Abstract
A growing body of research supports a prominent role for the bed nucleus of the stria terminalis (BST) in the expression of adaptive and perhaps even pathological anxiety. The traditional premise that the BST is required for long-duration responses to threats, but not for fear responses to distinct, short-lived cues may, however, be oversimplified. A thorough evaluation of the involvement of the BST in cued and contextual fear is therefore warranted. In a series of preregistered experiments using male Wistar rats, we first addressed the involvement of the BST in cued fear. Following up on earlier work where we found that BST lesions disrupted auditory fear while the animals were in a rather high stress state, we here show that the BST is not required for the expression of more specific fear for the tone under less stressful conditions. In the second part, we corroborate that the same lesion method does attenuate contextual fear. Furthermore, despite prior indications for an asymmetric recruitment of the BST during the expression of anxiety, we found that bilateral lesioning of the BST is required for a significant attenuation of the expression of contextual fear. A functional BST in only one hemisphere resulted in increased variability in the behavioral outcome. We conclude that, in animals that acquired a fear memory with an intact brain, the bilateral BST mediates the expression of contextual fear, but not of unambiguous cued fear.
Keywords: bed nucleus of the stria terminalis, cued fear conditioning, contextual fear conditioning, fear, anxiety, rats, c-Fos
Introduction
Accumulating evidence in rodents, healthy participants and patients implicates the bed nucleus of the stria terminalis (BST) in anxiety and stress1–6. Early models put forward that the BST is involved in sustained anxiety responses, but not in phasic fear responses1,7,8. Over the years, this emphasis on response duration has been challenged and different groups have highlighted the importance of threat proximity9, or its temporal (un)predictability10–13. Regardless of diverging theoretical frameworks, the general consensus is that the bilateral BST is involved in sustained anxiety states, but that BST inactivation does not affect fear responses in a classic cued fear conditioning paradigm.
In line with this prevailing view, we previously found that bilateral electrolytic lesioning of the BST (medial division, anterior part) in between context conditioning and test, significantly reduced freezing and startle potentiation in the conditioned context14. Much to our surprise, and in contrast with most of the literature7,15–19, we recently found that such lesions also abolished fear responses to a conditioned tone20. We argued that our animals may have experienced an overall heightened stress state, rendering them vulnerable to the effect of BST lesions. Given the translational potential of BST-based treatments4,21, as well as the existing discordance about the conditions that recruit this brain area10, further research seemed warranted.
In the first part of the present study, we first investigated whether changes in the behavioral procedure, aimed at reducing overall stress and any residual background context conditioning, would allow us to replicate the canonical finding that the BST is not required for the expression of cued fear. In-depth behavioral analyses in Luyck et al. 2018 had indicated that, at test, rats showed freezing levels in between the tone presentations that were statistically indistinguishable from those during the conditioned tones. We suggested that conditioned fear to the tone may have been entangled with a generalized stress response prompted by the rather strong conditioning parameters22, as well as the presentation of (potentially aversive) startle probes throughout the test session23. Note that the use of startle probes in itself is not necessarily problematic, given that prior studies have found that BST lesions do not disrupt cued fear-potentiated startle1. Nevertheless, it is possible that differences between our and their protocols or animals accounted for the surprising findings in Luyck et al. (2018). In a series of experiments, we therefore adapted the behavioral protocol in order to obtain more specific fear for the conditioned tone, and to assess the effects of the post-training lesioning method that we have used hitherto.
In the second part, a separate experiment aimed to replicate our prior finding that electrolytic lesioning of the BST does reduce contextual fear14,24. At the same time, we wanted to follow up on recent observations from ourselves (18, Luyck et al. in preparation) and others6,25,26 suggesting asymmetric BST functioning. More specifically, there are indications that the left BST may be recruited to a greater extent than its right counterpart during the expression of responses to unpredictable threats. We therefore wanted to evaluate if unilateral lesions of the left BST might be equally effective as those of the bilateral BST in reducing contextual fear, and concurrently asses their neural signature using c-Fos quantification in several parts of the anxiety network (basolateral amygdala (BLA), nucleus accumbens (NAc) core and shell, infralimbic (IL) and prelimbic cortex (PL))27–31.
Taken together, this series of preregistered experiments investigated the role of the BST in cued and contextual fear in more detail. A crucial difference between both procedures lies in the (un)certainty about when a shock may occur: only at the end of a 10-s tone in the former, or at any point during the 20-min test session in the latter. Our auditory fear conditioning studies thus focused on the involvement of the bilateral BST in specific fear memories with high temporal predictability. Furthermore, a context conditioning study (with marked temporal unpredictability) characterized the effects of uni- versus bilateral BST lesions on a behavioral and c-Fos level.
Materials & Methods
Preregistration
All experiments, including protocols, planned sample sizes and analysis plans, were registered on the Open Science Framework (OSF) before the start of data collection (https://doi.org/10.17605/OSF.IO/6JSNC). Raw data can be found here as well.
Subjects
Male Wistar rats (± 250-300 g) were used in all experiments (Exp1: N = 16; Exp2: N = 16; Exp3: N = 24; Exp4: N = 56). Animals were housed in pairs with food and water available ad libitum. In Exp3 and 4, a plastic cage divider was used to prevent damage to the surgical wound by cage mates, while still allowing for social interaction. Animals were maintained on a 14/10 h light-dark cycle (lights on at 7:00 am), with a room temperature of ±19°C. This project was in accordance with the Belgian and European laws, guidelines and policies for animal experimentation, housing and care (Belgian Royal Decree of 29 May 2013 and European Directive 2010/63/EU on the protection of animals used for scientific purposes of 20 October 2010).
Surgery
In Exp3 and 4, animals were implanted with stainless-steel cannulas (23-gauge guide cannula C317G/5 mm and dummy stylet C317DC/5 mm, PlasticsOne, Roanoke, VA, USA) on the dura, directed towards the BST (anterior-posterior axis (AP): 0.0 mm, medio-lateral (ML): ±3.4 mm, 20° angle to the sagittal plane), under general anesthesia [ketamine hydrochloride (22.5 mg/kg, Anesketin, Eurovet nv/sa, Heusden-Zolder, Belgium) and 0.15 mg/kg medetomine HCL (Kela, Sint-Niklaas, Belgium)], as described previously14,24. Cannulas were placed on the dura mater, and then fixed on the skull with 4 stainless steel bone screws (Fine Science Tools, Heidelberg, Germany) and dental cement (Tetric EvoFlow, Ivoclar Vivadent, Mississauga, ON, Canada). Animals were allowed to recover for 6-7 days before the start of behavioral experiments.
