1447, 1439, 1350, 1296, 1273, 1216, 1192, 1077, 1006, 814 cm-1; 1H NMR (500 MHz, CDCl3) δ 1.78 (3H, s, H-5′), 2.19 (2H, ddd, J 5.0, 7.0, 11.0 Hz, H-2′), 2.73 (1H, ddd, J 5.0, 7.0, 10.5 Hz, Ha-1′), 2.81 (1H, ddd, J 5.0, 7.0, 10.5 Hz, Hb-1′), 3.75 (3H, s, H-2″), 3.80 (3H, s, H-3″), 3.83 (1H, d, J 3.0 Hz, H-12a), 4.17 (1H, d, J 10.0 Hz, Ha-6), 4.60 (1H, d, J 1.0 Hz, Ha-4′), 4.62 (1H, dd, J 3.0, 10.0 Hz, Hb-6), 4.66 (1H, d, J 1.0 Hz, Hb-4′), 4.88 (1H, ddd, J 1.0, 2.5, 3.0 Hz, H-6a), 5.88 (1H, s, OH-9), 6.45 (1H, s, H-4), 6.47 (1H, d, J 7.0 Hz, H-10), 6.77 (1H, s, H-1), 7.72 (1H, d, J 7.0 Hz, H-11); 13C NMR (125 MHz, CDCl3) δ 21.6 (C-1′), 22.6 (C-5′), 36.9 (C-2′), 44.4 (C-12a), 56.0 (C-3"), 56.5 (C-2"), 66.5 (C-6), 72.3 (C-6a), 101.0 (C-4), 104.9 (C-1a), 110.5 (C-4′), 110.5 (C-10), 110.7 (C-1), 113.1 (C-8), 116.1 (C-11a), 127.0 (C-11), 143.9 (C-2), 145.9 (C-3′), 147.7 (C-3), 149.6 (C-4a), 160.7 (C-7a), 160.9 (C-9), 190.2 (C-12); HRESIMS m/z 397.1636 [M + H]+ (calcd for C20H16O6, m/z 397.1651).
(6aS,12aS,5′R)- and (6aS,12aS,5′S)-4′,5′-Dihydro-5′-hydroxydeguelin (27).57 Prepared from 25 (180 mg, 0.455 mmol) by GP4, the mixture of diastereoisomers 27 was obtained as a white solid (116 mg, 62%, dr 57:43 unassigned): IR νmax 3600-3300, 1665, 1601, 1582, 1512, 1440, 1342, 1261, 1212, 1195, 1136, 1094, 1043, 1008, 817 cm-1; NMR data for the major diasteroisomer: δ H (500 MHz, CDCl3) 1.32 (3H, s, H-7′), 1.34 (3H, s, H-8′), 1.65 (1H, s, OH-5′), 2.69 (1H, dd, J 5.5, 16.0 Hz, Ha-4′), 2.95 (1H, dd, J 5.5, 16.0 Hz, Hb-4′) 3.77 (3H, s, H-2′), 3.81 (3H, s, H-3′), 3.83-3.90 (2H, m obsc, H-12a and H-5′), 4.19 (1H, d, J 11.0 Hz, Ha-6), 4.63 (1H, dd, J 3.5, 11.0 Hz, Hb-6), 4.92 (1H, ddd, J 1.0, 3.5, 4.0 Hz, H-6a), 6.45 (1H, s, H-4) 6.50 (1H, d, J 9.0 Hz, H-10) 6.79 (1H, s, H-1), 7.75 (1H, d, J 9.0 Hz, H-11); δ C (125 MHz, CDCl3) 21.8 (C-7′), 25.1 (C-8′), 26.0 (C-4′), 44.4 (C-12a), 56.0 (C-3′), 56.5 (C-2′), 66.4 (C-6), 69.1 (C-5′), 72.6 (C-6a), 78.3 (C-6′), 101.1 (C-4), 104.9 (C-1a), 107.2 (C-8), 110.5 (C-1), 112.2 (C-10), 112.4 (C-11a), 127.1 (C-11), 144.0 (C-2), 147.6 (C-4a), 149.6 (C-3), 160.0 (C-9), 160.5 (C-7a), 189.5 (C-12); NMR data for the minor diasteroisomer: δ H (500 MHz, CDCl3) 1.27 (3H, s, H-7′) 1.39 (3H, s, H-8′) 1.72 (1H, s, OH-5′), 2.77 (1H, dd, J 5.5, 16.0 Hz, Ha-4′) 2.90 (1H, dd, J 5.5, 16.0 Hz, Hb-4′), 3.77 (3H, s, H-2′), 3.81 (3H, s, H-3′), 3.83-3.90 (2H, m obsc, H-12a and H-5′) 4.19 (1H, d, J 11.0 Hz, Ha-6), 4.64 (1H, dd, J 3.5, 11.0 Hz, Hb-6), 4.92 (1H, ddd, J 1.0, 3.5, 4.0 Hz, H-6a), 6.45 (1H, s, H-4), 6.51 (1H, d, J 9.0 Hz, H-10) 6.80 (1H, s, H-1), 7.76 (1H, d, J 9.0 Hz, H-11); δ C (125 MHz, CDCl3) 22.7 (C-7′), 24.8 (C-8′), 26.0 (C-4′), 44.4 (C-12a), 56.0 (C-3′), 56.5 (C-2′), 66.5 (C-6), 68.7 (C-5′), 72.6 (C-6a), 78.2 (C-6′), 101.1 (C-4), 105.0 (C-1a), 106.9 (C-8), 110.5 (C-1), 112.3 (C-10), 112.5 (C-11a), 127.1 (C-11), 144.0 (C-2), 147.6 (C-4a), 149.6 (C-3), 160.0 (C-9), 160.7 (C-7a), 189.5 (C-12); HRESIMS m/z 435.1400 [M + Na]+ (calcd for C23H24O7Na, m/z 435.1414).
