Summary
Blind people report disturbances in alertness, mood and performance. In laboratory studies, these waking functions can only be maintained when the wake-dependent deterioration is opposed by appropriately-timed endogenous circadian rhythms. We aimed to quantify whether variations in waking function experienced by blind people living in society were dependent on the phase relationship between the sleep-wake cycle and the circadian pacemaker. The time course of alertness, mood and performance was assessed in 52 blind subjects with and without circadian rhythm disorders every 2 h for 2 days per week for 4 weeks. Sleep-wake timing and circadian phase were assessed from diaries and weekly measurements of urinary 6-sulphatoxymelatonin rhythms, respectively. In those subjects who woke at either a normal circadian phase (n = 26) or abnormally early (n = 5), alertness, mood and performance deteriorated significantly with increased time awake (P < 0.05). In 17 non-entrained (‘free-running’) subjects, waking function varied significantly with circadian phase such that subjects rated themselves most sleepy (P = 0.03) and most miserable (P = 0.02) when they were awake during the time of peak melatonin production. The internal phase relationship between sleep-wake behaviour and the circadian melatonin rhythm in entrained subjects contributed to predictable differences in the daily profile of alertness, mood and performance. Disruption of this phase relationship in non-entrained blind individuals with circadian rhythm sleep disorders resulted in impaired waking function during the day equivalent to that usually only experienced when awake during the night. Treatment for circadian rhythm disorders should be targeted in normalizing these phase relationships.
Keywords: alertness, blindness, circadian, mood, performance, sleep
Introduction
Ocular light exposure is the major environmental time cue for synchronization of endogenous circadian rhythms, for example those of sleep, some hormones and temperature (see Czeisler and Wright, 1999 for review). Consequently, many totally blind individuals exhibit abnormally timed circadian rhythms in physiology and behaviour, particularly sleep (Leger et al., 1999; Lockley et al., 1999; Tabandeh et al., 1998), as a result of light failing to reach the suprachiasmatic nuclei, the site of the primary circadian oscillator. In most totally blind subjects, the timing of circadian rhythms, including the sleep-wake cycle, reverts to the individual’s endogenous circadian period, which is usually slightly longer than 24 h (range: 23.9–25.0 h) (Lockley et al., 1997a; Miles et al., 1977; Sack et al., 1992; Skene et al., 1999). The resultant non-24-hour sleep-wake disorder (ICSD-2, 2005) is characterized by episodes of good sleep, followed by episodes of poor night-time sleep and excessive day-time napping, in a repetitive life-long cycle (Klein et al., 1993; Lockley et al., 1997a, 1999; Miles et al., 1977). Some totally blind people also exhibit either abnormally advanced or abnormally delayed 24-h circadian rhythms (Lewy and Newsome, 1983; Lockley et al., 1997a), symptomatic of advanced- and delayed sleep phase syndrome (ICSD-2, 2005; Lockley et al., 1999), respectively.
While these studies have characterized changes in circadian physiology and sleep, little is known about the consequences of these disorders for an individual’s ability to function when awake, as characterized by alertness, mood and performance. Several laboratory-based case studies of totally blind men with non-24-hour sleep-wake disorder have reported cyclic components to the variability in alertness, mood and performance that were highly correlated with the timing of the endogenous circadian rhythms of cortisol and body temperature (Klein et al., 1993; Miles et al., 1977; Nakagawa et al., 1992). The timing of day-time naps, a good indicator of sleepiness, is also closely associated with the timing of the circadian melatonin rhythm in blind people with non-24-hour and abnormally-phased circadian rhythms studied at home (Lockley et al., 1997b, 1999).
In addition to the endogenous circadian pacemaker, the duration of wakefulness also modulates alertness and performance such that these waking neurobehavioural functions can only be maintained when their wake-dependent deterioration is appropriately synchronized with an opposing circadian arousal rhythm (e.g. Boivin et al., 1997; Carskadon et al., 2004; Dijk and Czeisler, 1994; Dijk et al., 1992; Monk et al., 1989; Wyatt et al., 1999). The time course of diurnal mood changes in healthy subjects is also dependent on the same processes (Boivin et al., 1997), with subjective ratings of ‘cheerfulness’ and ‘happiness’ gradually declining during wakefulness, and exhibiting a distinct circadian rhythm, with the poorest mood occurring in the middle of the biological night. The implication of these studies is that the internal phase relationship between the timing of the sleep-wake cycle and endogenous circadian rhythmicity is a major determinant of the time course of alertness, mood and performance rhythms during wake episodes (see Dijk and Lockley, 2002 for review).
The aim of the current study was to measure the variations in alertness, mood and performance rhythms in blind people with and without circadian rhythm disorders while living in society and to examine the contribution of circadian and homeostatic processes in determining the time course of waking function previously observed only under highly controlled laboratory studies.
Methods
Subjects
Fifty-two registered blind subjects were studied (35 male, 17 female; mean age ± SD = 48 ± 12 years, range: 19–72 years), 22 of whom had light perception (LP) in at least one eye and 30 who had no conscious perception of light (NPL). The study was conducted at the subjects’ homes for at least 4 weeks and no restrictions were placed on their lifestyle. Subjects were excluded prior to study, however, if they were taking medications which could affect sleep or melatonin rhythms (i.e. β-blockers, benzodiazepines, monoamine oxidase inhibitors, tricyclic antidepressants, steroids). Subjects were screened for affective disorders using the Montgomery-Asberg Depression Rating Scale (Tabandeh et al., 1998). None of the subjects or their primary care physicians reported any affective disorder. The study was approved by the University of Surrey Advisory Committee on Ethics and subjects provided written informed consent.
Sleep and melatonin
Daily sleep and nap diaries were completed, including information on sleep onset, sleep offset and the timing, number and duration of day time naps (defined as any sleep outside the bedtime episode) (Lockley et al., 1997a,b, 1999). Circadian rhythm type was determined from analysis of 6-sulphatoxymelatonin (aMT6s) in urine collected sequentially every 4–8 h for 24–48 h per week for 4 weeks. aMT6s is considered a reliable marker of circadian phase (Arendt, 1995). aMT6s concentration (ng mL−1) was converted to ng h−1 for each collection period [ng mL−1 × volume (mL) / collection duration (h)] and subjected to cosinor analysis. Regression analysis of significant cosinor-derived acrophase (peak, ϕ) times was used to determine whether the aMT6s rhythms cycled at a period (τ) significantly different from 24 h [non-entrained or ‘free-running’ (FR); τ = 24 + slope ± 95% confidence limits]. When the period of the rhythm did not differ significantly from 24 h, normally (NE) and abnormally entrained (AE) rhythms were defined as those with average acrophase times within or outside the range for sighted subjects, respectively (mean ± 2SD for sighted subjects = 1.3–7.1 h; Lockley et al., 1997a). A total of 26 subjects were defined as having NE aMT6s rhythms (18 LP; 8 NPL), 9 were AE (4 LP, 5 NPL) and 17 were FR (τ range: 23.92–24.79 h; all NPL). AE subjects were further categorized into those in whom the melatonin rhythm was timed relatively early (advanced) compared with normal (AE-Advanced, n = 4; aMT6s ϕ mean range = 20.3–1.0 h) or in whom the timing of the melatonin rhythm occurred relatively late (delayed) (AE-delayed, n = 5; aMT6s ϕ mean range = 7.2–14.3 h) (Lockley et al., 1997a). Additional analysis details and results are described elsewhere (Lockley et al., 1997a; Skene et al., 1999).
Alertness, mood and performance
Subjects were asked to complete four 9-point alertness and mood scales on the urine sampling days approximately every 2 h while awake, from as soon as possible after waking until just before going to bed (approximately 6–8 times/day × 8 days). They were also asked to complete the scales once per day in the evening throughout the study (approximately 28 days). The alertness scale was defined as follows; 1–very alert; 3–alert; 5–neither alert nor sleepy; 7–sleepy; 9–very sleepy. The mood scales had similar denominators defining very cheerful-very miserable; very calm-very tense; and very depressed-very elated. Subjects were instructed to use the even numbers when appropriate.
