Abstract
Purpose
It remains unclear to what extent habitual physical activity and sedentary time are associated with visceral fat and liver fat. We studied substitution of sedentary time with time spent physically active and total body fat (TBF), visceral adipose tissue (VAT) and hepatic triglyceride content (HTGC) in middle-aged men and women.
Design
In this cross-sectional analysis of the Netherlands Epidemiology of Obesity study, physical activity was assessed in 228 participants using a combined accelerometer and heart rate monitor. Total body fat was assessed by the Tanita bio-electrical impedance, VAT by MRI and HTGC by proton-MR spectroscopy. Behavioural intensity distribution was categorized as sedentary time (ST), time spent in light physical activity (LPA), and moderate-to-vigorous physical activity (MVPA). To estimate the effect of replacing 30 minutes/day of ST with 30 minutes/day LPA or MVPA, we performed isotemporal substitution analyses, adjusted for sex, age, ethnicity, education, the Dutch Healthy Diet index, and smoking.
Results
Included participants (41% men) had a mean (SD) age of 56 (6) years and spent 88 (56) minutes in MVPA and 9.0 hours (2.1) of ST. Replacing 30 minutes/day of ST with 30 minutes of MVPA was associated with 1.3% less TBF (95% CI: -2.0, -0.7), 7.8 cm2 less VAT (-11.6, -4.0) and 0.89 times HTGC (0.82, 0.97). Replacement with LPA was not associated with TBF (-0.03 %; -0.5, 0.4), VAT (-1.7 cm2; -4.4, 0.9) or HTGC (0.98 times; 0.92, 1.04).
Conclusions
Reallocation of time spent sedentary with time spent in MVPA, but not LPA, was associated with less total body fat, and visceral and liver fat. These findings contribute to the development of more specified guidelines on sedentary time and physical activity.
Keywords: physical activity, sedentary time, adiposity, epidemiology, middle-aged men and women
Introduction
Abdominal obesity is a well-established risk factor for diabetes mellitus type 2, the metabolic syndrome and cardiovascular diseases (1, 2). It has been hypothesized that especially accumulation of fat in the visceral area and in and around the organs (2), such as the liver, increases cardiometabolic risk. Both visceral fat and liver fat have been associated with metabolic risk factors, insulin resistance and cardiovascular diseases (3-6), making these adipose depots important targets to prevent cardiometabolic diseases.
Previous meta-analyses of randomised controlled trials have shown that exercise can reduce both visceral fat (7) and liver fat (8). However, the included trials focused on structured exercise rather than habitual daily activities and sedentary time, and evidence on the association between habitual daily activity and different adipose depots is lacking. Most European guidelines on physical activity state to perform at least 150 minutes per week of moderate to vigorous physical activity and to limit sedentary time (9), as sedentary behaviour has been associated with increased risks of type 2 diabetes, cardiovascular disease, and cancer, even after adjustment for physical activity (10). It must be noted that, as a day contains 24 hours, less time spent sedentary means more time spent in other intensity categories. When examining the association of one particular type of activity with health outcomes, it is therefore important to take into account which behaviour this is being replaced with (e.g. replacing sedentary time with the same amount of time spent with light-intensity physical activity)(11). Many studies that applied this isotemporal substitution analysis to examine substitution of sedentary time by time spent with activities with higher intensity in relation to body fat used objectively assessed physical activity. However, most used surrogate outcomes for adiposity such as body mass index or waist circumference (12) instead of direct measures of adipose tissue. Only one previous study investigating isotemporal substitution of habitual activities used direct measurements of both visceral fat and physical activity, and found that replacing sedentary time with moderate physical activity was associated with less visceral fat (13). However, liver fat was not assessed in this study and adjustment for total body fat was not performed.
Knowing how different types of daily activities and their mutual substitutions are associated with different adipose depots, such as total body fat, visceral and liver fat, helps elucidating the underlying mechanism of how sedentary time and physical inactivity can lead to multiple adverse health outcomes. This may ultimately lead to more specific guidelines on sedentary time and physical activity. We therefore aimed to study the association between substitution of sedentary time with time spent in other intensity of activity and total body fat, visceral fat and liver fat in a population-based cohort of middle-aged participants. We hypothesised that replacing sedentary time with activity of any intensity is associated with reduced adipose depots and this reduction will be more pronounced when replacing it with activity of higher intensity
Methods
Study design and study population
The Netherlands Epidemiology of Obesity (NEO) study is a population-based, prospective cohort study designed to investigate pathways that lead to obesity-related diseases. The NEO study includes 6,671 individuals aged 45–65 years who were included between September 2008 and October 2012, with an oversampling of individuals with overweight or obesity. The study design and data collection are described in detail elsewhere (14). Men and women living in the greater area of Leiden (in the West of the Netherlands) were invited by letters sent by general practitioners and municipalities and by local advertisements. They were invited to respond if they were aged between 45 and 65 years and had a self-reported BMI of 27 kg/m2 or higher. In addition, all inhabitants aged between 45 and 65 years from one municipality (Leiderdorp) were invited to participate irrespective of their BMI, allowing for a reference distribution of BMI. The Medical Ethical Committee of the Leiden University Medical Center (LUMC) approved the design of the study. All participants gave their written informed consent.
Participants were invited to a baseline visit at NEO study centre of the LUMC after an overnight fast. Prior to this study visit, participants collected their urine over 24 hours and completed a general questionnaire at home to report demographic, lifestyle and clinical information. The participants were asked to bring all medication they were using to the study visit. At the study center, the participants completed a screening form, asking about anything that might create a health risk or interfere with magnetic resonance imaging (MRI) (most notably metallic devices, claustrophobia, or a body circumference of more than 1.70 m). A random selection of approximately 35% of all NEO study participants who were eligible for MRI underwent direct assessment of abdominal fat study participants.
The present study is a cross-sectional analysis of the baseline measurements of the NEO study. We excluded participants with missing data on objectively assessed physical activity, body fat measurements or potential confounding factors (see results).
Assessment of habitual physical activity
Daily levels of activity were objectively assessed in a random subsample of NEO study participants (n=955) using a combined uniaxial acceleration and heart rate monitor (Actiheart, CamNtech Ltd, UK). The device weighs less than 8 g and is worn on the chest. Two standard ECG electrodes (H98SG, Tyco Healthcare, Germany) were placed at the level of the second intercostal space; one on the sternum and one 10 cm to the left of the first electrode. Participants were instructed to wear the monitor continuously for four consecutive days and nights, except when showering, bathing or swimming, and to carry on with all normal activities during this time. The monitor was set-up to record at 15 second epochs.
