Abstract
Existing neuroanatomical models argue that the bed nucleus of the stria terminalis (BST) principally mediates sustained, long-lasting fear or anxiety responses, but not shorter, phasic fear responses, although recent studies paint a more complex picture. In the current study, we evaluated the effect of post-training electrolytic BST lesions in a cued fear conditioning protocol with relatively short (10 s) tones. We hypothesized that the BST would not play a crucial role in the expression of fear upon re-exposure to the conditioned tones. Tone fear memory was primarily assessed through fear-potentiated startle. In addition, freezing measurements were obtained throughout the test sessions. In a series of three experiments, we explored the effects of BST lesions, taking into consideration contextual influences on cued fear expression (using (dis)similar training and test contexts) and temporal involvement of the BST in the consolidation of fear learning (lesion induction 3 or 27 hours after fear conditioning). In all three experiments, we found that post-training electrolytic lesions of the BST significantly reduced fear-potentiated startle, implying a deficit in differentiation between tone and context. These results are surprising and challenge the general consensus on the lack of BST involvement in cued fear. We discuss several alternative explanations that may account for these unexpected findings.
Keywords: bed nucleus of the stria terminalis, fear, conditioning, auditory fear conditioning, rat, startle, freezing, electrolytic lesions
Introduction
Pavlovian fear conditioning protocols have been extensively used to investigate neural mechanisms underlying both phasic and sustained fear (Calhoon and Tye, 2015; Davis et al., 2010; Perusini and Fanselow, 2015; Tovote et al., 2015). In general, a discrete cue (e.g. a 10-s tone) that was previously paired with an aversive event (e.g. a shock) will elicit a phasic fear response, whereas re-exposure to a conditioned cue or context associated with temporally unpredictable shocks will result in sustained fear, also referred to as anxiety. Substantial evidence, mainly collected by Davis, Walker and colleagues, suggests that phasic and sustained fear are mediated through different, yet highly interconnected regions of the brain: the central amygdala (CeA) and the bed nucleus of the stria terminalis (BST), respectively (Davis et al., 1997; Davis et al., 2010; Gewirtz et al., 1998; Hitchcock and Davis, 1986; Hitchcock and Davis, 1991; Luyten et al., 2011; Luyten et al., 2012; Sullivan et al., 2004; Walker and Davis, 1997).
Recent studies, however, indicate that the involvement of and interactions between the CeA and BST may be more complex than initially proposed (Goode and Maren, 2017; Gungor and Paré, 2016; Shackman and Fox, 2016). For instance, Duvarci et al. showed that ibotenic acid lesions made in the BST prior to training enhance discrimination between a reinforced white noise and unreinforced tone, with a smaller proportion of animals freezing during tone presentations compared to SHAM animals (Duvarci et al., 2009; Radke, 2009). In addition, pre-training intraperitoneal injection of a selective serotonin reuptake inhibitor (SSRI) significantly increased freezing to a conditioned tone, along with increased early gene expression in the BST and CeA (Ravinder et al., 2013). Intriguingly, these effects were reproduced by direct SSRI infusion in the BST, but not the CeA. Recently, Daldrup et al. investigated anterolateral BST activity in a cued fear conditioning procedure in which both phasic and sustained components of fear were represented through a conditioned tone of variable length (Daldrup et al., 2015; Daldrup et al., 2016). They showed that two categories of BST neurons were significantly suppressed during the acute phase of cued fear expression. In another recording study, Haufler et al. demonstrated that 25% of anterior BST neurons displayed phasic responses to a conditioned tone during fear recall (Haufler et al., 2013).
Previously, we found a hypermetabolic cluster comprising the BST in context- but not cue-conditioned animals (Luyten et al., 2012). In addition, we showed that electrolytic lesions of the anterior BST abolished contextual fear, as indexed by freezing and startle responses (Luyck et al., 2017; Luyten et al., 2011). Based on these prior findings and the seminal model proposed by Davis and colleagues, we hypothesized that the BST is most likely not importantly involved in cued fear expression. However, the studies listed above paint a more complex picture and suggest to look beyond the strict segregation between the CeA and BST regarding their involvement in phasic versus sustained fear.
In the current study, we therefore evaluated the effect of post-training electrolytic BST lesions in a tone fear conditioning protocol that we previously used in the abovementioned neuroimaging study (Luyten et al., 2012). Freezing before each session’s first tone presentation was measured to quantify contextual fear. Fear for the tone was indexed by fear-potentiated startle. In a series of three experiments, we explored the effects of BST lesions, taking into consideration contextual influences on cued fear expression (Exp1 & Exp2) and temporal involvement of the BST in the consolidation of fear learning (Exp2 & Exp3).
Materials & Methods
Subjects
Male Wistar rats (±250 g) were used in experiment (Exp) 1 (n = 29), in Exp2 (n = 21) and Exp3 (n = 22). All animals were housed in pairs with food and water available ad libitum. A plastic cage divider was used to prevent damage to the surgical wound by cage mates, while still allowing for social interaction. Animals were maintained on a 14/10 h light–dark cycle (lights on at 7:00 am), with a room temperature of ±19°C. This project was in accordance with the Belgian and European laws, guidelines and policies for animal experimentation, housing and care (Belgian Royal Decree of 29 May 2013 and European Directive 2010/63/EU on the protection of animals used for scientific purposes of 20 October 2010).
