Abstract
Self-reactive T cells have long been known to cause autoimmune diseases. Recently, more precise characterization of self-reactive cells in the peripheral T cell repertoire underpinned two important notions. First, a surprisingly diverse high frequency of self-reactive T cells can be found in the peripheral T cell repertoire of healthy individuals and second, self-reactive T cells can perform essential beneficial physiological functions. In this review, we discuss recent developments regarding the identification and characterization of self-reactive T cells in the peripheral T cell repertoire. A better understanding of these populations, and subsequently a better way to define them, will be paramount for the development of strategies to manipulate self-reactive T cells and thereby reduce pathological and increase beneficial physiological functions.
Keywords: self-reactivity, tolerance, autoimmunity, T cells, TCR, affinity
Introduction
T cell antigen receptors (TCR) are randomly generated during T cell development and subjected to positive and negative selection on self-peptide/MHC (pMHC) complexes in the thymus. The role of the thymus and central tolerance in shaping the peripheral self-reactive T cell pool has been discussed in detail in recent review articles [1,2]. These quality control steps eventually result in a peripheral pool of mature naïve T cells which are both self-MHC restricted and self-tolerant [2,3]. This means that essentially all peripheral T cells are self-reactive. However, this definition is far too broad for useful discourse. Likewise, defining self-reactivity based on the presence of destructive effector function is too restrictive as it ignores both beneficial self-reactive T cell responses as well as the possibility that cells can change from beneficial to destructive (or vice versa) under certain conditions.
MHC-tetramer positivity for a defined self-antigen or a productive or functional response is the minimal requirement for inclusion into the self-reactive T cell discussion. For example, peripheral T cells continuously receive tonic survival signals in peripheral lymphoid organs that depend on recognizing self-pMHC complexes but they do not become fully activated under normal conditions would not qualify. In contrast, different T cell populations respond to self-pMHC complexes by proliferating and/or producing effector cytokines. This includes classic autoimmune disease-causing T cells, regulatory T (Treg) cells (discussed in Text Box 1) and, more recently described, T cells involved in tissue homeostasis. Multiple peripheral tolerance mechanisms have been installed to keep the first group of potentially harmful self-reactive T cell responses at bay, including ignorance, anergy and active suppression [4]. As we will discuss in Text Box 2, new immunotherapeutic strategies for fighting cancer result in significant changes in peripheral tolerance and the T cell activation threshold and thereby unleash these normally innocuous self-reactive peripheral T cells.
Text Box 1. Treg cells as part of the self-reactive T cell pool.
It is believed that the majority of Foxp3+ Treg cells are thymic derived and can recognize self-antigens [62]. In fact, diversion into the Treg fate could be a salvage pathway to rescue certain TCR-specificity during negative selection in the thymus. This is not to say that all Treg cells are self-reactive or that Tregs or TCR specificities that are prone to become Treg cells cannot be negatively selected in the thymus [63,64]. Once the Treg cell program is induced in the thymus and Foxp3 is expressed, these cells are surprisingly strongly hardwired to stay in the Treg lineage, even under challenged conditions in the periphery. This is in part due to epigenetic changes of the Foxp3 locus and other important Treg identity genes [65,66].
A very recent study by Legoux and colleagues highlighted that self-antigen specific Treg cells restricted against peripheral tissue antigens are necessary to control self-reactive Tconv cells that are specific for the same tissue antigens [25]. This study was done with the model self-antigen Cre-recombinase, which was expressed in mice through different tissue promoters (e.g., pancreas, lung and intestine specific expression). Endogenous Cre-specific Treg and Tconv cells were analyzed with Cre-specific MHC-tetramers. Cre-specific self-reactive Tconvs and Tregs were readily detectable in all model systems tested and no deletion of self-reactive Tconvs was observed. These Cre-specific self-reactive Tconvs could be reactivated to become effector cells by removing the Treg cells. These data indicate that at least for some tissue antigens, tolerance relies entirely on non-deletional peripheral mechanisms [25].
The expression of tissue-restricted self-antigens that are important for negative selection and Treg cell generation are at least, in part, controlled by the autoimmune regulator (AIRE). The important role of Aire in shaping the Treg cell repertoire was recently described by the Mathis group who identified a unique wave of Aire-dependent perinatal Treg cells that seed the periphery and cannot be generated later on in life again [67]. The importance of a specialized perinatal wave of Treg cells seeding peripheral organs has also been observed during establishing tolerance to commensal bacteria in the skin [68].
The propensity of Treg cells to be enriched for TCR specificities recognizing self-antigens stimulates the idea that Treg cells go beyond the classical modulation of immune responses and also play important functional roles in tissue-homeostasis or –regeneration. This view has recently been supported by several lines of observation in different tissues. The first report described the tissue specialization and non-classic immune function of Treg cells in the adipose tissue and insulin sensitivity [56]. The accumulation and specialization of visceral adipose tissue Treg cells was driven by the transcription regulator PPAR-γ. PPAR-γ expression in adipose Treg cells was necessary for complete restoration of insulin sensitivity in obese mice with the PPAR-γ agonist drug pioglitazone [57]. Although it was not formally proven, the very restricted and unique TCR-repertoire of adipose Treg cells suggests that they recognize fat specific self-antigens [56,69]. Two other organ systems where tissue–regeneration mediated by Treg cells was demonstrated are muscle repair after injury and lung repair after virus infection [58,59]. Interestingly, in both scenarios amphiregulin, a member of the epidermal growth factor family, was required to be expressed by Treg cells to mediate wound healing.
Text Box 2. New immunotherapeutic strategies.
With the rising number of reports on successful cancer treatment based on immune-checkpoint blockade inhibitor therapy [70–72], there has been a massive increase in the number of private companies and public institutions attempting to develop novel immunotherapeutic strategies for the treatment of cancer and infectious diseases. Interfering with peripheral tolerance is just one promising treatment strategy.
Immunotherapeutic strategies can be broadly divided into two distinct categories. The first are the antigen-specific strategies such as vaccination and adoptive cellular therapy [73]. These are primarily aimed at directly increasing the number of antigen-specific T cells in the patient and are, therefore, by nature highly personalized. Lymphocytes are removed from the patient, expanded in culture, potentially genetically engineered to express conventional TCRs or chimeric antigen receptors (CARs) and reinfused into the patient. While there have been impressive results in the treatment of certain hematological tumors, there are a number of shortcomings that need to be addressed before this type of therapy can be put into broad use. The most promising studies use CAR T cells expressing a receptor that recognizes CD19 expressed by B cell lymphomas (and normal B cells) [73]. Central to this approach is the judicious choice of the correct target for antigen-specific therapy, since many tumor-associated antigens are also expressed by healthy tissues. Importantly, developing these personalized therapies for widespread use might prove the need to establish a new infrastructure system to locally produce the personalized cellular therapy. Moreover, the success of these antigen-specific strategies will critically depend on peripheral tolerance mechanisms, which in turn could lead to important synergies with the second category of immuno-therapeutic strategies.
