Abstract
Iron is essential in many physiological processes, including DNA metabolism, oxygen transport, and cellular energy generation. Deregulated iron metabolism, which results in iron overload or iron deficiency, is observed in many different diseases. We here summarize recent progress in the pathophysiology and pharmacology of iron-overload diseases, such as hereditary hemochromatosis, as well as iron-deficiency disorders, which are typically associated with anemia. The role of iron in immunity and the connection between iron and cancer are also addressed. We finally summarize and discuss the current (pre-) clinical landscape of pharmacotherapies targeting key players involved in iron metabolism.
Keywords: Iron, Anemia, Hemochromatosis, Inflammation, Cancer
Iron imbalance – at the origin of numerous pathologies
Iron is used by almost all organisms and is essential for their development and survival [1]. It is a vital part of various enzymes involved in many biological processes, including DNA biosynthesis, oxygen transport, and cellular energy generation [2].
Under physiological conditions, iron occurs in different oxidative states, between which it oscillates. Ferric (III) iron is stable but poorly soluble in water. Whereas ferric iron is bound to proteins serving as ligands, such as transferrin, to overcome solubility issues and ensure good bioavailability. By this means, ferric iron can be transported safely in a redox-inactive state. On the other hand, ferrous (II) iron is water-soluble, and its high reactivity contributes to its destructive potential. When present in excess, ferrous iron leads to the generation of reactive oxygen species (ROS) via the so-called Fenton reaction, which eventually results in cell damage, cell death, and organ failure, primarily affecting the liver, heart, pancreas, thyroid, and central nervous system [3], [4].
Iron-induced damage is aggravated by the fact that there is no physiological way of iron excretion, apart from blood loss and (to a lesser extent) shedding of cells. To diminish the destructive potential of iron while at the same time enabling the exploitation of its essential role in protein function, the uptake, distribution, and utilization of iron needs to be tightly regulated [5]. Iron regulation depends on a small number of crucial players, such as ferroportin (Fpn) and hepcidin (see Figure 1). Genetic modifications in such key players result in deregulation of iron homeostasis, which leads to severe pathological conditions requiring intensive medical care, such as hereditary hemochromatosis (HH) or beta-thalassemia.
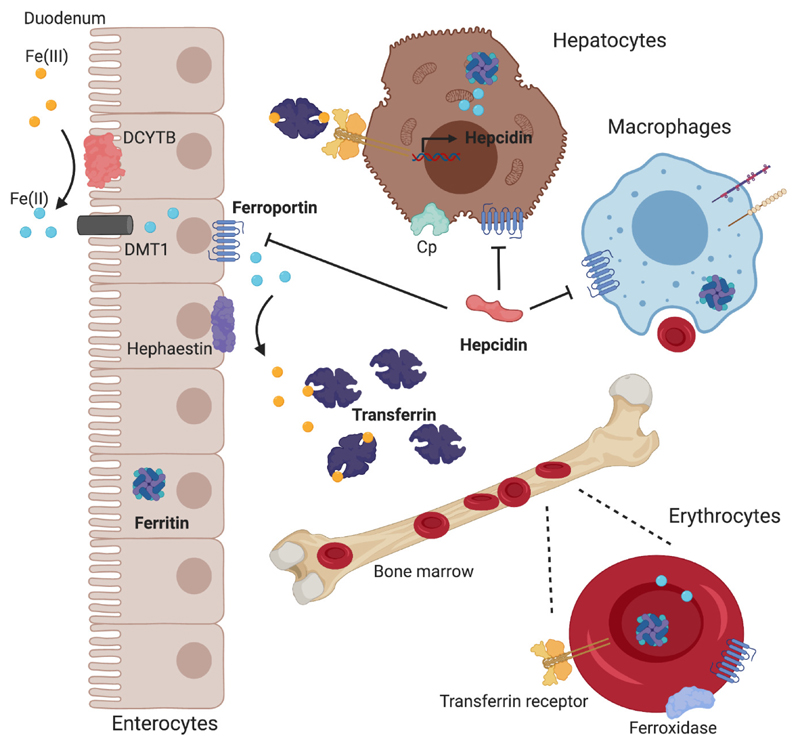
Figure 1. Key players in iron metabolism.
Iron is absorbed by enterocytes in the duodenum. Non-heme iron in ferric form is reduced by the duodenal cytochrome b (DCYTB) to ferrous iron, which can be transported into cells by divalent metal-ion transporter-1 (DMT1). Ferrous iron is released from enterocytes by ferroportin (Fpn) and oxidized by either membrane-bound hephaestin, ferroxidase or ceruloplasmin (Cp). In its ferric state, iron can be loaded onto transferrin, which allows for its transportation throughout the body to sites of high iron demand, such as the bone marrow, where the production of erythrocytes takes place. Senescent erythrocytes are recognized and phagocytosed by macrophages and degraded intracellularly. The iron obtained as part of this process is either secreted, stored inside ferritin, or employed as part of the labile iron pool. Hepcidin, the master regulator of iron metabolism, is produced and secreted by hepatocytes, where its production is regulated by iron stores and plasma iron levels. Hepcidin binds to Fpn and thereby initiates its internalization in and degradation by enterocytes, macrophages and hepatocytes, resulting in reduced plasma iron levels.
In this review, we summarize the involvement of deregulated iron homeostasis (i.e., iron deficiency and iron overload) in several prominent diseases’ pathophysiology. We furthermore provide an overview of recent advances in pharmacological modulation of iron metabolism, discussing therapeutic strategies that are either already employed in the clinic or currently under preclinical evaluation.
Key players in iron metabolism
The human body contains 3-4 grams of iron. The majority is utilized in erythrocytes to bind and shuttle oxygen throughout the body. Macrophages in the spleen, bone marrow, and liver recycle iron by taking up senescent erythrocytes and breaking them down to provide iron for processes such as erythropoiesis [5]. The remaining iron is stored in hepatocytes, which serve as a regulatory unit to control the systemic iron level. An alternative regulatory measure to control body iron is adjusting its absorption realized by enterocytes in the duodenum. In enterocytes, iron is either stored to minimize the amount of circulating iron as ferritin or transported to the basal side and released into the bloodstream by Fpn, the only known iron exporter on a cellular level [6], [7]. After the release, iron is loaded onto transferrin and distributed safely throughout the body [8]. Transferrin-bound iron is recognized by transferrin receptor (TfR), a membrane-bound protein that is widely expressed and commonly overexpressed on cells with a high iron demand, such as intermediate stages in red blood cell formation like erythroblasts [9] (see Figure 1).
The regulation of systemic iron homeostasis is primarily executed by hepcidin, a 25 amino acid peptide [10]. Hepcidin binds to Fpn, initiating the iron exporter’s internalization and degradation and thereby decreasing available iron in the circulation [11]. The expression of the HAMP gene, which encodes for hepcidin, is upregulated by a multitude of factors, including bone morphogenetic protein (BMP), hemojuvelin (HJV), and human hemochromatosis protein (HFE), targeting either the BMP receptor (BMPR) or the TfR on hepatocytes [12]–[14]. Additionally, inflammatory cytokines such as interleukin 6 (IL-6) induce hepcidin biosynthesis and reduce plasma iron levels as a preventive measure against bacterial growth [15]. To mobilize iron from its storages and increase its availability for processes like erythropoiesis, erythropoietin (EPO) stimulates erythroblasts to release the protein erythroferrone (ERFE), which inhibits hepcidin production by binding to BMPs [16]. Elevated EPO production is observed under oxygen-deprived conditions, mediated by the hypoxia-inducible factor (HIF), which leads to increased downregulation of hepcidin and consequently promotes iron release [17], [18].
At the cellular level, non-protein-bound iron is presented as the labile iron pool, consisting of ferrous iron bound by low-affinity iron chelators. The labile iron pool regulates the intracellular iron flux via iron-responsive proteins (IRP) interacting with iron-responsive elements (IRE) and controlling the translation of mRNA encoding for ferritin, TfR1, Fpn, and divalent metal-ion transporter-1 (DMT1) [19]–[22]. Moreover, the transcription of TfR, DMT1, and Fpn is also controlled by HIF via binding to their hypoxia-responsive element (HRE), serving as a potent transcription factor [23]–[26]. Prolyl-4-hydroxylase (PHD), which regulates the degradation of HIF, is highly Fe2+-dependent, making HIF and PHD suitable targets for the treatment of iron metabolism-related pathologies [27].
Generally, iron homeostasis is affected by the disturbance of one or more of its key players, causing either an excess or a shortage of systemic iron. Both conditions have detrimental effects on the overall health status of an individual.
Iron deficiency
Iron deficiency patients have improper systemic iron levels, not matching their demands for continuous processes like erythropoiesis. This can eventually lead to anemia, a condition where the body suffers from low hemoglobin levels causing oxygen shortage. Anemia is a major global healthcare problem, affecting 25% of the world population, with iron deficiency anemia and anemia of chronic disease being the most common forms. Prevalence is rising, especially for anemia of chronic disease, due to the global aging population, which comes with an increased risk to develop chronic pathological conditions.
Anemia is often addressed because of the apparent clinical presentation. However, iron deficiency also results in problems during pregnancy and delays in childhood development, affecting especially cognitive abilities, which need careful consideration regarding diagnostic measures and treatment options. Causes for iron deficiency include severe blood loss, elevated requirement occurring during pregnancy or periods of rapid growth, malassimilation, impaired iron absorption, and inflammatory flares; most of these conditions can be efficiently treated with iron supplementation [28], [29].
