Abstract
Whether depressed patients with evidence of inflammation are more appropriate candidates for immunotherapies is being tested in several clinical trials, which are selecting patients based on elevated C-reactive protein (CRP) and inflammation-related symptoms. However, studies of the clinical and phenotypic profile of depressed patients with elevated CRP are relatively scarce. We have investigated detailed clinical characteristics of 84 depressed patients, grouped as those with (CRP≥3 mg/L) and without (CRP<3 mg/L) inflammation. All patients met the International Classification of Diseases 10th Revision criteria for current depressive episode and had somatic symptoms of depression. We report that depressed patients with inflammation are more likely to be older (P=0.04), have higher body mass index (P<0.01), and be on non-selective serotonin reuptake inhibitor antidepressants (P=0.04). After adjusting for potential confounders, the inflammation group had higher depression severity (adjusted mean difference, 8.82; 95% CI, 3.91–13.72), somatic symptoms (adjusted mean difference, 3.25; 95% CI, 1.58–4.92), state anxiety (adjusted mean difference, 9.25; 95% CI, 3.82–14.67), perceived stress (adjusted mean difference, 4.58; 95% CI, 1.98–7.18), and fatigue (adjusted mean difference, 9.71; 95% CI, 3.09–6.33), but not anhedonia. The inflamed group also had poorer quality of life (adjusted mean difference, −0.18; 95% CI, −0.32–0.05). At individual depressive symptom level, the inflammation group had increased guilty feelings (adjusted odds ratio [OR], 7.28; 95% CI, 2.09–31.17), pessimism (adjusted OR, 5.38; 95% CI, 1.53–22.73), concentration difficulties (adjusted OR, 4.56; 95% CI, 1.32–19.02), and indecisiveness (adjusted OR, 4.21; 95% CI, 1.15–18.54). Our findings highlight the clinical features associated with inflammation in depressed patients with somatic symptoms, including poor quality of life, supporting the need for intervention targeting this group. These results could also aid patient and outcome selection in future clinical trials testing immunotherapies in depression. Replication of these findings in larger samples is required.
Keywords: C-Reactive protein, Inflammation, Depression, Fatigue, Anxiety, Quality of life
Highlights
-
•
We studied clinical features of depressed patients with somatic symptoms with/without evidence of inflammation .
-
•
Elevated CRP (≥3mg/L) was associated with higher age, higher BMI, and non-SSRI treatment.
-
•
Elevated CRP (≥3mg/L) was associated with higher depression severity, fatigue, state anxiety, and stress.
-
•
Elevated CRP (≥3mg/L) was associated with poorer subjective wellbeing and quality of life.
-
•
Elevated CRP (≥3mg/L) was associated with both somatic and psychological symptoms of depression.
1. Introduction
Accumulating evidence suggests an association between systemic low-grade inflammation and depression. Meta-analyses of cross-sectional studies confirm elevated concentrations of circulating inflammatory cytokines and acute phase proteins, like interleukin-6 (IL-6) and C-reactive protein (CRP), in patients with depression as compared to controls [19,49]. CRP is an archetypal inflammatory marker and has been used most extensively as a measure of inflammation in depression. A recent meta-analysis by Ref. [40] reported that evidence of low-grade inflammation (i.e., CRP >3 mg/L) is present in approximately 27% of depressed patients. Emerging evidence from population-based prospective studies and genetic Mendelian randomization (MR) studies suggest that inflammation may play a causal role in depression [29,30,55]. Inflammation is also clinically relevant and has been associated with poor response to antidepressants [8,36]. Additionally, anti-inflammatory drugs have antidepressant effects in patients with chronic inflammatory conditions, where novel anti-cytokine drugs improve depressive symptoms (at least partly) independently of improving physical illness symptoms [26,32,54]. These findings highlight the potential for therapeutic targeting of inflammation in patients with depression.
Currently, a number of randomised controlled trials (RCTs) are testing the effects of immunotherapies for depression outside the context of major physical illnesses. However, which depressed patients are likely to benefit from such treatment remains a key outstanding question. A RCT of infliximab [44], a tumour necrosis factor alpha (TNF-α) specific monoclonal antibody, reported that patients with evidence of inflammation may be more suitable candidates for immunotherapy trials. Consequently, a number of RCTs have begun evaluating immunotherapies on depressed patients with evidence of inflammation [2,28,51]. Typically, these trials have defined inflammation as elevated CRP or IL-6 levels. However, there is relatively limited research into potential characteristics of depressed patients with evidence of inflammation. Such characteristics could help inform patient selection in future RCTs.
Evidence suggests that elevated CRP levels in depressed patients are associated with higher depression severity [31] and somatic symptoms (e.g., fatigue, changes in appetite, and sleep), rather than psychological symptoms (e.g., hopelessness, excessive/inappropriate guilt) [24,39]. However, existing studies have often used single items on depression scales as measures of fatigue and anhedonia. In-depth assessment of these key inflammation-related symptom domains is required as fatigue and anhedonia are complex multi-faceted traits [12,53]. Anxiety symptoms are also often present in patients with depression, but studies testing the association between CRP levels and anxiety symptoms in patients with depression are scarce. Furthermore, little is known about associations of CRP levels with perceived stress, quality of life, and subjective wellbeing, which are key indicators of overall wellbeing.
To further understand the characteristics of depressed patients with somatic symptoms with and without evidence of inflammation, we have investigated a range of sociodemographic factors, clinical history, affective and somatic symptoms, quality of life, and wellbeing in these samples. We hypothesised that inflammation would be associated with a distinct phenotypic profile characterised by higher depression severity, increased fatigue, anhedonia, and poorer quality of life.
2. Materials and methods
2.1. Participants and study design
A case-control design was applied involving participants with International Classification of Diseases 10th Revision (ICD-10) diagnosis of depressive episode (ICD-10 code F32) and somatic symptoms (see below), grouped as with or without evidence of inflammation based on CRP levels. We aimed to recruit roughly equal number of participants with low and high CRP; recruitment strategy for both groups was identical. All patients were recruited through UK National Health Service Mental Health Trusts, primary care general practice surgeries, and self-referral in the East Anglia region between October 2018 and March 2020 as part of a RCT [28]. All participants were required to meet the following criteria regardless of their CRP levels: aged 20–65 years, ICD-10 criteria for depressive episode at time of assessment (confirmed by the Clinical Interview Schedule – Revised), somatic symptom score ≥7 (based on Beck Depression Inventory II, or BDI-II, items on pleasure, energy, sleep, appetite, concentration difficulty, tiredness/fatigue, and libido), currently taking an antidepressant at an adequate dose (as determined by British National Formulary or BNF) for at least four weeks. Exclusion criteria were current or lifetime diagnosis of bipolar disorder, psychotic disorder, personality disorder, eating disorder, history of alcohol or substance abuse/dependence within six months prior to assessment (nicotine and caffeine dependence were not exclusionary), current suicidal thoughts or wishes (assessed by BDI-II item 9 score of 3) or history of suicide attempt or deliberate self-harm within six months prior to assessment, any current infection, any infection requiring hospitalisation or treatment with intravenous antibiotics within four weeks prior to assessment, pregnancy or breast feeding, and physical illness and/or use of medication likely to compromise interpretation of immunological data. Self-reported information on key inclusion/exclusion criteria were verified by the participant's general practitioner prior to enrolment.
Demographic and medical history information was recorded and self-administered psychiatric evaluations were completed during the study visit. Additionally, all participants provided blood samples for serum high sensitivity CRP (hs-CRP) measurement. The sample was divided into two groups according to hs-CRP levels: those with evidence of low-grade inflammation (hs-CRP ≥3 mg/L), and those without evidence of low-grade inflammation (hs-CRP <3 mg/L). This cut-off was chosen based on the American Heart Association and Center for Disease Control and Prevention recommendations, which defined CRP levels of >3 mg/L as high [41].
All participants provided informed consent. The study was approved by the South Central – Oxford B Research Ethics Committee (Reference: 18/SC/0118).
