Abstract
The deficiency of micronutrients, including vitamins and minerals, is estimated to affect two billion people worldwide and can have devastating immediate and long-term consequences. Major causes range from inadequate micronutrient consumption mostly owing to a lack of dietary diversity, to poor nutrient absorption in the gastrointestinal tract as a result of clinical or pathological conditions. Recent studies in model organisms and humans demonstrated that intestinal microbiota plays an important role in the de novo biosynthesis and bioavailability of several micronutrients and might be a major determinant of human micronutrient status. Here, we address the importance of the gut microbiome for maintaining the balance of host vitamins and minerals and explore its potential therapeutic benefits and implications on human health.
Keywords: Microbiota, Micronutrient deficiency, Vitamins, Minerals, Bioavailability
Introduction
Vitamins and minerals, collectively termed micronutrients, are crucial for human health. These micronutrients are essential core regulators of fundamental biosynthetic cellular reactions such as immune, and energy functions [1], as well as growth, bone health, fluid balance, and other biological processes [2]. Micronutrient deficiency is considered a global health issue, as it is associated with severe health problems, particularly in children where it leads to poor physical and mental development and increased susceptibility to pathogen infections, development of allergies, and inflammatory diseases. Deficiency of micronutrients, such as that of vitamin D, may contribute to disordered immune response, both in children and adults, resulting in a higher risk of autoimmune disease development [3,4]. Minerals, particularly zinc, play a critical role in B and T cell-dependent immune activities [5]. Moreover, multiple studies show shreds of evidence that micronutrient deficiency may contribute to the progression of some human cancers [6]. Micronutrients also modulate the abundance and diversity of the gut microbiota resulting in beneficial or detrimental outcomes to the host [7,8].
Humans cannot synthesize all required micronutrients, which therefore need to be acquired exogenously from the three major resources of (i) dietary components, (ii) oral supplements, or (iii) synthesis by commensal gut bacteria. As a large percentage of the population does not meet recommended intakes of micronutrients, oral supplementation of micronutrients and food fortification programs have been implemented worldwide. Despite their effectiveness and positive impacts [9], a considerable number of studies have recently raised serious concerns for the negative health consequences of certain micronutrient supplements. For example, it has been shown that iron supplementation in individuals with an iron overload disease, such as hemochromatosis, iron supplementation/fortification, can lead to iron overload and liver disease, increased abundance of inflammation-associated bacterial species, and markers in the intestine [10], as well as higher risks of intestinal disorders such as colorectal cancer [10,11]. Moreover, folic acid (vitamin B9) supplementation was associated with adverse health outcomes including zinc deficiency caused by impaired absorption in the intestine, neurological damage owing to its role in masking the signs of B12 (cobalamin) deficiency, and increased risk of colorectal cancer [12].
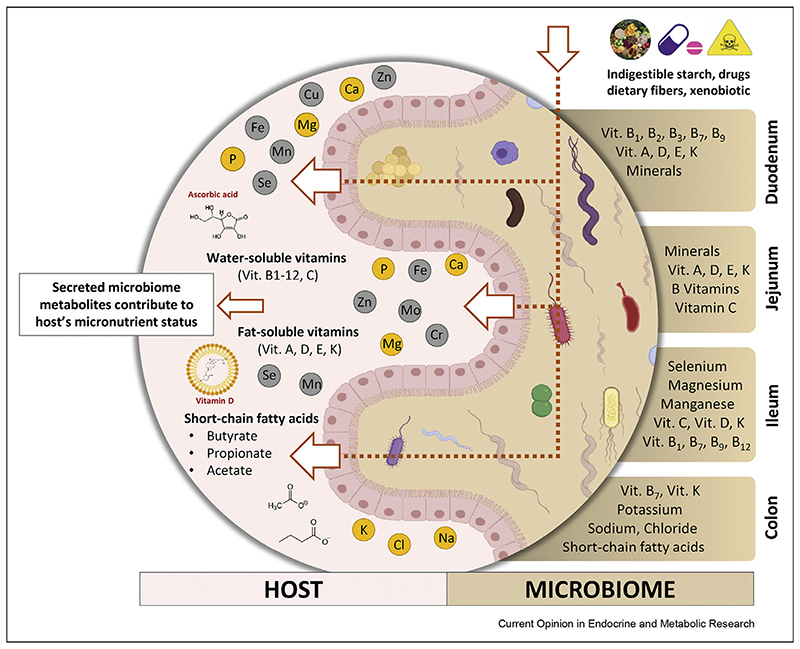
Micronutrient bioavailability is the fraction of a micronutrient that is available for use and storage in the body [13–15]. Micronutrients use various, and in some cases, specific absorption routes and mechanisms that can be both passive and active [16,17]. For example, absorption of both vitamin C and vitamin B7 (biotin) is mediated by Na + -dependent carriers, and vitamin B9 (folate) uses three folate-specific carriers. On the other hand, absorption of vitamins A and D occurs by passive diffusion in the small intestine (Figure 1). However, detailed absorption mechanisms and involved carriers of many micronutrients pend further research [16].
