Abstract
Context
Mutations in the KCNJ11 and ABCC8 genes encoding the pancreatic β-cell KATP channel have recently been shown to be the most common cause of permanent neonatal diabetes mellitus (PNDM). Information regarding the frequency of PNDM has been based mainly on nonpopulation or short-term collections only. Thus, the aim of this study was to identify the incidence of PNDM in Slovakia and to switch patients to sulfonylurea (SU) where applicable.
Design
We searched for PNDM patients in the Slovak Children Diabetes Registry. In insulin-treated patients who matched the clinical criteria for PNDM, the KCNJ11 or ABCC8 genes were sequenced, and mutation carriers were invited for replacement of insulin with SU.
Results
Eight patients with diabetes onset before the sixth month of life without remission were identified since 1981, which corresponds to the PNDM incidence in Slovakia of one case in 215,417 live births. In four patients, three different KCNJ11 mutations were found (R201H, H46Y, and L164P). Three patients with the KCNJ11 mutations (R201H and H46Y) were switched from insulin to SU, decreasing their glycosylated hemoglobin from 9.3–11.0% on insulin to 5.7–6.6% on SU treatment. One patient has a novel V86A mutation in the ABCC8 gene and was also substituted with SU.
Conclusions
PNDM frequency in Slovakia is much higher (one in 215,417 live births) than previously suggested from international estimates (about one in 800,000). We identified one ABCC8 and four KCNJ11 mutation carriers, of whom four were successfully transferred to SU, dramatically improving their diabetes control and quality of life. (J Clin Endocrinol Metab 92: 1276–1282, 2007)
PERMANENT NEONATAL DIABETES mellitus (PNDM) is a heterogeneous group of disorders with diabetes manifestation before 6 months of age with no remission (1, 2). The incidence of PNDM has not been precisely defined. It includes 40–50% of cases with neonatal diabetes mellitus, the frequency of which has been estimated at 1:400,000 to 1:500,000 live births (3–5).
The etiology of PNDM is different from that of type 1 diabetes, which is of autoimmune origin. PNDM, with the exception of IPEX syndrome, does not have an autoimmune background and results from genetic abnormalities of the pancreas and its β-cells in the islets of Langerhans (6). The minority of PNDM cases arise from mutations of both alleles of the glucokinase gene (7), insulin promoter factor 1 (8), EIF2AK3 (syndrome Wolcott–Rallison) (9), forkhead box-P3 (IPEX syndrome, i.e. immunodysregulation, polyendocrinopathy, enteropathy, X-linked) (10), mutations of the pancreatic transcription factor 1 α (11), or GLIS3 (neonatal diabetes with congenital hypothyroidism) (12).
It has been shown recently that the majority of PNDM cases are caused by activating mutations of genes coding subunits of the ATP-dependent potassium (KATP) channel (13). The pancreatic KATP channel is an important regulator of the β-cell membrane polarity, with influence on insulin secretion (14). The channel is composed of four Kir6.2 and four sulfonylurea (SU) receptor 1 (SUR1) subunits. Activating mutations of the KCNJ11 gene coding Kir6.2 and ABCC8 coding the SUR1 subunit can lead to neonatal diabetes (13, 15, 16).
KCNJ11 mutations are responsible for 26–64% of permanent diabetes diagnosed before the sixth month of age as reported in several large, but nonpopulation-based studies (1, 2, 13, 17–19). The majority of patients with a KCNJ11 mutation develop diabetes in the first 3 months (19), but no KCNJ11 mutations were detected in patients with diabetes onset after the sixth month of age (2, 19).
The clinical manifestation and prognosis of KCNJ11 mutation carriers depends on the location of the mutation. Mutations of the Kir6.2 region responsible for ATP binding (e.g. R201H) cause decreased sensitivity for ATP and manifest usually with diabetes only (13, 19). Mutations activating the KATP channel by altering channel gating are responsible for diabetes and additional neurological features including seizures, developmental delay, and facial dysmorphism. The most severe form is called DEND syndrome (developmental delay, epilepsy, and neonatal diabetes) (20). The V59M mutation is associated with an intermediate phenotype with diabetes and developmental delay (described as intermediate DEND syndrome) (19). Milder activating KCNJ11 mutations can also cause transient NDM with diabetes relapsing after some years (21).
Physiological studies showed that SU, after binding to the SUR1 subunit, closes the KATP channel and initiates the insulin secretion (14). In an international series of 49 patients with KCNJ11 mutations, 90% were switched from insulin to SU with an improvement in glycemia in all cases where glycosylated hemoglobin (HbA1c) was measured (22). Patients with mutations causing DEND syndrome or affecting KATP channel gating usually fail to respond (1, 23). Thus, the likelihood of success may be determined by the type of KCNJ11 mutation (14).
However, it has also recently been shown that mutations of the ABCC8 gene coding the SUR1 subunit are responsible for some cases of PNDM, with a clinical picture similar to that of the KCNJ11 mutations (15, 16). Some of these patients also respond to SU therapy (16). Interestingly, ABCC8 mutations also cause transient NDM and more frequently than KCNJ11 mutations (24).
The aim of this study was to identify the genetic etiology of PNDM cases in Slovakia, to establish their population-based incidence, and to initiate the SU therapy where applicable.
Patients and Methods
Patients with diabetes onset before 6 months of age were identified based on information from the Slovak National Children Diabetes Registry. This registry was established in 1981 at the First Department of Pediatric Medicine, Comenius University, with cooperation of the National Health Information Center. It contains information on all children with diabetes since 1981, i.e. data on 2812 patients encompassing date of birth, diabetes onset, symptoms at diabetes onset, and treatment at discharge (25).