In a random subgroup of participants who wore a monitor, an 8-min ramped step test was performed to calibrate the individual heart rate response to activity intensity. If individual calibration was not available, a group calibration equation based on age, sex, and sleeping heart rate (SHR) was applied (15). The following group calibration equation was derived from the valid step tests (n=132) in our population:
In this equation, age is displayed in years, sex coded as 1 for men and 0 for women, SHR in beats per minute, heart rate above sleep (HRaS) in beats per minute, and betablocker coded as 0 or 1 if the participant was taking betablocker medication.
A Gaussian process regression method was applied to the heart rate data to handle potential measurement noise (16). Using a branched equation algorithm the acceleration and heart rate information was summarised into calibrated estimates of physical activity energy expenditure (PAEE) and time spent at different activity intensities expressed as metabolic equivalents of task (MET) (17, 18). One MET was defined as an energy expenditure of 71 J/min/kg. When summarising the data we accounted for non-wear time and any potential diurnal imbalance of wear time by weighting all hours of the day equally in the summation (19), and excluding participants who had less than 4 hours of valid data in each quadrant of the day.
Sedentary time was defined as time spent in activities with an intensity ≤1.5 MET, excluding sleep time (assumed as time between 23:00h and 07:30h on weekdays and time between 23:30h and 08:30h on weekend days). Light intensity physical activity (LPA) was defined as any activity during wear time with an intensity >1.5 and ≤3 MET. Moderate-to-vigorous physical activity (MVPA) was defined as any activity >3 MET.
Participants with a valid total wear time <24 hours were excluded from the analyses. No minimum bout duration was set for all activity intensity categories.
Assessment of body fat
Body weight and percent body fat were assessed by the Tanita bio impedance balance (TBF-310, Tanita International Division, UK) without shoes and one kilogram was subtracted from the body weight to account for the weight of clothing.
Visceral adipose tissue (VAT) was quantified by a turbo spin echo imaging protocol using MRI. Imaging was performed on a 1.5 Tesla MR system (Philips Medical Systems, Best, the Netherlands). At the level of the fifth lumbar vertebra, three transverse images each with a slice thickness of 10 mm were obtained during a breath-hold. Visceral fat area was converted from the number of pixels to centimeters squared using in-house-developed software (MASS, Medis, Leiden, the Netherlands) and the average of three slices was used in the analyses (14).
Hepatic triglyceride content (HTGC) was quantified by proton-MR spectroscopy (1H-MRS) of the liver (20). An 8 ml voxel was positioned in the right lobe of the liver, avoiding gross vascular structures and adipose tissue depots. Sixty-four averages were collected with water suppression. Spectra were obtained with an echo time of 26 ms and a repetition time of 3,000 ms. Data points (1,024) were collected using a 1,000 Hz spectral line. Without changing any parameters, spectra without water suppression, with a repetition time of 10 s, and with four averages were obtained as an internal reference. 1H-MRS data were fitted using Java-based magnetic resonance user interface software (jMRUI version 2.2, Leuven, Belgium), as described previously (21). Hepatic triglyceride content relative to water was calculated as the sum of signal amplitudes of methyl and methylene divided by the signal amplitude of water and then multiplied by 100.
Covariates
On the questionnaire, participants reported ethnicity by self-identification in eight categories which we grouped into white (reference) and other (black, Turkish, Moroccan, South-east Asian, Hindus, or other). Tobacco smoking was reported in the three categories current, former, and never smoking (reference). Highest level of education was reported in 10 categories according to the Dutch education system and grouped into high (including higher vocational school, university, and post-graduate education) versus low education (reference). Participants reported their medical history of diabetes and cardiovascular diseases. Pre-existing cardiovascular disease was defined as myocardial infarction, angina, congestive heart failure, stroke, or peripheral vascular disease. BMI was calculated by dividing the weight in kilograms by the height in meters squared. Habitual dietary intake of all participants was estimated using a semi-quantitative self-administered 125-item food frequency questionnaire (FFQ) (22, 23). Based on these variables, an adapted version of the Dutch Healthy Diet Index (DHD-index) 2015 was calculated which consisted of 13 components (vegetables, fruit, wholegrain products, legumes, unsalted nuts, dairy, fish, tea, liquid to solid fat ratio, red meat, processed meat, sweetened beverages and alcohol). The score can range between 0 and 130, in which a higher score of reflects better adherence to the Dutch Guidelines for a Healthy Diet of 2015 (24).
Statistical analysis
In the NEO study there is an oversampling of persons with a BMI of 27 kg/m2 or higher. To correctly represent associations in the general population (25), adjustments for the oversampling of individuals with a BMI ≥ 27 kg/m2 were made. This was done by weighting individuals towards the BMI distribution of participants from the Leiderdorp municipality (26), whose BMI distribution was similar to the BMI distribution of the general Dutch population (27). All results were based on weighted analyses. Consequently, the results apply to a population-based study without oversampling of individuals with a BMI ≥ 27 kg/m2. Because of the weighted analyses, percentages and proportions are given instead of numbers of participants. Other baseline characteristics are expressed as mean with standard deviation.
We performed linear regression analyses and fitted several models. First, we examined the association between 30 minutes of daily activities (i.e. sedentary time, light, and moderate to vigorous physical activity) and total body fat, visceral fat and liver fat in a crude model. Second, a multivariable model was performed that was adjusted for sex, age, ethnicity, education, DHD-index, and smoking. This model describes the association between 30 minutes/day of each of the daily activities on top of the regular activity pattern for each outcome. This model was also performed for total PAEE per 10 kj/day/kg. Next, we performed an isotemporal substitution approach in our final model by adding total time awake and dropping sedentary time. Consequently, the regression coefficients of each activity represent the estimated difference in measure of body fat associated with replacing 30 minutes of sedentary time with this type of intensity of activity. We also performed this model with light physical activity as the intensity of activity to be substituted. This isotemporal substitution model on visceral fat and liver fat was additionally adjusted for total body fat to study whether physical activity had an extra effect on visceral fat and liver fat beyond effects via total body fat. Moreover, we performed sensitivity analyses in which we only included participants with at least 72 hours of valid data instead of 24 hours. Lastly, the associations between physical activity and total body fat were also estimated without exclusion of participants with no MRI measurement.
Regression coefficients of total body fat represent an absolute difference in TBF in % per 30 minutes of a certain intensity of activity, and those of VAT an absolute difference in VAT in cm2 per 30 minutes of a certain intensity of activity per day.