Lesion procedure
The lesioning procedure was performed as described previously14. Rats were briefly anesthetized with isoflurane (5% (induction) and 2% (maintenance) in 1.5-2.0 l/min oxygen). The dura was punctured through the cannula with a stainless steel needle (Acupro P20-3210, Medichin, Hasselt, Belgium). Next, custom-made insulated stainless steel electrodes (200 μm in diameter) (008SW/30S, PlasticsOne, Roanoke, VA, USA) with a transversally cut tip were inserted into the cannulas and lowered 6.3 mm below the dural surface, thereby bilaterally targeting the medial division of the anterior BST32. The electrodes were connected to a stimulator (DS8000 and DLS100, World Precision Instruments, Stevenage, UK) and an anodal direct current pulse of 1 mA (to create a lesion) or 0 mA (sham condition) was applied during 15 s. One minute later, the electrodes were removed and anesthesia was ended. A few minutes later, the animals were awake. The whole lesioning procedure took 10–15 min.
Equipment
Two different contexts (Med Associates Inc., Fairfax, VT, USA) were used for behavioral tests. Both contexts were located on opposite sides of the same room in ventilated, sound-attenuating boxes. Freezing behavior of the animals was recorded with a video camera (DCR-SR55E Super NightShot Plus, Sony Corporation) positioned in front of each cage. Freezing was scored continuously by an experienced observer, blinded to group division. Freezing scores were expressed as a percentage of the amount of time under investigation.
Context A consisted of a small animal cage (inner dimensions: 9.4 cm height, 8.2 cm width, and 16.5 cm length) with a grid floor, through which foot shocks can be delivered. The grid floor consisted of six 5-mm diameter stainless-steel rods spaced 10 mm apart. The cage was fixed on a response platform on which the startle reaction of the rats generated a pressure, and analog signals were amplified, digitized, and processed by Startle Reflex software (version 5.95; Med Associates). The presentation and sequencing of the acoustic stimuli and foot shocks were controlled by the same software. One of two speakers, both located 7 cm behind the rat holder, was used to deliver a continuous white background noise (55 dB); the other speaker delivered the startle stimuli (white noise, 100 dB, 50 ms). The startle response was defined as the first peak accelerometer voltage that occurred during the first 100 ms after onset of the startle probe and was measured on an arbitrary scale ranging from 0 to 2047. The startle platform and loudspeakers were calibrated before each experiment. A dim red light bulb was continuously on and the cage was cleaned with 70% ethanol in between rats.
Context B consisted of a larger cage (21 cm height, 24.1 cm width, 30.5 cm length), with a grid floor with 19 rods (4.8 mm diameter, spaced 16 mm center to center) and a black triangular ceiling. The cage was cleaned with a scented cleaning product between rats, the chamber was dimly lit with a white light of 50 lux, and presentation of tones and shocks was controlled by Video Freeze software (Med Associates).
Behavioral procedure
Experiments 1-3 employed auditory fear conditioning (including an unpaired control condition in the first two experiments). With the aim of reducing overall stress in the animals, startle probes were removed in comparison with prior studies20. Experiment 4 used a context conditioning procedure, as described previously14. All experimental steps were scheduled using ExpTimer software33.
Experiment 1
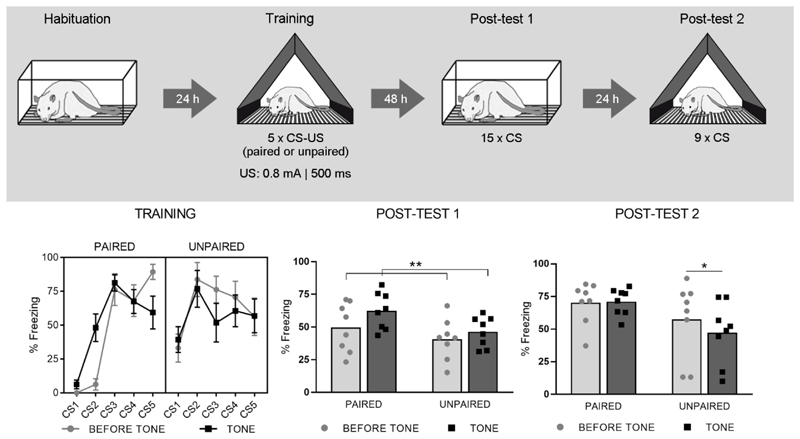
On day 1 (Habituation), animals were placed in context A for 23 minutes. On day 2 (Training), the animals were placed in a distinct context B. During the first five minutes of this training session (23 min in total), no stimuli were given. Following the 5-min acclimation phase, animals received five tones (Conditioned Stimulus (CS); 4000 Hz, 75 dB, 10 s, intervals of 3-4 min) and five shocks (Unconditioned Stimulus (US); 0.8 mA, 500 ms) in a paired or unpaired manner (Paired (n = 8) and Unpaired (n = 8) groups). Freezing was measured during each CS (measure of cued fear) and during the 10-s period preceding each CS presentation (measure of contextual fear). On day 4 (Post-test 1), the rats were again placed in context A for a duration of 23 min. After a 5-min acclimation phase, 15 CSs were presented (intertrial interval (ITI) 40-70 s). On day 5 (Post-test 2), all animals were returned to the training context (context B) and 9 CSs were presented. For both post-tests, freezing was measured during acclimation, during CSs and during the 10-s period preceding each CS.
Experiment 2
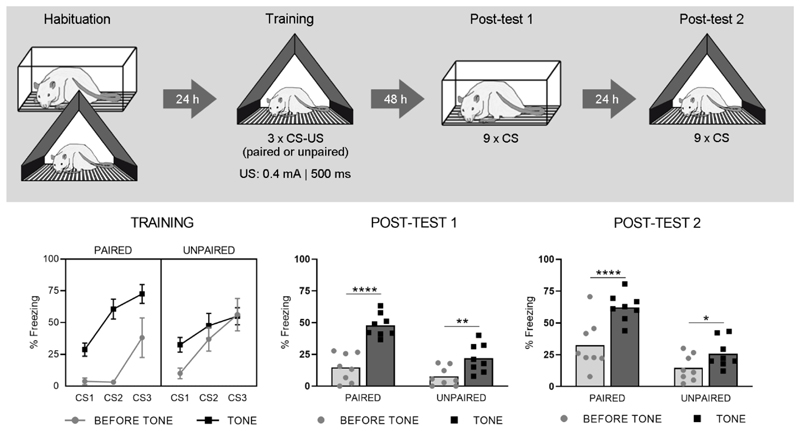
The protocol described for Exp1 was modified as follows with the aim of obtaining fear for the CS with less extensive spreading to the test context. On day 1, all animals underwent two Habituation sessions of 23 minutes each, both in context A and B, in a counterbalanced order. During Training, 3 tones (intervals of ±7 min) and 3 shocks (0.4 mA, 500 ms) were presented in a paired or unpaired manner (n = 8 per group). Finally, Post-test 1 contained only 9 tone presentations instead of 15, and both post-tests had 100-130-s ITIs.