2.2. Measurement of CRP
Blood samples were collected from non-fasting participants. Samples were promptly centrifuged and assayed for serum hs-CRP levels using an automated colorimetric immunoassay on the Siemens Dimension EXL analyser. The minimum detection limit was 0.1 mg/L. All samples were assayed at the Core Biochemical Assay Laboratory, located in Addenbrooke's Hospital, Cambridge, by staff blind to psychiatric measure outcomes. Acute infection was excluded by white blood cell count, antibody tests for TB, HIV, Hepatitis B, and Hepatitis C, and chest X-ray.
2.3. Diagnosis of depression
ICD-10 diagnosis of depression was assessed by the Clinical Interview Schedule – Revised (CIS-R), which was administered by trained research staff. The CIS-R is a widely used, standardised tool for measuring common mental health disorders in research settings [35]. The CIS-R is a fully structured assessment suitable for trained social survey interviewers and does not require any expert knowledge on the part of the interviewers. As such, it can also be administered using personal computers on which the subjects self-complete the questionnaire [35]. The CIS-R elicits responses to 14 areas of symptoms including fatigue, appetite, sleep problems, concentration difficulties, irritability, depression, depressive ideas, anxiety, worry, panic, phobia, compulsive behaviours, obsessive thoughts, and somatic symptoms. It can be used to generate diagnostic categories according to the ICD-10, including diagnosis of depression.
2.4. Assessment of outcome measures
Participants completed self-administered validated questionnaires for depression, state and trait anxiety, perceived stress, pleasure, fatigue, quality of life, and subjective wellbeing. Total scores for each of these were used as main outcomes (continuous variables), calculated by summing individual item scores according to user manuals. For all questionnaires higher scores represented greater symptom severity, with the exceptions of subjective wellbeing and quality of life (see below). We used Cronbach's alpha to quantify internal consistency in the current study [11]; Cronbach's alpha of ≥0.90 was considered as excellent, 0.8–0.9 as good, 0.7–0.8 as acceptable, 0.6–0.7 as questionable, 0.5–0.6 as poor, and <0.5 as unacceptable.
2.4.1. Depressive symptoms
Depressive symptoms were assessed using the BDI-II [1]. Each item on this 21-item questionnaire was coded on a 4-point scale ranging from 0 to 3 giving a total score of 0–63. Cronbach's alpha for the BDI-II was 0.88, indicating high internal consistency in this sample. In addition to total score, we created a categorical variable representing different degrees of depression severity using established thresholds for the total score as follows: 0–13 = minimal/no depression, 14–19 = mild depression, 20–28 = moderate depression, and 29–63 = severe depression [1,50]. Using the BDI-II cut-off of 14, sensitivity and specificity for depression were reported as 87.7% and 83.9% respectively in adults in primary care [50]. A cut-off of 20 has similar sensitivity and specificity for depression, 82% and 75% respectively, as reported in a sample of adult outpatients [50].
Furthermore, we calculated a somatic symptom score by summing seven relevant BDI-II items based on current literature suggesting a link between inflammation and somatic symptoms of depression [9,24,39], specifically: 4 = lack of pleasure, 15 = loss of energy, 16 = changes in sleeping pattern, 18 = changes in appetite, 19 = concentration difficulty, 20 = tiredness or fatigue, and 21 = loss of interest in sex. Cronbach's alpha for the somatic symptom score was 0.69, indicating acceptable internal consistency in this sample. For symptom-based analyses, we recoded each symptom as a binary variable by recoding scores 0 and 1 to represent no/mild symptoms and scores 2 and 3 to represent moderate/severe symptoms. In depression, sleep and appetite changes can occur in either direction. Therefore, in addition to a composite variable reflecting change, we created separate variables to represent an increase or decrease in both sleep and appetite.
2.4.2. Anxiety, stress, pleasure, and fatigue
The State-Trait Anxiety Inventory (STAI) [47] was used to measure state (STAI-S) and trait (STAI-T) anxiety. Both questionnaires demonstrated good internal reliability with a Cronbach's alpha of 0.92 and 0.88, respectively. Participants were presented with two 20-item questionnaires to assess these two forms of anxiety. Responses were recorded on a 4-point scale ranging from 1 to 4, giving a total score of 20–80 per questionnaire. Stress was assessed using the Perceived Stress Scale-10 (PSS) [10], a 10-item questionnaire where each item is coded as 0 to 4 with a total score of 0–40 (Cronbach's α = 0.83). The Snaith-Hamilton Pleasure Scale (SHAPS) [46] was used to measure anhedonia. Items on this 14-item scale were coded as 0 = agree and 1 = disagree with a total score of 0–14 (Cronbach's α = 0.82). Fatigue was assessed using the 20-item Multidimensional Fatigue Inventory (MFI) [45]. Item scores were coded on a 5-point scale ranging from 1 to 5 with a total score of 20–100 (Cronbach's α = 0.91). Higher scores indicated greater fatigue severity. The MFI evaluates five dimensions of fatigue, namely general fatigue (Cronbach's α = 0.71), physical fatigue (Cronbach's α = 0.84), mental fatigue (Cronbach's α = 0.84), reduced motivation (Cronbach's α = 0.63), and reduced activity (Cronbach's α = 0.86) with score ranges of 5–20. In addition to MFI total score, we calculated total scores for each of these five individual dimensions.
2.4.3. Subjective wellbeing and quality of life
Subjective well-being was assessed using the Visual Analogue Scales for Subjective Well-Being (VAS-W) [4]. Each item in this series of 16 analogue scales was assigned a score between 1 and 100, representing a participant's response to the item in question (Cronbach's α = 0.90). The VAS-W consists of three dimensions of wellbeing; alertness (Cronbach's α = 0.83), contentedness (Cronbach's α = 0.84), and calmness (Cronbach's α = 0.52, reflecting poor internal consistency for this dimension). Total scores for each dimension were calculated by summing the representative item scores for each scale.
Quality of life was assessed using the EQ-5D three-level version (EQ-5D-3L) [15]. The EQ-5D-3L assesses five dimensions of quality of life, namely, mobility, self-care, usual activities, pain/discomfort, and anxiety/depression (Cronbach's α = 0.65). Participants assigned each dimension a score ranging from 1 to 3, with higher scores indicating poorer quality of life. Combining these numbers in sequence resulted in a five-digit health state profile that represented the level of reported problems on each of the five dimensions. Health state profiles were converted into a single index value to reflect participants' overall quality of life, scored from around 0 to maximum 1, with 1 representing perfect health.
2.5. Assessment of covariates
We used self-report questionnaires to measure age, sex, ethnicity, relationship status, employment status, alcohol, tobacco and other drug use, physical comorbidity, number of previous depressive episodes, current antidepressant type and treatment duration. Body mass index (BMI) was calculated from height and weight, which were assessed during the study visit. Regression models were adjusted for age (years), sex (male/female), BMI, and type of current antidepressant medication (selective serotonin reuptake inhibitors/SSRIs or other) as these variables were statistically different between the two groups. Other covariates, including alcohol and tobacco use frequency, were not significantly different between groups, and thus were not adjusted for in the statistical models.
2.6. Statistical analysis
Statistical analyses were performed in R version 4.0.2 [43]. The sample comprised 84 participants, grouped as 40 with elevated CRP (≥3 mg/L) and 44 with low CRP (<3 mg/L). This sample size allowed 80% statistical power to detect moderate-to-large effect sizes of Cohen's d = 0.62 (two-sided tests; alpha = 0.05). Statistical significance was defined by P<0.05, with false discovery rate (FDR)-adjusted corrections for multiple comparisons for each set of analyses using the Benjamini-Hochberg method [3].
2.6.1. Sample characteristics
Sociodemographic and other characteristics were compared between two groups of patients with and without evidence of inflammation. Mean values for continuous variables were compared using independent samples t-test. For categorical variables, the proportion of participants between groups were compared using the Chi-squared test. Variables that violated the assumption of normality (i.e., BMI) were log-transformed before testing significance due to right skew.