Iron supplements are either given orally or administered intravenously (see Figure 2). The downside of oral iron administration is the necessity of high and frequent dosing, typically given over months to enable the re-establishment of physiological iron levels. This intense and prolonged treatment regimen is necessary because of inefficient intestinal iron absorption. Consequently, due to the significantly increased iron intake, hepcidin – the master regulator of iron metabolism (see Figure 1) – is upregulated, reducing plasma iron levels. To partially bypass this regulatory mechanism, an alternate day treatment regimen rather than everyday dosing is recommended, increasing the overall effectiveness of oral iron supplementation [30].
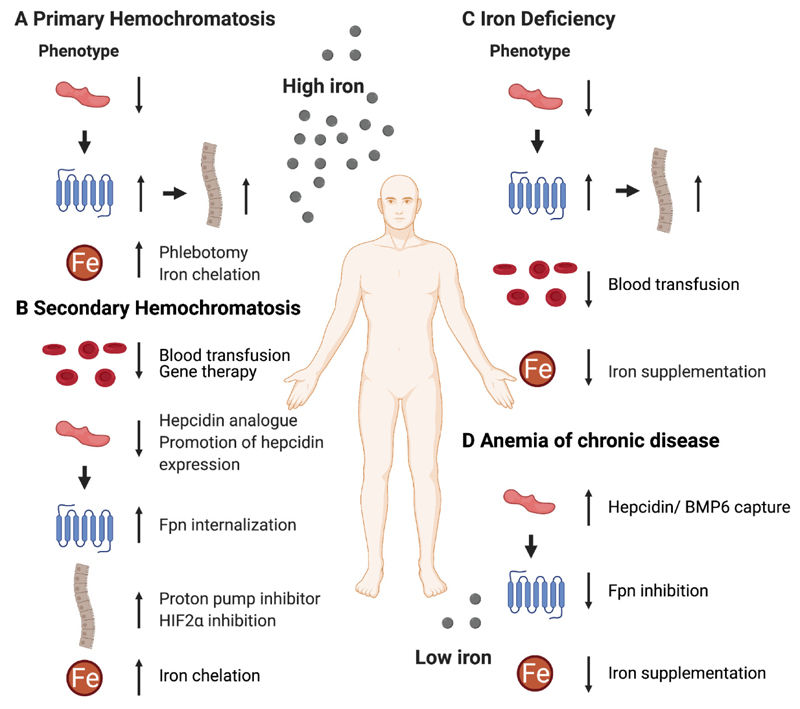
Figure 2. Therapeutic strategies targeting iron metabolism.
Deregulation of iron metabolism leads to hemochromatosis or iron deficiency. A) Primary hemochromatosis is often associated with low hepcidin expression resulting in high ferroportin (Fpn) occurrence and an increase in duodenal iron absorption. Therapeutic strategies include direct reduction of systemic iron by iron chelation or phlebotomy. B) Secondary hemochromatosis results from inefficient erythropoiesis leading to a possible undersupply with oxygen and an increased intestinal iron absorption leading to iron overload. Red blood cell transfusion or genetic engineering primarily targeting hemoglobin are employed to correct for the defective erythrocyte production. To reduce the occurring iron burden the hepcidin-Fpn axis or the intestinal iron absorption can be pharmacologically targeted. C) The increased demand of erythrocytes leads to iron deficiency which is accompanied by low systemic hepcidin levels, high Fpn expression, and increased duodenal iron absorption. Treatment includes direct elevation of iron levels via the administration of iron supplements or full blood. D) Anemia of chronic disease is provoked by an underlying inflammation reducing the circulating iron by hepcidin upregulation and Fpn downregulation. Therapeutically targeting hepcidin and Fpn or elevating the iron level by iron supplementation has been therapeutically exploited to ameliorate the anemic state.
In severe iron deficiency cases and depending on hemoglobin levels, intravenous administration is to be favored over oral administration, allowing significantly higher doses to be applied with a single injection [31]. There is ongoing controversy about the most optimal method for iron supplementation, ensuring efficient iron intake and uptake, as well as a good therapeutic outcome while keeping the incidence and the intensity of side effects under control [32].
Iron loading anemia
Besides absolute iron deficiency, iron loading anemia (see Glossary) is one of the leading causes of a pathological state where the lack of functional erythrocytes causes an undersupply with oxygen, also known as functional iron deficiency [33].
Iron loading anemia, such as beta-thalassemia and sickle cell disease, is commonly treated with blood transfusion combined with measures to prevent iron overload or allogeneic hematopoietic stem cell transplantation (HSCT) [34]. Treatment via red cell transfusion and subsequent chelation therapy to counteract the major side effect of the resulting iron accumulation is very elaborate. The orally administered iron chelator deferasirox (DFX or Exjade/ Jadenu; Novartis)i serves as a first-line treatment to lower iron and reduce blood transfusion-related side effects in iron loading anemia. Deferiprone (DFP or Ferriprox; ApoPharma) is being used as a second-line option. Prominent side effects of iron chelation therapy include cytopenia, neurotoxicity, and gastrointestinal dysfunction [35]. Moreover, it should be kept in mind that chelation therapy is not curative and just a supportive treatment. Vice versa, HSCT directly addresses the origin of the disease and intends to cure it. It is, however, a very costly and relatively invasive procedure, which comes with serious side effects and limited application. HSCT requires the health care infrastructure of an industrialized nation and is mostly employed in individuals under the age of 18 [36]. In recent years, targeted interventions, such as gene therapy, are being explored to directly interfere and cure the underlying condition (Box 1) (see Table 1). They are currently still largely in clinical trials, but promise broader applicability while at the same time being less invasive.
Box 1. Gene therapy in iron-related diseases.
New developments in the treatment of iron-related diseases originate from defective or impaired genes where monogenetic diseases like beta-thalassemia or sickle cell disease show the most promising curative gene therapy approaches. In such setups, transfusion-dependent beta-thalassemic patients receive genetically engineered autologous CD34-positive hematopoietic stem and progenitor cells (HSPC) [111]. Products currently undergoing clinical evaluation are ST-400 (NCT03432364) and CTX001 (NCT03655678), both in phase 1/2 trials targeting the BCL11A gene and fetal hemoglobin production [112], [113]. Two additional products are tested using a lentiviral vector encoding the normal human β-globin gene, the TNS9.3.55 currently in phase 1 (NCT01639690) and LentiGlobinR BB305 (Zynteglo) in phase 3 (NCT03207009) [114], [115]. The gene addition through lentiviral vectors bypasses the burden of massive side effects of the current standard HSCT treatment. However, the number of treated patients remains low due to this method’s complexity and cost. Some successful applications of Zynteglo already led to conditional market approval in the EU. However, the commercial use was partially delayed regarding the COVID-19 pandemiciv [116].
Table 1. Pharmacological agents targeting iron metabolism.
| Name | Type | Mechanism | Indication | Clinical status | Trial Number | Ref. |
|---|---|---|---|---|---|---|
| Therapeutics for iron deficiency conditions | ||||||
| Iron supplement for oral administration | ||||||
| Ferrous sulfate | Iron salt | Refills depleted iron stores | Iron deficiency anemia | Marketed | NCT01904864 | [28–32] |
| Ferrous gluconate | Iron salt | Refills depleted iron stores | Iron deficiency anemia | Marketed | NCT01528644 | [28–32] |
| Iron supplement for intravenous administration | ||||||
| Ferumoxytol | Iron oxide nanoparticle | Refills depleted iron stores | CKD | Marketed | NCT01227616 | [104] |
| Ferric carboxymaltose | Iron complex | Refills depleted iron stores | Iron deficiency anemia | Marketed | NCT03523117 | [28–32] |
| Sodium ferric gluconate | Macromolecule | Refills depleted iron stores | Iron deficiency anemia | Marketed | NCT00223964 | [28–32] |
| Hepcidin downregulation | ||||||
| LY3113593 | Ant¡-BMPΘ antibodies | Binds/inhibits BMP6 | CKD | Phase 1 | NCT02144285 | [47] |
| Hepcidin capture | ||||||
| Anticalin | Binds hepcidin | Binds/inhibits hepcidin | CKD | Phase 1/II |
NCT02754167 (Ph. 1) NCT03325621 (Ph. II) |
[49] |
| LY2787106 | Anti-hepcidin antibody | Binds/inhibits hepcidin | Anemia of chronic disease | Phase 1 | NCT01340976 | [48] |
| Stimulation of erythropoiesis | ||||||
| Luspatercept | Recombinant fusion protein | Ligand trap for TGF-beta superfamily | β-Thalassemia | Phase III | NCT02604433 | [37] |
| Sotatercept | Recombinant fusion protein | Ligand trap for TGF-beta superfamily | β-Thalassemia | Phase II | NCT01S71635 | [38] |
| Gene therapy | ||||||
| OTL-300 | Cells/Lentiviral vector | Cells genetically modified with a vector encoding human β-globin | β-Thalassemia | Marketed | NCT03275051 | [116] |
| LentiGlobinR BB305 | Cells/Lentiviral vector | Engineered hematopoietic stem cells with a functional version of human β-globin gene | β-Thalassemia | Phase III | NCT03207009 | [114] |
| ST-400, CTX001 | Cells/Vector | Modified patients’ blood stem cells with disrupted BCL11A gene, induction of fetal hemoglobin synthesis | β-Thalassemia | Phase I/ll | CTX001: NCT03655678 ST-400: NCT03432364 |
[111], [112] |
| Therapeutics for iron overload conditions | ||||||
| Iron absorption | ||||||
| Esomeprazole | Small molecule | Proton pump inhibitor | β-Thalassemia | Phase III | NL6659 (Netherlands trial Register) | [76] |
| PT-2385 | Small molecule | Inhibits HIF2α-dependent genes | Hemochromatosis | Preclinîcal | NCT03108066 | [77] |
| Ebselen | Small molecule | DMT1 inhibitor acting as a mimic of glutathione peroxidase | Alzheimer’s disease | Preclinical | Not listed yet | [91],[92] |
| Iron binding | ||||||
| Deferoxamine (DFO), | Small molecule | Iron chelation | Secondary | Marketed | NCT00001203 | [69] |
| Deferasirox (DFX), | Small molecule | Iron chelation | Hemochromatosis | NCT00171171 | [35] | |
| Deferiprone (DFP) | Small molecule | Iron chelation | NCT02189941 | [69] | ||
| Triapine | Small molecule | Iron chelation, RR inhibitor | Cancer | Phase II | NCT00293345 | [63] |
| Cefiderocol | Small molecule | Antibiotic-siderophore conjugate | Urinary tract infection | Phase II | NCT02321800 | [96] |
| Dpc | Small molecule | Iron chelation | Cancer | Phase 1 | NCT02688101 | [63] |