Figure 1. Micronutrient interexchange between the gut microbiota and host.
Differences in the physicochemical properties of various sections of the gastrointestinal tract, together with the presence of site-specific receptors, enable absorption of different vitamins and minerals along the tract. The colonization of each different section by different microorganisms can impact the local environment and thereby positively or negatively influence the bioavailability of micronutrients.
Commensal gut bacteria supply their host with essential nutrients and are the least explored resource for acquiring micronutrients. Gut microbiota is considered an effective bioreactor in the human intestinal tract, transforming various compounds into beneficial or harmful metabolites, thus having a crucial role in their bioavailability [18]. Although many efforts in microbiome research have been directed toward discovering effective means for modulating the microbiome to improve health, the contribution of gut microbes in the biosynthesis, uptake, absorption, and bioavailability of micronutrients remains less well studied. With the advances of meta-genomics technologies in microbiome research, there has been an increasing interest in understanding the contribution of different bacteria to human micronutrient status. In this review, we primarily focus on the in vivo studies and clinical trials addressing the association between bacterial classes/families with the host micronutrient balance, the microbiota role in biosynthesis and bioavailability of a given micronutrient, and the potential therapeutic benefits and implications of this interplay on human health.
Gut microbiota and micronutrients
The micronutrient–microbiome axis is bidirectional. On one hand, microbes in the gut are consumers of micronutrients for their growth and functioning. The host nutrition and micronutrient supplementation largely impact the gut microbiota composition and function (Figure 1). In particular, supplementation of vitamin A [19], vitamin C [20], vitamin B12 [21], and vitamin D [22] contribute to changes in the composition of the gut microbiota by promoting colonization of several beneficial species from the Bifidobacterium, Lactobacillus, and Roseburia genera. Assessing the effect of mineral deficiency or supplementation on the gut microbiota is an emerging field [23], and it has been shown that iron [24], calcium [25], zinc [26], and magnesium [27] supplementation modulate the gut microbiome (reviewed in the study by Yang et al. [8]).
On the other hand, the gut microbiota produces significant quantities of a wide range of vitamins, notably vitamin K and B group vitamins, and facilitates uptake and absorption of minerals such as iron and calcium. The Human Microbiome Project [28] provided high-resolution portrayal of the human gut microbiota, which enabled a wealth of in vitro studies evaluating the bacterial metabolic capabilities, including micronutrient biosynthesis, uptake, absorption, and secretion capabilities of some of the representative strains. Engevik et al. [29] assessed the folate (vitamin B9) biosynthesis capabilities of 512 gastrointestinal strains from six key phyla of the human microbiome (Actinobacteria, Bacteroidetes, Firmicutes, Proteobacteria, Fusobacteria, and Verrucomi crobia) and found that only 13% of the investigated strains, all belonging to Proteobacteria, encompass the required genes for a complete de novo folate biosynthesis. Another 39% of the strains (mostly from Firmicutes, Actinobacteria, and Verrucomicrobia) had the partial genetic capacity to synthesize folates. This group of bacteria required pABA, a biosynthesis pathway intermediate that can be obtained from the diet or other intestinal microbes, to completely generate folate de novo. These findings suggest a close cooperation amongst intestinal microbes to provide the required folate for both the host and the microbiome metabolic activities. Another in vitro study by González et al. [30] addressed the mechanisms by which probiotic bacteria increase iron absorption in the host. Fe(III) must be reduced to Fe(II) to be properly absorbed in the gastrointestinal tract. The authors demonstrated that Lactobacillus fermentum, one of the main probiotics of the microbiota, exhibits a remarkably high ferric-reducing activity. They also found that excretion of p-hydroxyphenyllactic acid by L. fermentum results in increasing Fe(II) bioavailability and its uptake by enterocytes. These in vitro studies advance our understanding of the gut microbiota contributions to the overall host micronutrient status. Nevertheless, considering the complexity of the human gut microbiota and its metabolic interactions with the host, in vivo studies are essential for probiotics-based nutritional counseling aiming to improve the host micronutrient states. The in vivo studies investigating the microbiome–micronutrient axis are reviewed in the following sections.
