Abstract
Purpose
Stellate nonhereditary idiopathic foveomacular retinoschisis (SNIFR) is a disorder characterized by splitting of the retina at the macula, without a known underlying mechanical or inherited cause. This study investigates demographic, anatomical and functional characteristics of subjects with SNIFR, to explore potential underlying mechanisms.
Methods
In this single-site, retrospective and cross-sectional, observational study, data were collected from 28 eyes from 24 subjects with SNIFR. Descriptive statistics were reported, based on the observed anatomico-functional features.
Results
Visual acuity remained stable (median 20/20) in all subjects over a median follow-up of 17 months. All cases demonstrated foveomacular retinoschisis within Henle’s fiber layer, at the junction of the outer plexiform and outer nuclear layers. This schisis cavity extended beyond the limits of the macular OCT temporally in all eyes. In the majority of affected eyes, there were documented features of peripheral retinoschisis and broad attachment of the posterior hyaloid at the macula. Functional testing in a cross-sectional subset demonstrated normal retinal sensitivity centrally but an absolute scotoma peripherally.
Conclusions
Stellate nonhereditary idiopathic foveomacular retinoschisis appears to be associated with peripheral retinoschisis and anomalous or incomplete posterior hyaloid detachment. Despite chronic manifestation, this does not significantly affect central visual function, but can manifest with profound loss of peripheral visual function.
Keywords: Peripheral retinoschisis, Posterior hyaloid attachment, Stellate nonhereditary idiopathic foveomacular retinoschisis
Introduction
Foveomacular retinoschisis (FRS) describes the presence of a localized separation of retinal layers affecting the central macula. Although FRS is typically associated with congenital X-linked retinoschisis (XLRS) (OMIM #312700),1 it is observed in other inherited disorders, such as enhanced S-cone syndrome (OMIM #268100) and CRB1-associated maculopathy (OMIM #604210).2,3 It is also a recognized manifestation of optic disc pit, myopic traction maculopathy, glaucomatous optic neuropathy, epiretinal membrane, vitreomacular traction syndrome and drug-related cystoid macular edema.4–10 These conditions often appear to have similar morphology, but display different anatomico-functional natural histories.
There are several reports of atypical FRS, some of which are suggestive of a possible inherited mechanism,11–15 while others remain unexplained.16 In 2014, Ober et al. coined the term ‘stellate nonhereditary idiopathic foveomacular retinoschisis’ (SNIFR), in an attempt to provide a unifying classification under which to categorize unusual cases, without an explanatory pathophysiological mechanism.17 Reported cases of SNIFR appear to have favorable functional profiles and share similar anatomical configurations, namely splitting at the level of Henle’s fiber layer, which is located within the parafoveal retina and comprised of horizontally-aligned photoreceptor axons and Müller cell processes. However, the precise pathoanatomical mechanism through which SNIFR arises remains elusive.
We present a retrospective study of the anatomico-functional characteristics in 28 eyes with presumed SNIFR, of which 7 eyes had additional, cross-sectional multimodal imaging and functional testing, to investigate the retinal function and explore potential underlying mechanisms.
Methods
A single site retrospective, observational study was performed to identify subjects with evidence of FRS without a known predisposing disorder. Subjects were included, who presented to a tertiary ophthalmic hospital trust between January 2010 and January 2020, with center-involving macular schisis. Cases were identified through review of electronic case notes, using the search terms “schisis”, “retinoschisis”, “maculoschisis” and “foveoschisis”, and correlation with historical optical coherence tomography (OCT) imaging. Exclusion criteria included subjects under 18-years-old; those with significant ocular co-pathology or alternative pre-disposing features (such as high myopia or posterior staphyloma, optic nerve anomalies, epiretinal membrane (ERM) or focal vitreomacular traction (VMT)); a family history or known genetic abnormality associated with foveomacular retinoschisis. Where documented, the following data were collected: demographic characteristics (age, gender, ethnicity), Snellen visual acuity (VA) at baseline and final visit, spherical equivalent (SE), axial length (AL), reported visual symptoms and ophthalmic examination findings, including evidence of peripheral retinoschisis (PRS). In cases where data for SE or AL were not available, high myopia was excluded if there was an absence of staphyloma on OCT or myopic retinal features on fundus imaging. Serial OCT imaging was reviewed to determine posterior vitreous detachment (PVD) status and peripheral extension of retinoschisis.
A subset of 5 subjects underwent further, cross-sectional studies, including OCT imaging (Cirrus 5000 HD-OCT, Carl Zeiss Meditec, Dublin, CA, USA or Spectralis SD-OCT, Heidelberg Engineering, Dossenheim, Germany), Optos California widefield scanning laser ophthalmoscopy (SLO) (Optos, Marlborough, MA, USA), microperimetry (MAIA, CentreVue, Padova, Italy), Humphrey perimetry (Carl Zeiss Meditec) and, where available, biometry (IOLMaster 700, Carl Zeiss Meditec) and autorefraction (ARK-510A, NidekCo., Aichi, Japan). Composite OCT images were created using open-source graphics editing software (GNU Image Manipulation Program). Health Research Authority approval was obtained and the study was carried out according to the tenets of the Declaration of Helsinki.
Results
1,219 of the subjects identified using the pre-specified search terms had macula-involving retinoschisis, of whom 1,194 (98%) were excluded for other predisposing factors (as detailed in Table 1). 33 eyes from the remaining 25 subjects were considered to meet the diagnostic criteria for SNIFR, of which 5 eyes were excluded from further analysis due to the existence of significant ocular co-pathology, including amblyopia, branch retinal vein occlusion and focal VMT.