Due to a skewed distribution of HTGC, we used the natural logarithm of this variable in the analyses. For interpretation of the results, we back transformed the regression coefficients of HTGC towards a ratio with 95% confidence interval, which is associated with 30 minutes per day spent in a certain activity. Such ratio, for example a ratio of 1.2, can be interpreted as each 30 minutes per day of a certain activity being associated with a 1.2-fold increased HTGC, which would reflect an increase in liver fat content from, for example, 5% to 6%.
“We tested interaction effects of age, sex, and BMI. For age and sex, we generated interaction terms of sex and age with the independent variables (e.g. sex*MVPA) and added them to the fully adjusted multivariable models (models 3). For BMI, we replaced TBF with BMI as covariate in the models for outcomes VAT and HTGC and added an interaction term of BMI in the fully adjusted models (e.g. BMI*MVPA). All p-values for the interaction terms were >0.10, except for PAEE*sex and PAEE*age for outcome TBF. Despite a statistically significant interaction term, stratified analyses yielded similar regression coefficients and therefore we present all analyses for total population.”
Data were analyzed using STATA v.16 (StataCorp LP, College Station, TX, USA).
Results
Physical activity was objectively assessed in 955 participants, of whom 932 had a successful measurement. The group of participants with a physical activity assessment was similar in sex distribution, age, and BMI to the group without a physical activity assessment, but were slightly more likely to have a history of CVD (5.6% versus 6.1%). We excluded participants with less than 24 hours of measurement (n=39) or for whom activity intensities could not be estimated (n=32), participants without an MRI of the abdomen (n=558) or 1H-MRS measurement of the liver (n=45), missing data on smoking status (n=1), education (n=7) or ethnicity (n=1), and participants who consumed 40 grams of alcohol or more (4 standard glasses) per day (n=21), leaving a total of 228 participants.
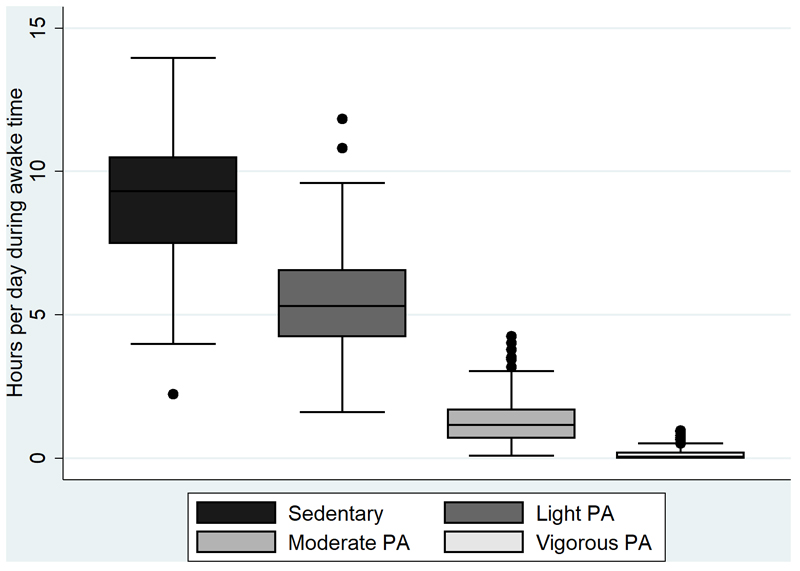
Included participants (41% men) had a mean (SD) age of 56 (6) years and BMI of 25 kg/m2 (4). Participants had a mean (SD) wear time of physical activity monitoring of 85 (11) hours and 87% of participants had more than 72 hours of valid wear time. Most time awake was spent sedentary, and least time in vigorous intensity physical activity (Figure 1). The baseline characteristics of the population stratified by sex-specifc tertiles of moderate to vigorous physical activity are shown in Table 1. Participants in the highest tertile were somewhat younger, more often had a high educational background, had a lower BMI and higher PAEE, and spent less time sedentary compared with participants in the lowest tertile. To provide additional insight into the characteristics of our study population, we also provide baseline characteristics stratified by BMI and sex-specific tertiles of TBF (see supplemental tables 1 and 2, Supplemental Digital Content, Appendix).
Figure 1.
Distribution of habitual activity intensities during awake time in participants with a physical activity and total body fat measurement in the Netherlands Epidemiology of Obesity study (N=228)
Table 1. Characteristics of participants of the Netherlands Epidemiology of Obesity study: men and women between 45 and 65 years of age with objectively measured physical activity and MRI of the abdomen, stratified by sex-specific tertiles of moderate to vigorous physical activity.
| Moderate to vigorous physical activity (min/day) | |||
|---|---|---|---|
| T1 (men: 5.7-53.2, women 5.5-52.2) | T2 (men: 53.8-103.5, women: 52.8-92.5) | T3 (men: 104.4-243.5, women: 93.5-284.8) | |
| Age (y) | 57 (7) | 55 (6) | 54 (5) |
| Sex (% men) | 40 | 40 | 42 |
| Ethnicity (% white) | 95 | 99 | 99 |
| Education level (% high)1 | 36 | 47 | 52 |
| BMI (kg/m2) | 26.9 (4.7) | 25.6 (3.5) | 23.8 (3.3) |
| Tobacco smoking (% current) | 13 | 17 | 15 |
| Dutch Healthy Diet Index | 71 (17) | 70 (15) | 72 (12) |
| HDL-Cholesterol (mmol/l) | 1.6 (0.5) | 1.5 (0.4) | 1.7 (0.5) |
| Triglycerides (mmol/l) | 1.1 [0.7, 1.6] | 1.0 [0.8, 1.4] | 0.8 [0.6, 1.2] |
| CVD (%) | 11 | 9 | 1 |
| Sedentary time (min/day) | 641 (92) | 548 (87) | 434 (85) |
| LPA (min/day) | 267 (87) | 332 (96) | 378 (81) |
| MPA (min/day) | 31 (13) | 71 (14) | 129 (38) |
| VPA (min/day) | 0 [0, 4] | 3 [0, 7] | 14 [3, 31] |
| PAEE (J/kg/min) | 30 (8) | 45 (8) | 69 (13) |
| Total body fat (%) | |||
| Men | 24.7 (7.6) | 24.5 (5.7) | 21.8 (6.8) |
| Women | 39.4 (6.3) | 35.5 (5.9) | 33.0 (4.3) |
| Visceral fat (cm2) | |||
| Men | 110 [69, 169] | 98 [87, 146] | 63 [38, 109] |
| Women | 64 [46, 116] | 57 [46, 80] | 37 [21, 57] |
| Liver fat (%) | |||
| Men | 3.6 [2.2, 13.1] | 5.1 [1.1, 11.0] | 3.6 [1.8, 5.8] |
| Women | 2.7 [1.2, 5.0] | 1.6 [0.9, 1.9] | 1.1 [0.8, 1.5] |
Results are based on analyses weighted toward the BMI distribution of the general population (n=228). Data are shown as mean (standard deviation), median [interquartile range] or percentage.