PNDM patients were contacted via their diabetologists, invited for a health check-up, and given information on genetic testing, and blood was withdrawn for DNA isolation. Samples of 8 ml venous blood were collected into PAXgene Blood DNA tubes (QIAGEN, Sussex, UK) for DNA analysis, and 5 ml were collected into EDTA tubes (Nümbrecht, Sarstedt, Germany) for autoantibodies measurement. DNA was isolated from peripheral lymphocytes using the Flexigene DNA Kit (QIAGEN) and divided into two aliquots containing 100 μg of DNA each. EDTA tubes were centrifuged 10 min at 2000 × g and 4 C. Plasma was stored at –30 C until assayed.
Autoantibodies against insulin, thyrosin phosphatase, and glutamate decarboxylase were determined using a RIA (DRG Diagnostics, London, UK) to proof the nonautoimmune nature of the diabetes.
Genetic analysis of the KCNJ11 gene
The single exon of KCNJ11 was amplified in three overlapping fragments by PCR using previously described primers (19). Sequencing was performed in both directions using M13 universal primers and a BigDye Terminator Cycler Sequencing Kit v1.1 (Applied Biosystems, Warrington, UK). Reactions were analyzed using an ABI 3100 capillary sequencer (Applied Biosystems). Sequences were compared with the published sequence (NM_000525.3) using Mutation Surveyor software (Softgenetics, State College, PA).
Genetic analysis of the ABCC8 gene
Analysis of the ABCC8 gene was undertaken in all patients who did not carry a KCNJ11 mutation. The full 39 exons were amplified in 38 fragments using previously described primers (15). Sequencing was performed in a single direction using M13 universal primers and a BigDye Terminator Cycler Sequencing Kit v3.1 (Applied Biosystems). Sequences were analyzed using an ABI 3730 capillary sequencer (Applied Biosystems) and compared with the published sequence (NM_000352.2) using Mutation Surveyor software (Softgenetics) (15).
Family studies
All mutations were tested for cosegregation with diabetes in other family members, and family relationships were confirmed using a panel of six microsatellite markers on chromosome 11 (19).
Clinical studies
Patients with KCNJ11 or ABCC8 mutations were invited for hospitalization to be switched from insulin to SUs. Before therapy change, patients underwent a clinical examination and developmental assessment carried out by a specialized pediatric endocrinologist. A pediatric neurologist evaluated the neurological status and psychomotor development. The pretransfer value of HbA1c was also measured.
Replacement of insulin with SU therapy
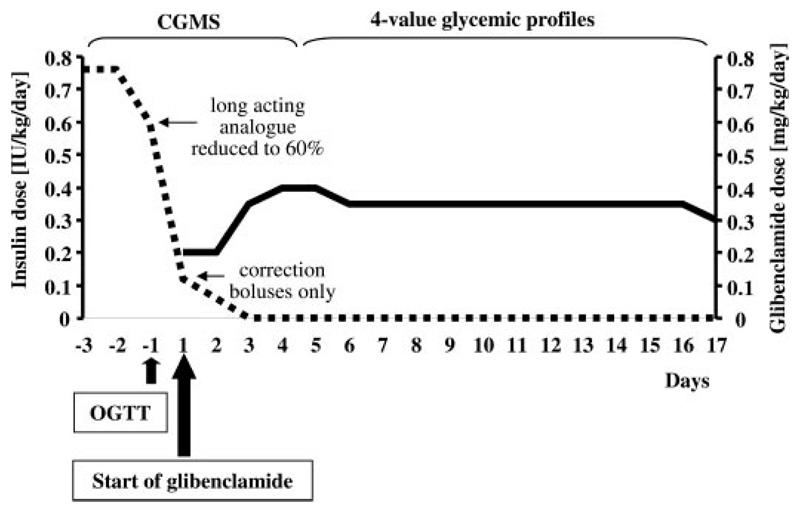
The oral SU glibenclamide (Maninil, Berlin Chemie, Germany) in standard 3.5- and 5-mg tablets was introduced according to a previously published protocol (19, 22) (Fig. 1). The initial dose of glibenclamide was 0.1 mg/kg (administered twice a day), which was increased by 0.1–0.2 mg/kg per day in a stepwise manner (17). The SU was increased simultaneously with the decrease of insulin. After glycemia stabilization, the insulin was stopped.
Fig. 1.
Short protocol for switching from insulin to SU. Major steps of the transfer protocol are displayed. Insulin dose (units per kilogram per day) is shown as a dotted line, and glibenclamide dose (milligrams per kilogram per day) is shown as a solid line. Insulin dose was reduced significantly already during the first day of therapy change without glycemia worsening (see also Fig. 2). OGTT, Oral glucose tolerance test.
Before and after the replacement tests
Before the therapy change and 1 month after the switch to SU, patients underwent continuous glucose monitoring (CGMS, Medtronic, Minneapolis, MN) for at least 48 h. The monitoring data were evaluated using the MiniMed Solutions software. In two patients (SK-4 and SK-6), oral glucose tolerance tests were carried out before and 1 month after SU introduction. After overnight fasting, 1.75 g of glucose per kg was given in 2 dl of water over 2 min. Blood samples were collected at 15, 30, 45, 60, 90, and 120 min for glucose, insulin, and C-peptide assays.
After replacement follow-up
The patients were invited to the diabetes outpatient clinic every 14 d. Weight and height were measured; results of the home self-monitoring were checked. HbA1c was evaluated in the first and sixth months after the therapy change. The glibenclamide dose was modified based upon the actual glycemia readings.
Hormonal and other analyses
Venous blood samples for glucose levels were taken into fluoride tubes (Nümbrecht) and C-peptide into the EDTA tubes (Nümbrecht). After centrifugation, the supernatant was taken and stored at –30 C. Plasma glucose concentrations were measured with the glucose oxidase method (Hitachi 911; Hitachi, Hitachinaka, Japan). C-peptide was determined using the Elecsys (Roche, Basel, Switzerland) automatic analyzer. HbA1c was evaluated by HPLC analyzer (Bio-Rad, Hercules, CA) in the SK-4 and SK-6 patients, by LPLC DiaSTAT analyzer (Bio-Rad) in the SK-1, and by Olympus AU640 (Olympus, Center Valley, PA) in the SK-2 and SK-5. All the values were transferred to the Diabetes Control and Complications Trial values using the National Glycohemoglobin Standardization Program equation (26, 27).