Vitamins
Human gut commensals such as Bacteroides, Enterococcus, and Bifidobacterium can synthesize vitamin K and most water-soluble B-vitamins de novo. Magnúsdóttir et al. [31] systematically assessed the in silico biosynthetic capability of common human gut bacteria for the production of eight B-vitamins and showed that 40–65% of each of those vitamins was produced by the studied gut microbes. To be available to the host, however, the bacterial de novo synthesis of micronutrients must take place upstream of their dedicated intestinal absorption zone. For example, because cobalamin is only absorbed in the ileum, B12-producing colonic bacteria will unlikely contribute to increasing the bioavailability of this vitamin for the host, except in the case of coprophagy seen in rodents and nonhuman primates [32]. Recent in vivo studies and clinical trials shed light on the role of microbiota in the host vitamin balance (Table 1). Using C. elegans, Maynard et al. [12] found that Escherichia coli assists the metabolism of vitamin B9 (folic acid) by increasing its bioavailability, through uptake of the breakdown product (PABA-glu), and via de novo synthesis of tetrahydrofolate, demonstrating that bacteria play an important role in the effective metabolism of micronutrients. In addition, E. coli acts as an indispensable conduit of vitamin B12 for the host, by scavenging exogenous vitamin B12 (cobalamine) through the tonB siderophore [12]. Several members of the Firmicutes phylum identified using 16S ribosomal RNA sequencing of human stool samples, such as Clostridia class, Clostridia order, and part of the Ruminococcacus, Coprococcus, Mogibacterium, Blautia genera, were positively associated with the vitamin D levels in serum [33] (Table 1). Butyrate-producing bacteria were linked to an increased vitamin D receptor protein expression in a human cohort. This is in line with an earlier study in mice [34], which demonstrated a relationship between the vitamin D receptor and gut microbiota, important for maintaining intestinal homeostasis and for the pathophysiology of inflammatory bowel disease.
Table 1. In vivo and clinical studies demonstrating association between the gut microbiota and host micronutrients.
| Vitamins | Studied in | Commensal bacteria | References |
|---|---|---|---|
| Vitamin B9 (folic acid)and vitamin B12 (cobalamine) | C. elegans | Escherichia coli | C. Maynard et al. (2020) [12] |
| Vitamin D | Humans |
Ruminococcacus genus
Coprococcus genus Mogibacterium genus Blautia genus |
R.L. Thomas et al. (2020) [33] |
| Humans | Lactobacillus reuteri | M.L. Jones etal. (2013) [49] | |
| Mice | Colonic butyrate producers | S. Wu et al. (2015) [34] | |
| Vitamin E | Mice | Antibiotic-mediated microbiota depletion | L. Ran et al. (2019) [35] |
| Vitamin C | Mice | Gram-negative bacteria overgrowth | V.S. Subramanian et al. (2018) [36] |
| Minerals | |||
| Iron | Humans | Lactobacillus plantarum | N. Scheers et al. (2016) [40] |
| Humans | Lactobacilli genus | R. Balamurugan et al. (2010) [39] | |
| C. elegans | Escherichia coli | B. Qi et al. (2018) [42] A K. Sewell (2018) [41] |
|
| Calcium | Rats |
Bacteroides genus
Butyricicoccus genus Oscillibacter genus Dialister genus |
C.M. Weaver (2015) [44] |
| Humans |
Bifidobacterium lactis
Lactobacillus acidophilus |
Z. Asemi et al. (2013) [46] | |
| Phosphate | Broilers |
Enterococcus faecium
Eubacterium genera Ruminococcaceae genera |
W. Wang et al. (2020) [47] |
Microbiota can also be negatively associated with vitamin bioavailability. A recent mouse study [35] suggested an increased bioavailability of vitamin E after an antibiotic treatment, likely owing to increased absorption of vitamin E, or its decreased degradation by gut microbes after microbiota depletion. In contrast, Subramanian et al. [36] have shown that an increase in the circulating lipopolysaccharide, which is produced by the microbiota, inhibits sodium-dependent transport of ascorbic acid within the intestine, and in some cases, could lead to vitamin C deficiency by reducing its absorption.