This second category, which is generally antigen-independent, can be further divided into pro-stimulatory and anti-inhibitory strategies. The now classic immune-checkpoint blockade inhibitors, such as CTLA-4 and PD-1 pathway blocking antibodies, are part of the latter group, while recent research is focusing on activating pro-stimulatory pathways through molecules such as CD137 (4-1BB), GITR, CD40 and OX40 [74]. The existence of multiple distinct therapeutic strategies for treating cancer is encouraging, especially since combination therapies prove most promising in clinical studies [74,75].
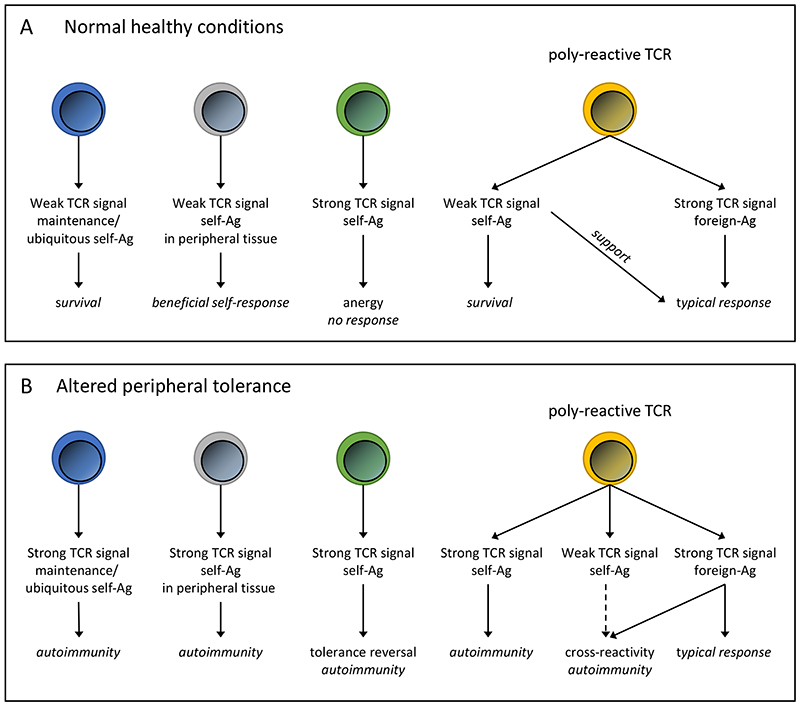
Check-point inhibitor blockade has been widely hailed as the future of cancer therapy [75–77]. Although it has been successful at boosting anti-tumor immunity, it has also been shown to promote the development of so called immune-related adverse events (irAEs). These irAEs emerge because interfering with peripheral tolerance can also result in the activation of many self-reactive T cells (Figure 1). In fact, recent clinical trials with check-point inhibitor antibodies uncovered that self-reactive T cells are unleashed by these treatments. This now constitutes a major limitation for therapies that interfere with tolerance and immune homeostasis [78]. Interestingly, many of the irAEs that develop following immunotherapy seem to differ from classic organ-specific autoimmune diseases in this broader range of affected organs and responding cells [78]. For example, in a murine model of manipulating peripheral tolerance through elimination of Treg cells, it has been shown that many different tissues are affected and a broad array of TCRs are responsible for this destruction [18–20]. While classic autoimmune diseases target a narrower range of specific tissue antigens such as pancreatic ß-cell antigens in type 1 diabetes or myelin sheath components in multiple sclerosis, these irAEs are more diverse and more reminiscent of the breakdown of major immune tolerance pathways [78–81]. The proposed first-line treatment with check-point inhibitor blockade in different cancer entities will result in an increased number of patients that will develop autoimmune diseases. This number might easily exceed the number of “classic” autoimmune patients and highlights the urgent medical need to understand the hidden self-reactive T cell repertoire.
Recent methodological advances, described in the following sections, have allowed us to more precisely define and delineate the self-reactive T cell population. Specifically, a surprisingly high frequency of self-reactive T cells has been found in the peripheral T cell repertoire of healthy individuals and these cells have a very broad range of antigen-specificities. Furthermore, in addition to classic pathogenic self-reactive T cells and Treg cells, self-reactive T cells with essential and beneficial functions have been discovered.
By the end of this review, we hope to initiate a conceptual framework to eventually define self-reactive T cells as pathological or physiological in the context of both normal healthy conditions as well as under conditions of altered peripheral tolerance. Research in this direction will allow the development of strategies to manipulate the self-reactive T cell pool thereby reducing the development of clinical autoimmune diseases and increase the potential beneficial contributions to tissue homeostasis and fighting infections.
The self-reactive T cell pool in healthy individuals
Paul Ehrlich coined the term horror autotoxicus, or “the horror of self-toxicity”, to describe the concept of destructive self-reactivity over 100 years ago. Since then, many self-antigen specific T (and B) cells have been identified in patients suffering from autoimmune diseases. The self-reactive cells in these patients occur at an elevated frequency and in a particular location and cause overt clinical symptoms; this allows them to be isolated and identified. In contrast, self-reactive T cells in healthy individuals are dispersed in the peripheral T cell pool at a low frequency for each individual specificity and functionally hidden by peripheral tolerance mechanisms. While self-reactive T cells were first serendipitously identified in healthy individuals over two decades ago [5] (reviewed in [6]), recent technological advancements have led to a rapid expansion of our knowledge of this interesting T cell subset.
Identification of individual self-reactive T cell specificities in healthy individuals
Early experiments that identified specific target antigens of self-reactive T cells such as myelin basic protein in patients with autoimmune disease also demonstrated that responsive cells were present in healthy subjects. Since then, many individual self-reactive T cell specificities have been identified in both mice and man [6,7]. During the last decade, significant advancements have been made in peptide-MHC (pMHC) tetramer technology that has allowed for the enrichment and identification of very rare antigen-specific lymphocyte populations. In contrast to previous techniques that used peptide stimulation to identify self-reactivity based on functional read outs (such as proliferation or cytokine production), pMHC tetramers can identify self-reactive T cells in their naïve state, which allows for a more precise ex vivo characterization of their phenotype, functional status and precursor frequency. For example, melanocyte-antigen-specific CD8+ T cells isolated from healthy individuals were shown to be in an anergic state that was maintained by Treg cells [8] and they were therefore functionally invisible in the peripheral T cell repertoire.