Subjects were asked to complete a four-choice auditory serial reaction time test on hand-held personal organizers (Psion Organizer II, Model LZ; Psion PLC, London, UK) (Totterdell and Folkard, 1993) after completion of the alertness and mood scales. Subjects were also asked to perform the test as often as possible prior to and between the urine sampling days to reduce potential practice effects. The test consisted of four tones that were presented in a random order a total of 40 times each (160 stimuli for each test). Each of the tones corresponded to one of four tactilely-marked buttons on the computer keyboard and subjects placed their first and second fingers of each hand on an individual button. They were asked to press the corresponding button in response to a continuous tone as accurately and quickly as possible. Once a response had been entered (whether correct or not), the next tone sounded immediately. The test was therefore self-paced. The tone continued indefinitely if no response was made. The tones were set two octaves apart at 33, 131, 523 and 2093 Hz. The performance parameters were recorded for each half of the test for each hand individually and consisted of reaction time (RT) in centiseconds of both correct and incorrect responses separately, RT of all responses, the percentage of incorrect responses and the percentage of ‘gaps’ or lapses, defined as responses >1 s. To control for gross practice effects, the first quartile of tests for each subject was excluded prior to analysis.
Data analysis
To analyse the contribution of circadian phase and time awake to alertness, mood and performance, each observation was assigned a circadian phase in degrees (0°/360° = aMT6s acrophase) and either a time elapsed since waking from night-time sleep, or time elapsed since waking from the last sleep, including day-time naps. Effects of circadian phase (45° bins; equivalent to approximately 3 h clock time) and time elapsed since waking (3 h intervals) were assessed using a mixed repeated measures linear model with random between-subjects effects and autoregressive correlations within-subjects (SAS Institute, Cary, NC, USA) (Littell et al., 1998). Analyses were completed on both raw data and on the data after being z-scored for each subject and averaged within subjects, within measures and within circadian rhythm type for the same circadian phase and elapsed time. Statistical significance was always reached for the z-scores if the raw data showed a significant effect therefore the statistics are reported for the raw data. All times are reported as decimals.
Results
Compliance
Of the 52 subjects reported, 38 completed both the 9-point scales and performance test (18 NE, 6 AE, 14 FR), 11 completed only the scales (6 NE, 3 AE, 2 FR) and 3 subjects completed only the performance test (2 NE, 1FR). Of the 11 subjects without performance data, only 6 failed to perform the test or could not distinguish between the tones; the other 5 were not asked to complete it.
Relative timing of circadian rhythms of sleep and melatonin
Representative examples of subjectively-recorded sleep-wake behaviour and its relationship with the timing and periodicity of aMT6s acrophases are shown in Fig. 1. In the NE subject (Subject 21, NE; top panel), the aMT6s acrophase occurred at approximately 4:00 hours during all four weekly phase assessments (mean ± SD = 4.28 ± 0.21 h) and, on average, sleep was initiated at approximately midnight (23.79 ± 0.72 h) and ended shortly after 8:00 hours (8.15 ± 0.56 h). This timing of the sleep and melatonin rhythms is well within the range observed for NE sighted subjects.
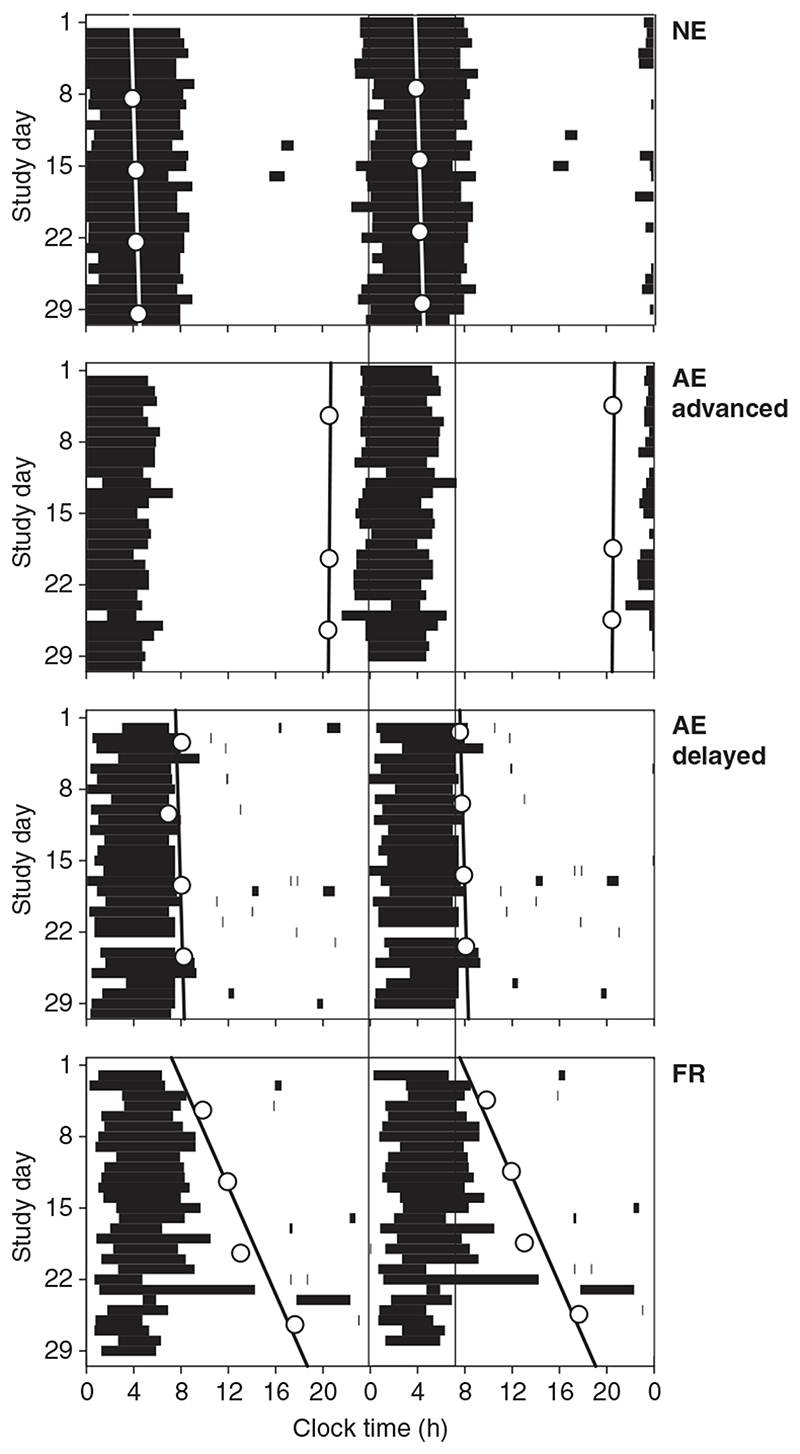
Figure 1.
Double raster plots of subjective sleep and nap episodes (black bars) for four blind subjects with differing circadian rhythm types: normally entrained (top panel), entrained but with an advanced phase (second panel), entrained with a delayed phase (third panel) and ‘free-running’ (lower panel) with a non-24-hour period (see text for definitions). Sequential study days are plotted vertically and horizontally with clock time on the horizontal axis. Weekly assessments of cosinor-derived aMT6s acrophase times are represented by asterisks with the associated lines of best fit (regression) used to determine circadian period shown for each subject. The average (± SD) night-time sleep onset (23.93 ± 0.92 h) and offset times (7.15 ± 0.81 h) for the blind subjects with light perception and normally-phased aMT6s acrophase times (4.57 ± 0.98 h; n = 22) are denoted to highlight the differences in sleep-wake behaviour. aMT6s, 6-sulphatoxymelatonin.