Table 1. Pathologies associated with foveomacular retinoschisis.
| Pathology subgroup | Precise pathology | No. of subjects |
|---|---|---|
| Mechanical | High myopia | 531 |
| Vitreo-retinal interface disorders | 243 | |
| Optic disc pit | 53 | |
| Other peri-papillary disorders | 15 | |
| Degenerative | Age-related macular degeneration | 13 |
| Degenerative retinoschisis-detachment | 12 | |
| Inherited | X-linked retinoschisis | 170 |
| Enhanced s-cone syndrome | 16 | |
| Macular dystrophy | 14 | |
| Retinitis pigmentosa | 5 | |
| Best disease | 5 | |
| Other inherited | 20 | |
| Inflammatory/vascular | Cystoid macular oedema | 27 |
| Diabetic macular oedema | 13 | |
| Central serous chorioretinopathy | 9 | |
| Macular telangiectasia | 5 | |
| Other inflammatory/vascular | 9 | |
| Neoplastic | Melanoma | 19 |
| Naevus | 7 | |
| Other intra-ocular tumours | 7 | |
| Iatrogenic | Nicotinic acid maculopathy | 1 |
| Idiopathic | Stellate non-hereditary idiopathic foveomacular retinoschisis | 25 |
| Total | 1219 |
In this group of 28 eyes (from 24 subjects), the mean (±SD) subject age at initial presentation was 63.6 (±11.7) years and 63% were female (Table 2). The median VA at baseline was 20/20, which remained stable over a median follow-up duration of 17 months (range: 2-134 months, in 23 eyes with follow-up data). 15/24 (63%) of subjects were asymptomatic throughout, while 9 (31%) reported mild to moderate distortion or blurring. 1 eye (subject 6) had reported long-standing unexplained poor vision, despite normal electrodiagnostic tests. 1 subject had a negative genetic test for RS1 mutation, while the remainder did not undergo testing based on a lack of anatomical or functional evidence for an inherited retinal disease phenotype, on specialist clinical assessment. 14/24 (58%) had a negative family history documented.
Table 2. SNIFR subject characteristics (28 eyes).
| No. | Sex | Baseline age (yrs) | Eye | Ethnicity | SE (D) | AL (mm) | FRS | PRS | Complete PVD | Baseline VA | Final VA | Follow-up duration (months) | Symptoms | Comment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 51 | OS | White | +1.00 | 22.15 | Y | Y | N | 20/16 | 20/20 | 53 | Mild distortion | Eccentric temporal mfERG abnormality |
| 2 | F | 58 | OS | Black | +0.50 | 22.55 | Y | Y | N | 20/16 | 20/20 | 22 | Asymptomatic | |
| 3 | M | 70 | OS | White | +2.00 | NR | Y | Y | N | 20/40 | 20/30 | 20 | Mild blurring | |
| 4 | F | 54 | OD | White | -5.00 | 24.12 | Y | Y | N | 20/20 | 20/30 | 5 | Asymptomatic | |
| OS | White | -4.75 | 24.32 | Y | Y | N | 20/16 | 20/20 | 5 | Asymptomatic | ||||
| 5 | M | 53 | OD | White | +2.50 | NR | Y | Y | N | 20/20 | 20/16 | 112 | Mild distortion | |
| OS | White | +2.50 | NR | Y | Y | N | 20/20 | 20/16 | 112 | Mild distortion | ||||
