Abstract
Objectives
To develop and validate a novel ex vivo model of conjunctival contraction.
Methods
Ex vivo segments of conjunctiva were maintained in culture for 4 weeks in permeable support plates. Digital images were obtained twice a week to monitor contraction using tissue area changes and weekly weight measurements. Investigated were the effects of known contraction stimulators (fetal bovine serum and transforming growth factor β2) and of the matrix metalloproteinase inhibitor GM6001. Microscopic contraction, tissue organization, and cell viability (using the cell vital dye carboxyfluorescein diacetate) were monitored by confocal reflection and 2-photon microscopy, revealing detailed real-time kinetics of tissue remodeling.
Results
Fetal bovine serum and transforming growth factor β2 induced significant tissue contraction in conjunctiva segments, with no changes in cell viability. This correlated with dramatic and specific degradation of the collagen component in the tissue. Contraction and collagen degradation were reduced in the presence of GM6001.
Conclusions
Ex vivo segments of conjunctiva can be used as an integral model system to provide a higher level of understanding about the efficacy of antiscarring therapies and can help bridge the current gap between in vitro and in vivo models.
Contraction is a fundamental part of the wound-healing response, yet this physiological process can lead to amultitude of problems. Scarring has a major part in many systemic diseasesandis linked to the pathogenesis or treatment failure of most blinding conditions. In the eye,manytypes of surgery fail due to contraction and scarring of the wound site. For example, subconjunctival scarring can severely affect the success of glaucoma filtration surgery.1,2 The few treatments available to prevent scarring do not target contraction directly but affect many facets of cell function, including cell proliferation, which often leads to cytotoxic effects and sight-threatening complications.3–6Therefore, amore detailed understanding of conjunctival healing and contraction processes, as well as the effects of various modulators of the scarring process, is desirable to facilitate the investigation of new drug therapies.
The well-established fibroblast-populated collagen lattice model7–9 is commonly used to study contraction in vitro. This model is simple to set up and has the advantage of allowing the study of cell-mediated contraction within a pseudophysiological 3-dimensional environment. However, this simplistic 3-dimensional environment using tissue-cultured cells artificially embedded in a loose collagen matrix fails to replicate the complexity and mechanical properties of the native tissue,10–12 and the fast contraction kinetics observed varies depending on the method used to cast the collagen gel13 and does not match in vivo wound healing. Several studies14–16 have focused on fibroblast-populated collagen lattice contraction induced by human Tenon fibroblasts (HTFs), which are believed to be the key cells involved in the subconjunctival wound-healing response. The cytokine transforming growth factor β2 (TGFβ2) has been shown to stimulate contraction by HTFs,15 which led to studies17,18 using anti-TGFβ2 antibodies for the prevention of contraction. Although these antibodies were successful at reducing conjunctival scarring in a rabbit model of glaucoma filtration surgery,17,18 clinical trial results were disappointing.19 Therefore, current contraction models that are used to predict antiscarring efficiency may be inadequate, and more information is needed to improve the understanding of the contraction process before new compounds can reach clinical trial status.
Videos available online at www.archophthalmol.com
The present in vitro model system involves removing fibroblasts from their natural surroundings and replacing that environment with an artificial loose collagen matrix. The alternative in vivo model of glaucoma filtration surgery provides valuable information about bleb morphologic structure and intraocular pressure in vivo but involves animal sacrifice. Therefore, it would be convenient and valuable to use a model that offers information about contraction without removal of cells from their natural environment and that reduces the number of in vivo experiments. This study aimed to develop and validate a novel ex vivo model of conjunctival contraction that can provide additional understanding of the contraction process and bridge the gap between the current in vitro and in vivo models to better evaluate novel potential therapeutic targets.
Methods
Cell Culture
Human Tenon fibroblasts were isolated from donor tissue in accord with the tenets of the Declaration of Helsinki and with local ethics approval. Briefly, Tenon capsule tissue was cut into segments and air-dried for approximately 15 minutes in 25cm2 flasks (Corning, Inc, Corning, New York). Segments were covered by 1mL of Dulbecco modified Eagle medium (DMEM), supplemented with 10% (vol/vol) fetal bovine serum (FBS) and antibiotics (described herein), and maintained at 37°C with 5% carbon dioxide for several weeks until fibroblast growth was visible. Segments were removed, and cells were expanded and frozen down for storage. Cells between passages 1 and 6 were used for this study.
Conditions used throughout the study included the following: DMEM containing 2mM L-glutamine, 100 U/mL penicillin G sodium, and 100 μg/mL streptomycin sulfate, supplemented with 0.7% bovine serum albumin (BSA) (hereafter serum free [SF]); 10% FBS (hereafter FBS); 10 ng/mL TGFβ2 plus 0.7% BSA (hereafter TGFβ2); 10% FBS plus 100 μM GM6001 (hereafter FBS+GM); or 10 ng/mL TGFβ2 plus 100 μM GM6001 and 0.7% BSA (hereafter TGFβ2+GM).
Macroscopic Fibroblast-Populated Collagen Lattice Contraction Assay
Human Tenon fibroblasts were resuspended in DMEM containing SF, FBS, TGFβ2, FBS + GM, or TGFβ2 + GM and were added to 1.5 mg/mL neutralized collagen solution (First Link [UK] Ltd, Birmingham, England) as previously described.14 Cell concentrations of 50 000/mL of gel were used for FBS stimulation or 200 000/mL for TGFβ2. The gels were detached from the edge of the well, and 2 mL of DMEM was added containing the appropriate conditions. Digital photographs were obtained daily over 7 days, and lattice areas were measured using available software (ImageJ [http://rsb.info.nih.gov/ij/]). Contraction was expressed as a percentage of gel area normalized to day 0.