Experiment 3
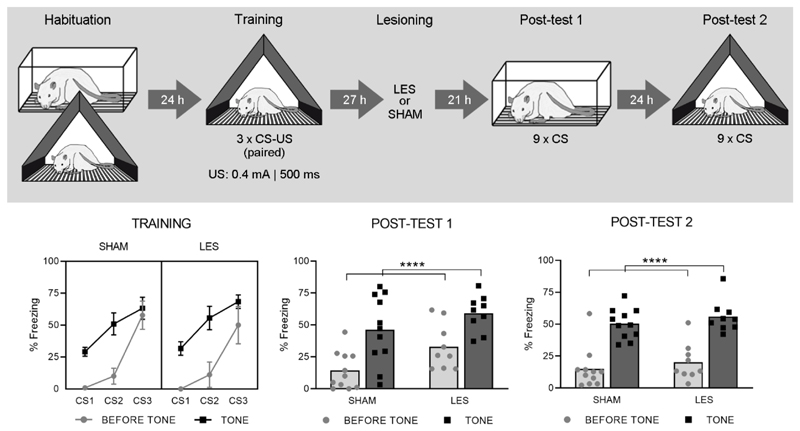
The ‘paired’ protocol described for Exp 2 was used for all animals. On day 3, 27 h following the training session, rats received bilateral sham (SHAM, n = 11) or BST lesions (LES, n = 13).
Experiment 4
All sessions took place in context A. On day 1 (Habituation), rats were placed in context A for a total of 20 minutes. After five minutes, 30 acoustic startle stimuli (100 dB, 50 ms, ITI 30 s) were administered. On day 2 (Pre-test), the rats underwent a session identical to Habituation on the day before. Based on their startle values on day 2, rats were matched into equivalent groups for all experiments. During Pre-test, baseline measurements of contextual fear were collected, i.e., freezing during the 5-min acclimation period and startle responses during the rest of the session. On day 3, after a 5-min acclimation phase, all rats received 10 electrical foot shocks (0.8 mA, 250 ms) with a variable ITI of 60 – 180 s. The total duration of the session was 30 minutes. On day 4 (Post-test), the animals were tested using the same protocol as during Pre-test.
Histology
For more details regarding the perfusion protocol, dissection and processing of the brains and immunohistochemical analyses, see Supplement.
Cresyl violet (Exp3-4)
Bregma slices were stained with cresyl violet (0.5% cresyl violet acetate in dH20, Merck KGaA, Darmstadt, Germany) with one slice every 100 μm in a radius of 500 μm anterior and posterior with respect to bregma. Microscopic analysis revealed the exact location of the largest lesion diameter, which was manually delineated and then transferred to the corresponding Paxinos coronal plate. Lesion animals were included when the largest diameter of the lesion comprised the anterior BST (between +0.48 and -0.48 mm with respect to bregma) and clear damage (including necrosis and edema) was visible on the bregma slice. Lesions included in this study mainly destroyed the medial and lateral divisions of the anterior BST (dorsal and often ventral part). SHAM animals were included when no damage, except for the electrode track, was observed.
Immunohistochemistry (Exp4)
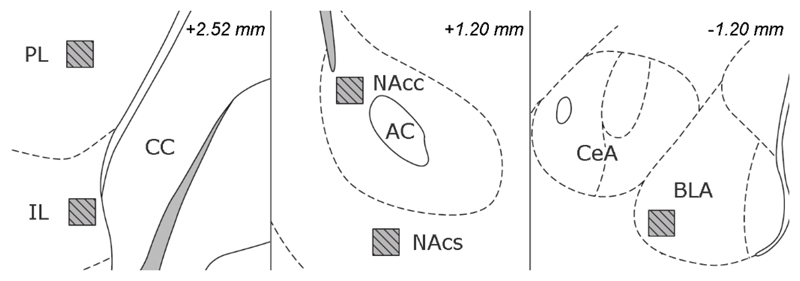
Six representative animals of each group were selected for immunofluorescent staining. For these 24 animals, we selected representative slides for each structure of interest, based on Paxinos coordinates: +2.40 mm (IL, PL; note that the atlas figure is at +2.52 mm), +1.20 mm (NAc, core and shell) and -2.40 mm (BLA), with respect to bregma. These slices were doublestained for c-Fos and NeuN with immunofluorescent markers34. Projection images of the IL/PL, NAc and BLA were acquired through an inverted C2+ confocal microscope (Eclipse Ti2, Nikon, Tokyo, Japan) using a 20X objective at a resolution of 512x512. We imaged the inner six μm section of each ROI, using predetermined intensity settings that remained fixed throughout all acquisitions. Neuronal c-Fos expression was quantified within 200 x 200 μm ROIs. Intensities were averaged over three consecutive slices and had an arbitrary scaling between 0 (black) and 4095 (white, highest activity).
Statistics
GraphPad Prism (version 8.02, GraphPad Software) was used for all statistical analyses and graphs. When assumptions for parametric testing were not met, non-parametric alternatives were used. Significance levels were set at p < 0.05 and corrected for multiple testing.
Experiments 1-3
Freezing during Training is shown for illustrative purposes only. Statistical analyses were performed for Post-test 1 and 2, using the averages of all (9 or 15) trials. Percentage freezing during acclimation was compared between groups using an unpaired t-test. Two-way repeated-measures ANOVA (RM-ANOVA) with factors ‘Group’ and ‘Trial’ were used to evaluate all other freezing measurements (i.e., freezing before or during tones).
Experiment 4
Animals were excluded from all analyses if Pre-test freezing exceeded 25%, or when ROUT testing (Q=1) revealed significant Pre-test startle outliers. For all graphs and statistical analyses, freezing during acclimation and startle responses are expressed as a difference score between Post- and Pre-test measurements. A one-way ANOVA was used to compare freezing and startle between groups. For each ROI, a ROUT test (Q=1) was used to detect significantly deviant c-Fos values which were then excluded, next a Grubbs’ test was conducted to detect outliers within each group. A two-way RM-ANOVA with factors ‘Hemisphere’ and ‘Group’ was conducted to evaluate c-Fos intensities. Bonferroni post-hoc tests were used to identify differences.
Results
Experiment 1
On Post-test 1 (Fig. 1, lower middle panel), freezing during acclimation was low in both groups (Paired: 8% ± 8%, mean ± SD; Unpaired: 4% ± 6%, t (14) = 1.05 ; p = 0.31). The RM-ANOVA on freezing before and during tones showed a main effect of Trial (F (1, 14) = 12.2; p < 0.01), but no effect of Group (F (1, 14) = 3.02; p = 0.10), nor interaction (F (1, 14) = 1.75; p = 0.21). On Post-test 2 (Fig. 1, lower right panel), which was conducted in the training context, contextual freezing during acclimation was high and did not differ between groups (Paired: 45% ± 20%; Unpaired: 58% ± 28%, t(14) = 1.04 ; p = 0.32). The RM-ANOVA showed a significant Group by Trial interaction (F (1, 14) = 5.6; p<0.05), but no main effects of Group (F (1, 14) = 3.17; p = 0.10) or Trial (F (1, 14) = 4.07; p = 0.06).
Fig. 1. Experiment 1.