Davis and colleagues, who pioneered the use of pMHC tetramers to track antigen-specific T cells in healthy mice and man, recently reported that the precursor frequency of CD4+ and CD8+ T cells specific for certain self-antigens (including gp100, fibrinogen, preproinsulin, fructose bisphosphate aldolase and keratin) was between one and ten cells per million T cells [9]. It was thus in the same range as T cells specific for foreign viral epitopes [10,11]. These results are consistent with a previous study showing that the frequency of T cells for a particular self-antigen (gp100) was in the range of 0.0001 percent [12]. In this study, the authors indirectly determined precursor frequency by comparing pMHC tetramer staining on endogenous T cells and a spiked-in titration of gp100-specific TCR transgenic T cells four weeks following irradiation, adoptive transfer and vaccination with gp100 peptide. Compared to these types of elaborate indirect and functional assays, the directly ex vivo pMHC tetramer-based enrichment techniques originally developed by Jenkins and colleagues [13] and further modified by Davis and colleagues will continue to accelerate the identification of self-reactive T cells in normal repertoire. For example, the frequency of self-reactive CD8+ T cells specific for a Y chromosome-encoded antigen, SMCY in humans and SMCY3 in mice, was compared between males and females: In both species, self-reactive T cells were identified and, interestingly, there was only three-fold reduction in the frequency in males as compared to females [9] suggesting a surprisingly limited imposition of clonal deletion on the self-reactive T cell pool.
Antigen-tetramer technology has also revealed NKT cells that recognize self-lipids presented by CD1a and CD1c in the peripheral blood of healthy individuals [14,15]. Likewise, self-reactive B cells form part of the healthy peripheral repertoire [16] (reviewed in [17]). The development of these techniques to directly and specifically label T and B cell antigen receptors has allowed for the identification of these self-reactive cells in their naïve state. Unfortunately, this approach is somewhat limited because each tetramer is peptide specific. The development of more unbiased tools, such as large peptide libraries, will be extremely useful to quantify and characterize the pool of self-reactive T cells at large.
Analysis of the entire self-reactive T cell population
How can the entire peripheral T cell pool be examined for the presence of self-reactive cells? Recent evidence showed that Treg cells can induce anergy in self-reactive T cells thereby making them essentially invisible in the peripheral T cell repertoire [8]. This suggests that the total self-reactive T cell repertoire can only be accurately assessed in the absence of Treg cell-mediated control (or perhaps other peripheral tolerance mechanisms). Transient depletion of Treg cells in adult Foxp3-DTR mice has been shown to result in systemic autoimmune disease [18–20]. By examining the expression of TCR signaling markers and cell division, it was shown that approximately four percent of both the peripheral CD4+ and CD8+ T cell populations are self-reactive and normally controlled by Treg cells [20]. Intriguingly, these data suggest that the size of the self-reactive peripheral T cell pool is in the range of the responses to allo-MHC complexes (1 to 10 percent) [21] or super-antigens (5 to 20 percent) [22], and similar to the proposed frequency of self-reactive B cells (10 to 20 percent) [17]. This surprisingly high proportion of self-reactive T cells so far escaped our attention because the population size of self-reactive T cells is only fully revealed in the absence of peripheral tolerance mechanisms, such as Treg cells [8,18–20]. This suggests that functional assays that abrogate peripheral tolerance mechanisms will provide a more accurate account of the entire self-reactive T cell pool. Since other mutations in essential tolerance mechanisms display a similar reactivation of self-reactive T cells, e.g., in CTLA-4 [23] or TGF-β signaling [24], it would be important to confirm the overall size of the self-reactive peripheral T cell pool in such model systems. For example, adoptive transfer of TGF–β receptor deficient T cells could be such a model. A very recent report by Legoux and colleagues supported the above described finding that Treg cells control a large variety of self-reactive T cells in healthy mice [25]. This report went a step further and showed that for some tissue-restricted self-antigens, immune tolerance entirely relies on non-deletional mechanisms [25]. The model system used the Cre-recombinase as a self-antigen. Since there is a large variety of mouse strains that express Cre through different tissue promoters (e.g., RIP in the pancreas), they could address how the tissue-restricted expression of the self-antigen Cre influenced the endogenous Cre-specific T cell pool with MHC-tetramers. Their data revealed that, in the four Cre lines tested, negative selection against self-reactive Cre-specific T cells was not detectable and these self-reactive T cells were frequently found in the periphery. These cells could be reactivated to become effector T cells either by Treg depletion or specific immunization strategies [25]. These data are in clear contrast to previous findings that self-antigen specific T cells are negatively selected in the thymus [26,27]. One needs to carefully address in which respect these different self-antigens were Aire-dependently or -independently expressed in the thymus in the model systems where negatively selected versus the non-deleting conditions is observed. Irrespective of this, these findings open the intriguing possibility that certain self-antigens, maybe specifically a subset of peripheral tissue antigens are somehow ignored in the thymus and those self-antigen specific T cells are allowed to seed the periphery. Whether those antigens are important drivers of physiological self-reactivity in the tissue is currently unclear. In addition to having a blind spot for certain self-antigens, it has also been shown that self-reactive T cells with low avidity TCRs can also escape thymic negative selection [7,26,28].
Antigen-specificities of self-reactive T cells
The high frequency of T cells that respond to self-antigens described to occur in the absence of Treg cell-mediated control and the systemic nature of the autoimmune disease that follows implies that a broad range of antigen specificities are involved. In the previous section we described some of the classical autoimmune-associated antigen-specificities that have been found in healthy individuals. In this section we will expand our discussion to a broader range of these pMHC ligands.