Macroscopic Ex Vivo Contraction Assay
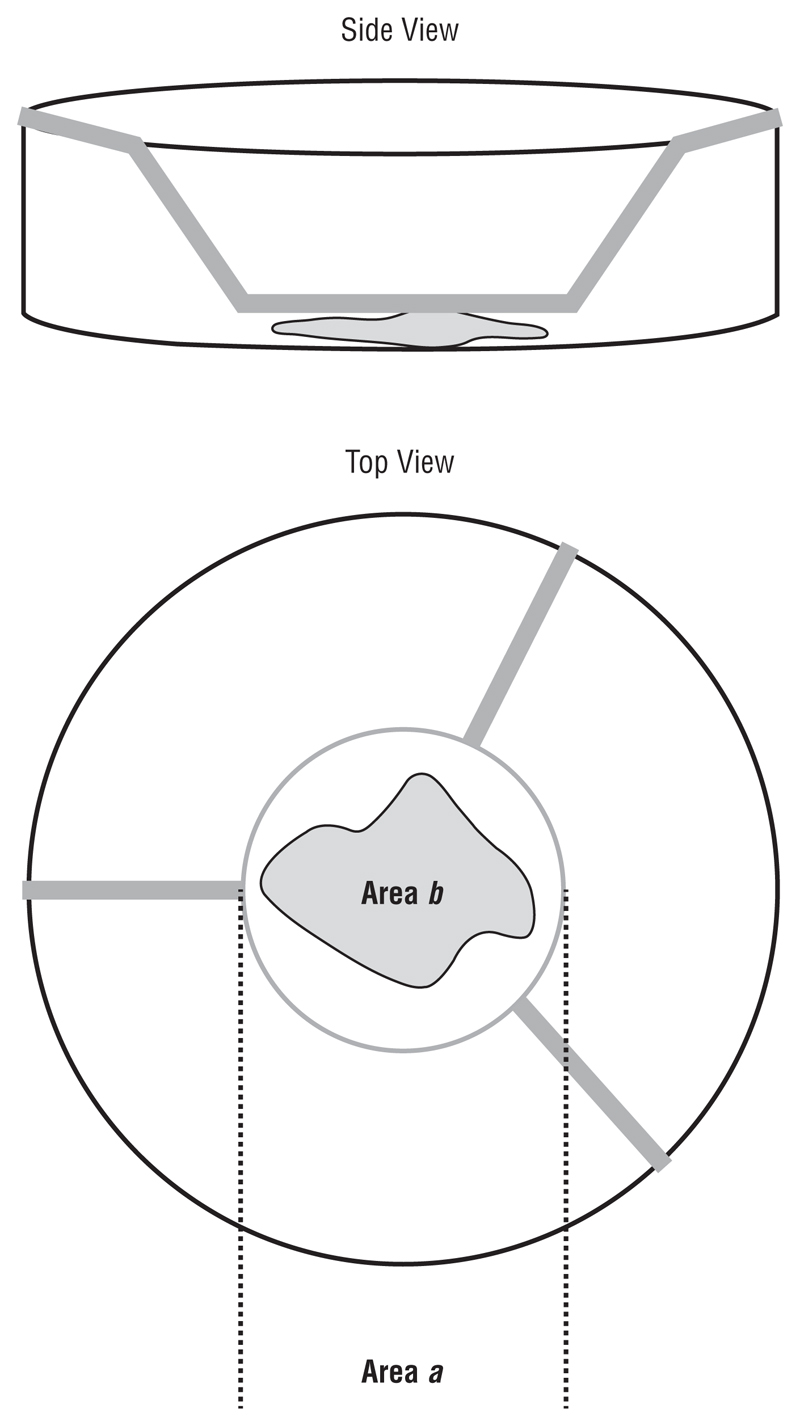
Porcine and rabbit eyes were obtained from the local abattoir within 5 hours of animal death and were used within 24 hours. The eyes were immersed in iodine disinfectant, followed by a rinse in phosphate-buffered saline containing 10× antibiotics (1000-U/mL penicillin, 1000 μg/mL streptomycin, 2.5 μg/mL amphotericin B, and 500 μg/mL gentamycin sulfate; Invitrogen, Carlsbad, California). Conjunctiva was dissected and cut into 5- to 10-mm2 pieces and placed in a 12-well plate. Polyester membrane inserts (3-μm pore size and 12-mm membrane diameter [Transwell; Corning, Inc) were placed on top of the segments, holding the tissue in place and maintaining their shape. Segments were submerged in 1.5 mL of DMEM with the appropriate conditions added (SF, FBS, TGFβ2, FBS + GM, or TGFβ2 + GM) and were kept at 37°C and 5% carbon dioxide for 4 weeks. Digital photographs were obtained, and media were changed twice a week. Changes in area were calculated for each segment by normalizing tissue area (area a in Figure 1) to Transwell area (area b). Percentage contraction was calculated by normalizing percentage area values to those at day 0.
Figure 1.
Segments of ex vivo conjunctiva held in place using a Transwell insert (Corning Inc, Corning, New York). Area (πr 2) is measured using ImageJ (http://rsb.info.nih.gov/ij/). A percentage area for each segment is calculated by normalizing the area of the tissue (Area b) to the area of the well (Area a). Percentage contraction was calculated by normalizing the percentage area for each time point to day 0.
Microscopic Ex Vivo Tissue Contraction
Porcine eyes were sterilized as aforedescribed. A fine-gauge needle was used to inject 50 μL of sterile phosphate-buffered saline solution containing BSA-coated polystyrene beads (10-μm beads coated as per the manufacturer’s instructions, 2.3 × 107 beads per milliliter; PolyScience, Niles, Illinois) and 2 μL of trypan blue, with or without 10 ng/mL TGFβ2. The injected area (approximately 10mm2) was then dissected, transferred into a glass-bottomed culture dish in L15 medium with 1× antibiotics with or without 10 ng/mL TGFβ2, and secured in place with a coverslip. The dish was then transferred to an environmental chamber on a confocal laser scanning microscope (Axiovert S100 [Zeiss, Welwyn Garden City, England]/ Radiance 2000 [BioRad, Hercules, California]) and allowed to equilibrate for 30 minutes to 1 hour before imaging. An area with beads was identified, and confocal z-stacks at 10-μm steps were acquired every hour for 18 hours using differential interference contrast imaging. The resulting images were registered then combined in a software program (Openlab; Improvision/Perkin Elmer, Norwalk, Connecticut) to generate a single-plane movie and were uploaded for analysis (Deformation Quantification and Analysis Web site [http://dqa.web.cmu.edu/]). A mask was inserted as a single dot at the center of the image for analysis in each set of data. All samples were analyzed using identical variables, and the strain per grid point within each sample was calculated as the mean of the individual eigenvalues for the principal strains using first harmonic generation data.20
Microscopy And Cell Staining
Porcine or rabbit conjunctiva segments were placed in a glass-bottomed culture dish in 1 mL of L15 medium containing 1μM carboxyfluorescein diacetate (Vybrant CFDA-SE Cell Tracker Kit, Invitrogen) and were secured with a glass coverslip. Live tissue imaging was performed on a confocal laser scanning microscope (Axiovert S100/Radiance 2000). The matrix was imaged by confocal reflection microscopy14 using a standard objective (Plan Neo-Fluar 20× 0.5NA, Zeiss). Simultaneous reflection and carboxyfluorescein diacetate images were acquired in parallel as z-stacks in 3-μm sections through the depth of the tissue. The acquired z-stacks were imported into a software program (Volocity; Improvision/ Perkin Elmer) and were compressed into a single projection image or converted into 3-dimensional projections.
Second Harmonic Generation
Second harmonic generation was performed on ex vivo porcine tissue segments to investigate collagen fiber degradation. Details of the experimental setup are described by Theodossiou et al.21
Histologic Evaluation Of Tissue Samples
Porcine segments were fixed in 4% formaldehyde for 30 minutes, embedded in paraffin wax, and cut into 5-μm sections. The sections were stained with picrosirius red to assess collagen deposition and were counterstained with hematoxylineosin. Slides were examined using conventional light microscopy and polarized light microscopy.