The upper panel shows the experimental design. The lower left panel shows percentage freezing during each CS (tone) and its preceding 10-s interval (before tone), for both Paired (n = 8) and Unpaired (n = 8) groups during Training (means ± SEM). The lower middle and right panels show freezing during these same intervals, for Post-test 1 and 2 respectively (shown as individual data points with mean). Main effect of Trial: **p < .01, Sidak’s post-hoc test: *p < .05.
Experiment 2
On Post-test 1 (Fig. 2, lower middle panel), freezing during acclimation was low in both groups (Paired: 0% ± 0%; Unpaired: 1% ± 3%, t (14) = 0.81 ; p = 0.43). The RM-ANOVA on freezing before and during tones showed main effects of Group (F (1, 14) = 14.36; p<0.01) and Trial (F (1, 14) = 99.01; p<0.0001), and a significant interaction (F (1, 14) = 15.42; p<0.01). On Post-test 2 (Fig. 2, lower right panel), which was conducted in the training context, contextual freezing during acclimation was higher and did not differ between groups (Paired: 22% ± 25%; Unpaired: 12% ± 12%, t(14) = 0.98 ; p = 0.35 ). The RM-ANOVA showed main effects of Group (F (1, 14) = 19,07; p<0.001) and Trial (F (1, 14) = 54,66; p<0.0001), and a significant interaction (F (1, 14) = 11,30; p<0.01).
Fig. 2. Experiment 2.
The upper panel shows the experimental design. The lower left panel shows percentage freezing during each CS (tone) and its preceding 10-s interval (before tone), for both Paired (n = 8) and Unpaired (n = 8) groups during Training (means ± SEM). The lower middle and right panels show freezing during these same intervals, for Post-test 1 and 2 respectively (shown as individual data points with mean). Sidak’s post-hoc tests: *p < .05, **p < .01, ****p < .0001.
Experiment 3
Exclusions
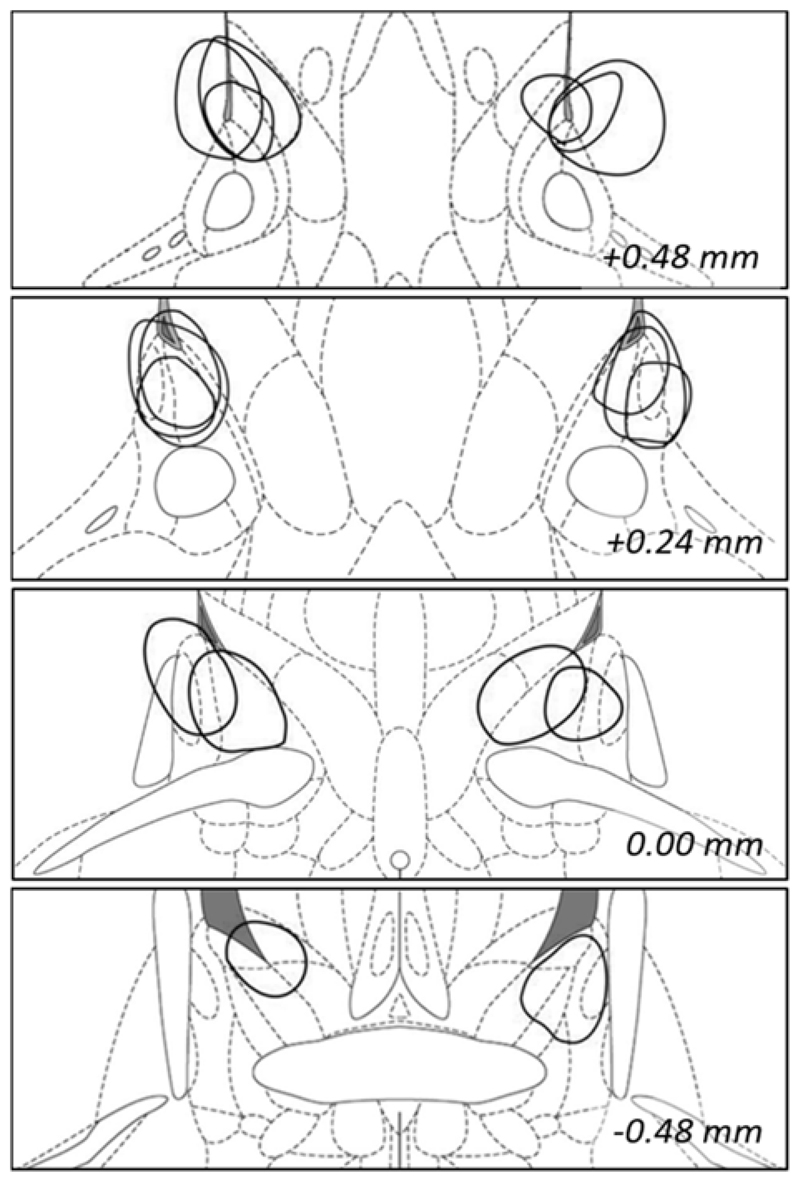
Four animals were excluded from the LES group due to insufficient damage to the bilateral medial BST. In total, this left 11 rats in the SHAM group and 9 animals in the LES group (Fig. 3).
Fig. 3. Reconstruction of electrolytic BST lesions in Experiment 3.
The maximal diameter of all included lesions is shown. Coronal slices shown from top to bottom are: +0.48, +0.24, 0.00 and -0.48 mm with respect to bregma. Adapted from 32.
Behavioral data
On Post-test 1 (Fig. 4, lower middle panel), freezing during acclimation was low in both groups (SHAM: 3% ± 4%; LES: 2% ± 1%, t (18) = 1.32; p = 0.21). The RM-ANOVA on freezing before and during tones showed a main effect of Trial (F (1, 18) = 78,9; p<0.0001), but no effect of Group (F (1, 18) = 3.86; p = 0.07), nor interaction (F (1, 18) = 0.73; p = 0.40). On Post-test 2 (Fig. 4, lower right panel), which was conducted in the training context, contextual freezing during acclimation was also low and did not differ between groups (SHAM: 10% ± 15% LES: 5% + 4%, t (18) = 1.03; p = 0.32). The RM-ANOVA again showed a main effect of Trial (F (1, 18) = 128,1; p<0.0001), but no effect of Group (F (1, 18) = 1.01; p = 0.33), nor interaction (F(1, 18) = 0.003; p = 0.95).
Fig. 4. Experiment 3.
The upper panel shows the experimental design. The lower left panel shows percentage freezing during each CS (tone) and its preceding 10-s interval (before tone), for both SHAM (n = 11) and LES (n = 9) groups during Training (means ± SEM). The lower middle and right panels show freezing during these same intervals, for Post-test 1 and 2 respectively (shown as individual data points with mean). Main effect of Trial: ****p < .0001.