| Polymeric DFO | Nanopartîcle | Iron chelation | Hemochromatosis | Preclinical | [69] | |
| Hepcidin upreguiotion | ||||||
| TMPRSS6-LRX | Antisense DNA oligonucleotide | Inhibition of TMPRSS6 expression | β-Thalassemia | Phase 1 | NCT03165864 | [70] |
| SLN124 | GalNAcdouble-stranded siRNA conjugate | Inhibition of TMPRSS6 expression | Hemochromatosis | Phase 1 | NCT04176653 | [71], [72] |
| Antî-ERFE 15.1 | Anti-ERFE antibody | Binds/inhibits ERFE | β-Thalassemia | Preclinical | No listed yet | [73] |
| Ferroportin downregulotion | ||||||
| VIT-2736 | Anti-Fpn antibody | Fpn inhibitor | β-Thalassemia | Phase 1 | NCT04364269 | [75] |
| Ferroptosis inducer | ||||||
| Sorafenib | Small molecule | Tyrosine kinase inhibitor, Inhibition of Xc-induced glutathione depletion and lipid peroxidation | Cancer | Marketed | [106] | |
| Erastîn | Small molecule | Inhibition of Xc-induced glutathione depletion and lipid peroxidation | Cancer | Preclinical | No listed yet | [106] |
| RSL3 | Small molecule | GPX4 inhibition, ROS production | Cancer | Preclinical | No listed yet | [104] |
| Ferumoxytol | Iron oxide nanoparticle | ROS production | AML | Preclinical | No listed yet | [105] |
Alternative treatments for iron loading anemia are based on subcutaneously self-administrable ligand traps for transforming growth factor-β (TGF-β) superfamily members, which mediate curative effects by enhanced erythroid differentiation. Representatives of this group of drugs are the ligand trap for TGF-β superfamily members called sotatercept (ACE-011) with an ongoing clinical trial in phase 2 (Clinical Trial Numberii: NCT01571635), and the recombinant fusion protein derived from human activin receptor type IIb named luspatercept (ACE-536), tested in phase 3 clinical trial (NCT02604433) [37], [38] (see Table 1). Luspatercept significantly reduces the transfusion burden in patients with transfusion-dependent beta-thalassemia compared to placebo, and demonstrates a novel treatment approach with a preferable dosing regimen of an subcutaneous injection every 21 days and low side effects [37]. Full recovery, however, is not achieved with luspatercept, and the need for red blood cell transfusion remains.
Taken together, multiple therapeutic options are available to alleviate disease symptoms or even cure patients suffering from iron loading anemia, several of which already approved and others close to being implemented in clinical practice.
Inflammation-associated diseases
Another form of functional iron deficiency up to the level of anemia can be caused by an underlying inflammatory disease, which, e.g., occurs in patients with inflammatory bowel disease (IBD), chronic kidney disease (CKD), autoimmune diseases such as rheumatoid arthritis, and cancer [39].
In IBD, iron deficiency results from reduced intestinal iron absorption, chronic inflammation, bowel resection, malnutrition, or blood loss. Iron deficiency in IBD being underdiagnosed is often untreated, even under anemic conditions, which can eventually worsen the patient’s well-being. Importantly, IBD patients with iron deficiency are often not suited for oral iron preparation and need to be treated with intravenous iron to improve quality of life [40].
Patients diagnosed with CKD are anemic due to impaired renal EPO production, consequently decreasing erythrocyte formation. Iron supplementation or direct induction of erythropoiesis with erythropoiesis-stimulating agents (ESA) such as EPO (recombinant or derivatives) have been used to ease the anemic condition in these patients [41]. The administration of ESA is considered standard-of-care but can come with relatively serious side effects, including cardiovascular events [42]. Other therapy approaches for CKD patients include PHD inhibitors to stabilize HIF, leading to increased EPO formation and simultaneously inhibiting hepcidin production [43].
Iron deficiency is often overlooked in the case of underlying inflammatory disease, where it can significantly impact patients’ quality of life. Therefore, new markers and distinct thresholds for distinguishing both conditions are urgently needed. It has been proposed to not solely look at ferritin serum level but also use transferrin saturation [44]. However, both markers might not indicate iron unavailability in an inflammatory state with functional iron deficiency. Consequently, it has been proposed to include further measures such as hepcidin serum level determination to evaluate the inflammatory condition [45].
Multiple pharmacological strategies have been explored in the last couple of years for addressing inflammation-associated anemia. These, e.g., include treatment with the natural monoterpenoid hinokitiol, which affects iron distribution by restoring the ability to transport iron across membranes [46], as well as approaches targeting the main iron regulator hepcidin (see Figure 2). Examples of the latter are hepcidin inhibition with anti-BMP6 antibodies (LY3113593) [47] and hepcidin capturing with either anti-hepcidin antibodies (LY2787106) [48] or anticalin (PRS-080) [49] (see Table 1). Anti-BMP6 antibodies combined with anti-Fpn antibodies (and in a separate study also with anticalin) have been assessed in healthy individuals and CKD patients as a therapeutic intervention against anemia of chronic disease, evaluating particularly tolerability and iron mobilization efficiency [47], [49]. Analogously, the tolerability and hepcidin binding capability of anti-hepcidin antibodies have been tested in patients with cancer-induced anemia of chronic disease [48]. In all cases, the formulations were found to be safe and induced iron redistribution mediated via hepcidin inhibition. Therapeutic strategies targeting the hepcidin-Fpn axis hold great potential. However, the results obtained thus far are from early-stage clinical investigations and need to be confirmed by more extensive efficacy assessment.
Iron deficiency and cancer
In cancer progression, iron metabolism is often altered at the systemic and cellular levels. Cancer is typically accompanied by anemia of chronic disease, mediated by an upregulated hepcidin production, and worsens due to therapeutic interventions such as chemotherapy. Patients undergoing chemotherapy may receive iron supplementation to increase systemic iron levels and improve their quality of life.
At the cellular level, the expression of proteins responsible for iron uptake, storage, and export is altered in cancer, albeit with considerable variation between different cancer types. Iron uptake is mainly organized by TfR1 and DMT1 [22], [50]. TfR1 is highly overexpressed in a wide range of tumor types, increasing the intracellular iron amount [51]–[53]. DMT1 responsible for intestinal iron absorption and endosomal transport of iron into the cytosol is upregulated, especially in colorectal cancer [54]. Ferritin, the iron storage protein, is commonly cytosolic but can be excreted by macrophages and serve as an additional iron source [55]. Receptors, like scavenger receptor class A member 5 (SCARA5) or TfR1, bind ferritin and internalize it, and are known to be altered in cancer cells [56]–[58]. Fpn is responsible for iron release and has been widely described to be downregulated in prostate and breast cancer [59], [60]. Furthermore, proteins such as lipocalin 2 (LCN2), which sequester iron, are upregulated in certain malignancies, such as breast cancer (Box 2) [61].
Box 2. Lipocalin and cancer.
As part of the innate immune system, lipocalin 2 (LCN2) helps in host defense against iron-dependent pathogens and simultaneously provides cells with iron. LCN2 binds characteristic iron-loaded catecholate siderophores, such as enterochelin (also known as enterobactin), a molecule released by bacteria for iron sequestration. The LCN2-enterochelin-iron complex returns to the secreting cell and is taken up for degradation, resulting in elevated intracellular iron levels and bacterial iron depletion, eventually leading to bacterial growth inhibition [117]. Furthermore, LCN2 is associated with the onset, progression, and metastatic spread of various cancer types, such as breast, prostate, and esophageal cancer. It has been shown in a breast cancer model that LCN2 is employed to deliver iron to cancer cells via tumor-associated macrophages [118]. Additionally, a recently published study reports that LCN2 was expressed by cancer cells rather than macrophages in leptomeningeal metastasis, utilizing it for iron capture in a poorly nutritious environment [119]. Consequently, the iron-shuttling function of LCN2 contributes to reduction of ROS-induced damage and inhibition of apoptosis, promoting cancer cell survival in the tumor microenvironment [120]. Moreover, certain cytokines secreted by immune cells induce activation and binding of transcription factors such as NF-κB, AP-1, and C/EBPβ to the LCN2 promoter, thereby stimulating its expression. As a result, LCN2-enterochelin-iron complexes re-enter tumor cells via specific receptors, thereby helping cells to resist hypoxic conditions and ROS-induced damage and cell death [120].