Subthreshold ligands
What was once broken down into simplified binary cognate or non-cognate ligands (or interactions) is now understood as a sliding analog scale of T cell responses. All the diverse pMHC complexes that can bind to a single TCR are now often labeled with a wide range of ambiguous terms, such as selection, maintenance, subthreshold or activation/stimulatory ligands. Importantly, all of these TCR:pMHC interactions result in a range of qualitatively different intracellular signaling outcomes. It has recently been proposed that the peripheral T cell repertoire could be subdivided into small “colonies” that share common non-stimulatory sub-threshold ligands, but have otherwise independent cognate ligands [29]. It is tempting to speculate that the activation of self-reactive T cells that emerge following alterations in peripheral tolerance could conceivably be due to shared subthreshold ligands that are derived from ubiquitous self-antigens and therefore widely expressed by APCs. In this scenario, a large, but limited, population of T cells would vigorously respond to a previously non-stimulatory subthreshold self-antigen due to changes in the activation status of APCs or the absence of environmental suppressive effectors, like IL-10 or TGF-β (Text Box 2 and Figure 1).
Figure 1. Functional responses following TCR:pMHC engagement in healthy conditions and situations with altered peripheral tolerance.
(A) In healthy individuals, weak signals produced by engagement of TCRs with self-peptide/MHC complexes (self-Ag) can result in multiple beneficial responses ranging from survival (maintenance ligand; on the single cell level) to supporting tissue homeostasis and foreign antigen responses (poly-reactive TCRs). While strong self-peptide/MHC induced TCR signals lead to anergy. (B) In situations where peripheral tolerance mechanisms have been altered, such as cancer immunotherapy or Treg cell depletion, the functional avidity of TCR:pMHC interactions will be changed. This might shift the activation threshold and lead to the emergence of destructive autoimmune diseases. For example, a previously non-functional T cell can now be activated by a ubiquitous self-Ag or a physiological self-reactive T cell could be converted into a pathological self-reactive T cell.
Poly-reactive TCRs
It is now well documented that an individual TCR can recognize a broad range of pMHC complexes. This phenomenon has been loosely termed mimicry, degeneracy, cross-reactivity or poly-reactivity (as is commonly used in the B cell field) (Figure 1). In fact, it was determined that nonamer peptides that bind to the same class II MHC molecule only need to share five amino acids to cross-react with the same TCR [30]. Furthermore, probing a highly diverse yeast-displayed pMHC (class II MHC) library with soluble TCRs showed that 100s of peptides could react with each of the human or mouse TCRs [31]. TCR:pMHC binding was dependent on conserved recognition motifs in the TCR contact amino acids but independent of class II MHC anchor amino acids. By probing TCR libraries with single antigens or, conversely, peptide libraries with single TCRs, the poly-reactivity of human self-reactive CD4+ T cells has been firmly established [31,32]. Likewise in the class I MHC system, using a peptide library and a diabetes patient-derived preproinsulin-specific CD8+ T cell clone, it was shown that a single autoimmune TCR could potentially recognize over one million different pMHC complexes [33]. While this is probably an extreme example, it nevertheless highlights the poly-reactivity of peripheral self-reactive T cells. Furthermore, a very elegant report using an unbiased cloning and retroviral expression system of pre-selection TCRs to study the fates of specific TCRs in vivo, recently demonstrated that negatively selected TCRs were more poly-reactive than positively selected or non-selected TCRs [34]. Collectively these data suggest that self-reactive T cells can respond to a wider than usual range of pMHC complexes. It remains to be determined if this can be used to differentiate pathological from physiological self-reactive T cells or if the breadth of the reactivity is more critical than the ability to respond to different pMHC complexes.
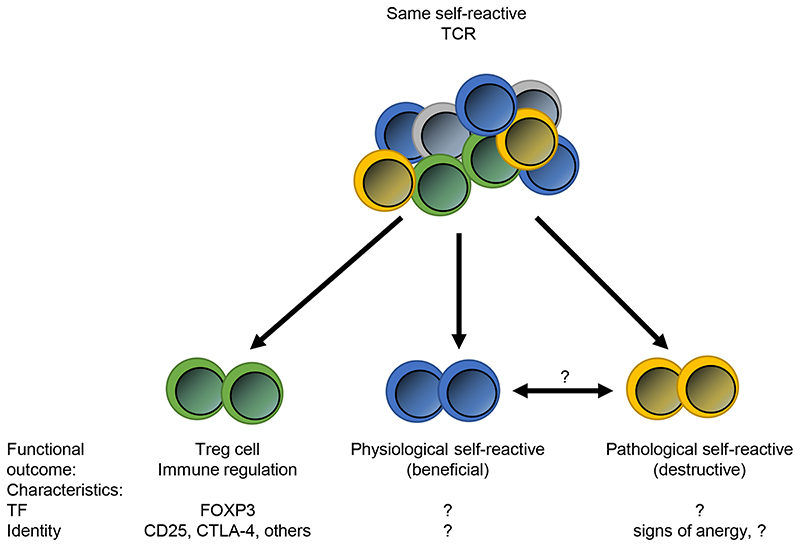
It will most likely be impossible to differentiate pathological from physiological self-reactive T cells based on antigen-specificity. It is becoming clear that the same TCR:pMHC interaction can stimulate distinct responses, for example when Treg cells suppress effector T cells sharing the same TCR (Figure 2) [25]. In this study, Treg cells and self-reactive conventional T (Tconv) cells were both specific against the same Cre antigen (as introduced earlier in this review). In the end, further identification of antigen specificities of self-reactive T cells might be useful to appreciate the breath of the antigens that are recognized and the target tissues that might be effected. It may not answer the question of the functional outcome of those self-reactive T cells, e.g. if they are Treg cells, physiological or potential pathological self-restricted T cells. That can only be done with additional characteristics as the expression of Foxp3, or yet unidentified factors that could differential or predict the function of the self-restricted T cells (Figure 2).
Figure 2. Differentiation between self-reactive T cells.
While the different groups of self-reactive T cells may be impossible to discriminated based on TCR specificity, they have unique functional outcomes. Except for Treg cells, little is known about the unique phenotypic characteristics of physiological and pathological self-reactive T cells, such as transcription factors (TF) or surface protein expression patterns. The most promising line of research could involve looking for evidence of anergy in potential pathological self-reactive T cells.
Alterations in peripheral tolerance and functional TCR avidity
A further complication of the matter is that potential dangerous self-reactive T cells can be functionally disarmed by being tolerized (anergic). This, however, can be overcome and that could be related to the functional TCR avidity. The importance of TCR affinity/avidity and the consequences of TCR:pMHC engagement have been thoroughly reviewed in two recent publications [1,35]. The concept of poly-reactive TCRs, with multiple pMHC ligands, in the context of changes in the activation threshold is intimately connected to TCR affinity and, more critically, functional avidity (Text Box 2). Functional avidity refers to the amount of antigen that is necessary to activate a T cell. For example, it was shown that T cell responsiveness to a specific pMHC increases more then 50-fold during the early stages of viral infection, indicating that T cells can undergo extensive functional maturation, increasing the functional avidity, which is independent of changes to the TCR affinity [36]. Do changes in peripheral tolerance result in significant changes in functional avidity and does this maturation result in activation of self-reactive T cells that otherwise would receive a TCR signal that is too weak to respond on the mere basis of TCR affinity (Figure 1)? In addition, could this change in functional avidity turn a physiological self-reactive T cell into a pathological one (or vice versa)?