Experiment 4
Exclusions
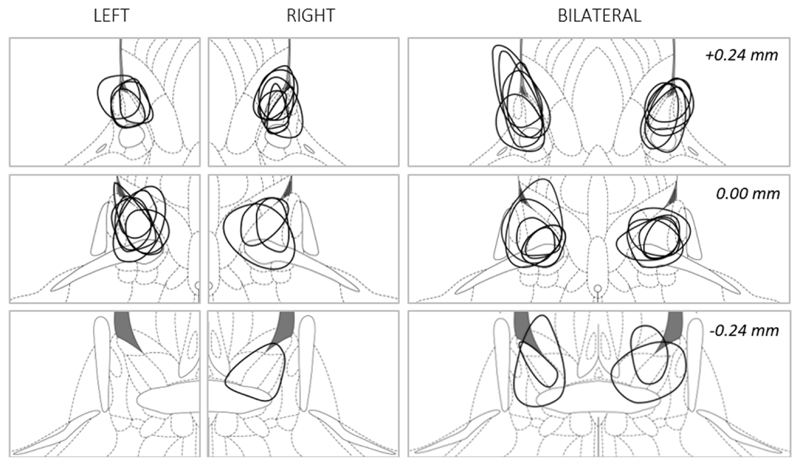
From the 56 rats that underwent surgery, two died peri- or post-operatively, four lost their headstage and two other animals had damaged cannulas, making electrode insertion impossible. Five animals were excluded based on incorrect positioning of the lesions. Six additional animals were excluded based on Pre-test behavioral data, using the criteria specified above. Thirty-seven animals were included for behavioral analysis: SHAM, n = 9; BILAT, n = 11; LEFT, n = 8; RIGHT, n = 9 (Fig. 5).
Fig. 5. Reconstruction of electrolytic BST lesions in Experiment 4.
The maximum diameter of each lesion is shown for included animals of the LEFT (n = 8), RIGHT (n = 9) and BILAT (n = 11) groups. Coronal sections from top to bottom are +0.24, 0.00 and -0.24 with respect to bregma. Adapted from 32.
Behavioral data
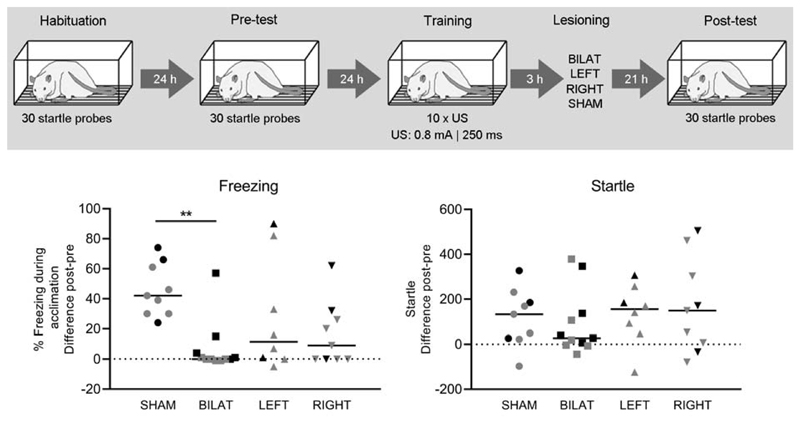
Pre-test freezing was low and did not differ between groups (SHAM: 2% ± 5%; BILAT: 1% ± 1%; LEFT: 1% ± 3%; RIGHT: 1% ± 2%; F(3,33) = 0.84, p = 0.48). Matching using Pre-test startle values resulted in 4 comparable groups (SHAM: 138 ± 110, BILAT: 99 ± 71; LEFT: 158 ± 132, RIGHT: 148 ± 93; F(3,33) = 0.65; p = 0.59).
Kruskall-Wallis analysis of freezing showed a main effect of Group H(4) = 12.63; p<0.01. Dunn’s post-hoc analysis revealed a significant difference between SHAM and BILAT (p<0.01), but not between other groups (Fig. 6, lower left panel). Startle data did not show any significant group differences H(4) = 1.72; p = 0.63), but, animals with bilateral BST lesions did have (nominally) the lowest startle amplitudes (Fig. 6, lower right panel).
Fig. 6. Behavioral data of Experiment 4.
The upper panel shows the experimental design. In the lower panels, data are shown as individual data points and medians. Animals indicated in grey were used for subsequent c-Fos analysis. Post-hoc analysis:**p < .01.
In addition, visual inspection of the freezing data (Fig. 6, lower left panel) showed nominally lower freezing in animals with unilateral lesions versus sham animals, a difference that failed to reach significance because of quite large within-group variability. Indeed, in the lesioned groups, freezing variability appears to be larger in animals that received unilateral lesions, versus those that received bilateral lesions. This is reflected by the interquartile range, which is a measure of the spread of the data within each group. For animals with bilateral lesions, the IQR was only 4%, whereas for animals with unilateral lesions, the IQR was 29% (RIGHT) or 70% (LEFT).
c-Fos data
In each group, six animals were selected for further c-Fos analysis (animals shown in grey on Fig. 6, ROIs are depicted in Fig. 7). For analysis of the basolateral amygdala, one RIGHT and one BILAT animal had to be excluded, since slices were collected too anterior from the targeted position of -2.40 mm with respect to bregma. For IL/PL analysis, one SHAM animal had to be excluded due to poor diffusion of the mounting medium and subsequent drying of the tissue. Additionally, for IL, Grubbs’ analysis identified an outlier in the SHAM group (Z = 1.74, p<.05) and two more outliers were detected in the PL (LEFT group: Z = 1.96, p<.05; RIGHT group: Z = 1.94, p<.05). In NAc, no outliers were detected for core or shell regions.
Fig. 7. Regions of interest (ROIs) for immunohistochemistry.
ROIs are indicated with hatched squares and were consistently positioned as shown above. Abbreviations: AC: anterior commissure, BLA: basolateral amygdala, CC: corpus callosum, CeA: central amygdala, IL: infralimbic cortex, NAcc: core of the nucleus accumbens, NAcs: shell of the nucleus accumbens, PL: prelimbic cortex. Adapted from 32.
No significant differences were detected between groups or between hemispheres for any of the regions of interest (Table 1 and Supplement).