| 6 | F | 41 | OD | White | NR | NR | Y | NR | N | 20/200 | 20/600 | 134 | Poor vision | Unexplained poor vision |
| 7 | M | 74 | OS | Black | +2.25 | NR | Y | NR | N | 20/16 | 20/20 | 10 | Mild blurring | |
| 8 | F | 60 | OD | White | NR | NR | Y | Y | N | 20/16 | 20/16 | 117 | Asymptomatic | |
| 9 | F | 65 | OS | White | NR | NR | Y | N | N | 20/20 | 20/20 | 77 | Asymptomatic | Normal ERG |
| 10 | M | 70 | OS | White | NR | NR | Y | NR | Y | 20/16 | 20/30 | 2 | Difficulty in dim light | |
| 11 | M | 74 | OS | NR | NR | NR | Y | Y | Y | 20/20 | N/A | 0 | Asymptomatic | mfERG abnormalities peripherally |
| 12 | F | 61 | OS | NR | NR | NR | Y | N | N | 20/16 | 20/20 | 47 | Asymptomatic | |
| 13 | F | 60 | OD | Asian | NR | NR | Y | Y | N | 20/20 | N/A | 0 | Asymptomatic | |
| 14 | F | 37 | OS | Chinese | Emmetropia | 23.27 | Y | Y | N | 20/30 | 20/30 | 4 | Asymptomatic | Normal ERG |
| 15 | M | 70 | OD | Black | Hyperopia | NR | Y | NR | N | 20/20 | 20/30 | 22 | Mild distortion | |
| 16 | F | 56 | OD | White | +1.50 | NR | Y | Y | N | 20/20 | N/A | 0 | Asymptomatic | |
| 17 | F | 84 | OD | White | NR | NR | Y | NR | N | 20/40 | 20/40 | 24 | Blurred vision | |
| 18 | M | 67 | OD | NR | NR | NR | Y | NR | N | 20/30 | N/A | 0 | Asymptomatic | |
| OS | NR | NR | Y | NR | Y | 20/30 | N/A | 0 | Asymptomatic | |||||
| 19 | M | 63 | OD | Black | NR | NR | Y | NR | N | 20/30 | 20/30 | 3 | Asymptomatic | |
| OS | NR | NR | Y | NR | N | 20/30 | 20/20 | 3 | Asymptomatic | |||||
| 20 | F | 60 | OD | White | NR | NR | Y | Y | N | 20/20 | 20/20 | 13 | Asymptomatic | |
| 21 | F | 84 | OD | NR | Pseudophakia | NR | Y | Y | N | 20/40 | 20/60 | 17 | Mild blurred vision | |
| 22 | F | 71 | OD | NR | Pseudophakia | 23.77 | Y | NR | N | 20/30 | 20/20 | 7 | Asymptomatic | |
| 23 | F | 77 | OS | Asian | NR | NR | Y | Y | Y | 20/60 | 20/60 | 47 | Blurred vision | |
| 24 | M | 66 | OS | White | Mild myopia | NR | Y | Y | N | 20/16 | 20/20 | 4 | Asymptomatic | Normal ERG |
NR: not recorded, SE (D): spherical equivalent (dioptres), AL: axial length, FRS: foveomacular retinoschisis, DRS: degenerative retinoschisis, PVD: posterior vitreous detachment, mfERG: multifocal electroretinogram
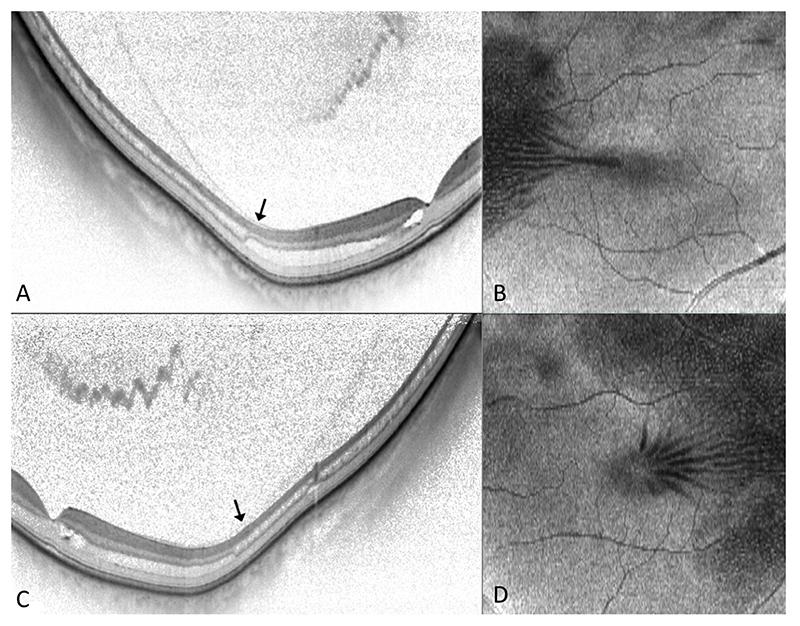
All affected eyes had OCT evidence of FRS, which, in each case, extended beyond the limits of the macular cube scan temporally (Figure 1). 18 affected eyes had a contemporaneous comment regarding examination of the peripheral retina, of which 16 (89%) had recorded features of PRS on examination, one of which had a stable schisis-detachment.
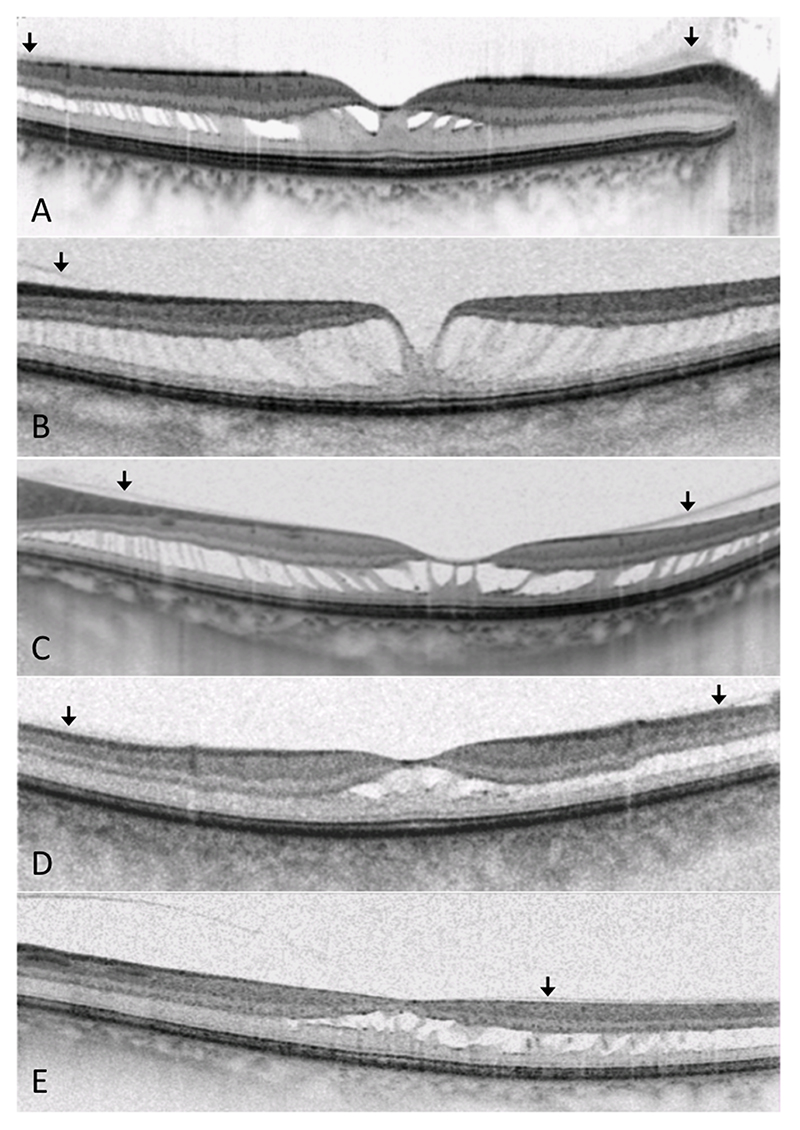
Figure 1. Images from subjects 6 (OD), 12 (OS), 14 (OS), 19 (OS) and 24 (OS). Foveomacular retinoschisis is demonstrated, on macular OCT (A-E), involving HFL and extending peripherally. There is evidence of incomplete posterior vitreous separation (arrows).