(6aS,12aS,6′R)- and (6aS,12aS,6′S)-4′,5′-Dihydro-7′-hydroxydeguelin (28). Prepared from 26 (180 mg, 0.455 mmol) by GP4, the mixture of diastereoisomers 28 was obtained as a white solid (129 mg, 69%, dr 53:47 unassigned): IR νmax 3600-3300, 1664, 1599, 1580, 1511, 1439, 1335, 1249, 1212, 1196, 1093, 1056, 1003, 817 cm-1; NMR data for the major diasteroisomer: δ H (500 MHz, CDCl3) 1.22 (3H, s, H-8′), 1.70-1.75 (1H, m obsc, Ha-5′), 1.86 (1H, s, OH-7′), 1.93-1.97 (1H, m obsc, Hb-5′), 2.59-2.65 (1H, m obsc, Ha-4′), 2.77-2.81 (1H, m obsc, Hb-4′), 3.58-3.67 (2H, m obsc, Ha-7′ and Hb-7′), 3.77 (3H, s, H-2′), 3.81 (3H, s, H-3′), 3.84 (1H, d, J 4.5 Hz, H-12a), 4.20 (1H, d, J 12.0 Hz, Ha-6), 4.64 (1H, dd, J 3.0, 12.0 Hz, Hb-6), 4.91 (1H, ddd, J 1.0, 3.0, 4.0 Hz, H-6a), 6.46 (1H, s, H-4), 6.48 (1H, d, J 9.0 Hz, H-10), 6.81 (1H, s, H-1), 7.74 (1H, d, J 9.0 Hz, H-11); δ C (125 MHz, CDCl3) 16.0 (C-4′), 20.5 (C-8′), 26.5 (C-5′), 44.4 (C-12a), 56.0 (C-3′), 56.4 (C-2′), 66.5 (C-6), 69.3 (C-7′), 72.6 (C-6a), 78.2 (C-6′), 101.1 (C-4), 105.0 (C-1a), 109.4 (C-8), 110.5 (C-1), 112.1 (C-11a), 112.3 (C-10), 126.7 (C-11), 144.0 (C-2), 147.6 (C-4a), 149.6 (C-3), 160.1 (C-7a), 160.7 (C-9), 189.6 (C-12); NMR data for the minor diasteroisomer: δ H (500 MHz, CDCl3) 1.29 (3H, s, H-8′), 1.70-1.75 (1H, m obsc, Ha-5′), 1.80 (1H, s, OH-7′), 1.93-1.97 (1H, m obsc, Hb-5′), 2.59-2.65 (1H, m obsc, Ha-4′), 2.77-2.81 (1H, m obsc, Hb-4′), 3.58-3.67 (2H, m obsc, Ha-7′ and Hb-7′), 3.77 (3H, s, H-2′), 3.81 (3H, s, H-3′), 3.84 (1H, d, J 4.5 Hz, H-12a), 4.20 (1H, d, J 12.0 Hz, Ha-6), 4.63 (1H, dd, J 3.0, 12.0 Hz, Hb-6) 4.93 (1H, ddd, J 1.0, 3.0, 4.0 Hz, H-6a), 6.45 (1H, s, H-4), 6.48 (1H, d, J 9.0 Hz, H-10), 6.81 (1H, s, H-1), 7.73 (1H, d, J 9.0 Hz, H-11); δ C (125 MHz, CDCl3) 16.0 (C-4′), 20.8 (C-8′), 26.7 (C-5′), 44.4 (C-12a), 56.0 (C-3′), 56.5 (C-2′), 66.5 (C-6), 68.9 (C-7′), 72.4 (C-6a), 78.1 (C-6′), 101.0 (C-4), 105.0 (C-1a), 109.3 (C-8), 110.5 (C-1), 112.1 (C-11a), 112.2 (C-10), 126.7 (C-11), 144.0 (C-2), 147.6 (C-4a), 149.6 (C-3), 160.0 (C-7a), 160.6 (C-9), 189.6 (C-12); HRESIMS m/z 435.1398 [M + Na]+ (calcd for C23H24O7Na, m/z 435.1414).