Low education: none, primary school, or lower vocational education as highest level of education. BMI, body mass index; CVD, cardiovascular disease; HDL, high density lipoprotein; LPA, light physical activity; MPA, moderate physical activity; PAEE, physical activity energy expenditure; VPA, vigorous physical activity.
Physical activity in relation to total body fat
After adjustment for potential confounding factors, PAEE was inversely associated with body fat (-1.3%, 95% -1.8, -0.9). With respect to intensity, each 30 minutes per day of sedentary time was associated with 0.5% more total body fat (95% CI 0.2, 0.7), whereas 30 minutes per day of light physical activity (-0.4, 95% CI -0.8, 0.04) and moderate to vigorous physical activity (-1.4, 95% CI -1.8, -0.9) were associated with less total body fat (Table 2). In the isotemporal substitution model, replacing 30 minutes of sedentary time per day with 30 minutes of moderate to vigorous physical activity was associated with less total body fat (-1.3%, 95% CI -2.0, -0.7), whereas light physical activity (-0.03, 95% CI -0.5, 0.4) was not. Replacing 30 minutes of light physical activity with sedentary time was not associated with total body fat, whereas replacement with 30 minutes of moderate to vigorous physical activity was associated with less total body fat (-1.3%, 95% CI -2.3, -0.3). Results were similar in participants with at least 72 hours of objectively measured physical activity data (see Supplemental table 3, Supplemental Digital Content, Appendix). Results on total body fat without exclusion of participants with no MRI measurement can be found in Supplemental table 4 (see Supplemental Digital Content, Appendix).
Table 2. Associations of daily activities per 30 minutes/day with total body fat (%) in participants with an MRI of the abdomen and objectively measured physical activity.
| Per 30 min/day | Crude % TBF (95% CI) |
Multivariable1
% TBF (95% CI) |
Substitution model2
% TBF (95% CI) |
Substitution model3
% TBF (95% CI) |
|---|---|---|---|---|
| Sedentary time | 0.1 (-0.3, 0.5) | 0.5 (0.2, 0.7) | substituted | 0.03 (-0.4, 0.5) |
| Light physical activity | 0.2 (-0.3, 0.7) | -0.4 (-0.8 0.04) | -0.03 (-0.5, 0.4) | substituted |
| Moderate to vigorous physical activity | -1.4 (-2.3, -0.5) | -1.4 (-1.8, -0.9) | -1.3 (-2.0, -0.7) | -1.3 (-2.3, -0.3) |
Results reflect regression coefficients from linear regression analyses weighted toward the BMI distribution of the general population (n=228).
Adjusted for sex, age, ethnicity, education, Dutch Healthy Diet-index and smoking
Additionally adjusted for total time awake, and for all other daily activities but not for sedentary time. Coefficients represent the association between substitution of 30 minutes sedentary time with 30 minutes of either light or moderate to vigorous physical activity and total body fat (%).
Same as model 2, but coefficients represent the association between substitution of 30 minutes light physical activity with 30 minutes of either sedentary time or moderate to vigorous physical activity and total body fat (%).
Physical activity in relation to visceral fat area
After adjustment for potential confounding factors, PAEE was inversely associated with visceral fat (-7.5 cm2, 95% CI -11.4, -3.7). With regard to intensity, each 30 minutes per day of sedentary time was associated with 2.6 cm2 more visceral fat (95% CI 0.6, 4.6), whereas 30 minutes per day of moderate to vigorous physical activity was associated with 7.8 cm2 less visceral fat (95% CI -11.6, -4.0) (Table 3). Light physical activity was not associated with visceral fat (-1.7 cm2, 95% CI -4.4, 0.9). In the isotemporal substitution model, replacing 30 minutes per day of sedentary time with 30 minutes per day of moderate to vigorous physical activity was associated with 8.0 cm2 less (95% CI -11.9, -4.1) visceral fat, whereas replacement with light physical activity was not associated with visceral fat (0.0 cm2, 95% CI -2.8, 2.8). The association with moderate to vigorous physical activity attenuated after additional adjustment for total body fat. After additional adjustment total body fat, isotemporal substitution of light physical activity with sedentary time or moderate to vigorous physical activity was not associated with visceral fat. Results were similar in participants with at least 72 hours of objectively measured physical activity data (Supplemental table 1, Supplemental Digital Content, Appendix).
Table 3. Associations of daily activities per 30 minutes/day with visceral fat (cm2) in participants with an MRI of the abdomen and objectively measured physical activity.
| Per 30 min/day | Crude cm2 VAT (95% CI) |
Multivariable1
cm2 VAT (95% CI) |
Substitution model2
cm2 VAT (95% CI) |
Substitution model3
cm2 VAT (95% CI) |
Substitution model4
cm2 VAT (95% CI) |
|---|---|---|---|---|---|
| Sedentary time | 3.9 (2.2, 5.7) | 2.6 (0.6, 4.6) | substituted | substituted | -0.2 (-2.5, 2.1) |
| Light physical activity | -3.2 (-5.3, -1.1) | -1.7 (-4.4, 0.9) | 0.0 (-2.8, 2.8) | 0.2 (-2.1, 2.5) | substituted |
| Moderate to vigorous physical activity | -8.8 (-12.2, -5.3) | -7.8 (-11.6, -4.0) | -8.0 (-11.9, -4.1) | -1.4 (-5.1, 2.3) | -1.6 (-6.7, 3.6) |
Results reflect regression coefficients from linear regression analyses weighted toward the BMI distribution of the general population (n=228).
Adjusted for sex, age, ethnicity, education, DHD-index and smoking
Additionally adjusted for total time awake, and for all other daily activities but not for sedentary time. Coefficients represent the association between substitution of 30 minutes sedentary time with 30 minutes of either light or moderate to vigorous physical activity and visceral fat (cm2).
Additionally adjusted for total body fat
Same as model 3, but coefficients represent the association between substitution of 30 minutes light physical activity with 30 minutes of either sedentary time or moderate to vigorous physical activity and visceral fat (cm2).