Taken together, these processes lead to an increase in iron sequestration and retention, and result in an elevated labile iron pool. Iron-induced ROS generation results in oxidative stress when the amounts of ROS exceed the amounts of ROS scavengers. In general, iron is needed for proliferation, especially as a cofactor in enzymes taking part in DNA synthesis and repair, such as ribonucleotide reductase, and for enzymes producing adenosine triphosphate (ATP) during oxidative phosphorylation. As observed in cancer cells, the iron demand increases with a higher proliferation rate [62].
Upon iron restriction, cancer cells respond with a cell cycle arrest in the G1/S phase, underlining the proliferative nature of iron-depending processes. Thus, chelation therapy has been tested using already approved iron chelators, such as deferoxamine (DFO or Desferal; Novartis) and deferasirox (DFX or Exjade/Jadenu; Novartis). These iron chelators have been intensively studied in preclinical settings; the number of clinical trials has been low. DFO was investigated in a phase 1 clinical trial for unresectable HCC, together with transarterial chemoembolization (NCT03652467). However, the short blood half-life of DFO requires a labor- (and cost-) intensive procedure involving parenteral administration over hours and frequent re-administration [35]. DFX is given orally, which is more favorable, and has been under clinical investigation for acute myeloid leukemia as a single treatment as well as in combination with cholecalciferol and azacitidine (NCT02341495).
More promising newly developed iron chelators, such as di-2-pyridyl ketone thiosemicarbazone (DpC) and triapine, have been intensively investigated in clinical trials indicating a higher success rate to be finally approved compared to standard iron chelators used for iron overload conditions [63] (see Figure 3).
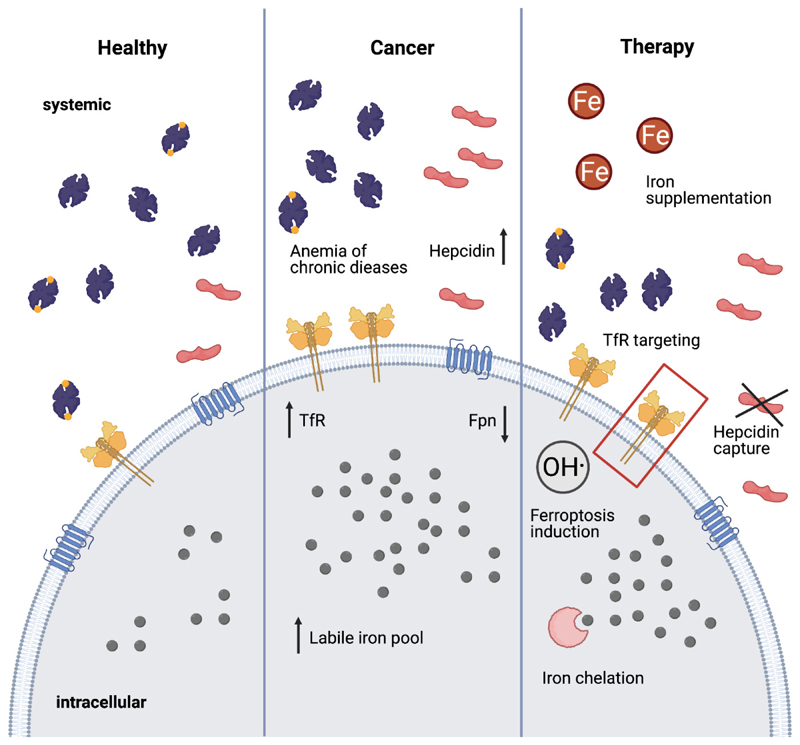
Figure 3. Therapeutic strategies targeting iron metabolism in cancer.
Cancer is often accompanied by anemia of chronic disease mediated by hepcidin upregulation. This can be ameliorated by iron supplementation or hepcidin capture. Moreover, TfR and Fpn expression are altered, increasing the intracellular labile iron pool. Pharmacological strategies explored for cancer therapy include targeting iron transporters overexpressed on certain cancer cells, ferroptosis induction as well as iron depletion to inhibit proliferation.
Multiple TfR-targeted therapies have been investigated for oncological applications in the past two decades. This include agents specifically recognizing TfR1, such as anti-TfR antibodies or transferrin-drug conjugates [64], [65]. Antibodies against TfR1 have also been tested, in preclinical and clinical settings, but showed severe side effects due to off-target binding to other cell types with high iron demand, such as erythroblasts [66].
To address challenges related to fast blood clearance of iron chelators such as DFO as well as DFO-induced induction of HIF1α expression, novel nanoformulations consisting of Tfr1-targeted liposomes co-loaded with DFO and the HIF1α inhibitor YC-1 have been assessed, proving their suitability for cancer combination therapy [67]. This approach seems to reduce the available iron in the tumor but potentially worsens the patient’s anemic condition and overall well-being. Careful evaluation of such novel concepts is required to help decide which therapeutic option is most suitable in a given situation.
The exploration of the systemic iron regulator hepcidin and its relevance to cancer progression has been the objective of a preclinical study. In hepcidin knockout mice, prolonged overall survival and reduction in metastasis have been observed compared to wild-type animals, suggesting a pro-proliferative effect of hepcidin. In in vivo experiments, the same study showed a reduction in cancer cell proliferation and tumor growth in mice with Fpn overexpression [68].
Together, these findings have led to the conclusion that intracellular iron retention worsens disease outcomes in cancer, making the hepcidin-Fpn axis a target for cancer therapy. Potential pharmacological agents available for capturing and binding hepcidin include the above-mentioned anti-hepcidin antibodies [48], anticalin [49], and anti-BMP6 antibodies [47] (see Table 1, Figure 3).
Iron overload
Excess iron and iron overload are also known as hemochromatosis. Iron overload has several underlying causes of which genetic mutation of the HFE gene is the most common one. We further differentiate between primary and secondary hemochromatosis, with far more individuals suffering from primary hemochromatosis. In the United States, ~1 million people are affected by iron-related diseases, primarily individuals with Northern European descents. In contrast, non-HFE hemochromatosis disorders are rare.
Secondary iron overload associated with iron loading anemia, such as beta-thalassemia and sickle cell disease, is indirectly induced by the underlying pathology and is commonly diagnosed at a young age. Worldwide, 40,000-60,000 infants are born annually with beta-thalassemia, and 300,000 with sickle cell disease.
Phlebotomy, or bleeding, has been employed to treat patients diagnosed with primary hemochromatosis. In certain severe non-HFE-associated hemochromatosis cases, a combination of phlebotomy and iron chelation is implemented. Patients suffering from secondary hemochromatosis, in which phlebotomy is not an option, benefit from iron chelation therapy. The iron chelators DFO, DFX, and deferiprone (DFP or Ferriprox; ApoPharma) are approved for clinical use and are extensively employed to reduce the iron burden in patients suffering from secondary hemochromatosis (see Table 1 and Figure 2). New DFO formulations with prolonged circulation times are under development to overcome the rapid clearance of these drugs from the bloodstream, which is a major disadvantage with an enormous impact on the patient’s quality of life [69].
Novel treatment approaches of iron overload include the modulation of hepcidin expression by targeting proteins that regulate HJV or BMP activity, with a positive effect on BMP-SMAD signaling. An example of this is silencing MT2 (i.e., TMPRSS6) with the antisense DNA oligonucleotide drug Ionis TMPRSS6-LRX, which is currently under clinical phase 1 investigation (NCT03165864) [70]. Along the same line of thinking, a compound called SLN124, which is a GalNAc double-stranded siRNA conjugate targeting TMPRSS6 for the treatment of beta-thalassemia and iron overload [71], [72], has recently entered phase 1 clinical trials (NCT04176653). Another pharmacological approach relies on targeting ERFE, which functions as a negative regulator of hepcidin. An anti-ERFE antibody was explicitly developed to inhibit the interaction between ERFE and BMP, thereby activating BMP-SMAD signaling and hepcidin expression. As a result, lower iron levels in the liver and plasma accompanied by a recovery of erythropoiesis are observed. Overall, the redistribution of iron together with improvements in anemia conditions make this antibody-based therapy suitable for beta-thalassemia therapy. However, its safety and efficacy still need to be tested in humans [73].
The recently discovered ROS-protective role of Nuclear factor erythroid 2-related factor (NRF2) and its ability to induce BMP6-mediated hepcidin expression to protect against excessive iron makes this an exciting therapeutic strategy in iron-overloaded individuals [74]. Instead of increasing hepcidin expression, Fpn targeting has also been employed to lower plasma iron levels. VIT-2736 is an oral Fpn inhibitor developed by Vifor Pharma to treat beta-thalassemia intermedia, and it has recently completed a phase 1 clinical trial (NCT04364269; see Table 1 and Figure 2) [75].
An alternative approach to reduce systemic iron levels, especially useful for patients with non-transfusion-dependent secondary iron overload such as beta-thalassemia, is restricting intestinal iron absorption. To this end, the proton pump inhibitor (PPI) esomeprazole (see Figure 2) is currently under investigation in phase 3 clinical trial (Netherlands Trial Registeriii: NL6659) [76]. Also tested as an iron overload therapeutic, but still at the preclinical level, is a compound called PT2385, a HIF2α antagonist. HIF2α regulates intestinal iron absorption in enterocytes by activating DMT1 and Fpn production at the transcriptional level [26], [77]. HIF2α furthermore downregulates hepcidin synthesis in the liver via EPO [78]. By targeting the dysfunctional hepcidin-Fpn axis and reducing dietary iron absorption via HIF2α inhibition, laboratory animals’ plasma iron levels were lowered [79]. Testing in humans with iron overload and hepcidin deficiency will show its potential as a therapeutic agent.