In the second panel, the abnormally advanced subject (Subject 22) had an aMT6s acrophase in the early evening (20.57 ± 0.06 h), an advance of approximately 8 h relative to normal. The sleep episode was not shifted by the same amount, however (average sleep onset and offset = 23.33 ± 0.06 h and 5.31 ± 0.06 h, respectively), and therefore occurred relatively late in the melatonin cycle.
In the third panel of Fig. 1, the opposite phase relationship is illustrated in an abnormally delayed subject (Subject 38): while the aMT6s acrophase was delayed by approximately 3 h (7.88 ± 0.59 h), sleep was not (onset 1.03 ± 0.88 h, offset 7.68 ± 0.68 h), and therefore occurred at a relatively earlier time in the melatonin cycle than normal. In fact, the subject’s sleep termination coincided with the aMT6s acrophase.
In the bottom panel, non-entrained (FR) melatonin and sleep-wake cycles are illustrated (Subject 44). During the first circadian phase assessment (days 5–6), the aMT6s acrophase occurred at 9.9 h and became progressively delayed week by week so by days 26–27, the acrophase occurred at 17.7 h. The resultant circadian period (τ ± 95% c.l.) was 24.35 ± 0.31 h, a daily delay of approximately 20 min. Although the variance in night-time sleep was greater than in the entrained subjects illustrated above, sleep still occurred primarily during the night, with sleep initiation at 1.69 h (± 1.01) and a wake time of 7.83 h (± 1.94). As noted previously (Lockley et al., 1997b), sleep duration was curtailed during the latter half of the study, and the frequency and duration of day-time naps increased as the aMT6s rhythm drifted further into the day-time hours (day 15–29, Fig. 1, FR).
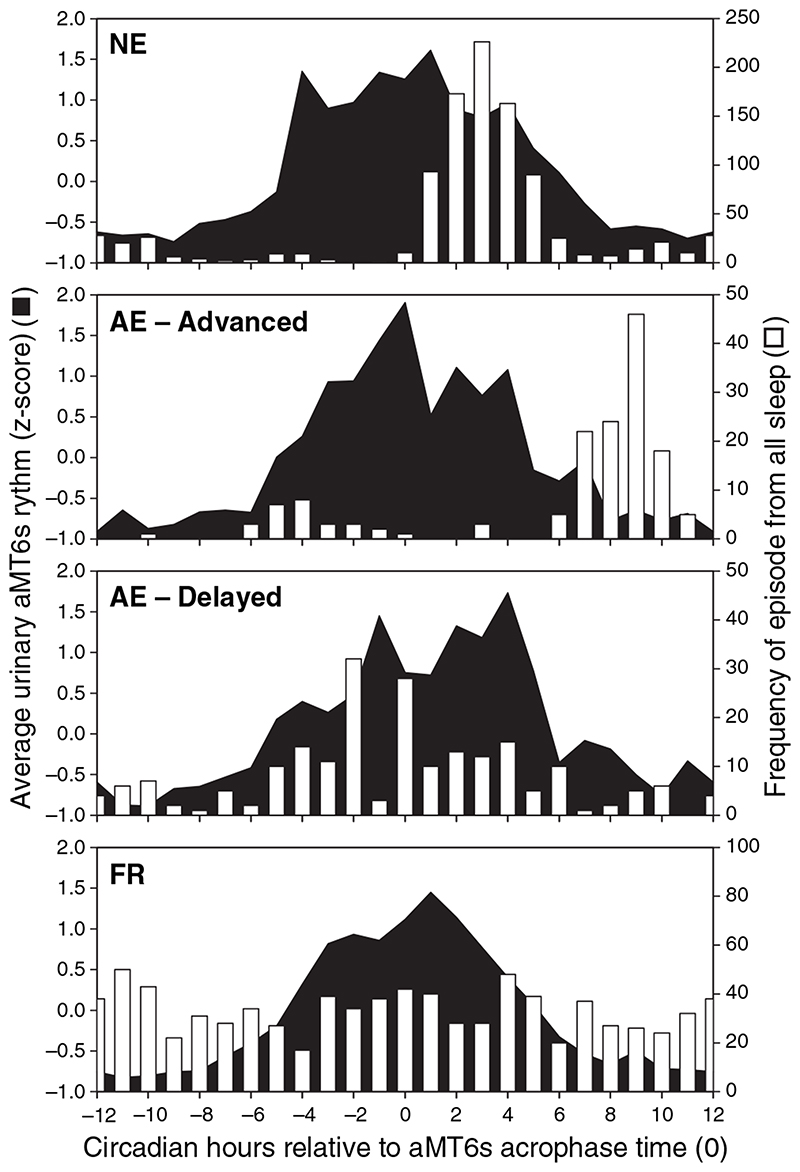
The distribution of wake times with respect to melatonin phase and circadian rhythm type for all subjects is shown in Fig. 2. In the NE subjects (top panel, NE), awakenings from night-time sleep (Fig. 2, white bars) clustered on the initial part of the falling limb of the aMT6s rhythm. In the AE advanced individuals, waking generally occurred at the very end of the falling limb of the aMT6s rhythm, at a later circadian phase than the NE subjects. In the AE delayed individuals, although wake time tended to be more variable, it occurred predominantly on the rising limb of the aMT6s rhythm at an earlier circadian phase. Finally, as expected, the distribution of wake times in relation to the melatonin rhythm for FR subjects who sleep and wake across all circadian phases was near-uniform. Thus, while all subjects attempted to sleep at night, their underlying differences in endogenous circadian phase resulted in systematic variations in the phase relationship between the timing of sleep and wake and circadian phase.
Figure 2.
Average aMT6s profiles for each circadian rhythm type (filled profile) plotted concurrently with the frequency of waking from all sleep episodes (white columns) as a function of aMT6s phase for different circadian rhythm types (see Fig. 1 and text for definitions). aMT6s, 6-sulphatoxymelatonin.
There were significant differences in the average duration of night-time sleep and total sleep time (TST) between the circadian rhythm groups (night-time sleep, F 3,47 = 5.91, P < 0.05; TST, F 3,45 = 4.48, P < 0.05). Normally entrained subjects slept the longest (night-time sleep mean ± SD = 6.70 ± 1.00 h; TST = 6.88 ± 0.96 h; n = 25) and abnormally delayed subjects the least (night-time sleep = 5.05 ± 1.56 h; TST = 5.59 ± 0.79 h; n = 5; Table 1).
Table 1. Average sleep duration, alertness, mood and performance according to circadian rhythm type.
| Parameter | Mean raw data (SEM) | ||||
|---|---|---|---|---|---|
| Abnormally entrained advanced n = 4 | Normally entrained n = 26 | Abnormally entrained delayed n = 5 | Non-entralned n = 17 | Group | |
| n = 4 | n = 25 | n = 5 | n = 17 | ||
| Night-time sleep duration (h)* | 5.64 (0.55) | 6.70 (0.20) | 5.05 (0.70) | 5.49 (0.27) | 6.05 (0.18) |
| Total sleep time (h)* | 6.00 (0.49) | 6.88 (0.19) | 5.59 (0.39) | 5.96 (0.26) | 6.38 (0.15) |
| n = 4 | n = 24 | n = 5 | n = 16 | ||
| Alert – sleepy (1–9) | 4.36 (0.40) | 4.71 (0.20) | 4.64 (0.59) | 4.38 (0.26) | 4.57 (0.14) |
| Cheerful – miserable (1–9) | 3.82 (0.48) | 4.08 (0.13) | 4.79 (0.53) | 4.07 (0.20) | 4.13 (0.11) |
| Calm – tense (1–9) | 3.79 (0.56) | 4.22 (0.16) | 4.97 (0.53) | 4.27 (0.32) | 4.28 (0.15) |
| Depressed – elated (1–9) | 5.49 (0.43) | 5.20 (0.07) | 4.88 (0.32) | 5.08 (0.15) | 5.15 (0.08) |
| n = 3 | n = 20 | n = 3 | n = 15 | ||
| RT all responses (cs) | 55.22 (0.80) | 54.01 (2.21) | 61.53 (5.48) | 51.75 (3.03) | 53.82 (1.60) |
| RT incorrect responses (cs) | 33.90 (5.62) | 38.25 (3.68) | 38.39 (6.06) | 32.40 (3.34) | 35.80 (2.24) |
| RT correct responses (cs) | 55.16 (0.77) | 53.71 (2.15) | 61.89 (5.88) | 51.85 (2.99) | 53.73 (1.58) |
| % Incorrect | 3.02 (0.72) | 9.86 (3.99) | 5.35 (1.74) | 4.67 (0.96) | 7.13 (2.00) |
| % Lapses >1 s | 6.21 (1.22) | 6.70 (2.03) | 15.51 (4.02) | 4.14 (1.64) | 6.37 (1.25) |
RT, reaction time.