2.6.2. Association of inflammation with psychiatric measures and quality of life
Independent samples t-test and analysis of covariance (ANCOVA) were used to assess mean difference in scores for depression, other psychiatric, and quality of life measures between groups before and after adjusting for potential confounders, including age, sex, BMI, and type of current antidepressant medication. For categorical variables (e.g., depression category), proportion of participants between groups were compared using Chi-squared test. Variables that violated the assumption of normality (i.e., MFI total score, EQ-5D-3L index total score, and MFI general fatigue score) were square-transformed before testing significance due to right skew.
2.6.3. Association between CRP and depressive symptoms
Logistic regression was used to estimate the odds ratio (OR) and 95% confidence interval (CI) for each BDI-II depressive symptom, coded as binary variables, for participants with evidence of inflammation compared to those without. Regression models were adjusted for age, sex, BMI, and current antidepressant medication type. Adjusted ORs (95% CIs) were visualised using forest plots.
3. Results
3.1. Sample characteristics
This study included 84 patients: 40 with evidence of low-grade inflammation (median hs-CRP = 7.32 mg/L; interquartile range = 4.45, 10.64) and 44 without (median hs-CRP = 0.66 mg/L; interquartile range = 0.38, 1.39). The group with evidence of inflammation was older (P = 0.04), had higher BMI (P<0.01), and was more likely to be on a non-SSRI antidepressant (P=0.04) (Table 1).
Table 1.
Characteristics of patients with current ICD-10 depressive episode and somatic symptoms included in the study (N=84).
| Characteristic | Evidence of inflammation |
Test statistic (P-valuea) | |
|---|---|---|---|
| Yes (hs-CRP ≥3mg/L) |
No (hs-CRP <3mg/L) |
||
| Sample, no. (%) | 40 (48) | 44 (52) | - |
| hs-CRP, mean (SD) | 8.22 (4.95) | 0.92 (0.75) | - |
| hs-CRP, median (IQR) | 7.32 (4.45, 10.64) | 0.66 (0.38, 1.39) | - |
| Age, mean (SD) | 41.30 (11.16) | 35.97 (12.08) | 2.10 (0.04) |
| Female sex, no. (%) | 28 (70) | 32 (73) | 0.08 (0.78) |
| BMI, mean (SD)b | 35.93 (8.07) | 25.62 (6.45) | 7.21 (<0.01)c |
| Ethnicity, no. (%) | |||
| White | 37 (93) | 41 (93) | 0.02 (0.90) |
| Other | 3 (7) | 3 (7) | |
| Relationship status, no. (%) | |||
| Relationship | 24 (60) | 25 (57) | 0.09 (0.77) |
| Single | 16 (40) | 19 (43) | |
| Employment status, no. (%) | |||
| Employed | 32 (80) | 31 (70) | 1.02 (0.31) |
| Unemployed | 8 (20) | 13 (30) | |
| Previous depressive episodes, no. (%) |
|||
| 2 or less | 10 (25) | 11 (25) | 0.02 (1.00) |
| 3 – 4 | 6 (15) | 7 (16) | |
| 5 or more | 16 (40) | 17 (39) | |
| Don’t know | 8 (20) | 9 (20) | |
| Current antidepressant duration, no. of months (SD)b | 20.84 (37.19) | 23.65 (38.26) | 0.34 (0.74) |
| Total past antidepressant medication, no. (%) | |||
| 1 | 18 (45) | 23 (52) | 4.69 (0.10) |
| 2 | 18 (45) | 11 (25) | |
| 3 or more | 4 (10) | 10 (23) | |
| Current antidepressant type, no. (%) |
|||
| SSRI | 25 (62) | 36 (82) | 3.93 (0.04) |
| Other | 15 (38) | 8 (18) | |
| Alcohol use frequency, no. (%) | |||
| Never | 12 (30) | 8 (18) | 2.60 (0.27) |
| Monthly/Yearly | 19 (48) | 20 (46) | |
| More than once per week | 9 (22) | 16 (36) | |
| Tobacco use frequency, no. (%) | |||
| Never | 28 (70) | 26 (59) | 2.21 (0.33) |
| Less than daily | 3 (8) | 8 (18) | |
| Daily | 9 (22) | 10 (23) | |
| Other drug use, no. (%) | 6 (15) | 13 (30) | 2.53 (0.11) |
| Physical comorbidity, no. (%)d | |||
| 0 | 9 (26) | 18 (41) | 2.31 (0.31) |
| 1 | 13 (37) | 15 (34) | |
| 2 or more | 13 (37) | 11 (25) | |
ICD-10, International Classification of Diseases 10th Revision; hs-CRP, high-sensitivity C-reactive protein; SD, standard deviation; IQR, interquartile range; BMI, body mass index; SSRI, selective serotonin reuptake inhibitors.
Mean values for continuous variables were compared using independent samples t-test; For categorical variables proportion of participants between groups were compared using Chi-squared test.
N=83
Variables that violated the assumption of normality (i.e., BMI) were log-transformed before testing significance due to right skew.
N=79
3.2. Association of inflammation with psychiatric measures
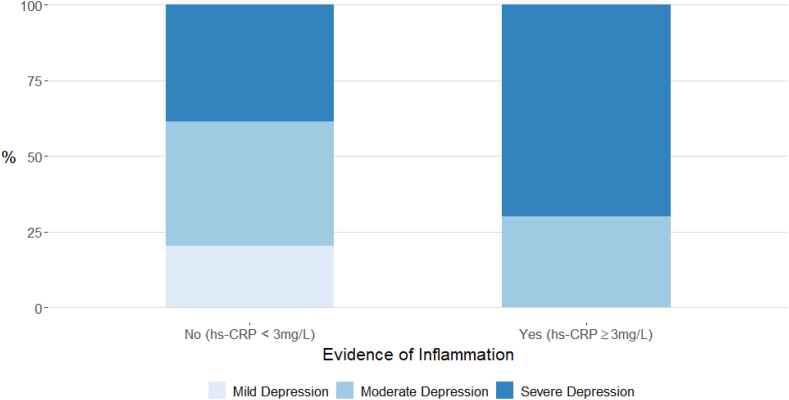
The group with inflammation had higher BDI-II total scores (adjusted mean difference, 8.82; 95% CI, 3.91, 13.72; adjusted P=0.02), somatic symptom scores (adjusted mean difference, 3.25; 95% CI, 1.58, 4.92; adjusted P=0.02), state anxiety (adjusted mean difference, 9.25; 95% CI, 3.82, 14.67; adjusted P=0.02), perceived stress (adjusted mean difference, 4.58; 95% CI, 1.98, 7.18; adjusted P=0.02) (Table 2), and increased depression severity (χ2 = 7.78; adjusted P=0.03) (Fig. 1). The group with inflammation also had increased scores for total fatigue (adjusted mean difference, 9.71; 95 % CI, 3.09, 6.33; adjusted P=0.02), general fatigue (adjusted mean difference, 1.82; 95 % CI, 0.51, 3.13; adjusted P=0.03), and physical fatigue (adjusted mean difference, 3.99; 95 % CI, 1.99, 5.98; adjusted P=0.03) (Table 3). The inflamed group also had significantly higher scores for mental fatigue (unadjusted mean difference, 1.73; 95 % CI, 0.32, 3.13), reduced motivation (unadjusted mean difference, 1.76; 95 % CI, 0.51, 3.01), and reduced activity (unadjusted mean difference, 2.61; 95 % CI, 0.82, 4.40) in the unadjusted analysis.
Table 2.