Cardiovascular diseases
Iron overload has been associated with vascular diseases, which pose a major threat to the western world’s population considering their high morbidity and mortality rates [80], [81]. It is controversially discussed how iron metabolism imbalance, particularly the high circulating free iron levels, affects vascular diseases such as atherosclerosis. Inflammatory processes potentially link cardiovascular diseases and iron, as iron might worsen inflammation by catalyzing ROS generation [82]. Iron deposition in atherosclerotic plaques has been observed, but it is not clear if iron accumulation is the cause or consequence of atherosclerotic plaques. Iron is also involved in the oxidation of low-density lipoprotein (LDL), a hallmark for atherosclerosis. It has become increasingly clear that iron acts as a risk factor in the onset progression of cardiovascular disease. It seems that not high systemic iron concentrations but rather elevated intracellular iron levels in macrophages drive the inflammatory process [81]. Accordingly, preventive measures to reduce the risk of atherosclerosis include a reduction of dietary iron [83]. As therapeutic strategies, iron chelating agents and apotransferrin administration are being considered to reduce the iron burden [84]. These agents primarily bind systemic iron, even though most of the iron-chelating agents are effective intracellularly too. Intracellular reduction of iron can occur either by direct iron-binding, or indirectly by reducing circulating iron and initiating redistribution.
Neurodegenerative diseases
Analogously, neurodegenerative diseases, such as Parkinson’s disease (PD), Alzheimer’s disease (AD), Huntington’s disease (HD), or Friedreich’s ataxia (FA), are associated with elevated iron levels in pathognomonic brain regions resulting in diverse pathologic patterns. It indicates that they might be amenable to iron restriction treatments induced, e.g., by chelation therapy to regain the balance between low and high iron deposit regions in FA or hepcidin targeting [85]–[87]. Iron chelation might also mitigate the effects of iron dyshomeostasis and neurotoxicity in dopaminergic neurons, which are mainly affected in PD. This proteinopathy is also characterized by alpha-synuclein aggregates, where the postulated malfunction of alpha-synuclein as a ferrireductase potentially increases levels of ferrous iron and aggravates ROS formation [88]–[90]. An additional pharmacological approach in this regard is treatment with ebselen (see Table 1), which is known for its inhibitory action on the crucial iron transporter DMT1. Promising results have been obtained in vitro with regard to reducing iron intake, and the compound has already been successfully employed as a therapeutic agent for Alzheimer’s disease [91], [92]. However, it remains unclear if iron overload is a cause or consequence of neurodegenerative disease, as comprehensively discussed in a recent review on this topic [93]. In the years to come, this needs to be systematically studied for each disease entity.
Immunity
As almost all living beings, microorganisms such as bacteria and viruses rely on iron for growth and survival [94]. Numerous studies report a correlation between a high systemic iron level and an increased risk of infection [95]. To fight iron-dependent infections, reducing the pool of available iron is anticipated to be an attractive strategy. Direct iron chelation, as well as the targeting of hepcidin or BMP/IL-6 signaling, could serve as a therapy in infectious diseases. Furthermore, to specifically address gram-negative bacteria’s iron sequestration mechanism, antibacterial drugs conjugated to siderophores have been tested. The drug candidate cefiderocol belonging to the cephalosporin family of medications completed a phase 2 clinical trial (NCT02321800; see Table 1) to treat urinary tract infection [96].
The ongoing pandemic provoked by the severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) impacts all levels of daily life, affecting besides healthcare also economic and societal aspects. Since the beginning of the outbreak, we have gradually learned how the disease presents and progresses. There are, however, still many unknowns with regard to understanding SARS-CoV-2 pathophysiology. In terms of iron metabolism, hemoglobinopathy and hyperferritinemia have been proposed to accelerate its progression [97]. SARS-CoV-2 has been shown to bind to erythrocytes and induce hemoglobin denaturation, which causes an oxygen-deprived condition. Furthermore, the hyperferritinemic state of COVID-19 patients is associated with an increased risk of mortality, but it is not yet clear if hyperferritinemia serves as a biomarker for progressive disease or can be considered a cause of infection [98].
Several studies have recently shown that excessive iron induces a shift towards a pro-inflammatory phenotype in macrophages, contributing to inflammatory responses in diseases such as atherosclerosis [81], [84], steatohepatitis, and liver fibrosis [99], [100]. Also, inflammatory conditions occur in patients with iron loading anemia, such as beta-thalassemia, possibly mediated by the high intracellular iron levels found in macrophages [2]. These observations indicate that iron mediates macrophage-mediated inflammatory responses. However, the opposite is the case in hemochromatosis, where no increase in intracellular iron levels in macrophages and no association with inflammatory conditions has been reported. Together with the notions mentioned earlier on iron’s role in supporting bacterial and viral infections, these insights suggest that pharmacological reduction of iron levels can help to dampen inflammatory and immune responses.
Iron overload and cancer
Strikingly, in patients with iron overload, such as HH, the risk of HCC is increased by up to 200-fold. Besides HCC, the evidence of increased cancer incidence in HH patients is inconclusive, with papers reporting conflicting outcomes. Several studies indicate an increase in cancer incidence, e.g., in breast, colon, and prostate cancer, whereas others report no effect of iron overload on cancer development. Further investigations and particularly prospective clinical trials will help elucidate the role of iron in cancer initiation and progression [101], [102].
Evading programmed cell death is one of the hallmarks of cancer and plays a crucial role in cancer initiation and progression. Therefore, a major focus in cancer therapy has been on cell death mechanisms’ reactivation. Ferroptosis is a recently discovered form of non-apoptotic cell death mediated by ROS and lipid peroxidation induction [103]. Several substances, including iron itself, promote ferroptosis and have been used to induce cell death in cancer cells, which are relatively susceptible to such interventions because of their altered iron metabolism [88], [103], [104]. In a preclinical study, Feraheme® (ferumoxytol), a clinically approved anti-anemia iron oxide nanoparticle formulation, has been employed as a drug for acute myeloid leukemia (AML) treatment. It was found that low Fpn expression levels on leukemia cell lines and primary cells lead to an increased susceptibility to Feraheme®-induced ferroptosis [105]. Also, sorafenib, a tyrosine kinase inhibitor approved for HCC, thyroid cancer, and advanced renal cancer treatment, has shown ferroptotic activity, inhibiting cellular cysteine intake (see Table 1, Figure 3) [106]. Cysteine is a building block of glutathione, and it is part of the cellular defense mechanism against ROS. Alternative ways to increase the labile iron pool and to induce ROS generation are stimulation of ferritin degradation, a process known as ferritinophagy. For instance, targeting either the L-chain or the H-chain of ferritin with small hairpin RNA (shRNA) has been shown to reduce the growth of cancer stem cells [107]. Another ferritinophagy-related ferroptosis strategy includes the upregulation of NCOA4, which induces ferritin transport to the lysosome, resulting in ferritin degradation and iron liberation.
Iron oxide nanoparticles such as Feraheme® have furthermore been shown to modulate the polarization of tumor-associated macrophages (TAM), thereby supporting the outcome of established cancer therapies. Several studies have been published in this regard, showing, e.g., that iron delivery to TAM mediates a shift from an M2-like to an M1-like phenotype, which is accompanied by an enhanced anti-tumoral effect [108]–[110]. These and some of the other iron-based therapeutic strategies outlined above open up promising new avenues to improve anticancer therapy, particularly in combined modality settings, e.g., via enhancing immunotherapy efficacy.
The role of iron and its therapeutic potential in cancer strongly depends on the tumor stage. It can either enhance proliferation by providing the necessary iron for enzymes taking part in oxidative phosphorylation and DNA synthesis. Conversely, excess iron can also catalyze ROS generation and induces ferroptosis, leading to cell death, and potentially providing a powerful therapeutic strategy. At early stages, excess iron may worsen the disease, by causing (additional) DNA damage driving cell cycle deregulation and tumor initiation. Altogether, as already alluded above, there is still much to be learnt about the involvement of iron in cancer initiation, progression and treatment, and systematic future investigations are needed to elucidate iron’s role of specific malignancy types and stages.
Concluding Remarks
Iron is involved in fundamental biological processes. Therefore, control over iron homeostasis is crucial, and an iron imbalance results in various pathological conditions.
Even with the major advances in better understanding iron metabolism in recent years, iron deficiency and anemia are still major global health problems. New formulations, especially for intravenous administration and new therapeutic routes have been developed, but the need for controlled clinical trials remains (see Outstanding Questions). Moreover, targeting iron metabolism via hepcidin inhibition holds great potential. More advanced clinical studies are needed to translate the so far promising preclinical results and make them accessible for clinical practice.
Iron overload leads to an increased risk of infection, an increased inflammatory response, and may even increase the risk of cancer. Iron overload furthermore induces ferroptosis, which can ultimately result in organ failure. Several neurodegenerative disorders have been linked to iron excess, even though it has remained largely elusive if increased iron levels are the cause or the consequence of the disease. Treatment options such as iron chelation or pharmacological stimulation of hepcidin production reduce systemic iron levels and prevent pathological effects.
The wide variety of different disease conditions associated with deregulated iron metabolism calls for a better and broader understanding of iron homeostasis and novel iron-targeted pharmacotherapies and biomarkers to facilitate disease diagnosis staging and treatment monitoring. Concerted future actions, in which basic scientists working on iron biology and iron (patho-) physiology closely collaborate with industrial and clinical scientists on the development and translation of iron-metabolism-modulating pharmacotherapies, are considered to be crucial for promoting progress in the field, eventually resulting in the generation of several new compounds for targeting iron metabolism in the clinic.