CD5 expression as a marker of functional TCR avidity and self-reactivity
The degree of T cell self-reactivity is thought to be linked to pMHC recognition during positive selection in the thymus. CD5 expression levels on T cells reflect affinity, or signaling strength, of TCR:pMHC engagement during positive selection and these “tuned” levels become hard-wired and seem to reflect the levels of self-reactivity in the mature peripheral T cell population [2,37,38]. This could be due to the correlation between CD5 expression and basal CD3 ζ chain phosphorylation levels and/or expression of a number of genes linked to the ability of CD5high T cells to better respond to initial activation [2,38,39]. This is supported by recent evidence showing that self-reactive T cells express TCRs that sit near the activation threshold and are therefore primed for developing overt disease [40].
While these data suggest that CD5high T cells are more prone to self-reactivity, it is not implicit that all self-reactive T cells are CD5high. To test this hypothesis, CD5high and CD5low cells were sorted and transferred into Foxp3DTR mice followed by Treg cell depletion. A similar number of self-reactive T cells were found within both the CD5high and CD5low populations suggesting that in the context of eliminating Treg-mediated peripheral tolerance, CD5 high expression was not an exclusive marker of self-reactivity [20]. Intriguingly, it has also been suggested a correlation between CD5 expression and development of peripherally derived Treg cells [41].
Using Nur77 to monitor functional TCR avidity
A second model system that can report functional TCR avidity in a more dynamic way is the Nur77-GFP reporter mouse [42]. Nur77 is an immediate early gene upregulated by TCR stimulation in T cells and importantly not by inflammatory stimuli. This model has been used to trace the functional avidity of T cells in the thymus as well as the periphery [1,43]. In addition, a modified model has been used to define the very sharp functional TCR avidity, or signaling, threshold that is required for commitment to cell division both in vitro and in vivo. [44]. In this elegant system, the Nur77-GFP reporter was crossed to a Zap70 mutant (Zap70(AS) inhibitor system) that can be selectively inhibited in order to manipulate and then detect TCR signal strength in a variety of conditions [44]. It will be informative to use this system to investigate the self-reactive T cell pool. Intriguingly, naïve peripheral T cells express a basal level of GFP that is rapidly lost in the absence of self-MHC [42]. In addition, it has been shown that CD5high T cells also express higher levels of GFP, consistent with the notion that the CD5high pool more readily responds to (self)-stimulation [39].
Collectively, these results show that the functional TCR avidity, as measured by CD5 or Nur77 expression, is an important component of the self-reactive T cell response. It is clear that therapies manipulating peripheral tolerance mechanisms result in the emergence of pathological self-reactive T cell responses (Text Box 2 and Figure 1). However, it remains to be determined if functional TCR avidity can be used to manipulate self-reactive T cells, thereby decreasing the destructive and increasing the beneficial self-reactive T cell responses.
Is there a beneficial physiological function for self-reactive T cells?
Why do such a high number of self-reactive T cells pass through thymic selection and patrol the periphery? Is this incomplete negative selection only potentially harmful (pathological) or can it also be beneficial (physiological)? Treg cells are an obvious example of a self-reactive T cell population with beneficial physiological functions (described in Text Box 1). In this section, we will describe the beneficial physiological functions for self-reactive T cells that act on, or through, non-immune cells. In this case, the large array of self-reactive T cells in the peripheral repertoire could conceivably support anti-pathogen responses, organ function and tissue homeostasis.
No gaps in anti-pathogen repertoire
It can be argued from a theoretical standpoint that too stringent negative selection would result in a restricted repertoire that would be less capable of recognizing a broad array of pathogens. Recent evidence in both mice and man lend support to this notion [9,30,45,46]. In mouse CD4+ T cells it was shown that clonal deletion by certain self-peptides also reduces the size of the T cell pools specific for foreign-peptides thus reducing the repertoire size [30]. In humans, tetramer staining identified CD8+ T cells that respond to peptides with every natural amino acid substituted at position 5 of a hepatitis C virus protein including a peptide that was previously reported to represent a gap in the TCR repertoire caused by thymic deletion [9]. Additionally, the poly-reactive nature of self-reactive T cells could lower the required functional TCR avidity threshold for anti-pathogen responses and thus allow productive responses with fewer viral pMHC complexes [46]. Finally, it was shown in mice that CD5high naïve CD8+ T cells, described in the previous section, are able to respond better than CD5low cells to infection [39].
Although this is not a direct benefit of self-reactive T cells, it is thought to be a benefit derived from having these cells present in the peripheral repertoire. It is also unclear if this effect is a product of potential pathological or physiological self-reactive T cells. On one hand, there are many reports of autoimmune diseases following infections [47,48]. On the other hand, it is more difficult to observe “the absence of a pathological response” that would, in this case, characterize the response of physiological self-reactive T cells.
Tissue homeostasis
An interesting example of directly beneficial self-reactive T cell responses can be found in the central nervous system (CNS) [49–51]. CNS-specific, self-reactive T cells seem to play an important role in maintaining homeostasis in the central nervous system. For example, self-reactive T cells specific for myelin basic protein (MBP) can protect injured CNS neurons from secondary degeneration [52]. This is an interesting observation since self-reactive MBP-specific T cells have long been known to be able to cause destruction, for example, the paralytic disease, EAE [53]. Therefore, as mentioned before, the mere specificity of a self-reactive T cell may not predict the function consequence of this cell. This may complicate the diagnostic situation, but it will also create a window of opportunity to manipulate these pathological responding cells, to convert into either neutral or in the best case to beneficial self-reactive T cells, which would not further destroy the tissue, but could support the regeneration phase. There is quite a large number of publications that support the physiological functions of self-reactive T cells in the brain. This includes many diverse functions, ranging from recovery from injury, neurogenesis, to learning and brain memory [49–51]. Not only the brain seems to benefit from physiological functions of self-reactive T cells. Other examples of beneficial self-reactive T cell responses are being identified in other tissues. Self-reactive T cells play important roles in hematopoiesis [54] and skin wound healing [55]. For example, human epidermal T cells are able to produce insulin-like growth factor 1 (IGF-1) upon activation and promote wound healing [55]. This is an area that is still under-investigated and will likely yield in the future existing surprises.