PNDM frequency data
The data on live births rates during the years 1981 to 2004 in Slovakia were taken from the annual reports of the National Health Information Center (28). These numbers were used to compare the numbers of children with diabetes before 3 and 6 months and the number of the KCNJ11 or ABCC8 mutation carriers.
Ethics committee approval
All steps of this study (DNA analysis, oral glucose tolerance tests, and switching from insulin to SU) were approved by the Faculty Hospital Ethics Committees in Bratislava and Kosice. They comply with the ethical guidelines of the Helsinki Declaration as revised in 2000. All patients and/or their parents had signed the informed consent.
Results
Based on information from the Slovak National Children Diabetes Registry, from 1981 to 2004 eight patients with diabetes manifestation under 6 months [the recently accepted cutoff point for neonatal diabetes (1, 2, 22)] of age without remission were identified (Table 1). Six of them were diagnosed at less than 3 months of age and also met the previous criteria for PNDM (6). Newly diagnosed patients in the years 2005 and 2006 were excluded to get patients with diabetes duration of at least 18 months, assuring the permanent character of the neonatal diabetes.
Table 1. Clinical data of Slovak PNDM patients with diabetes manifestation before the sixth month of life.
| Patient no. | SK-1 | SK-2 | SK-3 | SK-4 | SK-5 | SK-6 | SK-7 | SK-8 |
|---|---|---|---|---|---|---|---|---|
| Diagnosis | KCNJ11 mutation R201H | KCNJ11 mutation L164P, VHC | PNDM, unknown etiology a | KCNJ11 mutation H46Y | Wolcott-Rallison syndrome | KCNJ11 mutation R201H | Intermediate DEND, not analyzed | ABCC8 mutation V86A |
| Gender | Female | Female | Male | Female | Male | Male | Female | Male |
| Birth weight (g) | 3000 | 2600 | 2450 | 3500 | 3750 | 2480 | 1450 | 2900 |
| Gestation (wk) | 39 | 40 | 40 | 40 | 42 | 40 | 37 | 40 |
| DM onset (wk) | 18 | 5 | 10 | 15 | 11 | 4 | <1 | 9 |
| Dysmorphic features | No | No | No | Mild | No | No | Yes | No |
| Seizures | No | No | No | No | No | No | No | No |
| Developmental delay | No | No | No | No | No | No | Yes | No |
| Autoantibodies | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| Therapy after onset | Insulin | Insulin | Insulin | Insulin | Insulin | Insulin | Insulin | Insulin |
| Insulin (IU/kg·d) | 0.6 | 1.24 | 0.8 | 1 | 0.8 | 0.66 | 0.5 | 0.45 |
| C-peptide (ng/ml) | 0.01 | 0.01 | 0.42 | 0.04 | 0.18 | 0.11 | 0.12 | 0.01 |
| Current status | ||||||||
| Current age (yr) | 25 | 19 | 13 | 12 | 11 | 11 | Deceased at 18 months | 5 |
| Current therapy | SU | Insulin | Insulin | SU | Insulin | SU | Insulin | SU |
| Current HbA1c (%) | 7.0 | 15.2 | 12.2 | 6.6 | 15.1 | 5.7 | Not available | 6.9 |
DM, Diabetes mellitus; VHC, virus hepatitis C.
Negative for mutations in KCNJ11, SUR1, IPF1, and NEUROD1 genes.
One of the eight patients, subject SK-7, developed diabetes at the age of 2 d with additional symptoms (i.e. facial and cranial dysmorphy, ear deformities, developmental delay, central tonus disorder with acral hypertonus, agenesis of corpus callosum) and died 18 months after delivery due to severe hypoglycemia (Table 1).
Patient SK-5 is mentally retarded and has deformities of the bones due to epiphyseal dysplasia. In 2002, he was diagnosed with the Wolcott-Rallison syndrome with mutations in the EIF2AK3 gene (compound heterozygous for W898C and L1057P mutations) (29). In five of the remaining six patients, mutations in the KCNJ11 gene (n = 4) or ABCC8 gene (n = 1) were found. The SK-3 patient who also has a diabetic father (with diabetes onset in the seventh year of life, treated with insulin) was negative for mutations in KCNJ11, ABCC8, IPF1, and NEUROD1 genes.
The incidence of permanent diabetes as diagnosed before the sixth month of life in Slovakia between the years 1981 to 2004 was one in 215,417 live births. Using the more strict criteria for PNDM definition (6) (i.e. diabetes manifestation until 3 months of age), the incidence was one in 287,223.
The most common cause was mutations in the KCNJ11 gene coding Kir6.2 subunit, with 57% of all living diabetes cases. The incidence of KCNJ11 mutation in children with diabetes onset up until 6 months of age was one in 430,834 live births. The KCNJ11 mutation carriers developed diabetes at a median of 2.5 months, with only 50% diagnosed before 3 months of age. This number is lower than reported in other studies (1, 19).
Characteristics of KCNJ11 and ABCC8 gene mutations
We found three different heterozygous de novo KCNJ11 mutations in four patients; two had R201H, one H46Y, and one L164P [previously reported by Flanagan et al. (19)]. V86A (c.257T>C) is a novel mutation in exon 2 of the ABCC8 gene that results in the substitution of alanine for valine at codon 86 (p.Val86Ala). It arose de novo in patient SK-8 and is located in the second transmembrane helix of the transmembrane domain TMD0. This residue is conserved from human to mouse and rat (30) and has not been found in 100 normal chromosomes.