Acknowledgments
The authors gratefully acknowledge financial support by the German Research Foundation (DFG: GRK/RTG 2375 Tumor-targeted Drug Delivery (project number 331065168), SFB1066, and SFB/TRR57) and by the European Research Council (ERC: Consolidator Grant Meta-Targeting; project number 864121).
Footnotes
Disclaimer Statement:
All authors declare no conflict of interest.
- i)
- ii)
- iii)
- iv)
References
- [1].Aisen P, Enns C, Wessling-Resnick M. Chemistry and biology of eukaryotic iron metabolism. Int J Biochem Cell Biol. 2001 Oct;33(10):940–959. doi: 10.1016/s1357-2725(01)00063-2. [DOI] [PubMed] [Google Scholar]
- [2].Crielaard BJ, Lammers T, Rivella S. Targeting iron metabolism in drug discovery and delivery. Nature Reviews Drug Discovery. 2017 Jun;16(6) doi: 10.1038/nrd.2016.248. Art. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dixon SJ, Stockwell BR. The role of iron and reactive oxygen species in cell death. Nature Chemical Biology. 2014 Jan;10(1):9–17. doi: 10.1038/nchembio.1416. [DOI] [PubMed] [Google Scholar]
- [4].Papanikolaou G, Pantopoulos K. Iron metabolism and toxicity. Toxicology and Applied Pharmacology. 2005 Jan;202(2):199–211. doi: 10.1016/j.taap.2004.06.021. [DOI] [PubMed] [Google Scholar]
- [5].Ganz T. Systemic Iron Homeostasis. Physiological Reviews. 2013 Oct;93(4):1721–1741. doi: 10.1152/physrev.00008.2013. [DOI] [PubMed] [Google Scholar]
- [6].McKie AT, et al. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell. 2000 Feb;5(2):299–309. doi: 10.1016/s1097-2765(00)80425-6. [DOI] [PubMed] [Google Scholar]
- [7].Donovan A, et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000 Feb;403(6771):776–781. doi: 10.1038/35001596. [DOI] [PubMed] [Google Scholar]
- [8].Laurell C-B. Analytical Review: What is the Function of Transferrin in Plasma? Blood. 1951 Feb;6(2):183–187. doi: 10.1182/blood.V6.2.183.183. [DOI] [PubMed] [Google Scholar]
- [9].Horton MA. Expression of transferrin receptors during erythroid maturation. Experimental Cell Research. 1983 Apr;144(2):361–366. doi: 10.1016/0014-4827(83)90415-9. [DOI] [PubMed] [Google Scholar]
- [10].Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a Urinary Antimicrobial Peptide Synthesized in the Liver*. Journal of Biological Chemistry. 2001 Mar;276(11):7806–7810. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- [11].Nemeth E, et al. Hepcidin Regulates Cellular Iron Efflux by Binding to Ferroportin and Inducing Its Internalization. Science. 2004 Dec;306(5704):2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- [12].Babitt JL, Huang FW, Xia Y, Sidis Y, Andrews NC, Lin HY. Modulation of bone morphogenetic protein signaling in vivo regulates systemic iron balance. J Clin Invest. 2007 Jul;117(7):1933–1939. doi: 10.1172/JCI31342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Babitt JL, et al. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet. 2006 May;38(5):531–539. doi: 10.1038/ng1777. [DOI] [PubMed] [Google Scholar]
- [14].Gao J, Chen J, Kramer M, Tsukamoto H, Zhang A-S, Enns CA. Interaction of the hereditary hemochromatosis protein HFE with transferrin receptor 2 is required for transferrin-induced hepcidin expression. Cell Metab. 2009 Mar;9(3):217–227. doi: 10.1016/j.cmet.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nemeth E, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004 May;113(9):1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Arezes J, et al. Erythroferrone inhibits the induction of hepcidin by BMP6. Blood. 2018 Oct;132(14):1473–1477. doi: 10.1182/blood-2018-06-857995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Peyssonnaux C, et al. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs) J Clin Invest. 2007 Jul;117(7):1926–1932. doi: 10.1172/JCI31370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mastrogiannaki M, et al. Hepatic hypoxia-inducible factor-2 down-regulates hepcidin expression in mice through an erythropoietin-mediated increase in erythropoiesis. Haematologica. 2012 Jun;97(6):827–834. doi: 10.3324/haematol.2011.056119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hentze MW, et al. Identification of the iron-responsive element for the translational regulation of human ferritin mRNA. Science. 1987 Dec;238(4833):1570–1573. doi: 10.1126/science.3685996. [DOI] [PubMed] [Google Scholar]
- [20].Casey JL, et al. Iron-responsive elements: regulatory RNA sequences that control mRNA levels and translation. Science. 1988 May;240(4854):924–928. doi: 10.1126/science.2452485. [DOI] [PubMed] [Google Scholar]
- [21].Abboud S, Haile DJ. A Novel Mammalian Iron-regulated Protein Involved in Intracellular Iron Metabolism *. Journal of Biological Chemistry. 2000 Jun;275(26):19906–19912. doi: 10.1074/jbc.M000713200. [DOI] [PubMed] [Google Scholar]
- [22].Gunshin H, et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997 Jul;388(6641) doi: 10.1038/41343. Art. 6641. [DOI] [PubMed] [Google Scholar]
- [23].Lok CN, Ponka P. Identification of a hypoxia response element in the transferrin receptor gene. J Biol Chem. 1999 Aug;274(34):24147–24152. doi: 10.1074/jbc.274.34.24147. [DOI] [PubMed] [Google Scholar]
- [24].Tacchini L, Bianchi L, Bernelli-Zazzera A, Cairo G. Transferrin receptor induction by hypoxia. HIF-1-mediated transcriptional activation and cell-specific post-transcriptional regulation. J Biol Chem. 1999 Aug;274(34):24142–24146. doi: 10.1074/jbc.274.34.24142. [DOI] [PubMed] [Google Scholar]
- [25].Qian Z-M, et al. Divalent metal transporter 1 is a hypoxia-inducible gene. J Cell Physiol. 2011 Jun;226(6):1596–1603. doi: 10.1002/jcp.22485. [DOI] [PubMed] [Google Scholar]
- [26].Taylor M, et al. Hypoxia-Inducible Factor-2α Mediates the Adaptive Increase of Intestinal Ferroportin During Iron Deficiency in Mice. Gastroenterology. 2011 Jun;140(7):2044–2055. doi: 10.1053/j.gastro.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jaakkola P, et al. Targeting of HIF-α to the von Hippel-Lindau Ubiquitylation Complex by O2-Regulated Prolyl Hydroxylation. Science. 2001 Apr;292(5516):468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- [28].Camaschella C. New insights into iron deficiency and iron deficiency anemia. Blood Rev. 2017;31(4):225–233. doi: 10.1016/j.blre.2017.02.004. [DOI] [PubMed] [Google Scholar]
- [29].Camaschella C. Iron-Deficiency Anemia. New England Journal of Medicine. 2015 May;372(19):1832–1843. doi: 10.1056/NEJMra1401038. [DOI] [PubMed] [Google Scholar]
- [30].Moretti D, et al. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood. 2015 Oct;126(17):1981–1989. doi: 10.1182/blood-2015-05-642223. [DOI] [PubMed] [Google Scholar]
- [31].De Franceschi L, Iolascon A, Taher A, Cappellini MD. Clinical management of iron deficiency anemia in adults: Systemic review on advances in diagnosis and treatment. Eur J Intern Med. 2017 Jul;42:16–23. doi: 10.1016/j.ejim.2017.04.018. [DOI] [PubMed] [Google Scholar]
- [32].Muñoz M, et al. Current misconceptions in diagnosis and management of iron deficiency. Blood Transfus. 2017 Sep;15(5):422–437. doi: 10.2450/2017.0113-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sankaran VG, Weiss MJ. Anemia: progress in molecular mechanisms and therapies. Nature Medicine. 2015 Mar;21(3) doi: 10.1038/nm.3814. Art. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gattermann N. The Treatment of Secondary Hemochromatosis. Dtsch Arztebl Int. 2009 Jul;106(30):499–504. doi: 10.3238/arztebl.2009.0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Saliba AN, Rassi FE, Taher AT. Clinical monitoring and management of complications related to chelation therapy in patients with β-thalassemia. Expert Review of Hematology. 2016 Feb;9(2):151–168. doi: 10.1586/17474086.2016.1126176. [DOI] [PubMed] [Google Scholar]
- [36].Angelucci E, et al. Hematopoietic stem cell transplantation in thalassemia major and sickle cell disease: indications and management recommendations from an international expert panel. Haematologica. 2014 May;99(5):811–820. doi: 10.3324/haematol.2013.099747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cappellini MD, et al. A Phase 3 Trial of Luspatercept in Patients with Transfusion-Dependent β-Thalassemia. N Engl J Med. 2020;382(13):1219–1231. doi: 10.1056/NEJMoa1910182. [DOI] [PubMed] [Google Scholar]
- [38].Cappellini MD, et al. Sotatercept, a novel transforming growth factor β ligand trap, improves anemia in β-thalassemia: a phase II, open-label, dose-finding study. Haematologica. 2019 Mar;104(3):477–484. doi: 10.3324/haematol.2018.198887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Weiss G, Goodnough LT. Anemia of Chronic Disease. New England Journal of Medicine. 2005 Mar;352(10):1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- [40].Cappellini MD, et al. Iron deficiency across chronic inflammatory conditions: International expert opinion on definition, diagnosis, and management. Am J Hematol. 2017 Oct;92(10):1068–1078. doi: 10.1002/ajh.24820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Webster AC, Nagler EV, Morton RL, Masson P. Chronic Kidney Disease. The Lancet. 2017 Mar;389(10075):1238–1252. doi: 10.1016/S0140-6736(16)32064-5. [DOI] [PubMed] [Google Scholar]