Surgery
All animals were implanted with stainless steel cannulas (23-gauge guide cannula C317G/5mm and dummy stylet C317DC/5mm, PlasticsOne, Roanoke, VA, USA) on the dura, directed towards the BST (anterior-posterior axis (AP): 0.0 mm, medio-lateral (ML): ± 3.4 mm, 20° angle to the sagittal plane), under general anesthesia (ketamine hydrochloride (22.5 mg/kg, Anesketin, Eurovet nv/sa, Heusden-Zolder, Belgium) and 0.15 mg/kg medetomine HCL (Kela, Sint-Niklaas, Belgium)), as described previously (Luyten et al., 2011). Animals were allowed to recover for 6-7 days before the start of behavioral experiments.
Equipment
Two different contexts were used for the behavioral experiments. Both contexts were located in different, ventilated, sound-attenuating Med Associates (Fairfax, VT, USA) chambers, positioned on opposite sides of the same room. Context A consisted of a small animal cage (inner dimensions: 9.4 cm height, 8.2 cm width, and 16.5 cm length) with a grid floor with six 5 mm-diameter stainless-steel rods, through which foot shocks could be delivered. A dim red light was continuously on. The freezing behavior of the animals was recorded by a video camera (DCR-SR55E Super NightShot Plus, Sony Corporation, Minato, Tokyo, Japan) positioned in front of the cage. The startle reaction of the rats generated a pressure on the response platform onto which the cage was fixed and analog signals were amplified, digitized, and processed by software (Startle Reflex, version 5.95; Med Associates). The presentation and sequencing of the acoustic stimuli and foot shocks were controlled by the same software. One of two loudspeakers, both located 7 cm behind the cage, was used to deliver a continuous white background noise (55 dB), the other speaker delivered the startle (white noise, 100 dB, 50 ms) and tone stimuli (4000 Hz, 75 dB, 10 s, 5 ms rise/fall). The startle response was defined as the first peak accelerometer voltage that occurred during the first 100 ms after onset of the startle probe and was measured on an arbitrary scale ranging from 0 to 2047. The startle platform and loudspeakers were calibrated before each experiment. The cage was cleaned with 70% ethanol between rats.
Context B consisted of a larger cage (21 cm height, 24.1 cm width, 30.5 cm length), with a grid floor with 19 rods (4.8 mm diameter, spaced 16 mm center to center) and a black triangular ceiling. The chamber was dimly-lit with a white light of 50 lux and the cage was cleaned with a scented cleaning product between rats. Behavior was recorded with a built-in infrared video camera and presentation of tones and shocks was controlled by software (Video Freeze, Med Associates).
Fear conditioning protocol
Exp1
All rats underwent four experimental sessions in context A, which were separated by 24h intervals (Fig. 1). Between the sessions, the animals were returned to their home cages. All experimental steps were strictly timed using ExpTimer software (Luyten and Van Cappellen, 2013), so each rat was tested at the exact same time every day.
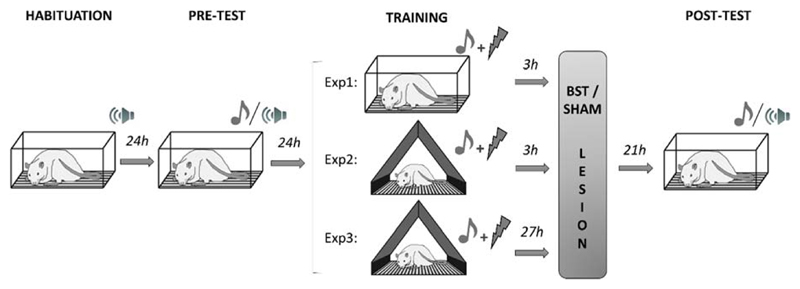
Fig. 1.
Experimental design for Exp1-3. On day 1 and 2, all animals underwent a habituation phase and baseline behavioral measurements (pre-test). On day 3, rats received ten tones (10 s each) that were paired with a foot shock, either in the same context as used during the test sessions (Exp1; SHAM, n=13; LES, n=10), or in a different context (Exp2; SHAM, n=9; LES, n=8) (Exp3; SHAM, n=9; LES, n=11). Rats in the LES group received lesions in the bed nucleus of the stria terminalis either 3 hours (Exp1-2) or 27 hours (Exp3) after the end of the training session. The next day, cued fear expression was evaluated during post-test.
Habituation
On day 1, the rats were placed in the startle chamber for a total of 20 minutes. During the first 5 minutes (acclimation phase), only background noise (55 dB) was presented. Afterwards, 30 acoustic startle stimuli (100 dB, 50 ms) were administered with a fixed inter-trial interval (ITI) of 30 s. This habituation session was added to stabilize startle responses, before any experimental manipulations took place. The data from this habituation session were not included in the analyses.
Pre-test
On the second day, the rats underwent a baseline test session with a total duration of 23 minutes. During the first 5 minutes, referred to as the acclimation phase, only background noise was presented. Percentage freezing during acclimation was scored manually by a blinded observer and served as a measure of contextual fear. Next, 30 startle stimuli were presented with an ITI of 30 s. Half of these startle stimuli were preceded by a 10-s tone (tone-noise trials), whereas the other half was presented as noise-alone trials. Startle measurements on noise-alone trials were a measure of contextual fear, whereas tone-noise trials indexed fear conditioned to the tone. The order of trial types was counterbalanced and randomized while no more than two trials of the same type occurred in a row. Rats were matched into equivalent groups (SHAM and LES) based upon their mean startle response during noise-alone trials on the pre-test.