(6aS,12S,12aR)-12-Deoxo-12-hydroxydeguelin (29).58 Prepared from 2 (120 mg, 0.305 mmol) by GP1 and obtained as a white solid (108 mg, 90%): [α]D 20 −79 (c 0.1, acetone); IR νmax 3600-3300, 1612, 1586, 1509, 1462, 1444, 1216, 1191, 1152, 1094, 1083, 994, 808 cm-1; 1H NMR (500 MHz, acetone-d 6) δ 1.35 (3H, s, H-7′), 1.36 (3H, s, H-8′), 3.45 (1H, app t, J 5.0 Hz, H-12a) 3.70 (3H, s, H-2′), 3.73 (3H, s, H-3′), 4.18 (1H, dd, J 3.5, 10.0 Hz, Ha-6), 4.54 (1H, d, J 3.5 Hz, OH-12), 4.59 (1H, dd, J 8.5, 10.0 Hz, Hb-6), 4.84 (1H, ddd, J 1.0, 5.0, 10.0 Hz, H-6a), 5.12 (1H, d, J 3.5, 4.0 Hz, H-12), 5.63 (1H, d, J 10.0 Hz, H-4′), 6.30 (1H, d, J 8.0 Hz, H-10), 6.36 (1H, s, H-4), 6.63 (1H, d, J 10.0 Hz, H-5′), 7.08 (1H, d, J 8.0 Hz, H-11), 7.19 (1H, s, H-1); 13C NMR (125 MHz, acetone-d 6) δ 27.9 (C-7′), 28.1 (C-8′), 38.3 (C-12a), 55.8 (C-3′), 56.8 (C-2′), 66.6 (C-6), 67.6 (C-12), 71.4 (C-6a), 76.3 (C-6′), 101.3 (C-4), 109.2 (C-10), 109.9 (C-8), 111.5 (C-1a), 114.6 (C-1), 117.5 (C-4′), 117.6 (C-11a), 129.4 (C-11), 129.6 (C-5′), 144.4 (C-2), 149.2 (C-3), 150.2 (C-4a), 150.3 (C-9), 154.2 (C-7a); HRESIMS m/z 419.1450 [M + Na]+ (calcd for C23H24O6Na, m/z 419.1465).
(6aR,12R,12aS)-12-Deoxo-12-hydroxytephrosin (30). Prepared from 8 (20.0 mg, 0.049 mmol) by GP1 and obtained as a white solid (18.5 mg, 92%): [α]D 20 −87 (c 0.1, CHCl3); IR νmax 3600-3300, 2924, 1614, 1585, 1508, 1445, 1266, 1217, 1197, 1151, 1114, 1056, 732 cm-1; 1H NMR (500 MHz, CDCl3) 1.40 (3H, s, H-7′) δ 1.41 (3H, s, H-8′) 2.22 (1H, d, J 2.5 Hz, OH-12), 2.46 (1H, s, OH-12a), 3.82 (3H, s, H-2′), 3.82 (3H, s, H-3′), 4.34 (1H, dd, J 4.0, 10.5 Hz, Ha-6), 4.62 (1H, dd, J 4.0, 9.5 Hz, H-6a), 4.67 (1H, dd, J 9.5, 10.5 Hz, Hb-6), 4.80 (1H, app s, H-12), 5.56 (1H, d, J 10.0 Hz, H-5′), 6.39 (1H, s, H-4), 6.44 (1H, d, J 8.5 Hz, H-10), 6.63 (1H, d, J 10.0 Hz, H-4′), 7.09 (1H, d, J 8.5 Hz, H-11), 7.23 (1H, s, H-1); 13C NMR (125 MHz, CDCl3) δ 28.0 (C-7′), 28.1 (C-8′), 56.0 (C-2′), 56.6 (C-3′), 64.9 (C-6), 68.5 (C-12a), 72.0 (C-12), 75.2 (C-6a), 76.2 (C-6′), 100.4 (C-4), 109.1 (C-1), 109.7 (C-8), 110.1 (C-10), 112.3 (C-1a), 113.0 (C-11a), 116.5 (C-4′), 129.3 (C-11), 129.3 (C-5′), 144.0 (C-3), 147.4 (C-7a), 149.4 (C-4a), 150.6 (C-2), 154.3 (C-9); HRESIMS m/z 435.1419 [M + Na]+ (calcd for C23H24O7Na, m/z 435.1414).