- [42].Hayat A, Haria D, Salifu MO. Erythropoietin stimulating agents in the management of anemia of chronic kidney disease. Patient Prefer Adherence. 2008 Feb;2:195–200. doi: 10.2147/ppa.s2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Soni H. Prolyl hydroxylase domain-2 (PHD2) inhibition may be a better therapeutic strategy in renal anemia. Medical Hypotheses. 2014 May;82(5):547–550. doi: 10.1016/j.mehy.2014.02.008. [DOI] [PubMed] [Google Scholar]
- [44].Dignass A, Farrag K, Stein J. Limitations of Serum Ferritin in Diagnosing Iron Deficiency in Inflammatory Conditions. Int J Chronic Dis. 2018 Mar;2018 doi: 10.1155/2018/9394060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Weiss G, Ganz T, Goodnough LT. Anemia of inflammation. Blood. 2019;133(1):40–50. doi: 10.1182/blood-2018-06-856500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Grillo AS, et al. Restored iron transport by a small molecule promotes absorption and hemoglobinization in animals. Science. 2017 May;356(6338):608–616. doi: 10.1126/science.aah3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Sheetz M, et al. Targeting the hepcidin-ferroportin pathway in anaemia of chronic kidney disease. Br J Clin Pharmacol. 2019;85(5) doi: 10.1111/bcp.13877. Art. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Vadhan-Raj S, et al. A first-in-human phase 1 study of a hepcidin monoclonal antibody, LY2787106, in cancer-associated anemia. J Hematol Oncol. 2017 Mar;10 doi: 10.1186/s13045-017-0427-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Renders L, et al. First-in-human Phase I studies of PRS-080#22, a hepcidin antagonist, in healthy volunteers and patients with chronic kidney disease undergoing hemodialysis. PLoS ONE. 2019;14(3):e0212023. doi: 10.1371/journal.pone.0212023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Jandl JH, Inman JK, Simmons RL, Allen DW. Transfer of Iron from Serum Iron-Binding Protein to Human Reticulocytes*. J Clin Invest. 1959 Jan;38(1):161–185. doi: 10.1172/JCI103786. Pt 1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Rychtarcikova Z, et al. Tumor-initiating cells of breast and prostate origin show alterations in the expression of genes related to iron metabolism. Oncotarget. 2017 Jan;8(4):6376–6398. doi: 10.18632/oncotarget.14093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kindrat I, et al. MicroRNA-152-mediated dysregulation of hepatic transferrin receptor 1 in liver carcinogenesis. Oncotarget. 2016 Jan;7(2):1276–1287. doi: 10.18632/oncotarget.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Horniblow RD, et al. BRAF mutations are associated with increased iron regulatory protein-2 expression in colorectal tumorigenesis. Cancer Sci. 2017 Jun;108(6):1135–1143. doi: 10.1111/cas.13234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Xue X, et al. Iron Uptake via DMT1 Integrates Cell Cycle with JAK-STAT3 Signaling to Promote Colorectal Tumorigenesis. Cell Metabolism. 2016 Sep;24(3):447–461. doi: 10.1016/j.cmet.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Cohen LA, et al. Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood. 2010 Sep;116(9):1574–1584. doi: 10.1182/blood-2009-11-253815. [DOI] [PubMed] [Google Scholar]
- [56].Li JY, et al. Scara5 is a Ferritin Receptor Mediating Non-Transferrin Iron Delivery. Dev Cell. 2009 Jan;16(1):35–46. doi: 10.1016/j.devcel.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].You K, et al. SCARA5 plays a critical role in the progression and metastasis of breast cancer by inactivating the ERK1/2, STAT3, and AKT signaling pathways. Mol Cell Biochem. 2017 Nov;435(1):47–58. doi: 10.1007/s11010-017-3055-4. [DOI] [PubMed] [Google Scholar]
- [58].Li L, et al. Binding and uptake of H-ferritin are mediated by human transferrin receptor-1. Proc Natl Acad Sci U S A. 2010 Feb;107(8):3505–3510. doi: 10.1073/pnas.0913192107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Xue D, Zhou C-X, Shi Y-B, Lu H, He X-Z. Decreased expression of ferroportin in prostate cancer Corrigendum in /10.3892/ol.2021.12518. Oncology Letters. 2015 Aug;10(2):913–916. doi: 10.3892/ol.2015.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Pinnix ZK, et al. Ferroportin and iron regulation in breast cancer progression and prognosis. Sci Transl Med. 2010 Aug;2(43):43ra56. doi: 10.1126/scisignal.3001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Yang J, et al. Lipocalin 2 promotes breast cancer progression. PNAS. 2009 Mar;106(10):3913–3918. doi: 10.1073/pnas.0810617106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Bystrom LM, Guzman ML, Rivella S. Iron and Reactive Oxygen Species: Friends or Foes of Cancer Cells? Antioxid Redox Signal. 2014 Apr;20(12):1917–1924. doi: 10.1089/ars.2012.5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lui GYL, Kovacevic Z, Richardson V, Merlot AM, Kalinowski DS, Richardson DR. Targeting cancer by binding iron: Dissecting cellular signaling pathways. Oncotarget. 2015 Aug;6(22):18748–18779. doi: 10.18632/oncotarget.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Taetle R, Castagnola J, Mendelsohn J. Mechanisms of growth inhibition by anti-transferrin receptor monoclonal antibodies. Cancer Res. 1986 Apr;46(4):1759–1763. Pt 1. [PubMed] [Google Scholar]
- [65].Faulk WP, Taylor CG, Yeh CJ, McIntyre JA. Preliminary clinical study of transferrin-adriamycin conjugate for drug delivery to acute leukemia patients. Mol Biother. 1990 Mar;2(1):57–60. [PubMed] [Google Scholar]
- [66].Daniels-Wells TR, Penichet ML. [accessed Apr. 18, 2021];Transferrin receptor 1: a target for antibody-mediated cancer therapy. 2016 Jul 04; doi: 10.2217/imt-2016-0050. https://www.futuremedicine.com/doi/abs/10.2217/imt-2016-0050. [DOI] [PubMed]
- [67].Lang J, et al. Targeted Co-delivery of the Iron Chelator Deferoxamine and a HIF1α Inhibitor Impairs Pancreatic Tumor Growth. ACS Nano. 2019 Feb;13(2):2176–2189. doi: 10.1021/acsnano.8b08823. [DOI] [PubMed] [Google Scholar]
- [68].Guo W, et al. An important role of the hepcidin-ferroportin signaling in affecting tumor growth and metastasis. Acta Biochim Biophys Sin. 2015 Sep;47(9):703–715. doi: 10.1093/abbs/gmv063. [DOI] [PubMed] [Google Scholar]
- [69].Kang H, et al. Renal clearable nanochelators for iron overload therapy. Nature Communications. 2019 Nov;10(1) doi: 10.1038/s41467-019-13143-z. Art. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].McCaleb M, et al. Transmembrane Protease, Serine 6 (TMPRSS6) Antisense Oligonucleotide (IONIS-TMPRSS6-LRX) Reduces Plasma Iron Levels of Healthy Volunteers in a Phase 1 Clinical Study. Blood. 2018 Nov;132(1):3634–3634. doi: 10.1182/blood-2018-99-115339. [DOI] [Google Scholar]
- [71].Altamura S, et al. SLN124, a Galnac-siRNA Conjugate Targeting TMPRSS6, for the Treatment of Iron Overload and Ineffective Erythropoiesis Such As in Beta-Thalassemia. [10.1182/blood-2018-99-110163];Blood. 2018 Nov;132(1):2340–2340. [Google Scholar]
- [72].Altamura S, et al. SLN124, a GalNAc-siRNA Conjugate Targeting TMPRSS6, Efficiently Prevents Iron Overload in Hereditary Haemochromatosis Type 1. Hemasphere. 2019 Oct;3(6) doi: 10.1097/HS9.0000000000000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Arezes J, et al. Antibodies against the erythroferrone N-terminal domain prevent hepcidin suppression and ameliorate murine thalassemia. Blood. 2020 Feb;135(8):547–557. doi: 10.1182/blood.2019003140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Lim PJ, et al. Nrf2 controls iron homeostasis in haemochromatosis and thalassaemia via Bmp6 and hepcidin. Nat Metab. 2019 May;1(5):519–531. doi: 10.1038/s42255-019-0063-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Richard F, van Lier JJ, Roubert B, Haboubi T, Göhring U-M, Dürrenberger F. Oral ferroportin inhibitor VIT-2763: First-in-human, phase 1 study in healthy volunteers. Am J Hematol. 2020;95(1):68–77. doi: 10.1002/ajh.25670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Van Vuren AJ, et al. Proton Pump Inhibition for Secondary Hemochromatosis in Hereditary Anemia, a Phase III Placebo Controlled Randomized Cross-over Trial in Progress. [10.1182/blood-2019-124059];Blood. 2019 Nov;134(1):960–960. [Google Scholar]