Physical activity in relation to liver fat content
After adjustment for potential confounding factors, PAEE was inversely associated with liver fat (0.88 times, 95% CI 0.80, 0.95). As for intensity, each 30 minutes per day of sedentary time was associated with more liver fat (1.05 times, 95% CI 1.01, 1.10) whereas 30 minutes per day of moderate to vigorous physical activity was associated with less liver fat (0.88 times, 95% CI 0.81, 0.96)(Table 4). Light physical activity was not associated with liver fat (0.96, 95% CI 0.91, 1.02). In the isotemporal substitution model, replacing 30 minutes per day of sedentary time with 30 minutes per day of moderate to vigorous physical activity was associated with less liver fat (0.89 times, 95% CI 0.82, 0.97), whereas replacement with light physical activity was not associated with liver fat (0.98, 95% CI 0.92, 1.04). The association with moderate to vigorous physical activity attenuated after additional adjustment for total body fat. After this additional adjustment, isotemporal substitution of light physical activity with sedentary time or moderate to vigorous physical activity was not associated with liver fat. Results were similar in participants with at least 72 hours of objectively measured physical activity data (Supplemental table 3, Supplemental Digital Content, Appendix).
Table 4. Associations of daily activities per 30 minutes/day with hepatic triglyceride content (%) in participants with an MRI of the abdomen and objectively measured physical activity.
| Per 30 min/day | Crude Relative change in HTGC (95% CI) |
Multivariable1
Relative change in HTGC (95% CI) |
Substitution model2
Relative change in HTGC (95% CI) |
Substitution model3
Relative change in HTGC (95% CI) |
Substitution model4
Relative change in HTGC (95% CI) |
|---|---|---|---|---|---|
| Sedentary time | 1.08 (1.03; 1.12) | 1.05 (1.01; 1.10) | Substituted | Substituted | 1.02 (0.97, 1.07) |
| Light physical activity | 0.94 (0.89; 0.99) | 0.96 (0.91; 1.02) | 0.98 (0.92; 1.04) | 0.98 (0.94; 1.03) | Substituted |
| Moderate to vigorous physical activity | 0.87 (0.80; 0.94) | 0.88 (0.81; 0.96) | 0.89 (0.82; 0.97) | 1.00 (0.92; 1.09) | 1.02 (0.91, 1.14) |
Results reflect regression coefficients from linear regression analyses weighted toward the BMI distribution of the general population (n=228). Due to a skewed distribution of HTGC, we used the natural logarithm of this variable in the analyses. For interpretation of the results, we back transformed the regression coefficients of HTGC towards a ratio with 95% confidence interval, which is associated with 30 minutes per day spent in a certain activity. Such ratio, for example a ratio of 1.2, can be interpreted as each 30 minutes per day of a certain activity being associated with a 1.2-fold increased HTGC, which would reflect an increase in liver fat content from, for example, 5% to 6%.
Adjusted for sex, age, ethnicity, education, DHD-index and smoking
Additionally adjusted for total time awake, and for all other daily activities but not for sedentary time. Coefficients represent the association between substitution of 30 minutes sedentary time with 30 minutes of either light or moderate to vigorous physical activity and hepatic triglyceride content.
Additionally adjusted for total body fat
Same as model 3, but coefficients represent the association between substitution of 30 minutes light physical activity with 30 minutes of either sedentary time or moderate to vigorous physical activity and hepatic triglyceride content.
Discussion
In this population-based cohort study of middle-aged men and women, overall physical activity was associated with less total body fat, visceral fat and liver fat. Regarding the underlying intensity distribution, sedentary time was associated with more total body fat, visceral fat and liver fat, whereas moderate to vigorous physical activity was associated with less total body fat, visceral fat and liver fat. Light physical activity only seemed associated with less total body fat but not with visceral fat or liver fat. Within the range of physical activity in our study population, replacement of 30 minutes of sedentary time with moderate to vigorous physical activity, but not light physical activity, was associated with less total body fat, visceral fat and liver fat. These associations with visceral fat and liver fat attenuated after additional adjustment for total body fat. It therefore seems that the associations with visceral fat and liver fat are mainly mediated by total body fat.
To our knowledge, in only one previous study using isotemporal substitution analysis direct measures of adiposity in combination with objectively measured physical activity were investigated (13). This study observed that isotemporal substitution of one hour per day of sedentary and light intensity physical activity with other types of physical activity was associated with less visceral fat, and this association attenuated after additional adjustment for BMI (13), which is in line with our findings. However, in the previous study liver fat was not assessed and analyses were adjusted for BMI rather than for total body fat.
In another study with objectively measured levels of sedentary time and physical activity in 82 overweight or obese adults, there was no relationship between physical activity and liver fat and a weak positive association between time spent in moderate physical activity and visceral fat (28). These results could be explained by the fact that BMI was already included in the prediction model before measures of physical activity were added. These findings are consistent with our results, which show that sedentary time is positively associated with liver fat, and moderate to vigorous activity negatively. However, these associations also attenuated after the additional adjustment for total body fat, which suggests that they are mainly mediated by total body fat. A twin study of both monozygotic and dizygotic twins who were discordant for physical activity based on questionnaires during a follow-up of more than 30 years showed that habitual physical activity potentially prevents accumulation of visceral and ectopic fat (29). Whereas several previous studies observed associations between time spent in sedentary time and the risk of non-alcoholic fatty liver (30-33), in only one study sedentary time was measured objectively (33).
Besides abdominal adiposity, increased sedentary time has also been associated with other adverse metabolic consequences. Recent studies have shown that a high amount of sedentary time is associated with multiple adverse health outcomes, including 2 diabetes (34). This association was also present in physically fit individuals (35). Moreover, time spent sedentary has been shown to predict higher levels of fasting insulin after adjusting for time spent in moderate to vigorous physical activity (36). This indicates that the detrimental effects of sedentary time are not merely due to a lack of sufficient physical activity. Large meta-analyses have also shown that high sedentary time was associated with all-cause mortality (37), type 2 diabetes, cardiovascular disease, and cancer risk, even after adjustment for physical activity (10). However, the underlying biological pathways via which sedentary time may lead to disease remain largely unknown. It has been suggested that replacing sitting with standing and LPA improves insulin sensitivity and decreases plasma triglycerides, thereby leading to a decrease in triglyceride storage in the liver (38, 39). It has also been suggested that exercise results in preferential reductions in visceral fat compared with caloric restriction when the amount of exercise is sufficient to result in weight loss (40). This might be due to the fact that visceral fat is more metabolically active and more sensitive to lipolytic activation by the adrenal system (41), that occurs during vigorous exercise. We observed that habitual replacement of sedentary time with time spent in moderate to vigorous physical activity was associated with less total body fat, visceral fat and liver fat, whereas replacement of sedentary time with light physical activity was not. A recent systematic literature review of multiple observational studies on physical activity in relation to adiposity by means of isotemporal substitution analyses described that replacement of 30 min/day of sedentary time with moderate to vigorous physical activity resulted in a decreased waist circumference, body mass index and body fat percentage in healthy adult populations (12). Moreover, reallocating sedentary time to light or moderate to vigorous physical activity was associated with multiple favourable cardiometabolic biomarkers, such as insulin sensitivity (12). Although most of the included studies used body mass index or waist circumference as an outcome, the results are in line with our own findings, although we did not find an association between replacing sedentary time with time spent performing light physical activity and measures of adiposity.