(6aS,12aS,4′R,5′R)- and (6aS,12aS,4′S,5′S)-4′,5′-Dihydro-4′,5′-cisdihydroxydeguelin (31) were prepared by modifying a literature procedure.58 OsO4 (20 μL of a 2.5 wt% solution in t-BuOH, 0.002 mmol) was added to a solution of 2 (40 mg, 0.102 mmol), N-methylmorpholine N-oxide (14 mg, 0.122 mmol), and citric acid (39 mg, 0.203 mmol) in acetone (4.0 mL) and H2O (1.0 mL). The mixture was stirred at rt for 28 h. EtOAc (10 mL) was then added followed by saturated aqueous Na2SO3 solution (10 mL) and the two phases were mixed vigorously for 10 min. The organic layer was separated, washed with H2O (10 mL) and brine (10 mL), dried (MgSO4), filtered, and concentrated. The yellow oily residue was subjected to flash chromatography (SiO2, 1:2 hexanes/EtOAc) to give the mixture of diastereoisomers 31 as a white solid (32 mg, 78%, dr 89:11): IR νmax 3600-3100, 1662, 1601, 1582, 1512, 1441, 1259, 1213, 1195, 1096, 1042, 817 cm-1; NMR data for the major diastereoisomer [tentatively assigned as (6aS,12aS,4′S,5′S)-4′,5′-dihydro-4′,5′-cis-dihydroxydeguelin as the convex face of 2 is more accessible to reagents than the concave face57]: δ H (500 MHz, CDCl3) 1.30 (3H, s, H-7′), 1.47 (3H, s, H-8′), 3.03 (1H, d, J 4.5 Hz, OH-4′), 3.67 (1H, d, J 2.0 Hz, OH-5′), 3.78 (3H, s, H-2′), 3.79 (1H, m obsc, H-5′), 3.81 (3H, s, H-3′), 3.90 (1H, d, J 4.5 Hz, H-12a), 4.23 (1H, d, J 12.5 Hz, Ha-6), 4.61 (1H, dd, J 3.5, 12.5 Hz, Hb-6), 5.04 (1H, dd, J 3.0, 4.5 Hz, H-4′), 5.07 (1H, ddd, J 1.0, 3.5, 4.5 Hz, H-6a), 6.45 (1H, s, H-4), 6.54 (1H, d, J 9.0 Hz, H-10), 6.78 (1H, s, H-1), 7.82 (1H, d, J 9.0 Hz, H-11); δ C (125 MHz, CDCl3) 22.7 (C-8′), 24.6 (C-7′), 44.4 (C-12a), 56.0 (C-3′), 56.5 (C-2′), 62.5 (C-4′), 66.4 (C-6), 70.6 (C-5′), 73.2 (C-6a), 79.1 (C-6′), 101.1 (C-4), 104.6 (C-1a), 109.6 (C-8), 110.5 (C-1), 112.6 (C-11a), 113.0 (C-10), 129.1 (C-11), 144.2 (C-2), 147.5 (C-4a), 149.7 (C-3), 160.1 (C-9), 161.2 (C-7a), 188.7 (C-12); NMR data for the minor diastereoisomer [tentatively assigned as (6aS,12aS,4′R,5′R)-4′,5′-dihydro-4′,5′-cis-dihydroxydeguelin]: δ H (500 MHz, CDCl3) 1.35 (3H, s, H-7′), 1.43 (3H, s, H-8′), 3.10 (1H, d, J 4.5 Hz, OH-4′), 3.73 (1H, d, J 2.0 Hz, OH-5′), 3.78 (3H, s, H-2′), 3.79 (1H, m obsc, H-5′), 3.82 (3H, s, H-3′), 3.90 (1H, d, J 4.5 Hz, H-12a), 4.23 (1H, d, J 12.5 Hz, Ha-6), 4.61 (1H, dd, J 3.5, 12.5 Hz, Hb-6), 5.00 (1H, dd, J 3.0, 4.5 Hz, H-4′), 5.07 (1H, ddd, J 1.0, 3.5, 4.5 Hz, H-6a), 6.44 (1H, s, H-4), 6.54 (1H, d, J 9.0 Hz, H-10), 6.77 (1H, s, H-1), 7.82 (1H, d, J 9.0 Hz, H-11); δ C (125 MHz, CDCl3) 22.6 (C-8′), 24.7 (C-7′), 44.6 (C-12a), 56.0 (C-3′), 56.5 (C-2′), 62.3 (C-4′), 66.4 (C-6), 70.7 (C-5′), 73.1 (C-6a), 79.2 (C-6′), 101.2 (C-4), 104.7 (C-1a), 109.3 (C-8), 110.3 (C-1), 112.8 (C-11a), 113.4 (C-10), 129.1 (C-11), 144.2 (C-2), 147.5 (C-4a), 149.7 (C-3), 160.1 (C-9), 161.2 (C-7a), 188.6 (C-12); HRESIMS m/z 429.1539 [M + H]+ (calcd for C23H25O8, m/z 429.1549).