Affective symptoms, subjective wellbeing, and quality of life measures in depressed patients with somatic symptoms, grouped as with or without inflammation based on CRP.
|
Measures |
Evidence of inflammation |
Mean Difference (95% CI)a |
T-valueb (P-value) for final model | CorrectedcP-value for final model | |||
|---|---|---|---|---|---|---|---|
| Yes (n = 40; hs-CRP ≥3 mg/L) Mean (SD) |
No (n = 44; hs-CRP <3 mg/L) Mean (SD) |
Unadjusted | Adjusted for age, sex, BMI | Additional adjustment for antidepressant type | |||
| BDI-II total score | 34.63 (9.02) | 27.55 (9.19) | 7.08 (3.12, 11.04) | 9.25 (4.22, 14.29) | 8.82 (3.91, 13.72) | 3.58 (<0.01) | 0.02 |
| BDI-II somatic symptom score | 12.23 (2.96) | 9.16 (3.26) | 3.07 (1.71, 4.42) | 3.40 (1.69, 5.11) | 3.25 (1.58, 4.92) | 3.88 (<0.01) | 0.02 |
| Trait anxiety (STAI-T) total score | 62.50 (7.73) | 60.11 (9.21) | 2.39 (−1.33, 6.10) | 4.21 (−0.51, 8.94) | 4.05 (−0.69, 8.80) | 1.70 (0.09) | 0.11 |
| State anxiety (STAI-S) total score | 60.00 (10.12) | 53.27 (9.58) | 6.73 (2.45, 11.00) | 9.45 (4.04, 14.86) | 9.25 (3.82, 14.67) | 3.39 (<0.01) | 0.02 |
| PSS total score | 28.53 (4.65) | 25.18 (5.26) | 3.34 (1.18, 5.51) | 4.82 (2.15, 7.48) | 4.58 (1.98, 7.18) | 3.51 (<0.01) | 0.02 |
| SHAPS total score | 5.35 (3.49) | 4.14 (3.46) | 1.21 (−0.30, 2.72) | 1.02 (−0.88, 2.92) | 0.87 (−1.00, 2.73) | −0.93 (0.36) | 0.36 |
| MFI total score | 82.75 (9.52) | 70.45 (13.96) | 12.30 (7.14, 17.45) | 10.17 (3.47, 16.86) | 9.71 (3.09, 16.33) | 2.92 (<0.01)d | 0.02 |
| EQ-5D-3L total index score | 0.49 (0.29) | 0.69 (0.22) | −0.20 (−0.32, −0.09) | −0.20 (−0.34, −0.60) | −0.18 (-0.32, -0.05) | 2.89 (<0.01)d | 0.02 |
| VAS-W alertness total score | 61.64 (10.58) | 57.05 (10.66) | 4.59 (−0.03, 9.21) | 5.43 (−0.54, 11.39) | 4.90 (−0.91, 10.70) | 0.68 (0.10) | 0.11 |
| VAS-W contentedness total score | 62.54 (13.20) | 56.93 (12.12) | 5.61 (0.11, 11.10) | 7.03 (0.01, 14.05) | 6.43 (−0.43, 13.28) | 1.87 (0.07) | 0.09 |
| VAS-W calmness total score | 33.78 (8.52) | 41.45 (6.75) | −7.68 (−11.00, −4.36) | −8.64 (−12.88, −4.40) | −8.48 (-12.73, -4.22) | 3.97 (<0.01) | 0.02 |
hs-CRP, high-sensitivity C-reactive protein; SD, standard deviation; BMI, body mass index; BDI-II, Beck Depression Inventory II; STAI-T, State-Trait Anxiety Inventory – Trait; STAI-S, State-Trait Anxiety Inventory – State; PSS, Perceived Stress Scale-10; SHAPS, Snaith-Hamilton Pleasure Scale; MFI, Multidimensional Fatigue Inventory; EQ-5D-3L, EQ-5D three-level version; VAS-W, Visual Analogue Scales for Subjective Well-Being.
Total sample for adjusted models is N = 83.
Mean values for continuous variables were compared using independent samples t-test and ANCOVA.
P-values corrected for multiple testing using Benjamini & Hochberg’s False Discovery Rate method.
Variables that violated the assumption of normality (i.e., MFI total score and EQ-5D-3L index total score) were square-transformed before testing significance due to left skew.
Fig. 1.
Cases of ICD-10 current mild, moderate, and severe depressive episode grouped by serum hs-CRP level.
ICD-10, International Classification of Diseases 10th Revision; hs-CRP, high-sensitivity C-reactive protein.
Table 3.
Fatigue dimension scores in depressed patients with somatic symptoms, grouped as with or without inflammation based on CRP.
|
MFI Fatigue Dimensions |
Evidence of inflammation |
Mean Difference (95% CI)a |
Test Statisticb (P-value) for final model | CorrectedcP-value for final model | |||
|---|---|---|---|---|---|---|---|
| Yes (n = 40; Mean (SD) |
No (n = 44; Mean (SD) |
Unadjusted | Adjusted for age, sex, BMI | Additional adjustment for antidepressant type | |||
| General fatigue | 18.40 (1.43) | 16.55 (2.94) | 1.86 (0.84, 2.87) | 1.88 (0.57, 3.19) | 1.82 (0.51, 3.13) | 2.85 (<0.01)d | 0.03 |
| Physical fatigue | 16.80 (2.78) | 12.45 (4.20) | 4.35 (2.78, 5.91) | 4.12 (2.10, 6.13) | 3.99 (1.99, 5.98) | 3.98 (<0.01) | 0.03 |
| Mental fatigue | 15.60 (2.54) | 13.84 (3.16) | 1.73 (0.32, 3.13) | 1.44 (−0.34, 3.23) | 1.35 (−0.43, 3.13) | 1.51 (0.14) | 0.20 |
| Reduced motivation | 15.23 (3.54) | 12.61 (4.59) | 1.76 (0.51, 3.01) | 1.00 (−0.60, 2.59) | 0.90 (−0.68, 2.49) | 1.14 (0.26) | 0.26 |
| Reduced activity | 16.73 (2.78) | 15.00 (3.58) | 2.61 (0.82, 4.40) | 1.73 (−0.56, 4.02) | 1.65 (−0.64, 3.95) | 1.43 (0.16) | 0.20 |
MFI, Multidimensional Fatigue Inventory; hs-CRP, high-sensitivity C-reactive protein; SD, standard deviation; BMI, body mass index.
Total sample for adjusted models was N = 83.
Mean values for continuous variables were compared using independent samples t-test and ANCOVA.
P-values corrected for multiple testing using Benjamini & Hochberg’s False Discovery Rate method.
Variables that violated the assumption of normality (i.e., MFI general fatigue) were square-transformed before testing significance due to left skew.
3.3. Association of inflammation with subjective wellbeing and quality of life
The group with inflammation had lower scores for calmness on the VAS-W (adjusted mean difference, −8.48; 95 % CI, −12.73, −4.22; adjusted P=0.02), and poorer quality of life (adjusted mean difference, −0.18; 95 % CI, −0.32, −0.05; adjusted P=0.02) (Table 2).
3.4. Association between CRP and individual depressive symptoms
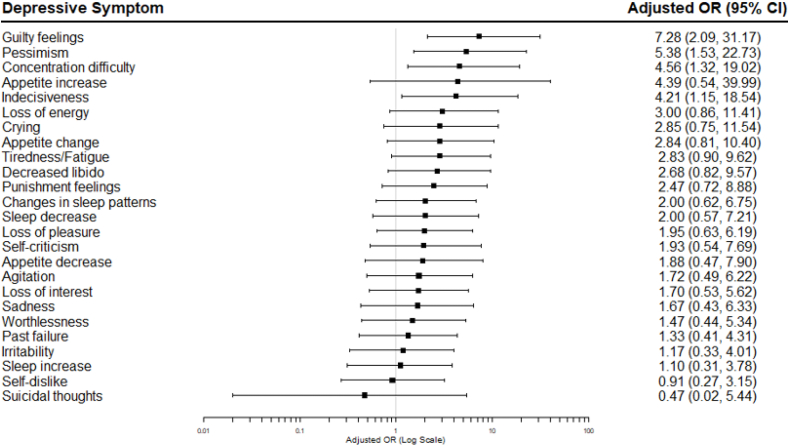
At symptom level, the group with inflammation had higher odds for guilty feelings (adjusted OR, 7.28; 95% CI, 2.09, 31.17; unadjusted P<0.01), pessimism (adjusted OR, 5.38; 95% CI, 1.53, 22.73; unadjusted P=0.01), concentration difficulty (adjusted OR, 4.56; 95% CI, 1.32, 19.02; unadjusted P=0.02), and indecisiveness (adjusted OR, 4.21; 95% CI, 1.15, 18.54; unadjusted P=0.04) (Table 4; Fig. 2). However, these no longer remained significant following FDR correction. The group with inflammation also had higher odds for loss of energy (unadjusted OR, 3.00; 95% CI, 1.21,7.83), appetite change (unadjusted OR, 4.07; 95% CI, 1.56, 11.42), tiredness/fatigue (unadjusted OR, 2.92; 95% CI, 1.22, 7.23), punishment feelings (unadjusted OR, 3.52; 95% CI, 1.45, 9.19), loss of pleasure (unadjusted OR, 2.95; 95% CI, 1.23, 7.33), and appetite decrease (unadjusted OR, 4.20; 95% CI, 1.42, 14.30) in the unadjusted analysis, before FDR correction. It is worth noting that after adjusting for potential confounders, while the point estimates remained similar, confidence intervals widened and included the null. The inflammation group also showed higher odds for appetite increase (adjusted OR, 4.21; CI, 0.54, 39.99), but sample sizes for this symptom were low and the difference between groups was not significant.