A strength of this study is the extensive phenotyping, including direct assessment of visceral adipose tissue and hepatic triglyceride using MRI and 1H-MRS. Moreover, physical activity was objectively assessed using combined accelerometry and heart rate monitoring, which has been shown to classify physical activity more accurately than either of the two measures (42-44). Another strength is that we applied isotemporal substitution analysis to be able to investigate replacement of sedentary time with time spent in light and moderate to vigorous physical activity.
A few limitations should also be discussed. Due to the fact that both the objective measurement of physical activity and the body fat measurements by MRI were only performed in a random subset of the participants of the NEO study, the number of participants in the analyses on visceral fat and liver fat were relatively small. It must therefore also be noted that our results apply to people that were able to undergo an MRI of the abdomen and wear a monitor during free-living. Nevertheless, this sample size seems sufficient since we were able to detect associations with visceral fat and liver fat, as were previous studies that reported on the associations between objectively measured daily activities and visceral fat and liver fat and included even smaller sample sizes (N<100) (28, 33). Additionally, precision of combined sensing estimates of intensity is improved by individual calibration and not everybody in our sample performed the step test. Consequently, misclassification of activity intensity may have occurred for whom this was estimated based on the group calibration. Whereas we aimed to prevent such misclassification with our population specific calibration, if present, this may have resulted in an overestimation of our results. Furthermore, our definition of sedentary time was purely based on intensity as used in many other studies but ideally information on sitting posture per se would have enhanced this classification. We also have no information in which domain the physical activity was performed. In addition, participants were not instructed to keep a log to record sleep and waking times and therefore these were not available. Instead, we used general times during which we assumed most participants were asleep. This may have led to an over- or underestimation of sedentary time in certain participants. However, we do not expect that such misclassification would be related to the amount of body fat. Further, the amount of daily MVPA in our study population was relativity high. Whereas the Dutch population is known to be amongst the most active in Europe (45), a high level of MVPA has also been commonly reported in other studies where PA was assessed by Actiheart (46). Nevertheless, even if the absolute amount of MVPA was overestimated in our study we do not expect that this would have affected our results in relation to the different measures of adiposity Moreover, our population consisted mainly of white participants, so results need to be confirmed in other ethnic groups. Lastly, the observational cross-sectional study design precludes from any causal inference, and therefore larger prospective studies are needed to confirm these associations. In the present cross-sectional analyses, reverse causation, e.g. people engaging in less MVPA due to their high total body fat, cannot be ruled out. Nevertheless, the results on visceral fat and liver fat are less likely affected by reverse causation because people are not aware of the amount of visceral fat or liver fat in their body.
To conclude, in this population-based study of middle-aged men and women, sedentary time was associated with more total body fat, visceral fat, and liver fat. Replacement of 30 minutes of sedentary time per day with moderate to vigorous physical activity was associated with less total body fat, visceral fat and liver fat. In our analyses, replacement of sedentary time with light activities was not associated with total body fat, visceral fat or liver fat. The associations for visceral fat and liver fat attenuated after additional adjustment for total body fat, which suggests that the associations with visceral fat and liver fat mainly flow via total body fat.
This study provides knowledge on how reducing sedentary time by replacing it with moderate to vigorous physical activity is negatively associated with multiple total body fat and abdominal fat, which is important for the prevention of abdominal obesity, and ultimately cardiometabolic diseases.
Supplementary Material
Acknowledgements
The authors’ responsibilities were as follows: RdM and FR designed the study; RdM and HL conducted the study, EW-vE, SCB, JvdV, KW and SB performed data collection and Actiheart data processing EW-vE analysed data; EW-vE, drafted the manuscript; EW-vE and RdM had primary responsibility for final content. All authors read and approved the final manuscript.
We express our gratitude to all individuals who participate in the Netherlands Epidemiology in Obesity study. We are grateful to all participating general practitioners for inviting eligible participants. We furthermore thank Stefanie Hillebrand, Pat van Beelen and all research nurses for collecting the data, Petra Noordijk and her team for sample handling and storage, and Ingeborg de Jonge for data management of the NEO study. We further thank Kristel Schaap from the Biomedical Data Sciences of the Leiden University Medical Center and Lewis Griffiths and Antonia Smith from the MRC Epidemiology Unit, University of Cambridge for their help with cleaning and processing the objective physical activity data.
The NEO study is supported by the participating departments, the Division and the Board of Directors of the Leiden University Medical Center, and by the Leiden University, Research Profile Area ‘Vascular and Regenerative Medicine’. We acknowledge the support from the Netherlands Cardiovascular Research Initiative, an initiative with support from the Dutch Heart Foundation (CVON2014-02 ENERGISE). The work of KW and SB is supported by the UK Medical Research Council (MC_UU_12015/3) and NIHR Cambridge Biomedical Research Centre (IS-BRC-1215-20014).
Footnotes
Clinical Trial Registry Number: NCT03410316 at clinicaltrials.gov
Conflict of Interest.