Measurement of Total Protein Content in Tissue Samples
Segments were fixed in 4% formaldehyde for 30 minutes and then stained in Coomassie blue for 30 minutes. Dye was extracted from the tissue samples using 70% ethanol for 1 hour, and samples of the dye were measured for their absorbance at 550 nm (Fluostar Optima; BMG Biotech, Cary, North Carolina). For whole-tissue mass evaluation, conjunctiva fragments were weighed in sterile Petri dishes following brief blotting onto presterilized filter paper.
Measurement Of Lactate Dehydrogenase Release
A cytotoxicity assay (Roche, Mannheim, Germany) was performed on media samples collected twice a week from the ex vivo contraction assay to measure the percentage of lactate dehydrogenase activity present in the samples. The assay was performed according to the manufacturer’s protocol, and absorbances were read at 490 nm (Fluostar Optima).
Results
Growth Factor Stimulation Is Sufficient To Induce Ex Vivo Tissue Contraction
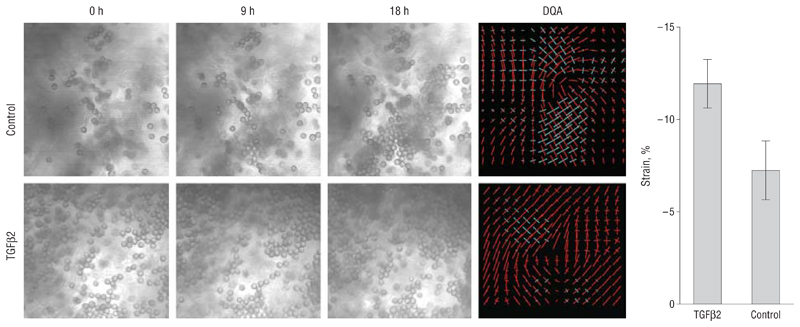
Figure 2 shows differential interference contrast images extracted from a representative movie for a control sample (top) (video 1, http://www.archophthalmol.com) and from a TGFβ2-treated sample (bottom) (video 2), demonstrating local porcine tissue movement over time as tracked by the bead position. The Deformation Quantification and Analysis plots show the local forces at each analysis grid point as the principal strains (eigenvector values for the direction and magnitude of the strain), with compaction strains in red, expansion in blue, and the length of the arms on the cross proportional to the value of the force. Control conjunctiva fragments demonstrated a minimal level of contraction over 18 hours (contraction of the tissue by 7.25% of its original size, expressed as the value per analysis unit). However, tissue contraction was enhanced 1.6 times in the samples treated with TGFβ2 (mean [SD] strain value, −11.93% [−1.32%]), confirming the direct role of TGFβ2 in the contraction process.
Figure 2.