Table 1. Experiment 4, neuronal c-Fos intensities in each region of interest (mean ± standard deviation).
| SHAM | BILAT | LEFT | RIGHT | |
|---|---|---|---|---|
| BLA | ||||
| Left | 1301 ± 144 | 1322 ±194 | 1377 ± 59 | 1401 ± 180 |
| Right | 1243 ± 73 | 1321 ± 115 | 1271 ± 79 | 1322 ± 86 |
| Bilateral | 1272 ± 98 | 1322 ± 94 | 1324 ± 51 | 1361 ± 103 |
| IL | ||||
| Left | 1328 ± 5 | 1417 ± 146 | 1352 ± 143 | 1418 ± 102 |
| Right | 1307 ± 65 | 1393 ± 153 | 1419 ± 106 | 1294 ± 158 |
| Bilateral | 1318 ± 31 | 1405 ± 131 | 1386 ± 107 | 1356 ± 126 |
| PL | ||||
| Left | 1193 ± 147 | 1209 ± 93 | 1197 ± 78 | 1163 ± 81 |
| Right | 1090 ± 177 | 1203 ± 165 | 1171 ± 33 | 1074 ± 86 |
| Bilateral | 1142 ± 148 | 1206 ± 112 | 1184 ± 41 | 1119 ± 25 |
| NAc core | ||||
| Left | 1287 ± 143 | 1326 ± 71 | 1187 ± 128 | 1300 ± 73 |
| Right | 1262 ± 135 | 1294 ± 106 | 1253 ± 101 | 1253 ± 121 |
| Bilateral | 1274 ± 137 | 1310 ± 59 | 1220 ± 70 | 1277 ± 73 |
| NAc shell | ||||
| Left | 1343 ± 181 | 1377 ± 125 | 1223 ± 149 | 1297 ± 68 |
| Right | 1295 ±151 | 1337 ± 85 | 1264 ± 91 | 1237 ± 94 |
| Bilateral | 1319 ± 165 | 1357 ± 102 | 1244 ± 112 | 1267 ± 64 |
Discussion
The precise function of the BST in fear and anxiety responses is the subject of ongoing debate and investigation. Here, we aimed to characterize the role of the BST in the expression of cued and contextual fear using an electrolytic lesion and immunohistochemical approach in rats that underwent Pavlovian conditioning. In the first part of this paper, we showed that the BST (medial division, anterior part) was not required for the expression of auditory fear in a behavioral protocol that minimized potentially confounding stress and background context conditioning. In the second part, we found that the bilateral BST was necessary for the expression of contextual fear. Unilateral lesions only had partial effects on contextual freezing and, overall, resulted in more behavioral variability than bilateral lesions, suggesting that it takes two functioning BSTs to produce full expression of contextual fear, but also that it takes two lesions, one in each hemisphere, to have a significant reduction of contextual fear on the group level. To further characterize the effects of BST lesions, c-Fos activity was quantified in the basolateral amygdala, infra- and prelimbic cortex, as well as nucleus accumbens (core and shell).
The first three experiments focused on cued fear and were inspired by the surprising observation that (bilateral) BST lesions completely blocked fear-potentiated startle in a previous study20. This finding was unexpected and diametrically opposed to traditional models that state that the BST has no role in cued fear responses, which is supported by several reports using the startle reflex or freezing as an index of fear learning and expression1,15. An alternative explanation that was elaborated upon in Luyck et al. (2018) centers around overall elevated stress levels as an important confounding factor. Animals, although expressing distinct fear-potentiated startle to the tones, may be in a continuously stressed state and uncertain about the temporal contiguity between the tones and shocks, thereby putting them de facto in a situation with high temporal unpredictability, which has been shown to recruit the BST, even in the presence of 10-s tones10. This line of reasoning is supported by in-depth behavioral analyses in Luyck et al. (2018) which indicated that freezing remained elevated in between tones, suggesting a sustained fear response throughout the test session. It is noteworthy that such freezing measurements are not typically reported, as researchers will often only analyze freezing during the CS, sometimes accompanied by a contextual freezing measurement in the period that precedes the first tone (often called ‘baseline freezing’). Whereas such baseline freezing quantifies the initial fear response to the test context, it may provide limited information about how the test session and context are experienced once the CSs are presented. Even when they are perceptually dissimilar, training and test contexts undeniably share features (if only for the fact that, in both cases, the animal is removed from its home cage and put in a different environment), and generalization between these contexts may increase in the presence of CSs, resulting in sustained anxiety or stress responses, which in turn, may recruit the BST1–3. Why cued fear was nevertheless picked up by one behavioral measure (startle, in this case), but not the other (freezing), remains an open question. Freezing may be more sensitive to lower levels of fear than the startle reflex, resulting in a failure to discriminate between cue and context in the presence of intermediate-to-high stress levels, when indexed with freezing but not startle35,36. This is a cautionary tale that underlines the importance of thorough behavioral assessment, as well as the added value of combining different behavioral measures. Of note, in Exp4 of this paper, freezing and startle responses correlated positively (Spearman r = 0.53, p = .0008, exploratory analysis), supporting that both indices tap into the same construct (i.e. a fearful animals shows more freezing and higher startle responses). However, including startle reflex measurements in the behavioral protocol is not always possible or desirable, as it requires the repeated presentation of loud bursts of noise which can be perceived as quite aversive by the subject23. This is the main reason why we removed the startle probes from the procedures used in the first three experiments of this paper. In addition, we lowered the number of shocks, in comparison with the 10 CS-US pairings in Luyck et al. (2018). Note that a common analysis of freezing during the tones in Exp1 of this paper would have led to the conclusion that there was clear auditory fear (8±8% baseline freezing, and 62±14% freezing during the tones), whereas our additional contextual freezing measurements between tones (50±19%), as well as the unpaired control group (which behaves similarly) suggested otherwise. The protocol used in Exp2 did result in fear that seemed quite specific for the CS, with decent discrimination between CS and context, even when tested in the original training context on Test 2. This implies good temporal predictability during the test with regard to the anticipated time of shock delivery. The protocol of Exp2 was then used to test the effects of bilateral post-training BST lesions in Exp3. In line with the canonical view that the BST is not required for the expression of cued fear, we indeed found no effect of bilateral BST lesions in this experiment. Interestingly, the lack of effect in Exp3 is in contrast with the findings of Luyck et al. (2018), which used the same lesioning technique, strain of animals, tones and contexts, but found a disruptive effect of BST lesions on fear-potentiated startle. Note that some lesions in Exp3 were located slightly more anterior or dorsal than in Exp4, where we did observe anxiolytic effects. It is unlikely that this may explain the null effect of the lesions in Exp3, as only animals with sufficient damage to the anterior BST were included, and moreover, lesion location and spread was in line with prior studies finding anxiolytic effects14,24.
In other words, the precise training and testing conditions, and potentially the accompanying stress levels, may have a profound impact on recruitment of the BST. Future studies can directly compare the effects of BST lesions in more and less stressful auditory fear protocols, e.g., with the behavioral procedures used in the current study or in Luyck et al. (2018), or by adding external stressors to the moderate fear procedure (at training or at test) used in Exp3. This will permit well-founded statements about the importance of stress levels regarding BST recruitment during acquisition and retrieval of fear memories. In conclusion, our findings indeed suggest that the BST may play a role in the expression of cued fear in circumstances with high stress and/or (perceived) elevated temporal unpredictability11, but not during the expression of specific, moderate cued fear. To make a cautious clinical translation, this may implicate that the BST is involved in responses to trauma-related cues in highly anxious PTSD patients, but not when a healthy person is confronted with a mildly threatening, temporally predictable event. Future research in clinical populations will have to put this to the test, but existing data do seem to support this5,26,37.