The authors declare no conflict of interest. The results of the present study do not constitute endorsement by ACSM. The results of this study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
References
- 1.Stevens GA, Singh GM, Lu Y, Danaei G, Lin JK, Finucane MM, Bahalim AN, McIntire RK, Gutierrez HR, Cowan M. National, regional, and global trends in adult overweight and obesity prevalences. Popul Health Metr. 2012;10(1):22. doi: 10.1186/1478-7954-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–7. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 3.Lim S, Meigs JB. Links between ectopic fat and vascular disease in humans. Arteriosclerosis, thrombosis, and vascular biology. 2014:ATVBAHA. 114.303035. doi: 10.1161/ATVBAHA.114.303035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gast KB, den Heijer M, Smit JWA, Widya RL, Lamb HJ, de Roos A, Jukema JW, Rosendaal FR, de Mutsert R. Individual contributions of visceral fat and total body fat to subclinical atherosclerosis: The NEO study. Atherosclerosis. 2015;241(2):547–54. doi: 10.1016/j.atherosclerosis.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 5.Kaess BM, Pedley A, Massaro JM, Murabito J, Hoffmann U, Fox CS. The ratio of visceral to subcutaneous fat, a metric of body fat distribution, is a unique correlate of cardiometabolic risk. Diabetologia. 2012;55(10):2622–30. doi: 10.1007/s00125-012-2639-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nazare J-A, Smith JD, Borel A-L, Haffner SM, Balkau B, Ross R, Massien C, Alméras N, Després J-P. Ethnic influences on the relations between abdominal subcutaneous and visceral adiposity, liver fat, and cardiometabolic risk profile: the International Study of Prediction of Intra-Abdominal Adiposity and Its Relationship With Cardiometabolic Risk/Intra-Abdominal Adiposity–. American Journal of Clinical Nutrition. 2012;96(4):714–26. doi: 10.3945/ajcn.112.035758. [DOI] [PubMed] [Google Scholar]
- 7.Ismail I, Keating S, Baker M, Johnson N. A systematic review and meta‐analysis of the effect of aerobic vs. resistance exercise training on visceral fat. Obesity reviews. 2012;13(1):68–91. doi: 10.1111/j.1467-789X.2011.00931.x. [DOI] [PubMed] [Google Scholar]
- 8.Keating SE, Hackett DA, George J, Johnson NA. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. Journal of hepatology. 2012;57(1):157–66. doi: 10.1016/j.jhep.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 9.Kahlmeier S, Wijnhoven TM, Alpiger P, Schweizer C, Breda J, Martin BW. National physical activity recommendations: systematic overview and analysis of the situation in European countries. BMC Public Health. 2015;15:133. doi: 10.1186/s12889-015-1412-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biswas A, Oh PI, Faulkner GE, Bajaj RR, Silver MA, Mitchell MS, Alter DA. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta-analysis. Annals of Internal Medicine. 2015;162(2):123–32. doi: 10.7326/M14-1651. [DOI] [PubMed] [Google Scholar]
- 11.Mekary RA, Willett WC, Hu FB, Ding EL. Isotemporal substitution paradigm for physical activity epidemiology and weight change. Am J Epidemiol. 2009;170(4):519–27. doi: 10.1093/aje/kwp163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grgic J, Dumuid D, Bengoechea EG, Shrestha N, Bauman A, Olds T, Pedisic Z. Health outcomes associated with reallocations of time between sleep, sedentary behaviour, and physical activity: a systematic scoping review of isotemporal substitution studies. International Journal of Behavioral Nutrition and Physical Activity. 2018;15(1):69. doi: 10.1186/s12966-018-0691-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dahl-Petersen IK, Brage S, Bjerregaard P, Tolstrup JS, Jørgensen ME. Physical Activity and Abdominal Fat Distribution in Greenland. Medicine and science in sports and exercise. 2017;49(10):2064–70. doi: 10.1249/MSS.0000000000001337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Mutsert R, den Heijer M, Rabelink TJ, Smit JW, Romijn JA, Jukema JW, de Roos A, Cobbaert CM, Kloppenburg M, le Cessie S, et al. The Netherlands Epidemiology of Obesity (NEO) study: study design and data collection. Eur J Epidemiol. 2013;28(6):513–23. doi: 10.1007/s10654-013-9801-3. [DOI] [PubMed] [Google Scholar]
- 15.Brage S, Ekelund U, Brage N, Hennings MA, Froberg K, Franks PW, Wareham NJ. Hierarchy of individual calibration levels for heart rate and accelerometry to measure physical activity. Journal of applied physiology (Bethesda, Md : 1985) 2007;103(2):682–92. doi: 10.1152/japplphysiol.00092.2006. [DOI] [PubMed] [Google Scholar]
- 16.Stegle O, Fallert SV, MacKay DJ, Brage S. Gaussian process robust regression for noisy heart rate data. IEEE Transactions on Biomedical Engineering. 2008;55(9):2143–51. doi: 10.1109/TBME.2008.923118. [DOI] [PubMed] [Google Scholar]
- 17.Brage S, Brage N, Franks PW, Ekelund U, Wong M-Y, Andersen LB, Froberg K, Wareham NJ. Branched equation modeling of simultaneous accelerometry and heart rate monitoring improves estimate of directly measured physical activity energy expenditure. Journal of applied physiology. 2004;96(1):343–51. doi: 10.1152/japplphysiol.00703.2003. [DOI] [PubMed] [Google Scholar]
- 18.Brage S, Brage N, Franks P, Ekelund U, Wareham N. Reliability and validity of the combined heart rate and movement sensor Actiheart. Eur J Clin Nutr. 2005;59(4):561. doi: 10.1038/sj.ejcn.1602118. [DOI] [PubMed] [Google Scholar]
- 19.Brage S, Westgate K, Wijndaele K, Godinho J, Griffin S, Wareham N. Evaluation of a method for minimising diurnal information bias in objective sensor data; Int Conf Amb Mon Phys Act Mov; 2013. [Google Scholar]
- 20.Van Der Meer RW, Hammer S, Lamb HJ, Frolich M, Diamant M, Rijzewijk LJ, De Roos A, Romijn JA, Smit JW. Effects of short-term high-fat, high-energy diet on hepatic and myocardial triglyceride content in healthy men. The Journal of Clinical Endocrinology & Metabolism. 2008;93(7):2702–8. doi: 10.1210/jc.2007-2524. [DOI] [PubMed] [Google Scholar]
- 21.Naressi A, Couturier C, Devos J, Janssen M, Mangeat C, De Beer R, Graveron-Demilly D. Java-based graphical user interface for the MRUI quantitation package. Magnetic resonance materials in physics, biology and medicine. 2001;12(2–3):141. doi: 10.1007/BF02668096. [DOI] [PubMed] [Google Scholar]
- 22.Siebelink E, Geelen A, de Vries JH. Self-reported energy intake by FFQ compared with actual energy intake to maintain body weight in 516 adults. Br J Nutr. 2011;106(2):274–81. doi: 10.1017/s0007114511000067. [DOI] [PubMed] [Google Scholar]
- 23.Verkleij-Hagoort AC, de Vries JH, Stegers MP, Lindemans J, Ursem NT, Steegers-Theunissen RP. Validation of the assessment of folate and vitamin B12 intake in women of reproductive age: the method of triads. European journal of clinical nutrition. 2007;61(5):610–5. doi: 10.1038/sj.ejcn.1602581. [DOI] [PubMed] [Google Scholar]
- 24.Looman M, Feskens EJ, de Rijk M, Meijboom S, Biesbroek S, Temme EH, de Vries J, Geelen A. Development and evaluation of the Dutch Healthy Diet index 2015. Public Health Nutr. 2017;20(13):2289–99. doi: 10.1017/s136898001700091x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korn EL, Graubard BI. Epidemiologic studies utilizing surveys: accounting for the sampling design. American Journal of Public Health. 1991;81(9):1166–73. doi: 10.2105/ajph.81.9.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lumley T. Analysis of complex survey samples. Journal of Statistical Software. 2004;9(1):1–19. [Google Scholar]
- 27.Ministerie van VWS. [accessed February 20 2017]; Internet: https://www.volksgezondheidenzorg.info/onderwerp/overgewicht/cijfers-context/huidige-situatie.