P < 0.05 between circadian rhythm groups.
Alertness, mood and performance – data distribution and averages
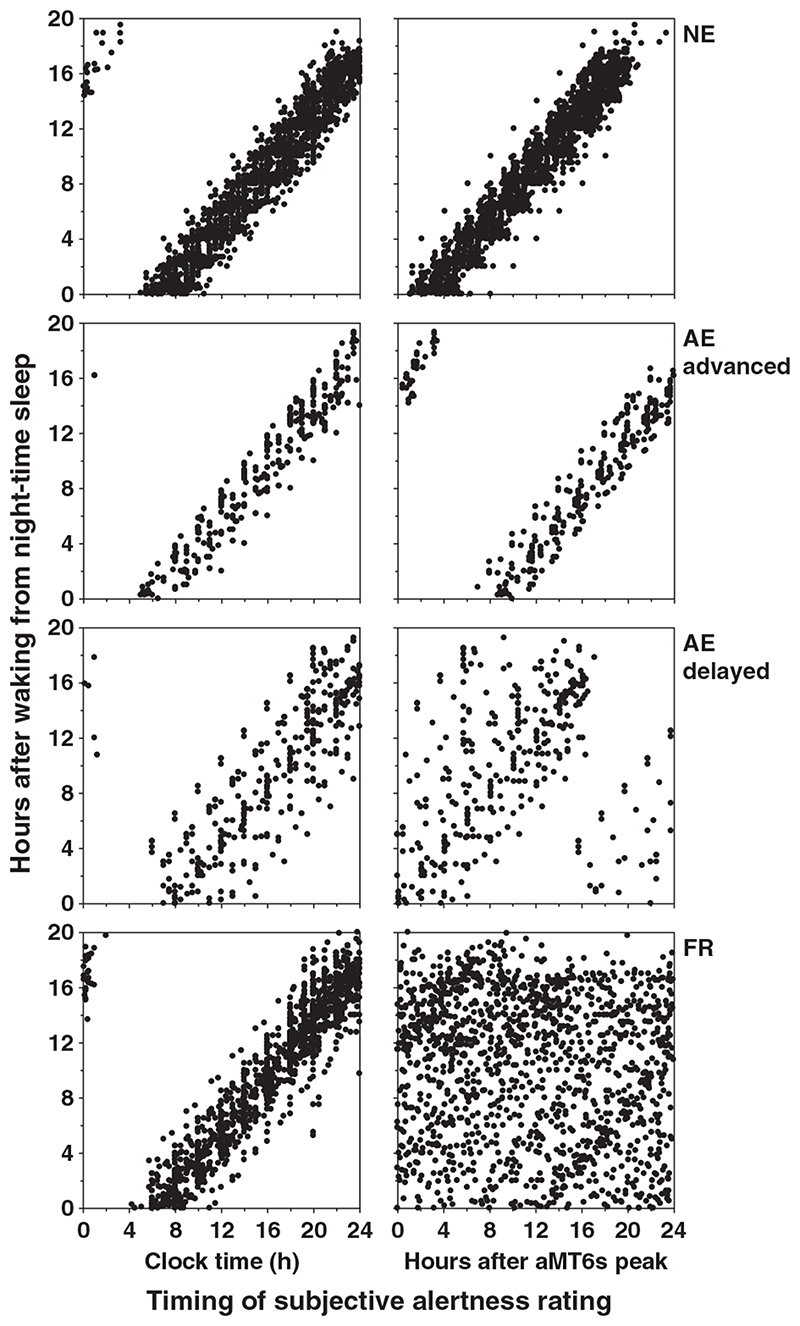
From the 52 subjects reported herein, the total number of observations for each circadian rhythm type for alertness (4273), cheerfulness (4228), calmness (4199), depression (4065) and performance (2871) was 2144, 2100, 2074, 1987, 1372 (NE); 1401, 1399, 1398, 1351, 1086 (FR); and 728, 729, 727, 727, 413 (AE), respectively. The data were distributed near-uniformly with respect to time since waking and circadian phase for the non-entrained (FR) subjects (Fig. 3, bottom right panel) as expected. By definition, the entrained subjects were only awake at a particular circadian phase and therefore had a limited data distribution (Fig. 3).
Figure 3.
Distribution of subjective alertness ratings during wakefulness in relation to clock time (left panel) and circadian phase (right panel) for normally entrained (NE), abnormally entrained (AE) advanced, AE delayed, and non-entrained (FR, ‘free-running’) subjects. When plotted (inappropriately) relative to clock time, there appears to be little difference between the data distributions of the four groups. When plotted appropriately against circadian time, however, clear differences are observed in the data distribution in the AE and FR subjects with respect to time awake and circadian phase. As most of these subjects attempted to sleep on a normal 24-h day, we were able to make observations of waking behaviour at particular interactions of time awake (Process S) and circadian phase (Process C) that are never observed in NE subjects (NE, top right panel). The group data from non-entrained subjects are distributed near-equally across all possible interactions of time awake and circadian phase because of the natural ‘forced desynchrony’ caused by desynchronization between the near-24-hour sleep-wake pattern and the non-24-hour circadian pacemaker (bottom right panel). The performance data also show similar distributions in relation to time wake and circadian phase for each circadian rhythm type (data not shown).
The overall averages for alertness, mood and performance for each circadian rhythm type were computed (Table 1). There were no significant differences in average alertness, mood or performance parameters when grouped by circadian rhythm type (F 3,45 < 1.45, P > 0.24; Table 1).
