Abstract
Microbubbles (MB) are routinely used ultrasound (US) contrast agents that have recently attracted increasing attention as stimuli-responsive drug delivery systems. In order to better understand MB-based drug delivery, we studied the role of drug hydrophobicity and molecular weight on MB loading, shelf-life stability, US properties and drug release. Eight model drugs, varying in hydrophobicity and molecular weight, were loaded into the shell of poly(butyl cyanoacrylate) (PBCA) MB. In the case of drugs with progesterone as a common structural backbone (i.e. for corticosteroids), loading capacity and drug release correlated well with hydrophobicity and molecular weight. Conversely, when employing drugs with no structural similarity (i.e. four different fluorescent dyes), loading capacity and release did not correlate with hydrophobicity and molecular weight. All model drug-loaded MB formulations could be equally efficiently destroyed upon exposure to US. Together, these findings provide valuable insights on how the physicochemical properties of (model) drug molecules affect their loading and retention in and US-induced release from polymeric MB, thereby facilitating the development of drug-loaded MB formulations for US-triggered drug delivery.
Keywords: Drug delivery, Theranostics, Microbubbles, Ultrasound, PBCA, Corticosteroids
Graphic abstract.
1. Introduction
Microbubbles (MB) are 1-10 μm sized air-filled vesicles that are shell-stabilized by lipids, proteins or polymers. They are widely used as contrast agents for functional and molecular ultrasound (US) imaging1–3. Apart from US imaging, MB have recently gained increasing attention for drug delivery purposes: when MB are exposed to US, they oscillate (at low mechanical index) or implode (at high mechanical index) resulting in a number of biophysical phenomena which are known to permeabilize blood vessel and cellular membranes, thereby facilitating drug delivery into tissues and cells4–10.
In the context of MB- and US-mediated drug delivery, strategies have focused on both co-administration and co-formulation, the former being applied more frequently so far11–13. This may be due to the fact that co-formulation is somewhat more demanding, since it requires the loading of drugs or drug delivery systems into or onto the MB shell14–15. A particular advantage of using drug-loaded MB is that upon US exposure, drug release from the MB shell is specifically facilitated at target site, thereby improving efficacy, while at the same time reducing off-target drug localization and side effects. This strategy has shown promise in thrombolysis and tumor therapy16.
For co-formulation, polymer-based hard shell MB have advantages compared to lipid-based soft shell MB, as they can encapsulate higher amounts of drug molecules in their much thicker shell17–18. Among polymers used for MB synthesis, poly(butyl cyanoacrylate) (PBCA) is a well known and biocompatible polymer that is synthesized via the anionic polymerization and that has been used not only only for MB preparation, but also for generating chemotherapy-loaded nanoparticles, several of which have even made it to evaluation in patients19. Moreover, Sulheim et al. showed that nanoparticles made of PBCA can be internalized and efficiently degraded inside cells, potentially facilitating intracellular drug release. However, longer alkyl side-chain cyanoacrylates, as in poly(octyl cyanoacrylate) nanoparticles, remained intact. This notion support the use of PBCA as a polymeric material for drug delivery applications20–21. Previously, we showed that PBCA MB could efficiently encapsulate ultrasmall superparamagnetic iron oxide (USPIO) nanoparticles, as well as fluorophores such as rhodamine B and coumarin 6 into the polymeric shell22–23. Wheatley and colleagues found that paclitaxel, a highly hydrophobic drug, was encapsulated in the polymeric shell more efficiently than doxorubicin, which is more hydrophilic24. However, systematic investigations on how drug properties influence drug loading into and drug release from the shell of polymeric MB are lacking, which is essential for translating such systems to the clinic.
In this manuscript, we set out to better understand the effect of drug hydrophobicity (i.e. log P) and molecular weight on the loading, stability, US properties and drug release profile of PBCA MB. To this end, we loaded eight different model drugs into the shell of PBCA MB and characterized the resulting formulations using spectroscopy, high performance liquid chromatography (HPLC) and US imaging (Figure 1). We observed that for corticosteroids with progesterone as common structural derivative (left panel Figure 2), loading capacity and release correlated with concurrently increasing hydrophobicity and molecular weight. For structurally different dyes, whose hydrophobicity and molecular weight did not concurrently increase (right panel Figure 2), loading capacity and release did not correlate with hydrophobicity and molecular weight. These findings provide important new insights on how drug properties affect the loading, stability, US properties and release of polymeric MB, which may facilitate the development of drug-loaded MB formulations.
Figure 1. Schematic study setup.
Poly(butyl cyanoacrylate)-based (PBCA) polymeric microbubbles (MB) were synthesized by anionic polymerization of BCA. Upon loading of eight different (model) drugs into the shell of PBCA MB, several characterization techniques were employed to study drug loading capacity, drug retention, ultrasound (US) properties and US-induced drug release.
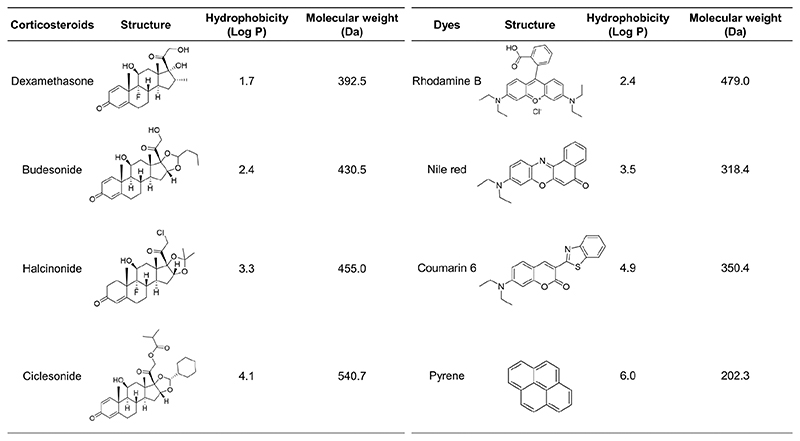
Figure 2. Overview of the (model) drugs used in this study.