Table 4.
Odds Ratios (95% CI) for individual depressive symptoms in depressed patients with somatic symptoms and evidence of inflammation.
| Depressive Symptom | Evidence of inflammation |
Odds Ratio (95% CI) for Depressive Symptoma |
Test Statistic (P-value) | CorrectedbP-value for final model | |||
|---|---|---|---|---|---|---|---|
| Yes (hs-CRP ≥3 mg/L) N (%) with symptom |
No (hs-CRP <3 mg/L) N (%) with symptom |
Unadjusted | Adjusted for age, sex, BMI | Additional adjustment for antidepressant type | |||
| Guilty feelings | 28 (70) | 6 (14) | 4.08 (1.67, 10.48) | 7.33 (2.11, 31.31) | 7.28 (2.09, 31.17) | 2.93 (< 0.01) | 0.13 |
| Pessimism | 23 (58) | 15 (34) | 2.62 (1.09, 6.45) | 5.25 (1.54, 21.09) | 5.38 (1.53, 22.73) | 2.48 (0.01) | 0.13 |
| Concentration difficulty | 28 (70) | 22 (50) | 2.33 (0.96, 5.86) | 4.61 (1.34, 19.19) | 4.56 (1.32, 19.02) | 2.27 (0.02) | 0.17 |
| Appetite increase | 5 (13) | 3 (7) | 1.95 (0.45, 10.07) | 4.38 (0.59, 36.21) | 4.39 (0.54, 39.99) | 1.36 (0.17) | 0.39 |
| Indecisiveness | 30 (75) | 26 (59) | 2.08 (0.83, 5.44) | 4.20 (1.18, 17.92) | 4.21 (1.15, 18.54) | 2.06 (0.04) | 0.25 |
| Loss of energy | 30 (75) | 22 (50) | 3.00 (1.21, 7.83) | 3.03 (0.89, 11.32) | 3.00 (0.86, 11.41) | 1.69 (0.09) | 0.34 |
| Crying | 15 (38) | 11 (25) | 1.80 (0.71, 4.68) | 2.73 (0.79, 10.23) | 2.85 (0.75, 11.54) | 1.53 (0.13) | 0.36 |
| Appetite change | 19 (48) | 8 (18) | 4.07 (1.56, 11.42) | 2.88 (0.84, 10.31) | 2.84 (0.81, 10.40) | 1.62 (0.10) | 0.34 |
| Tiredness/Fatigue | 25 (63) | 16 (36) | 2.92 (1.22, 7.23) | 2.86 (0.92, 9.47) | 2.83 (0.90, 9.62) | 1.74 (0.08) | 0.34 |
| Decreased libido | 23 (58) | 19 (43) | 1.78 (0.75, 4.28) | 2.71 (0.83, 9.64) | 2.68 (0.82, 9.57) | 1.59 (0.11) | 0.34 |
| Punishment feelings | 19 (48) | 9 (20) | 3.52 (1.38, 9.54) | 2.45 (0.72, 8.80) | 2.47 (0.72, 8.88) | 1.43 (0.15) | 0.38 |
| Changes in sleep patterns | 25 (63) | 22 (50) | 1.67 (0.70, 4.04) | 2.04 (0.65, 6.75) | 2.00 (0.62, 6.75) | 1.15 (0.25) | 0.48 |
| Sleep decrease | 13 (33) | 10 (23) | 1.64 (0.63, 4.39) | 2.03 (0.58, 7.28) | 2.00 (0.57, 7.21) | 1.09 (0.28) | 0.50 |
| Loss of pleasure | 26 (65) | 17 (39) | 2.95 (1.23, 7.33) | 1.98 (0.64, 6.27) | 1.95 (0.63, 6.19) | 1.15 (0.25) | 0.48 |
| Self-criticism | 28 (48) | 31 (53) | 0.98 (0.38, 2.52) | 1.96 (0.55, 7.81) | 1.93 (0.54, 7.69) | 0.99 (0.32) | 0.53 |
| Appetite decrease | 14 (35) | 5 (11) | 4.20 (1.42, 14.30) | 1.87 (0.46, 7.84) | 1.88 (0.47, 7.90) | 0.89 (0.37) | 0.54 |
| Agitation | 11 (28) | 12 (27) | 1.01 (0.38, 2.65) | 1.77 (0.52, 6.26) | 1.72 (0.49, 6.22) | 0.85 (0.40) | 0.56 |
| Loss of interest | 20 (50) | 17 (39) | 1.59 (0.67, 3.82) | 1.75 (0.57, 5.59) | 1.70 (0.53, 5.62) | 0.90 (0.37) | 0.54 |
| Sadness | 13 (33) | 8 (18) | 2.17 (0.80, 6.18) | 1.72 (0.47, 6.39) | 1.67 (0.43, 6.33) | 0.76 (0.45) | 0.59 |
| Worthlessness | 25 (63) | 28 (64) | 0.95 (0.39, 2.32) | 1.49 (0.45, 5.41) | 1.47 (0.44, 5.34) | 0.61 (0.54) | 0.68 |
| Past failure | 26 (65) | 23 (52) | 1.70 (0.71, 4.14) | 1.37 (0.43, 4.39) | 1.33 (0.41, 4.31) | 0.48 (0.63) | 0.72 |
| Irritability | 17 (43) | 15 (34) | 1.43 (0.59, 3.49) | 1.23 (0.37, 4.06) | 1.17 (0.33, 4.01) | 0.25 (0.81) | 0.88 |
| Sleep increase | 12 (30) | 12 (27) | 1.14 (0.44, 2.97) | 1.14 (0.33, 3.85) | 1.10 (0.31, 3.78) | 0.15 (0.88) | 0.89 |
| Self-dislike | 26 (65) | 28 (64) | 1.06 (0.43, 2.62) | 0.98 (0.30, 3.24) | 0.91 (0.27, 3.15) | −0.14 (0.89) | 0.89 |
| Suicidal thoughts | 2 (5) | 3 (7) | 0.72 (0.09, 4.57) | 0.53 (0.03, 5.74) | 0.47 (0.02, 5.44) | −0.57 (0.57) | 0.68 |
hs-CRP, high-sensitivity C-reactive protein; BMI, body mass index.
Total sample for adjusted models was N = 83.
P-values corrected for multiple testing using Benjamini & Hochberg's False Discovery Rate method.
Fig. 2.
Adjusted (i.e., age, sex, BMI, and current antidepressant medication type) Odds Ratios (95% CI) for individual depressive symptoms in depressed patients with somatic symptoms and evidence of inflammation.
OR, Odds ratio.
4. Discussion
We report the characteristics of depressed patients with somatic symptoms and evidence of inflammation, including an in-depth investigation into affective symptoms, fatigue, perceived stress, quality of life, and wellbeing. We replicated previous reports of inflammation being associated with higher depression severity. At symptom level, inflammation was found to be associated with both psychological and somatic symptoms of depression. Our work highlights particular domains of fatigue as being more strongly associated with inflammation, namely general and physical fatigue. We also show that depressed patients with somatic symptoms and evidence of inflammation have increased stress, lower subjective wellbeing, and poorer quality of life. These are clinically relevant findings that highlight the need for interventions targeting groups with inflammation-related depression.