Growth factor stimulation induces ex vivo microscopic tissue contraction. Images were extracted at the times indicated from movies for a representative control sample (Control) and a sample injected with transforming growth factor β2 (TGFβ2). On the right is the corresponding Deformation Quantification and Analysis (DQA) of strain distribution at individual analysis grid points, with the length of the vectors reflecting the absolute value of the strain and the color coding indicating the direction of the strain (red, contraction; blue, expansion). While the control sample is largely equilibrated in red and blue, the TGFβ2 sample is mostly red, reflecting the greater contractile strain in the sample. The graph shows the SEM strain value per analysis grid point for 4 control samples and for 3 TGFβ2 samples. P< .001, t test.
Whole Ex Vivo Tissue Contraction Mimics In Vivo Contraction
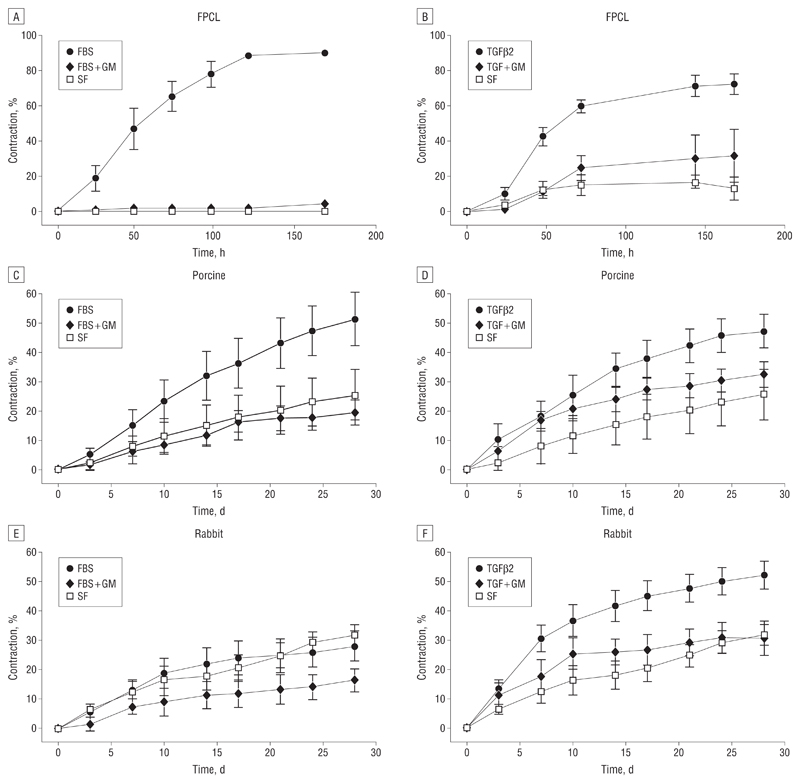
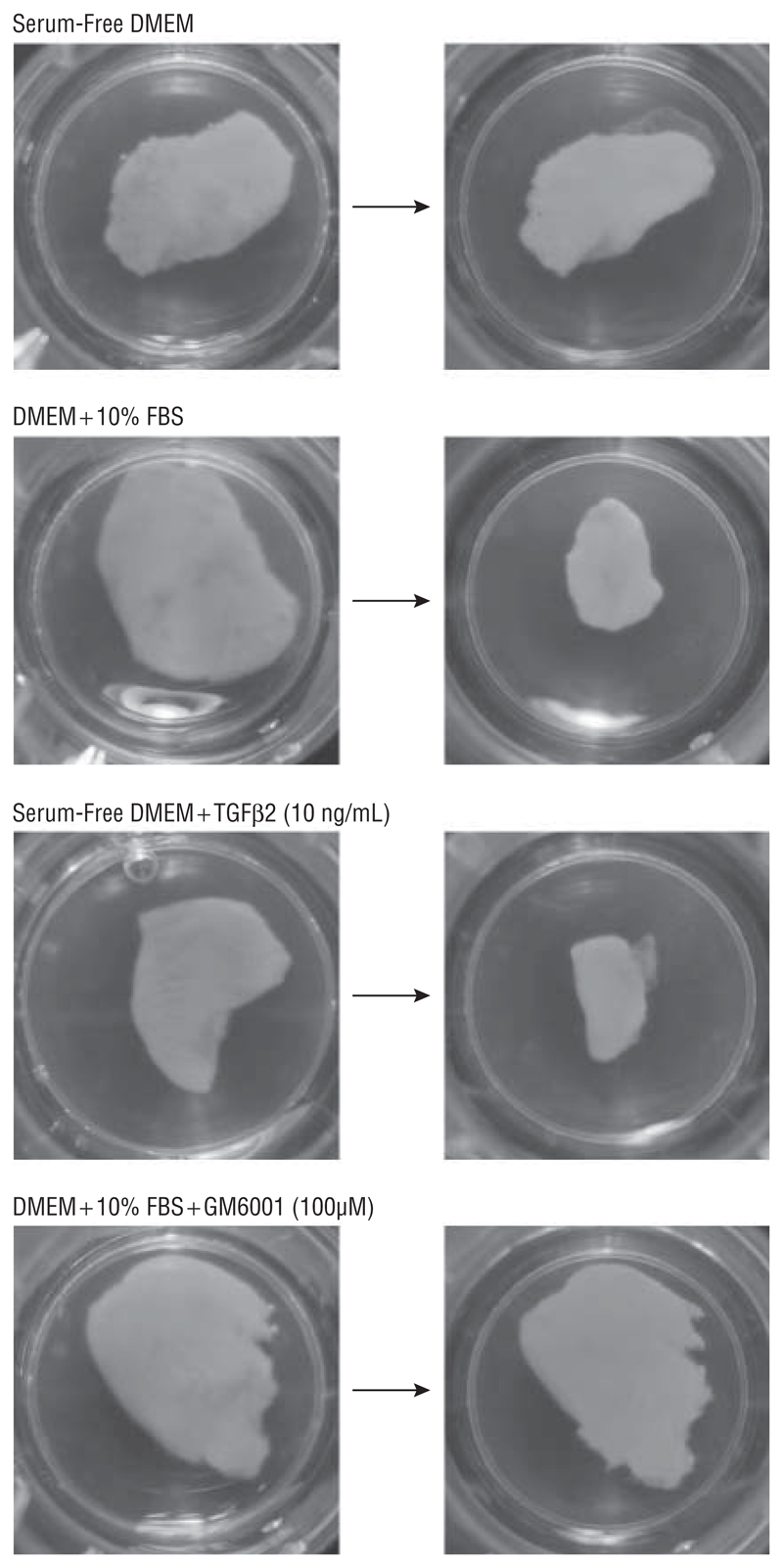
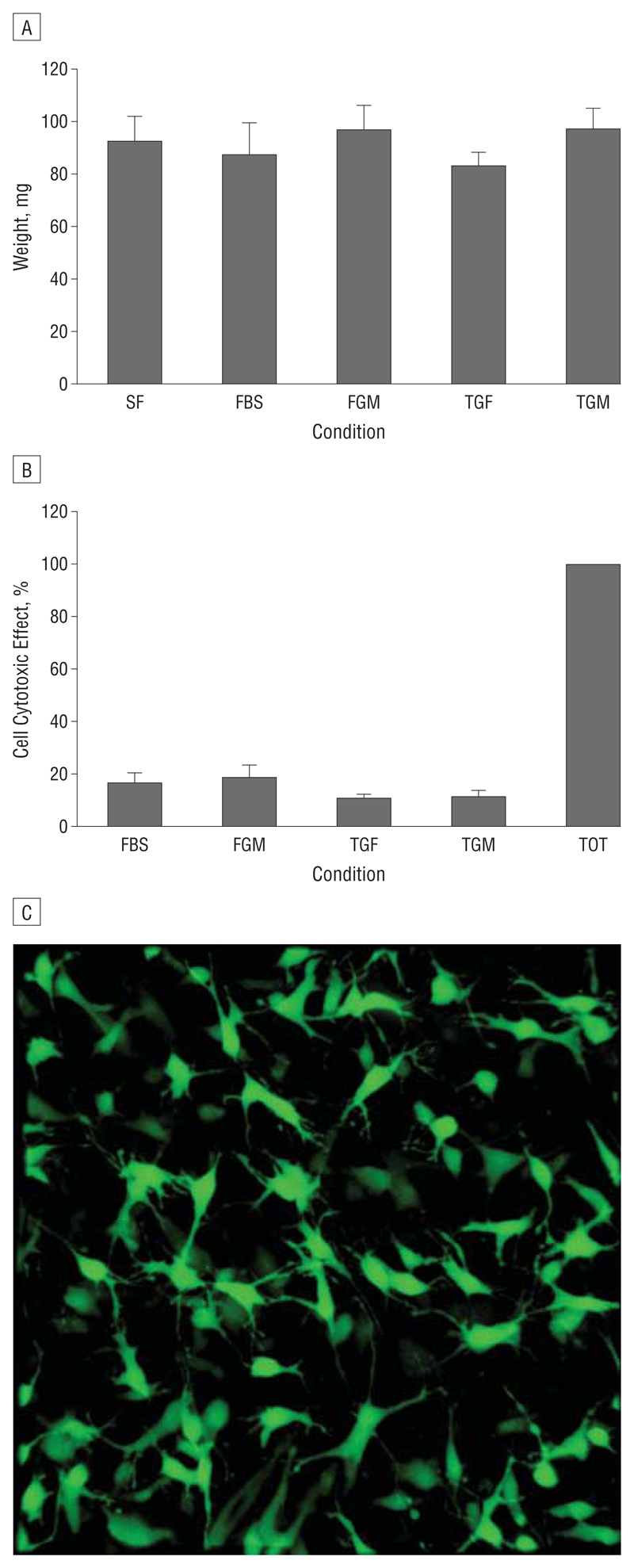
Fetal bovine serum dramatically triggered the contraction of gels populated with HTFs, with little or no contraction in SF conditions (Figure 3A). Human Tenon fibroblasts were less responsive to TGFβ2, as the number of cells per gel had to be increased 4 times to obtain an overall gel contraction matching that of FBS, but in both cases (FBS and TGFβ2) GM6001 completely blocked contraction, as expected.14 A novel system was devised to record contraction on a macroscopic level over the course of 4 weeks, which is approximately the time required for contraction in vivo.22 When porcine conjunctiva fragments were analyzed using the same stimuli, significant contraction was observed after a month in both cases (Figure 4), even though the tissue segments were perfectly homogeneous in size and weight at the start of the experiments (Figure 5A). In contrast, the fragments remained unchanged in SF conditions or in the presence of GM6001. No significant differences in cell cytotoxic effects were found in porcine samples with vs without GM6001 (Figure 5B), and live cells were readily visible following 5 weeks in culture with GM6001 (Figure 5C).