Clinical characteristics of the KCNJ11 mutation carriers
Selected clinical features of the KCNJ11 mutation carriers (SK-1, SK-2, SK-4, and SK-6) are summarized in Table 1. The mutation carriers were born in the 40th gestational week (39–40 confidence limits) with a median birth weight of 2800 g (2480–3500). Diabetes started in 2.5 months (range, 1–4 months) with symptoms of polyuria, polydipsia, and failure to thrive. All of them were treated with multiple injections of insulin.
Clinical characteristics of the ABCC8 mutation carrier
Patient SK-8 with the V86A mutation was born in the 40th gestational week with birth weight of 2800 g and developed diabetes in his second month of life as manifested with polyuria, polydipsia, and failure to thrive during a respiratory tract infection. Hyperglycemia reached 28 mmol/liter, yet without changes in acidobasic balance. Axial hypotonus required rehabilitation lasting for 36 months. Other laboratory test results are shown in Table 1.
Switching of the KCNJ11 mutation carriers to SU
Three of our patients (SK-1, SK-4, and SK-6) changed treatment from insulin to glibenclamide (nonselective SU) using an inpatient-based short transfer protocol (17), www.diabetesgenes.org. Before therapy change, the subjects had an insulin requirement of 0.66, 1.00, and 0.6 U/kg·d, respectively. The maximal glibenclamide dose reached 0.5 in SK-1 and 0.8 mg/kg·d in SK-4 and SK-6 at the end of the first week. After this, the SU dose was reduced to 0.3 mg/kg·d in response to capillary blood glucose values. Insulin was reduced during the first day of SU substitution to less than 20% of its pretransfer dose and then used only as a bolus for hyperglycemia correction (Fig. 1). Patients came off insulin completely within 3–7 d.
Glycemia decreased after the first glibenclamide dose (Fig. 2) but was not stable until the end of the first week. With lowering of the SU dose, the glycemic fluctuations reduced, and after another week the patients reached normoglycemia. Evening hypoglycemia resulted in patients SK-1 and SK-6, omitting the lunch dose of glibenclamide after the second month of treatment.
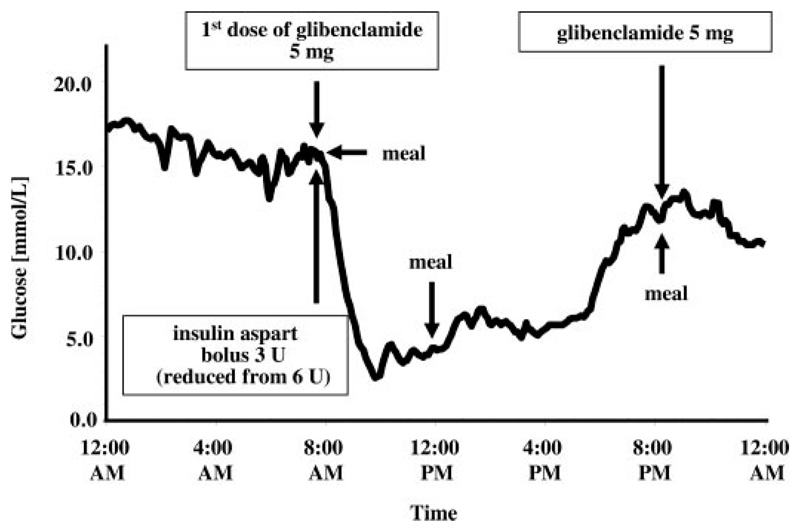
Fig. 2.
First day of switching from insulin to glibenclamide in the SK-1 patient (CGMS data). Glycemia decreased remarkably already after the first glibenclamide dose despite a 60% and 50% reduction of the basal insulin analog and the short acting analog, respectively.
The CGMS monitoring was used to evaluate the glycemic trends before and 1 month after the therapy change. During insulin treatment, glycemia exceeded 10 mmol/liter threshold in 51, 67, and 77% (SK-1, SK-4, and SK-6) of the monitoring time; decreased to less than 3.3 mmol/liter in 2, 0, and 2% and between 3.3 and 10 mmol/liter only in 47, 33, and 21% of the monitoring time. After 1 month on glibenclamide, the times with glycemia above 10 mmol/liter decreased to 10, 17, and 17%, less than 3.3 mmol/liter reached glycemia 8, 2 and 0%. The times in which glycemia was ranging between 3.3 and 10 mmol/liter increased to 83, 81, and 83%.
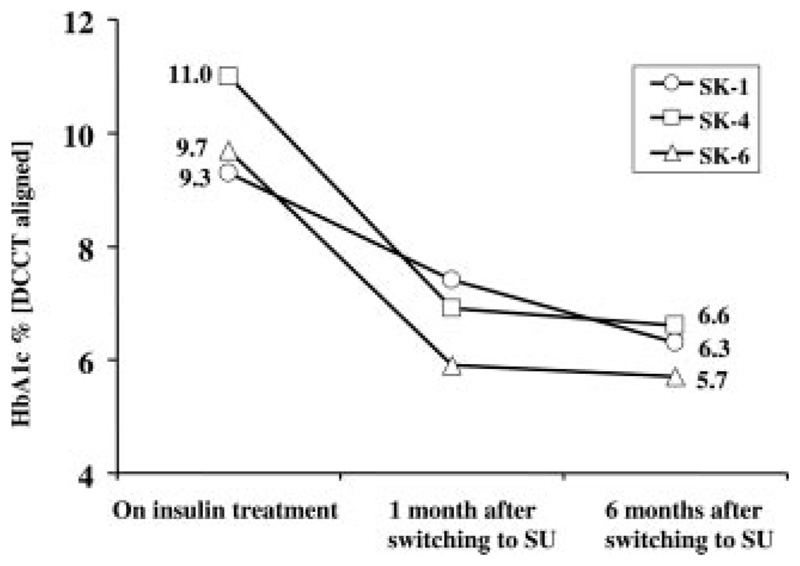
Long-term glycemia was evaluated by measurements of HbA1c, which ranged in the presubstitution stage between 9.3 and 11.0%. After 1 month off insulin, HbA1c decreased to 7.3% in SK-1, 5.9% in SK-4, and 6.9% in SK-6, respectively. This trend also continued in the further follow-up (Fig. 3).