24/28 (86%) affected eyes were also noted to have incomplete or anomalous separation of the posterior hyaloid on OCT (Figure 1). 12 subjects had fellow eyes unaffected by FRS or other macular pathology, 6 (50%) of which also had documented evidence of PRS while only 5/12 (42%) had incomplete separation of the posterior hyaloid on OCT. Of note, 2 subjects had FTMH and 1 had a lamellar macular hole in their respective fellow eyes.
A minority of eyes (11/27) had data for refractive spherical equivalent or axial length, but in all documented cases, these fell within the normal range. 6 eyes also underwent ancillary electrodiagnostic testing, all of which were reported as grossly normal, although 2 reports mentioned patchy irregularity on eccentric multifocal testing of the temporal retina, presumably in the vicinity of the PRS.
7 eyes (from subjects 1-5) underwent further cross-sectional examination, multimodal imaging and functional testing. On biomicroscopic examination, all these subjects demonstrated a stellate appearance at the macula and had peripheral features suggestive of PRS, including microcystoid degeneration or absolute scotoma. On the composite widefield OCT scans, the FRS was evident at the level of Henle’s fiber layer (HFL) and was continuous with PRS or schisis-detachment, at which point the schisis cavity appears to widen, involving different or multiple retinal layers (Figures 2-4). All affected eyes had evidence of hyaloid attachment, to varying degrees, at the posterior pole, while the fellow eye in the 3 unilaterally affected subjects showed complete vitreous separation, but in conjunction with features of PRS. This functional peripheral loss was further demonstrated on 5 eyes with 60-4 ± 30-2 static VF testing. In all cases, microperimetry demonstrated normal macular function, with a mean (±SD) average sensitivity 28.5 (±1.0) dB (Figures 2 & 4).
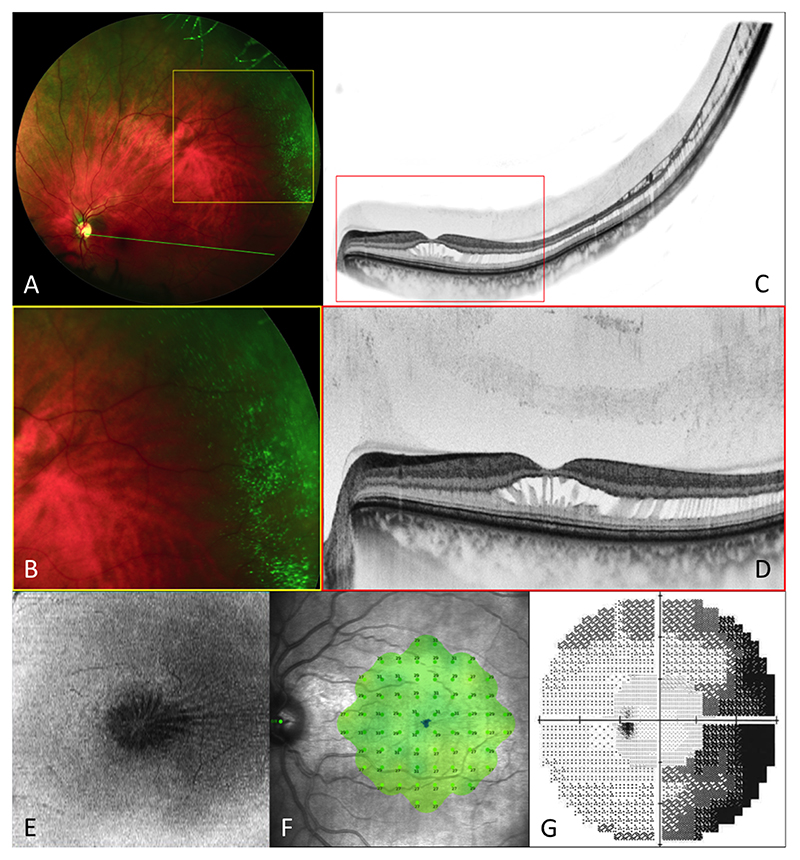
Figure 2.
Images from subject 1 (OS). Optos widefield SLO imaging (A&B) reveals microcystoid changes in the temporal peripheral retina. Widefield composite OCT (C) demonstrates continuity between the central foveomacular schisis and peripheral retinoschisis, with incomplete posterior vitreous separation (D). En face projection of the mid-retina (E) shows the ‘spoke-wheel’ distribution of the schisis cavity. Microperimetry (F) is normal, with evidence of scotoma in the nasal peripheral visual field (corresponding to the temporal retinal changes) on 60-4 static perimetry (G).