Alertness, mood and performance – sleep/wake-dependent effects (Process S)
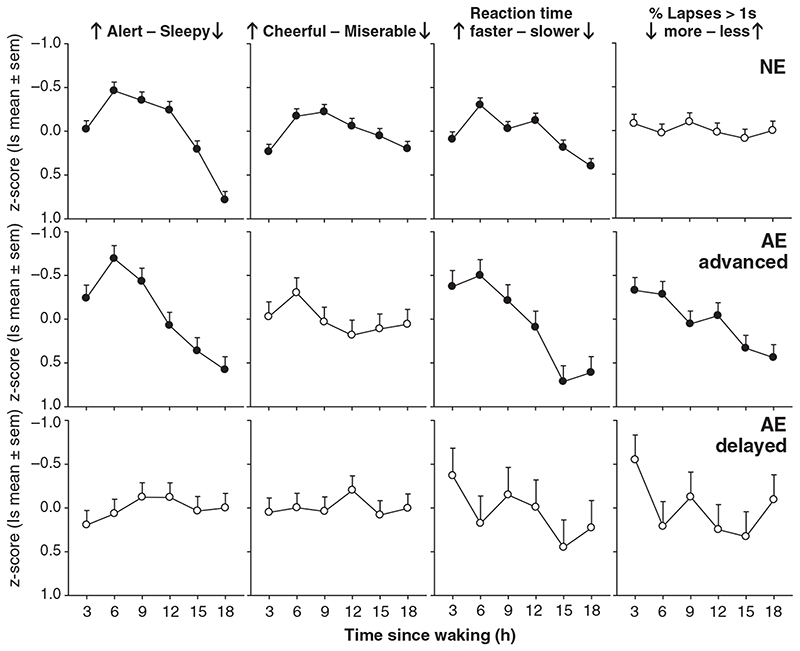
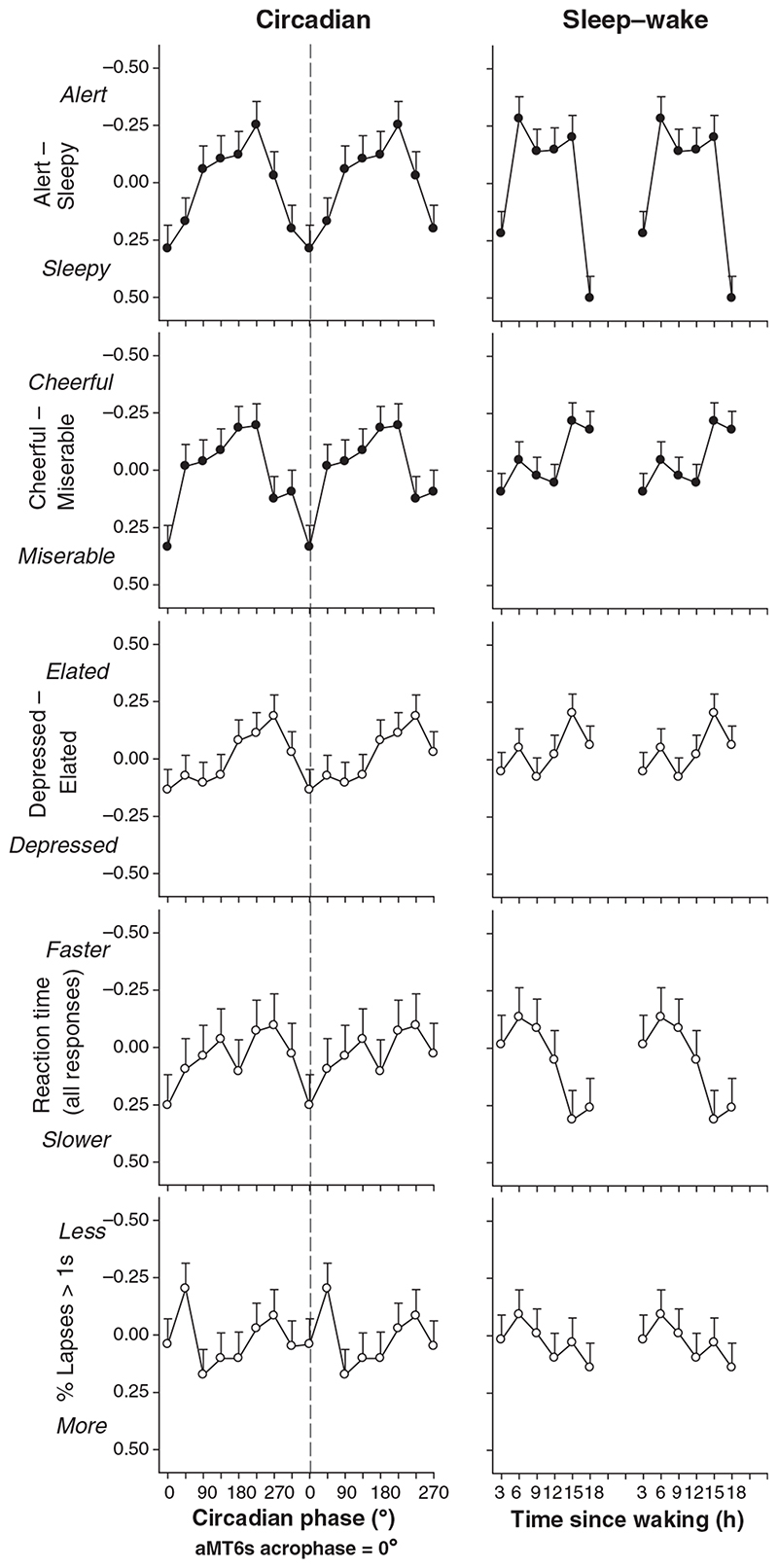
Subjective alertness demonstrated significant changes with time since waking for NE and FR subjects from night-time sleep only (NE: F 5,113 = 12.60, P < 0.0001, Fig. 4; FR: F 5,75 = 11.55, P < 0.0001) and from both day- and night-time sleeps (NE: F 5,113 = 17.32, P < 0.0001; FR: F 5,75 = 9.95, P < 0.0001; Fig. 5). Alertness profiles were characterized by a peak in alertness 3–6 h after waking which remained at a plateau for up to 12 h after which a marked reduction in alertness occurred (Fig. 4–6). A robust sleep inertia effect was also observed with reduced alertness in the first 3-h bin after waking. Abnormally advanced subjects showed a similar significant change with time awake from all sleeps (F 5,15 = 5.79, P < 0.004) and from night-time sleep (F 5,15 = 5.92, P = 0.003; Fig. 4) but did not exhibit the prolonged plateau of stable alertness observed in the NE group. Alertness peaked 3 h after waking but then showed a rapid decline in alertness through the remainder of the day (Fig. 4). Group data of abnormally delayed subjects did not exhibit a significant change in alertness across the waking day (P > 0.05; Fig. 4). Significant changes in subjective cheerfulness in relation to time awake after all sleeps and night-time sleep followed a similar profile to alertness in NE subjects (last sleep: F 5,111 = 2.38, P = 0.043; night sleep: F 5,113 = 3.72, P = 0.004; Fig. 4). FR subjects only demonstrated significant changes in cheerfulness after waking from night sleep episodes (night sleep: F 5,75 = 2.44, P = 0.042; last sleep: F 5,75 = 1.97, P = 0.093) with a general increase in cheerfulness rating across the waking day (Fig. 5). No significant changes were observed for cheerfulness in the other circadian types or in subjective measures of calmness and depression with time since waking.
Figure 4.
Average alertness, mood and performance (least squares mean z-score ± SEM) plotted in relation to time elapsed since waking from night-time sleep episodes for normally and abnormally entrained subjects (see Fig. 1 and text for definitions). Parameters with significant changes with respect to time since waking are shown by the filled symbols.
Figure 5.
Average alertness, mood and performance (least squares mean z-score ± SEM) double-plotted in relation to circadian phase (left panel) and time elapsed since waking from all sleep episodes (right panel) for free-running subjects. Parameters with significant changes with respect to time since waking and circadian phase are shown by the filled symbols.
Figure 6.