Fig. 3.
HbA1c before and after therapy change. After the first month on SU treatment, HbA1c level decreased significantly, and this improvement remained.
Insulin secretion was evaluated by measuring plasma C-peptide levels (Table 1). On insulin treatment, C-peptide in the presence of glucose values greater than 8 mmol/liter was less than 0.11 ng/ml. After the first glibenclamide dose, postprandial plasma C-peptide increased in SK-4 to 3.0 (H46Y mutation carrier) and in SK-6 to 2.4 ng/ml (mutation R201H). After 1 month off insulin, basal C-peptide concentrations increased to 0.61, 0.79, and 0.79 ng/ml, respectively. The insulin secretory response to oral glucose during an oral glucose tolerance test was higher on glibenclamide than on the insulin treatment: the AUC for C-peptide during oral glucose tolerance test on insulin vs. glibenclamide reached 12.3 vs. 260.8 ng/ml·min in SK-4 and 21.6 vs. 151.4 ng/ml·min in SK-6, respectively.
Side effects
The only side effects seen were transitory and of gastro-intestinal origin in the SK-1 patient. Mild loss of appetite caused transitory weight loss of 4 kg during the first 3 months of treatment, and thereafter weight became normalized.
Switching of the ABCC8 mutation carrier to SU
The patient with ABCC8 mutation was recently switched from insulin to glibenclamide, following the same short protocol as used in KCNJ11 mutations. Before SU therapy, he required 0.45 U/kg·d of insulin with C-peptide plasma level of 0.01 ng/ml. After the first glibenclamide dose, postprandial plasma C-peptide increased to 3.6 ng/ml, and insulin was discharged. The SU dose at discharge was 0.09 mg/kg·d, and fasting plasma C-peptide was 0.75 ng/ml. The gliben-clamide treatment achieved normoglycemia.
Discussion
These are the first neonatal diabetes incidence data based on a nationwide collection using a national diabetes registry. Previous studies estimated the PNDM incidence be one in 800,000 live births (5, 31, 32). These studies have not been population based; thus, the number of cases may have been underestimated (1). Our study is population based, using a diabetes registry with data from more than 23 yr. We found that the incidence of PNDM was one case in 215,417 live births, which is much higher than 1 in 800,000 live births (Table 1).
The Slovak National Children Diabetes Registry includes all children diagnosed with all forms of diabetes treated continuously with insulin. The data were collected from 1981 onward. Medical logistics of data collection in that period was already sufficient to identify the majority of children with neonatal diabetes. Moreover, our data seem not to be influenced by population migration because all of the KCNJ11 or ABCC8 mutations are de novo mutations and are equally distributed throughout Slovakia. This is the first study using a diabetes registry to search for PNDM cases. The method has proven to be effective, but this has not been reported before even in the few countries that have nation-wide diabetes registries for childhood diabetes (i.e. Refs. 25 and 33–38).
There are earlier studies on PNDM incidence in the literature (5). In 1997, Shield et al. (4) reported the total neonatal diabetes incidence of 1:400,000 based on a nationwide collection covering 1 yr only. This corresponds to estimates of von Muhlendahl and Herkenhoff (5) from Germany (1:450,000 live births). Nevertheless, in both of these studies PNDM contributed to less than 50% of the total neonatal diabetes. Thus, incidence of PNDM was lower than 1:800,000. The shortcoming of the first study (4) was a short period (i.e. 1 yr) of data collection. The second study (5) did not cover the whole German population. Criteria for neonatal diabetes were hyperglycemia occurring within the first 6 (4) or 4 wk (5), respectively, that requires insulin treatment and lasts for at least 2 wk. Recent studies from both human leukocyte antigen studies and from Kir6.2 both support 6 months as a more appropriate cutoff when trying to classify by etiology, i.e. genetic vs. autoimmune (19, 39).
We have been able to define a monogenic etiology in all except for one (SK-3) of the living patients, which corresponds to 87% of monogenic disorders in patients alive. This number is much higher than estimated in other recent studies (18, 19). The incidence of KCNJ11 and/or ABCC8 mutations is high (Table 2) and greater than former estimations for the whole PNDM (31). Finally, the etiology also remains uncertain in patient SK-7 (deceased at the age of 18 months), who developed diabetes and additional neurological features. His clinical picture corresponds thus to intermediate DEND syndrome, and the likelihood of a mutation in either the KCNJ11 or ABCC8 genes is high. No patients have autoimmune type 1 diabetes in the group of children with diabetes onset under 6 months, in keeping with recent studies (19).
Table 2. Incidence of PNDM and its subgroups to live births.
| Subgroup of PNDM | Slovakia frequency data | Other sources | ||
|---|---|---|---|---|
| Frequency data | Nationality of cohort | Ref. | ||
| PNDM < 3 months | 1:287,223 | 1:800,000 | International | 4, 5 |
| PNDM < 6 months | 1:215,417 | Not available | ||
| MDM < 6 months | 1:287,223 | Not available | ||
| KCNJ11 and ABCC8 mutations < 6 months | 1:344,668 | Not available | ||
| KCNJ11 mutations < 6 months | 1:430,835 | Not available | ||
| KCNJ11 mutations < 3 months | 40% (2 in 5) | 54% (7 in 13) | French | 18 |
| KCNJ11 mutations < 6 months | 57% (4 in 7) | 34% (10 in 29) | International | 13 |
| 57% (8 in 14) | Italian | 2 | ||
| 64% (7 in 11) | International | 17 | ||
| KCNJ11 mutations < 3–6 months | 100% (2 in 2) | 50% (2 in 4) | French | 18 |
MDM, Monogenic diabetes mellitus.