- [77].Shah YM, Matsubara T, Ito S, Yim S-H, Gonzalez FJ. Intestinal Hypoxia-Inducible Transcription Factors Are Essential for Iron Absorption following Iron Deficiency. Cell Metabolism. 2009 Feb;9(2):152–164. doi: 10.1016/j.cmet.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Rankin EB, et al. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest. 2007 Apr;117(4):1068–1077. doi: 10.1172/JCI30117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Schwartz AJ, et al. Hepatic hepcidin/intestinal HIF-2α axis maintains iron absorption during iron deficiency and overload. J Clin Invest. 2019 Jan;129(1):336–348. doi: 10.1172/JCI122359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014 Oct;13(10):1045–1060. doi: 10.1016/S1474-4422(14)70117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Cornelissen A, Guo L, Sakamoto A, Virmani R, Finn AV. New insights into the role of iron in inflammation and atherosclerosis. EBioMedicine. 2019 Sep;47:598–606. doi: 10.1016/j.ebiom.2019.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Ruparelia N, Chai JT, Fisher EA, Choudhury RP. Inflammatory processes in cardiovascular disease: a route to targeted therapies. Nat Rev Cardiol. 2017 Mar;14(3):133–144. doi: 10.1038/nrcardio.2016.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Kraml P. The role of iron in the pathogenesis of atherosclerosis. Physiol Res. 2017 Apr;66(1):S55–S67. doi: 10.33549/physiolres.933589. [DOI] [PubMed] [Google Scholar]
- [84].Vinchi F, et al. Atherosclerosis is aggravated by iron overload and ameliorated by dietary and pharmacological iron restriction. Eur Heart J. 2020 Apr; doi: 10.1093/eurheartj/ehz112. [DOI] [PubMed] [Google Scholar]
- [85].Ward RJ, Crichton RR. Ironing out the Brain. Met Ions Life Sci. 2019 Jan;19 doi: 10.1515/9783110527872-010. [DOI] [PubMed] [Google Scholar]
- [86].Qian Z-M, Ke Y. Hepcidin and its therapeutic potential in neurodegenerative disorders. Medicinal Research Reviews. 2020;40(2):633–653. doi: 10.1002/med.21631. [DOI] [PubMed] [Google Scholar]
- [87].Boddaert N, et al. Selective iron chelation in Friedreich ataxia: biologic and clinical implications. Blood. 2007 Jul;110(1):401–408. doi: 10.1182/blood-2006-12-065433. [DOI] [PubMed] [Google Scholar]
- [88].Xie Y, et al. Ferroptosis: process and function. Cell Death Differ. 2016 Mar;23(3):369–379. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Patel D, et al. Alpha-synuclein inhibits Snx3-retromer-mediated retrograde recycling of iron transporters in S. cerevisiae and C. elegans models of Parkinson’s disease. Hum Mol Genet. 2018 May;27(9):1514–1532. doi: 10.1093/hmg/ddy059. [DOI] [PubMed] [Google Scholar]
- [90].Davies P, Moualla D, Brown DR. Alpha-synuclein is a cellular ferrireductase. PLoS One. 2011 Jan;6(1):e15814. doi: 10.1371/journal.pone.0015814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Xie L, et al. DMT 1 inhibitor ebselen inhibits iron-induced amyloidogenic APP processing. 2018 [Google Scholar]
- [92].Xie Y, Tan Y, Zheng Y, Du X, Liu Q. Ebselen ameliorates β-amyloid pathology, tau pathology, and cognitive impairment in triple-transgenic Alzheimer’s disease mice. J Biol Inorg Chem. 2017 Aug;22(6):851–865. doi: 10.1007/s00775-017-1463-2. [DOI] [PubMed] [Google Scholar]
- [93].Ndayisaba A, Kaindlstorfer C, Wenning GK. Iron in Neurodegeneration – Cause or Consequence? Front Neurosci. 2019 Mar;13 doi: 10.3389/fnins.2019.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Ganz T, Nemeth E. Iron homeostasis in host defence and inflammation. Nature Reviews Immunology. 2015 Aug;15(8) doi: 10.1038/nri3863. Art. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Ganz T. Iron and infection. Int J Hematol. 2018 Jan;107(1):7–15. doi: 10.1007/s12185-017-2366-2. [DOI] [PubMed] [Google Scholar]
- [96].Portsmouth S, et al. Cefiderocol versus imipenem-cilastatin for the treatment of complicated urinary tract infections caused by Gram-negative uropathogens: a phase 2, randomised, double-blind, non-inferiority trial. Lancet Infect Dis. 2018;18(12):1319–1328. doi: 10.1016/S1473-3099(18)30554-1. [DOI] [PubMed] [Google Scholar]
- [97].Cavezzi A, Troiani E, Corrao S. COVID-19: hemoglobin, iron, and hypoxia beyond inflammation. A narrative review. Clin Pract. 2020 May;10(2) doi: 10.4081/cp.2020.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Edeas M, Saleh J, Peyssonnaux C. Iron: Innocent bystander or vicious culprit in COVID-19 pathogenesis? Int J Infect Dis. 2020 Aug;97:303–305. doi: 10.1016/j.ijid.2020.05.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Handa P, et al. Iron alters macrophage polarization status and leads to steatohepatitis and fibrogenesis. Journal of Leukocyte Biology. 2019;105(5):1015–1026. doi: 10.1002/JLB.3A0318-108R. [DOI] [PubMed] [Google Scholar]
- [100].Abe N, Tsuchida T, Yasuda S-I, Oka K. Dietary iron restriction leads to a reduction in hepatic fibrosis in a rat model of non-alcoholic steatohepatitis. Biology Open. 2019 May;8(5) doi: 10.1242/bio.040519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Milman NT, Schioedt FV, Junker AE, Magnussen K. Diagnosis and Treatment of Genetic HFE-Hemochromatosis: The Danish Aspect. Gastroenterology Res. 2019 Oct;12(5):221–232. doi: 10.14740/gr1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Torti SV, Manz DH, Paul BT, Blanchette-Farra N, Torti FM. Iron and Cancer. Annual Review of Nutrition. 2018;38(1):97–125. doi: 10.1146/annurev-nutr-082117-051732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Dixon SJ, et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell. 2012 May;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Sui X, et al. RSL3 Drives Ferroptosis Through GPX4 Inactivation and ROS Production in Colorectal Cancer. Front Pharmacol. 2018 Nov;9 doi: 10.3389/fphar.2018.01371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Trujillo-Alonso V, et al. FDA-approved ferumoxytol displays anti-leukaemia efficacy against cells with low ferroportin levels. Nature Nanotechnology. 2019 Jun;14(6) doi: 10.1038/s41565-019-0406-1. Art. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Dixon SJ, et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife. 2014 May;3:e02523. doi: 10.7554/eLife.02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Schonberg DL, et al. Preferential Iron Trafficking Characterizes Glioblastoma Stem-like Cells. Cancer Cell. 2015 Oct;28(4):441–455. doi: 10.1016/j.ccell.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Zanganeh S, et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol. 2016 Nov;11(11):986–994. doi: 10.1038/nnano.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Vinchi F, et al. Hemopexin therapy reverts heme-induced proinflammatory phenotypic switching of macrophages in a mouse model of sickle cell disease. Blood. 2016 Jan;127(4):473–486. doi: 10.1182/blood-2015-08-663245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Costa da Silva M, et al. Iron Induces Anti-tumor Activity in Tumor-Associated Macrophages. Front Immunol. 2017;8 doi: 10.3389/fimmu.2017.01479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].de Dreuzy E, Bhukhai K, Leboulch P, Payen E. Current and future alternative therapies for beta-thalassemia major. Biomed J. 2016 Feb;39(1):24–38. doi: 10.1016/j.bj.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Holmes MC, et al. A Potential Therapy for Beta-Thalassemia (ST-400) and Sickle Cell Disease (BIVV003) Biology of Blood and Marrow Transplantation. 2018 Mar;24(3):S172. doi: 10.1016/j.bbmt.2017.12.105. [DOI] [Google Scholar]
- [113].Zipkin M. CRISPR’s ‘magnificent moment’ in the clinic. Nature Biotechnology. 2019 Dec; doi: 10.1038/d41587-019-00035-2. [DOI] [PubMed] [Google Scholar]
- [114].Boulad F, et al. Safe mobilization of CD34+ cells in adults with β-thalassemia and validation of effective globin gene transfer for clinical investigation. Blood. 2014 Mar;123(10):1483–1486. doi: 10.1182/blood-2013-06-507178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Lal A, et al. Northstar-3: Interim Results from a Phase 3 Study Evaluating Lentiglobin Gene Therapy in Patients with Transfusion-Dependent β-Thalassemia and Either a β0 or IVS-I-110 Mutation at Both Alleles of the HBB Gene. Blood. 2019 Nov;134(1):815. doi: 10.1182/blood-2019-128482. [DOI] [Google Scholar]
- [116].Naldini L. Genetic engineering of hematopoiesis: current stage of clinical translation and future perspectives. EMBO Mol Med. 2019 Mar;11(3) doi: 10.15252/emmm.201809958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Flo TH, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004 Dec;432(7019) doi: 10.1038/nature03104. Art. 7019. [DOI] [PubMed] [Google Scholar]
- [118].Mertens C, et al. Macrophage-derived lipocalin-2 transports iron in the tumor microenvironment. Oncoimmunology. 2017 Dec;7(3) doi: 10.1080/2162402X.2017.1408751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Chi Y, et al. Cancer cells deploy lipocalin-2 to collect limiting iron in leptomeningeal metastasis. Science. 2020 Jul;369(6501):276–282. doi: 10.1126/science.aaz2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Santiago-Sánchez GS, Pita-Grisanti V, Quiñones-Díaz B, Gumpper K, Cruz-Monserrate Z, Vivas-Mejía PE. Biological Functions and Therapeutic Potential of Lipocalin 2 in Cancer. IJMS. 2020 Jun;21(12):43–65. doi: 10.3390/ijms21124365. [DOI] [PMC free article] [PubMed] [Google Scholar]