Training
On the third day, the rats were conditioned to the tone. The total duration of the training session was 30 min. After a 5 min acclimation period, 10 tones (4000 Hz, 75 dB, 10 s) were presented (variable ITI of 100-140 s), each co-terminating with a foot shock (0.4 mA, 500 ms).
Post-test
On day 4, rats underwent a 23 min protocol identical to that described for pre-test.
Exp2
The fear conditioning protocol described in Exp1 was followed, with the exception that rats were trained in context B to reduce conditioned or generalized fear to the context during post-test.
Exp3
The behavioral protocol of Exp2 was followed, with the exception of the post-test taking place 48h after training to exclude any effects of the BST lesions on the consolidation of the long-term tone fear memory.
Lesion procedure
The lesioning procedure was performed as described by Luyten et al. (Luyten et al., 2011). Rats were briefly anesthetized with isoflurane (5% (induction) and 2% (maintenance) in 1.5–2.0 l/min oxygen). The dura was punctured through the cannula with a stainless steel needle (Acupro P20-3210, Medichin, Hasselt, Belgium). Next, custom-made insulated stainless steel electrodes (200 μm in diameter) (008SW/30S, PlasticsOne, Roanoke, VA, USA) with a transversally cut tip were inserted into the cannulas and lowered 6.3 mm below the dural surface, thereby bilaterally targeting the medial division of the anterior bed nucleus of the stria terminalis. The electrodes were connected to a stimulator (DS8000 and DLS100, World Precision Instruments, Stevenage, UK) and an anodal direct current pulse of 1 mA (LES) or 0 mA (SHAM) was applied during 15 s. One minute later, the electrodes were removed and anesthesia was ended. A few minutes later, the animals were awake. The whole lesioning procedure took 10–15 min.
Histology
Approximately one week after testing, all rats were given a lethal intraperitoneal injection of pentobarbital (2 ml; Nembutal, CEVA Santé Animale, Brussels, Belgium). The animals were perfused with a solution of 10% sucrose (D(+)-Saccharose, VWR international bvba, Leuven, Belgium), and subsequently with a 4% formaldehyde solution (37% dissolved in water, stabilized with 5-15% methanol, Acros organics, Geel, Belgium, 10x diluted in DI water). The brains were dissected and stored in 4% formaldehyde, processed and embedded in paraffin. Five-μm thick coronal slices were collected with the microtome (Leica Biosystems GmbH, Nussloch, Germany), and stained with Cresyl-Violet (0.5% cresyl violet acetate in dH2O, Merck KGaA, Darmstadt, Germany). Microscopic analysis revealed the exact location of electrode tips and lesions, which were transferred to a Paxinos coronal plate (Paxinos and Watson, 2005). Lesion animals were included when the largest diameter of the lesion comprised the anterior BST and clear damage (including necrosis and edema) was visible on the bregma slice. Lesions included in this study mainly destroyed the medial and lateral divisions of the anterior BST (dorsal and often ventral part). Additional damage was observed to parts of the septal nuclei, posterior BST and internal capsule. Exceptionally, partial damage to the caudate putamen and to the posterior part of the nucleus accumbens was observed. SHAM animals were included when no damage was observed in the BST or surrounding areas (e.g. through electrode insertion).
Statistics
All analyses and graphs were generated using Graphpad Prism (version 4.03, GraphPad Software). Significance levels were set at p < 0.05. Pre-test startle measurements were analyzed using an unpaired t-test, to verify successful matching between groups. Post-test freezing and startle measurements were corrected for baseline values acquired during pre-test and are therefore shown as absolute difference scores (post minus pre).
Difference scores (post minus pre) for startle response were analyzed by means of a 2-way repeated measures ANOVA (RM-ANOVA), using factors “Trial Type” (noise-alone vs tone-noise) and “Group” (SHAM vs LES). Bonferroni’s post-hoc test was used to specify group differences. In addition, planned comparisons (paired t-tests) were performed within each group to compare difference scores (post minus pre) of startle responses between noise-alone and tone-noise trials. To correct for multiple testing, significance levels for planned comparisons were set at p < 0.025. The assumption of normality was not met for freezing data during acclimation (difference scores, post minus pre), which were therefore analyzed with a non-parametric Mann-Whitney U (MWU) test. Startle responses are represented as means ± SEM, freezing data as individual scores with group medians. Note that, throughout the text, when discussing post-test startle amplitudes or freezing scores, we always refer to the corrected post-test value, i.e., the absolute difference of post-test minus pre-test, unless a direct comparison between raw pre-test and post-test values is explicitly mentioned.
Finally, to evaluate the presence of contextual fear in SHAM and LES animals, paired t-tests were conducted to directly compare raw pre- and post-test measurements of freezing during acclimation, as well as raw pre- and post-test startle responses on noise-alone trials. Significance levels were adjusted to correct for multiple testing (p < 0.025).