Minerals
The gut microbiota affects the mineral metabolism by (i) directly influencing mineral absorption in the gastrointestinal (GI) tract during digestion and by (ii) producing an array of enzymes, which are exclusive for the colonic microbes and that help releasing minerals from foods [37]. Such are the bacterial phytases, which catalyze hydrolysis of the phytic acid found in many plant tissues, releasing useable forms of minerals such as calcium, magnesium, and phosphate [38].
In iron-deficient women, the gut microbiome is relatively depleted of genus Lactobacilli compared with controls [39]. Although it is not clear whether less abundant Lactobacilli contribute to iron deficiency, or the iron deficiency instead results in reduction of the Lactobacilli genus, further studies in humans demonstrated that Lactobacillus plantarum increases the amount of hydrated ferric iron Fe(III) via lactic fermentation, which leads to enhanced iron absorption [40]. Moreover, two studies conducted in C. elegans showed that secretion of the bacterially produced siderophore enterobactin facilitates host uptake of iron by promoting mitochondrial iron uptake [41,42]. This finding introduced an unexpected benefit from commensal bacteria to the host by uncovering a distinct beneficial role of a bacteria-generated molecule in promoting the host’s iron homeostasis.
The dynamic relationship between the gut microbiota and mineral bioavailability is well illustrated in the bone field. Increased vitamin D intake can enhance the calcium active absorption, in part mediated by vitamin D-regulated calcium-binding proteins, calbindin D9k [43]. However, under conditions of low calcium intake, microbiota plays an important role in calcium bioavailability. Weaver et al. [44] showed that prebiotics, which alter the gut microbiome in genera known to enhance short-chain fatty acid production (such as Bifidobacteria, Lactobacilli, Eubacterium, and others), correlated with increased calcium absorption (in humans and animal models) and bone density and strength (animal models) (Table 1). Mineo et al. [45] showed an increase in the production of short-chain fatty acids and organic acids by dietary fiber fermenters, resulting in a decrease of the cecal pH. This in turn led to solubilization of calcium and increase in its passive absorption in the large intestine of rats. Similarly, Asemi et al. [46] found that consumption of probiotic yogurt containing Bifidobacterium lactis and Lactobacillus acidophilus in pregnant women resulted in maintaining serum calcium levels compared with subjects consuming conventional yogurt. Wang et al. [47] also showed that Enterococcus faecium treatment increases the mRNA expression of the IIb sodium-dependent phosphate cotransporter (NaP-IIb) and enhances bone’s phosphate content. E. faecium supplementation also induced changes in the microbiota by promoting development of butyrate producers from the genus Eubacterium and family Ruminococcaceae. Although this is in line with several other studies demonstrating the microbiota importance in preventing bone loss [23,48], it further demonstrates that microbiota may regulate bone via various mechanisms, including micronutrient bioavailability.
Gut microbiota as sustainable therapeutic targets for improving host micronutrient status
The composition of the human gut microbiome largely contributes to the host’s micronutrient status. The aforementioned studies demonstrate that several micronutrient deficiencies could be positively or negatively associated with gut microbiota. Therefore, an alternative solution for increasing the micronutrient bioavailability could be applied by correcting the microbiome (in the case of the pathologic role of gut microbiota) or by promoting a specific microbiome (in the case of the positive contribution of gut microbiota), thereby addressing the underlying cause of the problem rather than its symptoms. Further research should explore the actions of specific bacterial species and their effects on improving or preventing micronutrient deficiency.
Similarly, a better understanding of the underlying mechanisms by which intestinal microbiota impacts host micronutrient uptake and absorption would enable postbiotic approaches for tailoring the micronutrient availability relative to the host demands. In this light, it would be interesting to assess how endogenous bacterially derived micronutrients contribute to the host micronutrient status compared to exogenous micronutrients provided by the diet and supplementations. Such studies would help in developing physiological and effective interventions against micronutrient deficiency.