Corticosteroids with progesterone as a common structural derivative (whose hydrophobicity and molecular weight increased simultaneously; left panel) and fluorescent dyes with no common structural derivative (whose hydrophobicity and molecular weight did not increase simultaneously; right panel) were loaded into the shell of PBCA MB.
2. Materials and methods
2.1. Materials
Butyl cyanoacrylate (BCA) was purchased from Special Polymer Ltd (Bulgaria). Dexamethasone, budesonide, coumarin 6, pyrene, nile red, triton X-100, gelatin, dimethylsulfoxide (DMSO), acetonitrile (ACN) and methanol were bought from Sigma-Aldrich (Germany). Halcinonide was obtained from United States Pharmacopeia (Italy) and rhodamine B from AppliChem GmbH (Germany). Deionized (DI) water was used for all experiments and all other reagents were of appropriate analytical grade.
2.2. Synthesis of PBCA-based polymeric MB
Poly(butyl cyanoacrylate) (PBCA) MB were synthesized using a previously established protocol22,25. Briefly, 3 ml of BCA monomer was added drop-wise to a 300 ml aqueous solution containing 1% (w/v) triton X-100 at pH 2.5. Upon drop-wise addition of BCA, the mixture was homogenized at 10000 rpm for 1 h using an Ultra-turrax (IKA-Werke, Germany). This led to the formation of air-filled polydispersed MB. Subsequently, these MB were purified by multiple centrifugation steps at 500 rpm for 20 mins and were finally suspended in 0.02 % (w/v) triton X-100 at pH 7. These purification steps resulted in a relatively monodisperse MB size population with a mean size of approximately 2.5 μm13,22.
2.3. Quantification of size, size distrubution and concentration of PBCA MB
The size distribution and concentration of PBCA MB were analyzed using a Multisizer 3 (Beckmann Coulter, Germany) which was equipped with a 30 μm sensor orifice. Briefly, 20 ml of isotone solution was poured into an acuvette and was placed on the sample platform. This was used as a blank measurement in order to correct for background noise. Subsequently, 10 μl of PBCA MB was diluted with 20 ml of isotone solution and the sample was measured in volumetric mode with a diltuion factor of 2000. These steps were repeated in triplicates to obtain mean ± standard deviation. Further details of the experimental parameters used in the Multisizer 3 measurements are listed in Table S1.
2.4. Drug loading into the shell of PBCA-based polymeric MB
The corticosteroids dexamethasone, budesonide, halcinonide, ciclesonide and the fluorescent dyes rhodamine B, nile red, coumarin 6, pyrene were used as model compounds for studying drug loading into the shell of PBCA-based polymeric MB. Different (model) drug amounts, i.e. 0.1, 1, 5, 10 and 20 mg, were dissolved in 200 μl of DMSO and subsequently mixed in a 10 ml of an aqueous suspension containing 1010 MB. These drug amounts corresponded to feed ratios (drug:MB) w/w of 1:200, 1:20, 1:4, 1:2 and 1:1 respectively. The mixtures were stirred at 250 rpm for 19 h at room temperature (RT). To remove unloaded drug, the MB were allowed to float due to their bouyant force and the aqueous solution underneath the MB was replaced multiple times until there was no free drug present anymore13. Finally, the drug-loaded MB were suspended in 0.02 % (w/v) triton X-100 at pH 7 and stored at RT for further characterization.
2.5. Confocal laser scanning microscopy
Coumarin 6 was utilized to visualize encapsulation into the shell of PBCA MB. A Leica TCS SP8 X inverted confocal microscope (Leica Microsystems, Wetzlar, Germany) equipped with a plan-apochromat 100x/1.40 oil-immersion objective was used to visualize coumarin 6-loading in PBCA MB. The samples were applied on a high precision cover glass (170 μm, No. 1.5H) from Marienfeld (Lauda-Königshofen, Germany). The excitation wavelength was set at λ = 470 nm via a filtered white light laser and the resulting emission was detected at λ = 491 - 556 nm. Deconvolution of the images was processed using the Huygens Professional software via the Classic Maximum Likelihood Estimation (CMLE) method.
2.6. Quantification of drug loading
All samples were prepared by diluting 10 μl of drug-loaded MB with 90 μl of DMSO, which destroyed the MB and facilitated the release of the encapsulated model drugs. The amount of drug incorporated into the MB shell was then quantified either using a microplate reader TECAN Infinite M200 Pro (Tecan Group Ltd, Germany) or high performance liquid chromatography (HPLC). The details of the experimental parameters used in the plate reader and HPLC are summarized in table S1 (supplementary section). Finally, encapsulation efficiency (EE) and loading capacity (LC) of all MB formulations were determined using the formulas: EE = (weight of drug incorporated / weight of drug added during the formulation) × 100 %; and LC = (weight of drug incorporated / weight of drug-loaded MB) × 100 %.
2.7. Stability assessment of drug-loaded MB
MB formulations containing 109 MB/ml (i.e. a relevant range for in vivo application; equivalent to 2 mg/ml) were stored in screw-cap glass vials at RT for stability analysis. In particular, retention of drugs inside the MB shell were studied over a period of 12 weeks and quantified using the formula: Drug retention at week x = (amount of drug retained inside the MB shell at week x / amount of incorporated drug) × 100 %.