Results
Experiment 1
Histology
Four rats were excluded due to insufficient damage to the bilateral medial BST (for details see Suppl. Fig. 1). In addition, on post-test, one animal of the LES group was not sufficiently recovered from lesion induction (porphyrin discharge around eyes and nose, puffy appearance, immobile) and was therefore omitted from all analyses. Finally, one LES animal was excluded due to equipment malfunction during lesion induction, which resulted in a unilateral lesion in the right BST. In total, 13 rats were included in the SHAM group and 10 animals in the LES group (Fig. 2A).
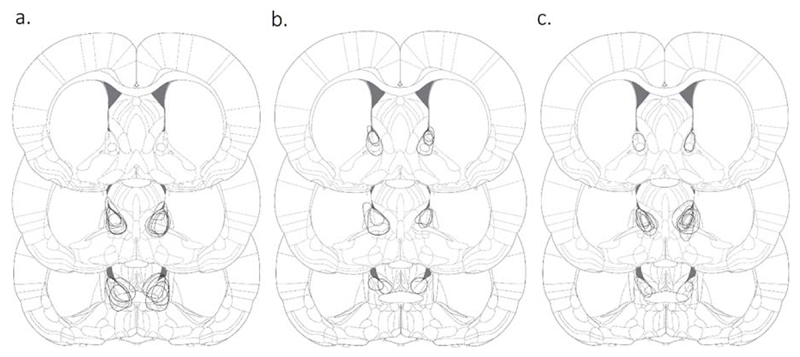
Fig. 2.
Reconstruction of electrolytic lesions in the bed nucleus of the stria terminalis. The maximal diameter of each lesion is shown for Exp1 (A), Exp2 (B) and Exp3 (C). Coronal slices shown from top to bottom are +0.24 mm, 0.00 mm and -0.24 mm, with respect to bregma. Figure adapted from (Paxinos and Watson, 2005).
Behavior
Pre-test freezing levels during acclimation were low (1% ± 2%, mean ± SD) and startle amplitudes on noise-alone trials were comparable between groups (SHAM: 159 ± 139, LES: 141 ± 105) (t (21) = 0.33; p = 0.74), indicating that matching was effective. In addition, pre-test startle amplitudes on tone-noise trials did not differ significantly between SHAM (226 ± 245) and LES (172 ± 153) groups (t(21) = 0.65; p = 0.52).
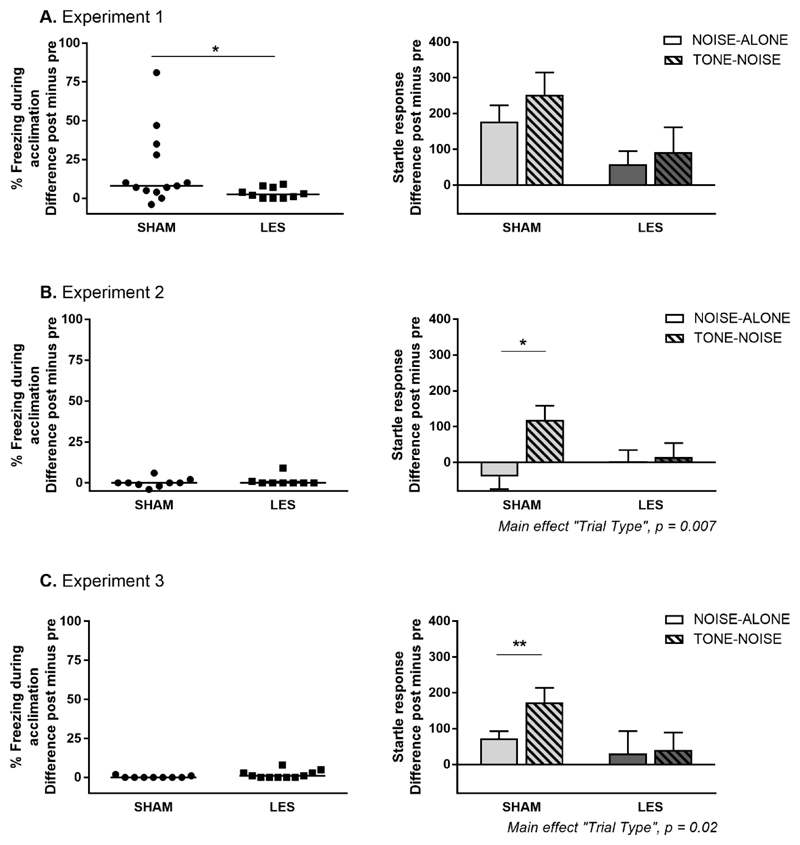
On post-test, contextual freezing during acclimation was significantly lower in the LES group than in the SHAM group (MWU: 31.50, p = 0.036) (Fig. 3A, left panel). A two-way RM-ANOVA of startle response revealed a borderline significant main effect of “Trial Type” (F (1,21) = 4.25; p = 0.05) and “Group” (F (1,21)= 3.52; p = 0.07), but no interaction (F (1,21) = 0.62, p = 0.44) (Fig. 3A, right panel). Due to relatively high startle amplitudes on noise-alone trials in the SHAM group, the difference between noise-alone and tone-noise trials just failed to reach significance (planned comparison: t(12) = 2.22; p = 0.047, not significant after correction for multiple testing, α = 0.025). For LES rats, startle responses did not differ between both trial types (t (9) = 0.82; p = 0.43). Exploratory analyses (Suppl. Fig. 2) suggest that the overall startle attenuation in the LES group was specific to lesions in the anterior BST, as opposed to adjacent brain areas.