Inflammation has previously been reported to be associated with various clinical features in patients with depression. Several studies have supported associations of higher CRP or IL-6 levels with increased depression severity [20,48]. A study of 231 depressed patients reported that prevalence of moderate/severe depressive episode was higher in those with elevated CRP (7–10 mg/L), as compared to those with low CRP (<1 mg/L) [31]. Our results for higher BDI-II total score and higher prevalence of categorically defined ICD-10 moderate/severe depression in the group with evidence of inflammation are consistent with these studies.
Using the BDI-II, we calculated a somatic symptom score comprising fatigue, loss of energy, anhedonia, changes in sleep or appetite, concentration difficulty, and decreased libido. This somatic symptom score was found to be higher in the group with inflammation-related depression, even though entry criteria required somatic symptoms to be present in all participants. Immune activation in cancer patients after treatment with interferon-alpha (IFN-α) has been shown to be associated with rapid development of somatic symptoms (namely fatigue and impaired sleep) in most patients, while other affective symptoms and cognitive dysfunction arise more slowly and in fewer patients [6,37]. Similarly, population-based studies have reported that elevated IL-6 and CRP levels are associated with fatigue and sleep disturbance, but not with psychological symptoms, such as hopelessness [24,38]. Similar findings have also been reported from the NESDA cohort, where inflammatory markers were found to be specifically associated with somatic symptoms of depression, such as fatigue, weight gain, and sleep disturbance [13]. However, in our symptom-level analysis, we found that elevated CRP was associated with both somatic (e.g., fatigue, loss of energy, concentration difficulties) and psychological symptoms (e.g., guilty feelings, pessimism, indecisiveness), before FDR correction. This could be due to overall higher depression severity in our sample, which comprised individuals meeting ICD-10 criteria for current depressive episode. It is possible that during early stages of depression inflammation is more relevant for somatic symptoms, while in established/chronic illness it is associated with both somatic and psychological symptoms of the syndrome. Of note, the current study excluded participants with severe suicidal thoughts or wishes (i.e., BDI II item 9 score = 3, “I would kill myself if I had the chance”). This may have led to lower severity of suicidal thoughts in our sample and introduced a bias towards the null for the results for suicidality in our symptom-level analysis.
While an association between inflammation and fatigue is well established [12,33], previous studies have typically used single items on depression scales rather than providing an in-depth assessment of specific dimensions of fatigue. We used the multidimensional fatigue inventory to examine specific fatigue domains. Our findings highlight that elevated CRP is associated with overall higher fatigue scores in depressed individuals, the domains of general and physical fatigue being most strongly associated with inflammation. Mental fatigue (i.e., cognitive symptoms of fatigue) was significantly higher in the inflamed group in the unadjusted model. A recent study reported that higher TNF-α levels were associated with cognitive fatigue, but not somatic or psychosocial fatigue in depressed individuals [42]. Taken together, these results suggest that inflammation may exacerbate not only a general feeling of fatigue, but also, specifically, physical and possibly cognitive fatigue in depressed individuals with somatic symptoms.
Anxiety symptoms are common in depression [23,27], yet few studies have examined the association of inflammation with anxiety in people with depression. We assessed state and trait anxiety, showing that elevated CRP is more strongly associated with state anxiety in depressed individuals. Of note, we excluded individuals with a primary diagnosis of anxiety disorder and any anxiety symptoms reported were in addition to the participants’ primary diagnosis of depression. These results support findings of an anxious-depression phenotype reported at transition to depression in a large cohort of IFN-α-treated patients [52]. More recently, higher concentrations of CRP were also found to be associated with increased prevalence of anxiety symptoms [55]. However, this association was fully attenuated after adjusting for depressive symptoms. Taken together, these results suggest that CRP is associated with anxiety, but these associations are strongly related to the presence of underlying depression. The results for increased state anxiety in our sample are consistent with that for stress, subjective wellbeing, and quality of life measures. We report that depressed individuals with evidence of inflammation had higher perceived stress, lower calmness scores, and poorer quality of life. A previous study also reported that physical quality of life was lower in depressed patients with chronic inflammation [16]. Our findings, together with existing evidence, suggest that inflammation may negatively impact the overall standard of health, comfort, and happiness experienced by depressed individuals with somatic symptoms and highlight the need for intervention.
In our sample of depressed individuals with somatic symptoms, elevated CRP was not associated with anhedonia as measured by the Snaith Hamilton Pleasure Scale. These findings are at odds with previous experimental studies reporting an association between inflammation and anhedonia/pleasure perception. In non-human primates, chronic low-dose infusion of IFN-α has been reported to decrease striatal dopamine release and increase anhedonia-like behaviour [18]. In patients with depression, plasma CRP concentration was reported to be associated with left basal ganglia glutamate levels, which, in turn, was associated with psychomotor slowing and anhedonia [17,21]. In healthy volunteers, experimental immuno-activation was reported to alter activation of reward-related brain regions [5,22], including reduction in the ventral striatum responses to hedonic reward [7,14]. Therefore, replication of our findings in larger samples is required.
Strengths of this work include in-depth assessments for affective symptoms, including depression, anxiety, anhedonia, and fatigue, as well as measures for stress, subjective wellbeing, and quality of life. We adjusted regression models for several relevant confounders including age, sex, BMI, and current antidepressant type. The primary limitation of this study is the relatively small sample size, limiting statistical power. This reduced the power of identifying small effect sizes, particularly after correcting for confounding and multiple comparisons. Nevertheless, this deep phenotyping study provides a useful starting point for the characterisation of depressed patients with evidence of inflammation and somatic symptoms. Second, over 70% of our sample was female and over 90% was White, reducing potential generalisability of our findings. Moreover, only depressed patients with somatic symptoms were recruited and so this sample is not representative of all cases of depression. Fourth, lack of a control group means that we cannot compare our results with non-depressed individuals. However, it is already known that affective symptoms and stress are higher and subjective wellbeing and quality of life is lower in depressed patients [25,34]. This study adds to current evidence by showing that some of these features are particularly relevant to depressed patients with evidence of inflammation and somatic symptoms.
5. Conclusion
In conclusion, our findings highlight that depressed individuals with somatic symptoms and evidence of low-grade systemic inflammation may have a distinct clinical profile, which includes higher depression severity, physical fatigue, state anxiety, and stress levels, as well as poorer quality of life and subjective wellbeing. These results add to our understanding of the phenotypic profile of inflammation-related depression and could help inform selection of patients and selection of key outcome measures in future RCTs of immunotherapies for depression. Replication of our findings in larger and more diverse samples is required.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Role of the funding source
This work was funded by a Wellcome Trust fellowship to GMK (grant code: 201486/Z/16/Z). GMK also acknowledges funding support from Cambridgeshire and Peterborough NHS Foundation Trust R&D Department (Grant code: G101481), the BMA Foundation (J Moulton grant 2019); the MQ: Transforming Mental Health (grant code: MQDS17/40); and the Medical Research Council UK (grant codes: MC_PC_17,213 and MR/S037675/1). The BMA Foundation J Moulton grant supports ÉMF and the MRC grant MC_PC_17,213 supports JTP. NK is supported by the International Max Planck Research School of Translational Psychiatry (IMPRS-TP). The funding sources had no role in study design; collection, analysis, and interpretation of data; writing of the report; and the decision to submit the paper for publication.
CRediT authorship contribution statement
Éimear M. Foley: Methodology, Formal analysis, Investigation, Data curation, Writing – original draft, Visualization, Project administration. Joel T. Parkinson: Formal analysis, Investigation, Writing – review & editing. Nils Kappelmann: Formal analysis, Writing – review & editing. Golam M. Khandaker: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Acknowledgements
We would like to thank all study participants. We are grateful to former research assistant Bianca Oltean for their help with study approval, set up, and data collection. We are grateful to our colleagues at the University of Cambridge Department of Psychiatry (Sarah Ayerst, Professor Peter Jones, Professor Ed Bullmore, Dr Muzaffer Kaser, Dr Ben Perry); National Institute for Health Research (NIHR) East of England Clinical Research Network (Siobhan Campbell, Clare Fletcher); Cambridgeshire and Peterborough NHS Foundation Trust (Heidi Rice, Codie Fahey, Marian Shiyanbade, Siobhan Coleman, Dr Emanuele Osimo); Norfolk and Suffolk NHS Foundation Trust (Louise McCarthy, Dr Camilo Zapata, Dr Claire Dibben); and the NIHR Cambridge Biomedical Research Centre (Dr Lori Turner, Natalia Savinykh) for their support. This study was supported by the NIHR, specifically NIHR Cambridge Clinical Research Facility, and NIHR Cambridge Biomedical Research Centre.