2.8. US analysis of drug-loaded MB
To study the effect of drug encapsulation on US properties, MB with and without model drugs were exposed to low and high power US, and based on these responses the percentage of MB destruction was quantified. For this purpose, 3 × 105 MB were suspended in 4.5 ml of 2% w/v gelatin solution, and the mixture was embedded in 10% w/v gelatin solution. A preclinical US device (Vevo 2100, VisualSonics, Canada) was employed to image the MB in gelatin phantoms. US imaging was performed in non-linear contrast mode at a center frequency of 18 MHz, initially with a 4% power (mechanical index 0.2), followed by 100% power (mechanical index 0.9) in order to destroy the MB in the gelatin phantom. A region-of-interest (ROI) of approximately 500 mm2 was drawn at the middle of all images and the resulting contrast intensity was analyzed using the Vevo LAB software. Finally, % MB destruction was quantified as: ((US contrast at 4% power - US contrast at 100% power) / US contrast at 4% power) × 100 %.
2.9. Quantification of US-mediated drug release
An ultrasonic cleaner device (UCS 200TH, VWR) was employed to destroy the MB and to induce the release of encapsulated model drugs from the MB shell22,26. To ensure that all the released model drugs retained in the supernatant, we diluted 109 MB with 0.02 % (w/v) triton X-100 such that the final drug concentration was lower than its solubility. All MB samples were subjected to US for 10 min. The samples were subsequently centrifuged at 10000 rpm for 10 min to separate the polymer fragments (as a result of MB destruction) from the released drugs (supernatant). The amount of released drugs was quantified in a similar way as described in section 2.4, and upon analyses drug release was determined as percentage of the total encapsulated drug.
2.10. Statistical analysis
Three different batches of drug-loaded MB formulations were prepared from three different batches of preformed MB, and each formulation was measured thrice. All values are presented as mean ± standard deviation. Statistical analyses were performed using GraphPad Prism 5. The results in Figures 3, 4, 5 and 6 were analyzed using two-way ANOVA, corrected for multiple comparisons (Bonferroni). The correlation analyses in Figures 7 and 8 were performed using Pearson’s r coefficients. A p value of less than 0.05 was considered to be statistically significant.
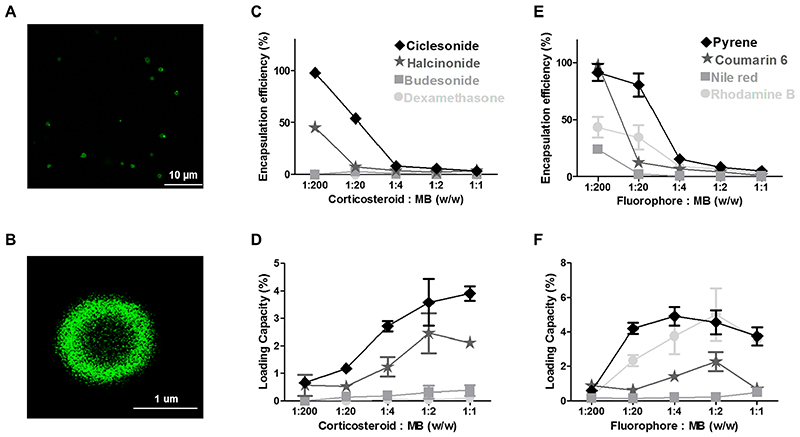
Figure 3. Loading capacity and encapsulation efficiency of PBCA MB.
A-B: Representative fluorescence microscopy image exemplifying successful loading of coumarin 6 into the shell of PBCA MB. C-D: Quantification of corticosteroids in MB showing that an increase in hydrophobicity and molecular weight results in an increased encapsulation efficiency and loading capacity. E-F: Quantitative analysis of fluorophore-loaded MB showing that with increasing hydrophobicity, loading capacity and encapsulation efficiency also do tend to increase, with the exception of rhodamine B. Note that the color-coding of the plotted lines corresponds to the degree of hydrophobicity. Values represent mean ± standard deviation of three different batches of drug-loaded MB, measured in triplicates.
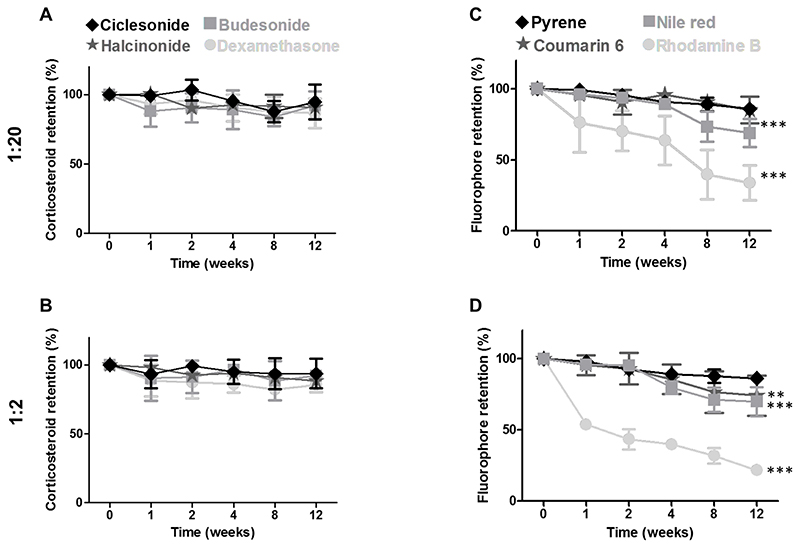
Figure 4. Shelf-life stability of drug-loaded PBCA MB.
A-B: Quantitative analysis of drug retention, showing that at both the 1:20 and 1:2 feed ratios, corticosteroid content did not decrease significantly over time and exemplifying that all four drugs were efficiently retained in the MB shell even after 12 weeks of storage. C-D: Quantitative analysis of fluorophore retention showing that fluorescent dyes were less efficiently retained at the higher feed ratio of 1:2 as compared to lower feed ratio of 1:20. Additionally, less hydrophobic model drugs were less efficiently retained in the MB shell and vice-versa. Note that the color-coding of the plotted lines corresponds to the degree of hydrophobicity. Values represent mean ± standard deviation of three different batches of drug-loaded MB, measured in triplicates. All statistical comparisons were made with respect to week 0. ** and *** indicate p < 0.01 and p < 0.001, respectively.