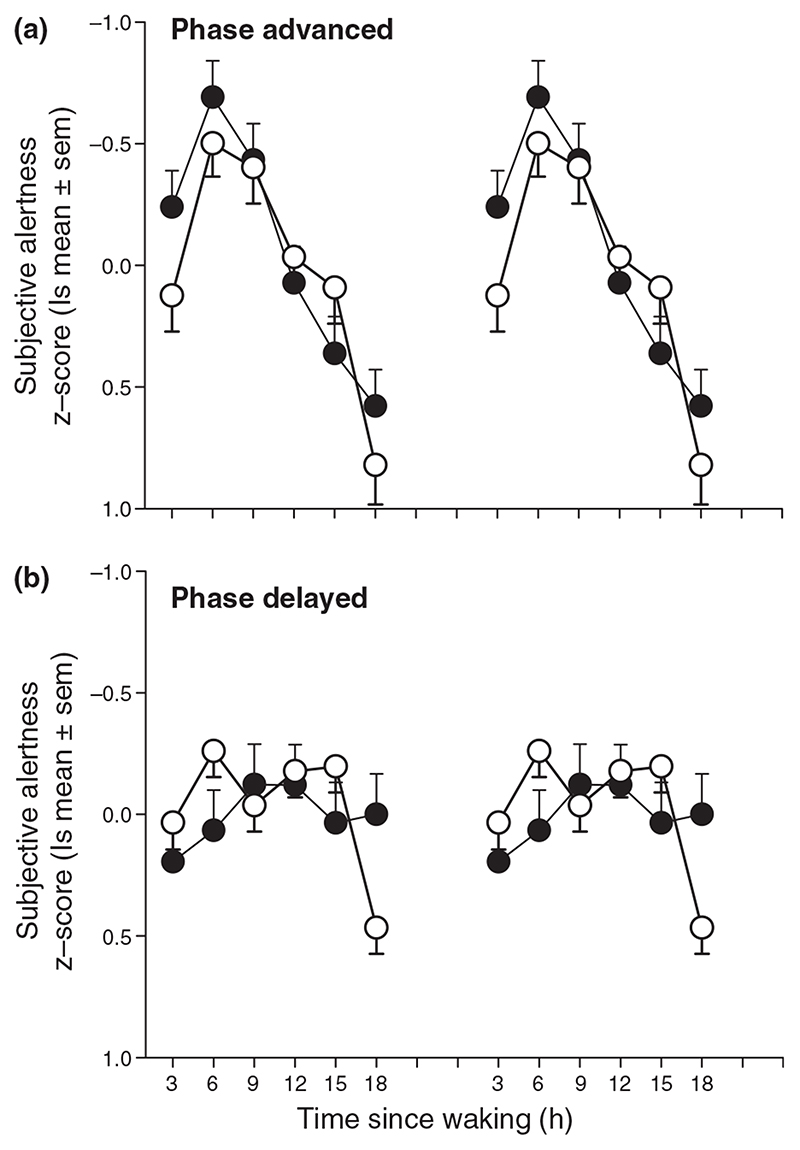
Average alertness (least squares mean z-score ± SEM) double-plotted in relation to time elapsed since waking from night-time sleep episodes for AE subjects (●, panel a) and AD subjects (●, panel b). Alertness data from free-running subjects for the corresponding circadian phase and time elapsed since waking are also shown (◯) for the two circadian phase types and demonstrate similar time courses.
Performance measures also demonstrated significant changes with time since waking. RT for all responses was fastest 3–6 h after waking followed by a gradual decline with increasing hours awake in NE subjects (last sleep: F 5,90 = 3.05, P = 0.014; night sleep: F 5,90 = 4.47, P < 0.001; Fig. 4) with a similar but non-significant pattern in FR subjects (Fig. 5). Relatively advanced and delayed subjects showed a deterioration in RT with increasing hours awake (Fig. 4) although only abnormally advanced subjects showed significant changes (last sleep: F 5,10 = 8.51, P = 0.002; night sleep: F 5,10 = 7.24, P = 0.004, Fig. 4). Changes in RT for correct responses only were virtually identical to RT for all responses and there were no consistent changes in RT of incorrect responses with time awake (data not shown). Only abnormally advanced subjects showed a significant increase in lapses of attention with increased time awake (last sleep: F 5,10 = 5.51, P = 0.011; night sleep: F 5,10 = 3.04, P = 0.063, Fig. 4).
Regression analysis did not show a significant relationship between daily mean alertness, mood and performance with sleep the night before or total sleep time per 24 h.
Alertness, mood and performance – circadian-dependent effects (Process C)
By definition, the distribution of data with respect to circadian phase was dependent on circadian rhythm type and therefore only FR subjects had a near-equal distribution of data with respect to both circadian phase and time since waking (Fig. 3). We therefore analysed the effects of circadian phase for FR subjects only. FR subjects showed a significant effect of circadian phase on alertness (F 7,104 = 2.36, P = 0.028) and cheerfulness (F 7,104 = 2.57, P = 0.017) with minimal alertness and cheerfulness coincident with aMT6s acrophase time (0 °). The peak occurred at 135 ° (≡ 9 h) earlier (Fig. 5), equivalent under entrained conditions to a clock time for minimal alertness at approximately 4:00 hours and maximal alertness at approximately 19:00 hours. A similar but non-significant change in the depressed-elated scale was also observed, with maximal depression close to the aMT6s peak (Fig. 5). There were no significant circadian-phase dependent changes in performance in FR subjects although RT was slowest at aMT6s peak time (0 °) (NS, P > 0.05; Fig. 5).
To explore further the impact of an abnormal phase relationship between the timing of the wake episode and endogenous circadian phase on alertness, we selected data from the FR subjects such that the combination of circadian phase and time awake was identical to either that observed in the abnormally advanced or abnormally delayed subjects. The resultant alertness profiles matched closely to those observed in the stably advanced or delayed subjects (Fig. 6).
Discussion
Sleep disturbances, fatigue, impaired performance and mood variations have often been reported in otherwise healthy blind people. In the current study, we have investigated the mechanisms underlying such symptoms in the absence of a clinical mood disorder and have demonstrated that the patterns of alertness and mood of blind people living in society are modulated by both internal biological time and duration of prior wakefulness. Under normal conditions, the circadian and homeostatic processes interact to optimize waking function across the day. In totally blind people with circadian rhythm sleep disorders, however, these two processes can become desynchronized because of a lack of light input to the circadian pacemaker, resulting in an abnormal phase angle between the circadian system and sleep-wake behaviour and subsequently altering the pattern of alertness, mood and performance across the day. Subjects felt most sleepy and most miserable when their wake episode occurred during their biological night (as defined by aMT6s production). The time course of alertness, mood and performance reflected the circadian phase at which the subjects woke: phase-advanced subjects exhibited robust profiles which declined rapidly through the day whereas phase-delayed subjects had a reduced amplitude across the day and a shorter duration decline. Remarkably, these profiles persist under societal conditions despite the presence of other uncontrolled factors that influence waking function (e.g. caffeine-, alcohol-, and medication use, employment, family and social interactions etc), indicating the fundamental nature of the circadian and homeostatic oscillatory processes in determining alertness, mood and neurobehavioural performance.
We have extended our previous work describing changes in sleep timing in relation to circadian phase in the blind (Lockley et al., 1997b, 1999) by establishing the impact of circadian rhythm sleep disorders on waking function. First, the profiles of alertness, mood and performance in NE blind subjects were similar to those of sighted individuals (e.g. Cajochen et al., 1999; Dijk et al., 1992; Monk et al., 1989, 1997), suggesting that visual impairment per se has no major impact on the time course of these rhythms. Changes in the circadian phase at which waking occurs, however, has a dramatic effect on the time course of waking function. Advanced subjects, despite waking at an earlier clock time, woke at a later phase of their aMT6s rhythm and exhibited advanced alertness and performance profiles with an earlier peak and a prolonged decline. Delayed subjects showed an opposite tendency, although they woke at a later clock time, sleep offset occurred at an earlier phase of the aMT6s rhythm and waking function remained relatively stable throughout the day and was generally higher at the end of the day than advanced subjects (Fig. 6). Furthermore, the observation that the diurnal alertness pattern is nearly identical in FR subjects when they wake at the same circadian phases as stably advanced- or delayed subjects demonstrates that the time course of alertness is determined to a large extent by the circadian phase at which waking occurs (Fig. 6).