Figure 3.
In vitro and ex vivo contraction analysis. A and B, Fibroblast-populated collagen lattices (FPCL) were used to study contraction by human Tenon fibroblasts. The fetal bovine serum (FBS) induced contraction to approximately 90% of the original gel size and transforming growth factor β2 (TGFβ2) to approximately 75%. In both cases, GM6001 significantly inhibited contraction. C and D, The FBS induced contraction of porcine conjunctiva up to approximately 50% of the original tissue size and TGFβ2 to approximately 45%, and both are inhibited by GM6001. Significant differences between samples in FBS compared with FBS + GM can be seen from day 10 (P<.05), increasing to P<.01 on day 24. Differences between the means of contraction with TGFβ2 and TGF + GM were found to be significant from day 21 (P<.05). E and F, The TGFβ2 increased rabbit conjunctiva contraction to approximately 50%. Differences between the means of contraction with TGFβ2 and TGF + GM were found to be significant from day 14 (P< .05). Gel and tissue areas were expressed as the mean of triplicates, and data are expressed as the mean (SEM) of 5 independent experiments.
Figure 4.
The effects of different conditions on tissue area are compared in porcine segments at day 0 (left) and 28 days later (right). While serum-free Dulbecco modified Eagle medium (DMEM) had little or no effect on contraction, fetal bovine serum (FBS) and transforming growth factor β2 (TGFβ2) significantly increased contraction of the conjunctiva. This contraction is blocked by GM6001.
Figure 5.
Porcine conjunctiva tissue samples remain viable over time. A, Samples were weighed preceding each experiment to ensure that the sample size remained constant. No significant variations in tissue size were found between conditions. B, Cell cytotoxic effects were minimal for all conditions. GM6001 did not increase lactate dehydrogenase levels released into the extracellular medium surrounding the samples. Media samples collected throughout the 4-week period were measured for lactate dehydrogenase activity at different time points. The data were combined to give overall percentage lactate dehydrogenase release, as no differences between the time points were seen. C, Three-dimensional projection shows cells stained with the vital dye carboxyfluorescein diacetate following 4 weeks in culture in the presence of GM6001. Data represent the mean (SEM) (n=3). SF indicates serum free; FBS, fetal bovine serum; FGM, FBS + GM6001; TGF, transforming growth factor β2; TGM, TGF plus GM6001; and TOT, total lactate dehydrogenase release.
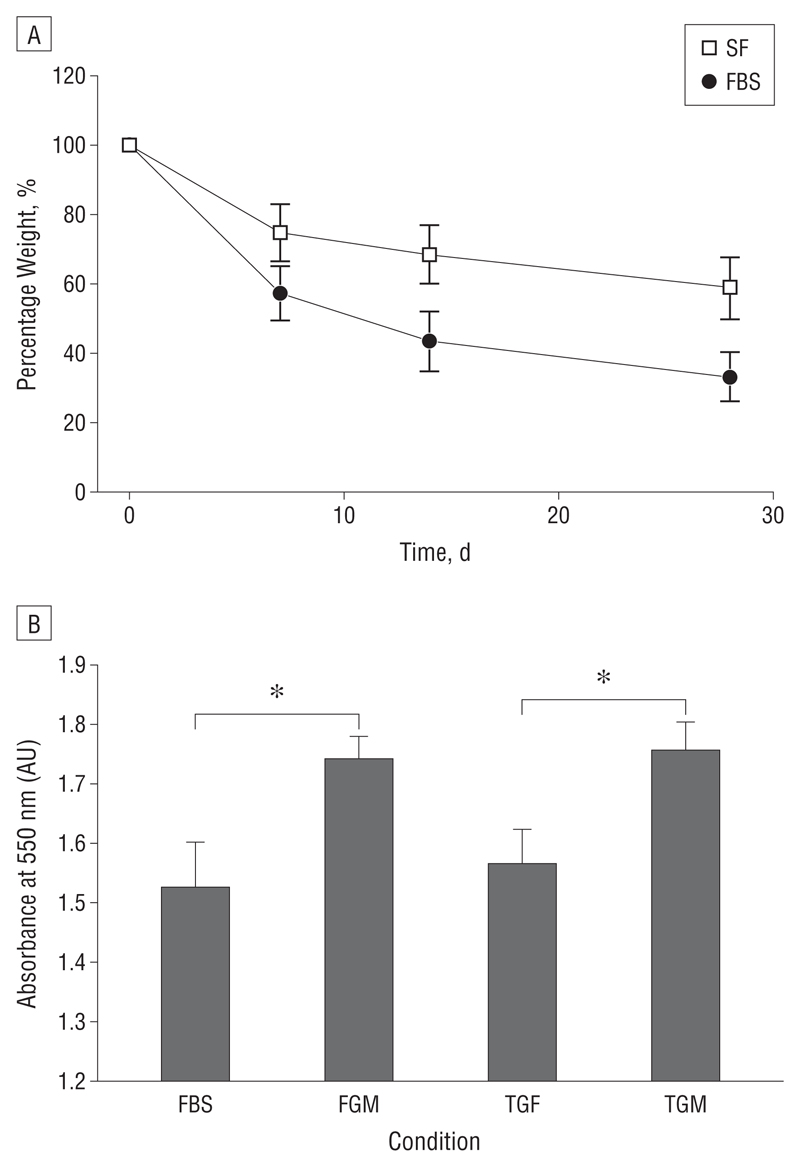
Significant differences were found between contraction kinetics of the rabbit and porcine conjunctiva (Figure 3C-F). Porcine conjunctiva contracted to approximately 50% in the presence of TGFβ2 and FBS (Figure 3C and D). Rabbit conjunctiva did not respond well to FBS, showing contraction curves similar to SF baseline levels of 25% (Figure 3E) but contracted at a higher initial rate in response to TGFβ2 (Figure 3F). In all cases, GM6001 significantly reduced contraction (Figure 3C and E), although it was not as efficient following TGFβ2 stimulation (Figure 3D and F). Baseline contraction in SF medium was similar for rabbit and porcine segments (25%-30%), suggesting that when nothing else is involved these pieces of tissue in different species behave in a similar manner. Tissue contraction was matched by a significant decrease in tissue weight (Figure 6A) and total protein concentration (Figure 6B), suggesting that loss of tissue mass contributed to the contraction process.
Figure 6.
Ex vivo segments of porcine conjunctive loose net tissue mass and decrease in total protein as they contract. A, Weight measurements were obtained weekly to monitor the mass over 4 weeks in the serum-free (SF) vs fetal bovine serum (FBS) conditions (n=4). B, The graph shows the absorbance values (AU, arbitrary units) of extracted Coomassie blue from these samples at day 28. Samples in the presence of GM6001 had a significantly higher concentration of total protein compared with samples without GM6001 in the media (*P<.05) (n=3). Data represent the mean (SEM). FGM indicates FBS + GM6001; TGF, transforming growth factor β2; and TGM, TGF plus GM6001.