Preparation of and Kinetic Measurements on Mitochondrial Membranes
Bos taurus (bovine) heart mitochondrial membranes were prepared at 4 °C as described previously.72 Assays were performed in a SpectraMax 96-well plate reader at 32 °C. NADH:O2 oxidoreduction by complexes I, III, and IV in membranes was measured using 50 μg mL−1 membranes in 10 mM Tris-HCl (pH 7.4) and 250 mM sucrose using 120 μM NADH and supplemented with 1.5 μM horse heart cytochrome c (Sigma-Aldrich). The reaction was monitored using ε340–380(NADH)= 4.81 mM −1 cm−1. Catalysis was initiated by addition of NADH and maximal rates determined by linear regression.
OCR Measurements on Cultured Human Cells
PNT2, C4-2, and C4-2B cells were obtained from the Cancer Research UK Cambridge Research Institute and tested for mycoplasma before use. Cells were grown in RPMI 1640 (Gibco) supplemented with 10% FBS (Themo Fisher Scientific), 25 mM HEPES and 1 mM pyruvate. Seahorse cell plates were coated with poly-L-lysine hydrobromide (Sigma-Aldrich), washed with PBS and dried prior to cell seeding. 20,000 cells per well were plated into coated 96-well Seahorse plates and incubated for 16 h at 37 °C and 5% CO2. The medium was exchanged for assay buffer containing RPMI without carbonate, supplemented with 4.5 g L-1 glucose, 1 mM pyruvate, 32 mM NaCl, 2 mM GlutaMAX and 15 mg L-1 Phenol Red (pH 7.4) and the cells placed in a CO2-free incubator at 37 °C for 60 min. OCRs (oxygen consumption rates) were measured in a Seahorse extracellular flux analyser; after ~ 1 h of baseline measurements, 1 μM of rotenoids from ethanolic stocks diluted in media (as well as ethanol-only controls wells) were added and incubated for a further hour. After this, a mixture of 1 μM rotenone and 1 μM antimycin was added to inhibit mitochondrial respiration. To calculate the normalized rotenone-sensitive OCRs, the rotenone-insensitive rates (determined at the end of the experiment) were subtracted and the traces baselined to 100% before addition of rotenoids. The measured basal OCR prior to baselining for PNT2, C4-2, and C42B cells were 45, 71, and 79 pM min-1.
Cell Growth Curves
PNT2, C4-2, and C4-2B cells were each seeded to a density of 1,000 cells per well in a 96 well plate in RPMI 1640 (Gibco) supplemented with 10% FBS (Themo Fisher Scientific), 25 mM HEPES and 1 mM pyruvate. After 24 h, 1 μM of rotenoid compounds from ethanolic stocks diluted in media (as well as ethanol-only controls) were added and growth monitored in an Essen Bioscience Incucyte until confluence was reached in the untreated control. Eight replicates were used per condition.
Cell Viability
Cell viability was tested with the acridine orange/DAPI method using a chemometec NC-3000. Cells were seeded to a density of 150,000 cells per well in 6-well plates and grown overnight. Rotenoids were added to 1 μM concentration from ethanolic stocks diluted in media, as well as ethanol controls wells and incubated for 48 hours. Cells were trypsinized using TrypLe Express (Gibco), quenched with complete media and cells pelleted by centrifugation at 400 x g for 5 mins. Cells were resuspended in PBS and mixed with staining solution containing acridine orange and DAPI (Chemometec), then loaded onto slides and measured according to the manufacturer’s instructions. Prior to trypsinization, no floating cells were visible in the wells and the viability of the control PNT2, C4-2, and C4-2B cells were 89, 87, and 83% respectively.
Statistical Methods
Data are presented as mean averages. IC50 values were calculated in Prism version 7.0e from normalized kinetic data using a log(inhibitor) vs. normalized response fit with variable slope. Error is presented as either a 95% confidence interval or standard error of the mean and statistical significance between samples were tested in Prism via an unpaired, two-tailed t-test. * P ≤ 0.1, ** P ≤ 0.01, *** P ≤ 0.001,**** P ≤ 0.000.1.
Acknowledgements
We thank Jo Hutchings (MRC MBU) for her support with tissue culture work, Dr. Mohammad Asim (Cancer Research UK, Cambridge Institute) for advice and encouragement, and Dr. Michael Pollak (McGill University) for his critical comments on the manuscript. D.A.R thanks Cancer Research UK for funding. H.R.B, R.S, and J.H were supported by the Medical Research Council (MC_U105663141 and MC_UU_00015/2 to J. H.). S.L.K. thanks AstraZeneca for funding. N.M.S. thanks the EU for a Marie Curie Fellowship (2013-IEF-626191). T.J.O., H.F.S., and D.R.S. acknowledge support from the EPSRC (EP/P020291/1). D.R.S. also thanks the Royal Society for a Wolfson Research Merit Award and the ERC, EPSRC, BBSRC, MRC, and Royal Society for general laboratory support.