Fig. 3.
3 Effects of BST lesions on contextual freezing and fear-potentiated startle. Absolute difference scores (i.e., post-test values minus pre-test values) are shown in each graph. Percentage freezing during acclimation is shown in the left panels as individual data and median per group for Exp1 (A), Exp2 (B) and Exp3 (C). Startle responses during noise-alone and tone-noise trials are shown in the right panels as means ± SEM, * p < 0.05, ** p < 0.01.
The experimental protocol used for this first study produced substantial contextual fear, as indicated by the strong increase in freezing during acclimation (t (12) = 2.77; p = 0.02) and startle responses on noise-alone trials (t(12) = 3.90; p = 0.002) from pre- to post-test in SHAM animals. LES animals also showed an increase in freezing during acclimation (t(9) = 3.10; p = 0.01) from pre- to post-test, but with very low residual contextual freezing during post-test (4% ± 4%, mean ± SD). In line with this, there was no significant increase in startle responses on noise-alone trials in LES animals (t(9) = 1.61; p = 0.14). Since the presence of contextual fear may have confounded the interpretation of the lesion effect on cued fear, training sessions were conducted in a perceptually different context in the next experiments.
Experiment 2
Histology
Four rats were excluded due to incorrect lesion location. In two of these animals, lesions were situated too dorsally and mainly affected the lateral septal nucleus and the most dorsal extension of the BST. In one animal, bilateral lesions were observed in the nucleus accumbens, but not in the BST. Finally, one animal displayed unilateral damage focused on the septohypothalamic nucleus rather than the BST. In total, we included 9 animals in the SHAM group and 8 in the LES group (Fig. 2B).
Behavior
Pre-test freezing levels during acclimation were low (1% ± 1%, mean ± SD) and startle amplitudes on noise-alone trials were comparable between groups (SHAM: 216 ± 105, LES: 251 ± 125) (t (15) = -0.64; p = 0.53). In addition, pre-test startle amplitudes on tone-noise trials did not differ significantly between SHAM (207 ± 110) and LES (312 ± 169) groups (t (15) = 1.54; p = 0.14).
On post-test, contextual freezing during acclimation did not differ between LES and SHAM groups (MWU = 26; p = 0.34) (Fig. 3B, left panel). Startle responses showed significant effects of “Trial Type” (F (1,15) = 9.82; p = 0.007) and an interaction between “Group” and “Trial Type” (F (1,15) = 7.26; p = 0.017), but no main effect of “Group” (F (1,15) = 0.47; p = 0.50) (Fig. 3B, right panel). Post-hoc Bonferroni analysis revealed a significant difference between noise-alone and tone-noise trials in SHAM group (p = 0.001), but not in the LES group (p = 0.95).
Experiment 3
Histology
One animal was excluded based on incorrect lesion location, as damage in the right hemisphere targeted the septal nuclei rather than the BST. One additional animal was omitted from further analyses due to insufficient recovery after lesion induction. Nine subjects were included in the SHAM group and 11 in the LES group (Fig. 2C).
Behavior
Pre-test freezing levels during acclimation were low (1% ± 2%, mean ± SD) and startle amplitudes on noise-alone trials were comparable between groups (SHAM: 200 ± 108, LES: 164 ± 170) (t (18) = 0.55; p = 0.59). In addition, pre-test startle amplitudes on tone-noise trials did not differ significantly between SHAM (213 ± 101) and LES (200 ± 188) groups (t (18) = 0.18; p = 0.86).
On post-test, contextual freezing levels during acclimation did not differ between SHAM and LES groups (MWU = 30.5; p = 0.10) (Fig. 3C, left panel). Startle responses showed a significant effect of “Trial Type” (F (1,18) = 6.84; p = 0.02) and an interaction between “Group” and “Trial Type” (F (1,18) = 4.57; p = 0.046), but no main effect of “Group” (F (1,18) = 1.81; p = 0.20) (Fig. 3C, right panel). Post-hoc Bonferroni analysis revealed a significant difference between noise-alone and tone-noise trials in SHAM group (p = 0.01), but not in the LES group (p = 0.93).
Discussion
In this study, three experiments were conducted to evaluate the effect of post-training electrolytic lesions in the anterior BST on the expression of cued fear.
In Exp1, conditioning to a 10-s tone and subsequent testing occurred in the same context. We found that differentiation of fear responses to tone and context was suboptimal in the SHAM group due to relatively high levels of contextual fear. LES animals showed lower freezing and startle levels than SHAM animals, regardless of trial type, confirming our previous finding that BST lesions reduce contextual fear (Luyten et al., 2011). In the second experiment, animals were conditioned in a different context to reduce conditioned or generalized fear to the testing context. As anticipated, this approach resulted in low contextual freezing during acclimation in both groups. In addition, SHAM rats discriminated between both trial types in terms of startle response, whereas LES animals did not. In a third experiment, lesions were made 27 hours instead of 3 hours after training, to ensure that our observations were not confounded by interference with fear memory consolidation. Results were similar to Exp2, since SHAM, but not LES, animals showed differential noise-alone and tone-noise startle responses.