In the second part of this paper, we turned to the widely-recognized role of the BST in the expression of contextual fear and replicated the attenuating effects of post-training, bilateral BST lesions14,15,24. To our knowledge, we were the first to directly compare the effects of unilateral (left/right) versus bilateral lesions in the BST on the expression of contextual fear. This research interest was rooted in observations of hemispheric differences in neuronal activity in the BST in anxious animals (Luyck et al., in preparation). Even though our hypothesis (left lesions are as effective as bilateral lesions) was not confirmed, our data do provide new insights in the contribution of the BST in contextual fear expression, and suggest that the BST is bilaterally involved. Although not formally tested, bilateral lesions appeared to have stronger effects than unilateral lesions in reducing contextual fear. When comparing contextual freezing (Fig. 6, lower left panel) between SHAM and BILAT, the 95% confidence interval was [-61%;-26%], whereas the difference between SHAM and UNILAT (i.e., LEFT and RIGHT groups combined) had a 95% confidence interval of [-45%;-9%], suggesting larger effects with bilateral than unilateral lesions. The freezing data also support increased variability in unilaterally lesioned groups versus rats with bilateral lesions. In addition, bilateral lesions have repeatedly been shown to attenuate startle potentiation in the conditioned context (note that this reduction was statistically significant in prior studies, but in the current study failed to reach significance). Conversely, we found that unilateral lesions did not affect startle responses at all. Visual inspection of the data (Fig. 6, lower right panel) suggests that it is unlikely that this is a power problem.
In Exp4, we also evaluated c-Fos intensity levels in different regions of the anxiety network. In line with our pre-registered hypothesis, c-Fos intensity in the right BLA was nominally higher in lesioned animals than in SHAM rats, but this difference was statistically negligible. Moreover, intensities were also higher in the left BLA. Comparison with prior studies is difficult, as they typically contrasted conditioned and non-conditioned animals, often used cued instead of contextual fear conditioning, and more commonly looked at counts of c-Fos positive cells rather than their intensity levels. An early study found significantly more c-fos-positive nuclei in the BLA in context-conditioned animals compared to controls, but did not distinguish between hemispheres38. Another study also found an increased number of c-Fos immunoreactive neurons in the BLA in rats expressing contextual freezing versus controls. In addition, they differentiated between both hemispheres and found more active neurons in the right versus the left BLA39. Others have specifically focused on a role of the BLA during extinction. One can argue that the expression of contextual fear and concomitant c-Fos expression that we observed during the 20-min test session was presumably intermixed with extinction learning. Cued fear conditioning experiments have indicated a role for the BLA during extinction in procedures with freezing (increased number of c-Fos nuclei)40 and fear-potentiated startle41, but information from context conditioning studies is scarce. In addition to the BLA, we examined two cortical areas with established importance for the up- and downregulation of fear responses, i.e., the pre- and infralimbic cortex42, but again found no significant group differences. Differences in the amount of anxiety as quantified by contextual freezing, e.g. when comparing anxious SHAM animals and non-anxious BILAT animals, were thus not accompanied by higher c-Fos intensities in the prelimbic cortex and lower intensities in the infralimbic cortex in SHAM animals. Finally, we also looked at the nucleus accumbens (core and shell), where we expected no group differences (cf Luyck et al., in preparation). However, the observed absence of such differences in the present study is difficult to interpret in the light of the general lack of statistically significant c-Fos group differences. Note that for this study, we deliberately focused on the analysis of a limited number of predefined ROIs. Of course, it is possible that other brain regions (e.g., other portions of the amygdala) may show group differences in c-Fos expression under the same testing conditions. As a final point, it is noteworthy that our data suggest the existence of c-Fos differences between hemispheres in several brain areas (Suppl. Fig. 4-6). These differences were not significant in the present study, but sometimes surpassed nominal between-group differences. Although the current sample size and power do not allow us to draw any conclusions regarding these hemispheric differences, this may be an important caveat to keep in mind when interpreting the large majority of the c-Fos literature, which is typically based on data from only one hemisphere or from both hemispheres combined.
We conclude that bilateral lesions of the BST do not interfere with the expression of moderate fear that is tightly connected to a specific conditioned cue. Conversely, bilateral functioning BSTs do seem to be required for the expression of contextual fear in a conditioned context, and unilateral lesions in the BST only partially reduced the observed anxiety-like behavior.
Supplementary Material
Acknowledgements
We thank Phaedra Lebegge, Asuka Nakajima, Victoria Aurora Ossorio Salazar and Eline Princen for their help with the data collection, as well as Benjamin Pavie and Nikky Corthout from the Light Microscopy and Imaging Network LiMoNe, VIB Bio Imaging Core Leuven. We acknowledge the financial support of the Research Foundation – Flanders (FWO) (Research Project G0C9817N), Medtronic Chair for Stereotactic Neurosurgery in Psychiatric Disorders (B. Nuttin), European Research Council (CoG 648176), KU Leuven Research Council (Grant C14/16/048, L. Arckens) and Hercules Foundation (Grant AKUL-09-005, L. Arckens).