Footnotes
The authors declare no competing financial interest.
References
- 1.Rawla P. World J Oncol. 2019;10:63–89. doi: 10.14740/wjon1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taitt HE. Am J Men’s Health. 2018;12:1807–1823. doi: 10.1177/1557988318798279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong YNS, Ferraldeschi R, Attard G, de Bono J. Nat Rev Clin Oncol. 2014;11:365–376. doi: 10.1038/nrclinonc.2014.72. [DOI] [PubMed] [Google Scholar]
- 4.Gartrell BA, Saad F. Nat Rev Clin Oncol. 2014;11:335–345. doi: 10.1038/nrclinonc.2014.70. [DOI] [PubMed] [Google Scholar]
- 5.Naguib A, Mathew G, Reczek CR, Watrud K, Ambrico A, Herzka T, Salas IC, Lee MF, El-Amine N, Zheng W, Di Francesco EM, et al. Cell Rep. 2018;23:58–67. doi: 10.1016/j.celrep.2018.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Udeani GO, Gerhäuser C, Thomas CF, Moon RC, Kosmeder JW, Kinghorn AD, Moriarty RM, Pezzuto JM. Cancer Res. 1997;57:3424–3428. [PubMed] [Google Scholar]
- 7.Gerhäuser C, Lee SK, Kosmeder JW, Moriarty RM, Hamel E, Mehta RG, Moon RC, Pezzuto JM. Cancer Res. 1997;57:3429–3435. [PubMed] [Google Scholar]
- 8.Lee H-Y, Suh Y-A, Kosmeder JW, Pezzuto JM, Hong WK, Kurie JM. Clin Cancer Res. 2004;10:1074–1079. doi: 10.1158/1078-0432.ccr-0833-3. [DOI] [PubMed] [Google Scholar]
- 9.Jin Q, Feng L, Behrens C, Bekele BN, Wistuba II, Hong W-K, Lee H-Y. Cancer Res. 2007;67:11630–11639. doi: 10.1158/0008-5472.CAN-07-2401. [DOI] [PubMed] [Google Scholar]
- 10.Thamilselvan V, Menon M, Thamilselvan S. Int J Cancer. 2011;129:2916–2927. doi: 10.1002/ijc.25949. [DOI] [PubMed] [Google Scholar]
- 11.Boreddy SR, Srivastava SK. Oncogene. 2013;32:3980–3991. doi: 10.1038/onc.2012.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindahl PE, Öberg KE. Nature. 1960;187:784. doi: 10.1038/187784a0. [DOI] [PubMed] [Google Scholar]
- 13.Öberg KE. Exptl Cell Res. 1961;24:228–237. doi: 10.1016/0014-4827(61)90033-7. [DOI] [PubMed] [Google Scholar]
- 14.Burgos J, Redfearn ER. Biochim Biophys Acta. 1965;110:475–483. [Google Scholar]
- 15.Garcia J, Barluenga S, Gorska K, Sasse F, Winssinger N. Bioorg Med Chem. 2012;20:672–680. doi: 10.1016/j.bmc.2011.09.064. [DOI] [PubMed] [Google Scholar]
- 16.Hirst J. Annu Rev Biochem. 2013;82:551–575. doi: 10.1146/annurev-biochem-070511-103700. [DOI] [PubMed] [Google Scholar]
- 17.Bridges HR, Jones AJY, Pollak MN, Hirst J. Biochem J. 2014;462:475–487. doi: 10.1042/BJ20140620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zi F, Zi H, Li Y, He J, Shi Q, Cai Z. Oncol Lett. 2018;15:683–690. doi: 10.3892/ol.2017.7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molina JR, Sun Y, Protopopova M, Gera S, Bandi M, Bristow C, McAfoos T, Morlacchi P, Ackroyd J, Agip A-NA, Al-Atrash G, et al. Nat Med. 2018;24:1036–1046. doi: 10.1038/s41591-018-0052-4. [DOI] [PubMed] [Google Scholar]
- 20.Caboni P, Sherer TB, Zhang N, Taylor G, Na HM, Greenamyre JT, Casida JE. Chem Res Toxicol. 2004;17:1540–1548. doi: 10.1021/tx049867r. [DOI] [PubMed] [Google Scholar]
- 21.Varughese RS, Lam WS-T, bin Hanifah Marican AA, Viganeshwari SH, Bhave AS, Syn NL, Wang J, Wong AL-A, Kumar AP, Lobie PE, Lee SC, et al. Cancer. 2019;125:1789–1798. doi: 10.1002/cncr.32069. [DOI] [PubMed] [Google Scholar]
- 22.Hansch C, Björkroth JP, Leo A. J Pharm Sci. 1987;76:663–687. doi: 10.1002/jps.2600760902. [DOI] [PubMed] [Google Scholar]