In contrast with the present findings, several research groups previously reported that post-training BST lesions do not affect cued fear, as quantified with startle or freezing responses. The neural pathways that mediate these behavioral responses to conditioned stimuli are largely common, until the point where they diverge to two different brain stem regions, the caudal pontine reticular nucleus and ventral periaqueductal gray, respectively (Walker et al., 2003). Hitchcock et al. performed electrolytic BST lesions 1 to 2 days after 10 light-shock pairings (Hitchcock and Davis, 1991). When testing 7 days later, they observed no effect on fear-potentiated startle. Likewise, Walker et al. found no effect of chemical BST lesions made 48h after light-shock training, immediately followed by fear-potentiated startle measurements (Walker and Davis, 1997). Note that, in both studies, lesion coordinates were slightly posterior (approximately 500 μm) to the ones used in the current study. On the other hand, Sullivan et al. made extensive electrolytic BST lesions along a large portion of the AP-axis, with lesion sites at 0.0 mm, -0.5 mm and -1.0 mm with respect to bregma (Sullivan et al., 2004). They induced the lesions 48h post-training and animals were tested 5 days later. No effects were found on cortisol levels and freezing to the conditioned tone. Finally, Zimmerman and Maren found no effect on freezing to the tone with NMDA-lesions in the BST made 24h after tone-shock pairings (Zimmerman and Maren, 2011).
The findings of the studies described above are seemingly inconsistent with the current study. In this regard, it is important to note that our electrolytic BST lesions most likely did not affect motor behavior. While the startle increase from pre- to post-test is abolished in LES animals, we still detected a substantial startle reflex during post-test, implying an intact startle circuitry. In addition, electrolytic BST lesions made with the exact same technique at the same position, did not affect motor behavior in an open field test in a previous experiment, as measured by the total distance travelled and the percentage of movement (Luyck et al., 2017). Apart from a lack of motor deficits resulting from the BST lesions, the open field data also indicate that LES animals are not completely free of any fear whatsoever, which could have precluded them from expressing cued fear in the current study if this would have been the case. However, we found that LES animals still show substantial thigmotaxis (about 3% of the test time spent in the center of the open field, versus 5% in SHAM animals) (Luyck et al., 2017; Treit and Fundytus, 1988).
Although somewhat surprising given the existing lesion literature, our data do suggest that lesions of the BST can interfere with cued fear retrieval. This may be in line with more recent evidence suggesting that the functional distinction between BST and CeA involvement may be not that straightforward (Daldrup et al., 2016; Duvarci et al., 2009; Gungor and Paré, 2016; Haufler et al., 2013; Ravinder et al., 2013; Shackman and Fox, 2016). It is also interesting in this regard that (Goode et al., 2015) found that BST lesions prevented reinstatement (which is presumably mediated by context conditioning (Waddell et al., 2006; 2008)) of extinguished fear to a 10-s tone. Nevertheless, a profound disruption of cued fear by BST lesions is unexpected, and therefore we here present two alternative explanations that might have, in combination or alone, contributed to our results.
A first alternative account hinges on the assumption that our animals experienced overall elevated stress and sustained fear levels during post-test, which may have played a role in all three experiments. Startle probe presentation during post-test may activate a previously formed association between tones and startle probes, which, in turn, results in recall of the shocks, and thus in generalized fear and stress. To further investigate this hypothesis, we evaluated cued fear using a second parameter, i.e. freezing before probes (see Supplement). Whereas startle responses are reflexive reactions, freezing during the 10-s interval before each startle probe provides information over a longer time period.
While contextual freezing before onset of the first startle probe was still low and hardly seemed to indicate any contextual fear, the first tones and startle probes appear to recollect the shock memory, resulting in a significant increase in overall freezing (see Supplement). Remarkably, freezing during tones versus context intervals did not differ significantly in SHAM animals in any experiment. In addition, freezing before probes was particularly high in Exp1, in agreement with heightened overall stress and contextual fear levels on post-test when the physical context is the same as during conditioning. Taken together, fear to the tone on the post-test may be substantially influenced by a generalized stress response prompted by the presentation of startle probes (and tones). Given the demonstrated involvement of the BST in stress responses (Crestani et al., 2013; Daniel and Rainnie, 2016), we cannot exclude that the observed effects of BST lesions on fear-potentiated startle largely reflect a non-specific reduction of general stress levels.
On a side note, we would like to mention that, in prior experiments, we did find significant differentiation between the tone and context with the behavioral protocol used in Exp1 (Luyten et al., 2012). However, several slight procedural changes (use of operated animals, different experimenter, housing conditions, and experimental room) may explain the higher overall stress levels in the current experiment. Note that the presumably less confounded fear to the tone in our prior PET study may account for the observed absence of BST involvement, i.e. no metabolic differences in this brain area in tone-conditioned rats compared to control conditions. Additionally, the lack of BST involvement in our imaging study may be partially explained by the limited exposure to the conditioned tone (fifteen 10-s tones during a 23-min test session) during radioactive tracer uptake, although this remains speculative.