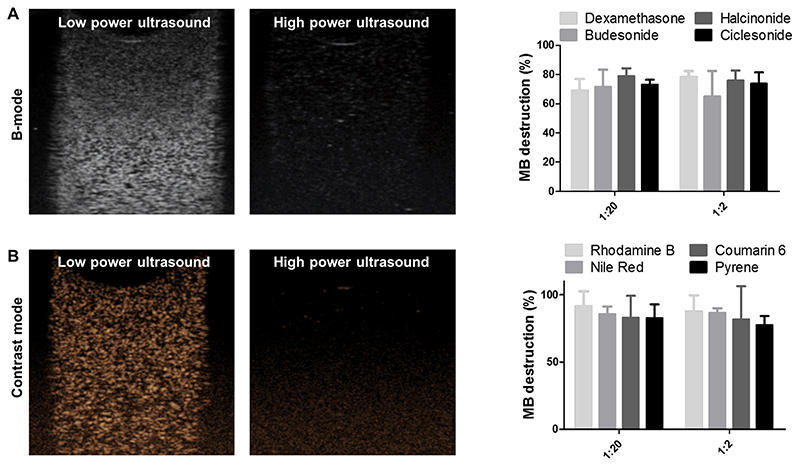
Figure 5. Ultrasound analysis of drug-loaded PBCA MB.
A-B: A representative B-mode and contrast-mode image of all drug-loaded PBCA MB showing that ultrasound (US) contrast is efficiently generated at low powers, while at high powers, MB are destroyed (as evidenced by the lack of US contrast). C-D: Quantitative analysis of MB destruction showing that there are no significant differences between the different drug-loaded PBCA MB, and demonstrating that US properties are independent of the hydrophobicity and molecular weight of the encapsulated drug. Note that the color-coding of the plotted lines corresponds to the degree of hydrophobicity. Values represent mean ± standard deviation of three different batches of drug-loaded MB, measured in triplicates.
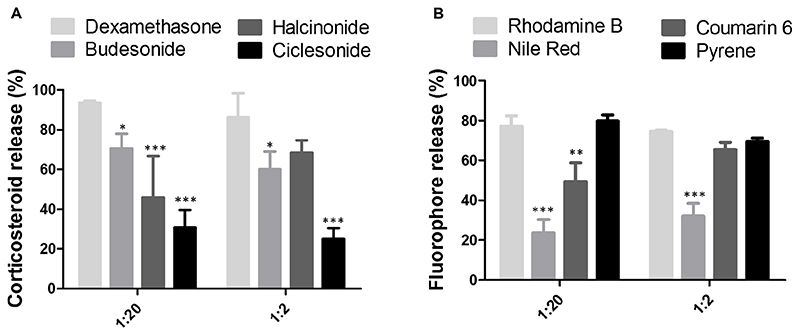
Figure 6. US-induced drug release from PBCA MB.
A: Quantitative analysis of corticosteroid release showing that hydrophobicity and molecular weight negatively affect US-induced drug release. B: Fluorophore release from the MB shell did not show a relationship to hydrophobicity and molecular weight. Note that color-coding corresponds to the degree of hydrophobicity. Values represent mean ± standard deviation of three different batches of drug-loaded MB, measured in triplicates. All statistical comparisons were made respect to dexamethasone and rhodamine B in panel A and B respectively. *, ** and *** indicate p < 0.05, p < 0.01 and p < 0.001 respectively.
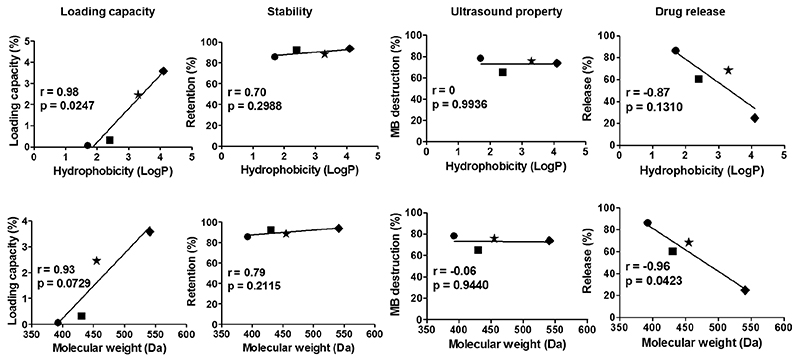
Figure 7. Correlating the hydrophobicity (top) and molecular weight (bottom) of corticosteroid drugs with the characteristics of shell-loaded PBCA MB.
Loading capacity and drug release of corticosteroid-loaded MB showed a good correlation with both hydrophobicity and molecular weight. Symbols: ● = Dexamethasone. ■ = Budesonide. ★ = Halcinonide. ◆ = Ciclesonide.
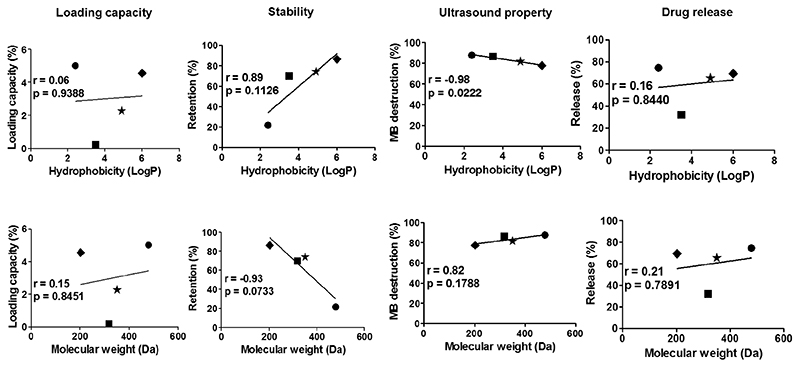
Figure 8. Correlating hydrophobicity (top) and molecular weight (bottom) of fluorophores with the characteristics of shell-loaded PBCA MB.