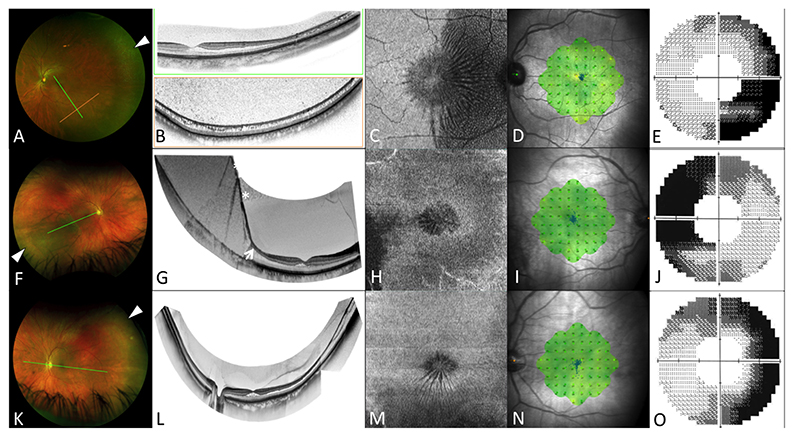
Figure 4.
Images from subjects 2 (OS) and 4 (OU). Optos widefield SLO imaging (A,F,K) demonstrates peripheral microcystoid changes (white arrow heads). Widefield OCT composites (B,G,L) reveal continuity with peripheral retinoschisis (B&J) and schisis-detachment (F, arrow); asterisks denote mirror artefacts on OCT. En face projections of the mid-retina demonstrating the ‘spoke-wheel’ distribution of the schisis, extending peripherally, where it takes on a ‘speckled’ appearance (C,H,M). Microperimetry (D,I,N) is normal, while 60-4 static perimetry (E,J,O) shows loss of sensitivity in the nasal visual field.
Discussion
We present a combination of retrospective and cross-sectional, observational data from 28 eyes affected with stellate non-hereditary idiopathic foveomacular retinoschisis (SNIFR), many of which exhibit peripheral retinal manifestations and posterior vitreous hyaloid features that have not been widely reported previously. A mean age at presentation of 63.6 years and slight female preponderance, albeit non-significant, is in keeping with previous reports and supports the notion that vitreous liquefaction and anomalous PVD, which is known to occur earlier and more frequently in female subjects, may play a role.17,18
In 2014, Ober et al. published a retrospective study of 22 eyes from 17 patients, all of which had FRS reported within the outer plexiform and outer nuclear layers, with no alternative predisposing disorder.17 In this study, 6 eyes from 4 patients in this series were demonstrated to have concurrent PRS, while a total of 19 eyes (86%) were reported not to have evidence of PVD (despite an average age of 61 in a predominantly myopic cohort). Several subsequent case studies have attributed findings of FRS to SNIFR, some of which have evidence of concurrent extramacular schisis, with associated features suggestive of both vitreoretinal adhesion and PRS (although these was not always considered of primary relevance in these reports). 19–22 Considering these factors, along with our findings, we hypothesize that there may be both a tractional element at the vitreoretinal interface resulting in FRS in eyes with SNIFR, as well as an association with PRS.
We found that, of those with contemporaneous documentation, 89% had evidence of PRS in addition to FRS. Moreover, in those who underwent cross-sectional functional testing, a discrepancy was noted between the areas affected by retinoschisis centrally and peripherally. All of our patients’ microperimetric findings support the consensus in the literature that SNIFR does not, for the most part, lead to significant loss of macular function.17 However, it appears that there is a transition zone, in the mid-periphery, where both the anatomical and functional characteristics of the retinoschisis changes, from a cavitation solely within HFL to one including the inner nuclear layer (INL) and, in some cases, also the nerve fiber layer (Figures 2-4). At approximately this point, the 60-4 static perimetry demonstrates the presence of a dense visual field defect. Here, the retinoschisis is behaving in a functional manner that we would traditionally expect with acquired PRS. While it is reassuring that the central retina appears to be spared such degeneration, the loss of peripheral field could challenge the purportedly benign course of SNIFR. The precise mechanism by which acquired retinoschisis causes absolute scotoma in the periphery is unclear, but may be attributable to erosion of the neuroretinal and glial support elements during coalescence of microcystoid cavities.23,24 Natural history studies of PRS have previously shown central involvement to be extremely rare, however these studies pre-date the widespread use of high-resolution OCT.25,26
Another novel observation is the large proportion of eyes with anomalous or incomplete PVD (86%) compared to those unaffected fellow eyes (42%). Furthermore, the presence of vitreomacular interface abnormalities in 5 excluded or fellow eyes lends further support to the possible role of anomalous PVD in subjects predisposed to developing the features of SNIFR. It was also noted that the unaffected fellow eyes of several subjects had evidence of PRS, but with complete PVD and no FRS. This asymmetric finding has been described previously in a single case by Ahmed et al., who ascribed it to possible “early stage of stellate nonhereditary idiopathic retinoschisis without foveal involvement”.21 In fact, in our study, spontaneous improvement of FRS was observed in 2 subjects following separation of the posterior hyaloid (Figure 5) and, in one of these cases, residual shallow PRS was detected on OCT. The observation of concurrent PRS and FRS, as in these cases of SNIFR, lends credence to the plausibility of a common pathophysiological mechanism. While acquired PRS is a common disorder, the concomitant manifestation of FRS might only be rarely observed, due to the necessary co-existence of tightly adherent cortical vitreous at the macula.