Similar observations have recently been made with Treg cells (Text Box 1). Subsets of Treg cells can perform non-immune functions including stabilizing metabolic homeostasis [56,57] and supporting tissue repair [58,59]. Subsets of Treg cells can produce soluble factors such as amphiregulin, which promote tissue-repair in the injured muscle or in virus-infected lungs [58,59]. These functions are independent of their classic immune-suppressive function.
In parallel, beneficial physiological functions of self-reactive B cells have also been reported. Natural IgM (nIgM) produced by CD5+ B1 B cells, can be poly-reactive and a major fraction of nIgM has been reported to recognize self-antigens [60]. Natural IgM is involved in tissue homeostasis by promoting the removal of apoptotic cells via binding to oxidation-associated phosphorylcholine and malondialdehyde epitopes expressed on apoptotic cells. This in turn will enhance the clearance of such labeled apoptotic cells by phagocytes [61]. These observations emphasize that beneficial self-recognition is also a hallmark of the B cell compartment.
The field of beneficial, or physiological, self-reactive T cell immunology is rapidly evolving and many open questions remain. Many of the reported functions seem to involve tissue resident T cell populations suggesting that the environment plays an important role. It will be interesting to determine if the pathological self-reactive T cells that can also be found in these peripheral tissues are converted from physiological cells when the environment changes or if they compete against them. Determining the stability of these self-reactive T cell populations will be critically important.
Concluding remarks
It is now widely recognized that self-reactivity is not just associated with autoimmune diseases. Self-reactive T and B cells are present at relative high numbers in virtually every healthy individual. Several issues arise from these new insights.
Are all self-reactive T cells potentially dangerous? This question is too broad to be answered. With the knowledge of today we can at least subdivide self-reactive T cells into three groups: Treg cells, physiological and pathological self-reactive T cells. Currently we have very little knowledge to use to discriminate between these more functional definitions. Antigen-specificity as measured by MHC-tetramer binding will likely be not sufficient, as all three might recognize the same self-antigen, but respond quite differently. While we have a fairly good idea how Treg cells look (e.g., expression of Foxp3, CTLA-4, GITR, CD25 and others), we have virtually no markers to differentiate the other two groups. The exception might be that a fraction of potentially pathological self-reactive T cells may show signs of anergy (again a more functional readout). It might be even more complicated, as we currently do not know if these are stable “lineage-like” compartments or if they can be converted from one to the other depending on the environment changes. We need to understand the molecular programs that enable the functional discrimination. This will allow us to potentially reprogram these self-reactive T cells, e.g., in an autoimmune setting towards beneficial tissue repair or in a tumor situation towards destruction. Although, one has to be careful with the latter, as a broad reactivation of self-reactive T cells, by disturbing immune tolerance, can be quite devastating as currently seen in a fraction of cancer patients treated with checkpoint inhibitors.
Finally, the notion that self-reactive T cells can also be beneficial is still in its infancy. Currently, it is unclear which non-immune functions are performed by T cells in order to support organ function. Is there a division of labor between Foxp3+ Treg cells and conventional self-reactive T cells? While this is possible for CD4+ T cells, we currently do not know the counterpart on the CD8 side, since there are very few Foxp3 expressing CD8+ T cells. Is there an alternative salvage pathway for CD8+ T cells to rescue certain TCR-specificities during negative selection in the thymus?
Trends Box.
Self-reactive T cells are present in significant numbers in otherwise healthy men and mice. These cells are normally contained but have the potential to cause pathological autoimmune disease when peripheral tolerance mechanisms are alleviated (e.g., novel cancer immunotherapeutic strategies).
T cell receptors are poly-reactive. One TCR can recognize a wide array of pMHC complexes with different functional outcomes. Changes in the functional avidity of TCR:pMHC interactions may unleash previously tolerized self-reactive T cells.
Not all self-reactive T cells are destructive. Self-reactive T cells can support such diverse processes as maintaining tissue homeostasis in the central nervous system, wound healing and boosting immunity against infections. These functions can be summarized as physiological (beneficial) self-reactive. In this respect, these cells can perform similar functions as regulatory T cells.
The mechanisms that restrain self-reactive T cells and instruct them to perform physiological functions are not understood.
Outstanding Questions.
Currently, fundamental aspects of self-reactive T cells are unknown. What are the self-antigens that they recognize?
Are tissue-restricted antigen specific self-reactive T cells more abundant compared to ubiquitous self-antigen specific?
How can alterations in peripheral tolerance settings change the functional TCR avidity of the self-reactive T cells and allow them to be unleashed?
Does the contained self-reactive T cell repertoire contribute to the anti-cancer effect of the new immunotherapeutic strategies? Or are they just responsible for the immune-related adverse events?
Which mechanisms control physiological (beneficial) self-reactive T cells in peripheral tissues to not induce auto-aggression but still allow the production of factors that support tissue homeostasis?
Can a pathological self-reactive T cell be re-programmed to become physiological or vice versa?
How poly-reactive are individual TCRs? Exception or common feature of peripheral T cells?
Acknowledgments
This review was supported by grants from the Helmholtz Association of German Research Centers (Grant HZ-NG-505) and the European Research Council (ERC-2015-CoG) to M.F.