These data extend previous studies in sighted subjects examining the effect of diurnal preference or ageing on internal phase relationships and their impact on alertness, mood and performance. Morning-types have an earlier circadian phase than evening types (Baehr et al., 2000; Duffy et al., 2001; Kerkhof and Van Dongen, 1996) and wake up relatively later in their circadian cycle (Baehr et al., 2000; Duffy et al., 1999; Mongrain et al., 2004), resulting in an earlier peak and decline in alertness (Foret et al., 1982; Kerkhof and Van Dongen, 1996; Watts et al., 1983), mood (Kerkhof, 1998; Totterdell et al., 1994) and performance (Horne et al., 1980) compared with evening types. Older adults are phase-advanced compared with younger adults but wake up earlier in their circadian cycle (Duffy and Czeisler, 2002; Duffy et al., 1998, 2002) and exhibit an earlier increase in mood (Monk et al., 1992) and performance (Duffy et al., 1998; Monk et al., 1992). In adolescents who generally exhibit a tendency to eveningness, there is also an uncoupling of the timing of sleep relative to circadian phase, as in the abnormally delayed blind subjects, that causes them to wake at a relatively early circadian phase, compromising their ability to remain awake and perform during the day (Carskadon et al., 2004). While the change in phase relationship in these populations results in significant deterioration of waking function, none exhibit the complete reversal in the timing of sleep relative to circadian phase experienced by totally blind people with non-24-hour sleep wake disorder. When night-time sleep occurs at an abnormal circadian phase (i.e. at a time when melatonin levels are low; e.g. days 18–26, Fig. 1, bottom panel), wake coincides with the biological night (i.e. at a time when melatonin levels are high) and therefore alertness, mood and performance are equivalent to that experienced by normally-phased subjects at 3:00–6:00 hours. Unfortunately, this adverse phase relationship can persist continuously for many weeks (Fig. 1, bottom panel), resulting in day after day of debilitating sleepiness, poor mood and inability to perform.
We did not detect differences in average alertness, mood and performance between the circadian rhythm types or in relation to sleep duration but rather differences in their time course due to the circadian phase at which wake time occurred. This is not surprising as subjective scales tend to be sensitive for assessing changes within an individual but relatively insensitive at detecting differences between individuals because of the lack of a common external reference when gauging their response. The differences in the time course of waking function, however, are consistent with the changes observed in sleep-wake behaviour in totally blind subjects with circadian rhythm sleep disorders. While the timing and duration of both night-time sleep and day-time naps change dramatically but predictably with respect to the circadian phase at which sleep occurs (Lockley et al., 1999), average total sleep duration per day does not (Lockley et al., 1999), highlighting the interaction between circadian and homeostatic processes in determining overall behaviour. Similarly, the time course of alertness, mood and performance differs with respect to circadian phase, being worst when wake coincides with the biological night (Fig. 5), without necessarily being poorer between circadian rhythm types on average. In AE subjects, these disturbances in the time course of waking behaviours represent a functional disorder identical to advanced- or delayed sleep phase syndrome wherein they are unable to optimally be alert when desired (ICSD-2, 2005); advanced subjects are unable to remain alert in the evening whereas delayed subjects struggle to remain alert in the morning. Assessment of day-time dysfunction resulting from circadian rhythm disorders should therefore focus on the pattern of these behaviours, rather than absolute decrements.
The detrimental effects of blindness on sleep and waking function are often inadequately recognized by physicians, families, friends and employers, making it difficult for blind people to obtain the treatment and support required to deal with this unavoidable condition. Our data confirm the anecdotal accounts from subjects, who describe fighting to stay awake at work, having problems maintaining concentration and memory during the day, or being overwhelmed with the prospect of further training or responsibility while suffering cyclic sleep disturbances. These circadian rhythm sleep disorders are chronic, unrelenting and currently difficult to treat. Simply treating the sleep-wake symptoms conventionally, for example with a combination of day-time stimulants and night-time hypnotics indicates an inappropriate diagnosis and a failure to address the underlying cause of the condition. Correcting the underlying misalignment between circadian and sleep-wake cycles, for example using appropriately-timed melatonin treatment (Lockley et al., 2000; Sack et al., 2000) is fundamental for the successful treatment of circadian rhythm sleep disorders. While melatonin’s effects on day-time function have not yet been quantified in the blind we would hypothesize that realignment of the circadian system with the social day would realign sleep, alertness, mood and performance rhythms in a similar manner, in addition to directly facilitating sleep and consequently day-time function. The timing of melatonin treatment with respect to both internal circadian time and social time is therefore important, and for optimal scheduling, should be based on evaluations similar to those previously described (Lockley, 2005).
In summary, our results show that the abnormal sleepiness, mood and performance patterns experienced by blind people are not simply a passive consequence of poor sleep but are determined by an active endogenous process which promotes sleepiness and sleep according to internal circadian time, regardless of the social day.
Acknowledgements
The authors thank Professor Emeritus Simon Folkard, DSc, and Peter Totterdell, PhD, for development and programming the auditory performance tests. This work was supported by South Thames Regional Health Authority, Institut de Recherches Internationale Servier, Stockgrand Ltd. and The Wellcome Trust (Grants 048197/Z/96/Z and 060018/B/99/Z).
References
- Arendt J. Melatonin and the Mammalian Pineal Gland. Chapman and Hall; London: 1995. [Google Scholar]
- Baehr EK, Revelle W, Eastman CI. Individual differences in the phase and amplitude of the human circadian temperature rhythm: with an emphasis on morningness-eveningness. J Sleep Res. 2000;9:117–127. doi: 10.1046/j.1365-2869.2000.00196.x. [DOI] [PubMed] [Google Scholar]
- Boivin DB, Czeisler CA, Dijk DJ, Duffy JF, Folkard S, Minors DS, Totterdell P, Waterhouse JM. Complex interaction of the sleep-wake cycle and circadian phase modulates mood in healthy subjects. Arch Gen Psychiatry. 1997;54:145–152. doi: 10.1001/archpsyc.1997.01830140055010. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Khalsa SBS, Wyatt JK, Czeisler CA, Dijk DJ. EEG and ocular correlates of circadian melatonin phase and human performance decrements during sleep loss. Am J Physiol. 1999;277:R640–R649. doi: 10.1152/ajpregu.1999.277.3.r640. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C, Jenni OG. Regulation of adolescent sleep: implications for behaviour. Ann NY Acad Sci. 2004;1021:276–291. doi: 10.1196/annals.1308.032. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Wright KP., Jr . In: Regulation of Sleep and Circadian Rhythms. Turek FW, Zee PC, editors. Marcel Dekker Inc; New York: 1999. Influence of light on circadian rhythmicity in humans; pp. 149–180. [Google Scholar]