The majority of our KCNJ11 and ABCC8 mutation carriers have isolated diabetes, with only SK-4 having mild dysmorphic features. In two of our patients (SK-1 and SK-6), the R201H mutation and in one case (SK-4) the H46Y in the KCNJ11 gene were identified. Both mutations are situated in the binding site of the Kir6.2 for the ATP molecule, decreasing the sensitivity to ATP (13) and responding well to the SU treatment both in vitro (19) and in vivo. The L164P mutation has been identified in two additional patients to date (Flanagan, S. E., S. Ellard, and A. T. Hattersley, unpublished data). L164P is a gating mutation (23), and the carriers usually fail to respond to SU (14, 20). Unfortunately, this teenage female has chronic active hepatitis C with high readings of liver enzymes (e.g. aspartate amino transferase, alanine amino transferase, and also D-glutamyl transferase). Due to a potential worsening of the liver function by introduction of SU treatment, we have not yet initiated her replacement of insulin with SU.
There are some remarkable benefits of SU treatment. This includes improved glycemic control also seen in other KCNJ11 mutation carriers after switching to SU (17, 22). A further advantage of the SU treatment is the flexibility to respond with appropriate insulin secretion, which is very important for a normal lifestyle. In addition, the side effects of SU treatment (i.e. diarrhea and abdominal pain) are only mild and transitory. Similar SU treatment benefits may also be achieved for patients with ABCC8 mutations (16).
In conclusion, the high incidence of PNDM in Slovakia (1 in 215,417 live births) indicates that PNDM is probably more frequent than indicated from previous international estimates (1 in 800,000). In the majority of cases of PNDM, the monogenic etiology can be defined. The commonest etiologies are mutations of the KATP channel, and we identified one ABCC8 and four KCNJ11 mutation carriers. All of them were classified and treated as type 1 diabetes until the molecular genetic diagnosis was made. Four patients were subsequently switched to SU treatment resulting in a dramatic improvement in their diabetes control and quality of life. Identifying PNDM and defining the underlying genetic etiology is a priority.
Acknowledgments
We thank Mrs. Alica Mitkova and Brigita Kramplova as well as Dr. Ann-Marie Patch and Mr. Andrew Parrish for technical assistance.
This work was supported by research grants MZ.2005/15-NEDU-01, SP 51/0280800/ 028 0802-2003, APVV-51-014205 Slovakia (to the Institute of Experimental Endocrinology, Slovak Academy of Sciences, Bratislava, Slovakia) and by funds from the Wellcome Trust and Diabetes UK (to the Institute of Biomedical and Clinical Science, Peninsula Medical School, Exeter, UK). A.T.H. is a Wellcome Trust Research Leave Fellow.
Abbreviations
- HbA1c
Glycosylated hemoglobin
- KATP
ATP-dependent potassium
- PNDM
permanent neonatal diabetes mellitus
- SU
sulfonylurea
Footnotes
Disclosure Summary: The authors have nothing to disclose.
Contributor Information
Juraj Stanik, DIABGENE and the Diabetes Research Laboratory, Institute of Experimental Endocrinology, 833 06 Bratislava, Slovakia; Children Diabetes Center at the First Department of Pediatrics, comenius University Medical School, 833 40 Bratislava, Slovakia.
Daniela Gasperikova, DIABGENE and the Diabetes Research Laboratory, Institute of Experimental Endocrinology, 833 06 Bratislava, Slovakia.
Magdalena Paskova, Second Department of Pediatrics, Safarik University Medical School, 040 11 Kosice, Slovakia.
Lubomir Barak, Children Diabetes Center at the First Department of Pediatrics, Comenius University Medical School, 833 40 Bratislava, Slovakia.
Jana Javorkova, Department of Pediatrics, Jessenius Faculty Medicine, Comenius University, 036 59 Martin, Slovakia.
Emilia Jancova, Children Diabetes Center at the First Department of Pediatrics, Comenius University Medical School, 833 40 Bratislava, Slovakia.
Miriam Ciljakova, Department of Pediatrics, Jessenius Faculty Medicine, Comenius University, 036 59 Martin, Slovakia.
Peter Hlava, National Health Registries Division, National Health Information Center, 811 09 Bratislava, Slovakia.
Jozef Michalek, National Institute for Diabetes and Endocrinology, 034 91 Lubochna, Slovakia.
Sarah E. Flanagan, Institute of Biomedical and Clinical Science, Peninsula Medical School, Exeter EX2 5DW, United Kingdom
Ewan Pearson, Ninewells Hospital, Medical School, Dundee DD1 9SY, United Kingdom.
Andrew T. Hattersley, Institute of Biomedical and Clinical Science, Peninsula Medical School, Exeter EX2 5DW, United Kingdom
Sian Ellard, Institute of Biomedical and Clinical Science, Peninsula Medical School, Exeter EX2 5DW, United Kingdom.
Iwar Klimes, DIABGENE and the Diabetes Research Laboratory, Institute of Experimental Endocrinology, 833 06 Bratislava, Slovakia.