- 28.Keating SE, Parker HM, Pavey TG, Baker MK, Caterson ID, George J, Johnson NA. Objectively quantified physical activity and sedentary behavior in predicting visceral adiposity and liver fat. Journal of obesity. 2016 doi: 10.1155/2016/2719014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leskinen T, Sipilä S, Alen M, Cheng S, Pietiläinen KH, Usenius JP, Suominen H, Kovanen V, Kainulainen H, Kaprio J, et al. Leisure-time physical activity and high-risk fat: a longitudinal population-based twin study. International Journal Of Obesity. 2009;33:1211. doi: 10.1038/ijo.2009.170. [DOI] [PubMed] [Google Scholar]
- 30.Ryu S, Chang Y, Jung HS, Yun KE, Kwon MJ, Choi Y, Kim CW, Cho J, Suh BS, Cho YK, et al. Relationship of sitting time and physical activity with non-alcoholic fatty liver disease. J Hepatol. 2015;63(5):1229–37. doi: 10.1016/j.jhep.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 31.Wei H, Qu H, Wang H, Deng H. Associations between sitting time and non-alcoholic fatty liver diseases in Chinese male workers: a cross-sectional study. BMJ open. 2016;6(9):e011939. doi: 10.1136/bmjopen-2016-011939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helajärvi H, Pahkala K, Heinonen OJ, Juonala M, Oikonen M, Tammelin T, Hutri-Kähönen N, Kähönen M, Lehtimäki T, Mikkilä V. Television viewing and fatty liver in early midlife. The Cardiovascular Risk in Young Finns Study. Annals of medicine. 2015;47(6):519–26. doi: 10.3109/07853890.2015.1077989. [DOI] [PubMed] [Google Scholar]
- 33.Hallsworth K, Thoma C, Moore S, Ploetz T, Anstee QM, Taylor R, Day CP, Trenell MI. Non-alcoholic fatty liver disease is associated with higher levels of objectively measured sedentary behaviour and lower levels of physical activity than matched healthy controls. Frontline gastroenterology. 2015;6(1):44–51. doi: 10.1136/flgastro-2014-100432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Berg JD, Stehouwer CD, Bosma H, van der Velde JH, Willems PJ, Savelberg HH, Schram MT, Sep SJ, van der Kallen CJ, Henry RM, et al. Associations of total amount and patterns of sedentary behaviour with type 2 diabetes and the metabolic syndrome: The Maastricht Study. Diabetologia. 2016;59(4):709–18. doi: 10.1007/s00125-015-3861-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Velde JH, Schaper NC, Stehouwer CD, van der Kallen CJ, Sep SJ, Schram MT, Henry RM, Dagnelie PC, Eussen SJ, van Dongen MC. Which is more important for cardiometabolic health: sedentary time, higher intensity physical activity or cardiorespiratory fitness? The Maastricht Study. Diabetologia. 2018;61(12):2561–9. doi: 10.1007/s00125-018-4719-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Helmerhorst HJF, Wijndaele K, Brage S, Wareham NJ, Ekelund U. Objectively Measured Sedentary Time May Predict Insulin Resistance Independent of Moderate- and Vigorous-Intensity Physical Activity. Diabetes. 2009;58(8):1776–9. doi: 10.2337/db08-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Rezende LFM, Rey-López JP, Matsudo VKR, do Carmo Luiz O. Sedentary behavior and health outcomes among older adults: a systematic review. BMC public health. 2014;14(1):333. doi: 10.1186/1471-2458-14-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duvivier BM, Schaper NC, Bremers MA, Van Crombrugge G, Menheere PP, Kars M, Savelberg HH. Minimal intensity physical activity (standing and walking) of longer duration improves insulin action and plasma lipids more than shorter periods of moderate to vigorous exercise (cycling) in sedentary subjects when energy expenditure is comparable. PloS one. 2013;8(2):e55542. doi: 10.1371/journal.pone.0055542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duvivier BM, Schaper NC, Hesselink MK, van Kan L, Stienen N, Winkens B, Koster A, Savelberg HH. Breaking sitting with light activities vs structured exercise: a randomised crossover study demonstrating benefits for glycaemic control and insulin sensitivity in type 2 diabetes. Diabetologia. 2017;60(3):490–8. doi: 10.1007/s00125-016-4161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy JC, McDaniel JL, Mora K, Villareal DT, Fontana L, Weiss EP. Preferential reductions in intermuscular and visceral adipose tissue with exercise-induced weight loss compared with calorie restriction. Journal of Applied Physiology. 2011;112(1):79–85. doi: 10.1152/japplphysiol.00355.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arner P. Differences in lipolysis between human subcutaneous and omental adipose tissues. Annals of medicine. 1995;27(4):435–8. doi: 10.3109/07853899709002451. [DOI] [PubMed] [Google Scholar]
- 42.Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Research quarterly for exercise and sport. 2000;71(sup2):1–14. doi: 10.1080/02701367.2000.11082780. [DOI] [PubMed] [Google Scholar]
- 43.Freedson PS, Miller K. Objective monitoring of physical activity using motion sensors and heart rate. Research quarterly for exercise and sport. 2000;71(sup2):21–9. doi: 10.1080/02701367.2000.11082782. [DOI] [PubMed] [Google Scholar]
- 44.Treuth MS. Physical activity assessments for health-related research. Champaign: Human Kinetics; 2002. Applying multiple methods to improve the accuracy of activity assessments; pp. 213–25. [Google Scholar]
- 45.Lera-López F, Marco R. Sports participation, physical activity, and health in the European regions. Journal of Sports Sciences. 2018;36(15):1784–91. doi: 10.1080/02640414.2017.1418810. [DOI] [PubMed] [Google Scholar]
- 46.Lindsay T, Westgate K, Wijndaele K, Hollidge S, Kerrison N, Forouhi N, Griffin S, Wareham N, Brage S. Descriptive epidemiology of physical activity energy expenditure in UK adults (The Fenland study) International Journal of Behavioral Nutrition and Physical Activity. 2019;16(1):1–13. doi: 10.1186/s12966-019-0882-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.