Contributor Information
Éimear M. Foley, Email: ef423@medschl.cam.ac.uk.
Joel T. Parkinson, Email: jp820@medschl.cam.ac.uk.
Nils Kappelmann, Email: nils_kappelmann@psych.mpg.de.
Golam M. Khandaker, Email: golam.khandaker@bristol.ac.uk.
References
- 1.Beck A.T., Steer R.A., Brown G.K. 1996. Manual for the BDI-II. San Antonio, TX. [Google Scholar]
- 2.Bekhbat M., Chu K., Le N.-A., Woolwine B.J., Haroon E., Miller A.H., Felger J.C. Glucose and lipid-related biomarkers and the antidepressant response to infliximab in patients with treatment-resistant depression. Psychoneuroendocrinology. 2018;98:222–229. doi: 10.1016/j.psyneuen.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing yoav Benjamini. Yosef Hochberg Journal of the Royal Statistical Society . Series B ( Methodological ) 1995;57(No . 1):289–300. ( 1995), pp . 57. [Google Scholar]
- 4.Bond A., Lader M. The use of analogue scales in rating subjective feelings. Br. J. Med. Psychol. 1974;47:211–218. doi: 10.1111/j.2044-8341.1974.tb02285.x. [DOI] [Google Scholar]
- 5.Brydon L., Harrison N.A., Walker C., Steptoe A., Critchley H.D. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biol. Psychiatr. 2008;63:1022–1029. doi: 10.1016/j.biopsych.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capuron L., Gumnick J.F., Musselman D.L., Lawson D.H., Reemsnyder A., Nemeroff C.B., Miller A.H. Neurobehavioral effects of interferon-α in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 7.Capuron L., Pagnoni G., Drake D.F., Woolwine B.J., Spivey J.R., Crowe R.J., Votaw J.R., Goodman M.M., Miller A.H. Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Arch. Gen. Psychiatr. 2012;69:1044–1053. doi: 10.1001/archgenpsychiatry.2011.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carvalho L.A., Torre J.P., Papadopoulos A.S., Poon L., Juruena M.F., Markopoulou K., Cleare A.J., Pariante C.M. Lack of clinical therapeutic benefit of antidepressants is associated overall activation of the inflammatory system. J. Affect. Disord. 2013;148:136–140. doi: 10.1016/j.jad.2012.10.036. [DOI] [PubMed] [Google Scholar]
- 9.Chu A.L., Stochl J., Lewis G., Zammit S., Jones P.B., Khandaker G.M. Longitudinal association between inflammatory markers and specific symptoms of depression in a prospective birth cohort. Brain Behav. Immun. 2019;76:74–81. doi: 10.1016/j.bbi.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen S., Kamarck T., Mermelstein R. A global measure of perceived stress. J. Health Soc. Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 11.Cronbach L.J. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16:297–334. doi: 10.1007/BF02310555. [DOI] [Google Scholar]
- 12.Dantzer R., Heijnen C.J., Kavelaars A., Laye S., Capuron L. The neuroimmune basis of fatigue. Trends Neurosci. 2014;37:39–46. doi: 10.1016/j.tins.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duivis H.E., Vogelzangs N., Kupper N., de Jonge P., Penninx B.W.J.H., Jonge P. De, Penninx B.W.J.H. Differential association of somatic and cognitive symptoms of depression and anxiety with inflammation : findings from The Netherlands Study of Depression and Anxiety ( NESDA ) Psychoneuroendocrinology. 2013;38:1573–1585. doi: 10.1016/j.psyneuen.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Eisenberger N.I., Berkman E.T., Inagaki T.K., Rameson L.T., Mashal N.M., Irwin M.R. Inflammation-Induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol. Psychiatr. 2010;68:748–754. doi: 10.1016/j.biopsych.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.EuroQol Research Foundation . 2018. EQ-5D-3L User Guide. [Google Scholar]
- 16.Faugere M., Micoulaud-Franchi J., Faget-Agius C., Lançon C., Cermolacce M., Richieri R. Quality of life is associated with chronic inflammation in depression. A cross-sectional study. 2018;227:494–497. doi: 10.1016/j.jad.2017.11.061. [DOI] [PubMed] [Google Scholar]
- 17.Felger J.C., Li Z., Haroon E., Woolwine B.J., Jung M.Y., Hu X., Miller A.H. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol. Psychiatr. 2016;21:1358–1365. doi: 10.1038/mp.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Felger J.C., Mun J., Kimmel H.L., Nye J.A., Drake D.F., Hernandez C.R., Freeman A.A., Rye D.B., Goodman M.M., Howell L.L., Miller A.H. Chronic interferon-a decreases dopamine 2 receptor binding and striatal dopamine release in association with anhedonia-like behavior in nonhuman primates. Neuropsychopharmacology. 2013;38:2179–2187. doi: 10.1038/npp.2013.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haapakoski R., Mathieu J., Ebmeier K.P., Alenius H., Kivimäki M. Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav. Immun. 2015;49:206–215. doi: 10.1016/j.bbi.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Häfner S., Baghai T.C., Eser D., Schüle C., Rupprecht R., Bondy B., Bedarida G., Schacky C. von. C-reactive protein is associated with polymorphisms of the angiotensin-converting enzyme gene in major depressed patients. J. Psychiatr. Res. 2008;42:163–165. doi: 10.1016/j.jpsychires.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Haroon E., Fleischer C.C., Felger J.C., Chen X., Woolwine B.J., Patel T., Hu X.P., Miller A.H. Conceptual convergence: increased inflammation is associated with increased basal ganglia glutamate in patients with major depression. Mol. Psychiatr. 2016;21:1351–1357. doi: 10.1038/mp.2015.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrison N.A., Cercignani M., Voon V., Critchley H.D. Effects of inflammation on Hippocampus and substantia nigra responses to novelty in healthy human participants. Neuropsychopharmacology. 2015:831–838. doi: 10.1038/npp.2014.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirschfeld R.M.A. The comorbidity of major depression and anxiety disorders: recognition and management in primary care. Prim. Care Companion J. Clin. Psychiatry. 2001;3:244–254. doi: 10.4088/pcc.v03n0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jokela M., Virtanen M., Batty G.D., Kivimäki M. Inflammation and specific symptoms of depression. JAMA Psychiatry. 2016;73:87. doi: 10.1001/jamapsychiatry.2015.1977. [DOI] [PubMed] [Google Scholar]
- 25.Kanter J.W., Busch A.M., Weeks C.E., Landes S.J. The nature of clinical depression: symptoms, syndromes, and behavior analysis. Behav. Anal. 2008;31:1–21. doi: 10.1007/BF03392158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kappelmann N., Lewis G., Dantzer R., Jones P.B., Khandaker G.M. Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol. Psychiatr. 2018;23:335–343. doi: 10.1038/mp.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kennedy S.H. Core symptoms of major depressive disorder: relevance to diagnosis and treatment. Dialogues Clin. Neurosci. 2008;10:271–277. doi: 10.31887/dcns.2008.10.3/shkennedy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khandaker G.M., Oltean B.P., Kaser M., Dibben C.R.M., Ramana R., Jadon D.R., Dantzer R., Coles A.J., Lewis G., Jones P.B. Protocol for the insight study: a randomised controlled trial of single-dose tocilizumab in patients with depression and low-grade inflammation. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2018-025333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khandaker G.M., Pearson R.M., Zammit S., Lewis G., Jones P.B. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life a population-based longitudinal study. JAMA Psychiatry. 2014;71:1121–1128. doi: 10.1001/jamapsychiatry.2014.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khandaker G.M., Zammit S., Burgess S., Lewis G., Jones P.B. Association between a functional interleukin 6 receptor genetic variant and risk of depression and psychosis in a population-based birth cohort. Brain Behav. Immun. 2018;69:264–272. doi: 10.1016/j.bbi.2017.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Köhler-Forsberg O., Buttenschøn H.N., Tansey K.E., Maier W., Hauser J., Dernovsek M.Z., Henigsberg N., Souery D., Farmer A., Rietschel M., McGuffin P., Aitchison K.J., Uher R., Mors O. Association between C-reactive protein (CRP) with depression symptom severity and specific depressive symptoms in major depression. Brain Behav. Immun. 2017;62:344–350. doi: 10.1016/j.bbi.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 32.Köhler-Forsberg O., Lydholm C.N., Hjorthøj C., Nordentoft M., Mors O., Benros M.E. Efficacy of anti-inflammatory treatment on major depressive disorder or depressive symptoms: meta-analysis of clinical trials. Acta Psychiatr. Scand. 2019;139:404–419. doi: 10.1111/acps.13016. [DOI] [PubMed] [Google Scholar]