Contraction Involves Specific Matrix Metalloproteinase–Mediated Collagen Degradation
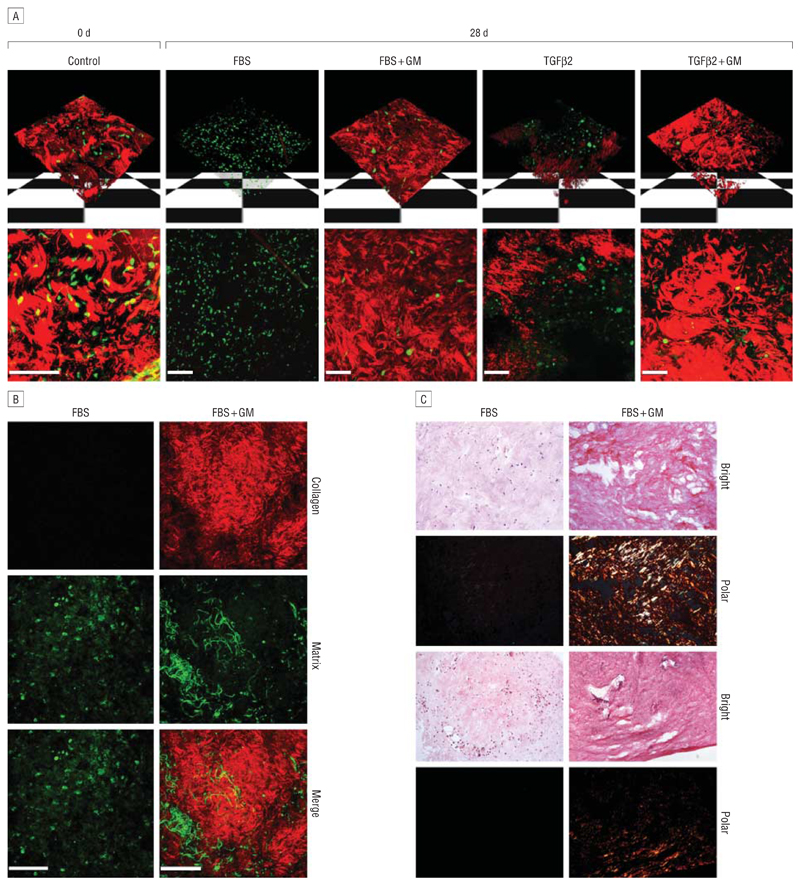
A combination of confocal and multiphoton microscopy was used to further investigate the organization of the conjunctival tissue during contraction. In freshly isolated live segments of porcine conjunctiva, a dense network of matrix fibrils could clearly be seen (Figure 7A) using confocal reflection (red), intermingled with numerous carboxyfluorescein diacetate–labeled cells (green). After 28 days in FBS, thick fibrils were completely absent, leaving only a background haze, suggesting that most fibers had been degraded. Segments in the presence of GM6001 (FBS + GM) showed a notable absence of fiber degradation, with numerous thick fibers. In line with the contraction results, porcine conjunctiva showed less degradation in the presence of TGFβ2 compared with FBS (Figure 3D), and GM6001 had slightly less of an inhibitory effect on TGFβ2-induced degradation compared with FBS-induced degradation. The correlation between matrix degradation and overall tissue contraction was even more striking in the rabbit samples, where the lack of contraction in response to FBS was matched by an absence of matrix degradation, while strong matrix degradation (completely reversed by GM6001 treatment) was observed in the TGFβ2-treated samples (Figures 3E and F and Figure 8).
Figure 7.
Matrix metalloproteinase inhibitor GM6001 (GM) prevents collagen fiber breakdown in porcine conjunctiva. A, The upper panel shows 3-dimensional reconstruction of z-stack confocal images, and the lower panel shows a flat projection of all sections (red, matrix; green, live cells). In fresh tissue (Control), thick masses of collagen fibers can clearly be seen. After 4 weeks in FBS or TGFβ2, fiber degradation is observed, which is significantly reduced in the presence of GM6001. Viable cells are present in the segments in all conditions. B, Second harmonic generation shows that FBS degrades collagen fibers (red), which is prevented in the presence of GM. The green panel represents the 2-photon autofluorescence of the sample, showing cells and other fibers of the connective tissue. C, Picrosirius red staining of porcine tissue samples after 4 weeks in culture. Brightfield images were obtained (Bright), showing total collagen (red-pink) in the tissue. A polarizing filter (Polar) was also used to monitor the birefringence of the thick collagen fibers (orange-yellow), which are detectable in samples that have been maintained in GM but are undetectable in those without GM. Shown are 2 representative fields for each condition. Scale bar, 100 μm.
Figure 8.
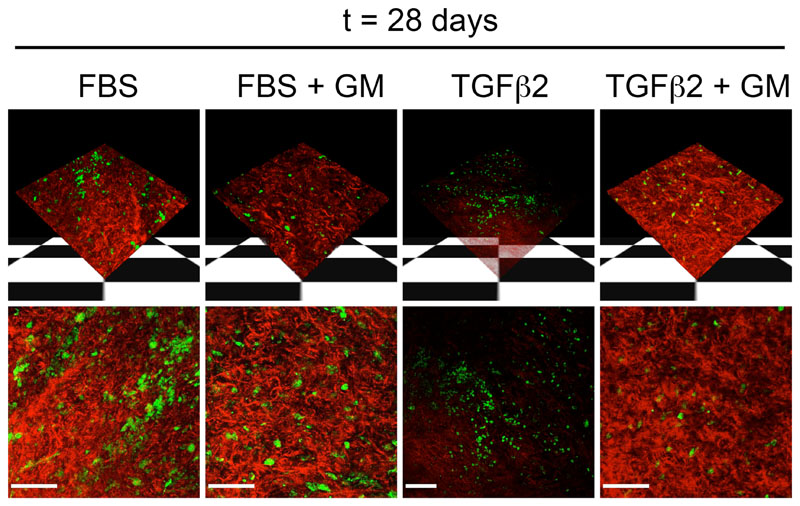
Matrix metalloproteinase inhibitor GM6001 (GM) prevents matrix degradation in rabbit conjunctiva. Images were take of live rabbit conjunctiva using reflection confocal microscopy (red) to observe matrix conditions at the end of the ex vivo contraction assay. Live cells (green) were visualized using the cell vital dye carboxyfluorescein diacetate. The upper panel shows 3-dimensional reconstruction of z-stack confocal images, and the lower panel shows a flat projection of all sections. After 4 weeks in fetal bovine serum (FBS), no fiber degradation is observed. However, fiber degradation is seen in tissue exposed to 10 ng/mL transforming growth factor β2 (TGFβ2), and this effect is inhibited by GM6001 (TGFβ2 + GM). Viable cells are present in the segments in all conditions. Bar indicates 100 μm.