Acknowledgements
This work is part of a project that has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (ERC Consolidator Grant agreement No. 815962, Healthybiota), and from the Clayton Foundation for biomedical research.
Footnotes
Conflict of interest statement
Nothing declared.
References
Papers of particular interest, published within the period of review, have been highlighted as:
-
*
of special interest
-
**
of outstanding interest
- 1.Yoshii K, Hosomi K, Sawane K, Kunisawa J. Front Nutr. Vol. 6. Frontiers Media S.A; Apr 17, Metabolism of dietary and microbial vitamin b family in the regulation of host immunity; p. 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huskisson E, Maggini S, Ruf M. The role of vitamins and minerals in energy metabolism and well-being. J Int Med Res. 2007;35 doi: 10.1177/147323000703500301. [DOI] [PubMed] [Google Scholar]
- 3.Correale J, Ysrraelit MC, Gaitn MI. Immunomodulatory effects of Vitamin D in multiple sclerosis. Brain. 2009;132 doi: 10.1093/brain/awp033. [DOI] [PubMed] [Google Scholar]
- 4.Prietl B, Treiber G, Pieber TR, Amrein K. Vitamin D and immune function. Nutrients. 2013;5 doi: 10.3390/nu5072502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hojyo S, Fukada T. Roles of zinc signaling in the immune system. J Immunol Res. 2016;2016 doi: 10.1155/2016/6762343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson IT. Micronutrients and cancer. Proc Nutr Soc. 2004;63 doi: 10.1079/pns2004389. [DOI] [PubMed] [Google Scholar]
- 7.Biesalski HK. Nutrition meets the microbiome: micronutrients and the microbiota. Ann N Y Acad Sci. 2016 May;1372:53–64. doi: 10.1111/nyas.13145. [DOI] [PubMed] [Google Scholar]
- 8.Yang Q, Liang Q, Balakrishnan B, Belobrajdic DP, Feng QJ, Zhang W. Nutrients. Vol. 12. MDPI AG; 2020. Jan, Role of dietary nutrients in the modulation of gut microbiota: a narrative review; p. 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tam E, Keats EC, Rind F, Das JK, Bhutta ZA. Nutrients. Vol. 12. MDPI AG; Feb 01, Micronutrient supplementation and fortification interventions on health and development outcomes among children under-five in low-and middleincome countries: a systematic review and meta-analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rescigno M. Science. Vol. 368. American Association for the Advancement of Science; Apr 10, The ‘iron will’ of the gut; pp. 129–130. [Google Scholar]
- 11.Yilmaz B, Li H. Pharmaceuticals. Vol. 11. MDPI AG; Dec 01, Gut microbiota and iron: the crucial actors in health and disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maynard C, Weinkove D. Genes Nutr. Vol. 15. BioMed Central Ltd; Mar 05, Bacteria increase host micronutrient availability: mechanisms revealed by studies in C. elegans; p. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hambidge KM. Am J Clin Nutr. Vol. 91. Oxford Academic; May 01, Micronutrient bioavailability: dietary reference intakes and a future perspective; pp. 1430S–1432S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibson RS. The role of diet- and host-related factors in nutrient bioavailability and thus in nutrient-based dietary requirement estimates. Food Nutr Bull. 2007 Dec 1;28 doi: 10.1177/15648265070281S108. [DOI] [PubMed] [Google Scholar]
- 15.Melse-Boonstra A. Frontiers in Nutrition. Vol. 7. Frontiers Media S.A; Jul 24, Bioavailability of micronutrients from nutrient-dense whole foods: zooming in on dairy, vegetables, and fruits; p. 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiela PR, Ghishan FK. Best Pract Res Clin Gastroenterol. Vol. 30. Bailliere Tindall Ltd; Apr 01, Physiology of intestinal absorption and secretion; pp. 145–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graaf SFJ, Bindels RJ, Hoenderop JGJ. Physiology of epithelial Ca2+ and Mg2+ transport. Rev Physiol Biochem Pharmacol. 2007;158:77–160. doi: 10.1007/112_2006_0607. [DOI] [PubMed] [Google Scholar]