As a second alternative explanation for our set of data, particularly for Exp2 and 3, we suggest that BST lesions may interfere with contextual generalization of the fear memory. Generalization is a crucial aspect of learning as it allows individuals to adapt their behavior in changing situations (Hermans, 2013). More specifically, knowledge acquired during a prior experience (e.g. tone-shock association) should be transferred to other situations in order to be useful (e.g. a different physical context at a different point in time) (Bouton, 1993). It is not unlikely that a functional BST is necessary for such successful generalization of cued fear to different situations (see also Goode et al., 2015). This may be especially applicable to Exp2 and 3, where the animals had to transfer the acquired fear memory to a physically different context, 24 or 48 hours later. In addition, we note that tones were already presented during pre-test to obtain baseline behavioral measurements and to increase comparability with our previous studies (Luyten et al., 2011; Luyten et al., 2012). This procedural choice, however, may have enhanced the relative importance of the context (i.e. tones predict startle probes in the test context, and tones predict shocks in the training context), thereby fostering the integration of the context in the tone fear memory. As a result, the predictive value of the CS may become dependent on the context, thereby making generalization of cued fear from the training to the test context more difficult. Whereas this may not be a problem for animals with an intact BST, it may be detrimental to retrieval of the tone fear memory in lesioned animals.
Generalization from training to test context may have been less challenging for animals in earlier studies which concluded that BST lesions did not affect cued fear. Many of these experiments used identical chambers for training and testing (e.g., Walker and Davis, 1997; Gewirtz et al., 1998; Zimmerman et al., 2011, but see Hitchcock et al., 1991). Others used the same chambers for training and testing but changed some contextual features (for instance floor and odor, e.g. Duvarci et al., 2009) or used completely different chambers (for instance Med Associates and Coulbourn, e.g. Sullivan et al., 2004), but, in each of these cases, pre-exposed the animals to both contexts for 20 minutes on day 0, which has been found to make contexts more similar (Luyten, unpublished observations).
To further explore the avenue of a potential generalization deficit in BST-lesioned animals, future experiments might, for example, use a cued fear conditioning procedure that does not require extensive contextual generalization. More specifically, all sessions should take place in the same physical context, which may also include startle probe presentations during training to enhance similarity on all days. Modifications to the duration of the inter-trial intervals or the number of tone-shock pairings (as compared with the procedure applied in the current studies) should ensure that SHAM animals show statistically significant startle potentiation to the tone as compared with the context (which in itself should elicit very low levels of fear). If LES animals would then still show impaired tone fear memory, this could not be attributable to a generalization deficit.
In conclusion, we showed that post-training electrolytic lesions of the BST significantly reduced fear-potentiated startle in three experiments. These results are rather surprising and challenge the general consensus on the lack of BST involvement in cued fear. We note that alternative explanations may (partially) account for these unexpected findings, although further research is necessary to confirm this. We suggest that BST lesions may have reduced overall elevated stress and contextual fear levels in our procedure, thereby also disrupting cued fear. Additionally, a functional BST might be required for generalization of a cued fear memory from the training to the test context. Our data open up interesting perspectives for further research to address these questions, for example through experiments with lower overall stress levels (e.g. weaker fear conditioning, no startle measurements) or experiments with increased similarity of training and test contexts.
Supplementary Material
Acknowledgements
We acknowledge the financial support of the Medtronic Chair for Stereotactic Neurosurgery in Psychiatric Disorders, of which Bart Nuttin is chair holder, the Research Foundation - Flanders (FWO) (Research Projects G072909N and G0C9817N, and Postdoctoral Fellowship 1295613N (to Laura Luyten)), the European Research Council (CoG 64817), as well as the KU Leuven Center for Excellence on Generalization Research Grant PF/10/005. We would like to thank Marjolijn Deprez, Steve Ravelingien, Kelly Pelsmaekers, Ineke Pillet and Anna-Elisabeth Schnell for their contributions to the behavioral and histological analyses. We also thank Prof. Tom Beckers for the helpful discussions during the preparation of this manuscript.