Second harmonic generation was performed on a multiphoton system to specifically localize collagen fibers. Porcine conjunctiva in the presence of FBS with GM6001 presented a typical connective tissue profile, with collagen fibers (second harmonic generation [red]) representing most of the fibers detected (Figure 7B). By contrast, no collagen signal could be detected in contracted conjunctival fragments after 4 weeks in the presence of FBS, with the tissue consisting of a dense matrix of thin autofluorescent fibers and cells. This was confirmed by histologic analysis of the tissue following staining with picrosirius red to visualize collagen (Figure 7C). Brightfield images showed a much stronger collagen signal in the presence of GM6001. Similarly, when looking through a polarizing filter, the yelloworange birefringence that characterizes thick collagen fibers was clearly seen in GM6001-treated samples but was largely absent in samples maintained in FBS.
Comment
This study describes the first ex vivo model system to date that can be used for the assessment of conjunctival tissue contraction on a microscopic scale and a macroscopic scale. The model paves the way for future in vivo studies by providing a more “real-time” analysis of cell and matrix remodeling in a “living” complex tissue and by offering a means to directly monitor drug effects on a complex matrix in real time. Using this model, we have shown for the first time that growth factor stimulation can directly trigger intact tissue contraction, as predicted.11,23,24 It has also allowed us to identify a direct correlation between tissue contraction and specific degradation of the collagen component of the matrix, which could not be identified in the collagen gel models. The degradation and contraction were consistently prevented by the matrix metalloproteinase (MMP) inhibitor GM6001, suggesting that MMPs (particularly collagenases) have an important role in tissue contraction. Because MMPs have been shown to be essential in the wound-healing response25,26 and because several studies16,22,27 have focused on using MMP inhibitors to modulate the effects of postoperative scarring in the eye, our results suggest that collagen degradation through MMP activation may be a good target to control contraction and may provide an ideal model to study the process further.
Porcine conjunctiva was chosen for this study because of its similarity to the human eye in size and anatomy. We observed common features in the behavior of porcine tissue and HTFs in collagen matrices, as both responded more to serum stimulation than to TGFβ2, and in both cases GM6001 was more efficient in preventing contraction mediated by serum rather than by TGFβ2. In contrast, rabbit conjunctiva was unresponsive to serum but contracted well in the presence of TGFβ2, consistent with the strong effect of anti-TGFβ2 therapy in vivo in the rabbit model of glaucoma filtration surgery.17–19 These interspecies differences suggest that, while TGFβ2 may have an important role in rabbit conjunctiva contraction, porcine and human conjunctiva contraction may be driven by other factors, which could also partly explain why TGFβ2 antibody treatment was ineffective in human trials.19 Furthermore, our findings suggest that, although the GM6001 MMP inhibitor seems to be a promising tool in modulating tissue contraction in vivo, there may be differences in its efficiency depending on the tissue or species. Such differences may be due to variations in drug receptor expression or mechanisms of contraction, and results of recent studies suggest that porcine ocular tissue seems more similar to human tissue than rabbit tissue in structure and function28 and in receptor composition.29 Given that current in vivo studies for conjunctiva contraction are performed in rabbit models, it would be useful to have a comparative analysis of ex vivo tissue contraction in rabbit vs human systems. This validated model could be used to provide a better prediction of how rabbit in vivo studies may translate to the human. Therefore, future studies will examine the differences between species more closely and will particularly focus on the use of human tissue, which so far has had limitations of availability, ethical approval, and cost.
In summary, the present study introduces a novel ex vivo tissue contraction model that uses whole ex vivo segments of conjunctiva as a means of investigating the effects of potential drug therapies. This model provides more accurate data in terms of mimicking in vivo contraction, as it does not involve the removal of cells from their natural surroundings but instead uses a segment of intact tissue with its resident cells. Therefore, it is a more efficient and convenient way of obtaining data on intact tissue contraction, which will lessen the requirement for in vivo testing and will help better predict the outcome of potential antiscarring therapies in vivo.
Clinical Relevance.
Scarring leads to the failure of several ocular surgical procedures. This novel ex vivo model recapitulates tissue contraction (with kinetics close to that of in vivo scarring) and allows for a more physiological analysis of conjunctival scarring, which could better evaluate potential therapeutic targets.
Funding/Support
This work was supported by grants G0100195, G0100200, and G0801049 from the Medical Research Council (Drs Khaw and Bailly) and by the Royal Society (Dr Bailly). Laboratories and imaging facilities were supported by the Wellcome Trust and the Medical Research Council. Dr Dahlmann-Noor’s work was funded by a Wellcome Trust Research Training Fellowship, and studies by Drs Dahlmann-Noor and Khaw were also supported by Fight for Sight, the Helen Hamlyn Trust (in memory of Paul Hamlyn), Freemasons Grand Charity, and Moorfields Trustees, as well as by the National Institute for Health Research Biomedical Research Centre at Moorfields Eye Hospital and by the University College London Institute of Ophthalmology.
Footnotes
Financial Disclosure: Dr Khaw hold patents through his institution for the use of MMP inhibitors and novel formulations to prevent scarring and facilitate regeneration.
Online-Only Material
The videos are available at http://www.archophthalmol.com.
Additional Contributions: Christopher Thrasivoulou, PhD, performed second harmonic generation and Sumit Dhingra helped with tissue embedding and sectioning.
Contributor Information
Victoria E. Tovell, Departments of Cell Biology, National Institute for Health Research Biomedical Research Centre at Moorfields Eye Hospital, University College London Institute of Ophthalmology, London, England.
Maryse Bailly, Departments of Cell Biology, National Institute for Health Research Biomedical Research Centre at Moorfields Eye Hospital, University College London Institute of Ophthalmology, London, England.