Only shelf-life stability showed a good correlation with both hydrophobicity and molecular weight. Symbols: ● = Rhodamine B. ■ = Nile red. ★ = Coumarin 6. ◆ = Pyrene.
3. Results and discussions
3.1. Loading capacity and encapsulation efficiency of PBCA MB
We loaded four different corticosteroids and four different fluorescent dyes into the shell of PBCA MB (Figure 2). As a proof-of-concept, Figure 3A-B shows that the fluorescent model drug coumarin 6 can indeed be successfully loaded into the shell of PBCA MB. We furthermore evaluated five different feed ratios, i.e. 1:200, 1:20, 1:4, 1:2 and 1:1, in order to achieve maximal levels of drug amounts inside the MB shell. We observed that model drug loading at different feed ratios does not influence the size and and size distribution of PBCA MB (see Figure S1 and Table S2).
The selected corticosteroids had progesterone as a common structural backbone, and their hydrophobicity and molecular weight increased simultaneously. Upon loading them into the shell of PBCA MB, we observed that as the hydrophobicity of these drugs increased, MB payload increased. This is shown in Figure 3C, where dexamethasone, budesonide, halcinonide and ciclesonide, in the order of increasing hydrophobicity, resulted in increasing encapsulation efficiencies, e.g. at a 1:20 feed ratio, values of 0.02, 2.75, 7.05 and 53.86 % were obtained. Similarly, at a feed ratio of 1:2, corticosteroid loading capacity increased in the order of increasing hydrophobicity, with values of 0.05, 0.31, 2.46 and 3.58 %, respectively (Figure 3D). These values were significantly different from each other (p < 0.001) except when comparing the two least hydrophobic drugs, i.e. dexamethasone vs. budesonide. Analogously, since hydrophobicity and molecular weight concurrently increased for corticosteroids that had common progesterone backbone, employing these molecules with increasing molecular weight also led to an increase in MB loading.
On the other hand, the fluorescent dyes selected for this study were structurally different, and their hydrophobicity and molecular weight did not increase in the same order. Their order of increasing hydrophobicity was rhodamine B, nile red, coumarin 6 and pyrene; while their order of increasing molecular weight was pyrene, nile red, coumarin 6 and rhodamine B (see Figure 2). When studying the impact of hydrophobicity on MB payload, Figure 3E-F demonstrates that increasing hydrophobicity led to an increase in encapsulation efficiency and loading capacity, with the exception of rhodamine B. Rhodamine B, which was the least hydrophobic dye, was still encapsulated quite efficiently into the MB shell. This may be due to the relatively large molecular weight of rhodamine B, allowing for more efficient retention in the PBCA shell even after several washing steps during the experiment. Similarly, and along the same line of thinking, fluorescent dyes with increasing molecular weight were more efficiently retained and loaded into the MB shell, with the exception of pyrene. Pyrene, being the smallest in molecular weight, was still efficiently loaded into the PBCA shell, as it was the most hydrophobic dye molecule (Figure 3E and 3F).
These findings can also be explained from a structural point of view. PBCA has a carbonyl oxygen and a cyanate nitrogen, which are both partially electronegatively charged, making it possible to have an n-Pi* type of interaction in whih they donate electron ion pairs to electropositive sites in corticosteroids and dyes27. For corticosteroids, the 4 drugs used in this study had a similar progesterone-based structural backbone, the only variations being in substitutions of the exocyclic side chains. The presence of such substituted groups can change the electronegativity of neighboring molecules and thus influence its interaction with PBCA. Examples of this are the presence of fluorine in dexamethasone versus hydrogen in budesonide, or the presence of the hydroxyl group in dexamethasone versus a diether group in the other corticosteroids. Along the same line of thinking, also increasing the side chain (as in ciclesonide) resulted in increased affinity towards PBCA. As opposed to the steroids, for the dyes, the molecular structures greatly differed. All have a benzene ring sub-unit, which provide free electrons for exchange and interaction with the MB shell. Coumarin 6 has more free electons than nile red, which potentially explains its enhanced interaction with PBCA. Rhodamine B not only has free and flexible electrons in its rings, but also contains electropostively charged oxygen, which may interact with both carbonyl oxygen and cyanate nitrogen on the PBCA28, and which potentially explain its relatively efficient loading.
The results in Figure 3 and the above molecular interactions directly relate to shell composition of PBCA MB, which is highly hydrophobic and which has a thickness of 50-200 nm13, thereby allowing hydrophobic drug molecules to be incorporated efficiently. Smaller molecular weight drugs are likely washed out of the PBCA MB shell more easily than larger molecular weight drugs during the washing steps, explaining the more efficient retention and higher loading of larger molecular weight drug molecules. Similar findings have been reported for poly(lacticacid) (PLA)-based MB, which were shown to encapsulate paclitaxel, a hydrophobic and relatively large anticancer drug more efficiently than doxorubicin, which is less hydrophobic and smaller24. Overall, these findings indicate that the loading of PBCA MB increases with increasing drug hydrophobicity and molecular weight.
3.2. Shelf-life stability of drug-loaded PBCA MB
We employed the formulations that had minimal and maximal levels of drug molecules in the MB shell for analysis of shelf-life stability, US properties and drug release. Given the negligible differences and similar amounts of drugs were loaded for the 1:200 and 1:20 feed ratios, we opted to use the 1:20 feed ratio as the minimal one. For the maximal one, saturated levels of drug amounts were observed at 1:2 feed ratio.