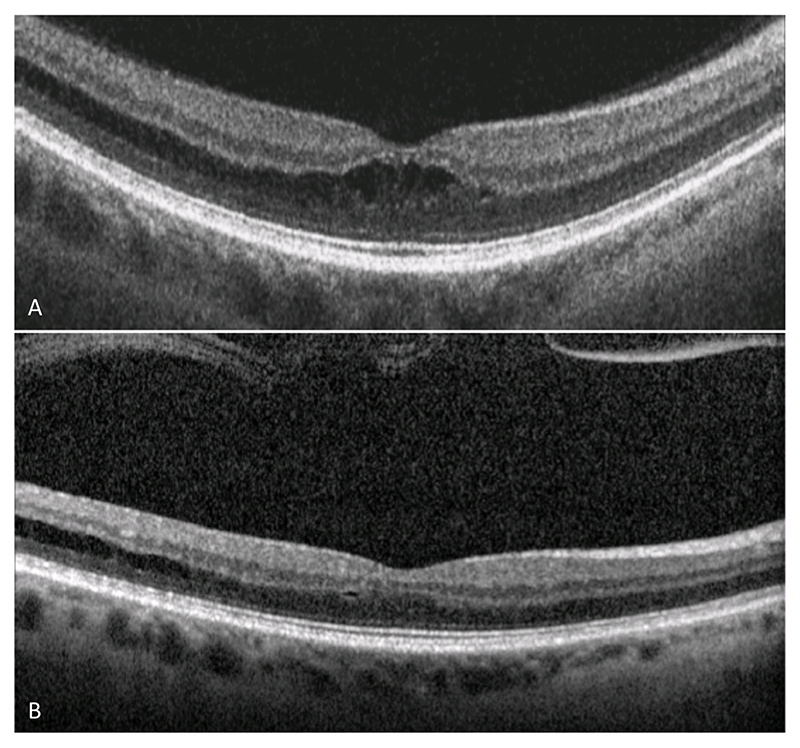
Figure 5. Images from subject 8 (OD). Serial macular OCT (A&B) demonstrating partial resolution of foveomacular retinoschisis following spontaneous posterior vitreous detachment.
The pathoanatomical mechanism by which tractional macular disorders, such as ERM or VMT, lead to the formation of foveomacular retinoschisis has previously been explored.27–30 It is proposed that, under normal conditions, the combined action of a specialized Müller cell (MC) subpopulation in the foveola (termed the ‘Müller cell cone’) and ‘typical’ z-shaped MCs in the foveal walls, form and maintain the foveal ultrastructure.30 The outer processes of the z-shaped MCs run in HFL with the photoreceptor axons in a horizontal orientation, thereby rendering this layer mechanically vulnerable to separation in response to inward tractional forces.28,29 In fact, the morphology of these MCs appear to provide a degree of anatomical compliance, allowing the retention of function in the presence of significant foveal deformation. On OCT, anteroposterior and tangential traction (such as those observed in tractional disorders of the vitreoretinal interface) appear to manifest with progressive beveling of columnar retinal elements (thought to be the MC processes), which obliquely span the schisis cavity. This anatomical phenomenon is thought to be responsible for the radiating ‘spoke-wheel’ pattern, as seen on en face imaging.27–29 Visual acuity is preserved at the point that the MC processes are in a beveled orientation, only deteriorating once the processes become fully verticalized.29 At this stage, it is presumed that the tensile capacity of the MCs is overcome and, as a result, mechanical disruption of the fovea may occur.31–34 While our cases do not have angiographic data to support an absence of exudative macular edema, the OCT and en face images are highly supportive of a similar pathoanatomical mechanism in SNIFR. Furthermore, the discrepancy observed between the anatomico-functional behavior of the retina centrally and peripherally could potentially be explained by a difference in MC morphology. Outside the macula, MCs become verticalized early in response to traction, resulting in the observed multi-layer retinoschisis and associated functional decline. This anatomic variability is best observed on the en face images (e.g. Figure 4C), where the ‘spoke-wheel’ pattern of the beveled MC processes, centered on the fovea, progressively verticalizes into the perifoveal and mid-peripheral retina, giving rise to a ‘speckled’ appearance.
In view of this potential mechanism, it is important to distinguish SNIFR from other causes of tractional FRS, which may share morphological characteristics. In particular, the presence of high myopia, VMT or an ERM may indicate an alternative mechanism;7–9 continuity with the optic nerve ought to raise suspicion of optic disc pit maculopathy or glaucoma-associated maculopathy.4–6 Inherited retinal disease should be considered as a possible cause in all young patients with cystoid spaces and inner/outer segment disruption on OCT.1–3 In 2019, Sun et al. reported a series of 17 eyes from 10 young, moderately myopic and predominantly female patients.35 In the first report of its kind, they described a condition, distinct from SNIFR, which manifests with FRS and leads to rapid functional deterioration with the development of subfoveal fluid and FTMH. At the time of surgery, they noted “remarkable liquefaction of the core vitreous” and had difficulty inducing PVD due to tight attachment to the macula. We agree that this is a distinct entity to SNIFR, but postulate that they may share common features, in particular the lack of complete PVD and tight vitreomacular adherence. Perhaps the difference in subject demographic reflects the premature vitreous liquefaction in Sun et al.’s cohort, compared to the normal age of liquefaction at which those patients with SNIFR seem to be affected. Despite Sun et al.’s description of a lack of PRS in their group, they published 2 images demonstrating extramacular schisis and it would be interesting to know the results of both anatomical and functional investigations of the peripheral retina in this cohort.
Despite a probable tractional etiology, there is currently no evidence to support surgical intervention in uncomplicated cases of SNIFR. Despite chronicity, most cases appear to maintain good macular function, and subjects are generally not aware of peripheral scotomata. Moreover, it is likely that surgical induction of PVD and removal of the posterior hyaloid would be hampered by both the retinoschisis itself and tight vitreoretinal adhesion, which could increase the rate of intra-operative complication or surgical failure.22
This study is limited by the retrospective design, resulting in incomplete collection of data, such as refractive error, axial length or investigations, including genetic testing or fluorescein or OCT angiography. In this regard, we are not in a position to explore certain associations, such as the relationship between refractive error and SNIFR, or confirm a definite absence of inherited or exudative pathology. Nevertheless, we have shown that SNIFR appears to be an under-recognized clinical phenomenon, accounting for up to 2% of all recorded cases of FRS. Additionally, we have demonstrated an apparent association between SNIFR and both PRS and anomalous or incomplete PVD, in the largest study of this disorder to date. It is conceivable that FRS, and by association, PRS, in SNIFR may represent an acquired mechanical process. In these cases, retinoschisis manifests with different anatomico-functional behavior at the macula to the periphery, exhibiting apparent long-term stability of visual acuity, despite peripheral absolute scotoma. The reason for this observed discrepancy remains unclear, but may relate to ultrastuctural variations in the retina between the macula and the periphery, such as the anatomical conformation of glial support cells (e.g. Müller cells). Further identification and characterization of such cases using prospective multimodal anatomico-functional testing may shed more light on the causative mechanism of this interesting and unusual disorder.