- Dijk DJ, Czeisler CA. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci Lett. 1994;166:63–68. doi: 10.1016/0304-3940(94)90841-9. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Lockley SW. Integration of human sleep-wake regulation and circadian rhythmicity. J Appl Physiol. 2002;92:852–862. doi: 10.1152/japplphysiol.00924.2001. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Duffy JF, Czeisler CA. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J Sleep Res. 1992;1:112–117. doi: 10.1111/j.1365-2869.1992.tb00021.x. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Czeisler CA. Age-related change in the relationship between circadian period, circadian phase, and diurnal preference in humans. Neurosci Lett. 2002;318:117–120. doi: 10.1016/s0304-3940(01)02427-2. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Dijk DJ, Klerman EB, Czeisler CA. Later endogenous circadian temperature nadir relative to an earlier wake time in older people. Am J Physiol. 1998;275:R1478–R1487. doi: 10.1152/ajpregu.1998.275.5.r1478. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Dijk DJ, Hall EF, Czeisler CA. Relationship of endogenous circadian melatonin and temperature rhythms to selfreported preference for morning or evening activity in young and older people. J Investig Med. 1999;47:141–150. [PMC free article] [PubMed] [Google Scholar]
- Duffy JF, Rimmer DW, Czeisler CA. Association of intrinsic circadian period with morningness-eveningness, usual wake time, and circadian phase. Behav Neurosci. 2001;115:895–899. doi: 10.1037//0735-7044.115.4.895. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Zeitzer JM, Rimmer DW, Klerman EB, Dijk DJ, Czeisler CA. Peak of circadian melatonin rhythm occurs later within the sleep of older subjects. Am J Physiol. 2002;282:E297–E303. doi: 10.1152/ajpendo.00268.2001. [DOI] [PubMed] [Google Scholar]
- Foret J, Benoit O, Royant-Parola S. Sleep schedules and peak times of oral temperature and alertness in morning and evening ‘types’. Ergonomics. 1982;25:821–827. doi: 10.1080/00140138208925038. [DOI] [PubMed] [Google Scholar]
- Horne JA, Brass CG, Pettitt AN. Circadian performance differences between morning and evening ‘types’. Ergonomics. 1980;23:29–36. doi: 10.1080/00140138008924715. [DOI] [PubMed] [Google Scholar]
- ICSD-2. The International Classification of Sleep Disorders Diagnostic and Coding Manual. 2nd. American Academy of Sleep Medicine; Westchester, IL, USA: 2005. [Google Scholar]
- Kerkhof GA. The 24-hour variation of mood differs between morning- and evening-type individuals. Percept Mot Skills. 1998;86:264–266. doi: 10.2466/pms.1998.86.1.264. [DOI] [PubMed] [Google Scholar]
- Kerkhof GA, Van Dongen HPA. Morning-type and eveningtype individuals differ in the phase position of their endogenous circadian oscillator. Neurosci Lett. 1996;218:153–156. doi: 10.1016/s0304-3940(96)13140-2. [DOI] [PubMed] [Google Scholar]
- Klein T, Martens H, Dijk DJ, Kronauer RE, Seely EW, Czeisler CA. Chronic non-24-hour circadian rhythm sleep disorder in a blind man with a regular 24-hour sleep-wake schedule. Sleep. 1993;16:333–343. doi: 10.1093/sleep/16.4.333. [DOI] [PubMed] [Google Scholar]
- Leger D, Guilleminault C, Defrance R, Domont A, Paillard M. Prevalence of sleep/wake disorders in persons with blindness. Clin Sci. 1999;97:193–199. [PubMed] [Google Scholar]
- Lewy AJ, Newsome DA. Different types of melatonin circadian secretory rhythms in some blind subjects. J Clin Endocrinol Metab. 1983;56:1103–1107. doi: 10.1210/jcem-56-6-1103. [DOI] [PubMed] [Google Scholar]
- Littell RC, Henry PR, Ammerman CB. Statistical analysis of repeated measures data using SAS procedures. J Anim Sci. 1998;76:1216–1231. doi: 10.2527/1998.7641216x. [DOI] [PubMed] [Google Scholar]
- Lockley SW. Timed melatonin treatment for delayed sleep phase syndrome: the importance of knowing circadian phase. Sleep. 2005;28:1214–1216. doi: 10.1093/sleep/28.10.1214. [DOI] [PubMed] [Google Scholar]
- Lockley SW, Skene DJ, Arendt J, Tabandeh H, Bird AC, Defrance R. Relationship between melatonin rhythms and visual loss in the blind. J Clin Endocrinol Metab. 1997a;82:3763–3770. doi: 10.1210/jcem.82.11.4355. [DOI] [PubMed] [Google Scholar]
- Lockley SW, Skene DJ, Tabandeh H, Bird AC, Defrance R, Arendt J. Relationship between napping and melatonin in the blind. J Biol Rhythms. 1997b;12:16–25. doi: 10.1177/074873049701200104. [DOI] [PubMed] [Google Scholar]
- Lockley SW, Skene DJ, Butler LJ, Arendt J. Sleep and activity rhythms are related to circadian phase in the blind. Sleep. 1999;22:616–623. doi: 10.1093/sleep/22.5.616. [DOI] [PubMed] [Google Scholar]
- Lockley SW, Skene DJ, James K, Thapan K, Wright J, Arendt J. Melatonin administration can entrain the free-running circadian system of blind subjects. J Endocrinol. 2000;164:R1–R6. doi: 10.1677/joe.0.164r001. [DOI] [PubMed] [Google Scholar]
- Miles LEM, Raynal DM, Wilson MA. Blind man living in normal society has circadian rhythms of 24.9 hours. Science. 1977;198:421–423. doi: 10.1126/science.910139. [DOI] [PubMed] [Google Scholar]
- Mongrain V, Lavoie S, Selmaoui B, Paquet J, Dumont M. Phase relationships between sleep-wake cycle and underlying circadian rhythms in morningness-eveningness. J Biol Rhythms. 2004;19:248–257. doi: 10.1177/0748730404264365. [DOI] [PubMed] [Google Scholar]
- Monk TH, Moline ML, Fookson JE, Peetz SM. Circadian determinants of subjective alertness. J Biol Rhythms. 1989;4:393–404. doi: 10.1177/074873048900400401. [DOI] [PubMed] [Google Scholar]
- Monk TH, Buysse DJ, Reynolds CF, III, Jarrett DB, Kupfer DJ. Rhythmic vs. homeostatic influences on mood, activation, and performance in young and old men. J Gerontol. 1992;47:P221–P227. doi: 10.1093/geronj/47.4.p221. [DOI] [PubMed] [Google Scholar]
- Monk TH, Buysse DJ, Reynolds CF, III, Berga SL, Jarrett DB, Begley AE, Kupfer DJ. Circadian rhythms in human performance and mood under constant conditions. J Sleep Res. 1997;6:9–18. doi: 10.1046/j.1365-2869.1997.00023.x. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Sack RL, Lewy AJ. Sleep propensity free-runs with the temperature, melatonin and cortisol rhythms in a totally blind person. Sleep. 1992;15:330–336. doi: 10.1093/sleep/15.4.330. [DOI] [PubMed] [Google Scholar]
- Sack RL, Lewy AJ, Blood ML, Keith LD, Nakagawa H. Circadian rhythm abnormalities in totally blind people: incidence and clinical significance. J Clin Endocrinol Metab. 1992;75:127–134. doi: 10.1210/jcem.75.1.1619000. [DOI] [PubMed] [Google Scholar]
- Sack RL, Brandes RW, Kendall AR, Lewy AJ. Entrainment of free-running circadian rhythms by melatonin in blind people. N Engl J Med. 2000;343:1070–1077. doi: 10.1056/NEJM200010123431503. [DOI] [PubMed] [Google Scholar]
- Skene DJ, Lockley SW, Thapan K, Arendt J. Effects of light on human circadian rhythms. Reprod Nutr Dev. 1999;39:295–304. doi: 10.1051/rnd:19990302. [DOI] [PubMed] [Google Scholar]
- Tabandeh H, Lockley SW, Buttery R, Skene DJ, Defrance R, Arendt J. Disturbance of sleep in blindness. Am J Ophthamol. 1998;126:707–712. doi: 10.1016/s0002-9394(98)00133-0. [DOI] [PubMed] [Google Scholar]
- Totterdell P, Folkard S. In situ repeated measures of affect and cognitive performance facilitated by use of a hand-held computer. Behav Res Methods Instrum Comput. 1993;24:545–553. [Google Scholar]
- Totterdell P, Reynolds S, Parkinson B, Briner RB. Associations of sleep with everyday mood, minor symptoms and social interaction experience. Sleep. 1994;17:466–475. doi: 10.1093/sleep/17.5.466. [DOI] [PubMed] [Google Scholar]
- Watts C, Cox T, Robson J. Morningness-eveningness and diurnal variations in self-reported mood. J Psychol. 1983;113:251–256. doi: 10.1080/00223980.1983.9923583. [DOI] [PubMed] [Google Scholar]
- Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Circadian temperature and melatonin rhythms, sleep, and neurobehavioural function in humans living on a 20-h day. Am J Physiol. 1999;277:R1152–R1163. doi: 10.1152/ajpregu.1999.277.4.r1152. [DOI] [PubMed] [Google Scholar]