References
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychological Bulletin. 1993;114:80. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Calhoon GG, Tye KM. Resolving the neural circuits of anxiety. Nat Neurosci. 2015;18:1394–404. doi: 10.1038/nn.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani CC, Alves FH, Gomes FV, Resstel LB, Correa FM, Herman JP. Mechanisms in the bed nucleus of the stria terminalis involved in control of autonomic and neuroendocrine functions: a review. Curr Neuropharmacol. 2013;11:141–59. doi: 10.2174/1570159X11311020002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daldrup T, Remmes J, Lesting J, Gaburro S, Fendt M, Meuth P, Kloke V, Pape HC, Seidenbecher T. Expression of freezing and fear-potentiated startle during sustained fear in mice. Genes Brain Behav. 2015;14:281–91. doi: 10.1111/gbb.12211. [DOI] [PubMed] [Google Scholar]
- Daldrup T, Lesting J, Meuth P, Seidenbecher T, Pape HC. Neuronal correlates of sustained fear in the anterolateral part of the bed nucleus of stria terminalis. Neurobiol Learn Mem. 2016;131:137–46. doi: 10.1016/j.nlm.2016.03.020. [DOI] [PubMed] [Google Scholar]
- Daniel SE, Rainnie DG. Stress Modulation of Opposing Circuits in the Bed Nucleus of the Stria Terminalis. Neuropsychopharmacology. 2016;41:103–25. doi: 10.1038/npp.2015.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Lee Y. Amygdala and bed nucleus of the stria terminalis: differential roles in fear and anxiety measured with the acoustic startle reflex. Philos Trans R Soc Lond B Biol Sci. 1997;352:1675–87. doi: 10.1098/rstb.1997.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–35. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S, Bauer EP, Paré D. The bed nucleus of the stria terminalis mediates inter-individual variations in anxiety and fear. J Neurosci. 2009;29:10357–61. doi: 10.1523/JNEUROSCI.2119-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz JC, McNish KA, Davis M. Lesions of the bed nucleus of the stria terminalis block sensitization of the acoustic startle reflex produced by repeated stress, but not fear-potentiated startle. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:625–48. doi: 10.1016/s0278-5846(98)00028-1. [DOI] [PubMed] [Google Scholar]
- Goode TD, Kim JJ, Maren S. Reversible Inactivation of the Bed Nucleus of the Stria Terminalis Prevents Reinstatement But Not Renewal of Extinguished Fear(1,2,3) eNeuro. 2015;2 doi: 10.1523/ENEURO.0037-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode TD, Maren S. Role of the bed nucleus of the stria terminalis in aversive learning and memory. Learn Mem. 2017;24:480–491. doi: 10.1101/lm.044206.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gungor NZ, Paré D. Functional Heterogeneity in the Bed Nucleus of the Stria Terminalis. J Neurosci. 2016;36:8038–49. doi: 10.1523/JNEUROSCI.0856-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haufler D, Nagy FZ, Pare D. Neuronal correlates of fear conditioning in the bed nucleus of the stria terminalis. Learn Mem. 2013;20:633–41. doi: 10.1101/lm.031799.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans D, Baeyens F, Vervliet B. In: Handbook of Cognition and Emotion. Robinson M, Watkins E, Harmon-Jones E, editors. The Guilford Press; 2013. Generalization of acquired emotional responses; pp. 117–134. [Google Scholar]
- Hitchcock J, Davis M. Lesions of the amygdala, but not of the cerebellum or red nucleus, block conditioned fear as measured with the potentiated startle paradigm. Behav Neurosci. 1986;100:11–22. doi: 10.1037//0735-7044.100.1.11. [DOI] [PubMed] [Google Scholar]
- Hitchcock JM, Davis M. Efferent pathway of the amygdala involved in conditioned fear as measured with the fear-potentiated startle paradigm. Behav Neurosci. 1991;105:826–42. doi: 10.1037//0735-7044.105.6.826. [DOI] [PubMed] [Google Scholar]
- Luyck K, Tambuyzer T, Deprez M, Rangarajan J, Nuttin B, Luyten L. Electrical stimulation of the bed nucleus of the stria terminalis reduces anxiety in a rat model. Transl Psychiatry. 2017;7:e1033. doi: 10.1038/tp.2017.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyten L, van Kuyck K, Vansteenwegen D, Nuttin B. Electrolytic lesions of the bed nucleus of the stria terminalis disrupt freezing and startle potentiation in a conditioned context. Behav Brain Res. 2011;222:357–62. doi: 10.1016/j.bbr.2011.03.066. [DOI] [PubMed] [Google Scholar]
- Luyten L, Casteels C, Vansteenwegen D, van Kuyck K, Koole M, Van Laere K, Nuttin B. Micro-positron emission tomography imaging of rat brain metabolism during expression of contextual conditioning. J Neurosci. 2012;32:254–63. doi: 10.1523/JNEUROSCI.3701-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyten L, Van Cappellen F. ExpTimer: timer software to facilitate complex, multi-step procedures. Journal of Open Research Software. 2013;1 [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5 Elsevier Academic; Amsterdam: 2005. [Google Scholar]
- Perusini JN, Fanselow MS. Neurobehavioral perspectives on the distinction between fear and anxiety. Learn Mem. 2015;22:417–25. doi: 10.1101/lm.039180.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke AK. The role of the bed nucleus of the stria terminalis in learning to fear. J Neurosci. 2009;29:15351–2. doi: 10.1523/JNEUROSCI.5194-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravinder S, Burghardt NS, Brodsky R, Bauer EP, Chattarji S. A role for the extended amygdala in the fear-enhancing effects of acute selective serotonin reuptake inhibitor treatment. Transl Psychiatry. 2013;3:e209. doi: 10.1038/tp.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Fox AS. Contributions of the Central Extended Amygdala to Fear and Anxiety. J Neurosci. 2016;36:8050–63. doi: 10.1523/JNEUROSCI.0982-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GM, Apergis J, Bush DE, Johnson LR, Hou M, Ledoux JE. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004;128:7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Tovote P, Fadok JP, Lüthi A. Neuronal circuits for fear and anxiety. Nat Rev Neurosci. 2015;16:317–31. doi: 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- Treit D, Fundytus M. Thigmotaxis as a test for anxiolytic activity in rats. Pharmacol Biochem Behav. 1988;31:959–62. doi: 10.1016/0091-3057(88)90413-3. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci. 1997;17:9375–83. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Zimmerman JM, Maren S. The bed nucleus of the stria terminalis is required for the expression of contextual but not auditory freezing in rats with basolateral amygdala lesions. Neurobiol Learn Mem. 2011;95:199–205. doi: 10.1016/j.nlm.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.