References
- 1.Hattersley AT, Ashcroft FM. Activating mutations in Kir6.2 and neonatal diabetes: new clinical syndromes, new scientific insights, and new therapy. Diabetes. 2005;54:2503–2513. doi: 10.2337/diabetes.54.9.2503. [DOI] [PubMed] [Google Scholar]
- 2.Massa O, Iafusco D, D’Amato E, Gloyn AL, Hattersley AT, Pasquino B, Tonini G, Dammacco F, Zanette G, Meschi F, Porzio O, et al. KCNJ11 activating mutations in Italian patients with permanent neonatal diabetes. Hum Mutat. 2005;25:22–27. doi: 10.1002/humu.20124. [DOI] [PubMed] [Google Scholar]
- 3.Polak M, Shield J. Neonatal diabetes mellitus: genetic aspects 2004. Pediatr Endocrinol Rev. 2004;2:193–198. [PubMed] [Google Scholar]
- 4.Shield JP, Gardner RJ, Wadsworth EJ, Whiteford ML, James RS, Robinson DO, Baum JD, Temple IK. Aetiopathology and genetic basis of neonatal diabetes. Arch Dis Child Fetal Neonatal Ed. 1997;76:F39–F42. doi: 10.1136/fn.76.1.f39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Muhlendahl KE, Herkenhoff H. Long-term course of neonatal diabetes. N Engl J Med. 1995;333:704–708. doi: 10.1056/NEJM199509143331105. [DOI] [PubMed] [Google Scholar]
- 6.Shield JP, Temple IK. Neonatal diabetes mellitus. Pediatr Diabetes. 2002;3:109–112. doi: 10.1034/j.1399-5448.2002.30208.x. [DOI] [PubMed] [Google Scholar]
- 7.Gloyn AL. Glucokinase (GCK) mutations in hyper- and hypoglycemia: maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemia of infancy. Hum Mutat. 2003;22:353–362. doi: 10.1002/humu.10277. [DOI] [PubMed] [Google Scholar]
- 8.Stoffers DA, Zinkin NT, Stanojevic V, Clarke WL, Habener JF. Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nat Genet. 1997;15:106–110. doi: 10.1038/ng0197-106. [DOI] [PubMed] [Google Scholar]
- 9.Brickwood S, Bonthron DT, Al Gazali LI, Piper K, Hearn T, Wilson DI, Hanley NA. Wolcott-Rallison syndrome: pathogenic insights into neonatal diabetes from new mutation and expression studies of EIF2AK3 . J Med Genet. 2003;40:685–689. doi: 10.1136/jmg.40.9.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wildin RS, Smyk-Pearson S, Filipovich AH. Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Genet. 2002;39:537–545. doi: 10.1136/jmg.39.8.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sellick GS, Barker KT, Stolte-Dijkstra I, Fleischmann C, Coleman RJ, Garrett C, Gloyn AL, Edghill EL, Hattersley AT, Wellauer PK, Goodwin G, et al. Mutations in PTF1A cause pancreatic and cerebellar agenesis. Nat Genet. 2004;36:1301–1305. doi: 10.1038/ng1475. [DOI] [PubMed] [Google Scholar]
- 12.Senee V, Chelala C, Duchatelet S, Feng D, Blanc H, Cossec JC, Charon C, Nicolino M, Boileau P, Cavener DR, Bougneres P, et al. Mutations in GLIS3 are responsible for a rare syndrome with neonatal diabetes mellitus and congenital hypothyroidism. Nat Genet. 2006;38:682–687. doi: 10.1038/ng1802. [DOI] [PubMed] [Google Scholar]
- 13.Gloyn AL, Pearson ER, Antcliff JF, Proks P, Bruining GJ, Slingerland AS, Howard N, Srinivasan S, Silva JM, Molnes J, Edghill EL, et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med. 2004;350:1838–1849. doi: 10.1056/NEJMoa032922. [DOI] [PubMed] [Google Scholar]
- 14.Proks P, Antcliff JF, Lippiat J, Gloyn AL, Hattersley AT, Ashcroft FM. Molecular basis of Kir6.2 mutations associated with neonatal diabetes or neonatal diabetes plus neurological features. Proc Natl Acad Sci USA. 2004;101:17539–17544. doi: 10.1073/pnas.0404756101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Proks P, Arnold AL, Bruining J, Girard C, Flanagan SE, Larkin B, Colclough K, Hattersley AT, Ashcroft FM, Ellard S. A heterozygous activating mutation in the sulphonylurea receptor SUR1 (ABCC8) causes neonatal diabetes. Hum Mol Genet. 2006;15:1793–1800. doi: 10.1093/hmg/ddl101. [DOI] [PubMed] [Google Scholar]
- 16.Babenko AP, Polak M, Cave H, Busiah K, Czernichow P, Scharfmann R, Bryan J, Aguilar-Bryan L, Vaxillaire M, Froguel P. Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. N Engl J Med. 2006;355:456–466. doi: 10.1056/NEJMoa055068. [DOI] [PubMed] [Google Scholar]
- 17.Sagen JV, Raeder H, Hathout E, Shehadeh N, Gudmundsson K, Baevre H, Abuelo D, Phornphutkul C, Molnes J, Bell GI, et al. Permanent neonatal diabetes due to mutations in KCNJ11 encoding Kir6.2: patient characteristics and initial response to sulfonylurea therapy. Diabetes. 2004;53:2713–2718. doi: 10.2337/diabetes.53.10.2713. [DOI] [PubMed] [Google Scholar]
- 18.Vaxillaire M, Populaire C, Busiah K, Cave H, Gloyn AL, Hattersley AT, Czernichow P, Froguel P, Polak M. Kir6.2 mutations are a common cause of permanent neonatal diabetes in a large cohort of French patients. Diabetes. 2004;53:2719–2722. doi: 10.2337/diabetes.53.10.2719. [DOI] [PubMed] [Google Scholar]
- 19.Flanagan SE, Edghill EL, Gloyn AL, Ellard S, Hattersley AT. Mutations in KCNJ11, which encodes Kir6.2, are a common cause of diabetes diagnosed in the first 6 months of life, with the phenotype determined by genotype. Diabetologia. 2006;49:1190–1197. doi: 10.1007/s00125-006-0246-z. [DOI] [PubMed] [Google Scholar]