References
- 1.Addicks EM, Quigley HA, Green WR, Robin AL. Histologic characteristics of filtering blebs in glaucomatous eyes. Arch Ophthalmol. 1983;101(5):795–798. doi: 10.1001/archopht.1983.01040010795021. [DOI] [PubMed] [Google Scholar]
- 2.Hitchings RA, Grierson I. Clinico pathological correlation in eyes with failed fistulizing surgery. Trans Ophthalmol Soc U K. 1983;103(pt 1):84–88. [PubMed] [Google Scholar]
- 3.Dahlmann AH, Mireskandari K, Cambrey AD, Bailly M, Khaw PT. Current and future prospects for the prevention of ocular fibrosis. Ophthalmol Clin North Am. 2005;18(4):539–559. doi: 10.1016/j.ohc.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Khaw PT, Doyle JW, Sherwood MB, Grierson I, Schultz G, McGorray S. Prolonged localized tissue effects from 5-minute exposures to fluorouracil and mitomycin C. Arch Ophthalmol. 1993;111(2):263–267. doi: 10.1001/archopht.1993.01090020117035. [DOI] [PubMed] [Google Scholar]
- 5.Crowston JG, Akbar AN, Constable PH, Occleston NL, Daniels JT, Khaw PT. Antimetabolite-induced apoptosis in Tenon’s capsule fibroblasts. Invest Ophthalmol Vis Sci. 1998;39(2):449–454. [PubMed] [Google Scholar]
- 6.Mietz H, Addicks K, Bloch W, Krieglstein GK. Long-term intraocular toxic effects of topical mitomycin C in rabbits. J Glaucoma. 1996;5(5):325–333. [PubMed] [Google Scholar]
- 7.Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol. 1994;124(4):401–404. doi: 10.1083/jcb.124.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bell E, Ivarsson B, Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci U S A. 1979;76(3):1274–1278. doi: 10.1073/pnas.76.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Occleston NL, Alexander RA, Mazure A, Larkin G, Khaw PT. Effects of single exposures to antiproliferative agents on ocular fibroblast-mediated collagen contraction. Invest Ophthalmol Vis Sci. 1994;35(10):3681–3690. [PubMed] [Google Scholar]
- 10.Even-Ram S, Yamada KM. Cell migration in 3D matrix. Curr Opin Cell Biol. 2005;17(5):524–532. doi: 10.1016/j.ceb.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Sawhney RK, Howard J. Slow local movements of collagen fibers by fibroblasts drive the rapid global self-organization of collagen gels. J Cell Biol. 2002;157(6):1083–1091. doi: 10.1083/jcb.200203069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandran PL, Barocas VH. Microstructural mechanics of collagen gels in confined compression: poroelasticity, viscoelasticity, and collapse. J Biomech Eng. 2004;126(2):152–166. doi: 10.1115/1.1688774. [DOI] [PubMed] [Google Scholar]
- 13.Dallon JC, Ehrlich HP. A review of fibroblast-populated collagen lattices. Wound Repair Regen. 2008;16(4):472–479. doi: 10.1111/j.1524-475X.2008.00392.x. [DOI] [PubMed] [Google Scholar]
- 14.Dahlmann-Noor AH, Martin-Martin B, Eastwood M, Khaw PT, Bailly M. Dynamic protrusive cell behaviour generates force and drives early matrix contraction by fibroblasts. Exp Cell Res. 2007;313(20):4158–4169. doi: 10.1016/j.yexcr.2007.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cordeiro MF, Bhattacharya SS, Schultz GS, Khaw PT. TGF-β1, -β2, and -β3 in vitro: biphasic effects on Tenon’s fibroblast contraction, proliferation, and migration. Invest Ophthalmol Vis Sci. 2000;41(3):756–763. [PubMed] [Google Scholar]
- 16.Daniels JT, Cambrey AD, Occleston NL, et al. Matrix metalloproteinase inhibition modulates fibroblast-mediated matrix contraction and collagen production in vitro. Invest Ophthalmol Vis Sci. 2003;44(3):1104–1110. doi: 10.1167/iovs.02-0412. [DOI] [PubMed] [Google Scholar]
- 17.Mead AL, Wong TT, Cordeiro MF, Anderson IK, Khaw PT. Evaluation of anti-TGF-β2 antibody as a new postoperative anti-scarring agent in glaucoma surgery. Invest Ophthalmol Vis Sci. 2003;44(8):3394–3401. doi: 10.1167/iovs.02-0978. [DOI] [PubMed] [Google Scholar]
- 18.Cordeiro MF, Gay JA, Khaw PT. Human anti-transforming growth factor-β2 antibody: a new glaucoma anti-scarring agent. Invest Ophthalmol Vis Sci. 1999;40(10):2225–2234. [PubMed] [Google Scholar]
- 19.Khaw P, Grehn F. Hollo’ G, et al; CAT-1520102 Trabeculectomy Study Group. A phase III study of subconjunctival human anti-transforming growth factor β(2) monoclonal antibody (CAT-152) to prevent scarring after first-time trabeculectomy. Ophthalmology. 2007;114(10):1822–1830. doi: 10.1016/j.ophtha.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 20.Vanni S, Lagerholm BC, Otey C, Taylor DL, Lanni F. Internet-based image analysis quantifies contractile behavior of individual fibroblasts inside model tissue. Biophys J. 2003;84(4):2715–2727. doi: 10.1016/s0006-3495(03)75077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Theodossiou TA, Thrasivoulou C, Ekwobi C, Becker DL. Second harmonic generation confocal microscopy of collagen type I from rat tendon cryosections. Biophys J. 2006;91(12):4665–4677. doi: 10.1529/biophysj.106.093740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong TT, Mead AL, Khaw PT. Matrix metalloproteinase inhibition modulates postoperative scarring after experimental glaucoma filtration surgery. Invest OphthalmolVisSci. 2003;44(3):1097–1103. doi: 10.1167/iovs.02-0366. [DOI] [PubMed] [Google Scholar]
- 23.Meshel AS, Wei Q, Adelstein RS, Sheetz MP. Basic mechanism of threedimensional collagen fibre transport by fibroblasts. Nat Cell Biol. 2005;7(2):157–164. doi: 10.1038/ncb1216. [DOI] [PubMed] [Google Scholar]
- 24.Sawhney RK, Howard J. Molecular dissection of the fibroblast-traction machinery. Cell Motil Cytoskeleton. 2004;58(3):175–185. doi: 10.1002/cm.20004. [DOI] [PubMed] [Google Scholar]
- 25.Ravanti L, Kähäri VM. Matrix metalloproteinases in wound repair [review] Int J Mol Med. 2000;6(4):391–407. [PubMed] [Google Scholar]
- 26.Wong TT, Sethi C, Daniels JT, Limb GA, Murphy G, Khaw PT. Matrix metalloproteinases in disease and repair processes in the anterior segment. Surv Ophthalmol. 2002;47(3):239–256. doi: 10.1016/s0039-6257(02)00287-4. [DOI] [PubMed] [Google Scholar]
- 27.Wong TT, Mead AL, Khaw PT. Prolonged antiscarring effects of ilomastat and MMC after experimental glaucoma filtration surgery. Invest Ophthalmol Vis Sci. 2005;46(6):2018–2022. doi: 10.1167/iovs.04-0820. [DOI] [PubMed] [Google Scholar]
- 28.Nicoli S, Ferrari G, Quarta M, et al. Porcine sclera as a model of human sclera for in vitro transport experiments: histology, SEM, and comparative permeability. Mol Vis. 2009;15:259–266. [PMC free article] [PubMed] [Google Scholar]
- 29.Turner HC, Alvarez LJ, Candia OA, Bernstein AM. Characterization of serotonergic receptors in rabbit, porcine and human conjunctivae. Curr Eye Res. 2003;27(4):205–215. doi: 10.1076/ceyr.27.4.205.16600. [DOI] [PubMed] [Google Scholar]