- 23.Waterhouse RN. Mol Imaging Biol. 2003;5:376–380. doi: 10.1016/j.mibio.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 24.Acree F, Jacobsen M, Haller HL. J Org Chem. 1943;8:572–574. [Google Scholar]
- 25.Claisse J, Crombie L, Peace R. J Chem Soc. 1964:6023–6036. [Google Scholar]
- 26.Adinarayana D, Radhakrishniah M, Rao JR, Campbell R, Crombie L. J Chem Soc C. 1971:29–32. [Google Scholar]
- 27.Crombie L, Kilbee GW, Moffatt F, Proudfoot G, Whiting DA. J Chem Soc, Perkin Trans 1. 1991:3143–3148. [Google Scholar]
- 28.Kasymov AU, Kondratenko ES, Abubakirov NK. Chem Nat Compds. 1972;8:109–110. [Google Scholar]
- 29.Kasymov AU, Kondratenko ES, Rashkes YV, Abubakirov NK. Chem Nat Compds. 1970;6:192–195. [Google Scholar]
- 30.Kasymov AU, Kondratenko ES, Abubakirov NK. Chem Nat Compds. 1974;10:470–473. [Google Scholar]
- 31.Fukami J, Yamamoto I, Casida JE. Science. 1967;155:713–716. doi: 10.1126/science.155.3763.713. [DOI] [PubMed] [Google Scholar]
- 32.Fukami J, Shishido T, Fukunaga K, Casida JE. J Agric Food Chem. 1969;17:1217–1226. [Google Scholar]
- 33.Wager TT, Chandrasekaran RY, Hou X, Troutman MD, Verhoest PR, Villalobos A, Will Y. ACS Chem Neurosci. 2010;1:420–434. doi: 10.1021/cn100007x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghose AK, Herbertz T, Hudkins RL, Dorsey BD, Mallamo JP. ACS Chem Neurosci. 2012;3:50–68. doi: 10.1021/cn200100h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Latorre AO, Borghi GA, Lopes PL, Higa KC, Lopes LMX, Maiorka PC, Gorniak SL, Haraguchi M. J Anim Vet Adv. 2011;10:291–294. [Google Scholar]
- 36.Heinz S, Freyberger A, Lawrenz B, Schladt L, Schmuck G, Ellinger-Ziegelbauer H. Sci Rep. 2017;7:45465. doi: 10.1038/srep45465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li L, Wang H-K, Chang J-J, McPhail AT, McPhail DR, Terada H, Konoshima T, Kokumai M, Kozuka M, Estes JR, Lee K-H. J Nat Prod. 1993;56:690–698. doi: 10.1021/np50095a005. [DOI] [PubMed] [Google Scholar]
- 38.Konoshima T, Terada H, Kokumai M, Kozuka M, Tokuda H, Estes JR, Li L, Wang H-K, Lee K-H. J Nat Prod. 1993;56:843–848. doi: 10.1021/np50096a006. [DOI] [PubMed] [Google Scholar]
- 39.Fang N, Casida JE. J Agric Food Chem. 1999;47:2130–2136. doi: 10.1021/jf981188x. [DOI] [PubMed] [Google Scholar]
- 40.Blatt CTT, Chávez D, Chai H, Graham JG, Cabieses F, Farnsworth NR, Cordell GA, Pezzuto JM, Kinghorn AD. Phytother Res. 2002;16:320–325. doi: 10.1002/ptr.889. [DOI] [PubMed] [Google Scholar]
- 41.Liang Y, Li X, Gu Z, Qin P, Ji M. Molecules. 2015;20:3238–3254. doi: 10.3390/molecules20023238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ji M, Liang Y, Gu Z, Li X. Int J Mol Sci. 2015;16:19713–19727. doi: 10.3390/ijms160819713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muharini R, Díaz A, Ebrahim W, Mándi A, Kurtán T, Rehberg N, Kalscheuer R, Hartmann R, Orfali RS, Lin W, Liu Z, et al. J Nat Prod. 2017;80:169–180. doi: 10.1021/acs.jnatprod.6b00809. [DOI] [PubMed] [Google Scholar]
- 44.Deyou T, Marco M, Heydenreich M, Pan F, Gruhonjic A, Fitzpatrick PA, Koch A, Derese S, Pelletier J, Rissanen K, Yenesew A, et al. J Nat Prod. 2017;80:2060–2066. doi: 10.1021/acs.jnatprod.7b00255. [DOI] [PubMed] [Google Scholar]
- 45.Unai T, Yamamoto I, Cheng H-M, Casida JE. Agric Biol Chem. 1973;37:387–401. [Google Scholar]
- 46.Bhandari P, Crombie L, Kilbee GW, Pegg SJ, Proudfoot G, Rossiter JT, Sanders M, Whiting DA. J Chem Soc, Perkin Trans 1. 1992:851–863. [Google Scholar]