- 18.Zhang F, et al. Bioavailability based on the gut microbiota: a new perspective. Microbiol Mol Biol Rev. 2020 Apr;84 doi: 10.1128/MMBR.00072-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lv Z, et al. Vitamin A deficiency impacts the structural segregation of gut microbiota in children with persistent diarrhea. J Clin Biochem Nutr. 2016;59 doi: 10.3164/jcbn.15-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li L, Krause L, Somerset S. Associations between micronutrient intakes and gut microbiota in a group of adults with cystic fibrosis. Clin Nutr. 2017;36 doi: 10.1016/j.clnu.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 21.Degnan PH, Barry NA, Mok KC, Taga ME, Goodman AL. Human gut microbes use multiple transporters to distinguish vitamin B 12 analogs and compete in the gut. Cell Host Microbe. 2014;15 doi: 10.1016/j.chom.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanhere M, et al. Bolus weekly Vitamin D3 supplementation impacts gut and airway microbiota in adults with cystic fibrosis: a double-blind, randomized, placebo-controlled clinical trial. J Clin Endocrinol Metab. 2018;103 doi: 10.1210/jc.2017-01983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skrypnik K, Suliburska J. Association between the gut microbiota and mineral metabolism. J Sci Food Agric. 2018 May;98:2449–2460. doi: 10.1002/jsfa.8724. [DOI] [PubMed] [Google Scholar]
- 24.Rusu IG, et al. Nutrients. Vol. 12. MDPI AG; Jul 01, “Iron supplementation influence on the gut microbiota and probiotic intake effect in iron deficiency—a literature-based review; pp. 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaplin A, Parra P, Laraichi S, Serra F, Palou A. Calcium supplementation modulates gut microbiota in a prebiotic manner in dietary obese mice. Mol Nutr Food Res. 2016;60 doi: 10.1002/mnfr.201500480. [DOI] [PubMed] [Google Scholar]
- 26.Zackular JP, et al. Dietary zinc alters the microbiota and decreases resistance to Clostridium difficile infection. Nat Med. 2016;22 doi: 10.1038/nm.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winther G, et al. Dietary magnesium deficiency alters gut microbiota and leads to depressive-like behaviour. Acta Neuropsychiatr. 2015;27 doi: 10.1017/neu.2015.7. [DOI] [PubMed] [Google Scholar]
- 28.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449 doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engevik MA, et al. Microbial metabolic capacity for intestinal folate production and modulation of host folate receptors. Front Microbiol. 2019;10 doi: 10.3389/fmicb.2019.02305. [*Here authors investigate the contribution of microbial folate to overall host folate status by examining the folate bioynthesis capability of a large cohort of human intestinal microbes in vitro The work suggests www.sciencedirect.com Current Opinion in Endocrine and Metabolic Research 2021, 20:100285 that probiotics-based approches may provide supplemental sources of folate for preventing folate deficiency] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.González A, Gálvez N, Martín J, Reyes F, Pérez-Victoria I, Dominguez-Vera JM. Identification of the key excreted molecule by Lactobacillus fermentum related to host iron absorption. Food Chem. 2017 doi: 10.1016/j.foodchem.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Magnúsdóttir S, Ravcheev D, de Crécy-Lagard V, Thiele I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front Genet. 2015 Apr;6:148. doi: 10.3389/fgene.2015.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rowley CA, Kendall MM. “To B12 or not to B12 : five questions on the role of cobalamin in host-microbial interactions. PLoS Pathog. 2019 Jan;15:e1007479. doi: 10.1371/journal.ppat.1007479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas RL, et al. Vitamin D metabolites and the gut microbiome in older men. Nat Commun. 2020 Dec;11:1–10. doi: 10.1038/s41467-020-19793-8. [**Large scale clinical study on the association of the gut microbiota and micronutrients status of the host, showing possitive/negative associations between certain bacterial phyla and the serum vitamin D levels in men] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu S, et al. Intestinal epithelial vitamin D receptor deletion leads to defective autophagy in colitis. Gut. 2015;64:1082–1094. doi: 10.1136/gutjnl-2014-307436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ran L, Liu AB, Lee MJ, Xie P, Lin Y, Yang CS. Effects of anti biotics on degradation and bioavailability of different vitamin E forms in mice. Biofactors. 2019 doi: 10.1002/biof.1492. [*This study investigates the potential effect of bacteria on the vitamin E level in the host using microbiota depletion strategies. They reported an increased bioavailability of vitamin E in antibiotics-treated mice, which could be explianed either by the increased absorption of vitamin E, or its decreased degradation by gut microbes] [DOI] [PubMed] [Google Scholar]