For stability studies, MB formulations were stored at RT and drug retention was quantified over a period of 1, 2, 4, 8 and 12 weeks. We observed only very minute changes (< 5 %) in the size of MB formulations after 12 weeks of storage (see table S3, supplementary section). In the case of corticosteroids, there was no statistically significant decline in drug retention over time at a feed ratio of 1:20 (p > 0.05; Figure 4A), exemplifying that all four drugs were efficiently retained in the MB shell even after 12 weeks of storage. Similar findings were also observed at 1:2 feed ratio (Figure 4B), highlighting that all corticosteroid-loaded MB were stable up to 12 weeks upon storage at RT. Importantly, these experiments only demonstrate proper shelf-life stability of drug-loaded MB. To be able to draw conclusions on stable in vivo drug retention in the shell of PBCA MB, experiments in more complex media (e.g. in full blood or serum) are needed.
As opposed to the corticosteroid case, fluorophore retention varied quite a bit with feed ratio and hydrophobicity. Figure 4C-D shows that fluorophores at higher feed ratio (i.e. 1:2) are less efficiently retained in the MB shell than those at lower feed ratio (i.e. 1:20). In the context of hydrophobicity, fluorophores with low hydrophobicity were found to be poorly retained in the MB shell. This is illustrated in Figure 4C, showing that rhodamine B, nile red, coumarin 6 and pyrene, in order of increasing hydrophobicity, gave rise to more efficient dye retention upon 12 weeks of storage, with values of 33.97 % (p < 0.001), 68.94 % (p < 0.001), 85.12 % (p > 0.05) and 85.85 % (p > 0.05), respectively. Conversely, fluorophore retention in the MB shell was not depending on flurophore molecular weight (Figure 4C and 4D). Together, these analyses demonstrate that all corticosteroids were efficiently retained in the MB shell. In the case of fluorophores, retention in the MB shell increased with dye hydrophobicity.
3.3. US properties of drug-loaded PBCA MB
To study the US properties of the MB formulations upon drug encapsulation, they were embedded in tissue-mimicking gelatin phantoms and exposed to US. Figure 5A and 5B show representative US B-mode and contrast-mode images, respectively, illustrating that all drug-loaded loaded MB generated contrast at low power US and were efficiently destroyed at high power US.
In the case of corticosteroid-loaded MB, there were no differences in the extent of MB destruction between the four formulations (p > 0.05; Figure 5C), exemplifying that the destruction of the MB was independent of the hydrophobicity and molecular weight of the encapsulated drug. Analogously, also in the case of the dye-loaded MB, the destruction levels were similar for rhodamine B, nile red, coumarin 6 and pyrene-loaded MB (independent of the feed ratio; p > 0.05; Figure 5D). One plausible explanation is that due to the relatively low loading capacity of PBCA MB (< 5 %), the overall effect of molecular interactions between the loaded agents and the PBCA shell is not very substantial.
Overall, it can be concluded that drug-loaded MB can be efficiently destroyed by US, indicating that drug loading does not negatively affect MB response to US. Additionally, all drug-loaded MB were equally efficient in getting destroyed upon US exposure, highlighting that the destruction of MB formulations has no dependency on the properties of the model drug.
3.4. US-induced drug release from PBCA MB
For corticosteroids, US-induced drug release from the MB shell decreased as the hydrophobicity of the drug increased. This is exemplified in Figure 6A for the 1:20 feed ratio, with dexamethasone, budesonide, halcinonide and ciclesonide - in the order of increasing hydrophobicity - showing release percentages of 93.62, 70.64, 45.99 and 30.81 %, respectively. All of these values were significantly lower than dexamethasone. Similarly, US-induced drug release was decreased as the molecular weight of the corticosteroid increased. Also at the 1:2 feed ratio, these drugs with lower hydrophobicity and smaller molecular weight were more efficiently released from the MB shell (Figure 6A). These findings can be explained by taking the thick and hydrophobic shell of PBCA MB into account, which allows for a stronger interaction with more hydrophobic and larger molecular weight drugs, eventually resulting in less efficient release as compared to for hydrophilic and smaller molecular weight drugs.
In the case of the fluorophores, increasing hydrophobicity of the encapsulated model drugs did not result in any clear relationship to drug release from the MB shell (Figure 6B). Similarly, also when studying the effect of model drug molecular weight on its US-induced release from the MB shell, there appeared no clear relationship (Figure 6B). In summary, for corticosteroids with a common progesterone backbone, triggered drug release was inversely correlated with hydrophobicity and molecular weight. Vice versa, for structurally different fluorescent dyes, drug release did not show any relationship with hydrophobicity and molecular weight.
3.5. Correlating drug hydrophobicity and molecular weight with PBCA MB characteristics
Taking all of the above results together, we finally correlated both hydrophobicity and molecular weight of the selected (model) drugs with key characteristics of resulting MB formulations, i.e. with loading capacity, shelf-life stability, US properties and US-induced drug release at the 1:2 feed ratio. In the case of corticosteroids, which had progesterone as a common structural backbone and whose hydrophobicity and molecular weight increased concurrently, several key characteristics showed a good correlation to both hydrophobicity and molecular weight. This is exemplified in Figure 7, illustrating that loading capacity correlated reasonably well with hydrophobicity (r = 0.98) and molecular weight (r = 0.93). Also drug release correlated relatively well with hydrophobicity (r = -0.87) and molecular weight (r = -0.96), but in an inverse manner. Two of the these correlations were of borderline insignificance which can be due to the relatively low sample size.
Also shelf-life stability correlates relatively well with hydrophobicity and molecular weight, but the almost horizontal slope shows that the overall impact is very small. US properties did not correlate with hydrophobicity and molecular weight (Figure 7). These findings are favorable, because it shows that independent of drug properties, drug entrapment in the MB shell is stable. Corticosteroid-loaded MB such as the ones described here may find applications in priming tumors for improved delivery of drugs and drug delivery systems, by permeabilizing tumor vessels via the oscillation of MB and by at the same time reducing the extent of extracellular matrix deposition in tumors through the release and action of corticosteroids29–30.