- 33.Lee C.H., Giuliani F. The role of inflammation in depression and fatigue. Front. Immunol. 2019;10:1696. doi: 10.3389/fimmu.2019.01696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lenox-Smith A., Macdonald M.T.B., Reed C., Tylee A., Peveler R., Quail D., Wildgust H.J. Quality of life in depressed patients in UK primary care: the FINDER study. Neurol. Ther. 2013;2:25–42. doi: 10.1007/s40120-013-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewis G. Assessing psychiatric disorder with a human interviewer or a computer. J. Epidemiol. Community Health. 1994;48:207–210. doi: 10.1136/jech.48.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Y., Blackwood D.H., Caesar S., de Geus E.J.C., Farmer A., Ferreira M.A.R., Ferrier I.N., Fraser C., Gordon-Smith K., Green E.K., Grozeva D., Gurling H.M., Hamshere M.L., Heutink P., Holmans P.A., Hoogendijk W.J., Hottenga J.J., Jones L., Jones I.R., Kirov G., Lin D., McGuffin P., Moskvina V., Nolen W.A., Perlis R.H., Posthuma D., Scolnick E.M., Smit A.B., Smit J.H., Smoller J.W., St Clair D., van Dyck R., Verhage M., Willemsen G., Young A.H., Zandbelt T., Boomsma D.I., Craddock N., O’Donovan M.C., Owen M.J., Penninx B.W.J.H., Purcell S., Sklar P., Sullivan P.F., Wellcome Trust Case-Control Consortium Meta-analysis of genome-wide association data of bipolar disorder and major depressive disorder. Mol. Psychiatr. 2011 doi: 10.1038/mp.2009.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyers C.A., Albitar M., Estey E. Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer. 2005;104:788–793. doi: 10.1002/cncr.21234. [DOI] [PubMed] [Google Scholar]
- 38.Milaneschi Y., Kappelmann N., Ye Z., Lamers F., Moser S., Jones P.B., Burgess S., Penninx B.W.J.H., Khandaker G.M. Association of inflammation with depression and anxiety: evidence for symptom-specificity and potential causality from UK biobank and NESDA cohorts. medRxiv. 2021 doi: 10.1101/2021.01.08.20248710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milaneschi Y., Lamers F., Berk M., Penninx B.W.J.H. Depression heterogeneity and its biological underpinnings: toward immunometabolic depression. Biol. Psychiatr. 2020;88:369–380. doi: 10.1016/j.biopsych.2020.01.014. [DOI] [PubMed] [Google Scholar]
- 40.Osimo E.F., Baxter L.J., Lewis G., Jones P.B., Khandaker G.M. Prevalence of low-grade inflammation in depression: a systematic review and meta-Analysis of CRP levels. Psychol. Med. 2019;49:1958–1970. doi: 10.1017/S0033291719001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pearson T.A., Mensah G.A., Alexander R.W., Anderson J.L., Cannon R.O., Criqui M., Fadl Y.Y., Fortmann S.P., Hong Y., Myers G.L., Rifai N., Smith S.C., Taubert K., Tracy R.P., Vinicor F. Markers of inflammation and cardiovascular disease: application to clinical and public health practice. A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.CIR.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 42.Pedraz-Petrozzi B., Neumann E., Sammer G. Pro-inflammatory markers and fatigue in patients with depression: a case-control study. Sci. Rep. 2020;10:1–12. doi: 10.1038/s41598-020-66532-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.R Core Team . 2020. R: A Language Environment for Statistical Computing. [Google Scholar]
- 44.Raison C.L., Rutherford R.E., Woolwine B.J., Shuo C., Schettler P., Drake D.F., Haroon E., Miller A.H. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smets E.M., Garssen B., Bonke B., De Haes J.C. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J. Psychosom. Res. 1995;39:315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 46.Snaith R.P., Hamilton M., Morley S., Humayan A., Hargreaves D., Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br. J. Psychiatry. 1995;167:99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- 47.Speilberger C.D., Gorsuch R.L., Lushene R., Vagg P.R., Jacobs G.A. Consulting Psychologists Press; Palo Alto, CA: 1983. Manual for State-Trait Anxiety Inventory. [Google Scholar]
- 48.Stewart J.C., Rand K.L., Muldoon M.F., Kamarck T.W., Karmack T.W. A prospective evaluation OF the directionality OF the depression-inflammation relationship. Brain Behav. Immun. 2009;23:936–944. doi: 10.1016/j.bbi.2009.04.011.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang A.K., Miller B.J. Meta-analysis of cerebrospinal fluid cytokine and tryptophan catabolite alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder, and depression. Schizophr. Bull. 2018;44:75–83. doi: 10.1093/schbul/sbx035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y.P., Gorenstein C. Assessment of depression in medical patients: a systematic review of the utility of the Beck Depression Inventory-II. Clinics. 2013;68:1274–1287. doi: 10.6061/clinics/2013(09)15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weinberger J.F., Raison C.L., Rye D.B., Montague A.R., Woolwine B.J., Felger J.C., Haroon E., Miller A.H. Inhibition of tumor necrosis factor improves sleep continuity in patients with treatment resistant depression and high inflammation. Brain Behav. Immun. 2015;47:193–200. doi: 10.1016/j.bbi.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whale R., Fialho R., Field A.P., Campbell G., Tibble J., Harrison N.A., Rolt M. Factor analyses differentiate clinical phenotypes of idiopathic and interferon-alpha-induced depression. Brain Behav. Immun. 2019;80:519–524. doi: 10.1016/j.bbi.2019.04.035. [DOI] [PubMed] [Google Scholar]
- 53.Winer E.S., Jordan D.G., Collins A.C. Conceptualizing anhedonias and implications for depression treatments. Psychol. Res. Behav. Manag. 2019;12:325–335. doi: 10.2147/PRBM.S159260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wittenberg G.M., Stylianou A., Zhang Y., Sun Y., Gupta A., Jagannatha P.S., Wang D., Hsu B., Curran M.E., Khan S., Chen G., Bullmore E.T., Drevets W.C., Vértes P.E., Cardinal R., Richardson S., Leday G., Freeman T., Hume D., Regan T., Wu Z., Pariante C., Cattaneo A., Zunszain P., Borsini A., Stewart R., Chandran D., Carvalho L., Bell J., Souza-Teodoro L.H., Perry H., Harrison N., Jones D., Henderson R.B., Chen G., Bullmore E.T., Drevets W.C. Effects of immunomodulatory drugs on depressive symptoms: a mega-analysis of randomized, placebo-controlled clinical trials in inflammatory disorders. Mol. Psychiatr. 2020;25:1275–1285. doi: 10.1038/s41380-019-0471-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ye Z., Kappelmann N., Moser S., Smith G.D., Jones P.B., Khandaker G.M. Role of inflammation in depression and Anxiety : tests for disorder specificity , linearity and potential causality of association in the UK biobank. medRxiv. 2021:1–25. doi: 10.1101/2021.02.02.21250987. [DOI] [PMC free article] [PubMed] [Google Scholar]