References
- 1.Hogquist KA, Jameson SC. The self-obsession of T cells: how TCR signaling thresholds affect fate ‘decisions’ and effector function. Nat Immunol. 2014;15:815–823. doi: 10.1038/ni.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein L, et al. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see) Nat Rev Immunol. 2014;14:377–391. doi: 10.1038/nri3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stritesky GL, et al. Selection of self-reactive T cells in the thymus. Annu Rev Immunol. 2012;30:95–114. doi: 10.1146/annurev-immunol-020711-075035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mueller DL. Mechanisms maintaining peripheral tolerance. Nat Immunol. 2010;11:21–27. doi: 10.1038/ni.1817. [DOI] [PubMed] [Google Scholar]
- 5.Ota K, et al. T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature. 1990;346:183–187. doi: 10.1038/346183a0. [DOI] [PubMed] [Google Scholar]
- 6.Rosenblum MD, et al. Mechanisms of human autoimmunity. J Clin Invest. 2015;125:2228–2233. doi: 10.1172/JCI78088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito Y, et al. Detection of T cell responses to a ubiquitous cellular protein in autoimmune disease. Science. 2014;346:363–368. doi: 10.1126/science.1259077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maeda Y, et al. Detection of self-reactive CD8(+) T cells with an anergic phenotype in healthy individuals. Science. 2014;346:1536–1540. doi: 10.1126/science.aaa1292. [DOI] [PubMed] [Google Scholar]
- 9.Yu W, et al. Clonal Deletion Prunes but Does Not Eliminate Self-Specific alphabeta CD8(+) T Lymphocytes. Immunity. 2015;42:929–941. doi: 10.1016/j.immuni.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su LF, et al. Virus-specific CD4(+) memory-phenotype T cells are abundant in unexposed adults. Immunity. 2013;38:373–383. doi: 10.1016/j.immuni.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jenkins MK, Moon JJ. The role of naive T cell precursor frequency and recruitment in dictating immune response magnitude. J Immunol. 2012;188:4135–4140. doi: 10.4049/jimmunol.1102661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rizzuto GA, et al. Self-antigen-specific CD8(+) T cell precursor frequency determines the quality of the antitumor immune response. J Exp Med. 2009;206:849–866. doi: 10.1084/jem.20081382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moon JJ, et al. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Jong A, et al. CD1a-autoreactive T cells are a normal component of the human alphabeta T cell repertoire. Nat Immunol. 2010;11:1102–1109. doi: 10.1038/ni.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Lalla C, et al. High-frequency and adaptive-like dynamics of human CD1 self-reactive T cells. Eur J Immunol. 2011;41:602–610. doi: 10.1002/eji.201041211. [DOI] [PubMed] [Google Scholar]
- 16.Taylor JJ, et al. Deletion and anergy of polyclonal B cells specific for ubiquitous membrane-bound self-antigen. J Exp Med. 2012;209:2065–2077. doi: 10.1084/jem.20112272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meffre E, Wardemann H. B-cell tolerance checkpoints in health and autoimmunity. Curr Opin Immunol. 2008;20:632–638. doi: 10.1016/j.coi.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Kim JM, et al. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 19.Nystrom SN, et al. Transient Treg-cell depletion in adult mice results in persistent self-reactive CD4(+) T-cell responses. Eur J Immunol. 2014;44:3621–3631. doi: 10.1002/eji.201344432. [DOI] [PubMed] [Google Scholar]
- 20.Richards DM, et al. The Contained Self-Reactive Peripheral T Cell Repertoire: Size, Diversity, and Cellular Composition. J Immunol. 2015;195:2067–2079. doi: 10.4049/jimmunol.1500880. [DOI] [PubMed] [Google Scholar]
- 21.Suchin EJ, et al. Quantifying the frequency of alloreactive T cells in vivo: New answers to an old question. J Immunol. 2001;166:973–981. doi: 10.4049/jimmunol.166.2.973. [DOI] [PubMed] [Google Scholar]
- 22.Li HM, et al. The structural basis of T cell activation by superantigens. Annu Rev Immunol. 1999;17:435–466. doi: 10.1146/annurev.immunol.17.1.435. [DOI] [PubMed] [Google Scholar]
- 23.Waterhouse P, et al. Lymphoproliferative disorders with early lethality in mice deficient in ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 24.Marie JC, et al. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity. 2006;25:441–454. doi: 10.1016/j.immuni.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 25.Legoux FP, et al. CD4(+) T Cell Tolerance to Tissue-Restricted Self Antigens Is Mediated by Antigen-Specific Regulatory T Cells Rather Than Deletion. Immunity. 2015;43:896–908. doi: 10.1016/j.immuni.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zehn D, Bevan MJ. T cells with low avidity for a tissue-restricted antigen routinely evade central and peripheral tolerance and cause autoimmunity. Immunity. 2006;25:261–270. doi: 10.1016/j.immuni.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moon JJ, et al. Quantitative impact of thymic selection on Foxp3+ and Foxp3-subsets of self-peptide/MHC class II-specific CD4+ T cells. Proc Natl Acad Sci U S A. 2011;108:14602–14607. doi: 10.1073/pnas.1109806108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Enouz S, et al. Autoreactive T cells bypass negative selection and respond to selfantigen stimulation during infection. J Exp Med. 2012;209:1769–1779. doi: 10.1084/jem.20120905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh NJ, et al. Subsets of nonclonal neighboring CD4(+) T cells specifically regulate the frequency of individual antigen-reactive T cells. Immunity. 2012;37:735–746. doi: 10.1016/j.immuni.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson RW, et al. T cell receptor cross-reactivity between similar foreign and self peptides influences naive cell population size and autoimmunity. Immunity. 2015;42:95–107. doi: 10.1016/j.immuni.2014.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Birnbaum ME, et al. Deconstructing the peptide-MHC specificity of T cell recognition. Cell. 2014;157:1073–1087. doi: 10.1016/j.cell.2014.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eugster A, et al. High diversity in the TCR repertoire of GAD65 autoantigen-specific human CD4+ T cells. J Immunol. 2015;194:2531–2538. doi: 10.4049/jimmunol.1403031. [DOI] [PubMed] [Google Scholar]
- 33.Wooldridge L, et al. A single autoimmune T cell receptor recognizes more than a million different peptides. J Biol Chem. 2012;287:1168–1177. doi: 10.1074/jbc.M111.289488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDonald BD, et al. Crossreactive alphabeta T Cell Receptors Are the Predominant Targets of Thymocyte Negative Selection. Immunity. 2015;43:859–869. doi: 10.1016/j.immuni.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grossman Z, Paul WE. Dynamic tuning of lymphocytes: physiological basis, mechanisms, and function. Annu Rev Immunol. 2015;33:677–713. doi: 10.1146/annurev-immunol-032712-100027. [DOI] [PubMed] [Google Scholar]
- 36.Slifka MK, Whitton JL. Functional avidity maturation of CD8(+) T cells without selection of higher affinity TCR. Nat Immunol. 2001;2:711–717. doi: 10.1038/90650. [DOI] [PubMed] [Google Scholar]