Summary Statement.
The precise pathophysiological mechanism of stellate nonhereditary idiopathic foveomacular retinoschisis (SNIFR) is unknown. This study demonstrates an association between SNIFR and incomplete posterior hyaloid detachment, indicating a possible tractional etiology, as well as a relationship with peripheral retinoschisis and extramacular absolute scotoma, despite retention of good central visual function.
Figure 3. Images from subject 5 (OU). Widefield OCT (A&C) reveals a transition from HFL to the INL, with persistent attachment of the posterior hyaloid (arrows). En face projection of the mid retina demonstrates the ‘spoke-wheel’ distribution of the schisis cavity, extending temporally (B&D).
Acknowledgements
We would like to thank Mr Vincent Rocco for his support and expertise in ophthalmic imaging.
Funding sources
This work was carried out in the NIHR-funded Biomedical Research Centre of UCL Institute of Ophthalmology and Moorfields Eye Hospital. OAM is supported by the Wellcome Trust (206619/Z/17/Z).
References
- 1.Yoshida-Uemura T, Katagiri S, Yokoi T, et al. Different foveal schisis patterns in each retinal layer in eyes with hereditary juvenile retinoschisis evaluated by en-face optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2017;255(4):719–23. doi: 10.1007/s00417-016-3552-2. [DOI] [PubMed] [Google Scholar]
- 2.Audo I, Michaelides M, Robson AG, et al. Phenotypic variation in enhanced S-cone syndrome. Invest Ophthalmol Vis Sci. 2008;49(5):2082–93. doi: 10.1167/iovs.05-1629. [DOI] [PubMed] [Google Scholar]
- 3.Khan KN, Robson A, Mahroo OAR, et al. A clinical and molecular characterisation of CRB1-associated maculopathy. Eur J Hum Genet. 2018;26(5):687–94. doi: 10.1038/s41431-017-0082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bloch E, Georgiadis O, Lukic M, da Cruz L. Optic Disc Pit Maculopathy: New Perspectives on the Natural History. Am J Ophthalmol. 2019;207:159–69. doi: 10.1016/j.ajo.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Steel DHW, Suleman J, Murphy DC, et al. Optic disc pit maculopathy: A two-year nationwide prospective population-based study. Ophthalmology. 2018;125(11):1757–64. doi: 10.1016/j.ophtha.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Fortune B, Ma KN, Gardiner SK, et al. Peripapillary Retinoschisis in Glaucoma: Association With Progression and OCT Signs of Müller Cell Involvement. Invest Ophthalmol Vis Sci. 2018;59(7):2818–27. doi: 10.1167/iovs.18-24160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panozzo G, Mercanti A. Optical coherence tomography findings in myopic traction maculopathy. Arch Ophthamol. 2004;122(10):1455–60. doi: 10.1001/archopht.122.10.1455. [DOI] [PubMed] [Google Scholar]
- 8.Shimada N, Tanaka Y, Tokoro T, Ohno-Matsui K. Natural course of myopic traction maculopathy and factors associated with progression or resolution. Am J Ophthalmol. 2013;156(5):948–957. doi: 10.1016/j.ajo.2013.06.031. [DOI] [PubMed] [Google Scholar]
- 9.Johnson MW. Tractional cystoid macular edema: a subtle variant of the vitreomacular traction syndrome. Am J Ophthalmol. 2005;140(2):184–92. doi: 10.1016/j.ajo.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 10.Makri OE, Georgalas I, Georgaopoulos CD. Drug-induced macular edema. Drugs. 2013;73(8):789–802. doi: 10.1007/s40265-013-0055-x. [DOI] [PubMed] [Google Scholar]
- 11.Yassur Y, Nissenkorn I, Ben-Sira I, et al. Autosomal Dominant Inheritance of Retinoschisis. Am J Ophthalmol. 1982;94(3):338–43. doi: 10.1016/0002-9394(82)90359-2. [DOI] [PubMed] [Google Scholar]
- 12.Lewis RA, Lee GB, Martonyi CL, et al. Familial foveal retinoschisis. Arch Ophthamol. 1977;95(7):1190–6. doi: 10.1001/archopht.1977.04450070088006. [DOI] [PubMed] [Google Scholar]
- 13.Shimazaki J, Matsuhashi M. Familial retinoschisis in female patients. Doc Ophthalmol Adv Ophthalmol. 1987;65(3):393–400. doi: 10.1007/BF00149946. [DOI] [PubMed] [Google Scholar]
- 14.Han DP, Sieving PA, Johnson MW, Martonyi CL. Foveal retinoschisis associated with senile retinoschisis in a woman. Am J Ophthalmol. 1988;106(1):107–9. doi: 10.1016/s0002-9394(14)76406-2. [DOI] [PubMed] [Google Scholar]
- 15.Kabanarou SA, Holder GE, Bird AC, et al. Isolated foveal retinoschisis as a cause of visual loss in young females. Br J Ophthalmol. 2003;87(6):801–3. doi: 10.1136/bjo.87.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen FK, McAllister IL, Chelva ES. Thirteen-year follow up of isolated foveal retinoschisis in a 24-year-old woman. Clin Experiment Ophthalmol. 2006;34(6):600–5. doi: 10.1111/j.1442-9071.2006.01283.x. [DOI] [PubMed] [Google Scholar]