In the case of the fluorophores, which were structurally very different and whose hydrophobicity and molecular weight did not increase concurrently, most characteristics studied did not show a good correlation with hydrophobicity and molecular weight. This is depicted in Figure 8, exemplifying that loading capacity and drug release did not correlate with the hydrophobicity and molecular weight (r < 0.2). MB destructability did correlate with hydrophobicity and molecular weight, but the overall impact seems minimal, since the slope is close to horizontal. Only the shelf-life stability of fluorophore-loaded MB correlated well with hydrophobicty (r = 0.89) and molecular weight (r = -0.93) of the encapsulated dyes. In general, fluorophore-loaded MB may find application in different multimodal imaging setups, for instance in experiments aiming to verify the in vivo binding of ligand-targeted MB to tumor or inflamed blood vessels in an ex vivo microscopy setup, or in studies looking at the ex vivo distribution of polymer shell fragments at pathological (target) sites after US-mediated MB destruction13,31–33.
4. Conclusion
We show that PBCA-based polymeric MB can be used to encapsulate a variety of model drugs. For drugs with progesterone as a common structural derivative, we found that compounds with increasing hydrohobicity and molecular weight can be loaded and retained more efficiently in the shell of PBCA MB, but are less efficiently released upon US-mediated MB destruction. Conversely, when employing structurally different fluorescent dyes, loading capacity and compound release did not correlate with hydrophobicity and molecular weight. Taking eveything together, the results presented here demonstrate that the physicochemical properties of (model) drugs play an important role in determining loading in and release from PBCA-based MB.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge financial support by the Chinese Scholarship Council (grant number: 201506910070), the European Commission (EuroNanoMed-III: NSC4DIPG), the European Research Council (ERC-CoG 864121) and the German Research Foundation (DFG: GRK2375 (grant #331065168), SFB1066 and SFB/TRR57). Image templates from Servier Medical Art (http://smart.servier.com) were used for the preparation of Figure 1.
Footnotes
Competing interests
FK and TL hold a patent on multimodal US imaging using PBCA-based polymeric microbubbles.
References
- 1.Lindner JR. Microbubbles in medical imaging: current applications and future directions. Nat Rev Drug Discov. 2004;3:527–532. doi: 10.1038/nrd1417. [DOI] [PubMed] [Google Scholar]
- 2.Klibanov AL. Ligand-carrying gas-filled microbubbles: ultrasound contrast agents for targeted molecular imaging, Bioconjug. Chem. 2005;16:9–17. doi: 10.1021/bc049898y. [DOI] [PubMed] [Google Scholar]
- 3.Kiessling F, Huppert J, Palmowski M. Functional and molecular ultrasound imaging: concepts and contrast agents. Curr Med Chem. 2009;16:627–642. doi: 10.2174/092986709787458470. [DOI] [PubMed] [Google Scholar]
- 4.Unger EC, Hersh E, Vannan M, Matsunaga TO, McCreery T. Local drug and gene delivery through microbubbles. Prog Cardiovasc Dis. 2001;44:45–54. doi: 10.1053/pcad.2001.26443. [DOI] [PubMed] [Google Scholar]
- 5.Mitragotri S. Healing sound: the use of ultrasound in drug delivery and other therapeutic applications. Nat Rev Drug Discov. 2005;4:255–260. doi: 10.1038/nrd1662. [DOI] [PubMed] [Google Scholar]
- 6.Dasgupta A, Liu M, Ojha T, Storm G, Kiessling F, Lammers T. Ultrasound-mediated drug delivery to the brain: principles, progress and prospects. Drug Discov Today: Technologies. 2016;20:41–48. doi: 10.1016/j.ddtec.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frenkel V. Ultrasound mediated delivery of drugs and genes to solid tumors. Adv Drug Delivery Rev. 2008;60:1193–1208. doi: 10.1016/j.addr.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernot S, Klibanov AL. Microbubbles in ultrasound-triggered drug and gene delivery. Adv Drug Delivery Rev. 2008;60:1153–1166. doi: 10.1016/j.addr.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lentacker I, De Smedt SC, Sanders NN. Drug loaded microbubble design for ultrasound triggered delivery. Soft Matter. 2009;5:2161–2170. [Google Scholar]
- 10.Klibanov AL, Shevchenko TI, Raju BI, Seip R, Chin CT. Ultrasound-triggered release of materials entrapped in microbubble-liposome constructs: a tool for targeted drug delivery. J Control Release. 2010;148:13–17. doi: 10.1016/j.jconrel.2010.07.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deckers R, Yudina A, Cardoit LC, Moonen CT. A fluorescent chromophore TOTO-3 as a ‘smart probe’ for the assessment of ultrasound-mediated local drug delivery in vivo. Contrast Media Mol Imaging. 2011;6:267–274. doi: 10.1002/cmmi.426. [DOI] [PubMed] [Google Scholar]
- 12.Treat LH, McDannold N, Vykhodtseva N, Zhang Y, Tam K, Hynynen K. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. Int J Cancer. 2007;121:901–907. doi: 10.1002/ijc.22732. [DOI] [PubMed] [Google Scholar]
- 13.Koczera P, Appold L, Shi Y, Liu M, Dasgupta A, Pathak V, Ojha T, Fokong S, Wu Z, van Zandvoort M, Iranzo O, et al. PBCA-based polymeric microbubbles for molecular imaging and drug delivery. J Control Release. 2017;259:128–135. doi: 10.1016/j.jconrel.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang X, Xu Y, Gao C, Zhou YM, Zhang NS, Dai ZF. Ultrasound contrast agent microbubbles with ultrahigh loading capacity of camptothecin and floxuridine for enhancing tumor accumulation and combined chemotherapeutic efficacy. NPG Asia Mater. 2018;10:761–774. [Google Scholar]