- 37.Persaud SP, et al. Intrinsic CD4+ T cell sensitivity and response to a pathogen are set and sustained by avidity for thymic and peripheral complexes of self peptide and MHC. Nat Immunol. 2014;15:266–274. doi: 10.1038/ni.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mandl JN, et al. T cell-positive selection uses self-ligand binding strength to optimize repertoire recognition of foreign antigens. Immunity. 2013;38:263–274. doi: 10.1016/j.immuni.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fulton RB, et al. The TCR’s sensitivity to self peptide-MHC dictates the ability of naive CD8(+) T cells to respond to foreign antigens. Nat Immunol. 2015;16:107–117. doi: 10.1038/ni.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koehli S, et al. Optimal T-cell receptor affinity for inducing autoimmunity. Proc Natl Acad Sci U S A. 2014;111:17248–17253. doi: 10.1073/pnas.1402724111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henderson JG, et al. CD5 instructs extrathymic regulatory T cell development in response to self and tolerizing antigens. Immunity. 2015;42:471–483. doi: 10.1016/j.immuni.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 42.Moran AE, et al. T cell receptor signal strength in T-reg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stritesky GL, et al. Murine thymic selection quantified using a unique method to capture deleted T cells. Proc Natl Acad Sci U S A. 2013;110:4679–4684. doi: 10.1073/pnas.1217532110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Au-Yeung BB, et al. A sharp T-cell antigen receptor signaling threshold for T-cell proliferation. Proc Natl Acad Sci U S A. 2014;111:E3679–3688. doi: 10.1073/pnas.1413726111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolfl M, et al. Hepatitis C virus immune escape via exploitation of a hole in the T cell repertoire. J Immunol. 2008;181:6435–6446. doi: 10.4049/jimmunol.181.9.6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stefanova I, et al. Self-recognition promotes the foreign antigen sensitivity of naive T lymphocytes. Nature. 2002;420:429–434. doi: 10.1038/nature01146. [DOI] [PubMed] [Google Scholar]
- 47.Blank M, et al. Molecular mimicry and auto-immunity. Clin Rev Allergy Immunol. 2007;32:111–118. doi: 10.1007/BF02686087. [DOI] [PubMed] [Google Scholar]
- 48.Horai R, et al. Microbiota-Dependent Activation of an Autoreactive T Cell Receptor Provokes Autoimmunity in an Immunologically Privileged Site. Immunity. 2015;43:343–353. doi: 10.1016/j.immuni.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baruch K, Schwartz M. CNS-specific T cells shape brain function via the choroid plexus. Brain Behavior and Immunity. 2013;34:11–16. doi: 10.1016/j.bbi.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 50.Kipnis J, et al. Pro-cognitive properties of T cells. Nat Rev Immunol. 2012;12:662–668. doi: 10.1038/nri3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwartz M, Raposo C. Protective Autoimmunity: A Unifying Model for the Immune Network Involved in CNS Repair. Neuroscientist. 2014;20:343–358. doi: 10.1177/1073858413516799. [DOI] [PubMed] [Google Scholar]
- 52.Moalem G, et al. Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nat Med. 1999;5:49–55. doi: 10.1038/4734. [DOI] [PubMed] [Google Scholar]
- 53.Zamvil SS, Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]
- 54.Monteiro JP, et al. Normal hematopoiesis is maintained by activated bone marrow CD4+ T cells. Blood. 2005;105:1484–1491. doi: 10.1182/blood-2004-07-2856. [DOI] [PubMed] [Google Scholar]
- 55.Toulon A, et al. A role for human skin-resident T cells in wound healing. J Exp Med. 2009;206:743–750. doi: 10.1084/jem.20081787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Feuerer M, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cipolletta D, et al. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–553. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burzyn D, et al. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155:1282–1295. doi: 10.1016/j.cell.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arpaia N, et al. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell. 2015;162:1078–1089. doi: 10.1016/j.cell.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Elkon K, Casali P. Nature and functions of autoantibodies. Nat Clin Pract Rheumatol. 2008;4:491–498. doi: 10.1038/ncprheum0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaveri SV, et al. Natural IgM in immune equilibrium and harnessing their therapeutic potential. J Immunol. 2012;188:939–945. doi: 10.4049/jimmunol.1102107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hsieh CS, et al. Selection of regulatory T cells in the thymus. Nat Rev Immunol. 2012;12:157–167. doi: 10.1038/nri3155. [DOI] [PubMed] [Google Scholar]
- 63.Perry JS, et al. Distinct contributions of Aire and antigen-presenting-cell subsets to the generation of self-tolerance in the thymus. Immunity. 2014;41:414–426. doi: 10.1016/j.immuni.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feuerer M, et al. Enhanced thymic selection of FoxP3+ regulatory T cells in the NOD mouse model of autoimmune diabetes. Proc Natl Acad Sci U S A. 2007;104:18181–18186. doi: 10.1073/pnas.0708899104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ohkura N, et al. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity. 2012;37:785–799. doi: 10.1016/j.immuni.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 66.Delacher M, et al. Transcriptional control of regulatory T cells. Curr Top Microbiol Immunol. 2014;381:83–124. doi: 10.1007/82_2014_373. [DOI] [PubMed] [Google Scholar]
- 67.Yang S, et al. Immune tolerance. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science. 2015;348:589–594. doi: 10.1126/science.aaa7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scharschmidt TC, et al. A Wave of Regulatory T Cells into Neonatal Skin Mediates Tolerance to Commensal Microbes. Immunity. 2015;43:1011–1021. doi: 10.1016/j.immuni.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kolodin D, et al. Antigen- and cytokine-driven accumulation of regulatory T cells in visceral adipose tissue of lean mice. Cell metabolism. 2015;21:543–557. doi: 10.1016/j.cmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wolchok JD, et al. Nivolumab plus Ipilimumab in Advanced Melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–68. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Melero I, et al. Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat Rev Cancer. 2015;15:457–472. doi: 10.1038/nrc3973. [DOI] [PubMed] [Google Scholar]
- 75.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Littman DR. Releasing the Brakes on Cancer Immunotherapy. Cell. 2015;162:1186–1190. doi: 10.1016/j.cell.2015.08.038. [DOI] [PubMed] [Google Scholar]
- 77.Medler TR, et al. Immune response to cancer therapy: mounting an effective antitumor response and mechanisms of resistance. Trends Cancer. 2015;1:66–75. doi: 10.1016/j.trecan.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kong YC, Flynn JC. Opportunistic autoimmune disorders potentiated by immune-checkpoint inhibitors anti-CTLA-4 and anti-PD-1. Front Immunol. 2014;5:206. doi: 10.3389/fimmu.2014.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Josefowicz SZ, et al. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Klatzmann D, Abbas AK. The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat Rev Immunol. 2015;15:283–294. doi: 10.1038/nri3823. [DOI] [PubMed] [Google Scholar]
- 81.Mathis D, Benoist C. Aire. Annu Rev Immunol. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]