- 47.Yamamoto Y, Fujikawa R, Umemoto T, Miyaura N. Tetrahedron. 2004;60:10695–10700. [Google Scholar]
- 48.Russell DA, Fong WJS, Twigg DG, Sore HF, Spring DR. J Nat Prod. 2017;80:2751–2755. doi: 10.1021/acs.jnatprod.7b00527. [DOI] [PubMed] [Google Scholar]
- 49.Büchi G, Crombie L, Godin PJ, Kaltenbronn JS, Siddalingaiah KS, Whiting DA. J Chem Soc. 1961:2843–2860. [Google Scholar]
- 50.Crombie L, Godin PJ. J Chem Soc. 1961:2861–2876. [Google Scholar]
- 51.Rossi M, Fule PZ, Taylor MR. Bioorg Chem. 1988;16:376–387. [Google Scholar]
- 52.Ravanel P, Tissut M, Douce R. Plant Physiol. 1984;75:414–420. doi: 10.1104/pp.75.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miyoshi H. Biochim Biophys Acta. 1998;1364:236–244. doi: 10.1016/s0005-2728(98)00030-9. [DOI] [PubMed] [Google Scholar]
- 54.Russell DA, Freudenreich JJ, Ciardiello JJ, Sore HF, Spring DR. Org Biomol Chem. 2017;15:1593–1596. doi: 10.1039/c6ob02659a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Unai T, Yamamoto I, Casida JE. Agr Biol Chem. 1973;37:897–901. [Google Scholar]
- 56.Carson D, Crombie L, Whiting DA. J Chem Soc, Chem Commun. 1975:851–852. [Google Scholar]
- 57.Bhandari P, Crombie L, Harper MF, Rossiter JT, Sanders M, Whiting DA. J Chem Soc, Perkin Trans 1. 1992:1685–1697. [Google Scholar]
- 58.Chang D-J, An H, Kim K-S, Kim HH, Jung J, Lee JM, Kim N-J, Han YT, Yun H, Lee S, Lee G, et al. J Med Chem. 2012;55:10863–10884. doi: 10.1021/jm301488q. [DOI] [PubMed] [Google Scholar]
- 59.Ueno H, Miyoshi H, Ebisui K, Iwamura H. Eur J Biochem. 1994;225:411–417. doi: 10.1111/j.1432-1033.1994.00411.x. [DOI] [PubMed] [Google Scholar]
- 60.Unai T. J Pestic Sci. 1980;5:453–461. [Google Scholar]
- 61.Okun JG, Lümmen P, Brandt U. J Biol Chem. 1999;274:2625–2630. doi: 10.1074/jbc.274.5.2625. [DOI] [PubMed] [Google Scholar]
- 62.Fendel U, Tocilescu MA, Kerscher S, Brandt U. Biochim Biophys Acta. 2008;1777:660–665. doi: 10.1016/j.bbabio.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 63.Baradaran R, Berrisford JM, Minhas GS, Sazanov LA. Nature. 2013;494:443–448. doi: 10.1038/nature11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu J, Vinothkumar KR, Hirst J. Nature. 2016;536:354–358. doi: 10.1038/nature19095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fiedorczuk K, Letts JA, Degliesposti G, Kaszuba K, Skehel M, Sazanov LA. Nature. 2016;538:406–410. doi: 10.1038/nature19794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Agip A-NA, Blaza JN, Bridges HR, Viscomi C, Rawson S, Muench SP, Hirst J. Nat Struct Mol Biol. 2018;25:548–556. doi: 10.1038/s41594-018-0073-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Surin AM, Sharipov RR, Krasil’nikova IA, Boyarkin DP, Lisina OY, Gorbacheva LR, Avetisyan AV, Pinelis VG. Biochemistry (Moscow) 2017;82:737–749. doi: 10.1134/S0006297917060104. [DOI] [PubMed] [Google Scholar]
- 68.Crombie L, Holden I, Kilbee GW, Whiting DA. J Chem Soc, Perkin Trans 1. 1982:789–797. [Google Scholar]
- 69.Abe F, Donnelly DMX, Moretti C, Polonsky J. Phytochemistry. 1985;24:1071–1076. [Google Scholar]
- 70.Carson D, Crombie L, Kilbee GW, Moffatt F, Whiting DA. J Chem Soc, Perkin Trans 1. 1982:779–788. [Google Scholar]
- 71.Crombie L, Freeman PW, Whiting DA. J Chem Soc, Perkin Trans 1. 1973:1277–1285. [Google Scholar]
- 72.Sharpley M, Shannon R, Draghi F, Hirst J. Biochemistry. 2006;45:241–248. doi: 10.1021/bi051809x. [DOI] [PubMed] [Google Scholar]