- 17.Ober MD, Freund KB, Shah M, et al. Stellate nonhereditary idiopathic foveomacular retinoschisis. Ophthalmology. 2014;121(7):1406–13. doi: 10.1016/j.ophtha.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi K, Sato T, Manabe S-I, Hirata A. Sex-Related Differences in the Progression of Posterior Vitreous Detachment with Age. Ophthalmol Retina. 2019;3(3):237–43. doi: 10.1016/j.oret.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 19.Mandell JB, Kim AY, Shahidzadeh A, et al. Widefield OCT findings of a patient with stellate nonhereditary idiopathic foveomacular retinoschisis. Ophthalmic Surg Lasers Imaging Retina. 2016;47(8):774–7. doi: 10.3928/23258160-20160808-12. [DOI] [PubMed] [Google Scholar]
- 20.Weiss SJ, Adam MK, Hsu J. En face optical coherence tomography of stellate nonhereditary idiopathic foveomacular retinoschisis. JAMA Ophthalmol. 2017 doi: 10.1001/jamaophthalmol.2017.4280. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed D, Stattin M, Glittenberg C, et al. Stellate nonhereditary idiopathic foveomacular retinoschisis accompanied by contralateral peripheral retinoschisis. Retin Cases Brief Rep. 2019;13(2):135–40. doi: 10.1097/ICB.0000000000000544. [DOI] [PubMed] [Google Scholar]
- 22.Moraes BRM, Ferreira BFA, Nogueira TM, et al. Vitrectomy for stellate nonhereditary idiopathic foveomacular retinoschisis associated with outer retinal layer defect. Retin Cases Brief Rep. 2020 doi: 10.1097/icb.0000000000000966. [DOI] [PubMed] [Google Scholar]
- 23.Foos RY. Senile retinoschisis. Relationship to cystoid degeneration. Trans - Am Acad Ophthalmol Otolaryngol Am Acad Ophthalmol Otolaryngol. 1970;74(1):33–51. [PubMed] [Google Scholar]
- 24.Engstrom R, Glasgow BJ, Foos R, Straatsma B. In: Duane’s Ophthalmology. Tasman W, Jaeger EA, editors. Vol. 3. Lippincott Williams & Wilkins; 2006. Chapter 26: Degenerative Diseases of the Peripheral Retina. 2006. [Google Scholar]
- 25.Byer NE. Long-term natural history study of senile retinoschisis with implications for management. Ophthalmology. 1986;93(9):1127–37. doi: 10.1016/s0161-6420(86)33601-7. [DOI] [PubMed] [Google Scholar]
- 26.Buch H, Vinding T, Nielsen NV. Prevalence and long-term natural course of retinoschisis among elderly individuals: the Copenhagen City Eye Study. Ophthalmology. 2007;114(4):751–5. doi: 10.1016/j.ophtha.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 27.Govetto A, Sarraf D, Hubschman J-P, et al. Distinctive mechanisms and patterns of exudative versus tractional intraretinal cystoid spaces as seen with multimodal imaging. Am J Ophthalmol. 2020;212:43–56. doi: 10.1016/j.ajo.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 28.Govetto A, Hubschman J-P, Sarraf D, et al. The role of Müller cells in tractional macular disorders: an optical coherence tomography study and physical model of mechanical force transmission. Br J Ophthalmol. 2019;104:466–472. doi: 10.1136/bjophthalmol-2019-314245. [DOI] [PubMed] [Google Scholar]
- 29.Bringmann A, Unterlauft JD, Weidemann R, et al. Two different populations of Müller cells stabilize the structure of the fovea: an optical coherence tomography study. Int Ophthalmol. 2020;11:2931–2948. doi: 10.1007/s10792-020-01477-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gass JD. Müller cell cone, an overlooked part of the anatomy of the fovea centralis; hypotheses concerning its role in the pathogenesis of macular hole and foveomacular retinoschisis. 1999;6:821–823. doi: 10.1001/archopht.117.6.821. [DOI] [PubMed] [Google Scholar]
- 31.Govetto A, Dacquay Y, Farajzadeh M, et al. Lamellar macular hole: two distinct clinical entities? Am J Ophthalmol. 2016;164:99–109. doi: 10.1016/j.ajo.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Govetto A, Bhavsar KV, Virgili G, et al. Tractional abnormalities of the central foveal bouquet in epiretinal membranes: clinical spectrum and pathophysiological perspectives. Am J Ophthalmol. 2017;184:167–180. doi: 10.1016/j.ajo.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 33.Bringmann A, Unterlauft JD, Weidemann R, et al. Morphology of partial-thickness macular defects: presumed roles of Müller cells and tissue layer interfaces of low mechanical stability. Int J Retina Vitreous. 2020;6:28. doi: 10.1186/s40942-020-00232-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bringmann A, Duncker T, Jochmann C. Spontaneous closure of small full-thickness macular holes: Presumed role of Müller cells. Acta Ophthalmologica. 2020;4:e447–e456. doi: 10.1111/aos.14289. [DOI] [PubMed] [Google Scholar]
- 35.Sun Z, Gao H, Wang M, et al. Rapid progression of foveomacular retinoschisis in young myopics. Retina. 2019;39(7):1278–88. doi: 10.1097/IAE.0000000000002203. [DOI] [PubMed] [Google Scholar]