- 36.Subramanian VS, Sabui S, Moradi H, Marchant JS, Said HM. Inhibition of intestinal ascorbic acid uptake by lipopolysac charide is mediated via transcriptional mechanisms. Biochim Biophys Acta - Biomembr. 2018 doi: 10.1016/j.bbamem.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bielik V, Kolisek M. Bioaccessibility and bioavailability of minerals in relation to a healthy gut microbiome. Int J Mol Sci. 2021;22:6803. doi: 10.3390/ijms22136803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bohn L, Josefsen L, Meyer AS, Rasmussen SK. Quantitative analysis of phytate globoids isolated from wheat bran and characterization of their sequential dephosphorylation by wheat phytase. J Agric Food Chem. 2007;55:7547–7552. doi: 10.1021/jf071191t. [DOI] [PubMed] [Google Scholar]
- 39.Balamurugan R, Mary RR, Chittaranjan S, Jancy H, Shobana Devi R, Ramakrishna BS. Low levels of faecal lactobacilli in women with iron-deficiency anaemia in south India. Br J Nutr. 2010 doi: 10.1017/S0007114510001637. [DOI] [PubMed] [Google Scholar]
- 40.Scheers N, Rossander-Hulthen L, Torsdottir I, Sandberg AS. Increased iron bioavailability from lactic-fermented vegetables is likely an effect of promoting the formation of ferric iron (Fe3+) Eur J Nutr. 2016 doi: 10.1007/s00394-015-0857-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sewell AK, Han M, Qi B. Microbial Cell. Vol. 5. Shared Science Publishers OG; Oct 01, An unexpected benefit from E. Coli: how enterobactin benefits host health; pp. 469–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qi B, Han M. Microbial siderophore enterobactin promotes mitochondrial iron uptake and development of the host via interaction with ATP synthase. Cell. 2018 Oct;175:571–582.:e11. doi: 10.1016/j.cell.2018.07.032. [**This study shows a beneficial role of commensal bacteria in facilitating iron uptake by the host. Bacterially produced enterobactin promotes mitochondrial iron uptake by binding to the ATP synthase α subunit, which acts inside of mitochondria and independently of ATP synthase] [DOI] [PubMed] [Google Scholar]
- 43.Bronner F, Pansu D. Nutritional aspects of calcium absorption. J Nutr. 1999 Jan;129:9–12. doi: 10.1093/jn/129.1.9. [DOI] [PubMed] [Google Scholar]
- 44.Weaver CM. Curr Osteoporos Rep. Vol. 13. Current Medicine Group LLC; 2015. Diet, gut microbiome, and bone health; pp. 125–130. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mineo H, Hara H, Tomita F. Short-chain fatty acids enhance diffusional Ca transport in the epithelium of the rat cecum and colon. Life Sci. 2001 Jun;69:517–526. doi: 10.1016/s0024-3205(01)01146-8. [DOI] [PubMed] [Google Scholar]
- 46.Asemi Z, Esmaillzadeh A. Effect of daily consumption of probiotic yoghurt on serum levels of calcium, Iron and liver enzymes in pregnant women. Int J Prev Med. 2013 Jul;4:949–955. [PMC free article] [PubMed] [Google Scholar]
- 47.Wang W, et al. Enterococcus faecium modulates the gut microbiota of broilers and enhances phosphorus absorption and utilization. Animals. 2020 Jul;10:1–13. doi: 10.3390/ani10071232. [*Dietary supplementation with Enterococcus faecium increases the relative abundance of SCFA-producing bacteria in broilers, improves intestinal phosphorus absorption and bone forming metabolic activities, and decreases phosphorus excretion] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chevalier C, et al. Warmth prevents bone loss through the gut microbiota. Cell Metabol. 2020;32:575–590.:e7. doi: 10.1016/j.cmet.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones ML, Martoni CJ, Prakash S. Oral supplementation with probiotic L. reuteri NCIMB 30242 increases mean circulating 25-hydroxyvitamin D: a post hoc analysis of a randomized controlled trial. J Clin Endocrinol Metab. 2013 Jul;98:2944–2951. doi: 10.1210/jc.2012-4262. [DOI] [PubMed] [Google Scholar]