- 15.Chen PY, Yeh CK, Hsu PH, Lin CY, Huang CY, Wei KC, Liu HL. Drug-carrying microbubbles as a theranostic tool in convection-enhanced delivery for brain tumor therapy. Oncotarget. 2017;8:42359–42371. doi: 10.18632/oncotarget.16218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong Q, Gao X, Liu W, Hong T, Chen C. Drug-Loaded Microbubbles Combined with Ultrasound for Thrombolysis and Malignant Tumor Therapy. Biomed Res Int. 2019;2019:6792465. doi: 10.1155/2019/6792465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cavalieri F, Zhou M, Tortora M, Lucilla B, Ashokkumar M. Methods of Preparation of Multifunctional Microbubbles and their In Vitro / In Vivo Assessment of Stability, Functional and Structural Properties. Curr Pharm Des. 2012;18:2135–2151. doi: 10.2174/138161212800099874. [DOI] [PubMed] [Google Scholar]
- 18.Sirsi SR, Borden MA. Microbubble compositions, properties and biomedical applications. Bubble Sci, Eng Technol. 2009;1:3–17. doi: 10.1179/175889709X446507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Q, Sun X, Zeng L, Liu J, Zhang Z. Randomized Multicenter Phase II Clinical Trial of Mitoxantrone-Loaded Nanoparticles in the Treatment of 108 Patients with Unresected Hepatocellular Carcinoma. Nanomedicine. 2009;5:419–423. doi: 10.1016/j.nano.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 20.El-Egakey MA, Bentele V, Kreuter J. Molecular Weights of Polycyanoacrylate Nanoparticles. Int J Pharm. 1983;13:349–352. [Google Scholar]
- 21.Sulheim E, Baghirov H, von Haartman E, Bøe A, Åslund AK, Mørch Y, de Lange Davies C. Cellular uptake and intracellular degradation of poly (alkyl cyanoacrylate) nanoparticles. J Nanobiotechnol. 2016;14:1. doi: 10.1186/s12951-015-0156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fokong S, Theek B, Wu Z, Koczera P, Appold L, Jorge S, Resch-Genger U, van Zandvoort M, Storm G, Kiessling F, Lammers T. Image-guided, targeted and triggered drug delivery to tumors using polymer-based microbubbles. J Control Release. 2012;163:75–81. doi: 10.1016/j.jconrel.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Liu Z, Lammers T, Ehling J, Fokong S, Bornemann J, Kiessling F, Gätjens J. Iron oxide nanoparticle-containing microbubble composites as contrast agents for MR and ultrasound dual-modality imaging. Biomaterials. 2011;32:6155–6163. doi: 10.1016/j.biomaterials.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 24.Cochran MC, Eisenbrey J, Ouma RO, Soulen M, Wheatley MA. Doxorubicin and paclitaxel loaded microbubbles for ultrasound triggered drug delivery. Int J Pharm. 2011;414:161–170. doi: 10.1016/j.ijpharm.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fokong S, Siepmann M, Liu Z, Schmitz G, Kiessling F, Gatjens J. Advanced characterization and refinement of poly N-butyl cyanoacrylate microbubbles for ultrasound imaging. Ultrasound Med Biol. 2011;37:1622–1634. doi: 10.1016/j.ultrasmedbio.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Appold L, Shi Y, Rütten S, Kühne A, Pich A, Kiessling F, Lammers T. Physicochemical Characterization of the Shell Composition of PBCA - Based Polymeric Microbubbles. Macromol Biosci. 2017;17:1700002. doi: 10.1002/mabi.201700002. [DOI] [PubMed] [Google Scholar]
- 27.Yang SC, Ge HX, Hu Y, Jiang XQ, Yang CZ. Formation of positively charged poly (butyl cyanoacrylate) nanoparticles stabilized with chitosan. Colloid Polym Sci. 2000;278:285–292. [Google Scholar]
- 28.Wan Y, Wang X, Gu Y, Guo L, Xu Z. Surface-binding through polyfunction groups of Rhodamine B on composite surface and its high performance photodegradation. Appl Surf Sci. 2016;366:59–66. [Google Scholar]
- 29.Barnes PJ. How corticosteroids control inflammation: quintiles prize lecture 2005. Br J Pharmacol. 2006;148:245–254. doi: 10.1038/sj.bjp.0706736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L, Su H, Liu Y, Pang N, Li J, Qi XR. Enhancing solid tumor therapy with sequential delivery of dexamethasone and docetaxel engineered in a single carrier to overcome stromal resistance to drug delivery. J ControlRelease. 2019;294:1–16. doi: 10.1016/j.jconrel.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Liu Z, Koczera P, Doleschel D, Kiessling F, Gatjens J. Versatile synthetic strategies for PBCA-based hybrid fluorescent microbubbles and their potential theranostic applications to cell labelling and imaging. Chem Commun. 2012;48:5142–5144. doi: 10.1039/c2cc18048k. [DOI] [PubMed] [Google Scholar]
- 32.Koczera P, Wu Z, Fokong S, Theek B, Appold L, Jorge S, Möckel D, Liu Z, Curaj A, Storm G, van Zandvoort M, et al. Fluorescently labeled microbubbles for facilitating translational molecular ultrasound studies. Drug Deliv Transl Res. 2012;2:56–64. doi: 10.1007/s13346-011-0056-9. [DOI] [PubMed] [Google Scholar]
- 33.Fernandes AC, Liu M, Sorbo T, Appold L, Ilbert CM, Ferracci G, Kiessling F, Branco RJF, Lammers T, Iranzo O. A computational and experimental study to develop E-selectin targeted peptides for molecular imaging applications. Future Med Chem. 2018;10:2695–2711. doi: 10.4155/fmc-2018-0244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.