References
- 1.Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crestani CC, et al. Mechanisms in the bed nucleus of the stria terminalis involved in control of autonomic and neuroendocrine functions: a review. Curr Neuropharmacol. 2013;11:141–159. doi: 10.2174/1570159X11311020002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daniel SE, Rainnie DG. Stress Modulation of Opposing Circuits in the Bed Nucleus of the Stria Terminalis. Neuropsychopharmacology. 2016;41:103–125. doi: 10.1038/npp.2015.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luyten L, Hendrickx S, Raymaekers S, Gabriёls L, Nuttin B. Electrical stimulation in the bed nucleus of the stria terminalis alleviates severe obsessive-compulsive disorder. Mol Psychiatry. 2016;21:1272–1280. doi: 10.1038/mp.2015.124. [DOI] [PubMed] [Google Scholar]
- 5.Brinkmann L, et al. Dissociation between amygdala and bed nucleus of the stria terminalis during threat anticipation in female post-traumatic stress disorder patients. Human brain mapping. 2017;38:2190–2205. doi: 10.1002/hbm.23513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Somerville LH, Whalen PJ, Kelley WM. Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biological psychiatry. 2010;68:416–424. doi: 10.1016/j.biopsych.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci. 1997;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daldrup T, Lesting J, Meuth P, Seidenbecher T, Pape HC. Neuronal correlates of sustained fear in the anterolateral part of the bed nucleus of stria terminalis. Neurobiol Learn Mem. 2016;131:137–146. doi: 10.1016/j.nlm.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 9.Perusini JN, Fanselow MS. Neurobehavioral perspectives on the distinction between fear and anxiety. Learn Mem. 2015;22:417–425. doi: 10.1101/lm.039180.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goode TD, Ressler RL, Acca GM, Miles OW, Maren S. Bed nucleus of the stria terminalis regulates fear to unpredictable threat signals. Elife. 2019;8 doi: 10.7554/eLife.46525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goode TD, Maren S. Role of the bed nucleus of the stria terminalis in aversive learning and memory. Learn Mem. 2017;24:480–491. doi: 10.1101/lm.044206.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luyten L, et al. Micro-positron emission tomography imaging of rat brain metabolism during expression of contextual conditioning. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:254–263. doi: 10.1523/JNEUROSCI.3701-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clauss JA, Avery SN, Benningfield MM, Blackford JU. Social anxiety is associated with BNST response to unpredictability. Depress Anxiety. 2019;36:666–675. doi: 10.1002/da.22891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luyten L, van Kuyck K, Vansteenwegen D, Nuttin B. Electrolytic lesions of the bed nucleus of the stria terminalis disrupt freezing and startle potentiation in a conditioned context. Behav Brain Res. 2011;222:357–362. doi: 10.1016/j.bbr.2011.03.066. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan GM, et al. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004;128:7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 16.Davis M, Walker DL, Lee Y. Amygdala and bed nucleus of the stria terminalis: differential roles in fear and anxiety measured with the acoustic startle reflex. Philos Trans R Soc Lond B Biol Sci. 1997;352:1675–1687. doi: 10.1098/rstb.1997.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hitchcock J, Davis M. Lesions of the amygdala, but not of the cerebellum or red nucleus, block conditioned fear as measured with the potentiated startle paradigm. Behav Neurosci. 1986;100:11–22. doi: 10.1037//0735-7044.100.1.11. [DOI] [PubMed] [Google Scholar]
- 18.Luyten L, et al. Micro-positron emission tomography imaging of rat brain metabolism during expression of contextual conditioning. J Neurosci. 2012;32:254–263. doi: 10.1523/JNEUROSCI.3701-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gewirtz JC, McNish KA, Davis M. Lesions of the bed nucleus of the stria terminalis block sensitization of the acoustic startle reflex produced by repeated stress, but not fear-potentiated startle. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:625–648. doi: 10.1016/s0278-5846(98)00028-1. [DOI] [PubMed] [Google Scholar]
- 20.Luyck K, Nuttin B, Luyten L. Electrolytic post-training lesions of the bed nucleus of the stria terminalis block startle potentiation in a cued fear conditioning procedure. Brain Struct Funct. 2018;223:1839–1848. doi: 10.1007/s00429-017-1591-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lebow MA, Chen A. Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Molecular psychiatry. 2016;21:450–463. doi: 10.1038/mp.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laxmi TR, Stork O, Pape HC. Generalisation of conditioned fear and its behavioural expression in mice. Behav Brain Res. 2003;145:89–98. doi: 10.1016/s0166-4328(03)00101-3. [DOI] [PubMed] [Google Scholar]
- 23.Lissek S, et al. Airpuff startle probes: an efficacious and less aversive alternative to white-noise. Biological psychology. 2005;68:283–297. doi: 10.1016/j.biopsycho.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Luyck K, et al. Electrical stimulation of the bed nucleus of the stria terminalis reduces anxiety in a rat model. Transl Psychiatry. 2017;7:e1033. doi: 10.1038/tp.2017.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Straube T, Mentzel HJ, Miltner WH. Waiting for spiders: brain activation during anticipatory anxiety in spider phobics. Neuroimage. 2007;37:1427–1436. doi: 10.1016/j.neuroimage.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 26.Alvarez RP, Chen G, Bodurka J, Kaplan R, Grillon C. Phasic and sustained fear in humans elicits distinct patterns of brain activity. Neuroimage. 2011;55:389–400. doi: 10.1016/j.neuroimage.2010.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laurent V, Marchand AR, Westbrook RF. The basolateral amygdala is necessary for learning but not relearning extinction of context conditioned fear. Learns Mem. 2008;15:304–314. doi: 10.1101/lm.928208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denys D, et al. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2010;67:1061–1068. doi: 10.1001/archgenpsychiatry.2010.122. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Romaguera J, Do-Monte FH, Tanimura Y, Quirk GJ, Haber SN. Enhancement of fear extinction with deep brain stimulation: evidence for medial orbitofrontal involvement. Neuropsychopharmacology. 2015;40:1726–1733. doi: 10.1038/npp.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huff W, et al. Unilateral deep brain stimulation of the nucleus accumbens in patients with treatment-resistant obsessive-compulsive disorder: Outcomes after one year. Clin Neurol Neurosurg. 2010;112:137–143. doi: 10.1016/j.clineuro.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Baker KB, Kim JJ. Amygdalar lateralization in fear conditioning: evidence for greater involvement of the right amygdala. Behav Neurosci. 2004;118:15–23. doi: 10.1037/0735-7044.118.1.15. [DOI] [PubMed] [Google Scholar]
- 32.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5. Elsevier Academic; 2005. [DOI] [PubMed] [Google Scholar]
- 33.Luyten L, Van Cappellen F. ExpTimer: timer software to facilitate complex, multi-step procedures. Journal of Open Research Software. 2013;1 [Google Scholar]
- 34.Van Der Gucht E, Vandenbussche E, Orban GA, Vandesande F, Arckens L. A new cat Fos antibody to localize the immediate early gene c-fos in mammalian visual cortex after sensory stimulation. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2000;48:671–684. doi: 10.1177/002215540004800511. [DOI] [PubMed] [Google Scholar]
- 35.McNish KA, Gewirtz JC, Davis M. Evidence of contextual fear after lesions of the hippocampus: a disruption of freezing but not fear-potentiated startle. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:9353–9360. doi: 10.1523/JNEUROSCI.17-23-09353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richardson R, McNally GP. Effects of an odor paired with illness on startle, freezing, and analgesia in rats. Physiol Behav. 2003;78:213–219. doi: 10.1016/s0031-9384(02)00974-5. [DOI] [PubMed] [Google Scholar]
- 37.Brinkmann L, et al. Inter-individual differences in trait anxiety shape the functional connectivity between the bed nucleus of the stria terminalis and the amygdala during brief threat processing. Neuroimage. 2018;166:110–116. doi: 10.1016/j.neuroimage.2017.10.054. [DOI] [PubMed] [Google Scholar]
- 38.Beck CH, Fibiger HC. Conditioned fear-induced changes in behavior and in the expression of the immediate early gene c-fos: with and without diazepam pretreatment. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15:709–720. doi: 10.1523/JNEUROSCI.15-01-00709.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scicli AP, Petrovich GD, Swanson LW, Thompson RF. Contextual fear conditioning is associated with lateralized expression of the immediate early gene c-fos in the central and basolateral amygdalar nuclei. Behavioral neuroscience. 2004;118:5–14. doi: 10.1037/0735-7044.118.1.5. [DOI] [PubMed] [Google Scholar]
- 40.Herry C, Mons N. Resistance to extinction is associated with impaired immediate early gene induction in medial prefrontal cortex and amygdala. Eur J Neurosci. 2004;20:781–790. doi: 10.1111/j.1460-9568.2004.03542.x. [DOI] [PubMed] [Google Scholar]
- 41.Falls WA, Miserendino MJ, Davis M. Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.