- 20.Proks P, Girard C, Haider S, Gloyn AL, Hattersley AT, Sansom MS, Ashcroft FM. A gating mutation at the internal mouth of the Kir6.2 pore is associated with DEND syndrome. EMBO Rep. 2005;6:470–475. doi: 10.1038/sj.embor.7400393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gloyn AL, Reimann F, Girard C, Edghill EL, Proks P, Pearson ER, Temple IK, Mackay DJ, Shield JP, Freedenberg D, Noyes K, et al. Relapsing diabetes can result from moderately activating mutations in KCNJ11 . Hum Mol Genet. 2005;14:925–934. doi: 10.1093/hmg/ddi086. [DOI] [PubMed] [Google Scholar]
- 22.Pearson ER, Flechtner I, Njolstad PR, Malecki MT, Flanagan SE, Larkin B, Ashcroft FM, Klimes I, Codner E, Iotova V, Slingerland AS, et al. Hattersley AT for the Neonatal Diabetes International Collaborative Group Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med. 2006;355:467–477. doi: 10.1056/NEJMoa061759. [DOI] [PubMed] [Google Scholar]
- 23.Haider S, Antcliff JF, Proks P, Sansom MS, Ashcroft FM. Focus on Kir6.2: a key component of the ATP-sensitive potassium channel. J Mol Cell Cardiol. 2005;38:927–936. doi: 10.1016/j.yjmcc.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Ashcroft FM. ATP-sensitive potassium channelopathies: focus on insulin secretion. J Clin Invest. 2005;115:2047–2058. doi: 10.1172/JCI25495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michalkova DM, Cernay J, Dankova A, Rusnak M, Fandakova K. Incidence and prevalence of childhood diabetes in Slovakia (1985-1992). Slovak Childhood Diabetes Epidemiology Study Group. Diabetes Care. 1995;18:315–320. doi: 10.2337/diacare.18.3.315. [DOI] [PubMed] [Google Scholar]
- 26.Dhatt GS, Agarwal MM, Bishawi B. HbA1c: a comparison of NGSP with IFCC transformed values. Clin Chim Acta. 2005;358:81–86. doi: 10.1016/j.cccn.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Penttila IM, Halonen T, Punnonen K, Tiikkainen U. Best use of the recommended IFCC reference method, material and values in HbA1C analyses. Scand J Clin Lab Invest. 2005;65:453–462. doi: 10.1080/00365510510025809. [DOI] [PubMed] [Google Scholar]
- 28.Health statistics yearbook of the Slovak republic, 1981-2004. Bratislava, Slovakia: National Health Information Center; [Google Scholar]
- 29.Senee V, Vattem KM, Delepine M, Rainbow LA, Haton C, Lecoq A, Shaw NJ, Robert JJ, Rooman R, Diatloff-Zito C, Michaud JL, et al. Wolcott-Rallison syndrome: clinical, genetic, and functional study of EIF2AK3 mutations and suggestion of genetic heterogeneity. Diabetes. 2004;53:1876–1883. doi: 10.2337/diabetes.53.7.1876. [DOI] [PubMed] [Google Scholar]
- 30.Chan KW, Zhang H, Logothetis DE. N-terminal transmembrane domain of the SUR controls trafficking and gating of Kir6 channel subunits. EMBO J. 2003;22:3833–3843. doi: 10.1093/emboj/cdg376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polak M, Shield J. Neonatal and very-early-onset diabetes mellitus. Semin Neonatol. 2004;9:59–65. doi: 10.1016/S1084-2756(03)00064-2. [DOI] [PubMed] [Google Scholar]
- 32.Fosel S. Transient and permanent neonatal diabetes. Eur J Pediatr. 1995;154:944–948. doi: 10.1007/BF01958635. [DOI] [PubMed] [Google Scholar]
- 33.Kilkkinen A, Virtanen SM, Klaukka T, Kenward MG, Salkinoja-Salonen M, Gissler M, Kaila M, Reunanen A. Use of antimicrobials and risk of type 1 diabetes in a population-based mother-child cohort. Diabetologia. 2006;49:66–70. doi: 10.1007/s00125-005-0078-2. [DOI] [PubMed] [Google Scholar]
- 34.Rami B, Waldhor T, Schober E. Incidence of type I diabetes mellitus in children and young adults in the province of Upper Austria, 1994-1996. Diabetologia. 2001;44(Suppl 3):B45–B47. doi: 10.1007/pl00002953. [DOI] [PubMed] [Google Scholar]
- 35.Stene LC, Midthjell K, Jenum AK, Skeie S, Birkeland KI, Lund E, Joner G, Tell GS, Schirmer H. Prevalence of diabetes mellitus in Norway. Tidsskr Nor Laegeforen. 2004;124:1511–1514. [PubMed] [Google Scholar]
- 36.Nordly S, Jorgensen T, Andreasen AH, Hermann N, Mortensen HB. Quality of diabetes management in children and adolescents in Denmark. Diabet Med. 2003;20:568–574. doi: 10.1046/j.1464-5491.2003.00983.x. [DOI] [PubMed] [Google Scholar]
- 37.Schoenle EJ, Molinari L, Bagot M, Semadeni S, Wiesendanger M. Epidemiology of IDDM in Switzerland. Increasing incidence rate and rural-urban differences in Swiss men born 1948-1972. Diabetes Care. 1994;17:955–960. doi: 10.2337/diacare.17.9.955. [DOI] [PubMed] [Google Scholar]
- 38.Cernay J, Rusnak M, Michalkova D, Raisova A. The central register for diabetes mellitus in children in Slovakia. Cesk Pediatr. 1989;44:385–388. [PubMed] [Google Scholar]
- 39.Edghill EL, Gloyn AL, Gillespie KM, Lambert AP, Raymond NT, Swift PG, Ellard S, Gale EA, Hattersley AT. Activating mutations in the KCNJ11 gene encoding the ATP-sensitive K+ channel subunit Kir6.2 are rare in clinically defined type 1 diabetes diagnosed before 2 years. Diabetes. 2004;53:2998–3001. doi: 10.2337/diabetes.53.11.2998. [DOI] [PubMed] [Google Scholar]